General obstetric emergencies
The intensive care unit (ICU) will receive obstetric patients with the usual range of medical and surgical emergencies, and also provide supportive care for patients who suffer specific obstetric complications. The pattern of admission varies widely among countries with different standards of obstetric care. A recent prospective cohort study in the Netherlands reported the incidence of ICU admission to be 2.4 per 1000 deliveries. The most common reasons for ICU admission were major obstetric haemorrhage, hypertensive disorder of pregnancy and sepsis.1 Data from the Centre for Maternal and Child Enquiries (CMACE) in the United Kingdom showed that the overall maternal mortality rate for the triennium 2006–2008 was 11.39 per 100 000 maternities, with the commonest cause of direct death now being sepsis (1.13 per 100 000 maternities).2 Over half of the mothers that die will spend some time in an ICU, and many die there. This latest report highlighted the need for early consultation by obstetricians to intensivists, and discussed in more detail the management of sepsis and haemorrhage.
Pathophysiology
Two important points to recognise in treating obstetric patients are:
1. During pregnancy, the normal ranges for physiological variables change (Table 64.1).3 This may modify the presentation of the problem, variables used to guide treatment, and the response to treatment. The majority of physiological changes revert to normal several days after delivery.
Table 64.1
Changes in physiological variables during pregnancy
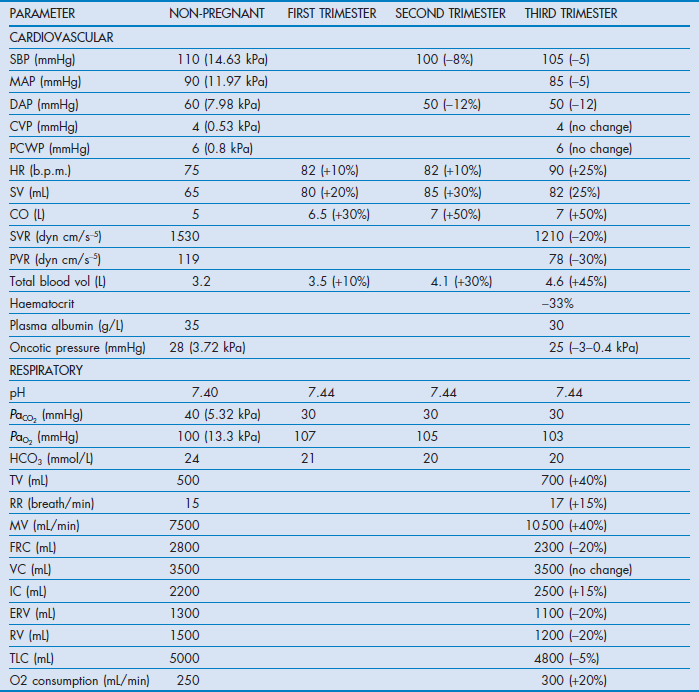
SBP = systolic arterial pressure; MAP = mean arterial pressure; DBP = diastolic arterial pressure; CVP = central venous pressure; PCWP = pulmonary capillary wedge pressure; HR = heart rate; SV = stroke volume; CO = cardiac output; SVR = systemic vascular resistance; PVR = pulmonary vascular resistance; TV = tidal volume; RR = respiratory rate; MV = minute volume; FRC = functional residual capacity; VC = vital capacity; IC = inspiratory capacity; ERV = expiratory reserve volume; RV = residual volume; TLC = total lung capacity.
2. Both mother and fetus are affected by the pathology and subsequent treatment.
Airway and ventilation
Several factors may complicate tracheal intubation in pregnancy:
Intensivists must be familiar with the difficult airway algorithm and the use of the laryngeal mask airway.4,5 Avoidance of intubation and the use of non-invasive ventilation may, in selected cases, be a good option.
Circulation
Tachycardia, low blood pressure, increased cardiac output and warm peripheries are normal in late pregnancy. After 20 weeks' gestation, aortocaval compression by the gravid uterus can decrease uterine perfusion and venous return to the heart. This is best prevented by using the full left-lateral position, but a left-lateral tilt or manual displacement of the uterus may be more practicable.
Haemodynamic support should generally start with good hydration, and assessment should take into account the altered cardiovascular variables in pregnancy. Non-invasive cardiac output monitoring is inaccurate and invasive monitoring with pulmonary artery catheters may be helpful in severe pre-eclampsia, pulmonary oedema and cardiac disease.6 The uterine vascular bed is considered maximally dilated but still responsive to stimuli that cause vasoconstriction, such as circulating catecholamines. Ephedrine has traditionally been the vasoconstrictor used in obstetrics because it was thought to preserve uterine blood flow better than pure alpha agonists. However, alpha agonists such as phenylephrine are more effective and not associated with fetal acidosis when used to manage hypotension during caesarean delivery.7 There is no evidence favouring any particular inotrope.
Coagulation
Pregnancy is associated with a fivefold increase in thromboembolism, and patients should be given thromboembolic prophylaxis, typically with unfractionated heparin or low-molecular-weight heparin (LMWH) and elastic compression stockings.
Mother and fetus
In the critically ill it is important to monitor the fetus because of the problems associated with:
An obstetric opinion should be sought as soon as possible regarding cardiotocography, ultrasound examination and timing of delivery. Nutrition is also very important for the fetus and adequate maternal feeding should be started as soon as possible. Neither perineal nor breast nursing care should be neglected.
Cardiopulmonary resuscitation8,9
Despite being younger than the more usual cardiac arrest patients, pregnant patients have a poor survival rate.10,11 The obstetrician and neonatologist should be notified when the cardiac arrest call is activated, and preparations be made for perimortem caesarean delivery.
Modification of CPR
The 2010 International Liaison Committee on Resuscitation (ILCOR) guideline emphasises the importance of good-quality chest compression, and suggests rescuers may consider starting CPR with chest compression rather than ventilations (the sequence changes from ‘ABC’ to ‘CAB’).
After about 20 weeks' gestation, the gravid uterus can compress the inferior vena cava and impede venous return and cardiac output from CPR. Positioning for obstetric CPR should aim at relieving aortocaval compression and optimising the quality of chest compression. It can be done by manual left uterine displacement in the supine position first, either at the patient's left side with a two-handed technique, or at the patient's right side with a one-handed technique. The mother can also be placed in left-lateral tilt of 27–30° using a firm wedge to support the pelvis and thorax. Chest compression should be performed with a slightly higher hand position (slightly above the centre of the sternum). Airway management in pregnant patients is generally more difficult. Bag-mask ventilation with high-flow oxygen before intubation is especially important. Early tracheal intubation after cricoid pressure will facilitate ventilation and decrease the risk of acid aspiration. During advanced life support (ALS), drugs are given and defibrillation performed according to the normal protocols. Apical placement of the paddle may be difficult because of position and breast enlargement, and adhesive defibrillation pads are preferred. Fetal or uterine monitors should be removed before defibrillation.
Case reports at advanced gestation indicate that both maternal and fetal survival from cardiac arrest may depend on prompt caesarean delivery to relieve the effects of aortocaval compression. The ILCOR advisory statement suggests that the resuscitation team leaders should activate the protocol for an emergency caesarean delivery as soon as cardiac arrest is identified in a pregnant woman with an obviously gravid uterus. Emergency caesarean section may be considered at 4 minutes after onset of maternal cardiac arrest if there is no return of spontaneous circulation.
Post-resuscitation care
Therapeutic hypothermia has been shown to improve outcome in post-arrest adult patients. There is one case report of hypothermia in early pregnancy without emergency caesarean section, but not in patients after perimortem caesarean section. The ILCOR 2010 guideline suggests hypothermia may be considered on an individual basis in comatose pregnant patients.
Trauma12,13
More than 10% of pregnancy-related traumas are associated with the use of illicit drugs or alcohol. Most injuries occur as the result of motor vehicle accidents, and other common causes are suicide (usually postpartum), falls and assaults.
Special considerations of trauma in obstetric patients include the potential for miscarriage, preterm rupture of membrane, placenta abruptio, inadequate uteroplacental blood flow, preterm labour, and fetal distress and demise. The gravid uterus displaces the bowel cephalad so visceral injuries are less common after lower abdominal injuries. The dilated pelvic vasculature increases the risk of retroperitoneal hemorrhage following pelvic injuries. Head injuries and haemorrhagic shock account for most maternal deaths, whereas placental abruption and maternal death are the most frequent causes of fetal death.14
Primary and secondary survey
Initial resuscitation should follow the normal plan of attention to airway, breathing and circulation.15 Unless a spinal injury is suspected, the pregnant patient should be transported and examined in the left-lateral tilt position or with manual left uterine displacement. Blood volume is increased during pregnancy and hypotension may not be evident until 35% or more of total blood volume is lost. Uterine blood flow is not autoregulated and may be decreased despite normal maternal haemodynamics, so that slight overhydration may be preferred to underhydration. Excessive resuscitation with crystalloids or non-blood colloids may increase the mortality from severe haemorrhage. Drug treatment of modest hypotension can be started with ephedrine, but more potent vasopressors should be used if necessary. Continuous fetal heart rate monitoring should be used.
In trauma assessment the increased significance of pelvic fractures in terms of uterine injury and retroperitoneal haemorrhage should be considered.
• Ultrasound is the investigation of choice.
• Diagnostic peritoneal lavage, if performed, should be through an open surgical incision above the fundus.
• Chest drains are placed slightly higher than normal, in the third or fourth intercostal space.
• It is important to exclude herniation of abdominal contents through a ruptured diaphragm.
• Vaginal examination to look for either leak of amniotic fluid and/or vaginal bleeding is vital.
Necessary radiological investigations should be performed as indicated as the radiation hazard to the fetus is very small, except in the early first trimester when exposure to more than 50–100 mGy is a cause for concern. A chest X-ray delivers less than 5 mGy to the lungs and very little to the shielded abdomen. The fetal radiation dose from abdominal examinations can range from 1 mGy for a plain film up to 20–50 mGy for an abdominal pelvic computed tomography (CT) with fluoroscopy.16
Definitive care
Cardiotocographic monitoring is considered essential, but there is wide variation in practice and recommended duration of monitoring. Premature labour and placental abruption may not be diagnosed unless regular monitoring is continued for at least 6 hours and even 24 hours if indicated.17 Rh immune globulin 300 µg should be considered for all Rh D-negative within 72 hours of injury to prevent future Rh alloimmunisation of the newborn. The Kleihauer–Betke test can be used to detect fetal blood in the maternal circulation and give an estimate of the volume of feto-maternal haemorrhage.
Burns
Approximately 7% of women of reproductive age are seen for treatment for major burns.18 Although the women are usually young and healthy, pregnancy is already a hypermetabolic state and the fetus is at great risk from many complications.19 Factors influencing morbidity and mortality include the depth and size of burn wound, the underlying health and age of the pregnant women, the estimated gestational age of the fetus, associated inhalational injury, and significant secondary complications. Preterm labour and stillbirth are more likely in the first few days after the burn injury. The presumed pathogenesis is due to the high levels of prostaglandins and maternal acidosis.20
Special considerations in pregnant burn patients20
Fluid resuscitation
Replacement of fluid loss from burns must keep in mind the normally increased circulating volume of pregnancy. Pregnant burn patients usually require significantly more fluid to maintain haemodynamic stability. Pregnancy affects the total body surface area (TBSA) as the abdomen increases in size. The Lund–Browder chart is still the commonly used method for burn area estimation.
Definitive burn care
Continuous enteral feeding should be initiated as soon as possible and can be within the first 24 hours of burn. Canadian Clinical Practice Guidelines suggest enteral glutamine should be considered in burn patients, but its role in pregnant burn patients is unclear.21 Topical povidone-iodine solution should be avoided because the iodine may be absorbed and affect fetal thyroid function. Inhalational injuries with hypoxia and carbon monoxide are especially detrimental to the fetus, because carbon monoxide has a greater affinity and longer half-life when it is bound to fetal haemoglobulin.
Severe obstetric haemorrhage
Obstetric haemorrhage accounts for 25–30% of all maternal mortality.22 The aim of management is rapid restoration of circulating volume, correct coagulopathy and remedy the underlying cause of haemorrhage.
Antepartum haemorrhage is defined as bleeding from the genital tract after 24 weeks of gestation. Its incidence is 2–5% of all pregnancies.23 The common causes are placenta praevia and placental abruption; both are ultimately managed by delivery. In placenta praevia, the placenta implants in advance of the presenting part and classically presents as painless bleeding during the second or third trimester. Placenta praevia is relatively common (1 in 200 pregnancies) but severe haemorrhage is relatively rare. In placental abruption, a normally implanted placenta separates from the uterine wall. The incidence of placental abruption is low (0.5–2%) but perinatal mortality may be as high as 50%. In concealed abruption, vaginal bleeding can be absent and several litres of blood may be concealed in the uterus.
Postpartum haemorrhage is most commonly due to uterine atony and abnormal placentation, with retained tissues, trauma to the genital tract and coagulopathy as other causes. In an emergency, the aorta can be compressed against the vertebral column by a fist pressed on the abdomen above the umbilicus.
Uterine atony is managed initially with bimanual compression, uterine massage and drugs that include:24
• oxytocin: 5 units slow intravenous injection, or infusion (40 units in 500 mL Ringer's lactate solution at 125 mL/h)
• ergonovine (ergometrine): 0.5 mg slow intravenous or intramuscular injection (contraindicated in patients with hypertension)
• carboprost: 250 µg intramuscular injection, repeated at intervals of not less than 15 minutes to a maximum of 8 doses (contraindicated in patients with asthma) or 500 µg direct intramyometrial injection
If bleeding persists, tamponade techniques using gauze packs or balloons can be useful. Otherwise, specific invasive management such as angiographic arterial embolisation, laparotomy for uterine haemostatic suturing techniques such as B-lynch and multiple square sutures, surgical bilateral uterine artery ligation, or definitive hysterectomy may be required. Recombinant factor VIIa has also been used; the recommended initial dose is 90 µg/kg, and a second dose can be given 20–60 minutes after the first dose if there is no response.
For patients with known and suspected risk factors for postpartum haemorrhage, prophylactic interventional radiology can be employed. Balloons are placed in the internal iliac or uterine arteries before delivery. The balloons then can be inflated to occlude the vessels in the event of postpartum haemorrhage.25
With massive bleeding and transfusion, there should be no need to wait for a coagulation profile before giving coagulation factors. Disseminated intravascular coagulation still develops in 10–30% of cases, partly because tissue thromboplastin is released during abruption.
Sepsis and septic shock26
Maternal sepsis and septic shock are relatively uncommon. However, sepsis should always be carefully considered because the underlying physiological changes in pregnancy and the response to labour can confound the presentation of sepsis and make its diagnosis difficult. The most common sources of infection are chorioamnionitis, postpartum endometritis, urinary tract infections, pyelonephritis and septic abortion. Animal studies suggest that pregnancy increases the susceptibility to endotoxin, and that metabolic acidosis and cardiovascular collapse occur earlier.
Management of septic shock follows the same guidelines as for non-pregnant population, with initial resuscitation, source control and prompt antibiotics. Gram-negative organisms are the frequent causative organisms, but streptococci and Bacteroides may also be present. Antimicrobial therapy is dependent on hospital prevalence and susceptibility patterns; empirical broad-spectrum therapy should be started early. Typical combinations are ampicillin, gentamicin and clindamycin, or carbapenem and vancomycin. Tetracyclines and quinolones should not be used in pregnancy.
Other treatment modalities, including insulin therapy, corticosteroids and activated protein C were never evaluated in pregnancy.
Venous thromboembolism27
The incidence of venous thromboembolism (VTE) ranges from 0.6 to 1.3 episodes per 1000 deliveries, which is a fivefold to tenfold increase in risk compared with those reported in non-pregnant women of comparable age. Pulmonary thromboembolism is a common cause of maternal death, accounting for 15–25% of maternal mortality. Pregnancy is associated with increased risk of VTE because of:
Accurate diagnosis of venous thromboembolism is crucial because of the long-term implications for therapy. Symptoms of dyspnoea or pain in the leg or chest require accurate diagnosis, especially in the immediate postpartum period. Although contrast venography is the gold-standard test for diagnosing deep-vein thrombosis, the radiation exposure and invasive nature of the test mean that duplex Doppler ultrasonography is the usual first choice. D-dimer testing may also be useful but a positive test must be interpreted carefully because D-dimer values normally increase throughout pregnancy. Venography, perfusion lung scanning, pulmonary angiography and helical CT scan should not be avoided if indicated.
Treatment of VTE during pregnancy28
Treatment is either subcutaneous LMWH or intravenous unfractionated heparin. LMWH has gradually replaced unfractionated heparin, based on the results of large trials in non-pregnant patients showing that LMWHs are at least as safe and effective as unfractionated heparin. In addition, the risk of heparin-induced thrombocytopenia and osteoporosis appears lower with LMWH than unfractionated heparin. The need for dose adjustments in proportion to change in weight over the course of pregnancy remains controversial. Full-dose anticoagulation should be continued during pregnancy. Subcutaneous LMWH can be discontinued 24–36 hours, and intravenous unfractionated heparin 4–6 hours, before elective induction of labour or caesarean section. A temporary retrievable venous filter can be inserted and removed postpartum. LMWH or unfractionated heparin should be restarted in the postpartum patient for at least 6–12 weeks. Neuraxial anaesthesia should not be used on patients with full anticoagulation.
With life-threatening massive pulmonary embolus, surgical treatment or thrombolysis must be considered. Thrombolysis was thought to be relatively contraindicated during pregnancy because of the risk of maternal and fetal haemorrhagic complications. No controlled trials are feasible and outcome data must be extracted from case reports. A review found that thrombolytic therapy was associated with a low maternal mortality rate of 1%, with a 6% rate of fetal loss and 6% rate of premature delivery.29 The fetal risks appear to be lower than that obtained with surgical intervention in pregnant patients, and maternal risks lower than reported risks for surgery, thrombolysis or transvenous filters in non-pregnant patients. Although heparin remains the treatment of choice, thrombolysis appears to be a viable option except during the immediate postpartum period.
Amniotic fluid embolism (AFE)30
The incidence of AFE has been reported as 1 in 13 000 deliveries in the United States, and 1 in 50 000 deliveries in the UK.31,32 The maternal mortality rate in developed countries ranges from 13% to 61%.
Pathophysiology
The pathophysiology of AFE remains uncertain. Traditionally, it is thought that amniotic fluid and fetal debris enter the maternal circulation by forceful uterine contraction. However, recent studies show that fetal cells are commonly found in the maternal circulation. AFE has more in common with anaphylaxis and septic shock than other embolic diseases. The exact trigger is unknown. There is an abnormal maternal immune response to fetal antigen exposure, and inflammatory mediators cause pulmonary vasoconstriction, complement activation and coagulopathy. Recently, the term ‘anaphylactoid syndrome of pregnancy’ has been proposed.
Clinical presentation
AFE is a clinical diagnosis. Classically, patients present with severe dyspnoea, cyanosis, sudden cardiovascular collapse, and coma or convulsions during labour, but AFE may occur earlier during pregnancy, during delivery or in the early puerperium. Some patients may present with bleeding and most patients eventually develop a coagulopathy.
Animal studies indicate that AFE causes a biphasic haemodynamic response. The early phase probably lasts less than half an hour and is characterised by severe hypoxia and right heart failure as a result of pulmonary hypertension from vasoconstriction or vessel damage. Patients who survive this first phase develop left ventricular failure with return of normal right ventricular function. Left ventricular failure may be a result of the initial hypoxia or the depressant effects of mediators. Disseminated intravascular coagulation is present in most patients. It could be caused by a specific activator of factor X, tissue factor or other substances, such as trophoblasts, in the amniotic fluid.
Treatment
Treatment is supportive. Assessment of central venous pressure may be misleading, and early pulmonary artery catheterisation has been advocated in a series reporting 100% survival in 5 patients by treating left ventricular failure aggressively.33 The differential diagnoses must be evaluated quickly. Survivors of AFE regain normal cardiorespiratory function but may have neurological sequelae.
Acute respiratory failure
Pregnant women should receive medication for optimal asthma control. There is no evidence that appropriate use of inhaled glucocorticosteroids, beta agonists and leukotriene modifiers is associated with increased incidence of fetal abnormalities. In contrast, there is a substantial risk of preterm delivery posed by poorly controlled maternal asthma.34 Acute exacerbation should also be treated aggressively with systemic glucocorticosteroids when necessary.
Pulmonary oedema is more likely in the pregnant patient (1 : 1000 pregnancies) because of the increased cardiac output and blood volume, and the decreased plasma oncotic pressure. The principles of treatment are relatively straightforward in terms of determining the cause of the oedema and improving oxygenation, although it can be difficult to distinguish between hydrostatic and permeability oedema.
The prevalence of acute respiratory distress syndrome during pregnancy has been estimated at 16–70 cases per 100 000 pregnancies, with mortality rates from 23 to 50%.35 Important causes of ARDS in pregnancy are sepsis, aspiration and pre-eclampsia. Non-invasive positive pressure ventilation has not been evaluated for treatment of hypoxaemic respiratory failure in pregnancy. Clinical criteria for intubation are similar to those in non-pregnant patients, but arterial blood gases must be interpreted carefully because of the underlying respiratory alkalosis in pregnancy. The ARDS Network lung protective strategy can be adopted in pregnant patients by using the non-pregnant predicted body weight and gestational age-appropriate blood gas targets. Permissive hypercapnia may cause fetal respiratory acidosis that will reduce the ability of fetal haemoglobin to bind oxygen. Furthermore, transfer of CO2 across the placenta requires a gradient of 10 mmHg (1.33 kPa). Therefore, PaCO2 should probably be kept below 45 mmHg (6.0 kPa) and PaO2 above 70 mmHg (9.3 kPa). If conventional ventilation fails, the effectiveness of alternative strategies is unknown.35 There are case reports of the use of extracorporeal membrane oxygenation (ECMO) in pregnant patients with severe ARDS.36
Acid aspiration (mendelson's syndrome)
Obstetric patients are at increased risk of acid aspiration because of decreased gastric emptying, increased gastric acidity and volume and increased intra-abdominal pressure. Aspiration of acidic material will cause acute lung injury, the severity being related to the amount, content and acidity of the aspirate.
The initial presentation is hypoxaemia and bronchospasm. Chemical pneumonitis and increased permeability pulmonary oedema develop over several hours.
Treatment includes standard respiratory support. Rigid bronchoscopy may be required to remove large food particles. Bronchoalveolar lavage and steroids are not useful and antibiotics should only be given for proven infection.
Peripartum cardiomyopathy (PPCM)37
Peripartum cardiomyopathy is characterised by new onset of heart failure between 1 month before and 5 months after delivery in previously healthy women. Diagnosis requires echocardiographic evidence of left ventricular dysfunction (ejection fraction <45%). The incidence varies from 1 : 100 to 1 : 10 000 pregnancies. PPCM often presents as acute heart failure. The echocardiogram usually shows features of dilated cardiomyopathy.
The exact aetiology of PPCM remains controversial. A number of mechanisms have been proposed, including genetic factors, volume overload, viral illness, autoimmune, and hormonal imbalance. Treatment is largely supportive with diuretics, inotropes and non-invasive positive-pressure ventilation (NIPPV). Intra-aortic balloon pump and left ventricular assist device may be used in severe case. Recently, 16 kDa prolactin has been shown to have detrimental effects on the maternal heart. A small-scale randomised study showed that addition of bromocriptine to standard heart failure therapy can improve left ventricular ejection fraction.38
Tocolytic therapy and pulmonary oedema39
Pulmonary oedema is an uncommon (1 in 400 pregnancies) but serious complication of tocolytic therapy with beta-adrenergic agonists. The underlying mechanism for pulmonary oedema is unclear, but it is probably related to fluid overload and the cardiovascular effects of beta-adrenergic agonists leading to increased pulmonary capillary hydrostatic pressure. The initial management of pulmonary oedema is discontinuation of the beta-adrenergic agonist and oxygen therapy, with further monitoring, diuretics and respiratory support as necessary. This problem should disappear as alternative tocolytics such as nifedipine and atosiban are used.
Cocaine toxicity40
Cocaine abuse during pregnancy has become a significant problem in the USA, affecting more than 30 million people, with 90% of the women of child-bearing age. Cocaine has local anaesthetic and sympathomimetic actions and can cause:
• hypertension, dysrhythmias, myocardial ischaemia and infarction
• tachycardia and increased cardiac output, but decreased uterine blood flow
Patients commonly present with chest pain, cardiovascular complications, or placental abruption and fetal distress. Acute toxicity may also mimic pre-eclampsia by presenting with cerebral haemorrhage or convulsions.
For the treatment of hypertension, the drug of choice is controversial. Although hydralazine is often used in obstetrics to treat maternal hypertension, labetalol is also widely used. However, concerns about beta blockers allowing unopposed alpha-adrenergic stimulation may also apply to a lesser extent with labetalol, and calcium channel antagonists and nitroglycerin have also been advocated. Nitroglycerin and benzodiazepines have been recommended for the treatment of cocaine-related myocardial ischaemia and infarction.
Ovarian hyperstimulation syndrome41
Ovarian hyperstimulation syndrome (OHSS) is a rare iatrogenic complication of ovarian stimulation usually occurring during the luteal phase or early part of pregnancy. The prevalence of severe OHSS is low, at 0.5–5% of stimulated ovarian cycles. However, it is becoming more recognised owing to the increasing number of women undergoing assisted reproductive technique.
The exact aetiology and pathogenesis of OHSS remain uncertain. It appears that exogenous or endogenous human chorionic gonadotropin (hCG) is the central triggering factor. OHSS usually occurs a few days after follicular rupture or follicular aspiration, after follicular growth has been medically stimulated or induced with either gonadotropins or clomiphene citrate. The stimulated ovaries become markedly enlarged with overproduction of ovarian hormones and vasoactive substances, including cytokines, angiotensin and vascular endothelial growth factor (VEGF). As a result, the capillary permeability increases leading to hypovolaemia with haemoconcentration, oedema and accumulation of fluid in the abdomen and pleural spaces.
OHSS can be classified as mild, moderate, severe or life threatening. For the life-threatening form, patients could have oliguria, renal failure, tense ascites, hydrothorax, thromboembolism, pericardial effusion, liver derangement, ovarian torsion, cerebral oedema and ARDS.
The treatment of OHSS is supportive until the condition resolves. In most cases, the syndrome follows a self-limiting course that parallels the decline in serum hCG level. Invasive haemodynamic monitoring is necessary to assist fluid management. Abdominal and pleural tapping is needed to release the accumulated fluid. Albumin can be used as a plasma expander. Prophylaxis for thromboembolism with anticoagulant should be given, and surgical intervention may be necessary for ovarian torsion.
References
1. Zwart, JJ, Dupuis, JR, Richters, A, et al. Obstetric intensive care unit admission: a 2-year nationwide population-based cohort study. Intensive Care Med. 2010; 36(2):256–263.
2. Centre for Maternal and Child Enquiries (CMACE). Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer: 2006–08. The Eighth Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011; 118(Suppl. 1):1–203.
3. Chamberlain, G, Broughton-Pipkin, F. Clinical Physiology in Obstetrics, 3rd ed. Oxford: Blackwell Science; 1998.
4. Vasdev, GM, Harrison, BA, Keegan, MT, et al. Management of the difficult and failed airway in obstetric anesthesia. J Anesth. 2008; 22(1):38–48.
5. Kuczkowski, KM, Reisner, LS, Benumof, JL. Airway problems and new solutions for the obstetric patient. J Clin Anesth. 2003; 15:552–563.
6. Fujitani, S, Baldisseri, MR. Hemodynamic assessment in a pregnant and peripartum patient. Crit Care Med. 2005; 33(Suppl. 10):S354–S361.
7. Ngan Kee, WD. Prevention of maternal hypotension after regional anaesthesia for caesarean section. Curr Opin Anaesthesiol. 2010; 23(3):304–309.
8. Vanden Hoek, TL, Morrison, LJ, Shuster, M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guideline for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010; 122(18 Suppl. 3):S829–S861.
9. Hazinski, MF, Nolan, JP, Billi, JE, et al. Part 1: Executive Summary: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendation. Circulation. 2010; 122(16 Suppl. 2):S250–S275.
10. Department of Health, Welsh Office. Scottish Office Department of Health, Department of Health and Social Services, Northern Ireland. Why mothers die. Report on confidential enquires into maternal deaths in the United Kingdom 2000–2002. London, UK: The Stationery Office; 2004.
11. Dijkman, A, Huisman, CM, Smit, M, et al. Cardiac arrest in pregnancy: increasing use of perimortem caesarean section due to emergency skills training? BJOG. 2010; 117:282–287.
12. Barraco, RD, Chiu, WC, Clancy, TV, et al. Practice management guidelines for the diagnosis and management of injury in the pregnant patient: the EAST Practice Management Guidelines Work Group. J Trauma. 2010; 69(1):211–214.
13. Oxford, CM, Ludmir, J. Trauma in pregnancy. Clin Obstet Gynecol. 2009; 52(4):611–629.
14. Rogers, FB, Rozycki, GS, Osler, TM, et al. A multi-institutional study of factors associated with fetal death in injured pregnant patients. Arch Surg. 1999; 134(11):1274–1277.
15. Tsuei, BJ. Assessment of the pregnant trauma patient. Injury. 2006; 37:367–373.
16. Goldman, SM, Wagner, LK. Radiological management of abdominal trauma in pregnancy. Am J Radiol. 1996; 166:763–767.
17. Curet, MJ, Schermer, CR, Demarest, GB, et al. Predictors of outcome in trauma during pregnancy: identification of patients who can be monitored for less than 6 hours. J Trauma. 2000; 49(1):18–25.
18. Graves, CR. Thermal and electrical injury. In: Dildy GA, Belfort MA, Saade GR, et al, eds. Critical Care Obstetrics. 4th ed. Malden, MA: Blackwell Science; 2004:506–511.
19. Polko, LE, McMahon, MJ. Burns in pregnancy. Obstet Gynecol Surv. 1998; 53(1):50–56.
20. Kennedy, BB, Baird, SM, Troiano, NH. Burn injuries and pregnancy. J Perinat Neonat Nurs. 2008; 22(1):21–30.
21. Criticalcarenutrition. com. Canada: Clinical Evaluation Research Unit, The Clinical Practice Guideline. Update. [cited 2012 Jun 8] Online. Available http://www. criticalcarenutrition. com, 2009.
22. World Health Organization. The World Health Report 2005: Make Every Mother and Child Count. Geneva, Switzerland: WHO Press; 2005.
23. Calleja-Agius, J, Custo, R, Brincat, MP, et al. Placental abruption and placenta praevia. Eur Clin Obstet Gynaecol. 2006; 2:121–127.
24. Royal College of Obstetricians and Gynaecologists. Green-top Guideline No. 52. Prevention and Management of Postpartum Haemorrhage. Online. Available http://www. rcog. org. uk, 2009.
25. Royal College of Obstetricians and Gynaecologists. Good Practice No. 6. The role of Emergency And Elective Interventional Radiology In Postpartum Haemorrhage. Online. Available http://www. rcog. org. uk, 2007.
26. Barton, JR, Sibai, BM. Severe sepsis and septic shock in pregnancy. Obstet Gynecol. 2012; 120:689–706.
27. Stone, SE, Morris, TA. Pulmonary embolism during and after pregnancy: maternal and fetal issues. Crit Care Med. 2005; 33(Suppl. 10):S294–S300.
28. Bates, SM, Greer, IA, Pabinger, I, et al. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th edn. Chest. 2008; 133:S844–S886.
29. Ahearn, GS, Hadjiliadas, D, Govert, JA, et al. Massive pulmonary embolism during pregnancy successfully treated with recombinant tissue plasminogen activator: a case report and review of treatment options. Arch Intern Med. 2002; 162(11):1221–1227.
30. Clark, SL. Amniotic fluid embolism. Clin Obstet Gynecol. 2010; 53(2):322–328.
31. Aberhaim, HA, Axoulay, L, Kramer, MS, et al. Incidence and risk factors of amniotic fluid embolism: a population-based study on 3 million births in the United States. Am J Obstet Gynecol. 2008; 1999:49. e1–49. e8.
32. Knight, M. Amniotic fluid embolism: active surveillance versus retrospective database review. UKOSS. Am J Obstet Gynecol. 2008; 199:e9.
33. Clark, SL, Cotton, DB, Gonik, B, et al. Central hemodynamic alterations in amniotic fluid embolism. Am J Obstet Gynecol. 1988; 158(5):1124–1126.
34. Bakhireva, LN, Schatz, M, Jones, KL, et al. Asthma control during pregnancy and the risk of preterm delivery or impaired fetal growth. Ann Allergy Asthma Immunol. 2008; 101:137–143.
35. Cole, DE, Taylor, TL, McCullough, DM, et al. Acute respiratory distress syndrome in pregnancy. Crit Care Med. 2005; 33(Suppl. 10):S269–S278.
36. Cunningham, JA, Devine, PC, Jelic, S. Extracorporeal membrane oxygenation in pregnancy. Obstet Gynecol. 2006; 108:792–795.
37. Silwa, K, Tibazarwa, K, Hilfiker-Kleiner, D. Management of peripartum cardiomyopathy. Curr Heart Fail Rep. 2008; 5(4):238–244.
38. Silwa, K, Blauwer, L, Tibazarwa, K, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010; 121(13):1465–1473.
39. Lamont, RF. The pathophysiology of pulmonary oedema with the use of beta-agonists. BJOG. 2000; 107(4):439–444.
40. Kuczkowski, KM. Peripartum care of the cocaine-abusing parturient: are we ready? Acta Obstet Gynecol Scand. 2005; 84(2):108–116.
41. Budev, MM, Arroliga, AC, Falcone, T. Ovarian hyperstimulation syndrome. Crit Care Med. 2005; 30(Suppl. 10):S301–S306.