Fetal Assessment during Labor
• Identify typical signs of normal (reassuring) and abnormal (nonreassuring) fetal heart rate (FHR) patterns.
• Compare FHR monitoring performed by intermittent auscultation with external and internal electronic methods.
• Explain the baseline FHR and evaluate periodic changes.
• Describe nursing measures that can be used to maintain FHR patterns within normal limits.
• Differentiate among the nursing interventions used for managing specific FHR patterns, including tachycardia and bradycardia, absent or minimal variability, and late and variable decelerations.
• Review the documentation of the monitoring process necessary during labor.
Infusion of normal saline or lactated Ringer's solution through an intrauterine catheter into the uterine cavity in an attempt to increase the fluid around the umbilical cord and prevent compression during uterine contractions
Average FHR during a 10-minute period that excludes periodic and episodic changes and periods of marked variability; normal FHR baseline is 110-160 beats/min
Slowing of FHR attributed to a parasympathetic response and described in relation to uterine contractions. Types of decelerations include:
A visually apparent gradual decrease of FHR before the peak of a contraction and return to baseline as the contraction ends; caused by fetal head compression
A visually apparent gradual decrease of FHR, with the lowest point of the deceleration occurring after the peak of the contraction and returning to baseline after the contraction ends; caused by uteroplacental insufficiency
A visually apparent decrease (may be either gradual or abrupt) in FHR of at least 15 beats/min below the baseline and lasting more than 2 minutes but less than 10 minutes
A visually apparent abrupt decrease in FHR below the baseline occurring any time during the uterine contracting phase; caused by compression of the umbilical cord
Reduction in arterial oxygen pressure resulting in metabolic acidosis by forcing anaerobic glycolysis, pulmonary vasoconstriction, and direct cellular damage
Listening to fetal heart sounds at periodic intervals using nonelectronic or ultrasound devices placed on the maternal abdomen
Inhibition of uterine contractions through administration of medications; used as an adjunct to other interventions in the management of fetal compromise related to increased uterine activity
Web Resources
Additional related content can be found on the companion website at ![]()
http://evolve.elsevier.com/Lowdermilk/Maternity/
• Critical Thinking Exercise: Fetal Monitoring
• Nursing Care Plan: Electronic Fetal Monitoring during Labor
T he ability to assess the fetus by auscultation of the fetal heart was initially described more than 300 years ago. With the advent of the fetoscope and stethoscope after the turn of the twentieth century the listener could hear clearly enough to count the fetal heart rate (FHR). When electronic FHR monitoring made its debut for clinical use in the early 1970s, the anticipation was that its use would result in fewer cases of cerebral palsy and be more sensitive than stethoscopic auscultation in predicting and preventing fetal compromise (Garite, 2007). Consequently, the use of electronic fetal monitoring rapidly expanded. However, the rate of cerebral palsy has risen slightly since that time and is not likely to improve (Gilbert, 2007). Moreover, in 2006 the cesarean birth rate in the United States reached an all-time high of 31.1% (Collard, Diallo, Habinsky, Hentschell, & Vezeau, 2008/2009).
Still, electronic fetal monitoring (EFM) is a useful tool for visualizing FHR patterns on a monitor screen or printed tracing and continues to be the primary mode of intrapartum fetal assessment. Currently in the United States, approximately 85% of women giving birth have continuous EFM during labor, making it the most commonly performed obstetric procedure (American College of Obstetricians and Gynecologists [ACOG], 2009; Tucker, Miller, & Miller, 2009). Pregnant women should be informed about the equipment and procedures used and the risks, benefits, and limitations of intermittent auscultation (IA) and EFM. This chapter discusses the basis for intrapartum fetal monitoring, the types of monitoring, and nursing assessment and management of abnormal fetal status.
Basis for Monitoring 
Fetal Response
Because labor is a period of physiologic stress for the fetus, frequent monitoring of fetal status is part of the nursing care during labor. The fetal oxygen supply must be maintained during labor to prevent fetal compromise and to promote newborn health after birth. The fetal oxygen supply can decrease in several ways:
• Reduction of blood flow through the maternal vessels as a result of maternal hypertension (chronic hypertension, preeclampsia, or gestational hypertension), hypotension (caused by supine maternal position, hemorrhage, or epidural analgesia or anesthesia), or hypovolemia (caused by hemorrhage)
• Reduction of the oxygen content in the maternal blood as a result of hemorrhage or severe anemia
• Alterations in fetal circulation, occurring with compression of the umbilical cord (transient, during uterine contractions [UCs], or prolonged, resulting from cord prolapse), placental separation or complete abruption, or head compression (head compression causes increased intracranial pressure and vagal nerve stimulation with an accompanying decrease in the FHR)
• Reduction in blood flow to the intervillous space in the placenta secondary to uterine hypertonus (generally caused by excessive exogenous oxytocin) or secondary to deterioration of the placental vasculature associated with maternal disorders such as hypertension or diabetes mellitus
Fetal well-being during labor can be measured by the response of the FHR to UCs. A group of fetal monitoring experts have recently recommended that FHR tracings that demonstrate the following characteristics be described as normal (Macones, Hankins, Spong, Hauth, & Moore, 2008):
• A baseline FHR rate of 110 to 160 beats/min
• Moderate baseline FHR variability
• Late or variable decelerations absent
Uterine Activity
Table 11-1 describes normal uterine activity (UA) during labor.
TABLE 11-1
Normal Uterine Activity during Labor
| CHARACTERISTIC | DESCRIPTION |
| Frequency | Contraction frequency overall generally ranges from two to five per 10 minutes during labor, with lower frequencies seen in the first stage of labor and higher frequencies (up to five contractions in 10 minutes) seen during the second stage of labor. |
| Duration | Contraction duration remains fairly stable throughout the first and second stages, ranging from 45-80 seconds, not generally exceeding 90 seconds. |
| Intensity (peak less resting tone) | Intensity of uterine contractions generally range from 25-50 mm Hg in the first stage of labor and may rise to over 80 mm Hg in second stage. Contractions palpated as “mild” would likely peak at less than 50 mm Hg if measured internally, whereas contractions palpated as “moderate” or greater would likely peak at 50 mm Hg or greater if measured internally. |
| Resting tone | Average resting tone during labor is 10 mm Hg; if using palpation, should palpate as “soft” (i.e., easily indented, no palpable resistance). |
| Montevideo units (MVUs) | Ranges from 100-250 MVUs in the first stage, may rise to 300-400 in the second stage. Contraction intensities of 40 mm Hg or more and MVUs of 80-120 are generally sufficient to initiate spontaneous labor. |
Source: Tucker, S. M., Miller, L. A, & Miller, D. A. (2009). Mosby's pocket guide to fetal monitoring: A multidisciplinary approach (6th ed.). St. Louis: Mosby.
Fetal Compromise
The goals of intrapartum FHR monitoring are to identify and differentiate the normal (reassuring) patterns from the abnormal (nonreassuring) patterns, which can be indicative of fetal compromise. Although the 2008 National Institute of Child Health and Human Development workshop (Macones et al., 2008) and a recent ACOG Practice Bulletin (2009) both recommend use of the terms normal and abnormal to describe FHR tracings, the terms reassuring and nonreassuring are still frequently used clinically.
Abnormal FHR patterns are those associated with fetal hypoxemia, which is a deficiency of oxygen in the arterial blood. If uncorrected, hypoxemia can deteriorate to severe fetal hypoxia, which is an inadequate supply of oxygen at the cellular level. Examples of abnormal FHR patterns include the following (Macones et al., 2008).
Monitoring Techniques 
The ideal method of fetal assessment during labor continues to be debated. When performed at prescribed intervals, especially during and immediately after contractions, IA has been shown to be as valuable as EFM at predicting fetal outcomes (Gilbert, 2007).
Intermittent Auscultation
Intermittent auscultation (IA) involves listening to fetal heart sounds at periodic intervals to assess the FHR. IA of the fetal heart can be performed with a Pinard stethoscope, a Doppler ultrasound device (Fig.11-1, A) an ultrasound stethoscope (Fig. 11-1, B) or a DeLee-Hillis fetoscope (Fig. 11-1, C). The fetoscope is applied to the listener's forehead because bone conduction amplifies the fetal heart sounds for counting. The Doppler ultrasound device and ultrasound stethoscope transmit ultrahigh-frequency sound waves reflecting movement of the fetal heart and convert these sounds into an electronic signal that can be counted. Box 11-1 describes how to perform IA.
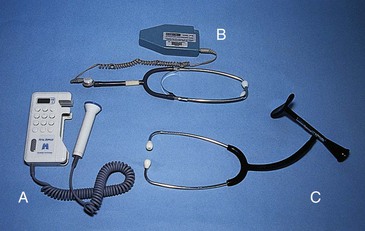
IA is easy to use, inexpensive, and less invasive than EFM. It is often more comfortable for the woman and gives her more freedom of movement. Other care measures, such as ambulation and the use of baths or showers, are easier to carry out when IA is used. On the other hand, IA may be difficult to perform in women who are obese. Because IA is intermittent, significant events may occur during a time when the FHR is not auscultated. Also, IA does not provide a permanent documented visual record of the FHR and cannot be used to assess visual patterns of the FHR variability or periodic changes (Albers, 2007; Tucker et al., 2009). By using IA the nurse can assess the baseline FHR, rhythm, and increases and decreases from baseline. The recommended optimal frequency for IA in low risk women during labor has not been determined (Nageotte & Gilstrap, 2009).
Every effort should be made to use the method of fetal assessment the woman desires, if possible. However, adherence to the frequency guides can make using IA difficult in today's busy labor and birth units because when used as the primary method of fetal assessment, auscultation requires a 1 : 1 nurse-to-patient staffing ratio. If acuity and census change such that auscultation standards are no longer met, then the nurse must inform the physician or nurse-midwife that continuous EFM will be used until staffing can be arranged to meet the standards.
The woman can become anxious if the examiner cannot readily count the fetal heartbeats. The inexperienced listener often needs time to locate the heartbeat and find the area of maximal intensity. To allay the mother's concerns, she can be told that the nurse is “finding the spot where the sounds are loudest.” If the examiner cannot locate the fetal heartbeat, assistance should be requested. In some cases, ultrasound can be used to help locate the fetal heartbeat. Seeing the FHR on the ultrasound screen will be reassuring to the mother if locating the best area for auscultation was initially difficult.
When using IA, UA is assessed by palpation. The examiner should keep his or her hand placed over the fundus before, during, and after contractions. The contraction intensity is usually described as mild, moderate, or strong. The contraction duration is measured in seconds, from the beginning to the end of the contraction. The frequency of contractions is measured in minutes, from the beginning of one contraction to the beginning of the next. The examiner should keep his or her hand on the fundus after the contraction is over to evaluate uterine resting tone or relaxation between contractions. Resting tone between contractions is usually described as soft or relaxed.
Accurate and complete documentation of fetal status and UA is especially important when IA and palpation are being used because no paper tracing record or computer storage of these assessments is provided, as is the case with continuous EFM. Labor flow records or computer charting systems that prompt notations of all assessments are useful for ensuring such comprehensive documentation.
Electronic Fetal Monitoring
The purpose of electronic FHR monitoring is the ongoing assessment of fetal oxygenation. The goal is to detect fetal hypoxia and metabolic acidosis during labor so that interventions to resolve the problem can be implemented in a timely manner before permanent damage or death occur (Garite, 2007).
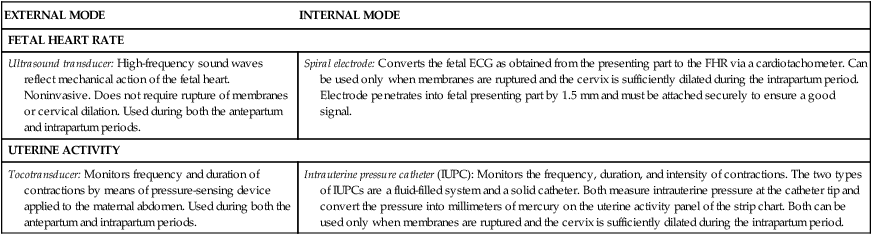
The two modes of EFM include the external mode, which uses external transducers placed on the maternal abdomen to assess FHR and UA, and the internal mode, which uses a spiral electrode applied to the fetal presenting part to assess the FHR and an intrauterine pressure catheter (IUPC) to assess UA and pressure. The differences between the external and internal modes of EFM are summarized in Table 11-2.
TABLE 11-2
External and Internal Modes of Monitoring
| EXTERNAL MODE | INTERNAL MODE |
| FETAL HEART RATE | |
| Ultrasound transducer: High-frequency sound waves reflect mechanical action of the fetal heart. Noninvasive. Does not require rupture of membranes or cervical dilation. Used during both the antepartum and intrapartum periods. | Spiral electrode: Converts the fetal ECG as obtained from the presenting part to the FHR via a cardiotachometer. Can be used only when membranes are ruptured and the cervix is sufficiently dilated during the intrapartum period. Electrode penetrates into fetal presenting part by 1.5 mm and must be attached securely to ensure a good signal. |
| UTERINE ACTIVITY | |
| Tocotransducer: Monitors frequency and duration of contractions by means of pressure-sensing device applied to the maternal abdomen. Used during both the antepartum and intrapartum periods. | Intrauterine pressure catheter (IUPC): Monitors the frequency, duration, and intensity of contractions. The two types of IUPCs are a fluid-filled system and a solid catheter. Both measure intrauterine pressure at the catheter tip and convert the pressure into millimeters of mercury on the uterine activity panel of the strip chart. Both can be used only when membranes are ruptured and the cervix is sufficiently dilated during the intrapartum period. |
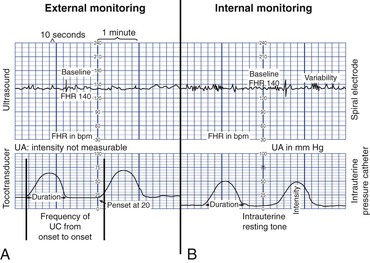
External monitoring
Separate transducers are used to monitor the FHR and UCs (Fig. 11-2). The ultrasound transducer works by reflecting high-frequency sound waves off a moving interface: in this case the fetal heart and valves. Reproducing a continuous and precise record of the FHR is sometimes difficult because of artifacts introduced by fetal and maternal movement. The FHR is printed on specially formatted monitor paper. The standard paper speed used in the United States is 3 cm/min. Once the area of maximal intensity of the FHR has been located, conductive gel is applied to the surface of the ultrasound transducer, and the transducer is then positioned over this area and held securely in place using an elastic belt.
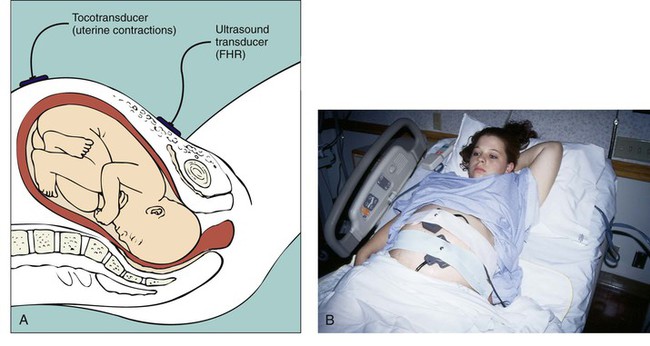
The tocotransducer (tocodynamometer) measures UA transabdominally. The device is placed over the uterine fundus and held securely in place using an elastic belt (see Fig. 11-2). UCs or fetal movements depress a pressure-sensitive surface on the side next to the abdomen. The tocotransducer can measure and record the frequency and approximate duration of UCs but not their intensity. This method is especially valuable for measuring UA during the first stage of labor in women with intact membranes or for antepartum testing. Because the tocotransducer of most electronic fetal monitors is designed for assessing UA in the term pregnancy, it may not be sensitive enough to detect preterm UA. When monitoring the woman in preterm labor, remember that the fundus may be located below the level of the umbilicus. The nurse may need to rely on the woman to indicate when UA is occurring and to use palpation as an additional way of assessing contraction frequency and validating the monitor tracing.
The external transducer is easily applied by the nurse, but it must be repositioned as the woman or fetus changes position (see Fig. 11-2, B). The woman is asked to assume a semi-sitting or a lateral position. Use of an external transducer confines the woman to bed. Portable telemetry monitors allow observation of the FHR and UC patterns by means of centrally located electronic display stations. These portable units permit the woman to walk around during electronic monitoring.
Internal monitoring
The technique of continuous internal FHR or UA monitoring allows a more accurate appraisal of fetal well-being during labor than external monitoring because it is not interrupted by fetal or maternal movement or affected by maternal size (Fig. 11-3). For this type of monitoring the membranes must be ruptured and the cervix sufficiently dilated (at least 2-3 cm) to allow placement of the spiral electrode or IUPC or both. Internal and external modes of monitoring may be combined (i.e., internal FHR with external UA or external FHR with internal UA) without difficulty.
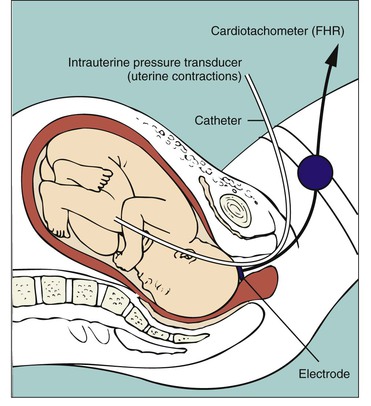
Internal monitoring of the FHR is accomplished by attaching a small spiral electrode to the presenting part. For UA to be monitored internally, a solid IUPC is introduced into the uterine cavity. The catheter has a pressure-sensitive tip that measures changes in intrauterine pressure. As the catheter is compressed during a contraction, pressure is placed on the pressure transducer. This pressure is then converted into a pressure reading in millimeters of mercury. The average pressure during a contraction ranges from 50 to 85 mm Hg. The IUPC can measure the frequency, duration, and intensity of UC, as well as uterine resting tone.
The FHR and UA are displayed on the monitor paper, with the FHR in the upper section and UA in the lower section. Fig. 11-4 contrasts the internal and external modes of electronic monitoring. Note that each small square on the monitor paper or screen represents 10 seconds; each larger box of six squares equals 1 minute (when paper is moving through the monitor at the rate of 3 cm/min).
Fetal Heart Rate Patterns 
Characteristic FHR patterns are associated with fetal and maternal physiologic processes and have been identified for many years. Because EFM was introduced into clinical practice before consensus was reached in regard to standardized terminology, however, variations in the description and interpretation of common fetal heart rate patterns were often great. In 1997 the National Institute of Child Health and Human Development (NICHD) published a proposed nomenclature system for EFM interpretation with standardized definitions for FHR monitoring. The NICHD recommendations were not widely incorporated into clinical practice, however, until they were endorsed by the American College of Obstetricians and Gynecologists (ACOG) in 2005. Shortly thereafter, use of the NICHD standard terminology was also endorsed by the Association of Women's Health, Obstetric, and Neonatal Nurses (AWHONN), and the American College of Nurse-Midwives (ACNM) (Tucker et al., 2009). All three organizations cited concerns regarding patient safety and the need for improved communication among caregivers as reasons for using standard EFM definitions in clinical practice.
In April 2008 the NICHD, ACOG, and the Society for Maternal-Fetal Medicine partnered to sponsor another workshop to revisit the FHR definitions recommended by the NICHD in 1997. The 1997 FHR definitions were reaffirmed at this workshop. In addition, new definitions related to UA were recommended, as well as a three-tier system of FHR pattern interpretation and categorization (Macones et al., 2008). ACOG (2009) has recently published a practice bulletin which supports use of the 2008 NICHD workshop recommendations.
Baseline Fetal Heart Rate
The intrinsic rhythmicity of the fetal heart, the central nervous system (CNS), and the fetal autonomic nervous system control the FHR. An increase in sympathetic response results in acceleration of the FHR, whereas an increase in parasympathetic response produces a slowing of the FHR. A balanced increase of sympathetic and parasympathetic response usually occurs during contractions, with no observable change in the baseline FHR.
The baseline fetal heart rate is the average rate during a 10-minute segment that excludes periodic or episodic changes, periods of marked variability, and segments of the baseline that differ by more than 25 beats/min (Macones et al., 2008). The normal range at term is 110 to 160 beats/min. The baseline rate is documented as a single number, rather than a range (Tucker et al., 2009).
Variability
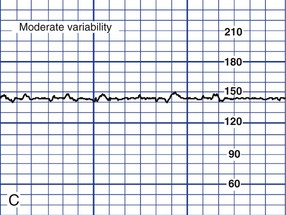
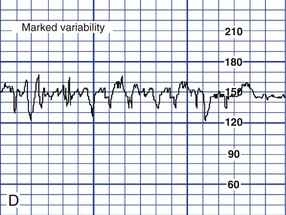
Variability of the FHR can be described as irregular waves or fluctuations in the baseline FHR of two cycles per minute or greater (Macones, et al., 2008). It is a characteristic of the baseline FHR and does not include accelerations or decelerations of the FHR. Variability is quantified in beats per minute and is measured from the peak to the trough of a single cycle. Four possible categories of variability have been identified: absent, minimal, moderate, and marked (Fig. 11-5). In the past, variability was described as either long term or short term (beat to beat). The NICHD definitions do not distinguish between long- and short-term variability, however, because in actual practice, they are visually determined as a unit (NICHD, 1997).
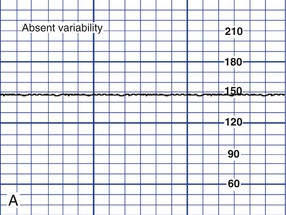
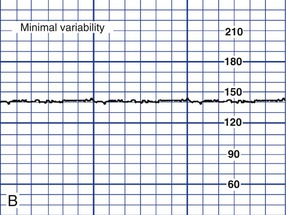
Depending upon other characteristics of the FHR tracing, absent or minimal variability is classified as either abnormal or indeterminate (Macones et al., 2008) (see Fig. 11-5, A and B). It can result from fetal hypoxemia and metabolic acidemia. Other possible causes of absent or minimal variability include congenital anomalies and preexisting neurologic injury. CNS depressant medications, including analgesics, narcotics (meperidine [Demerol]), barbiturates (secobarbital [Seconal] and pentobarbital [Nembutal]), tranquilizers (diazepam [Valium]), ataractics (promethazine [Phenergan]), and general anesthetics are other possible causes of minimal variability. In addition, minimal variability can occur with tachycardia, extreme prematurity, or when the fetus is in a sleep state (Tucker et al., 2009).
Moderate variability, on the other hand, is considered normal (see Fig. 11-5, C). Its presence is highly predictive of a normal fetal acid-base balance (absence of fetal metabolic acidemia). Moderate variability indicates that FHR regulation is not significantly affected by fetal sleep cycles, tachycardia, prematurity, congenital anomalies, preexisting neurologic injury, or CNS depressant medications (Macones et al., 2008; Tucker et al., 2009).
The significance of marked variability (see Fig. 11-5, D) is unclear (Macones et al., 2008).
A sinusoidal pattern—a regular smooth, undulating wavelike pattern—is not included in the definition of FHR variability. This uncommon pattern classically occurs with severe fetal anemia (Fig. 11-6) (Tucker et al., 2009).
Tachycardia
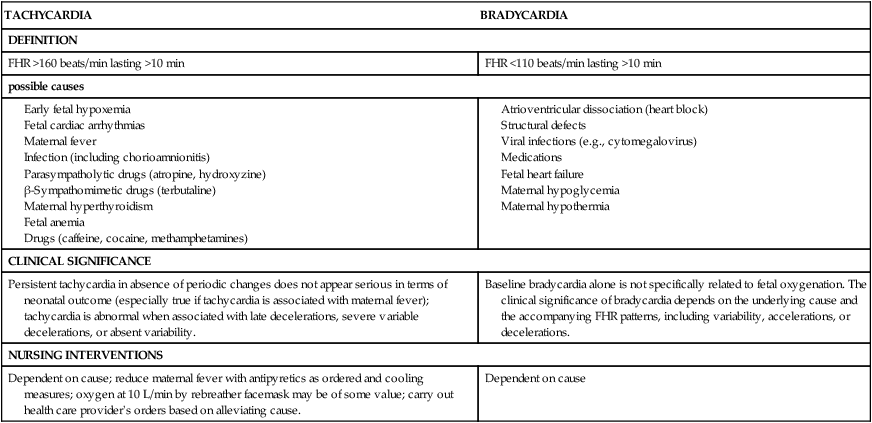
Tachycardia is a baseline FHR greater than 160 beats/min for a duration of 10 minutes or longer (Fig. 11-7). It can be considered an early sign of fetal hypoxemia, especially when associated with late decelerations and minimal or absent variability. Fetal tachycardia can result from maternal or fetal infection (e.g., prolonged rupture of membranes with amnionitis), from maternal hyperthyroidism or fetal anemia, or in response to medications such as atropine, hydroxyzine (Vistaril), terbutaline (Brethine), or illicit drugs such as cocaine or methamphetamines. Table 11-3 lists causes, clinical significance, and nursing interventions for tachycardia.
TABLE 11-3
| TACHYCARDIA | BRADYCARDIA |
| DEFINITION | |
| FHR >160 beats/min lasting >10 min | FHR <110 beats/min lasting >10 min |
| possible causes | |
| CLINICAL SIGNIFICANCE | |
| Persistent tachycardia in absence of periodic changes does not appear serious in terms of neonatal outcome (especially true if tachycardia is associated with maternal fever); tachycardia is abnormal when associated with late decelerations, severe variable decelerations, or absent variability. | Baseline bradycardia alone is not specifically related to fetal oxygenation. The clinical significance of bradycardia depends on the underlying cause and the accompanying FHR patterns, including variability, accelerations, or decelerations. |
| NURSING INTERVENTIONS | |
| Dependent on cause; reduce maternal fever with antipyretics as ordered and cooling measures; oxygen at 10 L/min by rebreather facemask may be of some value; carry out health care provider's orders based on alleviating cause. | Dependent on cause |
Bradycardia
Bradycardia is a baseline FHR less than 110 beats/min for a duration of 10 minutes or longer (Fig. 11-8). True bradycardia occurs rarely and is not specifically related to fetal oxygenation. True bradycardia must be distinguished from a prolonged deceleration because the causes and management of these two conditions are very different. Bradycardia is often caused by some type of fetal cardiac problem such as structural defects involving the pacemakers or conduction system or fetal heart failure. Other causes of bradycardia include viral infections (e.g., cytomegalovirus), maternal hypoglycemia, and maternal hypothermia. The clinical significance of the bradycardia depends on the underlying cause and accompanying FHR patterns, including variability and the presence of accelerations or decelerations (Tucker et al., 2009). (See Table 11-3 for a list of causes, clinical significance, and nursing interventions for bradycardia.)
Periodic and Episodic Changes in Fetal Heart Rate
Changes in FHR from the baseline are categorized as periodic or episodic. Periodic changes are those that occur with UCs. Episodic changes are those that are not associated with UCs. These patterns include both accelerations and decelerations (NICHD, 1997).
Accelerations
Acceleration of the FHR is defined as a visually apparent abrupt (onset to peak <30 seconds) increase in FHR above the baseline rate (Fig. 11-9). The peak is at least 15 beats/min above the baseline, and the acceleration lasts 15 seconds or more, with the return to baseline less than 2 minutes from the beginning of the acceleration. Before 32 weeks gestation the definition of an acceleration is a peak of 10 beats/min or more above the baseline and a duration of at least 10 seconds. Acceleration of the FHR for more than 10 minutes is considered a change in baseline rate (Tucker et al., 2009).
Accelerations can be periodic or episodic. They may occur in association with fetal movement or spontaneously. If accelerations do not occur spontaneously, they can be elicited by fetal scalp stimulation or vibroacoustic stimulation. Similar to moderate variability, accelerations are considered an indication of fetal well-being. Their presence is highly predictive of a normal fetal acid-base balance (absence of fetal metabolic acidemia) (Tucker et al., 2009). Box 11-2 lists causes, clinical significance, and nursing interventions for accelerations.
Decelerations
FHR decelerations are categorized as early, late, variable, or prolonged. FHR decelerations are defined according to their visual relationship to the onset and end of a contraction and by their shape.
Early decelerations.
Early deceleration of the FHR is a visually apparent gradual (onset to lowest point 30 seconds or more) decrease in and return to the baseline FHR associated with UCs (Macones et al., 2008). Generally the onset, nadir, and recovery of the deceleration correspond to the beginning, peak, and end of the contraction (Fig. 11-10). For this reason, early decelerations are sometimes called the “mirror image” of a contraction.
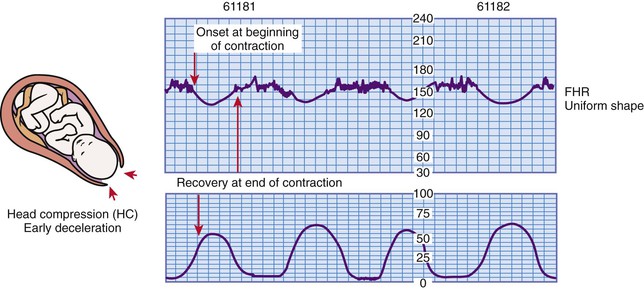
Early decelerations are thought to be caused by transient fetal head compression and are considered a benign finding (Tucker et al., 2009). Early decelerations may also occur during vaginal examinations, as a result of fundal pressure, and during placement of the internal mode of fetal monitoring. When present, they usually occur during the first stage of labor when the cervix is dilated 4 to 7 cm but can also be seen during the second stage when the woman is pushing.
Because early decelerations are considered to be benign, interventions are not necessary. Early decelerations should be identified, however, so that they can be distinguished from late or variable decelerations, for which interventions are appropriate. Box 11-3 lists cause, clinical significance, and nursing interventions for early decelerations.
Late decelerations.
Late deceleration of the FHR is a visually apparent gradual (onset to lowest point 30 seconds or more) decrease in and return to baseline FHR associated with UCs (Macones et al., 2008). The deceleration begins after the contraction has started, and the lowest point of the deceleration occurs after the peak of the contraction. The deceleration usually does not return to baseline until after the contraction is over (Fig. 11-11).
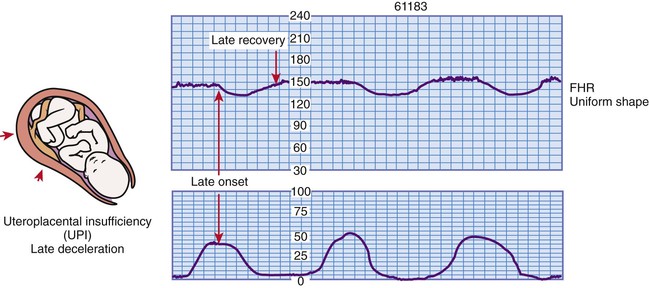
Uteroplacental insufficiency causes late decelerations. Persistent and repetitive late decelerations indicate the presence of fetal hypoxemia stemming from insufficient placental perfusion during UCs. If recurrent or sustained, late decelerations can lead to metabolic acidemia (Tucker et al., 2009). They should be considered an ominous sign when they are uncorrectable, especially if they are associated with absent or minimal variability and tachycardia. Several factors can disrupt oxygen transfer to the fetus, including maternal hypotension, uterine tachysystole (e.g., more than five contractions in 10 minutes, averaged over a 30-minute window), preeclampsia, postdate or postterm pregnancy, amnionitis, small-for-gestational-age fetuses, maternal diabetes, placenta previa, abruptio placentae, conduction anesthetics, maternal cardiac disease, and maternal anemia. The clinical significance and nursing interventions are described in Box 11-4.
Variable decelerations.
Variable deceleration of the FHR is defined as a visually abrupt (onset to lowest point <30 seconds) decrease in FHR below the baseline. The decrease is at least 15 beats/min or more below the baseline, lasts at least 15 seconds, and returns to baseline in less than 2 minutes from the time of onset (Macones et al., 2008). Variable decelerations are not necessarily associated with UCs. Variable decelerations are caused by compression of the umbilical cord (Fig. 11-12).
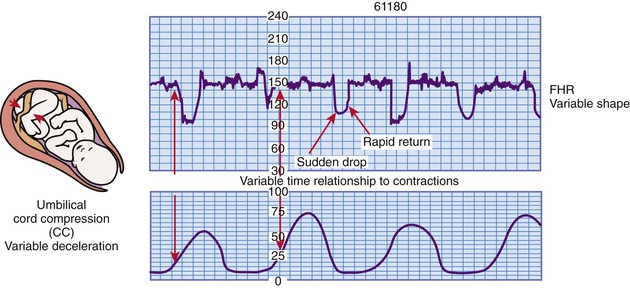
The appearance of variable decelerations differs from those of early and late decelerations, which closely approximate the shape of the corresponding UC. Instead, variable decelerations often have a U, V, or W shape characterized by a rapid descent to and ascent from the nadir (lowest point) of the deceleration (see Fig. 11-12). Some variable decelerations are preceded and followed by brief accelerations of the FHR, known as “shoulders,” which is an appropriate compensatory response to compression of the umbilical cord.
Occasional variable decelerations have little clinical significance. Repetitive variable decelerations, on the other hand, indicate recurrent disruption in the fetus' oxygen supply. This disruption can result in hypoxemia and eventually metabolic acidemia (Tucker et al., 2009). Variable decelerations are most commonly found during the transition phase of first stage labor or the second stage of labor as a result of umbilical cord compression and stretching during fetal descent (Garite, 2007). Box 11-5 lists causes, clinical significance, and nursing interventions for variable decelerations.
Prolonged decelerations.
A prolonged deceleration is a visually apparent decrease (may be either gradual or abrupt) in FHR of at least 15 beats/min below the baseline and lasting more than 2 minutes but less than 10 minutes. A deceleration lasting more than 10 minutes is considered a baseline change (Macones et al., 2008) (Fig. 11-13).
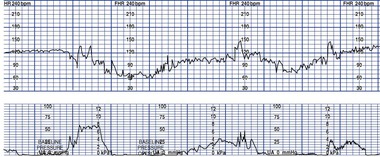
Prolonged decelerations are caused by a disruption in the fetal oxygen supply. They usually begin as a reflex response to hypoxia. If the disruption continues, however, the fetal cardiac tissue itself will become hypoxic, resulting in direct myocardial depression of the FHR (Tucker et al., 2009). Prolonged decelerations may be caused by prolonged cord compression, profound uteroplacental insufficiency, or perhaps sustained head compression. The presence and degree of hypoxia present are thought to correlate with the depth and duration of the deceleration, how abruptly it returns to the baseline, how much variability is lost during the deceleration, and whether rebound tachycardia and loss of variability occur after the deceleration (Garite, 2007).
Significant stimuli that may result in prolonged decelerations are a prolapsed umbilical cord or other forms of prolonged cord compression, prolonged uterine tachysystole, hypotension after spinal or epidural anesthesia or analgesia, abruptio placentae, eclamptic seizure, and rapid descent through the birth canal. Other more benign causes of prolonged decelerations include pelvic examination, application of a spiral electrode, and sustained maternal Valsalva maneuver (Garite, 2007).
Care Management 
Care of the woman receiving EFM in labor begins with evaluation of the EFM equipment. The nurse must ensure that the monitor is recording FHR and UA accurately and that the tracing is interpretable. If external monitoring is not adequate, changing to a fetal spiral electrode or IUPC may be necessary. A checklist for fetal monitoring equipment can be used to evaluate the equipment functions (Box 11-6).
After ensuring that the monitor is recording properly, the FHR and UA tracings are evaluated regularly throughout labor. Guidelines for Perinatal Care published jointly by the American Academy of Pediatrics (AAP) and ACOG (2007) recommends that the FHR tracing be evaluated at least every 30 minutes during the first stage of labor and every 15 minutes during the second stage of labor in low risk women. If risk factors are present, then the FHR tracing should be evaluated more frequently, every 15 minutes in the first stage of labor and every 5 minutes in the second stage of labor.
Based on assessment findings the nurse identifies relevant nursing diagnoses and expected outcomes of care, implements appropriate interventions, and evaluates the care provided (see Nursing Process box). Assessing FHR and UA patterns, implementing independent nursing interventions, documenting observations and actions according to the established standard of care, and reporting abnormal patterns to the primary care provider (e.g., physician, certified nurse-midwife) are the responsibilities of the nurse providing care to women in labor.
Electronic Fetal Monitoring Pattern Recognition
Nurses must evaluate many factors to determine whether an FHR pattern is normal or abnormal (see Nursing Process box). Nurses evaluate these factors based on the presence of other obstetric complications, progress in labor, and use of analgesia or anesthesia. They must also consider the estimated time interval until birth. Interventions are therefore based on clinical judgment of a complex, integrated process (Simpson & James, 2005).
Nursing Management of Abnormal Patterns
The five essential components of the fetal heart rate tracing that must be evaluated regularly are baseline rate, baseline variability, accelerations, decelerations, and changes or trends over time (Tucker et al., 2009). Whenever one of these five essential components is assessed as abnormal, corrective measures must immediately be taken (see Nursing Process box). The purpose of these actions is to improve fetal oxygenation (Tucker et al.). The term intrauterine resuscitation is sometimes used to refer to specific interventions initiated when an abnormal FHR pattern is noted. Basic corrective measures include providing supplemental oxygen, instituting maternal position changes, and increasing intravenous fluid administration. The purpose of these interventions is to improve uterine and intervillous space blood flow and increase maternal oxygenation and cardiac output (Simpson & James, 2005). Box 11-7 lists basic interventions to improve maternal and fetal oxygenation status.
Depending on the underlying cause of the abnormal FHR pattern, other interventions, such as correcting maternal hypotension, reducing uterine activity, and altering second stage pushing techniques, may also be instituted (Tucker et al., 2009). Box 11-7 also lists interventions for these specific problems. Realize that some of the items listed are not independent nursing interventions. Any medications administered, for example, must be authorized either through inclusion in a specific unit protocol or by a specific order. Some interventions are specific to the FHR pattern. See Table 11-3 and Boxes 11-4 and 11-5 for nursing interventions for tachycardia, late decelerations, and variable decelerations. Based on the FHR response to these interventions the primary health care provider decides whether additional interventions should be instituted or whether immediate vaginal or cesarean birth should be performed.
Other Methods of Assessment and Intervention
A major shortcoming of EFM is its high rate of false-positive results. Even the most abnormal patterns are poorly predictive of neonatal morbidity. Therefore other methods of assessment have been developed to evaluate fetal status. Fetal scalp stimulation and vibroacoustic stimulation and umbilical cord acid-base determination are frequently performed assessments. On the other hand, fetal scalp blood sampling and fetal pulse oximetry are assessments that are rarely performed in the United States. Amnioinfusion and tocolytic therapy are two interventions often used in an attempt to improve abnormal FHR patterns.
Assessment techniques
Fetal scalp stimulation and vibroacoustic stimulation.
Several research studies undertaken in the 1980s found that a FHR acceleration in response to digital or vibroacoustic stimulation was highly predictive of a normal scalp blood pH. The two methods of fetal stimulation currently used most often in clinical practice are scalp stimulation (using digital pressure during a vaginal examination) and vibroacoustic stimulation (using an artificial larynx or fetal acoustic stimulation device on the maternal abdomen over the fetal head for 1 to 5 seconds). The desired result of both methods of stimulation is an acceleration in the FHR of at least 15 beats/min for at least 15 seconds (Tucker et al., 2009). A FHR acceleration indicates the absence of metabolic acidemia. If the fetus does not respond to stimulation with an acceleration, fetal compromise is not necessarily indicated; however, further evaluation of fetal well-being is needed. Fetal stimulation should be performed at times when the FHR is at baseline. Neither fetal scalp stimulation nor vibroacoustic stimulation should be instituted if FHR decelerations or bradycardia is present (Tucker et al.).![]()
Umbilical cord acid-base determination.
In assessing the immediate condition of the newborn after birth, a sample of cord blood is a useful adjunct to the Apgar score. The procedure is generally performed by withdrawing blood from both the umbilical artery and the umbilical vein. Both samples are then tested for pH, carbon dioxide pressure (PCO2), oxygen pressure (PO2), and base deficit or base excess (Garite, 2007; Tucker et al., 2009). Umbilical arterial values reflect fetal condition, whereas umbilical vein values indicate placental function (Tucker et al.).
ACOG (2006a) suggests obtaining cord blood values in the following clinical situations: cesarean birth for fetal compromise, low 5-minute Apgar score, severe intrauterine growth restriction, abnormal FHR tracing, maternal thyroid disease, intrapartum fever, and multifetal gestation. Normal umbilical artery and vein cord blood values are listed in Table 11-4. Normal findings preclude the presence of acidemia at, or immediately before, birth (Tucker et al., 2009). If acidemia is present (e.g., pH <7.20), then the type of acidemia is determined (respiratory, metabolic, or mixed) by analyzing the blood gas values (Table 11-5) (Tucker et al.).
TABLE 11-4
Approximate Normal Values for Cord Blood
| CORD BLOOD | pH | CARBON DIOXIDE PRESSURE (PCO2) (mm Hg) | OXYGEN PRESSURE (PO2) (mm Hg) |
BASE DEFICIT (mmol/L) |
| Artery | 7.2-7.3 | 45-55 | 15-25 | <12 |
| Vein | 7.3-7.4 | 35-45 | 25-35 | <12 |

From Tucker, S. M., Miller, L. A, & Miller, D. A. (2009). Mosby's pocket guide to fetal monitoring: A multidisciplinary approach (6th ed.). St. Louis: Mosby.
TABLE 11-5
| BLOOD GASES | RESPIRATORY | METABOLIC | MIXED |
| pH | <7.20 | <7.20 | <7.20 |
| Carbon dioxide pressure (PCO2) | Elevated | Normal | Elevated |
| Base deficit | <12 mmol/L | ≥12 mmol/L | ≥12 mmol/L |

From Tucker, S. M., Miller, L. A, & Miller, D. A. (2009). Mosby's pocket guide to fetal monitoring: A multidisciplinary approach (6th ed.). St. Louis: Mosby.
Fetal scalp blood sampling.
Sampling of the fetal scalp blood for pH determination was first described in the 1960s and performed extensively in the 1970s. The procedure is performed by obtaining a sample of fetal scalp blood through the dilated cervix after the membranes have ruptured. Its use is limited by many factors, including the requirement for cervical dilation and membrane rupture, technical difficulty of the procedure, need for repetitive pH determinations, and uncertainty regarding interpretation and application of results. This procedure is now seldom used in the United States but remains a common practice in other countries (Tucker et al., 2009).
Fetal pulse oximetry.
Fetal pulse oximetry or continuous monitoring of fetal oxygen saturation levels is a method of fetal assessment that indirectly measures the oxygen saturation of hemoglobin in fetal blood. An intrauterine sensor placed in contact with the fetal cheek or temple area provides a continuous estimation of fetal oxygen saturation. Fetal pulse oximetry was approved for clinical use by the U.S. Food and Drug Administration in May 2000. The hope was that this technology would help to interpret nonreassuring FHR patterns more accurately and perhaps decrease the number of cesarean births performed for nonreassuring FHR tracings (Garite, 2007). Several studies, however, found that although fetal pulse oximetry did decrease the incidence of cesarean births for fetal indications, it had no consistent impact on overall cesarean birth rates or newborn outcomes. Therefore, fetal pulse oximetry has not been proven to be a clinically useful test for determining fetal status (ACOG, 2009). Because the manufacturer no longer distributes the sensors, the product has in effect been taken off the market (Tucker et al., 2009).![]()
Interventions
Amnioinfusion.
Amnioinfusion is infusion of room-temperature isotonic fluid (usually normal saline or lactated Ringer's solution) into the uterine cavity if the volume of amniotic fluid is low. Without the buffer of amniotic fluid the umbilical cord can easily become compressed during contractions or fetal movement, diminishing the flow of blood between the fetus and placenta. The purpose of amnioinfusion is to relieve intermittent umbilical cord compression that results in variable decelerations and transient fetal hypoxemia by restoring the amniotic fluid volume to a normal or near-normal level (Tucker et al., 2009). Women with an abnormally small amount of amniotic fluid (oligohydramnios) or no amniotic fluid (anhydramnios) are candidates for this procedure. Conditions that can result in oligohydramnios or anhydramnios are uteroplacental insufficiency and premature rupture of membranes.
In the past, amnioinfusion was also used to dilute moderate to thick meconium in an attempt to prevent meconium aspiration syndrome. However, a recent large research study found that amnioinfusion did not significantly reduce the incidence of meconium aspiration syndrome or perinatal death (Fraser et al., 2005). Therefore routine amnioinfusion for meconium-stained amniotic fluid without the presence of variable decelerations is not recommended by ACOG (2006b).![]()
Risks of amnioinfusion are overdistention of the uterine cavity and increased uterine tone. Fluid will be administered through an IUPC either by gravity flow or by use of an infusion pump. Usually a bolus of fluid will be administered over 20 to 30 minutes, and then the infusion will be slowed to a maintenance rate. Likely no more than 1000 ml of fluid will need to be administered. The fluid may be warmed by infusing it through a blood warmer for the preterm fetus (Tucker et al., 2009).
Intensity and frequency of UCs should be continually assessed during the procedure. The recorded uterine resting tone during amnioinfusion will appear higher than normal because of resistance to outflow and turbulence at the end of the catheter. Uterine resting tone should not exceed 40 mm Hg during the procedure. The amount of fluid return must be estimated and documented during amnioinfusion to prevent overdistension of the uterus. The volume of fluid returned should be approximately the same as the amount infused (Tucker et al., 2009).
Tocolytic therapy.
Tocolysis (relaxation of the uterus) can be achieved through the administration of drugs that inhibit UCs. This therapy can be used as an adjunct to other interventions in the management of fetal stress when the fetus is exhibiting abnormal patterns associated with increased UA. Tocolysis improves blood flow through the placenta by inhibiting UCs. Tocolysis may be considered by the primary health care provider and implemented when other interventions to reduce UA, such as maternal position change and discontinuance of an oxytocin infusion, have no effect on diminishing the UCs. Tocolytics are often administered when women are having excessive UCs spontaneously. Tocolytics are also frequently administered after a decision for cesarean birth has been made while preparations for surgery are underway. The most commonly used tocolytic in these situations is probably terbutaline (Brethine), given subcutaneously. Terbutaline works quickly and has been demonstrated to improve Apgar scores and cord pH values without apparent complications (Garite, 2007). If the FHR and UC patterns improve, then the woman may be allowed to continue labor; if no improvement is seen, immediate cesarean birth may be needed.
Patient and family teaching
Although the use of EFM can be reassuring to many parents, it can be a source of anxiety to some. Therefore the nurse must be particularly sensitive and respond appropriately to the emotional, informational, and comfort needs of the woman in labor and those of her family (Fig. 11-14 and Box 11-8).
Part of the nurse's role includes acting as a partner with the woman to achieve a high-quality birthing experience. In addition to teaching and supporting the woman and her family with understanding of the laboring and birth process, breathing techniques, use of equipment, and pain-management techniques, the nurse can assist with two factors that have an effect on fetal status: postioning and pushing. The nurse should solicit the woman's cooperation in avoiding the supine position. Instead, the woman should be encouraged to maintain a side-lying position or semi-Fowler's position with a lateral tilt to the uterus. In addition, the nurse should instruct the woman to keep her mouth and glottis open and to let air escape from her lungs during the pushing process. Both of these interventions will help to improve fetal oxygenation. See Chapter 12 for further discussion of maternal positioning and pushing techniques.
Documentation
Clear and complete documentation in the woman's medical record is essential. Each FHR and UA assessment must be completely documented in the woman's medical record. Currently, more and more hospitals are moving to use of the electronic medical record and computerized charting. With computerized charting, each required component usually appears on the screen so that it will routinely be addressed. Computerized charting often includes forced choices that greatly increase the use of standardized FHR terminology by all members of the health care team. In the past, nurses were often encouraged to chart both on the monitor strip and in the medical record. However, charting directly on the monitor strip is unnecessary when an electronic medical record is used. Any information that is handwritten on the monitor strip will not be recorded in the computer record. Furthermore, given that the EFM tracing is stored on computer, the paper strips are destroyed after the woman is discharged. No permanent record of the handwritten charting exists.
In institutions that still use a paper chart, documentation on the woman's monitor strip is started before the initiation of monitoring and consists of identifying information plus other relevant data. This documentation is continued and updated according to institutional protocol as monitoring progresses. See Box 11-6 for a checklist that can be used for documentation in a paper medical record. In some institutions, observations noted and interventions implemented are recorded on the monitor strip to produce a comprehensive document that chronicles the course of labor and the care rendered. In other institutions, this documentation is confined to the labor flow record. Advocates of documenting on both the medical record and the EFM strip cite as advantages of this approach the ease of writing directly on the strip while at the bedside and the improved accuracy in documenting critical events and the interventions implemented. Others believe that charting on the EFM strip constitutes duplicate documentation of the same information noted in the medical record, and thus it is unnecessary additional paperwork for the nurse.
A disadvantage of documenting on both the EFM strip and the medical record is that the times noted for events and interventions on the EFM strip frequently do not correlate with what is later documented in the medical record. These inaccuracies can lead persons involved in the retrospective review process carried out during litigation to infer that documentation errors have occurred. Therefore, if institutional policy mandates documentation on both the monitor strip and the medical record, the nurse must make sure the times and notations of events and interventions recorded in each place agree. Many of the aspects of care and events that can be documented on the patient's medical record or the monitor strip are listed in Box 11-9.


