Preeclampsia and eclampsia
Pre-eclampsia is a syndrome specific to pregnancy. Although diagnostic criteria have varied among countries and over time, it is generally defined as new onset of hypertension and proteinuria after 20 weeks' gestation (Table 63.1). In Australian practice, pre-eclampsia is defined as new-onset hypertension after 20 weeks' gestation with organ involvement; proteinuria is not mandatory for establishing the clinical diagnosis.1 Many organisations have issued extensive national guidelines for the prevention, diagnosis and treatment of pre-eclampsia and other hypertensive disorders in pregnancy.1–5 Eclampsia describes the occurrence in a pre-eclamptic woman of seizures not attributable to other causes.
Table 63.1
Basic diagnostic criteria for pre-eclampsia

A rise in blood pressure above baseline and oedema are now not usually included.
*Korotkoff phase V.
**A positive dipstick test for proteinuria should be confirmed by 24-hour urine collection.
Pre-eclampsia complicates 2–10% of pregnancies worldwide.6 In the United Kingdom, the maternal mortality attributed to pre-eclampsia in 2006–2008 was 0.83 per 100 000 deliveries.7 In developed countries, eclampsia complicates 2–3 cases per 10 000 deliveries with mortality less than 1%.8 Despite the decline of maternal mortality from pre-eclampsia and related complications in developed countries over the past two decades, hypertensive disorders remain one of the three leading cause of maternal death. The incidence of eclampsia and maternal mortality are higher in developing countries.9 This may be related to poor antenatal care services, and lack of expertise and health care resources.10
Factors associated with increased maternal risk include:11,12
• onset at or less than 32 weeks' gestation
• greater maternal age and parity
• pre-existing hypertension or medical complications
• nausea and vomiting and epigastric pain
• abnormal laboratory tests, including raised liver enzymes, increased serum creatinine and increased serum uric acid.
Patients may be referred to the intensive care unit (ICU) for poorly controlled hypertension, convulsions, postoperative care, or following complications such as pulmonary oedema, renal failure, haemorrhage, coagulopathy and stroke.
Aetiology
The cause of pre-eclampsia is unknown, and there is no satisfactory single unifying hypothesis. Pre-eclampsia is more common in primigravidae, multiple gestation, obesity, African–American race, molar pregnancy, pre-existing hypertension, underlying disease (e.g. autoimmune disease, renal disease, diabetes and thrombophilia) and previous or family history of pre-eclampsia.13 Although there is a genetic predisposition, no particular gene or mode of inheritance has been strongly implicated. Pre-eclampsia may be a result of cumulative effects of variants at multiple genes, both fetal and maternal.14–15 Genetic factors could influence not only the aetiology of pre-eclampsia, but also the development of the disease and response to treatment. Some investigators differentiate early-onset (before 34 weeks' gestation) and late-onset pre-eclampsia (at or after 34 weeks) because of differences in outcomes, biochemical markers and clinical features.16
Pathogenesis
Pre-eclampsia is a systemic disease that affects most organ systems. Many theories of pathogenesis have been proposed,, but a common concept is that of a two-stage disease with an initial stage of abnormal placentation and placental ischaemia, followed by a second stage of clinical disease. Initially, there is inadequate endovascular invasion of fetal trophoblast into the spiral arteries with reduced dilatation of uterine spiral arteries and placental hypoxia. This acts as a precipitating factor that leads to an imbalance of angiogenic and antiangiogenic factors and a generalised inflammatory response. The exact link between placental triggering and the systemic response is unknown, but there is placental release of circulating antiangiogenic factors such as soluble Fms-like tyrosine kinase-1 (sFlt1) and soluble endoglin, and inflammatory cytokines. There are reduced concentrations of angiogenic factors such as vascular endothelial growth factor and placental growth factor.17 All this leads to diffuse endothelial dysfunction with an increase in sensitivity to vasoactive substances and activation of a key vasoconstrictor endothelin-1,18 a decrease in endothelial synthesis of vasodilator substances such as prostaglandin and nitric oxide, activation of platelets and coagulation, and an increase in capillary permeability. This then causes widespread vasoconstriction, fluid extravasation, proteinuria, decreased intravascular volume, haemoconcentration and decreased organ perfusion.
Clinical presentation
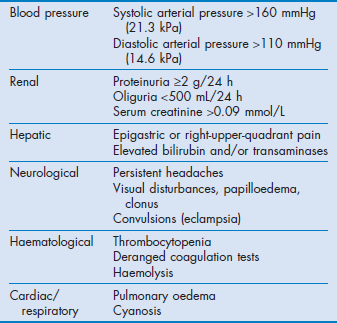
Pre-eclampsia is a syndrome with a spectrum of presentations. Although hypertension is the cardinal sign, some women present with convulsions, abdominal pain or general malaise and some complications may be life-threatening without a marked increase in blood pressure. Features typical of severe disease are listed in Table 63.2. Rarely, cocaine intoxication and phaeochromocytoma may be confused with pre-eclampsia.
Haemodynamic changes of pre-eclampsia consist of hypertension, increased systemic vascular resistance and decreased intravascular volume. Cardiac output is often decreased, usually secondary to changes in preload and afterload rather than contractility.19 Sympathetic activation occurs, and this may account for observations of increased cardiac output in the early stage.20 However early-onset and late-onset pre-eclampsia appear to have different patterns of cardiovascular change.21
Pulmonary oedema may occur because of iatrogenic fluid overload, decreased left ventricular function, increased capillary permeability and narrowing of the colloid osmotic–pulmonary capillary wedge pressure gradient; this is more likely to occur after delivery. Sudden ventricular tachycardia may occur during hypertensive crises.
Neurological complications include eclamptic convulsions, cerebral oedema and stroke. Intracranial haemorrhage is an important cause of death. In recent decades, posterior reversible encephalopathy syndrome has been recognised as a primary event in eclampsia.22
Renal changes include reduced glomerular filtration rate due to reduced renal plasma flow and filtration coefficient. These features, together with proteinuria, are associated with the characteristic lesion of glomeruloendotheliosis. Hyperuricaemia is associated with increased prenatal risk, particularly if serum uric acid concentration rises rapidly.
Haemostatic abnormalities include thrombocytopenia, which may be associated with decreased platelet function. Associated coagulation abnormalities may occur but are unlikely unless the platelet count is <100 000 × 109/L.23,24
Hepatic complications include liver oedema, hepatocellular necrosis, periportal and subcapsular haemorrhage, hepatic infarcts and rupture. Patients with HELLP syndrome (see below) are particularly at risk.
The leading causes of maternal death in pre-eclampsia/eclampsia are intracranial haemorrhage, pulmonary oedema and hepatic complications. Fetal morbidity results from placental insufficiency, prematurity and abruptio placentae.
Management
The principles of management include:
• timely delivery of the placenta and fetus
• supportive care before delivery, and during the immediate postpartum period, focusing on:
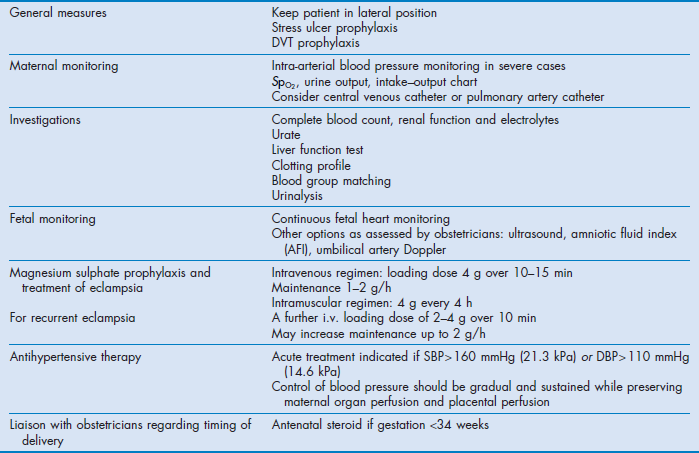
The management for severe pre-eclampsia is summarised in Table 63.3.
If complications are avoided, the disease normally resolves completely after delivery. Transfer of the mother to a tertiary centre before delivery should be considered if a level III neonatal unit is not available (see Ch. 1). Admission into an ICU before delivery may be appropriate in severe cases, or when the labour ward lacks the expertise or equipment for intensive monitoring. Because prematurity is a major cause of neonatal morbidity, expectant management of select patients with severe pre-eclampsia <34 weeks' gestation to prolong pregnancy may improve outcomes.25 This requires collaboration with obstetricians and careful balancing of the maternal and perinatal risks. In all cases, the maternal benefit should always outweigh the benefit of the fetus. Consulting a neonatologist for prenatal counselling is preferable if time is available. After delivery, severe cases should preferably be managed in an ICU for 24–72 hours.
Antihypertensive therapy
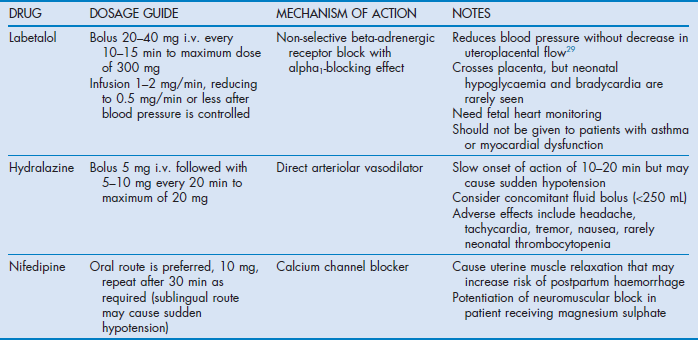
The aim of antihypertensive therapy is to prevent maternal complications (intracerebral haemorrhage, cardiac failure and abruptio placentae) while maintaining placental blood flow. It is important to appreciate that hypertension is a marker and not a causal factor in pre-eclampsia. Therefore, although controlling hypertension reduces the risk of complications, it does not ameliorate the underlying pathological process. Acute treatment is indicated when blood pressure is greater than 160 mmHg (21.3 kPa) systolic or 105–110 mmHg (14–14.6 kPa) diastolic. Reduction of systolic pressure is particularly important for the prevention of stroke.26 Initially, systolic blood pressure should only be reduced by about 20–30 mmHg (2.66–4 kPa) and diastolic pressure by 10–15 mmHg (1.33–2 kPa) while monitoring the fetus. Concomitant plasma expansion reduces the risk of sudden hypotension when vasodilators are used. Recommended antihypertensive drugs for acute treatment are summarised in Table 63.4.1,2,5,27 The most commonly used drugs are labetalol, hydralazine and nifedipine; insufficient data are available to show which of these is superior.28,29
Other agents
These agents are not routinely used and indicated only when hypertension is refractory to conventional treatment. Sodium nitroprusside (initial dose 0.25 µg/kg per min, maximum dose 5 µg/kg per min) can be used to reduce blood pressure rapidly in a hypertensive emergency but care should be taken in patients with depleted intravascular volume and duration should be limited to <4 hours to avoid fetal cyanide poisoning. Nitroglycerin infusion (initial dose 5 µg/min, maximum dose 100 µg/min) may be useful in cases complicated by pulmonary oedema.
Methyldopa has been used for mild cases, but its slow onset time makes it unsuitable for acute treatment. Diazoxide, ketanserin, nimodipine and magnesium are not recommended as first-line agents. Beta blockers other than labetalol may cause decreased uteroplacental perfusion, fetal bradycardia and decreased fetal tolerance to hypoxia. Angiotensin-converting enzyme inhibitors and angiotensin antagonists should not be used before delivery because of adverse fetal effects. Diuretics should generally be avoided because pre-eclamptic patients have reduced plasma volume.
Anticonvulsant therapy
Anticonvulsants are used to prevent recurrent convulsions in eclampsia or to prevent initial convulsions in pre-eclampsia. Magnesium sulphate should be used as first-line treatment for prophylaxis and treatment of eclamptic seizures, and for prophylaxis of seizures in severe pre-eclampsia. The Magpie trial conducted in 33 countries showed that women in the magnesium group had a 58% (95% CI 40–71%) lower risk of eclampsia and probably lower maternal mortality.30 Magnesium was more cost-effective in developing countries where pre-eclampsia is a more significant problem.31 However, the benefits of using magnesium in mild pre-eclampsia are uncertain, especially in developed countries. Adverse maternal events are increased, and there is no reduction in maternal or perinatal morbidity.32
The mechanism of action of magnesium for preventing eclamptic seizures is unknown. Although abnormal electroencephalograms are frequent in pre-eclampsia and eclampsia, they are not altered by magnesium sulphate. Part of magnesium's action may be reduction of cerebral vasospasm via antagonism of calcium at membrane channels or intracellular sites. However, the cerebral vasodilator nimodipine was ineffective for preventing seizures.33 Magnesium amplifies release of prostacyclin by vascular endothelium, and this may inhibit platelet aggregation and vasoconstriction. Doppler ultrasonography suggests that magnesium vasodilates smaller-diameter intracranial blood vessels, and some of its effects may be from relieving cerebral ischaemia. Part of the anticonvulsant activity of magnesium may be mediated by blockade or suppression of N-methyl-D-aspartate (NMDA) receptors. Magnesium has tocolytic effects and mild general vasodilator and antihypertensive actions, and increases renal and uterine blood flow.
Guidelines for administration of magnesium sulphate are summarised in Table 63.3. The kidney rapidly excretes magnesium. The half-life in patients with normal renal function is 4 hours and 90% of the dose is excreted by 24 hours after the infusion.34 When there is renal impairment or oliguria the dose should be reduced and serum concentration should be monitored. Suggested target serum concentration for severe pre-eclampsia is 2–3.5 mmol/L (4–7 mEq/L or 4.8–8.4 mg/dL). Magnesium toxicity is associated with muscle weakness and may lead to respiratory paralysis (>7.5 mmol/L). Increased conduction time with increased PR and QT intervals and QRS duration can lead to sinoatrial and atrioventricular block (>7.5 mmol/L) and cardiac arrest in diastole (>12.5 mmol/L). Toxicity is unlikely when deep tendon reflexes are present (the upper limb should be used during epidural analgesia). Magnesium toxicity can be treated with small intravenous doses of calcium and enhanced clearance with renal replacement therapy when it is associated with renal failure. Other reported adverse effects of magnesium include death from overdose, increased bleeding, slowed cervical dilatation and increased risk of pulmonary oedema. Magnesium crosses the placenta and can cause neonatal flaccidity and respiratory depression.
Eclampsia
With modern obstetric care, eclampsia may present without marked preceding hypertension or proteinuria, and up to 40% of cases occur postpartum, often more than 48 hours after delivery.35,36 Priorities in the management of eclamptic seizures are airway protection, oxygenation, and termination and prevention of seizures. Delivery of the fetus should be considered after maternal stabilisation. Patients should be placed in the left-lateral position and given oxygen. Magnesium should be given if not already started. A further 2 g loading dose of MgSO4 can be given if the patient is already on magnesium therapy (see Table 63.3). Approximately 10% of eclamptic patients will have a recurrent seizure despite receiving magnesium. Prolonged seizures can be terminated by diazepam 5–10 mg intravenously. If seizures are refractory, thiopental and suxamethonium should be given and the airway secured.
It is important to appreciate that eclampsia is not the only cause of seizure during pregnancy. Recurrent convulsions or prolonged unconsciousness may indicate additional cerebral pathology (e.g. cerebral oedema, intracerebral haemorrhage, venous thrombosis) and an urgent computed tomographic (CT) scan should be done whenever possible.
Other anticonvulsants
If repeated seizures occur despite therapeutic levels of magnesium, conventional anticonvulsants can be considered, but it is important to exclude other causes of convulsions. Although diazepam and phenytoin are inferior to magnesium for preventing eclamptic seizures, these agents may be considered when magnesium sulphate is contraindicated (e.g. renal failure, muscle weakness, allergy).
Diazepam as a bolus dose could be considered for immediate treatment of convulsions while preparing for magnesium therapy. Intravenous infusion of diazepam has been used (40 mg diazepam in 500 mL normal saline titrated to keep patients sedated but rousable) as prophylaxis when magnesium therapy is contraindicated.
Phenytoin is given as an initial intravenous loading dose of 10 mg/kg followed 2 hours later by 5 mg/kg. Doses are diluted in normal saline and given no faster than 50 mg/min. Electrocardiogram and arterial pressure should be monitored. Maintenance doses of 200 mg orally or intravenously are started 12 hours after the second bolus and given 8-hourly. However, there is no consensus on the optimal dosing regimen. Monitoring of phenytoin level is necessary to avoid toxicity.
Fluid balance
Fluid management in pre-eclampsia is controversial. Pre-eclamptic patients usually have reduced circulating intravascular volume. There may also be a significant decrease in blood pressure upon initiation of antihypertensive therapy, particularly with hydralazine. However, the effectiveness of fluid loading is uncertain,37 and there is a risk of pulmonary oedema. Oliguria is common in patients with pre-eclampsia, but this does not necessarily imply volume depletion. Hence fluid challenge should be considered for treatment of oliguria only when there are other signs of hypovolaemia. Renal failure in pre-eclampsia is uncommon. Some patients with persistent oliguria and a rising serum creatinine concentration may require a period of continuous renal replacement therapy, but the majority of cases recover. However, the risk of irreversible renal damage is greater when there is associated abruptio placentae, disseminated intravascular coagulation (DIC), hypotensive shock, or sepsis.
Low-dose dopamine (3 µg/kg per min) infusions have been used after correction of hypovolaemia to improve urine output, but they have not been shown to improve renal outcome and are no longer recommended.38
Furosemide could be considered if pulmonary oedema develops. Furosemide should not be used to treat oliguria without pulmonary oedema because this may exacerbate the fluid-depleted state.
There is controversy over the effectiveness of CVP and pulmonary capillary wedge pressure (PCWP) for assisting fluid management in pre-eclamptic patients. CVP and PCWP have poor correlation, especially when CVP is greater than 6 mmHg (0.8 kPa).39 Optimal CVP and PCWP values are unknown, but the measured response to fluid challenge may be informative and useful. Pulmonary artery catheterisation placement carries inherent risks and should be considered only when there are clear indications (e.g. refractory hypertension, pulmonary oedema, severe cardiac disease and refractory oliguria), and the obtained data will be used to guide decision making.39 Should pulmonary oedema develop, it is managed with oxygen therapy, positive end-expiratory pressure with or without ventilation, inotropes, vasodilators, morphine or diuretics as indicated. The echocardiogram can be used for assessment of cardiac function and guiding further therapy.40
Postpartum care
Patients are frequently referred to an ICU for postpartum care, particularly after caesarean delivery. Pre-eclampsia may persist or even develop post partum.41 The risk of pulmonary oedema is greatest after delivery. After delivery, there is often an initial improvement with a relapse in the first 24 hours. Magnesium should be continued for 24 hours after delivery or the last seizure. Ongoing monitoring for signs of severe pre-eclampsia should be continued in the postpartum period. Antihypertensive drugs may be reduced according to the blood pressure. Some patients may require a change to oral medication that may need to be continued for several weeks. Psychological support is important, especially if there has been an adverse neonatal outcome. Full recovery from the organ dysfunction of pre-eclampsia is normally expected within 6 weeks. However, patients are more likely to develop pre-eclampsia in subsequent pregnancies, and have double the risk of early cardiovascular disease and mortality.42
HELLP syndrome and hepatic complications
HELLP syndrome is a particularly high-risk form of pre-eclampsia characterised by more pronounced hepatic rather than cerebral or renal involvement. Diagnosis is based on laboratory findings showing haemolysis (microangiopathic haemolytic anaemia), elevated liver enzymes and low platelets, although exact criteria vary.43,44 The pathogenesis of HELPP is similar to that of early-onset pre-eclampsia, but biomarker derangements are more severe.45 Clinical presentation is variable. Many patients have non-specific signs such as right-upper-quadrant or epigastric pain, nausea, malaise or headache. Although most patients will have hypertension and proteinuria, these can be mild or absent. Important differential diagnoses include idiopathic thrombocytopenic purpura, systemic lupus erythematosus, thrombotic thrombocytopenic purpura, haemolytic–uraemic syndrome and acute fatty liver of pregnancy. Typically, HELLP syndrome presents at early gestational ages and is more common in white and multiparous women. In about 30% of cases, it first presents in the postpartum period, sometimes with no evidence of pre-eclampsia before delivery. After delivery, patients usually show a continuing deterioration in platelet count and liver enzymes, with a peak in severity 24–48 hours after delivery followed by gradual resolution and complete recovery if complications are avoided. Complications of HELLP syndrome include DIC, abruptio placentae, acute renal failure, pulmonary oedema, severe ascites, pleural effusion, liver haemorrhage or failure, acute respiratory distress syndrome (ARDS), sepsis and stroke.
Patients with HELLP should be managed aggressively, similarly to pre-eclampsia, with an emphasis on stabilisation and delivery.46 Expectant management of patients with gestation less than 34 weeks has been described but the relative benefits and risks of this are controversial.44
Corticosteroids are given to promote fetal lung maturation before premature delivery, but there is controversy concerning the use of high-dose corticosteroids for maternal benefit. The anti-inflammatory actions of corticosteroids are postulated to be beneficial for the disease, and the platelet count is increased. Although corticosteroids are part of a standardised protocol at one hospital,47 a recent Cochrane review of 11 trials concluded that there is no difference in maternal or perinatal death, and no evidence of significant maternal benefit.48 Plasma exchange with fresh frozen plasma has been described for postpartum patients with delayed resolution of HELLP, with variable response.49
Life-threatening hepatic complications may occur in pre-eclampsia, particularly in patients with HELLP syndrome. These include segmental hepatic infarction, parenchymal haemorrhage and subcapsular haemotoma with or without rupture. If suspected, patients should have an urgent CT scan. Hepatic rupture is a surgical emergency. Hepatic haemorrhage without rupture has been managed conservatively.
Anaesthesia and analgesia
Platelet count and coagulation tests should be checked before regional anaesthesia. Epidural analgesia in labour reduces fluctuations in arterial pressure and improves placental blood flow.50 For caesarean delivery, the use of epidural or spinal anaesthesia avoids the risks of aspiration, difficult intubation from airway oedema, exaggerated hypertensive response to intubation and magnesium-induced sensitivity to muscle relaxants associated with general anaesthesia.
If general anaesthesia is required, smaller-size endotracheal tubes may be required. The hypertensive response to intubation should be attenuated with drugs such as fentanyl (2.5 µg/kg), alfentanil (10 µg/kg), magnesium sulphate (40 mg/kg), a combination of alfentanil (7.5 µg/kg) with magnesium sulphate (30 mg/kg), or remifentanil (1 µg/kg). Occasionally, awake intubation under topical anaesthesia may be necessary when there is airway obstruction.
References
1. Society of Obstetric Medicine of Australia and New Zealand. Guidelines for the management of hypertensive disorders of pregnancy. Online. Available http://www. somanz. org, 2008.
2. National Collaborating Centre for Women's and Children's Health (UK). Hypertension in Pregnancy: The Management of Hypertensive Disorders during Pregnancy. National Institute for Health and Clinical Excellence (NICE) clinical guideline 107. 2010 Aug, updated 2011 Jan. Online. Available http://guidance. nice. org. uk/CG107/NICEGuidance/pdf/English.
3. ACOG Committee on Practice Bulletins – Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002; 99:159–167.
4. Lindheimer, MD, Taler, SJ, Cunningham, FG. Hypertension in pregnancy. J Am Soc Hypertens. 2008; 2:484–494.
5. Magee, LA, Helewa, M, Moutquin, JM, et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Hypertension Guideline Committee; Strategic Training Initiative in Research in the Reproductive Health Sciences (STIRRHS) Scholars. J Obstet Gynaecol Can. 2008; 30(Suppl. 3):S1–48.
6. Osungbade, KO, Ige, OK. Public health perspectives of preeclampsia in developing countries: implications for health system strengthening. J Pregnancy. 2011; 2011:481095.
7. Centre for Maternal and Child Enquiries (CMACE). Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011; 118(Suppl. 1):1–203.
8. Duley, L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009; 33:130–137.
9. Khan, KS, Wojdyla, D, Say, L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006; 367:1066–1074.
10. Ghulmiyyah, L, Sibai, B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012; 36:56–59.
11. Mattar, F, Sibai, BM. Eclampsia. VIII. Risk factors for maternal morbidity. Am J Obstet Gynecol. 2000; 182:307–312.
12. Martin, JN, Jr., May, WL, Magann, EF, et al. Early risk assessment of severe preeclampsia: admission battery of symptoms and laboratory tests to predict likelihood of subsequent significant maternal morbidity. Am J Obstet Gynecol. 1999; 180:1407–1414.
13. Duckitt, K, Harrington, D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. Br Med J. 2005; 330:565.
14. Staines-Urias, E, Paez, MC, Doyle, P, et al. Genetic association studies in pre-eclampsia: systematic meta-analyses and filed synopsis. Int J Epidemiol. 2012; 41:1764–1765.
15. Williams, PJ, Morgan, L. The role of genetics in pre-eclampsia and potential pharmacogenomic interventions. Pharmgenomics Pers Med. 2012; 5:37–51.
16. Raymond, D, Peterson, E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. 2011; 66:497–506.
17. Eiland, E, Nzerue, C, Faulkner, M. Preeclampsia 2012. J Pregnancy. 2012; 2012:586578.
18. George, EM, Granger, JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hyperten. 2011; 24:964–969.
19. Lang, RM, Pridjian, G, Feldman, T, et al. Left ventricular mechanics in preeclampsia. Am Heart J. 1991; 121:1768–1775.
20. Easterling, TR. The maternal hemodynamics of preeclampsia. Clin Obstet Gynecol. 1992; 35:375–386.
21. Valensise, H, Vasapollo, B, Gagliardi, G, et al. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008; 52:873–890.
22. Zeeman, GG. Neurologic complications of pre-eclampsia. Semin Perinatol. 2009; 33:166–172.
23. Leduc, L, Wheeler, JM, Kirshon, B, et al. Coagulation profile in severe preeclampsia. Obstet Gynecol. 1992; 79:14–18.
24. Sharma, SK, Philip, J, Whitten, CW, et al. Assessment of changes in coagulation in parturients with preeclampsia using thromboelastography. Anesthesiology. 1999; 90:385–390.
25. Publications Committee, Society for Maternal–Fetal MedicineSibai, BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011; 205:191–198.
26. Martin, JN, Jr., Thigpen, BD, Moore, RC, et al. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005; 105:246–254.
27. American College of Obstetricians and Gynecologists. Committee Opinion No. 514. Emergent therapy for acute-onset, severe hypertension with preeclampsia or eclampsia. Obstet Gynecol. 2011; 118:1465–1468.
28. Duley, L, Henderson-Smart, DJ, Meher, S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev. (3):2006.
29. Jouppila, P, Kirkinen, P, Koivula, A, et al. Labetalol does not alter the placental and fetal blood flow or maternal prostanoids in pre-eclampsia. Br J Obstet Gynaecol. 1986; 93:543–547.
30. Magpie Trial Collaborative Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002; 359:1877–1890.
31. Simon, J, Gray, A, Duley, L, Magpie Trial Collaborative Group. Cost-effectiveness of prophylactic magnesium sulphate for 9996 women with pre-eclampsia from 33 countries: economic evaluation of the Magpie Trial. Br J Obstet Gynaecol. 2006; 113:144–151.
32. Sibai, BM. Magnesium sulfate prophylaxis in preeclampsia: lessons learned from recent trials. Am J Obstet Gynecol. 2004; 190:1520–1526.
33. Belfort, MA, Anthony, J, Saade, GR, et al. Nimodipine Study Group. A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. N Engl J Med. 2003; 348:304–311.
34. Lu, JF, Nightingale, CH. Magnesium sulfate in eclampsia and pre-eclampsia: pharmacokinetic principles. Clin Pharmacokinet. 2000; 38:305–314.
35. Chames, MC, Livingston, JC, Ivester, TS, et al. Late postpartum eclampsia: a preventable disease? Am J Obstet Gynecol. 2002; 186:1174–1177.
36. Sibai, BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005; 105:402–410.
37. Duley, L, Williams, J, Henderson-Smart, DJ, Plasma volume expansion for treatment of pre-eclampsia. Cochrane Database Syst Rev 2000;(2):CD001805.
38. Steyn, DW, Steyn, P, Low-dose dopamine for women with severe pre-eclampsia. Cochrane Database Syst Rev 2007;(1):CD003515.
39. Clark, SL, Cotton, DB. Clinical indications for pulmonary artery catheterization in the patient with severe preeclampsia. Am J Obstet Gynecol. 1988; 158:453–458.
40. Mabie, WC, Hackman, BB, Sibai, BM. Pulmonary edema associated with pregnancy: Echocardiographic insights and implications for treatment. Obstet Gynecol. 1993; 81:227–234.
41. Sibai, BM. Etiology and management of postpartum hypertension-preeclampsia. An J Ostet Gynecol. 2012; 206:470–475.
42. McDonald, SD, Malinowski, A, Zhou, Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008; 156:918–930.
43. O'Brien, JM, Barton, JR. Controversies with the diagnosis and management of HELLP syndrome. Clin Obstet Gynecol. 2005; 48:460–477.
44. Sibai, BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 2004; 103:981–991.
45. Abildgaard, U, Heimdal, K. Pathogenesis of the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP): a review. Eur J Obstet Gynecol Reprod Biol. 2012.
46. Haram, K, Svendsen, E, Abildgaard, U. The HELLP syndrome: clinical issues and management. A review. BMC Pregnancy Childbirth. 2009; 9:8.
47. Martin, JN, Jr., Owens, MY, Keiser, SD, et al. Standardized Mississippi Protocol treatment of 190 patients with HELLP syndrome: slowing disease progression and preventing new major maternal morbidity. Hypertens Pregnancy. 2012; 31:79–90.
48. Woudstra, DM, Chandra, S, Hofmeyr, GJ, et al. Corticosteroids for HELLP. Cochrane Database Syst Rev. (9):2010.
49. Martin, JN, Jr., Files, JC, Blake, PG, et al. Postpartum plasma exchange for atypical preeclampsia–eclampsia as HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol. 1995; 172:1107–1125.
50. Jouppila, P, Jouppila, R, Hollmén, A, et al. Lumbar epidural analgesia to improve intervillous blood flow during labor in severe preeclampsia. Obstet Gynecol. 1982; 59:158–161.