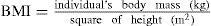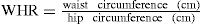Chapter 50 Drugs in Obesity
Obesity is a common and costly nutritional problem affecting a significant proportion of the Australian and New Zealand populations. Overweight and obese individuals with body mass index >25 have a higher long-term risk of morbidity and mortality. A wide range of interacting biopsychological factors contribute to obesity, which in some cases may not be simply solved by reducing food intake and increasing energy expenditure. In some individuals, refractory to weight-reduction interventions, bariatric or weight reduction surgery is undertaken. Alterations in diet, increased physical activity and behavioural modification are central to the prevention and treatment of obesity. Continued advances in understanding the pathophysiology of obesity are contributing to the development of more antiobesity drugs.
Key background
THE prevalence of obesity has reached epidemic proportions across the globe bringing with it an increased risk of cancer, cardiovascular disease, diabetes mellitus, dyslipi daemia, hypertension and sleep apnoea. In the last three decades self-interest in our weight and body shape has maintained a billion-dollar industry that ranges from glossy magazines to the sale of diet food, fitness centre exercise packages and sojourns at health resorts: ‘Australians spend in excess of $500 million on commercial weight control measures’ (Caterson 1999a). Without a doubt, Australians and New Zealanders are increasing in weight, and a proportion of each population is considered either overweight or obese (see Clinical Interest Box 50-1). A key issue, however, is the measurement of body weight. How do we define underweight, normal weight, overweight and obesity?
 Clinical interest Box 50-1 New zealand adult and child obesity statistics
Clinical interest Box 50-1 New zealand adult and child obesity statistics
Using standard adult BMI cut-offs for ‘overweight’ (≥26 for Maoris and Pacific Islanders; ≥25 for Europeans, Asians and ‘others’), the results from the 2006/07 New Zealand Health Survey indicate that one in three adult New Zealanders are overweight and one in four are obese (BMI ≥32 for Maoris and Pacific Islanders; ≥30 for Europeans, Asians and ‘others’). In children aged 2–14 years, one in five is overweight and one in twelve obese. Obesity was significantly more prevalent in children from lower socioeconomic areas; however, in adults only the most deprived areas showed a significant increase in obesity. Since 1997, the prevalence of obesity has increased, although this change has not been shown statistically.
For both males and females, the prevalence of obesity was highest in the Pacific ethnic group (2.5 times higher than the general population), followed by Maori (1.7 times higher than the general population), European/other and Asian ethnic groups, and the prevalence of obesity increased with age until 55–64 years, then declined slightly.
Adapted from: Ministry of Health 2007.
It has long been recognised that simply weighing an individual does not provide an indication of weight distribution or of risk factors. The measurement now most frequently used is the body mass index (BMI). However, it should be recognised that the BMI is highly variable: it does not account for high body mass due to muscle rather than fat, is unreliable in young people and the elderly and reflects poorly intra-abdominal fat, which is related to the metabolic syndrome and cardiovascular risk. The BMI is calculated from the following equation:
For a 100-kg person who is 1.7 metres tall, the BMI is 100 ÷ 1.72 = 100 ÷ 2.89 = 34.6, and this person would, according to World Health Organization (WHO) guidelines, be considered obese (Table 50-1).
Table 50-1 Body mass index: international standard (WHO 2000)
| CLASSIFICATION | BMI (kg/m2) |
| Underweight | <18.5 |
| Normal weight | 18.5–24.9 |
| Overweight (pre-obese) | 25–29.9 |
| Obese | ≥30 |
| Class I | 30–34.9 |
| Class II | 35–39.9 |
| Class III | >40.0 |
With the BMI used as the main indicator, the proportion of obese Australians increased for both men and women across all age groups between 1995 and 2004/05. During this period the average weight of an adult female and an adult male increased from 65 to 68 kg and from 80 to 84 kg, respectively. In 2004/05 20% of 45–54-year-old women were classed as obese as were 23% of males aged 25–34 years (Australian Bureau of Statistics 2007). Based on self-reports (2004–2005) ∼7.5 million Australian adults were overweight with more than one-third classed as obese (AIHW 2008). The National Aboriginal and Torres Strait Islander Health Survey (NATSIHS) conducted in 2004–2005 estimated that 35% of Indigenous people aged 18 years and over were in the normal weight range, 29% were classified as overweight and 31% were obese. Obesity is not unique to Australasia; it is common in industrialised countries throughout the world and is a serious public health problem.
Health risks associated with obesity
Obesity is well recognised as a disease in its own right. Health risks associated with obesity are enormous, and overweight individuals have a higher mortality rate than people with a normal BMI. Worldwide, approximately 1 billion adults and 10% of children are now classified as overweight or obese. In terms of contribution to the overall global burden of disease and disability, excessive weight (corpulence) is the sixth most important factor. Many studies have reported that obese individuals have a greater risk of:
The distribution of fat is also very important in relation to disease risk. A high proportion of visceral (e.g. abdominal) fat carries a greater risk of morbidity and mortality than does a peripheral distribution. Although large studies that have measured concomitantly body weight and glucose and insulin concentrations are rare, a strong association exists between abdominal adiposity, blood glucose concentration, insulin resistance and the development of type 2 diabetes. Within Australia, the higher prevalence of obesity has been identified as a major contributing factor to the increasing incidence of diabetes, specifically type 2 diabetes (AIHW 2008). A simple measurement of visceral fat is the waist:hip ratio (WHR), which is calculated from the following equation:
The WHR should be <0.9 in men and <0.8 in women. Although there is no standard cut-off for waist circumference that provides an indication of greater risk, the WHO (2000) suggests that a waist measurement >94 cm in men and ≥80 cm in women indicates some risk, and a waist circumference >102 cm in men and >88 cm in women indicates a substantially increased risk of health problems. These waist circumference measurement guidelines have been developed for Caucasians; similar guidelines have not yet been established for other populations. In 1995, waist measurements were >94 cm for 35% of Australian men and >102 cm for 19%. For women, waist measurements were >80 cm for 37% and >88 cm for 23% (AIHW 2003). In 2005 the AusDiab follow-up study surveyed people who had participated in the baseline study in 1999–2000. Over the period of the follow-up an increase in waist circumference was observed in all BMI groups. Of the people who were normal or overweight at baseline, 31.7% had progressed to a higher weight category over the follow-up period of 5 years (AusDiab 2006).
The need for prevention and intervention to reduce obesity has been recognised in both Australia and New Zealand. A range of strategies has been developed, including guidelines for food and nutrition in schools and national approaches to encouraging individuals to participate in physical activity. In September 2003, the National Health and Medical Research Council of Australia developed Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults (http://www.nhmrc.gov.au). In July 2006, a five-year, $500 million program called the Australian Better Health Initiative was launched by the Australian Government. This program is aimed at reducing the impact of chronic disease by promoting the concept of healthy weight. The management of obesity is complex, however, and increasing physical activity is just one strategy for weight control. To understand the disorder of obesity and to develop management strategies, it is essential to appreciate the complexity of the disorder.
Pathophysiology of obesity
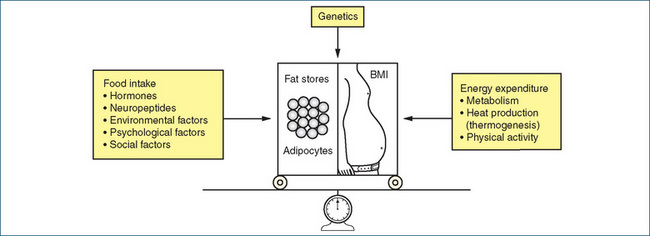
Obesity is a complex multifactorial disorder involving changes in energy balance (intake and expenditure), genetic factors, environmental factors (dietary and physical activity) and psychosocial factors (Figure 50-1). The complexity of these interacting forces has made the management of obesity difficult, and some individuals have resorted to surgical procedures such as gastric bypass and banded gastroplasty.
Primary obesity rarely results from endocrine disorders (e.g. Cushing’s syndrome, hypothyroidism or hypogonadism) or from neurological disorders or drug treatment. From the simplest viewpoint, obesity, which is manifest by increased fat storage, is a consequence of an imbalance between increased energy (food) intake and decreased energy expenditure. Although on the surface this is a simple relationship, various factors can modify the balance between energy intake and expenditure. ‘Overweight and obese individuals have four choices: accept their weight where it is, change their diet and physical activity behaviours, use a weight loss medication, or have weight loss surgery. These options go from low risk/low effectiveness to greater risk/greater effectiveness’ (Bessesen 2008).
Energy balance: integration involving the periphery and the hypothalamus
The control of food intake is not fully understood. It is regulated by a complex system of interacting monoamine and peptide neurotransmitters, involving both peripheral and central hypothalamic pathways. The involvement of the sympathetic nervous system (and its neurotransmitter noradrenaline) in regulating energy expenditure has been well documented scientifically, and it is popularly accepted that obese individuals have ‘slow metabolism’ and lean people have ‘high metabolism’. Stimulation of α-adrenoceptors by noradrenaline decreases food intake via an action in the feeding centre in the hypothalamus and increases energy expenditure via stimulation of peripheral β-adrenoceptors. Similarly, activation of central 5-hydroxytryptamine (5-HT, serotonin) receptors by an excess of 5-HT inhibits food intake (Figure 50-2).
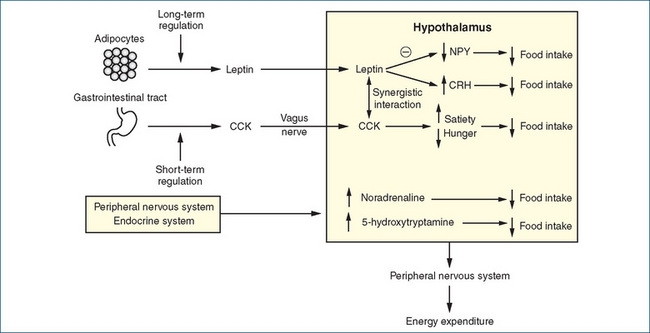
Figure 50-2 Schematic representation of food-regulating pathways. The long-term regulation of food intake involves the leptin system, which causes a reduction in food intake by decreasing hypothalamic neuropeptide Y (NPY) synthesis and release and increasing the release of corticotrophin-releasing hormone (CRH). Short-term control involves cholecystokinin (CCK) released from the duodenum; CCK stimulates receptors on the vagus nerve to signal the hypothalamus of reduced feelings of hunger and satiety. Signals are also received by the hypothalamus from the peripheral nervous system and various hormonal stimuli. Specialised nuclei in the hypothalamus integrate the various signals and modulate the release of chemicals that affect food intake and of impulses to the autonomic nervous system that regulate energy expenditure.
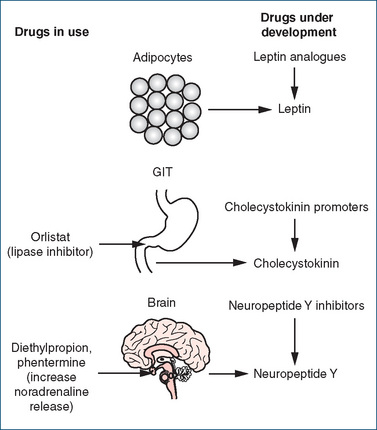
In addition to these monoamine transmitters, many central and peripheral peptide neurotransmitters are involved. These are classed as orexigenic (increasing food intake, e.g. neuropeptide Y) or anorexigenic (decreasing food intake, e.g. cholecystokinin). Interest has focused more recently on the role of peripherally released leptin and cholecystokinin, and anti-obesity drugs targeted at these sites are being developed.
Leptin
Leptin (derived from the Greek word leptos, meaning thin) is the protein product of the obesity (ob) gene identified in 1994 (Zhang et al 1994). This protein is released from adipocytes (fat cells) and signals the brain about the fat stores of the body. Generation of leptin is increased by oestrogen, glucocorticoids and possibly by insulin, and is reduced by β−adrenoceptor agonists. On reaching the brain, leptin reduces the production of neuropeptide Y, which normally stimulates food intake and causes a reduction in energy expenditure. Leptin also stimulates the production of corticotrophin-releasing hormone, which reduces food intake (Figure 50-2). Obese humans have higher levels of leptin that correlate with the amount of body fat, but the leptin in those individuals fails to normalise the fat stores. This has led some investigators to propose that obese individuals may have leptin resistance, analogous to the insulin resistance encountered in type 2 diabetes (Mertens & Van Gaal 2000).
Cholecystokinin
Cholecystokinin (CCK) is secreted from the duodenum in the presence of food. It inhibits gastric emptying, pyloric sphincter contraction and stimulation of pancreatic exocrine secretions, and significantly reduces feelings of hunger and increases satiety (feeling of fullness) (Figure 50-2). Stimulation of CCK-A receptors on the vagus nerve sends signals to the hypothalamus and an interaction appears to occur between leptin and CCK (Mertens & Van Gaal 2000). This interaction may play a role in both the short- and long-term regulation of body weight. Other peripheral and central pathways may also be involved in the regulation of food intake and energy storage, but our knowledge is still incomplete.
Genetic factors
There is now widespread acceptance of a genetic component to obesity. Studies with adopted children have shown that their weight is related to that of their biological parents and not to that of their adopted parents. Estimates of a contribution of genetic effects to obesity range from 25% to 80%, and these findings have undoubtedly given credibility to the widely held perception that overweight parents have overweight kids.
It has long been recognised that many people with diabetes are obese, but a link between the two disorders has been difficult to establish. In a hallmark study using mice, a team of researchers has shown a link between diabetes and a protein called resistin, which is produced in adipocytes. Their studies established that diabetic mice have higher resistin levels, and these are linked to both diet-induced and genetic obesity (Steppan et al 2001). Recent data on genetic linkage with obesity-related traits come from the fat mass and obesity-associated (FTO) gene. A number of variant alleles of FTO have been demonstrated and individuals who are homozygous for the high-risk alleles weigh on average 3 kg more than those individuals with the low-risk allele. This gene appears to encode for an enzyme in the hypothalamic nuclei that is involved in regulating energy balance (Gerken et al 2007). Determining associations between human obesity and genetic mutations is still in its infancy, but there is no doubt that obesity has a genetic basis in some individuals.
Environmental factors
The availability of ‘fast foods’ and foods high in fat is an ever-present problem, coupled with changing lifestyles and a tendency for more sedentary pursuits. In consequence, the average weight of Australian women has increased by 4.8 kg and that of men by 3.6 kg since 1980 (AIHW 2003). The tendency for us to obtain our daily energy from bread, milk and potatoes (mashed potato or hot chips) does not fit with the guidelines of the National Health and Medical Research Council for weight reduction (NHMRC 2003). Strategies are aimed at increasing the consumption of vegetables, fruit, grains and cereals in an attempt to control the pandemic of obesity. Increased physical activity is also being encouraged, again attempting to redress the imbalance between high dietary fat intake and reduced energy expenditure.
Management of obesity
Obesity is a complex disorder, and a complete understanding of all the interacting factors that lead to obesity is still to come. Management is difficult, and individuals’ compliance with one or all aspects of a program may vary enormously. Regulation of weight and the adoption of a healthy lifestyle are the main issues for many people, especially those whose genes predispose them to obesity: ‘A man, for example, with a marked family history of obesity who has been obese since childhood will probably never be thin, despite his best efforts’ (Wadden et al 1999). There is often initial difficulty in losing weight, and further difficulties in maintaining the weight reduction. Preferred strategies include dietary modification (low-fat diet), exercise programs and behavioural modification (Wadden et al 1999). Obesity is a lifelong problem for many people and too often the obese person is stigmatised as lacking the discipline to eat less and exercise more. Pharmacological agents could play an increasingly important role as knowledge of the biochemical and genetic factors contributing to obesity improves. Although drugs may produce an initial weight loss, maintaining the weight loss is still the key issue.
Drug therapy
Safe and effective drugs free of troublesome adverse effects for the treatment of obesity are not yet available, but several drugs targeted at specific sites are in development (Figure 50-3). Previously the main drugs used for the treatment of obesity were the centrally acting appetite suppressants that mimic the actions of noradrenaline.
Noradrenergic drugs
Dextroamphetamine was introduced during the 1930s for the treatment of narcolepsy (desire to sleep) and was found to produce weight loss. It was later established that the anorectic effect of amphetamine is mediated via release of noradrenaline in the hypothalamus, which enhances sympathetic neurotransmission, resulting in appetite suppression and increased energy expenditure. Because of actions on central dopaminergic pathways producing central excitatory effects, amphetamine unfortunately had very high addictive and abuse potential.
Other noradrenergic amphetamine-like drugs with reduced potential for drug abuse were developed specifically as appetite suppressants. These included benzphetamine, diethylpropion, phentermine, methamphetamine and phendimetrazine. Phentermine is the only one currently available in Australia and New Zealand but its role in the treatment of obesity is questionable given the availability of newer drugs with negligible addiction and abuse potential. This drug increases catecholamine concentration making it unsuitable for use in obese people with cardio- or cerebrovascular disease. Phentermine may be used for short-term treatment (about 3 months) of obesity in conjunction with a management plan of caloric restriction, an exercise program and behavioural modification. Tolerance to the anorectic effect of this drug develops at varying times and, as it is subject to misuse, long-term usefulness is limited. Common adverse effects include headache, insomnia, irritability, palpitations and nervousness.
Drugs acting via 5-HT
Dexfenfluramine and fenfluramine were drugs that enhanced 5-HT release and inhibited neuronal reuptake of 5-HT. This led to an increase in 5-HT concentration in the hypothalamus and a subsequent decrease in food intake. Both drugs had minimal effect on sympathetic and dopaminergic neurotransmission. These agents were often used in combination with phentermine but were found to cause pulmonary hypertension and thickening of cardiac valves, requiring valve replacement in some women. Both drugs were withdrawn voluntarily from the market.
Fluoxetine, a selective serotonin reuptake inhibitor used in the treatment of depression, produces a dose-related decrease in body weight but the effect is not long-term, lasting approximately 6 months in spite of continued use of the drug. Fluoxetine has not been approved for the treatment of obesity.
Sibutramine
Like many drugs acting on central neurotransmitters, sibutramine (Reductil®) was originally developed for the treatment of depression (Drug Monograph 50-1). In general, sibutramine is well tolerated but, as with other drug therapy for obesity, sibutramine use should be accompanied by caloric restriction, increased exercising and eating behaviour modification. Unfortunately, weight is often regained when treatment is stopped after <6 months duration.
Drug monograph 50-1 Sibutramine
Sibutramine is currently approved for long-term management of obesity and efficacy has been demonstrated for up to 2 years in obese adults aged 18 to 65 years. Beneficial changes following sibutramine-induced weight loss are observed in obesity-related risk factors such as hyperlipidaemia, serum uric acid concentration and control of blood glucose.
MECHANISM OF ACTION Sibutramine increases the central concentrations of noradrenaline, 5-HT and dopamine by inhibiting their reuptake mechanisms. This leads to weight loss via the suppression of appetite, induction of the sensation of satiety and increased thermogenesis. Sibutramine works predominantly through its two pharmacologically active metabolites: N-desmethylsibutramine (M1) and N-bisdesmethylsibutramine (M2). These metabolites are approximately 3 times more potent as inhibitors of noradrenaline and 5-HT uptake than of dopamine uptake.
PHARMACOKINETICS Sibutramine is rapidly absorbed from the GI tract and undergoes extensive first-pass metabolism by a number of CYP enzymes including CYP2B6, CYP3A4, CYP3A5 and CYP2C19 (Kim et al 2009), forming the two active metabolites M1 and M2. These metabolites are then further metabolised to pharmacologically inactive metabolites (M5 and M6). The majority (∼77%) of a dose of sibutramine is excreted in urine as the inactive metabolites and unchanged sibutramine. Steady state of the active metabolites is achieved within 4 days and the elimination half-lives of the active metabolites are 14 (M1) and 16 hours (M2).
DRUG INTERACTIONS Drugs that inhibit CYP2C19 and CYP3A4 (e.g. ketoconazole) may inhibit metabolism of sibutramine and its active metabolites increasing the risk of adverse effects. As sibutramine may cause serotonin toxicity, combination with phentermine, SSRIs and inhibitors of monoamine oxidase (MAOIs) should be avoided and in some instances is contraindicated.
ADVERSE REACTIONS These are most frequent during the first 4 weeks and include tachycardia, palpitations, raised BP, hot flushes, constipation, dry mouth and taste disturbances. Dizziness, insomnia, headache and anxiety have also been reported. Rare adverse effects include psychiatric disturbances, depression and sexual dysfunction and menstrual cycle disorders.
WARNINGS AND CONTRAINDICATIONS Sibutramine is contraindicated in any condition where an increase in BP or heart rate may cause a detrimental clinical outcome, e.g. coronary heart disease, congestive heart failure, history of stroke and uncontrolled hypertension. Use in persons with a history of eating disorders, bipolar disorder or bleeding disorders is contraindicated. BP and heart rate should be monitored at 2-week intervals for 12 weeks and then monthly for at least the next 3 months, thereafter at regular intervals. If BP increases, use of sibutramine should be ceased. Weight loss during pregnancy is generally inappropriate and, as there is limited human data, sibutramine is classed in ADEC Pregnancy Category C.
DOSAGE AND ADMINISTRATION The usual dose is 10 mg once daily in the morning. The dose may be increased to 15 mg daily (if tolerated) after 4 weeks if weight loss is less than 2 kg.
Source: 21 January 2010, EMA/808179/2009, EMEA/H/A-107/1256, http://www.ema.europa.eu/pdfs/human/referral/sibutramine/Sibutramine_Q&A_80817909en.pdf [3 March 2010].
Therapeutic Goods Administration (TGA):
Source: Reductil (sibutramine) product information (updated 22 January 2010), http://www.tga.gov.au/alerts/medicines/reductil.htm [3 March 2010].
The New Zealand Medicines and Medical Devices Safety Authority (Medsafe):
Source: http://www.medsafe.govt.nz/profs/datasheet/r/Reductilcap.pdf [28 April 2010].
Inhibition of nutrient absorption
Orlistat is synthesised from lipstatin, a natural product of Streptomyces toxytricini, and was approved for use in Australia in 2000 (Drug Monograph 50-2). In several clinical studies conducted over 1–2 years, the percentage of people losing weight with orlistat was 10%–20% higher than in the placebo-treated groups (Bray 1999). A number of clinical trials have demonstrated that use of orlistat results in a reduction in waist circumference and improvement in cardiovascular risk factors, e.g. reduction in total and LDL-C, blood pressure and blood glucose concentration and insulin resistance. Weight loss generally occurs within 2 weeks of commencement of drug therapy and is maintained with continued use. However, the value of orlistat in treating obese adolescents (12–16 years) is unclear and further studies are required to determine the efficacy and safety of orlistat in adolescents.
In addition to sibutramine, orlistat is the only other medication approved for long-term management of obesity.
MECHANISM OF ACTION Orlistat inhibits gastric and pancreatic lipase through the formation of a covalent bond with a serine residue in the active site of the enzyme. Inhibition of the action of the lipases prevents the breakdown of dietary triglycerides (fat) and inhibits the absorption of cholesterol and lipid-soluble vitamins. Orlistat therefore decreases the absorption of dietary fat by approximately 30% and promotes a reduction in body weight and plasma cholesterol (Carek & Dickerson 1999).
PHARMACOKINETICS Orlistat undergoes minimal systemic absorption and is essentially retained within the gastrointestinal tract. Plasma concentrations of the drug are either not detectable or exceeding low (<2 mcg/L). Metabolism occurs within the gastrointestinal wall, and five major metabolites have been identified. Less than 2% of orlistat is excreted in urine and most of the drug is eliminated in faeces (>96%), with unchanged drug accounting for 83% of the total dose excreted.
DRUG INTERACTIONS Drug interaction studies are limited with orlistat, and to date several studies have reported that coadministration of orlistat and cyclosporin may result in reduced plasma concentration of cyclosporin. As vitamin K absorption may also be decreased, people taking warfarin or phenindione should be monitored for changes in coagulation parameters. Additional contraceptive precautions are recommended when taking combined oral contraceptives and orlistat concurrently because of reports of breakthrough bleeding and contraceptive failure. In the absence of extensive drug interaction studies, concomitant administration of other antiobesity agents is not recommended.
ADVERSE REACTIONS The most commonly observed adverse reactions involve the gastrointestinal tract and include fatty or oily stools, oily spotting, flatulence, liquid stools, abdominal pain and faecal incontinence and urgency. Other commonly reported adverse reactions from clinical trials with orlistat included headache, nausea and dyspepsia.
WARNINGS AND CONTRAINDICATIONS Current drug information sources should be consulted with regard to warnings and contraindications. Orlistat should be used with caution in individuals with active peptic ulcer disease or significant cardiac, gastrointestinal, renal, hepatic or endocrine disorders. Use in people with cholestasis, chronic pancreatitis or pancreatic enzyme deficiency or chronic malabsorption syndrome is contraindicated. Although orlistat is classed in ADEC Pregnancy Category B1, its safety in pregnant women has not been established and, generally, weight loss during pregnancy is inappropriate.
DOSAGE AND ADMINISTRATION No advantage has been observed with doses greater than 120 mg three times daily with food. Orlistat should be taken either with each main meal or up to 1 hour after each meal.
Orlistat was re-scheduled from Schedule 4 (Prescription Only) to Schedule 3 (Pharmacist Only) by the National Drugs and Poisons Schedule Committee 2003/04. This decision was based on the outcome of clinical trials that indicated reasonable efficacy for weight reduction and a low incidence of adverse effects. It also recognised the role of pharmacists in providing high-quality advice on the management of obesity. Similarly, orlistat is scheduled Pharmacist Only in New Zealand.
The future
Weight gain after discontinuation of drug therapy is common. Maintaining a desirable and realistic weight on a long-term basis is a difficult, soul-destroying task for many individuals. The current antiobesity drugs can cause problems, and long-term use is not recommended. Investigations of the effectiveness of leptin analogues, neuropeptide Y antagonists, β3 adrenergic agonists, CB1 receptor antagonists, glucagon-like peptide 1, peptide YY(3–36) and lipolytic growth hormone fragments are ongoing. The search for safe and effective drugs continues but it is unlikely that there will ever be a drug that allows a person to lose weight despite increased food intake and reduced physical activity. Future drugs may help some individuals but until then ‘a supportive, knowledgeable medical environment in which individuals can be helped to understand what is necessary in the way of food intake and daily activity is vital ..... the aim is not to make everyone thin, but to prevent some becoming overweight or obese’ (Caterson 1999b).
Clinical interest Box 50-2 Rimonabant
There is tremendous interest in the development of safer, more efficacious drugs for the treatment of obesity. Currently at least 30 drugs are in various stages of research and development but in some areas progress has slowed because of adverse outcomes. Rimonabant, a novel selective cannabinoid-1 receptor (CB1) antagonist, was developed following evidence that the endocannabinoid receptor system is involved in the expression of mediators that control appetite. CB1 receptors are present in both the CNS and peripheral tissues. The endogenous ligands of the CB1 receptor (endocannabinoids) are thought to influence food intake through anorexic and orexigenic mediators. Hence, antagonists like rimonabant have been shown to decrease appetite and consumption of normal food (Patel & Pathak 2007).
In June 2006 the European Commission granted marketing authorisation for rimonabant throughout the European Union as an adjunct to diet and exercise for the treatment of overweight and obese individuals. Sanofi-Aventis voluntarily withdrew its marketing authorisation in December 2008, and in January 2009 the European Commission withdrew the marketing authorisation. Clinical trial data had highlighted a substantial increase in psychiatric adverse effects (e.g. depressed mood and anxiety). Development has slowed in relation to other drugs of this class.
Key points
 Obesity is a complex multifactorial disorder involving changes in energy balance, genetic factors, environmental factors and psychosocial factors.
Obesity is a complex multifactorial disorder involving changes in energy balance, genetic factors, environmental factors and psychosocial factors. The body mass index (BMI) is highly correlated with body fat and is calculated by dividing an individual’s body mass (kg) by the square of the height in metres (m2).
The body mass index (BMI) is highly correlated with body fat and is calculated by dividing an individual’s body mass (kg) by the square of the height in metres (m2). A waist:hip ratio >0.9 for men and >0.8 for women indicates significant visceral fat distribution, which is associated with a greater risk of morbidity and mortality.
A waist:hip ratio >0.9 for men and >0.8 for women indicates significant visceral fat distribution, which is associated with a greater risk of morbidity and mortality. The control of food intake is not fully understood. It is regulated by a complex system of interacting monoamine and peptide neurotransmitters involving both peripheral and central hypothalamic pathways.
The control of food intake is not fully understood. It is regulated by a complex system of interacting monoamine and peptide neurotransmitters involving both peripheral and central hypothalamic pathways. Interest has more recently focused on the role of peripheral modulators, which include leptin and cholecystokinin.
Interest has more recently focused on the role of peripheral modulators, which include leptin and cholecystokinin. There is widespread acceptance of a genetic component to obesity, and estimates of a genetic contribution range from 25% to 80%.
There is widespread acceptance of a genetic component to obesity, and estimates of a genetic contribution range from 25% to 80%. Management of obesity is difficult because of the complexity of the disorder. Preferred strategies include dietary modification (low-fat diet), exercise programs and behavioural modification.
Management of obesity is difficult because of the complexity of the disorder. Preferred strategies include dietary modification (low-fat diet), exercise programs and behavioural modification. Current drugs include the centrally acting noradrenergic appetite suppressant phentermine, the noradrenergic and 5-HT reuptake inhibitor sibutramine and the lipase inhibitor orlistat. Tolerance to the anorectic effect of the noradrenergic drugs has limited their usefulness. Long-term data on the safety and efficacy of both orlistat and sibutramine are still required.
Current drugs include the centrally acting noradrenergic appetite suppressant phentermine, the noradrenergic and 5-HT reuptake inhibitor sibutramine and the lipase inhibitor orlistat. Tolerance to the anorectic effect of the noradrenergic drugs has limited their usefulness. Long-term data on the safety and efficacy of both orlistat and sibutramine are still required.References and Further reading
AusDiab 2005 The Australian Diabetes Obesity and Lifestyle Study. Melbourne: International Diabetes Institute, 2006.
Australian Bureau of Statistics. Australian Social Trends 2007 Overweight and Obesity. Australian Bureau of Statistics cat. no. 4102.0. Commonwealth of Australia, 2007.
Australian Institute of Health and Welfare. A growing problem: trends and patterns in overweight and obesity among adults in Australia, 1980–2001. AIHW Bulletin 2003; Issue 8.
Australian Institute of Health and Welfare. Australia’s Health 2008 The Eleventh Biennial Health Report of the Australian Institute of Health and Welfare. cat. no. AUS 99. Canberra: AIHW, 2008.
Australian Institute of Health and Welfare (AIHW) and National Heart Foundation of Australia. The Relationship Between Overweight, Obesity and Cardiovascular Disease. AIHW cat. CVD 29. Canberra: AIHW (Cardiovascular Disease Series No. 23), 2004. in press.
Australian Medicines Handbook 2010. Adelaide, 2010.
Bessesen D.H. Update on obesity. Journal of Clinical Endocrinology and Metabolism. 2008;93:2027-2034.
Bray G.A. Drug treatment of obesity. Best Practice and Research Clinical Endocrinology and Metabolism. 1999;13:131-148.
Cameron A.J., Welborn T.A., Zimmet P.Z., et al. Overweight and obesity in Australia: the 1999-2000 Australian Diabetes. Obesity and Lifestyle Study (AusDiab). Medical Journal of Australia. 2003;178:427-432.
Caprio S., Daniels S.R., Drewnowski A., et al. Influence of race, ethnicity, and culture on childhood obesity: implications for prevention and treatment. A consensus statement of Shaping America’s Health and the Obesity Society. Diabetes Care. 2008;31:2211-2221.
Carek P.J., Dickerson L.M. Current concepts in the pharmacological management of obesity. Drugs. 1999;57:883-904.
Caterson I.D. Obesity and its management. Australian Prescriber. 1999;22:12-16.
Caterson I.D. What should we do about overweight and obesity? Medical Journal of Australia. 1999;171:599-600.
Gerken T., Girard C.A., Tung Y.C., et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469-1472.
Kim K.A., Song W.K., Park J.Y. Association of CYP2B6, CYP3A5, and CYP2C19 genetic polymorphisms with sibutramine pharmacokinetics in healthy Korean subjects. Clinical Pharmacology and Therapeutics. 2009;86:511-518.
Li M., Cheung B.M.Y. Pharmacotherapy for obesity. British Journal of Clinical Pharmacology. 2009;68:804-810.
Mertens I.L., Van Gaal L.F. Promising new approaches to the management of obesity. Drugs. 2000;60:1-9.
New Zealand Ministry of Health. A Portrait of Health: Key Results of the 2006/07 New Zealand Health Survey. Wellington: Ministry of Health; 2007.
National Health Medical Research Council of Australia. Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults. Canberra: NHMRC; 2003.
Patel P.N., Pathak R. Rimonabant: a novel selective cannabinoid-1 receptor antagonist for the treatment of obesity. American Journal Health-System Pharmacy. 2007;64:481-489.
Steppan C.M., Bailey S.T., Bhat S., et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-312.
Wadden T.A., Sarwer D.B., Berkowitz R.I. Behavioural treatment of the overweight patient. Best Practice and Research Clinical Endocrinology and Metabolism. 1999;13:93-107.
World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Technical Report Series 894 on Obesity. Geneva: WHO, 2000.
Zhang Y., Proenca R., Maffei M., et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432.