Otitis Media
Joseph E. Kerschner, Diego Preciado
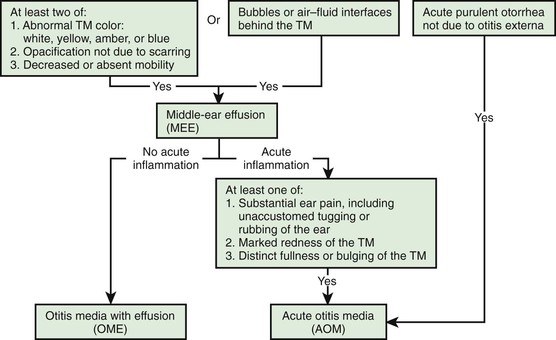
The term otitis media (OM) has 2 main categories: acute infection, which is termed suppurative or acute otitis media (AOM), and inflammation accompanied by middle-ear effusion (MEE), termed nonsuppurative or secretory OM, or otitis media with effusion (OME). These 2 main types of OM are interrelated: acute infection usually is succeeded by residual inflammation and effusion that, in turn, predispose children to recurrent infection. MEE is a feature of both AOM and of OME and is an expression of the underlying middle-ear mucosal inflammation. MEE results in the conductive hearing loss (CHL) associated with OM, ranging from none to as much as 50 dB of hearing loss.
The peak incidence and prevalence of OM is during the 1st 2 yr of life. More than 80% of children will have experienced at least 1 episode of OM by the age of 3 yr. OM is a leading reason for physician visits and for use of antibiotics and figures importantly in the differential diagnosis of fever. OM often serves as the sole or the main basis for undertaking the most frequently performed operations in infants and young children: myringotomy with insertion of tympanostomy tubes and adenoidectomy. OM is also the most common cause of hearing loss in children. OM has a propensity to become chronic and recur. The earlier in life a child experiences the first episode, the greater the degree of subsequent difficulty the child is likely to experience in terms of frequency of recurrence, severity, and persistence of middle-ear effusion.
Accurate diagnosis of AOM in infants and young children may be difficult (Table 640-1). Symptoms may not be apparent, especially in early infancy and in chronic stages of the disease. Accurate visualization of the tympanic membrane and middle-ear space may be difficult because of anatomy, patient cooperation, or blockage by cerumen, removal of which may be arduous and time consuming. Abnormalities of the eardrum may be subtle and difficult to appreciate. In the face of these difficulties, both underdiagnosis and overdiagnosis occur.
Table 640-1
Treatments for Otalgia in Acute Otitis Media
| TREATMENT MODALITY | COMMENTS |
| Acetaminophen, ibuprofen | Effective analgesia for mild to moderate pain. Readily available. Mainstay of pain management for AOM |
| Home remedies (no controlled studies that directly address effectiveness) Distraction External application of heat or cold Oil drops in external auditory canal | May have limited effectiveness |
| Benzocaine, procaine, lidocaine (topical) | Additional, but brief, benefit over acetaminophen in patients older than 5 yr |
| Naturopathic agents | Comparable to amethocaine/phenazone drops in patients older than 6 yr |
| Homeopathic agents | No controlled studies that directly address pain |
| Narcotic analgesia with codeine or analogs | Effective for moderate or severe pain. Requires prescription; risk of respiratory depression, altered mental status, gastrointestinal tract upset, and constipation |
| Tympanostomy/myringotomy | Requires skill and entails potential risk |
Epidemiology
Several factors have been demonstrated to affect the occurrence of OM, including age, gender, race, genetic background, socioeconomic status, breast milk feeding, degree of exposure to tobacco smoke, degree of exposure to other children, presence or absence of respiratory allergy, season of the yr, and vaccination status. Children with certain types of congenital craniofacial anomalies are particularly prone to OM.
Age
The age of onset of OM is an important predictor of the development of recurrent and chronic OM, with earlier age of onset having an increased risk for exhibiting these difficulties later in life. The development of at least 1 episode of OM has been reported as 63-85% by 12 mo and 66-99% by 24 mo of age. The percentage of days with MEE has been reported as 5-27% during the 1st yr of life and 6-18% during the 2nd yr of life. Across groups, rates were highest at 6-20 mo of age. After the age of 2 yr, the incidence and prevalence of OM decline progressively, although the disease remains relatively common into the early school-age years. The most likely reasons for the higher rates in infants and younger children include less-well–developed immunologic defenses and less-favorable eustachian tubal factors involving both the structure and function of the tube.
Gender
Epidemiologic data suggest an incidence of OM greater in boys than in girls, although some studies have found no gender-related differences in the occurrence of OM.
Race
OM is especially prevalent and severe among Native American, Inuit, and indigenous Australian children. Studies comparing the occurrence of OM in white children and black children have given conflicting results.
Genetic Background
That middle-ear disease tends to run in families is a commonplace observation, suggesting that OM has a heritable component. The degree of concordance for the occurrence of OM is much greater among monozygotic than among dizygotic twins.
Socioeconomic Status
Poverty has long been considered an important contributing factor to both the development and the severity of OM. Elements contributing to this relationship include crowding, limited hygienic facilities, suboptimal nutritional status, limited access to medical care, and limited resources for complying with prescribed medical regimens.
Breast Milk Compared to Formula Feeding
Most studies have found a protective effect of breast milk feeding against OM. This protective effect may be greater in socioeconomically disadvantaged than in more advantaged children. The protective effect is attributable to the milk itself rather than to the mechanics of breastfeeding.
Exposure to Tobacco Smoke
Exposure to tobacco smoke is thought to be an important preventable risk factor in the development of OM. Studies that have used objective measures to determine infant exposure to second-hand tobacco smoke, such as cotinine levels, have more consistently identified a significant linkage between tobacco smoke and OM.
Exposure to Other Children
Many studies have established that a strong, positive relationship exists between the occurrence of OM and the extent of repeated exposure to other children—measured mainly by the number of other children involved—whether at home or in out-of-home group daycare. Together, but independently, family socioeconomic status and the extent of exposure to other children appear to constitute 2 of the most important identifiable risk factors for developing OM.
Season
In keeping with the pattern of occurrence of upper respiratory tract infections in general, highest rates of occurrence of OM are observed during cold weather months and lowest rates during warm weather months. In OM, it is likely that these findings strongly depend on the significant association of OM with viral respiratory illnesses.
Congenital Anomalies
OM is universal among infants with unrepaired palatal clefts, and is also highly prevalent among children with submucous cleft palate, other craniofacial anomalies, and Down syndrome (see Chapter 81.2). The common feature in these congenital anomalies is a deficiency in the functioning of the eustachian tubes, which predisposes these children to middle-ear disease.
Vaccination Status
See “Immunoprophylaxis” below.
Other Factors
Pacifier use is linked with an increased incidence of OM and recurrence of OM, although the effect is small. Neither maternal age nor birthweight nor season of birth appears to influence the occurrence of OM once other demographic factors are taken into account. Very limited data are available regarding the association of OM with bottle feeding in the recumbent position.
Etiology
Acute Otitis Media
Pathogenic bacteria can be isolated by standard culture techniques from middle-ear fluid in a majority of well-documented AOM cases. Three pathogens predominate in AOM: Streptococcus pneumoniae (see Chapter 182), nontypeable Haemophilus influenzae (see Chapter 194), and Moraxella catarrhalis (see Chapter 196). The overall incidence of these organisms has changed with the use of the conjugate pneumococcal vaccine. In countries where this vaccine is employed, nontypeable H. influenzae initially overtook S. pneumoniae as the most common pathogen, being found in 40-50% of cases. However, over time, S. pneumoniae serotypes not covered in the conjugate vaccine have emerged, with S. pneumoniae again overtaking nontypeable H. influenzae as the most common pathogen in many studies. M. catarrhalis represents the majority of the remaining cases. Other pathogens include group A streptococcus (see Chapter 183), Staphylococcus aureus (see Chapter 181), and Gram-negative organisms. S. aureus and Gram-negative organisms are found most commonly in neonates and very young infants who are hospitalized; in outpatient settings, the distribution of pathogens in these young infants is similar to that in older infants. Molecular techniques to identify nonculturable bacterial pathogens have suggested the importance of other bacterial species such as Alloiococcus otitidis.
Evidence of respiratory viruses also may be found in middle-ear exudates of children with AOM, either alone or, more commonly, in association with pathogenic bacteria. Of these viruses, rhinovirus and respiratory syncytial virus are found most often. AOM is a known complication of bronchiolitis; middle-ear aspirates in children with bronchiolitis regularly contain bacterial pathogens, suggesting that respiratory syncytial virus is rarely, if ever, the sole cause of their AOM. Using more precise measures of viable bacteria than standard culture techniques, such as polymerase chain reaction assays, a much higher rate of bacterial pathogens can be demonstrated. It remains uncertain whether viruses alone can cause AOM, or whether their role is limited to setting the stage for bacterial invasion, and perhaps also to amplifying the inflammatory process and interfering with resolution of the bacterial infection. Viral pathogens have a negative impact on eustachian tube function, can impair local immune function, and increase bacterial adherence, and can change the pharmacokinetic dynamics, reducing the efficacy of antimicrobial medications.
Otitis Media with Effusion
Using standard culture techniques, the pathogens typically found in AOM are recoverable in only 30% of children with OME. However, in studies of children with OME using polymerase chain reaction assays, middle-ear effusions have been found to contain evidence of bacterial DNA and viral RNA in much larger proportions of these children. These studies suggest that these patients do not have sterile effusions as previously thought. Biofilms of pathogenic bacteria have been demonstrated to be present on the middle-ear mucosa and adenoid pad in a majority of children with chronic OM. Biofilms consist of aggregated and adherent bacteria, embedded in an extracellular matrix, allowing for protection against antimicrobials, and their presence may contribute to the persistence of pathogens and the recalcitrance of chronic OM to antibiotic treatment (see Chapter 171).
Pathogenesis
A multifactorial disease process, risk profile, and host–pathogen interactions have become recognized as playing important roles in the pathogenesis of OM. Such events as alterations in mucociliary clearance through repeated viral exposure experienced in daycare settings or through exposure to tobacco smoke may tip the balance of pathogenesis in less-virulent OM pathogens in their favor, especially in children with a unique host predisposition.
Anatomic Factors
Patients with significant craniofacial abnormalities affecting the eustachian tube function have an increased incidence of OM. During the pathogenesis of OM the eustachian tube demonstrates decreased effectiveness in ventilating the middle-ear space.
Under usual circumstances the eustachian tube is passively closed and is opened by contraction of the tensor veli palatini muscle. In relation to the middle ear, the tube has 3 main functions: ventilation, protection, and clearance. The middle-ear mucosa depends on a continuing supply of air from the nasopharynx delivered by way of the eustachian tube. Interruption of this ventilatory process by tubal obstruction initiates an inflammatory response that includes secretory metaplasia, compromise of the mucociliary transport system, and effusion of liquid into the tympanic cavity. Measurements of eustachian tube function have demonstrated that the tubal function is suboptimal during the events of OM with increased opening pressures.
Eustachian tube obstruction may result from extraluminal blockage via hypertrophied nasopharyngeal adenoid tissue or tumor, or may result from intraluminal obstruction via inflammatory edema of the tubal mucosa, most commonly as a consequence of a viral upper respiratory tract infection. Progressive reduction in tubal wall compliance with increasing age may explain the progressive decline in the occurrence of OM as children grow older. The protection and clearance functions of the eustachian tube may also be involved in the pathogenesis of OM. Thus, if the eustachian tube is patulous or excessively compliant, it may fail to protect the middle ear from reflux of infective nasopharyngeal secretions, whereas impairment of the mucociliary clearance function of the tube might contribute to both the establishment and persistence of infection. The shorter and more horizontal orientation of the tube in infants and young children may increase the likelihood of reflux from the nasopharynx and impair passive gravitational drainage through the eustachian tube.
In special patient populations with craniofacial abnormalities there exists an increased incidence of OM that has been associated with the abnormal eustachian tube function. In children with cleft palate, where OM is a universal finding, a main factor underlying the chronic middle-ear inflammation appears to be impairment of the opening mechanism of the eustachian tube. Possible factors include muscular changes, tubal compliance factors, and defective velopharyngeal valving, which may result in disturbed aerodynamic and hydrodynamic relationships in the nasopharynx and proximal portions of the eustachian tubes. In children with other craniofacial anomalies and with Down syndrome, the high prevalence of OM has also been attributed to structural and/or functional eustachian tubal abnormalities.
Host Factors
The effectiveness of a child's immune system in response to the bacterial and viral insults of the upper airway and middle ear during early childhood probably is the most important factor in determining which children are otitis prone. The maturation of this immune system during early childhood is most likely the primary event leading to the decrease in incidence of OM as children move through childhood. Immunoglobulin (Ig) A deficiency is found in some children with recurrent AOM, but the significance is questionable, inasmuch as IgA deficiency is also found not infrequently in children without recurrent AOM. Selective IgG subclass deficiencies (despite normal total serum IgG) may be found in children with recurrent AOM in association with recurrent sinopulmonary infection, and these deficiencies probably underlie the susceptibility to infection. Children with HIV infection have recurrent and difficult to treat episodes of AOM in the 1st and 2nd yr of life. Children with recurrent OM that is not associated with recurrent infection at other sites rarely have a readily identifiable immunologic deficiency. Evidence that subtle immune deficits play a role in the pathogenesis of recurrent AOM is provided by studies involving antibody responses to various types of infection and immunization; by the observation that breast milk feeding, as opposed to formula feeding, confers some protection against the occurrence of OM in infants with cleft palate; and by studies in which young children with recurrent AOM achieved a measure of protection from intramuscularly administered bacterial polysaccharide immune globulin or intravenously administered polyclonal immunoglobulin. This evidence, along with the documented decrease in incidence of upper respiratory tract infections and OM as children's immune systems develop and mature, is indicative of the importance of a child's innate immune system in the pathogenesis of OM (see Chapter 124).
Viral Pathogens
Although OM may develop and certainly may persist in the absence of apparent respiratory tract infection, many, if not most, episodes are initiated by viral or bacterial upper respiratory tract infection. In children in group daycare, AOM was observed in approximately 30-40% of children with respiratory illness caused by respiratory syncytial virus (see Chapter 260), influenzaviruses (see Chapter 258), or adenoviruses (see Chapter 262), and in approximately 10-15% of children with respiratory illness caused by parainfluenza viruses (see Chapter 259), rhinoviruses (see Chapter 263), or enteroviruses (see Chapter 250). Viral infection of the upper respiratory tract results in release of cytokines and inflammatory mediators, some of which may cause eustachian tube dysfunction.
Respiratory viruses also may enhance nasopharyngeal bacterial colonization and adherence and impair host immune defenses against bacterial infection.
Allergy
Evidence that respiratory allergy is a primary etiologic agent in OM is not convincing; however, in children with both conditions it is possible that the otitis is aggravated by the allergy.
Clinical Manifestations
Symptoms of AOM are variable, especially in infants and young children. In young children, evidence of ear pain may be manifested by irritability or a change in sleeping or eating habits and occasionally, holding or tugging at the ear. Pulling at the ear alone has a low sensitivity and specificity. Fever may also be present and may occasionally be the only sign. Rupture of the tympanic membrane with purulent otorrhea is uncommon. Systemic symptoms and symptoms associated with upper respiratory tract infections also occur; occasionally there may be no symptoms, the disease having been discovered at a routine health examination. OME often is not accompanied by overt complaints of the child but can be accompanied by hearing loss. This hearing loss may manifest as changes in speech patterns but often goes undetected if unilateral or mild in nature, especially in younger children. Balance difficulties or disequilibrium can also be associated with OME and older children may complain of mild discomfort or a sense of fullness in the ear (see Chapter 636).
Examination of the Tympanic Membrane
Otoscopy
Two types of otoscope heads are available: surgical or operating, and diagnostic or pneumatic. The surgical head embodies a lens that can swivel over a wide arc and an unenclosed light source, thus providing ready access of the examiner's instruments to the external auditory canal and tympanic membrane. Use of the surgical head is optimal for removing cerumen or debris from the canal under direct observation, and is necessary for satisfactorily performing tympanocentesis or myringotomy. The diagnostic head incorporates a larger lens, an enclosed light source, and a nipple for the attachment of a rubber bulb and tubing. When an attached speculum is fitted snugly into the external auditory canal, an airtight chamber is created comprising the vault of the otoscope head, the bulb and tubing, the speculum, and the proximal portion of the external canal. Although examination of the ear in young children is a relatively invasive procedure that is often met with lack of cooperation by the patient, this task can be enhanced if done with as little pain as possible. The outer portion of the ear canal contains hair-bearing skin and subcutaneous fat and cartilage that allow a speculum to be placed with relatively little discomfort. Closer to the tympanic membrane the ear canal is made of bone and is lined only with skin and no adnexal structures or subcutaneous fat; a speculum pushed too far forward and placed in this area often causes skin abrasion and pain. Using a rubber-tipped speculum or adding a small sleeve of rubber tubing to the tip of the plastic speculum may serve to minimize patient discomfort and enhance the ability to achieve a proper fit and an airtight seal, facilitating pneumatic otoscopy.
Learning to perform pneumatic otoscopy is a critical skill in being able to assess a child's ear and in making an accurate diagnosis of AOM. By observing as the bulb is alternately squeezed gently and released, the degree of tympanic membrane mobility in response to both positive and negative pressure can be estimated, providing a critical assessment of middle-ear fluid, which is a hallmark sign of both AOM and OME (Fig. 640-1). With both types of otoscope heads, bright illumination is also critical for adequate visualization of the tympanic membrane.
Clearing the External Auditory Canal
Many children's ears are “self-cleaning” because of squamous migration of ear canal skin. Parental cleaning of cerumen with cotton swabs often complicates cerumen impaction by pushing cerumen deeper into the canal compacting it. If the tympanic membrane is obscured by cerumen, the cerumen should be removed. This can be accomplished through direct visualization using a headlight or through the surgical head of the otoscope by using an ear curette or gentle suction with a No. 5 or 7 French ear suction tube. During this procedure it may be most advantageous to restrain the infant or young child in the prone position, turning the child's head to the left or right as each ear is cleared. In children old enough to cooperate, usually beginning at about 5 yr of age, clearing of the external canal may be achieved more easily and less traumatically by lavage than by mechanical removal, provided one can be certain that a tympanic membrane perforation is not present.
Tympanic Membrane Findings
Important characteristics of the tympanic membrane (TM) consist of contour, color, translucence, structural changes if any, and mobility. The TM is anatomically divided into the pars tensa and pars flaccida. The pars tensa comprises the lower two thirds of the drum inferior to the lateral process of the malleus. Its contour is normally slightly concave; abnormalities consist of fullness or bulging or, conversely, extreme retraction. The normal color of the pars tensa is pearly gray, with the pars flaccida being slightly more vascular in nature. Erythema may be a sign of inflammation or infection, but unless intense, erythema alone may result from crying or vascular flushing. Abnormal whiteness of the membrane may result from either scarring or the presence of effusion in the middle-ear cavity; this effusion also may impart an amber, pale yellow, or, rarely, bluish color. Rarely a persistent focal white area may be indicative of a congenital cholesteatoma in the middle-ear space. Normally, the membrane is translucent, although some degree of opacity may be normal in the 1st few mo of life; later, opacification denotes either scarring or, more commonly, underlying effusion. Structural changes include scars, perforations, and retraction pockets. Retractions or perforations, especially in the posterior-superior quadrant, or pars flaccida, of the TM may be a sign of cholesteatoma formation. Of all the visible characteristics of the TM, mobility is the most sensitive and specific in determining the presence or absence of MEE. Mobility is generally not an all-or-none phenomenon. A total absence of mobility does exist with a TM perforation that can develop following a substantial increase in middle-ear pressure associated with effusion. When a perforation is not present, substantial impairment of mobility is the more common finding with MEE. Bulging of the TM is the most specific finding of AOM (97%) but has lower sensitivity (51%) (Fig. 640-2).
Diagnosis
The 2013 guidelines from the American Academy of Pediatrics for diagnosis of AOM are more restrictive than were the earlier (2004) guidelines. The 2004 guidelines employed a 3-part definition: (1) acute onset of symptoms; (2) presence of an MEE; and (3) signs of acute middle-ear inflammation. This definition was thought by the 2013 American Academy of Pediatrics to lack sufficient precision and thereby liable to include cases of OME and/or enable the diagnosis of AOM to be made without visualizing the TM.
A diagnosis of AOM according to the 2013 guideline should be made in children who present with:
♦ moderate to severe bulging of the TM or new-onset otorrhea not caused by otitis externa
♦ mild bulging of the TM and recent (<48 hr) onset of ear pain or intense TM erythema
A diagnosis of AOM should not be made in children without MEE.
AOM and OME may evolve into the other without any clearly differentiating physical findings; any schema for distinguishing between them is to some extent arbitrary. In an era of increasing bacterial resistance, distinguishing between AOM and OME is important in determining treatment, because OME in the absence of acute infection does not require antimicrobial therapy. Purulent otorrhea of recent onset is indicative of AOM; thus, difficulty in distinguishing clinically between AOM and OME is limited to circumstances in which purulent otorrhea is not present. Both AOM without otorrhea and OME are accompanied by physical signs of MEE, namely, the presence of at least 2 of 3 TM abnormalities: white, yellow, amber, or (rarely) blue discoloration; opacification other than that caused by scarring; and decreased or absent mobility. Alternatively in OME, either air–fluid levels or air bubbles outlined by small amounts of fluid may be visible behind the TM, a condition often indicative of impending resolution (Fig. 640-3).
To support a diagnosis of AOM instead of OME in a child with MEE, distinct fullness or bulging of the TM may be present, with or without accompanying erythema, or, at a minimum, MEE should be accompanied by ear pain that appears clinically important. Unless intense, erythema alone is insufficient because erythema, without other abnormalities, may result from crying or vascular flushing. In AOM, the malleus may be obscured and the TM may resemble a bagel without a hole but with a central depression (see Fig. 640-3). Rarely, the TM may be obscured by surface bullae or may have a cobblestone appearance. Bullous myringitis is a physical manifestation of AOM and not an etiologically discrete entity. Within days after onset, fullness of the membrane may diminish, even though infection may still be present.
In OME, bulging of the TM is absent or slight or the membrane may be retracted (Fig. 640-4); erythema also is absent or slight, but may increase with crying or with superficial trauma to the external auditory canal incurred in clearing the canal of cerumen.
Both before and after episodes of OM and also in the absence of OM, the TM may be retracted as a consequence of negative middle-ear air pressure. The presumed cause is diffusion of air from the middle-ear cavity more rapidly than it is replaced via the eustachian tube. Mild retraction is generally self-limited, although in some children it is accompanied by mild conductive hearing loss. More extreme retraction is of concern, as discussed later in the section on sequelae of OM.
Conjunctivitis-Associated Otitis Media
Simultaneous appearance of purulent and erythematous conjunctivitis with an ipsilateral OM is a well-recognized presentation, caused by nontypeable H. influenzae in most children.
The disease often is present in multiple family members and affects young children and infants. Topical ocular antibiotics are ineffective. In an era of resistant organisms, this clinical association can be important in antibiotic selection, with oral antibiotics (see later) effective against resistant forms of nontypeable H. influenzae.
Asymptomatic Purulent Otitis Media
Rarely, a child will present during a routine exam without fever, irritability, or other overt signs of infection, but on exam, the patient will demonstrate an obvious purulent MEE and bulging TM. Although an uncommon presentation of “acute” OM, the bulging nature of the TM and the obvious purulence of the effusion do warrant antimicrobial therapy.
Tympanometry
Tympanometry, or acoustic immittance testing, is a simple, rapid, atraumatic test that, when performed correctly, offers objective evidence of the presence or absence of MEE. The tympanogram provides information about TM compliance in electroacoustic terms that can be thought of as roughly equivalent to TM mobility as perceived visually during pneumatic otoscopy. The absorption of sound by the TM varies inversely with its stiffness. The stiffness of the membrane is least, and accordingly its compliance is greatest, when the air pressures impinging on each of its surfaces—middle-ear air pressure and external canal air pressure—are equal. In simple terms, anything tending to stiffen the TM, such as TM scarring or middle-ear fluid, reduces the TM compliance, which is recorded as a flattening of the curve of the tympanogram. An ear filled with middle-ear fluid generally has a very noncompliant TM and, therefore, a flattened tympanogram tracing.
Tympanograms may be grouped into 1 of 3 categories (Fig. 640-5). Tracings characterized by a relatively steep gradient, sharp-angled peak, and middle-ear air pressure (location of the peak in terms of air pressure) that approximates atmospheric pressure (Fig. 640-5A) (type A curve) are assumed to indicate normal middle-ear status. Tracings characterized by a shallow peak or no peak are often termed “flat” or type B (Fig. 640-5B), and usually are assumed to indicate the presence of a middle-ear abnormality that is causing decreased TM compliance. The most common such abnormality in infants and children is MEE. Tracings characterized by intermediate findings—somewhat shallow peak, often in association with a gradual gradient (obtuse-angled peak) or negative middle-ear air pressure peak (often termed type “C”), or combinations of these features (Fig. 640-5C)—may or may not be associated with MEE, and must be considered nondiagnostic or equivocal with respect to OM. However, type C tympanograms do suggest eustachian tube dysfunction and some ongoing pathology in the middle ear and warrant follow-up.
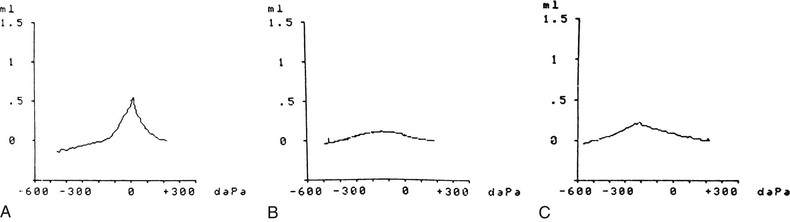
When reading a tympanogram it is important to look at the volume measurement. The type B tympanometric response has to be analyzed within the context of the recorded volume. A flat, “low”-volume (≤1 mL) tracing typically reflects the volume of the ear canal only, representing MEE, which impedes the movement of an intact ear drum. A flat, high-volume (>1 mL) tracing typically reflects the volume of the ear canal and middle-ear space, representing a perforation (or patent tympanostomy tube) in the TM. In a child with a tympanostomy tube present, a flat tympanogram with a volume <1 mL would suggest a plugged or nonfunctioning tube and middle-ear fluid, whereas a flat tympanogram with a volume >1 mL would suggest a patent tympanostomy tube.
Although tympanometry is quite sensitive in detecting MEE, it can be limited by patient cooperation, the skill of the individual administering the test, and the age of the child, with less-reliable results in very young children. Use of tympanometry may be helpful in office screening, may supplement the examination of difficult to examine patients, and may help identify patients who require further attention because their tympanograms are abnormal. Tympanometry also may be used to help confirm, refine, or clarify questionable otoscopic findings; to objectify the follow-up evaluation of patients with known middle-ear disease; and to validate otoscopic diagnoses of MEE. Even though tympanometry can predict the probability of MEE, it cannot distinguish the effusion of OME from that of AOM.
Prevention
General measures to prevent OM that have been supported by a number of investigations include avoiding exposure to individuals with respiratory infection; appropriate vaccination strategies against pneumococci and influenzae; avoiding environmental tobacco smoke; and breast milk feeding.
Immunoprophylaxis
Heptavalent pneumococcal conjugate vaccine (PCV7) reduced the overall number of episodes of AOM by only 6-8% but with a 57% reduction in serotype-specific episodes. Reductions of 9-23% are seen in children with histories of frequent episodes, and a 20% reduction is seen in the number of children undergoing tympanostomy tube insertion. A 13-valent pneumococcal polysaccharide-protein conjugate vaccine (PCV13) was licensed by the FDA in 2010. PCV13 contains the 7 serotypes included in PCV7 (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) and 6 additional serotypes (serotypes 1, 3, 5, 6A, 7F, and 19A). The effects of PCV13 on AOM incidence reduce pneumococcal nasopharyngeal carriage, including serotypes 19A, 7F, and 6C, in young children (younger than age 2 yr) with AOM. Given that 19A is a particularly invasive pneumococcal serotype, the effect of PCV13 on reducing complicated AOM will hopefully be of significance. Early data indicate a significant reduction in the number of invasive pneumococcal mastoiditis cases since the introduction of PCV13. With the widespread use of PCV13, continued surveillance will be necessary to detect other emerging serotypes, which are also demonstrating increasing resistance. Although the influenza vaccine also provides a measure of protection against OM, the relatively limited time during which individuals and even communities are exposed to influenzaviruses limits the vaccine's effectiveness in broadly reducing the incidence of OM. Limitation of OM disease is only a portion of the benefit realized from the vaccinations for pneumococci and influenza viruses. Support for these vaccination programs requires an understanding of the preventive benefit for OM in concert with the other benefits.
Treatment
Management of Acute Otitis Media
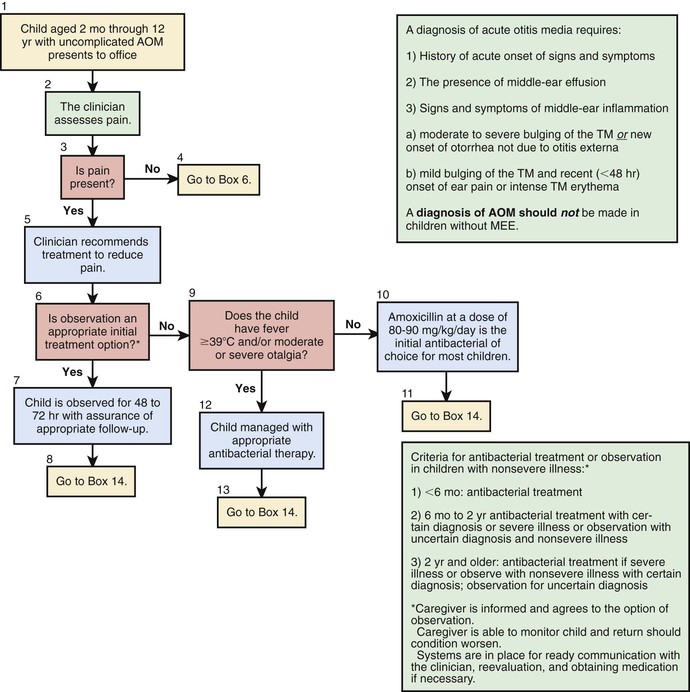
AOM can be very painful. Whether or not antibiotics are employed for treatment, pain should be assessed and if present, treated (see Table 640-1).
Individual episodes of AOM have traditionally been treated with antimicrobial drugs. Concern about increasing bacterial resistance has prompted some clinicians to recommend withholding antimicrobial treatment in some or most cases unless symptoms persist for 2 or 3 days, or worsen (Table 640-2). Three factors argue in favor of routinely prescribing antimicrobial therapy for children who have documented AOM using the diagnostic criteria outlined previously (see “Diagnosis” above). First, pathogenic bacteria cause a large majority of cases. Second, symptomatic improvement and resolution of infection occur more promptly and more consistently with antimicrobial treatment than without, even though most untreated cases eventually resolve. Third, prompt and adequate antimicrobial treatment may prevent the development of suppurative complications. The sharp decline in such complications during the last half-century seems likely attributable, at least in part, to the widespread routine use of antimicrobials for AOM. In the Netherlands, where initial antibiotic treatment is routinely withheld from most children older than 6 mo of age, and where only approximately 30% of children with AOM receive antibiotics at all, the incidence of acute mastoiditis, although low (in children younger than age 14 yr, 3.8 per 100,000 person-years), appears slightly higher than rates in other countries with higher antibiotic prescription rates by about 1-2 episodes per 100,000 person-years. Groups in other countries where initial conservative management of AOM is the standard in children older than 6 mo, such as Denmark, report acute mastoiditis rates similar to those of the Netherlands (4.8 per 100,000 person-years).
Table 640-2
Recommendations for Initial Management for Uncomplicated Acute Otitis Media*
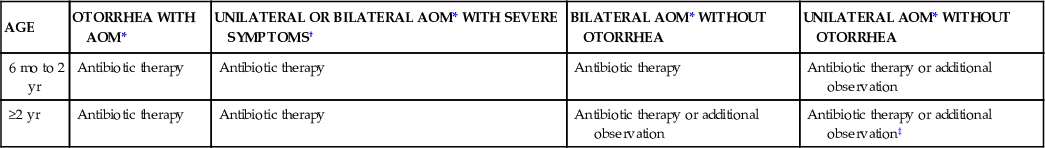
| AGE | OTORRHEA WITH AOM* | UNILATERAL OR BILATERAL AOM* WITH SEVERE SYMPTOMS† | BILATERAL AOM* WITHOUT OTORRHEA | UNILATERAL AOM* WITHOUT OTORRHEA |
| 6 mo to 2 yr | Antibiotic therapy | Antibiotic therapy | Antibiotic therapy | Antibiotic therapy or additional observation |
| ≥2 yr | Antibiotic therapy | Antibiotic therapy | Antibiotic therapy or additional observation | Antibiotic therapy or additional observation‡ |
Given that most episodes of OM will spontaneously resolve, consensus guidelines have been published by the American Academy of Pediatrics to assist clinicians who wish to consider a period of “watchful waiting” or observation prior to treating AOM with antibiotics (see Tables 640-2 and 640-3; Fig. 640-6). The most important aspect of these guidelines is that close follow-up of the patient must be ensured to assess for lack of spontaneous resolution or worsening of symptoms and that patients should be provided with adequate analgesic medications (acetaminophen, ibuprofen) during the period of observation. When pursuing the practice of watchful waiting in patients with AOM, the certainty of the diagnosis, the patient's age, and the severity of the disease should be considered. For younger patients, <2 yr of age, it is recommended to treat all confirmed diagnoses of AOM. In very young patients, <6 mo of age, even presumed episodes of AOM should be treated because of the increased potential of significant morbidity from infectious complications. In children between 6 and 24 mo of age who have a questionable diagnosis of OM but severe disease, defined as temperature of >39°C (102°F), significant otalgia, or toxic appearance, antibiotic therapy is also recommended. Children in this age group with a questionable diagnosis and nonsevere disease can be observed for a period of 2-3 days with close follow-up. In children older than 2 yr of age, observation might be considered in all episodes of nonsevere OM or episodes of questionable diagnosis, while antibiotic therapy is reserved for confirmed, severe episodes of AOM. Information from Finland suggests that the “watchful waiting” or delayed treatment approach does not worsen the recovery from AOM, or increase the complication rates. However, watchful waiting may be associated with transient worsening of the child's condition and longer overall duration of symptoms.
Table 640-3
Recommended Antibiotics for (Initial or Delayed) Treatment and for Patients Who Have Failed Initial Antibiotic Treatment
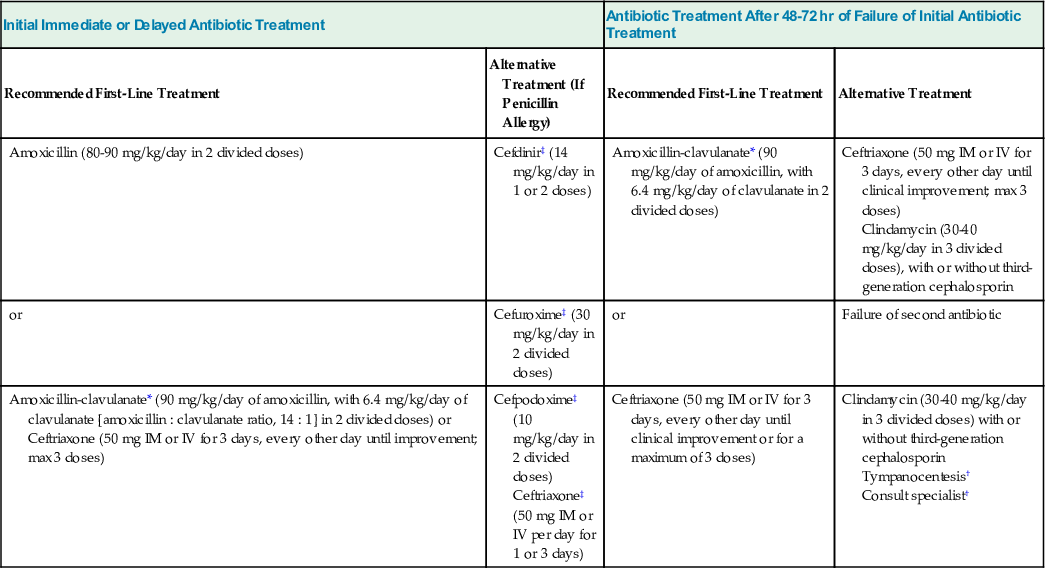
| Initial Immediate or Delayed Antibiotic Treatment | Antibiotic Treatment After 48-72 hr of Failure of Initial Antibiotic Treatment | ||
| Recommended First-Line Treatment | Alternative Treatment (If Penicillin Allergy) | Recommended First-Line Treatment | Alternative Treatment |
| Amoxicillin (80-90 mg/kg/day in 2 divided doses) | Cefdinir‡ (14 mg/kg/day in 1 or 2 doses) | Amoxicillin-clavulanate* (90 mg/kg/day of amoxicillin, with 6.4 mg/kg/day of clavulanate in 2 divided doses) | Ceftriaxone (50 mg IM or IV for 3 days, every other day until clinical improvement; max 3 doses) Clindamycin (30-40 mg/kg/day in 3 divided doses), with or without third-generation cephalosporin |
| or | Cefuroxime‡ (30 mg/kg/day in 2 divided doses) | or | Failure of second antibiotic |
| Amoxicillin-clavulanate* (90 mg/kg/day of amoxicillin, with 6.4 mg/kg/day of clavulanate [amoxicillin : clavulanate ratio, 14 : 1] in 2 divided doses) or Ceftriaxone (50 mg IM or IV for 3 days, every other day until improvement; max 3 doses) | Cefpodoxime‡ (10 mg/kg/day in 2 divided doses) Ceftriaxone‡ (50 mg IM or IV per day for 1 or 3 days) | Ceftriaxone (50 mg IM or IV for 3 days, every other day until clinical improvement or for a maximum of 3 doses) | Clindamycin (30-40 mg/kg/day in 3 divided doses) with or without third-generation cephalosporin Tympanocentesis† Consult specialist† |
Accurate diagnosis is the most crucial aspect of the treatment of OM. In studies utilizing stringent criteria for diagnosis of AOM the benefit of antimicrobial treatment is enhanced. Additionally, subpopulations of patients clearly receive more benefit from oral antimicrobial therapy than others. Younger children, children with otorrhea, and children with bilateral AOM have a significantly enhanced benefit from antimicrobial therapy in comparison to older children, children without otorrhea, or children with unilateral AOM.
Bacterial Resistance
Persons at greatest risk of harboring resistant bacteria are those who are younger than 2 yr of age, who are in regular contact with large groups of other children, especially in daycare settings, or who recently have received antimicrobial treatment. Bacterial resistance is a particular problem in relation to OM. The development of resistant bacterial strains and their rapid spread have been fostered and facilitated by selective pressure resulting from extensive use of antimicrobial drugs, the most common target of which, in children, is OM. Many strains of each of the pathogenic bacteria that commonly cause AOM are resistant to commonly used antimicrobial drugs.
Although antimicrobial resistance rates vary between countries, in the United States approximately 40% of strains of nontypeable H. influenzae and almost all strains of M. catarrhalis are resistant to aminopenicillins (e.g., ampicillin and amoxicillin). In most cases, the resistance is attributable to production of β-lactamase and can be overcome by combining amoxicillin with a β-lactamase inhibitor (clavulanate) or by using a β-lactamase–stable antibiotic. Occasional strains of nontypeable H. influenzae that do not produce β-lactamase are resistant to aminopenicillins and other β-lactam antibiotics by virtue of alterations in their penicillin-binding proteins. It is worth noting that bacterial resistance rates in northern European countries where antibiotic usage is less are comparatively exceedingly lower (β-lactamase resistance in 6-10% of isolates) than in the United States.
In the United States, approximately 50% of strains of S. pneumoniae are penicillin-nonsusceptible, divided approximately equally between penicillin-intermediate and, even more difficult to treat, penicillin-resistant strains. A much higher incidence of resistance is seen in children attending daycare. Resistance by S. pneumoniae to the penicillins and other β-lactam antibiotics is mediated not by β-lactamase production but by alterations in penicillin-binding proteins. This mechanism of resistance can be overcome if higher concentrations of β-lactam antibiotics at the site of infection can be achieved for a sufficient time interval. Many penicillin-resistant strains of S. pneumoniae are also resistant to other antimicrobial drugs, including sulfonamides, macrolides, and cephalosporins. In general, as penicillin resistance increases, so also does resistance to other antimicrobial classes. Resistance to macrolides, including azithromycin and clarithromycin, by S. pneumoniae has increased rapidly, rendering these antimicrobials far less effective in treating AOM. One mechanism of resistance to macrolides also results in resistance to clindamycin, which otherwise is generally effective against resistant strains of S. pneumoniae. Unlike resistance to β-lactam antibiotics, macrolide resistance cannot be overcome by increasing the dose.
First-Line Antimicrobial Treatment
Amoxicillin remains the drug of first choice for uncomplicated AOM under many circumstances because of its excellent record of safety, relative efficacy, palatability, and low cost. In particular, amoxicillin is the most efficacious of available oral antimicrobial drugs against both penicillin-susceptible and penicillin-nonsusceptible strains of S. pneumoniae. Increasing the dose from the traditional 40-45 mg/kg/24 hr to 80-90 mg/kg/24 hr will generally provide efficacy against penicillin-intermediate and some penicillin-resistant strains. This higher dose should be used particularly in children younger than 2 yr of age, in children who have recently received treatment with β-lactam drugs, and in children who are exposed to large numbers of other children because of their increased likelihood of an infection with a nonsusceptible strain of S. pneumoniae. A limitation of amoxicillin is that it may be inactivated by the β-lactamases produced by many strains of nontypeable H. influenzae and most strains of M. catarrhalis. Episodes of AOM caused by these pathogens often resolve spontaneously. Allergies to penicillin antibiotics should be categorized into type I hypersensitivity, consisting of urticaria or anaphylaxis, and those that fall short of type I reactions, such as rash formation. For children with a non–type I reaction in which cross reactivity with cephalosporins is less of a concern, first-line therapy with cefdinir would be an appropriate choice. In children with a type I reaction or known sensitivity to cephalosporin antibiotics there are far fewer choices. Resistance to trimethoprim-sulfamethoxazole by many strains of both nontypeable H. influenzae and S. pneumoniae and a reported high clinical failure rate in children with AOM treated initially with this antimicrobial argue against its use. Similarly, increasing rates of macrolide resistance argue against the efficacy of azithromycin. Although not approved by the FDA for use in children, many clinicians have employed quinolones in this patient population. Early alternative management in these allergic patients with tympanostomy tubes can allow for lessening of the severity of their disease and the utilization of topical antimicrobials.
Duration of Treatment
The duration of treatment of AOM has historically been set at 10 days and most efficacy studies examining antimicrobial treatment in AOM have utilized this duration as a benchmark. Studies comparing shorter with longer durations of treatment suggest that short-course treatment will often prove inadequate in children younger than 6 yr of age and particularly in children younger than 2 yr of age. Thus, for most episodes in most children, treatment that provides tissue concentrations of an antimicrobial for at least 10 days is advisable. Treatment for longer than 10 days may be required for children who are very young or are having severe episodes or whose previous experience with OM has been problematic.
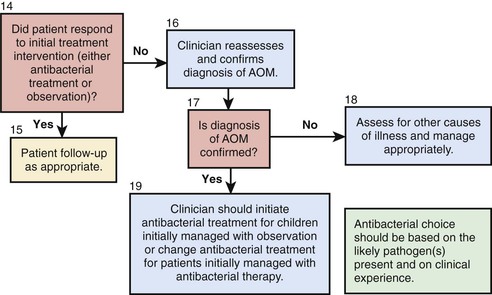
Follow-Up
The principal goals of follow-up are to assess the outcome of treatment and to differentiate between inadequate response to treatment and early recurrence. The appropriate interval for follow-up should be individualized. Follow-up within days is advisable in the young infant with a severe episode or in a child of any age with continuing pain. Follow-up within 2 wk is appropriate for the infant or young child who has been having frequent recurrences. At that point, the TM is not likely to have returned to normal, but substantial improvement in its appearance should be evident. In the child with only a sporadic episode of AOM and prompt symptomatic improvement, follow-up 1 mo after initial examination is early enough, or in older children, no follow-up may be necessary. The continuing presence of MEE alone following an episode of AOM is not an indication for additional or second-line antimicrobial treatment. However, persisting MEE does warrant additional follow-up to ensure that this resolves and does not lead to persisting hearing loss or other complications.
Unsatisfactory Response to First-Line Treatment
AOM is essentially a closed-space infection and its resolution depends both on eradication of the offending organism and restoration of middle-ear ventilation. Factors contributing to unsatisfactory response to first-line treatment, in addition to inadequate antimicrobial efficacy, include poor compliance with treatment regimens; concurrent or intercurrent viral infection; persistent eustachian tube dysfunction and middle-ear underaeration; reinfection from other sites or from incompletely eradicated middle-ear pathogens; and immature or impaired host defenses. The identification of biofilm formation in the middle ear of children with chronic OM also indicates that, in some children, eradication with standard antimicrobial therapy is likely to be unsuccessful. Despite these many potential factors, switching to an alternative or second-line drug is reasonable when there has been inadequate improvement in symptoms or in middle-ear status as reflected in the appearance of the TM, or when the persistence of purulent nasal discharge suggests that the antimicrobial drug being used has less-than-optimal efficacy. Second-line drugs may also appropriately be used when AOM develops in a child already receiving antimicrobial therapy, or in an immunocompromised child, or in a child with severe symptoms whose previous experience with OM has been problematic.
Second-Line Treatment
When treatment of AOM with a first-line antimicrobial drug has proven inadequate, a number of second-line alternatives are available (see Table 640-3). Drugs chosen for second-line treatment should be effective against β-lactamase–producing strains of nontypeable H. influenzae and M. catarrhalis and against susceptible and most nonsusceptible strains of S. pneumoniae. Only 4 antimicrobial agents meet these requirements: amoxicillin-clavulanate, cefdinir, cefuroxime axetil, and intramuscular ceftriaxone. Because high-dose amoxicillin (80-90 mg/kg/24 hr) is effective against most strains of S. pneumoniae and because the addition of clavulanate extends the effective antibacterial spectrum of amoxicillin to include β-lactamase–producing bacteria, high-dose amoxicillin-clavulanate is particularly well-suited as a second-line drug for treating AOM. The 14 : 1 amoxicillin-clavulanate formulation contains twice as much amoxicillin as the previously available 7 : 1 formulation. Diarrhea, especially in infants and young children, is a common adverse effect, but may be ameliorated in some cases by feeding active culture yogurt, and usually is not severe enough to require cessation of treatment. Cefdinir has demonstrated broad efficacy in treatment, is generally well tolerated with respect to taste, and can be given as a once-daily regimen. The ability to also utilize cefdinir in most children with mild type 1 hypersensitivity reactions has further added to its favorable selection as a second-line agent. Both cefuroxime axetil and intramuscular ceftriaxone have important limitations for use in young children. The currently available suspension of cefuroxime axetil is not palatable and its acceptance is low. Ceftriaxone treatment entails both the pain of intramuscular injection and substantial cost, and the injection may need to be repeated once or twice at 2 day intervals to achieve the desired degree of effectiveness. Nonetheless, use of ceftriaxone is appropriate in severe cases of AOM when oral treatment is not feasible, or in highly selected cases after treatment failure using orally administered second-line antimicrobials (i.e., amoxicillin-clavulanate or cefuroxime axetil), or when highly resistant S. pneumoniae is found in aspirates obtained from diagnostic tympanocentesis.
Clarithromycin and azithromycin have only limited activity against nonsusceptible strains of S. pneumoniae and against β-lactamase–producing strains of nontypeable H. influenzae. Macrolide use also appears to be a major factor in causing increases in rates of resistance to macrolides by group A streptococcus and S. pneumoniae. Clindamycin is active against most strains of S. pneumoniae, including resistant strains, but is not active against nontypeable H. influenzae or M. catarrhalis.
Other antimicrobial agents that have been traditionally utilized in the management of AOM have such significant lack of effectiveness against resistant organisms that employment seldom outweighs the potential side effects or complications possible from the medications. This includes cefprozil, cefaclor, loracarbef, cefixime, trimethoprim-sulfamethoxazole, and erythromycin-sulfisoxazole. Cefpodoxime has demonstrated reasonable effectiveness in some investigations but is generally poorly tolerated because of its taste.
Antimicrobial Prophylaxis
In children who have developed frequent episodes of AOM, antimicrobial prophylaxis with subtherapeutic doses of an aminopenicillin or a sulfonamide has been utilized in the past to provide protection against recurrences of AOM (although not of OME). However, because of the increased incidence of resistant organisms and the contribution of antimicrobial usage to bacterial resistance, the risks of sustained antimicrobial prophylaxis clearly outweigh potential benefits.
Myringotomy and Tympanocentesis
Myringotomy is a long-standing treatment for AOM but is not commonly needed in children receiving antimicrobials. Indications for myringotomy in children with AOM include severe, refractory pain; hyperpyrexia; complications of AOM such as facial paralysis, mastoiditis, labyrinthitis, or central nervous system infection; and immunologic compromise from any source. Myringotomy should be considered as third-line therapy in patients that have failed 2 courses of antibiotics for an episode of AOM. In children with AOM in whom clinical response to vigorous, second-line treatment has been unsatisfactory, either diagnostic tympanocentesis or myringotomy is indicated to enable identification of the offending organism and its sensitivity profile. Either procedure may be helpful in effecting relief of pain. Tympanocentesis with culture of the middle-ear aspirate may also be indicated as part of the sepsis work-up in very young infants with AOM who show systemic signs of illness such as fever, vomiting, or lethargy, and whose illness accordingly cannot be presumed to be limited to infection of the middle ear. Performing tympanocentesis can be facilitated by use of a specially designed tympanocentesis aspirator. Studies reporting the usage of strict, individualized criteria for the diagnosis of AOM that include office tympanocentesis with bacterial culture followed by culture-guided antimicrobial therapy demonstrate significant reduction in the frequency of recurrent AOM episodes and tympanostomy tube surgery. However, many primary care physicians do not feel comfortable performing this procedure, there is the potential for complications, and parents may view this procedure as traumatic. Often children requiring this intervention have a strong enough history of recurrent OM to warrant the consideration of tympanostomy tube placement, so that the procedure can be performed under general anesthesia.
Early Recurrence After Treatment
Recurrence of AOM after apparent resolution may be caused by either incomplete eradication of infection in the middle ear or upper respiratory tract reinfection by the same or a different bacteria or bacterial strain. Recent antibiotic therapy predisposes patients to an increased incidence of resistant organisms, which should also be considered in choosing therapy, and, generally, initiating therapy with a second-line agent is advisable (see Table 640-3).
Myringotomy and Insertion of Tympanostomy Tubes
When AOM is recurrent, despite appropriate medical therapy, consideration of surgical management of AOM with tympanostomy tube insertion is warranted. This procedure is effective in reducing the rate of AOM in patients with recurrent OM and in significantly improving the quality of life in patients with recurrent AOM. Individual patient factors, including the risk profile, severity of AOM episodes, child's development and age, presence of a history of adverse drug reactions, concurrent medical problems, and parental wishes, will affect the timing of a decision to consider referral for this procedure. When a patient experiences 3 episodes of AOM in a 6 mo period or 4 episodes in a 12 mo period with 1 episode in the preceding 6 mo, potential surgical management of the child's AOM should be discussed with the parents. Additionally, often patients with recurrent AOM may have persisting MEE between episodes with accompanying hearing loss, which may add to the indication for tympanostomy tube placement.
Tube Otorrhea
Although tympanostomy tubes often reduce the incidence of AOM in most children, patients with tympanostomy tubes may still develop AOM. One advantage of tympanostomy tubes in children with recurrent AOM is that if patients do develop an episode of AOM with a functioning tube in place, these patients will manifest purulent drainage from the tube. By definition, children with functioning tympanostomy tubes without otorrhea do not have bacterial AOM as a cause for a presentation of fever or behavioral changes and should not be treated with oral antibiotics. If tympanostomy tube otorrhea develops, ototopical treatment should be considered as first-line therapy. With a functioning tube in place, the infection is able to drain, there is usually negligible pain associated with the infection, and the possibility of developing a serious complication from an episode of AOM is extremely remote. The current quinolone otic drops approved by the U.S. Food and Drug Administration for use in the middle-ear space in children are formulated with ciprofloxacin/dexamethasone (Ciprodex) and ofloxacin (Floxin). The topical delivery of these otic drops allows them to utilize a higher antibiotic concentration than can be tolerated by administering oral antibiotics and they have excellent coverage of even the most resistant strains of common middle-ear pathogens as well as coverage of S. aureus and Pseudomonas aeruginosa. The high rate of success of these topical preparations, their broad coverage, the lower likelihood of their contributing to the development of resistant organisms, the relative ease of administration, the lack of significant side effects, and the lack of ototoxicity makes them the first choice for tube otorrhea. Oral antibiotic therapy should generally be reserved for cases of tube otorrhea that have other associated systemic symptoms, patients who have difficulty in tolerating the use of topical preparations, or, possibly, patients who have failed an attempt at topical otic drops. Despite these advantages of ototopical therapy, survey data have indicated that, compared to otolaryngologists, primary care practitioners are less likely to prescribe ototopicals as first-line therapy in tympanostomy tube otorrhea. As a result of the relative ease in obtaining fluid for culture and the possibility of the development of fungal otitis, which has shown an increase with the utilization of broad-spectrum quinolone ototopicals, patients that fail topical therapy should also have culture performed to rule out the development of fungal otitis. Other otic preparations are available; although these either have some risk of ototoxicity or have not received approval for use in the middle ear, many of these preparations were widely used prior to the development of the current quinolone drops and were generally considered reasonably safe and effective. In all cases of tube otorrhea, attention to aural toilet (e.g., cleansing the external auditory canal of secretions, and avoidance of external ear water contamination) is important. In some cases with very thick, tenacious discharge, topical therapy may be inhibited due to lack of delivery of the medication to the site of infection. Suctioning and removal of the secretions, often done through referral to an otolaryngologist, may be quite helpful. When children with tube otorrhea fail to improve satisfactorily with conventional outpatient management, they may require tube removal, or hospitalization to receive parenteral antibiotic treatment, or both.
Management of Otitis Media with Effusion
Management of OME depends on an understanding of its natural history and its possible complications and sequelae. Most cases of OME resolve without treatment within 3 mo. To distinguish between persistence and recurrence, examination should be conducted monthly until resolution; hearing should be assessed if effusion has been present for longer than 3 mo. When MEE persists for longer than 3 mo, consideration of referral to an otolaryngologist may be appropriate. For young children, this referral is warranted for the assessment of hearing levels. In older children (generally older than age 4 yr), and depending upon the expertise in the primary care physician's office, hearing screening may be achieved by the primary care physician. For any child who fails a hearing screening in the primary care physician's office, referral to an otolaryngologist is warranted. In considering the decision to refer the patient for consultation, the clinician should attempt to determine the impact of the OME on the child. Although hearing loss may be of primary concern, OME causes a number of other difficulties in children that should also be considered. These include predisposition to recurring AOM, pain, disturbance of balance, and tinnitus. In addition, long-term sequelae that have been demonstrated to be associated with OME include pathologic middle-ear changes; atelectasis of the TM and retraction pocket formation; adhesive OM; cholesteatoma formation and ossicular discontinuity; and conductive and sensorineural hearing loss. Long-term adverse effects on speech, language, cognitive, and psychosocial development have also been demonstrated. This impact is related to the duration of effusion present, whether the effusion is unilateral or bilateral, the degree of underlying hearing loss, and other developmental and social factors affecting the child. In considering the impact of OME on development, it is especially important to take into consideration the overall presentation of the child. Although it is unlikely that OME causing unilateral hearing loss in the mild range will have long-term negative effects on an otherwise healthy and developmentally normal child, even a mild hearing loss in a child with other developmental or speech delays certainly has the potential to compound this child's difficulties (Table 640-4). At a minimum, children with OME persisting longer than 3 mo deserve close monitoring of their hearing levels with skilled audiologic evaluation; frequent assessment of developmental milestones, including speech and language assessment; and attention paid to their rate of recurrent AOM.
Table 640-4
Sensory, Physical, Cognitive, or Behavioral Factors That Place Children Who Have Otitis Media with Effusion at an Increased Risk for Developmental Difficulties (Delay or Disorder)
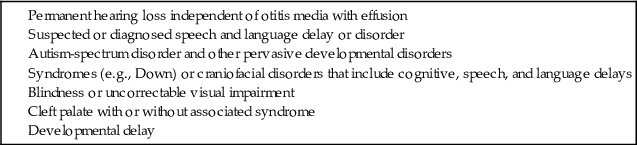
Variables Influencing Otitis Media with Effusion Management Decisions
Patient-related variables that affect decisions on how to manage OME include the child's age; the frequency and severity of previous episodes of AOM and the interval since the last episode; the child's current speech and language development; the presence of a history of adverse drug reactions, concurrent medical problems, or risk factors such as daycare attendance; and the parental wishes. In considering surgical management of OME with tympanostomy tubes, particular benefit is seen in patients with persisting OME punctuated by episodes of AOM, as the tubes generally provide resolution of both conditions. Disease-related variables that most otolaryngologists consider in the treatment of OME include whether the effusion is unilateral or bilateral; the apparent quantity of effusion; the duration, if known; the degree of hearing impairment; the presence or absence of other possibly related symptoms, such as tinnitus, vertigo, or disturbance of balance; and the presence or absence of mucopurulent or purulent rhinorrhea, which, if sustained for longer than 2 wk, would suggest that concurrent nasopharyngeal or paranasal sinus infection is contributing to continuing compromise of middle-ear ventilation.
Medical Treatment
In some studies, antimicrobials have demonstrated some efficacy in resolving OME, presumably because they help eradicate nasopharyngeal infection or unapparent middle-ear infection, or both. The most significant effects of antibiotics for OME have been shown with treatment durations of 4 wk and 3 mo. However, in the current era of bacterial antimicrobial resistance, the small potential benefit of antimicrobial therapy is outweighed by the negative potential of treatment and is not recommended. Instead, treatment should be limited to cases in which there is evidence of associated bacterial upper respiratory tract infection or untreated middle-ear infection. For this purpose, the most broadly effective drug available should be used as recommended for AOM.
The efficacy of corticosteroids in the treatment of OME is probably short term. The risk: benefit ratio for steroids would argue against their use. Antihistamine-decongestant combinations are not effective in treating children with OME. Antihistamines alone, decongestants alone, and mucolytic agents are unlikely to be effective. The risk profile for decongestants and antihistamines in children suggests that they are not indicated in the treatment of OME. Allergic management, including antihistamine therapy, might prove helpful in children with problematic OME who also have evidence of environmental allergies, although supporting data specifically analyzing this patient population are not conclusive. Recent randomized controlled trials do not support the usage of topical intranasal steroid sprays to treat the manifestations of eustachian tube dysfunction. Inflation of the eustachian tube by the Valsalva maneuver or other means has not demonstrated long-term efficacy but is unlikely to lead to significant harm. Other “alternative” therapies, including spinal manipulation, currently have no demonstrated efficacy or role in children with OME.
Myringotomy and Insertion of Tympanostomy Tubes
When OME persists despite an ample period of watchful waiting, generally 3-6 mo or perhaps longer in children with unilateral effusion, consideration of surgical intervention with tympanostomy tubes is appropriate. Myringotomy alone, without tympanostomy tube insertion, permits evacuation of middle-ear effusion and may sometimes be effective, but often the incision heals before the middle-ear mucosa returns to normal and the effusion soon reaccumulates. Inserting a tympanostomy tube offers the likelihood that middle-ear ventilation will be sustained for at least as long as the tube remains in place and functional. Tympanostomy tubes have a variable duration of efficacy based on design. Tubes that are designed for a shorter duration, 6-12 mo, have a lesser impact on disease-free middle-ear spaces in children. Some studies comparing the efficacy of tympanostomy tube types, including shorter-acting tubes, with watchful waiting provide a less helpful assessment of the differences between these approaches. Tubes that are somewhat longer acting, effective for 12-18 mo, are generally more appropriate for most children undergoing tube placement. Regardless of type, tympanostomy tube placement nearly uniformly reverses the conductive hearing loss associated with OME. Occasional episodes of obstruction of the tube lumen and premature tube extrusion may limit the effectiveness of tympanostomy tubes, and tubes can also be associated with otorrhea. However, placement of tympanostomy tubes is generally quite effective in providing resolution of OME in children. Tympanostomy tubes generally extrude on their own but rarely require surgical removal after several years in place. Sequelae following tube extrusion include residual perforation of the eardrum, tympanosclerosis, localized or diffuse atrophic scarring of the eardrum that may predispose to the development of atelectasis or a retraction pocket, or both, residual conductive hearing loss, and cholesteatoma. The more serious of these sequelae are quite infrequent. Recurrence of middle-ear effusion following the extrusion of tubes does develop, especially in younger children; most children without underlying craniofacial abnormalities only require 1 set of tympanostomy tubes, with developmental changes providing improved middle-ear health and resolution of chronic OME by the time of tube extrusion. Because even previously persistent OME often clears spontaneously during the summer mo, watchful waiting through the summer season is also advisable in most children with OME who are otherwise well. In considering surgical management of OME in children, primarily in those with bilateral disease and hearing loss, it has been demonstrated that placement of tympanostomy tubes results in a significant improvement in their quality of life.
Adenoidectomy
Adenoidectomy is efficacious to some extent in reducing the risk of subsequent recurrences of both AOM and OME in children who have undergone tube insertion and in whom, after extrusion of tubes, OM continues to be a problem. Efficacy appears to be independent of adenoid size and probably derives from removal of the focus of infection in the nasopharynx as a site of biofilm formation, chronic inflammation impacting eustachian tube function, and recurrent seeding of the middle ear via the eustachian tube. In younger children with recurrent AOM who have not previously undergone tube insertion, adenoidectomy is usually not recommended along with tube insertion, unless significant nasal airway obstruction or recurrent rhinosinusitis is associated, in which case, performing adenoidectomy might be considered.
Complications of Acute Otitis Media
Most complications of AOM consist of the spread of infection to adjoining or nearby structures or the development of chronicity, or both. Suppurative complications are relatively uncommon in children in developed countries but occur not infrequently in disadvantaged children whose medical care is limited. The complications of AOM may be classified as either intratemporal or intracranial.
Intratemporal Complications
Direct but limited extension of AOM leads to complications within the local region of the ear and temporal bone. These complications include dermatitis, TM perforation, chronic suppurative OM (CSOM), mastoiditis, hearing loss, facial nerve paralysis, cholesteatoma formation, and labyrinthitis.
Infectious Dermatitis
This is an infection of the skin of the external auditory canal resulting from contamination by purulent discharge from the middle ear. The skin is often erythematous, edematous, and tender. Management consists of proper hygiene combined with systemic antimicrobials and ototopical drops as appropriate for treating AOM and tube otorrhea.
Tympanic Membrane Perforation
Rupture of the TM can occur with episodes of either AOM or OME. Although damage to the TM from these episodes generally heals spontaneously, chronic perforations can develop in a small number of cases and require further surgical intervention in the future.
Chronic Suppurative Otitis Media
CSOM consists of persistent middle-ear infection with discharge through a TM perforation. The disease is initiated by an episode of AOM with rupture of the membrane. The mastoid air cells are invariably involved. The most common etiologic organisms are P. aeruginosa and S. aureus; however, the typical AOM bacterial pathogens may also be the cause, especially in younger children or in the winter months. Treatment is guided by the results of microbiologic investigation. If an associated cholesteatoma is not present, parenteral antimicrobial treatment combined with assiduous aural cleansing is likely to be successful in clearing the infection, but in refractory cases, tympanomastoidectomy can be required.
Acute Mastoiditis
Technically, all cases of AOM are accompanied by mastoiditis by virtue of the associated contiguous inflammation of the mastoid air cells. However, early in the course of the disease, no signs or symptoms of mastoid infection are present, and the inflammatory process usually is readily reversible, along with the AOM, in response to antimicrobial treatment. Spread of the infection to the overlying periosteum, but without involvement of bone, constitutes acute mastoiditis with periosteitis. In such cases, signs of mastoiditis are usually present, including redness and swelling in the postauricular area, often with protrusion and displacement of the pinna inferiorly and anteriorly (Fig. 640-7 and Table 640-5). Treatment with myringotomy and parenteral antibiotics, if instituted promptly, usually provides satisfactory resolution.
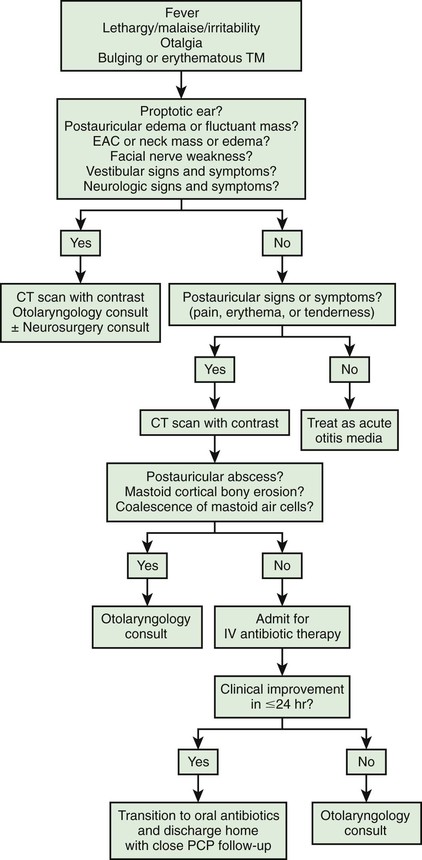
Table 640-5
Differential Diagnosis of Postauricular Involvement of Acute Mastoiditis with Periosteitis/Abscess
| DISEASE | Postauricular Signs and Symptoms | EXTERNAL CANAL INFECTION | MIDDLE-EAR EFFUSION | |||
| Crease* | Erythema | Mass | Tenderness | |||
| Acute mastoiditis with periosteitis | May be absent | Yes | No | Usually | No | Usually |
| Acute mastoiditis with subperiosteal abscess | Absent | Maybe | Yes | Yes | No | Usually |
| Periosteitis of pinna with postauricular extension | Intact | Yes | No | Usually | No | No |
| External otitis with postauricular extension | Intact | Yes | No | Usually | Yes | No |
| Postauricular lymphadenitis | Intact | No | Yes (circumscribed) | Maybe | No | No |
In acute mastoid osteitis, or coalescent mastoiditis, infection has progressed further to cause destruction of the bony trabeculae of the mastoid. Frank signs and symptoms of mastoiditis are usually, but not always, present. In acute petrositis, infection has extended further to involve the petrous portion of the temporal bone. Eye pain, a result of irritation of the ophthalmic branch of cranial nerve V, is a prominent symptom. Cranial nerve VI palsy is a later finding, suggesting further extension of the infectious process along the cranial base. Gradenigo syndrome is the triad of suppurative OM, paralysis of the external rectus muscle, and pain in the ipsilateral orbit. Rarely, mastoid infection spreads external to the temporal bone into the neck musculature that attaches to the mastoid tip, resulting in an abscess in the neck, termed a Bezold abscess.
When mastoiditis is suspected or diagnosed clinically, CT scanning of the temporal bones can be considered to further clarify the nature and extent of the disease. Bony destruction of the mastoid must be differentiated from the simple clouding of mastoid air cells that is found often in uncomplicated cases of OM. The most common causative organisms in all variants of acute mastoiditis are S. pneumoniae, group A streptococcus, and nontypeable H. influenzae. P. aeruginosa is also a causative agent, primarily in patients with CSOM. Children with acute mastoid osteitis generally require intravenous antimicrobial treatment and mastoidectomy, with the extent of the surgery dependent on the extent of the disease process. Early cases of mastoid osteitis may respond to myringotomy and parenteral antibiotics. Insofar as possible, choice of the antimicrobial regimen should be guided by the findings of microbiologic examination from cultures.
Each of the variants of mastoiditis may also occur in subacute or chronic form. Symptoms are correspondingly less prominent. Chronic mastoiditis is always accompanied by CSOM, and occasionally will respond to the conservative regimen recommended for that condition. In most cases, mastoidectomy also is required.
Facial Paralysis
The facial nerve, as it traverses the middle ear and mastoid bone, may be affected by adjacent infection. Facial paralysis occurring as a complication of AOM is uncommon, and often resolves after myringotomy and parenteral antibiotic treatment. Facial paralysis in the presence of AOM requires urgent attention as prolonged infection can result in the development of permanent facial paralysis, which can have a devastating effect on a child. Facial paralysis in an infant or child requires complete and unequivocal examination of the TM and middle-ear space. Any difficulty in examination requires urgent consultation with an otolaryngologist. Any examination that demonstrates an ear abnormality also requires urgent referral to an otolaryngologist. If facial paralysis develops in a child with mastoid osteitis or with chronic suppurative OM, mastoidectomy should be undertaken urgently.
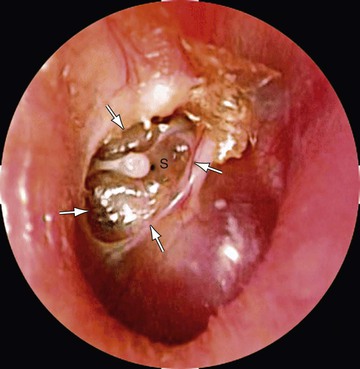
Cholesteatoma
Cholesteatoma is a cyst-like growth originating in the middle ear, lined by keratinized, stratified squamous epithelium and containing desquamated epithelium and/or keratin (see Chapter 638; Fig. 640-8).
Acquired cholesteatoma develops most often as a complication of long-standing chronic OM. The condition also may develop from a deep retraction pocket of the TM or as a consequence of epithelial implantation in the middle-ear cavity from traumatic perforation of the TM or insertion of a tympanostomy tube. Cholesteatomas tend to expand progressively, causing bony resorption, often extend into the mastoid cavity, and may extend intracranially with potentially life-threatening consequences. Acquired cholesteatoma commonly presents as a chronically draining ear in a patient with a history of previous ear disease. Cholesteatoma should be suspected if otoscopy demonstrates an area of TM retraction or perforation with white, caseous debris persistently overlying this area. Along with otorrhea from this area, granulation tissue or polyp formation identified in conjunction with this history and presentation should prompt suspicion of cholesteatoma. The most common location for cholesteatoma development is in the superior portion of the TM (pars flaccida). Most patients also present with conductive hearing loss on audiologic evaluation. When cholesteatoma is suspected, otolaryngology consultation should be sought immediately. Delay in recognition and treatment can have significant long-term consequences, including the need for more extensive surgical treatment, permanent hearing loss, facial nerve injury, labyrinthine damage with loss of balance function, and intracranial extension. The required treatment for cholesteatoma is tympanomastoid surgery.
Congenital cholesteatoma is an uncommon condition generally identified in younger patients (Fig. 640-9). The etiology of congenital cholesteatoma is thought to be a result of epithelial implantation in the middle-ear space during otologic development in utero. Congenital cholesteatoma most commonly presents in the anterior-superior quadrant of the TM but can be found elsewhere. Congenital cholesteatoma appears as a discrete, white opacity in the middle-ear space on otoscopy. Unlike patients with acquired cholesteatoma, there is generally not a strong history of OM or chronic ear disease, history of otorrhea, or changes in the TM anatomy such as perforation or retraction. Similar to acquired cholesteatoma many patients do have some degree of abnormal findings on audiologic evaluation, unless identified very early. Congenital cholesteatoma also requires surgical resection.
Labyrinthitis
This occurs uncommonly as a result of the spread of infection from the middle ear and/or mastoid to the inner ear (see Chapter 641). Cholesteatoma or CSOM is the usual source. Symptoms and signs include vertigo, tinnitus, nausea, vomiting, hearing loss, nystagmus, and clumsiness. Treatment is directed at the underlying condition and must be undertaken promptly to preserve inner-ear function and prevent the spread of infection.
Intracranial Complications
Meningitis, epidural abscess, subdural abscess, focal encephalitis, brain abscess (see Chapters 603 and 604), sigmoid sinus thrombosis (also called lateral sinus thrombosis), and otitic hydrocephalus each may develop as a complication of acute or chronic middle-ear or mastoid infection, through direct extension, hematogenous spread, or thrombophlebitis. Bony destruction adjacent to the dura is often involved, and a cholesteatoma may be present. In a child with middle-ear or mastoid infection, the presence of any systemic symptom, such as high spiking fevers, headache, or lethargy of extreme degree, or a finding of meningismus or of any central nervous system sign on physical examination should prompt suspicion of an intracranial complication.
When an intracranial complication is suspected, lumbar puncture should be performed only after imaging studies establish that there is no evidence of mass effect or hydrocephalus. In addition to examination of the cerebrospinal fluid, culture of middle-ear exudate obtained via tympanocentesis may identify the causative organism, thereby helping guide the choice of antimicrobial medications. Myringotomy should be performed to permit middle-ear drainage. Concurrent tympanostomy tube placement is preferable to allow for continued decompression of the “infection under pressure” that is the causative event leading to intracranial spread of the infection.
Treatment of intracranial complications of OM requires urgent, otolaryngologic, and, often, neurosurgical consultation, intravenous antibiotic therapy, drainage of any abscess formation, and tympanomastoidectomy in patients with coalescent mastoiditis.
Sigmoid sinus thrombosis may be complicated by dissemination of infected thrombi with resultant development of septic infarcts in various organs. With prompt recognition and wide availability of MRI, which facilitates diagnosis, this complication is exceedingly rare. Mastoidectomy may be required even in the absence of osteitis or coalescent mastoiditis, especially in the case of propagation or embolization of infected thrombi. In the absence of coalescent mastoiditis, sinus thrombosis can often be treated with tympanostomy tube placement and intravenous antibiotics. Anticoagulation therapy may also be considered in the treatment of sigmoid sinus thrombosis; however, otolaryngology consultation should be obtained before initiating this therapy to coordinate the possible need for surgical intervention prior to anticoagulation.
Otitic hydrocephalus, a form of pseudotumor cerebri (see Chapter 605), is an uncommon condition that consists of increased intracranial pressure without dilation of the cerebral ventricles, occurring in association with acute or chronic OM or mastoiditis. The condition is commonly also associated with lateral sinus thrombosis, and the pathophysiology is thought to involve obstruction by thrombus of intracranial venous drainage into the neck, producing a rise in cerebral venous pressure and a consequent increase in cerebrospinal fluid pressure. Symptoms are those of increased intracranial pressure. Signs may include, in addition to evidence of OM, paralysis of 1 or both lateral rectus muscles and papilledema with or without visual acuity loss. MRI can confirm the diagnosis. Treatment measures include the use of antimicrobials and medications such as acetazolamide or furosemide to reduce intracranial pressure, mastoidectomy, repeated lumbar puncture, lumboperitoneal shunt, and ventriculoperitoneal shunt. If left untreated, otitic hydrocephalus may result in loss of vision secondary to optic atrophy.
Physical Sequelae
The physical sequelae of OM consist of structural middle-ear abnormalities resulting from long-standing middle-ear inflammation. In most instances, these sequelae are consequences of severe and/or chronic infection, but some may also result from the noninfective inflammation of long-standing OME. The various sequelae may occur singly, or interrelatedly in various combinations.
Tympanosclerosis consists of whitish plaques in the TM and nodular deposits in the submucosal layers of the middle ear. The changes involve hyalinization with deposition of calcium and phosphate crystals. Uncommonly, there may be associated conductive hearing loss. In developed countries, probably the most common cause of tympanosclerosis is tympanostomy tube insertion.
Atelectasis of the TM is a descriptive term applied to either severe retraction of the TM caused by high negative middle-ear pressure or loss of stiffness and medial prolapse of the membrane as a consequence of long-standing retraction or severe or chronic inflammation. A retraction pocket is a localized area of atelectasis. Atelectasis is often transient and usually unaccompanied by symptoms, but a deep retraction pocket may lead to erosion of the ossicles and adhesive otitis, and may serve as the nidus of a cholesteatoma. For a deep retraction pocket, and for the unusual instance in which atelectasis is accompanied by symptoms such as otalgia, tinnitus, or conductive hearing loss, the required treatment is tympanostomy tube insertion and, at times, tympanoplasty. Patients with persisting atelectasis and retraction pockets should have referral to an otolaryngologist.
Adhesive OM consists of proliferation of fibrous tissue in the middle-ear mucosa, which may, in turn, result in severe TM retraction, conductive hearing loss, impaired movement of the ossicles, ossicular discontinuity, and cholesteatoma. The hearing loss may be amenable to surgical correction.
Cholesterol granuloma is an uncommon condition in which the TM may appear to be dark blue secondary to middle-ear fluid of this color. Cholesterol granulomas are rare, benign cysts that occur in the temporal bone. They are expanding masses that contain fluids, lipids, and cholesterol crystals surrounded by a fibrous lining and generally require surgical removal. Tympanostomy tube placement will not provide satisfactory relief. This lesion requires differentiation from bluish middle-ear fluid, which can also rarely develop in patients with the more common OME.
Chronic perforation may rarely develop after spontaneous rupture of the TM during an episode of AOM or from acute trauma, but more commonly results as a sequelae of CSOM or as a result of failure of closure of the TM following extrusion of a tympanostomy tube. Chronic perforations are generally accompanied by conductive hearing loss. Surgical repair of a TM perforation is recommended to restore hearing, prevent infection from water contamination in the middle-ear space, and prevent cholesteatoma formation. Chronic perforations are almost always amenable to surgical repair, usually after the child has been free of OM for an extended period.
Permanent conductive hearing loss (see Chapter 637) may result from any of the conditions just described. Rarely, permanent sensorineural hearing loss may occur in association with acute or chronic OM, secondary to spread of infection or products of inflammation through the round window membrane, or as a consequence of suppurative labyrinthitis.
Possible Developmental Sequelae
Permanent hearing loss in children has a significant negative impact on development, particularly in speech and language. The degree to which OM impacts long-term development in children is difficult to assess and there have been conflicting studies examining this question. Developmental impact is most likely to be significant in children that have greater levels of hearing loss, hearing loss that is sustained for longer periods of time, or hearing loss that is bilateral and in those children that have other developmental difficulties or risk factors for developmental delay (see Table 640-4).



