Transport of critically ill patients
Critical illness and injury are not necessarily defined by patient location. In addition, patients may overwhelm the level of care at their current location or require specific investigations or treatments not immediately available to them. For this reason, transport of critically ill and injured patients occurs frequently.
Critical care patient transport has traditionally been divided into two groups; patient movement within a hospital (intra-hospital) or movement between hospitals (inter-hospital or inter-facility). In addition, a select group of critically ill or injured patients not located in a hospital facility may be managed by physician-based medical teams prior to retrieval to a medical facility. Therefore, a third division (primary response or pre-hospital care) is well recognised.
The internationally widespread deployment of medical teams for critically ill patient management and retrieval from both health care facilities and pre-hospital locations has resulted in the developing recognition of pre-hospital and retrieval medicine as a distinct subspecialty.1
Intra-hospital transport
Transports are usually required to facilitate critical investigations and interventions or to move the patient from one critical care area to another. Critically ill or injured patients with limited or no physiological reserve undergoing such transports are at risk of clinical deterioration and adverse events are well reported.2,3 In order to reduce the mortality and morbidity associated with patient movement, a structured approach utilising high-level clinical personnel who have the correct equipment, training and sufficient planning time is required.
Moving the patient should be associated with little or no compromise in their condition. Unfortunately this is not the case with an adverse event occurring in up to 70% of transports. One-third of these events are equipment related,4 whereas deterioration in gas exchange5 and increased rates of ventilator-associated pneumonia are common.6 However, management is changed in 40–50% of patients, thus justifying the risk.
Patients with unstable physiology should not be transported for non-urgent interventions or investigations. However, where the intervention or investigation is deemed critical to achieving patient stability or providing definitive management, the benefits in patient outcome will outweigh the inherent risks of transport. The transport can therefore be seen as part of the patient's therapeutic requirement and stabilisation process. On occasions when the patient's need is so acute and/or the likelihood of irreversible deterioration in transit is so high consideration should be given to facilitating such interventions or investigations in the ICU rather than the locality where these procedures would normally occur.
When preparing for intra-hospital transport, the following structured approach is recommended:
• Clinical reassessment should occur swiftly, systematically and, whenever possible, with the patient already supported on the equipment that will be used during transport.
• The airway should be checked and secured, endotracheal suction performed, ventilation and oxygenation optimised, adequate and patent vascular access secured and drainage devices measured and emptied.
• Sedation and analgesic requirements should be addressed and any drugs required for transport (including additional infused agents) pre-drawn and labelled for immediate use.
• Ensure that the patient clinical record remains with the team caring for the patient.
Computed tomoraphy and magnetic resonance imaging scanning
CT scanning is the most common ICU diagnostic intervention requiring patient movement. Head injury patients and those requiring previous administration of oral contrast with decreased gut motility (and thus increasing risk of aspiration) require extra attention. The administration of i.v. contrast through standard multi-lumen central lines is not possible and a large-bore intravenous cannula needs to be inserted and well secured prior to leaving the ICU. Single-lumen large-bore central catheters are an alternative but should be used only as a last resort.
Repeated CT scanning of head-injured patients is common. In these patients with decreased cerebral compliance, movement or changes in body position or 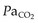 can result in significant elevations in intracranial pressure. Although movement-induced changes in ICP can be reduced with sedation, very little can be done about body position. Changes in
can result in significant elevations in intracranial pressure. Although movement-induced changes in ICP can be reduced with sedation, very little can be done about body position. Changes in 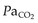 are usually due to variation between ICU and transport ventilators. Most transport ventilators are less precise at setting tidal volume, respiratory rate and PEEP compared with standard ICU ventilators. These changes can have significant effects on minute volume and lung compliance. If time permits,
are usually due to variation between ICU and transport ventilators. Most transport ventilators are less precise at setting tidal volume, respiratory rate and PEEP compared with standard ICU ventilators. These changes can have significant effects on minute volume and lung compliance. If time permits, 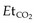 should be established using the transport monitor for 10–15 minutes. This sets the baseline
should be established using the transport monitor for 10–15 minutes. This sets the baseline 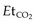 that must be maintained when the patient is connected to the transport ventilator. Respiratory rate or tidal volume is adjusted to maintain the
that must be maintained when the patient is connected to the transport ventilator. Respiratory rate or tidal volume is adjusted to maintain the 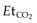 . Ideally the ICP should also be measured but at times this may not be possible.
. Ideally the ICP should also be measured but at times this may not be possible.
Radiation exposure for both the patient and staff needs to be considered. A stable patient who is adequately monitored with alarms activated can be observed by staff outside the room. The patient should be moved back to the ICU as soon as scanning is completed.
The use of MRI for ICU is increasing as a diagnostic and prognostic tool for a wide range of ICU patients. The major problems with MRI are the effect that the magnetic field may have on ventilators, monitors and infusion pumps and the potential for these items to become effectively a missile by being attracted to the magnet. The last 5–10 years have seen the development of monitoring and ventilators that are MRI compatible and the acceptance of standards for equipment in the MRI. These magnetic resonance standards are: MR safe, conditional or unsafe. Safe and unsafe are self-explanatory, while conditional relates to equipment that is safe when kept at a predetermined distance from the magnet. Although a lot of equipment has been developed as MR safe, it has been developed from anaesthetic practice for the provision of general anaesthesia during the MRI examination for some patients. This equipment is ‘foreign’ to most ICU staff and often is not used owing to lack of familiarity. As a result there has been a blending of practice: using some of the ‘anaesthesia’ equipment but also continuing to use ICU infusions and ventilators at some distance from the magnet. Distance from the magnet is achieved by the insertion of extension tubing but this must be balanced with the risk of disconnection.
Thermal dilution pulmonary artery catheters are probably safe, although opinion varies. Absolute contraindications for MRI scanning include pacemakers, internal defibrillators and cerebral aneurysm clips, whereas other clips may require a period of time, up to 6 weeks, to allow stabilisation within the tissues before scanning can occur. Prior discussion with the MRI unit must occur before the patient is moved from the ICU. Most MRI units will require an MRI checklist to be completed prior to scanning.
Staffing
A team consisting of at least one ICU medical officer and nurse should be free from other duties. Both team members should be thoroughly familiar with the transport process, equipment and environment. The team should possess the requisite skills and knowledge to independently manage critically ill patients in transit and to deal with anticipated emergencies. The more complex and unstable the patient is, the more capable the team must be. For very unstable patients an additional nurse and more senior doctors may be required. Assistance with safe patient, trolley and equipment movement will also be needed. Non-clinical hospital support staff are therefore part of the team and should be included in all briefs and contingency planning.
Equipment
Transport equipment should be regularly checked and serviceable. Powered devices should be fully charged, with power cords accessible to facilitate use of mains power in the event of delay. Where possible, equipment should be lightweight, robust and standardised throughout the ICU and hospital. In transit, equipment should be secured (not resting on the patient) but readily accessible. Dedicated transport bridges or gantries are commonly used. Dedicated transport packs or boxes ensure safe carriage of consumable items, resuscitation equipment and drugs. Equipment required for emergency airway management (e.g. bag valve mask, laryngoscope, airway devices and endotracheal tubes) should be immediately available.
Monitoring
As a minimum, intubated and ventilated patients requiring intra-hospital transport should have the following monitoring instituted:7
Ideally, a cardiac monitoring device should also provide cardiac defibrillation and external cardiac-pacing capacity. Patients requiring transport with more advanced monitoring in situ should be considered on a case-by-case basis. For example, ongoing ICP monitoring is critical to ensure avoidance of profound unmonitored falls in cerebral perfusion pressure in an ICU patient with a severe head injury, whereas pulmonary artery pressure monitoring may be excluded from the transport requirements in the haemodynamically stable patient.
Inter-hospital transport
Historical models of inter-hospital patient transfer utilising junior medical staff as ‘patient escorts’ have much higher rates of hypotension, acidosis and death.8 Thankfully this type of transport has become increasingly rare with the introduction of specialist retrieval services.
The general principles of patient transport, irrespective of the physical location of the patient, regarding equipment, patient monitoring and clinical requirements remain the same. Standards for transportation of the critically ill have been widely promulgated and must be followed whether it is a complex unstable patient being moved long distances or a semi-elective CT in a stable ICU patient.
With rising expectations for high-level care by the community in both metropolitan and rural locations and with the care for critically ill and injured patients becoming increasingly centralised in large, tertiary, metropolitan ICUs, the need to transfer patients between health care facilities has also increased. Such a demand has seen the development of dedicated specialist retrieval teams. These teams are trained to manage patients in the inter-hospital environment and have varied professional backgrounds. Although these teams can deal with most inter-hospital transfers there are a number where the patient complexity may be beyond the standard retrieval team and additional clinical personnel need to be added for the patient transfer. Inter-hospital transfer of a patient on ECMO (see below) is an example where a complex patient is being managed by a highly specialised team with little or no experience of moving patients in the inter-hospital environment. In these relatively rare cases the role of the retrieval team is to assist by providing the logistical and inter-hospital expertise to allow the ECMO team to concentrate on caring for the patient.
Retrieval clinical coordination and advice
Retrieval clinical coordination describes the process whereby specialist medical, nursing, paramedic and ambulance service staff are involved in direct supervision of the primary and inter-hospital transport or retrieval of patients. This is to ensure the:
• safe and efficient use of expensive transport and retrieval services
• high-level clinical advice is available prior to and during transport
• the patient is delivered in a timely manner to the most appropriate receiving hospital
safe and efficient use of expensive transport and retrieval services, that high-level clinical advice is available prior to and during transport, and that the patient is directed in a timely manner to the most appropriate receiving facility.
Not all patients who are referred for retrieval will require transport. Of those who do, not all will require emergency retrieval and not all will require a retrieval team. To ensure that this is addressed, an integrated systems approach is required. In general a retrieval service will be used when the complexity of the patient exceeds the ambulance service's ability to transport the patient. Patients requiring a retrieval response may be identified by:
• a diagnosis with the potential to deteriorate
• a clinical requirement for invasive physiological monitoring or acute intervention
• to facilitate continuity of already instituted critical care supports.
Tele-medicine is playing an important and increasing role in this process – not only in assisting decision making regarding retrieval activities (resulting in potential cost savings), but also in supporting remote and regional medical practitioners faced with acutely ill or injured patients and in supporting a retrieval team before and during patient transport.
When there is a requirement for a rapid medical response to a time-critical pre-hospital or retrieval incident, a retrieval service must be able to be activated swiftly and in a coordinated approach with other emergency services. For this reason, many retrieval coordination centres are co-located with ambulance service communication centres. In this way, clinical and logistic expertise is integrated.
Retrieval coordination centres should ideally be accessed by a single number and provide early teleconferencing of the referral agency, a senior critical care clinician (such as a receiving intensive care specialist, relevant specialist clinician or medical retrieval specialist), and occasionally the retrieval team.
Knowing where key assets (transport platforms such as road ambulances, helicopters and fixed wing aircraft) and retrieval teams are at any one time is crucial to effective retrieval clinical coordination. Real-time asset tracking or mapping systems and advanced radio or phone communication networks assist in this regard.
Retrieval team staffing
The aim of the team is to at least maintain, but ideally to increase the level of care during transport. This requires a team of sufficient size and skill to provide the full complement of care for the majority of patients being transported.
The minimum team should comprise two people; occasionally a very-low-acuity stable patient may be escorted by a single person. If multiple patients are to be transported a recommended staffing level is n + 1 where n equals the number of patients.10
Who makes up the team continues to be debated. In most cases a doctor will be one member while the other can be a person with either acute care nursing or ambulance background. For a primary pre-hospital response, the combination of a doctor and paramedic is the best mix; the paramedic is familiar with the pre-hospital scene environment and can often guide and support a doctor, especially one early in their retrieval career, while a doctor/nurse combination may be appropriate for complex inter-hospital transfers. The future second person will potentially be someone who has both an acute care nursing and paramedic background and will feel comfortable operating in both environments. Other requirements include the ability to work and communicate as a team, have reasonable body habitus and physical fitness and have no visual or auditory impairment or a susceptibility to motion sickness. In aviation transport the weight of the teams and their equipment is important as there is a maximum weight available. High team weights can limit the amount of fuel able to be carried, which may compromise some missions.
As discussed above there will be some highly complex cases that may be outside of the team's capability and supplementation of the retrieval team by additional specialist personnel may be required. An example of obstetricians or neurosurgeons11 depending on the type of mission may be added. It is mandatory that the specialist is added to a standard team because of the latter's familiarity with working in the retrieval environment.
Training
Training should cover the following:
• standard operating procedures for the service
• the use of scenarios to teach common procedures and also principles
• familiarity in the various transport platforms to be used; this would include safety briefings on aerial assets and may include helicopter underwater escape training (HUET) and crew (cockpit) resource management (CRM)
• understanding of the effects of altitude and flight on patient (and team) physiology.
Equipment
General considerations
Minimum equipment standards for supplies, equipment and monitoring have been published.7 The equipment carried is often a compromise between providing for every conceivable situation and lightweight and mobile. In some cases it is appropriate to have additional or procedure packs that are taken only when warranted by the clinical situation; for example, a Sengstaken–Blakemore tube or temporary transvenous pacing wire is taken only when a GI haemorrhage occurs in a patient who might have varices or the patient has symptomatic complete heart block. This requires a good communication and coordination process.
A suggested list of equipment is given in Box 4.1.
Transport monitors, infusion pumps and ventilators must work out of the transport vehicle. They must be battery powered whilst ideally allowing for utilisation of ambulance or aircraft power during transport. Batteries in most modern systems are either sealed lead acid or lithium. There is no place for the older-style Nicad battery, which needs to be totally discharged prior to recharging to overcome memory effect. Battery life is quoted for new batteries and with time this value decreases. Planning on a battery life of 50% of that quoted is prudent. Spare batteries can be carried but changing them usually result in temporary interruption of monitoring. With the newer, smaller defibrillators at least one spare battery is essential. During transport the equipment must be securely stowed. There are international standards in the ‘G force’ that securing systems must withstand in the event of a crash. In some modern road vehicles or helicopters the requirement is 20 G. The use of a suitability engineered ‘stretcher bridge’ attached to the patient's stretcher and to which the equipment can be secured provides the most safety.12
Monitoring
Clinical observation by experienced clinicians remains an important facet of monitoring.13 However, there are significant limitations to this approach. It is difficult to auscultate adequately in a moving vehicle and impossible in a helicopter. As a minimum, ECG, pulse oximetry and non-invasive blood pressure (BP) measurement must be provided with the addition of end-tidal CO2 (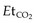 ) for any intubated patient. Non-invasive BP measurements are often subject to interference, and for critically ill patients invasive arterial access is essential, especially if the length of the transport is long.14 Newer defibrillators combine defibrillation and the monitoring aspects as outlined above may be an advantage. However, non-invasive BP and defibrillation uses a lot of battery power and spare batteries are essential or must be carried. The use of portable biochemical analysers provides additional management information in long transports.
) for any intubated patient. Non-invasive BP measurements are often subject to interference, and for critically ill patients invasive arterial access is essential, especially if the length of the transport is long.14 Newer defibrillators combine defibrillation and the monitoring aspects as outlined above may be an advantage. However, non-invasive BP and defibrillation uses a lot of battery power and spare batteries are essential or must be carried. The use of portable biochemical analysers provides additional management information in long transports.
Ventilators
A mechanical ventilator must be used on all intubated patients as manual ventilation cannot reliably deliver constant tidal volumes and a stable 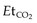 .15 However, a manual system must be available in the rare event of a ventilator failure. Transport ventilators are a compromise between portability and features. Over the last 5 years the desired features as listed in Box 4.2 have almost been met apart from the ability to ventilate neonates to large adults. Small neonates still require a specific ventilator.
.15 However, a manual system must be available in the rare event of a ventilator failure. Transport ventilators are a compromise between portability and features. Over the last 5 years the desired features as listed in Box 4.2 have almost been met apart from the ability to ventilate neonates to large adults. Small neonates still require a specific ventilator.
The provision of non-invasive ventilation (NIV) such as continuous positive airway pressure (CPAP) or BiPAP now possible on most modern transport ventilators. An improvement with inspiratory valve-triggering technology has resulted in substantial reductions in circuit work with concurrent reduction in the work of breathing. Although clapperboard CPAP systems provide the least circuit work and are optimal for patients with high work of breathing, the new transport ventilators are close enough to ideal to be used. Most patients will tolerate NIV with the modern ventilators, but it does require a different approach by retrieval teams. There needs to be a period of observation prior to transport as the ability to provide advanced airway support in transit is limited.
In most cases heat moisture exchangers (HME) will provide adequate humidification for intubated patients.
A suction system and reserve are required. In most transport vehicles this can be provided by electrically powered devices and a back up such as a gas powered venturi system as a back-up.
Infusions
Critically ill patients often need multiple infusions to be continued during transport. Some drugs that ideally should be given as infusions can be consolidated by combining sedation drugs, or the infusion stopped and given instead by frequent boluses. The older-style volumetric and drop-counting pumps have been superseded by lightweight syringe drivers, which should be the only method used for drug infusions in contemporary retrieval practice.
Intra-aortic balloon pump (IABP) and extracorporeal membrane oxygenation (ECMO)
Retrieval of patients with IABP in situ has been occurring for many years and, in general, a team with some experience in trouble shooting any pump alarms can manage these patients. The IABP machines are reasonably bulky and heavy with an internal battery life of 1–2 hours. The type of transport vehicle has to be considered to ensure that the pump can be safely secured and can be connected to an external power source either 12–28 V or mains power equivalent. Although the pump will run on external 12–28 V, most pumps require connection to mains power to recharge the battery, so it is essential to limit the time on internal batteries. Insertion of an IABP catheter requires some experience and some pre-departure consideration of the team's capabilities must be made. The addition of an extra doctor experienced in IABP insertion to the standard retrieval team should be considered.
The ‘swine flu’ epidemic in 2010 saw the rapid emergence of ECMO as a valid treatment for severe viral-induced respiratory failure.16 It was recognised that ECMO should be provided in a relatively small number of institutions and that ideally patients likely to need ECMO should be transferred early. However, significant numbers of patients deteriorated rapidly and rescue ECMO was instituted in many non-ECMO centres, hence requiring the patient to be transported on ECMO. Most ECMO centre staff will not be familiar with the retrieval environment. The principles of retrieval therefore need to be understood by the ECMO teams with the ideal solution being to combine the ECMO team with a standard retrieval team.
Mode of transport
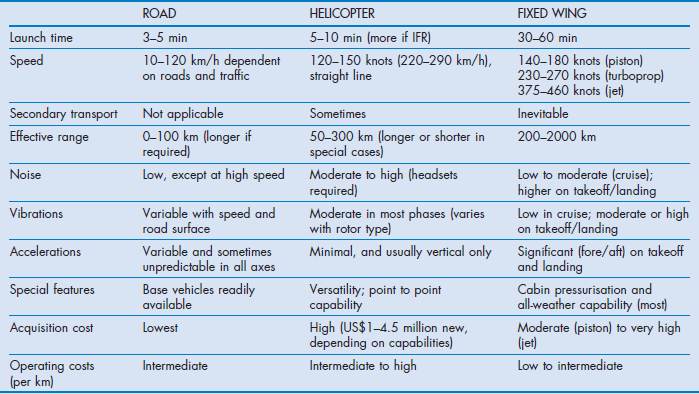
There are three common types of transport vehicle used: road vehicles, aeroplanes (fixed wing) and helicopters (rotary wing). The basic requirements are listed in Box 4.3 and their features and limitations are summarised in Table 4.1.
Ideally, dedicated vehicles should be used. Often the workload is insufficient to justify this and non dedicated vehicles needing to be reconfigured are used. The mode of transport is based on a number of criteria:
• the availability of the transport vehicle
• the weather, especially if flying
• the distance to be travelled
The coordination and tasking centre takes all these into consideration. All things being equal, road is used for distances up to 40–80 km, rotary wing for 60–200 km and fixed wing for over 200 km.
Road ambulance
This remains the most common form of transport and for some patients the safest even for long distances.
Fixed wing
Both propeller-driven and jet aircraft are used. Compared with helicopters, their faster speed needs to be offset with the need for a road leg at each end of the transfer. In comparison to helicopters lower noise and cabin pressurisation, often to sea level, and ability to fly in ‘icing’ conditions increase there utility. Most aeromedical fixed-wing aircraft are specifically configured with stretcher loading devices to assist in loading. Jets tend to be reserved for longer distance, greater than 800–1000 km.
Helicopters
These remain the most high-profile and expensive vehicles used for patient transport. Most will require significant adaption to provide a reasonable working space. The lack of space makes procedures such as intubation almost impossible. They are very noisy to work in, with conversation only possible via intercom systems. This makes communication with patients difficult. It is only recently that the benefits of using a helicopter have been mainly for longer distances. Whereas a mix of single-engine and twin-engine aircraft have been used in the past, changes by regulatory authorities in Europe and Australia mean that most helicopter transports are now being performed in more suitable, larger twin-engine aircraft. The optimal range for use is a ‘donut’ of 40–300 km and their main advantage is the ability to land on hospital helipads, removing an additional road leg. This, of course, requires the hospital to have a helipad. Helicopter also have a role in the delivery of retrieval teams to trauma cases in high traffic density areas such as London.
Safety
Any mode of transport involves some risk to patients and staff. In the aeromedical environment unfamiliar personnel perform clinical tasks poorly,17 so teams must be appropriately trained and equipped to function effectively and safely in each mode of transport. A senior member of the professional group concerned should train and mentor new personnel on their first few trips.
A safety brief encompassing the use of safety equipment carried on the aircraft, emergency egress and actions to take during an emergency is essential. Daily meetings with helicopter flight crews is essential to improve effective communication between members of the team. This leads to an enhancing the safety of missions understanding between individual team members.
Dangerous activities such as unsafe driving and flying below safe minima are unacceptable. For aviation missions the decision whether a mission proceeds rests entirely with the aircraft pilot, and the attempt to coerce pilots to take risks has been recognised as a contributor to air ambulance accidents.18 The pilot should be provided with only the details of where the team needs to go. Clinical details should generally not be given as this may influence the decision to proceed with the mission.
Altitude and transport physiology
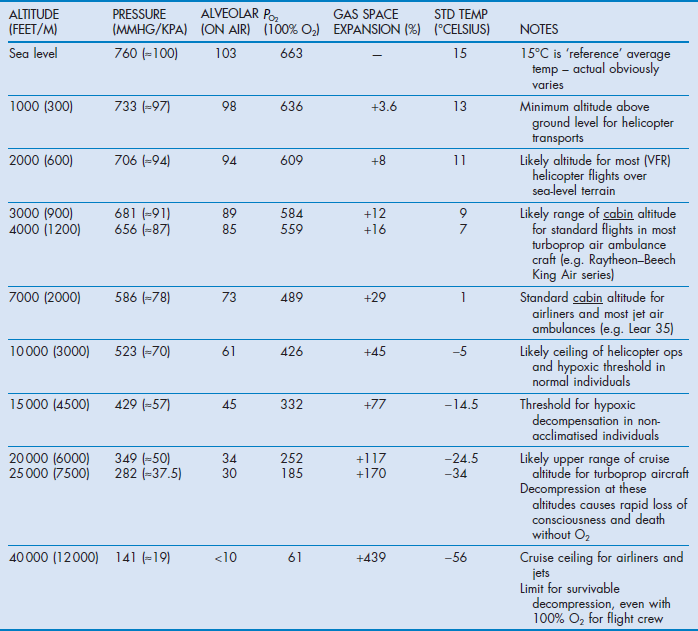
Teams need to be aware of altitude-related changes in gas, volume, temperature and partial pressures of oxygen (Table 4.2).
Patients already dependent on oxygen will be further compromised by even modest increases in height requiring further oxygen supplementation. It is the partial pressure not the percentage of oxygen that is critical. Monitoring of 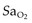 during ascent is essential.
during ascent is essential.
Gas expansion
Expansion of trapped gas in accordance with Boyle's law occurs in physiological and pathological air spaces and air-containing equipment such as endotracheal or tracheostomy tube cuffs, Sengstaken–Blakemore tubes and pulmonary artery balloons. Endotracheal tube cuff pressures will need to be adjusted during flight. Delivered tidal volumes may increase spontaneously in some ventilators, necessitating setting adjustments.
Physiological air spaces include the middle ear, nasal sinuses and GI tract. They can affect both patients and staff; consequently staff with upper respiratory tract infections or gastrointestinal disturbances should not fly.
Pathological air spaces include pneumothoraces, emphysematous lung bullae or cysts, intraocular and intracranial open injuries, bowel obstructions and gas emboli. These patients need to be transported at the lowest altitude possible. Most modern fixed-wing aircraft are capable of cabin pressurization, which decreases hypoxia and gas expansion. The pressurisation effectively replicates flying at a lower altitude – the so-called ‘cabin altitude’. The difference between actual altitude and cabin altitude varies, with most air ambulances providing about 350 mmHg (≈50 kPa) differential. This equates to a cabin pressure of 3000 ft (≈900 m) while flying at 20 000 feet (≈6000 m). Most commercial airliners fly with a cabin altitude of around 7–9000 ft (≈2000–2700 m). Once the maximum differential is reached a lower cabin pressure can only be achieved by flying lower, which is often associated with more turbulence and increased fuel consumption. The medical team should only request a lower cabin height if clinically indicated.
Temperature falls 2°C for every 1000 ft (≈300 m) increase in height, as does the partial pressure of water, which is not corrected by cabin pressurisation. This can lead to dehydration especially on long trips.
Preparation for transport
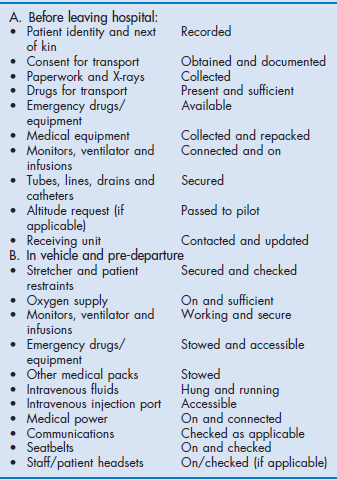
The preparation phase for transport will depend on the patient's clinical condition. The ideal is to spend sufficient time, including any urgent surgery, stabilising the patient so that the transport phase is uneventful. As with intra-hospital transport, this ideal may not be achievable, especially when the patient requires a time-critical intervention available only at the receiving destination. These missions are higher risk but are likely to be less futile than trying to stabilise an inevitably deteriorating patient. Prior to transport all patients must have a secure airway, either self-maintained or intubated and ventilated, and well-secured intravenous access. External bleeding should be controlled and investigations that may impact on the transport performed, if they can be performed in a short time frame. Heimlich-type valves may be attached to any chest drains rather than bulky UWSD (underwater seal drain) devices. The patient is transferred to the stretcher and secured with the patient harness. Any equipment is also attached securely to the stretcher bridge. Any documentation and copies of investigations need also to be taken. A checklist for departure and transport is recommended and provided in Box 4.4.
During the early stages of movement, special vigilance must be employed as this is the stage when equipment disconnections or physiological decompensation is likely to occur. During transport the patient is vulnerable to hypothermia and heating in the vehicle should be used.
Handover
Handover to the receiving hospital is critical. Unless the patient needs immediate resuscitation there should be an opportunity for the retrieval team to have the exclusive attention of the receiving hospital. Various handover tools such as MIST (Mechanism of injury, Injuries suspected or found, Signs (vital signs) and Treatment given) are useful for trauma cases. ISOBAR has a wider application to all patient groups and is being promoted as the standard handover tool in many areas:
Retrieval teams should use which handover tool is used in local practice. If one is not commonly used then ISOBAR will cover all the essential elements.
Pre-hospital care
Pre-hospital care of the acutely injured and critically ill is a complex and challenging field of medical practice. Ideally, patients should receive the most advanced required level of care at the earliest possible time, integrated with expedient transfer to the most appropriate definitive care facility. The ability to achieve this is both resource and system dependent with unique modifiers including transport platform logistics, environmental concerns and the need to integrate with other responding emergency services.
The benefits of adding a skilled critical care physician to the pre-hospital team are well recognised. Service models across Australasia and Europe reflect this belief.19 The potential care delivered by such a team approaches or matches that only usually available in a tertiary hospital environment and may represent a ‘definitive’ requirement for the patient. However, the benefit of a physician and the safety and effectiveness of the team are maximised only if staff involved in such activities have the requisite skills and knowledge and are familiar with the pre-hospital and retrieval environment.
Approach to the scene and scene safety
During the approach to a trauma scene an opportunity exists to:
Scene assessment is critical to ensuring team, scene and, ultimately, patient safety.
It begins as soon as details of the task become available. The tasking agency may have access to further information that may be forwarded to the team en route.
In any pre-hospital emergency situation, scene safety is the primary concern and, as detailed above, plans for approaching the scene should be made on or prior to arriving.
The pre-hospital retrieval (PHR) team should adopt the ‘safe self, safe team, safe scene, safe patient’ approach.
Pre-hospital plan
A pre-hospital plan is a continuously evolving mental plan of action that the team will make as soon as it is activated, using the information given by the tasking agency. In many cases, this initial information is vague or incomplete, which reflects the problems experienced when receiving early phone calls about an incident. Although making a plan prior to arrival with limited information has drawbacks, there are clear benefits in arriving at the incident with a strategy for scene and patient management already in place. The plan often develops as the team travels to the scene and therefore valuable time en route should not be wasted.
When at the scene, the PHR team must have the skill to listen to all members of the emergency services and weigh up their suggestions as part of the overall plan. The ambulance service is the primary provider of pre-hospital care and paramedics are likely to be very experienced. It is worthwhile remembering that the physician-based pre-hospital teams are an extension of the ambulance service and not a replacement for them.
A generic pre-hospital plan could be:
By having a pre-hospital plan, the team can add structure to their actions and, in doing so, develop a shared mental model inherent in teams that function in such high-acuity, high-consequence environments.
Entrapment and extrication
Relative entrapment is a situation in which patients are trapped because of their injuries (e.g. a broken leg with disabling pain), location (e.g. a cave) or the ambient environment (e.g. a blizzard). If it were not for these factors, they would not require help in extricating themselves.
Actual entrapment occurs when patients are physically held in a location by the structure itself – for example, a major vehicle deformation with cabin intrusion, or a building collapse.
The aim is to remove the patient from an entrapped situation as safely and as quickly as possible. The key determinant in this plan (apart from safety) is the condition of the patient. The PHR team must decide on how to compromise between the slower, methodical extrication with total spinal control and the quicker extrication of the less stable patient. Clearly, unstable patients will need rapid extrication but the ability to predict which patients are unsuitable for prolonged extrication due to the anticipated clinical course is more challenging. It may be better to compromise a degree of spinal protection earlier rather than have an emergency (‘crash’) extrication situation develop 30 minutes later.
The fire and rescue services will have access to the specialised equipment required. Without good teamwork between the services, the extrication will be significantly hindered.
Ultrasound in the field
The availability of robust portable ultrasound machines has resulted in increased use in the pre-hospital or retrieval environment with good success; however, their use must be limited to people accredited in its use and must not result in undue delays in the patient management.
References
1. Laird, C. Prehospital and retrieval medicine. Emerg Med J. 2005; 22(4):236.
2. Braman, S, Dunn, S, Amico, CA, et al. Complications of intrahospital transport in critically ill patients. Ann Intern Med. 1987; 107:469–473.
3. Ridley, S, Carter, R. The effects of secondary transport on critically ill patients. Anaesthesia. 1989; 44:822–827.
4. Waydhas, C. Intrahospital transport of critically ill patients. Crit Care. 1999; 3:R83–R89.
5. Marx, G, Vangerow, B, Hecker, H, et al. Predictors of respiratory function deterioration after transfer of critically ill patients. Intensive Care Med. 1998; 24:1157–1162.
6. Kollef, MH, Von Harz, B, Prentice, D, et al. Patient transport from intensive care increases the risk of developing ventilator-associated pneumonia. Chest. 1997; 112:765–773.
7. College of Intensive Care Medicine of Australia and New Zealand. Minimum standards for inter-hospital transport of critically ill patients. Joint Faculty of Intensive Care Medicine Policy Document IC10. Melbourne: College of Intensive Care Medicine of Australia and New Zealand; 2010.
8. Bellingan, G, Olivier, T, Batson, S, et al. Comparison of a specialist retrieval team with current United Kingdom practice for the transport of critically ill patients. Intensive Care Med. 2000; 26:740–744.
9. Lee, A, Lum, ME, Beehan, SJ, et al. Interhospital transfers: decision making analysis in critical care areas. Crit Care Med. 1996; 24:618–623.
10. International Society of Aeromedical Services Australasian chapter. Aeromedical Standards. Arncliffe, Sydney: ISAS Australasia; 1993.
11. Gilligan, JE, Griggs, WM, Jelly, MT, et al. Mobile intensive care services in rural South Australia. Med J Aust. 1999; 171:617–620.
12. Wishaw, KJ, Munford, BJ, Roby, HP. The CareFlight stretcher bridge: a compact mobile intensive care module. Anaesth Intensive Care. 1990; 18:234–238.
13. Goldsmith, JC. The US health care system in the year 2000. JAMA. 1986; 256:3371–3375.
14. Rutten, AJ, Isley, AH, Skowronski, GA, et al. A comparative study of mean arterial blood pressure using automatic oscillometers, arterial cannulation, and auscultation. Anaesth Intensive Care. 1986; 14:58–65.
15. Erler, CJ, Rutherford, WF, Rodman, G, et al. Inadequate respiratory support in head injury patients. Air Medical J. 1993; 12:223–226.
16. Burns, BJ, Habig, K, Reid, C, et al. Logistics and safety of extracorporeal membrane oxygenation in medical retrieval. Prehosp Emerg Care. 2011; 15(2):246–253.
17. Harris, BH. Performance of aeromedical crew members: training or experience? Am J Emerg Med. 1986; 4:409–413.
18. National Transportation Safety. Board (US) Safety study: commercial emergency medical services helicopter operations. SS/88/01. USA: NTSB; 1988.
19. Garner, A, Rashford, S, Lee, A, et al. Addition of physicians to paramedic helicopter services decreases blunt trauma mortality. Aust NZ J Surg. 1999; 69:697–700.
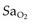
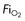 type), tubing, nebulisers
type), tubing, nebulisers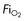 continuously variable from ambient air to 100% oxygen
continuously variable from ambient air to 100% oxygen