Research for health professionals
The material in this chapter will help you to:
 distinguish and describe various research paradigms used in psychological and health research
distinguish and describe various research paradigms used in psychological and health research
 describe how research shapes healthcare practice
describe how research shapes healthcare practice
 describe the role of health professionals as research consumers
describe the role of health professionals as research consumers
 critically appraise research reports and draw conclusions appropriate for practice
critically appraise research reports and draw conclusions appropriate for practice
 describe the ethics of research participation and in particular the advocacy role played by health professionals.
describe the ethics of research participation and in particular the advocacy role played by health professionals.
Introduction
This chapter examines how psychological and health research findings influence healthcare practice and provides an overview of research paradigms, methodologies and methods. It is not within the scope of this chapter to provide a detailed account of how to conduct research as this is covered in more detail and depth elsewhere in your course and in specific research textbooks. Rather, this chapter focuses on how research findings influence healthcare practice and how health professionals can use research in their day-to-day clinical practice and, thereby, be competent consumers of research (Schneider et al 2013). Being a competent consumer of research involves knowing how to access, critique and utilise research findings to inform your everyday clinical practice. Finally, the role of the health professional in the ethical conduct of research is addressed.
Healthcare research: an overview
Research is a process of inquiry that seeks to develop new knowledge or to expand existing knowledge. In the health arena research findings are used to: identify the health needs of populations; test and choose appropriate interventions and treatments for illness and health problems; plan and implement intervention strategies for illness prevention and health promotion; evaluate programs and interventions; and assist with resource allocation.
Research can be either basic or applied. Basic research aims to develop new theory and/or knowledge, while applied research examines the application of knowledge in certain circumstances. A study of the factors that influence an individual's decision to follow or disregard a recommended health treatment, for example, is basic research; and a randomised control trial (RCT) testing a new drug to treat cancer is an example of applied research.
A further important distinction between research studies is whether they are experimental, observational, interpretive or critical. Experimental studies utilise quantitative methods and are a powerful research method because people (subjects/participants) can be allocated to receive an exposure of interest, such as a new treatment or healthcare practice, or an intervention, such as allergen avoidance or dietary advice. In such studies, independent variables that distort the association between another independent variable and the problem (confounding factors) can be controlled and, therefore, the level of evidence obtained is high.
Observational research, also called descriptive studies, utilises either quantitative (e.g. census) or qualitative (e.g. ethnography) methods. They are less powerful for measuring associations; nevertheless, they are a valuable method for: measuring the effects of non-modifiable risk factors such as age or gender; measuring the effects of exposures to which people cannot be ethically randomised, such as breastfeeding or environmental tobacco smoke; or understanding human experience and social issues.
Interpretive and critical approaches are located within the qualitative research paradigm and aim to describe, explore and seek understanding of human and social phenomena. Interpretative research is focused on understanding or creating meaning, while critical research has the additional goal of bringing about social and political change.
Research paradigms
Various methods can be utilised to conduct research and the choice of method is driven by the methodology (or the philosophical and theoretical tradition that underpins the inquiry). Methodologies, also referred to as paradigms, are the theoretical and philosophical positions that underpin the research approach. They are a broad framework of perception, understanding and beliefs within which theory and practice operate.
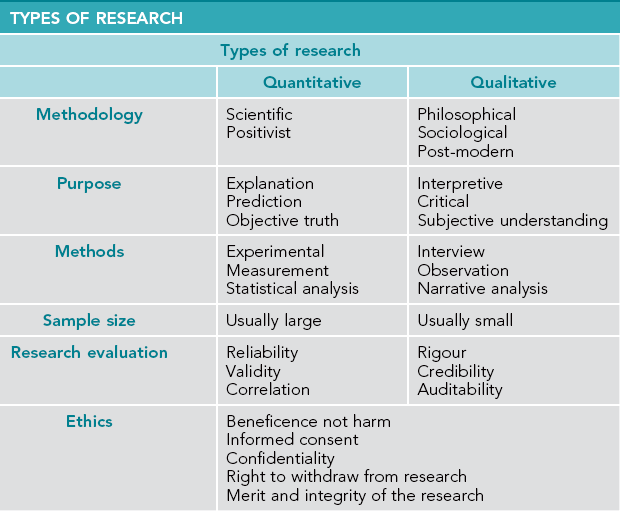
There are two main research paradigms: quantitative and qualitative. The quantitative research paradigm is scientific and positivist and seeks objective answers to the research question. It assumes that an objective answer to the question exists. The qualitative research paradigm is interpretative and critical and seeks greater meaning and understanding of the issue under investigation. It acknowledges the subjective nature of human experience.
Quantitative research
Quantitative research is steeped in the conventional scientific tradition. It involves collecting data that are quantifiable and measurable and, therefore, can be analysed and interpreted numerically. It takes a positivist philosophical position and is underpinned by the view that reality is objective, measurable and separate from the researcher. In this way quantitative research follows a scientific tradition of objective observation, prediction and testing of causal or correlational relationships (Bragge 2010, Swanwick 2010).
Quantitative research generally involves extensive data collection and, thereby, seeks a broad understanding to enable explanations and predictions to be made. Hypotheses can either be supported or rejected by applying statistical tests of significance to the data. The data collected in quantitative research can be: nominal, when the data distinguish categories (like male/female); ordinal, when the data distinguish degree like never, sometimes, always; or numerical, when the data measures numbers like how many cigarettes smoked per day. Examples of quantitative methods include experiments (e.g. RCTs, questionnaires and surveys), structured interviews and census collection.
Quantitative research designs include: experimental, which attempt to show that one thing causes another; quasi experimental, which is an experimental design but does not have random allocation to the control group or an experimental group; descriptive, which summarises and describes a set of measurements; and correlational, which explores the relationship between two variables.
Focus on quantitative research: randomised control trials (RCTs)
In RCTs with human subjects, participants agree to enrol following informed consent and are then randomly allocated to the experimental (receive the intervention) or control (don't receive the intervention) group. Depending on the type of study, the control group may receive a placebo intervention or may receive current best practice. Placebo interventions are usually only used when a new treatment method is being assessed and, for this reason, placebo-controlled trials usually have a small sample size because they are seen as an intermediary step in the process of showing the efficacy of a new treatment.
RCTs provide the highest level of evidence because the random allocation of participants to study groups minimises the influences of selection bias, of known and unknown confounders and of prognostic factors such as participant characteristics on the study results. Blinding (not informing the participants and/or the research team about which participants are in which group) can also reduce the effects of other biases.
The inclusion criteria are an important issue in RCTs. In trials to measure the effectiveness of a treatment or intervention, participants who have an identified health problem are enrolled. However, in interventions designed to prevent an illness or condition from developing, participants who are ‘at risk’ are enrolled before the illness or condition has developed. Although RCTs provide the most scientifically rigorous research method available, they are often difficult to conduct and low response rates may reduce the generalisability of the results. Table 6.1 summarises the strengths and weaknesses of RCTs.
Table 6.1
STRENGTHS AND WEAKNESSES OF RCTs
| Strengths | Weaknesses |
| Provide a high level of evidence | Selection bias may be an issue if potential participants have treatment preferences |
| Confounders, prognostic factors and exposures are balanced between groups | Follow-up bias may be influential if control group participants selectively drop out because they are receiving a placebo or existing treatment |
| Allocation, reporting and observer bias can be controlled by blinding | Low participation rates may reduce the generalisability of the results |
| Willingness to participate does not influence group allocation | Long-term outcomes may not be measured |
Qualitative research
Qualitative research proposes that there is no objective reality as assumed by the quantitative positivist paradigm. It is a constructivist approach in which the individual constructs the meaning and interpretation of reality from their own experience. Nevertheless, like quantitative studies, qualitative research utilises a range of methods and methodologies that facilitate the exploration of different phenomena, meaning and experience.
In qualitative research, data are collected from observation and interview within a population and describe the range of responses, as well as variation between responses. Narrative data are collected as opposed to numerical data in quantitative research and, thereby, a depth of understanding about the issue under investigation is provided in contrast to the breadth of understanding sought in quantitative research.
Examples of qualitative methodologies include:
 symbolic interactionism – that seeks to understand the social meaning that shapes human behaviour (see Beard & Fox 2008)
symbolic interactionism – that seeks to understand the social meaning that shapes human behaviour (see Beard & Fox 2008)
 phenomenology – that seeks the discovery of a phenomenon (European philosophical tradition) or the experience of a phenomenon or its meaning (North American / empirical tradition) (see Ranse & Arbon 2008)
phenomenology – that seeks the discovery of a phenomenon (European philosophical tradition) or the experience of a phenomenon or its meaning (North American / empirical tradition) (see Ranse & Arbon 2008)
 ethnography – that seeks to discover cultural meaning from a native perspective (see Larun & Malterud 2007)
ethnography – that seeks to discover cultural meaning from a native perspective (see Larun & Malterud 2007)
 grounded theory – that uses induction to develop theory (see Bahora et al 2009).
grounded theory – that uses induction to develop theory (see Bahora et al 2009).
Methods for collecting data in a qualitative study include but are not limited to: focus groups; unstructured or semi-structured interviews; participant observation; ethnography; case studies; and document analysis.
Focus on qualitative research: phenomenology
Phenomenology began as a philosophical mode of inquiry in continental Europe around the turn of the 20th century. Its acknowledged founder is the German philosopher Edmund Husserl (1859–1938). Throughout the latter part of the 20th century and early 21st century phenomenology was adapted as an approach in health research inquiry, particularly by nurse researchers (Dowling 2007). The goal of phenomenological health research is to understand a human or social issue by examining the human experience of the phenomenon under investigation, whereas the goal of philosophical phenomenology is to examine the phenomenon itself (Aspers 2010, Crotty 1996, Flood 2010).
Consequently, phenomenology is both a philosophy and a research method. As a philosophy it is interested in the person's perception of a phenomenon, that is, the subjective understanding of the meaning of the phenomenon being investigated, such as the phenomenon of sadness. And, as a research approach, phenomenology is interested in understanding the human (or lived) experience of a particular phenomenon, such as what it is like to be sad. It is the latter form of phenomenology that is prevalent in the health research literature. This type of phenomenological research generally takes the form of interviewing participants, followed by analysis of the data and the development of themes from which conclusions and recommendations are drawn.
Controversy surrounds the use of phenomenology as a method in health research. Crotty (1996) refers to the study of the lived experience as ‘new’ phenomenology, which he argued differs from philosophical phenomenology because it attempts to draw objective conclusions from subjective data. Paley (2005), too, is critical of phenomenological health research, stating that in attempting to make sense of subjective data, nurse researchers draw objective conclusions and in doing so ‘mimic science’ in assuming that an objective reality exists.
Aspers (2010), however, does not see a problem with there being two approaches. While he distinguishes philosophical phenomenology from what he calls empirical phenomenology, he describes the two approaches as having different purposes. Philosophical phenomenology seeks to understand the phenomenon itself, while empirical phenomenology is interested in the social meaning of the person's experience of the phenomenon. Finlay (2010) also observes that a variety of research methods and techniques are conducted under the banner of phenomenology. She argues that rather than debating the difference in approaches, the important issue for researchers should be to be clear about which philosophical and research traditions they are using and why. Nevertheless, despite the methodological debate surrounding phenomenological health research, phenomenological studies contribute to the body of knowledge about individuals' experiences of health and illness issues and thereby can influence healthcare practices.
Quantitative or qualitative?
In the planning phase of an investigation a decision is made early in the research process concerning which methods or methodology to use, namely, quantitative or qualitative, or whether to use multiple methods or methodologies – triangulation. Choosing a method is best addressed by considering the question to be investigated and the methodology that best informs the research question. For example, if a researcher wants to investigate the incidence of asthma in the community, then epidemiological (quantitative) data will provide that information. However, if the researcher wants to know what is the experience of a person living with asthma? then a semi-structured interview or focus group is an appropriate (qualitative) method for data collection. Table 6.2 summarises the similarities and differences between quantitative and qualitative research.
Triangulation
Triangulation, or the use of mixed methods and/or methodologies in psychological and social research, is a process whereby various forms of data are collected from different sources. Its justification is that no single method can provide a comprehensive explanation for the phenomenon under investigation and that collecting data from different sources provides multiple perspectives and enables better understanding (Denzin 2009, Torrance 2012). Triangulation may be applied to one or more of the following: methodology, method, data and/or investigator. It can utilise both quantitative and qualitative paradigms and methods for data collection, for example, by obtaining data from key informant interviews (qualitative) and questionnaires (quantitative). It can also include collecting data using different methods within the same research paradigm, for example, focus group and participant observation, which are both qualitative.
The purpose of triangulation is to validate the findings by collecting data on the same phenomenon from different sources (Howe 2012). Collecting data from various sources enables researchers to corroborate their findings, or not. And if both quantitative and qualitative methods are used, a broad and deep understanding of the issue is obtained. For example, in determining the needs of parents who have a child with a disability, a researcher could interview key informants (qualitative research) and use this data to design a questionnaire to canvass the opinions of a wider selection of the target population (quantitative research).
In summary, the question of whether to use quantitative or qualitative methods to conduct research relates not to what is the better method but to what is the more appropriate method of providing the information sought. If the identification of the magnitude – or the extent – of an issue is sought or a hypothesis is to be tested, then quantitative methods are required. On the other hand, if the researcher is concerned with understanding human experience from an informant's perspective, then a qualitative method is called for. Furthermore, by utilising both quantitative and qualitative methods – as in triangulation – researchers can obtain a greater understanding of the research question under investigation.
Health professionals as consumers of research
While most health professionals do not conduct research in their day-to-day work, all will use research findings on a daily basis. Schneider et al (2013 p 289) refers to this as being a consumer of research. Being a consumer of research requires health professionals to understand and utilise research evidence in clinical practice. Specifically this means being able to:
 access contemporary research reports related to your area of intended healthcare practice
access contemporary research reports related to your area of intended healthcare practice
 analyse and critique research findings and conclusions
analyse and critique research findings and conclusions
 underpin clinical practice with an evidence base, that is, articulate the evidence base for clinical practices
underpin clinical practice with an evidence base, that is, articulate the evidence base for clinical practices
 know the processes required to translate research findings into new clinical practices
know the processes required to translate research findings into new clinical practices
 know the processes required to change clinical practices that are not supported by contemporary evidence
know the processes required to change clinical practices that are not supported by contemporary evidence
 observe ethical issues of research participation (and advocate on behalf of participants should this be required).
observe ethical issues of research participation (and advocate on behalf of participants should this be required).
Accessing research findings
The findings of research studies need to be disseminated before they can influence practice. Research reports are published in a variety of genres including: as an article in a refereed journal; as an article in a non-refereed journal; as a systematic review; as a monograph; as a conference presentation or poster; as a report on an internet specialist website; or in the popular media, including newspapers, television and the internet.
Where a research report is published can be indicative of the degree of confidence the reader can place in the claims made by the researcher. For example, the conclusions drawn by the author of an article published in a scholarly refereed journal can be accepted with more confidence than claims posted on a news-based website or reported in a daily newspaper.
Refereed journals
Articles submitted to a refereed journal undergo peer review prior to being accepted for publication. This involves a process of subjecting the author's work to scholarly review by experts in the field of the study. For an article reporting on research findings the peer review process aims to ensure the research design is sound and ethical, and that the conclusions drawn and claims made by the authors can be substantiated by the process and results presented. One drawback, however, of disseminating research findings through the peer review process is that it can take months or even years for an article to be accepted by a scholarly journal, thereby delaying the time taken for the research findings to influence practice.
Systematic reviews
A systematic review of the literature identifies a single question and examines all of the published quality literature that relates to the question. They are commonly used to examine cause and effect and clinical effectiveness studies (Schneider et al 2013), for example, to identify risk factors for cancer or to ascertain the most effective drug treatment for osteoarthritis. In the health field systematic reviews are considered to provide the highest level of evidence.
The Cochrane Collaboration is the most widely known publisher of systematic reviews. The collaboration aims to provide independent evidence to inform healthcare decision making. Cochrane reviews combine the results of the world's best medical studies and are recognised as the ‘gold standard in evidence-based health care’ (Cochrane Collaboration 2011). In assessing systematic reviews Hagger (2012) suggests that the features of a ‘good’ review article are that it:
 is original – makes a unique contribution to the literature and field of study
is original – makes a unique contribution to the literature and field of study
 advances thinking and knowledge – challenges previous ideas and contributes to new and existing understandings of the issue
advances thinking and knowledge – challenges previous ideas and contributes to new and existing understandings of the issue
 is theory-based – takes into consideration what has gone before, and uses current thinking and evidence to develop new ideas
is theory-based – takes into consideration what has gone before, and uses current thinking and evidence to develop new ideas
 is evidence-based – takes into consideration previous research findings when developing new ideas
is evidence-based – takes into consideration previous research findings when developing new ideas
 is accurate, comprehensive and rigorous – uses the highest methodological standards when conducting the review, and includes all important studies in the field or provides justification for their exclusion
is accurate, comprehensive and rigorous – uses the highest methodological standards when conducting the review, and includes all important studies in the field or provides justification for their exclusion
 provides recommendations for future research – diligent in generating new questions to be addressed, and fosters future research enquiry and empirical work
provides recommendations for future research – diligent in generating new questions to be addressed, and fosters future research enquiry and empirical work
 stimulates debate – values scholarly debate between researchers and theorists on key questions related to theory, research and practice.
stimulates debate – values scholarly debate between researchers and theorists on key questions related to theory, research and practice.
Other publications
Research findings can also be disseminated through conference presentations and posters that may or may not be peer assessed and may or may not be published. As with journal articles that have been accepted through a review process, conference material will have met criteria with regard to methodological and interpretive soundness and, hence, the reader can place greater confidence in the conclusion(s) drawn by the researcher.
Another source of research reports is the popular media that, in the main, are not peer reviewed. Included in this category are newspaper articles, television programs and the internet. Less confidence can be placed in the reliability, validity and credibility of claims made in such reports if they have not undergone the scrutiny of professional review.
Government departments and non-government organisations frequently publish reports as a monograph, which is a small book or treatise on a particular subject. Governments, academics and organisations also publish reports and policies in hard copy and on the internet in what has become known as the ‘grey literature’. The credibility of these reports is influenced by who is the author and who published it. Non-refereed journals and newsletters are another source for research reports or updates. However, as these publications have not been subjected to the scrutiny of review by experts they do not carry the same authority as a report published in a scholarly refereed journal. Nevertheless, they can be informative and a discerning reader can critically evaluate the conclusions drawn by the authors just as they also would for a refereed article.
Access to research information by the general public has increased exponentially in recent years, principally through the internet but also through current affairs programs on television and from the print media. Many patients now research their conditions on the internet prior to visiting their general practitioner (GP). While this enables patients to be more informed about their condition and treatment options it is important to stress that any information sourced from the popular media must be critically assessed before being accepted as valid. To conclude, before accepting claims made by researchers the responsibility rests with readers to critically appraise the veracity of those claims, regardless of whether the research findings are peer assessed or not but particularly if they are not.
Analysis and critique of research reports
In analysing and critiquing research articles and reviews some questions apply to both quantitative and qualitative studies such as ‘how were ethical considerations addressed?’. There are also separate questions to be asked due to the different approaches of the two paradigms regarding research design, data collection, analysis, interpretation and conclusions drawn. The questions in Boxes 6.1 and 6.2 (adapted from Schneider et al 2013) provide a template for critically appraising quantitative and qualitative research reports.
Evidence-based healthcare practice
Evidence-based healthcare practice is premised on the notion that every clinical intervention needs to be supported by findings from contemporary research. In theory this means that health professionals will utilise effective practices, question practices that lack supporting evidence and cease practices that are harmful (Greenhalgh 2010). Such evidence needs to be both quantitative and qualitative because results, for example from RCTs alone, are not sufficient to change practice (Britten 2010, Zayas et al 2011).
Health professionals whose practice is underpinned by an evidence base, therefore, are able to articulate the rationale and refer to the research findings that support particular interventions and keep themselves up to date by regularly accessing the relevant quality literature in their area of practice or speciality. Importantly, practitioners will possess the skills to evaluate research findings and, where relevant, translate these into new practices, or cease existing practices that are not supported by contemporary evidence. Nevertheless, despite the rhetoric surrounding evidence-based best practice, barriers exist that prevent the transfer of identified best practice guidelines into everyday clinical care. Such barriers include resistance from health professionals as well as structural barriers within organisations (Avorn & Fischer 2010, Morrison et al 2012).
Research conducted by Forsner et al (2010) examining the barriers and facilitators to implementing clinical guidelines in psychiatry found there were three categories of barriers and facilitators to implementing clinical practice change: organisational resources; health professionals' individual characteristics; and perceptions of guidelines and implementation strategies. The researchers also found differences between the practitioners in the implementation team and the staff at the clinics, including: concern about control over clinical practice; beliefs about evidence-based practice; and suspicions about financial motives for introducing the guidelines. The researchers concluded that the adoption of new guidelines could be improved if staff at the local level were able to actively participate in the implementation process, and if the identified barriers were addressed at an organisational and individual level.
Earlier research by Grohl and Wensing (2004) examined the implementation of guidelines for diabetes care and found there were three categories of barriers to implementing clinical practice change, namely: individual, social and organisational/economic.
 cognitive – decision-making processes and risk–benefit analysis
cognitive – decision-making processes and risk–benefit analysis
 attitudinal – perceived behavioural control, self-efficacy
attitudinal – perceived behavioural control, self-efficacy
 motivational – the individual's motivational stages and barriers.
motivational – the individual's motivational stages and barriers.
 social network and influence – values and culture
social network and influence – values and culture
Organisational and economic factors included:
 innovativeness of the organisation – specialisation, decentralisation and professionalism
innovativeness of the organisation – specialisation, decentralisation and professionalism
 quality management – culture and leadership
quality management – culture and leadership
 complexity – interaction between system parts
complexity – interaction between system parts
 organisational learning – capacity and arrangements for ongoing learning
organisational learning – capacity and arrangements for ongoing learning
 economic – rewards and incentives for change or maintaining the status quo.
economic – rewards and incentives for change or maintaining the status quo.
The authors concluded that while the research identifies a range of factors that pose barriers to clinical practice change, the findings do not specify which of the factors are the most influential, nor in which circumstances they might have the most influence. Regardless, the findings are important because they highlight the complexity involved in bringing about change in clinical practice – even when there is evidence to support the change. Additionally, the findings identify areas for future research to explore these contributing factors further.
In summary, research provides the evidence on which best practice can be based. However, while health professionals are responsible for ensuring their practice is based on the available evidence, the responsibility for this does not rest with individual health professionals alone because the transfer of the research findings into clinical practice change is complex, political and influenced by social and organisational factors. Evidence-based practice, therefore, is not and cannot be merely an individual health professional's responsibility.
Research ethics in healthcare practice
Throughout the world any research conducted on humans must conform to ethical codes or guidelines. The primary ethical consideration of any research involving humans is that of beneficence, or the principle that on balance the potential good resulting from research participation must outweigh the potential harm.
Ethical codes and guidelines are intended to protect the rights of vulnerable people. The first code of medical ethics, the Nuremburg code, was developed subsequent to the Nuremburg Tribunal that investigated the human rights violations of the medical experiments carried out by doctors in Germany during the Second World War. In 1949 the World Medical Association released an international code of medical ethics, based on the Nuremburg code, which became known as the Declaration of Helsinki. The Helsinki declaration is a statement of ethical principles that provides guidance to physicians and other participants in medical research involving human subjects (World Medical Association 2008) and is the foundation on which worldwide codes of health research ethics are based.
Australian and New Zealand codes of ethics
In Australia the body that oversees the ethical conduct of research involving human subjects is the National Health and Medical Research Council (NHMRC) and in New Zealand it is the Health Research Council (HRC). Both councils have developed guidelines for the ethical conduct of research involving human subjects, namely the NHMRC National Statement on Ethical Conduct in Human Research (NHMRC et al 2009) in Australia, and HRC Guidelines on ethics in health research (2005) in New Zealand.
In 2007 (and updated in 2009) the NHMRC, Australian Research Council (ARC) and the Australian Vice-Chancellors' Committee (AVCC) released the National Statement on Ethical Conduct in Human Research, which contains Australia's primary guidelines for ethically conducting research involving human participants. The purpose of the statement is to: promote ethical human research; ensure that participants are accorded respect and protection; and foster research that benefits the community. The statement is based on four values that the design and conduct of all research involving human participants must follow. The central theme of these values is respect for all human beings and beneficence is the value that underpins the other three. The values are:
Research with Indigenous people
Following a history of colonisation and injustice Indigenous people are sensitive to the ethics of health research (Baum 2008 p 154). Hence further issues need to be considered for research that includes Indigenous people. Therefore, in addition to heeding the ethical principles outlined in the Guidelines on ethics in health research, New Zealand researchers, for example, must also take into consideration additional issues for M ori developed by the HRC (2010). The council directs that all research proposals involving M
ori developed by the HRC (2010). The council directs that all research proposals involving M ori must observe the principles of the Treaty of Waitangi and incorporate this in the proceedings and processes of ethics committees; particularly relevant are the principles of:
ori must observe the principles of the Treaty of Waitangi and incorporate this in the proceedings and processes of ethics committees; particularly relevant are the principles of:
 partnership– working together with iwi, hapu, whanau and M
partnership– working together with iwi, hapu, whanau and M ori communities to ensure M
ori communities to ensure M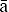 ori individual and collective rights are respected and protected
ori individual and collective rights are respected and protected
 participation – involving M
participation – involving M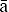 ori in the design, governance, management, implementation and analysis of research, especially research involving M
ori in the design, governance, management, implementation and analysis of research, especially research involving M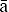 ori
ori
 protection – actively protecting M
protection – actively protecting M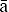 ori individual and collective rights, M
ori individual and collective rights, M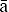 ori data, M
ori data, M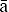 ori culture, cultural concepts, values, norms, practices and language in the research process (HRC 2010).
ori culture, cultural concepts, values, norms, practices and language in the research process (HRC 2010).
Australia, too, has developed guidelines for research with Indigenous people. In collaboration with the NHMRC the National Aboriginal and Islander Health Organisation developed guidelines for research in Indigenous communities. Key principles of the guidelines include:
 community engagement – consultation and negotiation with Aboriginal and Torres Strait Islander people and involvement of community members in the research
community engagement – consultation and negotiation with Aboriginal and Torres Strait Islander people and involvement of community members in the research
 benefit – the potential health benefit for Aboriginal and Torres Strait Islander people is evident in the research proposal
benefit – the potential health benefit for Aboriginal and Torres Strait Islander people is evident in the research proposal
 sustainability and transferability – how the result of the research can lead to achievable and effective health gains for Aboriginal people, beyond the research project is demonstrated in the proposal
sustainability and transferability – how the result of the research can lead to achievable and effective health gains for Aboriginal people, beyond the research project is demonstrated in the proposal
 building capacity – how Aboriginal communities, researchers and others will develop relevant capabilities and retain ownership of data and publication is demonstrated
building capacity – how Aboriginal communities, researchers and others will develop relevant capabilities and retain ownership of data and publication is demonstrated
 priority – the research and potential outcomes are a priority for Aboriginal communities
priority – the research and potential outcomes are a priority for Aboriginal communities
 significance – the research addresses important public health issues for Aboriginal people and takes account of the history of Indigenous colonisation, Indigenous values and respect for culture (NHMRC/ARC 2003, 2010).
significance – the research addresses important public health issues for Aboriginal people and takes account of the history of Indigenous colonisation, Indigenous values and respect for culture (NHMRC/ARC 2003, 2010).
Health professionals as patient advocates
As well as conducting research, health professionals also engage in health research either as participants or because the patients they care for are participants. Consequently, it is crucial that health professionals are aware of the ‘rights’ of research participants, including themselves (Schneider et al 2013 p 95).
With regard to caring for patients who are research participants, a health professional's role includes: ensuring: the patient fully understands what they are consenting to (informed consent); that the rule of beneficence applies; that the patient's anonymity, privacy and dignity is respected; and that the patient is aware that they can withdraw from the research at any time. At times this may involve acting as an advocate for the patient such as providing additional information to the patient or explaining the patient's right to not participate in the research should they not want to.
Extreme examples of the need to advocate on behalf of patients include the 1980s RCT of cervical cancer, conducted at the National Women's Hospital in Auckland, New Zealand, in which conventional cancer treatment was withheld from some participants in the study without their consent (Paul 1988, Skegg 2011) and the United States army-sponsored AIDS research in Thailand in the 1990s that tracked the natural course of vertical transmission of HIV in children born to sero-positive mothers. The infants were not given the antiretroviral drug (ARV) zidovudine (AZT) despite the drug being proven to be effective in reducing vertical HIV transmission in American and French studies. Thirty-seven children in the Thai study contracted the HIV virus (Hassani 2005, Robb et al 1998). Similar controversy surrounded trials of AZT in Africa in the 1990s, in which control groups were given a placebo, despite the results of other trials that convincingly demonstrated the effectiveness of ARV drug treatment, thereby making a control group unethical (Brewster 2011). Haire (2011) further argues that the debate that ensued following the exposure of the ARV trials in developing countries was important because it highlighted the need for health equity and access to care to redress health disparities. Researchers, she argues, have a duty of care and a moral responsibility to ensure that treatments for the diseases under investigation must be made available to the populations participating in the study, regardless of where they live.
In summary, codes of ethics and ethical research guidelines serve the purpose of protecting participants in health research. It is the responsibility of health professionals to ensure these principles are adhered to and to take action on behalf of themselves or the patient in their care should the health professional become aware that this is not the case.
Conclusion
In this chapter, the two major research paradigms – quantitative and qualitative – were presented. Differences and similarities of the two approaches were highlighted and the conclusion drawn that the selection of a particular research paradigm is influenced not by the intrinsic merits of either a positivist or a critical approach but by the question under investigation and the best way to seek an answer to the question.
The importance of research in the everyday practice of psychologists and health professionals was emphasised, including the notion that research provides the evidence on which all healthcare practice is based. The complexities of translating research findings into practice were recognised and the role of health professionals as consumers of research was emphasised. Finally, the important role played by health professionals in the ethical conduct of research was highlighted.
Bragge, P. Asking good clinical research questions and choosing the right study design. Injury, International Journal Care of Injured. 2010; 41S:S3–S6.
Brewster, D. Science and ethics of human immunodeficiency virus/acquired immunodeficiency syndrome controversies in Africa. Journal of Paediatrics and Child Health. 2011; 47:646–655.
Ministry of Health. Operational standard for ethics committees: updated edition. Wellington: NZ Ministry of Health; 2006.
National Health and Medical Research Council, Australian Research Council & Australian Vice-Chancellors’ Committee. National Statement on Ethical Conduct in Human Research. Canberra: NHMRC, AVCC and ARC; 2007.
Schneider, Z., Whitehead, D., LoBondio-Wood, G., et al. Nursing and midwifery research: methods and appraisals for evidence based practice, fourth ed. Sydney: Elsevier; 2013.
http://www3.interscience.wiley.com/cgi-bin/mrwhome/106568753/HOME
The Cochrane Library contains high-quality, independent evidence that can inform healthcare decision making. It includes reliable evidence from Cochrane and other systematic reviews, clinical trials and more. It includes the combined results of the world's best medical research studies, which are recognised as the gold standard in evidence-based healthcare.
Health Services Consumer Research
The HSCR website provides accurate, reliable and insightful information on consumers and providers of healthcare services in New Zealand to help monitor and improve healthcare service delivery.
The Joanna Briggs Institute is an international not-for-profit research and development organisation specialising in evidence-based resources for health professionals in nursing, midwifery, medicine and allied health. The institute is a recognised global leader in evidence-based healthcare.
National Health and Medical Research Council
The NHMRC is Australia's peak body for health and medical research, health advice and for ethics in healthcare and in health and medical research.
New Zealand Health Research Council/Te Kaunihera Rangahau Hauora o Aotearoa
This is the New Zealand Government's main funding agency for health research. Its mission is to benefit New Zealand through health research, with the goal of improving health for all.
References
Aldridge, J., Charles, V. Researching the intoxicated: informed consent implications for alcohol and drug research. Drug and Alcohol Dependence. 2008; 93:191–196.
Aspers, P. Empirical phenomenology: a qualitative research approach (The Cologne Seminars). The Indo-Pacific Journal of Phenomenology. 2010; 9(2):1–12.
Avorn, J., Fischer, M. Bench to behaviour: translating comparative effectiveness research into improved clinical practice. Health Affairs: At the intersection of health, health care and policy. 2010; 29(10):1891–1900.
Bahora, M., Sterk, C., Elifson, K. Understanding recreational ecstasy use in the United States: a qualitative inquiry. International Journal of Drug Policy. 2009; 20(1):62–69.
Barlow, J., Powell, L., Gilchrist, M., et al. The effectiveness of the training and support program for parents of children with disabilities: a randomized controlled trial. Journal of Psychosomatic Research. 2008; 64:55–62.
Baum, F. The new public health. Australia: Oxford University Press; 2008.
Beard, R., Fox, P. Resisting social disenfranchisement: negotiating collective identities and everyday life with memory loss. Social Science and Medicine. 2008; 66:1509–1520.
Bragge, P. Asking good clinical research questions and choosing the right study design. Injury, International Journal Care of Injured. 2010; 41(Suppl 1):3–6.
Brewster, D. Science and ethics of human immunodeficiency virus/acquired immunodeficiency syndrome controversies in Africa. Journal of Paediatrics and Child Health. 2011; 47:646–655.
Britten, N. Qualitative research and the take-up of evidence-based practice. Journal of Research in Nursing. 2010; 15(6):537–544.
Cochrane Collaboration, Cochrane Handbook for systematic reviews of interventions Online. Available, 2011. www.cochrane-handbook.org [19 Sep 2012].
Cox, J., De, P., Morissette, C., et al. Low perceived benefits and self-efficacy are associated with hepatitis C virus (HCV) infection-related risk among injection drug users. Social Science and Medicine. 2008; 66:211–230.
Crotty, M. Phenomenology and nursing research. Melbourne: Churchill Livingstone; 1996.
Denzin, N. The research act: a theoretical introduction to sociological methods. New Jersey: Transaction Publishers; 2009.
Dowling, M. From Husserl to van Manen. A review of different phenomenological approaches. International Journal of Nursing Studies. 2007; 44:121–142.
Finlay, L. Debating phenomenological research methods. Phenomenology and Practice. 2010; 3(1):6–25.
Flood, A. Understanding phenomenology. Nurse Researcher. 2010; 17(2):7–25.
Forsner, T., Hansson, J., Brommels, M., et al. Implementing clinical guidelines in psychiatry: a qualitative study of perceived facilitators and barriers. BMC Psychiatry. 2010; 10(8):1–20.
Greenhalgh, T. How to read a paper: the basics of evidence-based medicine. Chichester: Wiley-Blackwell; 2010.
Grohl, R., Wensing, M. What drives change? Barriers to and incentives for achieving evidence-based practice. Medical Journal of Australia Supplement. 2004; 180:57–60.
Hagger, M. What makes a ‘good’ review article? Some reflections and recommendations. Health Psychology Review. 2012; 6(2):141–146.
Haire, B. Because we can: clashes of perspective over researcher obligations in the failed PrEP trials. Developing World Bioethics. 2011; 11(2):63–74.
Hassani, B. Trials by fire: the case of unethical clinical trials in the countries of the south. University of Toronto Medical Journal. 2005; 82(3):212–216.
Health Research Council of New Zealand (HRC). Guidelines on ethics in health research. Auckland: HRC; 2005.
Health Research Council of New Zealand (HRC). Guidelines for researchers on health research involving. Auckland: M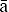 ori. HRC; 2010.
ori. HRC; 2010.
Howe, K. Mixed methods, triangulation and causal explanations. Journal of Mixed Methods Research. Thousand Oaks: Sage; 2012.
Karlsson, A., Arman, M., Wikblad, K. Teenagers with type 1 diabetes – a phenomenological study of the transition towards autonomy in self-management. International Journal of Nursing Studies. 2008; 45:562–570.
Larun, L., Malterud, K. Identity and coping experiences in chronic fatigue syndrome: a synthesis of qualitative studies. Patient Education and Counseling. 2007; 69:20–28.
Morrison, V., Bennett, P., Butow, P., et al. Introduction to health psychology in Australia, second edn. Sydney: Pearson Education; 2012.
National Health and Medical Research Council, Australian Research Council & Australian Vice-Chancellors’ Committee. National Statement on Ethical Conduct in Human Research, 2007. (updated 2009). Canberra: NHMRC, AVCC & ARC; 2009.
National Health and Medical Research Council, Australian Research Council (NHMRC/ARC). Values and ethics – guidelines for the ethical conduct in Aboriginal and Torres Strait Islander research. Canberra: NHMRC; 2003.
National Health and Medical Research Council, Australian Research Council (NHMRC/ARC), Criteria for health and medical research of Indigenous Australians. NHMRC, Canberra, 2010. Online, Available http://www.nhmrc.gov.au/your-health/indigenous-health [19 Sep 2012].
Paley, J. Phenomenology as rhetoric. Nursing Inquiry. 2005; 12(2):106–116.
Paul, C. The New Zealand Cervical Cancer Study: could it happen again? British Medical Journal. 1988; 297(6647):533–539.
Praphan, P. Ethical issues in studies in Thailand of the vertical transmission of HIV. The New England Journal of Medicine. 1998; 338(12):834–835.
Ranse, J., Arbon, P. Graduate nurses’ lived experience of in-hospital resuscitation: a hermeneutic phenomenological approach. Australian Critical Care. 2008; 21:38–47.
Robb, M., Khambaroong, C., Nelson, K. Studies in Thailand of the vertical transmission of HIV. New England Journal of Medicine. 1998; 338(12):843–844.
Schneider, Z., Whitehead, D., LoBondio-Wood, G., et al. Nursing and midwifery research: methods and appraisals for evidence based practice, fourth ed. Sydney: Elsevier; 2013.
Skegg, P. A fortunate experiment? New Zealand’s experience with a legislated code of rights. Medicine and Law Review. 2011; 19(Spring):235–266.
Swanwick, T., Quantitative research methods in medical education, Ch 21Norman G., Eva K., eds. Understanding medical education: Evidence, theory and practice. Wiley Online Library: 2010. Online Available http://onlinelibrary.wiley.com/doi/10.1002/9781444320282.ch21/summary [19 Sep 2012].
Torrance, H. Triangulation, respondent validation, and democratic participation in mixed methods research. Journal of Mixed Methods Research. Thousand Oaks: Sage; 2012. [pp. 1–23].
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Online Available http://www.wma.net/en/30publications/10policies/b3/, 2008. [19 Sep 2012].
Zayas, L., Drake, B., Jonson-Reid, J. Overrating or dismissing the value of evidence-based practice: consequences for clinical practice. Clinical Social Work. 2011; 39:400–405.