Assessment of Muscles Innervated by Cranial Nerves*
INTRODUCTION TO TESTING AND GRADING
Muscles innervated by the cranial nerves are not amenable to the classic methods of manual muscle testing and grading. In many, if not most, cases they do not move a bony lever, so manual resistance as a means of evaluation of their strength and function is not always the primary procedure.
The therapist needs to become familiar with the cranial nerve muscles in normal persons. Their appearance, strength, excursion, and rate of motion are all variables that are unlike the other skeletal muscles, which are more familiar. As for the infant and young child, the best way to assess the gross function of their muscles is to observe the child while crying or sucking, for example. In any event, experience with assessment requires considerable practice with both normal persons and a wide variety of patients with suspected and known cranial nerve motor deficits emanating from both upper and lower motor neuron lesions.
An anecdote from the personal experience of one of the authors (JM) involves a patient who was being evaluated for bulbar function because of a motor neuron disease. A “strange” structure appeared in the back of the throat when the patient opened wide to say “Ah-h-h.” As it turned out, there was no tumor, no foreign object, and no structural deformity. The “strange” structure was the epiglottis, not commonly observed in many people.
The issue of symmetry is particularly important in testing the ocular, facial, tongue, jaw, pharyngeal, and palate muscles. The symmetry of these muscles, except for the laryngeal muscles, is visible to the examiner. Asymmetry is more readily detected merely by observation in these muscles (in contrast to the limb muscles) and should always be documented.
In all tests in this chapter, the movements or instructions may not be entirely familiar to the patient, so each test should be demonstrated and the patient should be allowed to practice. In the presence of unusual or unexpected test results, the examiner should inquire about prior facial reconstructive (e.g., cosmetic) surgery.
General Grading Procedures
The distinction to be made in testing the muscles described in this chapter is to ascertain their relative functional level with respect to their intended activity. The scoring system, therefore, is a functional one, and motions or functions are graded as follows:
F: Functional; appears normal or only slight impairment.
WF: Weak functional; moderate impairment that affects the degree of active motion.
Universal Precautions in Bulbar Testing
In testing the muscles of the head, oral cavity, and throat the examiner frequently encounters body fluids such as saliva, tears, and bronchotracheopharyngeal secretions. The precaution of wearing gloves should always be followed. If the patient has any infectious disease or if there are copious secretions, the examiner should be masked and gowned as well as gloved.
The examiner should be cautious about standing directly in front of a patient who has been instructed to cough. This also is true in the case of the patient who has an open tracheostomy.
When a tongue blade is used, it should be sterile and care should be used about where it is placed between tests on a given patient.
Patient and Examiner Positions for All Tests
The short sitting position is preferred. The head and trunk should be supported as necessary to maintain normal alignment or to accommodate deformities. If the patient cannot sit for any reason, use the supine position, which will not influence testing of the head and eye muscles. When the muscles of the oral cavity and throat are tested, however, the head should be elevated. The examiner stands or sits in front of the patient but slightly to one side. A stool on casters is preferred so the therapist can move about the patient quickly and efficiently.
EXTRAOCULAR MUSCLES
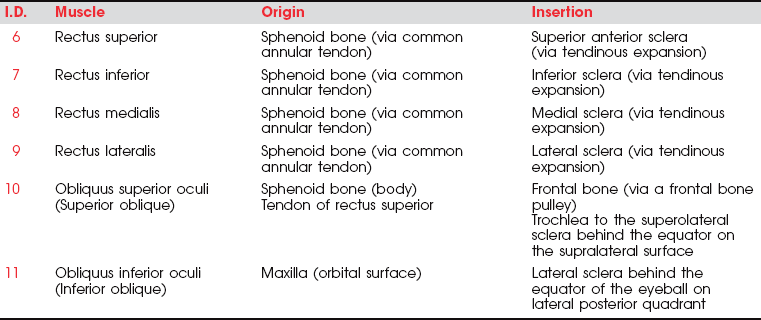
The six extraocular muscles of the eye (Figure 7-1 and Figure 7-2) move the eyeball in directions that depend on their attachments and on the influence of the movements themselves. It is probable that no muscle of the eye acts independently, and because these muscles cannot be observed, palpated, or tested individually, much of the knowledge of their function is derived from some variety of dysfunction. The extraocular muscles are innervated by cranial nerves III (oculomotor), IV (trochlear), and VI (abducent) (Figure 7-3).
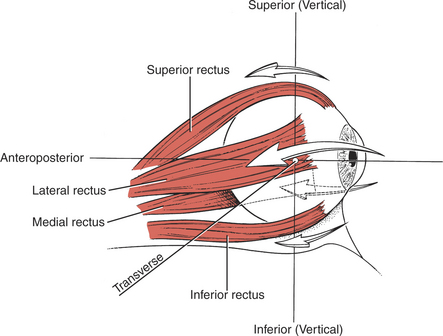
FIGURE 7-1
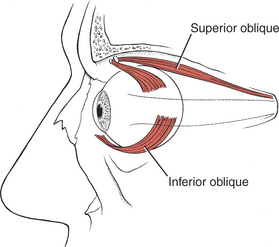
FIGURE 7-2
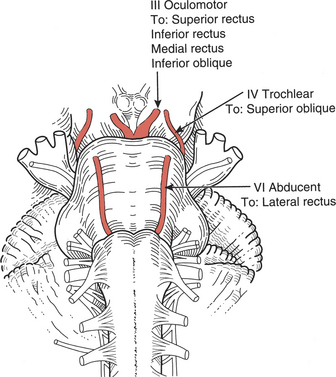
FIGURE 7-3
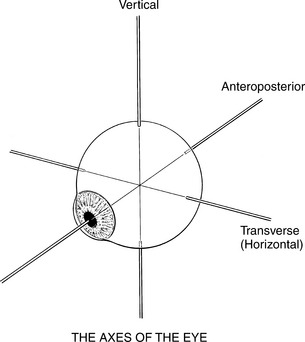
The Axes of Eye Motion
The eyeball rotates in the orbital socket around one or more of three primary axes (Figure 7-4), which intersect in the center of the eyeball.1
Vertical axis: Around this axis the lateral motions (abduction and adduction) take place in a horizontal plane.
Transverse axis: This is the axis of rotation for upward and downward motions.
Anteroposterior axis: Motions of rotation in the frontal plane occur around this axis.
The neutral position of the eyeball occurs when the gaze is straight-ahead and far away. In this neutral position, the axes of the two eyes are parallel. Normally, the motions of the two eyes are conjugate, that is, coordinated, and the two eyes move together.
Eye Motions
The extraocular muscles seem to work as a continuum; as the length of one changes, the length and tension of the others are altered, giving rise to a wide repertoire of movement.2,3 Despite this continuous commonality of activity, the function of the individual muscles can be simplified and understood in a manner that does not detract from accuracy but simplifies the test procedure.
Conventional clinical testing assigns the following motions to the various extraocular muscles1–3 (Figure 7-5):
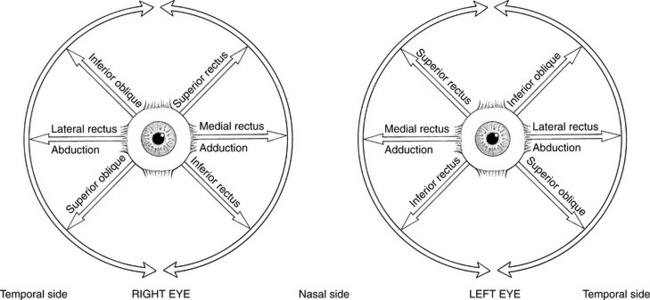
FIGURE 7-5 Extraocular muscles and their actions. The six extraocular muscles enable each eye to move in a circular arc, usually accompanied by head movements, though head position is static during testing. The traditional pairing of extraocular muscles is an oversimplification of their movement patterns. In any ocular rotation all six muscles change length. The reference point for description of the motions of the extraocular muscles is the center of the cornea.
6. Rectus superior (III, Oculomotor)
Primary Movement: Elevation of the eyeball; movement is upward and inward.
1. Rotation of the adducted eyeball so the upper end of the vertical axis is inward (see Figure 7-4).
7. Rectus inferior (III, Oculomotor)
Primary Movement: Depression of the eyeball; movement is downward and inward.
9. Rectus lateralis (VI, Abducent)
Primary Movement: Abduction of the eyeball.
Secondary Movements: None. VI nerve lesions limit lateral movement. In paralysis the eyeball is turned medially and cannot be abducted.
11. Obliquus inferior (III, Oculomotor)
Primary Movement: Elevation of the eye, particularly from adduction; movement is upward and outward.
2. Rotation of the eyeball so the vertical axis is outward.
3. Note: In paralysis the eyeball is deviated downward and somewhat laterally; it cannot move upward when in abduction.
4. Note: In a III nerve lesion, the eye is outward and cannot be brought in. (This is often referred to irreverently as the “bum’s eye,” that is, down-and-out.) Such a lesion also results in ptosis, or drooping, of the upper eyelid.2,3
Eye Tracking
Eye movements are tested by having the patient look in the cardinal directions (numbers in parentheses refer to tracks shown in Figure 7-6).2 All pairs in tracking are antagonists.
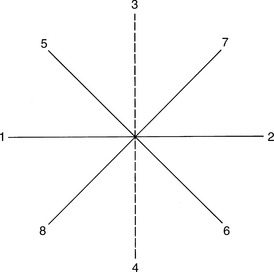
FIGURE 7-6
Ask the patient to follow the examiner’s slowly moving finger (or a pointer or flashlight) in each of the following tests. The object the patient is to follow should be at a comfortable reading distance. First, one eye is tested and then the other, covering the nontest eye. After single testing, both eyes are tested together for conjugate movements. Each test is started in the neutral position of the eye.
The range, speed, and smoothness of the motion should be observed as well as the ability to sustain lateral and vertical gaze.2–4 The physical therapist will not be able to use these observational methods to distinguish movement deviations accurately because accuracy requires the sophisticated instrumentation used in ophthalmology. The tracking movements will appear normal or abnormal, but little else will be possible.
Position of Patient: Head and eyeball in neutral alignment, looking straight-ahead at examiner’s finger to start. Head must remain static. If the patient turns the head while tracking the examiner’s finger, the head will have to be held still with the examiner’s other hand or by an assistant.
Instructions to Patient: “Look at my finger. Follow it with your eyes” (Figure 7-7).
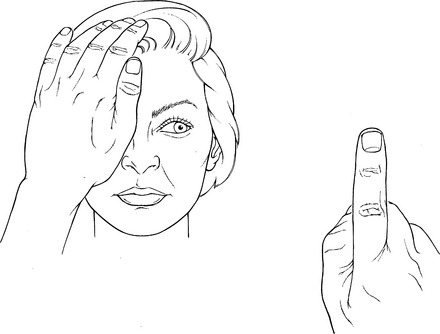
FIGURE 7-7
Test: Test each eye separately by covering first one eye and then the other. Then test both eyes together.
Examples of two bilateral tests show conjugate motion in the two eyes when tracking upward and to the right (Figure 7-8) and when tracking downward and to the left (Figure 7-9).

FIGURE 7-8 Patient tracks upward and to the right. The patient’s right eye shows motion principally with the superior rectus; the left eye shows motion principally with the inferior oblique.

FIGURE 7-9 Patient tracks downward and to the left. The right eye movement reflects principally the superior oblique; the left eye shows motion principally with the inferior rectus.
MUSCLES OF THE FACE AND EYELIDS
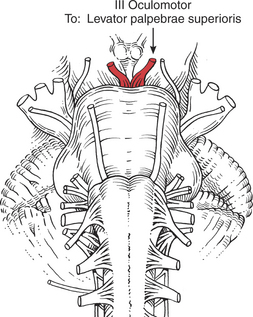
FIGURE 7-11
The face should be observed for mobility of expression, and any asymmetry or inadequacy of muscles should be documented. A one-sided appearance when talking or smiling, a lack of tone (with or without atrophy), the presence of fasciculations, asymmetrical or frequent blinking, smoothness of the face, or excessive wrinkling are all clues to VII nerve involvement.
The facial muscles (except for motions of the jaw) convey all emotions via voluntary and involuntary movements.
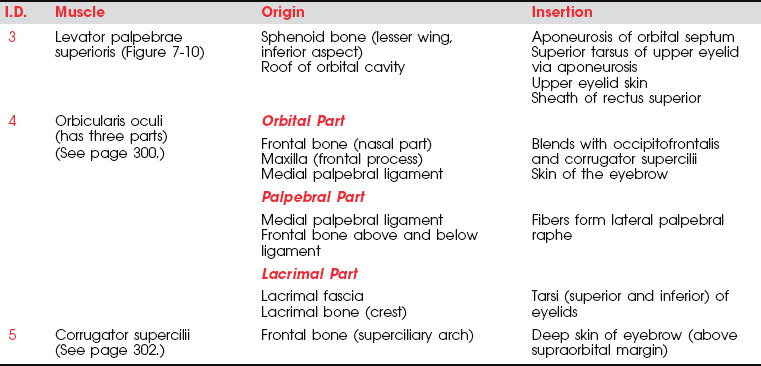
Eye Opening (3. Levator palpebrae superioris)
Opening the eye by raising the upper eyelid is a function of the levator palpebrae superioris (see Figure 7-10). The muscle should be evaluated by having the patient open and close the eye with and without resistance. The function of this muscle is assessed by its strength in maintaining a fully opened eye against resistance.
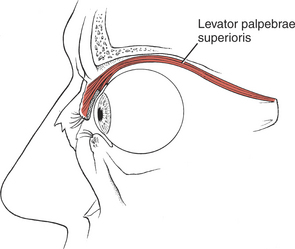
FIGURE 7-10
The patient with an oculomotor (III) nerve lesion will lose the function of the levator muscle, and the eyelid will droop in a partial or complete ptosis. (A patient with cervical sympathetic pathology may have a ptosis but will be able to raise the eyelid voluntarily.) Ptosis is evaluated by observing the amount of the iris that is covered by the eyelid.
In the presence of a facial (VII) nerve lesion, the levator sign may be present.2 In this case, the patient is asked to look downward and then slowly close the eyes. A positive levator sign is noted when the upper eyelid on the weak side moves upward because the action of the levator palpebrae superioris is unopposed by the orbicularis oculi.
Test: Patient attempts to keep the eyelids open against manual resistance (Figure 7-12). Both eyes are tested at the same time. NEVER PRESS ON THE EYEBALL FOR ANY REASON!
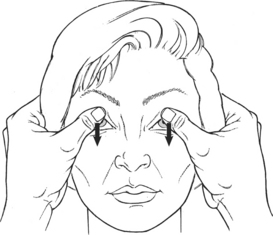
FIGURE 7-12
Manual Resistance: The thumb or index finger is placed lightly over the opened eyelid above the lashes, and resistance is given in a downward direction (to close the eye). The examiner is cautioned to avoid depressing the eyeball into the orbit while giving resistance.
Instructions to Patient: “Open your eyes wide. Hold them. Don’t let me close them.”
Criteria for Grading
F: Completes normal range of movement and holds against examiner’s light manual resistance. Iris will be fully visible.
WF: Can open eye but only partially uncovers the iris and takes no resistance. Patient may alternately open and close the lids, but excursion is small. The frontalis muscle also may contract as the patient attempts to open the eye.
NF: Unable to open the eye, and the iris is almost completely covered.
Closing the Eye (4. Orbicularis oculi)
The orbicularis oculi muscle is the sphincter of the eye1 (Figure 7-13). Its lids are innervated by the facial (VII) nerve (temporal branch and zygomatic branch) (Figure 7-14 and Figure 7-15). Its palpebral portion closes the eyelids gently, as in blinking and sleep. The orbital portion of the muscle closes the eyes with greater force, as in winking. The lacrimal portion draws the eyelids laterally and compresses them against the sclera to receive tears. All portions act to close the eyes tightly (Figure 7-16). Observation of the patient without specific testing will detect weakness of the orbicularis because the blink will be delayed on the involved side.
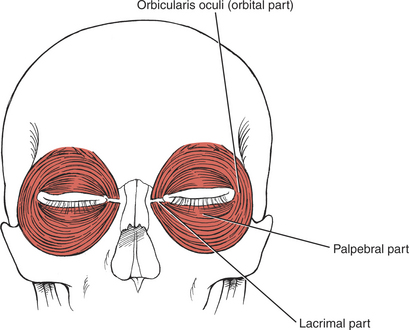
FIGURE 7-13
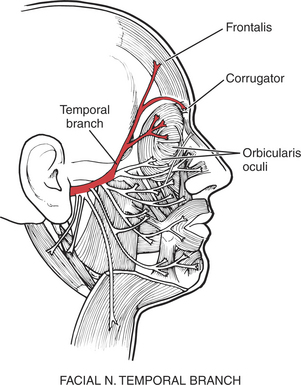
FIGURE 7-14
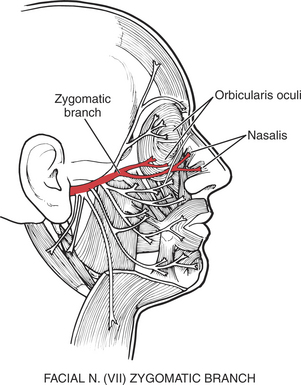
FIGURE 7-15

FIGURE 7-16
Test: Observe the patient opening and closing the eyes voluntarily, first together and then singly (Figure 7-16). (Single-eye closing is not a universal skill.) Patient closes eyes tightly, first together, then singly.
Rather than using resistance, the examiner may look at the depth to which the eyelashes are buried in the face when the eyes are closed tightly, noting whether the lashes are deeper on the uninvolved side.
Manual Resistance: Place the thumb and index finger below and above (respectively) each closed eye using a light touch (Figure 7-17). The examiner attempts to open the eyelids by spreading the thumb and index finger apart. REMINDER: NEVER PRESS ON THE EYEBALL FOR ANY REASON.
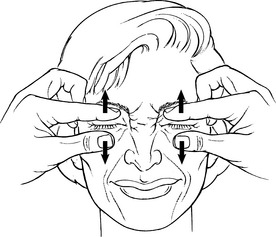
FIGURE 7-17
Instructions to Patient: “Close your eyes as tightly as you can. Hold them closed. Don’t let me open them.” OR “Close your eye against my finger.”
Criteria for Grading
F: Closes eyes tightly and holds against examiner’s resistance. Iris may not be visible.
WF: Takes no resistance to eye closure; closure may be incomplete, but only a small amount of the sclera and no iris should be visible. There may be closure of the eye, but the eyelid on the weaker side may be delayed in contrast to the quick closure on the normal side.
NF: Unable to close eyes so that the iris is completely covered. (These patients may need artificial eyedrops to prevent drying of the eye.)
Frowning (5. Corrugator supercilii)
To observe the action of the corrugator muscle (Figure 7-18; see also Figure 7-14), the patient is asked to frown. Frowning draws the eyebrows down and medially, producing vertical wrinkling of the forehead.
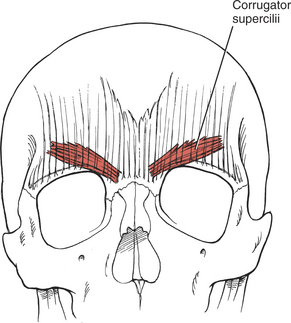
FIGURE 7-18
Test: Patient is asked to frown; the eyebrows are drawn down and together (Figure 7-19).

FIGURE 7-19
Manual Resistance: The examiner uses the thumb (or index finger) of each hand placed gently at the nasal end of each eyebrow and attempts to move the eyebrows apart (smooths away the frown) (Figure 7-20).

FIGURE 7-20
Raising the Eyebrows (1. Occipitofrontalis, frontalis part)
To examine the frontal belly of the occipitofrontalis muscle (Figure 7-21 and see Figure 7-14), the patient is asked to create an expression of surprise where the forehead skin wrinkles horizontally. The occipital belly of the muscle is not tested usually, but it draws the scalp backward.
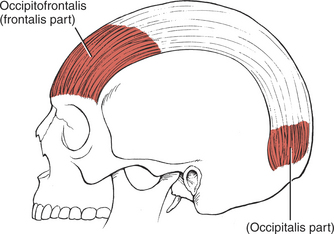
FIGURE 7-21
Test: Patient raises the eyebrows so that horizontal forehead lines appear (Figure 7-22).
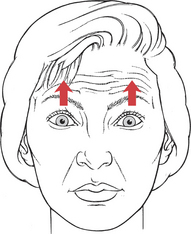
FIGURE 7-22
Manual Resistance: Examiner places the pad of a thumb above each eyebrow and applies resistance in a downward direction (smoothing the forehead) (Figure 7-23).
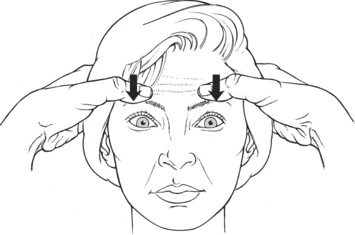
FIGURE 7-23
Instructions to Patient: “Raise your eyebrows as high as you can. Don’t let me pull them down.”
NOSE MUSCLES
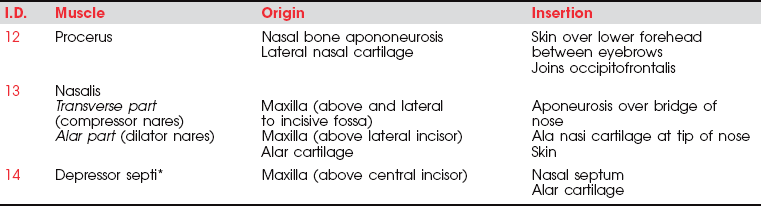
The three muscles of the nose are all innervated by the facial (VII) nerve. The procerus (Figure 7-24) draws the medial angle of the eyebrows downward, causing transverse wrinkles across the bridge of the nose. The nasalis (compressor nares) depresses the cartilaginous portion of the nose and draws the ala down toward the septum (see Figure 7-15). The nasalis (dilator nares) dilates the nostrils. The depressor septi draws the alae downward, constricting the nostrils.
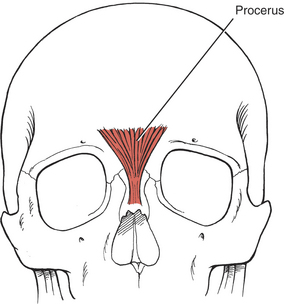
FIGURE 7-24
Of the three nose muscles only the procerus is tested clinically. The others are observed with respect to nostril flaring and narrowing in patients who have such talent.
Wrinkling the Bridge of the Nose (12. Procerus)
Test: Patient wrinkles nose as if expressing distaste (Figure 7-26).

FIGURE 7-26
Manual Resistance: The pads of the thumbs are placed beside the bridge of the nose, and resistance is given laterally (smoothing the creases) (Figure 7-27).
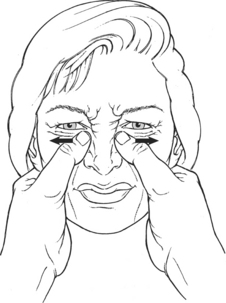
FIGURE 7-27
Instructions to Patient: “Wrinkle your nose as if to say ‘yuck’.”
MUSCLES OF THE MOUTH AND FACE
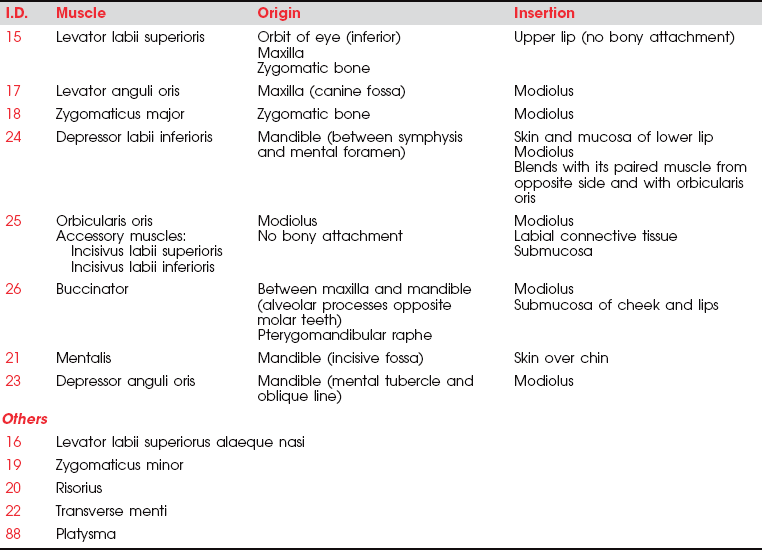
There are many muscles associated with the mouth, and all have some distinctive function, except perhaps the risorius. Rather than detail a test for each, only definitive tests will be presented for the buccinator and the orbicularis oris (the sphincter of the mouth). The function of the remaining muscles is illustrated, and individual testing is left to the examiner. All muscles of the mouth are innervated by the facial (VII) nerve.
Lip Closing (25. Orbicularis oris)
This circumoral muscle (see Figure 7-28 and Figure 7-29) serves many functions for the mouth. It closes the lips, protrudes the lips, and holds the lips tight against the teeth. Furthermore, it shapes the lips for such functional uses as kissing, whistling, sucking, drinking, and the infinite shaping for articulation in speech. (For innervation, see Figure 7-25.)
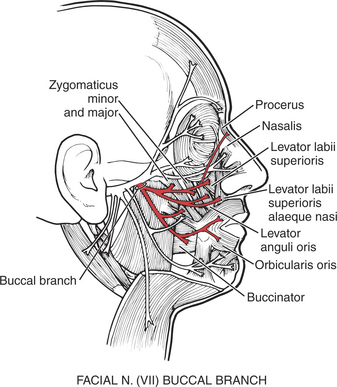
FIGURE 7-25
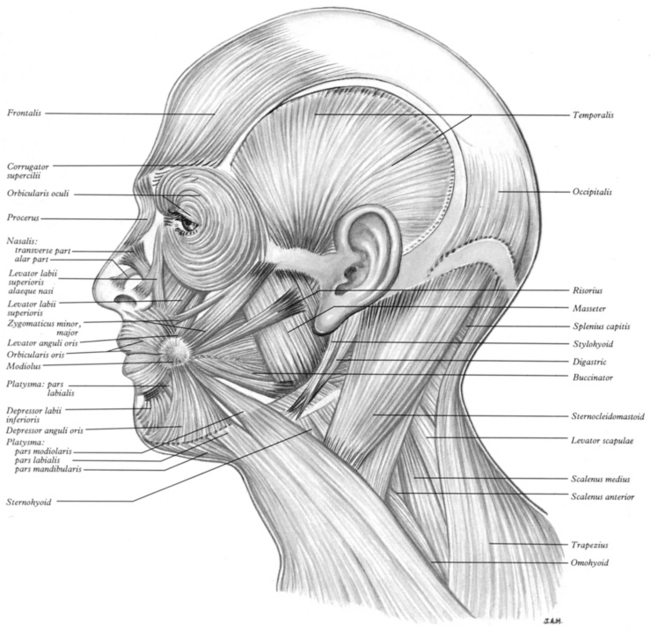
FIGURE 7-28 Muscles of the head and neck. (From Williams PL, Warwick R, Dyson M, Bannister LH [eds]. Gray’s Anatomy, 38th ed. New York: Churchill Livingstone, 1995.)
Churchill Livingstone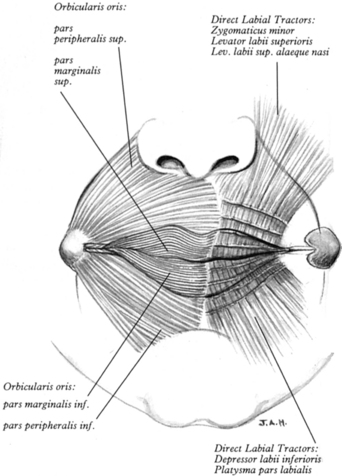
FIGURE 7-29 The disposition of the modiolus and orbicularis oris pars peripheralis and pars marginalis (on the left); the successively transected laminae of the direct labial tractors on both upper and lower lips (on the right). (From Standring S, Ellis H, Healy J et al [eds]. Gray’s Anatomy, 39th ed. New York: Churchill Livingstone, 2005.)
Churchill LivingstoneTest: Patient compresses and protrudes the lips (Figure 7-30).

FIGURE 7-30
Resistance: In deference to hygiene, a tongue blade rather than a finger is used to provide resistance. The flat side of the blade is placed diagonally across both the upper and lower lips, and resistance is applied inward toward the oral cavity (Figure 7-31).
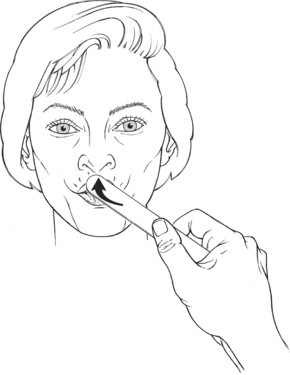
FIGURE 7-31
Instructions to Patient: “Purse your lips. Hold it. Push against the tongue blade.”
Cheek Compression (26. Buccinator)
The buccinator (see Figure 7-28) is a prime muscle used for positioning food for chewing and for controlling the passage of the bolus. It also compresses the cheek against the teeth and acts to expel air when the cheeks are distended (blowing). (For innervation, see Figure 7-25.)
Test: Patient compresses the cheeks (bilaterally) by drawing them into the mouth (Figure 7-32).
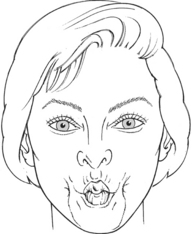
FIGURE 7-32
Resistance: A tongue blade is used for resistance. The blade is placed inside the mouth, its flat side lying against the cheek (Figure 7-33). Resistance is given by levering the blade inward against the cheek (at the angle of the mouth), which will cause the flat blade to push the test cheek outward.

FIGURE 7-33
Alternatively, the gloved index fingers of the examiner may be used to offer resistance. In this case, the index fingers are placed in the mouth (the left finger to the inside of the patient’s left cheek and vice versa). The fingers are used simultaneously to try to push the cheeks outward. Use caution in this form of the test for patients with cognitive impairment (lest they bite!) or with those who have a bite reflex.
Instructions to Patient: “Suck in your cheeks. Hold. Don’t let me push them out.”
Other Oral Muscles
17. Levator anguli oris (for innervation see Figure 7-25)
This muscle elevates the angles of the mouth and reveals the teeth in smiling. When used unilaterally, it conveys the expression of sneering (Figure 7-34). The muscle creates the nasolabial furrow, which deepens in expressions of sadness and with aging.

FIGURE 7-34
15. Levator labii superioris (see Figure 7-25, Figure 7-28, and Figure 7-29)
This muscle raises and pushes out the upper lip and modifies the nasolabial fold (or furrow), which runs from the end of the nose to flatten out over the cheek. It is a prominent feature of the subnasal area in many people and deepens in sadness and sometimes anger.
16. Levator labii superioris alaeque nasi (see Figure 7-25)
These two levator labii muscles (15 and 16) elevate the upper lip (Figure 7-35). The labii superioris also protracts the upper lip, and the alaeque nasi dilates the nostrils.
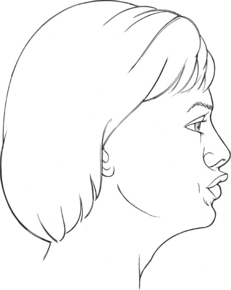
FIGURE 7-35
18. Zygomaticus major (see Figure 7-28)
The major zygomaticus muscles draw the angles of the mouth upward and laterally as in laughing (Figure 7-36).

FIGURE 7-36
21. Mentalis (see Figure 7-37)
The mentalis protrudes the lower lip, as in pouting or sulking (Figure 7-38).
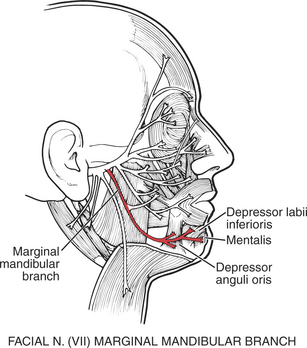
FIGURE 7-37
FIGURE 7-38
23. Depressor anguli oris (see Figure 7-37)
The depressor anguli oris crosses the midline to meet with its fellow muscles of the opposite side, forming the “mental sling.” It draws down the angle of the mouth, giving an appearance of deep sadness (see Figure 7-38).
88. Platysma
These muscles depress the lower lip and the buccal angle of the mouth to give an expression of grief or sadness (Figure 7-39). The platysma draws the lower lip backward, producing an expression of horror, and it pulls up the skin of the neck from the clavicle (evoking the expression of “egad!”). This muscle may be tested by asking the patient to open the mouth against resistance or bite the teeth together tightly (see also Figure 7-40).
FIGURE 7-39
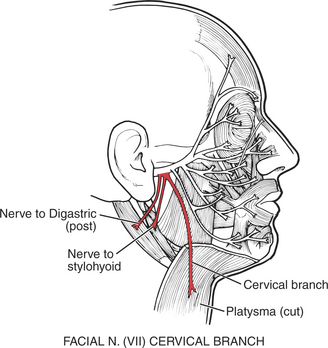
FIGURE 7-40
24. Depressor labii inferioris
This muscle draws the lower lip down and laterally, producing an expression of melancholy or irony (Figure 7-41).

FIGURE 7-41
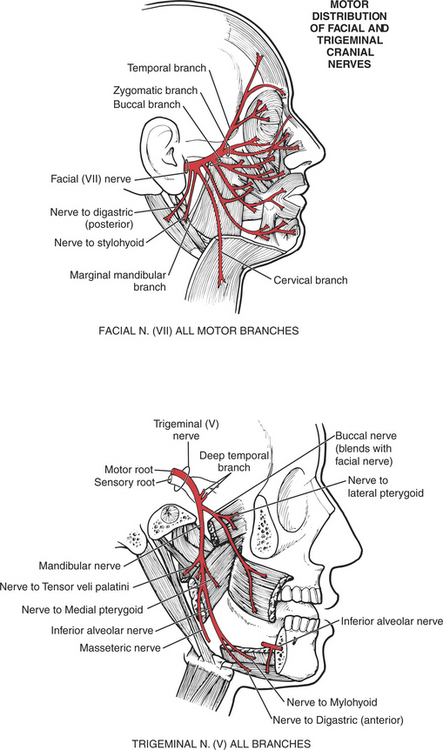
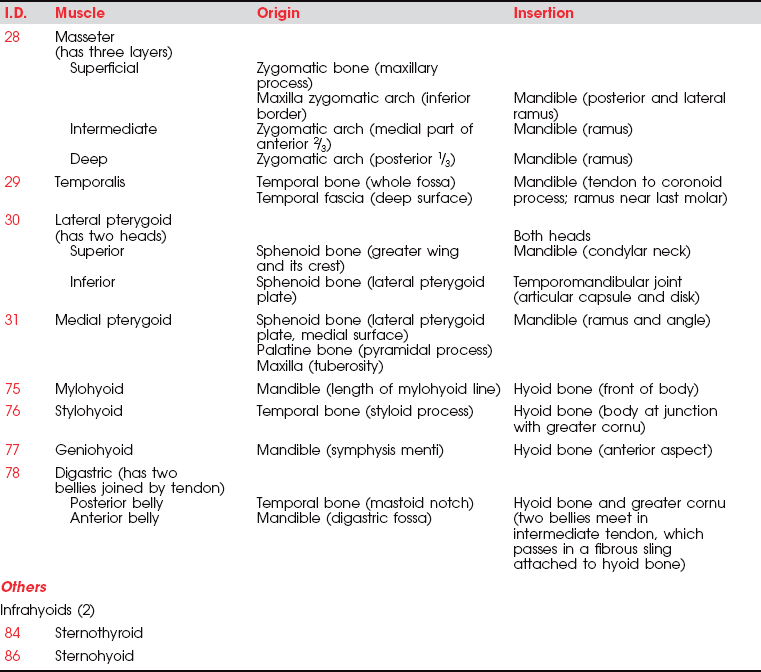
MUSCLES OF MASTICATION
Muscles of Mastication (28. Masseter, 29. Temporalis, 30. Lateral pterygoid, 31. Medial pterygoid)
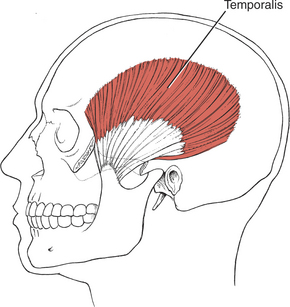
FIGURE 7-42
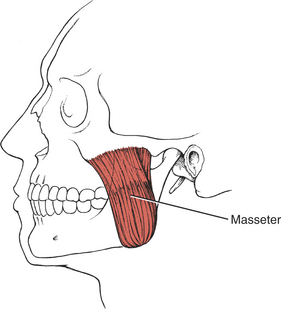
FIGURE 7-43
The mandible is the only moving bone in the skull, and mandibular motion is largely related to chewing and speech. The muscles that control the jaw are all near the rear of the mandible (on the various surfaces and processes of the ramus), where they contribute considerable force for chewing and biting.1 The muscles of mastication move the mandible forward (protraction) and backward (retraction), as well as shift it laterally. Excursion of the mandible is customarily limited somewhat, except in trained singers, who learn to open the mouth very wide to add to their vocal repertoire. The velocity of motions used for chewing is relatively slow, but for speech motions it is very rapid.
The muscles of mastication are all innervated by the motor division of cranial nerve V (trigeminal) (see Plate 8, page 299). The masseter elevates and protrudes the mandible. The temporalis elevates and retracts the mandible. The lateral pterygoids (Figure 7-44), acting in concert, protrude and depress the mandible; when one acts alone, it causes lateral movement to the opposite side. The medial pterygoids (see Figure 7-44) acting together elevate and protrude the mandible along with the lateral pterygoids, but acting alone they draw the mandible forward with deviation to the opposite side (as in chewing). The suprahyoid muscles (see Figure 7-45 and Figure 7-46), acting via the hyoid bone, aid in jaw depression when the hyoid is fixed. The infrahyoids are weak accessories to jaw depression.
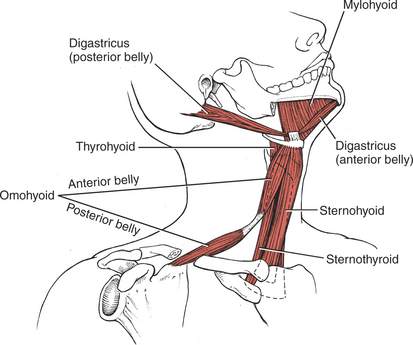
FIGURE 7-45
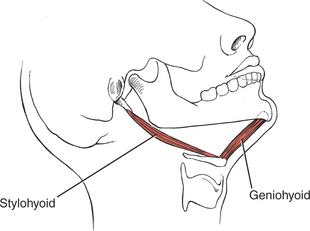
FIGURE 7-46
Lesions of the motor division result in weakness or paralysis of the motions of elevating, depressing, protruding, and rotating the mandible. In a unilateral lesion, the jaw deviates to the weak side; in a bilateral lesion, the jaw sags and is “paralyzed.” The jaw should be examined for muscle tone, atrophy (jaw contour), and fasciculations.
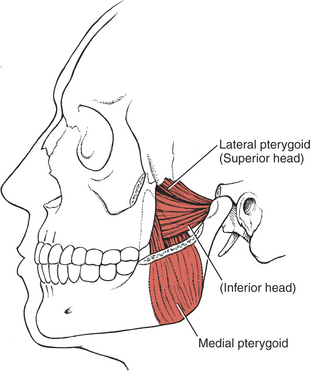
FIGURE 7-44
Jaw Opening (Mandibular Depression) (30. Lateral pterygoid, 75-78. Suprahyoid Muscles)
Note: Before testing the jaw muscles, the temporomandibular joint should be checked for tenderness and crepitus. If either is present, manual testing is avoided, and jaw opening and closing are simply observed.
Test: Patient opens the mouth as far as possible and holds against manual resistance.
Manual Resistance: One hand of the examiner is cupped under the chin; the other hand is placed on the crown of the head for stabilization (Figure 7-47). Resistance is given in a vertical upward direction in an attempt to close the jaw.
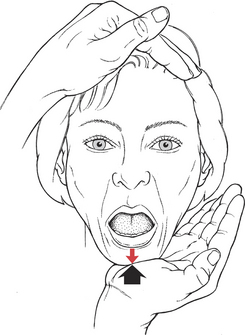
FIGURE 7-47
Instructions to Patient: “Open your mouth as wide as you can. Hold it. Don’t let me close it.”
Criteria for Grading
F: Completes available range and holds against strong resistance. Indeed, this muscle is so powerful that in the normal person it can rarely be overcome with manual resistance. The mouth opening should accommodate three (sometimes four) stacked fingers (in an average-sized person), or 35 to 40mm. There should be no deviation except downward.
WF: Can open mouth to accommodate two or fewer stacked fingers and can take some resistance.
NF: Minimal motion occurs. The lateral pterygoid can be palpated with a gloved finger inside the mouth, with the tip directed posteriorly past the last upper molar to the condyloid process of the ramus of the mandible. No resistance is tolerated.
Jaw Closure (Mandibular Elevation) (28. Masseter, 29. Temporalis, 31. Medial pterygoid)
Test: Patient clenches jaws tightly (for innervation, see Plate 8, page 299).
Manual Resistance: The chin of the patient is grasped between the thumb and index finger of the examiner and held firmly in the thumb web. The other hand is placed on top of the head for stability. Resistance is given vertically downward in an attempt to open the closed jaw (Figure 7-48).

FIGURE 7-48
Instructions to Patient: “Clench (or hold) your teeth together as tightly as you can, keeping your lips relaxed. Hold it. Don’t let me open your mouth.”
Criteria for Grading
F: Patient closes mouth (jaw) tightly. Examiner should not be able to open the mouth. This is a very strong muscle group. Consider circus performers who hang by their teeth!
WF: Patient closes jaw, but examiner can open the mouth with less than maximal resistance.
NF: Patient closes mouth but tolerates no resistance. The masseter and temporalis muscles are palpated on both sides. The masseter is palpated under the zygomatic process on the lateral cheek above the angle of the jaw. The temporalis muscle is palpated over the temple at the hairline, anterior to the ear and superior to the zygomatic bone.
O: Patient cannot completely close the mouth. This is more of a cosmetic problem (drooling, for example) than a significant clinical one.
In unilateral involvement, the jaw deviates to the strong side during attempts to close the mouth.
Alternate Test Procedure: The patient is asked to bite hard on a tongue blade with the molar teeth. Comparison of the depth of the bite marks from each side of the jaw is an indication of strength. If the examiner can pull out the tongue blade while the patient is biting, there is weakness of the masseter, temporalis, and lateral pterygoid muscles. (Note: This method of testing should never be used with a patient who has a bite reflex because the patient may break the blade and be injured by the splinters.)
Lateral Jaw Deviation (30. Lateral pterygoid, 31. Medial pterygoid)
When the patient deviates the jaw to the right, the acting muscles are the right lateral pterygoid and the left medial pterygoid. Deviation to the left is supported by the left lateral pterygoid and the right medial pterygoid.
With weakness of the pterygoids, when the patient opens the mouth there will be deviation to the side of the weakness.
The patient moves the jaw side to side against resistance. In V (trigeminal) nerve involvement, the patient can move the jaw to the paralyzed side but not to the unaffected side.
Test: Patient deviates jaw to the right and then to the left (for innervation, see Plate 8, page 299).
Manual Resistance: One hand of the examiner is used for resistance and is placed with the palmar side of the fingers against the jaw (Figure 7-49). The other hand is placed with the fingers and palm against the opposite temple to stabilize the head. Resistance is given in a lateral direction to move the jaw toward the midline.
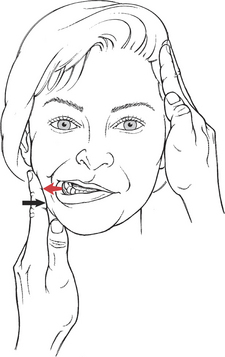
FIGURE 7-49
Criteria for Grading
F: The range of motion for jaw lateral deviation is variable. Deviation is assessed by comparing the relationship between the upper and lower incisor teeth when the jaw is moved laterally from the midline. Do not assess deviation by the position of the lips. A pencil or ruler lined up vertically with the center of the nose may indicate mandibular deviation.
Most people can move the center point of the lower incisors laterally over three upper teeth (approximately 10mm).5 The patient tolerates strong resistance.
W: Motion is decreased to lateral movement across one upper tooth, and resistance is minimal.
Jaw Protrusion (30. Lateral pterygoids, 31. Medial pterygoids)
The medial and lateral pterygoids act to protrude the jaw, which gives the face a pugnacious expression. The protrusion causes a malocclusion of the teeth, the lower teeth projecting beyond the upper teeth. With a unilateral lesion, the protruding jaw deviates to the weak side.
Test: Patient protrudes jaw so the lower teeth project beyond the upper teeth (for innervation, see Plate 8, page 299).
Manual Resistance: This is a powerful motion. The examiner stabilizes the head with one hand placed behind the head (Figure 7-50). The hand for resistance cups the chin in the thumb web with the thumb and index finger grasping the mandible. Resistance is given horizontally backward.
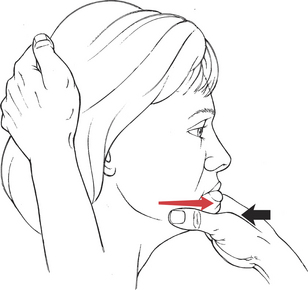
FIGURE 7-50
Instructions to Patient: “Push your jaw forward. Hold it. Don’t let me push it back.”
Criteria for Grading
F: Completes a range that moves the lower teeth in front of the upper teeth and can hold against strong resistance. There is sufficient space between the teeth in most people to see a gap between the upper and lower teeth.
WF: Moves jaw slightly forward but there is no discernible gap between the upper and lower teeth, and the patient tolerates only slight resistance.
NF: Minimal motion is detected, and the patient takes no resistance.
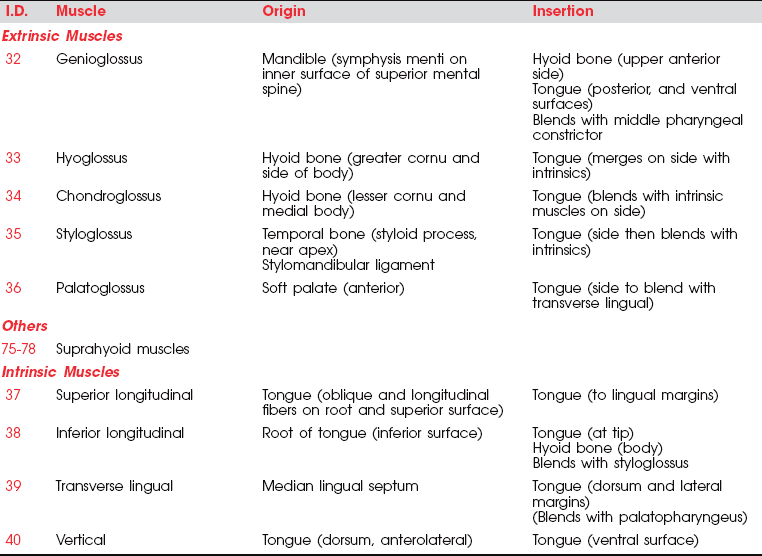
MUSCLES OF THE TONGUE
The extrinsic and intrinsic muscles of the tongue, except the palatoglossus, are innervated by the hypoglossal (XII) cranial nerve, a pure motor nerve. One XII nerve innervates half of the tongue (unilaterally). The hypoglossal nucleus, however, receives both crossed (mostly) and uncrossed (to a lesser extent) upper motor neuron fibers from the lowest part of the precentral gyrus via the internal capsule. Lesions of the XII nerve or its central connections may cause tongue paresis or paralysis.
Description of Tongue Muscles
The paired extrinsic muscles pass from the skull or the hyoid bone to the tongue. The intrinsic muscles rise and end within the tongue. The bulk of the tongue structure is muscle.
The principal muscle of the tongue is the genioglossus. It is a triangular muscle whose apex arises from the apex of the mandible, which is hard and immobile; its base inserts into the base of the tongue, which is soft and mobile. The genioglossus (Figure 7-51) is the principal tongue protractor, and it has crossed supranuclear innervation. The posterior fibers of the paired genioglossi draw the root of the tongue forward; a single genioglossus pushes the tongue toward the opposite side. The anterior fibers of the paired muscles draw the tongue back into the mouth after protrusion and depress it. The genioglossi acting together also depress the central part of the tongue, making it a tube.
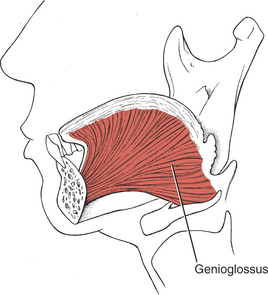
FIGURE 7-51
The hyoglossi (paired) (Figure 7-52) and the chondroglossi retract and depress the sides of the tongue, making the superior surface convex. The two styloglossi (see Figure 7-52) draw the tongue upward and backward and elevate the sides, causing a dorsal transverse concavity.
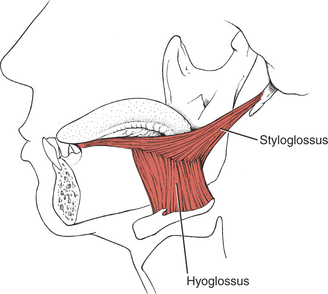
FIGURE 7-52
The suprahyoid muscles influence the movements of the tongue via their action on the hyoid bone.
The intrinsic tongue muscles (Figure 7-53) are similarly innervated by the XII nerve (Figure 7-54). The superior longitudinal muscle shortens the tongue and curls its tip upward; the inferior longitudinal shortens the tongue and curls its tip downward. Their combined function is to alter the shape of the tongue in almost infinite variations to provide the tongue with the versatility required for speech and swallowing.
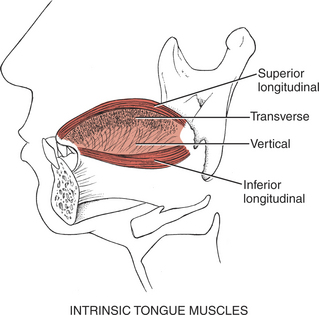
FIGURE 7-53
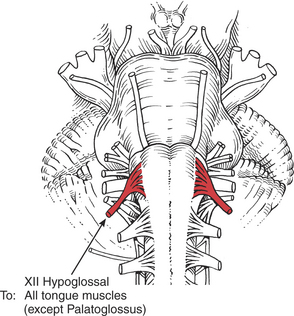
FIGURE 7-54
One test of a tongue motion used by therapists is called “channeling” in which the tongue is curled longitudinally; this motion may be considered to assist in sucking and directing the bolus of food into the pharynx. The difficulty presented with this motion, however, is that it is not a constant motion but rather a dominantly inherited trait that only 50% of the population can perform. Testing for channeling is acceptable as long as the inability to perform the motion is not considered a neurologic deficiency.
Examination of the Tongue
The tongue is a restless muscle, and when testing it, minor deviations are best ignored.4 The test should start with observation of the tongue at rest on the floor of the mouth and then with the tongue protruded. The tongue is observed as it is curled up and down over the lip and then when the margins are elevated; both motions should be performed both slowly and rapidly. In all tests the ability to change the shape of the tongue is observed, but especially in tipping and channeling. One listens for difficulty in enunciation, especially of consonants.
The examiner must become familiar with the contour and mass of the normal tongue. The tongue should be examined for atrophy, which is evidenced by decreased mass, corrugations on the sides, and longitudinal furrowing. Unilateral atrophy is easy to detect and is usually accompanied by deviation to that side. When there is bilateral atrophy, the tongue will protrude weakly, if at all, and deviation also will be weak.
Fasciculations are easily visible in the tongue at rest (the surface of the tongue appears to be in constant motion) and can be separated from the normal tremulous motions that occur in the protruded tongue. The “tremors” that are a part of supranuclear lesions disappear when the tongue is at rest in the mouth, whereas the fasciculations of motor neuron disease such as amyotrophic lateral sclerosis continue. The hyperkinesias of parkinsonism are exaggerated when the tongue is protruded or during talking.
The therapist proceeds to examine protrusion and deviation of the tongue at slow and fast speeds. The normal tongue can move in and out (in the midline) with vigor and usually protrudes quite far beyond the lips.11 The tongue deviates to the side of a weakness whether the cause of that weakness is a disturbance of the upper motor neuron (supranuclear disturbance) or the lower motor neuron (infranuclear disturbance).
Unilateral Weakness of the Tongue: At rest in the mouth, the tongue with a unilateral weakness may deviate slightly to the uninvolved side because of the unopposed action of the styloglossus.11 The protruded tongue will deviate to the weak side and show weakness or inability to deviate to the normal side. Tipping may be normal because the intrinsic muscles are preserved. These functions may be impossible to evaluate if the clinical picture includes facial and jaw muscle weakness.
Early in the course of the disorder, before the onset of atrophy, the weak side of the tongue may appear enlarged and may ride higher in the mouth. After the onset of atrophy, the weak side becomes smaller, furrowed, and corrugated on the lateral edge. A unilateral weakness of the tongue may result in few functional problems, and speech and swallowing may be minimally disturbed, if at all.
Bilateral Paresis: In persons with bilateral lesions, the tongue cannot be protruded or moved laterally. There will be indistinct speech, and swallowing may be difficult. Some patients experience interference with breathing when swallowing is impaired because the tongue may fall back into the throat. Total paralysis of the tongue muscles is rare (except in brain stem lesions or advanced motor neuron disease).
Supranuclear versus Infranuclear Lesions: In the presence of a supranuclear XII nerve lesion (central), the protruded tongue will deviate to the side of the weakness, which is the side opposite to the cerebral lesion. There is no atrophy of the tongue muscles. The tongue muscles also may evidence spasticity.11
In dyskinetic states (such as athetosis, chorea, or seizures), the tongue may protrude involuntarily as well as deviate to the opposite side. This is accompanied by other generally slow involuntary tongue movements that make speech thick and slow and difficult to understand.
Patients with hemiparesis following a vascular lesion (a unilateral corticobulbar lesion) may have a variety of bulbar symptoms, including tongue muscle dysfunction. In common with other bulbar manifestations, these symptoms generally are moderate and subside with time or are well compensated, so that little functional disability persists.5 Only in patients with a second stroke or a bilateral stroke (because these muscles have bilateral cortical innervation) will the bulbar signs persist.
Inability to flick the tongue in and out of the mouth quickly (after some practice) may indicate a bilateral supranuclear lesion. In an infranuclear (peripheral) nerve lesion, the tongue will deviate to the side of the weakness, which also is the side of the lesion. There will be atrophy of the tongue muscles. Bilateral atrophy most commonly is caused by motor neuron disease. The tongue also may be weak in myasthenia gravis (fatiguing after a series of protrusions), but there will be no atrophy.
The distinction between a lower motor neuron lesion and an upper motor neuron lesion of the XII nerve depends on the presence of supporting evidence of other upper motor neuron signs and on the presence of classic lower motor neuron signs such as hemiatrophy, unilateral fasciculations, and obvious deviation to the side of the paralysis when the tongue is protruded.4
Tongue Protrusion, Deviation, Retraction, Posterior Elevation, Channeling, and Curling
Test for Protrusion (32. Genioglossus, Posterior Fibers)
Patient protrudes tongue so that the tip extends out beyond the lips.
Manual Resistance: Examiner uses a tongue blade against the tip of the tongue and provides resistance in a backward direction to the forward motion of the tongue (Figure 7-55).

FIGURE 7-55
Instructions to Patient: “Stick out your tongue. Hold it. Don’t let me push it in.”
Test for Tongue Deviation (32. Genioglossus and Other Muscles)
Patient protrudes tongue and moves it to one side and then to the other.
Manual Resistance: Using a tongue blade, resist the lateral tongue motion along the side of the tongue near the tip (Figure 7-56). Resistance is given in the direction opposite to the attempted deviation.
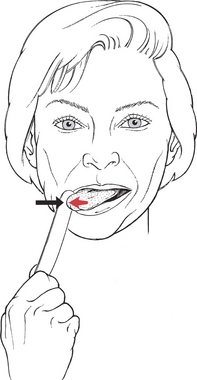
FIGURE 7-56
Instructions to Patient: “Stick out your tongue and move it to the right.” (Repeat for left side.)
Test for Tongue Retraction (32. Genioglossus [anterior fibers], 35. Styloglossus)
Patient retracts tongue from a protruded position.
Manual Resistance: Holding a 3 × 4-inch gauze pad, securely grasp the anterior tongue by its upper and under sides (Figure 7-57). Resist retraction by holding the tongue firmly and gently pulling it forward. (The tongue is very slippery, but be careful not to pinch.)
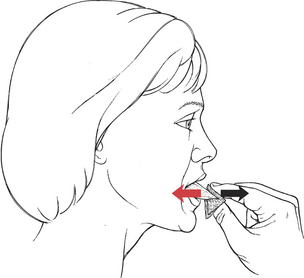
FIGURE 7-57
Instructions to Patient: (Tell patient you are going to grasp the tongue.) “Stick out your tongue. Now pull your tongue back. Don’t let me keep it out.”
Test for Posterior Elevation of the Tongue (36. Palatoglossus, 35. Styloglossus)
Patient elevates (i.e., “humps”) the dorsum of the posterior tongue.
Manual Resistance: Examiner places tongue blade on the superior surface of the tongue over the anterior one third. Placing the blade too far back will initiate an unwanted gag reflex (Figure 7-58). Resistance is applied in a down and backward direction, as in levering the tongue blade down, using the bottom teeth as a fulcrum (Figure 7-59).
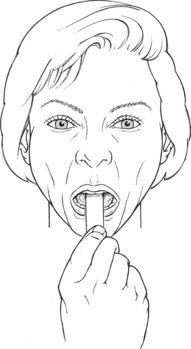
FIGURE 7-58

FIGURE 7-59
Instructions to Patient: This is a difficult motion for the patient to understand. After directions are given, time is allowed for practice.
Begin the test by rocking the tongue blade back and forth so the patient experiences pressure on the middle to the back of the tongue.
Test for Channeling the Tongue (32. Genioglossus, 37-40. Intrinsic tongue muscles)
The patient draws the tongue downward and rolls the sides up to make a longitudinal channel or tube, which is part of sucking and directing a bolus of food into the pharynx (Figure 7-60). Inability to perform this motion should not be recorded as a deficit because the motion is a dominantly inherited trait, and its presence or absence should be treated as such.

FIGURE 7-60
Instructions to Patient: Demonstrate tongue motion to the patient. “Make a tube with your tongue.”
Test for “Tipping” or Curling the Tongue (37, 38. Superior and Inferior longitudinals)
Patient protrudes tongue and curls it upward to touch the philtrum and then downward to the chin (Figure 7-61).
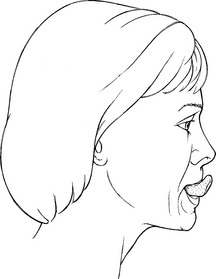
FIGURE 7-61
Instructions to Patient: “Touch above your upper lip with your tongue.”
Criteria for Grading Tongue Motions
F: Patient completes available range and holds against resistance.
Protrusion: Tongue extends considerably beyond lips.
Deviation: Tongue reaches some part of the cheek or the lateral sulcus (pocket between teeth and cheek).
Retraction: Tongue returns to rest position in mouth against resistance.
Elevation: Tongue rises so that superior surface reaches the hard palate against considerable resistance; it blocks the oral cavity from the oropharynx.
Tipping: Tongue protrudes and touches area between upper lip and nasal septum (philtrum).
Protrusion: Tongue reaches margin of lips.
Deviation: Tongue reaches corner(s) of mouth.
Retraction: Tongue returns to rest posture but with slight resistance.
Elevation: Tongue reaches hard palate with slight resistance, and oral cavity is blocked from oropharynx.
Tipping: Tongue protrudes and curls but does not reach philtrum.
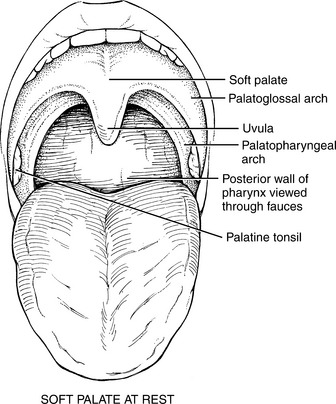
MUSCLES OF THE PALATE
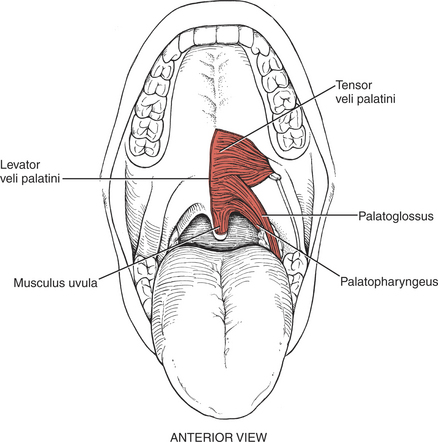
FIGURE 7-62
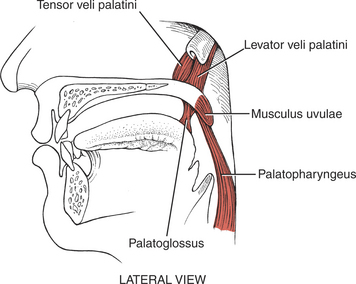
FIGURE 7-63
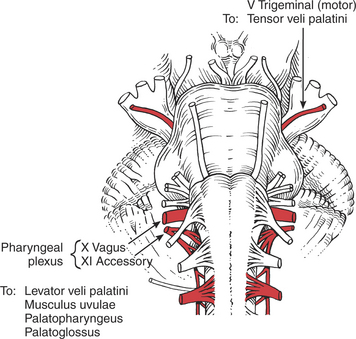
FIGURE 7-64
The muscles of the palate are innervated by the pharyngeal plexus (derived from the X [vagus] and XI [accessory] cranial nerves) (Figure 7-64), with the single exception of the tensor veli palatini, which derives its motor supply from the trigeminal (V) nerve (see Plate 8, page 299).
The tensor veli palatini elevates the soft palate, and paralysis of this muscle results in slight deviation of the uvula toward the unaffected side with its tip pointing toward the involved side. Weakness of the tensor as an elevator of the palate may be masked if the pharyngeal muscles innervated by the pharyngeal plexus are intact.1,11–13 In any event, the levator veli palatini is a more important elevator of the palate than the tensor.12,13
The levator veli palatini also pulls the palate upward and backward to block off the nasal passages in swallowing. The musculus uvulae shortens and bends the uvula to aid in blocking the nasal passages for swallowing. The palatopharyngeus draws the pharynx upward and depresses the soft palate.
In the presence of a unilateral vagus (X) nerve lesion, the levator veli palatini (Figure 7-64) and the musculus uvulae on the involved side are weak. There is a resultant lowering or flattening of the palatal arch, and the median raphe deviates toward the uninvolved side. With phonation, the uvula deviates to the uninvolved side.
With a bilateral vagus lesion, the palate cannot be elevated for phonation, but it does not sag because of the action of the tensor veli palatini (V nerve).12 The nasal cavity is not blocked off from the oral cavity with the bilateral lesion, which may lead to nasal regurgitation of liquids. Also, during speaking, air escapes into the nasal cavity, and the change in resonance gives a peculiar nasal quality to the voice. Dysphagia may be severe.
Elevation and Adduction of the Soft Palate (46. Levator veli palatini, 47. Tensor veli palatini, 36. Palatoglossus, 48. Musculus uvulae)
Test: Patient produces a high-pitched “Ah-h-h” to cause the soft palate to elevate and adduct (the arches come closer together, narrowing the fauces) (Figure 7-66).
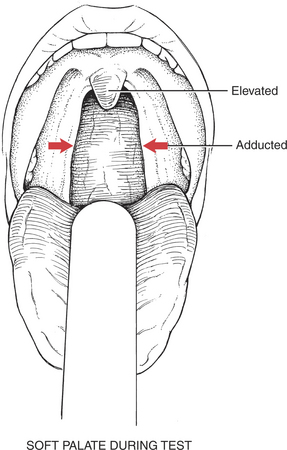
FIGURE 7-66
To see the palate and fauces adequately, the examiner may need to place a tongue blade lightly on the tongue and use a flashlight to illuminate the interior of the mouth. Placing the tongue blade too far back or too heavily on the tongue may initiate a disagreeable gag reflex.
When this test does not give the desired information, the examiner may have to stimulate a gag reflex. Light touch stimulation, done slowly and gradually with an applicator (preferably) or tongue blade placed on the posterior tongue or soft palate, will evoke a reflex and produce the desired motion when phonation fails to do so.
Remember that the gag reflex is not a constant finding. Some normal people do not have one, and many people have an exaggerated reflex.
Instructions to Patient: “Use a high-pitched (soprano) tone to say ‘Ah-a-a-a’.”
Criteria for Grading (Derived from Observation of Uvular and Arch Motion)
F: Uvula moves briskly and elevates while remaining in the midline. The palatoglossal and palatopharyngeal arches elevate and adduct to narrow the fauces.
WF: Uvula moves sluggishly and may deviate to one or the other side. Uvula deviation is toward the uninvolved side (Figure 7-67). The arches may elevate slightly and asymmetrically.
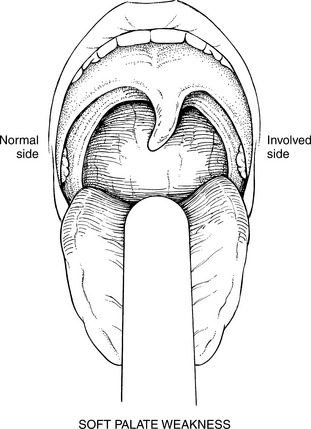
FIGURE 7-67
NF: Almost imperceptible motion of both the uvula and the arches occurs.
O: No motion occurs, and the uvula is flaccid and pendulous.
Occlusion of the Nasopharynx (49. Palatopharyngeus)
Test: Aiming at the examiner’s finger, the patient blows through the mouth with pursed lips to occlude the nasopharynx via the palatopharyngeus. Place a slim mirror above the upper lip (horizontally blocking off the mouth) to check for air escape from the nostrils (the mirror clouds). Alternatively, place a small feather fixed to a small plastic platform right under the nose; the motion of the feather is used to detect air leakage.
Nasal speech is a sign of inability to close off the nasopharynx.
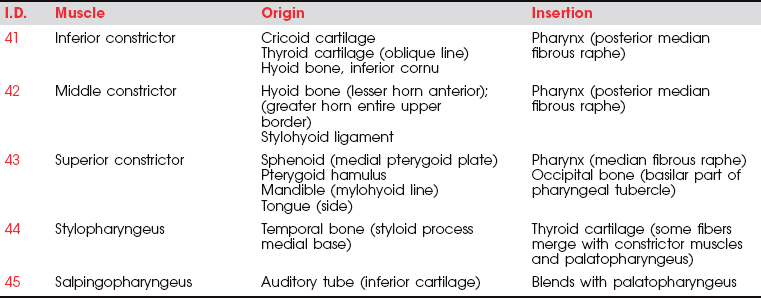
MUSCLES OF THE PHARYNX
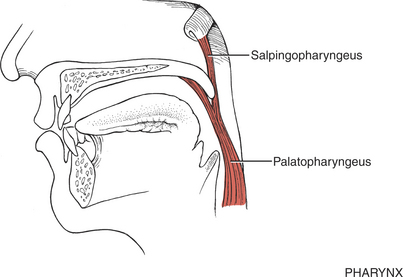
FIGURE 7-68
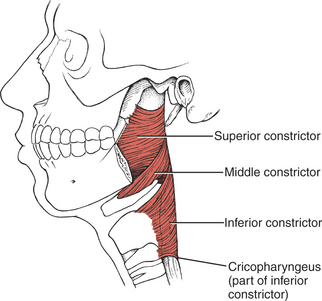
FIGURE 7-69
The function of the pharyngeal muscles is tested by observing their contraction during phonation and their elevation of the larynx during swallowing. The pharyngeal reflex also should be invoked and the nature of the muscle contraction noted. The manner in which the patient handles solid and liquid foods, as well as the quality and character of speech, should be described.
The motor parts of the glossopharyngeal (IX) cranial nerve (Figure 7-70) go to the pharynx but probably innervate only the stylopharyngeus muscle. The stylopharyngeus elevates the upper lateral and posterior walls of the pharynx in swallowing.18
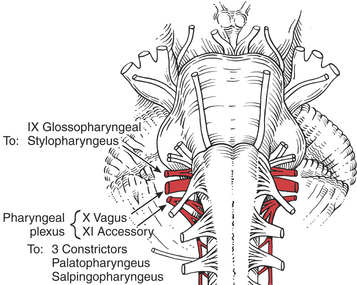
FIGURE 7-70
The remaining pharyngeal muscles (inferior, middle, and superior constrictors, palatopharyngeus, and salpingopharyngeus) are innervated by the pharyngeal plexus composed of elements from the vagus (X) and accessory (XI) cranial nerves. The three constrictor muscles flatten and contract the pharynx in swallowing and are important participants in forcing the bolus of food into the esophagus, thereby initiating peristaltic activity in the gut. The salpingopharyngeus blends with the palatopharyngeus and elevates the upper portion of the pharynx.1 Because the pharynx acts as a resonator box for sound, impairment of the pharyngeal muscles will alter the voice.
The inferior constrictor has two parts, which often are referred to as if they were separate muscles.1 One, the cricopharyngeus, blends with the circular esophageal fibers to act as a distal pharyngeal sphincter in swallowing. These fibers prevent air from entering the esophagus during respiration and reflux of food from the esophagus back into the pharynx. It has been reported that when the system is at rest, the cricopharyngeus is actively contracted to prevent air from entering the esophagus.15 When a swallow is initiated, some form of neural inhibition causes the cricopharyngeus to relax.15,16 At the same time, the hyoid bone and the larynx elevate and move anteriorly, and the constrictor muscles act in a peristaltic manner, the sum of which permits passage of the bolus.15
The upper part of the inferior constrictor is the thyropharyngeus, which acts to propel the bolus of food downward.1
In unilateral lesions of the vagus (X) nerve, laryngeal elevation is decreased on one side, and in bilateral lesions it is decreased on both sides.
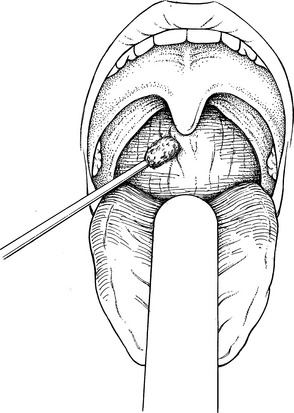
Constriction of the Posterior Pharyngeal Wall
Test: Patient opens mouth wide and says “Ah-h-h” with a high-pitched tone.
This sound causes the posterior pharyngeal wall to contract (the soft palate adducts and elevates as well).
Because it is difficult to observe the posterior wall of the pharynx, use a flashlight to illuminate the interior of the mouth. A tongue blade will probably be needed to keep the tongue from obstructing the view, but care must be taken not to initiate a gag reflex.
Patients with weakness may have an accumulation of saliva in the mouth. Ask the patient to swallow, or, if this does not work, use mouth suctioning. If the patient has a nasogastric tube, it will descend in front of the posterior wall and may partially obstruct a clear view.
If there is little or no motion of the pharyngeal wall, the examiner will have to stimulate the pharyngeal reflex to ascertain contractile integrity of the superior constrictor and other muscles of the pharyngeal wall. Patients do not like this reflex test.
The Pharyngeal Reflex Test: The pharyngeal reflex is tested by applying a stimulus with an applicator to the posterior pharyngeal wall or adjacent structures (Figure 7-71). The stimulus should be applied bilaterally. If positive, elevation and constriction of the pharyngeal muscles will occur along with retraction of the tongue.
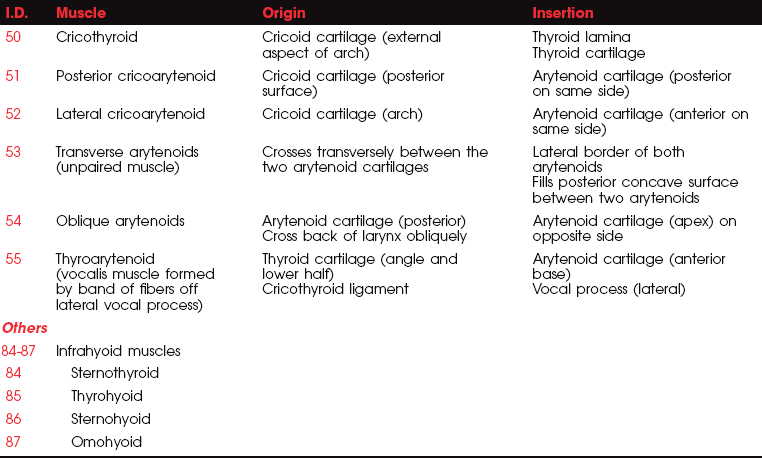
MUSCLES OF THE LARYNX
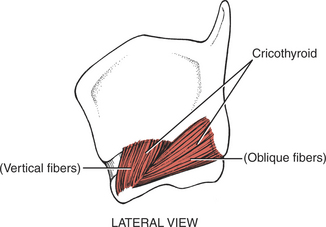
FIGURE 7-72
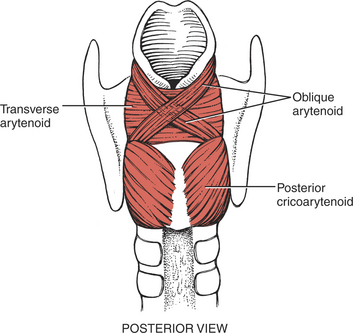
FIGURE 7-73

FIGURE 7-74

FIGURE 7-75
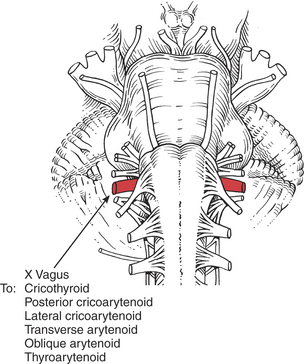
FIGURE 7-76
Examination of the muscles of the larynx includes assessing the quality and nature of the voice, noting any abnormalities of phonation or articulation; impairment of coughing (see accompanying sidebar); and any problems with respiration. Also important is the rate of opening and closing of the glottis.
Some general definitions are in order. Phonation is the production of vocal sounds without the formation of words; phonation is a function of the larynx.5 Articulation, or the formation of words, is a joint function of the larynx along with the pharynx, palate, tongue, teeth, and lips.
All the laryngeal muscles are innervated by the recurrent branches of the X (vagus) cranial nerve with the exception of the cricothyroid, which receives its motor innervation from the superior laryngeal nerve. The laryngeal muscles regulate the tension of the vocal cords and open and close the glottis by abducting and adducting the vocal cords. The vocal cords normally are open (abducted) during inspiration and adducted while speaking or during coughing.
The cricothyroids (paired) are the principal tensors owing to their action in lengthening the vocal cords.1,5,11 The posterior cricoarytenoids (paired) are the main abductors and glottis openers; the lateral cricoarytenoids (paired) are the main adductors and glottis closers. The thyroarytenoids (paired) shorten and relax the vocal cords by drawing the arytenoid cartilages forward. The unpaired arytenoid (transverse and oblique heads) draws the arytenoid cartilages together; the oblique head acts as the sphincter of the upper larynx (called the aryepiglottic folds), and the transverse head acts as the sphincter of the lower larynx.
Paralysis of the laryngeal muscles on one side does not cause an appreciable change in the voice, in contrast to the difficulty resulting from bilateral weakness. Loss of the cricothyroids leads to loss of the high tones and the voice sounds deep and hoarse and fatigues readily, but respiration is normal. Loss of the thyroarytenoids bilaterally changes the shape of the glottis and results in a hoarse voice, but again, respiration is normal.
With bilateral paralysis of the posterior cricoarytenoids, both vocal cords will lie close to the midline and cannot be abducted, leading to severe dyspnea and difficult inspiratory effort (inspiratory stridor).5 Expiration is normal.
In bilateral adductor paralysis (lateral cricoarytenoids), inspiration is normal because abduction is unimpaired. The voice, however, is lost or has a whisper quality.
With unilateral loss of both abduction and adduction, the involved vocal cords are motionless, and the voice is low and hoarse. In bilateral loss, all vocal cords are quiescent, and speech and coughing are lost. Marked inspiratory stress occurs, and the patient is dyspneic.
Elevation of the Larynx in Swallowing
Test: The larynx elevates during swallowing. The examiner lightly grasps the larynx with the thumb and index finger on the anterior throat to determine the presence of elevation and its extent (Figure 7-77).
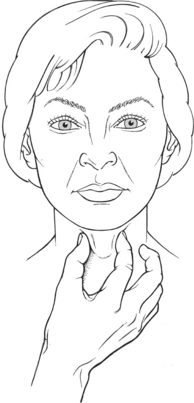
FIGURE 7-77
DO NOT PRESS DIRECTLY ON THE FRONT OF THE LARYNX, AND NEVER USE EXCESSIVE PRESSURE ON THE NECK.
Instructions to Patient: “Swallow.”
Criteria for Grading
F: Larynx elevates at least 20 mm in most people.18 The motion is quick and controlled.
WF: Laryngeal excursion may be normal or slightly limited. The motion is sluggish and may be irregular.
NF: Excursion is perceptible but less than normal. Aspiration may occur.
O: No laryngeal elevation occurs. (Aspiration will result in this event.)
Vocal Cord Abduction and Adduction (51. Posterior cricoarytenoids, 52. Lateral cricoarytenoids)
In this test the examiner is looking for hoarseness, pitch and tone range, breathlessness, breathiness, nasal-quality speech, dysarthria, and articulation or phonation disturbances.
Test and Instructions to Patient: The patient is asked to respond to four different commands to determine the nature of airflow control during respiration, vocalization, and coughing.
1. “State your name.” Patient should be able to say his or her name completely without running out of breath.
2. “Sing several notes in the musical scale,” (do, re, mi, etc.) “first at a low pitch and then at a higher pitch.” Patient should be able to sustain a tone (even if he or she “can’t carry a tune”) and vary the pitch.
3. “Repeat five times a hard staccato, interrupted sound: ‘Akh, Akh, Akh’.” Examiner must demonstrate this sound to the patient. Patient should make and break sounds crisply with a definite halt between each sound in the series.
Evaluation of Cough in the Context of Laryngeal Function: Refer to the accompanying sidebar on cough. Examiner determines whether the patient has a voluntary and effective cough. A voluntary cough is initiated on command. A reflex cough, because it cannot be initiated on command, must be evaluated when it occurs, which may be outside of the test session. The reflex cough occurs in response to irritation of the membranes of the postnasal air passages.
An effective or functional cough, voluntary or reflex, clears secretions from the lungs or airways. A functional cough is dependent on the coordination of the respiratory and laryngeal muscles.
Control of inspiration must be sufficient to fill the lungs with the necessary volume of air to produce a cough. Effective expiration of air during a cough is dependent on forceful contraction of the abdominal muscles. The vocal cords must adduct tightly to prevent air loss. Adduction of the vocal cords must be maintained before the expulsion of air.
A nonfunctional cough resulting from laryngeal deficiency sounds like clearing the throat or a low guttural sound, or there may be no cough sound at all.
SWALLOWING
The kinesiology of swallowing is the subject of continued controversy. Many of the rapid actions described as sequential are close to simultaneous events. The means of studying swallowing are limited to a great extent by the limitations inherent in palpation, following ingested food. Videofluoroscopy, manometry, and acoustic measures improve assessment accuracy.
MUSCLE ACTIONS IN SWALLOWING
Ingestion of Food and Formation of Bolus (Oral Preparatory Phase):
• The food or liquid is placed in the oral cavity, and the orbicularis oris contracts to maintain a labial seal and to prevent drooling. The palatoglossus maintains a posterior seal by maintaining the tongue against the soft palate, which prevents leak-age too early into the pharynx.19
• Foods are broken down mechanically by integrated action of the muscles of the tongue, jaw, and cheeks.
• Liquids: Intrinsic tongue muscles squirt fluids into the back of the mouth. The mylohyoid raises and bulges the back of the tongue into the oropharynx. Lips must be closed to retain fluids.
• Solids: Muscles of the tongue and cheek (buccinator) place the food between the teeth, which bite, crush, and grind it via action of the muscles of mastication (see Table 7-5). The food, when mixed with saliva (by the tongue intrinsics), forms a bolus behind the tip of the tongue.
• The tongue muscles (see Table 7-6) raise the an-terior tongue and press it against the hard palate, which pushes the bolus back into the fauces.
• In this phase of swallowing, the bolus is squeezed against the hard palate by the tongue, the lip seal is maintained, and the buccinator continues to prevent pocketing or lodging of food in the lateral sulci.
• The tongue is drawn up and back by the styloglossus.
• The palate muscles (see Table 7-7) depress the soft palate down onto the tongue to “grip” the bolus.
• The hyoid bone and the larynx are elevated and moved forward by the suprahyoid muscles.
• The palatal arches are adducted by the paired palatoglossi.
• The bolus is driven back into the oropharynx.
• As a prelude to the act of swallowing, the hyoid bone is raised slightly, and this action is accompanied by a quiescence of all muscle action: chewing, talking, food movement in the mouth, capital and cervical motion, facial movements. Even respiration is momentarily arrested.16,19
• The soft palate is raised (levator veli palatini) and tightened (tensor veli palatini) to be firmly fixed against the posterior pharyngeal wall. This leads to a tight closure of the pharyngeal isthmus (palatopharyngeus and superior constrictor), which prevents the bolus from rising into the nasopharynx.
The Engulfing Actions Through the Pharynx (Pharyngeal Phase):
• The epiglottis moves upward and forward, coming to a halt at the root of the tongue, and literally bends backward (possibly because of the weight of the bolus) to cover the laryngeal inlet. The bolus of food slides over its anterior surface. (The epiglottis in the human is not essential to swallow-ing, which is normal even in the absence of an epiglottis.1)
• The fauces narrows (palatoglossi).
Note: The pharyngeal isthmus is at the border of the soft palate and the posterior pharyngeal wall and is the communication between the nasal and oral parts of the pharynx. Its closure is effected by the approximation of the two palatopharyngeus muscles and the superior constrictor, which form a palatopharyngeal sphincter.
• The larynx and pharynx are raised up behind the hyoid (salpingopharyngeus, stylopharyngeus, thyrohyoid, and palatopharyngeus).
• The arytenoid cartilages are drawn upward and forward (oblique arytenoids and thyroarytenoids), and the aryepiglottic folds approximate, which prevents movement of the bolus into the larynx.
• During swallowing the thyroid cartilage and hyoid bone are approximated, and there is a general elevation of the pharynx, larynx, and trachea. This causes the many laryngeal folds to bulge posteriorly into the laryngeal inlet, thus narrowing it during swallowing.19,20
• The bolus then slips further over the epiglottis, and partly by gravity and partly by the action of the constrictor muscles it passes into the lowest part of the pharynx. Passage is aided by contraction of the palatopharyngei, which elevate and shorten the pharynx, thus angling the posterior pharyngeal wall to allow the bolus to slide easily downward.21
• The laryngeal passage is narrowed by the aryepi-glottic folds (posterior cricoarytenoids, oblique arytenoids, and transverse arytenoids), which close the laryngeal vestibule (glottis) and also form lateral channels to direct the bolus toward the esophagus.
• When the posterior cricoarytenoids are weak or paralyzed, the laryngeal inlet is not closed off in swallowing, the aryepiglottic folds move medially, and fluid or food enters the larynx (aspiration).
• At the beginning of this phase the compressed bolus is in the distal pharynx. The inferior constrictor pushes the bolus inferiorly (peristaltic action) to enter the esophagus. The distal fibers of the inferior constrictor, called the cricopharyngeus, are a distal sphincter and therefore must relax to allow the bolus to pass, but the mechanism of this action is in dispute.21,22
• After the passage of the bolus, the intrinsic tongue muscles move saliva around the mouth to cleanse away debris.
TESTING SWALLOWING
Swallowing is tested only when there is good cause to suspect that the swallowing mechanisms are faulty. Do not make an a priori assumption that the presence of a nasogastric tube, a gastrostomy, or a liquid diet precludes swallowing. The examiner also should review information from the patient’s history and current chart to identify the site of the lesion, the presence of upper respiratory tract infections, and similar facts, which will assist the direction of the evaluation.
When a patient has a tracheostomy, a suctioning machine is essential, and expertise with its use is required.
The examiner will have some prior information about patients from direct observation, such as how they handle saliva (swallowing it or drooling), whether and how they manage liquids and solids at mealtime, reports from nursing staff and family, and the nature of reported problems about swallowing. These all will suggest a starting point for testing.
In most swallowing tests, use a bib around the patient’s neck to prevent soiling. Remember to protect yourself from sudden aspirates! Damp washcloths or tissues should be available for clean-up.
Position of Patient: Sitting preferred, supine if necessary, but head and trunk should be elevated to at least 30°. Maintain head and neck in neutral position.
Position of Therapist: Sitting in front of and slightly to one side of the patient.
PRELIMINARY PROCEDURES TO DETERMINE CLINICALLY THE SAFETY OF INGESTION OF FOOD OR LIQUIDS
Laryngeal Elevation: Examiner lightly grasps the larynx between the thumb and index finger on the anterior surface of the throat. Ask the patient to swallow. Ascertain if there is laryngeal elevation and its extent (see Figure 7-77).
F: Larynx elevates at least 20 mm. Motion is quick and controlled.
WF: Laryngeal excursion may be normal or slightly limited. The motion may be sluggish or appear irregular.
NF: Elevation is perceptible but significantly less than normal.
O: No laryngeal elevation occurs.
Implications of Grade: If the patient is graded F (Functional) or WF (Weak functional), proceed with the swallowing assessment. If the patient is graded NF (Nonfunctional) or 0 and does not have a tracheostomy, discontinue the swallowing assessment. For patients with a tracheostomy, add a blue vegetable dye to the bolus to facilitate identification of any aspirated bolus during suctioning.
Test Sequence 2
Prerequisites: The patient has a grade of F or WF on Test Sequence 1.
There also must be at least a grade of WF or higher on the tests for posterior elevation of the tongue (see pages 332 and 333) and constriction of the posterior pharyngeal wall (see page 331).
Procedure: There are several ways to get water into the mouth to test swallowing. It does not matter which is used.
The first trial of swallowing begins with a small amount (1 to 3mL) of water. The rationale is that should the patient not be able to swallow the water correctly and it is aspirated, the lungs can absorb this small quantity without penalty. There also is increasing evidence that differences in the pH of water can cause damage to the lungs, so the small amount of water is very important. Each procedure should be repeated at least three or four times.
1. If the patient is cognitively clear, offer a glass or cup containing a tiny amount of water and allow the patient to sip. The test is successful if the water can be swallowed with one attempt, the swallow is inaudible, and the water is swallowed without any choking or coughing. If successful, proceed to Test Sequence 3.
2. If the patient cannot sip from a cup, offer a straw and ask the patient to suck a small amount. The shorter and wider the straw, the easier the task. If the swallowing attempt is successful as described in step 1, proceed to Test Sequence 3.
3. If the patient cannot sip or suck, trap water in a straw and place the straw in the side of the patient’s mouth between the cheek and lower teeth. Tell the patient you are going to release the water and request a swallow. If successful, proceed to Test Sequence 3.
4. If the patient is not cognitively clear, control the amount of water available. This is most readily done by trapping water in a straw to give to the patient.
5. For the patient who cannot handle fluid, try thickening the water with gelatin to a consistency of thin gruel or thick pea soup.
Outcomes: If any of these trials are successful, proceed cautiously to a trial of pureed food. If none of these tests are successful and the patient does not have a tracheostomy, DO NOT give the patient food by mouth until further testing (e.g., fluoroscopy) can be conducted.
If the procedures with water are not successful and the patient has a tracheostomy (through which aspirated food can be suctioned), proceed cautiously to the use of pureed food, which usually is easier to swallow than water.
Test Sequence 3
The most palatable commercial pureed foods are the pureed baby food fruits. The pureed meats and vegetables are totally unseasoned, which is unfamiliar and usually unpalatable to adults. Avoid milk products initially because they thicken the saliva. Ask about patient food preferences and try to use something enjoyable.
A suctioning machine is essential if the patient has a tracheostomy. It is recommended that the food be colored with vegetable dye (blue is readily seen and is not confused with body secretions or fluids) so that any aspiration can be readily detected as the color appears in tracheostomy secretions.
Criteria for Initiating Trials with Pureed Foods
1. Laryngeal elevation is Functional (F) or Weak functional (WF).
2. Posterior pharyngeal wall constriction is at least WF.
3. Patient has been successful in handling water in Test Sequence 2 or by observation.
4. Patient must have a functional cough (voluntary or reflex) or a tracheostomy. Some patients have a depressed gag reflex, but cough is the essential component in swallowing. The examiner cannot assume that a hyperactive gag reflex is synonymous with a functional cough.
5. The patient must have adequate cognition to attend to feeding.
6. There cannot be any respiratory problem present, such as aspiration pneumonia, that might be compromised by additional aspiration.
1. Place a small amount (½ teaspoon) of food on the front of the tongue. Ask the patient to swallow, and observe ability to manipulate food in the mouth to position it for swallowing. Allow the patient to place the food in the mouth if possible because this will better coordinate feeding with the respiratory cycle.
2. If the patient cannot move the food in the mouth, push it back slightly with a tongue blade, being careful not to initiate a gag reflex. Ask the patient to swallow, while lightly palpating the larynx to check laryngeal elevation.
3. Ask the patient to open the mouth, and check to see that food has indeed been swallowed and that none of it has pooled in the pharyngeal isthmus or oral cavity.
4. To check for a clear airway, ask the patient to repeat three sequential crisp sounds: “Agh, Agh, Agh.” Any gurgling indicates that food is in the airway and ask the patient to swallow again.
Repeat this procedure a number of times and check each response.
After four or five trials with pureed food, pause for about 10 minutes to ascertain that the patient does not have delayed coughing because of food collecting in the pharynx, larynx, or trachea. A blue aspirate from the tracheostomy tube may occur sometime after the actual ingestion of food.
Outcomes: If the patient has no immediate or delayed coughing, choking, or positive aspirate after swallowing and the airway is clear, the test is successful.
If the patient repeatedly coughs, chokes, or has a positive aspirate, this is solid evidence that there is inadequacy of swallowing, and the test should be terminated and no other food administered.
For patients who have been on a nasogastric tube and have demonstrated the ability to swallow water and pureed food without aspiration, proceed with feeding the pureed food until at least three fourths of the jar has been consumed. For the next meal, order a tray of pureed food. Observe the patient during eating; look for any problems and assess fatigue.
Use of a Mechanical Soft Diet: A mechanical soft diet (ground meat, ground vegetables if fibrous or hard) should be substituted for regular-consistency food for patients with any of the following: lack of teeth or dentures, poor intraoral control for chewing, fatigue during mastication (e.g., postpolio or Landry-Guillain-Barré syndrome), limited jaw range of motion, limited attention span to complete the oral preparatory phase.
REFERENCES
1. Williams, PL, Warwick, R, Dyson, M, et al. Gray’s Anatomy, 38th ed. New York: Churchill Livingstone, 1995.
2. Walsh, FB. Walsh & Hoyt’s Clinical Neuro Ophthalmology, 5th ed. Baltimore: Williams & Wilkins, 1998.
3. Bender, MB, Rudolph, SH, Stacy, CB. The neurology of the visual and oculomotor systems. In: Joynt RJ, ed. Clinical Neurology. Philadelphia: JB Lippincott, 1993.
4. Van Allen, MW. Pictorial Manual of Neurologic Tests. Chicago: Year Book, 1969.
5. Haerer, AF. DeJong’s The Neurologic Examination, 5th ed. Philadelphia: JB Lippincott, 1992.
6. Clemente, CD. Gray’s Anatomy, 30th ed. Philadelphia: Lea & Febiger, 1991.
7. Jenkins, DB. Hollingshead’s Functional Anatomy of the Limbs and Back, 7th ed. Philadelphia: WB Saunders, 1998.
8. DuBrul, EL. Sicher and DuBrul’s Oral Anatomy, 8th ed. St. Louis: Ishiyaku EuroAmerica, 1988.
9. Nairn, RI. The circumoral musculature: Structure and function. Br Dent J. 1975;138:49–56.
10. Lightoller, GH. Facial muscles: The modiolus and muscles surrounding the rima oris with remarks about the panniculus adiposus. J Anat. 1925;60:1–85.
11. Brodal, A. Neurological Anatomy in Relation to Clinical Medicine. London: Oxford University Press, 1981.
12. Misuria, VK. Functional anatomy of the tensor palatini and levator palatini muscles. Ann Otolaryngol. 1975;102:265.
13. Keller, JT, Saunders, MC, Van Loveren, H, Shipley, MT. Neuroanatomical considerations of palatal muscles: Tensor and levator palatini. J Cleft Palate. 1984;21:70–75.
14. Guyton, AC. Textbook of Medical Physiology, 10th ed. Philadelphia: WB Saunders, 2000.
15. Miller, AJ. Neurophysiological basis of swallowing. Dysphagia. 1986;1:91–100.
16. Doty, R. Neural organization of deglutition. In Handbook of Physiology, Section 6, Alimentary Canal. Washington, DC: American Physiologic Society; 1968.
17. Starr, JA. Manual techniques of chest physical therapy and airway clearance techniques. In: Zadai CC, ed. Pulmonary Management in Physical Therapy. Clinics in Physical Therapy. New York: Churchill Livingstone, 1992.
18. Jacob, P, Kahrilas, PJ, Logemann, JA, et al. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97:1469–1478.
19. Logemann, JA. Evaluation and Treatment of Swallowing Disorders. San Diego: College-Hill Press, 1997.
20. Bosma, J. Deglutition: Pharyngeal stage. Physiol Rev. 1957;37:275–300.
21. Buthpitiya, AG, Stroud, D, Russell, COH. Pharyngeal pump and esophageal transit. Dig Dis Sci. 1987;32:1244–1248.
22. Kilman, WJ, Goyal, RK. Disorders of pharyngeal and upper esophageal sphincter motor function. Arch Intern Med. 1976;136:592–601.
Cunningham, DP, Basmajian, JV. Electromyography of genioglossus and geniohyoid muscles during deglutition. Anat Rec. 1969;165:401–409.
Gates, J, Hartnell, GG, Gramigna, GD. Videofluoroscopy and swallowing studies for neurologic disease: A primer. Radiographics. 2006;26:22.
Hrycyshyn, AW, Basmajian, JV. Electromyography of the oral stage of swallowing in man. Am J Anat. 1972;133:333–340.
Isley, CL, Basmajian, JV. Electromyography of the human cheeks and lips. Anat Rec. 1973;176:143–147.
Miller, AJ. The Neuroscientific Principles of Swallowing and Dysphagia. (Dysphagia Series.). San Diego: Singular Publishing Group, 1998.
Palmer, JB, Drennan, JC, Baba, M. Evaluation and treatment of swallowing impairments. Am Fam Physician. 2000;61:2453–2462.
Palmer, JB, Tanaka, E, Ensrud, E. Motion of the posterior pharyngeal wall in human swallowing: A quantitative videofluorographic study. Arch Phys Med Rehabil. 2000;11:1520–1526.
Sonies, BC. Dysphagia and post-polio syndrome: Past, present, and future. Semin Neurol. 1996;16:365–370.
Vitti, M, Basmajian, JV. Electromyographic investigation of procerus and frontalis muscles. Electromyogr Clin Neurophysiol. 1976;16:227–236.
Vitti, M, Basmajian, JV, Ouelette, PL, et al. Electromyographic investigation of the tongue and circumoral muscular sling with fine-wire electrodes. J Dent Res. 1975;54:844–849.
Wolf, C, Meiners, TH. Dysphagia in patients with acute cervical spinal cord injury. Spinal Cord. 2003;41:347–353.
Zablotny, CM. Evaluation and management of swallowing dysfunction. In: Montgomery J, ed. Physical Therapy for Traumatic Brain Injury. New York: Churchill Livingstone, 1995.
Zafar, H. Integrated jaw and neck function in man. Studies of mandibular and head-neck movements during jaw opening-closing tasks. Swed Dent J Suppl. 2000;143:1–41.
*This chapter describes the muscles innervated by motor branches of the cranial nerves and describes test methods of assessing the muscles of the eyelid, face, jaw, tongue, soft palate, posterior pharyngeal wall, and larynx. It also covers the extraocular muscles. The tests are appropriate for patients whose neurologic deficits are either central or peripheral. The only requirement for the patient to participate in the test is the ability to follow simple directions.