Chancroid
Introduction
Chancroid is a sexually transmitted infection (STI) of the genitoinguinal area caused by the bacterium, Haemophilus ducreyi. It manifests clinically with single or multiple, superficial, tender ulcers over the anogenital region. Painful inguinal lymphadenitis with or without bubo formation may occur in half of the patients. Chancroid is one of the major causes of genital ulcer disease (GUD), and constitutes an important cofactor in the transmission of HIV infection in many resource-poor countries of Africa, Asia, and Latin America.1
Historical Aspects
According to Kampmeier, chancroid was first described in 1852 (France) by Ricord and Bassereau as ulcus molle differentiating it from the hard chancre of primary syphilis.2 In 1889 Augusto Ducrey, a bacteriologist at the University of Naples, performed a series of autoinoculations on the forearms of patients with purulent material from their own genital ulcers, and established H. ducreyi as the etiological agent of chancroid.3 Bezancon et al. are often credited for the first significant isolation of H. ducreyi.4 Unna described the histopathology of chancroid, and observed clumps and chains of gram-negative rods in tissues.5
Ito introduced intradermal testing with H. ducreyi obtained both from culture and with pus from a chancroidal bubo in 1913. The appearance of a papule 8 mm or more in diameter between the 3rd and 7th day was considered confirmatory.6 This was later validated by Reenstierna in 1921 at the Pasteur Institute, and the diagnostic value of the Ito-Reenstierna test was further evaluated by Greenblatt et al.7 However, this test is now only of historical importance as the antigens are no longer available.
Epidemiology
According to a World Health Organization (WHO) report, an estimated 7 million cases of chancroid occur annually across the globe.8 The true global epidemiology is unclear since chancroid cannot be reliably differentiated from other GUDs on clinical grounds and there is a lack of readily available diagnostic tests in most clinics. The disease however is prevalent worldwide, and endemic in many poor countries of tropical and subtropical regions with inadequate medical infrastructure, especially sub-Saharan Africa, Asia, and Latin America.9,10
The pattern of GUD is changing rapidly with studies from Africa revealing that GUD attributable to herpes simplex virus (HSV) type-2 infection is increasing, and that of H. ducreyi is decreasing in many areas.11 A gradual decline in the prevalence of H. ducreyi was noted among patients attending an STI clinic in Durban (South Africa), from 35% in 1995 to 6% in 1998. During the same period, HSV-2 infection rose from 11% to 45%.12 Since the mid-1960s, the incidence of chancroid has been decreasing in many parts of Africa and Asia, especially Kenya, Senegal, Philippines, and Thailand. This may partially be a consequence of intervention strategies targeting commercial sex workers (CSWs) and STI patients to prevent HIV transmission.1 In Nairobi, Kenya, chancroid was endemic but now accounts for less than 10% of genital ulcers. The number of reported cases of chancroid has declined steadily in the USA from 4986 cases in 1987 to 143 cases in 1999.13 More recent CDC statistics reveal that chancroid is disappearing in the United States, with only 23 cases reported nationwide in 2007.14 In the United Kingdom, 378 new episodes of tropical GUD (combining chancroid, LGV, donovanosis) were seen at the genitourinary medicine clinics in 2008. England accounted for 367 (97%) episodes, of which 183 (almost 50%) were seen in London. These figures were mainly due to the recent epidemic of LGV.15 Chancroid is rare in the Scandinavian countries and Australia.16,17 Chancroid is endemic in some parts of India, and its prevalence has varied from 1.6% to 51.9% based on reports from different STI clinics.18–20 The wide variation may be due to the lack of uniform diagnostic criteria besides diverse social, cultural, and religious practices prevailing in the local population. The prevalence of chancroid has been reported to be 22.4% among children below 14 years of age with STIs in Delhi.21
Chancroid is seen more frequently in the sexually active age group of 18–45 years. Men are more commonly affected, with the male to female ratio exceeding 10:1 (varying from 1.6:1 to 53:1).22–24 Important factors contributing to the gender disparity, may be the possibility of fewer women transmitting the infection to a large number of men through commercial sex, an asymptomatic carrier state in women,25 and the concealed and subclinical nature of lesions on the female genitalia resulting in unhampered sexual activity. It also appears that an occlusive environment provided by the prepuce makes men more susceptible. A recent meta-analysis revealed that circumcised men were clearly at a lower risk of chancroid.26
The disease is almost always contracted through sexual contact, barring minor episodes where medical professionals acquire the infection on fingers through accidental inoculation. Plummer et al. have reported that the chances of acquiring infection during a single act of unprotected exposure from man to woman are as high as 63%.27 In the absence of treatment, the duration of infection has been estimated to be 45 days in women, and it appears that the risk of transmission is much more with patients bearing visible lesions.28 Fomites do not play a role in the transmission of the disease. There is no nonhuman reservoir for H. ducreyi, and it depends for its survival and propagation on sexual transmission. Individuals with poor hygiene and CSWs constitute a major source of infection. Alcoholism and cocaine abuse in the smoked form ("crack") are also associated with an increased risk.29,30
Biology
H. ducreyi is a pleomorphic, slender (1.2 x 0.5 μm), gram-negative, nonmotile, nonspore forming, and facultative anaerobic streptobacillus with rounded ends. It is a fastidious organism with complex nutritional requirements for its growth. It has some characteristic biochemical properties, which include a reaction positive for oxidase and alkaline phosphatase activity, and negative for catalase activity. It reduces nitrate to nitrite and requires hemin (X-factor) for growth. In liquid culture or tissue the organisms form parallel chains resembling “railroad tracks”, while on solid agar “schools offish” and whorls appear which resemble “fingerprints”.31 Recent genetic sequencing data has revealed that H. ducreyi is more closely related to the animal pathogens Actinobacillus pleuropneumoniae and Mannheimia haemolytica than to human pathogens in the Pasteurellaceae family. However, animal models of chancroid do not adequately simulate human infection, suggesting that H. ducreyi has deviated from other organisms to establish itself as a unique human pathogen.32
Pathogenesis
The exact pathogenesis of ulcer formation in chancroid is not clear. The organisms appear to enter sites of trauma or abrasion where the integrity of skin is disrupted. The inoculum size has to be more than 104 to produce an infection.33 Adherence of bacteria to the epithelial cells is mediated by pili, and the subsequent production of hemolysins and cytotoxins results in cell damage. Both cell-mediated and humoral responses occur to infection with chancroid. A delayed hypersensitivity reaction and recruitment of peripheral blood mononuclear cells is seen in response to specific H. ducreyi antigens. The cell-mediated response to H. ducreyi infection is predominantly of Th-1 cells. Immunohistological studies have revealed a predominant mononuclear infiltrate consisting mainly of CD4+ and CD8+ T-lymphocytes, and monocytes with a conspicuous paucity of B-lymphocytes in the biopsy specimens of chancroid lesions. This predominant T-helper cell (Thl) response is consistent with the observation of increased levels of soluble interleukin-2 receptors in the urine and semen of chancroid patients.34 These cytokines are secreted by CD4+ T lymphocytes.
A humoral response with production of IgG, IgM, and IgA occurs but does not confer lasting immunity. Many antigens, including the major outer membrane protein (MOMP) with a molecular weight of 40 KDa, have been characterized and the cell wall bears pili. The exact role of the various cytotoxins and hemolysins in pathogenesis is not clear, though they are thought to participate in tissue damage and ulcer formation. The virulence factors associated with H. ducreyi are likely to be the lipooligosaccharide (LOS), pili, extracellular toxins, and hemolysins.33,35–37
Chancroidal lymphadenitis is predominantly a pyogenic inflammatory response, with an unknown pathogenesis; the paucity of organisms in the bubo pus is also unexplained. Virulence determinants in the organism include superoxide dismutase enzymes and hemolysin. Superoxide dismutase enzymes are thought to increase the survival and persistence of the pathogenic organism within the host, whereas hemolysin contributes to ulcer formation and the invasion of epithelial cells.35 The latter has immunogenic properties and is expressed both in vitro and in vivo by all known clinical strains of H. ducreyi. These properties make hemolysin a possible candidate for vaccine development.38
Clinical Features
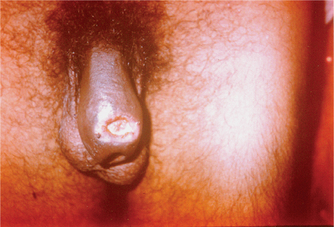
The incubation period is usually short and varies from 3 to 7 days, except in patients with concomitant HIV infection where it can be longer. The lesion may occur within 48 hours, if there are abrasions present over the genitalia at the time of intercourse. An erythematous papule appears at the site of entry of the organisms, which turns into a pustule, undergoes central necrosis and forms a characteristic, small, necrotic, tender, bleeding, nonindurated ulcer with ragged or undermined edges (Fig. 42.1). Autoinoculation may result in the formation of kissing ulcers or multiple ulcers in proximity to the primary ulcer (Fig. 42.2).
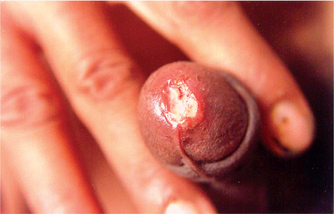
Fig. 42.1 Irregular, necrotic ulcer with ragged margins surrounded by an inflammatory margin over the urethral meatus is seen.
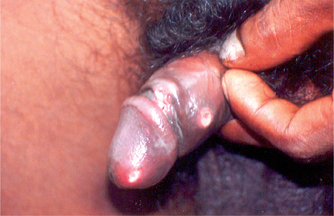
Fig. 42.2 Multiple, small, necrotic ulcers with ragged borders over the penile shaft and kissing chancroidal ulcer over the coronal sulcus are seen.
Men usually present with painful genital ulcer(s) and/or a painful inguinal mass, and at times, phimosis or paraphimosis. There is no prodromal phase. Rarely, the patient may present with purulent urethritis if the ulcer is located in the urethra.39 Genital lesions in women may be asymptomatic or may present with mild vaginal discharge, dyspareunia, pain on voiding, or defecation. Skin rash or systemic reactions are not encountered. The typical lesion of chancroid is a nonindurated (soft sore), painful ulcer with ragged or undermined edges and a necrotic base covered with purulent exudate. It bleeds easily on touch. Distinctively, the ulcer is variably painful depending upon the site of inoculation. The pain is more in men than in women. The ulcers are often multiple, however, not uncommonly, a solitary ulcer can occur. H. ducreyi seems to have a predilection for the stratified squamous epithelium, the mucosa being rarely affected. Ulcers in men usually occur over the preputial orifice, inner prepuce, frenulum, coronal sulcus, penile shaft, and anal orifice. The common sites affected in women are the labia, clitoris, fourchette, vestibule, anal margin, and cervix. Due to autoinoculation, the lesions may later spread to the peri-inguinal region, mons pubis, abdomen, and thighs. The rare occurrence of extragenital lesions over the fingers, tongue, lips, breasts, oral mucosa, and perianal area has been reported.33
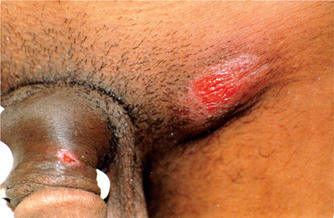
Various clinical patterns of genital ulcers in chancroid have been described.40–44 Dwarf chancroid consists of single or multiple herpetiform ulcers with or without associated inguinal lymphadenopathy. Giant chancroid, initially a small ulcer, extends rapidly to form a solitary destructive lesion (Fig. 42.3). This form is often associated with an inguinal bubo. In transient chancroid, the initial lesion resolves rapidly within 4–6 days, only to be followed by acute inguinal lymphadenitis with suppuration in 10 to 20 days. Follicular chancroid originates in the pilar apparatus, and therefore occurs on hair-bearing areas. Phagedenic chancroid is characterized by widespread necrosis with deep and often extensive destruction of the genitalia (Fig. 42.4). Pseudogranuloma inguinale is a variant of chancroid that bears close resemblance to granuloma inguinale.43 In serpiginous chancroid, multiple ulcers coalesce to form a serpiginous pattern. In mixed chancroid, nonindurated, tender ulcers occur along with an indurated, nontender ulcer of syphilis. Chancroidal ulcer is usually a tender, nonindurated, single large ulcer with the conspicuous absence of lymphadenopathy, and is caused by organisms other than H. ducreyi.
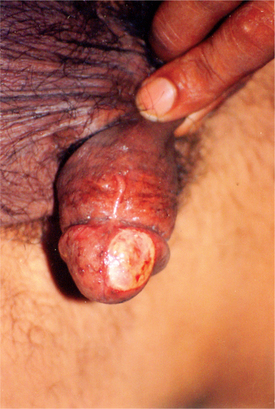
Fig. 42.3 Giant chancroid over the glans penis showing necrotic ulcer surrounded by erythematous borders.
Bubo
Subsequent to appearance of a genital ulcer, the regional lymph nodes become tender and inflamed. Lymphadenopathy occurs in 30–60% of patients (both in men and women), usually 1–2 weeks after the appearance of the genital ulcers. It is unilateral in two-thirds of the patients. The typical bubo consists of inguinal lymph nodes that have become fused and matted together by an acute inflammatory process of periadenitis forming a unilocular swellingwith erythematous overlying skin. The swelling is painful and causes difficulty in walking. The bubo softens and undergoes suppuration to form unilocular fluctuant swelling (Fig. 42.5), which may rupture spontaneously resulting in ulceration. The margins of the opening (ostium) are necrotic and inflammatory. In about half of the patients, the inguinal lymphadenitis may subside without suppuration. In contrast to the transient nature of the primary lesion seen in lymphogranuloma venereum (LGV), the genital ulcers in chancroid may persist when the patient presents with inguinal bubo.45 Features that differentiate the bubo of chancroid from that of LGV are summarized in Table 42.1.
Table 42.1
Differentiating Features of Lymphadenopathy in Chancroid and LGV
| Features | Chancroid | LGV |
| Frequency of involvement | 30–60% | ~100% |
| Incubation period (bubo) | 7–14 days | 10–30 days |
| Unilateral | 75% | 66.7% |
| Primary genital ulcer (when patient presents with bubo) | Present | Absent |
| Sinus formation from rupture of bubo | Single | Multiple |
| Constitutional symptoms | − | + |
| Systemic spread | − | + |
| Groove sign | − | + (20% cases) |
| Loculation | Unilocular | Multilocular |
Clinical Course and Complications
Chancroid is a self-limiting disease presenting with no serious problems in the majority of cases. Pain is the most important complaint. The primary lesions heal gradually or may spread locally through autoinoculation. Inguinal lymphadenitis may be minimal and subside gradually or progress to form a bubo that may rupture leaving behind large ulcerations. Complications occur more frequently in men that include balanitis, phimosis, paraphimosis, and the partial loss of tissue over the glans penis in phagedenic ulcers due to superinfection with anaerobic organisms. Discharging sinuses and large areas of ulceration at the site of the inguinal bubo may result in scar formation. Systemic infection or metastatic spread to distant sites has not been reported.46 However, mild constitutional symptoms may occur due to secondary infection of the ulcers.
Laboratory Diagnosis
The laboratory confirmation of chancroid is essential for accurate diagnosis, management, and epidemiological monitoring. In suspected cases of chancroid, testing for herpes simplex virus and syphilis should also be performed. It is interesting that the accuracy of the clinical diagnosis of chancroid is greatly affected by the prevalence of the disease.47 A comparison of studies that evaluated the accuracy of a clinical diagnosis of chancroid (Fig. 42.6) suggests that the diagnostic accuracy decreases with the prevalence of the infection declining in the given population.48–51 Therefore, with a continuous decline in the prevalence of the disease, the reliability of clinical diagnosis diminishes and laboratory confirmation becomes essential.
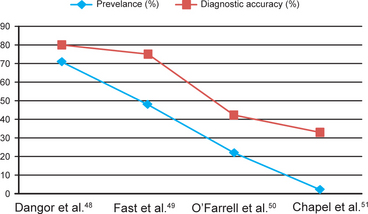
Fig. 42.6 Accuracy of clinical diagnosis of chancroid (in men) in relation to prevalence in various studies. Note, a consistent decline in the clinical diagnostic accuracy with a decline in the prevalence of chancroid among patients with genital ulcer disease.
Microscopic Examination of the Smears
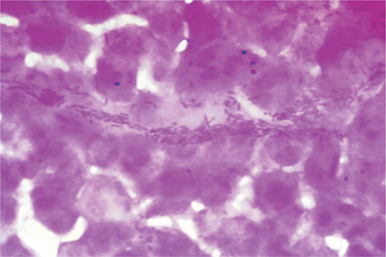
The ulcer is cleaned with saline and exudate from the undermined edge of the lesion is obtained by rolling a cotton or calcium alginate swab. This is then smeared onto a glass side and stained with Gram, Unna-Pappenheim, Wright or Giemsa stains. The presence of typical streptobacilli lying in clusters, either inside the leukocytes or outside in chains, paralleling the shreds of mucus with the appearance of “schools of fish,” “railroad tracks”, and “fingerprints” may suggest a diagnosis of chancroid (Fig. 42.7). Often, it is difficult to demonstrate H. ducreyi in smears due to the polymicrobial flora of genital ulcers, which may resemble the Ducreyi bacillus. The organisms are less frequently demonstrated in the pus aspirated from a bubo, and culture from bubo is mostly sterile unless it has ruptured.3,52 Some experts believe that direct microscopy of the smear is not reliable and should not be used in the routine diagnosis of chancroid.31 It is of limited value because of low sensitivity (5–63%) and specificity (51–99%).53
Culture and Identification
Although H. ducreyi is a fastidious microorganism which is difficult to isolate from genital ulcer specimens, culture remains the main diagnostic tool, and for many years has been the “gold standard” for evaluating newer methods of diagnosis. However, even with the optimal combination of media, it is only about 80% sensitive. The specimen of choice is a swab that has been taken from the base of the genital ulcer.53
Collection and Transport of Specimens
As H. ducreyi is a fastidious organism, it is essential that the specimens obtained from the base of ulcers or aspirate from the bubo should either be plated out directly onto the culture medium or sent to the microbiology laboratory rapidly (within 4 hours). The specimen should be obtained with a calcium alginate or plastic swab from the base of the ulcer after cleaning it with dry gauze. No selective transport medium is available, although Dangor et al. were able to grow relatively viable H. ducreyi for up to 4 days, from the specimens stored at 4° C in a thioglycolate-hemin based medium containing L-glutamine and bovine albumin.48 In swabs, H. ducreyi can survive for 2–4 hours only, and hence an alternative method (though less desirable) is to take the swab sample and place it in a transport media such as Amies.52–54 Culture from intact buboes has even lower detection rates compared with culture from the ulcer base or culture from ruptured buboes.
Culture Procedure
H. ducreyi grows best at 33° C in a microaerophilic, water saturated atmosphere containing 5% carbon dioxide, or in a traditional candle jar. Lenglet has been credited with the first successful in vitro culture of H. ducreyi in 1898.55 Teague and Debert described the successful culture of H. ducreyi using fresh clotted rabbit blood heated to 55° C.56 Numerous selective artificial media have been developed since. To date, the most widely used media are the gonococcal agar base enriched with 1% hemoglobin, 1% IsoVitaleX, and 5% foetal calf serum; Mueller-Hinton agar enriched with 5% horse blood and 1% IsoVitaleX; Columbia agar base with 5% lyzed horse blood, 2.5% yeast dialysate, and 1% IsoVitaleX; enriched protease peptone agar; and heart infusion agar enriched with 10% foetal calf serum.34 Nsanze et al. evaluated the potential benefit of using more than one medium to isolate H. ducreyi from clinical chancroid cases.57 A simple inexpensive medium containing gonococcal agar base supplemented with 5% foetal calf serum and noncoagulated horse blood, and also a medium containing 0.2% activated charcoal instead of foetal calf serum, have been recommended for use in resource poor countries.58,59 The success rate of H. ducreyi isolation in different culture media has been reported to range between 2% and 100%.
Cultures are incubated for 48 hours before noting the initial reading and kept for 5 days before reporting them negative. H. ducreyi exhibits an unusual tendency to autoagglutinate, when grown in a liquid medium or forms a cohesive colonial structure on agar plates. The colonies appear as nonmucoid, raised and granular, having a grayish-yellow color and can be pushed intact, across the surface of the agar with a bacteriologic loop. Gram staining of smears prepared from the colonies show gram-negative coccobacilli in short chains, clumps or whorls with individual bacterium showing bipolar staining. Identification can be done by (β-lactamase production, oxidase test, nitrate reduction, requirement for hemin (X factor), and negative catalase, indole and urease tests.
Polymerase Chain Reaction
Polymerase chain reaction (PCR) techniques have been developed to improve the sensitivity of diagnosis by DNA amplification60–66 using primers based on the H. ducreyi 16S rRNA gene,60,63,65 the rrs (16S)-rrl (23s) ribosomal intergenic spacer region,65 an anonymous fragment of cloned H. ducreyi DNA,61 the groEL gene,62 or the 27 kDa H. ducreyi-specific protein (p27).66 A multiplex PCR (M-PCR) assay has also been developed for the simultaneous amplification of DNA targets from H. ducreyi, pallidum, and HSV types 1 and 2, and appears more sensitive than the standard diagnostic tests for the detection of these etiological agents in genital ulcer specimens.64 Although PCR assays perform well with samples prepared from H. ducreyi cultures, they appear to be less sensitive when used to test genital ulcer specimens owing to the presence of Taq polymerase inhibitors in the DNA preparations extracted from the specimen.61
Antigen Detection by Immunofluorescence
Monoclonal antibodies (MAbs) have been raised against the 29 kDa outer membrane protein (OMP)67 for direct immunofluorescence, and the MAb MAHD 7 against H. ducreyi lipooligosaccharide (LOS) for indirect immunofluorescence assays.68 This assay can detect purified H. ducreyi LOS at a level of 25 pg/mL and could detect as few as 1000 CFU of in vitro grown H. ducreyi. The IF test identifies over 90% of the culture positive samples in addition to some culture negative chancroid cases. This technique has been shown to have a sensitivity of 100% and a specificity of 74% in comparison with culture, and a sensitivity of 81% in comparison with the PCR assay.
The immunolimulus assay combines the specificity of a MAb raised against H. ducreyi LOS, and the sensitivity of the chromogenic limulus amebocyte lysate test for endotoxin.69
Antibody Detection by Serological Tests
Various serologic tests are available for the diagnosis of chancroid which include the enzyme immunoassays (EIAs), dot immunobinding, agglutination, complement fixation, and precipitation.70–72 Antigens used for EIAs include the ultrasonicated whole cell antigen purified H. ducreyi LOS of OMPs.73 None of these tests are commercially available at present. Though less sensitive to detect the circulating antibodies to H. ducreyi in individual patients having symptoms, these tests are helpful for screening in large scale epidemiological studies at the community level.74
Other tests include nucleic acid probe technology and mass spectrometric methods. H. ducreyi DNA can be detected by the technique of DNA-DNA hybridization using labeled H. ducreyi-derived probes. These probes are reliable and can detect 104 CFU of H. ducreyi in pure and mixed cultures. Also, oligonucleotides complementary to different regions in the 16S and 23S rRNA molecules of H. ducreyi have been synthesised.75 Matrix assisted laser desorption-ionization time of flight mass spectrometry (MALDI-TOF MS) has been used for the rapid identification and speciation of Haemophilus.76
Histopathological Examination
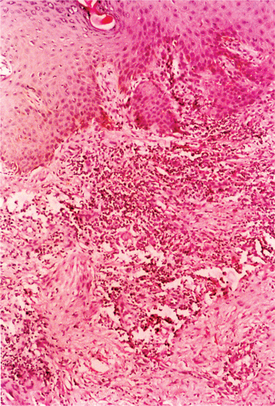
The histological examination of the ulcer may be helpful in arriving at a diagnosis, even though the changes observed are not pathognomonic. The histopathologic picture consists of three zones: the surface zone at the floor is narrow and consists of neutrophils, fibrin, erythrocytes, and necrotic tissues; the middle zone is wide, containing newly formed blood vessels showing marked proliferation of endothelial cells, thrombosis, and degenerative changes in the walls of the vessels; and the deep zone is composed of a dense infiltrate of plasma cells and lymphoid cells (Fig. 42.8).77 Histological examination is useful to exclude malignancy in nonhealing or atypical ulcers.
Differential Diagnosis
Chancroid has to be differentiated from other sexually transmitted GUDs, i.e., primary syphilis, genital herpes, granuloma inguinale, LGV and candidal balanitis, and non-STIs, such as traumatic ulcers, fixed drug eruption, and carcinoma. Coinfection of chancroid with T. pallidum or HSV may occur in up to 10% of patients.
Antimicrobial Susceptibility
Recent reports from different parts of the world have revealed that clinically relevant antimicrobial resistance in chancroid has become common and is spreading rapidly.78–81 More alarmingly, plasmid-mediated drug resistant strains of H. ducreyi to ampicillin, sulfonamides, chloramphenicol, tetracycline, streptomycin, and kanamycin have been reported.34 However, these strains are extremely sensitive to several other antibiotics, especially the macrolides (erythromycin and azithromycin), the quinolones (ciprofloxacin and fleroxacin), and the third-generation cephalosporins (ceftriaxone, cefotaxime, and cefixime).33
Treatment
Chancroid is an important cofactor in facilitating the transmission of HIV infection.1 Prompt and adequate therapy of affected patients and their contacts is vital, in addition to the aggressive implementation of appropriate control and prevention measures for eradicating the disease.
Earlier treatments, such as sulfonamides, ampicillin, tetracyclines, and streptomycin, are no longer being recommended because of poor response rates and widespread resistance developed by H. ducreyi to these antimicrobials. Trimethoprim-sulfamethoxazole has been used extensively for chancroid in the past; however, resistance to this drug has now been noted in many parts of the world (Thailand, Kenya, and Rwanda). Erythromycin is effective in a dose of 500 mg 4 times a day, orally for 7 days.82 Studies indicate that single-dose treatment with spectinomycin or ceftriaxone are also quite effective in the treatment of chancroid.80,83 Azithromycin 1 g single-dose is highly effective but quite expensive. Fluoroquinolones, especially ciprofloxacin 500 mg given as single-dose orally has been found to be safe, convenient, and effective in chancroid; however, treatment failures have occurred in patients with concomitant HIV infection.
Current therapies recommended for chancroid are summarized in Table 42.2, and the treatment guidelines can be accessed at:
Table 42.2
Comparative Analysis of the Current Standard Therapies Recommended for Chancroid by the World Health Organization (WHO 2003), the National Aids Control Organization (NACO, India 2005), the Centers for Disease Control (CDC, USA 2006), and the BASHH Clinical Effectiveness Group (CEG, UK 2007)
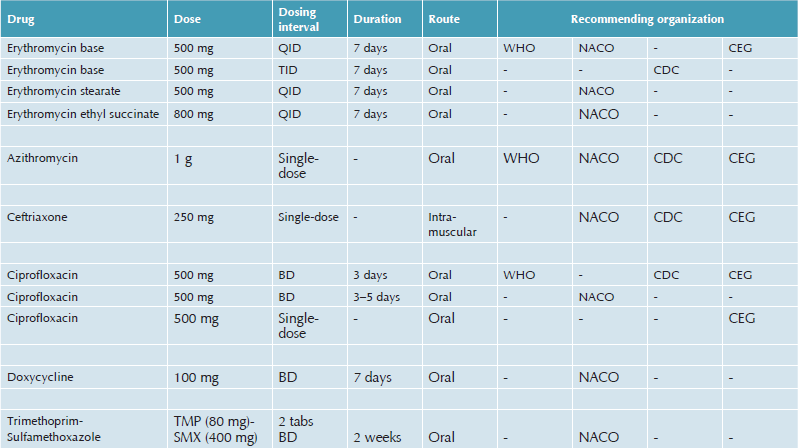
1. WHO: http://apps.who.int/medicinedocs/en/d/Jh2942e/4.5.html#Jh2942e.4.5
2. CDC: http://www.cdc.gov/std/treatment/2006/genitalulcers.htm#genulc2
3. CEG/BASHH: http://www.bashh.org/guidelines
Special Considerations
The safety of azithromycin in pregnant and lactating women has not been established. Ciprofloxacin is contraindicated in pregnancy, lactating women, children, and adolescents less than 18 years of age, hence erythromycin or ceftriaxone regimens should be used. No adverse effects of chancroid on pregnancy outcome or on the foetus have been reported. If associated with HIV infection, the patients have to be closely monitored. Healing of ulcers may be slower among HIV infected people and treatment failures have been reported with azithromycin, ceftriaxone, single-dose fleroxacin, low-dose erythromycin or ciprofloxacin. However, some recent studies have not shown any increased treatment failures in HIV-positive cases with low-dose erythromycin and single-dose ciprofloxacin. Currently, the data on therapeutic efficacy with the recommended ceftriaxone and azithromycin regimens among patients infected with HIV is limited. Hence, CDC recommends that these regimens should be used among persons known to be infected with HIV, only if a proper follow-up can be assured. However, some experts advocate the use of full doses of erythromycin for 7 days for treating HIV-infected persons.
Although not yet commercialized for human use, a promising potential vaccine technology has been developed. In this scenario, high-risk individuals in endemic areas could undergo vaccination against a heme-receptor essential for H. ducreyi survival in skin. Antibodies arising after this vaccination have prevented infection in vivo in a porcine model of chancroid.84
Management of Ulcers and Fluctuant Buboes
Bed rest is recommended early in the course of disease because of pain. Local antiseptics are contraindicated as they would interfere with the diagnosis of concomitant syphilis by dark-ground microscopy. Local cleansing with normal saline is advocated. Fluctuant buboes can be aspirated with a needle from the adjacent healthy skin, and should not normally be incised as there is a risk of autoinoculation. Although incision and drainage of fluctuant buboes under antibiotic cover has been recommended in the past,82 it is contraindicated according to the latest recommendations from WHO.
Follow-up
Re-examination of the patient should be carried out on the seventh day after initiation of therapy. With the administration of proper treatment, the ulcers should show improvement symptomatically in 3 days followed by substantial re-epithelialization after 7 days. The time required for complete healing is related to the size of the ulcer and possibly the HIV status of the individual; large ulcers may require more than 2 weeks. Also healing is slower for some uncircumcised men who have an ulcer under the foreskin. Resolution of lymphadenopathy is slower than that of the ulcer.
Treatment Failure
Coinfections with T. pallidum or HSV may result in partial or no improvement. During the past decade, high-level, plasmid-mediated drug resistance to sulfonamides, penicillins, kanamycin, streptomycin, tetracycline, chloramphenicol, and trimethoprim has been observed in H. ducreyi isolates. The most suitable medium to determine minimum inhibitory concentrations (MIC) is the Mueller-Hinton agar enriched with 1% hemoglobin, 5% foetal calf serum, and 1% HD supplement. Alternatively, a gonococcal agar base can be used.34 If drug resistance is suspected, it is important to do antimicrobial drug susceptibility testing of H. ducreyi by the agar dilution technique85 or the equally effective but simpler E-test.86
Management of Sexual Partner(s)
Sexual contacts within the last 10 days before the onset of patient's symptoms should be examined and treated, even in the absence of symptoms, in view of possible asymptomatic carriage.25 Serological tests for syphilis and HIV should be offered to all patients and their contacts.
Syndromic Approach
The clinical differentiation of chancroid from other GUDs is not reliable and culture of H. ducreyi is not routinely available, thereby imposing constraints on diagnosis, treatment, and control strategies.1 Several studies have established that GUDs facilitate the risk of acquisition and transmission of HIV infection.87 Hence, the current syndromic approach for the treatment of STIs, proposed by WHO is essential to manage GUDs and prevent HIV infection, especially in resource-poor nations where chancroid is endemic.88–91
HIV and Chancroid
Chancroid facilitates the transmission of HIV by increasing both the infectiousness of HIV and the susceptibility to infection by the virus. Subjects with HIV infection and a concomitant chancroid ulcer demonstrate increased infectiousness through the following:
1. There is increased HIV shedding into the genital tract from ulcer exudates. In Cote d'Ivoire, HIV-positive female sex workers with cervicovaginal ulcers had higher rates of HIV isolation from cervicovaginal lavage fluids than those without ulcer disease (66.8% vs. 21%). Adjustment for the level of immunosuppression did not alter these findings. Treatment of the chancroid ulcer reduced HIV detection to levels similar to those of individuals without GUD.
2. Genital ulcers bleed during intercourse, resulting in potential increases in viral shedding and HIV infectiousness.
3. In men with GUD, there is increased concentration of the virus in seminal fluid, especially in those who also have nongonococcal urethritis. This is attributed to an increased plasma viral load from advanced disease or systemic immune activation by H. ducreyi38
GUD, particularly chancroid, has been shown to increase the risk of acquiring HIV infection.17,92–94 Genital ulcers provide a portal of entry for both HIV-1 and HIV-2. H. ducreyi infection recruits CD4+ lymphocytes and macrophages to genital surfaces and these cells are the principal early targets for HIV infection. Concomitant HIV infection may alter the clinical presentation of chancroid lesions. The ulcers may present with atypical features, such as large size and number, and extragenital location. The ulcers may take a longer time to heal. HIV appears to reduce the responsiveness of chancroid to standard therapy, especially single-dose regimens, possibly requiring treatment for a longer period and/or increased dosing.84
Prevention and Control
H. ducreyi survives largely in networks with a high rate of sexual partner change, including MSM and CSWs. Control policies targeting these high risk groups will result in the rapid disappearance of chancroid from a community. Once endemic in Europe and North America, the incidence of chancroid declined steadily early in the twentieth century. Greater awareness and changes in the pattern of commercial sex probably disrupted the conditions necessary to sustain chancroid as an endemic disease among these communities.1 Several antibiotics, including some single-dose regimens, have the advantage of providing a rapid cure as well as ease of administration. Sporadic outbreaks can be controlled with effective treatment and preventive services targeted at high risk groups. More recently, the prevalence of chancroid has decreased in Thailand, Philippines, and Senegal with the implementation of intervention strategies targeting CSWs and their clients, a development that may contribute to the reduction of STIs and the stabilization of the HIV epidemic in these countries.
Greater awareness regarding diagnosis and treatment, strengthening diagnosis and treatment of STIs, widespread promotion of condom use, contact tracing, a targeted approach towards high risk groups, emphasis on maintaining local hygiene, and the use of a syndromic approach can all go a long way towards bringing down the incidence of this disease.
References
1. Steen, R. Eradicating chancroid. Bull World Health Organ. 2001; 79:818–826.
2. Kampmeier, R.H. The recognition of Haemophilus ducreyi as the cause of soft chancre. Sex Transm Dis. 1982; 9:212–213.
3. Ducrey, A. Experimentelle untersuchungen uber den Ansteckungsstof des weichen Schankers und uber die Bubonen. Monatsh Prakt Dermatol. 1889; 9:387–405.
4. Bezancon, F., Griffon, V., Sourd, L. Le. Culture du bacille du chancre mou. CR Soc Biol. 1900; 11:1048–1051.
5. Unna, P.G. Der streptobacillus des weichen Schankers. Monatsh Prakt Dermatol. 1982; 14:485.
6. Ito, T. Klinische und bacteriologische studien iber ulcus molle and ducreysche streptobazillen. Arch Dermatol Syphilol. 1913; 116:341.
7. Greenblatt, R.B., Sanderson, E.S. Diagnostic value of the intradermal chancroidal test. Arch Dermatol Syphilol. 1937; 36:486–493.
8. UNAIDS. Sexually transmitted diseases: policies and principles for prevention and care. New York: World Health Organization; 1997.
9. Le Bacq, F., Mason, P.R., Gwanzura, L., et al. HIV and other sexually transmitted diseases at a rural hospital in Zimbabwe. Genitourin Med. 1993; 69:352–356.
10. Behets, F.M., Liomba, G., Lule, G., et al. Sexually transmitted diseases and human immunodeficiency virus control in Malawi: a field study of genital ulcer disease. J Infect Dis. 1995; 171:451–455.
11. Chen, C.Y., Ballard, R.C., Beck-Saque, C.M., et al. Human immunodeficiency infection and genital ulcer disease in South Africa. The herpetic connection. Sex Transm Dis. 2000; 27:21–29.
12. Kharsany ABM, Mahabeer Y, Connolly C, et al. Changing etiology of genital ulcer disease (GUD) in STD clinic attenders with a rising HIV prevalence. 13th International AIDS Conference. Durban, 2000 (Abstract ThOrC728).
13. Centers for Disease Control and Prevention. Tracking the Hidden Epidemics: Trends in STDs in the United States. Division of Sexually Transmitted Diseases, 2000;25.
14. Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2007. Atlanta (GA), Department of Health and Human Services, 2009;45.
15. HPA. All new STI episodes seen at genitourinary medicine (GUM) clinics in the United Kingdom: 1999-2008. United Kingdom and country specific tables Chancroid/LGV/Donovanosis. 24 July, 2009: Page 4. http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1215589014474
16. Over, M., Piot, P. Human immunodeficiency virus infection and other sexually transmitted diseases in developing countries: public health importance and priorities for resource allocation. J Infect Dis. 1996; 174(Suppl 2):162–175.
17. Bong, C.T., Bauer, M.E., Spinola, S.M. Haemophilus ducreyi: clinical features, epidemiology, and prospects for disease control. Microbes Infect. 2002; 4:1141–1148.
18. Hira, S.K. Sexually transmitted diseases in the era of AIDS. WHO Southeast Asia Region Newsletter. AIDS Watch. 1997; 2:1–2.
19. Reddy, B.S.N., Garg, B.R., Rao, M.V. An appraisal of trends in sexually transmitted disease. IndianJSex Transm Dis. 1993; 14:1–4.
20. Kura, M.M., Hira, S., Kohli, M., et al. High occurrence of HBV among STD clinic attenders in Bombay, India. Int J STD AIDS. 1998; 9:231–233.
21. Pandhi, R.K., Khanna, N., Sekhri, R. Sexually transmitted diseases in children. Ind Paediatr. 1995; 32:27–30.
22. Lykke-Olesen, L., Larsen, L., Pedersen, T.G., et al. Epidemic of chancroid in Greenland 1977–78. Lancet. 1979; 1:654–655.
23. Kapur, L.T.R. Pattern of sexually transmitted diseases in India. Indian J Dermatol Venereol Leprol. 1983; 49:23–24.
24. Majumdar, S., Saha, S.S. Epidemiological survey of chancroid in Calcutta area. Indian J Sex Transm Dis. 1997; 18:9–11.
25. Hawkes, S., West, B., Wilson, S., et al. Asymptomatic carriage of Haemophilus ducreyi confirmed by the polymerase chain reaction. Genitourin Med. 1995; 71:224–227.
26. Weiss, H.A., Quigley, M.A., Hayes, R.J. Male circumcision and risk of syphilis, chancroid and genital herpes: a systematic review and meta-analysis. Sex Transm Infect. 2006; 82:101–110.
27. Plummer, F.A., Nsanze, H., Karasira, P., et al. Epidemiology of chancroid and Haemophilus ducreyi in Nairobi, Kenya. Lancet. 1983; 2:1293–1295.
28. Plummer, F.A., D'Costa, L.J., Nsanze, H., et al. Clinical and microbiological studies of genital ulcers in Kenyan women. Sex Transm Dis. 1985; 12:193–197.
29. DiCarlo, R.P., Armentor, B.S., Martin, D.H. Chancroid epidemiology in New Orleans men. J Infect Dis. 1995; 172:446–452.
30. Martin, D.H., DiCarlo, R.P. Recent changes in the epidemiology of genital ulcer disease in the United States. The crack cocaine connection. Sex Transm Dis. 1994; 21:S76–S80.
31. Albritton, W.L. Biology of Haemophilus ducreyi. Microbiol Rev. 1989; 53:377–389.
32. Gioia, J., Qin, X., Jiang, H., et al. The Genome Sequence of Mannheimia haemolytica A1: Insights into Virulence, Natural Competence, and Pasteurellaceae Phylogeny. J Bacteriol. 2006; 188:7257–7266.
33. Ronald, A.R., Albritton, W.L. Chancroid and Haemophilus ducreyi. In: Holmes K.K., Sparling P.F., Mardh P-A., et al, eds. Sexually Transmitted Diseases. New York: McGraw Hill; 1999:515–523.
34. Dyck, E.V., Meheus, A.Z., Piot, P. Chancroid. In: Laboratory Diagnosis of Sexually Transmitted Diseases. Geneva: World Health Organization; 1999:57–65.
35. Hobbs, M.M., Paul, T.R., Wyrick, P.B., et al. Haemophilus ducreyi infection causes basal keratinocyte cytotoxicity and elicits a unique cytokine induction pattern in an in vitro human skin model. Infect Immun. 1998; 66:2914–2921.
36. Brentjens, R.J., Ketterer, M., Apicella, M.A., et al. Fine tangled pili expressed by Haemophilus dureyi are a novel class of pili. J Bacteriol. 1996; 178:808–816.
37. Campagnari, A.A., Wild, L.M., Griffiths, G.E., et al. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect Immun. 1991; 59:2601–2608.
38. Mohammed, T.T., Olumide, Y.M. Chancroid and human immunodeficiency virus infection. Int J Dermatol. 2008; 47:1–8.
39. Kunimoto, D.Y., Plummer, F.A., Namaara, W., et al. Urethral infection with Haemophilus ducreyi in men. Sex Transm Dis. 1988; 15:37–39.
40. Sehgal, V.N., Shyam Prasad, A.L. Chancroid or chancroidal ulcers. Dermatologica. 1985; 170:136–141.
41. Sehgal, V.N. Chancroid. In: Venereal Diseases. New Delhi: Jaypee Medical Publishers; 1994:70–74.
42. Jonasson, J.A. Haemophilus ducreyi. Int J STD AIDS. 1993; 4:317–321.
43. Kraus, S.J., Werman, B.S., Biddle, J.W., et al. Pseudogranuloma inguinale caused by Haemophilus ducreyi. Arch Dermatol. 1982; 118:494–497.
44. Kumar, B. Giant chancroid. Indian J Dermatol Venereol Leprol. 1980; 46:309–310.
45. Goens, J.L., Schwartz, R.A., DeWolf, K. Mucocutaneous manifestations of chancroid, lymphogranuloma venereum and granuloma inguinale. Am Fam Physician. 1994; 49:415–418.
46. National guideline for the management of chancroid. Clinical Effectiveness Group (Association of Genitourinary Medicine and the Medical Society for the Study of Venereal Diseases). Sex Transm Infect. 1999; 75(Suppl 1):S43–S45.
47. Trees, D.L., Morse, S.A. Chancroid and Haemophilus ducreyi: an update. Clin Microbiol Rev. 1995; 8:357–375.
48. Dangor, Y., Ballard, R.C., Da L Exposto, F., et al. Accuracy of clinical diagnosis of genital ulcer disease. Sex Transm Dis. 1990; 17:184–189.
49. Fast, M.V., D'Costa, L.J., Nsanze, H., et al. The clinical diagnosis of genital ulcer disease in men in the tropics. Sex Transm Dis. 1984; 11:72–76.
50. O'Farrell, N., Hoosen, A.A., Coetzee, K.D., et al. Genital ulcer disease: accuracy of clinical diagnosis and strategies to improve control in Durban, South Africa. Genitourin Med. 1994; 70:7–11.
51. Chapel, T.A., Brown, W.J., Jeffres, C., et al. How reliable is the morphological diagnosis of penile ulcerations? Sex Transm Dis. 1977; 4:150–152.
52. Kumar, B., Sharma, V.K., V Bakaya, V, Ayyagiri, A. Isolation of anaerobes from bubo associated with chancroid. Genitourin Med. 1991; 67:47–48.
53. Kumar, B., Sharma, V.K., V Bakaya, V., Ayyagiri, A. Isolation of anaerobes from clinical chancroid associated with fluctuant bubo in men. Indian J Med Res. 1991; 93:236–239.
54. Alfa, M. The laboratory diagnosis of Haemophilus ducreyi. Can J Infect Dis Med Microbiol. 2005; 16:31–34.
55. Sullivan, M. Chancroid. Am J Syph Gonorrhea Ven Dis. 1940; 24:482–521.
56. Teague, O., Deibert, O. The value of the cultural method in the diagnosis of chancroid. J Urol. 1920; 4:543–550.
57. Nsanze, H., Plummer, F.A., Maggwa, A.B.N., et al. Comparison of media for the primary isolation of Haemophilus ducreyi. Sex Transm Dis. 1984; 11:6–9.
58. Lockett, A.E., Dance, D.A.B., Mabey, D.C.W., et al. Serum-free media for isolation of Haemophilus ducreyi. Lancet. 1991; 338:326.
59. Dangor, Y., Miller, S.D., Koornhof, H.J., et al. A simple medium for the primary isolation ofHaemophilus ducreyi. EurJ Clin Microbiol Infect Dis. 1992; 11:930–934.
60. Chui, L., Albritton, W., Paster, B., et al. Development of the polymerase chain reaction for diagnosis of chancroid. J Clin Microbiol. 1993; 31:659–664.
61. Johnson, S.R., Martin, D.H., Cammarata, C., et al. Development of polymerase chain reaction assay for the detection Haemophilus ducreyi. Sex Transm Dis. 1994; 21:13–23.
62. Parsons, L.M., Waring, A.L., Otido, J., et al. Laboratory diagnosis of chancroid using species-specific primers from Haemophilus ducreyi groEL and the polymerase chain reaction. Diagn Microbiol Infect Dis. 1995; 23:89–98.
63. West, B, Wilson, S.M., Changalucha, J., et al. Simplified PCR for detection of Haemophilus ducreyi and diagnosis of chancroid. J Clin Microbiol. 1995; 33:787–790.
64. Orle, K.A., Gates, C.A., Martin, D.H., et al. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum and Herpes simplex virus types 1 and 2 from genital ulcers. J Clin Microbiol. 1996; 34:49–54.
65. Gu, X.X., Rossau, R., Jannes, G., et al. The rrs (16S)-rrl (23S) ribosomal intergenic spacer region as a target for detection of Haemophilus ducreyi by a heminested-PCR assay. Microbiology. 1998; 144:1013–1019.
66. Tekle-Michael, T., Van Dyck, E., Abdellati, S., et al. Development of a heminested polymerase chain reaction assay for the detection of Haemophilus ducreyi in clinical specimens. IntJ STD AIDS. 2001; 12:797–803.
67. Karim, Q.N., Finn, G.Y., Easmon, C.S., et al. Rapid detection of Haemophilus ducreyi in clinical and experimental infections using monoclonal antibody: a preliminary evaluation. Genitourin Med. 1989; 65:361–365.
68. Ahmed, H.J., Frisk, A., Manssonc, J.E., et al. Monoclonal antibodies against Haemophilus ducreyi lipooligosaccharide and their diagnostic usefulness. EurJ Clin Microbiol Infect Dis. 1995; 14:892–898.
69. Hansen, E.J., Lumbley, S.R., Saxen, H., et al. Detection of Haemophilus ducreyi lipooligosaccharide by means of an immunolimulus assay. J Immunol Methods. 1995; 185:225–235.
70. Schalla, W.O., Sanders, L.L., Schmid, G.P., et al. Use of dot-immunobinding and immunofluorescence assays to investigate clinically suspected cases of chancroid. J Infect Dis. 1986; 153:879–887.
71. Museyi, K., Van Dyck, E., Vervoort, T., et al. Use of an enzyme immunoassay to detect serum IgG antibodies to Haemophilus ducreyi. J Infect Dis. 1988; 157:1039–1043.
72. Alfa, M.J., Olson, N., Degagne, P., et al. Use of an adsorption enzyme immunoassay to evaluate the Haemophilus ducreyi specific and cross-reactive humoral immune response of humans. Sex Transm Dis. 1992; 19:309–314.
73. Alfa, M.J., Olson, N., Degagne, P., et al. Humoral immune response of humans to lipooligosaccharide and outer membrane proteins of Haemophilus ducreyi. J Infect Dis. 1993; 167:1206–1210.
74. Lewis, D.A. Diagnostic tests for chancroid. Sex Transm Infect. 2000; 76:137–141.
75. Rossau, R., Duhamel, M., Janner, G., et al. The development of specific r-RNA derived oligonucleotide probes for Haemophilus ducreyi, the causative agent of chancroid. J Gen Microbiol. 1991; 137:277–285.
76. Haag, A.M., Taylor, S.N., Johnston, K.H., et al. Rapid identification and speciation of Haemophilus bacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Mass Spectrom. 1998; 33:750–756.
77. Lucas, S. Bacterial disease. In: Elder D., Elenitsas R., Jaworsky C., Johnson B., Jr., eds. Lever's Histopathology of the Skin. Philadelphia: Lippincott Raven Publishers; 1997:457–502.
78. D'Souza, P., Pandhi, R.K., Khanna, N., et al. A comparative study of therapeutic response of patients with clinical chancroid to ciprofloxacin, erythromycin, and cotrimoxazole. Sex Transm Dis. 1998; 25:293–295.
79. Knapp, J.S., Back, A.F., Babst, A.F., et al. In vitro susceptibilities of isolates of Haemophilus ducreyi from Thailand and the United States to currently recommended and newer agents for treatment of chancroid. Antimicrob Agents Chemother. 1993; 37:1552–1555.
80. Guzman, M., Guzman, J., Bernal, M. Treatment of chancroid with a single dose of spectinomycin. Sex Transm Dis. 1992; 19:291–294.
81. Rutanarugsa, A., Vorachit, M., Polnikom, N., et al. Drug resistance of Haemophilus ducreyi. South East Asian J Trop Med Public Health. 1990; 21:185–193.
82. Sehgal, V.N., Srivastava, G. Chancroid: contemporary appraisal. Int J Dermatol. 2003; 42:182–190.
83. Hartmann, A.A. Intravenous single-dose ceftriaxone treatment of chancroid. Dermatologica. 1991; 183:132–135.
84. Draft STD treatment recommendations. National AIDS Control organization, Ministry of health and family welfare, Govt of India, New Delhi, 2005.
85. Rosen, T., Vandergriff, T., Harting, M. Antibiotic use in sexually transmissible diseases. Dermatol Clin. 2009; 27:49–61.
86. Lewis DA, Dangor Y, Ballard RC. Comparison of E-test with agar dilution for antimicrobial susceptibility testing of Haemophilus ducreyi. Abstract P367. 12th meeting of the ISSTDR, International Congress of Sexually Transmitted Diseases, Seville, October 1997.
87. Gray, R.H., Wawer, M.J., Sewankambo, N.K., et al. Relative risks and population attributable fraction of incident HIV associated with symptoms of sexually transmitted diseases and treatable symptomatic sexually transmitted diseases in Rakai district, Uganda. Rakai Project Team. AIDS. 1999; 13:2113–2123.
88. Bogaerts, J., Vuylsteke, B., Martinez Tello, W., et al. Simple algorithms for the management of genital ulcers: evaluation in a primary health care center in Kigali, Rwanda. Bull World Health Organ. 1995; 73:761–767.
89. Adler, M.W. Syndromic management of genital ulcer disease. Genitourin Med. 1995; 71:416.
90. Htun, Y., Morse, S.A., Dangor, Y., et al. Comparison of clinically directed, disease specific and syndromic protocols for the management of genital ulcer disease in Lesotho. Sex Transm Infect. 1998; 74(Suppl 1):S23–S28.
91. van Dam, C.J., Beeker, K.M., Ndowa, F., et al. Syndromic approach to STD case management: where do we go from here? Sex Transm Infect. 1998; 74(Suppl 1):S175–S178.
92. Humphreys, T.L., Schnizlein-Bick, C.T., Katz, B.P., et al. Evolution of the cutaneous immune response to experimental Haemophilus ducreyi infection and its relevance to HIV-I acquisition. J Immunol. 2002; 169:6316–6323.
93. Dallabetta, G. HIV and AIDS: how are they linked? Afr Health. 1994; 17:19–20.
94. Laga, M. Interactions between STDs and HIV infection. STD Bulletin. 1992; 13:3–6.