Regressive Alterations of the Teeth
Regressive changes in the dental tissues include a variety of alterations that are not necessarily related either etiologically or pathogenetically. Some of the changes to be considered here are associated with the general aging process of the individual. Others arise as a result of injury to the tissues. Still other regressive changes of teeth occur with such frequency that there is some doubt whether they should actually be considered pathologic. None of the lesions discussed here can be regarded as developmental abnormalities or as inflammatory lesions. They are brought together in this chapter because they do represent what must be considered lesions of a retrograde nature.
Attrition, Abrasion and Erosion
Mechanical wear and tear of tooth substance is a consequence of both physiological and pathological means and therefore different adaptive strategies have evolved to tackle this situation. A disease state arises when this delicate balance goes awry resulting in early dissolution and loss of tooth substance with subsequent involvement of pulpal and periapical tissues. It is currently acknowledged that there are several mechanisms that contribute to tooth wear. These include abrasion resulting from the friction of exogenous material forced over tooth surfaces (e.g. masticating food) or the use of teeth as ‘tools’, erosion resulting from the chemical dissolution of tooth surfaces (e.g. effects of acid from various sources or from a highly acidic diet), and attrition from tooth-to-tooth contact (e.g. night grinding). These mechanisms most often occur together, each acting at different intensity and duration in a continuously changing salivary medium, producing immensely variable patterns and degrees of wear.
Attrition
Attrition may be defined as the physiologic wearing away of a tooth as a result of tooth-to-tooth contact, as in mastication. This occurs only on the occlusal, incisal, and proximal surfaces of teeth, not on other surfaces unless a very unusual occlusal relation or malocclusion exists. This phenomenon is physiologic rather than pathologic, and it is associated with the aging process. The older a person becomes, the more attrition is exhibited.
Attrition commences at the time contact or occlusion occurs between adjacent or opposing teeth. It may be seen in the deciduous dentition as well as in the permanent, but severe attrition is seldom seen in primary teeth because they are not retained normally for any great period of time. Occasionally; however, children may suffer from either dentinogenesis imperfecta or amelogenesis imperfecta, and in both diseases pronounced attrition may result from ordinary masticatory stresses.
The first clinical manifestation of attrition may be the appearance of a small polished facet on a cusp tip or ridge or a slight flattening of an incisal edge. Because of the slight mobility of the teeth in their sockets, a manifestation of the resiliency of the periodontal ligament, similar facets occur at the contact points on the proximal surfaces of the teeth. As the person becomes older and the wear continues, there is gradual reduction in cusp height and consequent flattening of the occlusal inclined planes. According to Robinson and his associates, there is also shortening of the length of the dental arch due to reduction in the mesiodistal diameters of the teeth through proximal attrition.
Only minor variation in the hardness of tooth enamel exists between individuals; nevertheless considerable variation in the degree of attrition is observed clinically. Men usually exhibit more severe attrition than women of comparable age, probably as a result of the greater masticatory force of men. Variation also may be a result of differences in the coarseness of the diet or of habits such as chewing tobacco or bruxism either of which would predispose to more rapid attrition. Certain occupations, in which the person is exposed to an atmosphere of abrasive dust and cannot avoid getting the material into his/ her mouth, also are important in the etiology of severe attrition.
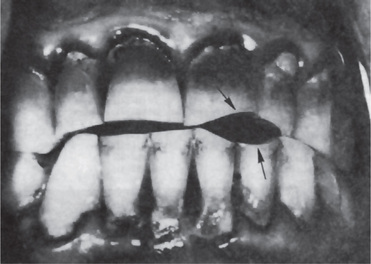
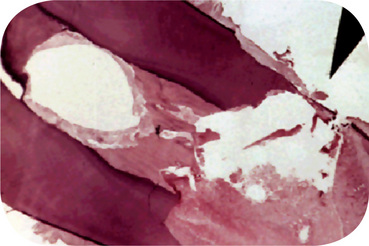
Advanced attrition, in which the enamel has been completely worn away in one or more areas, sometimes results in an extrinsic yellow or brown staining of the exposed dentin from food or tobacco (Figs. 13-1, 13-2). Provided there is no premature loss of the teeth, attrition may progress to the point of complete loss of cuspal interdigitation. In some cases the teeth may be worn down nearly to the gingiva, but this extreme degree is unusual even in elderly persons.
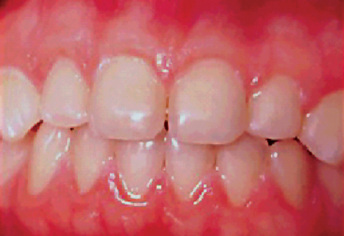
Figure 13-1 Advanced attrition.
This 14-year-old female exhibits total loss of surface characteristics and polished appearance of enamel on her maxillary incisors. The enamel layer was also very thin.
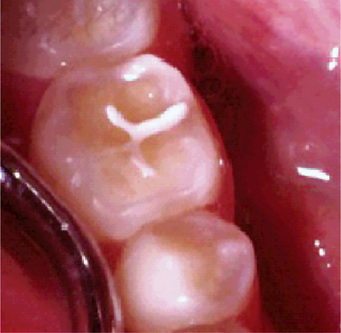
Figure 13-2 Advanced attrition.
The fissure sealant in this 14-year-old boy stands ‘raised’ from surrounding eroded occlusal enamel.
The exposure of dentinal tubules and the subsequent irritation of odontoblastic processes result in formation of secondary dentin (q.v.) pulpal to the primary dentin, and this serves as an aid to protect the pulp from further injury. The rate of secondary dentin deposition is usually sufficient to preclude the possibility of pulp exposure through attrition alone. Sometimes, as the teeth wear down by attrition, little tendrils of pulp horn remain and are exposed to the oral cavity. These can be seen only when the tooth is examined carefully under a magnifying lens.
Abrasion
Abrasion is the pathologic wearing away of tooth substance through some abnormal mechanical process. Abrasion usually occurs on the exposed root surfaces of teeth, but under certain circumstances it may be seen elsewhere, such as on incisal or proximal surfaces.
Robinson stated that the most common cause of abrasion of root surfaces is the use of an abrasive dentifrice. Although modern dentifrices are not sufficiently abrasive to damage intact enamel severely, they can cause remarkable wear of cementum and dentin if the toothbrush carrying the dentifrice is injudiciously used, particularly in a horizontal rather than vertical direction. In such cases abrasion caused by a dentifrice manifests itself usually as a V-shaped or wedge-shaped ditch on the root side of the cementoenamel junction in teeth with some gingival recession (Fig. 13-3). The angle formed in the depth of the lesion, as well as that at the enamel edge, is a rather sharp one, and the exposed dentin appears highly polished. It has been shown by Kitchin and by Ervin and Bucher that some degree of tooth root exposure is a common clinical finding, and a 66% incidence of abrasion among 1,252 patients examined was reported by Ervin and Bucher. The fact that abrasion was more common on the left side of the mouth in right-handed people, and vice versa, suggested that improper toothbrushing caused abrasion. The results of a study by the American Dental Association on the comparative abrasiveness of a number of popular dentifrices are shown in Table 13-1 and indicate the wide variation among the commercial products.
Tabel 13-1:
| Product | Manufacturer index | Abrasivity |
| T-Lak | Laboratories Cazé | 20 (20–21)+ (Lowest) |
| Thermodent | Chas. Pfizer & Co. | 24 (23–24) |
| Listerine | Warner-Lambert Pharm. Co. | 26 (22–30) |
| Pepsodent* | Lever Brothers Co. | 26 (23–29) |
| Amm-i-dent | Block Drug Co. | 33 (31–34) |
| Colgate with MFP | Colgate-Palmolive Co. | 51 (46–56) |
| Ultra-Brite | Colgate-Palmolive Co. | 64 (52–82) |
| Macleans,* Spearmint | Beecham Inc. | 66 (66) |
| Macleans,* regular | Beecham Inc. | 70 (68–72) |
| Pearl Drops | Cameo Chemicals | 72 (65–83) |
| Crest, mint* | Procter & Gamble Co. | 81 (71–90) |
| Close-up | Lever Brothers Co. | 87 (70–101) |
| Macleans, Spearmint | Beecham Inc. | 93 (85–99) |
| Macleans, regular | Beecham Inc. | 93 (74–103) |
| Crest, regular* | Procter & Gamble Co. | 95 (77–110) |
| Gleem | Procter & Gamble Co. | 106 (88–136) |
| Phillips | Sterling Drug Inc. | 114 (111–116) |
| Vote | Bristol-Myers Co. | 134 (112–162) |
| Sensodyne | Block Drug Co., Inc. | 157 (151–168) |
| Iodent No. 2 | Iodent Co. | 174 (172–176) |
| Smokers toothpaste | Walgreen Lab., Inc. | 202 (198–205) (Highest) |
Modified from American Dental Association Report of the Council on Dental Therapeutics: Abrasivity of current dentifrices. J Am Dent Assoc, 81:1177, 1970. Copyright by the American Dental Association. Reprinted by permission.
Figure 13-3 Toothbrush abrasion.
Severe gingival notching of the teeth as a result of improper toothbrushing habits is clearly seen.
Other less common forms of abrasion may be related to habit or to the occupation of the patient. The habitual opening of bobby pins with the teeth may result in a notching of the incisal edge of one maxillary central incisor (Fig. 13-4). Similar notching may be noted in carpenters, shoemakers, or tailors who hold nails, tacks, or pins between their teeth. Habitual pipe smokers may develop notching of the teeth that conforms to the shape of the pipe stem (Fig. 13-5). The improper use of dental floss and toothpicks may produce lesions on the proximal exposed root surface, which also should be considered a form of abrasion.
Figure 13-4 Abrasion of maxillary central incisor caused by habitual opening of bobby pins with tooth.
It is apparent that though the etiology of abrasion can be varied, the pathogenesis under these different conditions is essentially identical. The loss of tooth substance that occurs by one means or another is certainly pathologic but should present no problem in diagnosis.
The exposure of dentinal tubules and the consequent irritation of the odontoblastic processes stimulate the formation of secondary dentin similar to that seen in cases of attrition. Unless the form of abrasion is an extremely severe and rapidly progressive one, the rate of secondary dentin formation is usually sufficient to protect the tooth against pulp exposure.
Erosion
Dental erosion is defined as irreversible loss of dental hard tissue by a chemical process that does not involve bacteria. Dissolution of mineralized tooth structure occurs upon contact with acids that are introduced into the oral cavity from intrinsic (e.g. gastroesophageal reflux, vomiting) or extrinsic sources (e.g. acidic beverages, citrus fruits). This form of tooth surface loss is part of a larger picture of toothwear, which also consists of attrition, abrasion, and possibly, abfraction. Table 13-2 lists the definitions of each of these forms of tooth surface loss or tooth wear.
Table 13-2:
Definitions of tooth surface loss
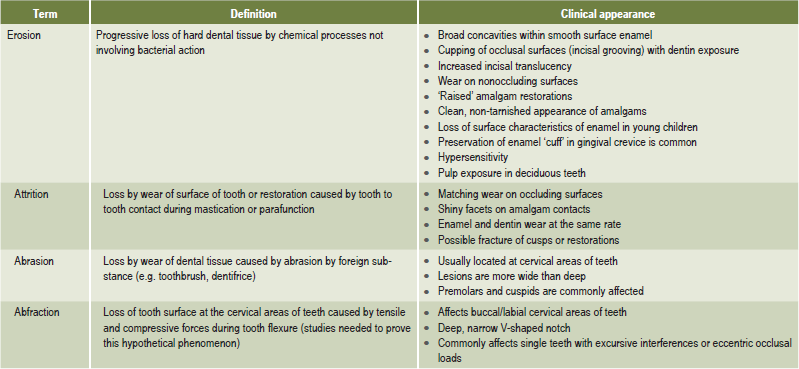
Milosevic A. Toothwear: etiology and presentation. Dent Update 25: 6–11, 1998.
Causes
Erosion of tooth substance is mainly due to contact with acidic media either by way of foodstuff or by iatrogenic exposure. There could be either extrinsic or intrinsic sources of acid that could cause this mode of tooth substance loss. Examples of extrinsic acids (source outside the body) are acidic beverages, foods, medications or environmental acids. The most common of these are dietary acids. It can be seen that most fruits and fruit juices have a very low pH (high acidity). Carbonated drinks and sports drinks are also very acidic. Several studies have found that the frequency of consumption of acidic drinks was significantly higher in patients with erosion than without. This finding is of concern, particularly since children and adolescents are the primary consumers of these drinks. With consumption of acidic drinks identified as a risk factor in erosion, this amount of soft drink consumption will likely lead to an increase in prevalence of erosion. The erosive potential of beverages does not depend on pH alone. Other components of beverages, such as calcium, phosphates, and fluoride may lessen erosive potential. Also, factors such as frequency and method of intake of acidic beverages as well as the toothbrushing frequency after intake may influence susceptibility to erosion.
Therefore, the role(s) of confounders like oral hygiene status, complicate the role of acids per se which necessitates further investigation to clarify the relationship between acidic beverage intake and dental erosion.
Medications that are acidic in nature can also cause erosion via direct contact with the teeth when the medication is chewed or held in the mouth prior to swallowing. Numerous case reports exist describing extensive erosion secondary to chewing vitamin C preparations or hydrochloric acid supplements. Less common sources of extrinsic erosive acids are related to occupational and recreational exposure. Chromic, hydrochloric, sulfuric and nitric acids have been identified as erosion-causing acid vapors. They are released into the work environment during industrial electrolytic processes. However current work safety standards make this type of erosion very rare. Dental erosion has been reported in swimmers who work out regularly in pools with excessive acidity as well as individuals who are occupational wine-tasters.
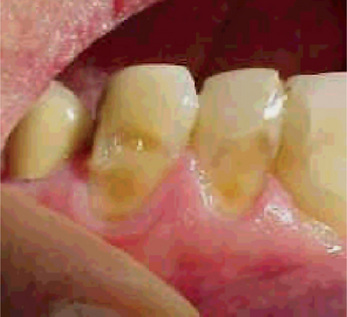
Intrinsic causes (acid source inside the body), for erosion are gastric acids regurgitated into the esophagus and mouth. Gastric acids, with pH levels that can be less than 1, reach the oral cavity and come in contact with the teeth in conditions such as gastroesophageal reflux and excessive vomiting related to eating disorders. The association of gastroesophageal reflux disease (GERD) with dental erosion has been established in a number of studies in adults (Figs. 13-6, 13-7). GERD is a common condition, estimated to affect 7% of the adult population on a daily basis and 36% at least one time a month. In this condition gastric contents pass involuntarily into the esophagus and can escape up into the mouth. This is caused by increased abdominal pressure, inappropriate relaxation of the lower esophageal sphincter or increased acid production by the stomach. However, GERD can also be ‘silent’ with the patient unaware of his or her condition until dental changes elicit assessment for the condition.
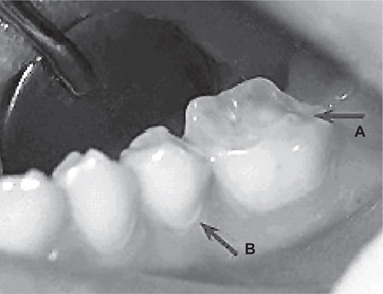
Figure 13-6 Gastroesophageal reflux disease (GERD) was discovered in this 19-year-old boy who exhibited early generalized erosion (arrow A).
Note the preservation of the enamel at the gingival crevice (arrow B).
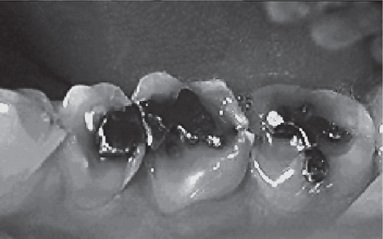
Figure 13-7 This 33-year-old male with GERD had severe asymptomatic erosion.
Note the amalgams ‘rising’ above the adjacent eroded occlusal surfaces.
Chronic, excessive vomiting has long been recognized as causing erosion of the teeth. The patient with an eating disorder such as anorexia nervosa or bulimia is the classic example. The problem was first reported by Hellstrom and Hurst in 1977. Many reports and reviews have been published on the topic since that time. Although erosion caused by vomiting typically affects the palatal surfaces of the maxillary teeth, it is also common for individuals with eating disorders to consume large amounts of acidic beverages and fresh fruits (Tables 13-3, 13-4). This results in another source of acid exposure, primarily affecting the labial surfaces of the teeth. In addition, treatment for bulimia may include use of antidepressants or other psychoactive medications that may cause salivary hypofunction. Therefore, the cause of erosion cannot be reliably determined from its location.
Table 13-3:
Acidity of common foods and beverages
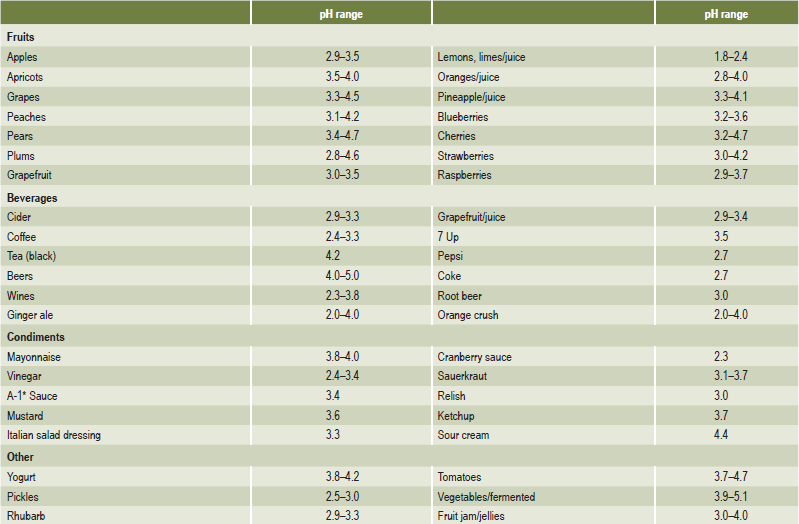
Clark DC, Woo G, Silver JG, et al. The influence of frequent ingestion of acids in the diet on treatment for dentin sensitivity. J Can Dent Assoc, 56: 1101–1103, 1990.
Table 13-4:
Risk factors for dental erosion

Jarvinen VK, Rytomaa II, and Heinonen OP. Risk factors in dental erosion. J Dent Res 70: 942–947, 1991.
Erosion associated with alcoholism is caused by frequent vomiting. Other causes of vomiting that may cause erosion include gastrointestinal disorders such as peptic ulcers or gastritis, pregnancy, drug side effects, diabetes or nervous system disorders.
The fluctuations in pH of saliva is mainly kept in balance by the buffering capacity of saliva. This property is largely due to the bicarbonate content of the saliva which is in turn dependent on the salivary flow rate. Bicarbonate concentration also regulates salivary pH. Therefore, there is a relationship between salivary pH, buffering capacity and flow rate, with pH and buffer capacity increasing as flow rate increases. Normally, when an acid enters the mouth, whether from an intrinsic or extrinsic source, salivary flow rate increases, along with pH and buffer capacity. Within minutes, the acid is neutralized and cleared from the oral cavity and the pH returns to normal. Patients with erosion were found to have lower salivary buffer capacity when compared with controls in several studies. In other studies, low whole salivary flow rates in patients with erosion were determined to be the major difference. Therefore, salivary function is an important factor in the etiology of erosion. Since many common medications and diseases can lower salivary flow rate (xerostomia), both whole and stimulated, it is important to assess salivary characteristics when evaluating a patient with erosion.
Management of Erosion
Identification of the etiology is important as a first step in management of erosion. If excessive dietary intake of acidic foods or beverages is discovered, patient education and counseling are important. If the patient has symptoms of GERD, then he/she should be referred to a medical doctor for complete evaluation and institution of therapy if indicated. A patient with salivary hypofunction may benefit with the use of sugarless chewing gum or mints to increase residual salivary flow. The use of oral pilocarpine (Salagen) may be beneficial in patients with dry mouth caused by Sjögren’s syndrome or post-therapeutic head and neck radiation. A patient suspected of an eating disorder should be referred to a medical doctor for evaluation.
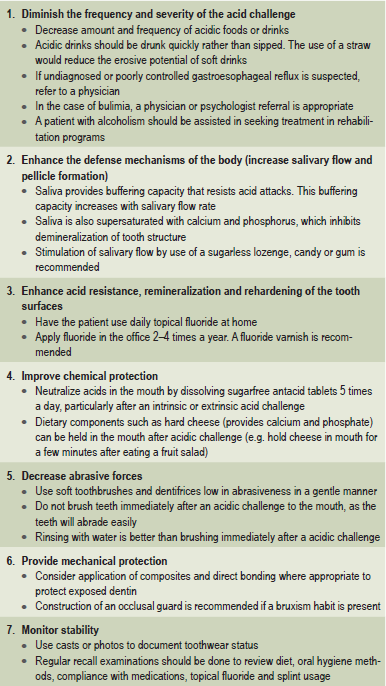
In some cases, an etiologic agent is not identifiable. In other cases, the etiologic agent may be difficult to control, such as the problem of alcoholism. However, regardless of the cause, it is important to follow preventive measures to prevent the progress of erosion. There are several preventive measures that can be taken to control tooth erosion. These are listed in Table 13-5. Patient education is of paramount importance. Much of erosion prevention depends on the compliance of the patient with dietary modification, use of topical fluorides, use of occlusal splint, etc.
Abfraction
Abfraction is currently described as a mode of tooth loss which has clinical and circumstantial evidence. Till recently this type of tooth loss which is mainly confined to the gingival third of the clinical crown was thought to be the result of toothbrush abrasion. It is proposed that with each bite, occlusal forces cause the teeth to flex though little. Constant flexing causes enamel to break from the crown, usually on the buccal surface. The physiological basis of this type of tooth substance loss held that, while not providing a complete explanation, does offer a significant clue to the real cause of this troubling phenomenon. Grippo, in 1991, coined the term abfraction to describe the pathologic loss of both enamel and dentin caused by biomechanical loading forces (Fig. 13-8). He stated that the forces could be static, such as those produced by swallowing and clenching; or cyclic, as in those generated during chewing action. The abfractive lesions were caused by flexure and ultimate material fatigue of susceptible teeth at locations away from the point of loading. The breakdown was dependent on the magnitude, duration, direction, frequency, and location of the forces.
Dentinal Sclerosis: (Transparent Dentin)
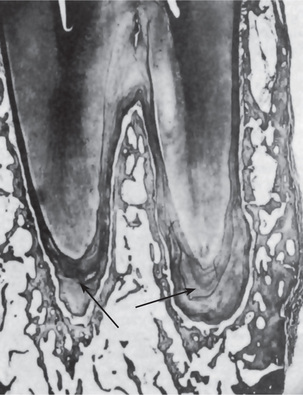
Sclerosis of primary dentin is a regressive alteration in tooth substance that is characterized by calcification of the dentinal tubules. It occurs not only as a result of injury to the dentin by caries or abrasion but also as a manifestation of the normal aging process. For many years it has been known that if a ground section of a tooth with a very shallow carious lesion of the dentin is examined by transmitted light, a translucent zone can be seen in the dentin underlying the cavity (Fig. 13-9). This was readily recognized as being due to a difference between the refractive indices of the sclerotic or calcified dentinal tubules and the adjacent normal tubules. Both Beust and Fish showed that dyes do not penetrate those dentinal tubules that are sclerotic as a result either of age or of a slowly progressive type of dental caries.
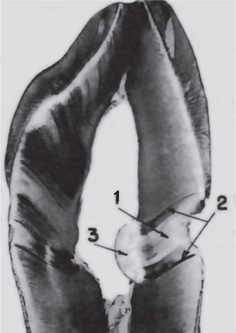
Figure 13-9 Dentinal sclerosis.
Sclerosed dentin (1) beneath a cervical cavity bounded on either side by ‘dead tracts’ (2) and below by secondary dentin (3).
The exact mechanism of dentinal sclerosis or the deposition of calcium salts in the tubules is not understood, although the most likely source of the calcium salts is the fluid or ‘dental lymph’ within the tubules. The increased mineralization of the tooth decreases the conductivity of the odontoblastic processes. In addition, the sclerosis slows an advancing carious process.
Sclerotic dentin under a carious lesion was shown by Hodge and McKay to be actually harder than adjacent normal dentin. Subsequently, Van Huysen, Hodge, and Warren confirmed the fact that sclerotic dentin is more highly calcified than normal dentin by employing a unique adaptation of the X-ray absorption technique in which ground slabs of teeth were photographed by X-rays and the degree of radiopacity in areas of normal and sclerotic dentin was measured.
Dead Tracts
‘Dead tracts’ in dentin are seen in ground sections of teeth and are manifested as a black zone by transmitted light but as a white zone by reflected light (Fig. 13-9). This optical phenomenon is due to differences in the refractive indices of the affected tubules and normal tubules. The nature of the change in the affected tubules is not known, although these tubules are not calcified and are permeable to the penetration of dyes.
Secondary Dentin
Secondary dentin is dentin that is formed and deposited in response to a normal or slightly abnormal stimulus, after the complete formation of the tooth.
There is a considerable variation in the composition of primary and secondary dentin. The dentinal tubules that make up dentin are generally irregular in secondary dentin and deposits contain less calcium, phosphorus, and collagenous matrix per unit volume than the primary dentin. Secondary dentin is less mineralized and contains 6–10% less mineral than primary dentin.
There are generally two types of secondary dentin, produced as a result of different stimuli. The two types of secondary dentin are physiological secondary dentin and reparative secondary dentin.
Physiological Secondary Dentin
This type of dentin is a regular, uniform layer of dentin around the pulp chamber that is laid down throughout the life of the tooth as a result of physiological factors generally thought to be age and tooth eruption. This type of secondary dentin is produced more slowly than primary dentin.
Reparative Secondary Dentin
Reparative secondary dentin is the dentin that forms around the pulp chamber as a result of irritation or attrition, which is a form of tooth wear. Attrition is due to tooth-to-tooth contact, which results from occlusal function, such as bruxism, and can cause loss of tooth structure. This gradual traumatic process stimulates the development of natural protective measures, such as secondary dentin.
Clinical Features
There are no significant clinical features that may be used to determine when teeth have formed secondary dentin, although there is an evident decrease in tooth sensitivity when secondary dentin formation is extensive, as it is in elderly persons. This type of dentin forms an additional insulating layer of calcified tissue between the pulp and the particular pathologic process that initiated the dentinal response. Thus the eventual pulp involvement is usually delayed. Although secondary dentin occurs in all teeth, including those of the deciduous dentition, a study by Bevelander and Benzer indicated that the anterior teeth exhibit a higher incidence of secondary dentin formation than the molar teeth.
Radiographic Features
Secondary dentin often may be visualized on the dental radiograph if it occurs in an area that is not overshadowed by other calcified tissue, either bone or tooth substance. Such secondary dentin formation may be noted particularly in the pulp horn areas as well as on the proximal walls of teeth with proximal caries.
The decrease in size of the pulp chamber and root canals that occurs with advancing age as a result of secondary dentin formation is obvious radiographically.
Histologic Features
Physiologic secondary dentin is usually similar in appearance to primary dentin, but in decalcified stained sections it often exhibits a different tinctorial reaction and may be rather well demarcated from the primary dentin by a deeply staining ‘resting line’. This type of secondary dentin exhibits somewhat fewer tubules, although their course is not especially irregular.
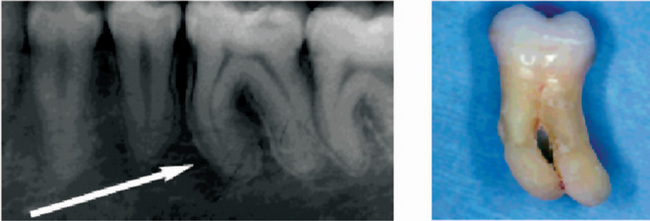
Adventitious secondary dentin arising in response to irritation is usually irregular in nature, being composed of few tubules that may be tortuous in appearance (Fig. 13-10). In some instances tubules are inconspicuous if not completely absent. Occasionally, this secondary dentin is formed at a rapid rate and odontoblasts may become entrapped, producing a superficial resemblance to bone. Such calcified tissue has been termed osteodentin.
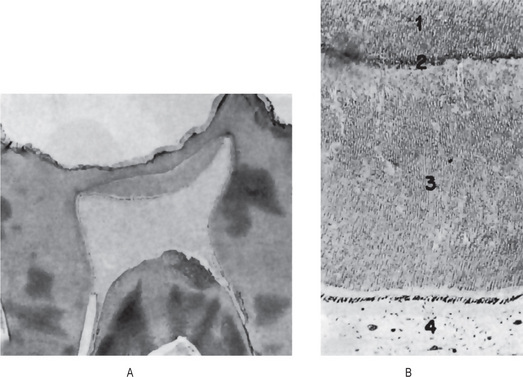
Figure 13-10 Secondary dentin.
Secondary dentin beneath carious lesion, (A). Normal dentin (1), resting line (2), secondary dentin (3), and pulp (4) are shown in higher magnification, (B).
Stanley and his associates have studied the rate of reparative dentin formation in human teeth following cavity preparation under a variety of clinical conditions. They found that reparative dentin formation was insignificant in the first postoperative month. Between one and one-and-a-half months, new dentin formation reached a maximum rate of approximately 3.5μ per day. After this period, new dentin formation rapidly decreased.
Reticular Atrophy of Pulp
Reticular atrophy of the pulp and more discrete vacuolization of the pulpal tissue and cells have often been described as degenerative or regressive changes of pulp, particularly when they occur as an age change in elderly persons. It has been stated that teeth so affected are clinically symptomless and respond normally to vitality tests. Histologically, reticular atrophy has been described as being characterized by the presence of large vacuolated spaces in the pulp, with a reduction in the number of cellular elements. Accompanying these changes are degeneration and disappearance of the odontoblasts.
It is most interesting and significant that alterations in the pulp tissue identical with those described above can be produced by improper fixation of the tooth and pulp after extraction preparatory to histologic sectioning. Most investigators now believe that this condition is purely an artifact brought about by autolysis of the pulp tissue and does not occur in vivo.
Pulp Calcification
Various forms of calcification within the pulps of teeth are found with such frequency that it may be questioned whether their presence represents a pathologic state or merely an occurrence within the range of normal biologic variation. These calcifications may be located in any portion of the pulp tissue, although certain types are more common in the pulp chamber and others in the root canal.
A number of studies have been carried out to determine the actual incidence of pulp calcification, and the results of these investigations are in essential agreement. For example, Willman reported that of a series of 164 teeth picked at random and examined histologically, 143 (or 87%) exhibited calcification in the pulp. Interestingly, only 15% of the areas of calcification were large enough to be seen on the dental radiograph. These findings confirm the investigations of Hill, who reported calcifications in 66% of all teeth examined in young persons between the ages of 10 and 20 years and in 90% of all teeth examined in persons between the ages of 50 and 70 years. There is no apparent difference in the frequency of occurrence either between the genders or among the various teeth in the dental arch.
The two chief morphologic forms of pulp calcifications are discrete pulp stones (denticles, pulp nodules) and diffuse calcification. Pulp stones have been classified as either true or false stones, depending upon their microscopic structure.
True denticles are made up of localized masses of calcified tissue that resemble dentin because of their tubular structure. Actually, these nodules bear greater resemblance to secondary dentin than to primary dentin, since the tubules are irregular and few in number. They are considerably more common in the pulp chamber than in the root canal.
True denticles may be subdivided further according to whether or not they are attached to the wall of the pulp chamber. Denticles lying entirely within the pulp tissue and not attached to the dentinal walls are called ‘free denticles’, while those that are continuous with dentinal walls are referred to as ‘attached denticles’. The latter type of calcification is somewhat more common than the former. It should be remembered that though a denticle may appear free in the one plane of section in which it is visualized, it may be attached in another plane. Thus, without serial section on an entire tooth pulp, one cannot state with any degree of assurance that a given denticle is free and not attached.
False denticles are composed of localized masses of calcified material, and unlike true denticles, do not exhibit dentinal tubules. Instead, the nodule appears to be made up of concentric layers or lamellae deposited around a central nidus (Fig. 13-11). The exact nature of this nidus is unknown, although Johnson and Bevelander believe that it is composed of cells, as yet unidentified, around which is laid down a layer of reticular fibers that subsequently calcify.
The false denticle also may be classified as free or attached. As the concentric deposition of calcified material continues, it approximates and finally is in apposition with the dentinal wall. Here it may eventually become surrounded by secondary dentin, and it is then referred to as an ‘interstitial denticle’. False denticles, which occur more commonly in the pulp chamber than in the root canal, are generally somewhat larger than true denticles. They may fill nearly the entire pulp chamber, while true denticles are seldom larger than a fraction of a millimeter in diameter.
Johnson and Bevelander concluded from their studies that a differentiation between ‘true’ and ‘false’ denticles should not be drawn, since all denticles originally show no tubules even though they subsequently may become surrounded by tissue containing dentinal tubules.
Diffuse calcification is most commonly seen in the root canals of teeth and resembles the calcification seen in other tissues of the body following degeneration. This type of calcification is frequently termed ‘calcific degeneration’. Its usual pattern is in amorphous, unorganized linear strands or columns paralleling the blood vessels and nerves of the pulp.
Etiology
The etiology of the various types of pulp calcification is unknown. Although the incidence appears to increase with the age of the persons, there is no definite association with pulpal irritation or inflammation such as that arising from caries or trauma. Since pulp calcifications have been described in unerupted teeth, it is doubtful whether pulpal disease such as inflammation is of any significance. Stafne and Szabo attempted to correlate pulp nodules with various local or systemic diseases including cholelithiasis, renal lithiasis, arteriosclerosis, gout, acromegaly, osteitis deformans, hyper-cementosis, and torus palatinus or mandibularis. Their data indicate that no clear-cut relation exists between any of these conditions and pulp calcification.
Kretschmer and Seybold reported that an extremely high percentage of pulp stones yield a pure growth of streptococci upon culture. On this basis it has been suggested that microorganisms are the cause of pulp calcifications. Since the pulps of the affected teeth were reportedly normal, aside from the calcification, and since it is well recognized that bacteria may be forced into the pulp tissue at the time of tooth extraction, it is most unlikely that bacteria are of any significance in the development of these pulp nodules.
More recently, Sundell and his associates have attempted to determine whether the degree of pulp response elicited by cutting procedures and restorative materials was capable of increasing the incidence of pulp stone nidi and pulp stones. They found no significant correlation between pulp stones or nidi and the age or gender of the patient, the thickness of remaining dentin beneath the cavity preparation, the preparation time, or the traumatic potential of the operative procedure. In addition, these workers have proposed the following schematic diagram incorporating several hypotheses from the literature, and concomitantly, including the mechanism of thrombosis or vascular wall injury or both, leading to pulp-stone formation.
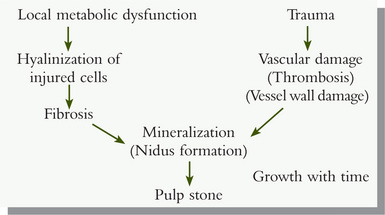
Clinical Significance
The clinical significance of pulp calcification is not completely understood. It has been reported upon numerous occasions that pulp stones are a cause of pain, varying from mild pulpal neuralgia to severe, excruciating pain resembling that of tic douloureux. The consensus is that though denticles may seem to impinge on nerves of the pulp, they probably do not. Most investigators now believe that seldom, if ever, are pulp stones the cause of dental pain. Therefore, the extraction of teeth with radiographically demonstrable pulp stones in the hope of relieving dental pain or vague facial neuralgia cannot be defended. Neither can the view that the presence of pulp stones indicates pulpal infection be condoned. In the light of our present knowledge, pulp calcification is a purely coincidental finding without clinical significance. Difficulty may be encountered in extirpating the pulp during root canal therapy if calcifications are present (Fig. 13-12).
Resorption of Teeth
Resorption of teeth occurs in many circumstances other than the normal process associated with the shedding of deciduous teeth. The roots of permanent teeth may undergo resorption in response to a variety of stimuli; moreover, it is recognized that root resorption in permanent teeth occurs to a slight degree even under apparently normal conditions. Since resorption of a tooth may begin either on the external surface (arising as a result of a tissue reaction in the periodontal or pericoronal tissue) or inside the tooth (from a pulpal tissue reaction), the general terms ‘external resorption’ and ‘internal resorption’ are used to distinguish between the two types. The chief causes or situations in which resorption may occur are as follows:
External Resorption
Resorption Associated with Periapical Inflammation
Resorption of calcified dental tissues occurs in the same fashion as that of bone, and in most instances, the presence of osteoclasts is an outstanding feature in areas of active resorption. It should be pointed out; however, that there is considerable evidence indicating that osteoclasts may not be essential for the resorption of calcified tissues.
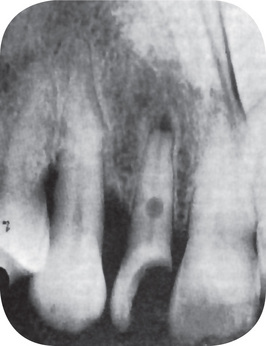
A periapical granuloma (q.v.) arising as a result of pulpal infection or trauma occasionally causes subsequent resorption of the root apex if the inflammatory lesion persists for a sufficient period of time (Fig. 13-13). Most teeth involved by a periapical granuloma; however, do not exhibit any significant degree of resorption. The reason for the occasional occurrence is not known. It is generally agreed that bone resorption occurs more readily in highly vascular areas than in relatively avascular areas, and since the periapical granuloma is quite vascular, it is surprising that resorption of the root is not more frequently seen. That bone is more readily resorbed than dental tissue is borne out by the fact that bone is always destroyed when a periapical granuloma develops, whereas resorption of the tooth root without loss of bone seldom occurs except at a microscopic level.
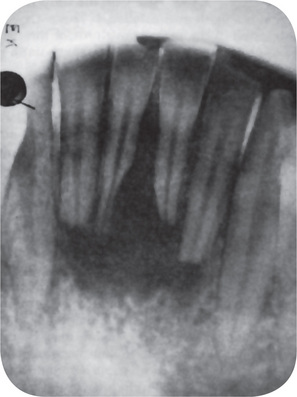
Figure 13-13 Root resorption.
Diffuse periapical granuloma resulting from death of pulp caused by traumatic injury has resulted in resorption of roots.
In those cases of periapical granuloma in which root resorption does occur, it is usually obvious on the dental radiograph. It appears as a slight raggedness or blunting of the root apex in the early stages, proceeding to a severe loss of tooth substance. In a tooth that has had the root canal treated and filled, but around which periapical inflammation persists, resorption of the root may occur and ultimately leave only the root canal filling projecting out of the shortened root. The radiographic picture of this phenomenon presents an unusual appearance and superficially resembles overfilling of the root canal.
Reimplanted Teeth
The reimplantation, or transplantation, of teeth (q.v.) almost invariably results in severe resorption of the root. The tooth substance, regardless of whether the root canal has been filled or not, must be considered nonvital tissue, except in the case of transplanted developing teeth when the vascular supply to the tooth may be reestablished and its vitality maintained. Thus the implanted tooth is analogous to a bone graft which acts only as a temporary scaffold and is ultimately resorbed and replaced. The tooth root is resorbed and replaced by bone, producing an ankylosis. If the tooth root does not become completely resorbed, the ensuing ankylosis may result in a functional tooth. Many reimplanted teeth; however, exhibit complete resorption of the root and are exfoliated.
Tumors and Cysts
The resorption of roots brought about by tumors is similar to that seen in teeth involved by cysts (Fig. 13-14). In many instances resorption by tumors or cysts appears to be essentially a pressure phenomenon. This is to be expected, since the phenomenon of resorption of bone under pressure is well recognized and actually forms the basis of orthodontic practice.
Both benign and malignant tumors may cause root resorption, although benign lesions are more likely to produce displacement than actual destruction of the tooth. In most cases connective tissue is present between the tumor and the tooth, and it is from this tissue that cells develop, chiefly osteoclasts, which appear responsible for the root resorption. This is particularly true of epithelial tumors that invade the jaws. Occasionally tumors are seen in which, histologically, the neoplastic cells are found adjacent to and within the ragged resorption lacunae on the root surface.
Cysts cause root resorption in a manner similar to resorption caused by benign tumors, that is, chiefly by pressure, although displacement of the tooth is more common than resorption. An apical periodontal cyst arising as a result of pulp infection may exert such pressure on the apex of the involved or adjacent tooth that the intervening connective tissue is stimulated, osteoclasts form, and resorption begins. This reaction may occur with any type of cyst that progressively expands, but it is more common with the periodontal cyst than with the dentigerous, primordial, or fissural cysts.
Excessive Mechanical or Occlusal Forces
The usual form of excessive mechanical force with which root resorption may be associated is that applied during orthodontic treatment. It has been recognized for many years, at least since the work of Ketcham, that patients who have undergone orthodontic treatment frequently exhibit multiple areas of root resorption irrespective of the manner of treatment, i.e. the type of appliance or the duration and degree of force exerted. In some patients this resorption is mild and involves only a few teeth; in others there may be loss of over 50% of the root length of most of the teeth.
The cause for this extreme variation under apparently similar clinical conditions is unknown. Becks reported that systemic disturbances, the chief among these being hypothyroidism, may predispose to root resorption, particularly in the patient who is receiving orthodontic treatment. The influence of the systemic factor remains to be confirmed, however.
It is fortunate that bone undergoes resorption far more readily than cementum when force is exerted upon the tooth by orthodontic appliances or by occlusal trauma. This pressure upon the supporting bone invariably results in destruction, primarily of bone. Small lacunae often appear on the surface of the cementum and ultimately extend into the dentin, indicating early tooth resorption. Probably most cases of this minor type of resorption are soon repaired by the deposition of bone or cementum in these ragged lacunae, particularly if the occlusal force or orthodontic pressure is relieved.
Some investigators have questioned the clinical significance of root resorption secondary to mechanical or occlusal forces since, regardless of the severity or degree of the resorption, seldom is a tooth ever exfoliated. There may be destruction of the apical two thirds of the root of a tooth with no evidence of looseness or other signs of impending difficulties.
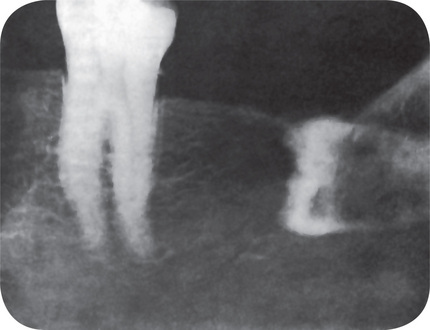
Impacted Teeth
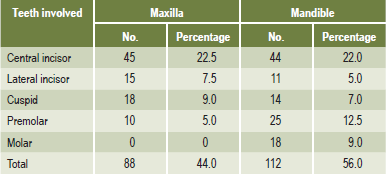
Teeth that are completely impacted or embedded in bone occasionally will undergo resorption of the crown or of both crown and root. Stafne and Austin pointed out that although this resorption most commonly begins on the crown of the tooth, destruction of all the enamel epithelium is not a prerequisite for the onset of resorption. In some cases only a limited amount of epithelium appears to be destroyed, allowing the connective tissue to come in contact with the crown and thus initiating the resorptive process. Stafne and Austin reported that teeth that are completely embedded are those most apt to undergo resorption. In a study of 226 embedded teeth in which resorption occurred, they found that 78% of the teeth were in the maxillary arch and that 60% of these maxillary teeth were cuspids (Table 13-6). This finding is unusual and significant because, although maxillary and mandibular third molars far outnumber maxillary cuspids in incidence of impaction, the cuspids undergo resorption more frequently than the third molars. The reason for this is unknown. Impacted supernumerary teeth, particularly mesiodens, also are prone to undergo resorption.
Table 13-6:
Distribution of embedded teeth exhibiting radiographic evidence of resorption
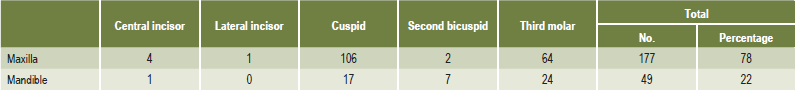
EC Stafne and LT Austin: Resorption of embedded teeth. J Am Dent Assoc, 32: 1003, 1945.
The radiographic picture presented by these teeth is an unusual one, particularly when the resorption occurs on the tooth crown. The irregular destruction frequently has suggested that the impacted or embedded tooth is involved by caries, an obvious impossibility (Fig. 13-15).
Impacted teeth also may cause resorption of the roots of adjacent teeth without being resorbed themselves. This is particularly common in the case of a horizontally or mesioangularly impacted mandibular third molar impinging on the roots of the second molar. There is always connective tissue interposed between the second and third molars, and the pressure of the third molar appears to activate the resorptive cells, thus setting the stage for tooth destruction.
Idiopathic Resorption
Many investigators have reported that the roots of permanent teeth may undergo a certain amount of resorption in apparently normal adults without any obvious cause (Fig. 13-16A). The term ‘idiopathic root resorption’ has been applied to this phenomenon. The actual incidence of this form of resorption was not appreciated until the study of Massler and Perreault, in which it was found that of 301 young (18–25 years old) male and female patients, all exhibited some degree of root resorption in four or more teeth, as judged by radiographic examination alone. Furthermore, it was reported that 82% of the teeth in the men and 91% of the teeth in the women showed some evidence of resorption. In less than 3% of the cases was there any indication as to the cause of this condition. The teeth most commonly involved by root resorption were the maxillary bicuspids, while the mandibular incisors and molars exhibited the least resorption.
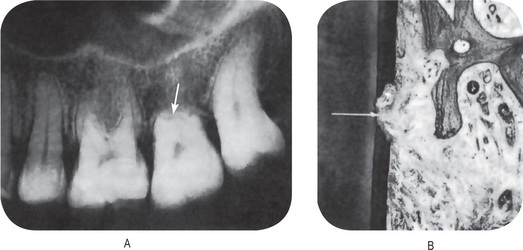
Figure 13-16 Root resorption.
(A) Severe idiopathic root resorption of maxillary second molar. (B) Mild root resorption, visible only microscopically.
Massler and Perreault pointed out; however, that this finding is in contradistinction to nearly all other reported studies, including those of Becks and of Stafne and Slocumb (Table 13-7), in which the maxillary and mandibular central incisors were shown to be most frequently involved.
It appears that root resorption is far more common than was formerly believed. The majority of cases of idiopathic resorption are mild (Fig. 13-16B). According to the data of Massler and Perreault, over 75% of the teeth exhibiting resorption showed loss of less than 4 mm of the root apex. Although the etiology remains unknown, several possibilities present themselves. The resorption may be related to one or more systemic disorders, the most obvious being some form of endocrine disturbance (Figs. 13-17–13-20). A genetic characteristic governing the resorption potential of bone and tooth substance has been demonstrated in animals and is conceivable in human beings. Finally, the possibility that root resorption, with subsequent repair, is no more pathologic than an analogous resorption and repair of bone must be considered.
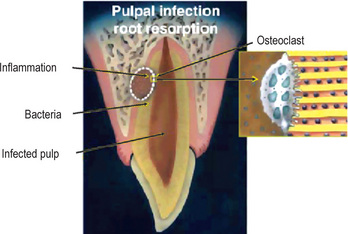
Figure 13-17 Graphical illustration of pulpal infection root resorption.
Root canal and dentinal tubules are necrotic and infected, and inflammatory response with osteoclastic activity is taking place in the dentin and the bone. Enlargement of osteoclast attached to dentin on the right demonstrates the stimulation factor of bacteria in the dentinal tubules.
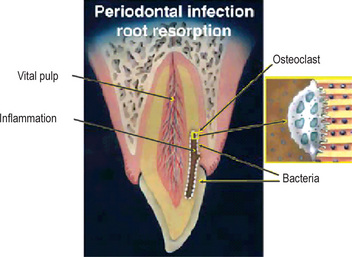
Figure 13-18 Graphical illustration of periodontal infection root resorption.
Pulp is vital and healthy, but the dentinal tubules adjacent to coronal cemental trauma are infected and inflammatory response with osteoclastic activity is taking place in dentin. Enlargement of osteoclast attached to dentin on the right demonstrates the stimulation factor of bacteria in the dentinal tubules. The bacteria originate from the periodontal tissues and not from the pulp space.
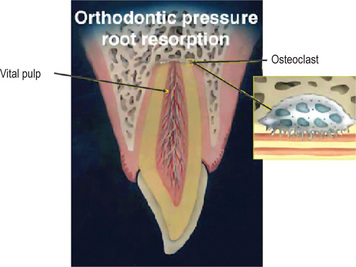
Figure 13-19 Graphical illustration of orthodontic pressure root resorption.
Pulp is vital and stimulation to the osteoclastic activity in the apex is related to extensive pressure during orthodontic treatment. Enlargement of an osteoclast attached to the dentin on the right demonstrates intact dentinal tubules with no bacteria.
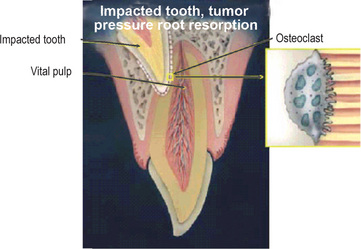
Figure 13-20 Graphical illustration of impacted tooth or tumor pressure root resorption.
Pulp is vital and stimulation to the osteoclastic activity is related to extensive pressure by the impacted tooth. Enlargement of an osteoclast attached to the dentin on the right demonstrates intact dentinal tubules with no bacteria.
A rare form of multiple idiopathic root resorption may occur that involves all or nearly all of the teeth. The resorption may begin at the cementoenamel junction or nearer to the root apex. This disease has been discussed by Kerr and his associates, who pointed out that these patients are medically normal and have no past history such as orthodontic treatment or radiation that might explain the phenomenon.
Internal Resorption: (Chronic perforating hyperplasia of pulp, internal granuloma, odontoclastoma, pink tooth of Mummery)
Internal resorption is an unusual form of tooth resorption that begins centrally within the tooth, apparently initiated, in most cases, by a peculiar inflammatory hyperplasia of the pulp. The cause of the pulpal inflammation and subsequent resorption of tooth substance is unknown, although an obvious carious exposure and accompanying pulp infection are sometimes present. It is even possible that true internal resorption does not exist but rather is a result of resorption of the tooth and invasion of the pulp by granulation tissue arising in the periodontium. An excellent discussion of the problem of idiopathic tooth resorption, both external and internal, has been published by Sullivan and Jolly, while Sweet has presented a historic review of the internal resorption of teeth, beginning with the first description of the problem by Bell in 1830.
Clinical Features
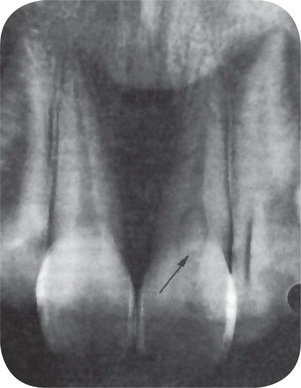
Most cases of internal resorption present no early clinical symptoms. The first evidence of the lesion may be the appearance of a pink-hued area on the crown of the tooth, which represents the hyperplastic, vascular pulp tissue filling the resorbed area and showing through the remaining overlying tooth substance (Fig. 13-21). In the event that the resorption begins in the root, there are no significant clinical findings.
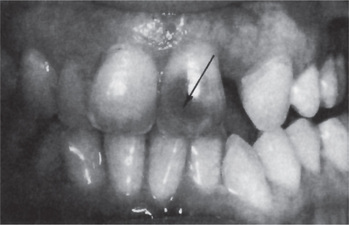
Figure 13-21 Internal resorption of maxillary central incisor.
The dark shadow represents pulp tissue visible through the tooth substance.
It is unusual for more than one tooth in any given patient to be affected by internal resorption, although cases of multiple tooth involvement have been recorded. There appears to be no specific predilection for occurrence in one jaw rather than the other, although no large enough series of cases has been reported to justify drawing any significant conclusions. The individual tooth involved may be any tooth, and examples of internal resorption in incisors, cuspids, bicuspids and molars have all been reported at one time or another.
Radiographic Features
Radiographic examination may provide the first revelation of pulpal disease when the patient appears for a routine check-up. The involved tooth exhibits a round or ovoid radiolucent area in the central portion of the tooth, associated with the pulp but not with the external surface of the tooth unless the condition is of such duration that perforation has occurred (Fig. 13-22). Complete perforation is not an uncommon finding if the tooth is left untreated.
Histologic Features
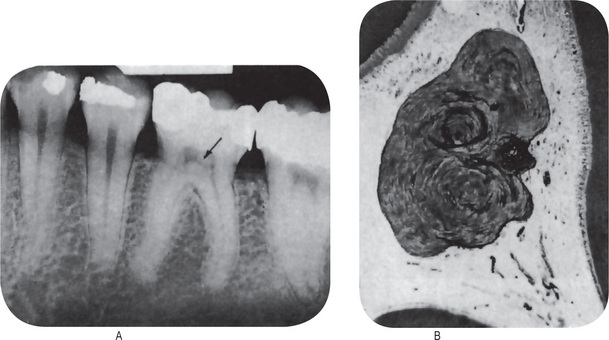
Microscopic examination of a tooth with internal resorption shows a variable degree of resorption of the inner or pulpal surface of the dentin and proliferation of the pulp tissue filling the defect. The resorption is of an irregular lacunar variety showing occasional osteoclasts or ‘odontoclasts’, hence the term ‘odontoclastoma’. The pulp tissue usually exhibits a chronic inflammatory reaction but little else to explain the cause for this unusual condition (Figs. 13-23, 13-24).
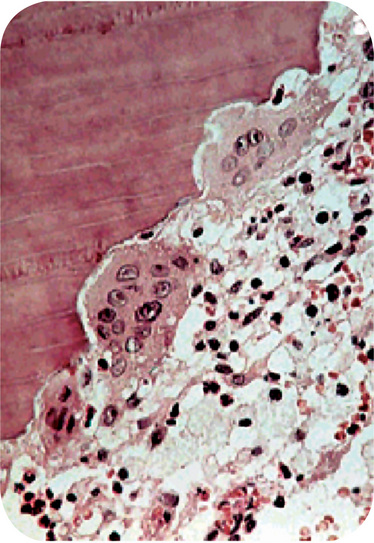
Figure 13-23 Internal resorption.
This section demonstrates the scalloped effect produced by the multinucleated odontoclasts as they resorb the dentinal surface.
Sometimes the tooth exhibits alternating periods of resorption and repair, as manifested by irregular lacuna-like areas in the dentin that are partially or completely filled in with irregular dentin or osteodentin, which itself is undergoing resorption. As the resorptive process advances, the dentin may be completely resorbed in a narrow segment. The enamel is also resorbed if the lesion is situated in the coronal portion of the tooth. If the lesion is in the root of the tooth, perforation of the dentin and cementum may occur, which, if left untreated, may eventually result in complete separation of the apical portion from the remainder of the tooth. When the root surface is perforated, it is impossible to determine whether the lesion began ‘externally’ or ‘internally’.
Treatment and Prognosis
If the condition is discovered before perforation of the crown or root has occurred, root canal therapy may be carried out with the expectation of a fairly high degree of success. Once perforation has occurred, the tooth must usually be extracted.
There are a few cases known in which spontaneous regression of the internal resorption occurred, with the lesion either remaining but showing no further progress or with actual repair by deposition of calcified tissue. The cause of the abrupt cessation of tissue destruction is as obscure as the cause of its initiation.
Hypercementosis: (Cementum Hyperplasia)
Hypercementosis is a non-neoplastic condition in which excessive cementum is deposited in continuation with the normal radicular cementum. Apart from the idiopathic nature of hypercementosis, this condition is associated with several local and systemic factors.
Hypercementosis may be regarded as a regressive change of teeth characterized by the deposition of excessive amounts of secondary cementum on root surfaces. This most commonly involves nearly the entire root area, although in some instances the cementum formation is focal, usually occurring only at the apex of a tooth.
Etiology
A variety of circumstances may favor the deposition of excessive amounts of cementum. These include:
In addition, hypercementosis of unknown etiology may occur either in a generalized form, involving all the teeth, or in a localized form, involving one tooth. Tooth function does not appear to favor the increased deposition of cementum on root surfaces. Actually, studies have indicated that the thickness of cementum is increased in nonfunctioning teeth, including embedded or impacted teeth. The stimulus in these instances is unknown.
Acceleration in the elongation of a tooth owing to loss of an antagonist is accompanied by hyperplasia of the cementum, apparently as a result of the inherent tendency to maintain the normal width of the periodontal ligament. This hypercementosis is most prominent at the apex of the root, although deposition of secondary cementum usually involves the entire root, tapering off in thickness toward the cervical portion of the tooth.
Inflammation at the apex of a tooth root, usually occurring as a result of pulpal infection, sometimes stimulates excessive deposition of cementum. This does not occur at the apex of the root directly adjacent to the area of inflammation, since the cementoblasts and their direct precursors in this area have been lost as a result of the inflammatory process. Instead, the cementum is laid down on the root surface at some distance above the apex, apparently being induced by the inflammatory reaction that, at some distance from its center, acts as a stimulus to cementoblasts. At the apex of the involved root itself it is not uncommon for actual resorption of cementum and dentin to occur. Thoma and Goldman pointed out that the periapical inflammation tends to cause some increase in eruption of the tooth, and this also favors the deposition of cementum in attempting to maintain the width of the periodontal ligament.
Tooth repair does not occasion the deposition of remarkable amounts of secondary cementum. Nevertheless, the cementum that is formed is often laid down with such rapidity that a mild form of hypercementosis is simulated. On occasion, occlusal trauma results in mild root resorption. Such resorption is repaired by secondary cementum. Root fracture is also repaired on occasion by deposition of cementum between the root fragments as well as on their periphery. Finally, cemental tears, detachment of strips of cementum from the root due to trauma, are repaired by cementum growing into and filling the defects and eventually uniting with the torn cementum.
Osteitis deformans or Paget’s disease of bone (q.v.) is a generalized skeletal disease characterized by deposition of excessive amounts of secondary cementum on the roots of the teeth and by the apparent disappearance of the lamina dura of the teeth, as well as by other features related to the bone itself. Although the bone changes are the most prominent features of the disease, generalized hypercementosis should always suggest the possibility of the presence of osteitis deformans.
Spike formation of cementum is an uncommon condition characterized by the occurrence of small spikes or outgrowths of cementum on the root surface. These cemental spikes appear in some cases of excessive occlusal trauma, probably as a result of deposition of irregular cementum in focal groups of fibers of the periodontal ligament. The exact mechanism of spike formation is unknown, and its significance is obscure.
Clinical Features
Hypercementosis produces no significant clinical signs or symptoms indicative of its presence. There is no increase or decrease in tooth sensitivity, no sensitivity to percussion unless periapical inflammation is present, and no visible changes in gross appearance in situ. When the tooth with hypercementosis is extracted, the root or roots appear larger in diameter than normal and present rounded apices.
Radiographic Features
On the periapical radiograph most cases of hypercementosis, at least of any significant degree, are distinguished by the thickening and apparent blunting of the roots. The roots lose their typical ‘sharpened’ or ‘spiked’ appearance and exhibit rounding of the apex (Fig. 13-25). It is generally impossible to differentiate the root dentin from the primary or secondary cementum radiographically; therefore the diagnosis of hypercementosis is established by the shape or outline of the root rather than by any differences in radiodensity of the tooth structure.
Histologic Features
The microscopic appearance of hypercementosis is a characteristic one in which an excessive amount of secondary or cellular cementum is found deposited directly over the typically thin layer of primary acellular cementum (Fig. 13-26). The area involved may be the entire root or only a portion, typically the apical region.
The secondary cementum has been termed ‘osteocementum’ because of its cellular nature and its resemblance to bone. This cementum typically is arranged in concentric layers around the root and frequently shows numerous resting lines, indicated by deeply staining hematoxyphilic lines parallel to the root surface.
Treatment and Prognosis
No treatment is indicated for teeth exhibiting hypercementosis, since the condition in itself is innocuous. In those cases in which the overproduction of cementum is due to inflammation of pulpal origin, treatment of the primary condition is obviously necessary. The extraction of teeth because of hypercementosis is contraindicated, since the prognosis of such teeth is excellent in the absence of concomitant infection.
Cementicles
Cementicles are small foci of calcified tissue, not necessarily true cementum, which lie free in the periodontal ligament of the lateral and apical root areas. The exact cause for their formation is unknown, but it is generally agreed that in most instances they represent areas of dystrophic calcification and thus are an example of a regressive or degenerative change.
It is recognized that actually a variety of calcified bodies may occur in the periodontal ligament, not all of which have the morphologic characteristics of cementum. Nevertheless, they have all been commonly known as cementicles. These various types of calcifications have been reviewed by Moskow.
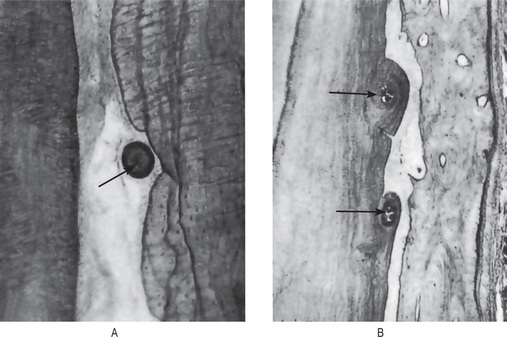
The most common manner in which cementicles develop is by calcification of nests of epithelial cells, i.e. epithelial rests, in the periodontal ligament as a result of degenerative change. These bodies enlarge by further deposition of calcium salts in the adjacent surrounding connective tissue (Fig. 13-27A). The continued peripheral calcification of the connective tissue may result in the eventual union of the cementicle with, and even inclusion in, root cementum or alveolar bone. The pattern of calcification often gives the appearance of a circular lamellated structure. When only partially embedded in the root cementum, the cementicle may impart a roughened globular outline to the root surface (Fig. 13-27B).
Cementicles may arise from focal calcification of connective tissue between Sharpey’s bundles with no apparent central nidus. This calcification occurs as small round or ovoid globules of calcium salts.
Small spicules of cementum torn from the root surface—i.e. cemental tears—or fragments of bone detached from the alveolar plate, if lying free in the periodontal ligament may resemble cementicles, particularly after they have undergone some remodeling through resorption and subsequent repair (Figs. 13-28, 13-29).
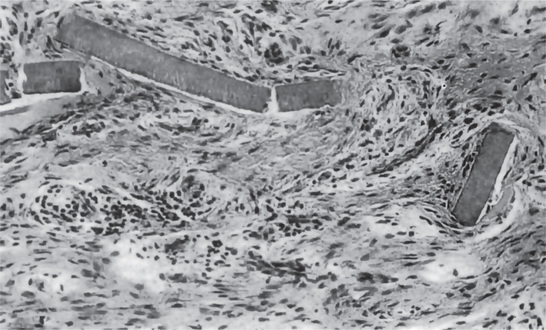
Figure 13-28 Cemental tears.
Small strips of primary cementum lying free in the periodontal membrane after traumatic injury.
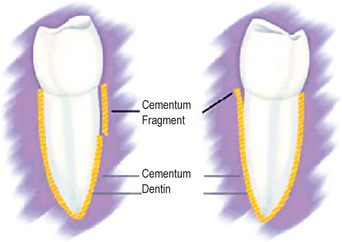
Figure 13-29 Complete and partial cemental tears. Courtesy of Barbara J Marquam. The Journal of Contemporary Dental Practice, Volume 4, No. 3, August 15, 2003.
Finally, cementicles appear to arise through calcification of thrombosed capillaries in the periodontal ligament, and as Mikola and Bauer pointed out in their excellent discussion of the formation of cementicles, are analogous to phleboliths that can occur elsewhere in the body. Although all cementicles are composed of calcified material, they are too small to be seen on the intraoral radiograph, seldom being larger than 0.2–0.3 mm in diameter. Clusters of cementicles may form, and at the apices of teeth these have sometimes been regarded as a cementoma (q.v.), particularly as the clusters unite through interstitial deposition of bone or cementum.
Cementicles are of no clinical significance and, as far as can be determined, are not detrimental to tooth function.
References
Applebaum, E. Internal resorption of teeth. Dent Cosmos. 1934; 76:847.
Baghdady, V.S, Ghose, L.J, Nahoom, H.Y. Prevalence of pulp stones in a teenage Iraqui group. J Endod. 1988; 14:309–311.
Becks, H, Cowden, R.C. Root resorptions and their relation to pathologic bone formation. Am J Orthod Oral Surg. 1942; 28:513.
Becks, H. Root resorptions and their relation to pathologic bone formation. Int J Orthod Oral Surg. 1936; 22:445.
Beust, T.B. Physiologic changes in the dentin. J Dent Res. 1931; 11:267.
Bevelander, G, Benzer, S. Morphology and incidence of secondary dentin in human teeth. J Am Dent Assoc. 1943; 30:1075.
Brady, W.F. The anorexia nervosa syndrome. Oral Surg. 1980; 50:509.
Browne, W.G. Idiopathic tooth resorption in association with metaplasia. Oral Surg. 1954; 7:1298.
Chan, L.R. Calcifications of the dental pulp. Dent Items Interest. 1926; 48:808.
Clark, D.C, Woo, G, Silver, J.G, et al. The influence of frequent ingestion of acids in the diet on treatment for dentin sensitivity. J Can Dent Assoc. 1990; 56:1101–1103.
Coolidge, E.D. The reaction of cementum in the presence of injury and infection. J Am Dent Assoc. 1931; 18:499.
Eccles, J.D. Dental erosion and diet. J Dent. 1974; 2:153–159.
Elvery, M.W, Savage, N.W, Wood, W.B. Radiographic study of the Broadbeach Aboriginal dentition. Am J Phys Anthropol. 1998; 107:211–219.
Ervin, J.C, Bucher, E.M. Prevalence of tooth root exposure and abrasion among dental patients. Dent Items Interest. 1944; 66:760.
Fish, E.W. Lesions of the dentine and their significance in the production of dental caries. J Am Dent Assoc. 1930; 17:992.
Gandara, B.K, Truelove, E.L. J Conte Dent Pract. 1999;1(1). [Fall Issue].
Gardner, B.S, Goldstein, H. The significance of hypercementosis. Dent Cosmos. 1931; 73:1065.
Gottlieb, B. Biology of the cementum. J Periodontol. 1942; 13:13.
Grippo, J.O. Abfraction. a new classification of hard tissue lesions of teeth. J Esth Dent. 1991; 3:14–18.
Hamasha, A A-H, Darwazeh, A. Prevalence of pulp stones in Jordanian adults. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 86:730–732.
Held, A.J. Cementogenesis and the normal and pathologic structure of cementum. Oral Surg. 1951; 4:53.
Hellstrom, I. Oral complications in anorexia nervosa. Scand J Dent Res. 1977; 85:71.
Henry, J.L, Weinmann, J.P. The pattern of resorption and repair of human cementum. J Am Dent Assoc. 1951; 42:270.
Heymann, H.O, Sturdevant, J.R, Bayne, S, Wilder, A.D, et al. Examining tooth flexure effects. J Am Dent Assoc. 122(41–47), 1991.
Hill, T.J. Pathology of the dental pulp. J Am Dent Assoc. 1934; 21:820.
Hodge, H.C, McKay, H. The micro-hardness of teeth. J Am Dent Assoc. 1933; 20:227.
Humerfelt, A, Reitan, K. Effects of hypercementosis on the movability of teeth during orthodontic treatment. Angle Orthod. 1966; 36:179–189.
Jarvinen, V, Meurman, J.H, Hyvarinen, H, et al. Dental erosion and upper gastrointestinal disorders. Oral Surg Oral Med Oral Pathol. 1988; 65:298–303.
Jarvinen, V.K, Rytomaa, I.I, Heinonen, O.P. Risk factors in dental erosion. J Dent Res. 1991; 70:942–947.
Johnson, P.L, Bevelander, G. Histogenesis and histochemistry of pulpal calcification. J Dent Res. 1956; 35:714.
Kerr, D.A, Courtney, R.M, Burkes, E.J. Multiple idiopathic root resorption. Oral Surg. 1970; 29:552.
Ketcham, A.H. A progress report of an investigation of apical root resorption of vital permanent teeth. Int J Orthod Oral Surg. 1929; 15:310.
Kitchin, P.C, Robinson, HBG. The abrasiveness of dentifrices as measured on the cervical areas of extracted teeth. J Dent Res. 1948; 27:195.
Kretschmer, O.S, Seybold, J.W. The bacteriology of dental pulp stones. Dent Cosmos. 1936; 78:292.
Kronfeld, R. The biology of the cementum. J Am Dent Assoc and Dent Cosmos. 1938; 25:1451.
Kuttler, Y. Classification of dentin into primary, secondary and tertiary. Oral Surg. 1959; 12:996.
Lee, W.C, Eakle, W.S. Possible role of tensile stress in the etiology of cervical erosive lesions of teeth. J Prosthet Dent. 1984; 52:374–380.
Leider, A.S, Garbarino, V.E. Generalized hypercementosis. Oral Surg Oral Med Oral Pathol. 1987; 63:375–380.
Lussi, A, Schaffner, M, Hotz, P, et al. Dental erosion in a population of Swiss adults. Comm Dent Oral Epidemiol. 1991; 19:286–290.
Manly, R.S. The abrasion of cementum and dentin by modern dentifrices. J Dent Res. 1941; 20:583.
Mannerberg, F. Salivary factors in cases of erosion. Odontol Revy. 1963; 14:156.
Marquam, B.J. J Cont Dent Prac. 2003;4(3). [Aug 15].
Massler, M, Perreault, J.G. Root resorption in the permanent teeth of young adults. J Dent Child. 1954; 21:158.
McClure, F.J, Ruzicka, S.J. The destructive effect of citrate vs lactate ions on rats’ molar tooth surfaces, in vivo. J Dent Res. 1946; 25:1.
McEvoy, S.A, Mitchell, R.J, Powell, M.L, Wedge-shaped cervical dental lesions in two prehistoric native American populations. Am J Phys Anthro 1996;(22 Suppl):162.
Mikola, O.J, Bauer, W.H. ‘Cementicles’ and fragments of cementum in the periodontal membrane. Oral Surg. 1949; 2:1063.
Milosevic, A, Young, P.J, Lennon, M.A. The prevalence of tooth wear in 14-year-old school children in Liverpool. Comm Dent Health. 1994; 11:83–86.
Milosevic, A. Toothwear. aetiology and presentation. Dent Update. 1998; 25:6–11.
Monahan, R. Periapical and localized radiopacities. Dent Clin North Am. 1994; 38:113–136.
Moskow, B.S. Origin, histogenesis and fate of calcified bodies in the periodontal ligament. J Periodontol. 1971; 42:131.
Mummery, J.H. The pathology of ‘pink spots’ on teeth. Br Dent J. 1920; 41:300.
Neville, B.W, Damm, D.D, Allen, C.M, Bouquot, J.E. Abnormalities of teeth. In: In Neville B.W, Damm D.D, Allen C.M, Bouquot J.E, eds. Oral and Maxillofacial Pathology. 2. Philadelphia: WB Saunders; 2002:49–106.
O’Brien, M. Children’s Dental Health in the United Kingdom 1993. Office of Population Censuses and Surveys Her Majesty’s Stationery Office. 1994.
Pindborg, J.J Pathology of the Dental Hard Tissue. 1970;
Rabinovitch, B.Z. Internal resorption. Oral Surg. 1957; 10:193.
Robinson, HBG, Boling, LR, Lischer, BE, Lischer BE. EV Cowdry: Problems on Ageing. 2. Williams and Wilkins, Baltimore, 1941.
Robinson, HBG. Abrasion, attrition and erosion of the teeth. Health Center J Ohio State Univ. 1949; 3:21.
Rudolph, C.E. A comparative study in root resorption in permanent teeth. J Am Dent Assoc. 1936; 23:822.
Shaw, L, Smith, A. Erosion in children: an increasing clinical problem? Dental Update. 1994; 21:103–106.
Shulman, E, Robinson, HBG. Salivary citrate content and erosion of teeth. J Dent Res. 1948; 27:541.
Sicher, H. The biology of attrition. Oral Surg. 1953; 6:406.
Siskos, G.J, Georgopoulou, M. Unusual case of general pulp calcification (pulp stones) in a young Greek girl. Endod Dent Traumatol. 1990; 6:282–284.
Smith, B.G, Knight, J.K. An index for measuring the wear of teeth. Br Dent J. 1984; 156:435–438.
Smith, B.G, Robb, N.D. The prevalence of tooth wear in 1007 dental patients. J Oral Rehabil. 1996; 23:232–239.
Stafne, E.C, Austin, L.T. Resorption of embedded teeth. J Am Dent Assoc. 1945; 32:1003.
Stafne, E.C, Lovestedt, S.A. Dissolution of tooth substance by lemon juice, acid beverages and acids from some other sources. J Am Dent Assoc. 1947; 34:586.
Stafne, E.C, Szabo, S.E. The significance of pulp nodules. Dent Cosmos. 1933; 75:160.
Stanley, H.R, White, C.L, McCray, L. The rate of tertiary (reparative) dentine formation in the human tooth. Oral Surg. 1966; 21:180.
Sullivan, H.R, Jolly, M. Idiopathic resorption. Aust Dent J. 1957; 2:193.
Sundell, J.R, Stanley, H.R, White, C.L. The relationship of coronal pulp stone formation to experimental operative procedures. Oral Surg. 1968; 25:579.
Sweet, APS. Internal resorption, a chronology. Dent Radiogr Photogr. 1965; 38:75.
Taintor, J.F, Biesterfeld, R.C, Langeland, K. Irritational or reparative dentin. Oral Surg. 1981; 51:442.
Tamse, A, Kaffe, I, Littner, M.M, Shani, R. Statistical evaluation of radiologic survey of pulp stones. J Endod. 1982; 8:455–458.
Ten, Bruggen Cate HJ. Dental erosion in industry. Br J Indust Med. 1968; 25:249.
Thoma, K.H, Goldman, H.M. The pathology of dental cementum. J Am Dent Assoc. 1939; 26:1943.
Van, Huysen G, Hodge, H.C, Warren, S.L. A quantitative roentgen-densitometric study of the changes in teeth due to attrition. J Dent Res. 1937; 16:243.
Van, Den Berghe JM, Panther, B, Gound, T.G. Pulp stones throughout the dentition of monozygotic twins. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 87:749–751.
Warner, G.R, Orban, B, Hine, M.K, Ritchey, B.T. Internal resorption of teeth: interpretation of histologic findings. J Am Dent Assoc. 1947; 34:468.
Weinberger, A. Attritioning of teeth. Oral Surg. 1955; 8:1048.
Willman, W. Calcifications in the pulp. Bur. 1934; 34:73.
Zipkin, I, McClure, F.J. Salivary citrate and dental erosion. J Dent Res. 1949; 28:613.
 . Attrition, Abrasion and Erosion
. Attrition, Abrasion and Erosion