CHLORINATED HYDROCARBONS (ORGANOCHLORIDES)
Etiology Poisoning by any of the group of insecticides including aldrin, hexachloride, chlordane, DDT, dieldrin, endrin, heptachlor, isodrin, lindane, methoxychlor, toxaphene.
Epidemiology Accidental or misinformed overdosing. Usage on animals now superceded by other less toxic compounds. Stored or leftover products may accidentally be accessed by animals. Importance now due to residues in animal products used in human food chain.
Clinical signs Excitement, tremor, intermittent convulsions, hyperthermia, death.
Clinical pathology Assay of compounds in animal tissues.
Necropsy lesions No consistent significant lesions; some animals show pale musculature.
Diagnostic confirmation
Chemical assay of liver or brain for acute poisoning; fat or other animal tissue for chronic poisoning.
Treatment Primary: nil. Supportive: by sedation, control of hyperthermia, removal of residual chemical; activated charcoal for oral detoxification.
Control Use alternative insecticides. Avoid mixed farming enterprises which include use of these insecticides for insect control in crops.
ETIOLOGY
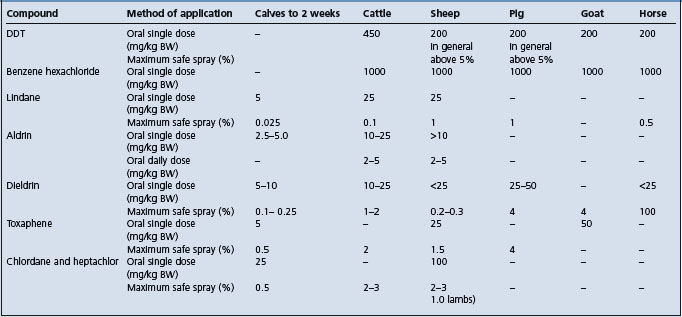
This group of poisons includes DDT, benzene hexachloride (and its pure gamma isomer – lindane), aldrin, dieldrin, chlordane, toxaphene, methoxychlor, DDD, isodrin, endrin, and heptachlor. General toxicity data for the more common compounds are given in Table 32.4. Methoxychlor is less toxic than DDT, and isodrin and endrin are more toxic than aldrin and dieldrin. Camphor (2-bornanone) is chemically similar to toxaphene and is associated with a similar syndrome when fed accidentally.
EPIDEMIOLOGY
Occurrence
Poisoning with these compounds has been recorded in all animal species. The chlorinated hydrocarbons have come under so much criticism as environmental contaminants that they are rarely used directly on animals nowadays, so that outbreaks of clinical illness associated with them are much less common than they were.
Source of toxin
Ingestion, inhalation, aspiration, and percutaneous absorption are all possible portals of entry so that contamination of feed and application of sprays and dips can all be associated with poisoning. Organochlorides are closely regulated and banned in some countries but still widely used in agriculture, principally on growing plants to control insect pests, and on stored seed grain to control fungi. If the plants or grain, even milled and by products, e.g. bran, are fed to animals they can be associated with problems of tissue residues; if they are fed in sufficient quantities they can be associated with clinical illness. Many outbreaks are associated with the application to animals of products intended for crops, e.g. endosulfan, and labelled specifically ‘Not For Animal Use’. These insecticides may also contaminate soil and persist there for many years. Rooting animals such as pigs are particularly susceptible to this source of poisoning. These compounds are also sometimes fed accidentally and in large amounts in lieu of feed additives, and are associated with acute poisoning. In feedlotted or shedded animals cases may continue for periods as long as a year because of repeated contamination from the environment. Insect baits, e.g. grasshopper baits containing toxaphene and chlordane, used on pasture and for leaf-eating insects on market gardens can be associated with poisoning in livestock, which may eat large quantities of them. These insecticides, especially heptachlor, are incorporated in the soil before the crop of potatoes or maize is sown to control soil pests. Subsequent grazing of the field will cause contamination of the livestock for several years.
Method of application
Dipping of animals is the most hazardous method of application because entry may occur through all portals. Spraying is safer, percutaneous absorption and inhalation being the only portals of entry. The small particle size of the compound and concentration of animals in confined spaces while spraying, increase the possibility of poisoning. Oily preparations are not used for animal treatment but are used inadvertently and are readily absorbed through the skin.
Formulation used
Concentrations of insecticide in formulations used for spraying barns are much higher than those used for animals. Amongst spray preparations simple solutions are most dangerous followed by emulsions and least of all suspensions of wettable powder. Dusting is safest and is preferred to other methods. Preparations for use on plants are often unstable emulsions, which come out of suspension quickly when they reach the plant. If these preparations are used in animal dips the first few animals through the dip can be heavily contaminated and suffer acute, lethal toxic effects. Although the treatment of pastures to control their insect pests is usually safe to animals grazing, the treated pasture or hay made from it can cause contamination of animal products. This contamination can be avoided by incorporating the insecticide into superphosphate granules (‘prills’) instead of applying it as sprays or dusts. The use of chlorinated hydrocarbons to protect stored seeds provides a hazard to animals if they are fed on the treated seed.
Risk factors
The compounds vary in their ability to pass the skin barrier. Benzene hexachloride, aldrin, dieldrin, and chlordane are readily absorbed. Species susceptibility to skin absorption also varies widely. Very young animals of any species are more susceptible than adults, and lactating and emaciated animals also show increased susceptibility.
When these compounds were first used in dips, skin and foot infections occurred frequently because of contamination of the dip in the absence of a bactericidal agent. Cases of otitis media have occurred for the same reason, but more rarely.
Importance
All of these compounds are capable of causing death due to acute poisoning but these are increasingly rare. Because the compounds are soluble in fat and accumulate in body stores of it they are formidable threats to the meat industry. They are also excreted in significant amounts in milk and enter the human food chain at this point. They are concentrated still further in cream and butter. They also represent a threat to sucklings, but the degree of contamination in fetuses and suckling animals is much less than in their dams.
The principal importance of organochlorine poisoning is the contamination of animal tissues at levels which are not acceptable by modern health standards. These contaminations become the subject of veterinary investigations, and are susceptible to standard techniques of epidemiological examination.
PATHOGENESIS
The mode of action of organochlorides is to induce repetitive discharge of motor and sensory neurons by interference with axonal transmission of nerve impulses. After absorption, cyclodiene insecticides are activated by the mixed function oxidase (MFO) system and any prior chemical or environmental exposures that increase the MFO system may exacerbate the onset of poisoning. The diphenyl aliphatic (DDT) organochlorines affect sodium channels, prolonging sodium influx and inhibiting potassium efflux at the nerve membrane. The cyclodiene organochlorines competitively inhibit the binding of gamma amino butyric acid (GABA) at receptor sites, resulting in loss of GABA inhibition and resultant stimulation of the neuron. In all organochlorine poisonings recovery may occur, but with smaller animals paralysis follows and finally collapse and death ensue.
Most of the substances accumulate in the fat depots, where they are a potential source of danger in that sudden mobilization of the fat may result in liberation of the compound into the bloodstream and the appearance of signs of toxicity.
CLINICAL FINDINGS
The speed of onset of illness after exposure varies from a few minutes to a few hours, depending on the portal of entry and the compound and its formulation, but it is never very long.
The toxic effects produced by the members of this group include complete anorexia, increased excitability and irritability followed by ataxia, muscle tremor, weakness and paralysis and terminal convulsions in severe cases. Salivation and teeth grinding occur in large animals and vomiting in pigs. Variations on this clinical syndrome which is common to all organochlorine intoxications include:
• DDT and methoxychlor chronic poisoning may be associated with moderate liver damage
• Benzene hexachloride, lindane, chlordane, toxaphene, dieldrin, endrin, aldrin, heptachlor are associated with an exaggerated syndrome including teeth grinding, champing of jaws, dyspnea, tetany, snapping of the eyelids, auricular spasms, opisthotonus, frequent micturition, frenzied movements, walking backwards, climbing walls, violent somersaults, and aimless jumping. Fever of 5–7% above normal may occur, possibly a result of seizure activity. Seizures may persist for 2 or 3 days if the animal does not die.
CLINICAL PATHOLOGY
Blood, hair, and ingesta can be assayed chemically for specific toxins. The removal of a biopsy from the fat pad near the cow’s tail offers a satisfactory means of providing samples for tissue analysis. Organochlorine residues in acutely poisoned animals may reach 4–7 ppm in brain or liver.
NECROPSY FINDINGS
At necropsy there are no specific major lesions in the nervous system but toxic hepatitis and tubular nephritis appear in some cases. For assay specimens of hair, if the portal is percutaneous, and of the ingesta, if oral intake is probable, are appropriate. Tissue levels need to be high to be good indicators of recent intoxication. If possible the specimens should be deep frozen and the suspected compound should be nominated as assay procedures are long and involved.
The predominantly nervous syndrome attracts attention to encephalitis, encephalomalacia and toxic and metabolic encephalomyelopathies in all species. Diagnostic confirmation depends on a positive assay on animal tissues.
• Lead poisoning, confirmed by a positive assay for lead on tissue
• Rabies confirmed by histopathological examination
• Pseudorabies of cattle in early stages with pruritis and the accompanying frenzy
• Polioencephalomalacia confirmed by histopathological examination
• Thromboembolic meningoencephalitis in cattle confirmed by the isolation of Histophilus somni
• Salt poisoning in pigs identifiable by the characteristic lesions of eosinophilic meningoencephalitis.
TREATMENT
There is no specific primary treatment.
Supportive treatment includes sedation with pentobarbital sodium; repeated doses until signs disappear are preferred, with intravenous injections of glucose and calcium and the administration of a non-oily purgative. Activated charcoal (2 g/kg) given early by stomach tube will bind pesticide in rumen and reduce further absorption. Residual chemical should be removed from the coat, and this may be facilitated by judicious washing with soap and copious water rinse.
Treatment to reduce the contamination of tissues is unsuccessful, and in most cases the time required for the contamination to subside naturally is long, of the order of 3–6 months, but varying between specific compounds. For example, cows fed DDT prepartum have required an average of 189 days from parturition for the level in the milk fat to decline to 125 ppm. Contamination in other species and with other chlorinated hydrocarbon compounds also tends to be persistent. After the source of contamination is removed drenching of cows with up to 2 kg of activated charcoal followed by daily incorporation in their feed for 2 weeks, or small amounts of mineral oil by mouth at short intervals, have been recommended for this purpose. Neither of these procedures is really practical in the average farm operation. The common procedure for reducing the level of contamination in animals is to put them in a feedlot without any contact with pasture and feed them on energy intensive rations. Sheep decontaminate much more quickly than cattle and animals on a high plane of nutrition eliminate the toxins more quickly.