Use of Electronic Medical Records in Pain Management
Designing Electronic Medical Record Systems for Pain Care
Pain-Related Electronic Medical Record Components
AN electronic health record is a broad term that includes portable electronic or web-based health records, electronic office-based care management systems, and electronic medical record (EMR) systems used by health care facilities such as acute, subacute, and long-term health care institutions. There are numerous synonyms for an electronic health record, with EMR being widely used. So, while some consider EMRs a subset of the electronic health record, others use the terms synonymously. The term EMR used in this Appendix applies to electronic systems implemented by institutions to store, share, and process patient information. While an EMR contains patient medical information found in a paper record, it also facilitates communication electronically and integrates clinical decision support. The latter is computer software consisting of a knowledge base that assists clinicians in making decisions about patient management. For example, clinical decision support systems include allergy checking, dose range checking, and drug interactions. See Box C-1 for terminology that will be used throughout this appendix and may be unfamiliar.
The implementation of an EMR system is expensive, resource intensive, and requires major changes in health care delivery processes, affecting routine daily activities of health care professionals. For this reason, EMRs have not been widely adopted. However, government, accrediting, and industry initiatives are providing the impetus for EMR implementation and moving the health care industry into the information age because of the numerous advantages over paper records (Pathways for medication safety, 2007; Leapfrog Group safety practices: Computerized physician order entry, 2009). EMR systems facilitate communication among health care professionals across care delivery sites, improve care coordination, increase patient safety, improve compliance with guidelines, and are useful in conducting research, auditing care process, assessing patient outcomes, and other activities (Cowell, 2007; Doran, Mylopoulos, Kushniruk, et al., 2007; Garg, Adhikari, McDonald, et al., 2005; Gruner, Ljutow, Schleinzer, et al., 2008; Hidle, 2007; Hunt, Haynes, Hanna, et al., 1998; Kaushal, Shojania, Bates, 2003; Nemeth, Wessell, Jenkins, et al., 2007; Wager, Lee, White, et al., 2000; Wolfstadt, Gurwitz, Field, et al., 2008).
Other than the use of pain diaries, little has been published about how the EMR can be used to safely and effectively assess and manage pain as well as meet accreditation requirements. Following is a discussion of how the EMR can help achieve these goals by using customized medication reconciliation; electronic dose range checking; medication order entry, preparation, and administration including range dose orders; pain documentation; and auditing pain processes.
Designing Electronic Medical Record Systems for Pain Care
Numerous EMR systems are available for purchase or as free open source systems, including one that was developed for and is used extensively in the Veterans Administration Health Care System (VHA Office of Information VistA Computerized Patient Record System, 2009; Yellowlees, Marks, Hogarth et al., 2008). Available features vary, with some EMRs having more flexibility allowing for customization of the system based on the user’s needs. Customization comes at an additional cost to the institution and may require hours of design work, testing, training, and evaluation prior to and after implementation. Fine-tuning may be necessary, and as system upgrades are available, design enhancements and new features may need implementation. However, anecdotal evidence suggests customization increases user compliance and satisfaction (Hidle, 2007; Whipple, Palchuk, Olsha-Yehiav et al., 2007).
Design and implementation teams consisting of professionals from multiple disciplines, such as nurses (e.g., advance practice, bedside, and pain nurses), information technology (IT) personnel, physicians, and pharmacists, are best suited to work through design decisions regarding pain management. IT personnel with a clinical background are invaluable members of the design team and should be requested as pain management is a high-volume and high-risk clinical phenomenon, regardless of the clinical setting. There are large percentages of patients in acute care settings, at home, in long-term care facilities, and in hospices who suffer from uncontrolled moderate to severe pain (see Chapter 1) (American Pain Foundation, 2008; Bercovitz, Decker, Jones, et al., 2008; Botti, Bucknall, Manias, 2004; Bradshaw, Empy, Davis, et al., 1087; Cohen, Easley, Ellis, et al., 2003; Dobratz, Burns, Oden, 1989; Green, 2008). Many require opioids, which have been identified by patient safety and accreditation organizations as high-risk, high-alert medications (Federico, 2007; The Joint Commission Sentinel Event #11 High-Alert Medications and Patient Safety, 2006). Even if opioids are not used, the numerous patient populations have differing needs and the varying responses to analgesics requires the ability to individualize pain assessment and management plans. Many pain therapies are complex. However, regardless of complexity, pain management is an interdisciplinary process. All of these increase the risk to the patient if there are errors. A well-designed EMR helps to address these issues.
It is important for the clinicians on the design team to understand paper-based system limitations, identify the safety measures developed to reduce error in paper-based systems, and translate these to IT professionals so that these aspects can be addressed in the various EMR components (e.g., pain assessment, order sets, and documentation). When developing safe, effective pain EMR components, design team members have to be able to think “out of the box,” talk and walk through potential scenarios using the proposed design, and consider if a Failure Effects Mode Analysis (FEMA) should be undertaken to determine the optimum design.
Similar to paper-based systems, EMRs also have limitations that design team members and clinicians need to appreciate, such as the inability to calculate maximum doses of acetaminophen administered over a 24-hour period when single-entity acetaminophen and a combination opioid plus acetaminophen product are given concurrently. The team may need to “push the envelope” utilizing existing EMR system features to address the issue and maximize safety. Two examples are given. First, in the case of two orders for acetaminophen, cautions to the person administering these medications can be developed. Second, to prevent prescription of multiple sedating medications in patients cared for by the pain service, a rule can be developed to prevent non–pain service prescribers from ordering opioids and sedatives for pain service patients. Ease of use, patient safety, and integration of evidence-based or best-practice guidelines are guiding principles for design team members.
Even customizable systems come with prebuilt features that can be implemented out of the box (e.g., a post-pain response field for “as needed” [PRN] analgesics that triggers a reminder to the nurse to obtain the postanalgesic pain score). Prebuilt features may also be customizable to a degree (e.g., adding times based on drug and route of administration to the post–pain response field). Customization may be performed even after initial system implementation. So if the initial product does not meet the end user’s needs, modifications should be discussed with IT specialists.
Although the institution’s EMR IT design and implementation specialists will develop a wealth of knowledge, the EMR system vendor’s staff and resources are more extensive and should be queried to answer difficult questions. Vendors also have user groups and Web sites where customized designs are shared. They can often provide contact information for individuals in comparable institutions who have grappled with similar design issues. E-mail list services such as is offered by the American Pain Society/American Society for Pain Management Nursing provide the ability to ask questions and receive screen shots of different designs as well as to dialogue with other users about how they addressed issues. These consultative mechanisms facilitate discussions about what individual institutions considered when making their design decisions, available options, and the pros and cons of these choices. List services and user groups also afford a mechanism to elicit support for submitting requests to the vendor about design changes. Vendors prioritize changes in their systems based on the number of design requests or concerns about system limitations from their users. So, if a system appears to lack desired features, has limitations, or is cumbersome, it is important to explore the avenues discussed above to create a user-friendly, safe, and efficient system.
Pain-Related Electronic Medical Record Components
Major EMR components integral to the assessment and management of pain are listed below. All are crucial to the provision of safe pain care.
• Medication order entry, preparation, and administration including range dose and interfaces with other electronic systems (medication dispensing cabinets)
Each EMR component has unique design and implementation challenges, some of which may be exclusive to a specific EMR system. Select examples of generic issues associated with these EMR components and potential solutions with a major focus on patient safety are discussed in the following paragraphs.
Medication Reconciliation
The Joint Commission 2006 safety initiative called for reconciling medications when there is a change in a patient’s care levels (The Joint Commission Addendum to Sentinel Event #35 Medication Reconciliation, 2006). The EMR can facilitate the process of medication reconciliation by reminding the clinician to perform the task and providing an accurate list of the patient’s prescribed medications. Ideally, such a list would include essential elements of the prescription as well as information about the frequency of use for PRN and scheduled medications. Well-designed EMR medication reconciliation can minimize the potential for withdrawal symptoms in individuals receiving long-term opioid therapy because between levels of care, the practitioner is provided with a list of active meds and their start dates and is required to make a decision about continuing or discontinuing each drug. For those knowledgeable in equi-analgesic conversions, medication reconciliation also provides information that can be used to determine dose conversions. This is an improvement over paper-based tracking of these changes that requires a review of several handwritten medication administration records, physician orders, or verbal reports. Obtaining this information prior to major status changes (e.g., preoperatively) is recommended.
Dose Range Checking
Dose range checking is one of the major error reduction features of EMR. It involves comparing the dose written by the prescriber against the dose in a drug database. Parameters such as minimum and maximum single dose limits and total daily dose can be compared. Database-recommended dose limits may be based upon parameters such as route of administration and patient-specific factors such as age, weight, and laboratory values (e.g., creatinine clearance). Doses exceeding database limits send an alert to the prescriber about the reason for the alert (e.g., dose exceeds the maximum recommended amount, or the intramuscular [IM] route is not recommended) and may suggest actions (e.g., use this dose only in opioid-tolerant individuals or consider another route of administration, respectively). Alerts that are overridden may also require the prescriber to provide a reason for overriding the alert (e.g., patient is opioid-tolerant).
Drug databases can include some inherent weaknesses that may or may not be corrected by customization. For example, they may contain routes of administration that are not used for pain management (e.g., hemodialysis catheters) or doses inconsistent with best-practice guidelines (e.g., a maximum single dose of 4 mg of intravenous [IV] hydromorphone, which exceeds guideline recommendations for opioid-naïve individuals) (American Pain Society, 2008). Some drug limitations may be set developing EMR rules outside of the dose range checking system, such as restricting the use of preservative-free morphine or other solutions to anesthesia prescribers or to the operating room (OR). Other major weaknesses in most, if not all, dose range–checking databases are the lack of automatic equianalgesic conversions during route or drug changes, inability to check for daily maximum cumulative doses when combination products (e.g., nonopioid + opioid formulations) are ordered, inability to customize limits based on patient status (e.g., higher dose limits allowed for opioid-tolerant individuals or for patients with ventilator-controlled respirations), and significant tolls on computer resources associated with multiple dose range–checking steps that can slow down the system. When the proposed change has more negative implications than benefits, practitioners should be alerted of the EMR limitation, and a request for modification should be sent to the vendor.
Depending upon the database and the EMR system, analgesic information may be customizable. For example, route limitations may be possible, such as restricting preservative-free morphine to intrathecal or epidural administration only. Minimum and maximum single dose and total maximum daily dose limits can also be changed. Dose limits may be designed to vary based on age, weight, and patient-specific laboratory values, such as a specific creatinine clearance level when ketorolac is ordered (American Pain Society, 2003). In such instances, customization may require the IT staff to write multiple rules. For example, Rule 1 checks that the patient’s age is within certain parameters, Rule 2 checks that the patient’s weight is within the rule’s specific parameters, and Rule 3 checks that a patient-specific laboratory value is within the designated range. To ensure safety, extensive testing and monitoring is recommended to determine what will happen if an individual rule and all possible combinations of rules are violated.
Attention to ensuring that customization is maintained during upgrades is imperative. Otherwise, as upgrades to the system or database occur, customized safety features may be lost. For example, if the IV hydromorphone single maximum dose limit is customized from 4 mg to 1 mg in an updated database, this customization can be overridden when upgrades are made. It is important to note that while dose range checking is a major safety feature, a phenomenon called “alert fatigue” whereby individuals are constantly bombarded with warnings (alerts) and begin to ignore them or breeze through them too quickly to comprehend and respond appropriately to them, is a real concern. Efforts to avoid unnecessary alerts are essential.
Medication Order Entry, Preparation, and Administration
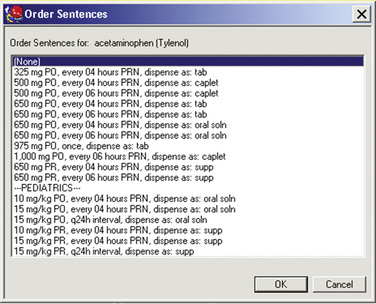
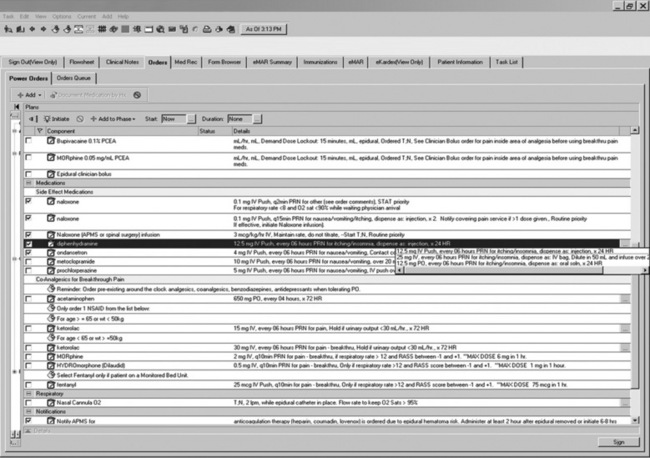
With EMRs, prescribers can build simple orders from a list of options for each field associated with the order (i.e., medication, dose, route, and frequency). Or, the prescriber can select prebuilt order sentences (Figure C-1) that include drug, dose, route, and frequency; specific fields can be modified as needed. Order sets (Figure C-2) can also be developed that include prebuilt pain and adverse effect medication sentences, as well as assessment and monitoring parameters. This is particularly advantageous for complex order sets, such as for those written for specialized analgesic therapies (e.g., IV PCA, epidural analgesia, and nerve blocks) where a missing order may compromise patient care. In some systems, all currently active orders in a specific order set can be collected, so that the numerous associated orders can easily be reordered during medication reconciliation or discontinued when the specialized analgesic therapy is stopped.
A pitfall of the EMR is that prescriber-built orders may be indistinguishable from prebuilt orders to the eyes of anyone but the original prescriber. Since EMR orders are typed, they have the appearance of being written correctly. This is likely, at least in part, because there is no issue with legibility and missing dosing parameters. As an example, although modified- release opioids should be administered in scheduled around-the-clock doses, a prescriber-built order for PRN administration of an modified-release opioid may be perceived as being correct because it is typed and legible. Staff must be knowledgeable of this phenomenon.
As with dose range checking, prebuilt or institution-built order sentences may also be inconsistent with best-practice or evidence-based guidelines. Similarly, order sets built by non–pain service providers may be inconsistent with dose range–checking parameters and evidence-based information. To prevent this, prior to implementation of the system, order sentences should be assessed for consistency with evidence-based guidelines, whether they are individual order sentences or part of an order set. Consistency is important for safety and to prevent alert fatigue. As examples, order sentences and order sets should be assessed for issues such as follows.
• Are long acting opioids scheduled for twice-daily (BID) dosing at unequal intervals or every 12 hours?
• Have the IM opioid administration sentences been deleted?
• Do the prebuilt IV opioid doses and frequencies use IM parameters, or is the dose and frequency divided in half as recommended (American Pain Society, 2008)?
• Are there low-dose naloxone sentences for opioid reversal?
The impact of evidence-based prebuilt analgesic order sets and sentences on patient outcomes (e.g., adverse effects and analgesic efficacy) is unknown, but if implemented, they should improve pain outcomes.
Customized Pain Management Orders and Alerts
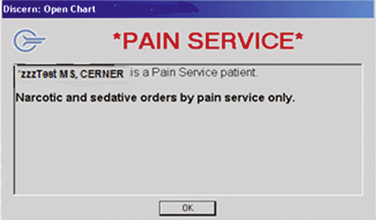
A distinct advantage for pain services is the ability to develop customized alerts reminding other prescribers not to order analgesics and sedatives for patients cared for by the pain service, a step that can help prevent oversedation and life-threatening respiratory depression (Figure C-3). Choices have to be made about whether the alert should function merely as a reminder or be tied to a hard stop, preventing other prescribers from ordering medications. These alerts require the EMR system to recognize differences betweenpain service prescribers and other prescribers, which requires collaboration between the pain service and the technology specialists to maintain an updated pain service prescriber list. Similarly, reminders for “No opioids or sedatives unless ordered by the Pain Service” can be constructed for nurses to see.
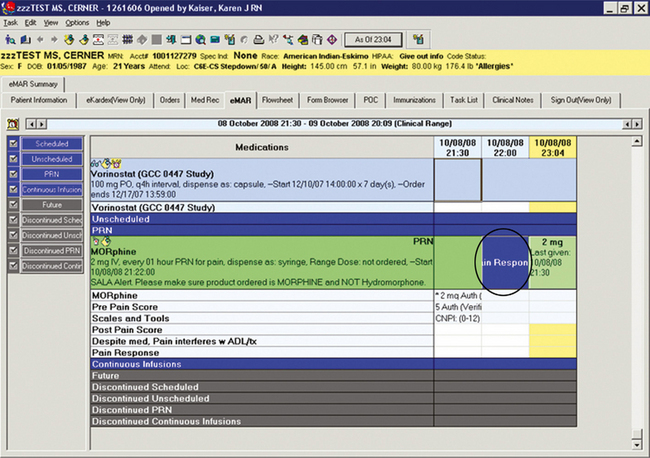
Order sentences can be customized as seen in Figure C-4. They can be constructed to require a reason for PRNmedication administration (such as for pain, itching, or nausea), which can increase compliance with accreditation standards. Orders can be developed to include comments, specialized instructions, or reminders about sound-alike look-alike (SALA) drug alerts (e.g., morphine and hydromorphone, hydrocodone and hydromorphone, codeine and hydrocodone). Other related systems (e.g., medication dispensing cabinets) should be programmed to carry similar relevant messages (e.g., SALA alerts) for reinforcement.

Figure C-4 PRN reason and sound alike look alike (SALA) reminder. University of Maryland Medical Center.
Specialized instructions can also be customized for different health care providers. For example, pharmacists may see special admixture directions, and bedside nurses may see instructions such as “not to exceed acetaminophen limits” or “do not crush or chew” or parameters for administration of certain drugs (Figure C-5). However, lengthy orders may not display in their entirety, and partsmay be obscured from the practitioner’s view, requiring the user to take extra steps for the information to display. For example, the nurse may need to “hover” the computer’s cursor over the order to see the complete order on the electronic medication administration record (eMAR).
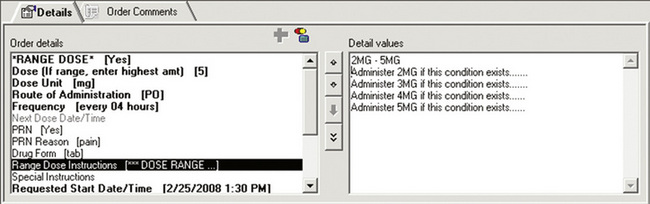
Range dose orders, which individualize the medication dose to a patient’s changing analgesic needs, may be constructed in accordance with policy and include guidance on how to consistently implement the order. For example, in Figure C-6, the prescriber is instructed to place the highest dose outside the parentheses, which facilitates EMR range dose checking of the highest amount of medication ordered; system limitations prevent dose range checking on information in the Comments section of an order. In Figure C-7, the prescriber is prompted to provide specific information about dosing.
Safety Issues
There are differences between the paper record and the EMR that may impact safety. Some of these changes are significant to specific types of users, but differences may be subtle to other health care disciplines. In the paper record, the same medication order is seen by the prescriber, the pharmacist, and the nurse. In the EMR, there are different views for different disciplines based on the process being executed. Each of the three disciplines sees something different when performing their primary functions of ordering, preparing, and administering, respectively. Although the views are distinct for each purpose, design decisions made for one process may impact the view for another purpose and cause potential safety issues. For example, in some systems, in order for the pharmacist to have access to drug compounding information within the system, the diluent, rather than the drug, is the first item listed on the eMAR. Designs best suited for the pharmacist’s drug verification and preparation may not result in the best display for the bedside nurse who administers the medication and vice versa. Regardless of the design decision made, one of the disciplines is put at risk for making an error. These “Catch-22” situations need to be reported to the vendor.
Views previously available on paper may not be readily available in the EMR, potentially resulting in patient harm or discomfort. For example, because there are no related pharmacy actions, the pharmacy may require that patient-controlled analgesia bolus orders be associated with the solution order instead of being placed as a separate order. In this case, the bolus order may be placed in the solution’s Comments section, which is hidden from the nurse’s view without hovering the computer’s cursor over the order to see the comment’s details. In response, the bolus dose may be delayed or not be given. In the case in which upper dose limits are programmed into the analgesic pump, such as in a computerized IV infusion with specialized features also known as a “Smart Pump,” there are anecdotal reports where nurses have not seen the bolus order and mistakenly utilized the programmed upper dose bolus limit as the bolus dose. This could lead to overmedication of the patient.
When any design team member is concerned about a potentially significant adverse safety event (such as described in the previous paragraph) for which a resolution is not evident, additional expertise should be enlisted. The team should be led by a provider with an understanding of the problem, knowledge of the system, and without a stake in the outcome. Similarly, other system and process experts should be enlisted. The multidisciplinary team of stakeholders must evaluate actual and potential clinician responses to an existing order, as well as evaluate potential resolutions and the various clinician responses to the proposed changes. Once the group has made a decision that maximizes safety and patient comfort, plans for making the change, dissemination strategies, and a timely evaluation of the change (i.e., determination of whether the change resolved the issue and if unanticipated problems occurred as a result of the change) must be arranged.
Changes in Work Processes
Changes in the hospital staff’s work processes are also required with the implementation of an EMR system (Kushniruk, Borycki, Kuwata, et al., 2006; Spence, Valenza, Taylor, 2007). These need to be well planned, communicated, and followed. To ensure safety while instituting these changes, best practices must be continued and not inadvertently abandoned. Some example of these work process changes and the potential impact follow.
Medications and the eMAR may be on separate portable utility carts. However, when preparing medications both carts need to be used concomitantly, which may be cumbersome. During administration of medications or double-checks of analgesic pump parameters, the order needs to be readily available, requiring access at the bedside to the computer or a current copy of the orders. Without the order and an identifiable medication at the bedside, it is difficult to assess the “5 Rights” (right patient, right drug, right dose, right time, and right route), which can increase the potential for error. Taking the required appropriate information to the bedside is a step that should not be abandoned, even if the patient is under isolation precautions. So, regardless of how cumbersome it is, computer access at the bedside with proper disinfection, as needed, or frequent downloads of current printed eMARs is necessary.
Some changes in processes are more subtle. For example, the nurse may receive an electronic notice that an opioid has been discontinued, but accepting this notice may take place on a screen that does not display the entire order, so the nurse may not readily recognize that an opioid the patient takes for chronic pain was discontinued prematurely without tapering the dose to prevent withdrawal. When the same discontinued medication is observed on the eMAR, the nurse may not be visually cued about the change, because discontinued medications drop to the bottom of the eMAR and are grayed out, making them more difficult to see than the text of current medications. In comparison, the visual cue of a crossed-out medication on a paper medication administration record that easily reminds all shifts about the medication change no longer exists. With an EMR, the nurse has to remember to actively look at all medication changes on the eMAR. Thus, it is important to make inspecting the eMAR during shift report a part of the normal routine with the advent of computerized prescriber order entry, which is the process of ordering, preparing, administering, and documenting medications using an electronic system instead of a paper system.
A major disadvantage of most EMR systems is the multistep process required for making changes or updating the system. For example, steps must be followed before the prescriber can order a nonformulary item. In some, if not all systems, first, the pharmacy or IT personnel must create a drug item that can be ordered by the prescriber. Next, the pharmacist must assure that the parameters (e.g., dose, route) of this item are correct. Then, the drug is made available by pharmacy or IT personnel for the prescriber to select (order). This process increases safety, but slows down the administration of care. In a fast-paced, quickly changing health care environment, a system that offers a quick response time and incorporates checks and balances to minimize harm from inadvertent errors is essential. When planning EMR implementation, expedited processes for adding a new formulary item at the point of care such as the one previously described must be developed. A decelerated, but similarly rigorous process can be developed for planned additions to the formulary.
Documentation
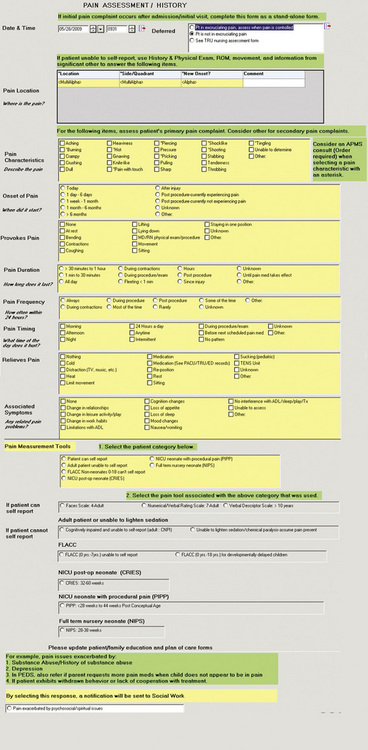
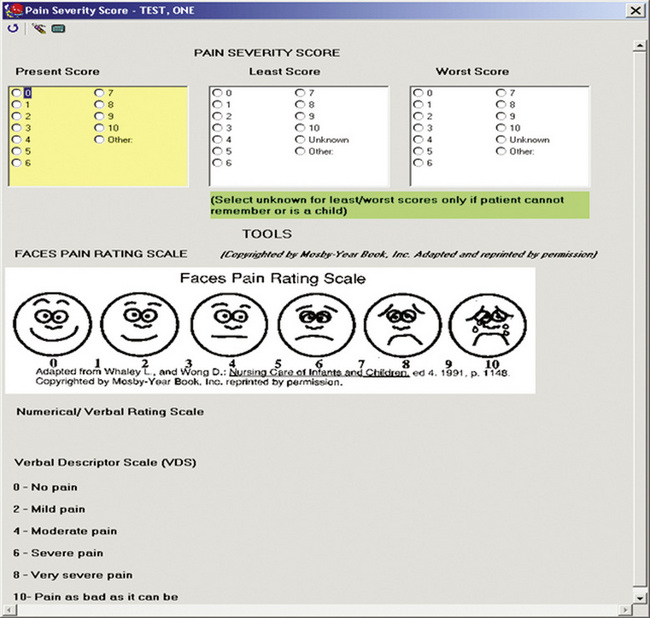
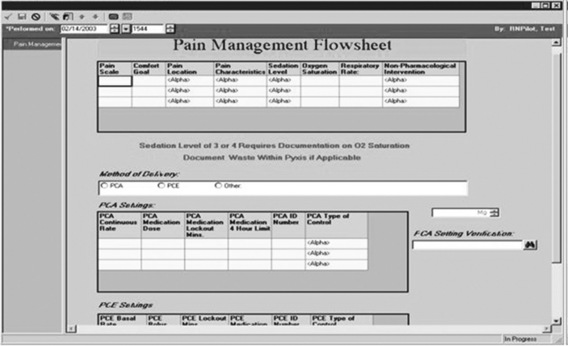
Pain documentation similar to those available in paper-based systems can be developed for EMR systems (Box C-2). A Pain Assessment/History form (Figure C-8), pain measurement tools (Figure C-9), and forms to document medication effectiveness such as a Pain Management Flow Sheet (Figure C-10) or graph depicting the pain score verses the pain management goal (Figure C-11) can be designed. Note that similar to paper-based tools, recreation of copyrighted information such as a pain measurement tool in the medical record requires the developer’s permission and a notation of permission on the electronic form as seen in Figure C-9.
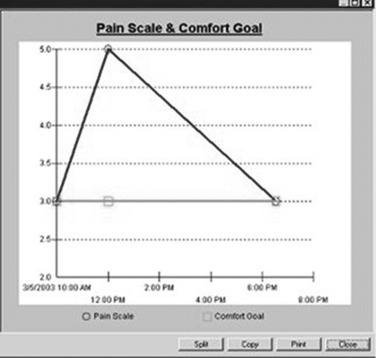
Figure C-11 Pain management score verses pain management goal. University of Maryland Medical Center.
To enhance usability, forms should be short, without redundancy, and easy to complete, yet flexible. Radio buttons, check boxes, drop-down boxes, and Comments sections are helpful to achieve these goals. Radio buttons are small open circles in front of (or sometimes after) items in a horizontal and vertical list that can be selected. They function similar to a check box in that the user clicks in the circle or check box next to the desired response(s) from a list of several items that may be displayed in several columns. Radio buttons are useful when a single item is selected. Check boxes are useful when multiple selections are possible, such as selecting pain characteristics or the quality of pain (e.g., hot, burning, and electrical shock). Both generally take up more space on a form than a drop-down box. Neither the radio button nor the check box requires the user to do anything to see the items in the list. This is in contrast to the drop-down box. A drop-down box appears as an open space on the same line next to a category, and when the drop-down box is clicked on, several responses drop down in a list, from which the user selects one response. For example, several pain measurement tools may be listed, but only one is chosen and shown in the box. Comments boxes provide the most flexibility and allow the user to type in pertinent information but take up considerable space if the entire text is viewable. Saving space is important when designing forms; even simple, easy-to-complete forms can seem overwhelming and burdensome if they appear to be long.
Enhancements can be made to electronic forms that are not possible with paper-based forms. It is important to realize that the capability to make the enhancements depends on the system and the choices the institution makes during implementation of the system. Several examples of possible enhancements follow.
• Referrals are often necessary for people with pain and can be generated easily with the EMR by pressing a button (Figure C-12).
• EMR users can be required to provide information by making fields mandatory (e.g., provoking factors, quality of pain, region/radiation/relief, severity/associated symptoms/medication side effects, and timing/onset/duration of pain).
• The nurse can be directed to select the appropriate pain measurement tool by choosing the category most appropriate for the patient (e.g., patient able to self-report, adult unable to self-report, neonate unable to self-report) on a Pain Assessment form as in Figure C-8.
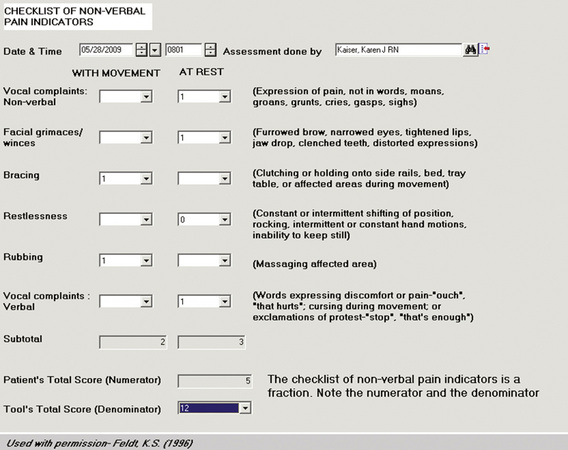
• Behavioral pain measurement tools can be designed to calculate total sums, using the information from the individual data elements entered by the nurse (Figure C-13).
• Information from the electronic vital sign sheet and the eMAR can be automatically entered into the patient’s pain management flow sheet.
• Documentation of analgesic pump parameter double-checks can be required.
• Reminders to obtain postanalgesic pain scores to evaluate medication effectiveness at specific time intervals based on drug formulation (e.g., injectable or transdermal) and route of administration (Figure C-14) can be programmed into the system.
Besides enhancements to electronic pain documentation, there are some limitations. Like the enhancements, these are also system dependent. Some EMR systems only allow simple figures, such as a picture of a FACES pain measurement tool. An interactive body diagram upon which notations can be made indicating pain location, severity, and quality is preferred, but this enhancement may make electronic auditing difficult. Medication effectiveness documentation may have system limitations as well. For example, a reminder to obtain a postanalgesic pain score may be limited to PRN analgesics. This can cause confusion if there is no similar reminder for postanalgesic pain score assessment for scheduled medications. There may be inflexibility when programming the time of the reminder (e.g., only one time allowed per route or drug; the time of the reminder must be less than 12 hours, which precludes programming an accurate peak effect for the fentanyl patch). There may also be limitations on the number of letters or words that can be displayed in drop-down box items and in a Comments section, respectively. When addressing the system limitations, the interdisciplinary team that develops pain documentation must carefully consider the effects of their choices on the clinician. In addition, the team must develop adequate ongoing clinician training and other strategies that address system limitations.
Pain Management Forms and Flow Sheets
Electronic pain management flow sheets that document preanalgesic and postanalgesic pain scores, pharmacologic and nonpharmacologic treatments, as well as the presence or absence of adverse effects using text or graphic display as shown in Figure C-10, had been designed but were not widely used at the time of this publication. Some parameters (e.g., respiratory rate, pain scores, sedation scores, medications, and doses) can be linked to other documentation forms in the EMR (e.g., vital sign record, eMAR) and may be pulled electronically into the pain management flow sheet or vice versa in some systems.
Paper-based pain management flow sheets and comprehensive pain assessment forms have been shown to increase the assessment of pain and effective management of pain (see Chapter 1) (McMillan, Williams, Chatfield, et al., 1988). Similar research is needed to demonstrate the effectiveness of electronic forms. One study in an outpatient clinic showed that compliance with documentation on an electronic pain assessment form by non-pain physicians was low because the form was burdensome(Saigh, Triola, Link, 2006). In contrast, a pain clinic EMR utilized by a variety of disciplines received positive reviews (Gruner, Ljutow, Schleinzer, et al., 2008). Similar to electronic reminders used to document postanalgesic pain scores as described in the next section, reminders to perform assessments for specialty analgesic therapy, such as IV PCA or epidural analgesia, that are frequently documented on a pain management flow sheet are not well described in the literature. A static notation on an electronic care plan (eKardex) is described as part of the University of Maryland Center experience below, but it is not as likely to ensure compliance the way a timed reminder would function.
Great strides in compliance with accreditation standards are possible with electronic pain documentation, especially if responses are mandatory or the nurse receives reminders to perform certain tasks. However, to reduce user burden, mandatory documentation requirements should be used only when necessary. Forms should be short and eliminate redundancy. User feedback should be sought and applied to enhance utilization of the forms and improve user satisfaction. Reminder systems should also be integrated into the electronic medical record to assist with compliance with accreditation standards.
Quality Improvement and Research
When designing pain-related EMR forms (orders, medication reconciliation forms, and documentation forms) and processes (mandatory or discretionary; documentation requirements of analgesic pump double-checks), one must consider the reports needed to monitor compliance with pain-related regulatory and accreditation standards, as well as the data needed for quality improvement initiatives and prospective or retrospective research. Mandatory items on forms eliminate missing data, which is desirable when information is required to meet accreditation standards, but as mentioned, they should not be used excessively. Predetermined options, which are available with drop-down boxes, check boxes, and radio buttons, provide data that are more easily retrieved electronically and aggregated into a report compared with data written in comments fields. Comments section information, otherwise known as free-text data, is difficult to consolidate from multiple notes, as the health care provider can write anything desired. Free-text data is also subject to misspellings. Both make it difficult to aggregate the data and perform calculations for quality improvement and research.
With paper-based records, auditing is conducted by reviewing each chart by hand and calculating the percent of overall compliance (e.g., multiple records, multiple units) with accreditation standards. This can be extremely time-consuming, even when the results are entered into a database and overall compliance is calculated by machine predesigned report. Electronic auditing, where data is pulled from the EMR and collated electronically, is more efficient and frees up nursing time previously spent collecting data from the medical record and aggregating (collating) the results. This is valuable time that can be used instead for developing process improvement actions.
To perform an electronic audit, an expert programmer, data analysis person, and report writer working in conjunction with a pain expert can develop the data extraction method, data calculations, and report format. Once each of these has been verified, repeat analyses using different time frames can be automated. This requires minimal nursing time expenditure compared with performing individual paper-based chart audits. All parties involved in the development of the audit need to be made aware of any changes to the electronic documentation forms, because the changes may affect future audit results.
Currently in the clinical setting, electronic auditing is limited primarily to use of data from fields where predetermined selections are available, such as radio buttons, check boxes, and drop-down boxes. Utilization of what is called “natural language” processing, which electronically searches rich narrative notes, determines the theme, and aggregates the data into a report, is time-consuming and requires sophisticated programming. Difficulties associated with natural language processing include searching and determining the meaning of free-text notes that contain misspellings or culturally specific nuances from notes written by multicultural health care providers. In addition, to audit a narrative record for pain information using natural language processing, the computer program must first identify that the nurse is writing about physical pain and not emotional pain. Once that task is complete, the program would have to examine what the nurse wrote, such as “the patient has 8/10 sharp, shooting, electrical shock pain in the left lower extremity.” From this note, natural language processing would have to identify “8/10” as the pain score; “sharp, shooting, electrical shock” as pain characteristics, and “left lower extremity” as a location before it could determine if each of these elements of a pain assessment were included in the documentation. In contrast, if separate items and specific selections are provided for pain score, pain characteristics, and pain location, an electronic audit program would only need to be programmed to search each item and see if the nurse selected one response per item. In summary, the use of natural language processing in the clinical arena is likely to be a time-consuming and expensive proposition for some time. It is in its infancy, particularly with regard to monitoring of nurse-sensitive outcomes (Pakhomov, Jacobsen, Chute, et al., 2008).
Regardless of how the data is obtained (natural language processing or fixed responses) or its purpose (quality improvement or research), validation of the data is required to ensure that the data that is captured is indeed the data that was intended to be captured. Validation activities, such as verification through real-time or retrospective assessment of patient records and discussions with health care providers (e.g., nurses, nurse practitioners, physicians), can be used to identify potential clinical practice issues that need clarification or improving. Well-designed forms with mandatory fields and standardized responses and reminders will facilitate the ability to obtain information retrospectively for quality improvement or research.
The University of Maryland Medical Center Experience
The author’s place of employment, the University of Maryland Medical Center (UMMC), has had an EMR system since the mid-1990s. However, it was limited in scope (e.g., obstetrical area, simple physician orders, and simple nursing documentation). Housewide implementation was planned, but the company that produced the original EMR system was sold, and system upgrades and support were discontinued during the planning phase. A replacement system was purchased, and the functionality of the previous system was closely replicated at the time of implementation. With system expansion, considerable hospital resources were dedicated to converting critical patient information such as nursing intake assessment and laboratory and radiology results to electronic data; replacing an outdated pharmacy system and trauma documentation system; and developing prescriber order entry, especially medication order entry. These processes can facilitate access to information, reduce duplication of medical tests, and promote safety in the medication process (Cowell, 2007; Kaushal, Shojania, Bates, 2003; Wolfstadt, Gurwitz, Field, et al., 2008).
Award Incentive
Electronic or computerized prescriber order entry is the process by which orders are written by the prescriber in the electronic medical record and are electronically transmitted via computer to other associated computers, particularly computers in locations where the work associated with the order is performed. In the literature, this is also referred to as a computerized physician order entry. A computerized prescriber order entry includes orders for processes traditionally conveyed on paper and telephone, such as a patient’s code status, dietary orders, laboratory, and x-rays. A specialized form of prescriber order entry is the computerized prescriber medication order entry, which is also known as the computerized physician medication order entry. In the computerized prescriber medication order entry, the prescriber’s order is electronically transmitted: to the pharmacy system for pharmacy review, preparation, and dispensing; to a nurse’s orders alert tab notifying the nurse of a new order; to the order’s tab for viewing in the usual order format; and to the eMAR for the nurse to administer and document that the medication was administered.
Literature about the medication safety benefits of EMR systems played an important part in making prescriber medication order entry a high priority at the UMMC. Attention to the Leapfrog group’s yearly computerized medication order entry safety enhancements was a contributing factor in the system customization process at the UMMC. The Leapfrog Group, which consists of numerous Fortune 500 companies, recognizes and rewards systems that meet the group’s yearly safety goals. Computerized prescriber order entry is integral to the organizational safety assessment that is required to be considered for the annual Leapfrog Award (Leapfrog Group safety practices: Computerized physician order entry, 2009). It is a contributing factor in the UMMC being a continued recipient of the award over consecutive years (Leapfrog Group safety practices: Computerized physician order entry, 2009).
Implementation
Introduction of EMR systems into the health care workplace is often completed in steps, particularly in the hospital setting. For example, dietary orders may precede laboratory orders, which may precede the computerized prescriber order entry. It may be months to years before the entire medical record is computerized. At the time of this publication, the University of Maryland was utilizing a combination of paper and electronic records. The nursing intake, patient care planning, and patient education are documented on computerized forms, but routine vital signs and daily nursing flow sheets (including pain management flow sheets and daily nursing documentation) are on paper.
Even when the system is completely implemented and there is no paper documentation, attention to the system is required. Similar to paper-based forms and associated processes, revisions are necessary. The UMMC’s experience with implementation of a pain-related EMR as of the publication date is detailed in the following paragraphs.
Three things were imperative to the success of our pain electronic documentation. First, the University selected a multidisciplinary design team with knowledge about the functionality of the system. For example, the PhD-prepared nurse pain expert (the Appendix author) was one of numerous support persons from many disciplines trained for the implementation of the computerized physician order entry system. Second, the team sought and utilized input from stakeholders during the design process. For example, when developing nursing documentation forms, the Pain Task Force, a preexisting housewide nursing group charged with implementation and adherence to national pain standards, was and continues to be consulted or actively involved with IT professionals in the development and revision of forms. Third, all of the design team’s work was conducted in a multidisciplinary fashion. For example, pain service nurse representatives, attending physicians, and a PhD-prepared pain expert worked with pharmacy and IT professionals to design and redesign prescriber order entry. The nursing representatives ensured that suggestions were evidence-based and applicable to all age groups and clinical settings as well as making sure data was in a format that could be easily extracted from the electronic record for auditing.
In conjunction with the IT Department, the Clinical Effectiveness Department at the UMMC, which consists of health analysts, the PhD-prepared pain expert, and other electronic documentation experts, initiated and validated electronic auditing for the following aspects of documentation and care to demonstrate compliance with accreditation requirements:
Successes
Great strides in compliance with accreditation standards have been made with electronic pain documentation at the UMMC. A major success was in the implementation of an electronic comprehensive pain assessment history form. In January 2002, approximately three months after implementation of a paper-based pain assessment history form, compliance with its completion was 28%. Over the course of a year and the use of various strategies, compliance increased to a steady state that ranged between 50% and 55%. In response to this low compliance rate, an electronic Pain Assessment/History Form was developed. The new form was designed to open up and display to the practitioner when a notation was made on the admission intake form stating the patient had pain on admission. Within two months after implementation of the compliance with the new Pain Assessment/History form, completion jumped to 85%, then dropped to 75% and plateaued. In an effort to improve compliance, additional enhancements were made over time based on suggestions from the Pain Task Force. Once all items on the form were required for patients without excruciating pain on admission, compliance rates increased and plateaued at 90%. Audits by units showed that some patients admitted on the day of surgery or with excruciating pain on admission had incomplete forms (nurses have the option of delaying a comprehensive pain assessment in patients with uncontrolled pain upon admission). Capturing comprehensive pain assessments in these patients required changes in clinical processes as well as implementing a reminder system. To expedite patient flow, the postanesthesia care units agreed to complete forms for all surgical patients. In addition, the system was programmed to place a boxed red flag in front of the Pain Assessment/History Form name on the “index tab” that lists all chart elements when the form is incomplete (Figure C-15). When the form is complete, the boxed flag is blue (Figure C-16).
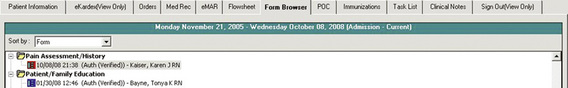
Figure C-15 Incomplete pain assessment history form. Box turns red to indicate task is incomplete. University of Maryland Medical Center.
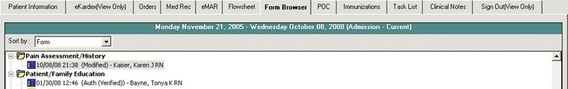
Figure C-16 Complete pain assessment history form. Box turns blue to indicate task is complete. University of Maryland Medical Center.
The UMMC has had similar success with preanalgesic pain score and postanalgesic pain score documentation. Preanalgesic pain score documentation has improved from 85% using paper documentation to 96% using electronic documentation. Postanalgesic pain score documentation has improved from approximately 79% using paper documentation to 87% with electronic documentation. The processes used to improve this documentation were analogous to those used to improve pain assessment history documentation. The Pain Task Force played an active role with these adaptations, required fields were used, reminder systems were implemented, nursing processes were changed, and the electronic form went through several adaptations. A synopsis of the changes is provided.
When computerized prescriber order entry was initiated at the UMMC, the vendor-developed preanalgesic and postanalgesic pain score forms were initiated. The preanalgesic form allows a preanalgesic pain score to be documented. The postanalgesic form provided a visual reminder to document a postanalgesic score. In this case, the visual cue is a blue flag posted on the eMAR for the time the nurse should obtain a postanalgesic pain score (at approximate peak effect of the administered analgesic) (see Figure C-14). The blue flag posts immediately after the nurse documents administration of the analgesic. The flag turns red, which indicates that the task is overdue if the nurse fails to document a postanalgesic pain score (Figure C-17). Due to system limitations at the time of implementation, this flag pertained only to PRN analgesics. Postanalgesic pain score documentation for PRN analgesics is consistently above 95%.
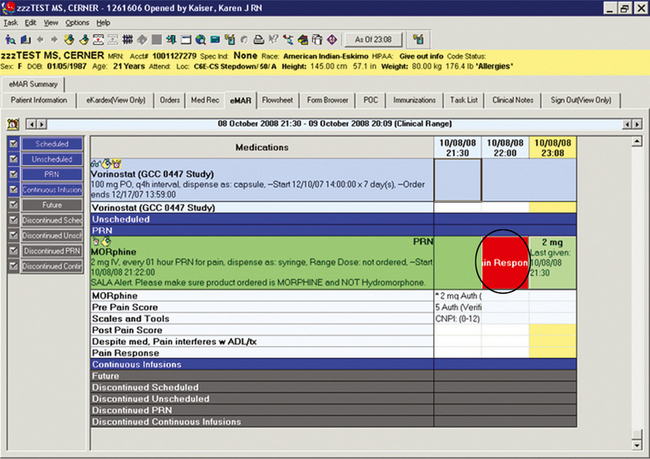
Figure C-17 Post pain response overdue task reminder. Response box is red, indicating task is overdue. University of Maryland Medical Center.
Pain Task Force members and staff notified the implementation team of the limitations of these forms. During the redesign process, the members of the Pain Task Force at the UMMC were instrumental in making several enhancements to the prebuilt tools for recording pain scores. The solution devised was more comprehensive and clinically useful than the original. This outcome was obtained by (a) face to face discussions with the information technology staff, (b) review of sequential animated drafts, and (c) evaluation of the final product.
For the preanalgesic form, the Pain Task Force requested the addition of an item that allows the selection and documentation of the pain measurement tool used to assess the presence of pain or pain severity. This encourages members of the health care team to consistently use the selected pain measurement tool for a particular patient, unless the patient’s cognition level changes, necessitating a more appropriate tool. For example, if the patient could not self-report pain due to head trauma, a behavioral tool would be used until the patient was able to self-report pain, at which time a self-report tool such as a numerical rating scale or verbal descriptor scale would be used.
Pain Task Force members felt that to evaluate the analgesic’s effectiveness, information on postanalgesic activities of daily living (ADL) was needed. So, for the postanalgesic form they requested a place to document pain interference with ADL and treatments (e.g., physical therapy, occupational therapy, respiratory treatment), which the nurse assesses when the postanalgesic pain score is obtained. The Pain Task Force also suggested reformatting the existing tool so that postanalgesic pain scores could be easily visualized for comparison with preanalgesic pain scores, when evaluating analgesic effectiveness (Figure C-18). Based on evaluation of the new forms, postanalgesic pain scores and documentation of pain interference have been made required fields for PRN analgesics. Besides encouraging assessment of the effectiveness of the analgesic and the overall pain treatment plan, the postanalgesic pain documentation form serves as a cue to remind the nurse to modify the care plan if pain interferes with ADLs or treatments.
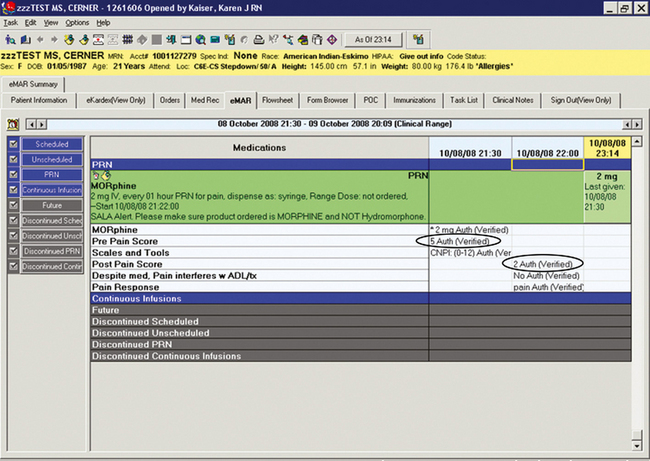
Figure C-18 Post pain response documentation allows comparison of pre and post pain scores. University of Maryland Medical Center.
Because of system limitations, postanalgesic pain score documentation fields used to assess PRN analgesic effectiveness are not yet required for scheduled analgesics. As previously noted, there is also no visual reminder (i.e., blue or red flag) to reassess pain for scheduled medications. Based on Pain Task Force recommendations that it would provide a visual cue for documentation and system requirements, the customized PRN and scheduled analgesic electronic documentation forms are similar. The presence of the postanalgesic pain assessment fields did initially improve compliance with documentation of postanalgesic pain scores on scheduled medications, but it was not sustainable.
Postanalgesic pain score documentation for scheduled medications averages around 70%. Until a vendor solution to this limitation can be initiated with a version upgrade, a static report was developed and is run several times daily by the clinical units. The report searches current patients’ EMRs for completion of required documentation fields. Nurses can use the report (particularly prior to or at change of shift or transfer) to ensure that their pain care and documentation are complete. Units that have integrated this into their daily clinical practice consistently score over 90% compliance with their postanalgesic pain scores for scheduled medications on their monthly audits.
Static reminders such as the one described above are not as likely to ensure compliance the way a timed reminder would. Currently, we utilize a static notation on an electronic plan (eKardex) for assessment of pain for patients on specialized analgesic therapy. While we have seen an improvement in pain documentation of specialized analgesic therapy since our last audit, the audits are not directly comparative.
At the UMMC, several of the processes that were initially used in developing pain-related electronic medical record features have been successfully applied to other electronic processes at our facility, such as required fields, order sets, red reminder flags, and drug database customization. Similarly, electronic processes used in other situations have been applied to support pain-related best practices such as requiring documentation of fentanyl patch placement site(s) and subsequent patch removal. The UMMC has also used the EMR or data from it in a variety of ways to support other best pain management practices. We have used the data in process improvement activities, guiding refinements in the EMR system, and associated processes. For example, data in the EMR system have been used to assess and reduce IM opioid administration and inappropriate medication use (e.g., meperidine and propoxyphene) as well as discourage high doses of opioids in opioid-naïve patients via range-checking alerts. Many other pain-related safe practices and best practices are also easily assessed, implemented, supported, and evaluated using EMR technology.
Safeguards
There are many benefits to implementing an EMR system. Although EMR systems have been shown to decrease some errors, new types or causes of errors can surface as well (Campbell, Sittig, Ash, et al., 2006; Koppel, Metlay, Cohen, et al., 2005). However, many of these errors offer opportunities to make the EMR system and associated processes safer. Evaluation of how dose range checking alerts fire can provide an excellent opportunity to improve the system. For example, it may be discovered that only one of several alerts fire (e.g., weight but not age for ketorolac), or that the dose range is set too high for opioid-naïve patients (e.g., 0.8 to 4 mg), or that prescribers can order a drug to be administered via a route inconsistent with its purpose (e.g., duramorph intravenously rather than intraspinally). In these cases, revisions are necessary. However, numerous overrides of an alert may also be dangerous, as practitioners may not read the alert and instead automatically dismiss it (van der Sijs, Aarts, Vulto, et al., 2006). These situations require education or revision of the alert so that it serves its original purpose. Unsafe or inappropriate practices can be discouraged by limiting the routes of administration that the prescriber can order for a particular drug, provider, or clinical location (see Box C-2).
The EMR is sometimes seen as a comprehensive error reduction strategy, leading some to implicitly trust the information generated in the system, including orders. Health care practitioners may lapse into a false sense of security even though errors exist. Prebuilt order sentences that undergo dose range checking using noncustomized databases can look correct even though they are inconsistent with best-practice guidelines and may be unsafe. Also, it may be impossible to detect a custom-built order from an order that was prebuilt and carefully reviewed in a nonrushed, nonclinical environment.
Health care practitioners may not be trained for, understand the need for, or integrate new processes required by the EMR into clinical practice (Kushniruk, Borycki, Kuwata, et al., 2006; Spence, Valenza, Taylor, 2007; Tuttelmann, Luetjens, Nieschlag, 2006). For example, pharmacists and physicians may not realize that nursing staff do not receive dose range–checking alerts, and nurses may falsely think prescribers and pharmacists will respond appropriately to all dose range–checking alerts. There may also be changes in processes for some disciplines that are not readily apparent. For example, as previously described, the pharmacist, the nurse, and the physician see different screen views of the same medication order during the ordering, preparation, and administration process and thus have access to different information on their computer screens. Health care practitioners may operate under the assumption that the checks and balances that were in place with the paper-based system are still available with the EMR system, when, in fact, they no longer exist. It is imperative for staff to adjust to work flow process changes upon EMR implementation and to understand the work flow and processes of other disciplines to reduce error and improve patient safety (Salas, Wilson, Murphy, et al., 2008; Scott, Poole, Jayathissa, 2008).
Conclusion
As demonstrated above, the EMR has great potential to improve documentation and adherence to regulatory and accreditation standards and best-practice guidelines, as well as improve patient safety. Nurses are the practitioners with the greatest understanding of evidence-based pain guidelines and effective strategies to manage pain and minimize potential associated safety issues such as those discussed in this appendix. Most if not all pain specialists have developed safe processes for managing pain using paper-based medical record systems that can be applied to the EMR system. However, pain specialists usually see only a small percentage of their institution’s patients. Based on the complexity of pain orders and the high-volume, high-risk (but low-potential) nature of errors associated with managing pain (some of which were demonstrated by examples provided in this appendix), it is recommended that pain practitioners take the initiative to become actively involved in and the primary owner of pain-related EMR development, implementation, and revisions. This includes creating a comprehensive medication reconciliation process, customized evidence-based dose range checking, safe and effective pain order sentences and order sets, appropriate alerts, electronic auditing procedures, and other processes that support best clinical practices. In addition, documentation processes that encourage thorough, adequate assessment and evaluation of pain relief interventions are essential. Pain practitioners should also ensure that there is consistency across institutional pain-related electronic technologies (e.g., Smart Pump technology, medication dispensing cabinets, and the EMR) to facilitate safety.
There is a general lack of research on pain-related EMRs. Until research can be conducted on this new technology, sharing information with colleagues about what strategies work best in the clinical setting and potential pitfalls associated with EMR is strongly encouraged. The overall goal is the development of streamlined safe, evidence-based, effective pain assessment and management from an interdisciplinary perspective using electronic technology.
References
American Pain Foundation. Overview of American pain surveys: 2005–2006. Journal of Pain & Palliative Care Pharmacotherapy. 2008;22(1):33–38.
American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain, 5th ed. Glenview, IL: American Pain Society, 2003.
American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain, 6th ed. Glenview, IL: American Pain Society, 2008.
Bercovitz, A., Decker, F.H., Jones, A., et al. End-of-life care in nursing homes: 2004 National Nursing Home Survey. Natl Health Stat Report. 2008;8(9):1–23.
Botti, M., Bucknall, T., Manias, E. The problem of postoperative pain: Issues for future research. International Journal of Nursing Practice. 2004;10(6):257–263.
Bradshaw, D.H., Empy, C., Davis, P., et al. Trends in funding for research on pain: A report on the National Institutes of Health grant awards over the years 2003 to 2007. The Journal of Pain. 2008;9(12):1077–1087.
Campbell, E.M., Sittig, D.F., Ash, J.S., et al. Types of unintended consequences related to computerized provider order entry. Journal of the American Medical Informatics Association. 2006;13(5):547–556.
Cohen, M.Z., Easley, M.K., Ellis, C., et al. Cancer pain management and the JCAHO’s pain standards: An institutional challenge. Journal of Pain and Symptom Management. 2003;25(6):519–527.
Cowell, J. Improving patient safety by eliminating unsafe abbreviations from medication prescribing. Alberta RN. 2007;63(8):8–9.
Dobratz, M.C., Burns, K.M., Oden, R.V. Pain in home hospice patients: An exploratory descriptive study. The Hospice Journal. 1989;5(3–4):117–133.
Doran, D.M., Mylopoulos, J., Kushniruk, A., et al. Evidence in the palm of your hand: Development of an outcomes-focused knowledge translation intervention. Worldviews on Evidence-Based Nursing. 2007;4(2):69–77.
Federico, F. Preventing harm from high-alert medications. Joint Commission Journal on Quality and Patient Safety. 2007;33(9):537–542.
Garg, A.X., Adhikari, N.K., McDonald, H., et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: A systematic review. JAMA. 2005;293(10):1223–1238.
Green, C.R. The healthcare bubble through the lens of pain research, practice, and policy: Advice for the new President and Congress. The Journal of Pain. 2008;9(12):1071–1073.
Gruner, A., Ljutow, A., Schleinzer, W., et al. Implementation of an electronic patient record. Experience in an interdisciplinary pain clinic. Schmerz (Berlin, Germany). 2008;22(1):24–33.
Hidle, U. Implementing technology to improve medication safety in healthcare facilities: A literature review. The Journal of the New York State Nurses’ Association. 2007;38(2):4–9.
Hunt, D.L., Haynes, R.B., Hanna, S.E., et al. Effects of computer-based clinical decision support systems on physician performance and patient outcomes: A systematic review. JAMA. 1998;280(15):1339–1346.
Kaushal, R., Shojania, K.G., Bates, D.W. Effects of computerized physician order entry and clinical decision support systems on medication safety: A systematic review. Archives of Internal Medicine. 2003;163(12):1409–1416.
Koppel, R., Metlay, J.P., Cohen, A., et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293(10):1197–1203.
Kushniruk, A., Borycki, E., Kuwata, S., et al. Predicting changes in workflow resulting from healthcare information systems: Ensuring the safety of healthcare. Healthcare Quarterly (Toronto, Ont.). 9, 2006. [Spec-8].
Leapfrog Group safety practices: Computerized physician order entry. Available at http://www.leapfroggroup.org/for_ hospitals/leapfrog_safety_practices/cpoe [Accessed March 15, 2009. Last update 2009].
McMillan, S.C., Williams, F.A., Chatfield, R., et al. A validity and reliability study of two tools for assessing and managing cancer pain. Oncology Nursing Forum. 1988;15(6):735–741.
Nemeth, L.S., Wessell, A.M., Jenkins, R.G., et al. Strategies to accelerate translation of research into primary care within practices using electronic medical records. Journal of Nursing Care Quality. 2007;22(4):343–349.
Pakhomov, S.V., Jacobsen, S.J., Chute, C.G., et al. Agreement between patient-reported symptoms and their documentation in the medical record. The American Journal of Managed Care. 2008;14(8):530–539.
Pathways for medication safety. Available at http://www.ismp.org/Tools/pathways.asp [Accessed March 15, 2009. Last update 2007].
Saigh, O., Triola, M.M., Link, R.N. Brief report: Failure of an electronic medical record tool to improve pain assessment documentation. Journal of General Internal Medicine. 2006;21(2):185–188.
Salas, E., Wilson, K.A., Murphy, C.E., et al. Communicating, coordinating, and cooperating when lives depend on it: tips for teamwork. Joint Commission Journal on Quality and Patient Safety. 2008;34(6):333–341.
Scott, I.A., Poole, P.J., Jayathissa, S. Improving quality and safety of hospital care: A reappraisal and an agenda for clinically relevant reform. Internal Medicine Journal. 2008;38(1):44–55.
Spence, J., Valenza, J.A., Taylor, D. Documentation of clinical workflow: A key step in a plan to facilitate implementation of an Electronic Patient Record. AMIA Annual Symposium Proceedings. 2007;11:1119.
The Joint Commission Addendum to Sentinel Event #35 Medication Reconciliation. Available at http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_35.htm, 2006. [Accessed March 17, 2009].
The Joint Commission Sentinel Event #11 High-Alert Medications and Patient Safety. Available at http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_11.htm, 2006. [Accessed March 18, 2009].
Tuttelmann, F., Luetjens, C.M., Nieschlag, E. Optimising workflow in andrology: A new electronic patient record and database. Asian Journal of Andrology. 2006;8(2):235–241.
van der Sijs, H., Aarts, J., Vulto, A., et al. Overriding of drug safety alerts in computerized physician order entry. Journal of AHIMA. 2006;13(2):138–147.
VHA Office of Information VistA Computerized Patient Record System. Available at http://www1.va.gov/CPRSdemo/VHAOffice of Information [Accessed March 21, 2009. Last updated 2009.].
Wager, K.A., Lee, F.W., White, A.W., et al. Impact of an electronic medical record system on community-based primary care practices. The Journal of the American Board of Family Practice. 2000;13(5):338–348.
Whipple, N.N., Palchuk, M.B., Olsha-Yehiav, M., et al. Supporting CMT and user customization in Clinical Documentation templates. AMIA Annual Symposium Proceedings. 2007:1153. [Oct 11].
Wolfstadt, J.I., Gurwitz, J.H., Field, T.S., et al. The effect of computerized physician order entry with clinical decision support on the rates of adverse drug events: A systematic review. Journal of General Internal Medicine. 2008;23(4):451–458.
Yellowlees, P.M., Marks, S.L., Hogarth, M., et al. Standards-based, open-source electronic health record systems: A desirable future for the U.S. health industry. Telemedicine Journal and E-health. 2008;14(3):284–288.