48 VAGINAL CYTOLOGY
2 What are basal cells?
Basal cells are located on the basement membrane and are the precursor cell for the other vaginal epithelial cells (parabasal, intermediate, superficial). Basal cells are seldom observed on vaginal cytology smears and appear as small cells with a small amount of basophilic cytoplasm.
3 What are parabasal cells?
Parabasal cells are the smallest and most immature of the epithelial cells typically observed on cytology smears. They appear as small round cells with a small amount of basophilic cytoplasm and a roundish nucleus. Parabasal cells with cytoplasmic vacuoles are called foam cells.
4 What are intermediate cells?
Intermediate cells are larger than parabasal cells, often being twice as large or larger. Depending on their size, intermediate cells are subclassified into small and large intermediate cells. The size of intermediate cells depends on the amount of their cytoplasm because the nucleus of both large and small intermediate cells is about the same size and typically roundish. The cytoplasm of small intermediate cells is usually smooth and round to slightly oval. The cytoplasm of large intermediate cells tends to be angular and occasionally may also be irregular and folded. The cytoplasm is typically blue to blue-green in both small and large intermediate epithelial cells. The terms superficial and transitional are occasionally used for large intermediate cells.
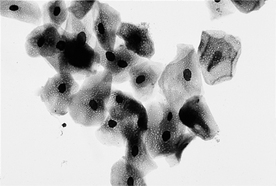
5 What are superficial cells?
Superficial cells are the most mature cells of vaginal epithelial stages. They are large cells with abundant, angular or folded, blue to blue-green cytoplasm. The cytoplasm may contain a few to many vacuoles, and some cells may contain dark-staining bodies of unknown significance. The nucleus is smaller than that of intermediate cells and may be faded or even absent (anucleate). The maturation process and development of superficial cells are often referred to as cornification, and superficial epithelial cells are sometimes referred to as cornified cells.
7 What are the cytologic features of proestrus in dogs?
8 Are the red blood cells observed on proestrus vaginal cytology smears from vaginal vessels?
RBCs observed during proestrus are not from vaginal vessels; they are erythrocytes exiting uterine capillaries by diapedesis.
9 What is the duration of proestrus in dogs?
In normal dogs, proestrus ranges from 2 to 17 days (depending on the reference source) with a mean duration of 9 days.
10 How do the cytologic features of proestrus in cats differ from those in dogs?
In cats, RBCs and neutrophils are absent, so only the epithelial cells are present. The epithelial cell changes are similar to those in dogs. Also, bacteria may be present.
11 What are the cytologic features of estrus in dogs?
12 How do the cytologic features of estrus in cats differ from those in dogs?
The cytologic features are similar except that superficial epithelial cells may comprise less than 90% (40%-88%) in feline estrus and RBCs are absent. Anucleate superficial epithelial cells generally make up 10% to 40% of the epithelial cells. Also, a prominent clearing of the background is observed in most cases of feline estrus, which some suggest is a sensitive indicator of estrus in the cat.
13 Is cytology a good predictor of the luteinizing hormone peak and time of ovulation in dogs?
Cytology is not a good predictor of LH peak and canine ovulation because too much variation exists. The occurrence of cytologic estrus may range from 6 days before to 3 days after the LH peak. Ovulation usually occurs 1 to 3 days after the LH peak.
14 What is the duration of estrus in dogs?
Duration of estrus in mature dogs has a broad range of 3 to 21 days, with an average duration of 9 days.
15 What is the duration of estrus in cats?
Duration of estrus in cats ranges from 3 to 16 days, with an average duration of 8 days.
16 Is the estrus cycle the same in dogs and cats?
The estrus cycle is not the same in cats and dogs. Cats are seasonally polyestrus, with the interval between estrus periods ranging from 4 to 22 days (average of 9 days) unless ovulation occurs. All stages other than estrus may occur during this interval period.
18 When ovulation occurs in a cat, does it delay onset of the next estrus?
Ovulation and pseudopregnancy, which generally follows ovulation, will delay the next estrus for about 45 days.
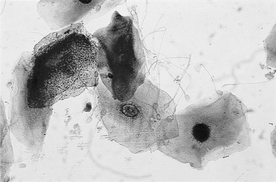
19 What are the cytologic features of diestrus in the dog?
In canine diestrus there is an abrupt decrease in superficial epithelial cells and an increase in parabasal cells and intermediate cells. Although the number is variable (absent to high numbers), neutrophils are often present in the cytology smear in moderate numbers. Neutrophils also may be observed within the cytoplasm of epithelial cells (see next question). RBCs may be present or absent during diestrus in the dog.
20 What are metestrous cells?
Metestrous cells are epithelial cells that have neutrophils within their cytoplasm. They may occur in any stage of the estrous cycle except estrus in normal dogs and therefore are not specific for diestrus (metestrus). Metestrous cells are more likely to be observed in diestrus because neutrophil migration across epithelial cells typically occurs during diestrus in dogs.
21 When does diestrus occur in the dog?
Canine diestrus occurs about 6 to 10 days after the LH peak, with a mean of 8 days.
22 How do the cytologic features of diestrus in cats differ from those in dogs?
Vaginal cytology smears from cats in diestrus do not contain RBCs. Neutrophils are usually absent, but a few neutrophils may be observed. The epithelial changes are similar to those in dogs.
23 Is the lack of neutrophils and red blood cells a problem in evaluating vaginal cytology smears from cats?
If serial smears are not evaluated, lack of neutrophils and RBCs may be a problem. Feline vaginal cytology smears made during the transition period from estrus to diestrus appear similar to those in proestrus. It is often necessary to evaluate smears daily for several days to identify the stage of estrus accurately.
24 What are the cytologic features of anestrus in the dog?
Vaginal cytology smears during canine anestrus consist primarily of parabasal and intermediate epithelial cells. Neutrophils and bacteria are absent or present only in low numbers.
25 Do the cytologic features of anestrus in the cat differ from those in the dog?
The cytologic features of anestrus in the cat are the same as those in the dog, except neutrophils are absent.
26 What is the cytologic appearance of vaginal smears from prepubertal dogs?
Vaginal cytology smears from prepubertal dogs are similar to those from anestrus dogs, except the smears may have very high numbers of parabasal cells, which occasionally exfoliate in sheets. When parabasal cells exfoliate in sheets, it is important not to confuse them with neoplastic cells.
27 Can spermatozoa be found on vaginal cytology to confirm mating?
The closer the sample collection time is to mating time, the higher the percentage of positive samples to confirm mating. Intact spermatozoa or sperm heads are reportedly present in approximately 65% of the vaginal cytology smears collected 24 hours after mating and 50% of samples collected 48 hours after mating (Figure 48-2). Negative findings cannot be used to rule out mating.
28 What is the significance of finding uterine glandular epithelial cells on a vaginal cytology?
Uterine glandular epithelial cells are occasionally found in vaginal smears, especially soon after whelping and in dogs with subinvolution of placental sites.
29 What is the cytologic appearance of a vaginal smear from a dog with an open pyometra?
The vaginal smear would be of high cellularity and consist primarily of degenerative neutrophils in a dog with an open pyometra. Bacteria may be observed within the cytoplasm of some or many of the neutrophils.
30 How would diestrus be differentiated from pyometra cytologically?
Cytologically, one would expect to see degenerative neutrophils and phagocytized bacteria in an open pyometra and nondegenerative neutrophils with or without phagocytized bacteria in diestrus. (Phagocytized bacteria may be observed in vaginal smears from some dogs during diestrus.)
Baker R, Lumsden JH. Cerebrospinal fluid. In: Baker R, Lumsden JH, editors. Color atlas of cytology of the dog and cat. St Louis: Mosby; 2000:95-115.
Baker R, Lumsden JH. The gastrointestinal tract: intestines, liver, and pancreas. In: Baker R, Lumsden JH, editors. Color atlas of cytology of the dog and cat. St Louis: Mosby; 2000:179-181.
Baker R, Lumsden JH. Infectious agents. In: Baker R, Lumsden JH, editors. Color atlas of cytology of the dog and cat. St Louis: Mosby; 2000:23-29.
Baker R, Lumsden JH. Pleural and peritoneal fluids. In: Baker R, Lumsden JH, editors. Color atlas of cytology of the dog and cat. St Louis: Mosby; 2000:159-164.
Baker R, Lumsden JH. The reproductive tract: vagina, uterus, prostate, and testicle. In: Baker R, Lumsden JH, editors. Color atlas of cytology of the dog and cat. St Louis: Mosby; 2000:235-238.
Baker R, Lumsden JH. The respiratory tract: nasal, bronchial, and tracheal wash and lung. In: Baker R, Lumsden JH, editors. Color atlas of cytology of the dog and cat. St Louis: Mosby; 2000:133-140.
Blue JT, French TW, Meyer DJ. The liver. In: Cowell RL, Tyler RD, Meinkoth JH, editors. Diagnostic cytology and hematology of the dog and cat. St Louis: Mosby; 1999:183-194.
Burkhard MJ, Valenciano A, Barger A. Respiratory tract. In: Raskin RE, Meyer DJ, editors. Atlas of canine and feline cytology. Philadelphia: Saunders; 2002:157-182.
Cowell RL. Cytology of neoplasia. Orlando, Fla: Eastern States Veterinary Association, 2002;154-155. Proceedings of the North American Veterinary Conference, Small Animal and Exotic
Cowell RL, Dorsey KE, Meinkoth JH. Lymph node cytology. Vet Clin North Am Small Anim Pract. 2003;33:47-67.
Cowell RL, Thrall MA, Rebar AH. Cytology of skin masses. Orlando, Fla: Eastern States Veterinary Association, 2003;171-172. Proceedings of the North American Veterinary Conference, Small Animal and Exotic
Cowell RL, Tyler RD, Baldwin CJ, Meinkoth JH. Transtracheal/bronchoalveolar washes. In: Cowell RL, Tyler RD, Meinkoth JH, editors. Diagnostic cytology and hematology of the dog and cat. St Louis: Mosby; 1999:159-173.
Cowell RL, Tyler RD, Meinkoth JH. Abdominal and thoracic fluid. In: Cowell RL, Tyler RD, Meinkoth JH, editors. Diagnostic cytology and hematology of the dog and cat. St Louis: Mosby; 1999:142-158.
Duncan JR. The lymph nodes. In: Cowell RL, Tyler RD, Meinkoth JH, editors. Diagnostic cytology and hematology of the dog and cat. St Louis: Mosby; 1999:97-103.
Freeman KP, Raskin RE. Cytology of the central nervous system. In: Raskin RE, Meyer DJ, editors. Atlas of canine and feline cytology. Philadelphia: Saunders; 2001:325-365.
Henson KL. Reproductive system. In: Raskin RE, Meyer DJ, editors. Atlas of canine and feline cytology. Philadelphia: Saunders; 2001:289-297.
Lumsden JH, Baker R. Cytopathology techniques and interpretation. In: Baker R, Lumsden JH, editors. Color atlas of cytology of the dog and cat. St Louis: Mosby; 2000:7-20.
Meinkoth JH, Crystal MA. Cerebrospinal fluid analysis. In: Cowell RL, Tyler RD, Meinkoth JH, editors. Diagnostic cytology and hematology of the dog and cat. St Louis: Mosby; 1999:125-141.
Meinkoth JH, Cowell RL. Recognition of basic cell types and criteria of malignancy. Vet Clin North Am Small Anim Pract. 2002;32:1209-1235.
Meinkoth JH, Cowell RL. Sample collection and preparation in cytology: increasing diagnostic yield. Vet Clin North Am Small Anim Pract. 2002;32:1187-1207.
Menard M, Papageorges M. Fine-needle biopsies: how to increase diagnostic yield. Comp Contin Educ Pract Vet. 1997;19:738-740.
Meyer DJ. The acquisition and management of cytology specimens. In: Raskin RE, Meyer DJ, editors. Atlas of canine and feline cytology. Philadelphia: Saunders; 2001:1-17.
Meyer DJ. The liver. In: Raskin RE, Meyer DJ, editors. Atlas of canine and feline cytology. Philadelphia: Saunders; 2001:231-252.
Raskin RE. General categories of cytologic interpretations. In: Raskin RE, Meyer DJ, editors. Atlas of canine and feline cytology. Philadelphia: Saunders; 2001:27-33.
Raskin RE. Lymphoid system. In: Raskin RE, Meyer DJ, editors. Atlas of canine and feline cytology. Philadelphia: Saunders; 2001:93-134.
Raskin RE. Skin and subcutaneous tissues. In: Raskin RE, Meyer DJ, editors. Atlas of canine and feline cytology. Philadelphia: Saunders; 2001:35-56.
Shelly SM. Body cavity fluids. In: Raskin RE, Meyer DJ, editors. Atlas of canine and feline cytology. Philadelphia: Saunders; 2001:187-205.
Taylor JA, Baker R. The lymphoid system: lymph nodes, spleen, and thymus. In: Baker R, Lumsden JH, editors. Color atlas of cytology of the dog and cat. St Louis: Mosby; 2000:71-94.
Thrall MA, Olson PN. The vagina. In: Cowell RL, Tyler RD, Meinkoth JH, editors. Diagnostic cytology and hematology of the dog and cat. St Louis: Mosby; 1999:240-248.
Tyler RD, Cowell RL, Baldwin CJ, Morton RJ. Introduction. In: Cowell RL, Tyler RD, Meinkoth JH, editors. Diagnostic cytology and hematology of the dog and cat. St Louis: Mosby; 1999:1-19.
Tyler RD, Cowell RL, Meinkoth JH. Cutaneous and subcutaneous lesions: masses, cysts, ulcers, and fistulous tracts. In: Cowell RL, Tyler RD, Meinkoth JH, editors. Diagnostic cytology and hematology of the dog and cat. St Louis: Mosby; 1999:23-51.
Weiss DJ, Moritz A. Liver cytology. Vet Clin North Am Small Anim Pract. 2002;32:1267-1291.