68 Rabies
Rabies has been recognized as a source of great human suffering and fear since ancient times. Characterized by a near-100% case-fatality rate, it is among the deadliest infectious diseases known to man. The rabies virus is present in the saliva of clinically ill mammals and is typically transmitted to humans through a bite. The incubation period is usually 1 to 3 months. After entering the central nervous system (CNS), the virus causes an acute, progressive encephalomyelitis. Although treatment options for rabies are currently limited, the disease is highly preventable with proper administration of rabies postexposure prophylaxis (PEP).
Geographic Distribution
Africa and Asia
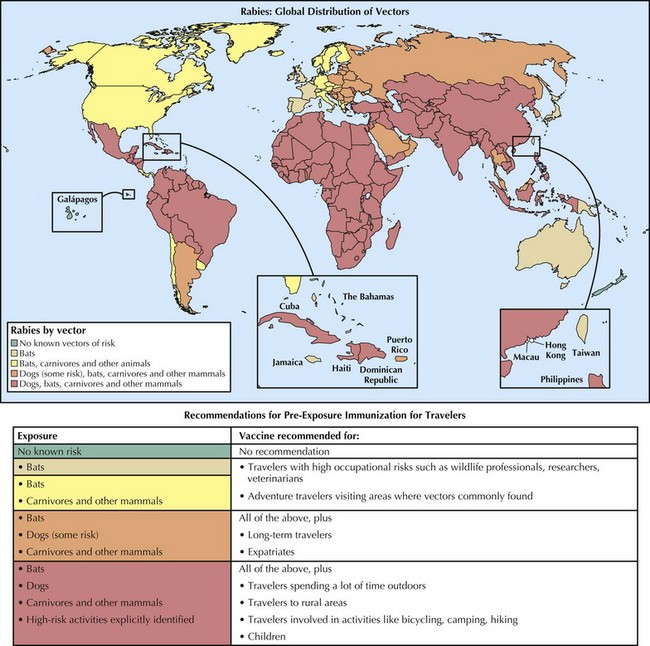
Of the estimated 35,000 to 55,000 rabies casualties every year, more than 95% occur as a result of dog bites in the developing countries of Africa and Asia. Exposure risk is highest in rural areas where free-roaming dogs are commonplace. Poor usage, accessibility, and affordability of medical care and rabies biologics are additional factors associated with human incidence. Children younger than age 15 years old are disproportionately infected owing to the high incidence of dog bites in this demographic group. Rabies and rabies-related viruses have also been isolated from African and Asian wildlife, including bats, mongooses, jackals, foxes, raccoon dogs, and other species. Travelers to rabies-endemic countries who anticipate prolonged stays in rural areas and extensive outdoor activities should consider preexposure immunization before travel (Figure 68-1).
Latin America and the Caribbean
In many Latin American and Caribbean countries, human rabies has declined in recent years as canine vaccination rates and use of rabies PEP have risen. From 1993 to 2002, dogs were implicated in 65% of human cases reported in the region; in 2004, 22% were attributed to dogs. In spite of this overall trend, canine rabies remains a concern in many places throughout the region. A growing proportion of human cases are also mediated by hematophagous (vampire) bats. Notable outbreaks caused by these animals have occurred in Brazil, Peru, and Columbia. Rabid vampire bats are particularly a threat to human and cattle populations in remote tropical areas within the Amazon. Although rabies is also present in nonhematophagous bats in the region, a complete understanding of their contribution to human disease is lacking because of the absence of taxonomic specificity when bats are identified and reported as a source of exposure, in addition to other surveillance limitations. In multiple instances, human rabies has been linked to monkeys, skunks, foxes, raccoons, and livestock. Domestic cats have also served as an important source of infection, with 3% of reported human cases in 1993 to 2002 attributed to these animals. Travelers who come in contact with animals—particularly wild or stray animals—should be mindful of rabies risks and take steps to avoid bites and other exposures.
Europe, Canada, and the United States
In Europe and temperate North America, human rabies is rare. From 1980 to 2008, an average of two people a year died from rabies in the United States, and in that time there were five human rabies deaths reported in Canada. Europe currently averages approximately nine reported cases a year, with most cases occurring in eastern Europe. The widespread availability of rabies vaccines and rabies immune globulin (RIG), a well-immunized dog population, and effective antistray programs are credited with the low human rabies incidence seen in most developed countries. Wildlife has the highest burden of rabies in North America and Europe. Most human cases in the United States and Canada are associated with insectivorous bats, whereas in eastern Europe rabies transmission is largely driven by the red fox, with dogs playing an important role as victims of fox-associated spillover infections. Insectivorous bats in Europe also serve as important reservoirs of European bat lyssaviruses (EBLs), which causes rabies in humans. Foreign-acquired rabies represents a significant portion of human cases reported in North America and western Europe, which are typically associated with dog exposures in rabies-endemic countries (Figure 68-2). From 1980 to 2008, 28% of cases reported in the United States and Canada were imported.
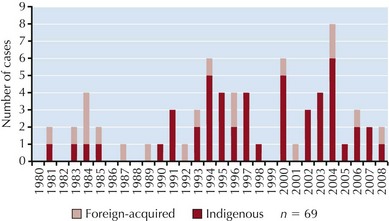
Figure 68-2 Human rabies cases in the United States, Puerto Rico, and Canada, 1980 to 2008. Four cases occurring in 2004 were the result of organ or tissue transplants from an infected donor, which accounts for the elevated count during this year. Of the indigenous cases, all but five were linked to bats either historically or through variant typing. Two indigenous U.S. cases occurring in 1991 and 1994 were associated with coyote or dog variants, and a skunk and a raccoon were linked to cases occurring in 1981 and 2003, respectively. In 2003, a man died from rabies in Puerto Rico as a result of a dog bite.
More than 65% of animal rabies reported in the United States is found in wild terrestrial carnivores such as raccoons, skunks, and foxes. However, insectivorous bats are considered higher risk vectors to humans because lesions inflicted by these mammals tend to be less conspicuous, less easily recognized, and/or taken less seriously and therefore are less likely to be treated than are bites and scratches from rabid carnivores. A majority of bat-associated human cases in the United States and Canada have been attributed to so-called cryptic bat exposures—characterized by the absence of an elicited bite or scratch history—which most often involve rabies virus variants associated with the silver-haired bat (Lasionycteris noctivagans) and the eastern pipistrelle bat (Pipistrellus subflavus). Because of the risks associated with undetected bat bites, the U.S Advisory Committee on Immunization Practices (ACIP) recommends that any suspected contact with a bat be evaluated for possible rabies virus exposure if a bite cannot be reasonably excluded.
Australia
In Australia, the emergence of Australian bat lyssaviruses (ABLs) in 1996 has elevated public health concerns in a country that historically has enjoyed “rabies-free” status. These bat-associated viruses—like the EBLs seen in Europe and the Lagos bat and Duvenhage viruses seen in African bats—although phylogenetically distinct from the classic rabies virus seen in the New World and most of the Old, produce a fatal encephalomyelitis indistinguishable from that caused by the rabies virus. In the late 1990s, two people acquired rabies after incurring bites from ABL-infected bats. Variants of the virus have been isolated from both frugivorous and insectivorous bats. Exposures to these animals should therefore be regarded the same way they would be in countries where bat rabies is present.
Rabies-Free Countries
The World Health Organization (WHO) may designate a country as “rabies free” if there have been no reports of indigenous cases in at least 2 consecutive years based on surveillance that is considered sufficiently sensitive. However, travelers should be aware that because surveillance for rabies often involves underreporting, an animal exposure may carry transmission risks even if the exposing animal is from a country considered “rabies free.”
Etiology
The rabies and rabies-related viruses belong to the Rhabdoviridae family as members of the Lyssavirus genus. Lyssaviruses are neurotropic, single-stranded ribonucleic acid (RNA) viruses characterized by a bullet-shaped morphology, a tightly coiled nucleocapsid, and five structural proteins. In keeping with other nonsegmented RNA viruses that have negative-sense polarity, genome replication and protein synthesis occur within the cytoplasm of infected cells under the direction of an RNA-dependent viral polymerase.
There are eleven recognized species of lyssaviruses that cause rabies, but only one species is formally called the rabies virus. Each species is further subdivided into phylogenetically distinct variants that are host-adapted to the mammalian reservoirs in which they circulate. The phenomenon known as spillover occurs when a variant adapted to one host species (such as the dog) infects another species (e.g., human) to which it is not adapted. Although disease in the newly infected host may result, spillover infrequently leads to sustained propagation of the variant in a new host population. Humans are poor conduits of disease transmission (naturally occurring human-to-human transmission has yet to be definitively established) and thus are considered dead-end hosts.
Pathophysiology
Rabies transmission usually occurs through the percutaneous bite of a rabid mammal shedding the virus in its saliva (Figure 68-3). Nonbite exposures such as scratches and licks can also lead to rabies infection, although less frequently than bites. Under atypical conditions, transmission may also occur through inhalation of highly concentrated aerosolized viral particles. Access to the nervous system is granted either through inoculation directly into peripheral nerves or via infection of surrounding tissue (e.g., muscle cells) with subsequent nerve entry at the neuromuscular junction (see Figure 68-3).
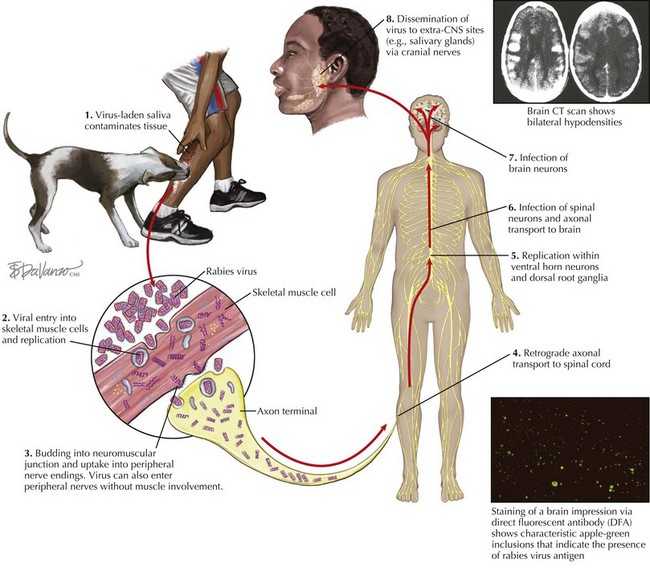
Figure 68-3 Pathophysiology of rabies.
(Computed tomography and direct fluorescent antibody courtesy Centers for Disease Control and Prevention.)
After peripheral nerve invasion, the rabies virus reaches the CNS via retrograde axoplasmic transport. Once the virus infects the ventral horn of the spinal cord and/or the dorsal root ganglia, viral amplification leads to rapid dissemination of the virus in the rostral gray matter of the spinal cord. Progression to the brain is achieved through axoplasmic transport within several ascending and descending fiber tracts, leading to early placement in the brainstem followed by retrograde diffusion into the rest of the brain. Resulting neurologic signs are considered to be primarily a product of nerve cell dysfunction as opposed to necrosis or apoptosis; however, the exact functional impairment involved is unclear.
Viral migration from the brain into peripheral sites such as the salivary glands provides a gateway for virus particles to escape from the body and invade a new host. After infection of brainstem nuclei, the facial and glossopharyngeal cranial nerves convey virus to the salivary glands via their associated ganglia. Subsequent infection of glandular epithelia results in considerable viral shedding into salivary secretions. Virions are also dispatched to ocular structures such as the cornea and retina and deposited in organs and tissues serviced by parasympathetic and sympathetic nerves, including the heart, kidneys, and liver. The iatrogenic implications of this latter phenomenon were demonstrated in 2004, when four people contracted rabies after receiving transplanted material from a then-undiagnosed rabies-infected organ donor. Rabies transmission via corneal transplants has also occurred on several occasions. Because the virus frequently accumulates in the free sensory nerve endings of nuchal tactile hair, a skin biopsy sample from this area is used as a standard diagnostic specimen.
Clinical Features
Onset of symptoms for most rabies patients occurs 3 to 12 weeks postexposure; however, incubation periods far outside of this timeframe do occur. Multiple bite wounds, severe wounds, and bite wounds to the head, face, and neck are associated with shorter incubation times and should therefore be considered when assessing the urgency of the need for PEP. Incubation periods a year or more have been described in a few cases of human infection, and therefore rabies PEP should be reasonably considered regardless of the amount of time that has elapsed since an exposure occurred.
Rabies has two clinical manifestations: the encephalitic form (or furious rabies) and the paralytic form (or dumb rabies). Encephalitic rabies is the most common form overall, although the majority of patients infected by vampire bats exhibit the paralytic form for reasons that remain unknown. Prodromal signs and symptoms for both are nonspecific and include fever, chills, malaise, and headache. Paresthesias on the part of the body that received the bite and pain and/or pruritus at the site of the bite wound that is unrelated to the injury itself are also common features.
Encephalitic rabies is characterized by altered mental status, agitation, hyperreactivity to sensory stimuli, intermittent consciousness, myoclonus, and muscle tremors. Also featured are signs indicative of autonomic neuropathy that include hypersalivation, mydriasis, and excessive lacrimation. Dysphagia and hydrophobia are cardinal sequelae; affected patients often react fearfully when offered water and exhibit inspiratory laryngeal spasms. Aerophobia is also frequently observed, as reflected by an exaggerated response to air currents passing over the skin (commonly referred to as the “fan test”). Seizures may occur but are not typical. Paralysis leading to coma usually occurs within 10 to 14 days, with death ensuing shortly thereafter and frequently precipitated by multiple organ failure.
In paralytic rabies, patients initially develop ascending muscle weakness that rapidly progresses to flaccid paralysis. Mental status is often unremarkable at the onset, hydrophobic spasms are less likely to be present, and patients may be unable to speak because of laryngeal muscle weakness—an occurrence that can interfere with obtaining an animal bite history and therefore complicate a clinical diagnosis. Peripheral neuropathy may be the cause of the muscle weakness seen in this form. Patients with the paralytic form overall tend to have longer periods of survival than patients exhibiting encephalitic rabies.
Differential Diagnosis
Viral encephalitides produce preliminary laboratory findings and clinical signs consistent with rabies. In particular, neuroinvasive arboviral diseases including those caused by California serogroup, equine encephalitis, and West Nile viruses are important differential diagnoses for rabies. Occurrence of these illnesses increases in late summer, which slightly resembles the seasonal pattern seen in bat-associated rabies in the United States. Serologic testing to rule out these diseases before testing for rabies (antemortem) is especially indicated if an encephalitic patient is older than age 50 years, lacks an animal exposure history, and resides in or has recently traveled to an area where West Nile disease and other mosquito-borne encephalitides are endemic.
Other acute neurologic diseases may be mistaken for rabies because of their epidemiologic link to animal exposures. Tetanus, which occurs secondarily to animal bites and other contaminated wounds, causes sustained muscle rigidity that differs from the spasms expressed in encephalitic rabies and the flaccidity expressed in paralytic rabies. In addition, altered mental status is not a typical feature of tetanus. Herpes B infection usually is the result of bites or scratches from macaque monkeys, and skin vesicles at the bite wound are a common manifestation. Incubation periods for both tetanus and herpes B are generally shorter than for rabies. Severe forms of brucellosis, leptospirosis, and toxoplasmosis are other zoonoses associated with encephalopathy but usually produce systemic sequelae that rabies does not.
Another differential diagnosis is acute disseminated encephalomyelitis (ADEM), which is an immune-mediated disease triggered by exanthematous viral and bacterial infections including measles, varicella-zoster, herpes simplex, and Rocky Mountain spotted fever. Less frequently, ADEM also occurs in association with certain vaccines, including those against rabies, smallpox, and measles. Signs of ADEM generally arise 1 to 20 days after a preceding illness or 1 to 3 weeks after vaccination is initiated. Rabies vaccines derived from neural tissue carry a higher risk for causing ADEM than do rabies cell-culture vaccines. Magnetic resonance imaging (MRI) findings suggestive of diffuse or multifocal demyelination in the CNS along with cortical signs such as aphasia, cortical blindness, and seizures are more characteristic of ADEM than rabies.
A condition that clinically mirrors paralytic rabies is Guillain-Barré syndrome (GBS). Demyelination of peripheral nerves and axonal degeneration are features of both. As with ADEM, patients with GBS usually have a recent history of vaccination or febrile illness. Patients with postinfection GBS may exhibit signs associated with the precipitating infection (e.g., gastroenteritis), whereas localized pruritus and pain occurring in the prodromal phase favor a rabies diagnosis.
A psychosomatic condition termed rabies hysteria has been ascribed to individuals whose belief in having the disease leads them to exhibit rabieslike signs of aggression, swallowing difficulty, and other behavioral patterns. Objective evidence, such as fever and cerebrospinal fluid pleocytosis, that points to an encephalomyelitic infection is usually absent in these patients, as is the steady progressiveness that exemplifies the clinical course of rabies disease.
Diagnostic Approach
Rabies should be suspected in any person with an animal exposure history who has an unexplained encephalitis or myelitis. It should be noted that the lack of an elicited exposure history should not preclude suspicion, because absent exposure histories are common in rabies patients. Recent travel to or emigration from a rabies-endemic area should also elevate suspicion. Progressive worsening of neurologic signs over a period of days is an important positive indicator of disease.
Facilities capable of conducting human rabies testing are limited to a few reference laboratories. Most human rabies testing in the United States and Canada is conducted by the rabies laboratories at the Centers for Disease Control and Prevention (CDC) and the Canadian Food Inspection Agency, respectively. Once rabies is suspected, consultation with state or provincial health departments is advisable. Other, more likely causes should be ruled out before resources are expended in rabies testing. However, laboratory testing should be pursued soon after the disease is suspected to ensure that persons potentially exposed to infectious material can take appropriate actions and safeguards.
For antemortem diagnosis, specimens used to confirm rabies include serum, cerebrospinal fluid, saliva, and neck skin biopsy specimen. A brain biopsy specimen may also be used for antemortem diagnosis; however, its collection is not recommended because its diagnostic value is outweighed by associated risks to the patient. Serum and cerebrospinal fluid are examined for the presence of rabies virus antibody via indirect fluorescent antibody and virus neutralization tests. Detection of viral antigen in a neck skin biopsy specimen is achieved through direct fluorescent antibody testing, and reverse transcription–polymerase chain reaction (RT-PCR) is used to detect the presence of viral RNA in both saliva and skin. Repeat testing may be necessary to rule out rabies if negative laboratory findings exist in the presence of strong clinical and epidemiologic evidence.
In deceased patients, brain tissue is the standard specimen for diagnosis. Direct fluorescent antibody testing is used to detect viral antigen in the brainstem, cerebellum, and hippocampus.
Treatment
There is no standard treatment for rabies besides palliative support, which includes appropriately applied analgesia, sedation, and assisted ventilation. Given the poor prognosis, careful consideration should be given before pursuing aggressive treatment measures. Experimental therapeutic approaches have been used to treat human cases, including one patient in Wisconsin who successfully recovered from the disease. Treatment for this patient included antiviral therapy using ribavirin and coma induction using benzodiazepines and barbiturates, with ketamine and amantadine used to prevent excitotoxicity. Neither rabies immunoglobulin nor vaccine was administered before or after illness. To date, this patient is the only documented survivor of rabies who had not received PEP or been previously vaccinated against rabies.
Prevention
Rabies in humans and animals is highly preventable through vaccination and, when applicable, passive immunization. There are currently two U.S. Food and Drug (FDA)–approved rabies vaccines available in North America: the human diploid cell vaccine (HDCV) and purified chick embryo cell vaccine (PCECV). These biologics are used for both preexposure prophylaxis and PEP.
Preexposure Prophylaxis
In areas where terrestrial animal rabies is present, occupational groups with frequent exposure to animals (e.g., veterinarians, wildlife workers, animal rehabilitators), or individuals engaged in activities that put them at risk of wildlife animal contact (e.g., ecotourists, cavers) should be vaccinated preventively, as should laboratory workers who work closely with the agent. Travelers who plan long-term travel to enzootic areas (e.g., expatriates and their young children) should also consider preexposure immunization. This recommendation also applies to travelers who are planning activities in settings where bats are abundant. Tiered recommendations for preexposure immunization for travelers are based on the global distribution of the principal reservoirs and vectors of rabies (see Figure 68-1).
For preexposure immunization, a 1-mL dose of rabies vaccine (either HDCV or PCECV) should be injected intramuscularly (IM) in the deltoid (or outer thigh, in children) on days 0, 7, and 21 (or 28). After primary immunization, boosters may be later indicated for individuals continuously or frequently at risk for inapparent rabies exposures, such as those encountered by rabies laboratory workers or bat handlers. For such occupational groups, antibody titers should be monitored using the rapid fluorescent focus inhibition test (RFFIT) every 6 months or 2 years depending on the individual’s risk category; a 1-mL booster is indicated if tested serum fails to exhibit complete virus neutralization at the 1 : 5 dilution. Periodic titer checks and booster shots are not recommended for individuals who are infrequently exposed to rabies and have a high likelihood of being aware of such exposures when they occur.
During 2007 to 2009, the supply of HDCV and PCEC was limited owing to production constraints. As a result, preexposure rabies vaccination was restricted for most people in the United States and Canada, including overseas travelers. Exceptions included high-risk occupational groups such as animal control officers and rabies laboratorians. However, vaccine remained available for PEP for individuals possibly exposed to rabies. For the latest update on availability and access to rabies vaccine, consult www.cdc.gov/rabies/news/RabVaxupdate.html.
Postexposure Prophylaxis
To appropriately manage a potential rabies exposure, the risk of infection should be thoroughly assessed. Administration of rabies PEP is generally considered a medical urgency, not a medical emergency. When feasible, rabies transmission should be ruled out by having the exposing animal either euthanized and tested for rabies or—in the case of dogs, cats, and ferrets—confined and observed for any neurologic signs that appear within 10 days. In the absence of a negative animal rabies diagnosis, any patient that has been bitten by a wild terrestrial carnivore (e.g. raccoons, skunks, foxes) should be suspected of rabies virus exposure and managed accordingly. State or local public health authorities can facilitate animal rabies testing and assist in conducting exposure risk assessments.
Travelers potentially exposed to rabies should contact local health authorities immediately for advice about the local availability of rabies PEP. Because RIG and/or rabies vaccine may not be available in the destination country, before travel the individual should have a strategy in place for responding to a possible exposure. This strategy may require the traveler to fly to a different country to obtain the appropriate care.
Any mammalian bite or scratch should receive prompt local first aid by thorough cleansing of the wound with copious amounts of soap, water, and a virucidal agent such as povidone iodine. Wound cleansing is considered an important component of rabies PEP, as it reduces tissue contact with infectious material. In unvaccinated patients with severe wounds, suturing should be delayed to allow infiltration of the wound with RIG and to prevent further dissemination of the virus throughout the traumatized tissue. The recommended protocol for PEP in immunized and nonimmunized patients differs (Figure 68-4).
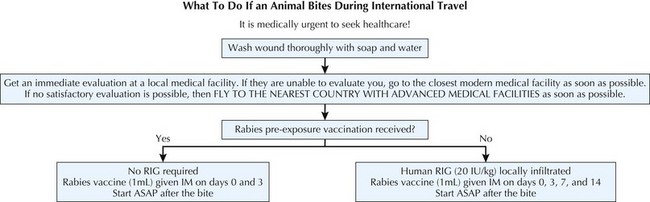
Figure 68-4 Algorithm for rabies postexposure management of travelers.
(Adapted from Keystone J, Kozarsky P, Freedman D, et al, eds: Travel medicine, ed 2, Philadelphia, 2008, Elsevier.)
Postexposure Prophylaxis for Previously Immunized Patients
PEP is indicated regardless of prior vaccination history. For patients who have previously recieved a full course of PreP or PEP with PCECV, HDCV, or a comparable vaccine, PEP consists of two booster doses of rabies vaccine each given IM in the deltoid on days 0 and 3 in addition to wound cleansing. RIG should not be administered to previously vaccinated patients. Patients that were last vaccinated before the year 1980 (when lower-potency rabies vaccines were used) may not have adequate immunity against the virus to safely qualify for a 2-dose course of PEP vaccination. In the absence of a documented history of an adequate antibody titer, it is advisable to manage this subset of patients identically to previously unvaccinated patients.
Postexposure Prophylaxis for Nonimmunized Patients
In previously unvaccinated patients, recommended PEP consists of one dose of human RIG (HRIG) (20 IU/kg body weight) given on day 0 and a series of four 1-mL injections of vaccine given IM on days 0, 3, 7, and 14 (see Figure 68-4). HRIG should be infiltrated in and around the wound(s), with any remaining volume given IM at a site distant to vaccine administration. The deltoid is the recommended injection site for vaccine; in children, the vastus lateralis is another acceptable location. Neither adults nor children should be given vaccine in the gluteus, and RIG and vaccine should not be administered in the same deltoid. The window for administering RIG can be extended up to day 7 if not given when vaccination was initiated, but after that time RIG is not indicated owing to its likely interference with active immunity. Minor deviations of the vaccine schedule have not been shown to adversely influence the effectiveness of prophylaxis; however, adherence to the recommended schedule is advised whenever possible. If substantial schedule deviations have occurred, serologic testing using the RFFIT 7 to 14 days after the last dose is indicated to ensure that an adequate antibody titer has been reached.
Immunocompromised patients should receive, in addition to RIG and wound cleansing, a 5-dose series of rabies vaccination on days 0, 3, 7, 14, and 28. Individuals that have HIV/AIDS or recipients of chemotherapy, antimalarials, and other immunosuppressive medications are included in this group. Postvaccination serological testing should be performed on these patients 7 to 14 days after the day 28 vaccine dose, to verify an adequate antibody response. Placement of RIG and vaccine in these patients is the same as it is in healthy patients.
Postexposure Prophylaxis Overseas
The first vaccines against rabies were derived from viruses extracted from the brains and spinal cords of infected animals. Some of these vaccines, also known as Semple rabies vaccines, are still in use in developing countries because of their low production costs relative to modern cell-culture vaccines. Therefore persons exposed to rabies while abroad may receive PEP with biologics that are not approved for use in the United States and Canada. Neural tissue vaccines are often administered in daily injections over a period of 14 to 21 days in the subcutaneous tissue overlying the stomach or upper back. Doses may be relatively high in volume (5-mL) and somewhat uncomfortable to receive. These vaccines pose a greater risk of vaccine-associated adverse events and are of lower potency than cell-culture vaccines. All attempts should be made to obtain modern cell-culture vaccines before accepting prophylaxis with these neural tissue vaccines. Other cell-culture–derived vaccines, including purified duck embryo and Vero cell vaccines, are also frequently used abroad, but they are not licensed for use in the United States. Purified equine RIG (ERIG) is frequently used in places where HRIG is unavailable. The frequency of reported adverse reactions associated with ERIG administration has been relatively low (0.8% to 6.0%), and most reactions reported are minor. Unpurified antirabies serum of equine origin may still be used in some countries where neither HRIG nor ERIG is available. More severe adverse reactions, including anaphylaxis, after antirabies serum administration have been reported.
If PEP is initiated with nonapproved biologics or regimens, a patient may require additional prophylaxis. State or provincial or local health departments should be contacted for specific advice in such cases. Serologic testing using the RFFIT may be indicated to determine whether the patient’s neutralizing antibody level precludes the need for additional vaccination.
Failure to prevent rabies in PEP recipients has not occurred in the United States since cell-culture vaccines and RIG have been in routine use; however, failures have occurred abroad when biologics of low potency were used, when some deviation occurred from the recommended PEP protocol, or when RIG was not administered, was administered in insufficient amounts, or was improperly administered. Inadequate wound cleansing or administration of vaccine at incorrect anatomic sites (e.g., the gluteal area) may also be associated with ineffective PEP. In addition, substantial delays between exposure and PEP initiation increase the likelihood that disease will occur before an adequate immune response has developed.
Adverse Reactions
Patients receiving preexposure prophylaxis or PEP should be advised that they may experience local reactions after vaccination, such as pain, erythema, swelling, or itching at the injection site, or mild systemic reactions, such as headache, nausea, abdominal pain, muscle aches, and dizziness. Approximately 6% of persons receiving booster vaccinations with HDCV have reported an immune complex–like reaction characterized by urticaria, pruritus, and malaise. Fewer adverse events have been reported in association with PCECV. If exposure to the rabies virus is a valid concern, rabies PEP should not be interrupted or discontinued because of local or mild systemic reactions to rabies vaccine.
Precautions and Contraindications
Pregnancy or age status are not contraindications for PEP. Known allergies to substances present in a particular vaccine (such as egg protein in the case of PCECV) may necessitate switching the vaccine to another type.
In immunocompromised individuals, rabies vaccination may fail to generate an adequate immune response. Such patients should postpone preexposure vaccinations and consider avoiding activities for which rabies preexposure prophylaxis is indicated. If preexposure prophylaxis and/or PEP must be administered to a person of poor immune status, serology is indicated to determine whether the patient obtained an adequate rabies virus neutralizing antibody titer. In the event that no acceptable antibody response is detected, the patient should be managed in consultation with his or her physician and appropriate public health officials.
Centers for Disease Control and Prevention (CDC). Rabies. Available at www.cdc.gov/rabies/ Accessed January 22, 2009. Useful online reference for rabies-related information, including how to collect and submit patient samples to the Rabies Laboratory at the CDC for diagnosis and a list of human rabies cases that have occurred in the United States and Puerto Rice since 1995
Freedman D, Virk A, Jong EC. Immunization of healthy adults. In: Keystone J, Kozarsky P, Freedman D, et al, editors. Travel medicine. ed 2. Philadelphia: Elsevier; 2008:85-121. Describes risk factors, epidemiology, and clinical management of rabies exposures in travelers
Jackson AC. Human disease. In: Jackson AC, Wunner WH, editors. Rabies. ed 2. London: Academic Press; 2007:309-340. Describes diagnostic approach and clinical manifestations of rabies disease in humans
Manning SE, Rupprecht CE, Fishbein D, et al. Human rabies prevention—2008: recommendations of the Advisory Committee for Immunization Practices. MMWR Recomm Rep. 2008;57:1-28. Presents an overview of current evidence pertaining to rabies prevention and management
Rupprecht CE, Briggs D, Brown CM, et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the Advisory Committee for Immunization Practices. MMWR Recomm Rep. 2010;59:1-9. Describes most current ACIP guidelines and supporting evidence for a 4-dose vaccination series in rabies PEP
Shlim D, Rupprecht C. Rabies. In Centers for Disease Control and Prevention (CDC). Health information for international travel 2010. U.S. Department of Health and Human Services, Public Health Service, Atlanta, 2009. Describes risk factors, epidemiology, and management of rabies exposures in travelers
World Health Organization (WHO). WHO Expert Consultation on Rabies. World Health Organ Tech Rep Ser. 2005;931:1-88. Describes the global picture of rabies with respect to disease burden, prevention activities, and emerging research areas, as well as outlines World Health Organization-approved regimens for rabies PreP and PEP
Belotto A, Leanes LF, Schneider MC, et al. Overview of rabies in the Americas. Virus Res. 2005;111:5-12. The authors summarize 1993 to 2002 rabies surveillance data in North and South America
Blanton JD, Palmer D, Christian KA, Rupprecht CE. Rabies surveillance in the United States—2007. J Am Vet Med Assoc. 2008;233:884-897. The authors describe 2007 rabies surveillance data from the United States
De Serres G, Daillaire F, Côte M, Skowronki DM. Bat rabies in the United States and Canada from 1950 through 2007: human cases with and without bat contact. Clin Infect Dis. 2008;46:1329-1337. The authors discuss bat-borne human rabies cases in the United States and Canada
Hemachudha T, Wacharapluesadee S, Mitrabhakdi E, et al. Pathophysiology of human paralytic rabies. J Neurovirol. 2005;11:93-100. The authors discuss key clinical features and pathologic changes associated with paralytic rabies
Huynh W, Cordato DJ, Kehdi E, et al. Post-vaccination encephalomyelitis: literature review and illustrative case. J Clin Neurosci. 2008;15:1315-1322. The authors discuss etiologic associations, clinical features, and pathologic changes associated with postvaccination ADEM
Jackson AC. Pathogenesis. In: Jackson AC, Wunner WH, editors. Rabies. ed 2. London: Academic Press; 2007:341-381. The author discusses current understanding of rabies pathophysiology
Noah DL, Drenzek CL, Smith JS, et al. Epidemiology of human rabies in the United States, 1980 to 1996. Ann Intern Med. 1998;128:922-930. The authors review human rabies cases in the United States from 1980 to 1996
Willoughby RE, Tieves KS, Hoffman GM, et al. Survival after treatment of rabies with induction of coma. N Engl J Med. 2005;352:2508-2514. The authors describe the approach used to treat a patient who survived rabies in the absence of any prior rabies vaccination or RIG administration