Viral diseases characterized by nervous signs
EASTERN AND WESTERN VIRAL ENCEPHALOMYELITIS OF HORSES
Etiology Eastern encephalitis and Western encephalitis viruses
Epidemiology Disease limited to the Americas. Arthropod, usually mosquito, borne virus. Mammals, including horses, are accidental hosts. Horse is dead-end host for EEE and WEE. Case fatality rate 5–70%. WEE and EEE occur as sporadic cases and as outbreaks
Clinical signs Fever, muscle fasciculation, severe depression, head pressing, incoordination, recumbency, opisthotonos and paddling, and death
Lesions Non-suppurative encephalomyelitis
Diagnostic confirmation Virus isolation and identification. Identification of viral antigen by indirect immunofluorescence. Serological confirmation of exposure, preferably demonstrating an increase in hemagglutination inhibition, virus neutralization, or complement fixation titer
Treatment No specific treatment. Supportive care
Control Vaccination with formalin-inactivated vaccines (EEE, WEE). Insect control
ETIOLOGY
Equine encephalomyelitis is associated with one of the two immunologically distinct arthropod borne alphaviruses (family Togaviridae): Eastern equine encephalomyelitis virus (EEE), Western equine encephalomyelitis virus (WEE).
• There is one EEE virus strain, but two antigenic variants of it: North American and South American1
• WEE likely arose as a recombinant of EEE and Sindbis virus.2 There are strains of WEE from Argentian, Brazil, and South Dakota that differ antigenically2 and there are 4 major lineages of WEE in California whose geographical distributions overlap.1,2
All the viruses are extremely fragile and disappear from infected tissues within a few hours of death. WEE is the least virulent of these viruses in horses and humans.
EPIDEMIOLOGY
These encephalitis viruses cause disease in horses, humans, pigs, and various birds including ratites and domestic pheasants.3
Distribution
Equine Eastern and Western encephalomyelitis viruses are restricted to the Americas. The two viruses have distinct geographical ranges that may overlap: EEE is restricted to South America and North America generally east of the Mississippi river whereas WEE is found west of the Mississippi river and predominantly in the western United States and Canada, although it also occurs in Florida and South America.
Viral ecology
Humans, horses, cattle, pigs, dogs, and ratites are accidental hosts of the virus. The EEE and WEE viruses are normally maintained in a host–vector relationship by cycling between mosquitoes, and some other hematophagous insects, and the definitive host. However, there are some important differences in the ecology of the different viruses.
Western equine encephalomyelitis
The definitive hosts of endemic WEE are wild birds, which are not clinically affected, and the vectors are the mosquitoes Culex tarsalis (in the western United States) and Culiseta melanura (in the eastern and southern United States).4 Infected mosquitoes bite susceptible birds, usually nestlings or fledglings, that then develop viremia. Mosquitoes are infected by feeding on viremic birds or by vertical transmission.5 Vertical transmission is likely an important over-wintering mechanism in WEE, and possibly EEE.
Epidemics of WEE are uncommon, but sporadic individual cases are not. Epidemics of WEE are associated with factors that increase the number of infected mosquitoes or their feeding on susceptible (unvaccinated) horses. The disease in horses occurs in mid-summer and fall, and is associated with a change in the feeding habits of Culex tarsalis.6 Horses, and humans, are dead-end hosts, as the viremia in these species is not sufficiently severe to allow infection of feeding mosquitoes.
Eastern equine encephalomyelitis
The primary maintenance cycle of EEE virus is transmission between passerine birds by the mosquito Culiseta melanura, an inhabitant of drainage ditches and swamps.4 However, other mosquitoes, including Aedes solicitans and A. vexans, can propagate the virus through infection of large shore birds. The Carolina chickadee and yellow-crowned night-heron are the most common avian hosts in the south-eastern United States.7 Horses are usually dead-end hosts, although viremia may be sufficiently severe in some horses to permit infection of mosquitoes.4 The reservoir of the virus during winter is not known, but may involve the vertical transmission of infection to larvae that survive the winter.
Epidemics of EEE have occurred in the provinces of Ontario and Quebec, in virtually all the states of the United States east of the Mississippi River, and in Arkansas, Minnesota, South Dakota, and Texas, in many of the Caribbean Islands, in Guatemala, Mexico, and Panama, and in Argentina, Brazil, Columbia, Ecuador, Guyana, Peru, Suriname, and Venezuela.1 Eastern equine encephalomyelitis continues to cause significant death losses annually in horses in Florida, primarily in unvaccinated horses.8 It is suggested that the incidence of clinical disease due to EEE in Florida is much higher than reported, and there is a need to increase public awareness about the importance of vaccination, particularly in foals.8 Unexpected epizootics occur in inland States of the United States, and frequently the source of the infection is undetermined, although meteorological factors that allow rapid movement of infected mosquitoes may be important. For instance, in 1972, outbreaks of EEE occurred in Quebec, Canada and in Connecticut, USA that originated with mosquitoes carried on surface winds from Connecticut to Quebec, a distance of 400 km, in 14–16 hours at a speed of 25–30 km/h and a temperature of 15°C.9 There may be a continual cycle of EEE virus in mosquitoes and birds in the southeastern US, from where the virus could be distributed by infected mosquitoes on the wind along the Gulf and Atlantic Coasts and up the Mississippi Valley.10
Animal risk factors
Recovered horses are resistant to infection for at least 2 years, and vaccination confers immunity of variable duration (see under ‘Control’). Unvaccinated horses are at increased risk of disease – the risk of a vaccinated horse contracting EEE is only 0.14 that of an unvaccinated horse.8,11 The disease is more severe, and mortality is higher, in unvaccinated horses than in vaccinated horses.12 The mortality in young foals from non-immune mares, that are infected with WEE, is always high, often as high as 100%.
Housing and exposure to mosquitoes are important risk factors for EEE, and presumably WEE. During an outbreak in 1831, only horses kept at pasture were affected.13 The use of insect repellants reduces the odds of a horse being infected with EEE to 0.04 that of an unprotected horse.11 Similarly, keeping horses at pasture near woods increases the risk of disease by almost four times, and the presence of swamp land increases the risk by over two times.11 Horses kept in areas with high precipitation have an increased risk of the disease, presumably because of the density of mosquitoes in these areas.14
Morbidity and case fatality
Morbidity varies widely depending upon seasonal conditions and the prevalence of insect vectors; cases may occur sporadically or in the form of severe outbreaks affecting 20% or more of a group. The prevalence of infections, as judged by serological examination, is much higher than the clinical morbidity. The case fatality rate differs with the strain of the virus; in infection with the WEE virus it is usually 20–30% and with the EEE it is usually between 40 and 80% and may be as high as 90%.
Zoonotic implications
The susceptibility of humans to the causative virus gives the disease great public health importance. Humans can become infected with the EEE virus and the WEE virus.1
PATHOGENESIS
Inapparent infection is the mildest form of the disease and may be characterized by only a transient fever. A more severe form of the disease is manifested by tachycardia, depression, anorexia, occasional diarrhea, and fever.
A transitory viremia occurs at the height of the fever. Penetration of the virus into the brain does not occur in all cases and the infection does not produce signs, other than fever, unless involvement of the central nervous system occurs. The lesions produced in nervous tissue are typical of a viral infection and are localized particularly in the gray matter of the cerebral cortex, thalamus and hypothalamus, with minor involvement of the medulla and spinal cord. It is this distribution of lesions that is responsible for the characteristic signs of mental derangement, followed at a later stage by paralysis. The early apparent blindness and failure to eat or drink appear to be cortical in origin. True blindness and pharyngeal paralysis occur only in the late stages.
CLINICAL FINDINGS
The diseases associated with EEE and WEE viruses are clinically indistinguishable. The incubation period for EEE is 1–3 days and 2–9 days for WEE. Uncomplicated disease usually lasts about 1 week. In the initial viremic stage there is fever, which may be accompanied by anorexia and depression, but the reaction is usually so mild that it goes unobserved. In the experimental disease, the temperature may reach 41°C (106°F) persisting for only 24–48 hours, with signs of neurologic dysfunction appearing at the peak of the fever. Animals that have signs of neurologic disease for more than 24 hours are often not pyrexic.
Initial signs of neurologic disease include hypersensitivity to sound and touch, and in some cases transient periods of excitement and restlessness, with apparent blindness. Horses can have a period of anorexia and colic before onset of signs of neurologic disease. Affected horses may walk blindly into objects or walk in circles and in severe cases can mimic signs of horses with catastrophic intestinal disease. Involuntary muscle movements occur, especially tremor of shoulder and facial muscles and erection of the penis. A stage of severely depressed mentation follows. Affected horses stand with the head hung low; they appear to be asleep and may have a half-chewed mouthful of feed hanging from the lips. At this stage the horse may eat and drink if food is placed in its mouth. The pupillary light reflex is still present. The animal can be aroused, but soon relapses into a state of somnolence.
A stage of paralysis follows. There is inability to hold up the head, and it is often rested on a solid support. The lower lip is pendulous and the tongue protrude from the mouth. Unnatural postures are adopted, the horse often standing with the weight balanced on the forelegs or with the legs crossed. Head-pressing or leaning back on a halter are often seen. On walking, there is obvious incoordination, particularly in the hindlegs, and circling is common. Defecation and urination are suppressed and the horse is unable to swallow. Complete paralysis is the terminal stage. The horse goes down, is unable to rise and usually dies within 2–4 days from the first signs of illness. A proportion of affected horses do not develop paralysis and survive, but have persistent neurological deficits.
CLINICAL PATHOLOGY
There are no characteristic hematological or biochemical abnormalities. The absence of biochemical indication of liver disease (hyperbilirubinemia, increased activity in serum of liver-specific enzymes such as sorbitol dehydrogenase or gamma glutamyl transferase, absence of hyperammonemia) rules out hepatic encephalopathy.
Diagnostic confirmation is achieved by one or more of several means:6
• Isolation of virus from an affected animal
• Detection of viral antigen or nucleic acid in an animal with appropriate clinical signs
• Seroconversion or an increase in serum titer of sick or recovered animal.
Virus isolation provides definitive proof of infection. However, viremia may have resolved by the time nervous signs have developed, and it may be advantageous to sample febrile animals instead of animals showing more advanced signs of the disease. Virus can be cultured in intracranially inoculated suckling mice, weanling mice, guinea pigs, cell culture, newly hatched chicks, or embryonated eggs.1 Virus isolates can be identified by complement fixation, hemagglutination inhibition, virus neutralization, PCR, IFA, and antigen capture ELISA.1,6 Acute and convalescent sera taken 10–14 days apart for the presence of neutralizing, hemagglutination-inhibiting, or complement-fixing antibodies in the serum of affected or in-contact horses, is of value in detecting the presence of the virus in the group or in the area. A four-fold increase in complement-fixing antibodies is considered positive.
Demonstration of viral nucleic acid in tissue, blood or insects by PCR test may be a useful indicator of the presence of the virus.15 There may be sufficient viral antigen to be detected by ELISA in clinical material, and this may provide a useful test in the early stages of an epidemic.6
The presence of a high hemagglutination-inhibition, complement fixation and neutralizing antibody in a single serum sample obtained from a horse during the acute phase of illness associated with the WEE virus can be used as presumptive evidence of infection with this virus.16 However, antibodies against the WEE virus can persist for years, are produced after vaccination with WEE or WEE/EEE bivalent vaccines, and in foals might be due to colostral immunity. Therefore, a single serum sample cannot be used to make a confirmed diagnosis of WEE using the hemagglutination-inhibition, complement fixation or neutralization tests. Horses infected experimentally or naturally with either the WEE or the EEE virus do not produce detectable hemagglutination-inhibition or neutralizing antibody for 5–10 days after infection.
Circulating antibody appears on or near the day of onset of clinical illness. Infection with the WEE virus results in the production of serum IgM specific to WEE, and the ELISA test is a rapid, sensitive, and specific test for IgM against WEE and EEE viruses.3 Additionally, the ratio of titers of EEE and WEE can be useful in detecting infection by EEE – ratios of >8:1 are highly suggestive of EEE infection.17
NECROPSY FINDINGS
The brain meninges may appear congested, but there are generally no gross changes. Histological examination of the brain reveals perivascular accumulations of leukocytes and damage to neurons.18 The gray matter of the forebrain and midbrain are the most severely affected areas. Lesions associated with EEE antigen are also present in myocardium, stomach, intestine, urinary bladder, and spleen.19
Cell culture and transmission experiments utilizing brain tissue as an inoculum are the traditional means of confirming a diagnosis, and require that the brain be removed within an hour of death. Transmission is by intracerebral inoculation of brain tissue into sucking mice or duck embryo tissue culture. Fluorescent antibody tests have been developed to detect EEE virus in brain tissue.20 A PCR-based diagnostic test is available for EEE virus.21 Lesions generally similar to those seen in horses have also been described in a beef cow infected with EEE.22 The disease in piglets is characterized by disseminated perivascular cuffing, gliosis, focal necrosis of the cerebral cortex, and multifocal myocardial necrosis.3
Samples for postmortem confirmation of diagnosis
• One half of midsagittally-sectioned brain and liver and spleen should be submitted for fluorescent antibody and PCR testing, virus isolation and bioassay
• One half of midsagittally-sectioned brain, fixed in formalin, should be submitted for light microscopic examination.
Note the zoonotic potential of these organisms when handling the carcass and submitting specimens.
Clinically, the disease has very great similarity to the other viral encephalomyelitides, from which it can often be discriminated by the geographical location of the horse, and to the hepatic encephalopathies and a number of other diseases (see below and in Table 22.1).
Table 22.1 Diseases of horses characterized by signs of intra-cranial or disseminated lesions of the central nervous system
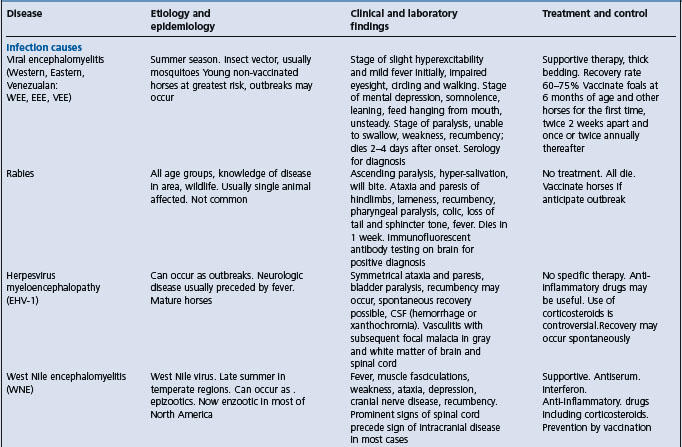
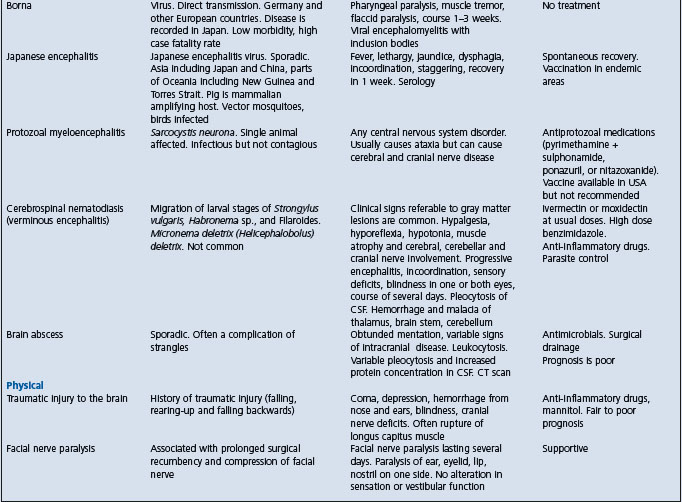

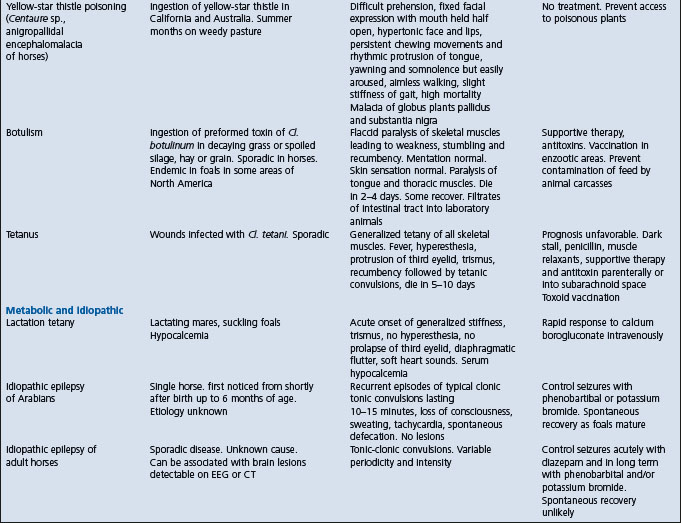
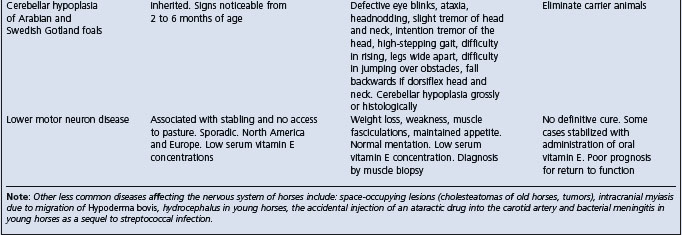
West Nile encephalitis – predominantly a myelitis with later development of signs of neurologic disease whereas EEE and WEE have predominant signs of encephalopathy.
• Borna disease – occurs in Europe
• Japanese encephalitis – occurs in Asia
• Various other viral infections that are geographically restricted
• Hepatic encephalopathy, such as that associated with poisoning by Crotalaria, Senecio, and Amsinckia spp.; acute serum hepatitis or hepatopathy
• Botulism causes weakness evident as muscle fasciculation, recumbency and dysphagia, but does not cause cerebral signs (irritation, behavioral abnormalities)
• Yellow star thistle poisoning (Centauria solstitialis, and poisoning by fumonisins (Fusarium moniliforme) can produce similar clinical signs to that of the encephalitides, with the exception of fever.
TREATMENT
There is no definitive or specific treatment. Supportive treatment may be given with the intention to prevent self-inflicted injury, and maintain hydration and nutritional status.
CONTROL
Control of viral encephalomyelitis of horses is based on:16,23,24
• Accurate clinical and laboratory diagnosis of the disease in horses
• Use of sentinel animals to monitor the presence of the virus in the region
• Quarantine of infected horses to stop movement of virus donors
Vaccination
Vaccination of horses is important not only because it minimizes the risk of disease in vaccinated horses but also because of the possibility that for EEE it prevents viremia, subsequent infection of feeding mosquitoes, and propagation of the epidemic.
Formalin-inactivated EEE and WEE virus vaccines are available and are effective although over 50% of horses with EEE had been vaccinated within the previous year.17,25 This apparent poor protection can be explained by many horses not developing a detectable change in antibody titer after vaccination with a bivalent vaccine and rapid decreases in antibody titer from a peak value achieved 2–4 weeks after vaccination.17 Vaccines are available as univalent or bivalent preparations and in combination with other antigens (for instance, tetanus toxoid). Horses should be vaccinated well in advance of the anticipated encephalomyelitis season in a given area. Vaccination against both strains of the virus is advisable in areas where the strain has not been identified or where both strains exist. The currently recommended vaccination schedule consists of two doses of the vaccine initially, 10 days apart, followed by annual revaccination using two doses. Annual revaccination is currently recommended because the duration of effective immunity beyond 1 year is not known. It is probable that the initial two-dose vaccination lasts for up to 3–4 years. The emphasis in a vaccination program should be on the young horses.
Colostral antibody can be detected in the blood of foals from vaccinated dams for up to 6–7 months, after which time it declines rapidly. Foals from vaccinated dams should be vaccinated at 6–8 months of age and revaccinated at 1 year of age. Foals from unvaccinated dams may be vaccinated at 2–3 months of age and again at 1 year of age. Colostral antibodies in the foal will prevent the development of autogenous antibodies, and foals vaccinated when less than 6 months should be revaccinated when they are 1-year-old or, in high-risk areas, foals from vaccinated mares should be vaccinated at 3, 4, and 6 months of age.
Experimental DNA vaccines hold promise for the prevention of WEE.26
Protection from insects
Housing of horses indoors at night, especially in flyproofed stables, and the use of insect repellents may restrain the spread of the virus. Use of insect repellents decreases the risk of EEE in horses to 0.04 that of unprotected horses.
Widespread spraying of insecticides to reduce the population of the vector insects has been used in the control of VEE, however, such measures are not practical for preventing sporadic cases of EEE or WEE, and the environmental impact of widespread insecticide use should be considered.
Complete eradication of the virus appears to be impossible because of the enzootic nature of the ecology of the virus. The horse being an accidental host for EEE and WEE virus make elimination of the virus impossible with methods currently available.
Zoonotic aspects of control
Control of the disease in humans in areas where the disease may occur is dependent on insect control, and a monitoring and surveillance early warning system is necessary to decide whether or not to take control measures. In areas where WEE occurs, clinical cases of the disease in unvaccinated horses usually precede the occurrence of the disease in human.27 The establishment of a reporting system whereby practicing veterinarians report all clinical cases of the disease in horses will also assist in predicting potential epidemics of WEE virus infection in the human population. Serological surveys of wildlife may also serve as good indicators of the geographical distribution and seasonality of circulation of these viruses and provide an early warning system prior to the detection of human cases.
Walton TE. Arboviral encephalomyelitis of livestock in the western hemisphere. J Am Vet Med Assoc. 1992;200:1385-1389.
Calisher CH, Walton TE. Japanese Western Eastern and Venezuelan encephalitides. In: Studdert MJ, editor. Virus infections of vertebrates: virus infections of Equines. Amsterdam: Elsevier; 1996:141-155.
1 Walton TE. J Am Vet Med Assoc. 1992;200:1385.
2 Kramer LD, Fallah HM. Am J Trop Med Hyg. 1999;60:708.
3 Elvinger F, et al. J Am Vet Med Assoc. 1994;205:1014.
4 Hayes CG, Wallis RC. Adv Virus Res. 1977;21:37.
5 Fulhorst CF, et al. Science. 1994;263:676.
6 Calisher CH, Walton TE. Studdert MJ, editor. Virus infections of vertebrates: virus infections of Equines 6. Amsterdam: Elsevier. 1996:141.
7 Hassan HK, et al. Am J Trop Med Hyg. 2003;69:641.
8 Wilson JH, et al. Prev Vet Med. 1986;4:261.
9 Sellers RF. Can J Vet Res. 1989;53:76.
10 Ross WA, Kaneene JB. Prev Vet Med. 1995;24:157.
11 Neufeld JL, Nayar GPS. In: Sekla L (ed) Western Equine encephalitis in Manitoba.
12 Winnipeg, Manitoba: Department of Health, 1982; pp. 62–79.
13 Chamberlain RW. Am J Trop Med Hyg. 1987;37:8S.
14 Ross WA, Kaneene JB. J Am Vet Med Assoc. 1996;208:1988.
15 Linssen B, et al. J Clin Micro. 2000;38:1527.
16 Calisher CH, et al. J Am Vet Med Assoc. 1983;183:438.
17 Waldridge BM, et al. Vet Therapeut. 2003;4:242.
18 Erickson GA, Mare CJ. Am J Vet Res. 1975;36:167.
19 Del Piero F, et al. Vet Pathol. 2001;38:451.
20 Weaver SC. Factors in the emergence of arbovirus diseases. Amsterdam: Elsevier, 1997;241.
21 Vodkin MH, et al. Am J Trop Med Hyg. 1993;49:772.
22 McGee ED, et al. Vet Pathol. 1992;29:361.
23 Wong FC, Lillie LE. Am J Publ Health. 1976;67(Suppl 1):15.
24 Omohundro RE. J Am Vet Med Assoc. 1971;161:1516.
25 Bryne RJ. Proc 3rd Intern Conf Equine Infect Dis 1973; 115.