Infection Prevention
Recommendations for Practice
STANDARD PRECAUTIONS AND SAFE INJECTION PRACTICES
ANESTHESIA MACHINE AND ANESTHESIA WORK SPACE
PREVENTION OF INFECTIOUS COMPLICATIONS
Device-Related Infectious Complications: Central Venous Catheters
Procedure-Associated Infectious Complications: Ventilator-Associated Pneumonia
Procedure-Associated Infectious Complications: Surgical Site Infections
MANAGING INFECTIOUS DISEASE RISKS TO ANESTHESIA PROFESSIONALS
Overview
Patients have a reasonable expectation that anesthesia care will not expose them to infectious disease. Anesthesia professionals, as part of the health care team, have a responsibility to limit the potential for patients to acquire an infection while receiving care. This chapter discusses infection-prevention recommendations and practices as they apply to the anesthesia professional (Box 20-1).
Health care–associated infections (HAIs), formerly termed nosocomial infections, are clinically important because they are the most common complication associated with hospital care. In 2002, investigators estimated an incidence of 1.7 million HAIs annually in the United States—1 of every 20 hospitalized patients—leading to 99,000 deaths.1
It is important to note that these HAI estimates are extrapolations based on generalizations. Nonetheless, if these 2002 estimates had been accurate, the annual incidence of HAIs would have exceeded that of many reportable diseases, such as hepatitis C and meningococcal meningitis.
Some infections may be inevitable. Harbarth and colleagues2 estimate that only 20% of all HAIs are likely preventable by using the latest technologies and recommended medical practices. The Study on the Efficacy of Nosocomial Infection Control (SENIC), an investigation conducted from 1971 through 1976, suggested that 6% of nosocomial infections could be prevented by minimal infection control efforts, and that “well-organized and highly effective programs” could forestall 32%.3
Nonetheless, some investigators have reported dramatic declines in the incidence of certain HAIs after implementing clinical processes derived from published recommendations. For example, after implementing a “bundle” of five initiatives designed to reduce the chance of infection, Pronovost and colleagues4 reported a 66% decrease in the rate of bloodstream infections associated with central venous catheters (CVCs). Prior to catheter insertion, the steps these researchers implemented included 1) avoiding the femoral site if possible; 2) performing proper hand hygiene; 3) preparing the skin with chlorhexidine; and 4) using full-barrier precautions prior to catheter insertion. After catheter insertion, the investigators provided ongoing surveillance of the need for the catheter and prompt removal when the central line was no longer essential for clinical care. Other studies have demonstrated that to achieve sustained results, these bundled practices as a whole must be continuously monitored for high compliance.5 Similar results were achieved using bundled practices to manage hospitalized patients with known or suspected infection with methicillin-resistant Staphylococcus aureus (MRSA).6
Listed in order of estimated direct socioeconomic costs, the following are the five most prevalent HAIs, comprising more than 80% of those reported: 1) surgical site infection (SSI); 2) Clostridium difficile–associated infections (CDIs); 3) central line–associated bloodstream infections (CLABSIs); 4) ventilator-associated pneumonia (VAP); and 5) catheter-associated urinary tract infections (CAUTIs).7 Direct cost estimates do not take into account indirect costs, such as loss of productivity. HAIs are categorized as 1) those associated with a medical device (e.g., CLABSI [CVCs]; CAUTI [urinary bladder catheters]); 2) those associated with a medical procedure (e.g., VAP [endotracheal intubation and mechanical ventilation]; SSI [surgical procedure]); and, 3) those associated with antibiotic use, such as CDIs and infections from multidrug-resistant organisms such as MRSA and vancomycin-resistant Enterococcus (VRE).
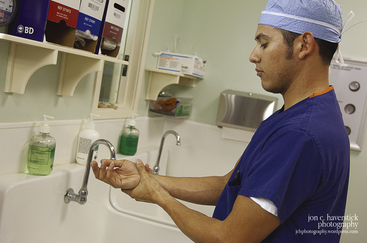
By following recommended infection-prevention practices, the incidence of infection transmission between patients, between health care personnel and their patients, and from equipment and other inanimate objects to patients can be decreased or eliminated. Timely and effective hand hygiene has frequently been cited as the single most important practice to reduce the transmission of infectious disease in health care settings.8 After making observations and deductions from clinical practices associated with infectious outcomes, Ignaz Semmelweis, in the mid-1800s, introduced a simple handwashing regimen. The incidence of puerperal sepsis was dramatically reduced when clinicians washed their hands. Since this time, hand hygiene has been a key element of every infection-prevention strategy. Handwashing with soap and water is recommended when hands are visibly soiled or contaminated (Fig. 20-1); hands may be decontaminated with an alcohol-based hand rub when they are not visibly soiled. Gloves do not substitute for or eliminate the need for hand hygiene. Compliance with the hand-hygiene guidelines published by the Centers for Disease Control and Prevention (CDC)9 and the World Health Organization (WHO)10 was one of The Joint Commission’s National Patient Safety Goals for hospitals in both 2011 and 2012.

FIGURE 20-1 If hands are visibly soiled, perform hand hygiene by washing vigorously with soap and water for 20 seconds. Wearing gloves does not eliminate the need for hand hygiene.
Injection practices are associated with the use of needles, syringes, medication and fluid containers (ampules, vials, bags, bottles), and infusion supplies (administration sets, ports, connectors, and flush solutions). Recent outbreaks of blood-borne infectious disease have underscored the need for anesthesia professionals to reexamine injection practices. Between 1998 and 2008, there were 33 reported outbreaks associated with the iatrogenic transmission of hepatitis B virus (HBV) and hepatitis C virus (HCV) to patients.11 These outbreaks were not associated with blood-product transfusions or tissue transplantations; each was the result of either reusing a syringe or needle between patients or reusing a syringe or needle to access a medication or fluid container that was subsequently reaccessed and administered to another patient. Delivery of anesthesia care was involved in 7 of the 33 outbreaks. In these 7 outbreaks associated with anesthesia professionals, more than 55,000 patients were identified “at risk,” and 144 patients acquired HBV or HCV infections.
Many of the devices, supplies, and equipment used in anesthesia care are labeled “single-use only.” After use for one patient, these items should be discarded and not reprocessed for reuse. For equipment and devices labeled as “reusable” by the manufacturer, anesthesia professionals should be familiar with reprocessing techniques appropriate for anesthesia equipment, including those applicable to the various components of the anesthesia machine.
Anesthesia professionals also should be knowledgeable of how infection prevention recommendations apply to the anesthesia work area, including anesthesia machines, work surfaces, and supply carts. Such knowledge can be extrapolated to other anesthesia care settings: offsite anesthetizing locations, critical care units, obstetric suites, pain management centers, and office-based surgery facilities. In all these settings, anesthesia professionals should be familiar with infection prevention recommendations and practices applicable to all patients (standard precautions) and those that apply to patients known or suspected to have infectious diseases of epidemiologic importance (expanded precautions). Diseases may be epidemiologically important because the infecting pathogen is either highly transmissible (Mycobacterium tuberculosis, varicella) or multidrug-resistant, and therefore difficult to treat (MRSA, VRE).
In addition to standard precautions, anesthesia professionals should be familiar with infectious occupational hazards associated with anesthesia care and recommendations intended to limit the potential for acquiring these infections from patients. This discussion will focus on anesthesia work practices, device and facility designs, postexposure prophylaxis, and vaccination.
Hand Hygiene
Hand hygiene refers to either handwashing with water and plain or antiseptic soap or rubbing hands with an alcohol-based product in the form of a gel, rinse, or foam (Fig. 20-2) followed by air drying. In the absence of visible soiling, approved alcohol-based hand rubs are recommended. Hand hygiene should be performed before and after direct patient contact, before and after wearing gloves, after touching patient care equipment or surfaces in the patient environment, and before and after performing invasive procedures.9,10
Standard Precautions and Safe Injection Practices
Because patient examination and medical history cannot reliably identify the presence of infectious disease, the CDC developed standard precautions to apply to all patients.8 Standard precautions require health care personnel to assume that all bodily fluids except sweat are potentially infectious regardless of the diagnosis or perceived risk of the patient. Barrier protection should be used whenever there may be contact with a patient’s nonintact skin, mucous membranes, and blood or other bodily fluids; this includes secretions and excretions, regardless of whether they contain visible blood.8
Safe injection practices are the application of standard precautions to injection equipment and procedures. Whenever a needle, syringe, medication or fluid container (ampules, vials, bags, bottles), or infusion supply (administration sets and flush solutions) is used, the anesthesia professional must adhere to the principle that each item is intended for use on a single patient. A syringe or needle that contacts a patient’s blood or body fluids or any part of the administration set connected to a patient’s vascular (intravenous or arterial) access should not be reused, either for another patient or to reaccess a fluid or medication container. This is the principle behind the CDC’s public health campaign entitled the “One and Only Campaign” to promote the “one needle, one syringe, one time” concept (Fig. 20-3).12 Safe injection practices also apply to the handling and disposal of injection devices and are designed to protect the anesthesia professional from accidental infection with blood-borne pathogens.
FIGURE 20-3 The Centers for Disease Control and Prevention’s “One and Only Campaign” targets the public and health care professionals. It is designed to reinforce the principle that syringes and needles should be used only once and discarded after use. Used needles and/or syringes should not be used to reaccess a multidose container of medication or fluid.
Two unsafe practices by anesthesia professionals have been associated with transmission of blood-borne pathogens: reusing a needle, cannula, or a syringe to administer intravenous (IV) medication to more than one patient and reaccessing a medication vial or flush preparation with a used needle or syringe, then subsequently administering medication or fluid from this contaminated container to another patient. Syringe and/or needle reuse may appear to be a glaring breach of basic infection prevention practices. Unfortunately, it is not universally understood that replacing only the needle on a contaminated syringe is not safe; it does not reliably eliminate the potential for transmission of pathogens from the residual syringe contents. Conversely, flushing a contaminated needle with saline and placing it on a new syringe is also not safe. A recent survey of clinicians in U.S. health care settings revealed that a small percentage of health care professionals continue to engage in syringe and needle reuse between patients.13
Vials designated for multiple-dose use may be reaccessed to administer residual medication or fluid. If a syringe or needle used for patient care is used again to reaccess a medication or fluid container, contamination occurs, and gross visual inspection is not a reliable means of determining its presence. Medication vials labeled for multidose use contain bacteriostatic and/or bactericidal agents; however, these agents cannot prevent transmission of infection after contamination from external sources and have no antiviral action. Because blood-borne infections have been transmitted through medication or fluid containers contaminated when accessed by used needles and syringes, the CDC recommends that health care professionals refrain from reusing syringes, needles, and cannulas for any purpose, even to access a medication or solution container for use for the same patient.
The CDC gives anesthesia work areas and similarly intense bedside areas the term “immediate patient care areas” (Fig. 20-4). In these areas, medications and fluids are sometimes prepared urgently for patients whose medical condition may be rapidly changing, and distracting events may increase the potential for these medications and fluids to become inadvertently contaminated. Within the immediate patient care area, the CDC recommends additional precautions. First, medication and fluid containers should be accessed only by a clean, sterile syringe, needle, or cannula. Second, medications and fluids used in these areas should be supplied as single-patient use only (single-dose) containers if possible. Medications in single-use-only containers, regardless of the location in which they are administered, should be discarded after access or when empty; residual medication should not be retained for later use. Third, if the manufacturer does not supply a medication in a single-dose container (e.g., neostigmine, succinylcholine), the multidose container should be discarded when empty or at the end of each patient’s anesthetic care. The latter procedure provides a second layer of safety to prevent the potential for transmission of blood-borne pathogens.
FIGURE 20-4 The “immediate patient care area,” as defined by the Centers for Disease Control and Prevention, is a “bedside” area where medications and fluids are sometimes prepared urgently for patients whose medical condition may be changing rapidly, such as within the operating room or delivery room suite or bedside in the critical care, dialysis, or oncology center. Within these locations, stock only single-dose medication and fluid containers if possible. If only multidose containers are available for certain medications and fluids, dispose of these containers after use on a single patient. (Courtesy Jon C. Haverstick Photography, Medcom, Inc., Cypress, CA.)
In accordance with these practices, two techniques are acceptable to the CDC to administer aliquots of medication or fluid to the same patient from a container labeled for multidose use. The entire contents of the container may be drawn into a sterile syringe, which may be used for sequential doses for the same patient. Alternatively, sequential doses may be obtained from the medication or fluid container for the same patient using a new, sterile syringe and needle or cannula for each access. The vial should be discarded when empty, or no later than the end of the procedure.
Prefilled syringes provide a well-labeled, ready-to-use medication that is sterile and stable for extended periods, often up to 6 to 8 weeks. The dosage supplied may be tailored to the end user. For example, a clinician may elect to have succinylcholine supplied either as 5 mL of a 20 mg/mL solution, for a total dose of 100 mg, or as 10 mL of a 20 mg/mL solution, for a total dose of 200 mg. Tamper-proof packaging makes it obvious when sterility has been compromised, and no supplies or handling is necessary to prepare the medication. Prefilled syringes are supplied by compounding pharmacies. The anesthesia professional should seek assurance that these sources follow current Good Manufacturing Practices and have a quality control program to test each lot for sterility.
Syringes, needles, medication and fluid containers, the internal surfaces of IV tubing, and any devices in contact with the vascular system or other normally sterile body areas (e.g., epidural, intrathecal, plexus, or peripheral nerve infusion catheters) are required to be sterile—that is, free from all bacteria, viruses, fungi, and bacterial spores—before use and maintained as such during use. These include stopcocks and other injection ports for medication injection, fluid infusion, and collection of blood samples and medication vial stoppers. All these represent potential entry sites for pathogens and should be kept free from contamination with a sterile cap and by wiping with 70% isopropyl alcohol during access. Hand hygiene is important before handling injection devices. All the aforementioned items are intended for a single patient and should be discarded after use.
Because of its higher specific gravity compared with IV fluid, blood can travel in a retrograde direction through tubing. A one-way valve does not prevent retrograde blood flow through IV administration tubing. In accordance with standard precautions, all syringes, needles, cannulas, injection equipment, medication and fluid containers, flush systems, and administration sets that contact a patient’s IV access should be discarded at the conclusion of the case except those that remain directly connected to the patient.
Multiday infusions should be purchased as premanufactured sterile products or compounded in the hospital pharmacy in accordance with United States Pharmacopoeia (USP) 797 guidelines.14 Anesthesia work areas do not meet these requirements, and medications mixed in this setting should be used or discarded within a period of hours. Infectious risk may be minimized by limiting the number of entries, including top-ups or bag changes, into the sterile infusion sets of continuous systems providing postoperative analgesia, such as epidural or peripheral nerve block infusion systems.
Upon further investigation of the largest outbreaks of blood-borne diseases associated with breaches of injection safety principles,15 the CDC published “Safe Injection Practices” recommendations (Box 20-2). These recommendations were subsequently adopted by the Healthcare Infection Control Practices Advisory Committee (HICPAC) of the CDC and were incorporated into the 2007 standard precautions.8 These practices have also been adopted by the American Society of Anesthesiologists (ASA), The Joint Commission, the Association of Professionals in Infection Control and Epidemiology (APIC), the Safe Injection Global Network of the WHO, the Centers for Medicare and Medicaid Services (CMMS), and several state and municipal health departments.
Safe injection practice recommendations also state that the proceduralist who inserts a needle, with or without a subsequent catheter, into the intrathecal or epidural space—such as for spinal or epidural anesthesia and/or analgesia, diagnostic lumbar puncture, myelography, or injection of intrathecal chemotherapeutic agents—should wear a face mask (Fig. 20-5).8 This recommendation stems from investigations into eight cases of meningitis following lumbar puncture.15a,15b,15c Blood and/or cerebrospinal fluid cultures obtained from the affected patients yielded streptococcal species consistent with the oropharyngeal flora of the proceduralist. Aseptic technique had otherwise been followed, and contamination of the equipment and medications had been excluded.
Reprocessing
If the manufacturer’s instructions indicate that reprocessing is acceptable, the requirements for cleaning, disinfection, and sterilization are based on a classification system developed in the mid to late 1970s known as the Spaulding scale. Spaulding and colleagues16 categorized medical devices based on the risk of infection associated with their clinical use.
Critical Items
These devices—syringes, needles, stopcocks, IV tubing, percutaneous cardiac interventional catheters, surgical instruments, and urethral catheters—either penetrate the skin or are in contact with normally sterile areas such as the bloodstream, tissue planes, neural sheaths, peritoneal or pleural cavity, and urinary bladder. These devices are either disposable or are reprocessed by cleaning followed by sterilization techniques that destroy all endospores, viruses, and vegetative bacteria.
Semicritical Items
Semicritical items contact mucous membranes (e.g., respiratory or alimentary tract) or nonintact skin. These devices—gastrointestinal endoscopes, esophageal echocardiography and temperature probes, laryngoscope blades, endotracheal tubes, laryngeal mask airways, and nasopharyngeal and oropharyngeal airways—require at least cleaning followed by high-level disinfection. Sterilization is not necessary; small numbers of bacterial spores are acceptable.
Limitations
The Spaulding system has limitations, and oversimplification is both its strength and its weakness. First, delicate equipment that requires sterilization under the Spaulding system may be heat sensitive, precluding steam autoclave use. Ethylene oxide gas sterilization may require prolonged turnover and may not be suitable for reprocessing equipment needed for sequential cases. Without evidence of demonstrable differences in outcome, the choice between sterilization and high-level disinfection for reprocessing some of this equipment remains challenging.17
Second, at the time the Spalding criteria were established, standard sterilization destroyed all known microorganisms and bacterial spores; however, in the late 1970s, prions were discovered. Prions are “misfolded” proteins that induce normal proteins to also fold abnormally. The word prion is derived from the words protein and infection. These pathogens affect the structure of the brain or other neural tissue; they are associated with currently untreatable and universally fatal transmissible spongiform encephalopathies such as Creutzfeldt-Jakob disease.
“Sterilizing” prions requires denaturation of the infectious protein structure such that the molecule is no longer able to exert its effect on adjacent proteins. Prions contain no nucleic acids and are resistant to standard sterilization techniques, including heat, radiation, and formalin. Effective prion decontamination relies on protein hydrolysis or destruction of the protein tertiary structure. First, the item is immersed in a sodium hydroxide (e.g., one normal NaOH) or a sodium hypochlorite (bleach) solution. Second, the item is steam autoclaved at 121° C to 134° C for 1 hour. Guidelines are available for reprocessing of heat-stable surgical instruments that have been used for patients with known or suspected spongiform prion disease.18 Single-use equipment should be used when possible; it may be necessary to sacrifice some equipment because of the extreme sterilization measures required to prevent prion transmission and the lack of treatment for the progressive and fatal diseases they cause.
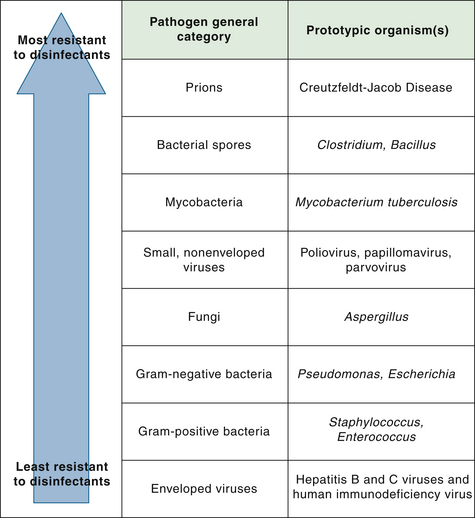
Microorganisms can be categorized on a continuum according to their innate resistance to disinfectants. Figure 20-6 illustrates the variation in resistance to disinfectants among general classes of organisms. Resistance to disinfectants is primarily associated with permeability barriers, such as the cell wall or the outer coat of a spore. For example, Mycobacteria have impermeable cell walls that act as a barrier to chemical disinfectants; Mycobacteria therefore are the most resistant of the vegetative microorganisms. In general, nonenveloped viruses such as poliovirus are more resistant to disinfectants than enveloped viruses (human immunodeficiency virus [HIV], herpesvirus) and vegetative microorganisms except Mycobacteria. Resistance to disinfectants does not equate with resistance to antibiotics. Antibiotic-resistant bacteria, such as MRSA and VRE, are just as susceptible to sterilization and disinfection as nonresistant bacteria of the same species.
Sterilization
As previously discussed, the Spaulding criteria are designed to determine the appropriate reprocessing technique based on the intended clinical use of the device. Devices that contact normally sterile tissues, potential spaces, or fluid chambers—the bloodstream, tissue planes, neural structures, and urinary bladder—require sterilization. The majority of critical items used in the delivery of anesthesia are single-use items; they are disposed of after use and do not undergo reprocessing.
If a hospital chooses to reprocess a single-use device for reuse, the Food and Drug Administration (FDA) holds the hospital to the same standards as it would the original manufacturer of the device. In the anesthesia care setting, reprocessing of disposable devices is not recommended because of the potential for adverse outcomes from device malfunction associated with reprocessing.
Thorough cleaning is an important and essential first step in reprocessing equipment. Cleaning removes soil, debris, and lubricants on the external and internal surfaces of equipment; this residual debris may act as a barrier to prevent disinfectants and sterilants from contacting pathogens.17 Cleaning involves washing with a detergent or enzymatic agent to remove blood, mucus, and foreign material. Rinsing also is important because residual detergents may inactivate the chemicals used to disinfect or sterilize the items.
Sterilization is the destruction of all forms of microbial life, exclusive of prions, including bacterial spores. Sterilization can be accomplished by either high-temperature steam autoclaving or low-temperature gas (ethylene oxide or ozone) or hydrogen peroxide gas plasma exposure; low-temperature sterilization is also possible by liquid immersion in chemical sterilants. Manufacturers’ instructions regarding cleaning of equipment always should be followed to enable sterilization methods to be effective and to avoid damage to the integrity and/or function of the device.
If a device will tolerate high temperatures (typically 134° C; the manufacturer’s labeling or documentation will indicate this), it may be processed by steam autoclaving. Steam autoclaving often is the first choice because it is the fastest and most effective method available. At a given temperature, moist heat is more effective than dry heat in penetrating and destroying organisms. When water vaporizes to steam, the change in physical state requires heat energy (the enthalpy of evaporation). Furthermore, the increased pressure effectively increases the steam saturation temperature, or the boiling point of water, within the autoclave. At temperatures of 125° C or higher, saturated steam under pressure readily penetrates and destroys microorganisms, and the amount of time to achieve sterilization is significantly reduced compared with dry heat or boiling at atmospheric pressures. After steam autoclaving, items must be cooled before handling and use.
Ethylene oxide (EtO), an alkylating agent, is a penetrating and reactive gas capable of destroying all known viruses, bacteria, and fungi, including bacterial spores; it is compatible with most materials, even when repeatedly applied. However, it is highly flammable, toxic, and carcinogenic. Gas autoclaving with EtO is useful for items that cannot tolerate exposure to high temperatures and/or water vapor, such as plastic and rubber devices and fiberoptic endoscopes. EtO sterilization requires at least 24 hours for exposure and subsequent aeration; aeration is essential to eliminate residual gas that would otherwise leach out into the tissues and potentially cause chemical burns. EtO sterilizers and aerators need to be installed in separate, well-ventilated areas that have dedicated air-extraction systems to evacuate gas residues to the outside.
Heat-sensitive objects may also be treated with oxidative processes; these include systems that use hydrogen peroxide gas plasma or ozone gas. Hydrogen peroxide plasma sterilization units require no venting (the by-products are water vapor and oxygen); shorter cycle times also are possible, and they avoid the explosive and carcinogenic risks of EtO units. However, they are not compatible with certain cellulose, packaging, dressing, and paper products. Furthermore, the penetrating ability of hydrogen peroxide is not as good as EtO and ozone, so there are limitations on the length and diameter of lumens that can be effectively sterilized and on the volume and complexity of the load.
Ozone is a toxic and unstable gas that has strong oxidizing properties capable of destroying a wide range of pathogens. Ozone is generated within the sterilizer from medical-grade oxygen, so there is no need for handling hazardous chemicals. Waste ozone is destroyed by exposure to a simple catalyst that reverts it back to oxygen. Penetration is excellent, and the cycle time is relatively short (about 270 minutes). However, ozone is immediately hazardous to life and health, so continuous monitoring must be in place to provide a rapid warning in the event of a leak.
Sterilization may also be achieved by exposure to liquid chemical sterilants, such as glutaraldehyde, ortho-phthalaldehyde, or peracetic acid. The FDA maintains a list of liquid chemical sterilants and high-level disinfectants that can be used to reprocess heat-sensitive medical devices; this listing is posted on the Internet.19 Liquid chemical sterilants reliably produce sterility only if cleaning precedes treatment and strict guidelines are followed regarding the contact time, concentration, temperature, and pH of the sterilant.
Disinfection
Semicritical devices contact mucous membranes or nonintact skin and therefore require high-level disinfection. Such disinfection destroys all viruses, bacteria, and fungi but does not destroy high numbers of bacterial spores (small numbers are acceptable), and it does not destroy prions. Intact mucous membranes, such as those of the lungs and gastrointestinal tract, generally are resistant to infection by common bacterial spores, but they may be susceptible to infection by other pathogens. As with sterilization, meticulous cleaning and rinsing must precede the high-level disinfection process.
Devices that require high-level disinfection include, but are not limited to, laryngoscopes, face masks, laryngeal airways, oral/nasal airways, lightwands, bronchoscopes, endotracheal tubes, transesophageal echocardiography probes, esophageal/rectal temperature probes, and the external anesthesia breathing circuit (distal to the one-way valves). Examples of high-level disinfection techniques include pasteurization and liquid immersion in high-level disinfectants. Depending on the contact time, concentration, temperature, and pH, liquid chemical disinfectants may effectively sterilize a device. For this reason, liquid chemical disinfectants may also be referred to as liquid chemical sterilants. Chemical disinfectants and sterilants used to reprocess critical or semicritical medical devices are regulated by FDA.17 The Environmental Protection Agency (EPA) regulates disinfectants and sterilants used on environmental surfaces.
High-level disinfection of some medical devices may be difficult because of long, narrow lumens and crevices or hinges. More outbreaks involving the spread of infectious disease from patient to patient have been associated with improperly reprocessed endoscopes than with any other medical device.20,21 Consequently, guidelines have been established for reprocessing these devices.17,22,23 The challenge is to achieve high-level disinfection without compromising the functionality of the device. Before high-level disinfection, the endoscope must be leak tested. If the device fails leak testing, it cannot undergo cleaning without risking further damage, and the manufacturer should be contacted regarding repair.
High-level disinfection with a liquid chemical generally requires five important steps: 1) cleaning of all surfaces, which includes brushing and flushing internal channels with water and a detergent or enzymatic cleaner; 2) disinfection by immersion and perfusion of all accessible channels (exposure time will vary with the product); 3) rinsing with sterile, filtered, or high-quality potable tap water that meets federal standards; 4) drying by rinsing with alcohol, which may necessitate directing forced air through channels in the device; and 5) storage in a manner that prevents recontamination and promotes drying.
As an important first step, items that require high-level disinfection reprocessing should undergo mechanical cleaning of all surfaces, including internal channels, with a low-sudsing enzymatic detergent as soon as possible after use. This action prevents drying of organic material that may later interfere with the effectiveness of disinfection/sterilization. Organic material retained in the internal channel is a major cause of transmission of infectious disease related to endoscope reprocessing.
After thorough cleaning, endoscopes should undergo a minimum immersion processing of high-level disinfection with a chemical disinfectant. Disinfecting solution should also perfuse the channels within the scope throughout the processing. Glutaraldehyde, hydrogen peroxide, ortho-phthalaldehyde, and peracetic acid with hydrogen peroxide are reliable high-level disinfectants, provided the conditions for their optimal activity are met.
After all surfaces and internal channels of the device have been exposed to chemical disinfectant at the temperature and for the duration recommended, the item should be rinsed thoroughly to remove any residual disinfectant and to reduce the chance of mucous membrane irritation from residual chemicals. After rinsing, the item must be dried both internally and externally. Flushing 70% ethyl alcohol and compressed air through the channel will facilitate drying. The item should be stored (e.g., packaged) in a manner that prevents recontamination and promotes drying (e.g., hung vertically).
Pasteurization is a method of high-level disinfection named after Louis Pasteur, the French chemist who discovered bacteria. After cleaning, a device is submerged in water at 77° C for 30 minutes. The item must then be transferred to a drying cabinet; once dry, it is packaged in plastic bags.
Medications that come in contact with mucous membranes (eye lubricants, topical anesthetics) or those used in conjunction with devices that contact mucosal surfaces (lubricants and topical anesthetics applied to endotracheal tubes) also must be free of pathogens. Contamination cannot always be determined visually; the use of unit-dose packages of these items therefore is recommended.
Noncritical devices contact only intact skin and require intermediate- or low-level disinfection. Noncritical items include blood pressure cuffs, pulse oximeters, stethoscopes, cables, and surfaces of the anesthesia machine and cart. Intermediate-level disinfectants kill vegetative bacteria and fungi, mycobacteria, and most viruses but are not efficient against small, nonenveloped viruses such as human papilloma virus (HPV), and they are ineffective against bacterial spores. The EPA generally classifies intermediate-level disinfectants as tuberculocidals. Low-level disinfectants kill vegetative bacteria and some fungi and viruses but not mycobacteria or spores. The EPA registers these agents as hospital disinfectants. The manufacturers’ instructions should be followed regarding concentration and contact time for these products, such as chlorine-based products, phenols, 70% to 90% alcohols, and quaternary ammonium products. Many of these products are supplied as wipes for convenient use (Fig. 20-7).
Anesthesia Machine and Anesthesia Work Space
The patient breathing circuit that connects to the fixed inspiratory and expiratory ports of the anesthesia machine (Fig. 20-8) and the reservoir bag are in close proximity to the patient and may be contaminated by secretions or exhaled droplets. If reprocessed, items that require high-level disinfection include, but are not limited to, the corrugated plastic tubing with extensions and adapters, the Y-piece and associated straight and elbow connectors, gas sampling ports, and the reservoir bag. It is difficult to clean, disinfect, rinse, dry, and store these items; furthermore, they are available as high-quality, prepackaged, single-patient-use units. For these reasons, anesthesia breathing circuits are not usually reprocessed in the United States. Even when a high-efficiency particulate air (HEPA) filter (Fig. 20-9) is used to protect the internal surface, a breathing circuit should not be reused without appropriate reprocessing because of the potential for contact and droplet contamination of its external surface.24 There are no reports to date of cross-contamination from reuse of the gas sampling line between patients, and there is little literature to address whether to replace the sampling line between patients.24a However, the sampling line may be contaminated by external droplet or contact exposure. It is theoretically possible for fluid and secretions to accumulate on the patient side of the sampling line and then be introduced into a new breathing circuit. Furthermore, gas sampling lines may be labeled “single patient use only.” For these reasons, it is recommended that the sampling line be replaced between each patient. The “trap,” or the disposable device to which the sampling line connects on the anesthesia machine, does not need replacement between procedures and should only be replaced periodically or if clogged or otherwise malfunctioning. Moisture that accumulates in the anesthesia breathing circuit may be a source of bacterial growth and should periodically be drained from the circuit (away from the patient).24 There is no recommendation to periodically change the anesthesia breathing circuit during the care for one patient; however, the circuit should be changed when it is visibly soiled or mechanically malfunctioning.24

FIGURE 20-9 A high-efficiency particulate air filter used to interconnect into a breathing circuit. The Centers for Disease Control and Prevention recommends a filter capable of trapping at least 95% of particles having a diameter of 0.3 μm, the measurement of the short axis of a tubercle bacterium, or greater. This means that no more than 5% of the particles  μm in diameter or larger penetrate the filter.
μm in diameter or larger penetrate the filter.
The fixed internal parts of the anesthesia machine that are exposed to respiratory gases—the interior of the unidirectional valves, flow sensors, internal conduits, interior of the ventilator bellows, water traps, nondisposable carbon dioxide absorbent chamber, and oxygen sensor—do not generally harbor or transmit infectious agents among patients.25 The CDC does not recommend routine sterilization or disinfection of the internal components of the anesthesia machine and does not recommend obtaining routine bacterial cultures from the anesthesia machine to monitor for contamination.24
Similarly, routine sterilization and disinfection are not recommended for nondisposable attachments to the anesthesia machine, such as the scavenging system and fixed suction apparatus (except the suction canister, tubing, and suction tip). Rather, these components are to be cleaned, sterilized, and/or disinfected in accordance with the manufacturer’s recommendations. If condensate forms within the tubing that connects the manual ventilation reservoir bag or the ventilator to the internal breathing circuit, it should be drained out, and the tubing should be replaced at the end of the case.24
No single reprocessing technique or agent is adequate for all components of and attachments to the anesthesia machine. Most modern anesthesia machines have internal breathing systems that can be disassembled into components, each of which may then be subjected to appropriate reprocessing techniques as specified by the manufacturer. Many of these components are heat stable, enabling sterilization by steam autoclaving (135° C for 8 minutes). Manufacturers have not established time intervals for reprocessing internal anesthesia machine components; rather, a statement is made to reprocess “as necessary,” and the determination of the need for and frequency of reprocessing of any particular component is deemed the responsibility of the institution, consistent with established principles of clinical microbiology and infection control. Because some manufacturers suggest performing routine maintenance on anesthesia machines every 6 months, many institutions choose to reprocess internal components following the same schedule.
The Apollo anesthesia machine (Dräger Medical, Telford, PA) has a breathing system that can be disassembled into three parts, each of which can be steam autoclaved.25 The ventilator bellows and the carbon dioxide absorber canister (if not disposable) can also be steam autoclaved. Other parts, such as the flow sensors, can be disinfected by pasteurization or by wiping with or immersion in a chemical disinfectant. The newer Datex-Ohmeda anesthesia machines (GE Healthcare, Waukesha, WI) can be similarly disassembled into parts, each of which can be subjected to the appropriate reprocessing technique.26 Most parts can be steam autoclaved; the flow sensors, the oxygen cell, and the scavenging system components are not autoclavable, and appropriate reprocessing techniques for these are described in the manufacturer’s manual.
The CDC makes no recommendation for the routine placement of a bacterial filter within the external breathing circuit of an anesthesia machine to protect the internal components exposed to respiratory gases. However, when caring for patients with known or suspected tubercular disease, the CDC does recommend placement of a bacterial filter within the external breathing circuit.27 This is also recommended for viral illnesses such as measles, chickenpox, smallpox, severe acute respiratory syndrome (SARS), and H1N1 influenza. These statements seem to contradict the principle of standard precautions; any patient might potentially harbor an airborne-transmissible respiratory pathogen. Respiratory pathogens, including multiple-drug–resistant tubercular bacteria, have been recovered and cultured from the inspiratory limb after nebulization into the expiratory limb of the anesthesia machine circuit.28 For these reasons, I recommend the routine use of a filter in the external breathing circuit; the CDC recommends a filter capable of trapping at least 95% of particles having a diameter of 0.3 μm, the measurement of the short axis of a tubercle bacterium, or greater. This means that no more than 5% of the particles 0.3 μm in diameter or larger penetrate through the filter. The filter should be placed in the anesthesia circuit, where it will protect both the machine and the ambient air from contamination.27,29 It should be placed either at the patient end of the Y-piece of the breathing circuit to filter respired gas returning from the patient (Fig. 20-10), or at the expiratory end of the corrugated breathing circuit before entry of gas through the flow sensor or the expiratory one-way valve of the anesthesia machine. Breathing circuit tubing often is supplied with a filter at both of the ends that connect to the anesthesia machine (Fig. 20-11). This is done to avoid the possibility that the filter may be inadvertently attached to the inspiratory port of the anesthesia machine, leaving the expiratory port unprotected. If a filter protects both ends, there is no chance for unfiltered, exhaled gases from the patient to enter the internal breathing circuit of the anesthesia machine. If possible, a filter should also protect the gas sampling port. Sampled gases should be scavenged and not returned to the ambient operating room (OR) air.
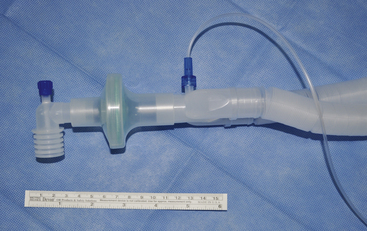
FIGURE 20-10 A filter recommended by the Centers for Disease Control and Prevention may be placed at the patient end of the Y-piece to filter respired gas returning from the patient.
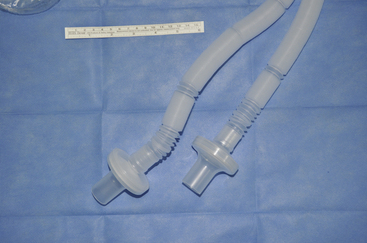
FIGURE 20-11 The Centers for Disease Control and Prevention recommended filter may be placed at the expiratory end of the corrugated breathing circuit before entry of gas through the expiratory port of the anesthesia machine. As shown, the breathing circuit tubing often is supplied with a filter at both of the ends that connect to the anesthesia machine to avoid the chance that the filter may inadvertently be placed on the inspiratory limb of the circuit.
The anesthesia work space is an area with many opportunities to apply principles of infection prevention. Injection safety and hand hygiene, perhaps the most important practices, have already been discussed. Cables and leads from patient monitors (Fig. 20-12) should be wiped with an intermediate-level disinfectant between cases and at the end of the day. Horizontal surfaces, such as the anesthesia machine and anesthesia cart (Fig. 20-13), and vertical surfaces contacted in the course of anesthesia care—including control knobs, buttons, dials, and handles (Fig. 20-14)—should also be wiped with an intermediate-level disinfectant between cases and at the end of the day. Box 20-3 summarizes the infection prevention recommendations for the anesthesia work area between and during patient cases and for periodic reprocessing of components of and attachments to the anesthesia machine.

FIGURE 20-12 Cables and leads from noninvasive and invasive patient monitors should be wiped with an intermediate-level disinfectant between cases and at the end of the day.
Expanded Precautions
Expanded precautions, also known as transmission-based precautions, are used in addition to standard precautions for patients with a known or suspected infection or colonization with either a highly transmissible or multidrug-resistant pathogen. For the anesthesia professional, knowledge of the management of patients with known or suspected infection with airborne and droplet pathogens is essential.
Airborne transmission occurs when airborne droplet nuclei, fine particles less than 5 μm in diameter, disseminate infectious agents. Air currents suspend droplet nuclei and may carry them for long distances. For this reason, diseases spread by airborne droplet nuclei—measles, chickenpox, tuberculosis, smallpox, and severe acute respiratory syndrome (SARS)—are considered highly transmissible.
Patients with known or suspected infection with airborne pathogens require specially designed facilities, equipment, and practices known as airborne infection isolation precautions.30,31 Routine care should be provided in rooms with special air handling and ventilation. Ambient air that contains infectious airborne droplet nuclei is removed from the room, so as not to enter other patient care areas, or it is circulated frequently through filtration capable of removing the infectious particles. Air-cleaning systems that use high-efficiency particulate aerosol (HEPA) filtration or ultraviolet germicidal irradiation (UVGI) technologies can be used in the room or surrounding areas to filter or decontaminate air evacuated from the patient’s room.
During routine care of patients on airborne precautions, health care personnel must wear a properly fitted N95 mask (Fig. 20-15) for protection from infectious droplet nuclei that may be inhaled from the ambient air. The N95 designation indicates that the mask filters out 95% of airborne particles, as certified by the National Institute for Occupational Safety and Health (NIOSH). Barrier protection with gowns, gloves, and eye protection also is required.
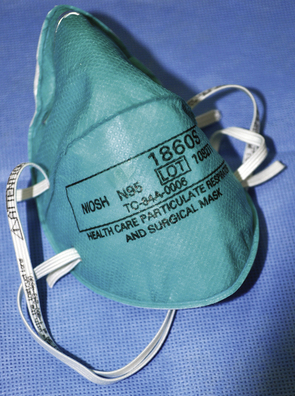
FIGURE 20-15 An N95 mask. The N95 designation indicates that that the mask filters out 95% of airborne particles, as certified by the National Institute for Occupational Safety and Health. During routine care of patients on airborne precautions, health care personnel should wear a properly fitted N95 mask for protection from infectious droplet nuclei that may be inhaled from the ambient air.
Droplets, not droplet nuclei, are generally larger than 5 μm and do not remain suspended in air currents. Droplets travel only a relatively short distance (~3 feet) from the source and infect others by depositing on the conjunctival, nasal, or oral mucosa. Therefore special air handling and ventilation are not required to prevent droplet transmission during routine care. Diseases generally transmitted through droplets include pertussis, viral influenza, and diphtheria.
Certain procedures can be associated with the generation of aerosols that create particles small enough to be considered droplet nuclei; these include bronchoscopy, endotracheal intubation and extubation, open suctioning of airways, and cardiopulmonary resuscitation (CPR). These droplet nuclei may become airborne particles capable of carrying pathogens long distances via air currents. If an anesthesia professional intends to perform an aerosol-generating procedure on a patient or to provide anesthesia care, and the patient has a known or suspected infection with a disease spread by airborne or droplet transmission, the CDC makes several recommendations to protect other patients and their caretakers: consult with the hospital’s infection control specialist for these. If possible the procedure should be performed in an airborne infection isolation room, which generates negative pressure inside the room relative to surrounding areas. In contrast, a typical OR is designed to provide positive pressure relative to the outside, with flow-directed, filtered, and temperature- and humidity-controlled incoming air. Therefore an OR does not provide protection for surrounding areas. Infection-control expertise should be sought to guide the management of these patients in an OR setting.31
When possible, the procedure should be postponed until the patient is either deemed noninfectious or is determined not to have the disease. When the procedure cannot be postponed, it should first be scheduled at a time when a minimum number of health care personnel and other patients are present in the suite, and when air turnover time between cases is maximized, usually at the end of the day. Second, the number of health care workers within the room during the procedure should be limited, and those present must wear properly fitted N95 respirators along with gowns, gloves, and eye protection. Third, when using a bag-valve-mask device, ventilator, or anesthesia delivery apparatus, a HEPA filter should be placed between the patient’s airway device (mask, endotracheal tube, laryngeal mask) and the breathing apparatus to reduce the risk of contaminating the anesthesia machine or discharging infectious particles into the ambient air. Postoperative recovery of a patient with suspected or confirmed disease spread by droplet nuclei should be in an airborne-infection isolation room. Box 20-4 lists the recommendations for anesthesia professionals caring for patients on airborne or droplet precautions.
Prevention of Infectious Complications
Device-Related Infectious Complications: Central Venous Catheters
By implementing “bundled” practices shown to reduce infectious complications, the incidence of bloodstream infections associated with CVCs has been reduced. These practices are associated with the planning, placement, ongoing care, and monitoring of CVCs. The “CVC bundle” includes hand hygiene prior to placement, even though sterile gloves are used (hand hygiene is a supplement to barrier protection using gloves); the use of full-barrier precautions (full-length draping of the patient and cap, gown, mask, and sterile gloves for the proceduralist); the use of 70% isopropyl alcohol and 2% chlorhexidine skin prep; avoidance of the femoral site, if possible; and the timely removal of catheters when they are no longer essential for patient care.32
Procedure-Associated Infectious Complications: Ventilator-Associated Pneumonia
Ventilator-associated pneumonia (VAP) affects between 9% and 27% of all intubated patients, carries a high mortality rate, and is among the most common infections acquired by adults in critical care units.33 In addition to hand hygiene, the measures described here may effectively reduce the incidence of VAP.33
If possible, noninvasive respiratory support should be used. Endotracheal intubation increases the risk of hospital-acquired pneumonia from 6-fold to 21-fold. Weaning and sedation protocols may be useful to reduce the duration of intubation and mechanical ventilation.33,34 Because nasotracheal intubation is associated with a higher incidence of sinusitis, orotracheal intubation is preferred.34,35 Unless contraindicated, the semirecumbent position (30- to 45-degree elevation of the head of the bed) is independently associated with lower rates of VAP than the supine position,36 possibly secondary to an increased risk of gastroesophageal reflux and aspiration when supine.37,38 Endotracheal cuff pressure should be greater than 20 cm H2O to limit microaspiration, and it should be less than 30 cm H2O to limit mucosal ischemia.39 If the expected duration of mechanical ventilation is longer than 3 days, consider endotracheal tubes with subglottic drainage capabilities.40 Routinely use topical oral antiseptics, such as chlorhexidine, to modulate colonization of the oral bacterial flora.41,42 The use of sterile water for nebulized medications and drainage of accumulated moisture away from the patient is also recommended. These recommendations are summarized in Box 20-5.
Procedure-Associated Infectious Complications: Surgical Site Infections
Of those patients who have inpatient surgery in the United States, 2% to 5% develop an SSI; when these infections occur, the length of the hospital stay is prolonged an average of 7 to 10 days, and the risk of perioperative death increases 2 to 11 times that of noninfected surgical patients. Anesthesia professionals have a role in the timing of perioperative antibiotic administration, if antibiotics are indicated. Current guidelines recommend the administration of prophylactic antibiotics within 1 hour of incision to maximize tissue concentrations.43 Because these drugs require up to 1 hour for infusion and have relatively long serum half-lives, vancomycin and fluoroquinolones may be administered up to 2 hours before incision.43 Perioperative prophylactic antibiotics such as cephalosporins exert bactericidal action only when therapeutic concentrations are maintained within the tissues. Eventually, the antibiotic diffuses back into the circulation and is metabolized by the liver and/or excreted by the kidneys. Periodic redosing is required to sustain the therapeutic effects of preventative antibiotics. When the duration of an operation is expected to exceed the time in which therapeutic levels of the antibiotic can be maintained, the anesthesia professional must determine a redosing time interval based on the approximate serum half-life of the drug, the tissue levels achieved in normal patients by the initial dosage, and the approximate minimum concentration of the antibiotic at which 90% inhibition is achieved, a concentration determined in vitro.44 For example, the redosing interval for cefazolin is estimated to be 3 to 4 hours. Because the serum half-life of cefazolin is 1.8 hours in normal adults, this represents approximately twice the half-life of the drug.
A randomized, controlled study of 200 patients undergoing colorectal surgery found that those treated with measures to maintain core temperature around 36.5° C, such as with forced-air convection surface warming and fluid warmers, had a significantly lower SSI rate than those provided normal intraoperative thermal care.45 This and one other study46 formed the basis for the current recommendation that all perioperative patients undergoing general or neuraxial anesthesia have active warming measures used or a body temperature of 36° C or greater recorded around the time of anesthesia emergence or the end of surgery. Currently, this recommendation is a core SSI prevention strategy of the CDC47 and has been implemented as part of the Surgical Care Improvement Project (SCIP).48 A recent retrospective, case-control study using data from the National Surgical Quality Improvement Program (NSQIP) showed no independent association between perioperative normothermia and infectious surgical site complications.49 However, no randomized, controlled trials refute the link between SSI and perioperative normothermia. Furthermore, perioperative hypothermia may be associated with adverse cardiac events, reduced drug metabolism, and altered coagulation function.50
A 1997 retrospective study showed that perioperative hyperglycemia (glucose >200 mg/dL) was associated with a greater risk of SSI in patients undergoing cardiovascular surgery.51 In addition, good preoperative glucose control (hemoglobin A1c <7%) was associated with fewer infectious complications following major noncardiac surgery.52 Recent studies have indicated that perioperative patients subjected to “tight control” (blood glucose concentrations between 80 and 120 mg/dL) suffer outcomes equivalent or worse than those managed with conventional glucose control (glucose <180 mg/dL).53 Maintaining perioperative glucose levels below 180 to 200 mg/dL may be beneficial, but aggressive glucose control requires further study.54
Managing Infectious Disease Risks to Anesthesia Professionals
For anesthesia professionals, caring for patients who have infectious disease is an unavoidable and often routine occurrence. The key to avoiding infection is to prevent direct exposure to pathogens. Most exposures do not result in infection; nonetheless, significant exposure, such as a needle-stick injury, may lead to anxiety and stress for the affected individual. Strategies to reduce the risk of transmission of disease among anesthesia professionals and their patients include 1) use of barrier precautions, 2) implementation of practices and use of devices that reduce exposure risk, 3) immunization against diseases for which vaccination is effective, and 4) expeditious and appropriate medical attention in the event of an exposure.
Barrier protections are required whenever there may be contact with a patient’s nonintact skin, mucous membranes, blood, bodily fluids, secretions, and excretions (except sweat). These measures are the essence of standard precaution and should be routinely used regardless of the diagnosis or perceived risk of the patient and the presence or absence of visible blood.
Hand hygiene is necessary not only to prevent transmission of pathogens to patients but also to protect the anesthesia professional. Hand hygiene should be performed, and gloves should be worn, before contact with a patient’s nonintact skin, mucous membranes, or bodily fluids. In most instances, it is not necessary that the gloves be sterile. Gloves should be removed promptly after contact, and hand hygiene should be repeated after glove removal. Anesthesia professionals should use a standard face mask and eye protection or a face shield to protect their mucous membranes during any procedure or patient care activity likely to generate splashes or sprays. A clean gown must be worn to protect the skin and to prevent soiling of clothing. The gown should be appropriate for the activity and the amount of fluid likely to be encountered. Remove a soiled gown as promptly as possible, and perform appropriate hand hygiene. Soiled patient care equipment should be handled in a manner that prevents skin or mucous membrane exposure, contamination of clothing, or transfer of pathogens.
When caring for patients on airborne precautions, anesthesia professionals should protect themselves by wearing a properly fitted N95 face mask and checking to ensure that appropriate air-handling measures have been instituted. Ordinary surgical face masks do not provide protection from airborne pathogens. If the patient is intubated, a HEPA filter should be used during mechanical or manual ventilation to prevent airborne droplet nuclei from contaminating the anesthesia machine or being discharged to the ambient environment. A patient who is not intubated should wear a mask when not enclosed within an airborne-infection isolation room.
Influenza viral infections are generally transmitted through droplets generated during talking, sneezing, and coughing. Droplets are larger than 5 μm in diameter and are generally not propelled more than 3 to 6 feet from their source. However, during procedures such as endotracheal intubation, suctioning of airway secretions, and bronchoscopy, droplets may become aerosolized into finer particles, and these particles are capable of traveling in air currents and traversing ordinary surgical masks. When performing these procedures on patients known or suspected of having infections that require droplet precautions, anesthesia professionals and those in the room should consider wearing N95 masks.55 If possible, these cases should be performed in an airborne-infection isolation room with only essential personnel present.
Anesthesia professionals are at a relatively high risk for needle-stick injuries compared with health care personnel in other medical and nursing disciplines. Greene and colleagues56 found that 69% of contaminated percutaneous, hollow-bore needle-stick injuries in anesthesia personnel may have been prevented. The investigators deemed a needle-stick injury preventable when it occurred after the needle had been used for its intended purpose; in such instances, percutaneous puncture may have been avoided by shielding the needle or refraining from recapping or disassembling the device harboring the needle.56 Tait and colleagues57 surveyed anesthesiologists and found that 31.8% had had a contaminated needle-stick injury within the previous year; of those who incurred such an injury, only 45% sought appropriate treatment and follow-up. An investigation targeting U.S. surgeons found that almost every surgeon experienced at least one needle-stick injury during his or her training.58 Unfortunately, health care personnel may not report many of these needle-stick injuries, because they downplay the risk, do not wish to take the necessary time, or fear stigmatization and professional consequences. This is especially dangerous, because timely postexposure prophylaxis is effective to prevent disease after percutaneous exposure to HIV and hepatitis B virus (HBV). Although needle-stick injuries have the potential of transferring bacteria, protozoa, viruses, and prions, from a practical standpoint, the transmission of HIV, HBV, and HCV are of greatest concern.59
The greatest risk of transmission of a blood-borne infection with HIV, HBV, or HCV is from a blood-contaminated percutaneous injury with a blood-filled hollow-bore needle. Efforts to reduce the risk of sharps injuries to health care personnel have taken the form of safety-engineered devices and modifications of work practices. Many devices designed to reduce the risk of needle-stick injury are available; these include needleless devices (stopcocks, needleless access ports, and valves), needle products with needle-stick protection safety features (self-sheathing needles, safety IV catheters, and recessed needles), and scalpels with safety-activated blade covers.
Recapping needles with a two-handed technique, in which the needle is directed toward the hand holding the cap, is strongly discouraged because it is one of the most common causes for accidental contaminated needle-stick injury among health care personnel. Puncture-resistant, leak-proof containers for disposal of used needles, syringes, scalpel blades, and other sharp items should be located as close as possible to the immediate area where sharps are used. Convenient access to these containers will facilitate the prompt disposal of contaminated needles without recapping or disassembling them from syringes.
Work practice modifications reduce or eliminate exposure risk by altering how a task is performed. For example, discouraging needle recapping is an effort to modify a work practice. If needle recapping is unavoidable, use of a mechanical device or a one-handed “scoop” technique is recommended; importantly, the sheath is not held in the contralateral hand. Unless required by a specific procedure, or no feasible alternative exists, bending sharps is not recommended. Shearing or breaking contaminated needles is not acceptable.
The injury rate from straight suture needles is more than seven times the rate associated with conventional, instrument-held curved suture needles.60 When suturing, anesthesia professionals should use a curved needle with a needle holder, rather than a straight needle held by hand, and forceps should be used, rather than fingers, to hold tissues when suturing or cutting.61 Double-gloving offers significantly reduced perforations to the innermost gloves62 and may decrease the risk of infection by decreasing the inoculum size from some types of needle-stick injuries.63,64 With regard to dried blood on needles and other inanimate objects, the infectiousness of HIV and HCV decreases within a couple of hours; however, even with desiccation, HBV remains stable and infectious for more than a week.65
After a needle-stick injury, the affected area should be rinsed and washed thoroughly with soap and water; the practice to “milk out” more blood is controversial and is not recommended by the CDC.59 Unless the source is known to be negative for HBV, HCV, and HIV, postexposure prophylactic measures should be initiated. Timeliness is important; if anti-HIV medications are indicated, for optimal effectiveness, they should be taken within hours; delay will result in a significant decline in effectiveness.59 Because needle-stick injuries do not always occur when employee health offices are open, serious consideration should be given to having postexposure prophylactic medications immediately available to anesthesia professionals at the work site. After phone consultation with appropriate infectious disease personnel, necessary prophylactic medication can then be given without delay. Following preventative medication, if indicated, the needle-stick recipient should then have blood tests drawn to determine his or her baseline serologic status; recipients who have been HBV immunized should have hepatitis B surface antibody titers drawn.59 Unless already known, the infectious status of the source individual should also be determined.
Following a specific exposure to a blood-borne pathogen, the risk of infection may vary with the pathogen, the type of exposure (e.g., superficial or deep, solid-bore or hollow-bore needle), the amount of blood involved in the exposure, and the amount of virus in the patient’s blood at the time of exposure. HBV carries the greatest risk of transmission. After a blood-contaminated percutaneous exposure, if the source patient is hepatitis B surface-antigen (HBsAg) positive and hepatitis B e-antigen (HBeAg) negative, the risk of HBV transmission to a nonimmune recipient is between 1% and 6%, and the risk of developing serologic evidence of HBV infection is between 23% and 37%. In comparison, if the source patient is positive for both HBsAg and HBeAg, the risk to a nonimmune recipient for acquiring clinical hepatitis is 22% to 31%, and the risk of developing serologic evidence of HBV infection is 37% to 62%. In nonimmunized recipients, appropriate and timely provision (within 24 hours of exposure) of hepatitis B immune globulin and initiation of the HBV vaccination series provides an estimated 75% protection from HBV infection.
The HCV transmission rate has been reported at 1.8%,59 but newer, larger surveys have shown only a 0.3% to 0.5% transmission rate.66,67 Immunoglobulin therapy for HCV prevention is not effective and is not recommended. In the absence of a currently available postexposure prophylactic treatment, recommendations for postexposure management for the recipient are early identification of disease. Theoretically, prompt treatment with antiviral agents when HCV ribonucleic acid first becomes detectable might prevent the development of chronic infection.59
The average risk of acquiring HIV infection after an accidental percutaneous exposure to blood from a known HIV-infected patient is estimated to be 0.3%.68 Although the average risk of HIV infection is 0.3%, the risk, although not quantified, exceeds 0.3% for an exposure in which there is a greater volume of blood transferred and/or a higher HIV titer in the blood of the source patient.69 If the source patient is known or suspected to have HIV infection, antiretroviral medications should be administered as soon as possible. The selection of a drug regimen for HIV postexposure prophylaxis must balance the risk for infection against the potential toxicities of any agent used. Antiretroviral medications used for this indication frequently have side effects such as nausea, fatigue, and rash; drug interactions are common and may be serious. For exposures that pose a negligible risk for transmission, the risk of preventative pharmacologic treatment may outweigh the benefit. HIV postexposure prophylactic regimens change periodically, and the NIOSH blood-borne pathogens page on the CDC website (www.cdc.gov)70 or another expert should be consulted for the most up-to-date guidelines.
The CDC publishes a list of immunizations strongly recommended for health care personnel.71 Like any other health care personnel who work with sharps, blood, or bodily fluids, anesthesia professionals should be vaccinated against HBV, unless antibody testing has demonstrated immunity the virus.59 If the anesthesia professional is an employee, the employer is required to offer the HBV vaccine free of charge. Anesthesia professionals should actively participate in periodic health assessments, vaccinations, and screening programs for tuberculosis and other potentially contagious diseases. Periodic mask fit testing is important to ensure that N95 masks provide sufficient protection from airborne pathogens. If the anesthesia professional contracts an acute illness (e.g., fever, upper respiratory illness, weepy rash), it would be appropriate to refrain from patient-care activities until the illness is resolved or adequately treated. Strategies to reduce the risk of infectious disease transmission from patients to anesthesia professionals are summarized in Box 20-6.
References
1. Klevens R.M., Edwards J.R., Richards C.L., et al. Estimating healthcare-associated infections in U.S. hospitals. Public Health Rep. 2002;2007(122):160–166.
2. Harbarth S., Sax H., Gastmeier P. The preventable proportion of nosocomial infections: an overview of published reports. J Hosp Infect. 2003;54:258–266.
3. Haley R.W., Culver D.H., White J.W., et al. The efficacy of infection surveillance and control programs in preventing nosocomial infections in U.S. hospitals. Am J Epidemiol. 1985;121:183–205.
4. Pronovost P., Needham D., Berenholtz S., et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732.
5. Furuya E.Y., Dick A., Perencevich E.N., et al. Central line bundle implementation in US intensive care units and impact on bloodstream infections. PLoS ONE. 2011;6(1):e15452.
6. Jain R., Kralovic S.M., Evans M.E., et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364:1419–1430.
7. Scott RD: The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Centers for Disease Control, Division of Healthcare Quality Promotion, March 2009.
8. Siegel J.D., Rhinehart E., Jackson M., Chiarello L. and the Healthcare Infection Control Practices Advisory Committee. 2007. http://www.cdc.gov/hicpac/pdf/isolation/Isolation2007.pdf Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. Centers for Disease Control and Prevention. Accessed online at
9. Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings: recommendations of the healthcare infection control practices advisory committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task force, MMWR Recommendations and Reports. October 25, 2002;51(RR-16):1–44. http://www.cdc.gov/mmwr/PDF/rr/rr5116.pdf Accessed online at
10. World Alliance for Patient Safety. WHO guidelines on hand hygiene in health care: a summary. http://www.who.int/patientsafety/events/05/HH_en.pdf, 2005. Accessed online at
11. Thompson N.D., Perz J.F., Moorman A.C., Holmberg S.D. Non-hospital health care–associated hepatitis B and C virus transmission: United States, 1998-2008, Ann Intern Med. 2009;150(1):33–39. http://www.annals.org/cgi/content/short/150/1/33 Accessed online at
12. Centers for Disease Control and Prevention: Injection Safety: May 16, 2008 Division of Healthcare Quality Promotion (DHQP). http://www.oneandonlycampaign.org. Accessed online 2011 Aug 6 at
13. Pugliese G., Gosnell C., Bartley J.M., Robinson S. Injection practices among clinicians in United States health care settings. Am J Infect Control. 2010;38(10):789–798.
14. USP 797 Guidebook to pharmaceutical compounding—sterile preparations. http://www.usp.org/products/797Guidebook. Accessed 2011 August 6 at
15. Centers for Disease Control and Prevention. Transmission of hepatitis B and C viruses in outpatient settings—New York, Oklahoma, and Nebraska, 2000–2002, MMWR. 2003;52(38):901–906. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5238a1.htm Accessed online at
15a. Centers for Disease Control and Prevention. Bacterial meningitis after intrapartum spinal anesthesia—New York and Ohio, 2008-2009. MMWR Morb Mortal Wkly Rep. 2010;59(3):65–69.
15b. Trautmann M., Lepper P.M., Schmitz F.J. Three cases of bacterial meningitis after spinal and epidural anesthesia. Eur J Clin Microbiol Infect Dis. 2006;21:43–45.
15c. Hebl J.R.: The importance and implications of aspectic techniques during regional anesthesia, Reg Anesth Pain Med 31(4):311–323
16. Spaulding E.H. Chemical disinfection of medical and surgical materials. In: Lawrence C., Block S.S., eds. Disinfection, sterilization, and preservation. Philadelphia: Lea & Febiger; 1968:517–531.
17. Rutala W.A., Weber D.J., the Healthcare Infection Control Practices Advisory Committee. Guideline for disinfection and sterilization in healthcare facilities, Centers for Disease Control and Prevention. 2008. http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf Accessed online at
18. Sutton J.M., Dickinson J., Walker JT, Raven N.D. Methods to minimize the risks of Creutzfeldt-Jakob disease transmission by surgical procedures: where to set the standard?, Clin Infect Dis. 2006;43:757–764. http://dx.doi.org/10.1086%2F507030 Accessed online at
19. Food and Drug Administration. FDA-cleared sterilants and high-level disinfectants with general claims for processing reusable medical and dental devices. March 2009. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/ReprocessingofSingleUseDevices/ucm133514.htm Accessed online 2012 Nov 5 at
20. Weber D.J., Rutala W.A. Lessons from outbreaks associated with bronchoscopy. Infect Control Hosp Epidemiol. 2001;22:403–408.
21. Srinivasan A., Wolfenden L.L., Song X., et al. An outbreak of Pseudomonas aeruginosa infections associated with flexible bronchoscopes. N Engl J Med. 2003;348:221–227.
22. Mehta A.C., Prakash U.B.S., Garland R., et al. Prevention of flexible bronchoscopy-associated infection. Chest. 2006;128:1742–1755.
23. Association of Perioperative Registered Nurses. Recommended practices for high-level disinfection. AORN J. 2005;81:402–412.
24. Centers for Disease Control and Prevention. Guidelines for preventing health-care associated pneumonia, MMWR. 2004;53(RR-03):1–36. 2003Accessed online at. http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/cdcpneumo_guidelines.pdf
24a. Cross-contamination vis gas sampling lines? Anesth Patient Saf News/. 2009:27–29. Summer
25. Dräger Medical. Operating Instructions, Apollo SW 4.n. Part Number 9039994, ed 2. Telford, PA: Dräger Medical; 2008. 203–228
26. Cleaning and sterilization. Aisys User Reference Manual, Chapter 2. Madison, WI: Datex-Ohmeda; 2008. a subsidiary of GE Healthcare
27. Du Moulin G.C., Saubermann A.J. The anesthesia machine and circle system are not likely to be sources of bacterial contamination. Anesthesiology. 1977;47:353–358.
28. Langevin P.B., Rand K.H., Layon A.J. The potential for dissemination of Mycobacterium tuberculosis through the anesthesia breathing circuit. Chest. 1999;115:1107–1114.
29. Demers R.R. Bacterial/viral filtration: let the breather beware!. Chest. 2001;120:1377–1389.
30. Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities. MMWR. 52(RR-10), 2003.
31. Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, MMWR. 2005;54(RR-17):20–21. 2005Accessed online at. http://www.cdc.gov/mmwr/PDF/rr/rr5417.pdf
32. O’Grady N.P., Alexander M., Burns L.A., et al. for the Healthcare Infection Control Practices Advisory Committee of the Centers for Disease Control and Prevention: Guidelines for the prevention of intravascular catheter-related infections. 2011. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf Accessed online at
33. Bouza E., Burillo A. Advances in the prevention and management of ventilator-associated pneumonia. Curr Opin Infect Dis. 2009;22:345–351.
34. American Thoracic Society and the Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
35. Coffin S.E., Klompas M., Classen D., et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29:S31–S40.
36. Drakulovic M.B., Torres A., Bauer T.T., et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851–1858.
37. Bassi G.L., Zanella A., Cressoni M., et al. Following tracheal intubation, mucus flow is reversed in the semi-recumbent position: possible role in the pathogenesis of ventilator-associated pneumonia. Crit Care Med. 2008;36:518–525.
38. Torres A., Serra-Batiles J., Ros E., et al. Pulmonary aspiration of gastric contents in patients receiving mechanical ventilation: the effect of body position. Ann Intern Med. 1992;116:540–543.
39. Rello J., Sonora R., Jubert P., et al. Pneumonia in intubated patients; role of respiratory airway care. Am J Respir Crit Care Med. 1996;154:111–115.
40. Dezfulian C., Shojania K., Collard H.R., et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia; a meta-analysis. Am J Med. 2005;118:11–18.
41. DeRiso A.J., 2nd., Ladowski J.S., Dillon T.A., et al. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556–1561.
42. Chan E.Y., Ruest A., Meade M.O., et al. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. Br Med J. 2007;334:861–862.
43. Anderson D.J., Kaye K.S., Classen D., Arias K.M. Strategies to prevent surgical site infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(S1):S51–S61.
44. Mangram A.J., Horan T.C., Pearson M.L., Silver L.C., Jarvis W.R. Guideline for prevention of surgical site infection. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;(20):250–278. 1999
45. Kurz A., Sessler D.I., Lenhardt R. Perioperative normothermia to reduce the incidence of surgical wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209–1215.
46. Melling A.C., Ali B., Scott E.M., Leaper D.J. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358:876–880.
47. Berrios-Torres S.I. Surgical Site Infection (SSI). Toolkit: CDC; 2009. http://www.cdc.gov/HAI/pdfs/toolkits/SSI_toolkit021710SIBT_revised.pdf Accessed online at
48. Fry D.E. Surgical site infections and the Surgical Care Improvement Project (SCIP): evolution of national quality measures. SurgInfect. 2008;9(6):579–584.
49. Lehtinen S.J., Onicescu G., Kuhn K.M., Cole D.J., Esnaola N.F. Normothermia to prevent surgical site infections after gastrointestinal surgery. Ann Surg. 2010;252:696–704.
50. Kurz A. Thermal care in the perioperative period. Best Pract Res Clin Anaesthesiol. 2008;22:39–62.
51. Zerr K.J., Furnary A.P., Grunkemeier G.L., Bookin S., et al. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63(2):356–361.
52. Dronge A.S., Perkal M.F., Kancir S., et al. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141:375–380.
53. Finfer S., Chittock D.R., Su S.Y., et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297.
54. Murray B.W., Huerta S., Dineen S., Anthony T. Surgical site infection in colorectal surgery: a review of the nonpharmacologic tools of prevention. J Am Coll Surg. 2010;211(6):812–822.
55. Centers for Disease Control and Prevention. Prevention Strategies for Seasonal Influenza in Healthcare Settings. 2010. http://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm Accessed online 2011 Aug 6 at
56. Greene E.S., Berry A.J., Arnold W.R., Jagger J. Percutaneous injuries in anesthesia personnel. Anesth Analg. 1996;83:273–278.
57. Tait A.R., Tuttle D.B. Prevention of occupational transmission of human immunodeficiency virus and hepatitis B virus among anesthesiologists: a survey of anesthesiology practice. Anesth Analg. 1994;79:623–628.
58. Makary M.A., Al-Attar A., Holzmueller C.G., et al. Needlestick injuries among surgeons in training. N Engl J Med. 2007;356(26):2693–2699.
59. Centers for Disease Control and Prevention. Updated U.S. Public Health Service Guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for post-exposure prophylaxis. MMWR. 50(RR-11), 2001.
60. Centers for Disease Control and Prevention. Evaluation of blunt suture needles in preventing percutaneous injuries among health-care workers during gynecologic surgical procedures: New York City, MMWR. March 1993–June 1994;(46):25–29. 1997
61. Centers for Disease Control and Prevention. Workbook for designing, implementing, and evaluating a sharps injury prevention program. 2008. http://www.cdc.gov/sharpssafety/pdf/sharpsworkbook_2008.pdf Accessed online at
62. Tanner J., Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2006 Jul 19;3:CD003087.
63. Bennett N.T., Howard R.J. Quantity of blood inoculated in a needlestick injury from suture needles. J Am Coll Surg. 1994;178:107–110.
64. Mast S.T., Woolwine J.D., Gerberding J.L. Efficacy of gloves in reducing blood volumes transferred during simulated needlestick injury. J Infect Dis. 1993;168:1589–1592.
65. Bond W.W., Favero M.S., Petersen N.J., et al. Survival of hepatitis B virus after drying and storage for one week [letter]. Lancet. 1981;1:550–551.
66. Jagger J., Puro V., De Carli G. Occupational transmission of hepatitis C virus. JAMA. 2002;288(12):1469–1470.
67. Chung H., Kudo M., Kumada T., et al. Risk of HCV transmission after needlestick injury, and the efficacy of short-duration interferon administration to prevent HCV transmission to medical personnel. J Gastroenterol. 2003;38(9):877–879.
68. Do A.N., Ciesielski C.A., Metler R.P., et al. Occupationally acquired human immunodeficiency virus (HIV) infection: national case surveillance data during 20 years of the HIV epidemic in the United States. Infect Control Hosp Epidemiol. 2003;24(2):86–96.
69. Centers for Disease Control and Prevention. Updated U.S. Public Health Service Guidelines for the management of occupational exposures to HIV and recommendations for post-exposure prophylaxis. MMWR. 2005;54(RR-09):1–17.
70. Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health: Workplace safety and health topics: diseases and injuries: bloodborne infectious diseases: HIV/AIDS, hepatitis B, hepatitis C. http://www.cdc.gov/niosh/topics/bbp. Accessed 2011 Aug 6 at
71. Centers for Disease Control and Prevention. Guidelines for infection control in dental health-care settings, 2003. Appendix B: immunizations strongly recommended for health care personnel. MMWR. 2003;52(RR-17):65.