Chapter 9 The medical literature
This chapter is presented in three parts:
This chapter may be utilised in several ways. Those trainees commencing their approach to the fellowship exam, who have the time and motivation to use EBM techniques as a cornerstone of their preparation, may find this a useful summary of what is most relevant to have in their ‘tool kit’. Alternatively, those closer to the event may choose to focus their efforts on using the material as a review of information that could be asked in the exam — EBM would lend itself well to being an SAQ or SCE topic. The important papers section has relevance to all parts of the exam for trainees at all stages of their preparation.
Part A: the emergency physician’s guide to basic statistics
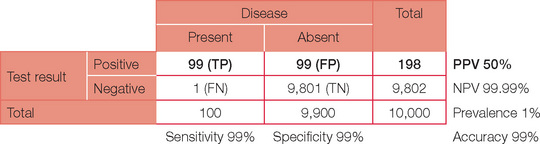
A test has a specificity of 99% and sensitivity of 99% in a population where the prevalence of disease is 1%. What is the positive predictive value (PPV) of this test?
If, like most clinicians, reading this question makes your eyes glaze over and your head ache, this section is for you. We will work our way through the answer step-bystep and by the end of this section, your headache will be gone!
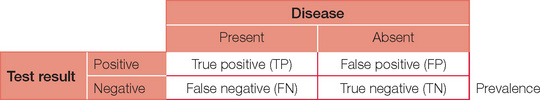
First, consider the possible results of a test (positive or negative) when a disease (or disorder) is present or absent. The possible combinations are shown below.
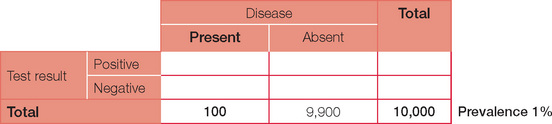
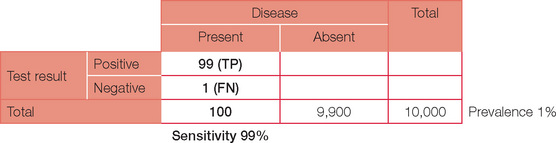
For ease of calculations, we will assume a population size of 10,000. Prevalence is the number of people in the population with the condition at a given time. So a prevalence of 1% in our population of 10,000 will therefore equal 100 people with the disease. Prevalence should not be confused with incidence, which is the number of presentations per unit of time. For example, the annual incidence of diabetes is the number being diagnosed each year, whereas the prevalence (the number of people who have diabetes) is much higher.
Sensitivity is the capacity to detect something when it is present — just like a sensitive person does. In statistical terms it is best thought of as ‘positivity in the presence of disease’ = TP/(TP + FN). Tests with high sensitivity are preferable if the desire is to ensure that a condition is detected or ‘ruled in’. For our case, a sensitivity of 99% will result in a total of 99 out of the 100 patients with the disease returning a (true) positive test and one returning a (false) negative test.
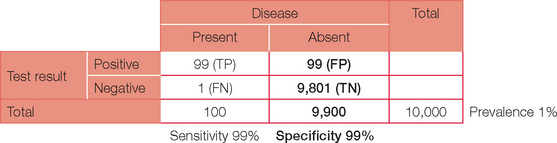
Specificity is the ability of a test to pick only the disease — just as being specific means not getting off the point. In statistical terms, specificity can be thought of as ‘negativity in the absence of disease’ = TN/(TN + FP). Tests with high specificity are preferable when it is important to ensure that a condition is not present, i.e. ‘ruled out’. For our example, a specificity of 99% will result in 99 (1%) of the 9,900 without the disease still returning a (false) positive test.
The table can now be completed with simple arithmetic.
So far, we have been working backwards from a given population with a known disease prevalence to calculate true and false negatives and positives. However, this is not the world of a clinician with a test result. The result returned will be either positive or negative (at least for the sake of this discussion). The question that the clinician must ask is: ‘What does a positive (or negative) test mean?’
Positive predictive value (PPV) and negative pre dictive value (NPV) are the likelihood that a positive (or negative) test is a true result, i.e. what pro portion of positive results are true positives and what proportion of negative results are true negatives.
This is of direct importance to you as the clinician, as it tells you whether or not you can rely on the result you have. Depending on whether the test was intended to ‘rule in’ or ‘rule out’ a particular condi tion, the focus will be more on the PPV or the NPV, respectively.
The final basic statistical value we can also now cal culate is accuracy. Accuracy is the proportion of time the test is correct (TP or TN) for the given population.
Accuracy = (TP + TN)/(TP + TN + FP + FN)
This now enables us to answer the original question. A test with a 99% sensitivity and specificity when applied to a population with a disease prevalence of 1% will have a positive predictive value of only 50%.
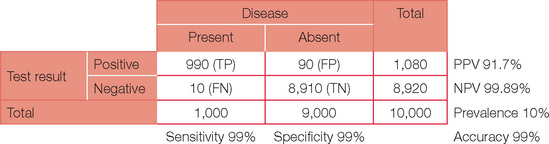
From this you should now be able to appreciate that prevalence of disease has a significant impact on the clinical interpretation of a test in addition to simply evaluating the sensitivity and specificity. A practical example of this is assigning pre-test prob abilities to ventilation/perfusion scanning for suspected pulmonary embolism. Assigning a pre-test probability defines the prevalence of disease and hence alters the interpretation of the test result, even though the same test has been performed!
Below is the original question with a prevalence of 10%.
Note, the PPV has now increased dramatically. If your confidence in basic statistics has now grown (and your headache has gone), try different combinations and permutations of parameters to confirm the effect on PPV and NPV. To start with, review a paper or even work through the detail for an investigation you perform on a regular basis. You may never view basic statistics with fear again!
Part B: an overview of EBM
The context
Doctors need to know about the studies that show whether new ideas work, but their volume has grown enormously. What’s more, many are published in inaccessible places, are not published at all, or are seriously flawed. Most busy doctors lack the time or skill to track down and evaluate this evidence. Although the skills of searching for evidence and critically appraising it are being mastered by growing numbers of doctors, many cannot keep up. Consequently there is a widening chasm between what we ought to do and what we actually do.
This excerpt from an editorial by Davidoff et al. in the BMJ in 1995 still holds true today. EBM comprises the latest information on the most effective or least harmful management for patients (Davidoff et al., 1995). The key processes in EBM are:
Critical appraisal is ‘the process of systematically examining research evidence to assess its validity, results and relevance before using it to inform a decision’ (Mark, 2008). It allows the reader to assess in a systematic way how strong or weak a paper is in answering a clinically relevant question or whether the paper can be relied on as the basis for making clinical decisions for patients.
Deficits in knowledge and understanding of the critical appraisal process restrict the implementation of the best available evidence into clinical practice. Recog nising the need to understand evidence regard ing treatment or diagnostic options for conten tious issues is the first step in the journey. This requires an acknowledgment of equipoise (one is not sure which is the better treatment or test). The clinician may then wish to explore the quality of evidence underpinning a proven treatment or test, even if they are aware of these.
Carefully formulating a precise, answerable question that is clinically relevant (i.e. that will provide improved care or a better diagnostic test) is the starting point (why). Without knowing the why, there is no point in starting the appraisal journey. The next step is to decide where to look for the evidence. Literature searches need to be efficient, comprehensive, unrestricted and unbiased, encompassing explicit search strategies of published, citable literature databases and sources of unpublished research. How to identify good-quality studies, critically appraise those selected and apply their findings to individual patient care completes the appraisal journey.
Most readers will not limit their literature search in terms of date of publication (when) unless they are confident that a treatment or test was developed only recently, or an unlimited search yields numerous citations, which become unmanageable. As there is often a time lag between study citations being added to searchable databases, it is worthwhile considering conducting a more recent targeted search in relevant journals if the topic area is rapidly evolving. Wang and Bakhai (2006) and Pocock (1983) provide excellent further reading in the area of clinical trials, as do Greenhalgh’s ‘Education and debate’ series from the BMJ (Greenhalgh, 1997a) and Gordon Guyatt’s 2000 focus series from the JAMA (Guyatt, 2000).
Before looking for individual studies, we recom mend a concerted search for metaanalyses, which offer a useful background perspective and, if one is lucky, may even answer the research question, using the summated ‘quality overall evidence’ available so far (Mark, 2008). Meta-analyses are formally designed and properly conducted critical appraisals of intervention trials that attempt to ‘aggregate’ outcome findings from individual studies if they show a consistent effect. The presence of compelling outcome effects, consistent in direction and size across individual non-clinically heterogeneous studies of acceptable methodological quality, will likely be enough to tell you whether a proposed treatment or diagnostic test will be suited to your patient.
Critics claim that such aggregated findings cannot be applied to an individual patient; on average, the treatment effect will be qualitatively similar (if it benefits meta-analysis patients, treatment would probably be effective in your patient) but quantitatively more variable (the magnitude of benefit will vary between patients). The conclusion reached by a meta-analysis will be applicable to your patient and specific clinical setting if these characteristics are comparable to those of the study patients or study settings included in the meta-analysis.
Critically appraising a meta-analysis will still save you time and effort if many intervention trials or studies of diagnostic performance of a certain test relevant to your objective have been carried out. You need to ascertain whether the methodological rigour and quality of the meta-analysis is sufficient for conclusions and recommendations contained in the meta-analysis to reliably fulfil your objectives. If a well-conducted and reported meta-analysis is available, re-examining individual studies in detail is less worthwhile, other than for personal interest. However, a meta-analysis will not include influential studies that become available after the date of publication of the metaanalysis. Carrying out a date-of-publication limited search for newly emerging studies is thus recommended, to see whether the conclusions reached in the meta-analysis remain consistent with the newer studies. For in-depth information on systematic reviews in health care, see the standard text by Matthias Egger et al. (2001).
There may be no relevant meta-analysis to inform your objectives. If there are too few studies, or the studies are relatively small, excessively clinically heterogeneous or dissimilar, or they show inconsistent and widely varying outcomes, an aggregated outcome in a meta-analysis may not be possible. In these situations, decisions regarding patient management or investigation are based on a critical appraisal of individual studies you believe to be relevant to your practice setting, with the clinical risk–benefit analysis tailored to suit the individual needs of your patient, as well as taking into account treatment feasibility, practicability and availability.
Critical appraisal and clinical practice
Standards of clinical care now demand identification and timely delivery of the most effective treatment available in order to achieve optimal outcomes. There are high expectations among colleagues, patients and health administrators that treatments are clinically effective, cost-effective and timely with minimal adverse effects. In emergency medicine this may also involve the further consideration of time-critical situations. Treatment selection by anecdote, eminence or prior personal experience is no longer acceptable. Emergency physicians must make active, informed decisions regarding treatment selection and not simply default to in-patient teams.
In emergency medicine, critical appraisal of the evidence is most pertinent to time-critical conditions that require non-established or contentious urgent treatments that may be highly beneficial but also lead to significant harm. For example, this situation arises in thrombolytic treatment for acute ischaemic stroke, where treatment administered within three hours of symptom onset gives better neurofunctional outcome, but remains little used for fear of causing intracranial bleeding. ECASS III, a recently published RCT comparing IV alteplase with a placebo in ischaemic stroke, found alteplase to remain beneficial at three to four and a half hours after symptom onset (Hacke et al., 2008). The most recent Cochrane meta-analysis of thrombolysis trials in stroke, published in 2003, did not include ECASS III (Wardlaw et al., 2003). Evidence is in a constant state of evolution, so critical appraisal is a continuing process that aligns itself with continuing medical education and professional development. Nowadays, studies informing on therapeutic (in) effectiveness are easily and rapidly accessible through user-friendly information technology media such as the 24-hour medical cybrary. With the exception of acute resuscitation, there is never an excuse not to evaluate effectiveness prior to patient treatment.
High-standard clinical care requires the clinician to correctly select and safely deliver the best available treatment or diagnostic test for each patient in a timely fashion. A poor outcome for a patient receiving the most effective available treatment or a consequential diagnosis missed despite use of the most reliable test is ethically and medico-legally more defensible than the same adverse events in a patient after suboptimal treatment or not receiving an appropriate diagnostic test. The clinician who knows that a poor patient outcome has not resulted in some way from a knowledge gap will sleep the better for it.
Within the realm of clinical research, an unbiased comprehensive literature search is able to identify whether a research question has been answered in previous studies, and therefore whether another study is necessary or even ethical in the presence of compelling evidence. The potential impact on improving patient care and clinical relevance of a proposed study is also assessable by critical examination of the available literature. Furthermore, peer review of medical research manuscripts requires critical appraisal of a study’s internal validity as it relates to methodological rigour and its capability to be generalised to various clinical settings.
Barriers and challenges remain in keeping up to date with the latest evidence. The time pressures of increasing demand for hands-on patient care discourage evidence appraisal. This is exacerbated by perceptions that clinical care is distant and divorced from medical research, engendering the negative connotation that critical appraisal by the ‘thinker’ clinician is a diversionary activity of little relevance to direct patient care rendered by the ‘doers’. Negative perceptions exist that medical research has become an industry with little relevance to clinical practice.
Exponential growth in the medical literature and the increasing ease of access to biomedical journals has produced a ‘noise to signal ratio’ that can easily overwhelm the time-pressured clinician. Successfully identifying, or conversely not missing, crucial studies can be a challenge. The following sections provide a framework to help with this important task.
Levels of evidence
Intervention and non-intervention studies can be stratified into several ‘levels of evidence’, according to their internal validity and dependability in informing treatment effects. A well-designed and conducted meta-analysis or randomised controlled blinded treatment trial is widely recognised as being able to offer the most reliable and least biased estimate of treatment benefit or harm (Wang et al., 2006), followed in descending order of quality of evidence by observational non-intervention studies such as case control studies and finally case series and case reports. This is variously graded (e.g. levels I–IV or grade A–C recommendations) depending on the body utilising this. Several issues should be apparent at this stage:
ACEM recognises the importance of EBM and research as being crucial for the future of the specialty. It is expected that fellowship exam candidates will be aware of major practice informing papers. The purpose of regulation 4.10 is to ensure that all trainees have exposure to research during their training. This is important so that individuals with a predilection for research can self-select and be supported in their future development.
For the working trainee who is approaching the exam, acquiring and applying EBM skills to each topic on the fellowship curriculum can be daunting. Some would say that this is unrealistic, when so many other priorities exist and time is short. Textbooks and exam-focused resources that have incorporated relatively recent clinical evidence provide an attractively efficient way to capture, in digestible portions, the latest controversy or ‘hot topic’ in emergency medicine. A great advantage to this approach is that someone else has already critically appraised the key papers for you, saving you from having to do this yourself. However, books are revised only periodically (usually every few years), so what was topical or controversial when a book was written may now be passé or resolved and no longer an attractive topic in the fellow ship exam.
In practice, the greatest proportion of a consultant emergency physician’s work time is spent ‘on the floor’, caring for patients directly or providing clinical supervision for registrars and residents. With multiple non-clinical tasks required for a functional Emergency Department, time for critical appraisal of EBM topics may be difficult to access. In terms of emergency medicine advanced training, the bread and butter of clinical emergency medicine remains the focus of the fellowship examination. The regulation 4.10 requirement and an occasional question during the fellowship examination on critical appraisal of the evidence are used to assess the trainee’s capacity for skilled self-directed learning and evidence analysis. In the process of achieving the latter aim, some trainees will aspire to becoming research leaders in the future, boosting the research credibility of our specialty.
A tool kit of EBM techniques
Critical appraisal of a single intervention study
It is assumed that you have already conducted an unbiased, reliable and comprehensive literature review. Having identified the article from major biomedical databases such as MEDLINE and EMBASE and others, you are now ready to appraise it. You wish to determine whether it has internal validity and is applicable to the patients you are looking after in the clinical setting in which you practise.
Critical appraisal requires the following questions to be satisfactorily answered.
What is the research question?
If the research question is not precisely stated and clearly defined, useful conclusions are unlikely. Within a critical appraisal process, using a PICOT structure is useful. The PICOT characteristics of a study allow you to determine whether its findings are generalisable:
P study participant characteristics at baseline, including disease severity; study setting
I experimental intervention or diagnostic test being investigated
C comparison or control group, usually the standard treatment/test, a placebo or usual care
O outcomes of interest; clinically meaningful for both the clinician and patient
T time period of the study observation or period of follow-up.
Are the study results likely to be valid?
A valid intervention trial addresses a clearly focused question with sufficient methodological rigour to enable the results to be trusted. We clearly need to avoid any bias, which occurs when the outcome is materially affected by factors other than the tested intervention. Key issues to assess pertain to trial design and conduct.
Was the trial design valid?
Was the conduct of the trial valid?
Was the analysis of the trial findings valid?
Intention to treat analysis
All patients should be analysed in the group to which they were randomised. Loss to follow-up greater than 20%, especially if differentially distributed between groups, will lead to post-randomisation bias if intention to treat (ITT) analysis is not used. ITT analysis means that patients are analysed according to the treatment group to which they were random ised, irrespective of whether they underwent the intended intervention or whether they adhered to protocol stipulations. ITT analysis results in an unbiased estimate of effect and more closely reflects what happens in reallife clinical practice, where patients have a range of compliance with treatment recommendations. In contrast, per protocol analysis is biased, since it includes only comparisons between patients who adhere to the treatment allocated to them. If the tested treatment works, the measured effect of the same treatment in the same study will be greater in magnitude for per protocol analysis (where only compliant patients are included in the analy sis) compared with ITT analysis (where all patient outcomes are included in the analysis whether patients comply with the treatment or not).
Statistical method
The statistical method used should be pre-specified and appropriate to the study objectives. The approp riate method depends on the outcome type (e.g. continuous variables such as BP results versus binary variables such as alive/dead) and the anticipated distribution of results (parametric tests for normally distributed data and non-parametric tests for non-normally distributed data; a transformation of raw data to approximate normality may be required). Pre-specified statistical methodology assures the reader that in the face of unimpressive or unexpected results, alternative analyses have not been used to achieve more impressive or desirable findings.
The use of post-hoc subgroup analyses, unjustified multiple outcome or interim comparisons will likely lead to a false positive finding in a small study subset (the more analyses are done, the more the risk of a false positive finding). However, it is reasonable to conduct post-hoc analysis adjustments if results indicate the method initially chosen is no longer valid. For example, a study may have been designed expecting normally distributed data, but non-normal distribution of data is unexpectedly encountered. In this situation, non-parametric methods will be required. Interested readers are referred to standard texts (Kirkwood et al., 2003) and user-friendly articles (Greenhalgh 1997b; 1997c).
What are the results?
Measures of treatment effect
Treatment effects of binary outcomes can be pre sented as an absolute difference (such as a risk difference), a relative difference (odds ratio or a risk ratio) or a relative risk (risk experimental group/risk standard group). Treatment effects of con tin uous measurements are usually analysed as absolute differences: for example, (mean blood pres sure experimental group) – (mean blood pressure standard group).
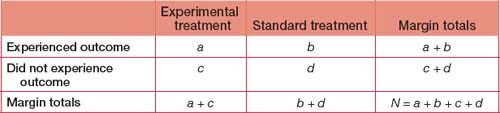
In a parallel group treatment trial an experimental treatment is being compared with standard treatment and patients are followed up for an outcome of interest (such as a specific benefit or harmful event such as death). Other metrics are used that are assisted by an outcomes matrix:
Absolute odds difference is not meaningful in a statistical sense. Odds ratios have similar character istics and application to the risk ratio, and although frequently used in intervention studies, are more valid to be applied to case control studies.
It is important that the results section presents both absolute and relative treatment effect measures. The latter usually gives an exaggerated impression of treatment effect if presented in isolation. In a hypothetical example comparing treatments X and Y, if beneficial outcome occurs in 4% on treatment X and 2% on treatment Y, the absolute risk difference is (4 – 2%) = 2% which is not particularly impressive. The relative risk of benefit of treatment X compared with Y is calculated as:
Risk treatment X/risk treatment Y = (4/2) = 2
If the author selectively presents only the relative risk, the reader will be led into thinking, and not incorrectly, that treatment X is twice as effective as treatment Y, but without any contextual awareness that only two out of every 100 patients attain greater benefit from treatment X. Although treatment X is better, its absolute impact is nowhere near as impres sive as the relative risk suggests.
Number needed to treat
Patients and health resource allocators need a more practical and intuitive way to understand the benefit or harm of a treatment offered to them or that they have been asked to fund. The number needed to treat (NNT) for benefit (or harm) translates previously discussed treatment effects into a more meaningful ‘How many patients do I need to treat before one of them experiences a benefit or harm?’
When we look at treatment benefit, the NNT to benefit is equivalent to (1/absolute risk difference). It is clear from this formula that the greater the absolute risk difference conferred by a treatment (the denominator), the smaller the number of patients required to be treated before one experiences a benefit. Using the previous example, where absolute risk difference is 2% benefit conferred by treatment X, the NNT for benefit is (1/0.02) = 50 for one patient to benefit. Although the relative risk of a benefit is 200% when X is compared with Y, 50 patients must be treated with X to obtain benefit for one, reflecting the small absolute risk benefit conferred by X.
Effect size
The higher the risk ratio, the more likely that the outcome will be different between the experimental and standard treatment in the real patient population. For example, for a given disease, a risk ratio of cure of 10 suggests a treatment is five times more likely to be beneficial compared to another treatment associated with a risk ratio of 2.
Precision of treatment estimates
The 95% confidence interval (95% CI) reflects the uncertainty of the study point estimate, informing the reader that the true population-level effect is likely to lie within this interval with 95% probability. The main results from a trial are best presented in terms of some treatment effect together with a confidence interval and (usually) a p-value.
How might the results be applied?
The final step is to consider the applicability of the results to your patients. This includes the similarity of their characteristics to trial patients and the practi calities of reproducing the study environment in your centre. The associated risks, costs, size and clinical significance of the treatment effect from both the patient’s and the institution’s viewpoints are practically important in deciding whether to intro duce a new therapy in your workplace.
An example of the application of these statistical factors and their clinical relevance is provided in Table 9.1 pertaining to the ECASS III study. A reasonable interpretation of this study could be that:
… for adult stroke patients similar to those enrolled in ECASS III (18–80 years admitted to a stroke centre with a diagnosis of acute ischaemic stroke able to receive the study drug within three to four hours after symptom onset), the number needed to treat to achieve a neurofunctional benefit (1 in 14) is far fewer than that required to harm (1 in 46 will suffer symptomatic intracranial haemorrhage). Despite increased risk of intracranial haemorrhage with alteplase, there was no mortality difference.
TABLE 9.1 Brief statistical analysis of ECASS III study
Source:Hacke W, Kaste M, Bluhmki E et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317−1329.
The patient could be told: ‘You have a much greater chance of recovering well from this stroke with alteplase than having a brain bleed from it.’
Critical appraisal of meta-analyses
Meta-analysis combines and summarises available research evidence quantitatively. If this is not pos sible, a more narrative systematic review is produced. Meta-analyses are most frequently and usefully employed to combine the effect estimates from multi ple randomised controlled intervention trials. An explicit, rational and comprehensive search strategy is applied to all sources of literature pertinent to a treatment topic. Unpublished or non-database cited trials should be identified and included to avoid publication bias (‘positive’ and English language trials are more likely to be published); this requires a search of ‘grey literature’ such as conference abstracts and theses and communication with researchers in the area. For single studies to be eligible for inclusion in a meta-analysis, they have to be independently evalu ated by two or more assessors as being of sufficient quality. These criteria are similar to those used to appraise single intervention studies.
The most valid meta-analyses obtain and analyse the disaggregated individual-level patient data from single trials rather than working only with aggregate data from single studies. The quality of design and conduct of meta-analyses must be appraised to ensure that their findings and conclusions are valid. The results of a well-designed and performed meta-analysis are likely to be most persuasive if it includes at least several good large-scale RCTs, the effect estimates from single studies are consistent, and the number of studies are sufficient and not clinically heterogeneous (i.e. are of similar clinical design). In cases where the available trials are small or poorly done, meta-analysis cannot compensate for the deficient primary trial data.
Forest plots are a visually excellent way to present results from meta-analyses. The general principles in assessing forest plots are:
We present two hypothetical meta-analyses for interventions to reduce the risk of failing the fellow ship examination in Figures 9.1 and 9.2.
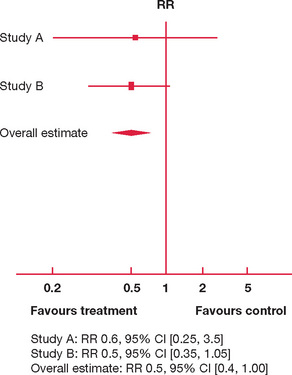
Figure 9.1 Hypothetical forest plot: 18-month versus 12-month (control) preparation time and success in the fellowship examination
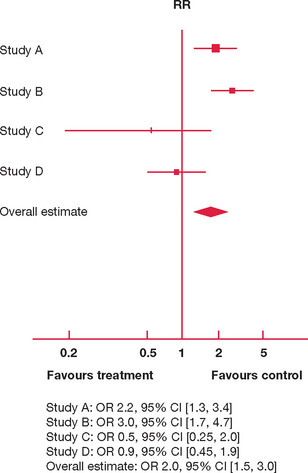
Figure 9.2 Hypothetical forest plot: overseas holiday versus weekly teaching sessions (control) and success in the fellowship examination
In Figure 9.1 there are only two studies available, A and B. Both studies have the majority of their 95% CI consistently to one side (left) of the vertical line of no effect. Both A and B are suggestive that an 18-month study phase reduces risk of failure (the risk ratio of failure is 0.6 and 0.5, respectively, with most of the 95% CI to the left of the vertical line). However, the upper limit of 95% CIs for A and B also extends to the right of the vertical line, so that an 18-month study phase may well increase the risk of failure by a factor of 3.5 and 1.05, respectively.
Avoiding failure using an 18-month study phase is not definitely proven, as the upper limit of the overall risk ratio is 1.0 (an 18-month study phase may not reduce your risk of failing the exam, but it is unlikely to exacerbate that risk); this is reflected in a p-value close to 0.05 (it achieves marginal statistical significance).
In Figure 9.2 there are four studies comparing the effect of attendance at weekly teaching sessions for six months and taking a three-month overseas holiday on failure in the fellowship examination. The individual study effects are inconsistent:
The overall effect is convincing though, with a three-month overseas holiday associated with a high odds of failure compared with attendance at weekly teaching sessions for six months.
However, since the effect estimations and 95% CI for individual studies are so variable, is it appropriate to aggregate their results in producing an overall estimate?
Part C: important papers
This section is divided into three groups of papers. First, brief synopses of a selection of important original studies and meta-analyses are presented together with their substantial contribution to evidence-based clinical practice in emergency medicine. These are not presented in any particular order, since all articles in this collection are deemed to be equally important. Next, some papers highlighting controversies and unanswered questions are provided to stimulate you to consider areas of ongoing debate. Some of the issues raised may surprise those who are unaware that the level of evidence supporting some widely employed therapies is not as robust as they once thought. Finally, useful resources that combine evidence from multiple sources into easy-to-use practice recommendations are presented. We encourage you to add to all of these sections during your exam preparations.
Lessons from original papers that have shaped emergency medicine
Validated decision rules have helped to rationalise emergency physicians’ approach to investigations and clinical risk stratification.
The greatest work has been done in low-risk trauma patients to reduce radiographic imaging (e.g. minor head injury, low-risk cervical spine injury, ankle injuries (Ottawa)); however, work is ongoing to develop validated decision rules for other patient cohorts (e.g. San Francisco Syncope rules). A recent international survey identified emergency physicians’ priorities:
The NOC is applicable to patients with a GCS of 15 and states that a CT scan of the head is indicated if there is at least one finding of either headache, vomiting, age over 60 years, drug or alcohol intoxication, persistent anterograde amnesia, visible trauma above the clavicle or seizure. The study found that for patients with a GCS of 15 the two rules have equivalent sensitivity of 100% for neurosurgical intervention and clinically important brain injury but CCHR was more specific. In the GCS 13–15 group, CCHR had 100% sensitivity for the same outcome.
Sepsis is now managed as a medical emergency and the concept of the ‘golden hour’ of aggressive goal-directed resuscitation commencing urgently in ED is firmly established.
Saline and albumin are both safe resuscitation fluids, although saline should be favoured in patients with traumatic brain injury.
The study did not involve patients with burns and those undergoing cardiac surgery or liver transplantation. Interestingly, the ratio of albumin to saline administered over the first four days ranged from 1:1.2 to 1:1.6, refuting previous dogmas suggesting much higher ratios are necessary for equivalent clinical effects. A post-hoc subgroup analysis alerted SAFE study investigators that patients with traumatic brain injury (TBI) resuscitated with albumin had a higher mortality, which instigated longer-term follow-up in this potentially at-risk patient group, a study published in 2007 (the next citation).
Two landmark studies were published in the same edition of the New England Journal of Medicine in 2002. They both employed specific interventions to improve cardiac outcome in all patients (i.e. reperfusion tech niques if appropriate) and concluded that there was a significant neurologic outcome benefit associated with active cooling. Ongoing work is being per formed to elucidate the most optimal therapeutic hypothermia regimen. The effects of this intervention on conventional prognostication strategies in ICU are also undergoing careful re-evaluation, with significant potential confounding effects of adjunctive sedation, particularly in the presence of renal and/ or liver dysfunction. There were a number of differences in the methods of each paper:
The Australian investigators are currently studying whether pre-hospital cooling by ambulance paramedics will add any additional benefit. The first of these trials was ceased during interim analysis for futility regarding benefit. With methodological changes, particularly earlier cooling, this remains an area of active research.
It is possible to identify patients with community-acquired pneumonia who can be safely managed as outpatients.
It has been more than two decades since the benefits of thrombolysis in acute myocardial infarction were established with the initial streptokinase studies, embedding the ‘unstable plaque theory’ (GISSI-1, ISIS-2). Subsequent studies compared streptokinase to tissue plasminogen activator (rt-PA) and examined the role of adjuvant heparin therapy (GISSI-2, ISIS-3, GUSTO-1). The subsequent era compared different agents/ regimens of thrombolytic agents (i.e. -teplases) and types of heparin (GUSTO-III, ASSENT-2 and 3). This era of ‘mega-trials’ paved the way for the ongoing wave of massive, high-quality randomised controlled trials driving the cardiology literature and clinical practice. It is interesting to review the original study:
A number of large studies have compared these two therapies, commencing with the GUSTO Angiographic Investigators study:
A Cochrane review in 2003 identified 10 relevant trials including 2,573 subjects and concluded that angioplasty provides a short-term clinical advantage over thrombolysis that may not be sustained:
Best-practice guidelines reflect that percutaneous interventions, when available in a timely fashion, are generally superior to lysis, particularly in patients with high-risk features (hypotension, elderly, recent surgery or trauma). Angioplasty has consistently been considered superior to lysis in the population with infarction-related cardiogenic shock:
N Engl J Med 1999; 341:625−634.
Patients with shock due to left ventricular failure complicating myocardial infarction were randomised to emergency revascularisation with coronary artery bypass grafting or angioplasty or received medical therapy with thrombolysis. Most patients in both groups (86%) were also supported with intra-aortic balloon pumps.
Evidence supporting a role for thrombolysis in patients with acute ischaemic strokes has been available for many years and yet application of this therapy has been limited with variable uptake, largely because patients frequently present late and some polarisation persists regarding the risks of intracranial haemor rhage compared with the actual clinical benefits of lysis. Arguably the European Cooperative Acute Stroke Study (ECASS) group has made the most substantial contribution over time to the still contro versial risk/benefit argument surrounding lysis in stroke, importantly igniting a more optimistic approach to stroke management:
It should be acknowledged, however, that a number of other stroke research groups have contributed, and the following systematic review is testament to this:
In contrast, a major development that has become widely recognised for stroke care is the role of dedi cated multidisciplinary stroke units with protocols for medical, nursing and therapy interven tions that energetically and optimistically embrace aggres sive rehabilitation. Improvements in mortality and reduced dependency have been demonstrated.
In the mid-1990s a number of trials established the safety and efficacy of aspirin after acute ischaemic stroke. References for the two leading landmark papers, presented in the same volume of the Lancet, are provided. This is followed by a recent meta-analysis on this issue:
Non-invasive ventilation has been investigated for a number of acute respiratory conditions, although its role in COPD has been established for the longest time.
A subsequent Cochrane review of studies per formed in and out of ICU settings also confirmed these benefits:
Low-molecular-weight heparin may safely replace unfractionated heparin for acute treatment of non-massive pulmonary embolism.
It is becoming indefensible to obtain central venous access without ultrasonic guidance as recommen dations for best practice strenuously advocate this technique.
Controversies and unanswered questions
There will always be areas where controversy exists and/or debate continues regarding the most appro priate therapy. This section outlines some of the most topical questions and includes a guide to further reading.
ATLS
At the time of writing, the 8th edition of ATLS (Advanced Trauma Life Support) was in the process of being released in Australasia. In addition to the wealth of references contained in the manual, a number of significant changes have occurred that will be relevant to clinical practice. Some of these are addressed further in this section. Major changes in the content include:
Interested readers may access further information regarding these most recent changes from http://web15.facs.org/atls_cr/atls_8thEdition_Update.cfm.
Should steroids be given to patients with traumatic spinal cord injuries?
Throughout the 1990s the use of steroids for traumatic spinal cord injury evolved from the NASCIS studies:
Major concerns have been raised about this paper and a general trend is away from the administration of methylprednisolone for acute spinal cord injury as the functional recovery purported to be associated with steroids lacks clinical significance:
Is hypertonic saline the fluid of choice for patients with traumatic brain injury?
What is the role for recombinant factor VIIa in ED patients?
Off-label use of this expensive drug is being explored widely in trauma patients with a possibility of it being cost-effective by virtue of a reduction in blood products transfused. For example:
The utility of this agent in intracranial haemorrhage remains unclear. Although it reduces haematoma size in patients with spontaneous intracranial haemor rhage, it does not appear associated with improvements in functional outcomes:
Is hypotensive resuscitation appropriate for some trauma patients?
Debate has continued to address the role of hypo tensive resuscitation in broader trauma cohorts. Of note, different algorithms for fluid resuscitation are now suggested in the Advanced Trauma Life Support (ATLS), Prehospital Trauma Life Support (PHTLS) and Battlefield Advanced Trauma Life Support (BATLS) protocols, depending on individual circum stances. Concerns must be explored regarding the appropriateness of hypotensive resuscitation in patients with traumatic brain injury where even tran sient hypotension has been associated with adverse effects on neurologic outcome. Some of these issues are addressed in a recent paper:
What is the best method for reducing anterior shoulder dislocations?
Is needle aspiration a safe and effective treatment of a primary spontaneous pneumothorax?
What is the influence of BLS and ALS by paramedics on outcome in traumatic and non-traumatic conditions?
A large amount of research material has been devel oped by the OPALS (Ontario Prehospital Advanced Life Support) study group. This group analysed the before and after-effects of introducing BLS and then ALS in a system-wide fashion in the province of Ontario. Publications continue to be produced from this work.
The really interesting results are seen following introduction of ALS skills (Phase III). Perhaps surprisingly, the effect on survival from cardiac arrest was an insignificant rise (5.0% to 5.1%).
Is chest compression alone better than full CPR in cardiac arrest?
The concept of chest compression alone for cardiac arrest instead of ‘standard’ CPR has attracted increasing interest in recent times. A number of papers have demonstrated equivalent or even superior outcomes. Combined with cooling, metabolic control and early reperfusion, this style of management has come to be known as ‘cardiocerebral resuscitation’.
While it is clear that any form of resuscitation is better than none and that untrained bystanders can not only perform chest compressions alone with minimal prompting, but they also seem more willing to do this than full CPR, further research is required to validate this approach. If proven successful, this may revolutionise the way we do BLS.
How should red-back spider (RBS) antivenom be administered?
How much antivenom is needed for brown snake envenomation?
Management of brown snake (and other snake) enven omation creates unique challenges for FACEMs in Australia. Much debate has been undertaken regard ing the amount and type of antivenom required. Some studies have suggested as many as 10 vials should be administered as an initial dose for severe envenomation.
More recently, this practice has been questioned. A number of the same researchers involved in RAVE have also collaborated in the Australian Snakebite Project (ASP):
In a similar pattern to the RAVE study, venom levels were measured following snake bites. Calculations from the full range of clinical presentations (severe envenomation to no antivenom required) confirmed that a single vial of brown snake antivenom is sufficient to neutralise all the venom from severe envenomation. However, it takes a number of hours for clotting factors to regenerate before coagulation studies return to normal. On reflection, it seems that the time taken to administer large amounts of antivenom was more important than the antivenom itself. Further research is now looking at the use of Fresh Frozen Plasma (FFP) after antivenom administration.
Does octreotide successfully decrease acute bleeding from oesophageal varices?
Should hyperbaric oxygen (HBO) therapy be used to treat patients with carbon monoxide poisoning?
A key Australian contribution to the world debate was provided in the form of evidence against the use of HBO:
Do hospital medical emergency teams (METs) improve the outcomes of critically ill patients?
Emergency physicians may be involved in managing cardiac arrest or MET calls. It is commonly believed that METs should replace cardiac arrest teams for rapid identification and treatment of patients before they deteriorate. Activation criteria for MET calls have included acute derangements in physiologic parameters as well as non-specific marked concern by staff members. Evidence supporting METs has been mixed.
Should EDs have a protocol regarding N-AC for prophylaxis against radiocontrast nephropathy?
Should thrombolysis be administered to patients with submassive PE?
Massive PE with shock has a high mortality and current recommendations are for aggressive treatment with thrombolysis, surgery or a percutaneous mechanical intervention. Thrombolysis may be considered in patients who experience a cardiac arrest with a high probability of PE as the cause. The situation for sub-massive PE with right ventricular dysfunction and normal blood pressure is less clear. The situation has been reviewed recently:
Is there a role for levosimendan in ED?
Despite recent enthusiasm for this new inotrope in patients with acute decompensation of chronic heart failure (LIDO), recent evidence concludes that levosimendan does not improve the survival of this patient group:
Is there a role for new ‘point-of-care’ tests such as brain natriuretic peptide (BNP) and procalcitonin in ED?
Procalcitonin is being explored in a number of ED patient cohorts to investigate its possible role as a diagnostic and/or prognostic marker in febrile paedi atric and adult patients, but definitive studies are necessary. For example:
Some authors support the use of BNP to increase the accuracy of the initial clinical impression regarding diagnosis of cardiac failure, as well as to improve patient disposition decisions:
Should all patients with bacterial meningitis be treated with high-dose steroids?
The neurological outcome of bacterial meningitis is related to the severity of the inflammatory process incited by the pathogen in the subarachnoid space. Improved neurological outcomes (particularly reduced risk of sensorineural deafness) from bacterial menin gitis have clearly been demonstrated in child ren given corticosteroids before the first dose of IV antibiotic is administered. Presumably harmful subarachnoid inflammation from the break down products of bacteriolysis is reduced by corticosteroids.
The evidence for benefit from corticosteroids in adults with bacterial meningitis is less certain. In van de Beek’s meta-analysis, 18 studies involving 2,750 patients were analysed. Adjuvant steroids were associated with lower mortality, reduced rates of severe hearing loss and long-term neurologic sequelae. In children the most significant effects were on preventing hearing loss and in adults the most significant effects were greater protection from death. With steroids, mortality reduction was most noticeable in patients with Streptococcus pneumoniae meningitis. Children with Haemophilus influenzae meningitis experienced the least risk of hearing loss:
In the most recent large adult study the regimen was dexamethasone 10 mg before or with the first dose of antibiotic, followed by 10 mg q6h for four days:
Concerns must remain about the wisdom of this regimen in patients who develop severe sepsis and septic shock, because high-dose steroids have been associated with increased mortality and even the use of low-dose steroids for ‘relative adrenal insufficiency’ remains highly contentious. The use of steroids in children in the era of HIB vaccination and in meningococcal sepsis is uncertain.
Is lactulose an important component of supportive treatment for patients with decompensated liver failure?
Evidence-based practice recommendations
Life support
The most recent ILCOR guidelines on ACLS in adults and paediatrics (2005) can be found in:
Acute coronary syndromes
Traumatic brain injury
Other topics
Altman DG. Practical Statistics for Medical Research, 1st edn. London: Chapman & Hall/CRC; 1991.
Davidoff F, Haynes B, Sackett D, Smith R. Evidence based medicine. BMJ. 1995;310:1085-1086.
Egger M, Davey Smith G, Altman DG. Systematic Reviews in Health Care. Metaanalysis in Context, 2nd edn. London: BMJ Publishing Group; 2001.
Greenhalgh T. Assessing the methodological quality of published papers. BMJ. 1997;315:305-308.
Greenhalgh T. How to read a paper. Statistics for the non-statistician. I: different types of data need different statistical tests. BMJ. 1997;315:364-366.
Greenhalgh T. How to read a paper. Statistics for the non-statistician. II: ‘significant’ relations and their pitfalls. BMJ. 1997;315:422-425.
Guyatt GH, Naylor D, Richardson WS, et al. What is the best evidence for making clinical decisions? JAMA. 2000;284:3127-3128.
Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329. Sep25
Kirkwood BR, Sterne JAC. Essential Medical Statistics, 2nd edn. Oxford: Blackwell Science; 2003.
Mark Daniel B. Chapter 3, ‘Decision-making in clinical medicine’. In Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors: Harrison’s Principles of Internal Medicine, 17th edn., New York: McGraw-Hill Medical, 2008.
Pocock SJ. Clinical Trials. A Practical Approach, 1st edn. Chichester, UK: John Wiley & Sons Ltd; 1983.
Wang D, Bakhai A. Clinical trials. A Practical Guide to Design, Analysis and Reporting, 1st edn. London: Remedica; 2006.
Wardlaw JM, del Zoppo GJ, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No. CD000213. DOI: 10.1002/14651858.CD000213.