BOVINE RESPIRATORY DISEASE: ACUTE UNDIFFERENTIATED BOVINE RESPIRATORY DISEASE
DEFINITION OF THE PROBLEM
A major problem which large animal clinicians commonly encounter is a group of cattle that are affected with an acute respiratory disease of uncertain diagnosis.
Acute undifferentiated bovine respiratory disease (UBRD) is characterized clinically by dyspnea, coughing, nasal discharge, varying degrees of depression, inappetence to anorexia, a fever ranging from 40–41°C, evidence of pneumonia on auscultation of the lungs, and a variable response to treatment. Some unexpected deaths may have occurred as the initial indication of the problem.
In most cases, pneumonia is the cause of the disease but determining the etiology is the formidable diagnostic problem. If lesions typical of any of the common diseases of the respiratory tract of cattle can be recognized clinically, like those of infectious bovine rhinotracheitis, then on a clinical basis a specific diagnosis can be made.
The affected group may be recently weaned beef calves in a farm feedlot; weaned beef calves or yearlings that have recently arrived in a feedlot; cattle that have been in the feedlot for varying periods of time; young growing cattle on summer pasture or mature cows that have recently been placed on a lush pasture; yearling or mature lactating dairy cattle; or a group of veal calves.
The morbidity rate can range from 10–50% depending on the age of animals affected, the immune status of the animals, the nature of the stressors involved and the nature of the disease. In a study of mortality ratios among US feedlots over a 5-year period (1994–1999), the relative risk of death attributable to respiratory tract diseases increased during most years of the study.1 Cattle entering the feedlots during 1999 had a significantly increased risk (relative risk, 1.46) of dying of respiratory tract diseases, compared with cattle entering in 1994. Respiratory tract diseases accounted for 57.1% of all deaths. Dairy cattle had a significantly increased risk of death of any cause, compared with beef steers, and beef heifers had a significantly increased risk of dying of respiratory tract diseases compared with steers.
In general, bovine respiratory disease accounts for 65–79% of the morbidity and 44–72% of mortality in feedlot cattle. In addition to direct costs associated with death loss and medical treatment, losses attributable to decreased growth performance associated with the incidence of bovine respiratory disease may also occur. Cattle with detectable lung lesions at slaughter had a reduced (0.08 kg/d) growth rate compared with cattle without lesions. Also, 68% of cattle with lung lesions at slaughter were never treated for bovine respiratory disease, suggesting that current methods of diagnosing bovine respiratory disease based on visual appraisal by feedlot pen riders may not always effectively identify sick animals. In general, medical treatment is more effective the earlier in the process it can be initiated. The limitations of identifying clinically affected animals that need therapy was a major factor in the development of metaphylactic use of antimicrobials.2
The primary goal of the clinician is to make the most accurate clinical diagnosis as rapidly as possible, based on the clinical and epidemiological findings that are identifiable on the farm, preferably when examining the animals on the first visit. Giving a prognosis and the formulation of rational and economic treatment that will minimize morbidity and mortality are the next goals. The outbreak may involve a herd of lactating dairy cows, and the selection of antimicrobials used can have a major impact on the length of time the milk from treated cows must be discarded. In any group situation, mass medication of each in-contact animal is a major consideration that will increase costs and must be balanced against the economic losses that might occur if all animals are not treated prophylactically. The clinical management of the outbreak, which includes treatment of the obvious cases and the prevention of new cases if possible, is dependent in part on the diagnosis. However, differentiation between the diseases based on clinical findings can be unreliable and it is usually necessary to begin antimicrobial therapy that will be effective against the bacterial pathogens most likely to be present. The specific cause may or may not be determined by laboratory examination or further clinical and epidemiological examination. Even after intensive clinical and laboratory investigation, the specific etiology will often not be determined and the clinician is left with a diagnosis of ‘acute undifferentiated respiratory disease of cattle’ or bovine respiratory disease.
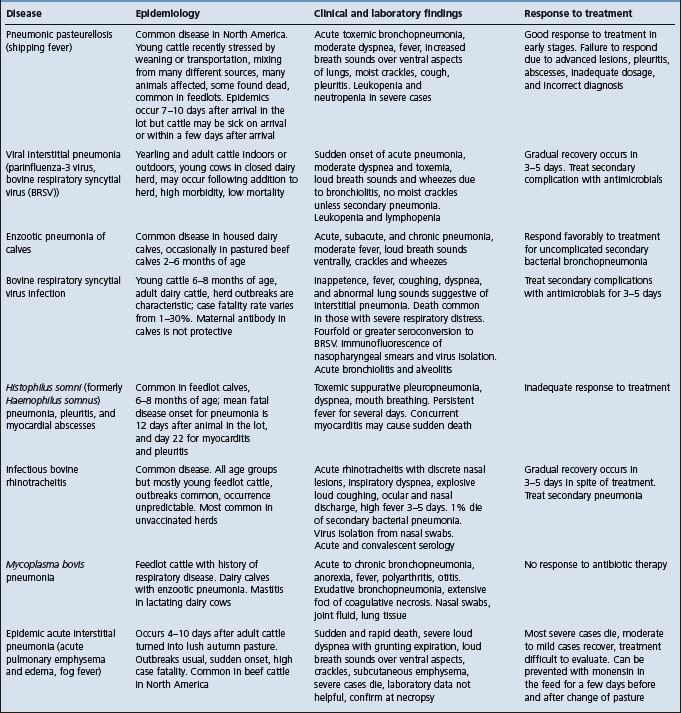
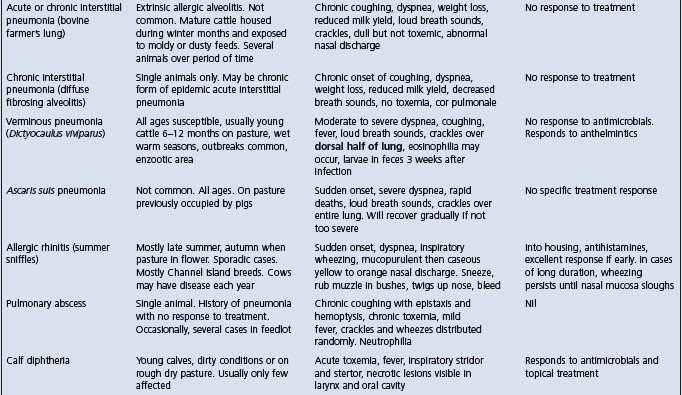
The salient clinical and epidemiological findings of the diseases included in the complex of bovine respiratory disease are summarized in Table 18.5. The common diseases of the respiratory tract of cattle can be broadly divided into those affecting the lower respiratory tract and those affecting the upper respiratory tract. Diseases of the lungs associated with either viruses or bacteria alone or in combination are difficult to distinguish from each other on the basis of clinical findings alone. Thus in a group of cattle with pulmonary disease it may be difficult to distinguish between pneumonic pasteurellosis, Haemophilus pleuropneumonia and the viral interstitial pneumonias with or without secondary bacterial infection. The presence of toxemia, which causes depression and anorexia in bacterial pneumonias, is a useful guide in categorizing the common diseases when making a differential diagnosis list. Cattle affected with uncomplicated viral diseases of the respiratory tract are usually not depressed and anorexic because bacterial toxemia is absent.
ETIOLOGY
The major etiological agents which cause or may be associated with acute UBRD include the following:
Role of etiological agents
The role of the etiological agents in the cause of acute undifferentiated respiratory disease in cattle has been controversial and often uncertain because the major pathogens of bovine respiratory disease are ubiquitous in clinically normal animals. The disease is considered to be the result of the effects of stressors causing immunosuppression, which allows colonization of the respiratory tract by opportunistic pathogens. The spectrum of the immune status of the animals is also a major factor. Animals vaccinated well before natural infection will usually be immune to specific pathogens. Animals that have had the natural infection and have adequate humoral immunity, or cell-mediated immunity in the case of some infections, will be immune to clinical disease.
Many epidemiological studies of bovine respiratory disease have attempted to correlate the level of serum antibodies in feedlot calves on arrival at the feedlot and over the first 30–50 days of the feeding period with morbidity and mortality due to bovine respiratory disease. A low level of antibody to a specific pathogen on arrival followed by significant seroconversion in animals that develop bovine respiratory disease in the first few weeks of the feeding period suggest that the pathogen was an important pathogen in the cause of cases of the disease. Conversely, those animals with a high level of antibody on arrival that do not develop bovine respiratory disease are considered immune. However, some animals with low levels of antibody may remain normal and seroconvert during the early part of the feeding period.
Feedlot cattle commonly seroconvert to the viruses of infectious bovine rhinotracheitis (IBRV), parainfluenza-3 virus (PI-3V), bovine virus diarrhea virus (BVDV), and bovine respiratory syncytial virus (BRSV),3 and to Mycoplasma bovis and Mycoplasma dispar, within the first month after arrival.4 Seroconversion to these pathogens occurs both in animals that develop respiratory disease and those that remain normal within the same group, but the relative importance of each agent and their causative nature is controversial. Seroconversion to M. haemolytica cytotoxin, BRSV and BVDV were predictive of approximately 70% of all respiratory disease cases in Ontario feedlots.3 Calves arriving in Ontario feedlots with high serum antibody levels to Histophilus somni (formerly Haemophilus somnus) had less bovine respiratory disease than calves with lower levels.5
Many respiratory pathogens that could cause disease are present in both individuals and groups of feedlot cattle after they are mixed and during outbreaks of bovine respiratory disease.3 Which ones are most important and causing disease is a major question. It has been suggested that undifferentiated bovine respiratory disease in weaned beef calves entering a feedlot is not a contagious disease and that, while respiratory pathogens may be necessary causes of the disease, they are not sufficient causes.3 This suggests that groups of animals experiencing a high incidence of bovine respiratory disease have been highly stressed and that it may be more important to identify and prevent the environmental and managemental risk factors.
The relationships between bacterial and viral antibody titers and undifferentiated fever and mortality in recently weaned beef calves in western Canada were examined.6 Feedlot calves are commonly exposed to M. haemolytica, H. somni, bovine herpesvirus (BHV)-1 G-IV glycoprotein, BVDV, M. bovis, and Mycoplasma alkalescens in the early feeding period.6 Seroconversion to M. haemolytica antileukotoxin was associated with a decreased risk of undifferentiated fever. Higher arrival BVDV antibody titer was associated with a decreased risk of undifferentiated fever. Higher arrival H. somni antibody titer and increases in H. somni antibody titer after arrival were both associated with a decreased risk of undifferentiated fever. The odds of overall mortality (OR 5.09) and hemophilosis mortality (OR 11.31) in clinical cases were higher than in the controls. In summary, protective immunity to M. haemolytica antileukotoxin H. somni, BHV-1 G-IV glycoprotein, BVDV, and Mycoplasma spp. may be necessary to reduce the occurrence of undifferentiated fever.
The association between exposure to H. somni and M. haemolytica and the risk of acute UBRD was examined using serological evidence of exposure and a vaccine field trial.7 Vaccination with H. somni in combination with M. haemolytica and with M. haemolytica alone reduced the risk of UBRD. There was no association between serological evidence of concurrent exposure to M. haemolytica and UBRD occurrence. Treated animals tend to have lower titers to H. somni compared to untreated animals.
Chronic, antibiotic-resistant pneumonia, sometimes with polyarthritis, occurs in feedlot cattle in western Canada.8,9 M. bovis, BVDV, and H. somni are commonly found in the tissues at necropsy. This coinfection suggests the possibility of synergism between the BVDV and M. bovis in the pneumonia with the arthritis syndrome.
BVDV has been identified as a contributor to respiratory disease in feedlot calves. On arrival in feedlots, 39% of animals were seropositive for BVDV, and those animals treated for UBRD had larger titer increases to the virus than nontreated animals.10 The virus has been demonstrated by experimental infections of the respiratory tract, isolation of the virus and/or identification of BVDV antigen in tissues and demonstration of active infection through seroconversion in groups of cattle with bovine respiratory disease. BVDV-1b strains have been associated with acute pneumonia in commingled calves that were not vaccinated with BVDV vaccines, and in which M. haemolytica and P. multocida were also present in the pneumonic lesions.11 Experimental infection of seronegative and immunocompetent calves with BVDV type resulted in primary respiratory disease.12
Bovine coronavirus (BCV) has been implicated as a cause of UBRD based largely on the isolation of the virus from the nasal cavities of cattle with respiratory disease. However, based on seroepidemiology of BCV titers in feedlot cattle, although higher antibody titers to the virus were associated with a decreased subsequent risk of treatment for UBRD, there was no association between evidence of recent infection (titer increase) and the occurrence of UBRD.13 Other studies have shown that BCV infections are not associated with an increased risk of treatment for UBRD.10
BCV is widespread in the cattle population and can be isolated from nasal swabs from cattle with bovine respiratory disease. The virus can be found in the feces and nasal swabs of recently arrived feedlot cattle and calves with and without clinical signs of bovine respiratory disease.14 Exposure to BCV prior to arrival in the feedlot is common, with 90% of animals being seropositive on arrival.10 BCV can be isolated from feedlot cattle in many different locations and most cattle seroconvert to the virus during the first 28 days after arrival in the feedlot.15 Cattle shedding the virus from the nasal cavity and developing an antibody response to the virus were 1.6 times more likely to require treatment for respiratory disease than cattle that did not shed the virus or develop an immune response. Cattle that shed the virus from the nasal cavity were 2.2 times more likely to have pulmonary lesions at slaughter than cattle that did not shed the virus.16 In natural outbreaks of shipping fever, more than 80% of affected cattle shed BCV at the beginning of the epidemic when the M. haemolytica infection rate was low.17
Intranasal vaccination of backgrounded feedlot cattle with a BCV vaccine reduced the risk of bovine respiratory disease.18 Vaccination had the greatest effect on calves with intranasal BCV, and on those with antibody titers less than 20, on entry.
The role of BCV in epidemics of shipping fever pneumonia in cattle was examined by the collection of nasal swabs and serum samples prior to the onset of the epidemic, during the course of the illness and after death when necropsies were done and samples of lung tissues were examined.19 Respiratory BCV (RBCV) was isolated from the nasal secretions before and after transport, from lung tissues of those cattle that died early in the epidemic but not later. Pasteurella spp. were isolated from all cattle that had severe pneumonia. All cattle were immunologically naive to both infectious agents at the onset of the epidemic but those that died after day 7 had rising antibody titers to RBCV and M. haemolytica. In contrast, the 18 clinically normal and RBCV isolation-negative cattle had high HAI antibody titers to RBCV from the beginning, while their antibody responses to M. haemolytica were delayed. Application of Evan’s criteria for causation were applied to the findings identified RBCV as the primary inciting cause in the epidemics. Because of the multifactorial nature of bovine respiratory disease, the criteria used are as follows.
Evan’s criteria for causation
1. The virus infects the mucosa of respiratory tract passages and lungs of affected cattle
2. The virus can be isolated in cell cultures at high rates from respiratory secretions and lung samples during the pathogenesis of bovine respiratory disease
3. Virus-specific immune responses are observed in cattle that recover from bovine respiratory disease. Rising titers of HAI antibodies against RBCV were detected in all surviving calves that had RBCV infections on days 0, 5 and later. They developed typical primary antibody responses to RBCV infections characterized by rises in IgG1 and IgG2. In contrast, the RBCV isolation-positive cattle with fatal outcomes had no or low titers of HAI antibodies against RBCV in the early stages of the epidemics. These cattle developed only initial IgM responses to RBCV infections before they died
4. Viruses isolated from cattle with bovine respiratory disease are not isolated from clinically normal cattle but they may be detected in the pathogenesis of other respiratory tract diseases
5. Cattle with significant antibody titers against the virus do not develop bovine respiratory disease, which occurs in cattle without such immune protection
6. Elimination of the virus factor prevents or decreases the severity of bovine respiratory disease
7. The whole thing should make biological and epidemiological sense.
CLINICAL CASE DEFINITION AND EPIDEMIOLOGY
Clinical case definition
The most important part of the clinical and epidemiological examination is to determine the case definition, which includes the following questions.
What is the clinical disease that is present in the affected animals?
• Which body system is affected and where in that body system is the lesion?
• Do the animals have pneumonia, rhinitis, laryngitis, tracheitis, bronchitis or combinations of these abnormalities?
The clinician should attempt to make a clinical diagnosis by closely examining several typically affected animals and determine if the lesions are in the lower or upper respiratory tract. The presence of toxemia, depression, fever, anorexia, and agalactia in lactating dairy cattle indicates a primary or secondary bacterial infection. The presence of loud breath sounds (consolidation) and abnormal lung sounds (crackles and wheezes) indicates the presence of pneumonia. Diseases of the upper respiratory tract are characterized by inspiratory dyspnea, stridor, loud coughing, sneezing, wheezing, and lesions of the nasal mucosa. When a single animal is involved, such as a dairy cow, or a group of dairy cows, a close physical examination, including auscultation of the lungs and inspection of the nasal mucosae and the larynx, is usually done.
In commercial feedlots, where large numbers of clinically affected animals are processed daily, and time is an economically important factor, the clinical examination of affected animals may be cursory and limited to taking the rectal temperature of animals that have been identified as sick based on recognition of depression and the absence of clinical findings suggestive of disease of some body system other than the respiratory tract. In such instances, if depressed animals have a rectal temperature above a predetermined level of, for example, 40°C, they are considered to have bovine respiratory disease if no other clinical findings are detectable that are referable to organ systems other than the respiratory system. In such cases, a diagnosis of acute undifferentiated fever of feedlot cattle has been suggested.20 In most cases, the thorax of these animals is not auscultated for evidence of pneumonia. As a result it is probable that the number of animals with respiratory disease is overestimated. Undoubtedly, some sick feedlot animals have a fever of undetermined origin unassociated with respiratory disease or any other inflammatory lesion. In a pen of recently arrived cattle in a feedlot, many animals that appear normal will have rectal temperatures above the critical temperature without any clinical evidence of bacterial infection that requires treatment. The elevated temperatures of these animals will return to within normal ranges within a few days.
The subjective clinical findings of distant examination that have been used by animal attendants in commercial feedlots to identify sick animals that need to be closely examined include: degree of ruminal fill (1, normal; 2, slightly gaunt; 3, moderately gaunt; 4, excessively gaunt), attitude (1, normal; 2, slight lethargy; 3, severe lethargy; 4, nonambulatory), ocular discharge (1, none; 2, slight; 3, moderate; 4, abundant), nasal discharge (1, none; 2, slight; 3, moderate; 4, abundant).
The sounds heard on auscultation of the lungs at three sites along a line extending from the cranioventral to caudodorsal lung fields (1, normal; 2, slightly harsh; 3, moderately harsh; 4, severely harsh).
The feeding and watering behavior of healthy and sick animals in a commercial feedlot has been examined using radiofrequency technology to record individual animal behaviors.21 Eating and drinking behaviors are associated with clinical signs of bovine respiratory disease but there is no obvious predictive association between signs of bovine respiratory disease in recently arrived weaned beef calves and eating and drinking behavior.22 Calves that were sick had greater frequency and duration of drinking 4–5 days after arrival than calves that were not sick. Sick calves had significantly lower frequency and duration of eating and drinking 11–27 days after arrival but had greater frequency of eating 28–57 days after arrival than calves that were not sick. Calves at slaughter that had a higher percentage of lung tissue with lesions had lower frequency and duration of eating 11–27 days after arrival but had greater frequency and duration of eating 28–57 days after arrival. Agreement of calves being sick and having pulmonary lesions at slaughter was adequate. Agreement for calves being removed and having pulmonary lesions at slaughter was low. Experimentally, the electronic acquisition of feeding behavior data for feedlot cattle, when analyzed using cumulative sums (CUSUM) procedures, offers the potential for predicting morbidity before conventional visual methods of appraisal. The feeding behavior during the first 30 days cattle are in a receiving pen may be used to detect animal morbidity approximately 4.1 days earlier than conventional methods typically employed in commercial feedlots.23 Overall accuracy, positive predictive value and sensitivity of the CUSUM prediction method were 87, 91, and 90%, respectively.23
There is a need to improve our clinical diagnostic techniques and to develop new ones that can be applied to making a rapid and accurate diagnosis beside the animal in the field situation. To base a diagnosis of acute respiratory disease on the presence of depression, which has a component of observer subjectivity, and a fever results in the unnecessary treatment of many animals, which is uneconomic and potentially promotes undesirable antimicrobial resistance and residues in milk and meat.
Who is affected?
This includes age of animals affected, a single animal or group of animals, and vaccination history. Recently arrived feedlot cattle mixed from many different origins are susceptible to fibrinous pneumonia associated with M. haemolytica.
Where are the affected animals?
Are they in the feedlot, on pasture, or housed in a barn with what quality of ventilation?
When were the animals affected?
• How soon after arrival in the feedlot did the animals become affected?
• What stressors may have recently preceded the outbreak?
• What risk factors could have predisposed to this outbreak?
• Have the animals been recently shipped and mixed with animals from another source?
Consideration of the clinical and epidemiological findings can then be correlated, and hypotheses formulated and tested to determine why the disease occurred.
Occurrence
Bovine respiratory disease occurs under many different situations, including all age groups, feedlot animals kept outdoors, housed dairy calves, nursing and recently weaned beef calves, dairy and beef cattle heifers, and adult lactating dairy cows. Epidemics of acute respiratory disease have been described in dairy calves from birth to 6 months of age.24,25 Outbreaks of BRSV can occur in dairy cattle heifers and adult dairy cattle.26
Pneumonic pasteurellosis is most common in recently arrived feedlot calves. In calves 3–5 weeks after arrival in the feedlot, H. somni pleuropneumonia may be more likely. Acute interstitial pneumonia (pasture-induced) is the most likely diagnosis when confronted with an outbreak of acute respiratory disease in mature beef cattle that have been moved from a summer pasture to a lush autumn pasture within the last 4–10 days. Acute pulmonary disease in a few head of feedlot animals several weeks or months after arrival in the lot is probably an acute interstitial pneumonia, which may be associated with 3-methlyindole (3MI) metabolites.27
Risk factors
The risk factors that have been identified in outbreaks of respiratory disease in feedlot cattle include the purchase of cattle from auction markets, whereby cattle arrive at the feedlot over an extended period of time, and mixing of cattle from many different sources.3 An epidemiological study of fatal fibrinous pneumonia in auction-market-derived feedlot calves in western Canada revealed that peak mortality occurred approximately 16 days after arrival at the feedlot.28 The risk of fatal fibrinous pneumonia was consistently greater for calves entering the feedlot in November, shortly after the auction sales had peaked, when the feedlot was reaching capacity.29 Mixing of calves from different farms was considerable, with a median of two calves per farm on truckloads arriving at the feedlot. Increased mixing at the auction markets was associated with increased fatal disease risk. The distance calves were transported by truck from the auction markets to the feedlot was not associated with fatal disease risk.3 When the incidence of fatal fibrinous pneumonia was high, the disease clustered within truckloads or pens. Risk factors positively associated with disease clustering included increased mixing of calves from different farms at the auction markets, month of purchase, number of calves passing through the auction markets, and weather conditions at arrival.28-31
Transportation of feedlot calves increases serum concentration of oxidative stress biomarkers, which are related to episodes of bovine respiratory disease.32 Transportation stress significantly decreases serum total antioxidant capacity and increases malondialdehyde concentrations in steer calves.33 It is proposed that stressors such as marketing (through an auction barn) and transportation to the feedlot precipitate oxidative stress, which reduces the antioxidant defense capacity and increases total body lipid peroxidation, resulting in increased susceptibility to bovine respiratory disease. These biomarkers may be useful to measure the oxidative stress of transported cattle. There is some experimental evidence that acidogenic diets and ketoacidosis may affect lymphocyte function, which may affect vaccine efficacy.34
The literature on how the adequacy of diets of recently arrived feedlot cattle may affect their health and immunity has been reviewed.35 Diets for newly arrived stressed beef cattle must be formulated to compensate for decreased feed intake and known nutrient deficiencies.
The literature on the risk factors for bovine respiratory disease in dairy heifers and the effect of the disease on productivity has been reviewed with relevance to commercial dairy farming in the Netherlands.26 Bovine respiratory disease in dairy heifers increases the risk of mortality directly after the disease episode by up to six times, reduces growth during the first 6 months of life by up to 10 kg, and increases the likelihood of dystocia after first calving. Both herd size and other diseases in dairy heifers are clearly associated with the risk of bovine respiratory disease. Season and colostrum feeding are important. The most important risk factors for mild and severe pneumonia in dairy calves aged birth to 3 months were inadequate air circulation and the purchase of cattle.24
An epidemic of acute respiratory disease associated with BRSV occurred during the winter and spring of 1995 in Norway.36 Data from 431 cattle herds were collected. The risk of acute respiratory disease occurring in cattle was related to herd size and type of production and an expressed interaction between these two variables. The risk of a herd outbreak in a mixed herd of 20 animals was estimated to be 1.7 times greater than in a dairy herd, and 3.3 times greater than in a beef herd of comparable size. On increasing herd size to 50 animals, the risk increased 1.3-fold for a mixed herd, 3.3-fold for a dairy herd and 2.1-fold for a beef herd, compared to a risk for a corresponding type of herd of 20 animals. The disease spread initially from one location to another during the first 6–9 weeks; the rate of transmission between neighboring farms seemed to be higher than for the other districts included in the study. It was hypothesized that one common source of infection was involved in the outbreak and the case herds were clustered in time as well as spatially.37 The average daily milk loss was estimated to be 0.70 kg per cow for 7 days after a herd outbreak compared with the period one week before.38
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
The clinician is limited in most situations to correlating clinical, epidemiological and necropsy findings in making a diagnosis. Diagnostic laboratories may not be readily available and their resources for microbiological and serological investigations may be much less than is needed for an accurate determination of causes. In the past, investigations of outbreaks of bovine respiratory disease have been incomplete and the interpretation of the findings almost impossible because one or more pieces of information were missing.
A number of important factors contribute to the difficulty of unraveling the etiologies in field outbreaks of respiratory disease. A review of the literature on the morbidity and mortality rates and disease occurrence in North American feedlots found differences in the definition of the terms used that makes the reports difficult to compare.39 In addition, the case definition or the clinical diagnosis is invariably inadequately defined. In feedlots, the morbidity rate will range from 15–45% of cattle within 3 weeks after arrival and the population mortality rate varies from 1–5%. Respiratory diseases account for about 75% of the diseases. Three important items are to be considered:
A systematic method of data collection from the customized records of large feedlots has been developed and validated for use in the National Animal Health Monitoring System. The current collection of data from large feedlots provides an acceptable level of sensitivity and specificity for the program but it is important that the veterinarian makes regular clinical observations to validate the data.
The course of the disease, especially when animals have been treated, alters the gross and microscopic appearance of tissues and the microbiological (bacteriological, virological) and serological findings so that the animal’s status is impossible to determine.
CLINICAL PATHOLOGY
Bacterial culture and antimicrobial sensitivity
Nasal swabs taken from clinical cases before treatment often yield a pure culture of pasteurellas but M. haemolytica biotype A serotype 1 is the most common isolate obtained from cattle with acute pneumonic pasteurellosis. The same serotype can usually be isolated from in-contact and apparently healthy calves. The antimicrobial sensitivity of the pasteurellas isolated can be done, but interpretation of the results is often difficult because it is not known whether the isolates from nasal swabs represent the organisms causing the lesions. Significant differences may exist between the antimicrobial sensitivities of isolates from nasopharyngeal swabs and those from the lung tissues. Thus it is not yet possible to recommend routine culturing and antimicrobial sensitivity determination of pasteurellas from nasal cavity or nasopharyngeal mucus from cattle with acute bovine respiratory disease. At the individual animal level, nasopharyngeal swabs and bronchoalveolar lavage reveal only moderate agreement; at the group level the isolation rates of various organisms are similar. In healthy calves monitored from the farm to the feedlot there was no relationship between the nasal flora and pulmonary lesions. The results of antimicrobial susceptibilities of bacterial pathogens isolated from the lung tissues of cattle with pneumonia over a period of years may provide some indication of trends in antimicrobial sensitivities but the results are not useful in making decisions about the selection of antimicrobial-affected animals.40
The literature on the principles of antimicrobial susceptibility testing of bacterial pathogens associated with bovine respiratory disease has been reviewed.41 Two different methods are used. The Kirby– Bauer method is the traditional in vitro test of bacterial susceptibility or resistance to antimicrobials, which uses a disk containing a standardized concentration of an antimicrobial. Bacteria grow or fail to grow surrounding the disk, and results are interpreted as resistance or susceptibility of the bacteria to certain antimicrobials. The serial-dilution testing uses a broth or agar medium with selected dilutions of antimicrobials in 1:2 dilution steps. Results are expressed as susceptible, intermediate susceptibility or resistant and also as minimum inhibitory concentrations (MICs), which are considered more reliable. The MIC is defined as ‘the lowest concentration of an antimicrobial that prevents visible growth of a microorganism in agar or broth dilution susceptibility test’.
It is important to adhere to standards set by the National Committee on Clinical Laboratory Standards/Veterinary Antimicrobial Susceptibility Testing Subcommittee (NCCLS/VASTS). Veterinary-specific breakpoints are determined by the NCCLS/VASTS through a consensus process based on reviewing pharmacokinetic, MIC, zone-diameter scattergram and clinical trial data relating to an antimicrobial application. The subcommittee selects MIC breakpoints and zone-interpretative criteria that best fit the definitions of susceptible, intermediate susceptibility and resistant.41
The most veterinary-specific breakpoints for pathogens in bovine respir-atory disease have been determined for five antibiotics: ceftiofur, enrofloxacin, florfenicol, spectinomycin, and tilmicosin.41 The breakpoints for oxytetracycline and chlortetracycline are adapted from human breakpoints developed for tetracycline.41
Virus isolation or detection
Nasal swabs may be submitted for isolation or detection of viruses such BVH-1, BRSV, bovine coronavirus.
Serology
Serum samples may be submitted for determination of the levels of specific antibody to suspected viral causes of the bovine respiratory disease. Paired acute and convalescent serum samples from both affected and normal animals in the herd are desirable. In a group of animals in a feedlot, or dairy or beef cattle herd, serology for a specific etiological agent may be followed over a period of time to determine seroconversion and its relationship to occurrence or absence of clinical disease.
Hematology
Plasma fibrinogen concentrations are elevated, paralleling the increase in body temperature, and are a more reliable indication of the presence of the lesion than clinical assessment. Young cattle with clinical signs of acute respiratory disease, a fibrinogen concentration greater than 0.7 g/dL, and a temperature greater than 40°C (104°F) are likely to have pneumonic pasteurellosis. Leukocyte counts are of little value, as a leukocytosis and neutrophilia occur in some animals but in others there may be a neutropenia or no significant change. Acute phase proteins are increased within 24 hours following experimental intratracheal inoculation of M. haemolytica into calves. The availability of a rapid test for acute-phase proteins could assist in the field diagnosis of the disease and its possible differentiation from similar diseases.
NECROPSY FINDINGS
Necropsies should be done on all animals that die with the disease. In feedlots this is a means of obtaining daily information on the occurrence of specific diseases and on the efficacy of the animal health management program. The most common pathological finding is fibrinous pneumonia and coagulation necrosis, with varying degrees of the common complications such as pleuritis and pulmonary abscesses. One of the major problems in the diagnosis of feedlot pneumonia has been to assess the age of the lesions. Determining the age of a bacterial pneumonia with some accuracy would help the health management to assess whether or not the pneumonia was already present in the animal on arrival or whether a treatment failure resulted from late detection or from inadequate drug therapy. An attempt to age the lesions of bacterial pneumonia of feedlot cattle indicated that the degree and extent of necrosis and fibrosis offers the best opportunity to age the pneumonia. In acute interstitial pneumonia, the lungs are diffusely red-tan, enlarged, and do not collapse. All lobes are rubbery, wet, and heavy. Emphysema is present mainly in the diaphragmatic lobes and there is white froth in the trachea. In fibrinous pleuritis there are thick sheets of fibrin over the visceral and parietal pleura. The thorax contains fibrin and straw-colored fluid. In caudal vena caval thrombosis, the lungs contain pulmonary arterial aneurysms and abscesses. Blood clots are present in the airways and the liver has an abscess near the vena cava that contains a thrombus.
Tissue samples are submitted for histopathology and bacteriological and virological examination depending on the specific disease suspected. However, the length of time usually required to do the diagnostic work and interpret the results means that the procedure is expensive and to an extent inconclusive because the results are available only when the outbreak is over.
A method has been described of recording pulmonary lesions of beef calves at slaughter and the association of lesions with average daily gain.42 Computer imaging technology can be employed to facilitate the capture of feedlot necropsy data.25 A digital camera is used to capture necropsy findings and the images are electronically transferred to a central reference laboratory for veterinary interpretation and diagnosis.
Interpretation of results of clinical pathology and necropsy findings
A large body of information has been generated on the microbiology, and more recently molecular microbiology, of specific pathogens associated with bovine respiratory disease but only a small amount is applicable clinically. Insufficient effort has been directed towards integrating the information and applying it to the effective control of respiratory disease on the farm. Ideally, investigations of outbreaks of bovine respiratory disease should consist of in-depth examinations of a representative sample of the affected group and normal in-contact animals using a multidisciplinary approach involving clinical, epidemiological, and laboratory investigation. These procedures, especially those requiring detailed virological and serological examinations, are expensive and in the light of the economic status of cattle industries not likely to be lightly borne. But it will only be when such a multidisciplinary approach is brought to bear on bovine respiratory disease that we will improve our position with respect to knowing what actually occurs in outbreaks of the disease. Of paramount importance is the identification of risk factors, which, if valid, gives the clinician a powerful clinical tool for the clinical management and control of respiratory disease in cattle. Of even greater importance is the necessity for the clinician to visit the farm and conduct those clinical and epidemiological investigations that are necessary to solve the problem and to monitor the problem and the herd until recovery occurs.
TREATMENT
The principles of the clinical management of outbreaks of acute undifferentiated bovine respiratory diseases are:
• The clinician must visit the farm and do the clinical and epidemiological investigations necessary to solve the problem, to assist the owner or the animal attendants with the clinical management of the disease, and to monitor the problem and the herd until recovery occurs. Simply dispensing antimicrobials to the owner without clinical examination of the animals is inadequate and contradicts the intention of the veterinarian–client relationship. The veterinarian is professionally obliged to provide explicit instructions about medication of affected animals and the drug withdrawal requirements, and to keep adequate records of affected animals, treatments given and the results of laboratory examinations. A final report should be prepared by the veterinarian and sent to the owner
• Unless otherwise determined, when toxemia and fever are present it is assumed that a primary bacterial pneumonia is present or, if a viral interstitial pneumonia is suspected, then a secondary bacterial pneumonia may occur. Therefore, antimicrobial therapy is of prime importance
• New cases must be identified as soon as possible. This will require increased surveillance of the group to detect affected animals as soon as clinical abnormalities such as depression, nasal discharge, and dyspnea are noticeable
• New cases must be treated as soon as they are detected. Each treated animal should be suitably identified and a record kept of the initial body temperature and the treatment administered. If the outbreak is due to pneumonic pasteurellosis, failure to respond favorably to antimicrobial therapy or relapse that occurs a few days after an initial apparent recovery is usually due to late treatment. Delaying treatment until 48 hours after an experimental aerosol infection of M. haemolytica can prolong the course of the disease and increase mortality
• Any of the common antimicrobials must be administered parenterally daily for at least 3 days. Oxytetracycline at 10 mg/kg BW intramuscularly, procaine penicillin G at 30 000–45 000 IU/kg BW intramuscularly, or trimethoprim– sulfadoxine at 3 mL/45 kg BW intramuscularly are effective when given early in the course of the disease. Tilmicosin at a dose of 10 mg/kg BW subcutaneously is also effective. Florfenicol at 20 mg/kg BW intramuscularly and repeated 48 h later is also highly effective.20 Florfenicol and tilmicosin are comparable for treatment of undifferentiated bovine respiratory disease in western Canada.43 Florfenicol is superior to tilmicosin for the treatment of undifferentiated fever in feedlot calves that have previously received metaphylactic tilmicosin upon arrival in the feedlot in western Canada.44 Enrofloxacin at 2.5–5.0 mg/kg BW intramuscularly daily for 3–5 days or a single dose of 7.5–12.5 mg/kg BW is effective for the treatment of bovine respiratory disease.45
In lactating dairy cows, antimicrobials with the shortest milk withdrawal times commensurate with effectiveness should be used. Ceftiofur with no withdrawal period is now available for use in lactating dairy cattle. Milk from treated cows must be kept from the bulk milk supply until the stated withdrawal time has elapsed. Other antimicrobials that have been evaluated for the treatment of acute undifferentiated bovine respiratory disease include sulbactam–ampicillin and the fluoroquinolones.
A beneficial response to therapy should be apparent within 12–24 hours. The body temperature should decline significantly and the appearance of the animal and its appetite should be improved.
The response to treatment, or lack of it, is valuable information in making a final decision on cause. Animals that do not respond to treatment and die should be submitted to intensive necropsy examination and culture of affected lungs. One of the emerging problems inherent in such broad policies in treatment is public health concern with the amount of antibiotic residue in meat. Pressure is now being applied to use antimicrobials only when necessary, which necessitates a more accurate diagnosis. A good example of this problem is when cattle are treated with antibiotics for bovine respiratory disease but the diagnosis is then refined in a day or two to interstitial pneumonia and emergency slaughter is then the appropriate course of action. The cattle cannot be slaughtered until the withdrawal period for the specific antibody used has expired, by which time many of the cattle will have died anyway. The regular use of a particular antimicrobial in feedlots may increase the level of resistance to M. haemolytica.
When confronted with an outbreak, one of the major decisions to be made is whether or not to recommend mass medication of the water or feed supplies for several days or to administer an antimicrobial to all in-contact animals in an attempt to treat cases in the preclinical stage. Veterinarians commonly recommend the use of medicated water supplies as an aid in the treatment of outbreaks of acute respiratory disease, and field observations claim beneficial results. However, there is no validated information available to support a recommendation for a medicated water supply for treatment or prophylaxis in the face of an outbreak. Depending on the water supply system it can be difficult to deliver and maintain a constant concentration of a drug in the water supply; palatability of certain drugs can also be a problem. The medication of the water or feed supplies can also create a false sense of security in the animal attendants, who may not be as efficient in the selection of affected animals in the early stages of the disease.
There are no validated reports of the use of medicated feed as an aid to treatment for outbreaks of acute respiratory disease in cattle.
In an outbreak of acute respiratory disease in feedlot cattle when the daily morbidity rate reaches 6–10% the parenteral administration of long-acting oxytetracycline to all in-contact cattle at a dose of 20 mg/kg BW intramuscularly is recommended. Tilmicosin at 10 mg/kg BW subcutaneously is also effective in reducing the morbidity rate when given to beef calves on arrival in the feedlot or 72 hours later.3 The intramuscular injection of two different formulations of oxytetracycline to high-risk feedlot calves on arrival can reduce the morbidity rate due to respiratory disease during the first 2 weeks on feed and for the entire feeding period by 15–19% and the mortality rate from fatal fibrinous pneumonia by 67–84%. Meta-analysis of field trials of antimicrobial mass medication for prophylaxis of bovine respiratory disease in feedlot cattle indicated that prophylactic parenteral mass medication of calves with long-acting oxytetracycline or tilmicosin on arrival at the feedlot would reduce morbidity rates.
CONTROL
The control of outbreaks of acute bovine respiratory disease will depend on mass medication or metaphylaxis, minimizing the effects of the risk factors and enhancing immunity by the judicious use of vaccines.
Mass medication or metaphylaxis
The parenteral administration of antimicrobials to each animal as a form of mass medication may assist in the reduction of morbidity and mortality rates due to respiratory disease. The use of long-acting oxytetracycline at a dose of 20 mg/kg BW intramuscularly to feedlot cattle on arrival significantly reduced morbidity and mortality rates. The combined use of long-acting oxytetracycline at a dose of 20 mg/kg BW intramuscularly on arrival followed by the oral administration of 25 g of sustained-release sulfadimethoxine on day 3 resulted in a 90% reduction in treatment days per calf purchased. Tilmicosin, given at a dose of 10 mg/kg body weight subcutaneously to calves on arrival in the feedlot or 72 hours later, can significantly reduce the morbidity rate due to respiratory disease and improve the rate of gain.46
A formulation of long-acting oxytetracycline (300 mg/mL) at a dose of 30 mg/kg BW intramuscularly is superior to the standard formulation of long-acting oxytetracycline (200 mg/mL) given at 20 mg/kg BW.47 The 300 mg/mL preparation of oxytetracycline is also more cost-effective than tilmicosin.48
Mass medication of cattle on entry into feedlots in Australia with tilmicosin at 10 mg/kg BW grew 0.08 kg/d faster than cattle medicated with oxytetracycline at 20 mg/kg BW and nonmedicated cattle.49 There was no significant difference in growth rate between oxytetracycline-medicated cattle and those not medicated on entry into the feedlot. Cattle medicated with tilmicosin had fewer treatments for all illnesses compared with cattle not given an antibiotic on entry to the feedlot and compared with cattle mass-medicated with oxytetracycline. Tilmicosin induces apoptosis in pulmonary neutrophils, leading to a reduction in leukotriene B4 synthesis, thereby reducing further amplification of the inflammatory injury of bovine respiratory disease. Preshipment medication with tilmicosin are not more effective than mass medication on arrival.50
The mass medication of feed supplies of newly arrived feedlot cattle has been investigated as a method of reducing the morbidity and mortality due to respiratory disease. The provision of chlortetracycline in the feed at a rate of 1, 2, or 4 g per head daily during the 2-week period after arrival reduced the number of calves that required treatment for respiratory disease.
Management of risk factors
As a general outline for the control of bovine respiratory disease the following factors are considered as contributing to disease and their effects must be minimized with suitable management and disease prevention techniques.
• Young growing cattle are more susceptible than mature cattle because of a lack of sufficient immunity. The mixing of young cattle of different origins requires increased surveillance to detect evidence of disease. Vaccination of calves at strategic times may be necessary
• Cattle purchased from various sources and mingled in a feedlot are more likely to develop bovine respiratory disease than cattle that have originated from one source. Some cattle will be highly susceptible and others relatively resistant because of differences in nasal flora and immunological, genetic, and nutritional backgrounds. A high level of management and constant surveillance are necessary to recognize, isolate, and treat clinical cases early in order to minimize morbidity and case mortality
• Rapid fluctuations in environmental temperatures and relative humidity, not only during the fall and winter months but also during warm seasons, will commonly precede outbreaks of respiratory diseases. Every practical and economical management technique must be used to provide as much comfort as possible and to avoid overcrowding
• Inadequate ventilation is a major predisposing cause of respiratory disease of cattle raised indoors. This is of major importance in dairy herds during the winter months in temperate climates
• The weaning of beef calves during inclement weather may exacerbate the stress of weaning and commonly results in an outbreak of respiratory disease
• The stress associated with the marketing of cattle is a major factor. The movement of cattle through saleyards – where they may be overcrowded, mixed with cattle of many different origins, temporarily deprived of adequate feed and water, handled roughly while being sorted, weighed, tagged, blood sampled, vaccinated or injected with antibiotics and/or vitamins and then loaded on to uncomfortable vehicles and transported long distances without adequate rest stops – is stressful. The practice of preconditioning cattle before they enter the feedlot must continue to be examined to determine which aspects are most profitable.
Presale vaccination programs are designed to establish an effective immune response to common respiratory tract pathogens well in advance of any natural exposure that may occur while calves travel through the auction market or after they arrive in the feedlot. These programs usually require calves to be castrated, dehorned, and vaccinated against IBRV, PI-3V, BRSV, and BVDV. Some programs also require vaccination against H. somni and M. haemolytica. Presale conditioning programs involve these procedures but also include weaning and nutritional components. Most such conditioning programs require calves to be weaned and adjusted to a roughage and concentrate diet for at least 30 days prior to sale.
Presale vaccination and conditioning programs have increased and decreased in popularity since their introduction in the 1970s. Producers tend to be reluctant to adopt these practices, because there is no assurance they will be rewarded, in terms of price premiums, for their efforts. However, the establishment of special auctions in Ontario that feature large numbers of vaccinated or conditioned calves resulted in an increased interest in these management practices. Producers selling lots of vaccinated or conditioned feeder calves through special auctions received a premium sale price compared with lots of feeder calves sold through conventional auctions.51 Vaccinated and conditioned calves were less likely to receive treatment for bovine respiratory disease during the first 28 days in the feedlot; but there was no difference in mortality.52 Calves that received antimicrobials on arrival at the feedlot had a reduced risk of treatment for bovine respiratory disease compared with calves that did not.
Vaccines
While vaccines are available for the control of acute respiratory disease associated with IBRV, PI-3V, and Pasteurella spp., there are almost no reports available of their efficacy determined under scientifically designed field trials. Based on current immunological technology, efficacious vaccines are considered to be feasible. The vaccines have been evaluated by experimental challenge of vaccinated animals with specific pathogens in a laboratory environment. However, there is little scientific evidence available that the vaccines are protective against acute UBRD as it occurs in the ‘real world’ situation. Preshipment vaccination of beef calves 3 weeks prior to weaning with vaccines containing IBRV, PI-3V, Pasteurella spp., and H. somni did not reduce the incidence of UBRD compared to those unvaccinated.
Pasteurella bacterins and respiratory viral vaccines have been used extensively in an attempt to control bovine respiratory disease. Many veterinarians and feedlot owners maintain that vaccination against respiratory disease is an essential component in their disease prevention programs, both to prevent specific disease of the respiratory tract such as clinical infectious bovine rhinotracheitis and to reduce losses due to respiratory disease in the first few weeks after arrival. However, a review of the literature on the efficacy of the vaccines available for the control of bovine respiratory disease concluded that there are few documented data to support the use of vaccines against respiratory disease under feedlot conditions. Efficacy refers to the ability of the vaccine to reduce overall treatment rate and/or increase weight gains economically.
In North America a large number of bacterial and viral vaccines are available for the control of bovine respiratory disease. There are single antigen or multiple antigen vaccines, modified live virus or inactivated virus vaccines containing one or more of the following antigens: M. haemolytica, H. somni, IBRV, PI-3V, BRSV, and BVDV. There are many multiple antigen vaccines containing combinations of the respiratory viruses, BVDV, H. somni, and M. haemolytica. In western Canada, it is more cost-effective to vaccinate auction-market-derived, fall-placed feedlot calves with a multivalent viral vaccine containing IBR, PI-3, BVD, and BRS viruses than a single univalent viral vaccine containing IBRV only.53
Selection of vaccines
The selection of which vaccine to recommend for the control of UBRD in feedlot cattle is currently not possible based on the efficacy information which is available to the veterinarian. The vaccines are used widely and many anecdotal claims for their effectiveness are made but there is little scientific evidence based on properly designed field trials that the vaccines are effective and economical in reducing the incidence or the consequences of respiratory disease such as suboptimal weight gain. The major problem has been that vaccine manufacturers have not conducted a sufficient number of well-designed field trials to evaluate the efficacy of the vaccine against naturally occurring disease in the field with vaccinated animals and unvaccinated animals as concurrent controls. In most cases the vaccines were approved for sale on the basis of tests for safety in animals, and the potency measured by a serological response to the vaccine or experimental challenge in animals under laboratory conditions.
The information available about the commercial vaccines currently used in Canada for protection against bovine respiratory disease has been reviewed.54 The available vaccines offer protection against only IBRV, BVDV, BRSV, PI-3V, M. haemolytica, and H. somni. Various combination vaccines containing modified live virus, killed virus, bacterins, and/or bacterial culture supernatants/surface extracts are available.
Efficacy of vaccines
Meaningful field trials to evaluate vaccines for the control of bovine respiratory disease are difficult to achieve. The case definition of what is a ‘case of respiratory disease’ has been very general, such as the presence of anorexia, depression, and a fever. Therefore, when testing a vaccine for the control of pneumonic pasteurellosis, the conclusions reached may be questionable if the cause of the sick animals in either the vaccinated or control group is not known – thus the importance of case definition. In contrast to field trials, the measures used by the manufacturer in the laboratory challenge of the vaccine have been specific. In a field trial, the control group and the vaccinated groups must be comparable. Where more than one vaccine is used to control respiratory disease in vaccinates and controls it is difficult to evaluate one of the vaccines or the components of a multiple antigen vaccine unless large numbers of animals are used. Another problem is the difficulty of having the controls and the vaccinates experience approximately the same risk of being affected with respiratory disease.
Field trials for bovine respiratory disease vaccines are often unsatisfactory because of inadequate planning, unsatisfactory experimental design and lack of monitoring. A check list of key elements to consider when assessing the clinical research published for a particular vaccine has been suggested. The following items should be considered:
• Has the vaccine been laboratory and field tested in randomized controlled field trials? If so, how many trials, and, in each case:
A field trial was conducted to compare the serological responses in weaned beef calves 6–8 months of age vaccinated against IBRV, PI-3V, BRSV, and BVDV. There were significant differences in serological responses among the various commercial vaccines. Antibody titers to IBRV were higher in calves vaccinated with modified-live virus IBRV vaccines than when the inactivated vaccine was used. Following double vaccination with modified-live virus IBRV and PI-3V vaccines, seroconversion rates and antibody titers were higher in calves vaccinated intramuscularly than in those vaccinated by the intranasal route. It is not known if these differences reflect differences in vaccine efficacy under field conditions. The effect of using multiple antigens in the same vaccine on the serological responses is not clear. In some cases, vaccines containing the BRSV antigen resulted in lower titers to BVDV and PI-3V than vaccines that did not contain the BRSV.
The following comments on the use of vaccines as an aid in the control of acute undifferentiated respiratory disease in feedlot cattle are based on the current information available.
Pasteurella vaccines
Because fibrinous pneumonia associated with M. haemolytica is the most common lesion associated with bovine respiratory disease in feedlot cattle, much of the emphasis has been on the development of effective vaccines for bovine pneumonic pasteurellosis. Based on the immunological and microbiological observations of both naturally occurring and experimentally induced pneumonic pasteurellosis it appears that effective artificial immunization of cattle is possible. High levels of naturally acquired antibody to M. haemolytica have been associated with protection against the disease.
Calves that recover from experimentally induced pneumonic pasteurellosis possess increased resistance to subsequent experimental challenge. Calves that were naturally exposed to M. haemolytica or exposed by vaccination subcutaneously or intradermally to the live organisms developed some resistance to experimental challenge and developed antibodies to all surface antigens and cytotoxin. Resistance to experimental challenge with the organism correlated directly with serum cytotoxin neutralizing titers. This supports the hypothesis that protection against experimental challenge with M. haemolytica may require an immune response to cytotoxin. This is supported by the observation that cattle that died from fibrinous pneumonia due to M. haemolytica had lower cytotoxin neutralizing activity in their sera than cattle from the same group that died from other causes.
Antibodies to leukotoxin and certain bacterial surface components appear to be important for resistance to disease. The basis of a recently introduced pasteurella vaccine is that vaccination of calves with a leukotoxic culture supernatant from pathogenic M. haemolytica provided some protection against experimental challenge with the organism. The vaccine has been evaluated in field trials in feedlots in Canada with variable results. In one trial, the vaccine was used on arrival in fall-placed calves and reduced the pull rate by 4%, the first relapse rate by 11.9% and the second relapse rate by 18.7%. There was an 8.2% decrease in the mean number of treatment days. Overall mortality was decreased by 49% and mortality rate due to fibrinous pneumonia was reduced by 50%. It appeared that vaccinated animals also responded more favorably than nonvaccinated animals. Vaccination of recently shipped nonpreconditioned calves with the vaccine in Ontario resulted in a slight decrease in morbidity, slight improvement in response rates and perhaps an important reduction in relapse rates. When the vaccine was combined with an intramuscular modified-live IBR/PI-3 virus vaccine, the morbidity rate was increased, the response rate was decreased and the mortality rate was increased in some groups. It appears that the use of modified-live virus vaccines in recently arrived calves is contraindicated; this is consistent with earlier observations in the Bruce County Project, where fall-placed calves were vaccinated on arrival with a modified-live virus vaccine.
The Pasteurella vaccine has also been evaluated in a field trial that compared its efficacy in calves vaccinated at the ranch 3 weeks prior to shipping to the feedlot, vaccination of ranch calves on arrival in the feedlot, and auction mart calves assigned to either receive or not receive the vaccine on arrival at the feedlot. The vaccine did not effect a change in morbidity rates or weight gain. Total mortality rates were increased significantly, and mortality rates from respiratory disease tended to be increased in ranch calves that were vaccinated at the ranch. In auction-mart-derived calves, the relapse rates were significantly lower in vaccinated calves. The source of calves was a major factor affecting the incidence and/or effects of bovine respiratory disease. Calves moved directly from the ranches to feedlots, regardless of vaccination status, had lower morbidity and mortality, and better weight gains, than calves purchased from auction marts.
Histophilus somni (formerly Haemophilus somnus) vaccine
A H. somni bacterin evaluated in a large number of feedlot cattle had no effect on the overall crude mortality rate; however, vaccination appeared to reduce the incidence rate of fatal disease and the mortality rate during the first 2 months in the feedlot, when risk of fatal disease onset was highest. The incidence of fatal disease onset was highest during the first week after arrival, which suggests that inclusion of a H. somni bacterin in a cow– calf preimmunization program might reduce the proportion of disease that occurs during the first week in the feedlot. Crude mortality and incidence of fatal disease onset during the second week were reduced significantly in the vaccinated steers. It was concluded that about 17% of fatal respiratory disease in the unvaccinated steers could have been prevented by vaccination with the H. somni bacterin. Fibrinous pneumonia was the most common pathological diagnosis. Vaccinating calves twice with a killed whole-cell H. somni bacterin reduced the clinical and pathological effects of experimentally induced H. somni pneumonia.
Viral vaccines
Because prior infection of the respiratory tract with either IBRV or PI-3V may predispose to pneumonic pasteurellosis, the vaccination of beef calves 2–3 weeks before weaning and feedlot cattle 2 weeks before shipment to a feedlot has been recommended as part of a preconditioning program. The results are variable, but vaccination of calves at 3–6 months of age with an intranasal modified-live IBR and PI-3 virus vaccine has provided protection against experimental pneumonic pasteurellosis induced by aerosol challenge with IBRV followed 4 days later by an aerosol of M. haemolytica. It is important to vaccinate the calves at least 2 weeks before they are weaned, stressed, or transported to a feedlot.
A modified-live virus BRSV vaccine given to beef calves prior to weaning, at weaning or immediately after arrival in the feedlot was associated with a significant reduction in the treatment rate in one of three groups immunized prior to weaning and in calves immunized after arrival in the feedlot.25 There was no significant effect of the vaccine on treatment rate in calves immunized at weaning, in calves immunized after arrival in a bull test station, or in yearlings immunized after arrival in the feedlot. It would appear that the vaccine did provide some protection but the small reduction may not justify the cost of the vaccination program.
Some feedlot veterinarians recommend that feedlot cattle be vaccinated on arrival with an M. haemolytica vaccine, the IBRV and PI-3V vaccine, an H. somni vaccine, and the BRSV vaccine. In some cases the BVDV vaccine is also used because some veterinarians feel that the virus is part of the respiratory disease complex. It is expected that control will be achieved if the animals are vaccinated against all the common pathogens that contribute to lesions of bovine respiratory disease. However, there is little, if any, published evidence based on controlled field trials that such blanket recommendations are justifiable.
Barrett DC. Cost-effective antimicrobial drug selection for the management and control of respiratory disease in European cattle. Vet Rec. 2000;146:545-550.
Bowland SL, Shewen PE. Bovine respiratory disease: commercial vaccines currently available in Canada. Can Vet J. 2000;41:33-48.
Coomber BL, Nyarko KA, Noyes TM, Gentry PA. Neutrophil-platelet interactions and their relevance to bovine respiratory disease. Vet J. 2001;161:41-62.
Cusack PMV, McMeniman N, Lean IJ. The medicine and epidemiology of bovine respiratory disease in feedlots. Aust Vet J. 2003;81:480-487.
1 Lonergan GH, et al. J Am Vet Med Assoc. 2001;219:1122.
2 Wittum TE, et al. J Am Vet Med Assoc. 1999;209:814.
3 Cusack PMV, et al. Aust Vet J. 2003;81:480.
4 Martin SW, et al. Can Vet J. 1999;40:560.
5 Martin SW, et al. Can J Vet Res. 1998;62:262.
6 Booker CW, et al. Can Vet J. 1999;40:40.
7 O’Connor A, et al. Can J Vet Res. 2001;65:143.
8 Haines DM, et al. Can Vet J. 2001;42:857.
9 Shahriar FM, et al. Can Vet J. 2002;43:863.
10 O’Connor A, et al. Can J Vet Res. 2001;65:137.
11 Fulton RW, et al. Can J Vet Res. 2002;66:181.
12 Baule C, et al. J Clin Microbiol. 2001;39:146.
13 Martin SW, et al. Can J Vet Res. 1998;62:257.
14 Hasoksuz M, et al. J Vet Diagn Invest. 2002;14:308.
15 Lathrop SL, et al. Am J Vet Res. 2000;61:1057.
16 Lathrop SL, et al. Am J Vet Res. 2000;61:1062.
17 Storz J, et al. J Am Vet Med Assoc. 2000;216:1599.
18 Plummer PJ, et al. J Am Vet Med Assoc. 2004;225:726.
19 Storz J, et al. J Clin Microbiol. 2000;38:3291.
20 Booker CW, et al. Can Vet J. 1997;38:555.
21 Sowell BF, et al. J Anim Sci. 1999;77:1105.
22 Buhman MJ, et al. Am J Vet Res. 2000;61:1163.
23 Quimby WF, et al. Can J Anim Sci. 2001;81:315.
24 Van der Fels-Klerx HJ, et al. Livestock Prod Sci. 2000;66:35.
25 Wildman BK, et al. Can Vet J. 2000;41:124.
26 Van der Fels-Klerx HJ, et al. Netherlands J Agric Sci. 2002;50:27.
27 Ayroud M, et al. Can Vet J. 2000;41:847.
28 Ribble CS, et al. J Am Vet Med Assoc. 1995;207:616.
29 Ribble CS, et al. Can J Vet Res. 1995;59:167.
30 Ribble CS, et al. J Am Vet Med Assoc. 1995;207:612.
31 Ribble CS, et al. Prev Vet Med. 1994;21:251.
32 Van Donkersgoed J. Can Vet J. 1992;33:786.
33 Chirase NK, et al. Am J Vet Res. 2004;65:860.
34 Donovan DC, et al. J Anim Sci. 2003;81:3088.
35 Galyean ML, et al. J Anim Sci. 1999;77:1120.
36 Norstrom M, et al. Prev Vet Med. 2000;44:87.
37 Norstrom M, et al. Prev Vet Med. 2000;47:107.
38 Norstrom M, et al. Prev Vet Med. 2001;51:259.
39 Kelly AP, Jansen ED. Aust Vet J. 1986;27:496.
40 Welsh RD, et al. J Vet Diagn Invest. 2004;16:426.
41 Apley MD. Vet Clin North Am Food Anim Pract. 2003;19:625.
42 Bryant LK, et al. Bovine Pract. 1999;33:163.
43 Hoar BR, et al. Can Vet J. 1998;39:161.
44 Jim GK, et al. Can Vet J. 1999;40:179.
45 Hamm M, et al. Bovine Pract. 1999;33:56.
46 Vogel GJ, et al. J Am Vet Med Assoc. 1998;212:1919.
47 Schunicht OC, et al. Can Vet J. 2002;43:940.
48 Schunicht OC, et al. Can Vet J. 2002;43:355.
49 Cusack PMV, et al. Aust Vet J. 2004;82:154.
50 Duff GC, et al. J Anim Sci. 2000;78:267.
51 Macartney JE, et al. J Am Vet Med Assoc. 2003;223:670.
52 Macartney JE, et al. J Am Vet Med Assoc. 2003;223:677.