SELENIUM AND/OR VITAMIN E DEFICIENCIES
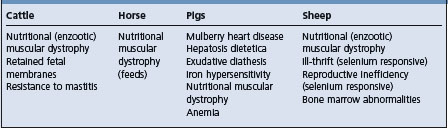
Several diseases of farm animals are associated with a deficiency of either selenium or vitamin E alone or in combination, usually in association with predisposing factors such as dietary polyunsaturated fatty acids, unaccustomed exercise and rapid growth in young animals. These are summarized in Table 30.5. All of these diseases are described under one heading because both selenium and vitamin E are important in the etiology, treatment and control of the major diseases caused by their deficiencies.
Table 30.5 Diseases considered to be associated with a deficiency of either selenium or vitamin E or both (including ‘selenium-responsive’ diseases)
They are also known as selenium-vitamin E-responsive diseases because, with some exceptions, they can be prevented by adequate supplementation of the diet with both nutrients.
The term ‘selenium-responsive disease’ has created some confusion relative to the selenium-deficiency diseases. In some regions of the world, particularly New Zealand and in parts of Australia and North America, diseases such as ill-thrift in sheep and cattle and poor reproductive performance respond beneficially to selenium administration. While these usually occur in selenium-deficient regions, they may not be due solely to selenium deficiency. Thus, there are some reasonably well-defined selenium-deficiency diseases and some ill-defined ‘selenium-responsive’ diseases.
ETIOLOGY
The selenium- and vitamin E-responsive or deficiency diseases of farm animals are caused by diets deficient in selenium and/or vitamin E, with or without the presence of conditioning factors such as an excessive quantity of polyunsaturated fatty acids in the diet. Almost all of the diseases that occur naturally have been reproduced experimentally using diets deficient in selenium and/or vitamin E. Conversely, the lesions can usually be prevented with selenium and vitamin E supplementation. In certain instances, as for example in hand-fed dairy calves, the incorporation of excessive quantities of polyunsaturated fatty acids was a major factor in the experimental disease and this led to the conclusion that certain myopathic agents were necessary to produce the lesion, which is no longer tenable. The presence of polyunsaturated fatty acids in the diet may cause a conditioned vitamin E deficiency because the vitamin acts as an antioxidant. In the case of naturally occurring muscular dystrophy in calves, lambs and foals on pasture, the myopathic agent, if any, is unknown and selenium is protective. However, selenium is not protective against the muscular dystrophy associated with the feeding of cod liver oil to calves.
Etiology Dietary deficiencies of selenium and vitamin E and conditioning factors like dietary polyunsaturated fatty acids.
• Enzootic muscular dystrophy occurs in young growing calves, lambs, goat kids, and foals born to dams in selenium-deficient areas and unsupplemented. Occurs worldwide and common in Australasia, UK, Great Plains of North America where soils are deficient in selenium. Vitamin E deficiency in animals fed poor quality forage and diets high in polyunsaturated fatty acids. Outbreaks of muscular dystrophy precipitated by exercise.
• Mulberry heart disease in finishing pigs.
• Selenium-responsive diseases occur in Australasia and are not obvious clinically but respond to selenium supplementation. Selenium and vitamin E deficiency may be involved in reproductive performance, retained placenta in cattle, resistance to infectious disease like bovine mastitis. Controversial.
Signs Muscular dystrophy characterized by groups of animals with stiffness, weakness, recumbency, severe in myocardial form. Mulberry heart disease characterized by outbreaks of sudden death in finishing pigs.
Clinical pathology Increased plasma levels of creatine kinase. Low serum levels of selenium and vitamin E. Glutathione peroxidase activity.
Necropsy findings Bilaterally symmetrical pale skeletal muscle, pale streaks in myocardial muscle. Hyaline degeneration of affected muscle.
Diagnostic confirmation Low selenium and vitamin E in diet and tissues, increased creatine kinase and muscle degeneration.
Acute muscular dystrophy in calves and yearlings
Subacute enzootic muscular dystrophy:
polyarthritis, traumatic or infectious myopathies (blackleg), osteodystrophy, and fractures of long bones
• Diseases of the nervous system:
spinal cord compression, Haemophilus somnus meningoencephalitis and myelitis, organophosphatic insecticide poisoning
• Diseases of the digestive tract:
carbohydrate engorgement resulting in lactic acidosis, shock, dehydration and weakness.
• Muscular dystrophy in lambs and kids:
• Muscular dystrophy in foals:
Traumatic injury to the musculoskeletal system and polyarthritis; meningitis; traumatic injury to the spinal cord.
Treatment Vitamin E selenium parenterally.
Control Selenium and vitamin E supplementation of diet, strategic oral and/or parenteral vitamin E and selenium to pregnant dams or young animals on pasture.
Selenium is an essential nutrient for animals and diseases due to selenium inadequacy in livestock are of worldwide distribution.1,2
Biological functions of selenium and vitamin E
Glutathione peroxidases and tissue peroxidation
Selenium is a biochemical component of the enzyme glutathione peroxidase (GSH-PX).3 The activity of the enzyme in erythrocytes is positively related to the blood concentration of selenium in cattle, sheep, horses, and pigs and is a useful aid for the diagnosis of selenium deficiency and to determine the selenium status of the tissues of these animals. The enzyme from the erythrocytes of both cattle and sheep contains 4 g atoms of selenium per mol of enzyme.1 Selenium is also a component of thyroid gland hormones.
Plasma GSH-PX protects cellular membranes and lipid-containing organelles from peroxidative damage by inhibition and destruction of endogenous peroxides, acting in conjunction with vitamin E to maintain integrity of these membranes.2 Hydrogen peroxide and lipid peroxides are capable of causing irreversible denaturation of essential cellular proteins, which leads to degeneration and necrosis. GSH-PX catalyzes the breakdown of hydrogen peroxide and certain organic hydroperoxides produced by glutathione during the process of redox cycling. This dependence of GSH-PX activity on the presence of selenium offers an explanation for the interrelationship of selenium, vitamin E and sulfur-containing amino acids in animals. The sulfur-containing amino acids may be precursors of glutathione, which in turn acts as a substrate for GSH-PX and maintains sulfhydryl groups in the cell. Selenium is also a component of several other proteins such as selenoprotein of muscle, selenoflagellin, Se-transport proteins and the bacterial enzymes, formate dehydrogenase, and glycine reductase. Selenium also facilitates significant changes in the metabolism of many drugs and xenobiotics. For example, selenium functions to counteract the toxicity of several metals such as arsenic, cadmium, mercury, copper, silver, and lead.
The arachidonic cascade, phagocytosis, and the immune response
Glutathione peroxidase may be involved in the arachidonic acid cascade.3 Eicosanoids are important mediators of immune and reproductive function.
Vitamin E
Vitamin E is an antioxidant that prevents oxidative damage to sensitive membrane lipids by decreasing hydroperoxide formation. The vitamin has a central role in protection of cellular membranes from lipoperoxidation, especially membranes rich in unsaturated lipids, such as mitochondria, endoplasmic reticulum, and plasma membranes.
Interrelationships between selenium and vitamin E
An important interrelationship exists between selenium, vitamin E and the sulfur-containing amino acids in preventing some of the nutritional diseases caused by their deficiency. If vitamin E prevents fatty acid hydroperoxide formation and the sulfur amino acids (as precursors of GSH-PX) and selenium are involved in peroxide destruction, these nutrients would produce a similar biochemical result, that is, lowering of the concentration of peroxides or peroxide-induced products in the tissues.1,2 Protection against oxidative damage to susceptible non-membrane proteins by dietary selenium, but not by vitamin E, might explain why some nutritional diseases respond to selenium but not to vitamin E. On the other hand, certain tissues or subcellular components may not be adequately protected from oxidant damage because they are inherently low in GSH-PX even with adequate dietary selenium. Damage to such tissues would be expected to be aggravated by diets high in unsaturated fatty acids and to respond adequately to vitamin E but not to selenium. The variations in GSH-PX activity between certain tissues, such as liver, heart, skeletal, and myocardial muscles, would explain the variations in the severity of lesions between species.
There are both selenium-dependent GSH-PX and non-selenium-dependent GSH-PX activities in the tissues and blood. The non-selenium-dependent enzyme does not contain selenium and does not react with hydrogen peroxide but shows activity toward organic hydroperoxide substrates. The spleen, cardiac muscle, erythrocytes, brain, thymus, adipose tissue, and striated muscles of calves contain only the selenium-dependent enzyme. The liver, lungs, adrenal glands, testes, and kidney contain both enzymes. Hepatic tissue contains the highest level of non-selenium-dependent enzyme.
Vitamin E can prevent a toxic reaction to oral iron (ferrous sulfate) or iron dextran IM. When 0.1 ppm of selenium and 50 IU vitamin E/kg are added to the gestation of sows, glutathione peroxidase activity increased in 2-day-old pigs, especially if the iron injection is given prior to colostrum ingestion.4
EPIDEMIOLOGY
Enzootic nutritional muscular dystrophy (NMD)
Occurrence
This muscular dystrophy occurs in all farm animal species, but most commonly in young, rapidly growing calves, lambs, goat kids, and foals born from dams that have been fed for long periods, usually during the winter months, on diets low in selenium and vitamin E.5 It is an important cause of mortality in goat kids from birth to about 3 months of age.1 Goat kids may require more selenium than lambs or calves, which may explain the higher incidence of the disease in kids. The disease in kids may also be associated with low α-tocopherol levels and normal selenium status. The literature on selenium and vitamin E deficiency in sheep and goats has been reviewed.2
NMD in horses occurs most commonly in foals to about 7 months of age.6 In reported cases, the concentration of selenium in the blood of the mares was subnormal, the concentrations of selenium and vitamin E in the feedstuffs were subnormal, the level of unsaturated fatty acids in the feed was high and vitamin E and selenium supplementation prevented the disease. The disease is not well-recognized in adult horses, but sporadic cases of dystrophic myodegeneration are recorded in horses from 5 to 10 years of age. Some baseline data for selenium and vitamin E concentration in horses from breeding farms is available.1
The disease also occurs in grain-fed yearling cattle. Stressors such as being turned outdoors after winter housing, walking long distances, the jostling and movement associated with vaccination, and dehorning procedures and the like are often precipitating factors. The disease has occurred in steers and bulls 12–18 months of age under feedlot conditions. There may even be laboratory evidence of subclinical myopathy in normal animals in a group from which an index case occurred. Outbreaks of severe and fatal NMD have occurred in heifers, at the time of parturition, which were previously on a diet deficient in both selenium and vitamin E. The disease may also occur sporadically in adult horses that are deficient in selenium. Muscular dystrophy has occurred in Bohemian Red Poll mature dairy cows in the Czech Republic moved from a stanchion barn into loose box housing which resulted in increased locomotor activity and stress associated with the change in housing conditions.7
Myopathy and hepatic lipidosis in weaned lambs deficient in vitamin E without concurrent selenium deficiency has been described.8
Geographical distribution
NMD occurs in most countries of the world but is common in the UK, the USA, Scandinavia, Europe, Canada, Australia, and New Zealand. In North America, it is common in the north-east and north-west and uncommon on the relatively high selenium soils of the Great Plains, where selenium toxicity has occurred. It is one of most common deficiency diseases of farm livestock in the USA.1 In the Czech Republic, the incidence of selenium deficiency in cattle is high and most frequently diagnosed in heifers, feeder bulls, grazed beef cattle, and dairy cows in the dry period.9 Surveys of live cattle in the Czech Republic and in cattle tissues obtained at slaughter have found significant deficiency of selenium.10,11 Poor selenium status, as assessed from blood, muscle, and liver selenium concentrations, was found in 80%, 70%, and 73% of the tested animals, respectively.11 White muscle disease has occurred in lambs in Turkey where the levels of selenium in the hay and soil are deficient.12 The mean values of selenium in the soil and hay were 0.03 ppm and 0.07 ppm, respectively.
NMD is endemic in grazing goats on the Mexican plateau because of selenium deficiency in the soil and forages.13 In two different locations of the plateau, the concentration of selenium in the soil was 0.047 and 0.051 ppm, in the forages 0.052 and 0.075 ppm, in the serum of goats, 0.02 and 0.21 ppm, respectively.13 The pH of the soil was 6.1 and 5.9, respectively. The mean concentration of selenium in the serum of kids with clinical signs of NMD was 36% lower compared with kids from the same farm which were normal.
Based on bulk tank milk selenium concentrations compared with serum selenium concentrations in dairy herds in Prince Edward Island, Canada, 59% of the herds were at some point marginal or deficient in selenium, which places them at risk of disease and suboptimal production.14 The periods of greatest risk were in the fall and winter when 5% and 4%, respectively, of herds fell in the range of true deficiency. Herds in which selenium supplementation was provided from a commercial dairy concentrate, were over 4 times more likely to be selenium-adequate than herds not using this method and adjusted average daily milk yield was 7.6% greater in herds determined to be selenium-adequate when compared with selenium-marginal herds.
Soils and therefore the pastures they carry, vary widely in their selenium content, depending largely on their geological origin. In general, soils derived from rocks of recent origin, e.g. the granitic and pumice sands of New Zealand, are notably deficient in selenium. Soils derived from igneous rocks are likely to be low in selenium. Sedimentary rocks, which are the principal parent material of agricultural soils, are richer in selenium. Forage crops, cereal grains, and corn grown in these areas are usually low in selenium content (below 0.1 mg/kg dry matter, DM), compared with the concentration in crops (above 0.1 mg/kg DM) grown in areas where the available soil selenium is much higher and usually adequate. The disease occurs in pigs, usually in association with other more serious diseases, such as mulberry heart disease and hepatosis dietetica.
Selenium in soil, plants and animals
Soils containing <0.5 mg/kg of selenium are likely to support crops and pastures with potentially inadequate selenium concentrations (<0.05 mg/kg DM).1,3
Plants vary in their uptake of selenium but selenium is not a requirement for plant growth.3 The selenium content of different pasture species on the same soil type does vary widely but slow growing and more deeply rooting species contained slightly higher concentrations.3 In New Zealand, the most deficient soils consist of rhyolitic pumice in the central volcanic plateau of the North Island. Peat soils in the Waikaito River Valley are also deficient. North Island coastal sands and stony soils in the several locations are considered to be selenium-responsive, while most of the South Island is at least marginally deficient.
In the USA, the States of the Pacific North-west and of the north-eastern and south-eastern seaboard are generally low in selenium.5 In Canada, western prairie grains generally contain relatively high levels of selenium, whereas in the eastern provinces, soils and feedstuffs usually have low selenium concentrations. Most soils in the Atlantic provinces of Canada are acidic and, consequently, the forages are deficient in selenium. Most forage samples contain less than 0.10 mg/kg DM of selenium and enzootic nutritional muscular dystrophy is common throughout the region.
Surveys in the UK found that the selenium status may be low in sheep and cattle fed locally produced feedstuffs without any mineral supplementation. In some surveys, up to 50% of farms are low in selenium, which places a large number of animals at risk. There are also differences in the selenium concentrations of different feeds grown in the same area. For example, in some areas 75% of cattle fed primarily corn silage, or 50% of the cattle fed sedge hay, might be receiving diets inadequate in selenium.
Several factors influence the availability of soil selenium to plants.
• Soil pH: alkalinity encourages selenium absorption by plants – and the presence of a high level of sulfur, which competes for absorption sites with selenium in both plants and animals, are two factors reducing availability
• Variation between plants in their ability to absorb selenium; ‘selector’ and ‘converter’ plants are listed under the heading of selenium poisoning; legumes take up much less selenium than do grasses
• Seasonal conditions also influence the selenium content of pasture, the content being lowest in the spring and when rainfall is heavy. Blood selenium in dairy cows in the USA were lower during the summer and fall than during the winter and spring.15 In this way, a marginally deficient soil may produce a grossly deficient pasture if it is heavily fertilized with superphosphate, thus increasing its sulfate content, if the rainfall is heavy and the sward is lush and dominated by clover as it is likely to be in the spring months.
Environmental sulfur from various anthropogenic activities has been suspected to be a significant factor in contributing to several health problems in livestock.1 Livestock producers near natural sour gas desulfurization plants have reported that sulfur emissions are responsible for an increased occurrence of nutritional muscular dystrophy, weak calves, and retarded growth. Experimentally, a moderate increase in dietary sulfur does not impair selenium and copper status, or cause related disease in cattle.
There may be wide variations in the serum selenium concentrations and glutathione peroxidase activities in cattle grazing forages of various selenium concentrations within the same geographical area. The selenium status of beef cows can vary between geographical areas within a region of a country, which is likely due to variations in selenium concentration of the soil and plants in these areas. Beef herds from areas with adequate soil levels of selenium, herds provided with supplemental feed on pasture and herds in which pregnancy diagnosis was done, had higher average herd blood selenium values than other herds.
Vitamin E
Vitamin E deficiency occurs most commonly when animals are fed inferior quality hay or straw or root crops. Cereal grains, green pasture, and well-cured fresh hay contain adequate amounts of the vitamin.
α-Tocopherol levels are high in green grasses and clovers, but there are wide variations in the concentrations from one area to another. The serum α-tocopherol levels are higher in calves born from cows fed grass silage than in those born from cows fed the same grass as hay. Many factors influence the α-tocopherol content of pasture and hence the animals’ intake. The level of α-tocopherol in pasture declines by up to 90% as it matures. Levels as low as 0.7 mg/kg DM have been reported in dry summer pastures grazed by sheep. The α-tocopherol content of ryegrass and clover pasture ranges from 22 to 350 mg/kg DM and 90–210 mg/kg DM, respectively. After harvesting and storage, the α-tocopherol content of pasture and other crops may fall further, sometimes to 0. Preservation of grain with propionic acid does not prevent the decline. Thus, the dietary intake of α-tocopherol by cattle and sheep may be expected to vary widely and lead to wide variations in tissue levels. The plasma vitamin E status of horses is highest from May to August in Canada when fresh grass is being grazed and lowest when the horses are being fed harvested or stored feed during the same period. Plasma vitamin E levels in dairy cows in the USA were higher during the summer and fall than during the winter and spring.15
Outbreaks of NMD may occur in yearling cattle fed on high-moisture grain treated with propionic acid as a method of inexpensive storage and protection from fungal growth. There is a marked drop in the vitamin E content of acid-treated grain and an increase in the levels of peroxides of fat, which is consistent with a loss of naturally occurring antioxidants such as the tocopherols (secondary vitamin E deficiency). In these situations, the levels of selenium in the feed were below 0.05 mg/kg DM, which is inadequate and emphasizes the interdependence of selenium and vitamin E. The α-tocopherol content of moist grain (barley and maize) stored for 6 months, with or without propionic acid, falls to extremely low levels compared with conventionally stored grain in which the α-tocopherol levels usually persist over the same length of time. Selenium-deficient barley treated with sodium hydroxide to deplete it of vitamin E can be used to induce NMD when fed to yearling cattle. The disease may occur in sucking lambs with low plasma α-tocopherol levels and an adequate selenium status, which indicates that the sparing effect of each nutrient may not occur over the broad spectrum of clinical deficiencies.
Polyunsaturated fatty acids (PUFAs) in diet
Diets rich in PUFA such as cod liver oil, other fish oils, fishmeal used as a protein concentrate, lard, linseed oil, soybean, and corn oils have been implicated in the production of NMD, particularly in calves fed milk replacers containing these ingredients. The disease can be reproduced experimentally in young ruminant cattle, 6–9 months of age, by feeding a diet low in vitamin E and selenium and adding a linolenic acid. There are widespread lesions of myodegeneration of skeletal and myocardial muscles.16 Fresh spring grass containing a sufficient concentration of linolenic acid to equal the amount necessary to produce NMD in calves may explain the occurrence of the naturally occurring disease in the spring months. The oxidation during rancidification of the oils causes destruction of the vitamin, thus increasing the dietary requirements (a conditioned vitamin E deficiency) and the presence of myopathic agents in the oils may also contribute to the occurrence of the disease. A secondary vitamin E deficiency occurs when NMD develops on rations containing vitamin E in amounts ordinarily considered to be adequate, but the disease is prevented by further supplementation with the vitamin. The lack of specificity of vitamin E in the prevention of muscular dystrophy in some circumstances is indicated by its failure and by the efficiency of selenium, as a preventive agent in lambs on lush legume pasture.
Other myopathic agents in diet
Not all of the myopathic agents that may be important in the development of NMD in farm animals have been identified. Unsaturated fatty acids in fish and vegetable oils may be myopathic agents in some outbreaks of NMD of calves and lambs. Lupinosis-associated myopathy in sheep is a substantial skeletal muscle myopathy encountered in weaner sheep grazing lupin stubbles infected with the fungus Phomopsis spp.17 Affected sheep have a stiff gait, walk reluctantly, stand with their back humped and their feet under the body, and have difficulty getting to their feet.
Unaccustomed exercise
Historically, NMD occurred most commonly in rapidly growing, well-nourished beef calves 2–4 months of age, shortly following unaccustomed exercise. This was commonplace in countries where calves were born and raised indoors until about 6–8 weeks of age when they were turned out onto new pasture in the spring of the year. This has been a standard practice in small beef herds in the UK, Europe, and North America. A similar situation applies for ewes that lambed indoors and the lambs were let out to pasture from 1 to 3 weeks of age. Thus, unaccustomed activity in calves and lambs running and frolicking following their turnout onto pasture is an important risk factor but is not necessarily a prerequisite for the disease. In lambs, the vigorous exertion associated with running and sucking may account for the peracute form of myocardial dystrophy in young lambs on deficient pastures and from deficient ewes. In older lambs up to 3 months of age, outbreaks of acute NMD and stiff-lamb disease may be associated with the driving of flocks long distances. A similar situation applies for calves that are moved long distances from calving grounds and early spring pastures to lush summer pastures. The wandering and bellowing that occurs in beef calves weaned at 6–8 months of age may precipitate outbreaks of subacute NMD. Degenerative myopathy of yearling cattle (feedlot cattle, housed yearling bulls and heifer replacements) is now being recognized with increased frequency. The disease resembles subacute NMD of calves and in the UK is often seen when yearlings are turned outdoors in the spring of the year after being housed during the winter and fed a poor quality hay or straw or propionic acid-treated grain. Unaccustomed exercise is a common precipitating factor. However, the disease has occurred in housed yearling bulls with no history of stress or unaccustomed exercise but whose diet was deficient in selenium and vitamin E.
In horses subjected to exercise, there is an increase in erythrocyte malondialdehyde, a product of peroxidation, but selenium supplementation has no beneficial effect. There is inconclusive evidence that a selenium-vitamin E deficiency causes NMD in adult horses. There is no evidence that paralytic myoglobinuria and the ‘tying-up’ syndrome are due to a deficiency of selenium and vitamin E.
Congenital nutritional muscular dystrophy
Congenital NMD is rare in farm animals. Isolated cases have been reported.18
Similarly, NMD can occur in calves and lambs only a few days of age but rarely. Selenium readily crosses the bovine placenta and fetal selenium is always higher than the maternal status.9 There is no evidence that the weak-calf syndrome is associated with selenium deficiency. Long-term parenteral supplementation with neither selenium alone nor in combination with vitamin E had any effect on the incidence of the weak-calf syndrome.
An investigation of aborted bovine fetuses with lesions of heart failure, specifically cardiac dilatation or hypertrophy along with a nodular liver and ascites compared with aborted fetuses without such lesions and non-aborted fetuses from the abattoir found myocardial necrosis and mean selenium levels of 5.5 μmol/kg in the fetuses with heart lesions, 6.5 μmol/kg in the fetuses without heart lesions and 7.5 μmol/kg selenium in the fetuses from the abattoir.19 This suggests that selenium deficiency in bovine fetuses may cause myocardial necrosis and heart failure. Normal levels of selenium in liver and kidney tissue of bovine fetuses derived from the abattoir were 7.5± 5.2 μmol/kg and 4.4± 1.1 μmol/kg, respectively.
In pigs, NMD has been produced experimentally on vitamin E- and selenium-deficient rations but is usually only a part of the more serious complex of mulberry heart disease and hepatosis dietetica.
Vitamin E-Selenium Deficiency (VESD) syndrome
Mulberry heart disease, hepatosis dietetica, exudative diathesis and nutritional myopathy, also known as the VESD syndrome (vitamin E and selenium deficiency) occur in pigs, usually as serious diseases. Nutritional muscular dystrophy may also occur in pigs. The occurrence of edema in various tissues has also been suggested as a possibility of Se or vitamin E deficiency. Impaired spermatogenesis and increased susceptibility to the effects of swine dysentery have also been suggested as responses to reduced levels of these two substances. There is a suspicion that the problems have become more common as the pig grows more quickly, the requirements have increased and the demands for anti-oxidants is increased at the same time that the provision of fat soluble vitamins is increasingly difficult. In addition, there is very small difference between the therapeutic and toxic levels of Se and Se toxicosis has occurred in an attempt to prevent Se deficiency. A more recent complication is the realization that we have been using inorganic Se to provide Se in the diet but that in the plant most of the Se is organic in the form of L-selenomethionine, a Se analogue of the amino acid methionine.20 In the pig as in other species they are believed to serve as antagonists to toxic free radicles and act in concert with other substances such as Vitamin C. Little is known about their metabolism in the pig. In the pig, there is very little transfer of fat soluble products across the placenta so there is very little reserve of vitamin E in the new born pig. Immediately after birth, the young pig gets its vitamin E from the colostrum and milk of the sow. If the sow has low body stores or is fed a ration low in vitamin E then the piglet will be very low in vitamin E when it is weaned. Each can substitute for the other in a limited way in the pig. In the pig the diet has the most influence. Diets rich in polyunsaturated fatty acids, copper, Vitamin A or mycotoxins may reduce the availability of vitamin E. As dietary vitamin A levels increase, serum and liver α-tocopherol concentrations decline, suggesting a reduced absorption and retention of α-tocopherol when weaned pigs were fed high dietary vitamin A levels.21 Se antagonists or crops from inherently low soil Se fields may also make the situation worse. In pigs, NMD has been produced experimentally on vitamin E- and Se-deficient rations but is usually only a part of the more serious complex of mulberry heart disease and hepatosis dietetica. Microangiopathy is most common in weaned pigs22 and may be particularly related to vitamin E deficiency.23
There is conflicting evidence on the effect of the anti-oxidative vitamins C and E on the reproductive performance of sows. In some studies,24,25 increasing dietary vitamin E in the diet during gestation may have increased the litter size and reduced the pre-weaning piglet mortality. A similar response has been seen following intra-muscular injection of sows with vitamin E and Se26,27 but the injection of vitamin C has produced no improvement.28,29 A recent study30 has confirmed that there was no effect on reproductive performance of sows and growth performance of piglets when supplemented by both vitamin E and C. Vitamin E and Se given to immature gilts for flushing purposes led to the formation of fewer but larger corpora lutea after ovulation, probably due to the progression of a smaller number of follicles to the ovulatory stage.31 Vitamin E and the Se increased the development of the uterus but did not influence the number of piglets at farrowing.
VESD occurs naturally in rapidly growing pigs, usually during the post-weaning period (3 weeks to 4 months), particularly during the early finishing period. The lowest concentration of vitamin E in piglets was day 45 after farrowing32 but it may be that the Se status of the newborn piglets may be more important for their health than their vitamin E status. The first 3–4 weeks following the move to the finishing house is the most dangerous period for a low vitamin E level33 and it is important to remember that there is considerable individual variation. Serum vitamin E declines after weaning and even with vitamin E supplementation it takes 2–3 days for levels to rise.34,35 There appears to be a temporary decreased absorption of the vitamin in the immediate post-weaning period and this in turn leads to the reduction of the stored vitamin E reserves. It is usually associated with diets deficient in both Se and vitamin E and those that may contain a high concentration of unsaturated fatty acids. Such diets include those containing mixtures of soybean, high-moisture corn and the cereal grains grown on soils with low levels of Se. The feeding of a basal ration of cull peas, low in Se and vitamin E, to growing pigs can cause the typical syndrome and low tissue levels of Se are present in pigs with spontaneously occurring hepatosis dietetica. It has been shown that feeding diets that contain linseed oil unfortunately reduced the vitamin E levels in the diet but increased the skatole levels.36 However, there are reports of naturally occurring mulberry heart disease of pigs in Scandinavia in which the tissue levels of Se and vitamin E are within normal ranges compared with normal pigs.37 In Ireland, in spite of supplementation of pig rations with vitamin E and Se at levels higher than that necessary to prevent experimental disease, spontaneous mulberry heart disease may still occur.23 Affected pigs have lower tissue vitamin E levels than control pigs, which suggests an alteration in α-tocopherol metabolism unrelated to dietary Se and PUFA contents.
Natural occurrence of the disease complex in pigs is not uncommonly associated with diets containing 50% coconut meal, fish-liver oil emulsion, fish scraps with a high content of unsaturated fatty acids, or flaxseed, which produces yellow and brown discoloration of fat preventable by the incorporation of adequate amounts of α-tocopherol or a suitable antioxidant. The quality of the dietary fat does not necessarily influence blood vitamin E levels, but the presence of oxidized fat reduces the resistance of the red blood cells against peroxidation. The higher requirement for vitamin E by pigs fed oxidized fat may be due to the low vitamin E content in such fat. It has recently been shown38 that the inclusion of 0.3 ppm Se to the diet of post-weaning piglets resulted in better performance than non-Se supplemented diets irrespective of the level of vitamin E in the ration (up to 200 ppm).
Mulberry heart disease
This is the most common form of Se and vitamin E deficiency of pigs. It occurs most commonly in rapidly growing feeder pigs (60–90 kg) in excellent condition being fed on a high-energy diet low in vitamin E and Se. The true causal mechanism is not known but it can be prevented by supplementation with vitamin E. It can also occur when it would appear that the level in the diet and in the serum or tissues appear to be satisfactory. The diets most commonly incriminated are soybean, corn, and barley. Mean liver concentrations of vitamin E were lower in pigs with MHD than in pigs that died of causes other than MHD.39 The α-tocopherol content of corn is usually low and it is virtually absent from solvent-extracted soybean meal. Both are low in Se. The use of high-moisture corn may further exacerbate the tocopherol deficiency. The level of PUFAs in the diet was thought to be an important etiological factor but this is now not considered to be a necessary prerequisite. Outbreaks of the disease may occur in which 25% of susceptible pigs are affected and the case mortality rate is about 90%. The disease has occurred in young piglets and in adult sows.
Hepatosis dietetica
Hepatosis dietetica appears to be less common than mulberry heart disease but the epidemiological characteristics are similar. It appears to be less common since the Se levels in supplements were raised to 0.3 ppm. It affects young growing pigs up to 3–4 months of age. NMD in pigs usually occurs in cases of mulberry heart disease and hepatosis dietetica but it has occurred alone in gilts.
Selenium-responsive disorders
A variety of diseases have been known as selenium-responsive disorders3,40 because they respond beneficially to the strategic administration of selenium. These include: ill-thrift in lambs and calves on pasture; lowered milk production in cows; white muscle disease in lambs, calves, and kids; lowered fertility and embryonic death in sheep and cattle; retained fetal membranes, metritis, poor uterine involution, and cystic ovaries in cows; subclinical mastitis and impaired immune function in cattle; and prematurity, perinatal death, and abortion in cattle.3 Of these, only ill-thrift, lowered fertility, lowered milk production, and white muscle disease have been reported in New Zealand.3 The literature on the roles of selenium deficiency in grazing ruminants in New Zealand and a rational approach to diagnosis and prevention has been reviewed.3
The pathogenesis of these selenium-responsive diseases is not well understood but it would appear that the selenium deficiency is only marginal. Most investigations into selenium-responsive diseases have occurred in selenium-deficient areas in which diseases such as NMD of calves and lambs occur. The evidence that selenium deficiency in breeding ewes can result in a decline in reproductive performance has not been substantiated experimentally. Reproductive performance was not affected in ewes on a selenium-depleted diet.
Selenium-responsive unthriftiness in sheep has received considerable attention in New Zealand where the response to selenium administration has been most dramatic compared with Australia where the syndrome has also been recognized but where the response is much smaller. The oral administration of selenium to lambs in these areas results in greater body weight gains from weaning to 1 year of age compared with lambs not receiving selenium supplementation. The mean fleece weight of selenium-treated lambs is also greater.
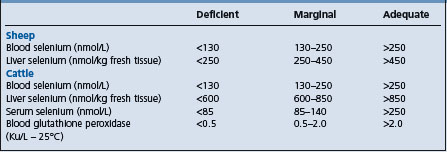
The diagnosis of selenium-responsive unthriftiness depends on analyses of the soil, pasture and animal tissues for selenium and response trials to selenium supplementation. A deficiency state might be encountered when the selenium content of the soil is below 0.45 mg/kg, the pasture content below 0.02 mg/kg DM, the liver content below 21 μg/kg (0.27 μmol/kg) (WW), and wool concentrations below 50–60 μg/kg (0.63–0.76 μmol/kg). For the blood in selenium-responsive unthriftiness of sheep the following criteria are suggested:
The GSH-PX activity is a good index of the selenium status of sheep with a selenium-responsive disease. If measured on a regular basis, it can provide an indication of the selenium status of grazing sheep in individual flocks. Single measurements of GSH-PX activity may fail to detect recent changes in grazing area, differences in pasture species and pasture composition and alterations in the physiological state of the animals.
Subclinical selenium insufficiency
Subclinical insufficiencies of selenium in grazing ruminants are widespread over large areas of southern Australia.1 The plasma concentrations of affected sheep flocks are low, there are no obvious clinical signs of insufficiency in the ewes and there are significant responses in wool production and fiber diameter to selenium supplementation. The incidence of estrus and fertility is not affected by selenium supplementation. Live weights at birth, in mid-lactation, and at weaning were increased in lambs born to selenium-supplemented and crossbred ewes and in lambs born as singletons. Clean fleece weight at 10 months of age was increased by 9.5% and fiber diameter by 0.3 μm in lambs born to ewes that had received supplementary selenium. Differences in fleece weight and live weight were not detected at 22 months, suggesting that subclinical selenium insufficiency in early life did not permanently impair productivity if selenium status subsequently increased.
Temporal variations in glutathione peroxidase activity in sheep can be used to identify seasons of the year with the highest risk of selenium deficiency.41 In the Mediterranean area, lambs born in the spring/summer are at higher risk to selenium-deficiency related diseases.41 Lambs born in autumn/winter are from ewes gestating during the summer, when supplementation with cereal grains is provided.
Selenium is a component of type-I iodothyronine deiodinase, which catalyzes the extrathyroidal conversion of thyroxine (T4) to the more active tri-iodothyronine (T3). Sheep grazing pastures low in selenium frequently have higher circulating T4 and lower circulating T3 concentrations than sheep receiving selenium supplementations.
When ewes grazing pastures low in selenium were supplemented thiocyanate (to cause iodine insufficiency), iodide and selenium, there was no evidence of clinical deficiencies. Growth rates of lambs were not affected by thiocyanate of their dams during mid-pregnancy, but plasma T3 and T4 concentrations were depressed in ewes receiving thiocyanate. The iodide supplementation increased thyroid hormone concentrations in ewes, but depressed plasma T3 concentrations in lambs. Supplementation of sheep grazing pastures low in selenium with both selenium and thyroid hormones improved wool characteristics, live weight gain, and blood selenium, but there was no evidence of an interaction between the selenium and the hormones. Thus, it seems unlikely that the decline in the quantity of T3 produced, or of T4 utilized for T3 production, in selenium-deficient sheep is responsible for the observed differences in the productivity of selenium-deficient and supplemented sheep. The thyroids have a major role in regulating thermogenesis and lambs born to ewes supplemented with iodide tend to have higher rectal temperatures during cold stress. The thermoregulatory ability of the perinatal lamb is not adversely affected by subclinical selenium deficiency.
In a survey of the status of vitamin E and selenium of the livers of cull ewes and market lambs raised in Ontario, selenium was present at marginal levels in 3.3% of cull ewe samples and in 43% of market lamb samples.16 Vitamin E was low to deficient in 10% of cull ewe samples and in 90% of market lamb samples. In cull ewes, there was a strong relationship between selenium and vitamin E. A large percentage of samples with marginal selenium values had adequate vitamin E, which may indicate that the sheep had access to high levels of vitamin E but received inadequate levels of supplement containing selenium.
An evaluation of the trace mineral status of beef cows in Ontario found that 96% of cull cows were deficient in blood selenium.42,43 Based on analysis of serum samples from cattle in Iowa and Wisconsin, subclinical selenium deficiency is common in the cattle population.44 The serum levels may be adequate for reproductive performance but marginal for optimal resistance to mastitis or for adequate transfer of selenium to the calf.
Reproductive performance
The published information on the effects of vitamin E and selenium deficiency or of dietary supplementation with one or the other or both on reproductive performance in farm animals are conflicting and controversial. Reproductive performance is complex and dependent on the interaction of many factors. Reproductive inefficiency is likewise complex and it is difficult to isolate one factor like a deficiency of vita min E or selenium as a cause of reproductive inefficiency. Conversely, it is difficult to prove that supplementation with these nutrients will ensure optimum reproductive performance.45
Sheep
The evidence about the effect of selenium and vitamin E deficiency on reproductive performance in sheep is conflicting. Observations in the 1960s concluded that selenium deficiency caused embryonic deaths 20–30 days after fertilization in ewes. But supplementation of ewes, low or marginal in selenium status, with selenium did not improve reproductive performance. Experimental studies using selenium-deficient diets in ewes have been unable to find any adverse effects of selenium depletion on ewe conception rates, embryonic mortality, or numbers of lambs born. The parenteral administration of selenium to pregnant ewes between 15 and 35 days after mating resulted in a reduced embryonic survival rate and is not recommended during the first month of pregnancy.46
Cattle
Vitamin E supplementation can have significant effects on the health and some aspects of fertility in lactating dairy cows.47 When used at four times the current recommendations. Vitamin E supplementation of dairy has its most beneficial effect of reducing the incidence of mastitis when used at rates of at least 1000 IU per day during the dry period and early lactation. The primary effect of vitamin E supplementation is on the immune system. The importance of selenium and vitamin E for the maintenance of optimum reproductive performance is not clear.47 The IM injection of dairy cattle with selenium and vitamin E 3 weeks prepartum did not have any effect on average days to first estrus or first service, average days to conception, services per conception, or number of uterine infusions required. The prepartum IM injection of vitamin E and selenium 3 weeks prepartum increased the percentage of cows pregnant to first service, reduced the number of services per conception, decreased the incidence of retained placenta and reduced the interval from calving to conception. In a randomized field trial in a large dairy herd in the USA, oral supplementation of pregnant first-calf dairy heifers with selenium using a commercially available sustained-release intra-ruminal selenium bolus, increased blood selenium concentrations in treated animals at 30 days after treatment until after calving.48 However, based on data analyzed mid-lactation and late lactation, there were no differences between treated and control groups in somatic cell count, days not pregnant, total milk production, or times bred. The use of an intra-ruminal pellet of selenium at two different levels in dairy herds in New Zealand was evaluated in yearling heifers.49 The recommended dose was effective in elevating whole blood GSH-PX activity and selenium concentrations to over 10 times those of control animals. Milk production was increased and there was a trend to decreased somatic cell counts. There were no differences in calving-first-service or calving-conception intervals, or in the percentage of animals pregnant to first or all services. In other observations, following the treatment of dairy cows with oral selenium pellets there was an improvement in first service conception rate and significantly higher blood levels of GSH-PX. The inconsistent results obtained following the use of selenium and vitamin E in pregnant cows may be related to the selenium status of the animals; in some herds the blood levels are marginal and in others the levels are within the normal range.
Winter-fed lactating Norwegian dairy cows were found to have an adequate plasma levels of vitamin E and marginal-to-adequate levels of blood selenium. Silage was the most important source of vitamin E and selenium-supplemented commercial concentrates the most important source of selenium. No significant differences in vitamin E or selenium status was found between cows with or without recorded treatments of mastitis, parturient paresis, or reproductive abnormalities.50
Retained fetal placenta
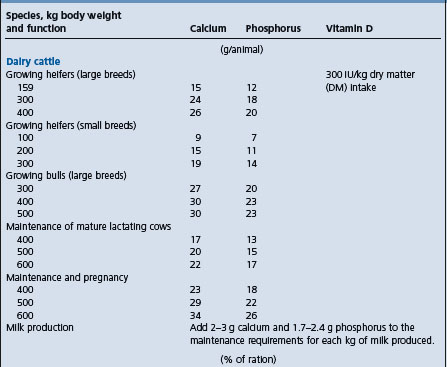
A high incidence (more than 10%) of retained fetal membranes has been associated with marginal levels of plasma selenium compared with herds without a problem. In some cases, the incidence could be reduced to below 10% by the injection of pregnant cattle with selenium and vitamin E about 3 weeks prepartum, while in other studies similar prepartum injections neither reduced the incidence nor improved reproductive performance. A single injection of selenium 3 weeks prepartum can reduce the number of days postpartum required for the uterus to reach minimum size and to reduce the incidence of metritis and cystic ovaries during the early postpartum period. The parenteral administration of a single injection of 3000 mg vitamin E prepartum to dairy cows of all ages decreased the incidence of retained placenta and metritis to 6.4% and 3.9%, respectively, in the treated group, compared with 12.5% and 8.8%, in the control group.51 The injection, 20 days pre-partum, of 50 mg of selenium and 680 IU of vitamin E reduced the incidence of retained fetal membranes in one series, but did not in another series. The plasma selenium concentration at parturition ranged from 0.02 to 0.05 ppm in control cows in which there was an incidence of 51% retained membranes and from 0.08 to 0.1 ppm in treated cows in which the incidence was reduced to 9%. A dietary level of 0.1 mg/kg DM selenium is recommended to minimize the incidence of the problem. The complex nature of the etiology of retained fetal membranes also requires a well-designed experimental trial to account for all of the possible factors involved.
Resistance to infectious disease
Many studies have examined the role of selenium and vitamin E resistance to infectious disease. Most of the evidence is based on in vitro studies of the effects of deficiencies of selenium or vitamin E or supplementation with the nutrients on leukocyte responses to mitogens, or on the antibody responses of animals to a variety of pathogens. The status of selenium and vitamin E in an animal can alter antibody response, phagocytic function, lymphocyte response and resistance to infectious disease. The administration of vitamin E and selenium during the dry period can influence mammary gland health and milk cell counts in dairy ewes.52 In general, a deficiency of selenium results in immunosuppression and supplementation with low doses of selenium augments immunological functions. A deficiency of selenium has been shown to inhibit:
• Resistance to microbial and viral infections
• Proliferation of T and B lymphocytes in response to mitogens
• Cytodestruction of T lymphocytes and natural killer lymphocytes.53
Vitamin E and selenium have interactive effects on lymphocyte responses to experimental antigens.54
Vitamin E supplementation of transport-stressed feedlot cattle is associated with reduced serum acute-phase protein concentrations compared with control animals.55 Supplementation of the diet of cattle arriving in the feedlot with vitamin E had beneficial effects on humoral immune response and recovery from respiratory disease.56
The parenteral administration of selenium and vitamin E during pregnancy in dairy cows has a positive effect on the increase of selenium and vitamin E concentrations in blood, increase of selenium and immunoglobulins concentrations in colostrum and an increase of T3 concentration in blood on the day of parturition.57 In addition, there was a trend toward a decreased incidence of clinical mastitis.
Neutrophil function
Selenium deficiency can affect the function of polymorphonuclear neutrophils (PMNs), which are associated with physiological changes in GSH-PX levels. In calves on an experimental selenium-deficient diet, the oxygen consumption and the activities of GSH-PX are lower than normal in neutrophils. The feeding of 80–120 mg of selenium/kg of mineral mixture provided ad libitum is an effective method of increasing blood selenium in a group of cattle and optimizing the humoral antibody response experimentally. It is suggested that blood selenium levels over 100 μg/L are necessary to maintain optimum immunocompetence in growing beef cattle.58 In selenium-deficient goats, the production of leukotriene B4, a product of neutrophil arachidonic acid lipoxygenation and a potent chemotactic and chemokinetic stimulus for neutrophils, is decreased, resulting in dysfunction of the neutrophils. A deficiency of selenium in pregnant sows impairs neutrophil function and vitamin E deficiency impairs function of both neutrophils and lymphocytes, which may result in increased susceptibility of their piglets to infectious diseases.59 It is suggested that selenium supplementation be maintained at 0.3 mg/kg of the diet.
Neutrophils from postparturient dairy cows with higher levels of selenium have greater potential to kill microbes and cattle with greater superoxide production may have higher milk production.49 Vitamin E is a fat-soluble membrane anti-oxidant which enhances the functional efficiency of neutrophils by protecting them from oxidative damage following intracellular killing of ingested bacteria. Peripartum immunosuppression in dairy cows is multifactorial but is associated with endocrine changes and decreased intake of critical nutrients. Decreased phagocytosis and intracellular killing by neutrophils occur in parallel with decreased dry matter intake and decreased circulating vitamin E. Since neutrophils are the primary mechanism of uterine defense and mammary health, the role of vitamin E on the health of dairy cows during the transition period have been examined. Compared with control cows given a placebo, the parenteral administration of vitamin E 1 week prepartum had no effect on the incidence of retained placenta, clinical mastitis, metritis, endometritis, ketosis, displaced abomasum or lameness.60 However, there was a decreased incidence of retained placenta in cows with marginal pretreatment vitamin E status. An increase in α-tocopherol of 1 μg/mL in the last week prepartum reduced the risk of retained placenta by 20%.61 In addition, serum non-esterified fatty acid concentration ≥ 0.5 mEq/L tended to increase the risk of retained placenta by 80% and in the last week pre-partum, a 100 ng/mL increase in serum retinol was associated with a 60% decrease in the risk of early lactation clinical mastitis.61
Immune response
The effects of selenium deficiency and supplementation on the immune response of cattle to experimental infection with the infectious bovine rhinotracheitis virus and sheep to parainfluenza-3 virus, indicates that a deficiency can affect the humoral response and supplementation enhances the response. The administration of selenium either alone or in combination with vitamin E can improve the production of antibodies against E. coli in dairy cows.62 Pigs fed a vitamin E- and selenium-deficient diet develop an impaired cell-mediated immunity as measured by lymphocyte response to mitogenic stimulations. Supplementation of the diets of young pigs with selenium at levels above those required for normal growth have increased the humoral response, but not in sows. The wide variations in antibody responses that occur in these experiments indicate that there is a complex relationship between the selenium status of the host, humoral immune responses and protective immunity. The concept of using selenium supplementation to enhance antibody responses in sheep to vaccines is probably unfounded. However, the administration of sodium selenite to sheep vaccinated against enzootic abortion (Chlamydophila abortus) increased the antibody response but not when given with vitamin E.63
Vitamin E can stimulate the immune defense mechanisms in laboratory animals and cattle, experimentally. In most cases, the immunostimulatory effects of additional vitamin E are associated with supplementation in excess of levels required for normal growth. The parenteral administration to calves of 1400 mg of vitamin E weekly increases their serum vitamin E concentrations and lymphocyte stimulation indices. Similarly in growing pigs, a serum vitamin E concentration above 3 mg/L was necessary to achieve a significant response of the lymphocytes to stimulation with mitogens.
General resistance
These changes may render selenium-deficient animals more susceptible to infectious disease, but there is no available evidence to indicate that naturally occurring selenium and vitamin E deficiencies are associated with an increase in the incidence or severity of infectious diseases. Neutrophils from selenium-deficient animals lose some ability to phagocytose certain organisms, but how relevant this observation is in naturally occurring infections is unclear. Field studies of the incidence and occurrence of pneumonia in housed calves found that selenium status was not a risk factor.
Transfer of selenium and vitamin E to the fetus, colostrum, and milk
In sheep, selenium is transferred across the placenta to the fetus and maternal selenium status during gestation is positively associated with fetal and newborn lamb selenium status.1 Supplementation of gestating ewes with selenium will improve the selenium status of the lambs at birth. However, after birth the selenium of the lamb is depleted quickly by about 18 days after birth. Thus, continued intake of selenium by the lamb is necessary to maintain normal selenium status during the postnatal period. The colostrum of ewes contains higher levels of selenium than ewe milk. The selenium content of ewe’s milk decreases rapidly after parturition, reaching a stable level by 1 week post partum. Supplementation of ewes during lactation results in higher milk selenium concentration and higher blood selenium in lambs. Supplementation of ewes has been shown to prevent nutritional myodegeneration in nursing lambs in selenium-deficient flocks.2
There is a highly significant relationship between blood selenium of cattle and milk selenium concentration.64 As in sheep, in cattle, selenium is transferred across the placenta to the fetus and across the mammary barrier into the colostrum and milk.9
The maternal intake of selenium affects fetal liver selenium and newborn piglets have lower liver selenium concentrations compared with their dams, regardless of selenium intake of sows during gestation.65 Thus compared with cattle and sheep, the relatively high concentration of selenium needed in the diets of young rapidly growing piglets may be partially a function of limited placental transport or hepatic deposition of selenium and why the piglet is more susceptible to selenium deficiency than the sow.
The transfer of vitamin E across the placenta to the fetus in sheep and cattle is limited.2 Plasma levels of vitamin E in the fetus and in newborn lambs (before ingestion of colostrum) are lower than in the ewe.2 Vitamin E supplementation of the ewe in late gestation results in insignificant increases of the serum vitamin E in the lamb. However, supplementation of the ewe in the last month of pregnancy increases the vitamin E content of colostrum and milk.2 Colostrum of the ewe is a rich source of vitamin E for the neonatal lamb, containing 5–11 times more vitamin E than milk at 1 week post partum. The parenteral administration of sodium selenite to ewes at lambing increases the vitamin E content of milk of ewes over the first 5 weeks of lactation, indicating a potential positive effect of selenium repletion on vitamin E transfer to milk.2
Neonatal morbidity and mortality
Based on some preliminary observations of the selenium content of hair samples of young calves, higher selenium levels in newborn calves may have some protective effect against morbidity due to neonatal disease. Similarly, neonatal piglets with high blood levels of GSH-PX activity may be more resistant to infectious diseases or other causes of neonatal mortality. Administration of vitamin E and selenium to dairy cows in late pregnancy resulted in the production of increased quantities of colostrum and the calves have increased quantities of GSH-PX at birth and 28 days of age, but the improved selenium status did not provide any improvement in passive immunity or growth.66 Supplementing selenium to beef cows grazing selenium-deficient pastures with a salt mineral mix containing 120 mg selenium/kg of mix increased the selenium status of the cows and increased the serum IgG concentration, or enhanced transfer of IgG from serum to colostrum and increased the selenium status of the calves.67 The parenteral administration of 0.1 mg Se and 1 mg of vitamin E/kg BW at mid-gestation did not affect the production of systemic or colostral antibodies. Supplementation of dairy cows at dry-off with selenium at 3 mg/d as selenite via an intra-ruminal bolus resulted in sufficient transfer of selenium to meet a target concentration of more than 2.2 μg of selenium/g of liver DM in newborn calves.68
Mastitis in dairy cattle
There is some evidence that a dietary deficiency of vitamin E may be associated with an increased incidence of mastitis in dairy cattle.18 An increased incidence of mastitis during the early stages of lactation coincides with the lowest plasma concentration of vitamin E. Supplementation of the diet of dairy cows beginning 4 weeks before and continuing for up to 8 weeks after parturition with vitamin E at 3000 IU/cow per day, combined with an injection of 5000 IU, 1 week before parturition, prevented the suppression of blood neutrophil and macrophage function during the early postpartum period compared with controls. The vitamin E prevented the suppression of blood neutrophils during the postpartum period.69 Cows in both the treated and control groups were fed diets containing selenium at 0.3 ppm of total dry matter. When selenium status in dairy cows is marginal, plasma concentrations of α-tocopherol should be at least 3 μg/mL.70 Cows receiving a dietary supplement of about 1000 IU/d of vitamin E had 30% less clinical mastitis than did cows receiving a supplement of 100 IU/d of vitamin E.70 The reduction was 88% when cows were fed 4000 IU/d of vitamin E during the last 14 days of the dry period.70
The selenium status of dairy cows may also have an effect on the prevalence of mastitis and mammary gland health.57 Dairy herds with low somatic cell counts had significantly higher mean blood GSH-PX and higher whole blood concentrations of selenium than in herds with high somatic cell counts. The prevalence of infection due to Streptococcus agalactiae and Staphylococcus aureus was higher in herds with the high somatic cell counts compared with those with the low somatic cell counts. This suggests that phagocytic function in the mammary gland may be decreased by a marginal selenium deficiency. In a survey of cattle in herds in Switzerland, those with chronic mastitis had lower serum levels of selenium than healthy control herds. Experimental coliform mastitis in cattle is much more severe in selenium-deficient animals than selenium-adequate animals. The severity was in part due to the increased concentrations of eicosanoids.
Milk neutrophils from cows fed a selenium-deficient diet have significantly reduced capacity to kill ingested Escherichia coli and Staph. aureus, compared with cells from cows fed a selenium-supplemented diet. However, other experimental results are not as convincing.
Blood abnormalities
In young cattle from areas where NMD is endemic and particularly at the end of winter housing, the erythrocytes have an increased susceptibility to hemolysis following exposure to hypotonic saline. During clinical and subclinical white muscle disease in calves, there is a significant increase in both the osmotic and the peroxidative hemolysis of the erythrocytes. This defect is thought to be the result of alterations in the integrity of cell membranes of which tocopherols are an essential component. Abnormalities of the bone marrow associated with vitamin E deficiency in sheep have been described and abnormal hematological responses have been described in young growing pigs on an experimental selenium- and vitamin E-deficient diet. Vitamin E deficiency in sheep results in increased hemolytic susceptibility of erythrocytes, which may provide a basis for a single functional test for vitamin E deficiency in sheep.
Anemia characterized by a decreased packed cell volume, decreased hemoglobin concentration and Heinz body formation has been observed in cattle grazing on grass grown on peaty muck soils in the Florida everglades. Selenium supplementation corrected the anemia, prevented Heinz body formation, increased the body weight of cows and calves and elevated blood selenium.
Equine degenerative myeloencephalopathy
Equine degenerative myeloencephalopathy, which may have an inherited basis, has been associated with a vitamin E deficiency. The vitamin E status is low in some affected horses and supplementation with the vitamin was associated with a marked reduction in the incidence of the disease. However, serum vitamin E and blood GSH-PX activities determined in horses with histologically confirmed diagnosis of the disease compared with age-matched controls failed to reveal any differences and the findings did not support a possible role for vitamin E deficiency as a cause. Foals sired by a stallion with degenerative myeloencephalopathy and with neurological deficits consistent with the disease during their first year of life had lower plasma levels of α-tocopherol when the levels were determined serially beginning at 6 weeks to 10 months of age than age-matched controls. Absorption tests with vitamin E revealed that the lower α-tocopherol levels were not due to an absorption defect.
Equine motor neuron disease
This is a neurodegenerative disease of the somatic lower motor neurons resulting in a syndrome of diffuse neuromuscular disease in the adult horse.71 Case-control studies found the mean plasma vitamin E concentrations in affected horses were lower than that of control horses. Adult horses are affected with the risk peaking at 16 years of age. In addition to the role of vitamin E depletion, other individual and farm-level factors, contribute to the risk of developing the disease.
Generalized steatitis
Steatitis in farm animals and other species may be associated with vitamin E and/or selenium deficiency. Most cases in horses have involved nursing or recently weaned foals. Generalized steatitis in the foal has been described as either generalized cachexia due to steatitis alone, or as a primary myopathy or myositis with steatitis of secondary importance. The terms used have included steatitis, generalized steatitis, fat necrosis, yellow fat disease, polymyositis, and muscular dystrophy. The relationships between steatitis and vitamin E and selenium deficiency in the horse are not clear and there may be none. Many more clinical cases must be examined in detail before a cause–effect relationship can be considered.
PATHOGENESIS
The literature on the antioxidant roles of selenium and vitamin E have has been reviewed.2 Dietary selenium, sulfur-containing amino acids and vitamin E act synergistically to protect tissues from oxidative damage.1,2 GSH-PX, which is selenium-dependent, functions by detoxifying lipid peroxides and reducing them to non-toxic hydroxy fatty acids. Vitamin E prevents fatty acid hydroperoxide formation. High levels of PUFAs in the diet increase the requirements for vitamin E and, with an inadequate level of selenium in the diet, tissue oxidation occurs, resulting in degeneration and necrosis of cells. Vitamin E protects cellular membranes from lipoperoxidation, especially membranes rich in unsaturated lipids, such as mitochondric, endoplasmic reticulum and plasma membranes. Thus, dietary PUFA are not a prerequisite for the disease. Diets low in selenium and/or vitamin E do not provide sufficient protection against the ‘physiological’ lipoperoxidation that occurs normally at the cellular level.
The relative importance of selenium, vitamin E and sulfur-containing amino acids in providing protection in each of the known diseases caused by their deficiency is not clearly understood. Selenium has a sparing effect on vitamin E and is an efficient prophylactic against muscular dystrophy of calves and lambs at pasture, but does not prevent muscular dystrophy in calves fed on a diet containing cod liver oil. The current understanding of the biochemical function of selenium and its relation to vitamin E and the mechanisms of action of selenium and vitamin E in protection of biological membranes has been reviewed.72
Nutritional muscular dystrophy
A simplified integrated concept of the pathogenesis of the NMD would be as follows. Diets deficient in selenium and/or vitamin E permit widespread tissue lipoperoxidation leading to hyaline degeneration and calcification of muscle fibers. One of the earliest changes in experimental selenium deficiency in lambs is the abnormal retention of calcium in muscle fibers undergoing dystrophy and selenium supplementation prevents the retention of calcium. Unaccustomed exercise can accelerate the oxidative process and precipitate clinical disease. Muscle degeneration allows the release of enzymes, such as lactate dehydrogenase, aldolase and creatine phosphokinase, the last of which is of paramount importance in diagnosis. Degeneration of skeletal muscle is rapidly and successively followed by invasion of phagocytes and regeneration. In myocardial muscle, replacement fibrosis is the rule.
In calves, lambs, and foals, the major muscles involved are skeletal, myocardial and diaphragmatic. The myocardial and diaphragmatic forms of the disease occur most commonly in young calves, lambs, and foals, resulting in acute heart failure, respiratory distress, and rapid death, often in spite of treatment. The skeletal form of the disease occurs more commonly in older calves, yearling cattle, and older foals and results in weakness and recumbency, is usually less severe and responds to treatment. The biceps femoris muscle is particularly susceptible in calves and muscle biopsy is a reliable diagnostic aid.
In foals with NMD, there is a higher proportion of type IIC fibers and a lower proportion of type I and IIA fibers than in healthy foals. The type IIC fibers are found in fetal muscle and are undifferentiated and still under development. During the recovery period, fibers of types I, IIA, and IIB increase and the proportion of type IIC fibers decreases. A normal fiber type composition is present in most surviving foals 1–2 months after the onset of the disease.
Acute NMD results in the liberation of myoglobin into the blood, which results in myoglobinuria. This is more common in horses, older calves, and yearling cattle, than in young calves whose muscles have a lower concentration of myoglobin. Hence, the tendency to myoglobinuria will vary depending on the species and age of animal involved.
Subclinical selenium insufficiency
Selenium deficiency affects thyroid hormone metabolism and may explain the cause of ill-thrift. The conversion of the iodine-containing hormone, thyroxine (T4) to the more potent triiodothyronine (T3) is impaired in animals with low selenium status and iodothyroninedeiodinase is a selenoprotein which mediates this conversion.72
VESD syndrome and others
The pathogenesis of mulberry heart disease, hepatosis dietetica, exudative diathesis, and muscular dystrophy of pigs is not yet clear. Vitamin E and Se are necessary to prevent widespread degeneration and necrosis of tissues, especially liver, heart, skeletal muscle, and blood vessels. Se and vitamin E deficiency in pigs results in massive hepatic necrosis (hepatosis dietetica), degenerative myopathy of cardiac and skeletal muscles, edema, microangiopathy, and yellowish discoloration of adipose tissue. Myocardial and hepatic calcium concentrations are increased in pigs with mulberry heart disease.73,74 In addition, there may be esophagogastric ulceration, but it is uncertain whether or not this lesion is caused by a Se and/or vitamin E deficiency. Anemia has also occurred and has been attributed to a block in bone marrow maturation, resulting in inadequate erythropoiesis, hemolysis or both. However, there is no firm evidence that anemia is a feature of Se and vitamin E deficiency in pigs. The entire spectrum of lesions has been reproduced experimentally in pigs with natural or purified diets deficient in Se and vitamin E, or in which an antagonist was added to inactivate vitamin E or Se. However, in some studies, the Se content of tissues of pigs that died from mulberry heart disease was similar to that of control pigs without the disease.
The extensive tissue destruction in pigs may account for the sudden death nature of the complex (mulberry heart disease and hepatosis dietetica) and the muscle stiffness that occurs in some feeder pigs and sows of farrowing time with muscular dystrophy. The tissue degeneration is associated with marked increases in serum enzymes related to the tissue involved. An indirect correlation between vitamin E intake and peroxide hemolysis in pigs on a deficient diet suggests that lipoperoxidation is the ultimate biochemical defect in pigs and that vitamin E and Se are protective.
CLINICAL FINDINGS
Acute enzootic muscular dystrophy
Affected animals may collapse and die suddenly after exercise without any other premonitory signs. The excitement associated with the hand-feeding of dairy calves may precipitate peracute death. In calves under close observation, a sudden onset of dullness and severe respiratory distress, accompanied by a frothy or blood-stained nasal discharge, may be observed in some cases. Affected calves, lambs, and foals are usually in lateral recumbency and may be unable to assume sternal recumbency even when assisted. When picked up and assisted to stand, they feel and appear limp. However, their neurological reflexes are normal. Their eyesight and mental attitude are normal and they are usually thirsty and can swallow unless the tongue is affected. The heart rate is usually increased up to 150–200/min and often with arrhythmia, the respiratory rate is increased up to 60–72/min and loud breath sounds are audible over the entire lung fields. The temperature is usually normal or slightly elevated. Affected animals commonly die 6–12 h after the onset of signs in spite of therapy. Outbreaks of the disease occur in calves and lambs in which up to 15% of susceptible animals may develop the acute form and the case fatality approaches 100%.
Subacute enzootic muscular dystrophy
This is the most common form in rapidly growing calves, ‘white muscle disease’ and in young lambs, ‘stiff-lamb disease’. Affected animals may be found in sternal recumbency and unable to stand but some make an attempt to stand. If they are standing, the obvious signs are stiffness, trembling of the limbs, weakness and, in most cases, an inability to stand for more than a few minutes. The gait in calves is accompanied by rotating movements of the hocks and in lambs a stiff, goose-stepping gait. Muscle tremor is evident if the animal is forced to stand for more than a few minutes. On palpation the dorsolumbar, gluteal and shoulder muscle masses may be symmetrically enlarged and firmer than normal (although this may be difficult to detect). Most affected animals retain their appetite and will suck if held up to the dam or eat if hand-fed. Major involvement of the diaphragm and intercostal muscles causes dyspnea with labored and abdominal-type respiration. The temperature is usually in the normal range but there may be a transient fever (41°C, 105°F) due to the effects of myoglobinemia and pain. The heart rate may be elevated, but there are usually no rhythmic irregularities. Following treatment, affected animals usually respond in a few days and within 3–5 days they are able to stand and walk unassisted.
In some cases, the upper borders of the scapulae protrude above the vertebral column and are widely separated from the thorax. This has been called the ‘flying scapula’ and has occurred in outbreaks in heifers from 18 to 24 months of age within a few days after being turned out in the spring following loose-housing throughout the winter. The abnormality is due to bilateral rupture of the serratus ventralis muscles and has been reported in a red deer.75 Occasionally, the toes are spread and there is relaxation of carpal and metacarpal joints or knuckling at the fetlocks and standing on tip-toe, inability to raise the head, difficulty in swallowing, inability to use the tongue and relaxation of abdominal muscles. Choking may occur when the animals attempt to drink. In ‘paralytic myoglobinuria’ of yearling cattle, there is usually a history of recent turning out on pasture following winter housing. Clinical signs occur within 1 week and consist of stiffness, recumbency, myoglobinuria, hyperpnea, and dyspnea. Severe cases may die within a few days and some are found dead without premonitory signs. In rare cases, lethargy, anorexia, diarrhea and weakness are the first clinical abnormalities recognized, followed by recumbency and myoglobinuria.
Congenital muscular dystrophy has been described in a newborn calf.18 The calf was still recumbent 13 h after birth, had increased serum creatine kinase and decreased serum vitamin E and selenium levels. Recovery occurred following supportive therapy and vitamin E and selenium.
Subcapsular liver rupture in lambs has been associated with vitamin E deficiency in lambs usually under 4 weeks of age.76 Affected lambs collapse suddenly, become limp, and die within a few minutes or several hours after the onset of weakness.
In foals, muscular dystrophy occurs most commonly during the first few months of life and is common in the first week.6 The usual clinical findings are failure to suck, recumbency, difficulty in rising and unsteadiness and trembling when forced to stand. The temperature is usually normal but commonly there is polypnea and tachycardia. The disease in foals may be characterized by an acute, fulminant syndrome, which is rapidly fatal, or a subacute syndrome characterized by profound muscular weakness. Failure of passive transfer, aspiration pneumonia, and stunting are frequent complications. In the subacute form, mortality rates may range from 30 to 45%.6
In adult horses with muscular dystrophy, a stiff gait, myoglobinuria, depression, inability to eat, holding the head down low, and edema of the head and neck are common. The horse may be presented initially with clinical signs of colic.
In pigs, muscular dystrophy is not commonly recognized clinically because it is part of the more serious disease complex of mulberry heart disease and hepatosis dietetica. However, in outbreaks of this complex, sucking piglets, feeder pigs and sows after farrowing, may exhibit an uncoordinated, staggering gait suggestive of muscular dystrophy.
Subclinical nutritional muscular dystrophy occurs in apparently normal animals in herds at the time clinical cases are present. The serum levels of creatine phosphokinase levels may be elevated in susceptible animals for several days before the onset of clinical signs; following treatment with vitamin E and selenium the level of serum enzymes returns to normal. Grossly abnormal electrocardiograms occur in some animals and may be detectable before clinical signs are evident.
Vitamin E selenium deficiency in pigs
Usually they occur separately but rarely MHD and HD occur together and even more rarely, you may find that there is NMD as well. There is a suspicion that the occurrence of two or more together has recently become more common but this in fact may be due to the greater awareness of both conditions. Two or more requires the supplementation with both Vitamin E and Se.
Mulberry heart disease
Usually seen in pigs from a few weeks to 4 months of age. These pigs are nearly always the best of the group and it may be that this rate of growth increases the demand for vitamin E and Se.
In mulberry heart disease, affected animals are commonly found dead without premonitory signs. More than one pig may be found dead. When seen alive, animals show severe dyspnea, cyanosis and recumbency and forced walking can cause immediate death. In some outbreaks, about 25% of pigs will show a slight inappetence and inactivity, these are probably in the subclinical stages of the disease. The stress of movement, inclement weather, or transportation will precipitate further acute deaths. The temperature is usually normal, the heart rate rapid and irregularities may be detectable. The feces are usually normal. A good ‘classical outbreak’ has been described.77
Hepatosis dietetica
In hepatosis dietetica, most pigs are found dead. Very few cases show other signs. In occasional cases, before death there will be dyspnea, severe depression, vomiting, staggering, diarrhea and a state of collapse. Some pigs are icteric. Outbreaks also occur similar to the pattern in mulberry heart disease. Muscular dystrophy is almost a consistent necropsy finding in both mulberry heart disease and hepatosis dietetica but is usually not recognized clinically because of the seriousness of the two latter diseases. Clinical muscular dystrophy has been described in gilts at 11 months of age. About 48 h after farrowing, there was muscular weakness, muscular tremors, and shaking. This was followed by collapse, dyspnea, and cyanosis. There were no liver or heart lesions. In experimental Se and vitamin E deficiency in young growing pigs, a subtle stiffness occurs along with a significant increase in the creatinine phosphatase (CPK) and serum glutamic-oxaloacetic transaminase (SGOT) values.
CLINICAL PATHOLOGY
Myopathy
Plasma creatine kinase (CK)
This is the most commonly used laboratory aid in the diagnosis of NMD. The enzyme is highly specific for cardiac and skeletal muscle and is released into the blood following unaccustomed exercise and myodegeneration. In cattle and sheep, its half-life is 2–4 h and plasma levels characteristically decline quickly unless there is continued myodegeneration, but remain a good guide to the previous occurrence of muscle damage for a period of about 3 days. The normal plasma levels of CK (IU/L) are: sheep 52± 10; cattle 26± 5; horses 58± 6; and pigs 226± 43. In cattle and sheep with NMD, the CK levels will be increased usually above 1000 IU/L, commonly increased to 5000–10 000 IU/L and not uncommonly even higher. Following turnout of housed cattle onto pasture the CK levels will increase up to 5000 IU/L within a few days. The CK levels will usually return to normal levels within a few days following successful treatment. Persistent high levels suggest that muscle degeneration is still progressive or has occurred within the last 2 days. Measurement of plasma CK activity could be used to monitor recovery of animals treated for nutritional myopathy.
Aspartate aminotransferase
Aspartate aminotransferase (AST) activity is also an indicator of muscle damage, but is not as reliable as the CK because increased AST levels may also indicate liver damage. The AST activity remains elevated for 3–10 days because of a much longer half-life than CK. In acute cases, levels of 300–900 IU/L in calves and 2000–3000 IU/L in lambs have been observed. In normal animals of these species, serum levels are usually less than 100 IU/L.
The magnitude of the increase in AST and CK is directly proportional to the extent of muscle damage. Both are elevated initially; an elevated AST and declining CK would suggest that muscle degeneration is no longer active. The levels of both enzymes will be increased slightly in animals that have just been turned out and subjected to unaccustomed exercise, horses in training and in animals with ischemic necrosis of muscle due to recumbency caused by diseases other than muscular dystrophy. However, in acute muscular dystrophy, the levels are usually markedly elevated.
Selenium status
Although information on the critical levels of selenium in soil and plants is accumulating gradually, the estimations are difficult and expensive. Most field diagnoses are made on the basis of clinicopathological findings, the response to treatment and control procedures using selenium. The existence of NMD is accepted as presumptive evidence of selenium deficiency, which can now be confirmed by analyses of GHS-PX and the concentrations of selenium in soil, feed samples, and animal tissues. Tentative critical levels of the element are as follows:
• Forages and grains: A content of 0.1 mg/kg DM is considered adequate
• Soil: Soils containing less than 0.5 mg/kg are likely to yield crops inadequate in selenium concentration5
• Animal tissues, blood and milk: The concentration of selenium in various tissues are reliable indicators of the selenium status of the animal. There is a positive correlation between the selenium content of feed and the selenium content of the tissues and blood of animals ingesting that feed and the values fluctuate with the dietary intake of the element.5
Three tests can be used to assess selenium status in cattle and sheep: serum and whole blood selenium and glutathione peroxidase activity.78 Serum selenium responds more rapidly to the administration of selenium than whole blood selenium. There is a similar delay in glutathione peroxidase activity to selenium supplementation. Blood or serum selenium status is most consistently measured at the herd-level. Interlaboratory differences in thresholds for deficiency exist and results should be considered based on laboratory-specific guidelines.
The recommended blood selenium reference ranges for New Zealand livestock have been used in several publications (see Table 30.7).40,79,80
Reference ranges for selenium and vitamin E in serum, blood, and liver of sheep and goat in the USA are available.2
Selenium status in horses
In New Zealand, the reference ranges for blood used for selenium status in horses are: adequate >1600 nmol/L (128 ng/mL); marginal 450–1600 nmol/L (36–128 ng/mL); and deficient <450 nmol/L (36 ng/mL).81
Kidney cortex and liver
Normal liver selenium concentrations range from 1.2 to 2.0 μg/g DM, regardless of species or age.82 Levels of 3.5–5.3 μg/g (44–67 nmol/g) DM in the kidney cortex and 0.90–1.75 μg/g (11–22 nmol/g) DM in the liver of cattle are indicative of adequate selenium. Levels of 0.6–1.4 μg/g (8–18 nmol/g) in the kidney cortex and 0.07–0.60 μg/g (0.9–8 nmol/g) in the liver represent a deficient state.
The selenium content of bovine fetal liver samples collected at an abattoir contained 0.77 μg/mL WW and 0.13 μg/mL WW, from dairy breeds and beef breeds of cattle, respectively.83 Mean liver selenium levels from aborted bovine fetuses with myocardial lesions were 5.5 μmol/kg, 6.5 μmol/kg in fetuses without myocardial lesions and 7.5 μmol/kg in fetuses from the abattoir, which suggests that selenium deficiency may be the cause of abortion.84
Blood and milk
Blood and milk levels of selenium are used as indicators of selenium status in cattle and the effect of dietary supplementation.85 Serum selenium values increase gradually with age from starting ranges for neonates of 50–80 ng/mL for calves and sheep and 70–90 for foals and pigs.82 Expected or normal values for adults are in the ranges of 70–100 for cattle, 120–150 for sheep, 130–160 for horses, and 180–220 for pigs.
Dams of affected calves have had levels of 1.7 ng/mL (22 nmol/L) (blood) and 4.9 ng/mL (62 nmol/L) (milk); their calves have blood levels of 5–8 ng/mL (63–102 nmol/L). Normal selenium-supplemented cows have 19–48 ng/mL (241–609 nmol/L) in blood and 10–20 ng/mL (127–253 nmol/L) in milk and their calves have blood levels of 33–61 ng/mL (419–774 nmol/L). Mean selenium concentrations in the blood of normal mares have been 26–27 ng/mL (329–342 nmol/L). In Thoroughbred horses, selenium concentrations in serum range from 39.5 to 118.5 mg/mL (40–160 ng/mL) (0.5–2.0 μmol/L) and there are significant differences between various stables of horses.
Bulk tank milk
The bulk tank milk selenium levels are closely related to the mean herd blood and milk levels and have the potential to be a low-cost, non-invasive means of evaluating herd selenium levels in order to determine selenium deficiency in the dairy herd.64,86 Bulk tank selenium concentrations are an accurate reflection of the herd selenium status over the range of selenium intakes typical of dairy herds in an area.
Glutathione peroxidase
There is a direct relationship between the GSH-PX activity of the blood and the selenium levels of the blood and tissues of cattle, sheep, horses, and pigs.1 The normal selenium status of cattle is represented by whole blood selenium concentration of 100 ng/mL (1270 nmol/L) and blood GSH-PX activity of approximately 30 mU/mg hemoglobin.
There is a high positive relationship (r = 0.87–0.958) between blood GSH-PX activity and blood selenium concentrations in cattle. Blood selenium levels less than 50 ng/mL are considered as selenium-deficient, while levels between 50 and 100 ng/mL (126.6 nmol/L) are marginal and greater than 100 ng/mL are adequate.1 Comparable whole blood levels of GSH-PX are deficient if less than 30 mU/mg hemoglobin, marginal if 30–60 mU/mg and adequate if greater than 60 mU/mg hemoglobin.1 There is some evidence of variation in GSH-PX activities between breeds of sheep; levels may also decrease with increasing age. Low levels in some breeds of sheep may also be a reflection of adaptation to low selenium intake because of low levels of selenium in the soil and forages.
The GSH-PX activity is a sensitive indicator of the level of dietary selenium intake and the response to the oral or parenteral administration of selenium.5 Because selenium is incorporated into erythrocyte GSH-PX only during erythropoiesis, an increase in enzyme activity of the blood will not occur for 4–6 weeks following administration of selenium. Plasma GSH-PX will rise more quickly and will continue to increase curvilinearly with increasing dietary selenium levels because it is not dependent on incorporation of the selenium into the erythrocytes. The liver and selenium concentration and serum GSH-PX activity may respond to changes in dietary selenium more rapidly than either whole blood selenium or erythrocyte GSH-PX activity. The response in GSH-PX activity may depend upon the selenium status of the animals at the time when selenium is administered. Larger increases in the enzyme activity occur in selenium-deficient animals. The GSH-PX activity in foals reflects the amount of selenium given to the mare during pregnancy.
The sandwich ELISA is a simplified method for the estimation of GSH-PX activity and selenium concentration in bovine blood and can be used for rapid screening of the selenium status of a large number of cattle.87 The GSH-PX activity of whole blood samples has been used to assess the selenium status of cattle in the Czech Republic.88
The GSH-PX activity can be determined rapidly using a spot test which is semiquantitative and can place a group of samples from the same herd or flock into one of three blood selenium categories: deficient, low marginal and marginal adequate.89 A commercial testing kit known as the Ransel Kit is now available. Because of the instability of GSH-PX plasma, GSH-PX activity in sheep, cattle, and pigs should be measured in fresh plasma or stored at –20°C (–4°F). For absolute measurements, it is suggested that pigs plasma GSH-PX activity be measured immediately after separation from the blood cells, or be assayed within 24 h under specified laboratory conditions.
Vitamin E status
Vitamin E occurs in nature as a mixture of tocopherols in varying proportions. They vary widely in their biological activity so that chemical determination of total tocopherols is of much less value than biological assay. Tocopherol levels in blood and liver provide good information on the vitamin E status of the animal. However, because of the difficulty of the laboratory assays of tocopherols, they are not commonly done and insufficient reliable data are available. Analysis of liver from clinically normal animals on pasture reveal a mean α-tocopherol level of 20 mg/kg WW for cattle and 6 mg/kg WW for sheep. The corresponding ranges were 6.0–53 mg/kg WW for cattle and 1.8–17 mg/kg WW in sheep. The critical level below which signs of deficiency may be expected are 5 mg/kg WW for cattle and 2 mg/kg WW for sheep. Tocopherol levels in the serum of less than 2 mg/L in cattle and sheep are considered to be critical levels below which deficiency diseases may occur. However, if the diet contains adequate quantities of selenium, but not an excessive quantity of PUFAs, animals may thrive on low levels of serum tocopherols. In growing pigs, the serum vitamin E levels are between 2 and 3 mg/L. In summary, there are insufficient reliable data available on the vitamin E status on animals with NMD to be of diagnostic value.
The mean plasma vitamin E levels in clinically normal horses of various ages and breeds were 2.8 μg/mL.90 The optimal method for storing equine blood prior to α-tocopherol analysis is in an upright position in the refrigerator for up to 72 h. If a longer period is needed, the serum or plasma should be separated, blanketed with nitrogen gas and frozen in the smallest possible vial; the α-tocopherol in these samples will be stable at –16°C (3°F) for at least 3 months.
A summary of the GSH-PX activity, tocopherol and selenium levels in blood and body tissues of animals deficient in selenium appears in Table 30.6. Normal values are also tabulated for comparison.91 Both the abnormal and normal values should be considered as guidelines for diagnosis because of the wide variations in levels between groups of animals. The level of dietary selenium may fluctuate considerably, which may account for variations in GSH-PX. Selenium reference ranges to determine selenium status of sheep and cattle in New Zealand are shown in Table 30.7.
Table 30.6 Glutathione peroxidase (GSH-PX) activity and selenium levels in blood and body tissues of animals deficient in selenium
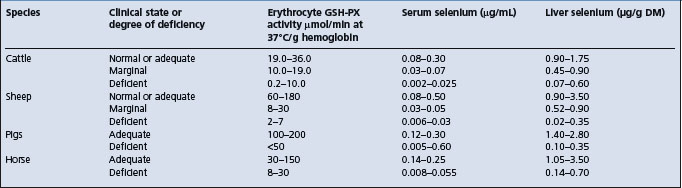
In the early stages of the subclinical form of NMD in lambs, there may be a decrease in serum selenium and glutathione peroxidase activity and an increase in the activity of aspartate aminotransferase (AST), creatine kinase (CK), and lactate dehydrogenase (LDH) compared with healthy lambs.92 The LDH-isoenzyme activity is useful for detection of subclinical forms of NMD because of significant increases in the activity of the LDH5-muscle fraction.
Farmed red deer
Reference range data for liver and blood selenium in red deer are limited.93,94 White muscle disease has occurred in young deer with blood and liver selenium concentrations of 84–140 nmol/L and 240–500 nmol/kg fresh tissue, respectively. No growth rate response to selenium supplementation occurred in 1-year-old deer when blood selenium concentrations were less than 130 nmol/L, the range in which a growth rate response would be expected in sheep.
Pigs
An increase in the activity of several plasma enzymes occurs in Se and vitamin E deficiencies of pigs. The measurement of AST, CPK, lactic acid dehydrogenase, and isocitrate dehydrogenase can be used to detect the onset of degeneration of skeletal and myocardial muscles and liver. However, these are not commonly used for diagnostic purposes because of the acuteness of the illness. The determination of the levels of Se in feed supplies, tissues, and blood of affected pigs is much more useful as an aid to diagnosis and for guidelines for supplementation of the diet.
In Se-vitamin E deficiency in pigs, serum Se values of less than 2.5 ng/mL (3.2 nmol/L), hepatic Se of less than 0.10 mg/kg (1.3 μmol/kg), plasma α-tocopherol values of <0.40 μg/mL and hepatic α-tocopherol concentrations of <0.75 μg/g of tissue are common. In a recent study, the vitamin E level was <2 ppm in 25% of pigs with gross and microscopic lesions of MHD.39 In a recent study results suggested that supplementation with a surfeit level of vitamin E reduced the response to endotoxin, i.e. a reduced response to the peak levels of IL-6.95
The diagnostic criteria for the VESD complex in pigs in New Zealand indicate that liver vitamin E concentrations >10 μmol/kg are adequate, with <2.5 μmol/kg associated with deficiency. Corresponding estimates for serum vitamin E are >2.5 μmol/L and <0.8 μmol/L, respectively. Liver Se concentrations of >2200 nmol/kg are adequate, with 1100–2200 nmol/kg being in the marginal range and <1100 nmol/kg being deficient. Deficiency levels for blood are in the range of 400–1500 nmol/L. These values must be interpreted along with the concentration of PUFAs in the diet.
There is a close relationship between blood vitamin E and resistance of erythrocytes against lipid peroxidation. The supplementation of the diet of pigs with vitamin E will increase both the serum levels of vitamin E and the resistance of the erythrocytes to lipid peroxidation.96
NECROPSY FINDINGS
The gross appearance of the muscle lesions is quite constant, but the distribution of affected muscles varies widely in different animals. Affected groups of skeletal muscle are bilaterally symmetrical and contain localized white or gray areas of degeneration and necrosis. These areas may be in streaks, involving a large group of muscle fibers that run through the center of the apparently normal muscle or as a peripheral boundary around a core of normal muscle. In the diaphragm, the distribution of damaged bundles gives the tissue a radially striated appearance. The affected muscle is friable and edematous and may be mineralized. Secondary pneumonia often occurs in cases where the muscles of the throat and chest are affected. In cases with myocardial involvement, white areas of degeneration are visible, particularly under the endocardium of the left ventricle in calves and of both ventricles in lambs. The lesions may extend to involve the interventricular septum and papillary muscles and have a gritty character consistent with mineralization. Pulmonary congestion and edema is common.
Histologically, the muscle lesions in all species are non-inflammatory. Hyaline degeneration is followed by coagulation necrosis and variable degrees of mineralization.
Other than a variable degree of muscular atrophy, gross lesions are not seen in horses with equine motor neuron disease. Confirmation of the diagnosis relies on histological identification of characteristic degeneration and loss of motor neurons of the spinal cord ventral horns. However, a very strong presumptive diagnosis can be achieved by microscopic confirmation of neurogenic atrophy in the sacrocaudalis dorsalis muscle or axonal degeneration in the spinal accessory nerve.97
A generalized steatitis has been described in newborn foals less than 2 months of age. The microscopic appearance of this yellow-brown fat consists of necrotic fat infiltrated by neutrophils, macrophages and giant cells. Steatitis and nodular panniculitis have also been reported in a 3-year-old vitamin E/selenium-deficient mare.98 Supplemental vitamin E is believed to protect against steatitis in foals.
In mulberry heart disease the carcass is in good condition. All body cavities contain excessive amounts of fluid and shreds of fibrin. In the peritoneal cavity, the fibrin is often in the form of a lacy net covering all the viscera. The liver is enlarged, mottled and has a characteristic nutmeg appearance on the cut surface. The lungs are edematous and excessive fluid in the pleural cavities is accompanied by collapse of the ventral lung field. The pericardial sac is filled with gelatinous fluid interlaced with bands of fibrin. Beneath the epicardium and endocardium are multiple hemorrhages of various sizes. Usually, this hemorrhage is more severe on the right side of the heart. This gives the heart the typical mottled appearance which is caused by areas of necrosis and areas of hemorrhage. Histologically, the characteristic lesion is widespread myocardial congestion, hemorrhage, and myofiber degeneration. Multiple fibrinous microthrombi are within the myocardial capillaries and, occasionally, degenerative changes are visible in walls of small arterioles in many organs, including the heart. Malacia of cerebral white matter, or more rarely the molecular layer of the cerebellum, may occur and is attributable to microvascular damage. It should be stressed that, in some cases, the disease course is so rapid that morphologic changes are not discernible in the myocardial cells. Since it can be extremely difficult to distinguish mulberry heart disease from S. suis septicemia histologically, it is prudent to also attempt bacteriologic culture when attempting to confirm the diagnosis.
In hepatosis dietetica, the liver is swollen and has a mottled to mosaic-like appearance throughout its lobes. Many of the lobules are distended and reddish in color. There is in fact an irregular distribution of hepatic necrosis and hemorrhage. The gall bladder may be edematous and there may also be myocardial necrosis and pulmonary edema. Typically, the disease course is so rapid that jaundice does not develop. Histologically, there is a distinct lobular distribution of hemorrhage, degeneration, and necrosis.
In NMD of pigs, the lesions are often only visible at the microscopic level and consist of areas of bilaterally distributed areas of muscular degeneration. The changes include hyalinization, loss of striations and fragmentation of myofibers. The sections are difficult to cut because of the presence of calcium in the myocytes. A mild degree of NMD may accompany some cases of hepatosis dietetica.
Samples for confirmation of diagnosis
• Toxicology – 50 g liver (ASSAY (Se) (Vitamin E))
• Histology – formalin-fixed skeletal muscle (multiple sites), heart (both left and right ventricular walls), brain (including cerebral hemisphere) (LM). May require special stains to show the presence of calcium in the sections
• Bacteriology (for mulberry heart disease only) – heart, liver, swab from pericardial sac (CULT).
Nutritional muscular dystrophy
NMD is most common in young rapidly growing animals fed a selenium-vitamin E-deficient ration or whose dams were on a deficient, unsupplemented ration throughout the winter months. Characteristically, the disease is sudden in onset and several animals are affected initially or within a few days, particularly following unaccustomed exercise. In the acute form, generalized weakness and a state of collapse are common. In the subacute form, the major clinical findings are stiffness in walking, long periods of recumbency or total recumbency, inability to stand, a normal mental attitude and appetite and no abnormal neurological findings to account for the recumbency. The CP levels are markedly elevated.
Calves and yearlings
Acute enzootic muscular dystrophy in calves with myocardial involvement must be differentiated from other diseases causing generalized weakness, toxemia and shock.
• Septicemias: Haemophilus septicemia resulting in weakness, recumbency and fever
• Pneumonia: Pneumonic pasteurellosis causing dyspnea, toxemia, fever and weakness.
Subacute enzootic muscular dystrophy in which skeletal muscle lesions predominate must be differentiated from other diseases of young calves and yearlings characterized clinically by paresis and paralysis. The subacute form is more common in yearlings and young cattle and is characterized by recumbency with other body systems being relatively within normal ranges. The other diseases include:
• Musculoskeletal diseases: Polyarthritis, traumatic or infectious myopathies (blackleg), osteodystrophy and fractures of long bones
• Diseases of the nervous system: Spinal cord compression, Hemophilus meningoencephalitis and myelitis, organophosphatic insecticide poisoning
• Diseases of the digestive tract: Carbohydrate engorgement resulting in lactic acidosis, shock, dehydration, and weakness.
Lambs and kids
In lambs with ‘stiff-lamb’ disease, there is stiffness and a stilted gait, affected animals prefer recumbency, they are bright and alert and will suck the ewe if assisted. The serum levels of CPK and SGOT are also markedly elevated. Differentiation may be necessary from enzootic ataxia and swayback, but in these two diseases, stiffness is not characteristic but rather weakness and paresis.
Foals
In foals, NMD must be differentiated from acute diseases of the musculoskeletal and nervous system causing abnormal gait, weakness and recumbency. They include:
TREATMENT
Because of the overlapping functions of selenium and vitamin E and because it is not always possible to know the relative etiological importance of one nutrient or the other in causing some of the acute conditions already described, it is recommended that a combined mixture of selenium and α-tocopherol be used in treatment. α-Tocopherol is the most potent form of the tocopherols and is available in a number of pharmaceutical forms, which also vary in their biological activity. It has become necessary to express the unitage of vitamin E in terms of international units of biological activity (1 IU:1 mg synthetic racemic a-tocopherol acetate. Natural D-α-tocopherol acetate 1 mg: 1 IU and natural D-α-tocopherol 1 mg: 0.92 IU).
Nutritional muscular dystrophy
For treatment of NMD in calves, lambs, and foals a mixture containing 3 mg selenium (as sodium or potassium selenite) and 150 IU/mL of DL-α-tocopherol acetate, given IM at 2 mL/45 kg BW is recommended. One treatment is usually sufficient. Animals with severe myocardial involvement will usually not respond to treatment and the case mortality rate is about 90%. However, all in-contact animals in the herd (calves, lambs, and foals) should be treated prophylactically with the same dose of selenium and vitamin E. They should be handled carefully during treatment to avoid precipitating acute muscular dystrophy. Animals with subacute skeletal muscular dystrophy will usually begin to improve by 3 days following treatment and may be able to stand and walk unassisted within 1 week.
Animals sometimes do not respond to either vitamin E or Se or treatment with both.
In outbreaks of mulberry heart disease, hepatosis dietetica and related Se and vitamin E deficiency diseases in pigs, all clinically affected pigs and all pigs at risk should be treated individually with a combination of Se and vitamin E parenterally at first to prevent any further sudden death. It can then be followed by oral administration.
CONTROL
The control and prevention of the major diseases caused by selenium and vitamin E deficiencies can generally be accomplished by the provision of both nutrients to susceptible animals fed on deficient rations. The following points are relevant and applicable to most situations:
• Provide selenium and vitamin E
• Maternal transfer to newborn
• Selenium is potentially toxic
• Dietary requirement of selenium
Provide selenium and vitamin E
Over the years, both the vitamin E levels and Se levels in the diets have increased but particularly the former. This is in response to the more rapid growth rates of pigs but also the realization that pigs are coping with many more oxidative disease states. Outdoor pigs usually have sufficient of both unless the soil is Se deficient. A recent Chinese paper99 has suggested that dietary zinc at 85 mg/kg, Se at 0.40 mg/kg, and vitamin E at 45 IU/kg is appropriate for crossbred sows.
While Se alone is protective against a greater spectrum of diseases than is vitamin E, there are situations in which vitamin E is more protective. Both Se and vitamin E should be provided when the diets are deficient in both nutrients, but this may not apply in every situation. Most of the emphasis has been on Se supplementation at the expense of vitamin E, which is more expensive and less stable. Most injectable vitamin E and Se preparations are adequate in Se but insufficient in vitamin E.
There have been several attempts to supplement weaner pigs with a vitamin E preparation. Besides individual injections, it is possible to supplement weaner pigs with water supplementation.100 Pigs will usually drink even if they are not eating. A recent study101 showed that the supplementation of drinking water with high doses of vitamin E (150 mg of Dl-α-tocopherol acetate) was effective in maintaining serum vitamin E levels over the weaning period. This was even true when over the weaning period the intake of food was very low (it can be as low as 0.2–0.3 kg) and there is a temporary malabsorption in the intestine. It takes about 100 IU/L of water to provide a good vitamin E blood serum value.
Maternal transfer to newborn
Diseases caused by selenium deficiency are preventable by the administration of selenium to the dam during pregnancy or directly to the young growing animal. Selenium is transported across the placenta and provides protection for the neonate. Oral supplementation with selenium in beef cattle will provide enough to maintain blood levels in the dam and for adequate transfer to the fetus, which can sequester selenium when the levels are low in the dam. The colostrum of selenium-supplemented cattle also contains an adequate amount of selenium to prevent severe selenium-deficiency diseases.68 However, by 7 days after parturition, the levels in milk may be inadequate to maintain adequate serum levels in calves. The strategic administration of selenium and vitamin E before the expected occurrence of the disease is also a reliable method of preventing the disease.
Selenium is potentially toxic
Se is toxic and any treatment and control program using it must be carefully monitored.102 Se injected into or fed to animals concentrates in liver, skeletal muscle, kidney, and other tissues and withdrawal periods before slaughter must be allowed. There is some concern that Se may be a carcinogen for man. The only tissues that appear likely to consistently accumulate more than 3–4 mg/kg of Se are the kidney and liver and these are very unlikely to constitute more than a very small part of the human diet. There have been no reports of untoward effects of Se on human health when it has been used at nutritional levels in food-producing animals. The incorporation of Se into commercially prepared feeds for some classes of cattle and pigs has been approved in some countries. A recent case in Norway showed the hazards of Se contamination in the case of an iron supplement.103 Se toxicosis has been fairly regularly reported.104-106
Pigs that are deficient may be more susceptible to other diseases. Pigs with NMD often have the appearance of pneumonic pigs because the diaphragm is weak and the pigs are dyspneic.
Deficient and small pigs may be more susceptible to the effects of iron and when this is given by injection there may be large numbers of dead piglets as a result of iron toxicity. In these cases, the heart lesions resemble those of MHD.
Selenium in milk supplies
The use of selenium in the diet of lactating dairy cows has caused concern about possible adulteration of milk supplies. However, the addition of selenium to the diets of lactating dairy cows at levels that are protective against the deficiency diseases does not result in levels in the milk that are hazardous for human consumption. The feeding of excessive quantities of selenium to dairy cattle would cause toxicity before levels became toxic for man.
Dietary requirement of selenium
The dietary requirement of selenium for both ruminants and non-ruminants is 0.1 mg/kg DM of the element in the diet. There may be nutritionally important differences in the selenium status between the same feeds grown in different regions and between different feeds within a region. Even within a region featuring high selenium concentrations, some feeds may contain levels of selenium below the 0.1 mg/kg minimum requirement for livestock. Thus a selenium analysis of feeds appears necessary in order to supplement livestock appropriately. Some geographical areas are known to be deficient in selenium and the feeds grown in these areas must be supplemented with selenium and vitamin E on a continuous basis. Some reports indicate that surveys have found that dairy producers are providing insufficient supplementary selenium in the ration to meet the recommended selenium intake for lactating dairy cows.14 Long-term administration of organic selenium in the form of selenium yeast provides higher blood and tissue concentrations than repeated parenteral administration of recommended therapeutic doses of inorganic selenium.71
High sulfate diets
Avoidance of high sulfate diets is desirable, but provision of adequate Se overcomes the sulfate effect.
Glutathione peroxidase activity
Whole blood GSH-PX activity is a way of monitoring Se status but is not as reliable in pigs as in sheep and cattle.
In growing pigs, both Se and vitamin E at 30 IU/kg DM of feed are necessary for the prevention of the diseases caused by diets deficient in vitamin E and Se. Supplementation of the diet of the sow will result in an adequate transfer to the piglets. Satisfactory protection of the diseases of pigs caused by vitamin E Se deficiency depends on the correct balance between Se, α-tocopherol, PUFAs in the diet and the presence of a suitable antioxidant to conserve the α-tocopherol.107
Different methods of supplementation
The prevention of the major diseases caused by selenium and vitamin E deficiencies can be achieved by different methods, including:
The method used will depend on the circumstances of the farm, ease of administration, cost, the labor available, severity of the deficiency that exists and whether or not the animals are being dosed regularly for other diseases such as parasitism. The subcutaneous injection of barium selenate, the administration of an intra-ruminal pellet and the addition of selenium to the water supply were compared in cattle; each method was effective for periods ranging from 4 to 12 months.
Dietary supplementation
The inclusion of selenium and vitamin E in the feed supplies or salt and mineral mixes has been generally successful in preventing the major diseases caused by deficiencies of these two nutrients.
Selenium dose
Individual injections
Injections of Se and vitamin E have been used successfully for prevention, particularly in circumstances where the diet cannot be easily supplemented. Following IM injections of sodium selenite into calves, lambs, and piglets, the Se concentration of the tissues, particularly the liver, increases and then declines to reach preinjection levels in 23 days in calves and 14 days in lambs, and piglets. Adequate sources of vitamin E also must be provided. Injectable preparations of Se and vitamin E are usually adequate in Se and deficient in vitamin E and it may not be possible to correct a marginal deficiency of vitamin E in pregnant beef cattle, for example, by IM injection of a Se and vitamin E preparation which contains an inadequate concentration of vitamin E.108 The current label dose of injectable Se, 0.055 mg Se/kg BW, which is therapeutically adequate for NMD, is not sufficient for long-term Se supplementation of cattle on a Se-deficient diet.109 Copper and Se supplementation by parenteral administration can be combined when both deficiencies are present.110
Subcutaneous injections
A slow-release preparation of barium selenate for SC injection is now available for use in cattle and sheep.89 A SC injection of 1 mg selenium/kg BW to ewes 3 weeks before breeding elevated the selenium level in milk during lactation and increased the selenium concentration and GSH-PX in the blood of the lambs during the period when they are at greatest risk from selenium-deficiency diseases.111 At a dose of 1 mg selenium/kg BW to pregnant ewes, the GSH-PX activity is increased and maintained at adequate levels for up to 5 months. There is adequate transfer of selenium to the lambs, providing protection for up to 12 weeks of age, which covers the period when lambs are at greatest risk. A dose of 1.2 mg selenium/kg BW provided adequate selenium status for as long as two consecutive lambing seasons. Barium selenate at 1 mg selenium/kg BW SC provides protection in young sheep for at least 3 months and is not associated with risk of selenium toxicity or unacceptable residues of selenium in tissues other than the site of injection.89 A dose of 1 mg selenium/kg BW (barium selenate) to cattle SC increased the GSH-PX activity within 4 weeks and was maintained at high levels for up to 5 months.
The SC injection of barium selenate of pregnant sows at 0.5–1.0 mg selenium/kg BW resulted in a significant difference in GSH-PX activity in the piglets from treated sows compared with untreated controls. The SC injection of barium selenate at 2.5 mg selenium/kg BW into pigs weighing 20 kg also maintained blood levels of selenium and GSH-PX activity during the most rapid growing period. The relative safety of barium selenate is due to its slow rate of release from the site of injection. By comparison, when selenium is administered as a soluble salt, such as sodium selenite, acute toxicity may occur at doses of 0.45 mg selenium/kg BW. Treatment with barium selenate increases the concentration of selenium in blood, liver and muscle and persists for at least 4 months. One disadvantage of barium selenate is that a large residue persists at the site of injection for long periods. The use of sodium selenite also increases tissue and blood concentrations of selenium, but they begin to decline by 23 days. The bovine liver rapidly removes approximately 40% of injected selenium salts (soluble) from the systemic plasma, binds it to a plasma component and within 1 h of injection releases it back into circulation.
A long-acting barium sulfate given subcutaneously to red deer on pasture, at 0.5, 1.0, or 2.0 mg Se/kg BW, elevated blood selenium concentrations from 105 nmol/L pre-injection for at least 377 days with peak levels of 1894, 1395, and 818 nmol/L for high, medium, and low doses, respectively.112 Pastures contained 10–30 mg Se/kg DM. There was no significant difference in growth rate between treated and control deer. The preparation produced fewer and less severe subcutaneous tissue reactions than previous preparations. Young growing deer seem less sensitive to selenium deficiency as measured by weight gain, than sheep and cattle, suggesting that reference ranges for those species are not appropriate for deer.
Oral selenium and anthelmintics
Oral dosing using sodium selenite is sometimes combined with the administration of anthelmintics and vaccinations. The dose should approximate 0.044 mg/kg BW. A routine program in a severely deficient area comprises three doses of 5 mg of selenium (11 mg sodium selenite) each to ewes, one before mating, one at mid-pregnancy and one 3 weeks before lambing and four doses to the lambs. The first dose to lambs (of 1 mg) is given at docking and the others (2 mg each) at weaning and then at 3-month intervals. A 100-day controlled release anthelmintic capsule containing 13.9 mg of selenium will protect lambs from selenium deficiency for at least 180 days.113
Both selenium and cobalt can be incorporated into an anthelmintic program. The levels of GSH-PX activity may be monitored on a regular basis following the drenching with selenium and provide a good indication of selenium availability and selenium status of grazing sheep.
Pasture top-dressing
The application of sodium selenate as a top-dressing to pasture is now practiced and permitted in some countries. Top-dressing at the approved rate of 10 g selenium/ha is effective for 12 months and has a toxicity margin of safety of about 20 times. Sodium selenate is now used in preference to sodium selenite because only about one-fifth is required to raise the pasture level of selenium to the same concentrations provided by sodium selenite. Top-dressing severely deficient pumice soils in New Zealand prevented deficiency for at least 12 months, sheep were protected against white muscle in lambs and reproduction performance and weight gains were improved. It is recommended that sodium selenate be applied annually to all selenium-deficient soils at the rate of 10 g selenium/ha added to the superphosphate fertilizer, or as prills of sodium selenate alone. Top-dressing is an economical alternative to individual animal dosing, particularly in severely deficient areas with a high stocking rate. At the approved rate, no adverse effects are anticipated in human or animal health or on the environment.
Muscular dystrophy
Under most conditions, NMD of calves and lambs can be prevented by providing selenium and vitamin E in the diets of the cow or ewe during pregnancy at 0.1 mg/kg DM of actual selenium and α-tocopherol at 1 g/d per cow and 75 mg/d per ewe. If possible, the supplementation should be continued during lactation to provide a continuous source of selenium to the calves and lambs. Under some conditions the level of 0.1 mg/kg DM may be inadequate. In some circumstances, the optimal selenium concentration in the feed is considerably higher than 0.1 mg/kg DM and levels up to 1.0 mg/kg DM in the feed result in increases in GSH-PX activity which may be beneficial; however, the cost-effectiveness has not been determined. Pregnant ewes being fed on alfalfa hay may require selenium at a level of up to 0.2 mg/kg DM to prevent white muscle disease in their lambs. Young growing cattle, particularly beef cattle likely to receive hay and straw deficient in selenium and those which are fed high-moisture grain, should receive a supplement of selenium at the rate of 0.1 mg/kg DM and α-tocopherol at 150 mg/d per head. If selenium-supplemented concentrates are used as part of a feeding program for dairy cows, it is not necessary to provide additional selenium by parenteral injection.
Lambs are born with a low serum level of vitamin E but the concentration increases rapidly after the ingestion of colostrum.1 Supplementation of pregnant ewes with α-tocopherol, either as a single IM dose (500 mg 2 weeks before lambing) or orally (150 mg daily during 3–4 weeks before lambing) results in a marked increase in the levels of the vitamin in the serum and colostrum. The vitamin E concentration in colostrum was 5–11 times higher than in milk 1 week after lambing.
Vitamin E supplementation of the feed of weaner sheep by oral drench or feed additive is effective in increasing plasma α-tocopherol concentrations. This is the most practical method for housed sheep and prevents subclinical myopathy.114 The IM oily injection was slow to increase plasma levels of tocopherols and did not prevent myopathy in grazing experiments. Vitamin E supplements have no beneficial effects on wool quality or quantity in grazing sheep and unless certain flocks are susceptible to vitamin E deficiency myopathy it is not recommended.
Beef cattle and sheep
NMD can be prevented in unweaned beef calves and lambs by the inclusion of selenium (14.8 mg/kg) and vitamin E (2700 IU/kg) in the mineral supplement provided ad libitum to the pregnant cows and ewes on a selenium-deficient ration during the latter two-thirds of gestation and for the first month of lactation. Under most conditions this will provide selenium at 0.1 mg/kg DM in the diet.
The provision of sodium selenite in a salt-mineral mixture to provide 90 mg of selenium/kg salt-mineral mixture on a year-round basis, even under range conditions, increased GSH-PX activity levels into normal ranges in beef cows for 3 months when fed to extremely deficient animals. Calves of these cows had increased weaning weights and decreased incidence of infectious diseases, but the trial was uncontrolled. The provision of 30 mg selenium/kg salt-mineral mixture was insufficient to raise the GSH-PX activity levels to normal ranges. Peak blood selenium levels were achieved in weaned beef calves supplemented with 80 and 160 mg selenium/kg in free-choice salt-mineral mixtures for a period of 108 days. In some jurisdictions, it may be necessary for the veterinarian to prescribe a supplement containing higher levels than those permitted by legislation. A level of 25 mg/kg selenium of a salt-mineral mixture provided ad libitum for sheep will result in sufficient levels of selenium in the dam’s blood and milk to prevent selenium deficiency diseases. Each ewe must consume from 8 to 12 g of the salt-mineral mixture per day.
Selenium deficiency in grazing and forage fed cattle is widespread in the United States and other countries.115 Calves may be severely depleted of selenium and selenium-dependent glutathione peroxidase but exhibit no clinical signs of deficiency unless they are subjected to an oxidant or other types of stress. Nursing beef calves may be at risk of selenium deficiency if their dams are not supplemented with selenium. Even when sodium selenite is used in a free-choice mineral supplement designed to deliver 2 mg of selenium daily, calves are still at risk for selenium deficiency for up to 90 days. Selenium supplementation of pregnant beef cows with seleno-yeast in a free-choice mineral mixture increased the whole blood selenium and GSH-PX activity of both cows and calves much superior to sodium selenite.115
The supplementation of beef cattle in late gestation with oral vitamin E, 1000 IU/head per day, influenced the vitamin E status of cows which calved in late winter to a greater extent than cows calving in late summer because of the high vitamin E content in the pasture-based summer diet.116 Calves from supplemented cows had higher serum vitamin E levels than calves from unsupplemented cows. Winter-born calves from supplemented Hereford cows had heavier 205-day adjusted weaning weights than did winter-born calves from unsupplemented cows. Supplementation did not affect vitamin E or IgG concentrations in cows which calved in late summer and it did not affect calf growth.
Dairy cattle
The legal commercial selenium supplementation of complete rations for dairy cattle in the USA has recently been increased from 0.1 to 0.3 mg/kg DM of complete feed.117 At this rate, a lactating cow consuming 20 kg of DM/d would consume about 6 mg supplemental selenium in addition to that naturally present in the feedstuffs. Current recommendations indicate that selenium intake for lactating and gestating dairy cattle should range from 5 to 7 mg/d for adequate concentrations in serum or plasma which would range from 70 to 100 ng of selenium/mL serum. Such supplementation should result in improved selenium status of the newborn, improved concentration of selenium in colostrum and improved health of the calves. The effects of selenium supplementation in dairy cattle on reproductive performance is equivocal. Some studies over a period of two lactations revealed no effect on reproductive performance, while others report an improvement in dairy cattle in a district considered to be marginally deficient in selenium. Intakes of inorganic selenium as sodium selenite in amounts of 50 mg/d for 90 days or 100 mg/d for 28 days by adult dairy cows (10–30 times the nutritional requirement) did not cause any health problems.118 The toxic dose for cattle ranges from 0.25 to 0.5 mg/kg BW.
Milk replacers for dairy calves should contain a suitable antioxidant and be supplemented with 300 IU/kg DM of α-tocopherol acetate at the rate of 0.1 mg/kg DM of the milk replacer.
Dietary or parenteral supplementation of vitamin E to dairy cows during the peripartum period has consistently improved the function of neutrophils and macrophages.117 However, the effects of supplementation of dry dairy cows with vitamin E in the feed or parenteral administration of vitamin E before parturition on the incidence of disease have been variable. The amount of supplemental vitamin E fed per day during the prepartum period has ranged from 1000 to 3000 IU/day. Feeding 1000 IU/day of supplemental vitamin E to dry cows when adequate selenium was supplemented reduced the incidence of retained placenta. The prepartum subcutaneous injection of dairy cows with 3000 IU of vitamin E, 1 week before expected calving had no significant difference on the incidence of retained placenta, clinical mastitis, metritis, endometritis, ketosis, displaced abomasum, or lameness.60 Vitamin E administered to cows with marginal pretreatment vitamin E status had a reduced risk of retained placenta. In cows, with adequate serum vitamin E, there was no reduction in the incidence of any disease.60,61
Based on health and immune function in cows, plasma concentrations of α-tocopherol in peripartum cows should be approximately 3 μg/mL. To maintain these blood values, dry cows and heifers fed stored forages during the last 60 days of gestation require approximately 1.6 IU of supplemental vitamin E/kg BW (approximately 80 IU/kg DMI). Increased intake of vitamin E of cows and heifers during the prepartum period also increases the vitamin E in colostrum. Milk is not a major source of vitamin E but colostrum contains high concentrations of α-tocopherol (3 to 6 μg/mL. For lactating cows, being fed stored forages, to reduce the incidence of mastitis, the recommendation for vitamin E is 0.8 IU/kg BW (approximately 20 IU/kg DMI).117 When fresh forage is fed, there is less need for supplemental vitamin E. The intake of polyunsaturated fatty acids increases the vitamin E requirement and additional vitamin E may be required when protected unsaturated fats are fed.
NMD in the neonate
The injection of Se 0.06 mg/kg BW into piglets under 1 week of age, repeated at weaning time and into the sow 3 weeks before farrowing will be effective. The minimum lethal dose of Se for piglets is 0.9 mg/kg BW, which provides a reasonably wide range of safety. A high concentration of Se in the diet of pregnant sows in the last half of gestation has been associated with hemorrhagic lesions on the claws of newborn piglets.119
While selenium alone is protective against a greater spectrum of diseases than is vitamin E, there are situations in which vitamin E is more protective. Both selenium and vitamin E should be provided when the diets are deficient in both nutrients, but this may not apply in every situation. NMD can occur in ruminants with vitamin E deficiency and an adequate selenium status. Most of the emphasis has been on selenium supplementation at the expense of vitamin E, which is more expensive and less stable. Most injectable vitamin E and selenium preparations are adequate in selenium but insufficient in vitamin E.
Selenium responsive reproductive performance and growth
Sheep
In selenium deficient situations, reproductive performance of ewes may be improved by selenium or selenium and vitamin E. Survival of lambs, live weights at birth and at weaning may be increased by selenium supplementation. Single injections of selenium before mating and lambing had no significant effects on estrus, fertility, prolificacy, and the number of lambs born and reared to 28 days in 2-year-old ewes.120 Two consecutive injections of selenium (before mating and lambing) significantly increased the incidence of estrus, fertility, and lamb body weight at 28 days and daily weight gains for 28 days in 3-year-old ewes compared with controls. The injection of selenium plus vitamin E did not significantly improve reproductive performance in 2- nor 3-year-old ewes in the flock not considered selenium deficient.
Weak-calf syndrome
The parenteral injection of selenium and iodine to pregnant cattle in Ireland did not significantly reduce the incidence of the weak-calf syndrome, which is often attributed to a selenium deficiency.
Pigs
The injection of selenium 0.06 mg/kg BW into piglets under 1 week of age, repeated at weaning time and into the sow 3 weeks before farrowing will be effective. The minimum lethal dose of selenium for piglets is 0.9 mg/kg BW, which provides a reasonably wide range of safety. A high concentration of selenium in the diet of pregnant sows in the last half of gestation has been associated with hemorrhagic lesions on the claws of newborn piglets.121
Horses
Little information is available on the need of horses for selenium but the optimum intake is 6 mg/week or 2.4 μg/kg BW daily. The oral supplementation of 1 mg selenium/d increases blood selenium concentrations above levels associated with myodegeneration in horses and foals. In New Zealand, for horses on pasture, the injection of barium selenate, at a dose of 0.5 mg Se/kg BW, aseptically at a deep intramuscular site was efficacious in correcting the selenium status of mares grazing pasture with a selenium content of 0.01 to 0.07 mg/kg DM.81 Some local swelling will occur.
To ensure nutritional adequacy and to have an adequate safety margin, adult Standardbred horses should receive 600–1800 mg DL-α-tocopherol daily in their feed. The parenteral administration of vitamin E and selenium to mares in late pregnancy and to their foals beginning at birth, will increase blood selenium to adequate levels. In selenium-deficient areas or when mares are fed selenium-deficient hay, the prepartum injections of selenium and vitamin E are indicated followed by intermittent injection of the foals, or supplementation of the diet with selenium at 0.1 mg/kg DM.
Intra-ruminal selenium pellets
Sheep
Intra-ruminal selenium pellets, similar to those used in cobalt deficiency, have produced satisfactory blood levels of selenium for up to 4 years in ewes at pasture.89 A satisfactory pellet is composed of 0.5 g elemental selenium and finely divided metallic iron. The technique is efficient, but not completely, due to wide variations between animals in the absorption rate of the selenium. The average delivery of selenium is 1 mg/d and there is no danger of toxicity. In sheep grazing selenium-deficient pastures, the ruminal pellets increase the selenium status and weight gains compared with controls. About 15% of treated sheep reject the pellets within 12 months and in varying degrees the pellets acquire deposits of calcium phosphate. Sheep fed pellets recovered from sheep have low selenium levels, which suggests a low release of selenium from pellets that have been in the rumen of other sheep for several months. The peak levels of selenium occur 3 months after administration; there is a rapid decline in activity between 5 and 13 months. Sustained-released boluses containing sodium selenite, cobalt sulfate, potassium iodide, manganese sulfate, zinc oxide, and sulfate and vitamins A, D, and E have also been formulated to provide long-term maintenance of selenium.
A zinc, cobalt, and selenium soluble glass bolus administered to ram lambs increased the selenium status of the animals and increased sperm motility, percentage of live sperm and sperm responding to hypo-osmotic swelling test (an assay to determine plasma membrane permeability).122
High density compressed pellets containing both sodium selenite and cobalt carbonate have been developed for cattle and sheep.43 The sheep pellet weighs 6 g and contains 276 mg Se and 765 mg Co. A 6 g bolus given to ewes before mating resulted in improved lambing performance, an increase in the percentage of twin lambs.43
Cattle
A selenium pellet containing 10% selenium and 90% iron grit is available for cattle and will maintain plasma selenium and GSH-PX activity above the critical level for up to 2 years.123 When given to beef cows in the last 3 months of pregnancy, the selenium levels in milk are higher than in controls and the selenium status of the calves was sufficient to prevent NMD. The use of these pellets at two, three, and four times the recommended dose in growing cattle weighing 300–350 kg did not cause toxicosis and the selenium levels in the tissues at slaughter were not a risk for humans.
Use of the intra-ruminal selenium pellets in dairy cattle in New Zealand resulted in improved growth and milk production in herds where the selenium status was below the adequate range, but there was no effect on udder health and reproductive performance.
High density compressed pellets containing both sodium selenite and cobalt carbonate have been developed for cattle and sheep.43 For cattle, the pellets weigh 18 g and contain 4.6% selenium and 12.75% cobalt (828 mg Se and 2295 mg Co). In both beef cows and growing cattle, the boluses increased blood glutathione peroxidase activity for at least 1 year.
A sustained-release intra-reticular bolus is an osmotic pump designed to release 3 mg selenium into the reticulorumen. It is intended to provide selenium supplementation for 120 days in grown heifers and pregnant beef cattle.
Selenium toxicity and residues
Selenium intoxication can occur following the administration of toxic amounts of a selenium salt. The use of selenium selenite instead of sodium selenate and giving a dose of five times the intended dose resulted in a high mortality within several hours after administration.1 Animals deficient in selenium are more susceptible to selenium toxicosis than those that are selenium-adequate. The pharmacokinetics of selenium toxicity in sheep given selenium selenite parenterally has been examined. When oral preparations of selenium and monensin are given concurrently as part of a routine dietary management practice, there is greater risk of selenium intoxication than if the selenium is given alone. Administration of monensin sodium at a constant, safe dosage enhanced the toxicity of selenium as demonstrated by increased severity of the signs of intoxication, fatalities, tissue selenium concentrations and intensified gross, histopathological, and biochemical changes. There is some concern about selenium supplementation of beef cattle being a potential source of contamination for nearby aquatic systems, but there is no evidence that this has occurred.
Selenium responsiveness
The response to selenium supplementation is proportional to the degree of deficiency and supplementation of animals that have adequate selenium intakes is unlikely to significantly improve growth rate. In New Zealand, for selenium-deficient lambs, the potential for a growth response to selenium supplementation is strongly related to blood selenium concentration.79 Economically significant live weight gains of >10 g/d can occur when initial blood selenium concentrations are <130 nmol/L. This is the basis for the development of reference curves using blood selenium concentration to diagnose selenium deficiency and predict growth responses to lambs.79
Although many methods of supplementation of selenium are efficacious, they can differ widely in their cost and convenience of administration. The objective of any micronutrient supplementation program should be to optimize the return on investment.40 The least cost option which provides adequate supplementation for the required period should be recommended initially.
Veterinarians are the professionals in the best position to offer advice on cost-effectiveness supplementation.40 To retain this position, they must provide sound recommendations based on micronutrient analysis of animal tissue and defensible reference ranges which are supported by production response data. Monitoring micronutrient status in animal tissue should be encouraged so as to ensure that regulatory requirements are met and that deficiency and excessive use are avoided. Circumvention of veterinary involvement in the diagnosis and treatment of micronutrient supplementation can lead to greater use of supplements when not indicated, higher costs to farmers and low cost-benefit ratios for the industry.
Depot and bolus preparations have revolutionized the treatment of deficiencies of cattle and sheep that are grazed extensively where there is little opportunity for frequent administration (Table 30.8). The relatively short duration of a single drench or injection of selenium salts such as sodium selenite should be noted. The use of fertilizer applications selenium prills is gaining widespread acceptance on farms with high stocking rates.
Table 30.8 Dose rates and duration for selected selenium supplements for adult cattle40
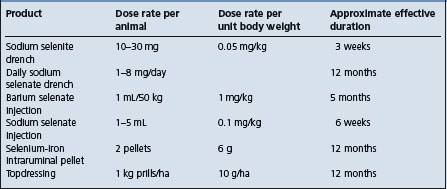
McDowell LR. Vitamin nutrition of livestock species. Nutrition Abstracts and Reviews Series B. Livestock Feeds and Feeding. 2001;71:33R-41R. Wallingford, Oxon: CAB International
Kim YY, Mahan DC. Biological aspects of Se in farm animals. Asian Aust J Anim Sci. 2003;16:435-444.
Underwood EJ, Suttle NF. Selenium. In The mineral nutrition of livestock, 3rd ed., Wallingford, Oxon: CAB International; 1999:421-475.
Wichtel JJ. A review of selenium deficiency in grazing ruminants. Part I: New roles for selenium in ruminant metabolism. N Z Vet J. 1998;46:47-52.
Wichtel JJ. A review of selenium deficiency in grazing ruminants. Part 2: Towards a more rational approach to diagnosis and prevention. N Z Vet J. 1998;46:54-58.
VanMetre DC, Callan RJ. Selenium and vitamin E. Vet Clin North Am Food Anim Pract. 2001;17:373-402.
Wilson PR, Grace ND. A review of tissue reference values to assess the trace element status of farmed red deer (Cervus elaphus). N Z Vet J. 2001;49:126-132.
Allison RD, Laven RA. Effect of vitamin E sup plementation on the health and fertility of dairy cows: a review. Vet Rec. 2000;147:703-708.
Lofstedt J. White muscle disease of foals. Vet Clin North Am Equine Pract. 1997;13:169-185.
Hemingway RG. The influences of dietary intakes and supplementation with selenium and vitamin E on reproduction diseases and reproductive efficiency in cattle and sheep. Vet Res Commun. 2003;27:159-174.
Clark, RG. Copper and selenium toxicity. In: Proceedings of the 25th Seminar of the Sheep and Beef Cattle Society of the New Zealand Veterinary Association. Massey University, Palmerston North: The Foundation for Veterinary Continuing Education, 1995;111–119.
. Subcommittee on Dairy Cattle Nutrition, Committee on Animal Nutrition, Board on Agriculture, National Research Council Subcommittee on Dairy Cattle Nutrition. Nutrient requirements of dairy cattle, 7th ed. 2000. Washington, DC: National Academy Press.
. Subcommittee on Beef Cattle Nutrition, Committee on Animal Nutrition, Board on Agriculture, National Research Council. Nutrient require ments of beef cattle, 7th revised ed. 2000. Washington, DC: National Academy Press.
. Subcommittee on Swine Nutrition, Committee on Animal Nutrition, Board on Agriculture, National Research Council. Nutrient requirements of swine, 10th revised ed. 1998. Washington, DC: National Academy Press.
1 Underwood EJ, Suttle NF. Selenium. In The mineral nutrition of livestock, 3rd ed., Wallingford, Oxon: CAB International; 1999:421-475.
2 VanMetre DC, Callan RJ. Vet Clin North Am Food Anim Pract. 2001;17:373.
3 Wichtel JJ. N Z Vet J. 1998;46:47.
4 Hill GM, et al. J Anim Sci. 1999;77:1762.
5 National Academy of Science. In Selenium in Nutrition. Washington, DC: National Research Council; 1983.
6 Lofstedt J. Vet Clin North Am Equine Pract. 1997;13:169.
7 Pavlata L, et al. Acta Vet Brno. 2001;70:269.
8 Menzies P, et al. Can Vet J. 2004;45:244.
9 Pavlata L, et al. Acta Vet Brno. 2003;72:639.
10 Pavlata L, et al. Acta Vet Brno. 2002;71:3.
11 Pavlata L, et al. Acta Vet Brno. 2001;70:277.
12 Beytut E, et al. Vet J. 2002;163:214.
13 Ramirez-Bribiesca JE, et al. Small Rum Res. 2001;41:81.
14 Wichtel JL, et al. Can Vet J. 2004;45:124.
15 Miller GY, et al. J Am Vet Med Assoc. 1995;206:1369.
16 Menzies PI, et al. Can Vet J. 2003;44:898.
17 Smith GM, Allen JG. Aust Vet J. 1997;75:341.
18 Abutarbush SM, Radostits OM. Aust Vet J. 2003;44:738.
19 Orr JP, Blakley BR. J Vet Diagn Invest. 1997;9:172.
20 Schrauzer GN. J Nutr. 2000;130:1653.
21 Ching S. J Anim Sci. 2002;80:2396.
22 Van Fleet JF, Kennedy S. Compendium on Continuing Education for the Practicing Veterinarian. 1989;11:662.
23 Rice DA, Kennedy S. Am J Vet Res. 1989;50:2101.
24 Cline JH, et al. J Anim Sci. 1974;39:974.
25 Mahan DC, et al. J Anim Sci. 1991;78:110.
26 Migdal W. Kaczmarcyk. J World Rev Anim Prod. 1993;28:68.
27 Mavromatis J, et al. J Vet Med. 1999;46:543.
28 Chavez ER. Can J Anim Sci. 1983;63:683.
29 Yen JT, Pond WG. J Anim Sci. 1983;56:621.
30 Pinelli-Saavedra A, et al. Proc 18th Int Pig Vet Soc, Hamburg, Germany, 2004;740.
31 de Alba C, et al. J Anim Fd Science. 2000;9:479.
32 Pherson B, et al. J Vet Med A. 2001;48:569.
33 Fellstrom C. Proc 15th Int Pig Vet Soc Cong. 1998;3:30.
34 Hidiroglou M, et al. Reprod Nutr Develop. 1995;35:443.
35 Wang YH, et al. Brit J Nutr. 1996;75:81.
36 Kovra M, et al. J Anim Sci. 2003;81:1967.
37 Nielsen TK, et al. Vet Rec. 1989;124:535.
38 Diaza A, et al. Invest Agraria Prod San Anim. 2000;15:21.
39 Pallares FJ, et al. J Vet Diag Inv. 2002;14:412.
40 Wichtel JJ. N Z Vet J. 1998;46:54.
41 Andres S, et al. Vet J. 1999;157:186.
42 Hoff B, et al. Can Vet J. 2001;42:384.
43 Hemingway RG, et al. Cattle Pract. 2003;11:167.
44 Villar D, et al. Bov Pract. 2002;38:73.
46 van Niekerk FE, et al. J South Afr Vet Ass. 1996;67:209.
47 Allison RD, Laven RA. Vet Rec. 2000;147:703.
48 Coe PH, et al. J Am Vet Med Assoc. 1993;202:875.
49 Cebra CK, et al. J Vet Intern Med. 2003;17:902.
50 Sivertsen T, et al. Acta Vet Scand. 2005;46:177-191.
51 Erskine RJ, et al. J Am Vet Med Assoc. 1997;211:466.
52 Morgante M, et al. J Dairy Sci. 1999;82:623.
53 Stabel JR, et al. Nutr Res. 1996;10:1053.
54 Pollock JM, et al. Res Vet Sci. 1994;56:100.
55 Carter JN, et al. Am J Vet Res. 2002;63:1111.
56 Rivera JD, et al. J Anim Sci. 2002;80:933.
57 Pavlata L, et al. Vet Med Czech. 2004;49:149.
58 Nicholson JWG, et al. Can J Anim Sci. 1993;73:355.
59 Wuryastuti W, et al. J Anim Sci. 1993;71:2464.
60 LeBlanc SJ, et al. J Dairy Sci. 2002;85:1416.
61 LeBlanc SJ, et al. J Dairy Sci. 2004;87:609.
62 Panousis N, et al. Vet Rec. 2001;149:643.
63 Giadinis N, et al. Comp Immunol Microbiol Infect Dis. 2000;23:129.
64 Grace ND, et al. N Z Vet J. 2001;49:24.
65 Hostetler CE, Kincaid RL. Biol Trace Elem Res. 2003;96:1.
66 Lacerta N, et al. Am J Vet Res. 1996;57:1176.
67 Swecker WSJr, et al. Am J Vet Res. 1995;56:450.
68 Abdelrahman MM, Kincaid RL. J Diary Sci. 1995;78:625.
69 Politis I, et al. Am J Vet Res. 1996;57:468.
70 Weiss WP, et al. J Dairy Sci. 1997;80:1728.
71 Pavlata L, et al. Acta Vet Brno. 2001;70:19.
72 David GP, Winterbottom AJ. Vet Rec. 1996;139:20.
73 Pallares FJ, et al. J Vet Diagn Invest. 2002;14:412.
74 Korpela HJ. Am Coll Nutr. 1991;10:127.
75 Macpherson A. Garnsworthy PC, Cole DJA, editors. Recent advances in animal nutrition. Nottingham: Nottingham Press. 1994:3-30.
76 Green LE, et al. Vet Rec. 1995;136:197.
77 Pettifer EBG. Pig J. 1998;42:144.
78 Waldner C, et al. Can Vet J. 1998;39:225.
79 Grace ND, Knowles SO. N Z Vet J. 2002;50:163.
80 Thompson JC, et al. N Z Vet J. 1998;46:65.
81 Wichtel JJ, et al. N Z Vet J. 1998;46:186.
82 Stowe HD, Herdt TH. J Anim Sci. 1992;70:3928.
83 Kirk JH, et al. Am J Vet Res. 1995;56:1460.
84 Orr JP, Blakley BR. J Vet Diagn Invest. 1997;9:172.
85 Zust J, et al. Vet Rec. 1996;139:391.
86 Lean IJ, et al. Cornell Vet. 1990;80:41.
87 Kinoshita C, et al. J Dairy Sci. 1996;79:1543.
88 Pavlata L, et al. Acta Vet Brno. 2000;69:281.
89 Archer JA, Judson G. Am J Exp Agric. 1994;34:581.
90 Steiss JE, et al. Equine Vet J. 1994;26:417.
91 Puls R. Veterinary trace mineral deficiency and toxicity information Publication, No. 5139. Ottawa: Information Series, Agriculture Canada, 1981;1-101.
92 Sobiech P, Kuleta Z. Small Rum Res. 2002;45:209.
93 Wilson PR, Grace ND. N Z Vet J. 2001;49:126.
94 Grace ND, Wilson PR. N Z Vet J. 2002;50:252.
95 Hoppeler H, Weibel ER. Acta Physiol Scand. 2000;168:445.
96 Jensen M, et al. Acta Vet Scand. 1990;31:129.
97 Diver TJ, et al. Vet Clin North Am: Equine Pract. 1997;13:97.
98 Menzies-Gow NJ, et al. Vet Rec. 2002;151:416.
99 Liang MZ, et al. Chin J Vet Sci. 2003;23:620.
100 Van Heugten E, et al. J Am Vet Med Assoc. 1997;211:1039.
101 Andersson M, et al. Proc 16th Int Pig Vet Soc Cong, Melbourne, Australia, 2000;259.
102 Clark, RG. Copper and selenium toxicity. In: Proceedings of the 25th Seminar of the Sheep and Beef Cattle Society of the New Zealand Veterinary Association. Massey University, Palmerston North: The Foundation for Veterinary Continuing Education, 1995;111–119.
103 Sivertsen T, et al. Vet Hum Toxicol. 2003;45:31.
104 Penrith ML, Robinson JTR. Am J Vet Res. 1996;63:171.
105 Davidson-York D, et al. J Vet Diag Invest. 1999;11:352.
106 Stowe HD, et al. J Am Vet Med Assoc. 1992;201:292.
107 Mahan D. Proc Maryland Nutrition Conf. 2000;47:26.
108 Cohen RDH, et al. Can Vet J. 1991;32:113.
109 Maas J, et al. J Vet Intern Med. 1993;7:342.
110 Van Niekerk FE, et al. J South Afr Vet Ass. 1995;66:11.
111 Zachara BA, et al. Small Rum Res. 1993;11:135.
112 Grace ND, et al. N Z Vet J. 2000;48:53.
113 Grace ND, et al. N Z Vet J. 1994;42:63.
114 Fry JM, et al. Aust J Agric Res. 1996;47:853. 869
115 Gunter SA, et al. J Anim Sci. 2003;81:856.
116 Bass II RT, et al. Am J Vet Res. 2001;62:821.
117 . Subcommittee on Dairy Cattle Nutrition, Committee on Animal Nutrition, Board on Agriculture, National Research Council Sub committee on Dairy Cattle Nutrition. Nutrient requirements of dairy cattle, 7th ed. 2000. Washington, DC: National Academy Press.
118 Ellis RA, et al. Am J Vet Res. 1997;58:760.
119 Mensink CG, et al. Vet Rec. 1990;126:620.
120 Gabryszuk M, Klewiec J. Small Rum Res. 2002;43:127.
121 Mensink CG, et al. Vet Rec. 1990;126:620.