CHAPTER 24 Meeting fluid and electrolyte needs
At the completion of this chapter and with some further reading, students should be able to:
• Discuss the fundamental differences in fluid volume content throughout the life span
• Discuss body fluid compartments and their significance in maintaining mechanisms of homeostasis
• Explain cations, anions and their associated movement
• Identify electrolytes and the common themes associated with their imbalances
• Discuss interventions utilised in the correction of electrolyte imbalances in the clinical environment
• Discuss the key physiological processes involved in fluid movement
• Explain the concept of tonicity and its relevance to the administration of intravenous fluids
• Discuss the key systems essential in regulating body fluid balance and composition
• Discuss common client presentations associated with fluid and electrolyte disturbances
• Discuss the homeostatic mechanisms involved in maintaining acid–base balance
• Discuss the implications of acid–base imbalance throughout the life span
• Describe the components of a comprehensive person-centred nursing assessment for clients throughout the life span
• Apply principles in planning, implementing and evaluating person-centred care for clients throughout the life span
The purpose of this chapter is to outline the theoretical foundations in the science of fluid and electrolyte homeostasis and acid–base balance. The opportunity to explore key physiological concepts and the pathophysiology of altered homeostasis is provided. This knowledge acquisition informs nursing practice, advancing skills in providing comprehensive person-centred care throughout the life span. Client interventions and management options are also explored to assist in developing best nursing practice.
As this chapter provides a brief overview of the subject material, it is recommended that you access the additional resources at end of the chapter to enhance your knowledge base.
My first graduate clinical position was on an oncology ward. I can remember caring for a client with lung cancer; the client was profoundly hyponatraemic. I wanted to know what the cause of the hyponatraemia was so I decided to ask one of the nurses I was working with. She explained to me that the patient had something called ‘SIADH’—at the time I found this to be a challenging concept. To help with my learning, I decided to present the client’s case to my colleagues, by doing this I was able to develop my understanding of this condition and provide care accordingly. My colleagues were also able to share this experience and gain new knowledge.
HOMEOSTASIS
Homeostasis can be referred to as ‘the state of equilibrium in the internal environment of the body, which is naturally maintained by adaptive responses that promote health survival’ (Brown & Edwards 2012). Fluids, electrolytes, acids and bases play a vital role in the maintenance of a healthy internal environment. It is critical for nurses to develop a sound understanding of the mechanisms involved in maintaining this environment. This knowledge will inform their ability to anticipate fluid, electrolyte and acid–base disturbances, initiate appropriate person-centred nursing assessment and management.
The significance of the nursing role in maintaining and restoring homeostasis can be demonstrated by the multitude of conditions clients present with that may result in fluid and electrolyte or acid–base disturbances. Clients might consume a variety of common medications that can also impact on maintenance of homeostasis. Nursing care includes the early identification of this client group, assessment and monitoring, and the development of a collaborative plan of care (LeMone et al 2011). Table 24.1 lists the common conditions that can affect fluid and electrolyte homeostasis.
Table 24.1 Common conditions affecting fluid and electrolyte homeostasis
| Skin losses | Excessive sweating, burns, fever |
| GIT losses | Diarrhoea, vomiting, nasogastric suctioning |
| Renal losses | Diuretics |
| Osmotic diuresis | Diabetic ketoacidosis (DKA) |
| Volume loss | Haemorrhage, diabetes insipidus (DI) |
| Third spacing | Burns, sepsis, bowel obstruction, pancreatitis |
| Others | IV fluid administration, chronic heart failure, chronic renal failure, crush injuries |
Fluid volume content of the body
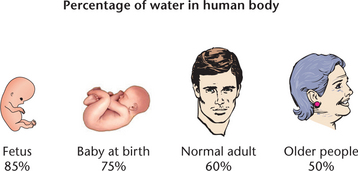
Fluids and electrolytes are usually discussed simultaneously because body fluid is the mode of transport for electrolytes and other substances. In a healthy adult total body water equates to approximately 60% of total body weight. This value changes quite significantly with age: in a newborn this figure can rise to approximately 70% and fall to 50–55% in an older person (Friedman 2009). Figure 24.1 illustrates the percentage of water content in the body.
The proportion of body water is dependent on the amount of lean body mass. As men generally tend to have more lean body mass than women, men naturally have greater body water content than women. Older people also have less lean mass and therefore a lower body water volume, rendering this client group more intolerant to fluid loss. Older clients require a particularly astute nursing assessment of their fluid volume status.
Fluid volume distribution
Body water is distributed between two major compartments, intracellular and extracellular (Figs 24.2 and 24.3). Intracellular fluid (ICF) is the fluid contained inside the cells which accounts for approximately 40% of total body weight (LeMone et al 2011). Electrolytes contained within the ICF include potassium, phosphorus and magnesium. Extracellular fluid (ECF) is located outside the cells and can be further divided into:
• Intravascular: contained within the blood vessels (plasma) accounting for 5% of body weight
• Interstitial: the fluid that cells bathe in accounting for approximately 15% of body weight
• Transcellular: includes fluid in spaces between joints (synovial) and other compartments such as pericardial, pleural, intraocular, cerebrospinal and secretions
• Sodium and chloride are the electrolytes found in the extracellular compartment (LeMone et al 2011).
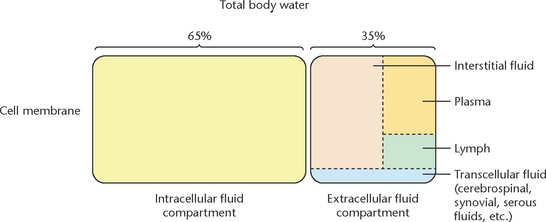
Figure 24.2 Body water distribution and compartments
(Body fluid distribution. Kenneth E. Saladin, Essential Study Partner V 2.0, Anatomy & Physiology: The Unity of Form & Function, 2e, McGraw-Hill Companies, NY, 2001. ISBN 007-24304-5. As at http://www.mhhe.com/biosci/esp/2001_saladin/folder_structure/ab/m3/s1/index.htm)
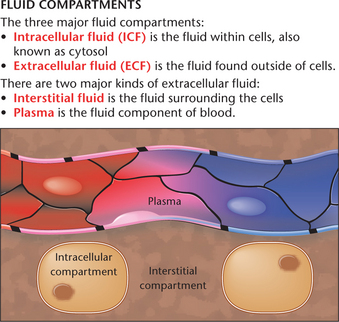
Figure 24.3 Body fluid compartments
(Humboldt State University. Content licensed under Creative Commons (ShareAlike 3.0 Unported License))
The volume of fluid within each compartment remains relatively unchanged in the healthy individual, although fluids and electrolytes move between compartments to maintain their relative compositions (McCance et al 2011). The fluids and electrolytes are able to move between compartments via a semi-permeable membrane, meaning only selected substances can cross it. This membrane is an example of a homeostatic mechanism (Caon 2008). It plays an integral role in maintaining fluid, electrolytes and other substances at their optimal levels and in the correct spaces for a healthily functioning body. The pathophysiology of diseases affecting the function of the semi-permeable membrane is explored later in this chapter.
Mechanisms of fluid and electrolyte movement to maintain homeostasis
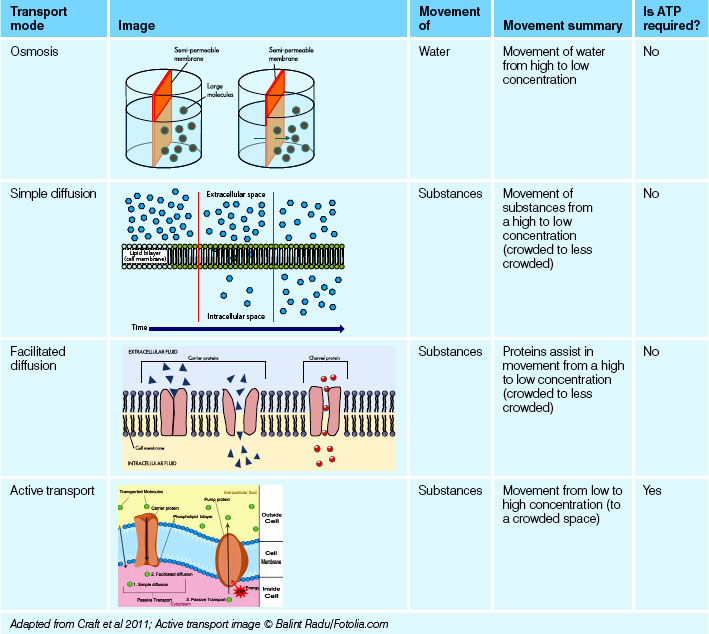
Fluid and electrolyte movement are critical in maintaining homeostasis of the internal environment. Movement across the semi-permeable membrane is facilitated to maintain the appropriate concentrations of fluids and electrolytes in their respective compartments; this is particularly essential for optimal nerve and muscle function. Several modes of transport are available for this process.
Simple diffusion
Diffusion is the movement of a substance, or solute, such as sodium (an electrolyte) or oxygen from an area of greater concentration to an area of lower concentration, resulting in an even distribution of the substance. This could be described as moving from a particularly crowded space to a less crowded space, termed as moving ‘down a concentration gradient’ (McCance et al 2011). Many substances in the body move by diffusion, such as electrolytes, amino acids and glucose. Sometimes diffusion of fluids occurs through a membrane, as in the movement of oxygen from alveoli in the lungs through the capillary membrane into the bloodstream.
Facilitated diffusion
Facilitated diffusion occurs when substances need to move but they need a little help to get to their destination. This assistance comes in the form of proteins, which transport substances through specific channels in the cell membrane. As a result of these substances moving down a concentration gradient (to the less crowded area) this process does not require energy (McCance et al 2011).
Active transport
Active transport is the movement of substances from an area of lower concentration to an area of higher concentration. Unlike diffusion, this process requires substances to move up the concentration gradient (McCance et al 2011). This process could be described as being similar to swimming against a strong current, therefore requiring more energy. Energy for this process comes in the form of adenosine triphosphate (ATP) (LeMone et al 2011). The sodium–potassium ATP pump is a familiar example of this type of movement. The concentrations of sodium and potassium are maintained within a tight range in the ECF and ICF; this is essential for optimal nerve conduction. As electrolytes have a tendency to want to reach equilibrium on either side of the membrane, they have to be pumped ‘against the current’ to maintain their concentrations. The sodium–potassium ATP pump is responsible for maintaining their concentrations and the cell ‘charge’, providing there is enough ATP available for this process (LeMone et al 2011). If ATP is depleted through disease processes, the consequences of homeostasis breakdown would potentially have a significant impact on the health of the client.
Osmosis
Osmosis is the movement of water through a semipermeable membrane from an area of high water concentration to an area of low water concentration (Caon 2008). This simply means water will move to where there is a stronger concentration of electrolytes—like adding more water to a strong cup of tea or coffee. This ultimately has a dilutional effect on the solution. This movement continues until the concentration reaches equilibrium on either side of the membrane. The rate at which osmosis occurs depends upon several factors, including:
• The concentration of electrolytes
• The temperature of the solution
• The electrical charge of the electrolytes
• The difference between the osmotic pressures exerted by the solutions.
See Table 24.2 for a summary of movement mechanisms for water and substances.
Tonicity
Tonicity can be thought of as the ‘concentration’ or ‘osmolality’ of a solution (McCance et al 2010). The terms osmolality and osmolarity are often used interchangeably. Osmolality refers to the solute concentration in osmoles per litre (per volume of solution), whilst osmolarity refers to the solute concentration in osmoles per kilogram. According to Caon (2008), the difference between the two terms with relevance to intravenous solutions encountered in nursing practice is so small it is acceptable to use either term.
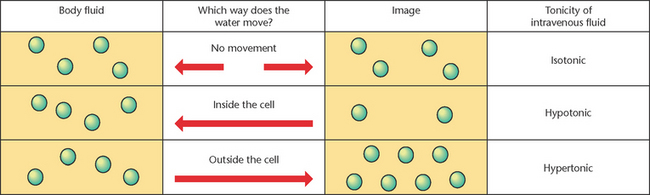
The tonicity or concentration of a solution refers to the concentration of one fluid compared to another (Caon 2008). It is measured against the normal osmolality fluid within, which is between 275 and 295 mOsm/kg (LeMone et al 2011). An isotonic solution has the same tonicity or concentration as body fluid, meaning this solution will not cause water movement into or out of cells if they were placed in this solution. A hypotonic solution is less concentrated than body fluid; therefore it will encourage water to move into the cell, causing it to swell and perhaps burst (haemolyse) if there is a significant influx of water. Hypertonic solution is more concentrated than body fluid and encourages water movement out of the cell, causing the cells to shrink (Caon 2008). The concept of tonicity is particularly relevant to nurses providing care and ongoing management for clients receiving treatment with intravenous fluids to monitor the effects of them on client health. Intravenous fluids will be discussed further at a later point in this chapter.
See Figure 24.4 for a visual representation of fluid tonicity.
Osmotic pressure and hydrostatic pressure
Osmotic pressure (also called oncotic pressure) refers to the concentration of a solution expressed as a pressure unit, usually mmHg (Caon 2008). Movement of water can be affected by the differences in osmotic pressure on opposite sides of the membrane. Plasma has a higher concentration of proteins compared to interstitial fluid; these proteins (especially albumin) exert osmotic pressure causing water to remain in the intravascular space (LeMone et al 2011).
Additionally, movement of water through a membrane can be affected by differences in hydrostatic pressure on either side of the membrane (Caon 2008). The process of water moving from an area of high hydrostatic pressure to low hydrostatic pressure is called filtration (LeMone et al 2011).
Hydrostatic and osmotic pressures greatly influence the movement of water between the interstitial and intravascular compartments. For example, hydrostatic pressure at the arterial end of the capillary pushes water into the interstitial space, until the hydrostatic pressure is finally reduced due to the movement of water. The osmotic pressure at the venous end of the capillary is then greater than hydrostatic pressure and so encourages water to move back into the capillary (LeMone et al 2011). Conditions affecting the integrity of the membrane or the amount of protein in the intravascular space will have a profound impact on health.
Oedema
Oedema (swelling) occurs due to the accumulation of fluid in the interstitial spaces (McCance et al 2011). Causes of oedema include increased capillary hydrostatic pressure, where fluid from the vascular space is forced into the interstitial spaces. This can occur in clients with heart failure. A lowered plasma oncotic pressure also causes fluid to shift into the interstitial spaces; this can occur in clients with a low albumin level. Increased capillary permeability affects the ability of the capillary membranes to maintain fluid within the vascular compartment, resulting in fluid shift to the interstitial space. Examples of conditions with increased capillary permeability include burns and systemic inflammatory response syndrome (SIRS). Figure 24.5 shows capillary hydrostatic and oncotic pressure.
FLUID BALANCE
Fluid intake
The normal daily turnover of water is equivalent to approximately 2500 mL (Welch 2010). See Table 24.3 to view the average adult fluid balance.
Table 24.3 Average adult fluid balance as millilitres of water
| Input | Amount (mL) |
|---|---|
| Drinks | 1500 |
| Food | 800 |
| Metabolism (breakdown of food) | 200 |
| Total | 2500 |
| Output | |
| Urine | 1500 |
| Breathing and sweating | 800 |
| Stools | 200 |
| Total | 2500 |
Adapted from Welch 2010
Fluid output
Insensible fluid losses can be referred to as invisible vaporisation; therefore, this type of fluid loss is difficult to measure. Insensible losses occur through the skin and lungs, amounting to approximately 800 mL daily. Sensible losses are those which can be measured. These losses are comprised of urine and faeces and amount to approximately 1700 mL daily (Welch 2010).
Increased water loss is associated with an accelerated metabolism. Factors that potentially increase fluid loss can include:
The antidiuretic hormone (ADH)
Several mechanisms are integral in the maintenance of fluid balance. They are briefly discussed in Table 24.4. The antidiuretic hormone (ADH) is the primary regulator of water balance. ADH is secreted when plasma concentration is elevated or when blood volume or blood pressure is decreased. When plasma concentration is increased, the osmoreceptors in the hypothalamus respond by stimulating the sensation of thirst to encourage fluid intake. The osmoreceptors also signal the posterior pituitary to release ADH, increasing the reabsorption of water by the kidneys (Fig 24.6). This reduces the amount of water lost in the urine and the reabsorbed water reduces plasma concentration (McCance et al 2011). Clinical Interest Box 24.1 lists other factors that may trigger thirst when fluid volume is normal.
Table 24.4 A systems approach to fluid balance
| Hypothalamus and pituitary | The primary regulator of fluid intake is the thirst mechanism. Thirst can result from fluid loss or an increase in plasma osmolality (an increase in plasma concentration). The thirst centre, located in the hypothalamus, is highly sensitive to changes in the concentration or osmolality of the blood. A change in the blood osmolality or a decrease in blood volume stimulates osmoreceptors in the hypothalamus to trigger the sensation of thirst and the release of the antidiuretic hormone (ADH). ADH is released from the posterior pituitary and acts in the renal tubules, causing water reabsorption. Through these mechanisms, there is an increase in water in the body and a decreased plasma osmolality (Craft et al 2011). |
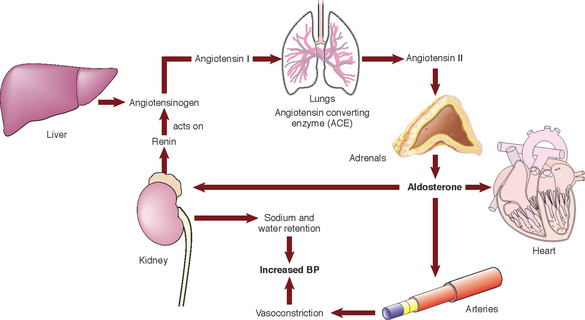
| Renal control Aldosterone Renin-angiotensin system (RAS) (See Fig 24.6.) |
In an average adult, total blood volume is filtered through the kidneys numerous times daily (Brown & Edwards 2012). Most of this filtrate (99%) is reabsorbed, resulting in the production of approximately 1500 mL of urine daily. Fluid and electrolytes are selectively reabsorbed in the renal tubules according to the plasma concentration. The renal tubules are where the ADH and aldosterone perform their actions. Aldosterone secretion can be stimulated by decreased renal perfusion: a client presenting with a low blood pressure, for instance. Aldosterone has the ability to retain sodium and excrete potassium. It forms part of the renin-angiotensin system. In response to reduced renal perfusion, renin is released by the kidneys. Angiotensinogen is produced by the liver and is also found in the blood. Renin is an enzyme; it reacts with angiotensinogen to produce angiotensin I which is converted to angiotensin II by angiotensin-converting enzyme (ACE). Angiotensin II activates the secretion of ADH and aldosterone, Aldosterone works to reabsorb both sodium and water in the renal tubules. The end result is the restoration of plasma volume and concentration. |
| Adrenal cortical control | Glucocorticoids and mineralocorticoids are secreted by the adrenal cortex and both play a role in fluid and electrolyte imbalance. Glucocorticoids have an anti-inflammatory effect and increase glucose levels in the plasma. Cortisol is an example of a glucocorticoid. Mineralocorticoids, such as aldosterone, work by increasing sodium retention and thus water reabsorption, and excretion of potassium. An increase in the secretion of these steroid hormones is associated with physical and psychological stress. |
| Cardiac control | An elevation in blood volume or blood pressure causes atrial natriuretic peptide hormone (ANP) to be released from cells located in the atria of the heart. ANP inhibits activation of the renin-angiotensin system (RAS), promoting excretion of sodium and water. |
| Gastrointestinal control | The gastrointestinal tract secretes approximately 800 mL of fluid a day and in a healthy adult most of this is reabsorbed (Brown & Edwards 2012). A small amount of fluid is eliminated in faeces. From this information, it is easy to imagine the impact diarrhoea and vomiting would have on the secretion and absorption of water. |
CLINICAL INTEREST BOX 24.1 Other factors that can trigger thirst when fluid volume is normal
Actions such as mouth breathing or eating dry or salty foods can be misinterpreted by the body as thirst. Situations such as these can cause additional fluid intake even though the osmolality of the blood or the blood volume is within normal range.
Electrolytes
Electrolytes are also commonly referred to as ‘solutes’; the two terms are used interchangeably and carry the same meaning. During this chapter the term electrolytes will be used to avoid any confusion for the reader.
Electrolytes are substances that dissociate into electrically charged particles called ‘ions’. These can be negatively or positively charged—known as anions and cations respectively. Electrolytes have an ‘osmotic potential’—their ability to pull water into a compartment. Sodium and dextrose are examples of electrolytes with high osmotic potentials. Movement of these electrolytes can result in major fluid shifts between the ICF and ECF (McCance et al 2011). Table 24.5 lists some familiar anions and cations.
Electrolytes vary in number depending on whether they belong inside or outside the cell (in the ICF or ECF). For example, sodium (Na+) is the most abundant cation outside the cells (in the ECF), whereas potassium (K+) is the most predominant cation inside the cell (in the ICF). It is critical for nerve function that electrolytes retain their numbers in their relative compartments to maintain a ‘charge’. If this charge is lost, it will ultimately impact on the transmission of electrical impulses across the cell membranes of nerve and muscle cells (McCance et al 2011). The detrimental impact electrolyte imbalances might have on maintaining health can be imagined.
Discussion of all the electrolytes is beyond the scope of this chapter. Electrolyte imbalances commonly encountered in the clinical environment will be discussed and a summary of common themes associated with electrolyte imbalances in general will be presented. See Table 24.6 for the blood values of electrolytes.
| Na+ | 135–145 mmol/L |
| Cl− | 98–110 mmol/L |
| K+ | 3.5–5.0 mmol/L |
| Ca+ | 2.2–2.6 mmol/L |
| Mg+ | 0.7–1.1 mmol/L |
| PO4− | 0.7–1.3 mmol/L |
| HCO3− | 22–26 mmol/L |
Fluid and electrolyte imbalance
Several factors can disturb the body’s fluid and electrolyte balance, resulting in fluid volume deficit or fluid volume excess. Water volume imbalances are usually accompanied by electrolyte imbalances, particularly sodium. It is important to note that the term ‘fluid volume deficit’ does not carry the same meaning as ‘dehydration’ (Brown & Edwards 2012). Dehydration refers to the loss of pure water alone without the associated loss of sodium.
Dehydration
Dehydration can be defined as a sudden loss of 3% or more of the individual’s body weight (Welch 2010). Dehydration is a common clinical presentation amongst older people and children. People over the age of 85 years are six times more likely to be admitted to hospital for dehydration, while dehydration in children is a leading cause of morbidity and mortality worldwide (Welch 2010). Special considerations for older clients include:
• Loss of appetite and thirst sensation
• Physical changes resulting in loss of independence
Comorbidities can also have a profound effect on hydration. For example, clients experiencing incontinence will frequently reduce their fluid intake; this can cause other problems and predispose the client to urinary tract infections (Welch 2010).
Fluid volume deficit
This term refers to the abnormal loss of body fluid. Causes can include:
• Decreased fluid intake as a result of unavailability of fluids, or secondary to other conditions; for example, unconsciousness
• Increased fluid output secondary to other conditions; for example, diarrhoea, vomiting, excessive urine output, hyperventilation, excessive perspiration, diabetes insipidus, prolonged fever, haemorrhage or inadequate replacement of aspirated or drained fluids
• Increased electrolyte load secondary to other conditions; for example, hyperglycaemia associated with uncontrolled diabetes mellitus; excessive intravenous infusion of highly osmotic or concentrated solutions of glucose or sodium bicarbonate.
Fluid volume deficit can also occur when fluid shifts abnormally from the plasma to the interstitial space. This is commonly referred to as ‘third-space fluid shifts’ in the clinical environment. There is not an absolute loss of fluid: it has simply moved to another compartment. Either disease processes causing this shift create an imbalance in hydrostatic and oncotic pressure and/or there is destruction of the semi-permeable membrane that usually contains fluids within their compartments. Examples of these disease processes include:
Fluid volume excess
This term refers to abnormal fluid excess. This could be as a result of excess intake or due to disease processes that result in an imbalance in hydrostatic and oncotic pressures causing fluid to shift into the vascular compartment or shift into the interstitial space. The latter exhibits signs such as oedema. Although the overall volume of the ECF does not change, it does affect the volume of fluid in the intravascular space. Examples of these disease processes include:
• Excessive administration of intravenous fluids or hypotonic solutions
• Disturbance of homeostatic mechanisms; for example, water retention resulting from kidney, cardiac or liver failure
• Insufficient sodium; for example, from use of diuretic medications
• Inappropriate ADH secretion due to cerebral infections, head injury or increased intracranial pressure (Brown & Edwards 2012).
ELECTROLYTE IMBALANCES
Sodium (Na+)
Normal plasma value: 135–145 mmol/L. Sodium imbalance is one of the most prevalent electrolyte imbalances encountered in the clinical environment. Sodium plays an integral role in the maintenance of irritability and conduction of nerve and muscle tissue. It impacts on volume and concentration of ECF, maintains acid–base balance and influences levels of other electrolytes such as potassium and chloride.
Sodium homeostasis
The kidneys are the primary organs responsible for maintaining the plasma concentration (ECF) of sodium within the tightly controlled limits of 135–145 mmol/L. Sodium balance is regulated by the hormone aldosterone which is secreted by the adrenal cortex (McCance et al 2011). If plasma sodium levels are decreased, aldosterone is secreted to increase sodium reabsorption in the kidney tubules, and as a result the plasma sodium level will rise.
The presentation of either hyponatraemia or hypernatraemia (low or elevated Na+ levels) is quite a complex clinical issue. Either presentation can be associated with either a loss or gain of water, or a loss or gain of sodium. To facilitate understanding of this challenging concept, imagine the body fluid as a cup of coffee. The coffee is the sodium and the body fluid is the water used to dilute the coffee. If the sodium is high (hypernatraemia), two things might have occurred. There is either too much coffee in the water or not enough water to dilute the strong-tasting coffee. In the case of low sodium (hyponatraemia) there may be either not enough coffee in the water or too much water and it has been over-diluted. The same principle can be applied to sodium imbalance; it can be associated with a loss or gain of sodium, making body fluid more or less concentrated (strong or weak coffee). Alternatively, it can be associated with a loss or gain of water. Hence, the most common cause of hypernatraemia is dehydration. See Table 24.7 for a list of conditions and signs and symptoms associated with hyper- and hyponatraemia. These will be dependent on whether there is an associated hypervolaemic or hypovolaemic state.
Table 24.7 Causes, signs and symptoms associated with hypo/hypernatraemia
| Causes of hyponatraemia | Associated signs and symptoms |
|---|---|
| Causes of hypernatraemia | Associated signs and symptoms |
|---|---|
Potassium (K+)
Normal plasma value: 3.5–5.0 mmol/L. Potassium is the chief intracellular cation and has a major role in the concentration of intracellular fluid and neural function. It is particularly essential for the neuronal function of cardiac, musculoskeletal and smooth muscle. Because of this, imbalances in the potassium levels in the intracellular or extracellular environment can be potentially life threatening and often present as a medical emergency (McCance et al 2011). Clinical Interest Box 24.2 outlines the nursing considerations for an individual taking potassium.
CLINICAL INTEREST BOX 24.2 Nursing considerations for potassium
• Foods rich in potassium may need to be restricted in patients with chronic renal failure. Such foods include dates and bananas
• IV K+ administration can be painful for clients
• Consider oral route of administration if appropriate to the clinical situation
• Preparations such as Resonium exchange K+ for calcium (Ca+) in the gut and have a long-lasting effect
• Administration of blood products can cause hyperkalaemia and hypocalcaemia
• K+ levels are usually kept in the high/normal range for cardiac patients i.e. ≥.4.0
• Digoxin is a medication commonly encountered in the clinical environment. Therapeutic levels of this drug are influenced by serum K+ levels.
Potassium homeostasis
Potassium is regulated by the kidneys. It is filtered by the nephrons, reabsorbed back into the blood and secreted according to bodily requirements and functioning. An alteration in the flow of urine also influences the secretion of potassium. For clients taking diuretics the urine flow will be increased, resulting in increased levels of potassium in the urine. Aldosterone also increases the secretion of potassium when levels are high. Potassium balance is also affected by acid–base disturbances. For example, an increase in hydrogen ions (H+) will cause potassium to shift outside the cell in exchange for H+ ions to maintain a balance of cations on either side of the cell. Table 24.8 outlines conditions and signs and symptoms associated with hyper/hypokalaemia.
Table 24.8 Causes, signs and symptoms associated with hypo/hyperkalaemia
| Causes of hyperkalaemia | Associated signs and symptoms |
|---|---|
| Causes of hypokalaemia | Associated signs and symptoms |
|---|---|
NURSING ASSESSMENT OF CLIENT WITH FLUID AND/OR ELECTROLYTE NEEDS
The nursing process is integral to the provision of person-centred care. The process encompasses the following:
The process is not merely a series of steps; a critical thinking approach is adopted by analysing at each stage and updating or changing plans according to the client’s needs (Crisp & Taylor 2009).
Clinical Scenario Box 24.1
Mary, an 84-year-old female, was brought into the emergency department from her nursing home. The staff at the nursing home reported that Mary has become increasingly confused and has developed a fever of 38.2ºC. Mary has a background of a previous stroke affecting her ability to self-care and Alzheimer’s disease. Mary has been commenced on IV fluids and antibiotics and she has been admitted to your ward for ongoing care.
• What risk factors could have contributed to Mary’s dehydration?
• What risk factors could have contributed to Mary developing a urinary tract infection?
• What are some possible nursing diagnoses for Mary?
• How will you manage Mary’s infusion of IV fluid? What are some important considerations?
• What will your ongoing assessment of Mary consist of and what complications will you be monitoring Mary for?
The assessment phase refers to the collection, validation, organisation and documentation of data retrieved from the client (Berman et al 2012). During this phase the nurse will gather information about the client’s health status. The use of a model to promote systematic data collection to prevent the omission of important relevant data is recommended. This data will include subjective and objective information, both of which are essential. This will provide the nurse with a holistic overview of the client’s health status to inform the provision of person-centred care.
With specific reference to fluid and electrolyte needs, obtaining an initial health history would be an appropriate starting point. This could include questioning the client about their present illness/condition/present complaint and their past history, including family history where relevant. Subjective data collection should include questioning the client about their lifestyle habits and psychosocial status. For the assessment of fluid and electrolyte needs it is particularly important to gather data on:
• Past medical history: any conditions associated with fluid/electrolyte disturbances
• Medications: any medications associated with fluid/electrolyte disturbances
• Diet: any specific requirements/restrictions, eating habits, supplements, factors limiting access to adequate nutrition/fluid intake
• Exercise: how often, type of exercise and the conditions; whether there are specific nutritional/fluid requirements associated with it
• Other personal habits: alcohol consumption, sports drinks
• Social data: cultural affiliation (some religious practices include fasting during certain periods)
• Psychological: mental health history, for example, eating disorders; anxiety, stress (Berman et al 2012).
Table 24.9 outlines assessment findings in clients with fluid volume deficit or excess.
Table 24.9 Assessment findings in clients with fluid volume deficit or excess
| Body system | Physical assessment findings—fluid volume excess | Physical assessment findings—fluid volume deficit |
|---|---|---|
| Central nervous system | ||
| Respiratory | ||
| Cardiovascular | ||
| Renal | ||
| Gastrointestinal | Distended abdomen | |
| Skin | Non-specific |
Gathering of objective data will include a thorough physical assessment of the client, frequently performed using a systems approach to facilitate comprehensive systematic data collection. These physical findings will be dependent on the associated disease process the client is experiencing. For example, a client with acute pulmonary oedema may be agitated and confused due to hypoxaemia caused by reduced ability of oxygen to diffuse into the pulmonary capillaries.
Urine
Where urine is concerned there are no absolutes. Urine tests may not be reliable in patients with medical conditions such as renal or heart failure or patients taking diuretics. Urine specific gravity (SG) refers to the density of urine compared with water. Osmolality refers to the concentration of the urine.
Normal urine specific gravity is between 1.005 and 1.030 and osmolality between 500 and 1200 mOsm/kg.
In states of volume deficit, urine SG can be elevated and the osmolality high, usually accompanied by a decrease in urine output (except in diabetic ketoacidosis). In volume excess, urine tends to be dilute with a low SG and osmolality.
See Clinical Interest Boxes 24.3 and 24.4 for more tips on undertaking a physical assessment and additional information to consider when assessing fluid needs of individuals.
CLINICAL INTEREST BOX 24.3 Physical assessment tips for nurses
• Physical examination is a critical element for assessing and treating disorders of fluid balance
• It is often more reliable than data obtained by invasive monitoring
• Weight gain is the most consistent sign of volume excess
• Gravity-dependent oedema is an important finding; not usually apparent until 2–4 kg of fluid have been retained
Paediatric assessment
There are some important issues to consider in paediatric assessments. These issues will obviously depend on the age of the child and a detailed account is beyond the scope of this chapter. However, an overview of questions to consider includes:
• Feeding regimen—are there problems with feeding, for example, lethargy, poor sucking, regurgitation, colic, irritability?
• What is the typical intake over 24 hours?
Important aspects of physical assessment include:
• Obtaining age-appropriate vital signs
• Assessment of state of alertness—how interactive is the child?
• Sunken eyes, pallor, reduced tears, dry arm creases, dry mucous membranes or depressed fontanel (age appropriate) may suggest dehydration
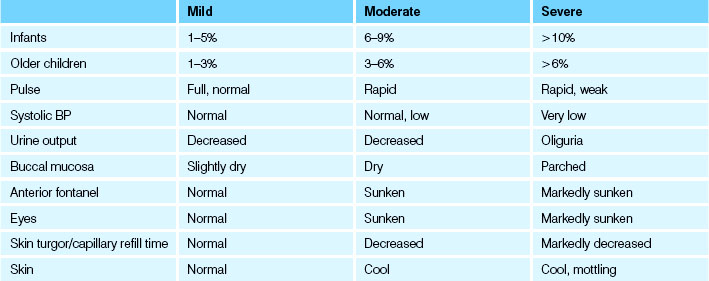
If the child’s current weight and pre-morbid weight are available, this information can be the most reliable indicator of the fluid volume deficit (The Royal Children’s Hospital Melbourne (nd)). There are also physical signs frequently encountered with varying degrees of fluid volume deficit. Volume loss in children is commonly expressed as a percentage of body weight. For example, a 10 kg child who is 5% dehydrated would have a fluid deficit of approximately 500 mL. See Table 24.10 for clinical signs of dehydration that might be observed in children less than 12 years old, depending on the severity of fluid loss.
See Clinical Interest Box 24.5 for further details on estimating volume loss in children.
CLINICAL INTEREST BOX 24.5 Assessment of dehydration in children
• Mild/no dehydration (<4%)—no physical signs
• Moderate dehydration (4–6%)—some physical signs
• Severe dehydration (>7%)—multiple physical signs present and child may also have acidosis and hypotension
(Adapted from The Royal Children’s Hospital Melbourne Clinical Practice Guideline—Intravenous Fluids (nd))
Care planning and intervention
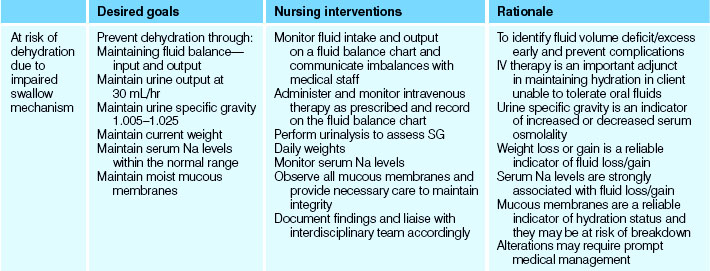
The data gathered from the comprehensive person-centred assessment will inform nursing diagnoses, subsequent planning of care and nursing interventions.
The formulation of nursing diagnoses requires critical thinking skills to be utilised by the nurse (Crisp & Taylor 2009). Diagnostic statements should be specific, including a ‘qualifier’ in the statement. These qualifiers include:
The statement should also include the causative factors.
Most healthcare organisations utilise pre-printed standardised care plans to some degree. Whether the plan is pre-written or not, an integral component of person-centred care is that the plan must be individually relevant to the particular client’s needs. The majority of plans frequently incorporate the key components of:
Table 24.11 is a sample care plan for a client with fluid and electrolyte needs.
Some important general aspects of care to assist the client who requires encouragement with their usual fluid intake, or requires assistance in meeting a higher than usual intake include:
• Providing assistance to clients who experience difficulty in pouring fluid or holding a cup or glass
• Explaining the importance of consuming the normal or increased fluid volume
• Providing the client’s preferred types of fluid if not contraindicated
• Ensuring that all fluids are presented attractively and at the correct temperature
• Encouraging smaller amounts of fluid at more frequent intervals
• Ensuring that about 75% of the total volume of fluid is taken by early evening so that sleep is undisturbed
• Ensuring that toilet facilities are easily accessible
• Documenting input and output to assess and maintain the client’s fluid and electrolyte balance.
Some clients may have restrictions on their fluid intake for medical reasons: those with renal failure, for example. Basic nursing actions to assist this client group might include:
• Explaining to the client and significant others the importance of consuming only the volume ordered
• Planning to distribute fluid evenly throughout the day, including fluid to swallow medications
• Encouraging small amounts at regular intervals to avoid thirst
• Providing thirst-quenching fluids; for example, water, and avoiding sweetened drinks that increase thirst
• Notifying ancillary staff, for example, dietitians and kitchen staff, of changes
• Ensuring that the client’s mouth does not become dry, by regular attention to oral hygiene
• If not contraindicated, the client may be permitted sweets or sugar-free gum to stimulate production of saliva to keep the mouth moist.
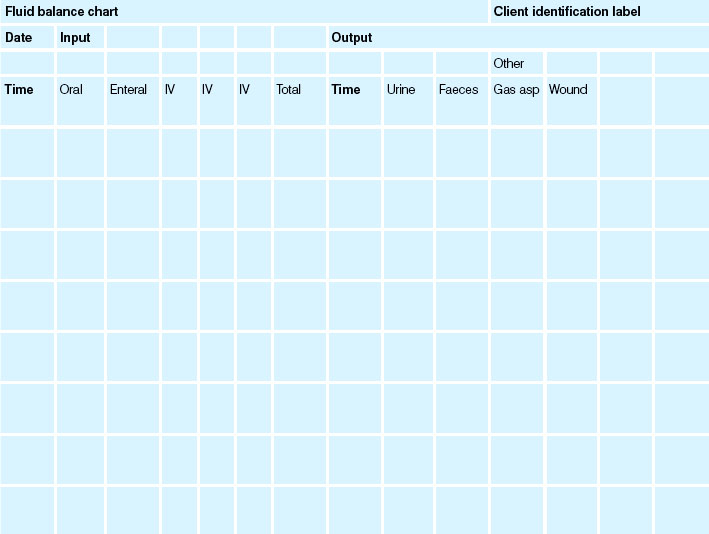
Weighing and fluid balance charts
Fluid balance charts (Table 24.12) are commonly utilised in clinical practice and are considered to be an important aspect of nursing documentation. The charts are used concurrently with daily client weights to assess hydration status. However, an evidence summary presented by the Joanna Briggs Institute (Menzies 2009) summarises some of the concerns with the use of fluid balance charts, which include omission or duplication of information, inappropriate chart design and arithmetical errors. Tang and Lee (2010) also report inconsistency and poor documentation with the use of fluid balance charts. The evidence currently recommends the use of daily weights without the use of fluid balance charts. However, fluid balance charts continue to be used in many healthcare facilities. If the decision is made to use a fluid balance chart, evidence strongly supports the use of client involvement in completing the chart to achieve more accurate charting (Menzies 2009).
The client should be weighed daily at the same time, wearing the same type of clothing and using the same set of scales. Weight can be measured with a standing scale or chair or bed scale and the measurement should be recorded on the appropriate form. The measurement can be affected by bowel and urine elimination pattern and this should be taken into account. Table 24.13 outlines the key aspects of care for clients with fluid imbalance.
Table 24.13 Key aspects of care for clients with fluid imbalance
INTRAVENOUS THERAPY
Management of a client who is receiving intravenous therapy incorporates:
• The prevention of complications occurring during and after completion of the intravenous (IV) infusion
Insertion of an intravenous cannula is performed by a registered medical officer, or a registered nurse (RN) who within their scope of practice is certified to insert IV cannulas. Prior to the insertion of a cannula, the most appropriate site is selected, which depends on several factors, including the condition of the client’s veins, avoiding areas that are oedematous, infected or otherwise impaired; and general comfort considerations (Fig 24.7). Other site determinants include:
• A vein in the non-dominant arm is preferably used
• The initial site should be in the most distal part of the vein, to allow more proximal replacement in the same vein in the event of cannula blockage, infiltration or phlebitis. A commonly selected site is one of the superficial veins on the dorsum of the hand
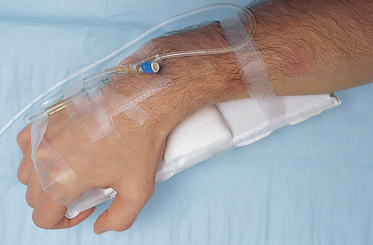
• If the site chosen is near a joint, extension and splinting of the limb may be necessary; for example, by back slab (Fig 24.8).
Healthcare facilities will have individual policies pertaining to the management and insertion of IV cannulas. As an overview they should be inserted by competent accredited healthcare professionals. They should be inserted utilising a sterile technique and the insertion site labelled with the corresponding date/time to allow for timely removal (usually replaced every 72 hours). Documentation in the patient’s chart should be completed. An extremely important aspect of care is monitoring the site for complications such as swelling and oedema. A site that is suspicious for infection should be reported and managed immediately. Infections from IV sites can have potentially lethal complications for patients, and preventing them is integral to hospital quality and risk programs.
The nurse caring for the client receiving IV therapy has the responsibility of ensuring the client care is within their scope of practice. IV fluid is prescribed depending on the desired treatment outcome for the client. We have previously discussed fluid tonicity in this chapter; it is an extremely important concept relevant to the administration of IV fluid. For example:
• Isotonic solution has the same tonicity as the internal environment of the body—it has approximately the same amount of Na+ concentration. Therefore the administration of this type of fluid is used to increase the circulating fluid volume or simply used for fluid/hydration maintenance. Normal saline (0.9%) is an example.
• Hypotonic solution has a lower tonicity or solute concentration than the internal environment. This type of solution will attract water into the cell—causing cells to swell. It is used for patients who are very dehydrated, patients with an elevated Na+ (depending on the cause) or increased serum osmolality. 5% dextrose is an example of a hypotonic solution. The dextrose is metabolised (used by the body for energy) so it leaves behind water.
Hypertonic solution has an increased tonicity compared to the internal environment. Therefore it draws water out of the cells and into the vascular space. 20% albumin is an example. Albumin is an important protein in the maintenance of plasma oncotic pressure and so assists with attracting fluid into the vascular compartment.
Care of the client with an intravenous infusion
As previously stated within this chapter, nurses have a professional accountability and responsibility to be aware of the scope of their role in relation to the management of IV infusions.
Of prime importance is careful observation of the client and the IV equipment used during the infusion, and accurate reporting of client assessment and vital signs. Complications that may occur when a client has an IV infusion, and which must be recognised and reported immediately, are:
• A flow rate that is too slow or too fast, or cessation of flow
• Infection, pain or infiltration at the insertion site
The nurse also has a responsibility to promote the general comfort of the client, to ensure that the infusion is administered as prescribed and to observe for and prevent complications. Key aspects related to the care of a client with an IV infusion are described as follows:
• Ensure that the flow rate is set as prescribed and adjusted when necessary to deliver the correct amount of fluid within the prescribed time. Various methods of determining the number of millilitres per minute are used to distribute the total amount of solution equally over the time period prescribed for the infusion.
• A calibrated burette (e.g. macrodrip or microdrip volume control set) can be incorporated into the infusion system. Such devices enable very small or precise amounts of fluid to be administered and can also be used when medications are to be given IV. A formula is used to determine the number of drops per minute at which the solution should flow. To calculate the flow rate, the following formula is used:
• When calculating the rate it is essential to know the calibration of the drip rate for each manufacturer’s product, as these vary from 10 drops/mL to 60 drops/mL
• The flow rate should be checked every 15 minutes to ensure that the drip rate is stable, then every hour or at the time intervals specified in the institution’s policy on IV infusions
• Check the level of solution to determine the volume of fluid remaining in the flask. It is important to ensure that a flask does not empty completely, as this can interfere with the flow rate and permit air to enter the tubing, which could lead to an air embolism. Air emboli can result in sudden decrease in blood pressure, rapid weak pulse, cyanosis and loss of consciousness
• Check the insertion site every 24 hours for signs of infection, inflammation or infiltration. Infection at the insertion site is indicated by redness, warmth, swelling and tenderness. The vein may become injured or irritated and phlebitis may occur. Signs and symptoms that indicate phlebitis are pain, swelling and inflammation along the course of the vein; the vein may also feel hard and warm to touch. Infiltration occurs if the needle becomes displaced, causing IV solution to flow into the surrounding tissues. Signs and symptoms of infiltration are cool skin in the site area, swelling and discomfort at the site, oedema of the limb, sluggish flow rate and absence of blood in the tubing when it is lowered
• Observe the client for signs of over-hydration or circulatory overload, caused by excessive or too rapid fluid administration. Signs and symptoms of circulatory overload include elevated blood pressure, venous dilation, especially of the neck veins, coughing, tachypnoea and shortness of breath
• Check the information on the label of the flask when each new flask is started and at the start of each shift. It is essential that the client receives the prescribed solution; the nurse should therefore follow the nursing protocol regarding checking of IV solutions
• The type, volume and expiry date of the solution is checked. Glass containers are inspected for chips and cracks and the seal over the opening is observed to ascertain if it is intact. Plastic containers should be squeezed gently to detect leaks. The solution is examined for particles, abnormal discolouration and cloudiness. The container is not to be used if any doubt exists, and the intact container is retained for further investigation
• Throughout the course of the infusion, document details of the flow rate, the type and amount of fluid and additives being administered and the client’s fluid input from other sources and fluid output. The vital signs (temperature, pulse, respirations and blood pressure) are also measured regularly and documented.
Electrolyte replacement
Nurses should be especially cautious when administering IV solutions containing electrolytes or fluids that have had electrolytes added to them. Potassium (K+) may require the client to be cardiac monitored. It will be important for the nurse to be aware of and monitor serum K+ levels. A common complaint from clients is that K+ administration causes significant pain at the IV site and sometimes to the whole limb. This is usually associated with larger doses and shorter administration times. Cold packs can be applied, but it might be necessary to liaise with the medical team for further problem resolution.
Intravenous fluid administration in paediatrics
Wherever it is appropriate, the enteral route (usually via a nasogastric tube) should be used. If using the enteral route is not achievable or appropriate in the clinical scenario, then intravenous fluid administration can be utilised. Nurses need to exercise caution prior to and during the administration of IV fluids in children and should ensure that it is within their scope of practice. If a nurse is uncertain of the fluid order for any reason, liaising with the prescribing doctor is critical and safe practice.
In a child with normal fluid volume status not consuming oral fluids, maintenance fluid will usually be administered. It is termed maintenance because it replaces insensible losses usually expected. A child’s maintenance fluid requirement decreases proportionately with increasing age (and weight). The calculations in Table 24.14 approximate the maintenance fluid requirements of well children according to weight in kilograms. Clinical Interest Box 24.6 outlines the treatment of gastroenteritis in children.
Table 24.14 Fluid requirements for paediatric clients
| Client’s weight | mL/day | mL/hour |
|---|---|---|
| 3–10 kg | 100 × wt | 4 × wt |
| 10–20 kg | 1000 plus 50 × (wt − 10) | 40 plus 2 × (wt − 10) |
| >20 kg | 1500 plus 20 × (wt − 20) | 60 plus 1 × (wt − 20) |
wt = weight in kg
Adapted from The Royal Children’s Hospital Melbourne (nd) Clinical Practice Guideline—Intravenous Fluids
CLINICAL INTEREST BOX 24.6 Paediatric gastroenteritis
Treatment of gastroenteritis in the young requires special attention to fluid and electrolyte balance. Fluids such as flat lemonade and mineral water are often used by clients’ significant others for rehydration. This treatment has potential life-threatening consequences. Lemonade has a high sugar content, and sugar acts as an osmotic agent, drawing water into the gastrointestinal tract (GIT) from the body’s fluid compartments. This fluid loss results in increased diarrhoea and dehydration. Sugar is also utilised by bacteria in the gastrointestinal tract as a form of nutrition, resulting in increased gas production by the bacteria, causing gaseous distension and abdominal pain.
Hydration in the young needs to be assessed by a medical officer. Clients often require rehydration with products such as Gastrolyte, by oral or nasogastric administration and, in more severe cases, intravenous therapy may be required.
Choice of IV fluid will depend on the clinical scenario. 0.45% NaCl with 5% glucose is commonly used as maintenance fluid, although it may not be appropriate in all situations.
If a child presents with dehydration then initial replacement of the loss is essential. The formula for this is 10–20 mL/kg administered as boluses until the desired clinical outcome is achieved. The fluid of choice in this situation is usually 0.9% saline.
The Royal Children’s Hospital Melbourne (nd) outlines the following recommendations in its guidelines:
UNDERSTANDING ACID–BASE BALANCE
Acids and bases
An acid is any substance that releases hydrogen ions (H+) when dissolved in water. Acids have chemical properties essentially opposite to those of bases. Examples of acids found in the body include hydrochloric, lactic, pyruvic, carbonic, citric, folic and fatty acids. A base is any substance that accepts hydrogen ions in chemical reactions. Alkalis are bases that are soluble in water. Examples of bases found in the body include hydrogen bicarbonate and sodium hydroxide (McCance et al 2011).
The meaning of pH
The pH scale is used to express the concentration of hydrogen ions (H+) in acids or bases. The ‘p’ stands for potential or power, and the ‘H’ stands for hydrogen. Therefore, pH represents the potential, or power, of hydrogen ions present. The pH of a solution is determined by measuring the amount of H+ present. The scale is numbered from 0–14; the lower the number on the scale, the more H+ is present, therefore the more acidic the solution is. A pH of 7 indicates a neutral solution, while a pH above 7 is termed basic, or alkaline, and a solution below 7 is acidic. Each integral step in the pH scale (i.e. 14, 13, 12 … to zero pH) represents a tenfold increase in H+ ion concentration; thus, the pH scale is an inverse logarithmic scale of the amount of hydrogen ions present.
Acid–base balance
In order to maintain optimal cellular function the hydrogen ion (H+) concentration of body fluid is maintained within a close range (LeMone et al 2011). The pH of body fluid is maintained between 7.35 and 7.45; this means it is slightly basic (alkaline) as neutral is a pH of 7. A number of buffering systems are required to maintain a normal pH; this is because the normal cellular metabolism of nutrients in the human body continuously produces acids (LeMone et al 2011).
The body’s capacity to form, excrete and ‘buffer’ acids and bases is known as the acid–base balance. The acid–base balance is critical to the survival of the human body. Variations outside the normal arterial range of a pH of 7.35–7.45 indicate serious dysfunction in the body. The normal metabolism of nutrients by cells produces large amounts of hydrogen ions (H+) that form acids. The concentration of hydrogen ions in the cells and tissues must, however, be kept low to prevent cellular damage. To maintain pH within normal limits, the body excretes acids at the same rate that they are produced and buffers any excess hydrogen ion (McCance et al 2011).
Buffer systems
A buffer is a chemical substance that resists changes in the pH of solutions by either removing or releasing hydrogen ions (LeMone et al 2011). When bloody fluid is too acidic buffers bind with hydrogen ions to keep the pH within the homeostatic range. When body fluid is alkaline buffers release hydrogen ions, again returning to pH to normal (LeMone et al 2011).
For example, CO2 is an end product of cellular metabolism and when combined with water, forms carbonic acid (CO2 + H2O = H2CO3), which drains back into the circulation. Excess carbonic acid in the blood is rapidly decomposed into carbon dioxide and water (H2CO3 = CO2 + H2O) and the CO2 is excreted by the lungs to maintain the pH at normal physiological levels. Conversely, if water is lost from the blood, it is replaced by the ionisation of carbonic acid, and a rise in pH is prevented. Thus, carbonic acid acts as a buffer in the blood. An example of the buffering action of bicarbonate ion is its reaction with lactic acid. Lactic acid is produced from glucose during muscle contraction, and the dissociation of lactic acid tends to lower the pH. As lactic acid is a stronger acid than carbonic acid, its conjugate base (lactic ion) is weaker. The stronger base (bicarbonate ion) combines with the hydrogen from lactic acid forming dissociated carbonic acid (McCance et al 2011).
Acidosis and alkalosis
Acidosis is a condition in which the blood becomes acidic (pH <7.35) due to an increase in hydrogen ion concentration as a result of an accumulation of an acid, or the loss of a base. Alkalosis is a condition characterised by an increased pH, above 7.45, due to a decrease in hydrogen ion concentration as a result of a loss of acids or the accumulation of a base.
An acid–base imbalance occurs when there is a deficit or excess of carbonic acid or base bicarbonate. In some situations in which a client cannot efficiently excrete enough CO2, for example, in a client with pneumonia, the buffer system may be unable to keep the pH within the normal range. In this situation the excess CO2 will make the blood more acidic, reducing the pH. If the pH falls below 7.35 the condition is termed acidosis; if the pH rises above 7.45 the condition is termed alkalosis.
Normally the body can soak up or neutralise acids as they are formed (buffering). This is done by:
• Blood: initial buffering of acids occurs in tissue, blood cells and the plasma
• Lungs: rapidly alter blood pH by increasing or decreasing the respiratory rate, increasing the removal of carbon dioxide
• Kidneys: excrete additional acids that cannot be excreted by any other method; they also reabsorb bicarbonate back into the bloodstream after acids have been excreted.
A pH above or below the normal pH of arterial blood (7.35–7.45) may result in severe dysfunction and potential death.
Causes of pH disturbances
Respiratory causes
Respiratory acidosis occurs with hypoventilation, when the body is unable to ventilate enough CO2 out of the lungs, as may occur in airway obstruction, emphysema, asthma or opiate overdose. (The kidneys compensate, e.g. by retaining bicarbonate.) Respiratory alkalosis occurs with hyperventilation, when rapid respirations blow off too much carbon dioxide, resulting in alkalosis of a respiratory nature, as may occur in panic attacks. (The kidneys compensate, e.g. by excreting more bicarbonate.)
Metabolic causes
Metabolic acidosis occurs when the body is unable to excrete enough acids because of a problem with malabsorption, such as diarrhoea; metabolism, for example, diabetic ketoacidosis (see Clinical Interest Box 24.7); or organ failure, such as kidney failure. (The lungs compensate, e.g. by increasing ventilation and increasing excretion of acidifying CO2.)
CLINICAL INTEREST BOX 24.7 Diabetic ketoacidosis
Clients with diabetes who have not been administered enough insulin or are unwell, or those with undiagnosed diabetes, are at risk of developing diabetic ketoacidosis. Without adequate insulin the body uses its muscle and fat for metabolism, producing ketone acids. To rid the body of acids, emesis and later hyperemesis occurs, resulting in a loss not only of acid but also of fluid and other electrolytes. The ketone acids can severely affect the pH of the blood, causing a metabolic acidosis. The body tries to compensate by excreting acid by the lungs (compensatory respiratory alkalosis), kidneys and skin.
Hyperglycaemia causes an osmotic-induced polyuria and a subsequent fluid and electrolyte imbalance and may result in severe dehydration. If insulin is not administered and the correct rehydration therapy instigated, in severe cases the condition can result in cerebral oedema, coma and death.
Metabolic alkalosis occurs when too much acid is lost, for example by vomiting, nasogastric drainage or use of some diuretics. (The lungs compensate, e.g. by decreasing ventilation and decreasing excretion of acidifying CO2.)
Compensation
A change in pH by one system of the body will tend to cause an opposite compensatory change in pH, known as respiratory or metabolic compensation. If respiratory acidosis exists, the body will compensate by producing a metabolic alkalosis. If metabolic alkalosis exists, the body will compensate by producing a respiratory acidosis.
Summary
This chapter provides an overview of the key physiological concepts implicated in fluid, electrolyte and acid–base homeostasis. Developing an understanding of these theoretical underpinnings is critical for nurses as this knowledge will inform the basis of evidence-based person-centred care. Knowledge acquisition will particularly assist nurses to utilise their critical thinking skills and strengthen their position as patient advocates. To consolidate learning, further reading and completion of the review and critical thinking exercises is highly recommended.
Several essential homeostatic processes are integral in the maintenance of fluid, electrolyte and acid–base balance within the body. Water is distributed within both intracellular and extracellular compartments. This fluid provides a transport medium for electrolytes and other substances. Movement of fluids and electrolytes is dynamic and constant and is essential for a healthily functioning body. Osmosis is the mechanism of movement for water; diffusion and active transport are mechanisms facilitating the movement of other substances. The semi-permeable membrane is a critical structure in the regulation of intracellular and extracellular fluid composition and in the maintenance of electrolytes within optimal levels in their respective compartments. Damage to the semi-permeable membrane through disease processes, such as burns and infections/sepsis, can result in fluid and electrolyte shifts detrimental to healthy function.
It is natural for the body to lose water through both sensible and insensible mechanisms. Sensible losses including those that are able to be measured, that is, urine and faeces. Insensible losses are not easily measurable, for example, perspiration. Under certain conditions fluid losses may increase; but in the healthily functioning body homeostatic mechanisms will act to restore body water volume and composition. Key systems for these mechanisms of action are the neural (hypothalamus and pituitary) system, the renal system (RAS—aldosterone) and the GIT. Disease processes resulting in fluid volume excess have also been discussed in this chapter. Bodily mechanisms that compensate for this also include the neural, renal and cardiovascular mechanisms.
Major cations and anions have been discussed with reference to their significance in maintaining nerve and muscle function. Electrolyte imbalance rarely occurs in isolation; there is usually more than one imbalance involved. Sodium is associated with water imbalance and it is important for the clinician to establish whether there has been a loss of sodium, for example, or an increase in water volume. Potassium is an extremely important electrolyte in the maintenance of an optimally functioning cardiac conduction system. It should be monitored closely and administered with caution as it is associated with potentially lethal complications.
The maintenance of the pH of blood within a closely regulated range is critical to survival. Maintenance is achieved through both renal and respiratory buffering capabilities. These buffering mechanisms can be overwhelmed by disease processes.
Person-centred care is aimed at treating the underlying clinical condition and providing supportive therapies during this process. An integral element of the nurse’s role is the completion of a comprehensive person-centred assessment, which includes considering additional risk factors associated with older adults and the paediatric population. The nurse is required to execute astute physical assessment skills; these accompanied by theoretical underpinnings will form the basis of planning, implementing and evaluating care and of timely reassessment of the client.
Maintaining client safety is paramount. The nurse is required to consider their individual scope of practice prior to the administration of intravenous fluids. Clients undergoing treatment with IV fluids must be monitored appropriately and continuously reassessed. It is vital for the nurse to understand the clinical reason for the particular IV fluid administration so the effects of the treatment can be evaluated accordingly.
Physiological concepts in fluid, electrolyte and acid–base homeostasis are likely to be frequently encountered in a multitude of clinical settings. The application of this knowledge in clinical practice not only promotes high quality, safe person-centred care but also renders satisfying and rewarding care delivery for the nurse.
1. Draw a table with three columns. Title the first column ‘Fluids’ and list the following fluids in that column:
Title the second column ‘Indications for use’ and the third ‘Tonicity of the fluid’. Complete the table.
2. An 18-month-old infant is admitted for gastroenteritis and is 7% dehydrated. The infant has been refusing all oral rehydration fluids. What clinical signs would you expect to find in your assessment of the client and what are the reasons for your findings?
3. John presents with a 5-day history of severe vomiting and diarrhoea on returning from an overseas trip.
4. While observing Table 24.1 think about clients you may have come into contact with. Reflect on what you would need to know to provide safe person-centred care. Make a note of your answers.
5. Fluid balance will be achieved if there is:
d. Normally functioning kidneys
Consider this list and client populations that may be affected by one or more of these factors. Make a note of your response.
6. What symptoms do you think might be present in a client with dehydration?
1. How is the pH of body fluid maintained within the range (7.35–7.45) for optimal cellular functioning?
2. Give one example of each of the following acid–base disturbances: respiratory acidosis, metabolic acidosis, respiratory alkalosis, and metabolic alkalosis.
3. Describe the different fluid compartments and what each compartment represents.
4. Define the terms: osmolality, osmotic pressure, hydrostatic pressure, filtration.
5. Describe the mechanisms of fluid movement.
6. Describe the mechanisms of electrolyte movement.
7. Discuss the physical assessment findings associated with fluid volume deficit and fluid volume excess.
8. Describe the appearance of phlebitis due to the presence of an IV cannula.
9. Describe the nursing measures that would be taken to minimise harm to a client undergoing IV therapy.
References and Recommended Reading
Australian Nursing & Midwifery Council. Nursing Practice Decision Flow Chart. Online. Available: www.nursingmidwiferyboard.gov.au/codes-guidelines-statements/codes-guidelines.aspx#decisionmakingframework, 2007.
Berman A, Snyder S, Kozier B, et al. Kozier and Erb’s Fundamentals of Nursing, 2nd edn. Pearson, Frenchs Forest, NSW, 2012.
Brown D, Edwards H. Medical–surgical Nursing. Assessment and management of clinical problems, 2nd edn. Sydney: Elsevier, 2012.
Caon M. Osmoles, osmolality and osmotic pressure: clarifying the puzzle of solution concentration. Contemporary Nurse. 2008;29(1):92–99.
Craft J, Gordon C, Tiziani A. Understanding Pathophysiology. Sydney: Elsevier, 2011.
Crisp J, Taylor C. Potter & Perry’s Fundamentals of Nursing, 3rd edn. Sydney: Elsevier, 2009.
Friedman A. Fluid and electrolyte therapy: a primer. Pediatric Nephrology. 2010;25:843–846.
LeMone P, Burke K, Dwyer T, et al. Medical–surgical Nursing: Critical thinking in client care. Frenchs Forest, NSW: Pearson, 2011.
McCance KL, Huether SE, Brashers VL, et al. Understanding Pathophysiology. Sydney: Elsevier, 2011.
Potter PA, Perry AG. Fundamentals of Nursing, 7th edn. St Louis: Mosby, 2008.
Rahman MA (2011) Evidence Summary: fluid balance charts: clinician information. The Joanna Briggs Institute Acute Care Practice Manual. Available from JBI connect
Tang VCY, Lee EWY. Fluid balance chart: do we understand it? Clinical Risk. 2010;16:10–13.
The Royal Children’s Hospital Melbourne. (nd) Clinical Practice Guideline—Intravenous Fluids. Online. Available: www.rch.org.au/clinicalguide/cpg.cfm?doc_id=5203.
Welch K. Fluid balance. Learning Disability Practice. 2010;13(6):33–38.
Acid–base terminology, www.acid–base.com/terminology.php.
Australian Health Practitioner Regulation Agency, www.ahpra.gov.au.
Nursing and Midwifery Board of Australia, www.nursingmidwiferyboard.gov.au.
Nursing Council of New Zealand, www.nursingcouncil.org.nz.
The Royal Children’s Hospital Melbourne Clinical Practice Guidelines, www.rch.org.au/clinicalguide/.