14
Chapter Outline
Before the discovery of the Rh system by Landsteiner in 1940, little was known about the etiology of erythroblastosis fetalis, a condition in which the fetus becomes edematous and often dies in the uterus from severe anemia and high-output cardiac failure. After this discovery, it was quickly learned that maternal Rh alloimmunization, with placental transfer of IgG antibodies, was the phenomenon responsible for the fetal red cell destruction. This was followed by the finding that spectrophotometric analysis of the amniotic fluid was an excellent index for measuring the severity of the fetal anemia and by the realization that early delivery and intrauterine transfusions (IUTs) could be lifesaving maneuvers for the compromised fetus. Finally, it was found that the administration of Dimmunoglobulin to mothers at risk is an extremely effective way of preventing the initial immune response responsible for the problem. In the last decade the main advances are the clarification of the genetics of the Rh factor and the discovery of a noninvasive method, the peak velocity of the fetal middle cerebral artery, or mid-cerebral artery (MCA), to detect the occurrence of fetal anemia. Despite these advances the incidence of Rh alloimmunization remains constant at about six cases per 1000 births. This incidence is significantly greater in countries with limited availability of D-immunoglobulin.
Pathophysiology
Erythroblastosis fetalis is a disease in which the red blood cells of the fetus and the newborn are hemolyzed by maternal alloantibodies (antibodies capable of reacting with red cells from the same species but not with red cells of the individual producing the antibodies) that have crossed the placenta. The resulting anemia leads to fetal heart failure, massive edema (hydrops fetalis), and intrauterine death. It also may cause varied degrees of neonatal hyperbilirubinemia (hemolytic disease of the newborn). Approximately 97% of all cases of erythroblastosis fetalis are caused by maternal antibodies directed against the RhD antigen present in the fetal red cells. The remaining cases are caused by immunization against other fetal antigenic groups such as C, c, E, e, K, k, Fya, M, and Jka (Box 14-1). Maternal alloimmunization may be also the result of the transfusion of Rh-positive blood to an Rh-negative female. In response to this immunologic stimulation, the mother develops 19S and 7S antibodies, the latter being able to cross the placenta and destroy the fetal red cells.
Box 14-1 Irregular antibodies associated with fetal or neonatal hemolytic disease
| Blood group system | Related antigen |
|---|---|
| Kell | K |
| k | |
| Ko | |
| Kpa | |
| Jsa | |
| Jsb | |
| Rh (non-D) | E |
| e | |
| C | |
| c | |
| Duffy | Fya |
| Fyb | |
| Kidd | Jka |
| Jkb | |
| Jk3 | |
| MNSs | M |
| N | |
| S | |
| s |
During normal pregnancy, fetal red cells cross the placenta in 5% of the cases during the first trimester and in 46% by the end of the third trimester (Bowman et al., 1986). However, in the majority of cases maternal Rh sensitization is the consequence of fetal–maternal bleeding happening at the time of delivery. Passage of fetal blood into the maternal circulation at the time of parturition is the rule rather than the exception, but only 10–15% of Rh-negative mothers who have Rh-positive husbands become sensitized at delivery. This happens because in most cases the amount of fetal cells transferred to the mother is small and insufficient to produce a primary immune response. Other factors also influence the probability of primary alloimmunization. One of them is the size of the inoculum; it is accepted that the greater the number of fetal cells entering the maternal circulation, the greater the possibility of maternal sensitization, although some mothers have been immunized with as little as 0.25ml of fetal Rh-positive cells. Another factor is the coexistence of ABO incompatibility between mother and fetus; if the mother is group O and the father A, B, or AB, the frequency of sensitization is decreased by 50–75% because the maternal anti-A or anti-B antibodies destroy the fetal red cells carrying the Rh antigen before they can elicit an immune response. Furthermore, 30–35% of Rhnegative subjects are non-responders (cannot be immunized) to the Rh-positive antigen, a characteristic that seems to be genetically controlled.
When an immune response is elicited during pregnancy (incidence less than 1%) or at delivery (incidence 10–15%) in an Rh-negative mother who carries an Rhpositive baby, the initial maternal response will be the development of anti-Rh IgM antibodies with a molecular weight too large to cross the placenta. This is followed by the synthesis of anti-Rh IgG antibodies that cross the placenta and stick to the fetal red cells, accelerating their destruction in the infant's reticuloendothelial system. The time between the fetal–maternal bleeding and the initiation of the primary immune response in the mother is not exactly known and probably has some biologic variation. Usually there is an interval of several weeks between the time of the fetal–maternal bleeding and the appearance of anti-Rh antibodies in the maternal serum. That is why prophylactic administration of D-immunoglobulin to the mother shortly after delivery inhibits the immune response. Even when the administration of Dimmunoglobulin is delayed up to 2 weeks after transfusion of Rh-positive cells, the procedure is protective in 50% of the cases.
Once Rh alloimmunization has started, the mother may produce large amounts of antibodies (secondary response) in response to small amounts of fetal Rh-positive blood leaking through the placenta. The anti-Rh antibodies cross the placenta and attach to the infant's red cells, making them susceptible to destruction in the fetal reticuloendothelial system. Depending on the severity of the hemolysis, the clinical picture may include congestive heart failure, hepatomegaly, splenomegaly, peripheral edema, and placental hypertrophy. The marked hepatomegaly and splenomegaly present in hydropic stillborns result not only of the development of large foci of compensatory extramedullary hematopoiesis but also of the accumulation of fluid because of congestive heart failure. If untreated, about 20–30% of fetuses affected by erythroblastosis die in utero. Kernicterus (bilirubin deposits in the basal nuclei of the brain) and jaundice are not components of erythroblastosis fetalis during intrauterine life, because accumulation of the pigment is prevented by its removal through the placental circulation and metabolism in the maternal liver. However, after birth the newborn liver cannot effectively handle the large amount of pigment released during the brisk hemolytic process, and this leads to rapid increases in serum bilirubin and eventual tissue deposition.
Genetics
The genetics of the Rh factor has been elucidated in the last 10 years. The Rh factor is codified by two genes the RHD and the RHCE that are in close proximity on the short arm of chromosome number 1 (Mouro et al., 1993). The nucleotide coding sequence of the two Rh genes is 96% identical. The RHD gene encodes only for the RhD antigen while the RHCE gene encodes for the other four antigens (E, e, C, c). A nucleotide difference in the RHCE gene—cytosine to thymine—determines the expression of the C instead of the c antigen. Another single nucleotide change results in the formation of the E rather than the e antigen. Rh-negative individuals are homozygous for a complete deletion of the RHD gene. Rh-positive individuals may have one copy (heterozygous) or two copies (homozygous) of the RHD gene. This has practical importance because a homozygous Rh-positive father (DD), if mated with an Rh-negative mother, will necessarily pass to his offspring a D gene and, as a result, the offspring will be Rh positive in 100% of the cases. If the father is heterozygous (D), the chances of his child receiving the paternal D gene and being Rh positive are 50%.
The majority of Caucasian Rh-negative mothers are “ccee.” For that reason Rh alloimmunization to antigens other than D, C, and E is rare. The C and E antigens usually cause immunization via blood transfusion rather than as a consequence of fetal–maternal bleeding during pregnancy. Other blood group systems different from the Rh have antigens with potential to cause fetal hemolytic disease. The most common are the K (Kell), Fya (Duffy), and Jka (Kidd). An antigen frequently found in routine antenatal testing is the Lewis group (Le-a and Le-b). The Lewis antigens do not cause fetal hemolytic disease and differ from all of the other red cell antigens in that they are not synthesized in the red cell membrane but are absorbed into it. Other rare antigenic groups may also cause mild to severe erythroblastosis fetalis (Table 14-1).
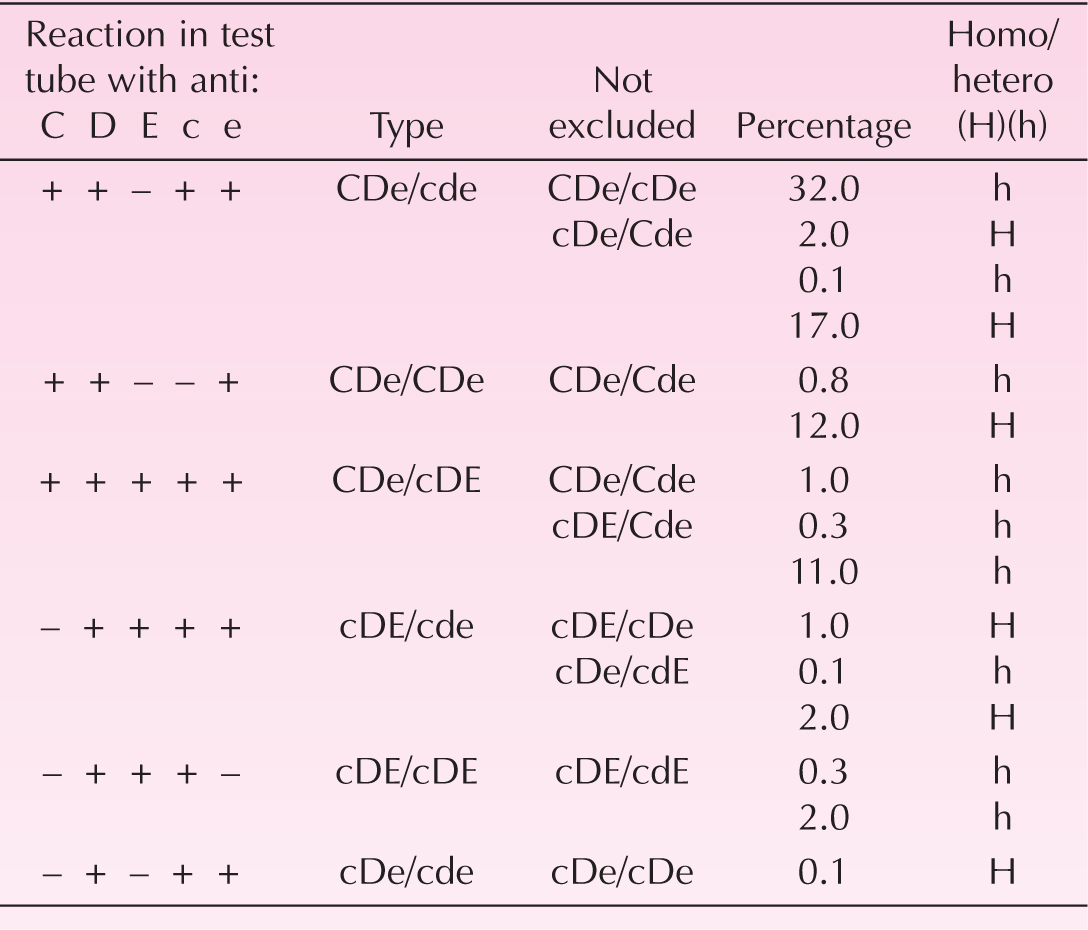
The general rules of Rh inheritance have exceptions. Some red cells react weakly with anti-D antibodies because they contain a gene that produces only a part of the D antigen. This variant is called Du and it should be absent (Du negative) in a given individual to be considered Rh-negative. A third allele of C and c has also been identified, most commonly in association with D and e, and has been called Cw. Some individuals have a rare state, termed Rh-null in which their red cells lack Rh antigens. As we will see later, some individuals of Asian and African ancestry have parts of nonfunctioning Rh genes that produce false positive Rh determinations with the polymerase chain reaction (PCR) technology.
Diagnosis
The blood type, Rh group, and antibody screening should be determined in all pregnant women at their first prenatal visit. The presence of anti-D antibodies in the serum is diagnostic of maternal Rh alloimmunization. To test for antibodies, maternal plasma is incubated with Rh-positive erythrocytes and with serum rich in antiglobulin antibodies (Coombs' serum) and the red cells will agglutinate if Rh antibodies are present in the maternal plasma. The IgG anti-Rh antibodies have a relatively small molecular weight and are not capable of bridging the intercellular distance of 250 A that exists between red cells in solution to cause agglutination. This distance results from red cells repelling each other because of their negative surface charge. The addition of Coombs' serum to the maternal plasma decreases the intercellular distance and facilitates the agglutination of red cells when anti-Rh antibodies are present.
The concentration of anti-D antibodies is determined by a titration procedure in which progressively double dilutions of the maternal serum are incubated with group O Rh-positive erythrocytes and the agglutination of the erythrocytes is used as the end point of the reaction. Titer values correspond to the greatest dilution with positive agglutination. For example, a titer of 32 indicates that the tube with the greatest dilution where agglutination was detected had a dilution of 1:32. In most first-immunized pregnancies, the concentration of antibodies is so low that they can be detected only in undiluted serum and may not appear until late in pregnancy. The explanation for those cases of late appearance of antibodies is that fetal–maternal bleeding generating a maternal antibody response is more common in the later stages of gestation.
There are variations in antibody titers among different laboratories, and the obstetrician managing an immunized pregnancy should use the same laboratory for all of the antibody titer determinations of a given patient. For maximal accuracy, serum samples should be stored and the procedure repeated in the original sample each time that a titer is determined in a subsequent sample. Also, it is important to know the critical titer level associated with intrauterine death for the reporting laboratory. For most laboratories the critical anti-D value is between 8 and 32.
All pregnant women should be screened for antibodies in the first prenatal visit. The screening should include Rh-negative women who have received anti-D immune globulin in a previous pregnancy because postpartum administration of anti-D immune globulin does not guarantee prevention of Rh alloimmunization. An antibody screening should also be obtained during the initial prenatal evaluation of Rh-positive mothers especially those who have had blood transfusions, unexplained fetal losses, or infants with unexplained jaundice.
Management
Rh-negative women presenting for obstetrical care can be categorized in two different groups: (a) Rh-negative nonimmunized women and (b) Rh-negative immunized women. To the last group it is necessary to add Rh-positive women immunized against non-D Rh antigens or against other blood group systems. These two sets of patients are managed differently. In Rh-negative nonimmunized women the objective of management is prevention of Rh alloimmunization. In women who are already immunized the objective of management is early detection and adequate treatment of fetal anemia.
Management of Rh-Negative Nonimmunized Women
Rh-negative nonimmunized women do not have detectable alloantibodies in the initial prenatal evaluation. In these cases the first thing to do is to determine the Rh phenotype of the baby's father. If the father is Rh negative the baby will be Rh negative, the possibility of alloimmunization does not exist, and the pregnancy should be managed like any other normal pregnancy without any further testing or treatment related to the Rh factor. If the baby's father is Rh positive, there is 50 (father heterozygous) to 100% (father homozygous) probability that the fetus will inherit one copy of the RHD gene and therefore Rh alloimmunization may occur during pregnancy. In these cases it is not necessary to determine the Rh genotype of the father because even in the best of the circumstances (father heterozygous for the D antigen), the probability that the fetus will be Rh positive is substantial and the plan of management will be identical to that for the homozygous father. If the father is Rh positive, it is necessary to design a strategy to detect Rh alloimmunization if it occurs during the first 28 weeks of pregnancy and to prevent its occurrence during the last 12 weeks and at the time of delivery when fetal–maternal bleeding is more common.
Detection of Rh Alloimmunization
The possibility that Rh sensitization may occur before delivery is small (about 1%). To identify the few Rh-negative women who will develop antepartum sensitization, the antibody screening should be repeated at 20, 24, and 28 weeks of gestation. If anti-D antibodies are detected, the woman has developed Rh alloimmunization and her management becomes similar to that of Rh-negative immunized women. If the antibody screening does not show evidence of alloimmunization, the patient should receive anti-D immune globulin at 28 weeks of gestation and further antibody screenings will be unnecessary. Also, at the time of delivery it will be necessary to determine the mother's eligibility for a second dose of anti-D immune globulin.
The need for antibody screening every 4 weeks in nonimmunized Rh-negative women is not universally accepted because Rh alloimmunization rarely happens during the antenatal period and because the first immunized pregnancy rarely produces severe fetal hemolytic disease. For these reasons some prefer to limit the testing to the antibody screening that is performed before the administration of Rhogam at 28 weeks. However, testing every 4 weeks will avoid missing the development of antibodies in the occasional patient who becomes immunized before delivery and will prevent the rare poor fetal outcome that may result from inadequate surveillance.
Prevention of Rh Alloimmunization
The antepartum administration of anti-D immune globulin at 28 weeks to Rh-negative women decreases the incidence of third trimester alloimmunization from 18 to 20/1000 to 2/1000 patients. After administration of antiD immune globulin, the antibody screening will detect anti-D antibodies in the patient's serum, but the titer should not be greater than 4 at term. An anti-D titer greater than 4 at term most probably results from alloimmunization rather than from anti-D-immunoglobulin administration. The antepartum administration of anti-D immune globulin is not cost-effective. Many women will receive one or two doses of a relatively expensive medication, and only a few will benefit from it. However, anti-D immune globulin administration decreases the incidence of Rh alloimmunization and is the procedure of choice.
The Rh-negative gravida who remains unsensitized (negative antibody screenings) during pregnancy and receives anti-D immune globulin antepartum should have her eligibility for postpartum administration determined immediately after delivery and anti-D immune globulin given when the following conditions are fulfilled:
2. The direct Coombs' test on umbilical cord blood is negative. This test reveals whether or not the infant's red cells are covered by irregular antibodies.
3. The crossmatch between anti-D immune globulin and the mother's red cells is compatible.
The usual dosage of anti-D immune globulin is 300mg. This amount is capable of neutralizing the antigenic potential of up to 30ml of fetal blood (about 15ml of fetal cells) and prevents Rh alloimmunization in 90% of the cases. In the other 10% of the cases D-immunoglobulin is ineffective, most probably as a consequence of insufficient antigenic neutralization following a large transfusion of fetal cells into the mother. A large fetal–maternal hemorrhage occurs in 1 out of every 300–500 deliveries, and it should be suspected with the birth of a pale baby, a fetal hemoglobin concentration of less than 10g, abruptio placentae, midforceps operations, and traumatic deliveries. These indicators are not completely reliable, and ideally the volume of fetal blood transfused to the mother should be quantified with the use of the Kleihauer-Betke stain. This method is based on the fact that an acid solution (citric acid phosphate buffer, pH 3.5) elutes the adult but not the fetal hemoglobin from the red cells; fetal erythrocytes appear in a smear stained dark red and surrounded by colorless ghosts that are adult erythrocytes without hemoglobin. This test can detect as little as 0.2ml of fetal blood diluted in 5L of maternal blood. The KleihauerBetke test is not useful and should not be used to determine the need for D-immunoglobulin administration. In fact, about 50% of Rh-negative mothers who become sensitized have negative postpartum Kleihauer-Betke testing. The Kleihauer-Betke test is difficult to perform and may produce false positive results as a consequence of multiple factors affecting the acid elution of hemoglobin from the red cells. Also, the presence of reticulocytes and adult red cells containing fetal hemoglobin may cause false positive results.
In USA, a crossmatch of the anti-D immune globulin against the mother's red cells is carried out before the administration of this product. This practice had its origin in the initial trials to determine the effectiveness of anti-D immune globulin prophylaxis, at which time there was a fear of causing hemolysis in the recipient. Today it is known that the infusion of plasma containing anti-D antibodies is innocuous and does not cause intravascular hemolysis. However, the practice continues because an important benefit of the anti-D immune globulin crossmatching is its ability to detect a large fetal–maternal hemorrhage. In fact, if more than 20ml of fetal blood has entered the maternal circulation, the anti-D immune globulin reacts and agglutinates the fetal erythrocytes present in the maternal blood and the crossmatch becomes incompatible. Because of its simplicity, the D-immunoglobulin crossmatch is widely used in place of the Kleihauer-Betke stain to screen mothers in need of high dosages of Dimmunoglobulin. However, the threshold sensitivity of the D-immunoglobulin crossmatch is high, and the Kleihauer-Betke test should be the procedure of choice to assess the volume of the fetal–maternal bleeding.
Anti-D immune globulin can be given any time up to 4 weeks after delivery. The maximal protective effect is obtained if the antibody is administered within 72 hours following delivery. This limit was chosen arbitrarily in the original experiments in which the value of Dimmunoglobulin in preventing Rh alloimmunization was proven. Other experiments have shown that administration of anti-D immune globulin several days and even weeks after delivery still has a protective effect although the efficiency of the protection is reduced. Therefore, antiD immune globulin should be given to any eligible Rhnegative mother as soon as possible after delivery, and treatment should not be withheld if more than 72 hours have passed in the postpartum period. The administration of anti-D immune globulin to eligible mothers after delivery decreases the incidence of Rh sensitization from 15 to 1 or 2%. The 1-in-10 failure rate results from undetected large fetal–maternal bleeding or from alloimmunization occurring before delivery. Anti-D immune globulin should also be given to all nonimmunized Rh-negative women after spontaneous or induced abortions, after amniocentesis, and after ectopic pregnancies. Since the half-life of anti-D immune globulin is 24 days, approximately 20% of women receiving treatment at 28 weeks will have no demonstrable antibodies at 40 weeks. For that reason some experts recommend a second dose of anti-D immune globulin if the pregnancy continues after 40 weeks.
A summary of the management of Rh-negative nonimmunized women is shown in Figure 14-1.
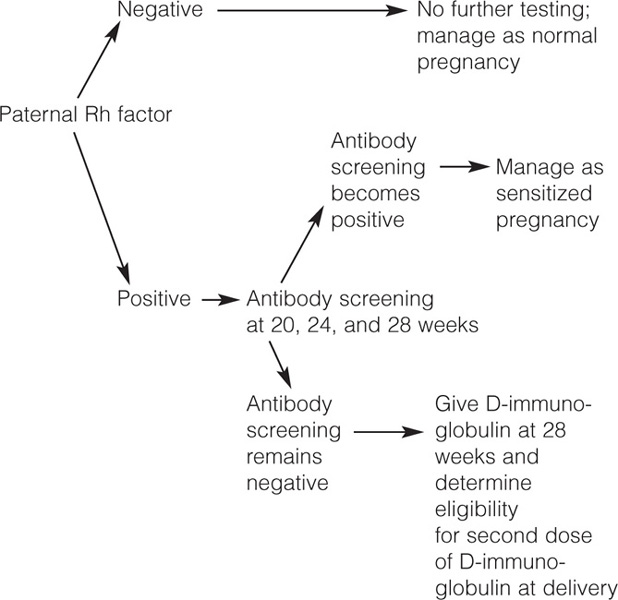
Figure 14-1. Management of Rh-negative nonimmunized women.
Management of Rh-Negative Immunized Women
The management of Rh-negative women immunized in a prior pregnancy or with immunization secondary to the administration of incompatible blood products requires, as a first step, the determination of the paternal and fetal Rh phenotype and genotype.
Paternal Rh Phenotype and Genotype
Determination of the paternal Rh phenotype is the first step. If the father of the baby is Rh negative the fetus will not be affected and further tests are unnecessary. If the father is Rh positive it is necessary to determine if he is homozygous or heterozygous for the RHD gene. The only exception to this rule is if the couple had an Rh-negative infant in a prior pregnancy, because in this case the father is heterozygous.
The father's genotype is indirectly determined by serologic testing for the antigens produced by the RHD and the RHCE genes and comparing the results with genotype frequency tables (Table 14-1). This is possible because of the closely linked inheritance of the RHD and RHCE genes. For example, a Caucasian Rh-positive father with positive serologic testing for Dcce, DCEe, or Dce antigens will have 90% or more probability of being heterozygous. The probability will decrease with the number of children he has parented that are Rh positive. There are mathematical models that take into consideration the ethnicity and the paternal history of Rh-positive neonates (Kanter, 1992) to determine the number of alleles in the paternal RHD gene. If the father is heterozygous, the fetus has a 50% probability of being Rh negative and determination of the fetal Rh becomes mandatory to avoid unnecessary testing in the 50% fetuses that will be Rh negative. If the father is homozygous, the fetus will be Rh positive and amniocentesis to determine the fetal Rh will be unnecessary.
Fetal Rh Determination
The fetal Rh genotype can be determined using cells collected by chorionic villous biopsy (CVS) or amniocentesis. The fetal Rh phenotype can be determined by serologic testing using fetal blood. CVS has the advantage of being done early in pregnancy and the potential disadvantage of increasing the severity of the alloimmunization if the fetus is Rh positive. Fetal blood sampling is a more complicated procedure and has greater morbidity and mortality than CVS or amniocentesis but it will allow the fetal Rh phenotype to be known rapidly by conventional blood bank serology. Amniocentesis is the method of choice to obtain fetal tissue for Rh factor determination because of its simplicity and safety,
To determine the fetal Rh genotype in amniocytes, it is necessary to use DNA technology. The fetal cells contained in the amniotic fluid are cultured in order to obtain an adequate amount of DNA. The genomic DNA is used for genetic amplification by PCR (Simsek et al., 1995). The primers for this reaction are highly specific for the RHD gene. The end point is whether or not the RHD gene is present in the amniocytes and can be amplified by the PCR. However, PCR technology cannot determine if there is one or two alleles of the RHD gene. A different DNA primer is used to amplify the RHCE gene to determine the C/c and E/e composition.
The PCR test is erroneous in 1–2% of the cases. The causes of PCR errors are contamination, failed amplification, and genetic rearrangements of the RHD gene. The worst consequence of this is when the PCR erroneously reveals an Rh-negative fetus because this will result in lack of surveillance for hemolytic anemia and potential fetal death. To avoid this problem it is prudent to repeat the antibody titer at 4 weeks' intervals in Rh-sensitized women with an Rh-negative fetus diagnosed with PCR. A fourfold increase in titer will make the accuracy of the PCR determination of the fetal Rh suspicious. There are also false Rh-positive results with the PCR. Some individuals of African ancestry possess only a part of the RHD gene (Singleton et al., 2000). The incomplete gene or pseudogene cannot codify for the D antigen and serologically they are Rh negatives. However, the incomplete gene will be copied by the PCR and the Rh-negative fetus may falsely appear to be Rh positive by amniocentesis and PCR.
In the near future the fetal Rh can be determined from the free fetal DNA that is present in maternal plasma in relatively high concentrations. This approach first requires determining that the DNA is fetal in origin. Then PCR is used to amplify RH gene-specific sequences to confirm or deny the presence of the RHD gene (Lo et al., 1998; Finning et al., 2002).
The management of sensitized Rh-negative women with an Rh-positive fetus is different if the pregnancy is the first affected one or not.
First Affected Pregnancy
The first affected pregnancy is the only pregnancy in which maternal anti-Rh antibody titers can be used to determine the risk of fetal anemia. The reason for this limitation is that the correlation between antibody titers and transfer of fetal cells into the maternal circulation that exists in the first affected pregnancy is lost during subsequent gestations. Also, in the majority of first immunized pregnancies the anti-Rh antibody concentration is low and rarely exceeds the critical level of most laboratories. The critical level means that no death due to fetal hemolytic disease has occurred within 1 week of delivery when the antibody titer was at that level or lower.
Serum Antibody Titers
Patients in the course of their first sensitized pregnancy should have antibody titers every 4 weeks unless the following occur:
1. The titer is found to be at or above the critical level (usually 32) on the initial evaluation.
2. The titer reaches or exceeds the critical level at any time during gestation.
3. There is a significant rise in titer (two-tube dilution) between two consecutive samples, even if the upper dilution does not reach the critical level (e.g., an increase from 4 to 32 with a critical level of 64).
If any of these conditions occur, there is no further use for antibody titers for following the first sensitized pregnancy and further management will be based on fetal assessment using the mid-cerebral artery peak systolic velocity (MCA PSV) and the concentration of bilirubin in the amniotic fluid.
If the antibody titer remains under the critical level up to 36 weeks of gestation, the patient with a first sensitized pregnancy should be delivered by elective induction of labor between 38 and 40 weeks, and the birth of a nonaffected (Rh-negative) or mildly affected Rh-positive infant should be anticipated. In these cases the neonatologist should be notified in advance of the induction of an Rhnegative immunized mother so that evaluation and treatment of the newborn can be started in the delivery room and continued without delay.
Women with a first sensitized pregnancy followed with antibody titers that have a sudden antibody titer elevation when they are more than 34 and less than 37 weeks' gestation should have amniocentesis and delivered if the fetal lung maturity is adequate. If the fetal lung maturity tests indicate pulmonary immaturity and the bilirubin level is low (less than 0.05), the pregnancy should be allowed to continue as long as weekly amniocentesis shows fetal pulmonary immaturity and a low bilirubin concentration. These babies usually have mild hemolytic disease and should be delivered as soon as their lungs reach adequate maturation.
Women with a Previous Affected Pregnancy
After the first affected pregnancy, the ability to predict fetal anemia from the maternal anti-D titers is lost and different methods are necessary to evaluate the likelihood of fetal anemia. In these cases the past obstetric history is the predominant indicator of the outcome. Sensitized patients with negative past obstetrical history and titers remaining at or below 64 have a 4% incidence of intrauterine death before 37 weeks. In contrast, patients with similar titers but with histories of delivering affected infants have a 32% stillbirth rate before 37 weeks. When the titer is above 64, patients with negative obstetrical histories have a stillborn incidence of 17.2%, whereas those with histories of affected infants have a stillborn rate of 67.8%. Since titers are not predictive of outcome, women with prior affected pregnancies should be followed with serial determinations of the PSV of the fetal middle cerebral artery (MCA PSV) and with amniocentesis to determine the concentration of bilirubin in the amniotic fluid.
Middle Cerebral Artery Peak Velocity
The MCA PSV is an accurate noninvasive method for the diagnosis of fetal anemia (Mari et al., 1995). The correlation between the MCA PSV and fetal anemia becomes stronger as the fetal hemoglobin decreases (Mari et al., 2000). Also, MCA peak velocity values can be used to predict the fetal hemoglobin concentration (Mari et al., 2002).
The technique for measuring the MCA peak velocity is not complicated and has a high index of reproducibility. Since the majority of fetuses are in cephalic presentation, the fetal head is relatively fixed and does not move excessively during the examination. The ultrasound probe is used to obtain a view adequate for the measurement of the biparietal diameter and the vascular structures are identified with color Doppler. The MCA of the cerebral hemisphere closer to the ultrasound transducer is interrogated a few millimeters after its origin from the internal carotid artery but without including any part of this artery and taking care that the angle of insonation is as close as possible to 0° (Figure 14-2). The fetus should be resting, since activity will cause falsely elevated PSV values (Sallout et al., 2004). The distal part of the MCA should not be used for this measurement and if it is not possible to interrogate the proximal part of the MCA closer to the ultrasound probe, it is better to examine the proximal part of the MCA from the cerebral hemisphere far from the ultrasound probe (Abel et al., 2003). Typical waveforms are obtained and the PSV of at least three of them is measured and averaged. The threshold for the diagnosis of fetal anemia is a value equal to or greater than 1.5 multiples of the median (MoM) for the gestational age (Figure 14-3). Abnormally elevated MCA PSV has a sensitivity of 100% and a false positive rate of 12% for the diagnosis of fetal anemia, and some investigators recommend cordocentesis and IUT as the next step following an abnormal MCA PSV. However, the false positive rate of the MCA PSV is at least 12% and a more conservative approach will be to perform amniocentesis to determine the concentration of amniotic fluid bilirubin. With this approach, cordocentesis and intravascular transfusion will be limited to fetuses showing abnormally elevated MCA PSV plus elevated amniotic fluid bilirubin.
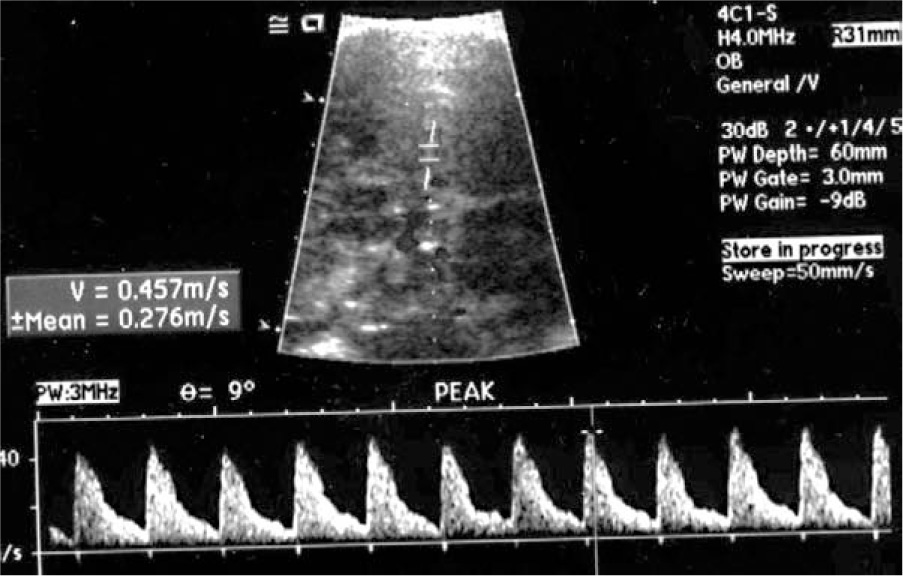
Figure 14-2. Determination of middle cerebral artery peak systolic velocity.
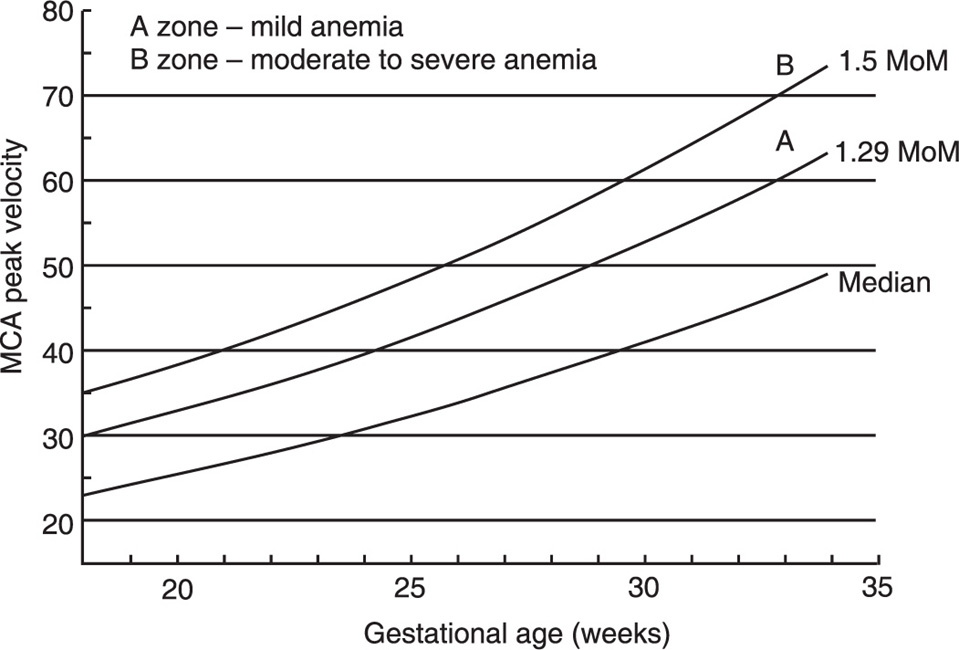
Figure 14-3. Normal values for middle cerebral artery peak systolic velocity with gestational age. From Moise KJ, Jr. Management of rhesus alloimmunization. Obstet Gynecol 2002; 100: 600–11.
Amniotic Fluid Bilirubin
Spectrophotometric analysis of the amniotic fluid to determine the concentration of bilirubin is the traditional method for evaluating the severity of the fetal hemolytic process and for determining the optimal time for IUT or for delivery of the infant. Usually the first amniocentesis is performed at 20 weeks but in women who start off with a high titer, who have had a baby that was hydropic or died in uterus, or whose ultrasound evaluation demonstrates early signs of fetal hydrops, the first amniocentesis may be done at 16 weeks. The decision regarding when to repeat the procedure will be dictated by the results of the amniotic fluid analysis, as will be discussed later.
Before performing an amniocentesis, the best site for the procedure should be determined using real-time ultrasound. The use of ultrasound has increased the success rate in obtaining adequate samples for amniotic fluid analysis to nearly 100%. Ultrasound allows identification of the placenta and delineation of the placental edges, and in the majority of cases amniocentesis can be performed safely without entering the placenta. Color Doppler is invaluable for visualization of the umbilical cord and gives the operator almost absolute certainty that the pocket selected for amniocentesis does not contain loops of cord. Once the tap site has been selected, the abdomen is prepared with aseptic technique using a Betadine solution. Local anesthesia is not necessary. Depending on the depth of the pocket, a 3.5–7.0-in. long, 22-G disposable needle is inserted to the depth previously determined. The tip of the needle should be visualized with ultrasound and the needle should be advanced until it reaches the center of the pocket of fluid.
Mild contractions occasionally occur following amniocentesis, but they usually subside after 30–40 minutes. In rare cases, contractions may continue and result in premature labor. Patients undergoing amniocentesis should be instructed to report if the contractions continue and become stronger, and if that is the case, therapy with tocolytic agents may be indicated.
A small button of red cells is frequently found after centrifugation of the amniotic fluid even when the amniocentesis yields clear fluid. This is not a “bloody tap,” a term that is reserved for gross, visible contamination of the fluid with fetal or maternal blood. Bloody taps are less common since amniocenteses are done under ultrasound guidance. In the large majority of cases, the blood is maternal in origin, but it may be a mixture of maternal and fetal cells, especially in cases of transplacental amniocentesis. If fluid grossly contaminated with blood comes out of the needle after amniocentesis, the best thing to do is to let the fluid escape to see if spontaneous clearing occurs. If the hematocrit value after clearing is more than 5%, the determination of bilirubin will be distorted and should not be used for patient management. If the fluid is grossly contaminated, the chances of adequate spontaneous clearing are low, and the amniocentesis should be postponed for 1 week.
Infection is a rare complication of amniocentesis. The resulting chorioamnionitis usually leads to preterm labor and results in preterm delivery. The treatment is evacuation of the uterus. Adherence to aseptic technique is the key to the prevention of this problem.
The peak absorption of meconium in amniotic fluid is similar to the Soret's band of hemoglobin and as a consequence, meconium causes a marked rise in bilirubin values, a change that does not disappear after centrifugation. For this reason, meconium-stained specimens should not be used for patient management. Another source of error in the evaluation of fetal anemia by amniocentesis is an increased amount of fluid. With polyhydramnios the bilirubin content of the amniotic fluid is diluted, resulting in falsely low bilirubin values.
In women with multiple pregnancy, each fetus should be evaluated separately. In the case of twin pregnancy, both, neither, or only one of the twins may be affected and each one of the amniotic sacs should be aspirated. In these cases, the help of ultrasound is invaluable. It allows (a) visualization of the membrane separating the sacs and (b) its penetration by the needle if a single puncture is chosen as the procedure of choice. If each sac is to be entered, 1ml of indigo carmine may be injected into the sac entered first. The fluid obtained from the second sac must be clear; if it shows a blue color, the tip of the needle is in the first sac.
About 5–10ml of amniotic fluid is required for spectrophotometric analysis. The fluid should be kept in a brown bottle to protect it from exposure to the sunlight, which destroys some of the bilirubin and causes false low readings. The fluid is centrifuged at 4000rpm for 20 minutes and analyzed by spectrophotometry. When normal amniotic fluid is examined in a spectrophotometer using water as a blank, the optical density (OD) readings between 350 and 650nm form an almost straight line. If the amniotic fluid contains bilirubin, the OD readings will show a peak at 450nm, and the size of the peak will be proportional to the amount of pigment in the fluid. Rather than using continuous scanning between 350 and 650nm, the majority of laboratories measure the OD at 375, 450, and 525nm. The results are plotted on semilogarithmic paper, and a straight line is drawn between the readings at 375 and 525nm. The difference between the point at which the line crosses the 450-nm mark (expected value) and the actual reading at this wavelength is the delta OD at 450nm (ΔOD450), which is used for patient management.
During normal pregnancy the ΔOD450 values change with gestational age and it is necessary to use adequate norms to correlate the laboratory values with the fetal situation. The original Liley curve has been used for this purpose for more than 40 years. In his original description, Liley (1961) recorded the ΔOD450 of 101 immunized patients on semilogarithmic paper (gestational age in weeks on the ordinate, ΔOD450 values on the abscissa) and divided the graph into three zones (Figure 14-4). The upper zone or zone 3 corresponded to severely affected infants, the low zone or zone 1 to nonaffected or mildly affected babies, and the middle zone or zone 2 included fetuses severely and mildly affected.
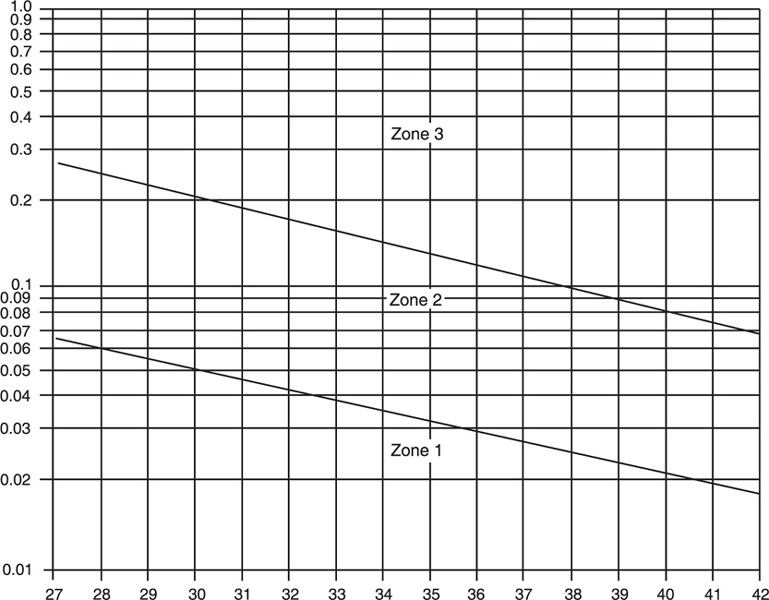
Figure 14-4. Liley graph to estimate severity of fetal hemolytic anemia.
If the amniotic fluid shows a ΔOD450 value in zone 1, there is no immediate danger of intrauterine fetal death, and the procedure should be repeated in 4 weeks. If the ΔOD450 values remain in zone 1 in repeated amniocentesis carried out every 4 weeks, the patient should be delivered at term, and the birth of a nonaffected (Rh-negative) or a mildly affected baby should be anticipated. If at any time the amniotic fluid shows a ΔOD450 value in zone 2, the procedure should be repeated in 1 week, since values in this zone may correspond to moderately or severely affected infants. If the following amniocentesis shows a ΔOD450 value in zone 1, there is no need to repeat the amniocentesis before 4 weeks. If the following amniocentesis shows a decreasing trend in ΔOD450 value but the lower value is still within zone 2, the amniocentesis should be repeated in 2 weeks. If the following amniocentesis shows a ΔOD450 value similar to the previous one and still within zone 2 (horizontal trend), the procedure should be repeated in another week, and if the horizontal trend continues, cordocentesis and evaluation of the fetal hematocrit are indicated with exception of those patients with adequate fetal lung maturity who are better managed by immediate delivery.
If the initial amniotic fluid examination shows a ΔOD450 in zone 3 or if any ΔOD450 value previously in zone 1 or 2 moves to zone 3 (rising trend), the infant may be in imminent danger of intrauterine death. In these cases, fetal blood sampling should be done and IUT performed if the fetal hematocrit is less than 30%. IUT may be avoided in fetuses with adequate indices of lung maturation because in these cases delivery is the management of choice. A summary of the management protocol using Liley zones is shown in Figure 14-5.
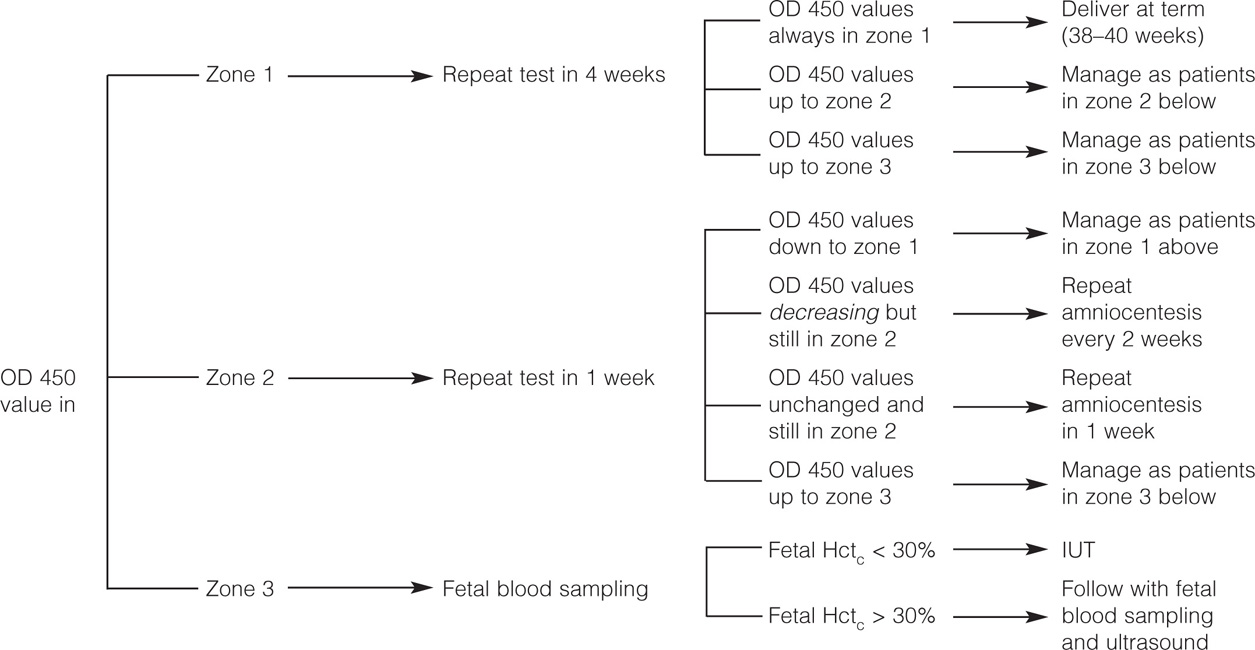
Figure 14-5. Management of Rh alloimmunization by amniotic fluid analysis.
The main limitation of the Liley curve is that it starts at 26 weeks' gestation, and extrapolation of the lines to earlier gestational ages is inaccurate. Other investigators (Queenan et al., 1993) have developed a curve for fetal assessment from 14 to 40 weeks, divided into 4 zones (Figure 14-6). The lower, first zone corresponds to nonaffected Rh-negative fetuses. Values in the second zone are indeterminate and do not permit a determination of whether the fetus is affected or not. The third zone corresponds to affected Rh-positive fetuses. The upper zone corresponds to fetuses at risk of intrauterine death. In general, a ΔOD450 greater than 0.15 indicates severe immunization and the need for cordocentesis and early transfusion. Values below 0.09 indicate mild disease or no disease. Values between 0.09 and 1.5 will require repeat amniocentesis in 1 week. After 26 weeks the need for intervention can be determined using the Liley graph.
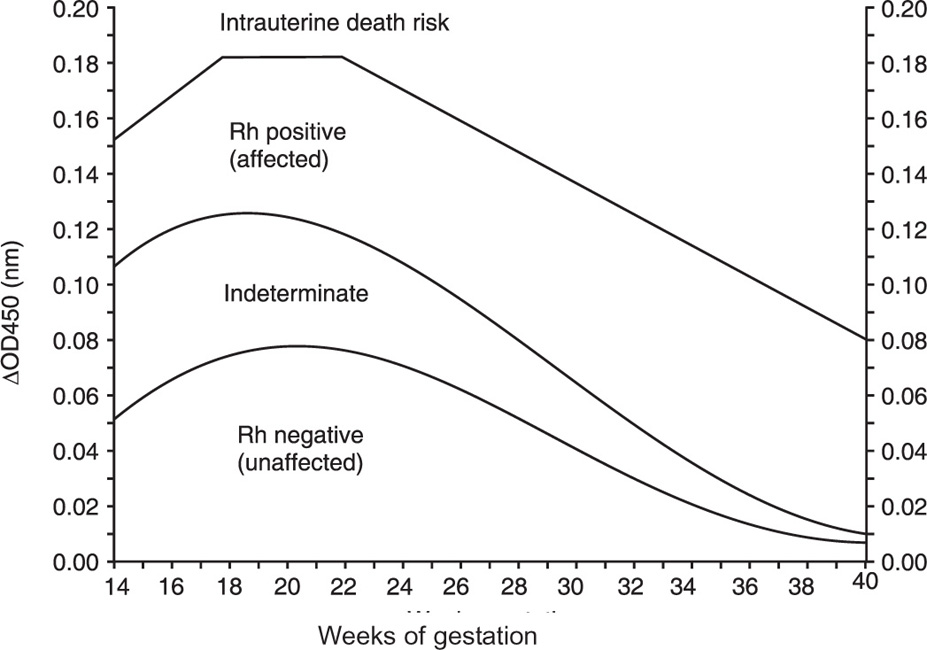
Figure 14-6. Curve for OD450 values from 14 to 40 weeks' gestation. From Queenan et al. Deviation in amniotic fluid optical density at a wavelength of 450nm in Rh-immunized pregnancies from 14 to 40 weeks' gestation: a proposal for clinical management. Am J Obstet Gynecol 1993; 168: 1370–6.
Amniotic fluid bilirubin and MCA PSV can be used together in the diagnosis of fetal anemia. PSV should be the initial test because it is not invasive and has better predictive values than amniotic fluid bilirubin concentration (Nishie et al., 2003; Pereira et al., 2003). When the MCA PSV is above normal limits, amniocentesis to determine the amniotic fluid bilirubin concentration should be performed before cordocentesis to avoid invasive procedures in nonanemic fetuses. In several of these cases the DOD450 will demonstrate values below the transfusion zone and IUT can be avoided or postponed.
Ultrasound Assessment
High-resolution ultrasound is a valuable tool in the management of sensitized Rh-negative patients. Modern equipment allows a clear visualization of the fetal structures and early diagnosis of the presence of fetal ascites, pericardial effusion, liver enlargement, and placental swelling. For these reasons, some investigators believe that the accuracy of ultrasound in detecting signs of fetal hemolytic disease is better than amniotic fluid bilirubin measurements (Frigoletto et al., 1986; Reece et al., 1989).
There are two important caveats in the use of ultrasound as the main indicator of fetal hemolytic disease. In the first place, fetal hydrops usually develops when the fetal hematocrit is below 20% and ultrasound will only detect advanced degrees of fetal anemia. Secondly, in many patients the onset of fetal hydrops is rather sudden and its detection by ultrasound will require the performance of frequent evaluations. Proponents of the use of ultrasound for the assessment of fetal anemia point out that ultrasound screening has no false positives like the MCA PSV and the amniotic fluid bilirubin. Once fetal ascites is detected, the fetus is anemic and IUT is necessary. However, MCA PSV and amniotic fluid bilirubin determinations can detect fetuses with moderate and severe hemolysis when ultrasound evaluation is still normal. Therefore, it is only in special circumstances that ultrasound should be used as the main criterion to judge the extension of the hemolytic process and the need for intervention.
Fetal Blood Sampling
The introduction of cordocentesis by Daffos et al. (1983) dramatically changed the management and the therapy of the Rh-sensitized patient. Umbilical cord blood sampling allows a precise measurement of the fetal hematocrit and hemoglobin concentration to determine the severity of the hemolytic process and the need for IUT. Also, the same technique may be used for direct intravascular transfusion to the fetus, a technique that has successfully allowed “in utero” treatment of hydropic fetuses unresponsive to transfusions into the peritoneal cavity.
For fetal blood sampling or transfusion the placental insertion of the umbilical cord is found using high resolution ultrasound and color flow mapping. Then, under ultrasound guidance a needle is introduced into the umbilical vein and fetal blood is drawn for determination of fetal blood group and Rh, direct Coombs' test, hemoglobin, and hematocrit. A hematocrit of less than 30% is an indication for IUT. Cordocentesis requires a degree of expertise and has the potential for serious complications. Cordocentesis is technically more difficult when performed between 16 and 20 weeks.
The main indication for fetal blood sampling in the Rhsensitized patient is a combination of a MCA PSV > 1.5MoMs above the mean and an amniotic fluid bilirubin values in the high middle zone or in zone 3. Another clear indication is the finding of fetal hydrops by ultrasonographic examination. When fetal blood sampling is limited to these two indications, only those fetuses that have clear indication of severe hemolytic disease are exposed to the risks of cordocentesis.
The most common complication of umbilical fetal blood sampling is bleeding which is usually transient. However, blood loss from cordocentesis may be severe and may cause fetal death. Cordocentesis may also cause fetal–maternal bleeding and a large increase in antibody concentration with worsening of the hemolytic process. Also, thrombosis of the umbilical vessels and severe vasospasm with secondary fetal bradycardia are procedural complications. The risk of fetal death following cordocentesis is approximately 1.5% after 24 weeks and it may be as high as 5% before 24 weeks.
Intrauterine Transfusion
Since its introduction in 1963, IUT has been instrumental in saving hundreds of infants affected by hemolytic disease secondary to maternal Rh alloimmunization. However, IUT results are not uniform. They are modified by variables such as the experience and skills of the obstetrician performing the procedure, the severity of the fetal disease, the placental implantation site, the maternal obesity, and the fetal lie, among many other factors.
There are two types of IUT: intraperitoneal and intravascular. In both methods, the procedure is carried out under visual control with real-time ultrasound. In intraperitoneal IUT the blood is injected into the peritoneal cavity and transported by the lymphatic system into the fetal bloodstream. In intravascular transfusion, the blood is injected directly into the umbilical circulation. The two types of IUT are not mutually exclusive. They complement each other and either one or both of them may be used depending on the circumstances. Although the direct intravascular approach is the procedure of choice, it is not without problems and it is preferable to perform an intraperitoneal transfusion if the approach to the umbilical cord is difficult (posterior placenta, maternal obesity) or if a sample of fetal blood cannot be obtained after several attempts. The overall survival rate of fetuses transfused intravascularly is 84.8%, 80.1% for hydropic, and 89.5% for nonhydropic fetuses.
In the severely hydropic fetus, the intravascular approach offers the best possibility for successful fetal therapy. If access to the umbilical cord is difficult, it is possible to transfuse through the intrahepatic portion of the umbilical vein. Another access to the intravascular compartment of the fetus when everything else fails is the right ventricle of the heart.
IUT has a potential for serious complications. Perinatal death was approximately 11.0% (7.4% fetal and 3.9% neonatal) in a series of 254 fetuses treated with 740IUTs (Van Kamp et al., 2005). Infection, rupture of membranes, and emergency delivery occur occasionally. The procedure-related complication rate is 3.1% per procedure.
Early Delivery and Glucocorticoids
The classical approach to the management of sensitized Rh-negative mothers was early delivery depending on the severity of the fetal hemolytic process. This has changed and at present most maternal–fetal medicine specialists perform the last transfusion at about 35–36 weeks and deliver at 37–38 weeks' gestation. If delivery before 36 weeks is necessary, the use of steroids to accelerate the maturation of the fetal lungs is recommended. Betamethasone 12mg IM daily for 2 consecutive days or dexamethasone 6mg every 12 hours for four doses are equally effective. We found (Arias et al., 1979) that glucocorticoid treatment has little immediate effect on the lecithin to sphingomyelin (L/S) ratio, and the studies by Caritis et al. (1977) show that RDS (respiratory distress syndrome) prevention occurs even if the infants with hemolytic disease treated with steroids are delivered when they still have immature L/S ratios.
Corticosteroids cause a decrease in ΔOD450 values. It is necessary to avoid the false sense of security given by the drop in delta ΔOD450 values that follows glucocorticoid treatment and to deliver the infant 24 hours after the second dose of betamethasone. Amniocentesis to assess fetal lungs maturity should be used generously after 34 weeks because delivery is the treatment of choice when the fetal lungs are mature.
Other Treatment Modalities
There are treatment modalities for women with Rh alloimmunization which have been used in cases of severe immunization with high initial titers and history of pregnancy losses due to fetal hydrops. They include plasmapheresis and administration of promethazine and IgG. The best evidence of benefit is for IgG administration. Voto et al. (1997) treated 69 women with extremely severe Rh alloimmunization. Thirty received IgG before 20 weeks, 400mg/kg/day for 5 consecutive days every 2–3 weeks, followed by IUTs after 20 weeks and 39 received IUTs only. They found a significantly lower incidence in the number of fetal deaths in women treated with the combination of IUT and IgG.
Alloimmunization to Rh Antigens Different from D
When the mother has alloimmunization by the E or C antigens as a consequence of prior childbirth and the father is the same, it is not necessary to find the paternal Rh genotype because obviously the E or C antigens in the fetal blood originated in the father. In contrast, when the mother develops Rh alloimmunization against E or C following transfusion of blood products, it is necessary to know the paternal genotype. If the father is negative for these antigens, there is no possibility of fetal hemolytic disease and no further testing or treatment is necessary. However, if the father is positive for the antigen or antigens causing maternal alloimmunization, the pregnancy should be followed similarly to the more common anti-D alloimmunization.
Alloimmunization against the E and C antigens is the most frequently found after D and Kell. The E antigen traditionally has been considered to be less immunogenic than D and cause less severe hemolytic disease of the newborn. The contrary was demonstrated in a study of 62 newborns with positive direct Coombs' test born to mothers sensitized against the E antigen, and 42 had mild, 8 modest, 5 severe, and 1 very severe hemolytic disease of the newborn. Overall 21% of affected infants required exchange transfusion (Moran et al., 2000). This study also found that there was no correlation between anti-E titers in the maternal serum and the severity of the fetal anemia, an observation that has been found in other studies (Babinzki and Berkowitz, 1999; To et al., 2003). There are no published reports about the value of using MCA PSV in anti-E cases. Based on this information, it seems prudent to manage cases of anti-E alloimmunization with serial amniocentesis.
Anti-c alloimmunization is another relatively frequent cause of hemolytic disease of the newborns. In a study of 46 newborns with positive direct Coombs' test born to mothers sensitized to c, 26% had severe hemolytic disease (Hackney et al., 2004). Contrary to anti-E, there was good correlation between anti-c titers and hemolytic disease and amniotic fluid spectrophotometry. Therefore, patients with anti-c can be followed similarly to patients with anti-D.
Indian Experience of Erythroblastosis Fetalis
The commonest cause of erythroblastosis fetalis in obstetric practice is Rh incompatibility. The incidence of Rhnegative in Western countries is about 15%. But its incidence in India varies between 3 and 5.7% (Bhakoo, 1986; Gupte and Kulkarni, 1994; Salvi, 1998). The incidence of Rh sensitization during pregnancy is about 1.9% (Salvi, 1998) and the perinatal loss due to Rh alloimmunization has been reported to be between 1 and 2.5% (Shah and Shroff, 2004). Factors protecting against Rh sensitization include maternal–fetal blood group ABO incompatibility and immunological nonresponder status. The main causes of Rh sensitization in present-day practice are the following: Lack of awareness in many places in India, particularly in rural set-ups where the practice of routinely testing all pregnant mothers for their ABO and Rh blood groups is not being observed. In small rural towns, facilities for laboratory testing for isoimmunization are nonexistent. Lastly the benefits of protecting nonimmune Rhnegative mothers from isoimmunization with the use of prophylactic inj. anti-D immunoglobulin is either unknown or ignored because of cost considerations.
Fetomaternal hemorrhage (FMH), fetomaternal leak (FML), or transplacental leak (TPL) is the cause of isoimmunization. As little as 0.1ml of leak can cause sensitization. The commonest antecedent event is delivery, but it is known to follow abortions, ectopic pregnancy, antepartum hemorrhage, etc. It is often precipitated during diagnostic obstetric procedures like chorion villus sampling (CVS) or amniocentesis. FMH has been reported during external version for breech presentations and following operative obstetric interventions (forceps delivery, cesarean section, and manual removal of placenta). In presentday practice of liberalized abortion laws and widespread acceptance of MTP (induced abortions), the incidence of Rh sensitization threatens to rise, unless the practice of protecting Rh-negative women undergoing MTP with anti-D prophylaxis is also universally accepted and practiced. Although sensitization commonly follows delivery, small asymptomatic and unsuspected fetomaternal leaks have been reported to occur during pregnancy. This led to the practice of offering all Rh-negative nonimmunized patients the benefit of antenatal inj. anti-D in the third trimester of pregnancy and again after delivery if indicated. Wherever facilities for assessing the quantum of FMH using the Kleihauer–Betke test are available, these should be availed of. In such patients, 20 μg/ml of FML calculated on the basis of the Kleihauer–Betke test would suffice to prevent isoimmunization. It also helps to save scarce resources.
Assessment of the incidence of FMH reveals that it occurs in 6.7% during the first trimester, in 13.9% during the second trimester, and in 29% during the third trimester (Reddy, 1999; Salvi, 1998). Its incidence following amniocentesis was reported to be 15–25%, and higher still after abortions. The incidence following MTP is higher than following spontaneous abortions, and the risks increase with gestation size (from first to second trimester) both following spontaneous and induced abortions (Desai, 1988). Reports from several Indian centers containing the incidence of TPH (transplacental hemorrhage) or FML following induced abortions (MTP) have been tabulated in Table 14-2.
Table 14-2. Incidence of FML following MTP (Indian Survey) |
||
| Author | Year | Incidence |
|---|---|---|
| Bakshi and Rosario-Pinto | 1978 | First trimester–1.0% Early second trimester (13–16 weeks)– 4.5% Late second trimester (17–20 weeks)–14.0% MTP accomplished by D&C–42.8% |
| Ambiye et al. | 1985 | First trimester–6.0% |
| Ramanan et al. | 1980 | First trimester–15% MTP using D&C–23.0% |
The risks of sensitization also depend on the quantum of leak. The estimated risk of sensitization following an FMH of 0.1ml was 1%, following FMH of 0.5–1.0ml the risk was 25.0%, and following FMH of > 5.0ml it was 65%. The quantum of FML was lower in women undergoing MTP by the method of suction evacuation as compared to those in whom a D&C was adopted to perform the MTP. The above data clearly established the role of the method of MTP and gestation size on the frequency of FMH and its effect on Rh sensitization (Daftary and Desai, 2006). Overall about 16% of Rhnegative women are at risk of isoimmunization, this risk is reduced to 1.5–2.0% if immunoprophylaxis with inj. anti-D immunoglobulin 300 μg is started antenatally at 28 weeks of gestation. A repeat top-up dose of inj. antiD prophylaxis at 34 weeks is desirable or soon after delivery if the newborn is Rh-positive and the direct Coombs test on the cord blood is negative, to reduce the risks of immunization to the minimum (Daftary and Desai, 2006). Should the newborn be Rh-negative, there is no need to administer the anti-D immunoprophylaxis. Deka et al. from New Delhi (2004) advocated determination of fetal Rh group by PCR on amniotic fluid obtained by amniocentesis. In case of Rh-negative pregnant women whose fetus is Rhnegative, the need for antenatal anti-D immunoprophylaxis is completely obviated (Deka et al. 2004).
The basic pathology of Rh isoimmunization results from the hemolysis of fetal RBCs as a result of maternal serum antibodies crossing the placental barrier into the fetal circulation and causing progressive hemolysis over time leading to fetal anemia and its grave consequences. A wide range of sonographic findings (Suresh, 1998) have been documented in response to fetal anemia. These changes not only include fetal organs and organ systems but also the fetal environment. These findings include hepatosplenomegaly, increase in portal venous diameter, and flow velocity (color Doppler flow studies). On sonography there may be evidence of presence of fluid in serous cavities, subcutaneous edema, disturbances of liquor distribution causing hydramnios or oligohydramnios, and some placentomegaly. Serial sonography is also useful in monitoring fetal growth pattern and wellbeing. Interventional sonography plays an important role in fetal cord blood testing, A fetal blood hematocrit of < 40% is indicative of fetal anemia. The lower the reading, the worse the prognosis. A hematocrit reading of < 30% after 26 weeks of gestation calls for prompt measures to improve fetal status or to save the fetus. In affected patients, serial testing of amniotic fluid and the charting of optical density at 450 μ on Liley's charts guides the clinician in deciding whether to continue keeping the patient under observation, to terminate pregnancy, or opt for performing an intrauterine fetal transfusion (cordocentesis followed by intravascular blood transfusion or intrauterine intraperitoneal transfusion). In earlier times the fetal prognosis following fetal hydrops was poor, but in present times with the facilities for intrauterine fetal transfusion being widely utilized, the prognosis has vastly improved. Successful intrauterine fetal blood transfusions have been achieved in India, but these cases are sporadic (Salvi, 1998).
Important Points
1. The Rh factor is codified by two genes, RHD and RHCE, located in close proximity to each other in the short arm of chromosome number 1. Rh-negative individuals lack the RHD gene. Rh-positive persons may have one (heterozygous) or two (homozygous) alleles in the RHD gene.
2. The RHCE gene contains the information for the synthesis of the Cc/Ee antigens. One nucleotide change is responsible for the synthesis of C or c and E or e antigens.
3. In the majority of cases Rh alloimmunization results from the passage of fetal Rh-positive cells into the bloodstream of Rh-negative mothers at the time of delivery.
4. The distinction between homozygous and heterozygous Rh-negative fathers is made by serologic testing against the five antigens produced by the RHD and RHCE genes and comparing the results with genotype frequency tables based on allelic frequencies or using mathematical models which take into consideration serology, ethnicity, and paternal reproductive history.
5. Some individuals have a gene that produces only a part of the D antigen. This variant is called Du and it should be absent for a given individual (Du-negative) to be considered to be Rh-negative.
6. Some antigens frequently found in routine antepartum testing belong to the Lewis group. The Lewis antigens do not cause fetal hemolytic disease.
7. Usually there is an interval of several weeks between the time of the fetal–maternal bleeding and the appearance of anti-Rh antibodies in the maternal serum. The administration of an adequate amount of D-immunoglobulin shortly after delivery inhibits this immune response.
8. In as many as 10% of the cases, postpartum administration of the usual dose (300 μg) of D-immunoglobulin is ineffective to prevent the development of Rh alloimmunization. In most cases this is the consequence of insufficient antigenic neutralization by the D-immunoglobulin of a large fetal–maternal transfusion. A large fetal–maternal transfusion should be suspected with the birth of a pale baby, a fetal hemoglobin of less than 10g/dl, abruptio placentae, midforceps operations, and traumatic deliveries.
9. Maternal antibody titers are useful for the follow-up of patients exclusively in their first immunized pregnancy. They are not useful during subsequent pregnancies.
10. There are two methods to indirectly determine the presence of fetal anemia: (a) measurement of the PSV of the fetal middle cerebral artery (MCA PSV) by Doppler ultrasound and (b) determination of the bilirubin concentration in the amniotic fluid. An MCA PSV > 1.5MoMs or a bilirubin concentration in the zone 3 of the Liley curve strongly suggest the presence of fetal anemia and should be followed by direct evaluation using umbilical cord blood. The advantage of using the peak MCA velocity is that it is a noninvasive procedure without risk to mother or fetus.
11. Traditionally, the first amniocentesis was carried out at 24–26 weeks because the Liley curve used to evaluate the severity of fetal hemolysis starts at this gestational age. More recently, the Queenan's curve may be used to assess the severity of the fetal hemolysis from 14 to 40 weeks.
12. Direct intravascular IUT can reverse fetal hydrops and result in better than 80% good outcomes in these seriously compromised fetuses.