Malignant Tumors of Muscle Tissue Origin
Leiomyosarcoma
The leiomyosarcoma is a malignant tumor of smooth muscle origin. It is very rare in the oral cavity and whether it develops through malignant transformation of a leiomyoma or de novo is not known. It probably arises at these sites from smooth muscle cells, especially those found in blood vessel walls, and from undifferentiated mesenchymal cells.
Clinical Features
Leiomyosarcoma typically presents as painful, lobulated, fixed mass of the submucosal tissues in a middle-aged or older individual. It is exceedingly rare in children. Lesions are usually less than 2 cm in diameter at diagnosis and are slow-growing, but secondary ulceration of the mucosal surface has been reported. The cheek and floor of mouth were the most common sites. No gender predilection was apparent in these cases.
Histologic Features
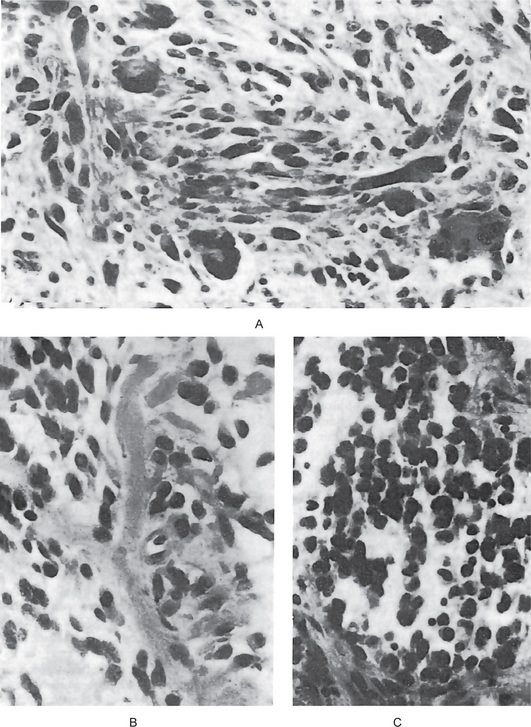
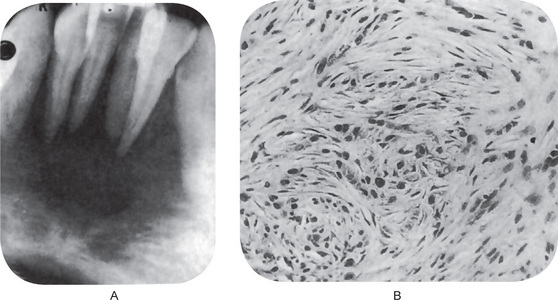
Leiomyosarcoma is composed of fascicles of interlacing spindle-shaped cells with abundant eosinophilic cytoplasm and moderately large, centrally located, cigar-shaped or blunt-ended nuclei, often with mild atypia. Cellularity and cellular differentiation can vary considerably between tumors and between different areas of the same tumor. The well-differentiated lesion shows the spindled cells streaming or interweaving in fascicles in a fashion similar to that seen in leiomyoma. Nuclear palisading may be seen in several areas of the tumor, ischemic areas show stromal fibrosis and hyalinization (Fig. 2-97).
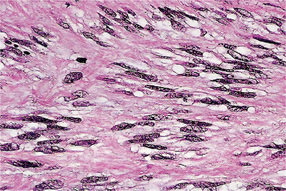
Figure 2-97 Leiomyosarcoma of oral cavity. Note the prominent cytoplasmic vacuoles indenting to the nuclear poles (Courtesy of Dr Juan Rosai).
The epithelioid variant, called malignant leiomyoblastoma or epithelioid leiomyosarcoma, is most prevalent in the gastrointestinal and genitourinary tracts and has rarely been reported in oral or pharyngeal locations.
Rhabdomyosarcoma
The rhabdomyosarcoma, the malignant tumor of striated muscle, is a relatively uncommon tumor in the oral cavity. According to Rubin, it is derived from primitive mesenchyme that retained capacity for skeletal muscle differentiation. The first published example of rhabdomyosarcoma was probably a tongue lesion reported in 1854.
There are four separate types of rhabdomyosarcoma based on histologic appearance, and many of the clinical features are quite characteristic of certain of these. The four forms of rhabdomyosarcoma are:
The embryonal form of rhabdomyosarcoma is recognized as having a marked predilection for occurrence in the head and neck area.
Clinical Features
This is the most common subtype observed in children, accounting for 60–70% of all rhabdomyosarcoma cases in this age group. These tumors can occur at any site, but they are most commonly observed either in the genitourinary region or the head and neck region. They occur more commonly in the head and neck area than any of the other forms. Stobbe and Dargeon have pointed out that this neoplasm arises chiefly from the orbital, facial, and cervical musculature. Of 15 cases reported by Stobbe and Dargeon, the sites of occurrence included the orbit and inner canthus, the tonsil, soft palate, mastoid, internal ear and parotid, zygoma, and temporal and cervical regions. The average age of this group of patients was six years, ranging from 16 months to 16 years, with no gender predilection. In another review of 37 cases of this tumor by Moore and Grossi, the cheek, mandible and gingiva were found to be additional sites of occurrence. The youngest patient in this series was seven weeks, although there is one case on record of an infant born with an embryonal rhabdomyosarcoma of the floor of the mouth. In a series of 48 patients with embryonal rhabdomyosarcoma reported by Lawrence and his associates, the ages at diagnosis ranged from 16 days to 14 years, with a mean of five years. Finally, a series of 11 cases of embryonal rhabdomyosarcoma of the oral soft tissues have been reported by O’Day and his associates. The age of these patients ranged between 2 and 41 years of age, with a mean of 16 years. Of these oral tumors, four were in the soft palate, three in the cheek and one each in the upper and lower labial folds, lower buccal fold and lateral aspect of the tongue (Fig. 2-98).
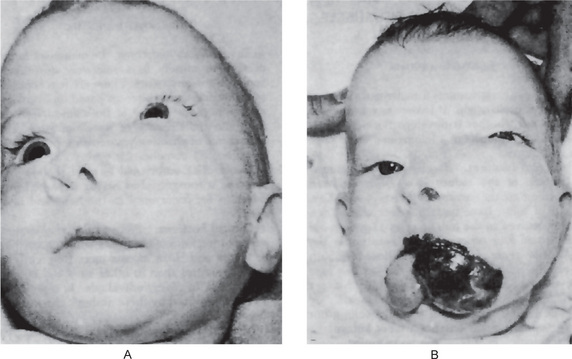
Figure 2-98 Embryonal rhabdomyosarcoma. The rapidity of growth of this type of neoplasm can be judged by the fact that the two illustrations of this lesion, originating in the buccal mucosa, were taken only 14 days apart.
Botryoid rhabdomyosarcoma (sarcoma botryoides) has been long recognized as a malignant tumor of the vagina, prostate, and base of the bladder in young children. Today, it is generally accepted as a variant of embryonal rhabdomyosarcoma and has been reported also involving the maxillary sinus, nasopharynx, common bile duct and middle ear. This tumor accounts for 10% of all rhabdomyosarcoma cases. It was formerly separated out as an entity because of its unusual clinical growth pattern.
This subtype making up about 20% of all rhabdomyosarcomas in an analysis of 110 cases by Enzinger and Shiraki, is reported to occur much earlier in life, generally between 10 and 20 years of age with a median of 15 years. However, the range in this group was five months to 58 years. While the majority of cases of this type also occurred in the extremities, approximately 18% were found in the head and neck region.
Pleomorphic rhabdomyosarcoma (least common of all rhabdomyosarcoma) is a form of the tumor which, according to Patton and Horn, occurs more frequently in the extremities than in other sites and is generally seen in older individuals. In a series of 19 cases reported by these authors, the average age was 53 years.
The chief presenting complaint of the patient with rhabdomyosarcoma, generally irrespective of the histologic type, is usually swelling, but pain may be present if there is nerve involvement. Depending upon the site of the lesion, the following phenomena may be recognized: divergence of an eye, abnormal phonation, dysphagia, cough, aural discharge or deviation of the jaw. The lesions are occasionally ulcerated and may invade underlying bone and develop distant metastases. The most common site of presentation is head and neck region (35 %).
Histologic Features
Embryonal rhabdomyosarcoma has been described by O’Day and his coworkers as exhibiting a mixture of four cell types:
• Eosinophilic spindle cells, usually arranged in interlacing fascicles.
• Round eosinophilic cells, large and intermediate in size, with a small nucleus and a granular eosinophilic cytoplasm, generally interspersed among other cell types.
• Broad elongated eosinophilic cells, occasionally with cross-striations.
• Small round and spindle cells with dark-staining nuclei and little cytoplasm (Fig. 2-99 C).
The more well-differentiated tumors demonstrate elongated, strap-shaped or tadpole-shaped rhabdomyoblasts. The background stroma consists of moderately loose to dense fibrous tissue and may be quite scant, myxoid zones are commonly seen in the stroma.
Pleomorphic rhabdomyosarcoma is composed chiefly of spindle cells in a haphazard arrangement. These cells are generally large and show considerable variation in appearance. The nuclei are ovoid or elongated with packed chromatin. A characteristic feature of this form of tumor is the large bizarre cells, the nuclei situated often in an expanded end of the cell, the ‘racquet’ cell. ‘Strap’ and ‘ribbon’ cells typically show processes of long streaming cytoplasm. Mitoses, particularly atypical, are common. The cytoplasm is eosinophilic, and intracytoplasmic longitudinal fibrils as well as transverse cross-striations may be seen. Cytoplasmic vacuoles are also present as a result of large amounts of glycogen in the cell (Fig. 2-99 A). This tumor is often so undifferentiated that the identification of the cell of origin is difficult or impossible. Positive immunostains for desmin and myoglobin are very helpful in such cases.
Alveolar rhabdomyosarcoma is comprised of relatively small, poorly differentiated round and oval cells aggregated into irregular clusters or nests separated by fibrous septa. Degenerated cells in the center of the clusters show lack of cohesiveness, while the peripheral cells adhere in a single layer to the septal walls. Multinucleated giant cells may be seen and mitotic figures are common and sometimes bizarre. It is differentiated from the alveolar soft part sarcoma by its less regular tissue pattern and more pleomorphic cells.
An occasional variant, referred to as the botryoid type, demonstrates a diffuse myxoid or mucoid matrix with sparsely scattered primitive mesenchymal cells. The characteristic feature of this type is a peripheral zone of increased cellularity, sometimes known as the ‘cambium layer’.
Regardless of the histologic subtype, special stains are often quite useful for differentiating rhabdomyosarcoma from other neoplasms. The trichrome stain colors rhabdomyoblasts bright red while myofilaments and cross-striations are stained by PTAH (deep purple color). Myxoid stroma may be positive for hyaluronidase with acid mucopolysaccharide staining, although many other tumors also have positive stroma with these stains.
Alveolar Soft-Part Sarcoma (Malignant granular cell myoblastoma)
The alveolar soft-part sarcoma is a tumor of uncertain histogenesis originally described under this name by Chris-topherson and his coworkers in 1952. It is a rare tumor, thought by some investigators to be of striated muscle origin, although it differs in some respects from the alveolar type of rhabdomyosarcoma. Other workers believe that it may be of neural origin and a variant of malignant granular cell tumor or a malignant nonchromaffin paraganglioma. Studies showed alveolar soft-part sarcomas are myogenic by immunophenotyping in a number of cases. In recent years, pathologists have shown this tumor to be a variant of a rhabdomyosarcoma, a sarcoma of skeletal muscle. Chromosome rearrangement at 17q25 and Xp11.2 in alveolar soft-part sarcoma was demonstrated.
Clinical Features
The initial report of Christopherson indicated that this is predominantly a tumor of females, occurring usually in the teens or early 20s. A study of 53 cases by Lieberman and his associates has confirmed these findings. Of the 53 patients, 34 were female and 19 were male. The average age of their male patients was 30 years but that of their female patients, only 20 years. Occasional cases in older adults are reported.
Approximately one in four lesions occur in the head and neck region, usually the oral cavity, pharynx and orbit. However, the greatest predilection is for the muscles of the extremities, although lesions in the tongue and floor of the mouth have been reported by Caldwell and his associates. Only one of the 53 cases reported by Lieberman and his associates was intraoral and this occurred in the tongue. Font and his associates have reported 17 cases involving the orbit. The lesions are usually slow-growing, well-circumscribed masses with no distinguishing gross features.
Histologic Features
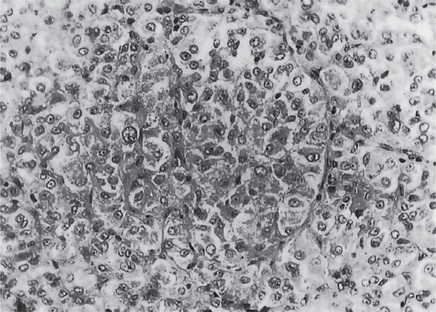
This tumor is composed of large cells with a finely granular cytoplasm that is not as eosinophilic as the cell of the rhabdomyosarcoma. The lesional cell is large and polygonal with a distinct cell border, a vesicular nucleus, and dense, abundant granular, eosinophilic or vacuolated cytoplasm. There is minimal variation in size and shape between cells and mitotic activity is sparse. These cells have a uniform pseudoalveolar or organoid pattern, arranged in relation to numerous delicate endothelium-lined vascular channels and septa (Fig. 2-100). The pattern is reminiscent of that seen in the nonchromaffin paraganglioma.
Vascular invasion is a frequent finding. Reticulin stains will enhance the organoid arrangement of the tumor cells.
Marshall and Horn reported that the alveolar soft-part sarcoma consistently showed a strongly positive periodic acid-Schiff (PAS) reaction before and after treatment with diastase, similar to the benign granular cell myoblastoma, but in contrast to the alveolar rhabdomyosarcoma in which the PAS-positive material is removed by digestion with diastase. This PAS-positive material in the cytoplasm of the alveolar soft-part sarcoma, described by Font and his coworkers as a highly characteristic and virtually pathognomonic finding of this lesion, represents crystalline structures composed of a protein-carbohydrate complex. They appear to form by coalescence of peculiar membrane-bound granules that exhibit acid-phosphatase activity.
Treatment and Prognosis
Radical surgical excision is the accepted treatment for this lesion because of the high frequency of recurrence, metastases and death of patients. Marshall and Horn reported the recurrence or metastatic rate as 70%, five-year survival being uncommon. Lieberman and his coworkers stated that they knew of no lifetime cures.
Benign Tumors of Nerve Tissue Origin
Traumatic Neuroma (Amputation neuroma)
Traumatic (post-traumatic) or amputation neuroma is not a true neoplasm, but rather an exuberant attempt at repair of a damaged nerve trunk, i.e. a hyperplasia of nerve fibers and their supporting tissues. It most frequently follows accidental or purposeful sectioning of a nerve and may be incidental to difficult extraction. Cases also have occurred after an accident in which the lip or tongue was deeply lacerated by the teeth and nerve fibers were inadvertently severed.
Degeneration of the distal portion of the nerve after severance of the nerve fibers begins with swelling, fragmentation and disintegration of the axis cylinders and myelin sheaths. Macrophages serve to remove this tissue debris. The neuri-lemmal sheaths or tubes shrink until the distal degenerated fibers consist only of strands of connective tissue and the neurilemma. The nerve does not disappear completely.
Repair of a damaged nerve begins with proliferation of the axis cylinders, the cells of the neurilemmal sheaths and the endoneurium. Regeneration is facilitated by the persistence of the neurilemmal tubes, since new fibers proliferate through them and Schwann cells multiply around them.
Reinnervation usually occurs; unless the proliferating proximal end meets some obstruction, such as scar tissue or a malaligned bone, in which case the nerve continues to proliferate into an unorganized bulbous or nodular mass of nerve fibers and Schwann cells in varying proportions. This constitutes a traumatic neuroma. The pathogenesis of this lesion has been reviewed by Swanson.
Clinical Features
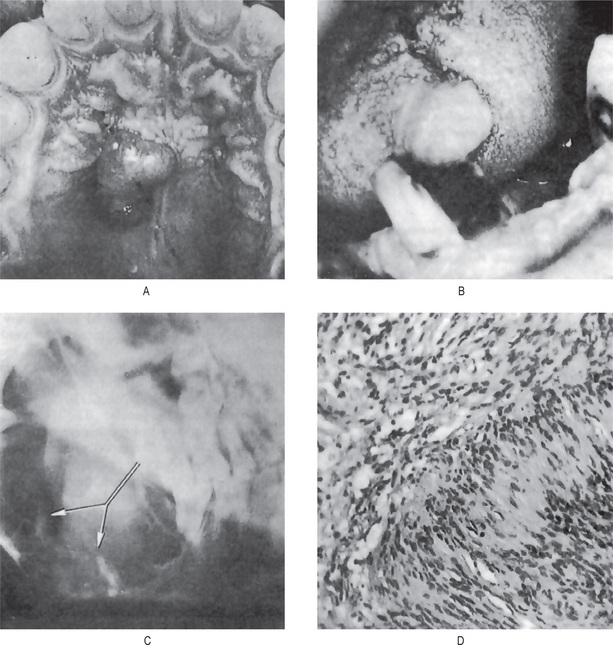
The oral traumatic neuroma usually appears as a small nodule or swelling of the mucosa, typically near the mental foramen, on the alveolar ridge in edentulous areas or on the lips or tongue (Fig. 2-101). A central lesion within the substance of the bone associated with a nerve trunk may also occur. This is a slowly growing lesion and seldom reaches a size greater than a centimeter in diameter (Fig. 2-101 A, B).
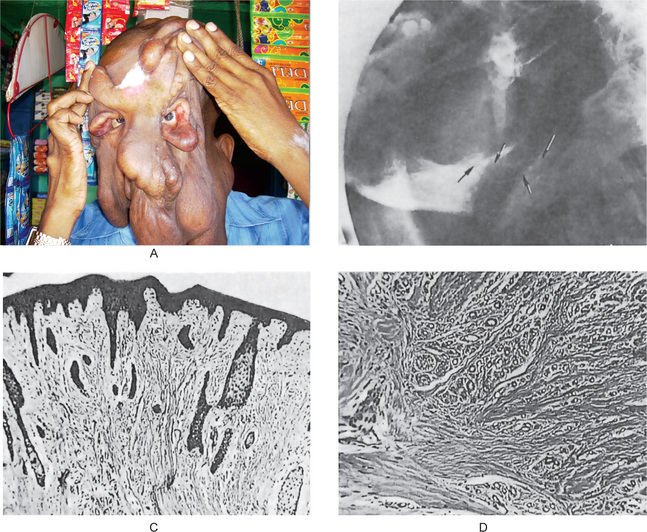
Figure 2-101 Traumatic neuroma. (A) The patient presented with several pedulous tumour masses of the facial skin. The patient had sustained a fracture of the mandible many years previously that had resulted in the abnormal course of the mandibular nerve seen in the lateral jaw radiograph (B). This nerve was subsequently sectioned for ‘relief of pain’. The photomicrographs (C, D) reveal the hyperplasia of nerve fibers in a fibrous stroma. (A, Courtesy of Dr K Murugesan, Maxillofacial Surgeon, Chennai)
Digital pressure may cause considerable pain locally, and in some instances along the course of the nerve involved. Reflex neuralgia with distant pain associated with the face, eyes and head has been recorded. Traumatic neuroma has been discussed in detail by Robinson and Slavkin and by Sist and Greene, who also reported 31 cases.
The palisaded, encapsulated neuroma is not a form of traumatic neuroma but may represent a primary hyperplasia of nerve fibers, the axons and their sheath cells. An alternative theory is that it represents a benign neoplasm. The lesion was first described by Reed and his coworkers as a clinically distinctive, solitary, benign cutaenous tumor occurring with equal frequency in both sexes and limited in its anatomic distribution (with rare exceptions) to areas bordering mucocutaneous junctions predominantly on the face. A case on the lower lip has been reported by Tomich and Moll.
Histologic Features
The histologic appearance of the neuroma is characteristic and shows a mass of irregular and often interlacing neurofibrils and Schwann cells situated in a connective tissue stroma scant or predominant. Much of this connective tissue is probably derived from the perineurium. The proliferating nerve fibers themselves may occur either in small discrete bundles or spread diffusely throughout the tissue (Fig. 2-101 C, D). Care must be taken to differentiate this lesion from both the neurofibroma and neurilemmoma. The histologic, histochemical and ultrastructural aspects of walle-rian degeneration of nerves have been described by Fisher and Turano and by Sist and Greene.
Treatment and Prognosis
Because of the progressive nature of this lesion and the associated pain, it is best treated by surgical excision along with a small proximal portion of the involved nerve. Recurrence is not common even though the sectioning of the nerve during treatment is similar to the injury that preceded the development of the tumor.
Multiple Endocrine Neoplasia Syndrome (MEN syndrome [MEN III, MEN IIb])
The multiple endocrine neoplasia (MEN) syndromes are characterized by tumors of neuroendocrine origin. The type, MEN III syndrome, also called MEN IIb syndrome or multiple mucosal neuroma syndrome, was initially described by Wagenmann in 1922.
The disease is associated with adrenal pheoc hromocytoma, medullary thyroid carcinoma, diffuse alimentary tract ganglio-neuromatosis, and multiple small submucosal neuroma nodules of the upper aerodigestive tract. The disease is inherited as an autosomal dominant trait, although many cases appear to be spontaneous mutations.
The affected individual has a tall, lanky, marfanoid body type, with a narrow face and perhaps with muscle wasting. The adrenal and thyroid tumors typically do not present until after puberty while the oral mucosal neuromas usually develop during the first decade of life. Mucosal neuromas are extremely rare, perhaps unheard of, outside of the MEN III syndrome. MEN syndromes are caused by mutations of the RET protooncogene, an important regulator of neural crest development and the receptor of glial derived neurotrophic factor (GDNF).
Clinical Features
The oral mucosal neuroma of this disease presents as a 2–7 mm yellowish-white, sessile, painless nodule of the lips, anterior tongue and buccal commissures. Usually there are two to eight (or more) neuromas, with deeper lesions having normal coloration. There may be enough neuromas in the body of the lips to produce enlargement and a ‘bumpy lip’ appearance. Similar nodules may be seen on the eyelids, sometimes producing eversion of the lid, and on the sclera. Facial skin, especially around the nose, may also be involved.
Abnormal laboratory values are part of this syndrome. When a medullary thyroid carcinoma is present, serum and urinary calcitonin levels are elevated. When a pheochromocytoma is present, there often is an increase in the serum levels of vanillylmandelic acid (VMA) and altered epinephrine/ norepinephrine ratios.
Histologic Features
The mucosal neuroma is comprised of a partially encapsulated aggregation or proliferation of nerves, often with thickened perineurium, intertwined with one another in a plexiform pattern. This tortuous pattern of nerves is seen within a background of loose endoneurium-like fibrous stroma. Individual nerves flow in fascicles of two or three fibers and are histologically normal except for occasional hyperplasias and bulbous expansions.
Inflammatory cells are not seen in the stroma and dysplasia is not present in the neural tissues. There may be close microscopic similarity with traumatic neuroma, but the streaming fascicles of mucosal neuroma are usually more uniform and the intertwining nerves of the traumatic neuroma lack the thick perineurium of the mucosal neuroma.
Treatment and Prognosis
The mucosal neuromas of this syndrome are asymptomatic and self-limiting, and present no problem requiring treatment. They may, however, be surgically removed for esthetic purposes or if they are being constantly traumatized. It is strongly suggested that other family members may also be evaluated for MEN III.
Neurofibroma (Neurofibromatosis, von Recklinghausen’s disease of the skin, fibroma molluscum)
The neurofibroma is a benign tumor of nerve tissue origin, derived from the cells that constitute the nerve sheath. Neurofibroma is seen either as a solitary lesion or as part of the generalized syndrome of neurofibromatosis (von Recklinghausen disease of the skin). The solitary form does not differ from the disseminated form or the multiple form of the disease except that systemic and hereditary factors present in the disseminated form are absent in the solitary type.
The cell of origin for neurofibroma has not been definitively identified, but is generally believed to arise from the perineural fibroblasts which are neuroectodermal in origin. The cause of solitary neurofibroma is unknown. However, neurofibromatosis is inherited as an autosomal dominant trait with a high degree of penetrance but variable expressivity. As many as 50% of cases are reported to be the result of spontaneous mutation. Recently, two subsets have been defined: one is associated with the neurofibromatosis type 1 (NF1), gene mutations of the tumor suppressor genes coding for neurofibromin on chromosome 17q11.2, and the other is associated with the neurofibromatosis type 2 (NF2), gene mutations of the tumor suppressor genes coding for schwannomin on chromosome 22q12.1.
Clinical Features
Neurofibromatosis, though not an exceedingly common disease, is by no means a clinical rarity. It has been reported in all races and does not exhibit a significant consistent sex predilection for occurrence. The hereditary nature of the disease has been recognized for many years and it is now known that it is inherited as a simple autosomal domi nant trait with variable penetrance and a 50% mutation rate. It occurs with a frequency of one case in approximately 3,000 births in the general population. The birth incidence of NF1 lies between 1 in 2,500–3,300 and its prevalence in the population is 1 in 5,000. The birth incidence of NF2 lies between 1 in 33,000–40,000 with a prevalence within the population of 1 in 210,000.
The great clinical significance of neurofibromatosis, aside from the cosmetic problem, lies in the fact that in some patients malignant transformation subsequently occurs in one or more of their lesions. The incidence of sarcomatous transformation in neurofibromatosis has been placed at approximately 15% of all cases by Hosoi and by Preston and his coworkers. The type of sarcoma has been variously described as fibrosarcoma, spindle cell sarcoma and neurogenic sarcoma. However, solitary neurofibromas seldom undergo malignant transformation. Preston and his coinvestigators have reported other associated pathologic lesions, including osseous changes, mental disorders, congenital defects and ocular disease occurring in approximately 20% of their patients.
Oral Manifestations
Oral lesions occur in patients with von Recklinghausen’s disease of skin, but the percentage of patients presenting such manifestations is not definitely known. In the series reported by Preston and his associates, intraoral neurofibromas were present in 7% of the patients. In contrast, Cherrick and Eversole reported that 20% of a series of 19 cases of intraoral neurofibroma occurred in association with von Recklinghausen’s disease.
Discrete, nonulcerated nodules, which tend to be of the same color as the normal mucosa, may be noted, usually occurring on the buccal mucosa, palate, alveolar ridge, vestibule and tongue (Fig. 2-102 A, B). Other cases exhibit diffuse masses of tissue which may involve the palate, buccal tissues and alveolar ridges and are composed of the same type of tissue as that seen in the isolated lesions. In addition, macroglossia due to diffuse involvement of the tongue is well recognized and has been reviewed by Ayres and his associates. Chen and Miller have also reported a series of 55 cases of benign nerve tumors of the oral cavity and noted the preponderance of neurofibroma over the neurilemmoma.
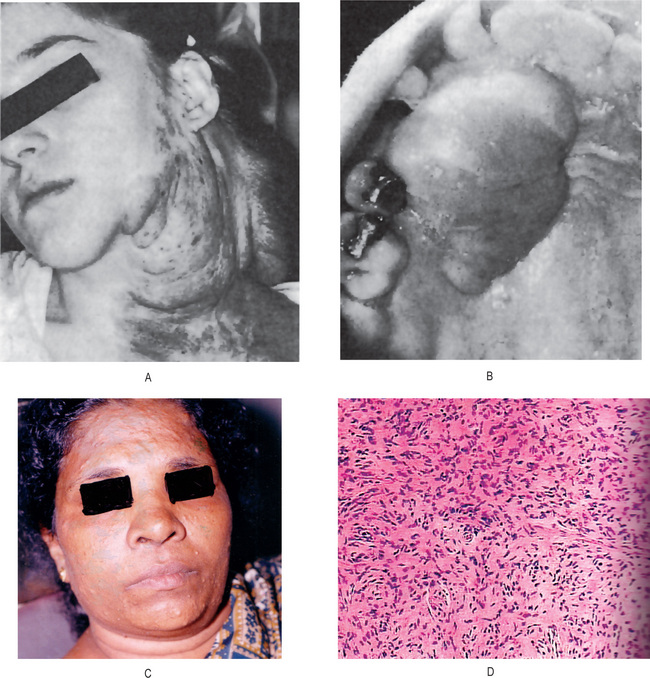
Figure 2-102 Neurofibroma. (A) The patient presented several pendulous, pigmented tumor masses of the skin. (B) Neurofibroma of the palate in a patient without apparent neurofibromatosis. (C) Multiple neurofibromas of the face. (D) Neurofibromatosis showing spindled, wavy nuclei in fascicular form.
Occasional cases of neurofibroma located centrally within the jaw are seen. These are generally in the mandible, associated with the mandibular nerve, and radiographically show a fusiform enlargement of the mandibular canal. Involvement of the trigeminal nerve may cause facial pain or paresthesia. Ellis and his associates have also discussed central nerve sheath tumors of the jaws and found that very few of these reported were associated with multiple neurofibromatosis.
Histologic Features
The neurofibroma exhibits considerable variation in histologic structure but is generally composed of a proliferation of delicate spindle cells with thin, wavy nuclei intermingled with neurites in an irregular pattern as well as delicate, intertwining connective tissue fibrils. Cellular and myxoid pat-terns predominate; organoid features are not present. Melanocytes may sometimes be found in the tumor and mast cells are common. The lesions may or may not be well circumscribed.
In plexiform neurofibroma, the pattern may be that of distorted masses of myxomatous peripheral nerve tissue still within the perineural sheath are scattered within a collagenrich matrix (Fig. 2-103). This histologic picture is considered to be virtually diagnostic of neurofibromatosis, even in the absence of other manifestations. There are reports of the existence of solitary plexiform neurofibromas unassociated with any established syndromes, occurring in the oral cavity (tumors on the buccal mucosa and gingiva) (Alatli C et al, 1996).
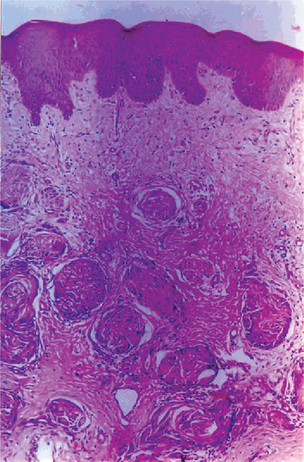
Figure 2-103 Plexiform neurofibroma of the gingiva. Abundant nerve tissue in fascicles and collagenous ibrous stroma in ordered arrangement.
The lesional cells are uniformly positive for S100 protein, signifying that they originate from neural crest-derived tissue. Antibodies to epithelial membrane antigen, CD57, and collagen IV are of secondary value and are used only when histologic differentiation with other neural tumors is difficult.
Treatment
Solitary oral neurofibromas are usually treated by surgical excision, depending on the extent and the site. Surgical removal may result in recurrence, and multiple recurrences have been associated with malignant transformation (5–15%). However, for neurofibromas associated with neurofibromatosis, surgical removal is attempted only for functional or cosmetic reasons. Genetic counseling and evaluation of other family members should be performed for those suspected to be affected by a syndrome.
Neurolemmoma (Neurilemmoma, perineural fibroblastoma, schwannoma, neurinoma, lemmoma)
The neurolemmoma is a rather common tumor accepted by most investigators today to be derived from Schwann cells. Neurites are not a component of the tumor as in the neurofibroma but may be found on the surface of the tumor. Tissue culture studies by Murray and Stout, who cultivated this tumor in vitro, lend credence to the idea of the Schwann cells as the source of origin.
Clinical Features
Available clinical evidence indicates that the neurolemmoma is a slowly growing lesion and is usually of long duration at the time of presentation by the patient. An occasional tumor does exhibit a relatively rapid course, however, the lesion does occur with some frequency in patients with neurofibromatosis. It may arise at any age, cases having been reported even during the first year of life as well as in elderly patients. There is no gender predilection.
Despite the fact that these tumors originate from nerve tissue, they are usually painless unless they are causing pressure on adjacent nerves rather than on the nerve of origin. The presenting symptom of the majority of patients is only the presence of a tumor mass.
Oral Manifestations
The head and neck are rather common regions for the development of this neoplasm, as shown by the report of Ehrlich and Martin, and a variety of oral and paraoral locations have been the site of development of the neurolemmoma. Furthermore, in a series of 303 patients with benign solitary neurolemmomas reported by Das Gupta and his associates, 136 occurred in the head and neck. Reported cases of intraoral soft-tissue neurolemmomas have been reviewed by Hatziotis and Asprides with the following frequency of occurrence: tongue, 59 cases; palate, 11 cases; floor of mouth, 10 cases; buccal mucosa, 9 cases; gingiva, 6 cases; lip, 6 cases; and vestibule, 5 cases. Other cases have involved the maxillary sinus and salivary glands, as well as the retropharyngeal, nasopharyngeal and retrotonsillar areas.
In addition, the neurolemmoma has been reported as a central lesion within bone, chiefly the mandible, apparently arising from the mandibular nerve. Eighteen such cases have been reviewed by Eversole, which Ellis and his coworkers have added additional cases.
The soft-tissue lesion is usually a single, circumscribed nodule of varying size that presents no pathognomonic features (Fig. 2-104 A, B). It may resemble any of a number of benign oral soft-tissue lesions. The central lesions in bone may produce considerable destruction of bone with expansion of the cortical plates and thus resemble a more serious lesion (Fig. 2-104 C). Pain and paresthesia may accompany these central lesions of bone.
Histologic Features
The microscopic picture of the neurolemmoma is characteristic and can seldom be confused with that of other lesions. The tumor is classically described as being composed of two types of tissue, Antoni type A and Antoni type B. Antoni type A tissue is made up of cells with elongated or spindle-shaped nuclei which are aligned to form a characteristic palisading pattern, while the intercellular fibers are arranged in parallel fashion between rows of nuclei. These fibers in some planes will give the impression of occurring in whorls or swirls. Antoni type B tissue does not exhibit this characteristic palisading, but rather a disorderly arrangement of cells and fibers with areas of what appears to be edema fluid and with the formation of microcysts. Verocay bodies, small hyaline structures, are also characteristically present in this tumor (Fig. 2-104 D). Of great importance is the fact that in nearly all instances the tumor is encapsulated.
Treatment and Prognosis
The treatment of the neuro-lemmoma is surgical excision. Like other nerve tumors, this lesion is not responsive to X-ray radiation. Since it is an en-capsulated tumor, little difficulty is usually encountered in its complete removal, but it has been suggested that in instances in which complete removal cannot be accomplished, a portion of tumor may be left without risk of recurrence. Such a pro-cedure is of poor clinical practice, however, except possibly in cases in which complete removal of the tumor would neces-sitate extensive sacrifice of structures and results in deformity. Recurrence is uncommon.
The neurolemmoma does not undergo malignant transfor-mation, as may the neurofibroma after numerous episodes of surgical tampering.
Melanotic Neuroectodermal Tumor of Infancy (Pigmented ameloblastoma, melanoameloblastoma, retinal anlage tumor, melanotic progonoma, melanotic epithelial odontoma, pigmented teratoma, atypical melanoblastoma, melanotic adamantinoma, pigmented epulis, retinal choristoma, retinoblastic teratoma, congenital melanocarcinoma)
The melanotic neuroectodermal tumor of infancy (MNTI) is a relatively uncommon osteolytic-pigmented neoplasm that primarily affects the jaws of newborn infants. Initially it was reported by Krompecker in 1918 as a congenital melanocarcinoma. Various theories suggested its origin from the odontogenic apparatus, the pigmented anlage of the retina, or the sensory neuroectodermal tissues.
In 1966, Borello and Gorlin reported a case with high urinary excretion of vanillylmandelic acid (VMA), suggesting a neural crest origin, and they proposed the term melanotic neuroectodermal tumor of infancy. Since then, numerous histochemical, immunohistochemical, electron microscopic, and tissue culture studies have supported the neural crest origin and confirmed the preferred term of melanotic neuroectodermal tumor of infancy.
Clinical Features
More than 90% of cases present within the first year of life, usually from age one to six months. The mean age of patients with MNTI is 4.3 months. Although extremely rare, cases of MNTI have been reported in adults. The sexual predilection is nearly equal, with a male-to-female ratio of 6 : 7.
More than 90% of MNTI occur in the head and neck region, with most on the anterior part of the maxillary ridge. Other common sites include the skull, the mandible, the epididymis, and the brain. Rare lesions have been reported in the shoulder, the skin, the femur, the mediastinum, and the uterus.
The majority of reported cases have been rapidly growing, nonulcerated, darkly pigmented lesions which have given a radiographic appearance of an invasive malignant neoplasm. In its typical premaxillary position, the tumor can displace or destroy the developing deciduous and permanent dentition. It can present as unilocular, or rarely as multiloculated radiolucency.
All the hematologic and blood chemistry values are within the normal range. The only finding in some but not all patients with MNTI is an increase in the urinary level of VMA, but shows no correlation with its clinical behavior. Elevated VMA has been reported in other tumors of neural crest origin, such as pheochromocytoma, ganglioneuroblastoma, retinoblastoma, and neuroblastoma.
Histologic Features
The microscopic appearance of this tumor is characteristic due to its distinct biphasic pattern. It is usually a nonencapsulated, infiltrating tumor mass of cells arranged in a pattern of alveolus-like spaces lined by cuboidal or large polygonal cells, which have pale abundant cytoplasm and nuclei with finely dispersed chromatin, many of which contain melanin pigment. Fontana stain can be used to demonstrate the melanin pigment. The central portions of the alveolar spaces contain many small round neuroblast-like cells which show little cytoplasm and exhibit a round, deeply staining nucleus. A moderately vascular fibrous stroma supports the tumor cells (Figs. 2-105, 2-106).
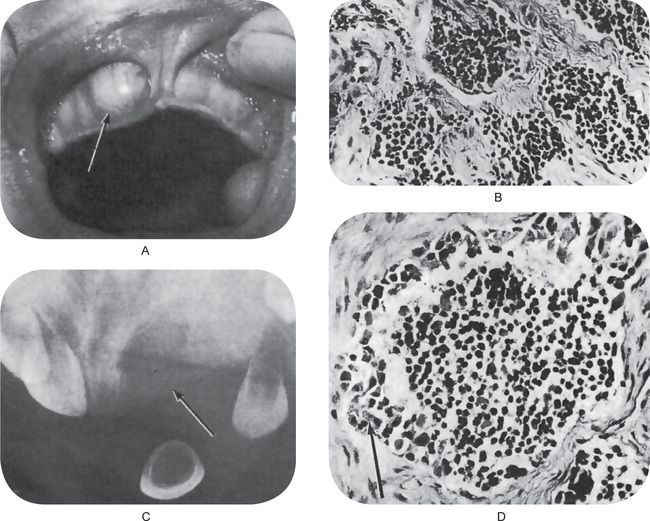
Figure 2-105 Melanotic neuroectodermal tumor of infancy. There is a rapidly growing mass present on the anterior maxilla. (A) The radiograph shows diffuse destruction of bone, suggestive of a malignant neoplasm. (B) The photomicrographs demonstrate typical alveolus-like structures lined by an irregular layer of cuboidal cells containing melanin pigment, (C) and (D) (A, Courtesy of Dr Jan L Silagi).
Treatment and Prognosis
The treatment of choice for MNTI is surgical excision, and it is usually curative. This treatment can usually be accomplished with a partial maxillectomy. Many clinicians advocate a 5 mm margin of healthy tissue to be included with the surgical specimen.
Local recurrence has been documented in 10–60% of patients. Overall, the average recurrence rate is 15–20%. Approximately 1% of tumors are malignant, with only rare tumors producing metastases.
Malignant Tumors of Nerve Tissue Origin
Malignant Peripheral Nerve Sheath Tumor (Malignant schwannoma, malignant neurilemmoma, neurogenic sarcoma, neurofibrosarcoma)
Malignant peripheral nerve sheath tumor (MPNST) is now the preferred name for the spindle cell malignancy of peripheral nerve Schwann cells. It represents approximately 10% of all soft tissue sarcomas and its diagnosis has been called ‘one of the most difficult and elusive diagnoses in soft tissue diseases’. Up to half of all cases of MPNST are diagnosed in persons with neurofibromatosis I (4% of patients with neurofibromatosis I). About one in 10 cases are associated with irradiation. The preferred site is the lower extremities, but a small percentage of these lesions occur in the head and neck region, usually associated with the large cranial nerves, especially the trigeminal nerve.
Clinical Features
These tumors commonly occur in per-sons of 20–50 years of age, but children and elderly persons may also be affected. Lesions which develop in persons with neurofibromatosis I typically occur a decade or more earlier than those in nonsyndrome patients. There is a slight predi-lection toward males in sporadic cases, but within the sub-group of patients with neurofibromatosis I, 80% of lesions are found in males. The most common head and neck area of involvement is the neck, but its occurrence in oral cavity is extremely rare. When it occurs in the oral cavity it is usually seen arising from the tongue or soft palate. The lip, gingiva, palate and buccal muosa have been sites of involvement. In the central tumors, the mandible or mandibular nerve is more frequently affected than the maxilla.
In some instances there is no complaint other than the presence of a mass, although in other cases pain and/or paresthesia, muscle weakness are present. At surgery, attachment to a major nerve trunk is not unusual, and the surgeon may notice cystic degeneration or hemorrhage within the lesional stroma.
Radiographic Features
The radiograph may reveal a diffuse radiolucency characteristic of a malignant infiltrating neoplasm (Fig. 2-107). On the other hand, the appearance may be that of only a smooth radiolucency, such as dilatation of the mandibular canal when the tumor is originating from this nerve. When this appearance prevails, the lesion may be mistaken on the radiograph for a benign one.
Histologic Features
The MPNST resembles fibrosarcoma in its overall organization, but the spindled lesional cells demonstrate the wavy or comma-shaped outline and nuclear contour of Schwann cells. The cytoplasm of lesional cells is usually indistinct and slightly eosinophilic. Cellular and nuclear pleomorphism may be quite pronounced and mitotic activity is usually high. Spindle cells are arranged in sweeping fascicles interspersed with hypocellular and myxoid regions.
Histologically MPNST may be classified into three major categories with epithelioid, mesenchymal or glandular characteristics.
The epithelioid variant demonstrates plump, rounded or ovoid epithelioid cells scattered throughout the spindled lesional cells, usually in rather small numbers and in well defined clusters. These cells may have vesicular or hyperchromatic nuclei and may bear slight resemblance to the cells of the amelanotic melanoma.
Some MPNST lesions show rhabdomyoblastic differentiation leading to the common use of the diagnostic term Triton tumor. The spindle cells are interspersed with large, plump, rounded or strap cells with eosinophilic, fibrillar cytoplasm and with cross-striations in the cytoplasm. These cells may be clustered and must be distinguished from simple entrapment of striated muscles fibers.
The glandular MPNST contains areas with usually welldifferentiated ductal structures lined by simple, stratified, cuboidal or columnar epithelial cells with occasional goblet cells. The lumen may contain PAS-positive, diastase-resistant mucus.
Rare MPNST cases contain multiple sarcomatous tissue types, especially osteosarcoma, chondrosarcoma and angiosarcoma. These have sometimes been indistinguishable from the malignant mesenchymoma of soft tissue.
MPNSTs may resemble fibrosarcoma and may require immunohistochemistry and EM evaluation to discern useful diagnostic differences. The other sarcomas most closely resembling this tumor are leiomyosarcoma and monophasic synovial sarcoma. In the oral cavity, the synovial sarcoma is so rare as to be excluded from the differential diagnosis, while the spindle cell of the leiomyosarcoma has a more distinct eosinophilic cytoplasm and a quite blunted nucleus.
Distinguishing the MPNST from a benign nerve sheath tumor is usually not difficult, but some neurofibromas may be quite cellular and may contain occasional pleomorphic cells. In such cases, presence or absence of mitotic activity is usually the determining feature.
Treatment and Prognosis
The MPNST of the oral region is treated by wide surgical excision, but local recurrences are common and hematogenous metastasis occurs in at least half of treated cases. The tumor is resistant to radiotherapy and chemotherapy, and those occurring in neurofibromatosis I behave in a more aggressive fashion than those not associated with the syndrome. Overall, the five-year survival for MPNST is 40–75%.
Olfactory Neuroblastoma (Esthesioneuroblastoma, esthesioneuroepithelioma)
The olfactory neuroblastoma is a rare tumor apparently originating from the olfactory apparatus, and therefore, found most frequently in the nasal cavity and nasopharynx. Occasional cases have been reported either originating in or invading the maxillary sinus. Such examples have been described by Church and Uhler and by Mashberg and his associates.
Clinical Features
The lesion generally appears as a painful swelling in the area of the nasal fossa. It is an invasive, destructive tumor, but only rarely metastasizes, chiefly to cervical lymph nodes and lungs. In contrast to other types of neuroblastoma, this lesion usually occurs in adults rather than children.
Histologic Features
The appearance of the tumor char-acteristically is one of densely packed masses of small darkly staining cells each with a poorly defined eosinophilic cyto-plasm and a regular round vesicular nucleus, sometimes with stippled chromatin. Rosette formation is common. This is a pseudoglandular structure lined by a single layer of nonciliated columnar cells with a basal nucleus and a cuticular border at the apex of the cell. These resemble the sustentacular and olfactory cells of the olfactory mucosa. Eosinophilic neurofibrils extend into the lumen from the cell borders. Pseudorosettes also occur. Mitotic figures are often present, but not in large numbers. The stroma has a fibrillar neuroid pattern. The difficulty in microscopic diagnosis, however, has been emphasized by Oberman and Rice.
Metastatic Tumors of Jaws (Metastatic tumors to oral cavity, metastatic tumors to the oral mucosa)
Metastatic tumors to the oral region are uncommon and may occur in the oral soft tissues or jawbones. Because of their rarity, metastatic tumors to the oral region are challenging to diagnose. Therefore, they should be considered in the differential diagnosis of inflammatory and reactive lesions that are common to oral region.
Even though the oral region is not a preferred site for metastatic deposits, approximately 30% of oral metastases are the first sign of the disease. In such cases, tumor cells bypass the filtration of the lungs, probably through the valveless vertebral venous plexus.
The pathogenesis of the metastatic process in the jawbones is not clear. In the skeleton, bones with red marrow are the preferred sites for metastatic deposits. Jawbones have little active marrow, especially in elderly persons. Remnants of hematopoietic active marrow can be detected in the posterior areas of the mandible. Since the mode of spread is usually hematogenous, tumor cells tend to be deposited in this vascular medullary tissue.
In dentulous patients, 80% of the metastatic tumors to the oral soft mucosa are found in the attached gingiva, whereas in edentulous patients, metastatic lesions are equally distributed between the tongue and the alveolar mucosa, and with much less frequency, the remaining mucosa. The rich capillary network of chronically inflamed gingiva has been suggested as a mechanism that entraps malignant cells. The proliferating capillaries have a fragmented basement membrane through which tumor cells can more easily penetrate.
Clinical Features
Most metastatic tumors to the oral region occur in patients aged 40–70 years. On average, patients with metastases to the jawbones are younger (aged 45 years) than those with metastases to the oral soft tissues (aged 54 years).
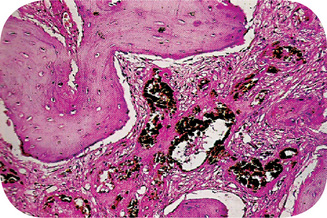
Metastatic tumors to the oral region are uncommon and account for approximately 1% of all malignant oral tumors. However, autopsies of patients with carcinoma reveal a higher frequency of metastatic deposits in the jawbones, which are not manifested clinically. Metastatic tumors to the jawbones are more frequently reported than those in the oral mucosa (Fig. 2-108).
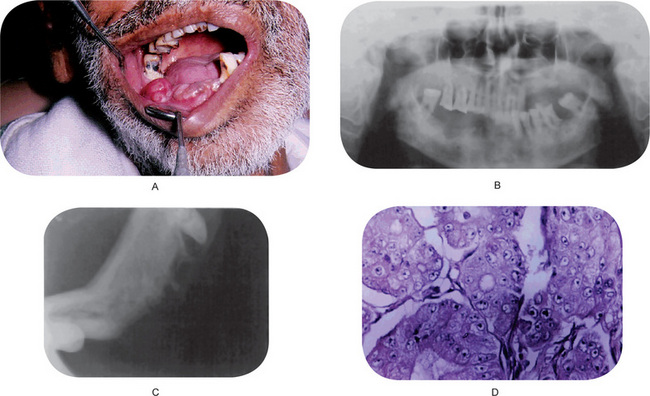
Figure 2-108 (A) Metastatic carcinoma of the lower alveolus. (B) Primary from prostate. (C) An osteolytic lesion of the mandible. (D) Photomicrograph of metastatic carcinoma of the alveolus.
The most common primary sources of metastatic tumors to the oral region are cancers in the breast, lung, kidney, bone, or colorectum. The breast is the most common primary site for tumors that metastasize to the jawbones, whereas the lung is the most common source for cancers that metastasize to the oral soft tissues (Tables 2-19, 2-20).
Table 2-19
Metastatic tumors to the oral region in men
| Metastatic site and origin | Percentage |
| Oral mucosa | |
| Lung | 35.0 |
| Kidney | 16.0 |
| Skin | 15.0 |
| Liver | 7.0 |
| Colorectum | 5.5 |
| Testis | 5.5 |
| Bone | 3.0 |
| Stomach | 3.0 |
| Rare tumors | 10.0 |
| Jawbones | |
| Lung | 22.0 |
| Prostate | 12.0 |
| Kidney | 10.0 |
| Bone | 9.0 |
| Adrenal gland* | 9.0 |
| Liver | 7.0 |
| Testis | 5.5 |
| Colorectum | 4.0 |
| Rare tumors | 21.5 |
Source: Adapted from: Abraham Hirshberg. eMedicine Speciaities. Diseases of the Oral Mucosa, 2002
*Cases of neuroblastoma, including cases from retroperitoneum and mediastinum.
Table 2-20
Metastatic tumors to the oral region in women
| Metastatic site and origin | Percentage |
| Oral mucosa | |
| Breast | 24.0 |
| Genital organs* | 17.0 |
| Lung | 12.0 |
| Kidney | 10.0 |
| Bone | 10.0 |
| Skin | 7.0 |
| Rare tumors | 20.0 |
| Jawbones | |
| Breast | 42.0 |
| Adrenal gland† | 8.5 |
| Colorectum | 8.0 |
| Kidney | 6.0 |
| Bone | 6.0 |
| Thyroid | 6.0 |
| Rare tumors | 23.5 |
*Uterus, ovaries, cervix, fallopian tubes.
†Cases of neuroblastoma, including cases from retroperitoneum and mediastinum.
In female patients, the most common primary cancers that metastasize to the oral region are those in the breasts, followed with much lower frequency by those in the female genital organs, colorectum, bone, and kidneys (Table 2-20).
In its early manifestation, gingival metastasis resembles hyperplastic or reactive lesions (e.g. pyogenic granuloma, peripheral giant cell granuloma, fibrous epulis) and in other oral soft tissue locations, especially in the tongue, the metastatic lesion manifests as a submucosal mass.
The metastatic lesions of the jaw may be completely asymptomatic. Usually, however, the patient is aware of slight discomfort or pain, followed in many cases by paresthesia or anesthesia of the lip or chin due to involvement of the mandibular nerve. The teeth in the affected area may become loose and extruded. Unfortunately, these teeth may be extracted simply because they are loose without an attempt by the dentist to learn the cause of this phenomenon. In such cases, the main symptom is a soft tissue mass extruding from a recent extraction wound and accompanied by pain. In some cases, pain described as a toothache has been the chief complaint of the patient. A definite swelling or expansion of the jaw is also an almost constant finding.
With the progression of the disease, oral metastatic lesions (especially those in soft tissues) cause progressive discomfort. Pain, bleeding, superinfection, dysphagia, interference with mastication, and disfigurement are some of the main patient complaints.
Radiographic Features
Metastatic lesions produce no pathognomonic radiographic appearance. Although most of such metastases produce osteolytic lesions and thus appear as a radiolucency on the radiograph, certain tumors produce osteoblastic lesions or lesions characterized by the production of bone. Such lesions are manifested as radiopaque or sclerotic areas and are most often associated with carcinoma of the prostate, and occasionally, of the breast and lung. The metastases in the radiograph may be relatively well demarcated and confined or they may exhibit diffuse, poorly outlined involvement of a considerable portion of bone. Because of the destruction of bone which may occur, pathologic fracture is occasionally seen. Lack of radiographic changes does not exclude the possible presence of a small metastatic deposit in the jawbone.
Histologic Features
The diagnosis is always based on histologic findings in the biopsy specimen. The clue to the diagnosis is the resemblance of the metastasis to the primary tumor. If history of a previous tumor exists, current histologic findings should compare with those of the preexisting primary malignant tumor. In some cases, histochemical staining, immunohistochemical tests, and electron microscopy should be performed to identify the primary source of the metastatic tumor.
Several primary intraoral malignancies (especially those originating from salivary glands) have histologic features similar to those of tumors in distant organs: for example, primary ductal carcinoma of a salivary gland origin versus metastatic breast carcinoma, primary intraoral clear cell carcinoma versus metastatic renal cell carcinoma, primary intraoral squamous cell carcinoma versus metastatic squamous cell carcinoma from the lung, or primary intraoral malignant melanoma versus metastatic malignant melanoma. Malignant soft tissue tumors may originate intraorally, but, because of their rarity, one should always consider a metastatic origin.
Treatment and Prognosis
Oral metastases usually indicate widespread disease. Treatment modalities are limited to palliation. In some cases, surgical treatment, sometimes combined with radiation therapy and/or chemotherapy, can improve the patient’s quality of life. Adequate surgical treatment can improve the prognosis in some cases in which the oral region is the only metastatic site.
References
Aalto, Y., Nordling, S., Kivioja, A.H. Among numerous DNA copy number changes, losses of chromosome 13 are highly recurrent in plasmacytoma. Genes Chromosomes Cancer. 1999; 25(2):104–107. [Jun].
Abbey, L.M., Page, D.G., Sawyer, D.R. The clinical and histopathologic features of a series of 464 oral squamous cell papillomas. Oral Surg. 1980; 49:419.
Abell, M.R., Hart, W.R., Olson, J.R. Tumors of the peripheral nervous system. Hum Pathol. 1970; 1:503.
Abels, J.C., Reckers, P.E., Martin, H., Rhoads, C.P. The relationship between dietary deficiency and the occurrence of papillary atrophy of the tongue and oral leukoplakia. Cancer Res. 1942; 2:381.
Abrams, B., Shear, M.A. Histological comparison of the giant cells in the central giant cell granuloma of the jaws and the gaint cell tumour of long bone. J Oral Path. 1974; 3:217.
Abrikossoff, A.J. Uber Myome, ausgehend von der quergestrieften willkurlichen. Muskulatur Virchows Arch Pathol Ant. 1926; 260:215.
del Regato, J.A., Ackerman, L.V. Cancer: Diagnosis, Treatment and Prognosis, 4. St Louis: CV Mosby, 1970.
Ackerman LV, Johnson R. Present day concepts of intraoral histopathology: Proceedings of the Second National Cancer Conference; American Cancer Society, Inc, 1954.
Ackerman, L.V. Verrucous carcinoma of the oral cavity. Surgery. 1948; 23:670.
Ahuja, S.C., Villacin, A.B., Smith, J., Bullough, P.G. Juxtacortical (parosteal) osteogenic sarcoma: histological grading and prognosis. J Bone Joint Surg. 1977; 59A:632.
Ainsworth, A.M., Folberg, R., Reed, R.J., Clark, W.H., Jr. Melanocytic nevi, melanocytomas, melanocytic dysplasis, and uncommon forms of melanoma. In: Clark W.H., Jr., Goldman L.I., Mastrangelo M.J., eds. Human Malignant Melanoma. New York: Grune and Stratton, 1979.
Al-Dewachi, H.S., Al-Naib, N., Sangal, B.C. Benign chondroblastoma of the maxilla: a case report and review of chondroblastomas in cranial bones. Br J Oral Surg. 1980; 18:150.
Alexiou, C., Kau, R.J., Dietzfelbinger, H. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999; 85(11):2305–2314.
Allan, C.J., Soule, E.H. Osteogenic sarcoma of the somatic soft tissues. Cancer. 1971; 27:1121.
Allen, C.M., Kapoor, N. Verruciform xanthoma in a bone marrow transplant recipient. Oral Surg Oral Med Oral Pathol. 1993; 75(5):591–594. [May].
Allen, A.C., Spitz, S. Malignant melanoma: a clinicopathological analysis of the criteria for diagnosis and prognosis Cancer. 1953; 6:1.
Allen, A.C. A reorientation on the histogenic sarcoma of the somatic soft tissues. Cancer. 1949; 2:28.
Al-Nafussi, A.I., Azzopardi, J.G., Salm, R. Verruciform xanthoma of the skin. Histopathology. 1985; 9(2):245–252. [Feb].
Amantea, A., Gaudio, E., Catricala, C. Verruciform xanthoma of the penis. G Ital Dermatol Venereol. 1989; 124(1–2):37–40. [Jan-Feb].
Andersen, L., Fejerskov, O., Philipsen, H.P. Oral giant cell granulomas: a clinical and histological study of 129 new cases. Acta Pathol Microbiol Scand A. 1973; 81(5):606–616. [No abstract available, Sep,].
Anderson WAD, Kissane JM. Pathology (7th ed). CV Mosby, St Louis, 1977.
Andrassy, R.J., Okcu, M.F., Despa, S. Synovial sarcoma in children: surgical lessons from a single institution and review of the literature. J Am Coll Surg. 2001; 192(3):305–313. [Mar].
Anneroth, G., Hansen, L.S. A methodologic study of histologic classification and grading of malignancy in oral squamous cell carcinoma. Scand J Dent Re. 1984; 92:448–468.
Angervall, L., Enzinger, F.M. Extraskeletal neoplasm resembling Ewing’s sarcoma. Cancer. 1975; 36:240.
Angervall, L., Kindblom, L.G., Nielsen, Jm, Stener, B. Hemangiopericytoma: a clinical clinicopathologic, angiographic and microangiographic study. Cancer. 1978; 42:2412.
A. Diagnosis, staging, and treatment of juvenile nasopharyngeal angiofibroma (JNA). Laryngoscope, 1319–25, 1 Nov, 1987, 97.
Aparacio, S.R., Lumsden, C.E. Light and electron microscopic studies on the granular cell myoblastoma of the tongue. J Pathol. 1969; 97:339.
Apostol, J.V., Frazell, E.L. Juvenile nasopharyngeal angiofibroma: a clinical study. Cancer. 1965; 18:869.
Archard, H.O., Carlson, K.P., Stanley, H.R. Leukoedema of the human oral mucosa. Oral Surg. 1968; 25:717.
Arlen, M., Higinbotham, N.L., Huvos, A.G., Marcove, R.C. Radiation-induced sarcoma of bone. Cancer. 1971; 28:1087.
Ash, C.L., Millar, O.B. Radiotherapy of cancer of the tongue and floor of the mouth. Am J Roentgenol Radium Ther Nucl Med. 1955; 73:611.
Austin, L.T., Dahlin, D.C., Jr., Royer, R.Q. Giantcell reparative granuloma and related conditions affecting the jawbones. Oral Surg. 1959; 12:1285.
Axéll, T., Henricsson, V. Leukoedema—an epidemiologic study with special reference to the influence of tobacco habits. Oral Epidemiol. 1981; 9:142.
Ayres, W.W., Delaney, A.J., Backer, M.H. Congenital neurofibromatous macroglossia associated in some cases with von Recklinghausen’s disease. Cancer. 1952; 5:721.
Azuma, H. Genetic and molecular pathogenesis of hereditary hemorrhagic telangiectasia. J Med Invest. 2000; 47(3–4):81–90. [Aug].
Backwinkel, K.D., Daddams, J.A. Hemangiopericytoma: report of a case and comprehensive review of the literature. Cancer. 1970; 25:896.
Baden, E., Newman, R. Liposarcoma of the oropharyngeal region: review of the literature and report of two cases. Oral Surg. 1955; 8:263.
Ballard, B.R., Suess, G.R., Pickren, J.W., Greene, G.W., Jr. Squamous-cell carcinoma of the floor of the mouth. Oral Surg. 1978; 45:568.
Balus, S., Breathnach, As, O’Grady, A.J. Ultrastructural observations on foam cells and the source of their lipid in verruciform xanthoma. J Am Acad Dermatol. 1991; 24(5):760–764. [May Pt 1].
Bang, G. Metastatic carcinoma of the mandible. Acta Odontol Scand. 1965; 23:103.
Bangle, R., Jr. A morphological and histochemical study of the granular-cell myoblastoma. Cancer. 1952; 5:950.
Banoczy, J., Sugar, L. Progressive and regressive changes in Hungarian oral leukoplakias in the course of longitudinal studies. Commuty Dent Oral Epidemiol. 1975; 3:194.
Banoczy, J. Follow-up studies in oral leukoplakia. J Maxillofac Surg. 1977; 5(1):69–75.
Barber, C.Z. Reactive bone formation in Ewing’s sarcoma. Cancer. 1951; 4:839.
Barcos, M., Herrmann, R., Pickren, J.W., Naeher, C. The influence of histologic type on survival in non-Hodgkin’s lymphoma. Cancer. 1981; 47:2894.
Barker, D.S., Lucas, R.B. Localised fibrous overgrowths of the oral mucosa. Br J Oral Surg. 1967; 5(2):86–92. [Nov].
Barnes, L., Eveson, J.W., Reichart, P., Sidransky, D. World Health Organization classification of Tumors: Pathology and Genetics of Head and Neck Tumours. IARC Press: Lyon; 2005.
Barnes, L. Tumors and tumorlike lesions of the soft tissues: in Barnes L. Surgical pathology of the Head and Neck. New York: Marcel Dekker, 1985; 725–780.
Barnes, R., Catto, M. Chondrosarcoma of bone. J Bone Joint Surg Br. 1996; 48:729.
Barnett, M.L., Bosshardt, L.L., Morgan, A.F. Double lip and double lip with blepharachalosis. Oral Surg. 1972; 34:929. [(Ascher’s syndrome)].
Barr, R.J., Plank, C.J. Verruciform xanthoma of the skin. J Cutan Pathol. 1980; 7(6):422–428. [Dec].
Barrock, J.J. Hereditary hemorrhagic telangiectasia. Wis Med J. 1944; 43:805.
Bataille, R., Sany, J. Solitary myeloma: clinical and prognostic features of a reivew of 114 cases. Cancer. 1981; 48:845.
Batsakis, J.G., Fries, G.T., Goldman, R.T., Karlsberg, R.C. Upper respiratory tract plasmacytoma. Arch Otolaryngol. 1964; 79:613.
Batsakis, J.G., Rice, D.H., Howard, D.R. The pathology of head and neck tumors: spindle cell lesions (sarcomatoid carcinomas, nodular fasciitis, and fibrosarcoma) of the aerodigestive tracts: part 14. Head Neck Surg. 1982; 4:499–513.
Batsakis, J.G. Tumors of the Head and Neck: Clinical and Pathological Considerations, 2. Baltimore: Williams and Wilkins; 1979.
Batsakis, J.G., Regezi, J.A., Solomon, A.R., Rice, D.H. The pathology of head and neck tumors: mucosal melanomas, part 13. Head Neck Surg. 1982; 4:404.
Batson, O.V. The function of the vertebral veins and their role in the spread of metastasis. Ann Surg. 1940; 112:138.
Bauer, W.H., Bauer, J.D. The so-called ‘congenital epulis’. Oral Surg. 1953; 6:1065.
Beltral, J., Simon, D.C., Levy, M. Aneurysmal bone cysts: MR imaging at 1.5 T. Radiology. 1986; 158(3):689–690. [Mar].
Berard, C., O’conor, G.T., Thomas, L.B., Torloni, H. Histopathological definition of Burkitt’s tumour Bull. WHO. 1969; 40:601.
Bernick S. Growths of the gingiva and palate I: chronic inflammatory lesions II— connective tissue tumors III. Epithelial growths. Oral Surg, 1: 1029, 2: 217, 1949, 1948.
Bernier, J.L., Bhaskar, S.N. Aneurysmal bone cysts of the mandible. Oral Surg. 1958; 11:1018.
Berquist, T.H., Ehman, R.L., King, B.F. Value of MR imaging in differentiating benign from malignant soft-tissue masses: study of 95 lesions. Am J Roentgenol. 1990; 155(6):1251–1255.
Berry, H.H., Landwerlen, J.R. Cigarette smoker’s lip lesion in psychiatric patients. J Am Dent Assoc. 1973; 86:657.
Bhansali, S.K., Desai, P.B. Ewing’s sarcoma. Observations of 107 cases. J Bone Joint Surg Am. 1963; 45:541.
Bhaskar, S.N., Jacoway, J.R. Peripheral fibroma and peripheral fibroma with calcification: report of 376 cases. J Am Dent Assoc. 1966; 73:1312.
Bhaskar, S.N., Akamine, R. Congential epulis (congenital granular cell fibroblastoma). Oral Surg. 1955; 8:517.
Bhaskar, S.N., Cutright, D.E. Multiple enostosis: report of 16 cases. J Oral Surg. 1968; 26:321.
Bhawan, J. Melanocytic nevi: a review. J Cutan Pathol. 1979; 6:153.
Bielamowicz, S., Dauer, M.S., Chang, B., Zimmerman, M.C. Noncutaneous benign fibrous histiocytoma of the Head and Neck. Otolaryngol Head Neck Surg. 1995; 113:140–146.
Biesecker, L.J., Marcove, R.C., Huvos, A.G., Miké, V. Aneurysmal bone cysts: a clinicopathological study of 66 cases. Cancer. 1970; 26:615.
Bill, A.H., Jr., Sumner, D.S. A unified concept of lymphangioma and cystic hygroma. Surg Gynecol Obstet. 1965; 120:79.
Bizzozero, O.J., Johnson, K.G., Jr., Ciocco, A. Radiation-related leukemia in Hiroshima Nagasaki, 1946–64 I Distribution, incidence, and appearance time. New Engl J Med. 1966; 274:1095.
Blanco, C., Miranda, C., Fernandez, F. Verruciform xanthoma of the lip: two lesions in a woman. Am J Dermatopathol. 1988; 10(2):176–178.
Blok, P., van Delden, L., van der Waal, I. Non Hodgkin’s lymphoma of the hard palate. Oral Surg. 1979; 47:445.
Bloom, S.M. Cancer, of the nasopharynx: with special reference to the significance of histopathology. Laryngoscope. 1979; 71:445.
Board, R.J., Shields, M.B. Combined trabeculotomy-trabeculectomy for the management of glaucoma associated with Sturge-Weber syndrome. Ophthalmic Surg. 1981; 2(11):813–817. [Nov].
Bodner, L., Peist, M., Gatot, A., Fliss, D.M. Growth potential of peripheral giant cell granuloma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997; 83(5):548–551. [May].
Boies, L.R., Peterson, R.G., Waldron, C.W., Stenstrom, K.W. Osteogenic sarcoma of the maxilla: report of a case. J Oral Surg. 1946; 4:56.
Bolek, T.W., Marcus, R.B., Mendenhall, N.P. Solitary plasmacytoma of bone and soft tissue. Int J Radiat Oncol Biol Phys. 1996; 36(2):329–333. [Sep].
Borello, E.D., Gorlin, R.J. Melanotic neuroectodermal tumor of infancy: a neoplasm of neural crest origin. Cancer. 1966; 19:196.
Borello, E.D., Sedano, H.O. Giant osteoid osteoma of the maxilla: report of a case. Oral Surg. 1967; 23:563.
Boston, H.C., Dahlin, D.C., Jr., Ivins, J.C., Cupps, R.E. Malignant lymphoma (so-called reticulum cell sarcoma) of bone. Cancer. 1974; 34:1131.
Bouquot, J., Speight, P.M., Farthing, P.M. Epithelial dysplasia of the oral mucosa – diagnostic problems and prognostic features. Curr Diagn Pathol. 2006; 12:11–22.
Bras, J., Batsakis, J.G., Luna, M.A. Malignant fibrous histiocytoma of the oral soft tissues. Oral Surg Oral Med Oral Pathol. 1987; 64:57–67.
Bras, J.M., Donner, R., van der Kwast, W.A.M., Snow, G.B., et al. Juxtacortical osteogenic sarcoma of the jaws. Oral Surg. 1980; 50:535.
Breslow, A. Thickness, cross sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970; 172:902.
Brinch, L., Hannisdal, E., Abrahamsen, A.F. Extramedullary plasmacytomas and solitary plasma cell tumours of bone. Eur J Haematol. 1990; 44(2):132–135. [Feb].
Broders, A.C. The grading of carcinoma. Minn Med. 1925; 8:726.
Broders, A.C. The microscopic grading of cancer. Surg Clin North Am. 1941; 21:947–961.
Brooks, J.S.J.Soft tissue lesions of the oral and maxillofacial region: Proceedings, Annual meeting of the American Academy of Oral and Maxillofacial Pathology. Texas: Dallas, 1998. [May].
Brothwell, D.J., Lewis, D.W., Bradley, G., Leong, I., Jordan, R.C., Mock, D., Leake, J.L. Observer agreement in the grading of oral epithelial dysplasia. Community Dent Oral Epidemiol. 2005; 31:300–305.
Brown, R.L., Suh, J.M., Scarborough, J.E., Wilkins, S.A. Snuff dippers’ intraoral cancer: clinical characteristics and response to therapy. Cancer. 1965; 18:2.
Browne, R.M., Rivas, P.H. Chondromyxoid fibroma of the mandible: a case report. Br J Oral Surg. 1977; 15:19.
Brownstein, M.H., Wolf Bikowski, J.B. Cowden’s disease: a cutaneous marker of breast cancer. Cancer. 1978; 41:2393.
Bruce, K.W., Royer, R.Q. Lipoma of the oral cavity. Oral Surg. 1953; 6:729.
Bruce, K.W. Solitary neurofibroma (neurilemmoma, schwannoma) of the oral cavity. Oral Surg. 1954; 7:1150.
Bryne, M., Boysen, M., Alfsen, C.G. The invasive front of carcinomas. The most important area for tumor prognosis? Anticancer Res. 1998; 18:4757–4764.
Buchner, A., Ficarra, G., Hansen, L.S. Peripheral odontogenic fibroma. Oral Surg Oral Med Oral Pathol. 1987; 64(4):432–438. [Oct].
Buchner, A., Hansen, L.S., Merrell, P.W. Verruciform xanthoma of the oral mucosa: report of five cases and review of the literature. Arch Dermatol. 1981; 117(9):563–565. [Sep].
Buchner, A., Hansen, L.S. The histomorphologic spectrum of peripheral ossifying fibroma. Oral Surg Oral Med Oral Pathol. 1987; 63(4):452–461. [Apr].
Buchner, A., Hansen, L.S. Pigmented nevi of the oral mucosa: a clinicopathologic study of 32 new cases and review of 75 cases from the literature Part I: a clinicopathologic study of 32 new cases. Oral Surg. 1979; 48:131.
Buchner, A., Hansen, L.S. Pigmented nevi of the oral mucosa: a clinicopathologic study of 32 new cases and review of 75 cases from the literature Part II: analysis of 107 cases. Oral Surg. 1980; 49:55.
Buirski, G., Watt, I. The radiological features of ‘solid’ aneurysmal bone cysts. Br J Radiol. 1984; 57(684):1057–1065. [Dec].
Bullough, P.G., Goodfellow, J.W. Solitary lymphangioma of bone: a case report. J Bone Joint Surg. 1976; 58A:418.
Bundens, W.D., Brighton, C.T. Malignant hemangioendothelioma of bone: report of two cases and review of the literature. J Bone Joint Surg Am. 1965; 47:762.
Bunting, H., Strauss, M.J., Banfield, W.G. Cytology of skin papillomas that yield virus-like particles. Am J Pathol. 1952; 28:985.
Burch, R.J. Metastasis of neuroblastoma to the mandible: report of case. J Oral Surg. 1952; 10:160.
Burford, W.N., Ackerman, L.V., Robinson, H.B.G. Leiomyoma of the tongue. Am J Orthod,Oral Surg. 1944; 30:395.
Burket, L.W. Oral Medicine Diagnosis and Treatment, 6. Philadelphia: JB Lippincott, 1971.
Burkitt, D., O’Conor, G.T. Malignant lymphoma in African children I: a clinical syndrome. Cancer. 1961; 14:258.
Burkitt, D.A. sarcoma involving the jaws in African children. Br J Surg. 1958; 46:218.
Burkitt DP, Wright DH. Burkitt’s Lymphoma. Edinburgh and London. ES Livingstone, 1970
Burrows, M.T. The mechanism of cancer metastasis. Arch Intern Med. 1926; 37:453.
Butler, J.J. Relationship of histological findings to survival in Hodgkin’s disease. Cancer Res. 1971; 31:1770.
Byard, R.W., Schliebs, J., Koszyca, B.A. Osler-Weber-Rendu syndrome—pathological manifestations and autopsy considerations. J Forensic Sci. 2001; 46(3):698–701. [May].
Byers, R.M. Squamous cell carcinoma of the oral tongue in patients less than thirty years of age. Am J Surg. 1975; 130:475.
Cadotte, M. Malignant granular-cell myoblastoma. Cancer. 1974; 33:1417.
Caldwell, J.B., Hughes, K.W., Fadell, E.J. Alveolar soft-part sarcoma of the tongue. J Oral Surg. 1956; 14:342.
Canale S.T., Jones L., Daugherty K., eds. Campbell’s Operative Orthopaedics, 9, St Louis: CV Mosby, 1998.
Capanna, R., Albisinni, U., Picci, P. Aneurysmal bone cyst of the spine. J Bone Joint Surg Am. 1985; 67(4):527–531.
Capanna, R., Van, Horn, Jr., Biagini, R. Aneurysmal bone cyst of the sacrum. Skeletal Radiol. 1989; 18(2):109–113.
Carney, J.A., Sizemore, G.W., Lovestedt, S.A. Mucosal ganglioneuromatosis, medullary thyroid carcinoma, and pheochromocytoma: multiple endocrine neoplasia, type 2b. Oral Surg. 1976; 41:341.
Carr, M.W. Congential bilateral hemangioendothelioma: clinicopathologic report. J Oral Surg. 1948; 6:341.
Cash, C.D., Royer, R.Q., Dahlin, D.C. Metastatic tumors of the jaws. Oral Surg Oral Med Oral Pathol. 1961; 14:897.
Casino, A.J., Sciubba, J.J., Ohri, G.L., Rosner, F. Oral-facial manifestations of the multiple endocrine neoplasia syndrome. Oral Surg. 1981; 51:516.
Rominger, C.J. Metastatic malignancy of the jaws. Am J Surg. 1954; 87:496.
Castro, L., de la Pava, S., Webster, J.H. Esthesioneuroblastomas: a report of 7 cases. Am J Roentgenol Radium Ther Nucl Med. 1969; 105:7.
Cataldo, E., Meyer, I. Solitary and multiple plasmacell tumors of the jaws and oral cavity. Oral Surg. 1966; 22:628.
Cataldo, E., Shklar, G., Meyer, I. Osteoma of the tongue. Arch Otolaryngol. 1967; 85:202.
Catlin, D. Mucosal melanomas of the head and neck. Am J Roentgenol Radium Ther Nucl Med. 1967; 99:809.
Cawson, R.A., Lehner, T. Chronic hyperplastic candidiasis—candidal leukoplakia. Br J Dermatol. 1968; 80:9.
Cawson, R.A. Chronic oral candidiasis and leukoplakia. Oral Surg. 1966; 22:582.
Chan, J.K.C., Hui, P.K., Ng, C.S. Epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia) and Kimura’s disease in Chinese. Histopathol. 1989; 15:557–574.
Chaudhry, A.P., Gorlin, R.J., Mosser, D.G. Carcinoma of the antrum. Oral Surg. 1960; 13:269.
Chaudhry, A.P., Hampel, A., Corlin, R.J. Primary malignant melanoma of the oral cavity Cancer. 1958; 11:923.
Chaudhry, A.P., Robinovitch, M.R., Mitchell, D.F. Chondrogenic tumors of the jaws. Am J Surg. 1961; 102:403.
Chen, S.Y., Fantasia, J.E., Miller, A.S. Myxoid lipoma of oral soft tissue: a clinical and ultrastructural study. Oral Surg Oral Med Oral Pathol. 1984; 57:300–307.
Chen, S.Y., Harwick, R.D. Ultrastructure of oral squamous-cell carcinoma. Oral Surg. 1977; 44:744.
Chen, S.Y., Miller, A.S. Neurofibroma and schwannoma of the oral cavity. Oral Surg. 1979; 47:522.
Chen, S.Y. Ultrastructure of a plasma-cell myeloma in the mandible. Oral Surg. 1979; 48:57.
Cheng, K.P. Ophthalmological Manifestations of Sturge-Weber Syndrome. In: Brodensteiner J.B., Roach E.S., eds. Sturge-Weber Syndrome St. Louis: CV Mosby, 1999.
Cherrick, H.M., Eversole, L.R. Benign neural sheath neoplasm of the oral cavity: report of thirty-seven cases. Oral Surg. 1971; 32:900.
Cherrick, H.M. Dunlap CL King, OH Jr. Leiomyomas of the oral cavity. Oral Surg. 1973; 35:54.
Choisser, R.M., Ramsey, E.M. Angioreticuloendothelioma (Kaposi’s disease) of the heart. Am J Pathol. 1939; 15:155.
Christensen, R.W. Lymphangioma of the tongue: report of case. Oral Surg. 1953; 6:593.
Christopherson, W.M., Foote, F.W., Stewart, F.W., Jr. Alveolar soft-part sarcomas. Cancer. 1952; 5:100.
Chung, E.B., Enzinger, F.M. Benign Lipoblastomatosis: an analysis of 35 cases. Cancer. 1973; 32:482.
Chung, E.B., Enzinger, F.M. Chondroma of soft parts. Cancer. 1978; 41:1414.
Chuong, R., Kaban, L.B., Kozakewich, H. Central giant cell lesions of the jaws: a clinicopathologic study. J Oral Maxillofac Surg. 1986; 44(9):708–713. [Sep].
Church, Le, Uhler, I.V. Olfactory neuroblastoma. Oral Surg. 1959; 12:1040.
Chyu, J., Medenica, M., Whitney, D.H. Verruciform xanthoma of the lower extremity— report of a case and review of literature. J Am Acad Dermatol. 1987; 17(4):695–698. [Oct].
Cibis, G.W., Tripathi, R.C., Tripathi, B.J. Glaucoma in Sturge-Weber syndrome. Ophthalmology. 1984; 91(9):1061–1071.
Clark, W.H., Ainsworth, A.M., Jr., Bernardino, E.A., Yang, C.H. The developmental biology of primary human malignant melanomas. Semin Oncol. 1975; 2:83.
Clark, W.H., Jr., Mihm, M.C., Jr. Lentigo maligna and lentigo-maligna melanoma. Am J Pathol. 1969; 55:39.
Clark, W.H., From, L., Jr., Bernardino, E.A., Mihm, M.C. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969; 29:705.
Clark, W.H., Goldman, L.I., Jr., Mastrangelo, M.J.Human Malignant Melanoma. New York: Grune and Stratton, 1979.
Clark, W.H., Reimer, R.R., Jr., Greene, M., Ainsworth, A.M. Origin of familial malignant melanomas from heritable melanocytic lesions. Arch Dermatol. 1978; 114:732.
Clarke, C.A., Howel-Evants, A.W., McConnell, R.B. Carcinoma of esophagus associated with tylosis. Br Med J. 1957; 1:945.
Clausen, F., Poulsen, H. Metastatic carcinoma to the jaws. Acta Pathol Microbiol Scand. 1963; 57:361.
Clearkin, K.P., Enzinger, F.M. Intravascular papillary endothelial hyperplasia. Arch Pathol Lab Med. 1976; 100:441–444.
1968. Clinical staging system for carcinoma of the oral cavity. CA Cancer J Clin, 81: 163, 1968
Clough, J.R., Price, C.H. Aneurysmal bone cyst: pathogenesis and long term results of treatment. Clin Orthop. 1973; 97:52–63. [Nov-Dec].
Cobb, C.M., Holt, R., Denys, F.R. Ultrastructural features of the verruciform xanthoma. J Oral Pathol. 1976; 5(1):42–51. [Jan].
Coburn, J.G., Morgan, J.K. Multiple idiopathic hemorrhagic sarcoma of Kaposi. Arch Dermatol Syph. 1955; 71:618.
Coffin Dehner, L.O’Shea P. Pediatric Soft Tissue Tumors: a clinical pathological, and therapeutic approach. Baltimore: Williams and Wilkins, 1997.
Cohan, W.G., Woddard, H.A., Higinbotham, N.L., Stewart, F.W. Sarcoma arising in irradiated bone. Cancer. 1948; 1:3.
Colberg, J.E. Granular cell myoblastoma. Surg Gynecol Obstet. 1962; 115:205.
Cole, L.J., Nowell, P.C. Radiation carcinogenesis: the sequence of events. Science. 1965; 150:1782.
Coleman, W.P., III., Loria, P.R., Reed, R.J., Krementx, E.T. Acral lentiginous melanoma. Arch Dermatol. 1980; 116:773.
Coley, B.L., Higinbotham, N.L. Groesbeck HP. Primary reticulum cell sarcoma of bone Radiology. 1950; 55:641.
Colonna, T.M., Fair, K.P., Patterson, J.W. A persistent lower lip lesion: verruciform xanthoma. Arch Dermatol. 2000; 136(5):665–666. [May].
Conley, J. Pack GT: Melanoma of the mucous membranes of the head and neck. Arch Otolaryngol. 1974; 99:315.
Connolly, S.B., Lewis, E.J., Lindholm, J.S. Management of cutaneous verruciform xanthoma. J Am Acad Dermatol. 2000; 42(2):343–347. [Feb Pt 2].
Cook, T.J., Zbar, K.J. Arteriovenous aneurysm of the mandible. Oral Surg. 1962; 15:442.
Cooke, B.E.C. Leukoplakia buccalis and oral epithelial naevi: a clinical and histological study. Br J Dermatol. 1974; 68:151.
Corio, R.L., Lewis, D.M. Intraoral rhabdomyomas. Oral Surg. 1956; 48:151.
Correa, J.N., Bosch, A., Marcial, V.A. Carcinoma of the floor of the mouth: review of clinical factors and results of treatment. Am J Roentgenol Radium Ther NuclMed. 1967; 99:302.
Corwin, J., Lindberg, R.D. Solitary plasmacytoma of bone vs extramedullary plasmacytoma and their relationship to multiple myeloma. Caner. 1979; 43:1007.
Cossu, S., Satta, R., Cottoni, F., Massarelli, G. Lymphangioma-like variant of Kaposi’s sarcoma – clinicopathological study of 7 cases with review of the literature. Am J Dermatopathol. 1997; 19:16–22.
Costanza, M.E., Dayal, Y., Binder, S., Nathanson, L. Metastatic basal cell carcinoma: review, report of a case, and chemotherapy. Cancer. 1974; 34:230.
Cotran, R.S. Metastasizing basal cell carcinomas. Cancer. 1961; 14:1036.
Coventry, M.B., Dahlin, D.C. Osteogenic sarcomas. J Bone Joint Surg Am. 1957; 39:741.
Cox, F.H., CHelwig, E.B. Kaposi’s sarcoma. Cancer. 1959; 12:289.
Cramer, L.M. Gardner’s syndrome. J Plast Reconstr Surg. 1959; 29:289.
Cross, J.E., Guralnick, E., Daland, E.M. Carcinoma of the lip Surg. Gynecol Obstet. 1948; 87:153.
Crowley, R.E. Neurofibroma. New York Dent J. 1951; 17:457.
Cundiff EJ. Peripheral ossifying fibroma: a review of 365 cases. MSD Thesis Indiana University, 1972.
Curkovic, M. Osteoma of the maxillary sinuses: report of case. Arch Otolaryngol. 1951; 54:53.
Custer, R.P., Fust, J.A. Congential epulis. Am J Clin Pathol. 1952; 22:1044.
Custer, R.P. The interrelationship of Hodgkin’s disease and other lymphatic tumors. Am J Med Sci. 1948; 216:625.
Cutler, S.J., Young, J.L., Jr. Third national cancer survey: incidence data. Natl Cancer Inst Monorg. 41, 1975. [(eds)].
D’Agostino, A.N., Soule, E.H., Miller, R.H. Primary malignant neoplasms of nerves (malignant neurilemmomas) in patients without manifestations of multiple neurofibromatosis (Von Recklinghausen’s disease). Cancer. 1963; 16:1003.
Dabelsteen, E., Roed-Petersen, B., Smith, C.J., Pindborg, J.J. The limitations of exfoliative cytology for the detection of epithelial atypia in oral leukoplakias. Br J Cancer. 1971; 25:21.
Dabska, M., Buraczewski, J. Aneurysmal bone cyst: pathology, clinical course and radiologic appearances. Cancer. 1969; 23:371.
Dahlin DC, McLeod RA. Aneurysmal bone cyst and other nonneoplastic conditions. Skel Radiol, 1982.
Dahlin, D.C., Ivins, J.C. Benigh chondroblastoma: a study of 125 cases. Cancer. 1972; 30:401.
Dahlin, D.C., Johnson, E.W., Jr. Giant osteoid osteoma. J Bone Joint Sure Am. 1954; 36:559.
Dahlin, D.C., Coventry, M.B., Scanlon, P.W. Ewing’s sarcoma. J Bone Joint Surg Am. 1961; 43:185.
Dahlin, D.C., Cupps, R.E., Johnson, E.W., Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970; 25:1061.
Dahnert, W. Bone Tumors General Aspects Data on 6,221 Cases, 3. Springfield: Charles C Thomas, 1978.
Dahnert, W. Bone soft-tissue disorders: in radiology review manual, (2rd ed), JB Lippincott: Williams and Wilkins; 1993:31–32.
Damm, D.D., Neville, B.W. Oral leiomyomas. Oral Surg. 1979; 47:343.
Danforth, R.A., Baughman, R.A. Chievitz’s organ: a potential pitfall in oral cancer diagnosis. Oral Surg. 1979; 48:231.
Das Gupta, T., Brasfield, R.D., Strong, E.W., Hajdu, S.I. Benign solitary schwannomas(neurilemomas). Cancer. 1969; 24:355.
Davis, G.B., Tideman, H. Chondromyxoid fibroma of the mandible: case report. Int J Oral Surg. 1978; 7:23.
Dayan, D., Buchner, A., Spirer, S. Bone formation in peripheral giant cell granuloma. J Periodontol. 1990; 61(7):444–446. [Jul].
De Lathouwer, C., Brocheriou, C. Sarcoma arising in irradiated jawbones. Possible relationship with previous non-malignant bone lesios. Report of 6 cases and review of the literature. J Maxillofac Surg. 1976; 4:8.
De Santos, L., Murray, J.A. The value of arteriography in the management of aneurysmal bone cyst. Skeletal Radiol. 1978; 2:137.
de Visscher, JGAM. Lipomas and fibrolipomas of the oral cavity. J Maxillofac Surg. 1982; 10:177–181.
Dehner, L.P., Enzinger, F.M., Font, R.L. Fetal rhabdomyoma: an analysis of nine cases. Cancer. 1972; 30:160.
Dehner, L.P., Sibley, R.K., Sauk, J.J., Vickers, R.A., Jr. Malignant melanotic neuroectodermal tumor of infancy: a clinical pathologic, ultrastructural and tissue culture study. Cancer. 1979; 43:1389.
Desforges, J.F., Rutherford, C.J., Piro, A. Hodgkin’s Disease. New Engl J Med. 1979; 301:1212.
DeVore, D.T., Waldron, C.A. Malignant peripheral nerve tumors of the oral cavity: review of the literature and report of a case. Oral Surg. 1961; 14:56.
Dimopoulos, M.A., Goldstein, J., Fuller, L. Curability of solitary bone plasmacytoma. J Clin Oncol. 1992; 10(4):587–590.
Dimopoulos, M.A., Moulopoulos, L.A., Maniatis, A. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000; 96(6):2037–2044.
Dolin, S., Dewar, J.P. Extramedullary plasmacytoma. Am J Pathol. 1956; 32:83.
Donaldson, D., Myall, R.W.T. Hereditary hemorrhagic telangiectasia, Raynaud’s disease, and the CREST syndrome. Oral Surg. 1973; 36:512.
Donaldson, S.S., Castro, J.R., Wilbur, J.R., Jesse, R.H., Jr. Rhabdomyosarcoma of head and neck in children: combination treatment by surgery, irradiation, and chemotherapy. Cancer. 1973; 31:26.
Dooling, E.C., Chi, J.G., Gilles, F.H. Melanotic neuroectodermal tumor of infancy: its histological similarities to fetal pineal gland. Cancer. 1977; 39:1535.
dorfman, H.D., Steiner, G.C., Jaffe, H.L. Vascular tumors of bone. Hum Pathol. 1971; 2:349.
Dupuy, D.E., Rosenberg, A.E., Punyaratabandhu, T. Accuracy of CT-guided needle biopsy of musculoskeletal neoplasms. Am J Roentgenol. 1998; 171(3):759–762. [Sep].
Durden, D.D. Skull base tumor surgery. Radiology of skull base neoplasms. Otolaryngol Clin North Am. 2001; 34(6):1043–1064. [1 Dec].
Echevarria, R., Ackerman, L.V. Spindle and epithelioid cell nevi in the adult: clinicopathologic report of 26 cases. Cancer. 1967; 20:175.
Eckenhoff, J.E. The physiologic significance of the vertebral venus plexus. Surg Gynecol Obstet. 1970; 131:72.
Ehrlich, H.E., Martin, H. Schwannomas (neurilemmomas) in the head and neck. Surg, Gynecol Obstet. 1943; 76:577.
Einhorn, J., Wersall, J. Incidence of oral carcinoma in patients with leukoplakia of the oral mucosa. Cancer. 1967; 20:2189.
Elder, D., Elenitsas, R., Jaworsky, C., Johnson, B., Jr. Lever’s Histopathology of the skin, 8. Lippincott-Raven: Philadelphia; 1997.
Ellis, G.L., Corio, R.L. Spindle cell carcinoma of the oral cavity: a clinicopathologic assessment of fifty-nine cases. Oral Surg Oral Med Oral Pathol. 1980; 50:523–533.
Ellis, G.L., Abrams, A.M., Melrose, R.J. Intraosseous benign neural sheath neoplasms of the jaws. Oral Surg. 1977; 44:731.
Ellis, G.L., Corio, R.L. Spindle cell carcinoma of the oral cavity. Oral Surg. 1980; 50:523.
Elwood, J.M., Lee, J.A.H. Recent data on epidemoilogy of malignant melanoma. Semin Oncol. 1975; 2:149.
Elzay, R.P., Dutx, W. Myxomas of the paraoral-oral soft tissue. Oral Surg. 1978; 45:246.
Enzinger, F.M., Shiraki, M. Alveolar rhabdomyosarcoma: an analysis of 110 cases. Cancer. 1969; 24:18.
Enzinger FM, Lattes R, Torloni H. Histological typing of soft tissue tumours. international histological classification of tumours monograph (3). World Health Organisation, 1969.
Epstein, M.A., Achong, B.G., Barr, Y.M. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet. 1964; 2:702.
Evans, H.L., Ayala, A.G., Romsdahl, M.M. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977; 40:818.
Eversole, L.R., Rovin, S. Reactive lesions of the gingiva. J Oral Pathol. 1972; 1:30.
Eversole, L.R., Schwartz, W.D., Sabes, W.R. Central and peripheral fibrogenic and neurogenic sarcoma of the oral regions. Oral Surg. 1973; 36:49.
Eversole, L.R. Central benign and malignant neural neoplasms of the jaws: a review. J Oral Surg. 1969; 27:716.
Ewing, J. Lymphoepithelioma. Am J Pathol. 1929; 5:99.
Farman, A.G., Kay, S. Oral leiomyosarcoma: report of a case and review of the literature pertaining to smooth-muscle tumors of the oral cavity. Oral Surg. 1977; 43:402.
Farman, A.G., Jortjé, C.J., Grotepass, F. Periosteal benign osteoblastoma of the mandible. Report of a case and review of the literature pertaining to benign osteoblastic neoplasms of the jaws. Br J Oral Surg. 1976; 14:12.
Faughnan, M.E., Hyl, R.H., Nanthakumar, K., Redelmeier, D.A. Screening in hereditary hemorrhagic telangiectasia patients. Chest. 2000; 118(2):566–567. [Aug].
Fawcett, K.J., Dahlin, D.C. Neurilemmoma of bone. Am J Clin Pathol. 1967; 47:759.
Feldman, F., Hecht, H.L., Johnston, A.D. Chondromyxoid fibroma of bone. Radiology. 1970; 94:249.
Ferlito, A., Recher, G. Ackerman’s tumor (verrucous carcinoma) of the larynx: a clinicopathologic study of 77 cases. Cancer. 1980; 46:1617.
Fernandez, C.H., Sutow, W.W., Merino, O.R., George, S.L. Childhood rhabdomyosarcoma. Analysis of coordinated therapy and results. 1975; 123:588.
Fetsch, J.F., Weiss, S.W. Observations concerning the pathogenesis of epithelioid hemangioma (angiolymphoid hyperplasia). Mod Pathol. 1991; 4:449–455.
Ficarra, G., Kaban, L.B., Hansen, L.S. Central giant cell lesions of the mandible and maxilla: a clinicopathologic and cytometric study. Oral Surg Oral Med Oral Pathol. 1987; 64(1):44–49.
Fisher, C. Synovial sarcoma. Ann Diagn Pathol. 1998; 2(6):401–421.
Fisher, E.R., Turano, A. A. Schwann cells in Wallerian degeneration. Arch Pathol. 1963; 75:517.
Fisher, E.R., Vuzevski, V.D. Cytogenesis of Schwannoma (neurilemoma), neurofibroma, dermatofibroma, and dermatofibrosarcoma as revealed by electron microscopy. Am J Clin Pathol. 1968; 49:141.
Fisher, E.R., Wechsler, H. Granular cell myoblastoma—a misnomer: electron microscopic and histochemical evidence concerning its Schwann cell derivation and nature (granular cell Schwannoma). Cancer. 1962; 15:936.
Fitzpatrick, T.B., Miyamoto, M., Ishikawa, K. The evolution of concepts of melanin biology. Arch Dermatol. 1967; 96:305.
Flamant, R., Hayem, M., Lazar, P., Denoix, P. Cancer of the tongue: a study of 904 cases. Cancer. 1964; 17:377.
Fletcher, C.Diagnostic Histopathology of Tumors (Vol I). Edinburg: Churchill Livingstone, 2000.
Fletcher, C.D., McKee, P.H. Sarcomas–a clinicopathologic guide with particular reference to cutaneous manifestations: III: angiosarcoma, malignant hemangiopericytoma, fibrosarcoma and synovial sarcoma. Clin Exp Dermatol. 1985; 10:332–349.
Fletcher, C.D. Benign fibrous histiocytoma of subcutaneous and deep soft tissue: a clinicopathologic analysis of 21 cases. Am J Surg Pathol. 1990; 14:801–809.
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995; 1(1):27–31. [Jan].
Font, R.L., Jurco, S., Zimmerman, L.E. Alveolar soft-part sarcoma of the orbit: a clinicopathologic analysis of seventeen cases and a review of the literature. Hum Pathol. 1982; 13:569.
Forman, G. Chondrosarcoma of the tongue. Br J Oral Surg. 1967; 4:218.
Forman, G.H., Wesson, C.M. Hodgkin’s disease of the mandible. Br J Oral Surg. 1970; 7:146.
Foss, R.D., Ellis, G.L. Myofibromas and myofibromatosis of the oral region: a clinicopathologic analysis of 79 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000; 89(1):57–65. [Jan].
Fowler, C.B., Hartman, K.S., Brannon, R.B. Fibromatosis of the oral and paraoral region. Oral Surg Oral Med Oral Pathol. 1994; 77(4):373–386. [Apr].
Franklin, C.D., Caraig, G.T., Smith, C.J. Quantitative analysis of histological parameters in giant cell lesions of the jaws and long bones. Histopathology. 1979; 3::511.
Frazell, E.L., Lucas, J.C., Jr. Cancer of the tongue. Report of the management of 1,554 patients. Cancer. 1962; 15::1085.
Freedman, P.D., Cardo, V.A., Kerpel, S.M., Lumerman, H. Desmoplastic fibroma (fibromatosis) of the jawbones: report of a case and review of the literature. Oral Surg. 1978; 46::386.
Freedman, P.D., Kerpel, S.M., Begel, H., Lumerman, H. Solitary intraoral keratoacanthoma. Oral Surg. 1979; 47::74.
Freiberger, R.H., Loitman, B.S., Helpern, M., Thompson, T.C. Osteoid osteoma: a report on 80 cases. Am J Roentgenol Radium Ther Nucl Med. 1959; 82::194.
Fritzler, M.J., Arlette, J.P., Behm, A.R. Hereditary hemorrhagic telangiectasia versus CREST syndrome: can serology aid diagnosis? J Am Acad Dermatol. 1984; 10(2 Pt 1):192–196. [Feb].
Fuhr, A.H., Krogh, P.H. Congential epulis of the newborn: centennial review of the literature and a report of case. J Oral Surg. 1972; 30:30.
Fujimura, N. Enomoto S. Lipoma of the tongue with cartilaginous change: a case report and review of the literature. J Oral Maxillofac Surg. 1992; 50:1015–1017.
Fust, J.A. Custer RP. On the neurogenesis of so-called granular-cell myoblastoma. Am J Clin Pathol. 1949; 19:522.
Galieni, P., Cavo, M., Pulsoni, A. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000; 85(1):47–51.
Gall, E.A., Mallory, T.B. lymphoma: a clincopathological survey of 618 cases. Am J Path. 1942; 18:381.
Gambardella, R.J. Kaposi’s sarcoma and its oral manifestations. Oral Surg. 1974; 38:591.
Gamez-Araujo, J.J., Toth, B.B., Luna, M.A. Central hemangioma of the mandible and maxilla: review of a vascular lesion. Oral Surg. 1974; 37:230.
Garancis, J.C., Komorowski, R.A., Kuzma, J.F. Granular cell myoblastoma. Cancer. 1970; 25:542.
Gardner, D.G., Mills, D.M. The widened periodontal ligament of osteosarcoma of the jaws. Oral Surg. 1976; 41:652.
Gardner, D.G., Paterson, J.C. Chondroma or metaplastic chondrosis of soft palate. Oral Surg. 1968; 26:601.
Gardner, D.G. The peripheral odontogenic fibroma: an attempt at clarification. Oral Surg. 1982; 54(1):40–48.
Garland, H.G., Anning, S.T. Hereditary hemorrhagic telangicetasia. Br J Dermatol Syph. 1950; 62:289.
Garrington, G.E., Scofield, H.H., Cornyn, J., Hooker, S.P. Osteosarcoma of the jaws: analysis of 56 cases. Cancer. 1967; 20:377.
Gerard-Marchant, R., Micheau, C. Microscopical diagnosis of olfactory esthesioneuromas: general review and report of five cases. J Natl Cancer Inst. 1965; 35:75.
Gerry, R.G., Williams, S.F. Primary reticulum-cell sarcoma of the mandible. Oral Surg. 1955; 8:568.
Geschickter, C.F., Copeland, M. Tumors of Bone, 3. Philadelphia: JB Lippincott, 1949.
Gettinger, R. Papilloma of the palate, tongue, retromolar area and cheek. Arch Clin Oral Pathol. 1939; 3:45. [51,56,63].
Ghadially, F.N., Barton, B.W., Kerridge, D.F. The etiology of keratoacanthoma. Cancer. 1963; 16:603.
Giansanti, J.S., Waldron, C.A. Peripheral giant cell granuloma: review of 720 cases. J Oral Surg. 1969; 27:787.
Gibbel, M.I., Cross, J.H., Ariel, I.M. Cancer of the tongue: review of 330 cases. Cancer. 1949; 2:411.
Gillespie, J.J., Roth, L.M., Wills, E.R., Einhorn, L.H. Extraskeletal Ewing’s sarcoma. Histologic and ultrastructural observations in three cases. Am J Surg Pathol. 1979; 3:99.
Ginsburg, L.D. Congenital aneurysmal bone cyst: case report with comments on the role of trauma in the pathogenesis. Radiology. 1974; 110(1):175–176. [Jan].
Gius, J.A., Boyle, D.E., Castle, D.D., Congdon, R.H. Vascular formations of the lip and pepticulcer. J Am Med Assoc. 1963; 183:725.
Goaz, P.W., White, S.C. Oral radiology, 2, St. Louis: CV Mosby; 1987:608–611.
Goette, D.K., Carson, T.E. Erythroplasia of Queyrat: treatment with topical 5-fluorouracil. Cancer. 1976; 38:1498.
Goldberg, M.H., Nemarich, A.N., Danielson, P. Lymphangioma of the tongue: medical and surgical therapy. J Oral Maxillofac Surg. 1977; 35:841–844.
Goldblatt, L.I., Edesess, R.B. Central leiomyoma of the mandible: report of a case with ultrastructural confirmation. Oral Surg. 1977; 43:591.
Goldenberg, R.R., Campbell, C.J., Bonfiglio, M. Giant-cell tumor of bone: an analysis of two hundred and eighteen cases. J Bone Joint Surg Am. 1970; 52:619.
Goldman, R.L., Klein, H.Z., Sung, M. Adenoid squamous cell carcinoma of the oral cavity. Report of the first case arising in the tongue. Arch Otolaryngol. 1977; 103:496.
Goldman, R.L. The periosteal counterpart of benign osteoblastoma. Am J Clin Pathol. 1971; 56:73.
Goldstein, B.H., Laskin, D.M. Giant cell tumor of the maxilla complicating Paget’s disease of bone. J Oral Surg. 1974; 32:209.
Gomes, M.M.R., Bernatx, P.E. Arteriovenous fistulas: a review and ten-year experience at the Mayo Clinic. Mayo Clin Proc. 1970; 45:81.
Gonzalez, S., Duarte, I. Benign fibrous histiocytoma of the skin: a morphologic study of 290 cases. Pathol Res Pract. 1982; 174:379–391.
Goodsell, J.O., Hubinger, H.L. Benign chondroblastoma of mandibular condyle: report of a case. J Oral Surg. 1964; 22:355.
Gordon, R.S. From the NIH Human wart virus found in many papillomas. J Am Med Assoc. 1980; 244:2041. [(ed)].
Gorlin, R.J., Sedano, H.O., Vickers, R.A., Cervenka, J. Multiple mucosal neuromas, pheochromocytoma and medullary carcinoma of the thyroid: a syndrome. Cancer. 1968; 22:293.
Gorlin, R.J. Bowen’s disease of the mucous membrane of the mouth. Oral Surg. 1950; 3:35.
Gorlin, R.L., Vickers, R.A., Kelln, E., Williamson, J.J. The multiple basal-cell nevi syndrome: an analysis of a syndrome consisting of multiple nevoid-basal-cell carcinoma, jaw cysts, skeletal anomalies, medulloblastoma, and hyporesponsiveness to parathormone. Cancer. 1965; 18:89.
Gowen, G.F., de Suto-Nagy, G. The incidence and sites of distant metastases in head and neck carcinoma Surg. Gynecol Obstet. 1963; 116:603.
Graham, S., Dayal, H., Rohrer, T., Swanson, M. Dentition, diet, tobacco, and alcohol in the epidemiology of oral cancer. J Natl Cancer Inst. 1977; 59:1611.
Granstein, R.D., Sober, A.J. Current concepts in ultraviolet carcinogenesis. Proc Soc Exp Biol Med. 1982; 170:115.
Gravanis, M.B., Giansanti, J.S. Benign chondroblastoma: report of four cases with a discussion of the presence of ossification. Am J Clin Pathol. 1971; 55:624.
Gray, P.B., Miller, A.S., Loftus, M.J. Benign fibrous histiocytoma of the oral/perioral regions: report of a case and review of 17 additional cases. J Oral Maxillofac Surg. 1992; 50:1239–1242.
Greene, G.W., Natiella, J.R., Jr., Spring, P.N., Jr. Osteoid osteoma of the jaws: report of a case. Oral Surg. 1968; 26:342.
Greer, R.O., Berman, D.N. Osteoblastoma of the jaws: current concepts and differential diagnosis. J Oral Surg. 1978; 36:304.
Greer, R.O., Goldman, H.M. Oral papillomas: clinicopathologic evaluation and retrospective examination for dyskeratosis in 110 lesions. Oral Surg. 1974; 38:435.
Greer, R.O., Richardson, J.F. The nature of lipomas and their significance in the oral cavity: a review and report of cases. Oral Surg. 1973; 36:551.
Griffith, J.G., Irby, W.B. Desmoplastic fibroma: report of a rare tumor of the oral structure. Oral Surg. 1965; 20:269.
Grossman, L.D., White, R.R., Arber, D.A. Angiomatoid fibrous histiocytoma. Ann Plast Surg. 1996; 36:649–651.
Grossman, H., Winchester, Ph, Bragg, D.G., Tan, C., et al. Roentenographic changes in childhood Hodgkin’s disease. Am J Roentgenol Radium. Ther Nucl Med. 1970; 108:354.
Grotepass, F.W., Farman, A.G., Nortje, C.J. Chondromyxoid fibroma of the mandible. J Oral Surg. 1976; 34:988.
Guillou, L., Dehon, A., Charlin, B. Pleomorphic lipoma of the tongue: case report and literature review. J Otolaryngol. 1986; 15:313–316.
Hagy, D.M., Halperin, V., Wood, C., III. Leiomyoma of the oral cavity: review of the literature and report of a case. Oral Surg. 1964; 17:748.
Hall, A.F. Relationships of sunlight, complexion and heredity to skin carcinogenesis. Arch Dermatol Syph. 1950; 61:589.
Hamner, J.E., III., Fullmer, H.M. Oxytalan fibers in benign fibro-osseous jaw lesions. Arch Pathol. 1966; 82:35.
Hamperl, H. Benign and malignant oncocytoma. Cancer. 1962; 15:1019.
Hansen, L.S., Buchner, A. Changing concepts of the junctional nevus and melanoma: review of the literature and report of case. J Oral Surg. 1981; 39:961.
Hansen, L.S. Diagnosis of oral keratotic lesions. J Oral Surg Anesth Hosp Dent Serv. 1959; 17:60.
Hardman, F.G. Keratoacanthoma on the lips. Br J Oral Surg. 1971; 9:46.
Harris, C.A. A physiological and pathological inquiry concerning the physical characteristics of the human teeth and gums, the salivary calculus, the lips and the tongue, and the fluids of the mouth. Am J Dent Sc. 1842; 3:20–132. [153–89].
Harrison, D.F. Use of estrogen in treatment of familial hemorrhagic telangiectasia. Laryngoscope. 1982; 92(3):314–320. [Mar].
Harsany, D.L., Ross, J., Fee, W.E., Jr. Follicular lymphoid hyperplasia of the hard palate simulating lymphoma. Otolaryngol Head Neck Surg. 1980; 88:349.
Hart, B., Schwartz, H.C. Cavernous hemangioma of the masseter muscle: report of a case. J Oral Maxillofac Surg. 1995; 53:467–469.
Hashimoto, K., Pritzker, M.S. Hereditary hemorrhagic telangiectasia. Oral Surg. 1972; 34:751.
Hatziotis, J.C., Asprides, H. Neurilemoma (schwannoma) of the cavity. Oral Surg. 1967; 24:510.
Haxel, O.G., Charles, G.W., Diamond, L.E. Leukoplakia buccalis. Arch Dermatol Syph. 1950; 61:781.
Hay, M.C., Paterson, D., Taylor, T.K. Aneurysmal bone cysts of the spine. J Bone Joint Surg Br. 1978; 60–B(3):406–411. [Aug].
Hazen, H.H., Eichenlaub, F.J. Leukoplakia buccalis. J Am Med Assoc. 1922; 79:1487.
Henderson, E.D., Kahlin, D.C. Chondrosarcoma of bone: a study of two hundred and eighty-eight cases. J Bone Joint Surg Am. 1922; 45:1450.
Henle, W. Evidence for viruses in acute leukemia and Burkitt’s tumor. Cancer. 1968; 21:580.
Herron, G.S., Rouse, R.V., Kosek, J.C. Benign lymphangioendothelioma. J Am Acad Dermatol. 1994; 31:362–368.
Hickey, M.J., Feinman, J. Chondrosarcoma of mandible. Dent J. 1949; 15:577. [New York].
Hicks, J.L., Nelson, J.F. Juvenile nasopharyngeal angiofibroma. Oral Surg. 1973; 35:807.
Hill, J.T., Briggs, J.D. Lymphangioma. West J Surg Obstet Gynecol. 1961; 69:78.
Hillerup, S., Hjorting-Hansen, E. Aneurysmal bone cyst—simple bone cyst, two aspects of the same pathologic entity? Int J Oral Surg. 1978; 7:16.
Hira, R.B. Diagnosis of common lesions of the oral cavity. J Oral Surg. 1957; 15:95.
Hirsch, R.J., Yousem, D.M., Loevner, L.A. Synovial sarcomas of the head and neck: MR findings. Am J Roentgenol. 1997; 169(4):1185–1188. Oct
Hirschl, S., Katx, A. Giant cell reparative granuloma outside the jaw bone. Diagnostic criteria and review of the literature with the first case described in the temporal bone. Hum Pathol. 1974; 5:171.
Hirshberg, A., Buchner, A. Metastatic tumours to the oral region: an overview. Eur J Cancer B Oral Oncol. 1995; 31B(6):355–360. [Nov].
Hirshberg, A., Leibovich, P., Buchner, A. Metastases to the oral mucosa: analysis of 157 cases. J Oral Pathol Med. 1993; 22(9):385–390. [Oct].
Hirshberg, A., Leibovich, P., Buchner, A. Metastatic tumors to the jawbones: analysis of 390 cases. J Oral Pathol Med. 1994; 23(8):337–341. [Sep].
Hobaek, A. Leukoplakia oris. Acta Odontol Scand. 1946; 7:61.
Hoffman, S., Martinez, M.G., Jr. Fibrous histiocytomas of the oral mucosa. Oral Surg Oral Med Oral Pathol. 1981; 52:277–283.
Holland, D.J. Metastatic carcinoma to the mandible. Oral Surg. 1953; 6:567.
Holman, C.B., Miller, W.E. Juvenile nasopharyngeal fibroma: roentgenologic characteristics. Am J Roentgenol Radium Ther Nucl Med. 1965; 94:292.
Holt, J.F. Neurofibromatosis in children. Am J Roentgenol. 1978; 130:615.
Horn, R.C., Jr., Enterline, H.T. Rhabdomyosarcoma: a clinicopathological study classification of 39 cases. Cancer. 1958; 11:181.
Hosoi, K. Multiple neurofibromatosis (von Recklinghausen’s disease): with special reference to malignant transformation. Arch Surg. 1931; 22:258.
Houston, G.D. The giant cell fibroma: a review of 464 cases. Oral Surg Oral Med Oral Pathol. 1982; 53(6):582–587.
Howell, J.B., Anderson, D.E., McClendon, J.L. Multiple cutaneous cancers in children:the nevoid basal cell carcinoma syndrome. J Pediatr. 1966; 69:97.
Hu, K., Yahalom, J. Radiotherapy in the management of plasma cell tumors. Oncology (Huntingt). 2000; 14(1):101–108. [111; discussion 111–12, 115, Jan].
Hubbard, E.M. Nasopharyngeal angiofibromas. AMA Arch Pathol. 1958; 65:192.
Hudson, T.M., Hamlin, D.J., Fitzsimmons, J.R. Magnetic resonance imaging of fluid levels in an aneurysmal bone cyst and in anticoagulated human blood. Skeletal Radiol. 1985; 13(4):267–270.
Hudson, T.M. Fluid levels in aneurysmal bone cysts: a CT feature. Am J Roentgenol. 1984; 142(5):1001–1004.
Hudson, T.M. Scintigraphy of aneurysmal bone cysts. Am J Roentgenol. 1984; 142(4):761–764.
Hutter, R.V.P., Stewart, F.W., Foote, F.W., Jr. Fasciitis: a report of 70 cases with follow-up proving the benignity of the lesion. Cancer. 1962; 15:992.
Hutter, R.V.P., Worcester, J.N., Jr., Francis, K.C., Foote, F.W., et al. Benign and malignant giant cell tumors of bone: a clinicopathological analysis of the natural history of the disease. Cancer. 1962; 15:653.
Huvos, A.G., Marcove, R.C., Erlandson, R.A., Miké, V. Chonodroblastoma of bone: a clinicopathologic and electron microscopic study. Cancer. 1972; 29:760.
Indignities, D.Z., Bailees, M., Papanayiotou, P. Concurrence of torus palatinus with palatal and buccal exostoses: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85:552–557.
Ingram, R.C., Coker, J.L., Jr. Palatal aneurysm. Oral Surg. 1958; 11:1158.
Ireland, D.C.R., Soule, E.H., Ivins, J.C. Myxoma of somatic soft tissues: a report of 58 patients, 3 with multiple tumors and fibrous dysplasia of bone. Mayo Clib Proc. 1973; 48:401.
Itonaga, I., Hussein, O., Kudo, A., Sabokbar, S. Athanasou. Cellular mechanisms of osteoclast formation and lacunar resorption in giant cell granuloma of the jaw. J Oral Pathol Med. 2003; 32(4):224. [April].
Ivey, D.M., Delfino, J.J., Sclaroff, A., Pritchard, L.J. Intramuscular hemangioma. Oral Surg. 1980; 50:295.
Iwach, A.G., Hoskins, H.D., Jr., Hetherington, J., Jr., Shaffer, R.N. Analysis of surgical and medical management of glaucoma in Sturge-Weber syndrome. Ophthalmology. 1990; 97(7):904–999. [Jul].
Jackson, A., Scarffe, J.H. Prognostic significance of osteopenia and immunoparesis at presentation in patients with solitary myeloma of bone. Eur J Cancer. 1990; 26(3):363–371. [Mar].
Jackson, H., Jr., Parker, F., Jr.Hodgkin’s Disease and Allied Disorders. New York: Oxford University Press, 1947.
Jacoway, J.R., Nelson, J.F., Boyers, R.C. Adenoid squamous-cell carcinoma (adenoacanthoma) of the oral labial mucosa: a clinicopathologic study of fifteen cases. Oral Surg. 1971; 32:444.
Jacqueline, M., Stevan, B.R., Joan, K.A., Mathers, M.H.B. Sorensen molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am J Surg Pathol. 2000; 24:937–946.
Jaffe, H.L. Benign osteoblastoma. Bull Hosp Joint Dis. 1956; 17:141.
Jakobi, P., Weiner, Z., Best, L., Itskovitz-Eldor, J. Hereditary hemorrhagic telangiectasia with pulmonary arteriovenous malformations. Obstet Gynecol. 2001; 97(5 Pt 2):813–814.
Jakobsson, P.A., Eneroth, C.M., Killander, D., Moberger, G., Mirtensson, B. Histologiclassification and grading of malignancy in carcinoma of the larynx. Acta Radiol Ther Phys Biol,. 1973; 12:1–8. [(a pilot study)].
James, J.N. Cavernous haemangioma of the mandible. Proc R Soc Med. 1964; 47:797.
Jarvi, O.H., Saxén, A.E., Hopsu-Havu, V.K., Wartiovaara, J.J. Elastofibroma: a degenerative pseudotumor. 1969; 23:42.
Jelinek, J.S., Murphey, M.D., Welker, J.A. Diagnosis of primary bone tumors with image-guided percutaneous biopsy: experience with 110 tumors. Radiology. 2002; 223(3):731–737. [Jun].
Jenson, A.B., Lancaster, W.D., Hartman, D.P., Shaffer, E.L., Jr. Fequency and distribution of papillomavirus structural antigens in verrucae, multiple papillomas, and condylomata of the oral cavity. Am J Pathol. 1982; 107:212.
Johnson, C.C., Gortin, R.J., Anderson, V.E. Torus mandibularis: A genetic study. Am J Hum Genet. 1965; 17:433–522.
Johnson Wc, Helwig Eb. Adenoid squamous cell carcinoma (adenoacanthoma). Cancer, 19: 1939, 1966.
Jones, A.C., Freedman, P.D., Kerpel, S.M. Oral myofibromas: a report of 13 cases and review of the literature. J Oral Maxillofac Surg. 1994; 52(8):870–875. [Aug].
Jones, B.C., Sundaram, M., Kransdorf, M.J. Synovial sarcoma: MR imaging findings in 34 patients. Am J Roentgenol. 1993; 161(4):827–830. [Oct].
Jones, R.E. Malignant melanomas mistaken histologically for junctional nevi: in AB Ackerman. In: Cash M.E., Jr., Ackerman A.B., eds. Pathology of Malignant Melanoma. USA, New York: Masson Publishing, 1981.
Ju, D.M.C. On the etiology of cancer of the lower lip. Plast Reconstr Surg. 1973; 52:151.
Kaffe, I., Ardekian, L., Taicher, S. Radiologic features of central giant cell granuloma of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996; 81(6):720–726. [Jun].
Kang, N., Ross, D., Harrison, D. Unilateral hypertrophy of the face associated with infiltrating lipomatosis. J Oral Maxillofac Surg. 1998; 56:885–887.
Kaplan, E.N. The risk of malignancy in large congenital nevi. Plast Reconstr Surg. 1974; 53:421.
Kauffman, S.L., Stout, A.P. Histocytic tumors (fibrous xanthoma and histiocytoma) in children. Cancer. 1961; 14:469.
Kay, S., Elzay, R.P., Wilson, M.A. Ultrastructural observations on a gingival granular cell tumor. Cancer. 1971; 27:674. [(congenital epulis)].
Kay, S., Gerszten, E., Dennison, S.M. Light and electron microscopic study of a rhabdomyoma arising in the floor of the mouth. Cancer. 1969; 23:708.
Kay, S. Subcutaneous pseudosarcomatous fibromatosis: report of 4 cases. Am J Clin Pathol. 1960; 33:433.
Keller, A.R., Kaplan, H.S., Lukes, R.J., Rappaport, H. Correlation of histopathology with other prognostic indicators in Hodgkin’s disease. Cancer. 1968; 22:487.
Keller, A.Z. Cirrhosis of the liver, alcoholism and heavy smoking associated with cancer of the mouth and pharynx. Cancer. 1967; 20:1015.
Kempson, R.L., McGavran, M.H. Atypical fibroxanthomas of the skin. Cancer. 1964; 17:1463.
Kennedy, T.L. Cystic hygroma-lymphangioma: a rare and still unclear entity. Laryngoscope. 1989; 99:1–10.
Kerr, D.A., Pullon, P.A. A study of the pigmented tumors of the jaws of infants (melanotic ameloblastoma, retinal anlage tumor, progonoma). Oral Surg. 1964; 18:759.
Kerr, D.A. Nicotine stomatitis. J Mich Dent Soc. 1948; 30:90.
Khairi, M.R.A., Dexter, R.N., Burzynski, N.J., Johnson, C.C., Jr. Mucosal neuroma, pheochromocytoma and medullary thyroid carcinoma: multiple endocrine neoplasia type 3. Medicine. 1975; 54:89.
Kingsley, T.C., Markel, S.F. Extrasketetal chondroblastoma: a report of the first recorded case. Cancer. 1971; 27:203.
Kjaerheim, A., Stokke, T. Juvenile xanthogranuloma of the oral cavity. Oral Surg. 1974; 38:414.
Klijanienko, J., Caillaud, J.M., Lagace, R. Cytohistologic correlations in 56 synovial sarcomas in 36 patients: the institut curie experience. Diagn Cytopathol. 2002; 27(2):96–102.
Klug, H., Gunther, W. Ultrastructural differences in human malignant melanomata. Br J Dermatol. 1972; 86:395.
Kohn, M.W., Eversole, L.R. Keratoacanthoma of the lower lip: report of cases. J Oral Surg. 1972; 30:522.
Kolas, S., Halperin, V., Jefferis, K., Huddleston, S.D. The occurrence of torus palatinus and torus mandibularis is 2,478 dental patients. Oral Surg. 1953; 6:1134.
Kollar, J.A., Finley, C.W., Jr., Nabers, J.M., Ritchey, B. Leukoplakia. J Am Dent Assoc. 1954; 49:538.
Kolmeier, K.H., Bayrd, E.D. Familial leukemia: report of instance and review of the literature. Mayo Clin Proc. 1963; 38:523.
Kostrubala, J.G., Thurston, E.W., Chapin, M.E. Metastatic dysgerminoma of the mandible. Oral Surg. 1950; 3:1184.
Kotner, L.M., Wang, C.C. Plasmacytoma of the upper air and food passages. Cancer. 1972; 30:414.
Koudstaal, J., Oldhoff, J., Panders, A.K., Hardonk, M.J. Melanotic neuroectodermal tumor of infancy. Cancer. 1968; 22:151.
Kraemer, B.B., Schmidt, W.A., Foucar, E., Rosen, T. Verruciform xanthoma of the penis. Arch Dermatol. 1968; 117:516.
Kragh, L.V., Dahlin, D.C., Erich, J.B. Cartilaginous tumors of the jaws and facial regions. Am J Surg. 1960; 99:852.
Kramer, I.R.H., El-Labban, N., Lee, K.W. The clinical features and risk of malignant transformation in sublingual keratosis. Br Dent J. 1978; 144:171.
Kramer, I.R.H. Carcinoma-in-situ of the oral mucosa. Int Dent J. 1973; 23:94.
Kransdorf, M.J. Malignant soft-tissue tumors in a large referral population: distribution of diagnoses by age, sex, and location. Am J Roentgenol. 1995; 164(1):129–134. [Jan].
Kreshover, S.J., Salley, J.J. Predisposing factors in oral cancer. J Am Dent Assoc. 1957; 54:509.
Krolls, S.O., Hoffman, S. Squamous cell carcinoma of the oral soft tissues: a statistical analysis of 14, 253 cases by age, sex and race of patients. J Am Dent Assoc. 1976; 92:571.
Krolls, S.O., Jacoway, J.R., Alexander, W.N. Osseous choristomas (osteomas) of intraoral soft tissues. Oral Surg. 1971; 32:588.
Krugman, M.E., Rosin, H.D., Toker, C. Synovial sarcoma of the head and neck. Arch Otolaryngol. 1973; 98:53.
Kumar, A., Bagewadi, A., Keluskar, V., Singh, M. Efficacy of lycopene in the management of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103(2):207–213.
Kwapis, B.W., Keubel, J.O. Bronchogenic carcinoma with probable metastasis to the gingiva: report of case. J Oral Surg. 1952; 10:255.
Kyle, R.A., Elveback, L.R. Management and prognosis of multiple myeloma. Mayo Clin Proc. 1976; 51:751.
Kyle, R.A. Multiple myeloma review of 869 cases. Mayo Clin Proc. 1975; 50:29.
Lack, E.E., Perez-Atayde, A.R., McGill, T.J., Vawter, G.F. Gingival granular cell tumor of the newborn (congenital ‘epulis’): ultrastructural observations relating to histogenesis. Hum Pathol. 1982; 13:686.
Ladanyi, M. Fusions of the SYT and SSX genes in synovial sarcoma. Oncogene. 2001; 20(40):5755–5762. [Sep,10].
Lain, E.S. Lesions of the oral cavity caused by physical and by physiochemical factors. Arch Dermatol Syph. 1941; 41:295.
Langdon, J.D., Rapidis, A.D., Patel, M.F. Ossifying fibroma—one disease of six? An analysis of 39 fibroosseous lesions of the jaws. Br J Oral Surg. 1976; 14:1.
Lapertico, P., Ibanez, K.L. Nasal glioma (encephalochoristoma nasfrontalis): report of a case. Arch Otolaryngol. 1964; 79:628.
Lapins, N.A., Helwig, E.B. Perineural invasion by keratoacanthoma. Arch Dermatol. 1980; 116:791.
Latchaw, RE-MR. CT Imaging of the Head, Neck and Spine. Louis: CV Mosby,St; 1991.
Lawrence, W., Jegge, G., Jr., Foote, F.W., Jr. Embryonal rhabdomyosarcoma: a clinicopathological study. Cancer. 1964; 17:361.
Lazova, R., Mynes, R., May, D., Scott, G. G. Ln-2 (CD74) — a marker to distinguish atypical fibroxanthoma from malignant fibrous histiocytoma. Cancer. 1997; 79:2115–2124.
Lehner, T. The jaws and teeth in Burkitt’s tumour (African lymphoma). J Path Bacteriol. 1964; 88:581.
Leifer, C., Miller, A.S., Butong, P.B., Min, B.H. Spindle-cell carcinoma of the oral mucosa: a light and electron microscopic study of apparent sarcomatous metastasis to cervical lymph nodes. Cancer. 1974; 34:597.
Lever, W.F., Schaumberg-Lever, G. Histopathology of the Skin, 5. JB Lippincott: Philadelphia, 1975.
Levin, L.S., Jorgenson, R.J., Jarvey, B.A. Lymphangiomas of the alveolar ridges in neonates. Pediatrics. 1976; 58:881–884.
Levine, G.D. Hibernoma.An electron microscopic study. Hum Pathol. 1972; 3:351.
Levine, P.H., Kamaraju, L.S., Connelly, R.R., Berard, C.W. The American Burkitt’s Lymphoma Registry: eight years’ experience. Cancer. 1982; 49:1016.
Levy, W.M., Miller, A.S., Bonakdarpour, A., Aegerter, E. Aneurysmal bone cyst secondary to other osseous lesions: report of 57 cases. Am J Clin Pathol. 1975; 63:1.
Lewis, J.J., Antonescu, C.R., Leung, D.H. Synovial sarcoma: a multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol. 2000; 18(10):2087–2094. [May].
Lichtenstein, L. Aneurysmal bone cyst observation on fifty cases. J Bone Joint Surg Am. 1957; 39:873.
Lichtiger, B., Mackay, B., Tessmer, C.F. Spindlecell variant of squamous carcinoma: a light and electron microscopic study of 13 cases. Cancer. 1970; 26:1311.
Lieberman, P.H., Foote, F.W., Stewart, F.W., Jr., Berg, J.W. Alveolar soft-part sarcoma. J Am Med Assoc. 1966; 198:1047.
Liebross, R.H., Ha, C.S., Cox, J.D. Solitary bone plasmacytoma: outcome and prognostic factors following radiotherapy. Int J Radiat Oncol Biol Phys. 1998; 41(5):1063–1067.
Lighterman, I. Hemangioendothelioma of the tongue: report of case. J Oral Surg. 1952; 10:163.
Lim, L., Gibbins, J.R. Immunohistochemical and ultrastructural evidence of a modified microvasculature in the giant cell granuloma of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995; 79:190–198.
Link, J.F. Chondrosarcoma of the maxilla. Oral Surg. 1954; 7:140.
Litzow, T.J., Lash, H. Lymphangiomas of the tongue. Mayo Clin Proc. 1961; 36:229.
Lloyd, G. Radiology in Focus. Imaging for juvenile angiofibroma. J Laryngol Otol. 2000; 114(9):727–730. [Sep].
Lloyd, G. Juvenile angiofibroma: the lessons of 20 years of modern imaging. J Laryngol Otol. 2000; 113(2):127–134. [Feb].
Lois, J.F., Fischer, H.J., Mirra, J.M., Gomes, A.S. Angiography of histopathologic variants of synovial sarcoma. Acta Radiol Diagn (Stockh). 1999; 7(4):449–454. [Feb].
Lowe, R.S., Robinson, D.W., Ketchum, L.D., Masters, F.W. Nasal gliomata. Plast Reconstr Surg. 1971; 47:1.
Lufkin, R., Borges, A., Nguyen, K., Anzai, Y. MRI of the Head and Neck (2nd ed). JB Lipincott: Williams and Wilkins; 2001.
Lukes, R.J., Collins, R.D. Immunological characterization of human malignant lymphomas. Cancer. 1974; 34:1488.
Lukes, R.J. Criteria for involvement of lymph node, bone marrow, spleen, and liver in Hodgkin’s disease. Cancer Res. 1971; 31:1755.
Lund, B.A., Dahlin, D.C. Hemangiomas of the mandible and maxilla. J Oral Surg. 1964; 22:234.
Lund, H.Z., Kraus, J.M.K.Melanotic Tumors of the Skin (Atlas of Tumor Pathology, Section I, Fascicle 3). Washington DC: Armed Forces Institute of Pathology, 1962.
MacDonald, I.M., Bech-Hansen, N.T., Britton, W.A., Jr. The phakomatoses: recent advances in genetics. Can J Ophthalmol. 1997; 32(1):4–11. [Feb].
MacGregor, A.B. Chondroma of the maxilla, Br Dent J. 1952; 94:39.
Mackenzie, I.C., Dabelsteen, E., Squier, C.A.Oral premalignancy. Iowa: University of Iowa Press, 1980.
Macmillan, A.R.G., Oliver, A.J., Reade, P.C. Regional macrodontia and regional bony enlargement associated with congenital infiltrating lipomatosis of the face presenting as unilateral facial hyperplasia. Int J Oral Maxillofac Surg. 1990; 19:283–286.
Maher, C.O., Piepgras, D.G., Brown, R.D., Jr., Friedman, J.A. Cerebrovascular manifestations in 321 cases of hereditary hemorrhagic telangiectasia. Stroke. 2001; 32(4):877–882. [Apr].
Mann, R.B., Jaffe, E.S., Berard, C.W. Malignant lymphomas—a conceptual understanding of morphologic diversity: a review. Am J Pathol. 1979; 94:105.
Marcove, R.C., Miké, V., Hajek, J.V., Levin, A.G. Osteogenic sarcoma under the age of twenty-one. J Bone Joint Surg Am. 1970; 52:411.
Mark, R.J., Sercarz, J.A., Tran, L. Fibrosarcoma of the head and neck. The UCLA experience. Arch Otolaryngol Head Neck Surg. 1991; 117:396–401.
Marsh, J., Vannier, M.Compreshensive Care for Craniofacial Deformities. St Louis: CV Mosby, 1985.
Marshall, R.B., Horn, R.C., Jr. Nonchromaffin paraganglioma. Cancer. 1961; 14:779.
Marti-Bonmati, L., Menor, F., Mulas, F. The Sturge-Weber syndrome: correlation between the clinical status and radiological CT and MRI findings. Childs Nerv Syst. 1993; 9:107–109.
Martin, H.E., Munster, H., Sugarbaker, E.D. Cancer of the tongue. Arch Surg. 1940; 41:888.
Martin, H.E., Sugarbaker, E.D. Cancer of the floor of the mouth. Surg Gynecol Obstet. 1940; 71:347.
Martin, H.E. Cancer of the gums (gingivae). Am J Surg. 1941; 54:765.
Mashberg, A., Meyers, H. Anatomical site and size of 222 early asymptomatic oral squamous cell carcinomas: a continuing prospective study of oral cancer II. Cancer. 1976; 37:2149.
Mashberg, A., Morrissey, J.B., Garfinkel, L. A study of the appearance of early asymptomatic oral squamous cell carcinoma. Cancer. 1973; 32:1436.
Mashberg, A., Thoma, K.H., Wasilewski, E.J. Olfactory neuroblastoma (esthesioneuro-epithelioma) of the maxillary sinus. Oral Surg. 1960; 13:908.
Massarelli, G., Tanda, F., Salis, B. Synovial sarcoma of the soft palate: report of a case. Hum Pathol. 1978; 9:341.
Masson, P. My conception of cellular nevi. Cancer. 1951; 4:9.
Mathis, H., Herrmann, D. Erythroplasie de Queyrat an der Mundschleimhaut Z Stomatol. 1963; 60:170.
Matsuo, M., Kanematsu, M., Kato, H., Kondo, H. Osler-Weber-Rendu disease: visualizing portovenous shunting with three-dimensional sonography. Am J Roentgenol. 2001; 176(4):919–920. [Apr].
Maurer, H.M., Moon, T., Donaldson, M., Fernandez, C. The Intergroup rhabdomysarcoma study: a preliminary report. Cancer. 1977; 40:2015.
McCaffery, T.V., Neel, H.B., III., Gaffey, T.A. Malignant melanoma of the oral cavity: review of 10 cases. Laryngoscope. 1980; 90:1329.
McCarthy, F.P. Etiology, pathology and treatment of leukoplakia buccalis, with report of 316 cases. Arch Dermatol Syph. 1936; 34:612–623.
McCarthy, W.P., Pack, G.T. Malignant blood vessel tumors. Surg Gynecol Obstet. 1950; 91:465.
McClathey, K.D., Batsakis, J.G., Young, S.K. Intravascular angiomatosis. Oral Surg. 1978; 46:70.
McComb, R.J., Trott, J.R. Spontaneous oral haemorrhage: arteriovenous aneurysm. An unusual cases. Br Dent J. 1970; 128:239.
McCormack, L.J., Gallivan, W.F. Hemangiopericytoma. Cancer. 1954; 7:595.
McCoy, J.M., Waldron, C.A. Verrucous carcinoma of the oral cavity: a review of forty-nine case. Oral Surg. 1981; 52:923.
McCrea, M.W., Miller, A.S., Rosenthal, S.L. Intraoral blue nevi. Oral Surg. 1968; 25:590.
McDaniel, R.K., Newland, J.R., Chiles, D.G. Intraoral spindle cell lipoma: case report with correlated light and electron microscopy. Oral Surg Oral Med Oral Pathol. 1984; 57:52–57.
McGovern, V.J. The nature of melanoma: a critical review. J Cutan Pathol. 1982; 9:61.
McGowan, D.A., Jones, J.H. Angioma (vascular leiomyoma) of the oral cavity. Oral Surg. 1969; 27:649.
McGrath, P.J. Giant-cell tumour of bone: an analysis of fifty-two cases. J Bone Joint Surg Br. 1972; 54:216.
McKenna, R.J., Schwinn, C.P., Soong, K.Y., Higinbotham, N.L. Osteogenic sarcoma arising in Paget’s disease. Cancer. 1964; 17:42.
McLeod, R.A., Dahlin, D.C., Beabout, J.W. The spectrum of osteoblastoma. Am J Roentgenol. 1976; 126:321.
Mehregan, A.H., Pinkus, H. Intraepidermal epithelioma: a critical study. Cancer. 1964; 17:609.
Meis, J.M., Butler, J.J., Osborne, B.M. Solitary plasmacytomas of bone and extramedullary plasmacytomas: a clinicopathologic and immunohistochemical study. Cancer. 1987; 59(8):1475–1485.
Meis-Kindblom, J.M., Kindblom, L.G., Enzinger, F.M. Sclerosing epithelioid fibrosarcoma–a variant of fibrosarcoma simulating carcinoma. Am J Surg Pathol. 1995; 19:979–993.
Meister, P. Malignant fibrous histiocytoma-histomorphological pattern or tumor type. Pathol Res Pract. 1996; 192:877–1811.
Melrose, R.J., Abrams, A.M. Juvenile fibromatosis affecting the jaws: report of three cases. Oral Surg. 1980; 49:317.
Merryweather, R., Middlemiss, J.H., Sanerkin, N.G. Malignant transformation of osteoblastoma. J Bone Joint Surg. 1980; 62:381.
Meyer, I., Abbey, L.M. The relationship of syphilis to primary carcinoma of the tongue. Oral Surg. 1970; 30:678.
Meyer, I., Shklar, G. Malignant tumors metastatic to mouth and jaws. Oral Surg. 1965; 20:350.
Miles, A.E.W. Chondrosarcoma of the maxilla. Br Dent J. 1950; 88:257.
Miller SC, Roth H. torus palatinus: a statistical study. J Am Dent Assoc, 1940; 27: 1950–57.
Miller, A.P., Owens, J.B., Jr. Teratoma of the tongue. Cancer. 1966; 19:1583.
Miller, A.S., Leifer, C., Chen, S.Y., Harwick, D. Oral granular-cell tumors: report of twenty-five cases with electron microscopy. Oral Rurg. 1980; 38:694.
Miller, R.L., Burzynski, N.J., Giammara, B.L. The ultrastructure of oral neuromas in multiple mucosal neuromas, pheochromocytoma, mekullary thyroid carcinoma syndrome. J Oral Pathol. 1977; 6:253.
Miller, W.B., Jr., Wirman, J.A., McKinney, P. Extraosseous osteogenic sarcoma of forearm Arch Pathol. 1974; 97:246.
Mincer, H.H., Spears, K.D. Nerve sheath myxoma in the tongue. Oral surg. 1974; 37:428.
Mintz, G.A., Abrams, A.M., Carlsen, G.D., Melrose, R.J. Primary malignant giant cell tumor of the mandible: report of a case and review of the literature. Oral Surg. 1981; 51:164.
Mitcherling, J.J., Collins, E.M., Tomich, C.E., Bianco, R.P. Synovial sarcoma of the neck: report of case. J Oral Surg. 1976; 34:64.
Mnaymneh, W.A., Dudley, H.R., Mnaymneh, L.G. Giant cell tumor of bone: an analysis nd followup study of the forty-one cases observed at the Massachusetts General Hospital between 1925 and 1961. J Bone Joint Surg Am. 1964; 46:63.
Modlin, J., Johnson, R.E. The surgical treatment of cancer of the buccal mucosa and lower gingivae. Am J Roentgenol. 1955; 73:620.
Moertel, C.G., Foss, E.L. Multicentric carcinomas of the oral cavity. Surg Gynecol Obstet. 1958; 106:652.
Monkman, G.R., Orwoll, G., Ivins, J.C. Trauma and oncogenesis. Mayo Clin Proc. 1974; 49:157.
Montgomery, H. Precancerous dermatoses and epithelioma in situ. Arch Dermatol Syph. 1939; 39:387.
Moore, C. Smoking and cancer of the mouth, pharynx, and larynx. J Am Med Assoc. 1965; 191:283.
Moore, O., Grossi, C. Embryonal rhabdomyosarcoma of the head and neck. Cancer. 1959; 12:69.
Moorman, W.C., Shafer, W.G. Metastatic carcinoma of the mandible. J Oral Surg. 1954; 12:205.
Moran, J.J., Enterline, H.T. Benign rhabdomyoma of the pharynx: a case report, review of the literature, and comparison with cardiac rhabdomyoma. Am J Clin Pathoo. 1964; 42:174.
Morrison, R., Deeley, T.J. Intra-alveolar carcinoma of the jaw: treatment by supervoltage radiotherapy. Br J Radiol. 1962; 35:321.
Morton, K.S. Aneurysmal bone cyst: a review of 26 cases. Can J Surg. 1986; 29(2):110–205. [Mar].
Moscovic, E.A., Azar, H.A. Multiple granular cell tumors (‘myoblastoma’); case report with electron microscopic observations and review of the literature. Cancer. 1967; 20:2032.
Mulay, D.N., Urbach, F. Local therapy of oral leukoplakia with vitamin: a Arch Dermatol Syph. 1958; 78:637.
Mulliken, J.B., Glowacki, J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982; 69(3):412–422. [Mar].
Mummery, Jh, Pitts, A.T. A melanotic epithelial odontome in a child. Br Dent J. 1926; 47:121.
Murray, M., Stout, A P. Characteristics of human Schwann cels in vitro. Ant Rec. 1942; 84:275.
Murray, M. Cultural characteristics of three granular-cell myoblastomas. Cancer. 1951; 4:857.
Mustard, R.A., Rosen, I.B. Cervical lymph node involvement in oral cancer. Am J Roentgenol Radium Ther Nucl Med. 1963; 90:978.
Nathanson, I.T., Weisberger, D.B. The treatment of leukoplakia buccalis and related lesions with estrogenic hormones. New Engl J Med. 1939; 221:556.
Nathwani, B.N. A critical analysis of the classifications of non-Hodgkin’s lymphomas. Cancer. 1979; 44:347.
Neumann-Jensen, B., Praetorius, F. Et usaedvanligt tilfaelde of aneurysmal knoglecyste Tandlaegebladet. 1977; 81:230.
Neville, B.W., Damm, D.D., Allen, C.M. Oral and maxillofacial pathology, 1, Philadelphia: WB Saunders; 1995:453–455.
Neville, B.W., Weathers, D.R. Verruciform xanthoma. Oral Surg. 1980; 49:429.
New, G.B., hallberg, O.E. The end-results of the treatment of malignant tumors of the palate. Surg Gynecol Obstet. 1941; 73:520.
Nieburgs, H.E., Herman, B.E., Reisman, H. Buccal cell changes in patients with malignant tumors. Lab Invest. 1962; 11:80.
Nix, J.T. Lymphangioma. Am Surg. 1954; 20:556.
1993. No authors listed. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 20–1993. A 23-year-old woman with a rapidly enlarging intraoral mass after a tooth extraction. New Engl J Med, 328(20): 1478–83, May, 1993.
Nuamah, I.K., Browne, R.M. Malignant fibrous histiocytoma presenting as perioral abscess. Internat J Oral Maxillofac Surg. 1995; 24:158–159.
O’Brien, J.E., Stout, A P. Malignant fibrous xanthomas. Cancer. 1964; 17:1445.
O’Brien, P.H., Brasfield, R.D. Hemangiopericytoma. Cancer. 1965; 18:249.
O’Connor, G.T., Rappaport, H., Smith, E.B. Childhood lymphoma resembling ‘Burkitt Tumor’ in the United States. Cancer. 1965; 18:411.
O’Conor, G.T. Malignant lymphoma in African children II: a pathological entity. Cancer. 1961; 14:270.
O’Day, R.A., Soule, E.H., Gores, R.J. Embryonal rhabdomyosarcoma of the oral soft tissues. Oral Surg. 1965; 20:85.
O’Malley, M., Pogrel, M., Stewart, J.C.B., Silva, R.G. Central giant cell granulomas of the jaws: phenotype and proliferation-associated markers. J Oral Pathol Med. 1997; 26:159–163.
Oakes, W.J. The natural history of patients with the Sturge-Weber syndrome. Pediatr Neurosurg. 1992; 18:287–290.
Oberman, H.A., Rice, D.H. Olfactory neurblastomas: a clinicopathologc study. Cancer. 1976; 38:2494.
Oberman, H.A., Holtz, F., Sheffer, L.A., Magielski, J.E. Chemodectomas (nonchromaffin paragangliomas) of the head and neck: a clinicopathologic study. Cancer. 1968; 21:838.
Odell, E.W., Morgan, P.R.Biopsy pathology of the oral tissues. London: Chapman and Hall Medical, 1998.
Oliver, W.M. Hereditary hemorrhagic telangiectasia. Oral Surg. 1963; 16:658.
Oppenheimer, R.W., Friedman, M. Fibrosarcoma of the maxillary sinus. Ear Nose Throat J. 1988; 67:193–198.
Orbach, S. Congenital arteriovenous malformations of the face. Report of a case oral Surg. 1976; 42:2.
Oringer, M.J. Neuroma of the mandible. Oral Surg. 1948; 1:1135.
Oshiro, Y., Fukuda, T., Tsuneyoshi, M. Atypical fibroxanthoma versus benign and malignant fibrous histiocytoma. Cancer. 1995; 75:1128–1134.
Paissat, D.K. Oral submucous fibrosis. Int J Oral Surg. 1966; 237:269.
Pandolfi, P.J., Felefli, S., Flaitz, C.M., Johnson, J.V. An aggressive peripheral giant cell granuloma in a child. J Clin Pediatr Dent, Summer. 1999; 23(4):353–355.
Parker, F., Jr., Jackson, H., Jr. Primary reticulum cell sarcoma of bone. Surg Gynecol Obstet. 1939; 68:45.
Parmentier. Essay on tumors in the palatine region. Am J Dent Sc, 7 (new series): 324–39, 456–65, 545–61, 1857
Patchefsky, A.S., Brodovsky, H.S., Menduke, H., Southard, M. Non-Hodgkin’s Lymphomas: a clinicopathologic study of 293 cases. Cancer. 1974; 34:1173.
Patchefsky, A.S., Brodovsky, H., Southard, M., Menduke, H. Hodgkin’s Disease: a clinical and pathologic study of 235 cases. Cancer. 1973; 32:150.
Patterson, C.N. Juvenile nasopharyngeal angiofibroma. Arch Otolaryngol. 1965; 81:270.
Patton, R.B., Horn, R.C., Jr. Rhabdomyosarcoma: clinical and pathological features and comparison with human fetal and embryonal skeletal muscle. Surgery. 1962; 52:572.
Pau, H., Carney, A.S., Murty, G.E. Hereditary haemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): otorhinolaryngological manifestations. Clin Otolaryngol. 2001; 26(2):93–98.
Peacock, E.E., Jr., Greenberg, B.G., Brawley, B.W. The effect of snuff and tobacco on the production of oral carcinoma: an experimental and epidemiological study. Ann Surg. 1960; 151:542.
Pearse, A.G. The histogenesis of granular-cell myoblastoma (granular-cell perineural fibroblastoma). J Pathol Bacteriol. 1950; 62:351.
Pesce, C., Valente, S., Gandolfo, A.M., Lenti, E. Intravascular lobular capillary hemangioma of the lip. Histopathol. 1996; 29:382–384.
Peterman, A.F., Hayles, A.B., Dockerty, M.B., Love, J.G. Encephalotrigeminal angiomatosis (Sturge-Weber disease). Clinical study of thirty-five cases. J Am Med Assoc. 1958; 167:2169.
Petit, V.D., Chamness, J.T., Ackerman, L.V. Fibromatosis and fibrosarcoma following irradiation therapy. Cancer. 1954; 7:149.
Pimpinella, R.J. The nasopharyngeal angiofibroma in the adolescent male. J Pediatr. 1964; 64:260.
Pindborg, J.J., Sirsat, S.M. Oral submucous fibrosis. Oral Surg. 1966; 22:764.
Pindborg, J.J., Reibel, J., Roed-Petersen, B., Mehta, F.S. Tobacco-induced changes in oral leukoplakic epithelium. Cancer. 1980; 45:2330.
Pindborg, J.J., Zachariah, J. Frequency of oral submucous fibrosis among 100 south Indians with oral cancer. Bull WHO. 1965; 32:750.
Pindborg, J.J., Chawla, T.N., Srivastava, A.N., Gupta, D. Epithelial changes in oral submucous fibrosis. Acta Odontol Scand. 1965; 23:277.
Pindborg, J.J., Chawla, T.N., Srivastava, A.N., Gupta, D. Clinical aspects of oral submucous fibrosis. Acta Odontol Scand. 1964; 22:679.
Pindborg, J.J.J., Olst, O., Renstrup, G., Roed-Petersen, B. Studies in oral leukoplakia: a report on the period prevalence of malignant transformation in leukoplakia based on a follow-up study of 248 patients. J Am Dent Assoc. 1968; 76:767.
Pindborg, J.J., Kramer, I.R.M., Torloni, H.Histological typing of odontogenic tumours, jaw cysts and allied lesions, international histological classification of tumours No 5. Geneva: World Health Organization, 1971.
Pindborg, J.J., Mehta, F.S., Gupta, P.C., Daftary, D.K. Prevalance of oral submucous fibrosis among 50,915 Indian villagers. Br J Cancer. 1968; 22:646.
Pindborg, J.J., Mehta, F.S., Daftary, D.K. Incidence of oral cancer among 30,000 villagers in India in a 7–year follow-up study of oral precancerous lesions. Community Dent Oral Epidemiol. 1975; 3:86.
Pindborg, J.J., Renstrup, G., Poulsen, H.E., Silverman, S., Jr. Studies in oral leukoplakias. Acta Odontol Scand. 1963; 21:407.
Pindborg, J.J. Fibrous dysplasia or fibro-osteoma. Acta Radiol. 1951; 36:196.
Pinkus, G.S., Said, J.W. Characterization of non-Hodgkin’s lymphomas using multiple cell markers. Immunologic, morphologic, and cytochemical studies of 72 cases. Am J Pathol. 1979; 94:349.
Pinkus, H. Keratosis senilis: a biologic concept of its pathogenesis and diagnosis based on the study of normal epidermis and 1730 seborrhiec and senile keratoses. Am J Clin Pathol. 1958; 29:193.
Platkajs, M.A. A clinicopathologic study of oral leukoplakia with emphasis on the keratinization pattern. J Can Dent Assoc. 1979; 3:107.
Pliskin, M.E. Malignant melanoma of the oral cavity. In: WH Clark, Jr., LI Goldman, MJ Mastrangelo, eds. Human Malignant Melanoma. New York: Grune and Stratton, 1979.
Poli, P., Floretti, G., Tessitori, G. Malignant fibrous histiocytoma of the floor of the mouth-case report. J Laryngol Otol. 1995; 109:680–682.
Pomeroy, T.C., Johnson, R.E. Prognostic factors for survival in Ewing’s sarcoma. Am J Roentgenol. 1975; 123:598.
Potdar, G.G. Ewing’s tumors of the jaws. Oral Surg. 1970; 29:505.
Potter, B.J., Tiner, B.D. Central giant cell granuloma: report of a case. Oral Surg Oral Med Oral Pathol. 1993; 75(3):286–289.
Praetorius-Clausen, F. Historadiographic study of oral leukoplakias, Scand J Dent Res. 1970; 78:479.
Prescott, G.H., White, R.E. Solitary, central neurofibroma of the mandible: report of case and review of the literature. J Oral Surg. 1970; 28:305.
Preston, F.W., Walsh, W.S., Clarke, T.H. Cutaneous neurofibromatosis (vonRecklinghausen’s disease): clinical manifestations and incidence of sarcoma in sixty-one male patients. Arch Surg. 1952; 64:813.
Price, C.H.G., Gldie, W. Paget’s sarcoma of bone: a study of eighty cases from the Bristol and the leeds bone tumour registries. J Bone Joint Surg Br. 1969; 51:205.
Quick, D., Cutler, M. Transitional cell epidermoid carcinoma: a radiosensitive type of intraoral tumor. Surg Gynecol Obstet. 1927; 45:320.
Quigley, L.F., Jr., Cobb, C.M., Schoenfled, S., Hunt, E.E. Reverse smoking and its oral consequences in Caribbean and South American peoples. J Am Dent Assoc. 1964; 69:427.
Rahausen, A., Sayago, C. Treatment of carcinoma of the tongue. Am J Roentgenol Radium Ther Nucl Med. 1954; 71:243.
Rahimi, A., Beabout, J.W., Ivins, J.C., Dahlin, D.C. Chondromyxoid fibroma: a clinicopathologic study of 76 cases. Cancer. 1972; 30:726.
Rajendran, R., Rani, V., Shaikh, S. Pentoxifylline therapy: a new adjunct in the treatment of oral submucous fibrosis. Ind J Dent Res. 2006; 17(4):190–198.
Ramani, P., Shah, A. Lymphangiomatosis: histologic and immunohistochemical analysis of four cases. Am J Surg Pathol. 1993; 17:329–335.
Rapidis, A.D. Lipoma of the oral cavity. Int J Oral Surg. 1982; 11:263–275.
Rappaport, H.Tumors of the Hematopoietic System (Atlas of Tumor Pathology, Section III, Fascicle 8). Armed Forces Institute of Pathology: Washington DC, 1966.
Rappaport, H.M. Neurofibromatosis of the oral cavity: report of case. Oral Surg. 1953; 6:599.
Reed, R.J., Fine, R.M., Meltzer, H.D. Palisaded, encapsulated neuromas of the skin. Arch Derm. 1972; 106:865.
Reed, R.J.New Concepts in Surgical Pathology of the Skin. New York: John Wiley and Sons, 1976.
Regezi, J.A., Sciubba, J.J. Oral pathology: clinical pathologic correlations, 3, Philadelphia: WB Saunders; 1993:368–370.
Regezi, J.A., Batsakis, J.G., Courtney, R.M. Granular cell tumors of the head and neck. J Oral surg. 1979; 37:402.
Regezi, J.A., Hayward, J.R., Pickens, T.N. Superficial melanomas of oral mucous membranes. Oral Surg. 1978; 45:703.
Reichart, P., Reznik-Schuller, H. The ultrastructure of an oral angiomyoma. J Oral Pathol. 1977; 6:25.
Renstrup, G. Leukoplakia of the oral cavity. Acta Odontol Scand. 1958; 16:99.
Resnick D Niwayama G. Tumors and tumor-like lesions of bone: imaging and pathology of specific lesions: in diagnosis of bone and joint disorders. 3831–42, 1988
Rezai, R.F., Jackson, J.T., Salamat, K. Torus palatinus, an exostosis of unknown etiology: review of the literature. Compend Contin Educ Dent. 1985; 6:149–152.
Ricciardelli, E.J., Richardson, M.A. Cervicofacial cystic hygroma: patterns of recurrence and management of the difficult case. Arch Otolaryngol Head Neck Surg. 1991; 117:546–553.
Richards, G.E. Radiation therapy of carcinoma of the buccal mucosa. In: Pack G.T., Livingston E.M., eds. Treatment of Cancer and Allied Diseases. New York: Paul B Hoeber, Inc, 1940. [(cheek) (eds)].
Rigor, V.U., Goldstone, S.E., Jones, J. Hibernoma: a case report and discussion of a rare tumor. Cancer. 1986; 57:2207–2211.
Ritch, R. Serous retinal detachment after glaucoma filtration surgery in Sturge-Weber Syndrome. J Glaucoma. 1992; 1(1):58–62.
Road-Petersen, B., Renstrup, G., Pindborg, J.J. Candida in oral leukoplakias: a histologic and exfoliative study. Scand J Dent Res. 1970; 78:323.
Robbins, S.L., Cotran, R.S. Pathologic Basis of Disease, 2. Philadelphia: WB Saunders, 1979.
Robinson, L.H., Unni, K.K., O Laughlin, S., Beabout, W.B. Surface chondromyxoid fibroma of bone. Mod Pathol. 1994; 7:10A. [(abstract)].
Robinson, L., Hukill, P.B. Hutchinson’s melanotic freckle in oral mucous membrane. Cancer. 1970; 26:297.
Robinson, M., Slavkin, H.C. Dental amputation neuromas. J Am Dent Assoc. 1965; 70:662.
Roca, A.N., Smith, J.L., Jing, B.S. Osteosarcoma and parosteal osteogenic sarcoma of the maxilla and mandible: study of 20 cases. Am J Clin Pathol. 1970; 54:625.
Rodriguez, H.A., Ackerman, L.V. Cellular blue nevus. Clinicopathologic study of forty-five cases. Cancer. 1968; 21:393.
Roed-Petersen, B. Cancer development in oral leukoplakia: follow-up of 331 patients. J Dent Res. 1971; 50:711.
Roed-Peterson, B., Pindborg, J.J. A study of Danish snuff-induced oral leukoplakias. J Oral Pathol. 1973; 2:301.
Roffo, A.H. Leucoplasie expérimentale produite par le tobac. Rev Stomatol. 1930; 32:699.
Rosen, G., Caparros, B., Mosende, C., McCormick, B. Curability of Ewing’s sarcoma and considerations for future therapeutic trials. Cancer. 1978; 41:888.
Rosenberg Gertzman, G.B., Clark, M., Gaston, G. Multiple hamartoma and neoplasia syndrome. Oral Surg. 1980; 49:314. [(Cowden’s syndrome)].
Rosenberg, S.A. National Cancer Institute Sponsored Study of Classification of Non-Hodgkin’s Lymphomas. Summary and description of a working formulation for clinical usage. Cancer. 1982; 49:2112.
Rossiter, J.L., Hendrix, R.A., Tom, L.W., Potsic, W.P. Intramuscular hemangioma of the head and neck. Otolaryngol Head Neck Surg. 1993; 108:18–26.
Roth, J.A., Enzinger, F.M., Tannenbaum, M. Synovial sarcoma of the neck: a follow-up study of 24 cases. Cancer. 1975; 35:1243.
Roth, S.I., Stowell, R.E., Helwig, E.B. Cutaneous ossification: report of 120 cases and review of the literature. Arch Pathol. 1963; 76:44.
Roux, M. On exostoses: there character. Am J Dent Sc. 1848; 9:133–134.
Roy, J.J., Klein, H.Z., Tipton, D.L. Osteochondroma of the tongue. Arch Pathol. 1970; 89:565.
Royster, H.P., Moyers, E.D., Williams, R.B., Jr., Horn, R.C., Jr. A study of cervical lymph node metastasis in squamous cell carcinoma of the oral cavity. Am J Roentgenol Radium Ther Nucl Med. 1953; 69:792.
Ruschak, P.J., Kauh, Y.C., Luscombe, H.A. Cowden’s disease associated with immunodeficiency. Arch Dermatol. 1981; 117:573.
Russell, D.S., Rubinstein, L.J. Pathology of Tumors of the Nervous System, 4. London: Arnold Ltd, 1977.
Sachs, W., Sachs, P.M. Erythroplasia of Queyrat: report of 10 cases. Arch Dermatol Syph. 1948; 58:184.
Saijo, M., Munro, I.R., Mancer, K. Lymphangioma: a long-term follow-up study. Plast Reconstr Surg. 1975; 56:642.
Salvador, A.H., Beabout, J.W., Dahlin, D.C. Mesenchymal chondrosarcoma: observations on 30 new cases. Cancer. 1971; 28:605.
Samter, T.G., Vellios, F., Shafer, W.G. Neurilemmoma of bone: report of 3 cases with a review of the literature. Radiology. 1960; 75:215.
Sandler, H.C. Morphological characteristics of malignant cells from mouth lesions. Acta Cytol. 1965; 9:282.
Sandstead, H.R., Lowe, J.W. Leukoedema and keratosis in relation to leukoplakia of buccal mucosa in man. J Natl Cancer Inst. 1953; 14:423.
Santa, Cruz D.J., Martin, S.A. Verruciform xanthoma of the vulva: report of two cases. Am J Clin Pathol. 1979; 71:224.
Sapp, J.P., Eversole, L.R., Wysocki, G.P.Contemporary oral and maxillofacial pathology. St. Louis: CV Mosby, 1997.
Sapp, J.P. Ultrastructure and histogenesis of periheral giant cell reparative granuloma of the jaws, Cancer. 1972; 30:1119.
Saunders, J.R., Jaques, D.A., Casterline, P.F., Percarpio, B. Liposarcomas of the head and neck: a review of the literature and addition of four cases. Cancer. 1979; 43:162.
Saunders, W.H. Nicotine stomatitis of the palate. Ann Otol Rhinol Laryngol. 1958; 67:618.
Schajowicz, F., Gallardo, H. Chondromyxoid fibroma (fibromyxoid chondroma) of bone. J Bone Joint Surg Br. 1971; 53:198.
Schajowicz, F., Lemos, C. Malignant osteoblastoma. J Bone Joint Surg. 1970; 58:205.
Schajowicz, F. Ewing’s sarcoma and reticulum-cell sarcoma of bone: with special reference to the histochemical demonstration of glycogen as an aid to differential diagnosis. Bone Joint Surg Am. 1959; 41:394.
Schirmer, R. Ein fall von telangiektasie. Albrecht von Graefes Arch Ophthalmol. 1860; 7:119–121.
Schreiber, M.M., Bozzo, P.D., Moon, T.E. Malignant melanoma in southern Arizona. Arch Dermatol. 1981; 117:6.
Schreiner, B.F., Christy, C.J. Results of irradiation treatment of the cancer of the lip: analysis of 636 cases from 1926–36. Radiology. 1942; 39:293.
Schuller, D.E., Lawrence, T.L., Newton, W.A., Jr. Childhood rhabdomyosarcomas of the head and neck. Arch Otolaryngol. 1979; 105:689.
Schutt, P.G., Frost, H.M. Chondromyxoid fibroma. Clin Orthop. 1971; 78:323.
Schwartz J. Atrophia Idiopathia (Tropica) Mucosae Oris Demonstrated at the Eleventh International Dental Congress, London, 1952.
Schwarz, E. Ossifying fibroma of the face and skull. Am J Roentgenol Radium Ther Nucl Med. 1964; 91:1012.
Scofield, H.H.Epidermoid carcinoma of the nasal and pharyngeal regions: a statistical and morphological analysis of two hundred and fourteen cases. Georgetown University: MS Thesis, 1952.
Seelig, C.A. Carcinoma of the antrum. Ann Otol Rhinol Laryngol. 1949; 58:168.
Seldin, H.M., Seldin, S.D., Rakower, W., Jarrett, W.J. Lipomas of the oral cavity: report of 26 cases. J Oral Surg. 1967; 25:270.
Sellevold, B.J. Mandibular torus morphology. Am J Phys Anthropol. 1980; 53:569.
Sessions, R.B., Zarin, D.P., Bryan, R.N. Juvenile nasopharyngeal angiofibroma. Am J Dis Child. 1981; 135:535.
Shafer, W.G., Hine, M.K., Levy, B.M. A textbook of oral pathology (4th edn). Philadelphia: WB Saunders, 1983; 146–149.
Shafer, W.G., Moorman, W.C. Traumatic (amputation) neuroma. J Oral Surg. 1957; 15:253.
Shafer, W.G., Waldron, C.A. A clinical and histopathological study of oral leukoplakia. Surg Gynecol Obstet. 1961; 112:411.
Shafer, W.G. Oral carcinoma in situ. Oral Surg. 1975; 39:227.
Shapiro, M.J., Mix, B.S. Heterotopic brain tissue of the palate: a report of two cases. Arch Otolaryngol. 1968; 87:96.
Sharp, G.S. Cancer of the oral cavity. Oral Surg. 1948; 1:614.
Shear, M. Lipoblastomatosis of the cheek. Br J Oral Surg. 1967; 5:173–179.
Shear, M., Pindborg, J.J. Verrucous hyperplasia of the oral mucosa. Cancer. 1980; 46:1855.
Shear, M. Erythroplakia of the mouth. Int Dent J. 1972; 22:460.
Shillitoe, E.J., Silverman, S., Jr. Oral cancer herpes simplex virus—a review. Oral Surg. 1979; 48:216.
Shillitoe, E.J., Greenspan, D., Greenspan, J.S., Hansen, L.S. Neutralizing antibody to herpes simplex virus type I in patients with oral cancer. Cancer. 1982; 49:2315.
Shklar, G., Cataldo, E. The gingival giant cell granuloma. Histochemical observations Periodont. 1967; 5:303.
Shklar, G., Meyer, I. Giant cell tumors of the mandible and maxilla. Oral Surg. 1961; 14:809.
Silverman, S., Jr., Griffith, M. Smoking characteristics of patients with oral carcinoma and the risk for second oral primary carcinoma. J Am Dent Assoc. 1972; 85:637.
Silverman, S., Jr., Renstrup, G., Pindborg, J.J. Studies in oral leukoplakias. Acta Odontol Scand. 1963; 21:271.
Sist, T.C., Jr., Greene, G.W. Traumatic neuroma of the oral cavity: report of thirty-one new cases and review of the literature. Oral Surg. 1981; 51:394.
Sivapathasundharam, B., Rohini, S. Adenoid sequamous cell carcinoma of gingiva. Proeedings of II International Congress on oral Cancer. 1992; II:142–146.
Sivapathasundharam B et al. Desimoplastic ameloblastoma in Indians: report of five cases and review of literature. Ind J Dent. Res, 18(4): 218–221.
Skolnik, E.M., Massari, F.S., Tenta, L.T. Olfactory neuroepithelioma. Arch Otolaryngol. 1966; 84:84.
Slaughter, D.P., Southwick, H.W., Smejkal, W. Field cancerization in oral stratified squamous epithelium: clinical implications of multicentric origin. Cancer. 1963; 6:693.
Slootweg, P.J., Roholl, P.J., Muller, H. Spindle-cell carcinoma of the oral cavity and larynx. Immunohistochemical aspects. J Cranio-Maxillofac Surg. 1989; 17:234–236.
Slootweg, P.J. Clear-cell chondrosarcoma of the maxilla: report of a case. Oral Surg. 1980; 50:233.
Small, I.A., Bloom, H.J. Hemangiopericytoma of the sublingual fossa: report of a case. J Oral Surg Anesth Hosp Dent Serv. 1959; 17:65.
Smith C, Pindborg JJ. Histological Grading of Oral Epithelial Atypia by the Use of Photographic Standards. World Health Organization’s International Reference Centre for Oral Precancerous Conditions, Copenhagen, 1969.
Smith, C. Carcinoma in situ. Hum Pathol. 1978; 9:373.
Smith, J.B. Cancer of the floor of the mouth. J Oral Surg. 1948; 6:106.
Sobel, H.J., Churg, J. Granular cells and granular cell lesions. Arch Pathol. 1964; 77:132.
Sobel, H.J., Schwartz, R. Marguet E. Light-and electron-microscope study of the origin of granular-cell myoblastoma. J Path. 1973; 109:101.
Soesan, M., Paccagnella, A., Chiarion-Sileni, V. Extramedullary plasmacytoma: clinical behavior and response to treatment. Ann Oncol. 1992; 3(1):51–57.
Solomon, M.P., Sutton, A.L. Malignant fibrous histiocytoma of the soft tissues of the mandible. Oral Surg. 1973; 35:653.
Soong, H.K., Pollock, D.A. Hereditary hemorrhagic telangiectasia diagnosed by the ophthalmologist. Cornea. 2000; 19(6):849–850.
Sordillo, P.P., Helson, L., Hajdu, S.I., Magill, G.B. Malignant schwannoma—Clinical characteristics, survival, and response to therapy. Cancer. 1981; 47:2503.
Sovadina, M. Ad leukoplacia oris. Czas Stomatol. 1955; 55:116.
Spouge, J.D. Odontogenic tumors: a unitarian concept. Oral Surg. 1967; 24:392.
Spraggs, P.D., Roth, J.J. Giant cell reparative granuloma of the maxilla. Ear Nose Throat J. 1997; 76(7):445–446. [Jul].
Stark, D., Bradley, W.Magnetic Resonance Imaging. CV Mosby: St Louis, 1999. [(Vol IV, 3rd ed)].
Stefansson, K., Wollmann, R.L. S-100 protein in granular cell tumors (granular cell myoblastomas). Cancer. 1982; 49:1834.
Stein, J.J., James, A.G., King, E.R. The management of the teeth, bone, and soft tissues in patients receiving treatment for oral cancer. Am J Roentgenol Radium Ther Nucl Med. 1970; 108:257.
Steiner, A.L., Goodman, A.D., Powers, S.R. Study of a kindred with pheochromocytoma, medullary thyroid carcinoma, hyperparathyroidism and Cushing’s disease: multiple endocrine neoplasia type 2. Medicine. 1968; 47:371.
Steiner, G.C., Mirra, J.M., Bullough, P.G. Mesenchymal chondrosarcoma: a study of the ultrastructure. Cancer. 1973; 32:926.
Steiner, G.C. Ultrastructure of osteoblastoma. Cancer. 1977; 39:2127.
Stern, M.H., Turner, J.E., Coburn, T.P. Oral involvement in neuroblastoma. J Am Dent Assoc. 1974; 88:346.
Stewart, R.E., Prescott, G.H.Oral Facial Genetics. CV Mosby: St Louis, 1976.
Stillman, F.S. Neurofibromatosis. J Oral Surg. 1952; 10:112.
Stobbe, G.D., Dargeon, H.W. Embryonal rhabdomyosarcoma of the head and neck in children and adolescents. Cancer. 1950; 3:826.
Stock, M.F. Hereditary hemorrhagic telagiectasia (Osler’s disease). Arch Otolaryngol. 1944; 40:108.
Stockdale, C.R. Metastatic carcinoma of the jaws secondary to primary carcinoma of the breast. Oral Surg. 1959; 12:1095.
Stoddart, T.G. Conference on cancer of the lip (based on a series of 3166 cases). Can Med Assoc J. 1964; 90:666.
Stout, A.P. Pathological aspects of soft part sarcomas. Ann New York Acad Sci. 1964; 114:1041–1046.
Stout, A.P., Kenney, F.R. Primary plasma-cell tumors of the upper air passages and oral cavity. Cancer. 1949; 2:261.
Stout, A.P., Lattes, R.Tumors of the Soft Tissues (Atlas of Tumor Pathology, Section II, Fascicle 1). Armed Forces Institute of Pathology: Washington DC, 1967.
Stout, A.P. Fibrosarcoma in infants and children. Cancer. 1962; 15:1028.
Stowens, D., Lin, T-H. Melanotic progonoma of the brain. Hum Patho. 1974; 5:105.
Strojan, P., Soba, E., Lamovec, J., et al. Extramedullary plasmacytoma: clinical and histopathologic study. Int J Radiat Oncol Biol Phys. 2002; 53(3):692–701.
Strong, E.W., McDivitt, R.W., Brasfield, R.D. Granular cell myoblastoma. Cancer. 1970; 25:415.
Struthers, P.J., Shear, M. Aneurysmal bone cyst of the jaws (I): clinicopathological features. Int J Oral Surg. 1984; 13(2):85–91.
Sturge, W.A. A case of partial epilepsy, apparently due to a lesion of one of the vaso-motor centers of the brain. Trans Clin Soc Lond. 1897; 12:162–167.
Sturgis, S.H., Lund, C.C. Leukoplakia buccalis and keratosis labialis. New Engl J Med. 1934; 210:996.
Sullivan, T.J., Clarke, M.P., Morin, J.D. The ocular manifestations of the Sturge-Weber syndrome. J Pediatr Ophthalmol Strabismus. 1992; 29(6):349–356.
Susac, J.O., Smith, J.L., Scelfo, R.J. The ‘tomatoe-catsup’ fundus in Sturge-Weber syndrome. Arch Ophthalmol. 1974; 92(1):69–70. [Jul].
Suzuki, M., Sakai, T. A familial study of torus palatinus and torus mandibularis. Am J Phys Anthropol. 1960; 18:263.
Svirsky, J.A., Freedman, P.D., Lumerman, H. Solitary intraoral keratoacanthoma. Oral Surg. 1961; 43:317.
Symmers, D. Lymphoid diseases. Arch Pathol. 1948; 45:73.
Syrop, H.M., Krantz, S. Kaposi’s disease. Oral Surg. 1951; 4:337.
Takagi, M., Ishikawa, G., Mori, W. Primary malignant melanoma of the oral cavity in Japan, with special reference to mucosal melanosis. Cancer. 1974; 34:538.
Takagi, M., Sakota, Y., Takayama, S., Ishikawa, G. Adenoid squamous cell carcinoma of the oral mucosa: report of two autopsy cases. Cancer. 1977; 40:2250.
Takahashi, K., Mulliken, J.B., Kozakewich, H.P. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest. 1994; 93(6):2357–2364. [Jun].
Tallini, G., Dalcin, P., Rhoden, K.J. Expression of Hmgi-C Hmgi (Y) in ordinary lipoma and atypical lipomatous tumors–immunohistochemical reactivity correlates with karyotypic alterations. Am J Pathol. 1997; 151:37–43.
Taylor, H.B., Helwig, E.B. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962; 15:717.
Tedeschi, C.G. Some considerations concerning the nature of the so-called sarcoma of Kaposi. Arch Pathol. 1958; 66:656.
Telles, N.C., Rabson, A.S., Pomeroy, T.C. Ewing’s sarcoma: an autopsy study. Cancer. 1978; 41:2321.
Tepperman, B.S., Fitzpatrick, P.J. Second respiratory and upper digestive tract cancers after oral cancer. Lancet. 1981; II:547.
Thoma, K.H. Rhabdomyoma of the tongue. Am J Orthod Oral Surg. 1941; 27:235.
Thomas, G. Solitary plasmacytoma of the upper air passages. J Laryngol Otolaryngol. 1965; 79:498.
Thompson, S.H., Shear, M. Fibrous histiocytomas of the oral and maxillofacial regions. J Oral Pathol. 1984; 13:282–294.
Tiecke, R.W., Bernier, J.L. Statistical and morphological analysis of four hundred and one cases of intraoral squamous cell carcinoma. J Am Dent Assoc. 1954; 49:684.
Tillman, B.P., Dahlin, D.C., Lipscomb, P.R., Stewart, J.R. Aneurysmal bone cyst: an analysis of 95 cases. Mayo Clin Proc. 1968; 43:478.
Toeg, A., Kermish, M., Grishkan, A., Temkin, D. Histiocytoid hemangioma of the oral cavity: a report of two cases. J Oral Maxillofac Surg. 1993; 51:812–814.
Tomich, C.E., Shafer, W.G. Lymphoproliferative disease of the hard palate: a clinicopathologic entity. Oral Surg. 1975; 39:754.
Tomich, C.E., Moll, M.C. Palisaded, encapsulated neuroma of the lip. J Oral Surg. 1976; 34:265.
Tomich, C.E. Oral focal mucinosis. Oral Surg. 1974; 38:714.
Tomich, E.C., Hutton, C.E. Adenoid squamous cell carcinoma of the lip: report of cases. J Oral Surg. 1972; 30:592.
Toto, P.D. Mucopolysaccharide keratin dystrophy of the oral epithelium. Oral Surg. 1966; 22:47.
Trieger, N., Ship, II., Taylor, G.W., Weisberger, D. Cirrhosis and other predisposing factors in carcinoma of the tongue. Cancer. 1958; 11:357.
Trieger, N., Taylor, G.W., Weisberger, D. The significance of liver dysfunction in mouth cancer. Surg Gynecol Obstet. 1959; 108:230.
Trodahl, J.N., Sprague, W.G. Benign and malignant melanocytic lesions of the oral mucosa: an analysis of 135 cases. Cancer. 1970; 25:812.
Tsang, R.W., Gospodarowicz, M.K., Pintilie, M. Solitary plasmacytoma treated with radiotherapy: impact of tumor size on outcome. Int J Radiat Oncol Biol Phys. 2001; 50(1):113–120.
Tsukada, Y., de la Pava, S., Pickren, J.W. Granularcell ameloblastoma with metastasis to the lungs: report of a case and review of the literature. Cancer. 1965; 18:916.
Tyldesley, W.R., Kempson, S.A. Ultrastructure of the oral epithelium in leukoplakia associated with tylosis and esophageal carcinoma. J Oral Pathol. 1975; 4:49.
Tyldesley, W.R., Osborne Hughes, R. Tylosis, leukoplakia and esophageal carcinoma. Br Med J. 1973; 4:427.
Unni, K.K. Dahlin’s bone tumors: general aspects and data on 11,087 cases (5th ed). Philadelphia: Lippincott, 1996; 382–390.
Unni, K.K., Dahlin, D.C., Beabout, J.W., Periosteal, C. Parosteal osteogenic sarcoma. Cancer. 1976; 37:2466.
Unni, K.K., Ivins, J.C., Beabout, J.W., Dahlin, D.C. Hemangioma, hemangiopericytoma, and heman gioendothelioma (angiosarcoma) of bone. Cancer. 1403; 27:1971.
Vally, I.M., Altini, M. Fibromatoses of the oral and paraoral soft tissues and jaws: review of the literature and report of 12 new cases. Oral Surg Oral Med Oral Pathol. 1990; 69(2):191–198. [Feb].
Van Wyck, W.C. The oral lesion caused by snuff: a clinicopathological study. Med Proc. 531, 1965. [11].
Vellios, F., Baez, J., Shumacker, H.B., Lipoblastomatosis:. a tumor of fetal fat different from hibernoma. Report of a case with observatins on the embryogenesis of human adipose tissue. Am J Pathol. 1958; 34:1149.
Waldron, C.A., Shafer, W.G. Current concepts of leukoplakia. Int Dent J. 350, 1960. [10].
Waldron, C.A. Giant cell tumors of the jawbones. Oral Surg. 1953; 6:1055.
Walsh, T.S., Jr., Tompkins, V.N. Some observations on the strawberry nevus of infancy. Cancer. 869, 1956. [9].
Wang, C.C., James, A.E., Jr. Chordoma: brief review of the literature and report of a case with widespread metastases. Cancer. 162, 1968. [22].
Warrington, R.D., Reese, D.J., Allen, G. The peripheral giant cell granuloma. Gen Dent. 1997; 45(6):577–579.
Watanabe, S. The metastasizability of tumor cells. Cancer,. 215, 1954. [7].
Watson, W.L., McCarthy, W.D. Blood and lymph vessel tumors: a report of 1. 056, cases. Surg Gynecol Obstet. 569, 1940. [71].
Wawro, N.M., Fredrickson, R.W., Tennant, R.W. Hemangioma of the parotid gland in the newborn and in infancy. Cancer. 1955; 8:595–599.
Wayte, D.M., Helwig, E.B. Melanotic freckle of Hutchinson. Cance. 1968; 893 [21].
Weary, P.E., Gorlin, R.J., Gentry, W.C., Jr., Comer, J.E., Greer, K.E. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 682, 1972. [106].
Weathers, D.R., Callihan, M.D. Giant-cell fibroma. Oral Surg,. 374, 1974. [37].
Weathers, D.R., Campbell, W.G. Ultrastructure of te giant-cell fibroma of the oral mucosa. Oral Surg,. 550, 1974. [38].
Weathers, D.R. Benign nevi of the oral mucosa: a report of six cases. Arch Dermatol. 688, 1969. [99].
Webb, H.E., Harrison, E.G., Masson, J.K., ReMine, W.H. Solitary extramedullary myeloma (plasmacytoma) of the upper part of the respiratory tract and oropharynx. Cancer. 1962; 1142. [15].
Webb, J.N. The histogenesis of nerve sheath myxoma: report of a case with electron microscopy. J Path. 35, 1979. [127].
Weber, F.P. Right-sided hemi-hypotrophy resulting from right-sided congenital spastic hemiplegia with a morbid condition of the left side of the brain, revealed by radiograms. Neurol Psychopathol. 1922; 3:134–139.
Weedon, D., Little, J.H. Spindle and epithelioid cell nevi in children and adults: a review of 211 cases of the Spitz nevus. Cancer. 217, 1977. [40].
Weiss, D.I. Dual origin of glaucoma in encephalotrigeminal haemangiomatosis. Trans Ophthalmol Soc UK. 1973; 93(0):477–493.
Weiss, S.W., Enzinger, F.M. Myxoid variant of malignant fibrous histiocytoma. Cancer. 1977; 1672. [39].
Weitzner, S., Lockey, M.W., Lockard, V.G. Adult rhabdomyoma of soft palate. Oral Surg. 70, 1979. [47].
Weitzner, S. Clear-cell acanthoma of vermilion mucosa of lower lip. Oral Surg. 911, 1974. [37].
Welbury, R.R. Congenital epulis of the newborn. Br J Oral Surg,. 238, 1980. [18].
Werning, J.T. Nodular fasciitis of the orofacial region. Oral Surg. 441, 1979. [48].
Wesley, R.K., Mintz, S.M., Wertheimer, F.W. Primary malignant hemangioendothelioma of the gingiva. Oral Surg. 103, 1975. [39].
Whitaker, B., Robinson, K., Hewan-Lowe, K., Budnick, S. Thyroid metastasis to the oral soft tissues: case report of a diagnostic dilemma. J Oral Maxillofac Surg. 1993; 51(5):588–593. [May].
Whitaker, S.B., Waldron, C.A. Central giant cell lesions of the jaws: a clinical, radiologic, and histopathologic study. Oral Surg Oral Med Oral Pathol. 1993; 75(2):199–208. [Feb].
Whitaker, S.B., Waldron, C.A. Central giant cell lesions of the jaws. Oral Surg Oral Med Oral Pathol. 1993; 75:199–208.
White, D.K., Miller, A.S., Gomez, L. Occurrence of oral squamous cell carcinoma in persons under 50 years of age. Phila Med. 442, 1978. [74].
Whitten, J.B. The fine structure of an intraoral granular cell myoblastoma. Oral Surg,. 202, 1968. [26].
WHO collaborating centre for oral precancerous lesions: definition of leukoplakia and related lesions: an aid to studies on oral precancer Oral Sur. 1978; 518 [46].
Widmann, B.P. Cancer of the lip. Am J Roentgenol Radium Ther Nucl Med. 13, 1950. [63].
Williamson, J.J. Erythroplasia of Queyrat of the buccal mucous membrane. Oral Surg,. 308, 1964. [17].
Wilson, S., Gould, A.R., Wolff, C. Multiple lymphangiomas of the alveolar ridge in a neonate: case study. Pediatr Dent,. 1986; 8231–8234.
Witschel, H., Font, R.L. Hemangioma of the choroid: a clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol. 1976; 20(6):415–431.
Wolbach, S.B. Pathologic changes resulting from vitamin deficiency. J Am Med Assoc. 7, 1937. [108].
Wolfe, J.J., Platt, W.R. Postirradiation osteogenic sarcoma of the nasal bone: a report of two cases. Cancer. 438, 1949. [2].
Wong, D.S., Fuller, L.M., Butler, J.J., Shullenberger, C.C. Extranodal non-Hodgkin’s lymphomas of the head and neck. Am J Roentgenol Radium Ther Nucl Med,. 471, 1975. [123].
Wood, J.S., Jr., Holyoke, E.D., Clason, W.P., Sommers, S.C., et al. An experimental study of the relationship between tumor size and number of lung metastases. Cancer. 437, 1954. [7].
Woodbridge, H. Carcinoma in situ. Oral Surg. 1950. [31447].
Woods, W.R., Tulumello, T.N. Management of oral hemangioma: review of the literature and report of a case. Oral Surg Oral Med Oral Pathol. 1977; 44:39.
Wright, B.A., Wysocki, G.P., Bannerjee, D. Diagnostic use of immunoperoxidase techniques for plasma cell lesions of the jaws. Oral Surg,. 615, 1981. [52].
Wynder, E.L., Bross, I.J., Feldman, R.M. A study of the etiological factors in cancer of the mouth. Cancer. 10, 1957. [1300].
Wysocki, G.P., Hardie, J. Ultrastructural studies of intraoral verruca vulgaris. Oral Surg. 58, 1979. [47].
Wysocki, G.P., Wright, B.A. Intraneural and perineural epithelial structures. Head Neck Surg,. 69, 1981. [4].
Yokota, J. Tumor progression and metastasis. Carcinogenesis. 2000; 21(3):497–503. [Mar].
Zachariades, N. Neoplasms metastatic to the mouth, jaws and surrounding tissues. J Craniomaxillofac Surg. 1989; 17(6):283–290. [Aug].
Zachariades, N., Papadakou, A., Koundouris, J., Constantinidis, J., et al. Primary hemangioendotheliosarcoma of the mandible: review of the literature and report of a case. J Oral Surg,. 288, 1980. [38].
Zegarelli, D.J., Zegarelli-Schmidt, E.C., Zegarelli, E.V. Verruciform xanthoma. Oral Surg,. 725, 1974. [38].
Zegarelli, E.V., Kutscher, A.H., Silvers, H.F. Keratotic lesions of the oral mucous membranes treated with high dosage topical systemic vitamin A. New York State Dent J. 244, 1959. [25].
Zelger, B.W., Zelger, B.G., Steiner, H., Ofner, D. Aneurysmal and hemangiopericytomalike fibrous histiocytoma. J Clin Pathol. 1996; 49:313–318.
Zelger, B.W.H., Zelger, B.G., Plorer, A., et al. Dermal spindle cell lipoma – plexiform and nodular variants. Histopathol. 1995; 27:533–540.
Zenker, W. Juxtaoral Organ (Chievitz’ Organ) Morphology and Clinical Aspects. Urban an Schwarzenberg: Baltimore; 1982.
Ziegler, J.L. Burkitt’s Lymphoma. New Engl J Med. 735, 1981. [305].
Zillmer, D.A., Dorfman, H.D. Chondromyxoid fibroma of bone: 36 cases with clinicopathological correlation. Hum Pathol. 1989; 20:952–964.
Ziskin, D.E., Blackberg, S.N., Slanetz, C.A. Effects of subScutaneous injections of estrogenic and gonadotrophic hormones on gums and oral mucous membranes of normal and castrated rhesus monkeys. J Dent ResS. 407, 1935-36. [15].
Ziskin, D.E. Effects of certain hormones on gingival and oral mucous membranes. J Am Dent Assoc. 422, 1938. [25].