Thyroid Gland
Functional anatomy
Gross anatomy
Thyroid gland is the largest endocrine gland in the body (weighing about 15−25 g in adults). It consists of two lobes joined together by a narrow isthmus and is located on either side of the trachea just below the larynx. It receives high blood supply (400–600 mL/100 g/min).
Histological structure (fig.8.3-1)
Histologically each lobe of thyroid gland is divided into various lobules by fibrous tissue septa. Each lobule is made up of an aggregation of several follicles. Each follicle is lined by follicular cells.
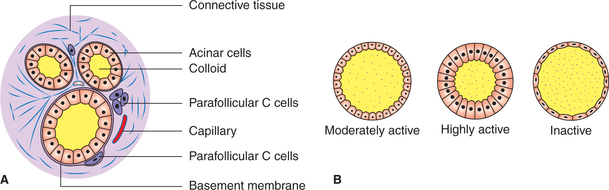
Fig. 8.3-1 Histological structure of thyroid gland (A) and variations in the follicular cell size with activity (B).
Follicular cells. These vary in shape with the degree of glandular activity. Normally (at an average level of activity) the cells are cuboidal and the colloid in the follicles is moderate in amount. During high degree of activity, the cells become columnar and flat when inactive (Fig.8.3-1B). These cells secrete thyroid hormones.
Parafollicular cells or C cells are scattered between follicular cells and basement membrane (Fig.8.3-1A), and secrete calcitonin, which is described in Chapter 8.4.
Colloid. This is a homogeneous material that fills the cavity of each follicle. The major constituent of the colloid is thyroglobulin, a glycoprotein with a molecular weight of 660,000. During active state the follicles are depleted of colloid; and in resting state, when unstimulated, the follicles accumulate colloid.
Thyroid hormones
Introduction
The two principal thyroid hormones include thyroxine (T4) and triiodothyronine (T3).
• Thyroxine or T4 (3,5,3′,5′-tetraiodothyronine) constitutes 90% of thyroid output.
• Triiodothyronine or T3 (3,5,3′-triiodothyronine) constitutes 10% of thyroid output.
• Calcitonin is a hormone secreted by the parafollicular cells of thyroid gland. It is concerned with calcium homeostasis.
Biosynthesis and storage of thyroid hormones
Thyroxine (T4) and triiodothyronine (T3) are synthesized from tyrosine and iodide by the enzyme complex, peroxidase.
Iodine metabolism
Dietary intake. Iodine is essential for the synthesis of thyroid hormones. It is ingested in the form of iodides. Sources of iodine are sea fish (richest), bread, milk and vegetables. Iodine is added to the table salt to prevent iodine deficiency.
Fate of dietary iodide. Most [80% (i.e. 400 μg/day)] of the iodides absorbed from the gastrointestinal tract (GIT) are selectively removed from circulation by cells of the thyroid gland.
Plasma iodide level is 0.15–0.3 μg%.
Thyroid iodide. Thyroid gland contains 5−8 mg of iodide, i.e. about 95% of total iodine content of the body. Thus the thyroid serves as a store of iodine. Of the total thyroid iodide only 5% is present within the cells of the follicular epithelium. The remaining 95% is present in the follicular lumen, stored in the colloid as thyroglobulin.
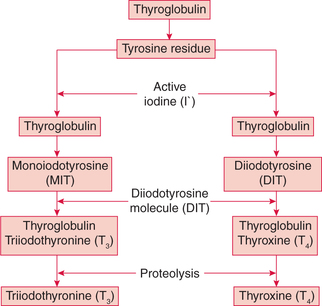
The steps involved in the synthesis of thyroid hormones are (Fig.8.3-2):
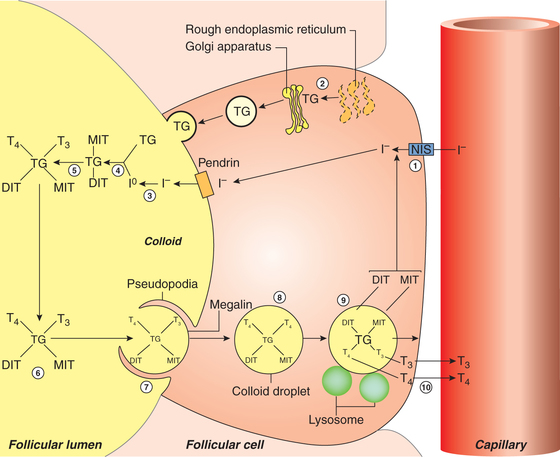
Fig. 8.3-2 Steps in synthesis and release of thyroid hormones: 1, iodine trapping; 2, synthesis of thyroglobulin; 3, oxidation of iodine; 4, organification of thyroglobulin; 5, coupling reaction; 6, storage of thyroid hormone in colloid; 7, take up of colloid by epithelial cell by endocytosis; 8, colloid vesicle; 9, release of T3 and T4 after proteolysis; and 10, diffusion of T3 and T4 into the capillary.
1. Iodine trapping. The first step in the synthesis of thyroid hormones is uptake of iodide by the thyroid gland, which occurs against the chemical (about 30:1) and electrical gradients. It is an energy-requiring process.
2. Synthesis and secretion of thyroglobulin. Thyroglobulin is a large glycoprotein that is synthesized on the rough endoplasmic reticulum of the thyroid epithelial cells and release into the lumen of follicle (Fig.8.3-2).
Each molecule of thyroglobulin contains about 140 tyrosine residues.
3. Oxidation of iodide. Once within the gland, iodide rapidly moves to the apical surface of the epithelial cells. From these, it is transported into the lumen of the follicles by a transporter called pendrin. The iodide (1) is then immediately oxidised to iodine (1°) by the enzyme peroxidase present near the apical border of the epithelial cells.
Thyroid is the only tissue that can oxidise iodide to iodine. Thyroid-stimulating hormone promotes this reaction while antithyroid drugs (thiourea, thiouracil, methimazole) inhibit.
4. Organification of thyroglobulin refers to the iodination of tyrosine residues present in the thyroglobulin molecule. This reaction occurs at the apical membrane of the cell and requires thyroid peroxidase. Tyrosine (of thyroglobulin) is first iodinated at position 3 to form monoiodotyrosine (MIT) and then at position 5 to form diiodotyrosine (DIT).
5. Coupling reaction. Two molecules of DIT couple to form thyroxine (T4). One molecule of MIT, when coupled with one molecule of DIT, triiodothyronine (T3) is produced (Fig.8.3-3). The enzyme peroxidase is required during coupling.
6. Storage. Once thyroglobulin has been iodinated it is stored in the lumen of the follicle as colloid for several months. It is estimated that the stored thyroid hormones can meet the body requirement for 1–3 months.
Secretion, transport and metabolism of thyroid hormones
Hormone secretion
Secretion of the thyroid hormone from the colloid stored in the lumen of follicle involves following steps (Fig.8.3-2):
Endocytosis. The colloid containing iodinated thyroglobulin enters from the lumen of the follicle into the epithelial cells through endocytosis. This process is facilitated by the TG receptor megalin located on the apical membrane.
Proteolysis. The lysosome vesicles containing proteolytic enzymes digest the thyroglobulin molecule releasing T4, T3, DIT, MIT and other amino acid constituent into the cytoplasm of the epithelial cells.
• T4 and T3 diffuse through the basal border of epithelial cells into the blood stream via adjacent rich capillary plexus.
• MIT and DIT are rapidly deiodinated within the follicular cells by the enzyme deiodinase. In this way, iodide is retrieved for recycling along with the tyrosine into T4 and T3 synthesis (Fig.8.3-2).
Transport of T4 and T3
Secreted T4 and T3 circulate in the blood stream in two forms: bound and free.
1. Bound form. Most of the circulating T4 (99.95%) and T3 (99.5%) is bound to specific binding protein.
2. Free form. Only about 0.05% of T4 and 0.5% of T3 circulate unbound (free form) in the plasma. These free, unbound hormones represent the biologically active hormone.
Regulation of thyroid hormone secretion
The secretion of thyroid hormones is regulated by:
A. Regulation through negative feedback mechanism
The negative feedback mechanism operating through hypothalamus–anterior pituitary–thyroid gland axis (Fig.8.3-4) plays the essential role in controlling secretion of thyroid hormones by:
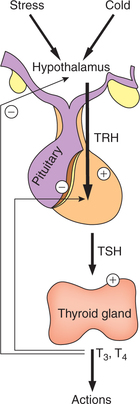
Fig. 8.3-4 Regulation of thyroid hormone secretion by negative feedback control mechanism through hypothalamus– anterior pituitary–thyroid gland axis.
Thyroid-stimulating hormone is a glycoprotein having molecular weight of 30,000. Average plasma level is 2.3 μIU/ mL (range 0.2−0.5 μIU/mL):
Action of TSH. TSH exerts following effects on the thyroid gland:
1. Increases the secretion of thyroid hormones by accelerating all the steps in biosynthesis.
2. Increases the number (hyperplasia) and size (hypertrophy) of the follicular epithelial cells.
1. Feedback control by plasma T4 and T3. Day-to-day secretion of TSH depends upon the negative feedback control exerted by the plasma levels of free T4 and T3 (Fig.8.3-4):
Since there exists an established inverse relationship between the plasma levels of thyroid hormones and TSH, therefore measurement of the plasma TSH levels is a reliable test for assessing the status of thyroid hormones.
2. Hypothalamic control of TSH. Hypothalamus exerts its effect by secreting thyrotropin-releasing hormone (TRH).
Thyrotropin-releasing hormone is a tripeptide secreted by the arcuate nucleus of hypothalamus, and is stored in median eminence from where it is released into the hypothalamo– hypophyseal portal vessels to reach the anterior pituitary. Thyrotropin-releasing hormone acts on the basophils (thyrotrophs) in the anterior pituitary and controls the release of TSH.
Control of TRH. Secretion of TRH by the hypothalamus is controlled by:
• Nervous stimuli like emotion, stress, exposure to cold etc. and also by
• Negative feedback control exerted by plasma T3 and T4 levels on the hypothalamus (Fig.8.3-4).
B. Autoregulation of thyroid gland
The secretions of thyroid gland are regulated by food iodine contents. If there is deficiency of iodine content in the diet then the iodine-trapping mechanism of the follicular cells becomes super efficient and vice versa is also true, i.e. when there is excess of iodine content in the food then iodine trapping becomes less efficient and organification of excess amount of iodine does not occur. In this way iodine availability for thyroxine synthesis remains constant, and this phenomenon is called autoregulation of thyroid gland.
Actions of thyroid hormone
The thyroid hormones do not have any discrete target organ. They effect cellular activity of almost all the tissues of the body. T3 acts by its effect on gene expression on the target cell.
The biochemical functions attributed to thyroid hormones are summarized:
1. Effects on growth and tissue development
Thyroid hormones are important for normal body growth and development
(i) Role in normal body growth and skeletal maturation. Thyroid hormones exert their effect directly by increasing protein synthesis and enzymes, and indirectly by increasing production of growth hormone and somatomedins. Some important effects are on:
(ii) Role in tissue differentiation and maturation.
(iii) Role in development of nervous tissue. T3 seems to be necessary for proper axonal and dendritic development as well as normal myelination in the nervous system.
2. Effect on the metabolic rate in general
The thyroid hormone in general stimulates the metabolic activities and increases the basal rate of oxygen consumption and heat production in most tissues of the body except the brain, retina, gonads, lungs and spleen.
3. Effects on metabolism
(i) Effect on carbohydrate metabolism. T4and T3 lead on to an overall increase in enzymes causing:
• Increased glucose absorption from the GIT and
• Acceleration in almost all aspects of glucose metabolism, i.e. rapid uptake of glucose by the cells, enhanced glycolysis, enhanced gluconeogenesis, and increased insulin secretion and its effects on the carbohydrate metabolism.
(ii) Effect on fat metabolism. Thyroid hormones cause:
• Mobilization of fat from the adipose tissue.
• Increase in the levels of fatty acids and enhanced oxidation of free fatty acids by cells.
• Decrease in the quantity of cholesterol, phospholipids and triglycerides in plasma; plasma cholesterol level is lowered due to increased excretion in bile.
(iii) Effect on protein metabolism. In physiological amounts, the thyroid hormones function as anabolic hormones. That is, they cause an increase in RNA and protein synthesis leading to positive nitrogen balance.
In high concentrations, thyroid hormones have catabolic effect leading to negative nitrogen balance. Therefore, muscle weakness and creatininuria are characteristic features of a hyperthyroid patient.
(iv) Metabolic effects through other hormones. T4 and T3 potentiate the respective stimulatory effects of epinephrine, norepinephrine, glucagon, cortisol and growth hormone on gluconeogenesis, lipolysis, ketogenesis and proteolysis of the labile protein pool.
(v) Effect on vitamin metabolism. Thyroid hormones increase the quantity of enzymes. Vitamins are the essential parts of some of the enzymes and coenzymes. Therefore, thyroid hormones cause an increased need for vitamins leading to relative vitamin deficiency in hyperthyroidism.
(vi) Effect on water and electrolyte balance. Thyroid hormones play role in the regulation of water and electrolyte balance.
4. Respiratory effects
Thyroid hormones could not stimulate O2 utilization for long without also enhancing oxygen supply, which is accomplished by following effects of T4 and T3.
(i) Increase in the resting respiratory rate, minute ventilation and ventilatory responses to hypercapnia and hypoxia. These actions maintain a normal pO2 when O2 utilization is increased and a normal pCO2 when CO2 production is increased.
(ii) Increase in oxygen-carrying capacity of blood by slightly increasing the red blood cell mass.
5. Cardiovascular effects
Thyroid hormone increases cardiac output, ensuring sufficient oxygen delivery in the tissues. In general, the thyroid hormones have the following effects on the cardiovascular system:
(iii) Tachycardia, i.e. increased heart rate (at rest, even during sleep) is an important physical sign, which is used by clinicians in assessing the function of thyroid gland.
(iv) Force of cardiac contraction is increased by moderate increase in the thyroid hormone. The cardiac inotropic effects are via adrenergic stimulation. Myocardial calcium uptake and adenylyl cyclase activity are increased and enhance the contractile force.
(v) Cardiac output is increased as a result of increased blood volume, increased heart rate and increased force of contraction.
(vi) Effect on blood pressure. Systolic blood pressure is increased due to increased strength and rate of heart beat; whereas, diastolic blood pressure is decreased due to peripheral vasodilatation. This results into an increased pulse pressure, but the mean arterial pressure is usually unchanged.
6. Effects on nervous system
(a) Effect on development of nervous system
Thyroid hormones play an essential role in the development of nervous system. Critical period for the development of nervous system is up to 1 year of life.
(b) Effect on functioning of nervous tissue in adults
(i) On central nervous system. T4 enhances wakefulness, alertness, responsiveness to various stimuli, auditory sense, awareness of hunger, memory and learning capacity. Normal emotional tone also depends on proper thyroid hormone availability.
(ii) Effects of thyroid hormones on peripheral nervous system. Thyroid hormone increases the speed and amplitude of peripheral nerve reflexes.
9. Effects on reproductive system
In both women and men, thyroid hormone plays an important permissive role in the regulation of reproductive functions.
In males, lack of thyroid hormones causes complete loss of libido and excess of hormones causes impotence.
10. Effects on other endocrine glands
Thyroid hormones also have significant effects on other parts of the endocrine system.
• Pituitary production of growth hormone is increased, whereas that of prolactin is decreased.
• Adrenocortical secretion of cortisol as well as metabolic clearance of this hormone is stimulated but plasma-free cortisol levels remain normal.
• Oestrogens and androgens ratio, in males, is increased. It accounts for occurrence of breast engorgement in males in hyperthyroidism.
• Parathyroid hormone and 1,25-(OH)2-vitamin D are decreased as a compensatory consequence of the effects of thyroid hormone on bone resorption.
Applied aspects of thyroid hormones
Abnormalities of thyroid gland
Hyperthyroidism
Hyperthyroidism refers to increased secretion of thyroid hormones. Its common causes are:
Graves’ disease
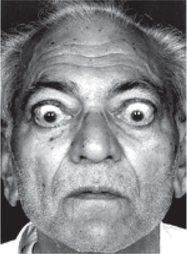
Graves’ disease or toxic goitre or thyrotoxicosis is the most common cause of hyperthyroidism (Fig.8.3-5).
It is an autoimmune disease characterized by the development of thyroid-stimulating antibodies (TSAb) against the TSH receptors, also called long-acting thyroid stimulator. These antibodies bind to TSH receptors and mimic TSH action on thyroid growth and hormone synthesis. The entire thyroid gland undergoes hyperplasia as a result of autoimmune stimulation.
• Marked increase in basal metabolic rate (BMR),
• Weight loss, despite an increased intake of food and
• Increased heat production causes discomfort, excessive sweating and a greater intake of water in warm environments.
2. Goitre. Goitre refers to the swelling of thyroid gland. Graves’ disease is characterized by diffuse goitre, while single or more nodules indicate toxic nodular goitre.
3. Cardiovascular features are:
4. Neuromuscular features are nervousness, irritability, restlessness, psychosis, tremors of hand, muscular weakness and exaggerated tendon reflexes.
5. Gastrointestinal features are diarrhoea or steatorrhoea and vomiting.
6. Dermatological features are perspiration (increased sweating or hyperhidrosis), loss of hair and redness of palm.
7. Reproductive features are impotence in males and oligomenorrhoea or amenorrhoea, abortions and infertility in females.
8. Ophthalmological signs are lid retraction producing staring look, and lid lag and exophthalmos, i.e. bulging out of eyeball.
Hypothyroidism
Hypothyroidism is a clinical syndrome caused by low levels of circulating thyroid hormones.
Depending upon the aetiology, hypothyroidism can be primary or secondary.
Primary hypothyroidism is caused by the disorder of thyroid gland.
Secondary hypothyroidism is caused by diseases of anterior pituitary and hypothalamus.
Clinical features depend upon the age at which deficiency manifests, duration and severity of the disease. Two different clinical entities are:
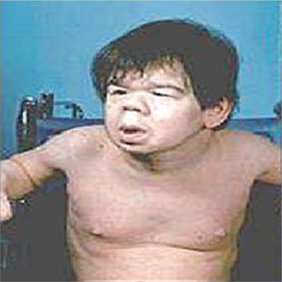
1. Infantile hypothyroidism (cretinism). It occurs when thyroid deficiency occurs during first year of life and is characterized by (Fig.8.3-6) mental retardation, marked retardation of growth, delayed milestones of development, pot belly, protruding tongue, flat nose, dry skin and sparse hairs.
Treatment should be prompt otherwise mental deficiency will persist.
2. Adult hypothyroidism is also called myxoedema because of characteristic infiltration of skin by myxoedematous tissue (Fig.8.3-7). Symptoms and signs include:
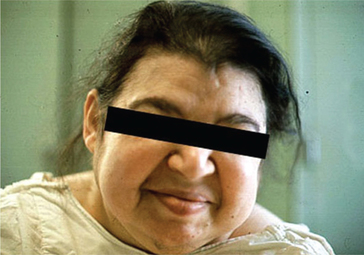
Fig. 8.3-7 Photograph of a patient with myxoedema showing puffy face, thick lips and periorbital oedema.
• General features: Tiredness and weight gain without an appreciable increase in caloric intake (due to lower than normal metabolic rate). Decreased heat production, lower body temperature, causes intolerance to cold and decreased sweating.
• Cardiovascular features. Adrenergic activity is decreased causing bradycardia. Other features include hypertension, pericardial effusion and precipitation of angina.
• Neuromuscular features. Movement, speech and thought are all slowed and lethargy, sleepiness, delayed relaxation of ankle jerks, aches and pain are common. Pressure palsy of peripheral nerves (e.g. carpal tunnel syndrome) due to entrapment in excess ground substance.
• Dermatological features. Dry thick skin (toad skin), sparse hair, non-pitting oedema due to infiltration by myxoedematous tissue (myxoedema).
• Reproductive features. Menorrhagia and infertility (common) galactorrhoea and impotence (less common).
• Gastrointestinal features. Constipation (common) and adynamic ileus (less common).
Goitre
Goitre refers to any abnormal increase in the size of the thyroid gland. The term goitre does not denote the functional status of thyroid gland because it may be associated with:
• Euthyroid, i.e. normal thyroid hormone level,
• Hypothyroidism, i.e. low thyroid hormone level, and
• Hyperthyroidism, i.e. high thyroid hormone levels; as seen in Graves’ disease and toxic nodular goitre.
Iodine deficiency goitre or endemic goitre occurs when the daily dietary intake of iodine falls below 10 μg (normal requirement 100−200 μg/day). It decreases the synthesis and secretion of thyroid hormone leading to increased TSH levels and proliferation of thyroid gland tissue (goitre). It is mostly found in the geographic regions away from the sea coast where the water and soil are low in iodine content. Consumption of iodized salt is advocated to overcome the problem of endemic goitre. In certain cases, administration of thyroid hormone is also indicated.
 Introduction
Introduction