Pharmacokinetics in herbal medicine
Pharmacokinetics can be defined as the study of the absorption, distribution, metabolism and elimination of pharmacologically active agents in the body. This discussion is not intended to be a primer on the principles of pharmacokinetics (there are appropriate texts for this purpose); however, certain basic issues will be discussed where there is particular relevance to plant chemicals.
One important issue that should underlie much of the study of herbal pharmacokinetics is that herbs are not usually directly introduced into the bloodstream by injection or other means, but rather the traditional oral or topical routes of administration are preferred. This renders the study of bioavailability of paramount importance for active constituents in plants. Bioavailability can be defined as the degree of absorption of active substances into the bloodstream after oral doses. Hence bioavailability is also a factor of the preparation that is used to deliver the dose of active substance. Conventional drugs intended for oral use are designed to have good bioavailability. In contrast, phytochemicals are of natural origin and may exhibit unusual or poor bioavailability that may be further compounded by the choice of dosage preparation.
In this discussion, the issue of the bioavailability of several of the archetypal plant constituents will be emphasised. For pharmacokinetic details related to specific herbs, see the monograph section (Part Three) of this book.
Herbal clinicians and students sometimes question the value of studying herbal pharmacokinetics. The following premises should be considered:
• If it is accepted that medicinal plants act at a chemical level in the body (as well as possibly other levels of activity), then knowledge of how their chemicals behave in the body is vital.
• Given that, with a few exceptions in some countries, oral and topical doses are used for herbal medicines, a better knowledge of bioavailability is critical to meet the challenge of future health problems.
Specifically the information derived from the detailed study of herbal pharmacokinetics can deliver:
• information to further assess the traditional and anecdotal uses of a medicinal plant
• better information on which to base rational dosages
• a better interpretation of scientific information, particularly in vitro research or in vivo studies where the active compounds are administered by injection. There is an abundance of misinformation in the herbal literature related to excessive extrapolation from such studies, with no consideration of bioavailability
• a better appreciation of the safety and toxicity of a plant
• anticipation of potential herb-drug interactions
• supporting evidence for the synergistic nature of herbal medicine
• ways to optimise the bioavailability and hence efficacy of herbal medicines.
The study of herbal pharmacokinetics is a unique and extraordinarily complex field. This is for the following reasons:
• The chemical complexity of plant medicines and the potential interactions between constituents
• The differing bioavailability of different compounds
• Often large polar molecules are involved that might be expected to have poor and unpredictable bioavailability
• The active components are often not known, so the components in the plant that should be studied cannot be identified
• Unlike drugs, herbal medicines are not designed for predictable pharmacokinetic behaviour, and in particular natural compounds are often metabolised in the digestive tract – that is, they are pro-drugs; this key feature is emphasised in this chapter.
But this does not mean to imply that the existing information is without value. On the contrary, many studies now provide a better understanding of this topic and inform clinical practice.
Before particular examples of the pharmacokinetics of plant constituents are discussed, it is worthwhile to examine some of the key issues pertaining to bioavailability. The bioavailability of a molecule depends on several factors that determine how it traverses the barrier of the gastrointestinal tract and survives into the bloodstream. These include:
• the pharmaceutical preparation
• the size of the molecule – very large molecules still have some bioavailability (about 1% or less) which may be due to pinocytosis
• the fat (lipid) solubility of the molecule – the more fat-soluble, the better the bioavailability (see Table 2.2515 for examples of how fat solubility influences bioavailability for cardioactive glycosides)
• the water solubility of the molecule – if a molecule is both water- and fat-soluble it will exhibit very good bioavailability because it will dissolve in the digestive juices and then cross lipid membranes; otherwise, purely water-soluble molecules can be expected to have poor bioavailability; ionisation of a molecule usually denotes poor bioavailability
• specific factors related to crossing the gut wall, such as active transport
• factors within the gut – interaction with food, stability in the gut, gastric emptying
• metabolism in the gut and first-pass metabolism by the liver
• individual factors in the patient, including the influence of genetic and pathological factors.
Table 2.2 Effect of lipid solubility on bioavailability515
| Cardiac glycoside | P | B% |
|---|---|---|
| g-Strophanthin | 0.01 | 6.6 |
| Convallatoxin | 0.33 | 13.6 |
| Digoxin | 18.2 | 26.4 |
| Digitoxin | 70 | 74.9 |
| Oleandrin | 338 | 86.0 |
B = bioavailability; P = partition between water and octanol, an indication of fat solubility.
Food is known to affect the bioavailability of conventional drugs (see Tables 2.3 and 2.4516 for examples). The presence or absence of food may also influence the absorption and bioavailability of plant constituents. For example, acetyl-11-keto-beta-boswellic acid (AKBA) from Boswellia serrata exhibits better bioavailability with a high fat meal,517 whereas a high fat meal reduces the bioavailability of resveratrol from Polygonum cuspidatum.66
Table 2.3 Drugs with absorption enhanced by food516
| Drug | Mechanism |
|---|---|
| Carbamazepine | Increased bile production; enhanced dissolution and absorption |
| Diazepam | Food enhances enterohepatic recycling; increased dissolution secondary to gastric acid secretion |
| Griseofulvin | Drug is lipid soluble; enhanced absorption |
| Metoprolol | Food may reduce first-pass extraction and metabolism |
| Phenytoin | Delayed gastric emptying and increased bile production improve dissolution and absorption |
Table 2.4 Drugs with absorption decreased by food516
| Drug | Mechanism |
|---|---|
| Isoniazid | Food raises gastric pH preventing dissolution and absorption; also delayed gastric emptying |
| Captopril | Mechanism unknown |
| Chlorpromazine | Drug undergoes first-pass metabolism in gut; delayed gastric emptying affects bioavailability |
| Tetracyclines | Bind with calcium ions or iron salts forming insoluble chelates |
Grapefruit juice (GFJ) is a plant substance that can exert a marked effect on bioavailability. For example, in early studies it increased the bioavailability of oral 17-beta-oestradiol and its metabolite oestrone.518 GFJ also substantially increased the bioavailability of the following drugs: felopidine,519 caffeine,520 nifedipine and similar drugs,521 cyclosporine522 and triazolam523 and similar drugs. The major interaction seems to be in the gut wall with enzymes belonging to various cytochrome P450 subfamilies.524 It also appears to inhibit renal 11-beta-hydroxysteroid dehydrogenase in humans and could therefore potentiate the side effects of licorice.525 Although the flavonoids naringin and naringenin were at first implicated, they are probably not the active components.526,527 In fact, the furanocoumarin bergamottin and related compounds appear to be largely responsible for this activity.528 This ability of plant furanocoumarins to inhibit drug-metabolising enzymes was first noted by Korean researchers in 1983.529 A 2011 review noted that GFJ demonstrated multiple interactions with drugs leading to loss of therapeutic effects or increased side effects.530 GFJ decreases presystemic metabolism through competitive or mechanism-based inhibition of gut wall CYP3A4 isoenzymes and P-glycoprotein. In addition multidrug resistance protein-2 or organic anion-transporting polypeptide inhibition may play a role. The review confirmed that, although GFJ contains high amounts of flavonoids (such as naringin, naringenin), furanocoumarins (especially 6′,7′-dihydroxybergamottin and bergamottin) are the main phytochemicals involved in the pharmacokinetic interactions. As compounds of GFJ show additive or synergistic effects, all the major furanocoumarins are necessary for the maximal inhibitory effect. Related citrus fruits or other plants containing furanocoumarins may also present pharmacological interactions yet to be discovered.
This phenomenon raises the question of how other plants and their phytochemicals might influence the bioavailability of both drugs and herbal constituents. The example of hyperforin in St John’s wort is now well studied in this regard (see the St John’s wort monograph).
Bioavailability is also affected by the preparation used to deliver the dose of the medicine. This area of study has been largely neglected for herbal medicines. However, some general statements about aqueous preparations such as infusions and decoctions can be proposed. Infusions and decoctions extract water-soluble compounds from plants. Many of these will have poor bioavailability. One important exception is plants containing essential oils taken by infusion. Here the hot water acts almost as a distillation medium and the oil will collect on the surface of the water. The addition of saponin-containing herbs to the mixture may increase the solubility of compounds not as water-soluble, which may then have better bioavailability. However, this advantage has been disputed (see previously under saponins). This practice is often observed in traditional Chinese medicine, a therapeutic system that relies heavily on aqueous preparations. Changes in water-soluble compounds in the digestive tract, most significantly the conversion of glycosides to aglycones in the caecum and large bowel, will render them more lipid-soluble. They can then be absorbed as the aglycone. In general, however, bioavailability and solubility considerations suggest that infusions and decoctions are inferior preparations for the extraction and delivery of herbal actives. The exception is where those actives are largely water-soluble anyway, such as some saponins, tannins, polysaccharides and proteins. However, only small quantities of these relatively large molecules will be absorbed as such (see later).
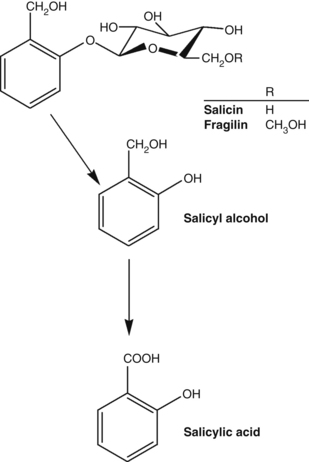
Salicin
A good starting point in the study of herbal pharmacokinetics is those archetypal plant constituents that have been relatively well investigated. The phenolic glycoside salicin and the anthraquinone glycosides are two such examples.
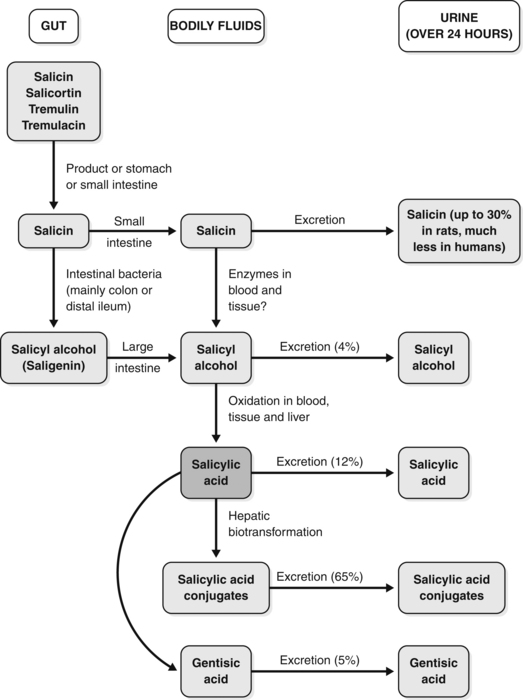
Salicin and its conversion products are illustrated in Figure 2.2 and a schematic diagram of the pharmacokinetics of salicin is provided in Figure 2.3.531,532 As shown in Figure 2.3, salicin derivatives are first converted into salicin in the stomach or small intestine. The salicin may then be absorbed in the small intestine, but in humans it is mainly carried to the distal ileum or colon where gut flora convert the glycoside into its aglycone, known as salicyl alcohol. The salicyl alcohol is absorbed and oxidised in the blood, tissue and liver to give salicylic acid, the main active form. The excretion of these various products is also outlined in Figure 2.3.
Despite the elaborate route by which salicin (and indeed willow bark) delivers salicylic acid into the bloodstream, the relative bioavailabilities of sodium salicylate and salicin are remarkably similar (Fig. 2.4531). The curve for salicin is slightly lower and flatter, indicating a greater half-life. The rapid absorption of salicin as salicylic acid additionally implies that its conversion is also rapid, suggesting the distal ileum or caecum as the site of conversion rather than the large intestine (orocaecal transit time is typically up to a few hours).
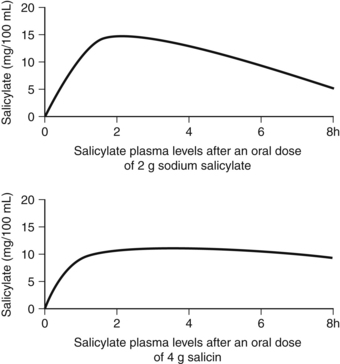
Figure 2.4 Relative bioavailabilities of salicin and salicylic acid (doses are approximately equivalent).531
At this point, it is worth reflecting upon the traditional use of willow bark and the history of aspirin. When scientists in the 19th century began to investigate the antipyretic and anti-inflammatory effects of willow bark, salicin was isolated as the active compound. Salicylic acid was more readily synthesised and was adopted into mainstream therapy, but had the drawback of being a strong irritant to the stomach. This led to the development of aspirin, a derivative of salicylic acid, in an attempt to minimise the gastric irritation. (Unfortunately, aspirin was still a gastric irritant, but it was more active than salicylic acid as an analgesic and antiplatelet agent.) Had the early scientists instead preferred salicin, they might have recognised that nature had already designed a derivative of salicylic acid that gave a good yield of bioavailable salicylic acid and was kind to the stomach.
This proposition is supported by an in vivo study.533 When salicin was administered orally to rats, salicylic acid appeared slowly in the plasma and levels increased gradually, in contrast to the rapid appearance observed after oral administration of sodium salicylate or saligenin. However, oral salicin still significantly reduced yeast-induced fever, producing a normal body temperature. Salicin did not induce gastric lesions, even at a high dose; conversely sodium salicylate and saligenin induced severe gastric lesions in a dose-dependent manner. When given to germ-free rats around 20% of the salicin was recovered intact from the digestive tract and no saligenin was found. These results provided clear evidence that salicin is a pro-drug, as discussed above, that is gradually transported to the lower part of the intestine and hydrolysed to saligenin by intestinal bacteria, producing its therapeutic effects without gastric injury.
Anthraquinone glycosides
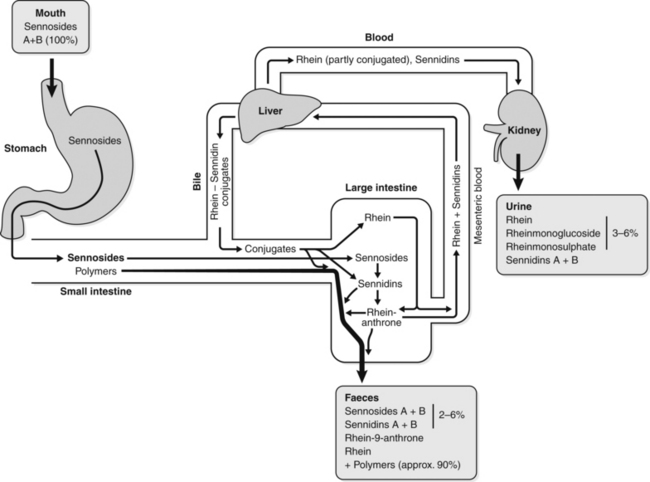
Early research on the laxative properties of anthraquinones and their glycosides baffled researchers. When equivalent doses were used, only the glycosides were active orally, not their aglycones. However, when equivalent doses were administered by injection, the reverse situation applied: the aglycones were more active. Equally intriguing were the observations that oral doses of the anthraquinone glycosides took 6 to 8 h to exert their laxative effect and the effective dose for laxation often varied dramatically from person to person.
The modern understanding of the pharmacokinetics of anthraquinone glycosides provides a simple explanation for these paradoxical observations. This is illustrated schematically in Figure 2.5.534
As anthraquinone glycosides pass through the digestive tract, a significant proportion undergoes polymerisation to inactive polymers. Any remaining glycoside is unchanged and unabsorbed until it reaches the large intestine. There the action of certain bowel flora converts the glycosides into their anthrone aglycones. These active aglycones then exert a laxative action in situ in the colon.534
Hence, the anthraquinone glycoside given by injection exerts less activity, because this is not the active form. On the other hand, the aglycone given orally also exerts little activity because it is broken down or absorbed before it reaches the colon. (Some modern laxative drugs are anthrone aglycones, but they are administered orally in quite high doses.) The 6 to 8 h lag-time for activity reflects the time it takes for ingested anthraquinone glycosides to reach the appropriate part of the colon for conversion into aglycones.
We can only wonder at this elegant and, when one considers the quantities of liberated anthrone aglycone involved, exquisitely sensitive mechanism designed by nature. One inference from this example is that the composition of an individual’s bowel flora is highly relevant to the pharmacokinetic equation and hence to the final pharmacological effect.534 This is a common theme for several of the archetypal plant constituents and emphasises the herbal clinician’s obsession with the health of the gut, not just as the central focus for restoring robust health, but also for delivering effective therapy.
Glycosides and gastric modification
A number of plant glycosides are modified by the action of gastric acid or the alkaline conditions of the duodenum. The following examples and their implications serve to illustrate this phenomenon.
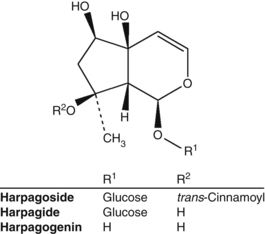
Harpagoside from devil’s claw (Harpagophytum procumbens) has oral anti-inflammatory activity (Fig. 2.6). However, when it is incubated with gastric acid, which generates harpagogenin from harpagoside, it tends to lose this activity in oral dose models.535 While harpagoside may not be the final bioavailable form, this research suggests that the action of gastric acid is detrimental to the anti-inflammatory activity of devil’s claw. Preparations of this herb should therefore be enterically coated or at least taken between meals to optimise the bioavailability of anti-inflammatory components. (See the devil’s claw monograph for a further discussion of this topic.)
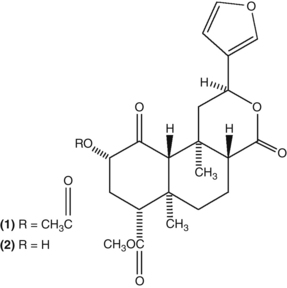
The valepotriates (Fig. 2.7) of valerian have cytotoxic activity when administered by injection, but do not exhibit this effect after oral doses. The former observation initially raised concerns about the safety of valerian, but it is now clear that valepotriates are quite unstable in aqueous solution and are decomposed by gastric acid. The breakdown products of the valepotriates still possess some sedative activity.536 (See the valerian monograph for more details.)
The following example is not of therapeutic significance but it does illustrate that the empirical basis for the traditional use of plants sometimes reflects an implicit understanding of pharmacokinetic issues. Salvia divinorum is a species of sage with hallucinogenic properties. Traditionally the fresh leaves are chewed for this effect, which led to the belief that the hallucinogenic component(s) were inactivated by drying the herb. Research has revealed that this is not the case. The active hallucinogenic component in Salvia divinorum is salvinorin A (Fig. 2.8).537 This is converted by gastric acid into salvinorin B, which is inactive. Hence the chewing of leaves by the shamans was in fact a delivery mechanism for salvinorin A that bypassed decomposition in the stomach. That the hallucinogenic quantities of salvinorin A can be absorbed through the oral mucosa underlines the potent activity of this compound.
Flavonoid glycosides and enteric modification
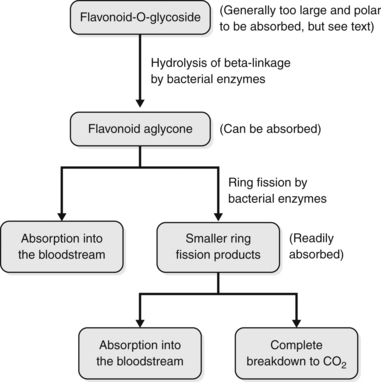
Flavonoid glycosides are a common component of many plants and are a significant class of archetypal plant constituents with pharmacological activity. However, in vitro models dominate the pharmacodynamic research on flavonoids and their glycosides. This is of concern because an understanding has existed for some time (and has been repeatedly confirmed) that flavonoids tend to have poor bioavailability as such, because they are largely decomposed by bowel flora.
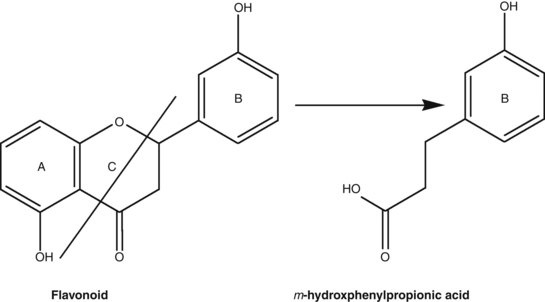
Studies have shown that flavonoid-O-glycosides are converted into the aglycone by bowel flora. But the decomposition can extend further than this; the aglycones undergo further breakdown by a process known as C-ring fission (the C-ring is the central ring in the flavonoid structure) to give two different phenolic products. An example of C-ring fission is illustrated in Figure 2.9. The ring fission products for several common flavonoids, flavonoid glycosides and related products are summarised in Table 2.5538 and a general scheme for the pharmacokinetics of many flavonoid glycosides is outlined in Figure 2.10.
Table 2.5 Flavonoid ring fission metabolites538
| Flavonoid administered | Ring fission products |
|---|---|
| Quercetin, rutin | 3,4-dihydroxphenylacetic acid,3-methoxy-4-hydroxyphenylacetic acid,m-hydroxyphenylacetic acid |
| Hesperidin, diosmin, eriodictyol, homoeriodictyol | m-hydroxyphenylpropionic acid,m-coumaric acid (rat), 3-hydroxy-4-methoxyphenyl-hydracrylic acid (human) |
| (+)-Catechin | delta-(hydroxphenyl)-gamma-valerolactones,m-hydroxyphenylpropionic acid,m-hydroxybenzoic acid,m-hydroxyhippuric acid |
| Kaempferol | delta-(p-hydroxyphenyl)-gamma-valerolactone,p-hydroxyphenylacetic acid |
| Myricetin, myrictrin | 3,5-dihydroxyphenylacetic acid |
| Tricetin, tricin | m-hydroxyphenylacetic acid,3,5-dihydroxyphenylpropionic acid,m-hydroxyphenylpropionic acid |
Studies have also shown the following:
• Oral doses of flavonoid aglycones are less bioavailable (as the flavonoid) than their glycosides because they are more susceptible to ring fission
• Levels of a flavonoid aglycone in the bloodstream will vary according to:
This picture of flavonoid pharmacokinetics needs to be modified in one specific area following the work of a German group of scientists early this century. Their study of the bioavailability of quercetin in various forms provided new and important information about the pharmacokinetics of flavonoids.540–542 The pharmacokinetics of two quercetin glycosides, and plant extracts containing these, were investigated in 12 healthy volunteers in a crossover study.540,542 This was because prior investigation had indicated that the bioavailability of quercetin may actually be improved by the presence of a sugar on the molecule. That is, certain quercetin glycosides can deliver quercetin more effectively into the bloodstream, presumably via active uptake by enterocytes. (Even this enhanced bioavailability was only about 5% of the amount ingested.)541
Each volunteer received an onion extract (containing quercetin-4′-O-glucoside) or pure quercetin-4′-O-glucoside, both equivalent to 100 mg of quercetin; or buckwheat tea (containing quercetin-3-O-rutinoside) or pure quercetin-3-O-rutinoside (rutin), both equivalent to 200 mg of quercetin. Pure quercetin was not detected in the human plasma samples. Instead, four different quercetin glucuronides (QG) were detected. These were presumably formed following uptake by the action of enterocytes.
The form of the flavonoid greatly influenced the rate and extent of QG appearance in the bloodstream. After administration of quercetin-4′-O-glucoside or onions, maximum plasma levels of QG were reached after 0.5 to 1 h. But only 5 to 10 h after administration of rutin or buckwheat tea did QG appear in volunteers’ bloodstreams. Despite the fact they were given at half the dose, the levels of QG achieved from onions and quercetin-4′-O-glucoside were about four times those achieved from rutin and buckwheat tea. The plant matrix enhanced the rate and extent of QG appearance, but to a much lesser extent than the form of the flavonoid.
These simple and elegant findings add a new dimension to our understanding of the pharmacokinetics of flavonoids:
• Aglycones (such as quercetin) have poor or even zero bioavailability
• There is an active uptake and metabolism of flavonoid glycosides by enterocytes that is determined by the nature of their sugar (with a preference for glucose)
• Before the flavonoid is taken up by the enterocyte, it is probably hydrolysed to the aglycone by a membrane-bound beta-glucosidase
• Quercetin-4′-O-glucoside is taken up in the small intestine, whereas rutin (which has a glucose and a mannose = rutinoside) is not
• Presumably rutin travels to the large intestine (hence the lag in QG appearance), where the terminal mannose is removed by bacteria, exposing the glucose which then results in uptake by enterocytes
• Measurement of the renal elimination of flavonoids suggested that at least 6% of the flavonoid dose was absorbed when given as onions.
QG have slightly more antioxidant activity than quercetin, but will not exert much effect in plasma as antioxidants because they are tightly bound to plasma proteins. However, they could well become active at target tissues, especially after removal of the glucuronide group.
The above study could provide no information on the uptake of C-ring fission compounds, since these are also metabolites naturally found in the bloodstream and it was therefore difficult to detect any changes in their levels above the natural background.
A 2011 review supported this perspective, albeit with some subtle modifications.543 As described above, lactase phlorizin hydrolase (LPH), a membrane-bound beta-glucosidase, is able to liberate aglycones into the intestinal lumen, where they passively diffuse across the small intestinal membrane. However, cytosolic beta-glucosidase may also be involved. As this particular enzyme is located intracellularly in the enterocyte, active transport of the flavonoid glycoside via the sugar transporter SGLT-1 (sodium-dependent glucose transporter) is first required. Flavonoid glycosides that are not substrates of LPH or SGLT-1 will pass to the colon where bacterial hydrolysis will produce the aglycone and C-ring fission products. Of course, the action of LPH or SGLT-1 is not quantitative, with much of the flavonoid glycosides that are their substrates still passing unchanged to the colon. In other words, the actions of LPH and SGLT-1 only render a small percentage of a flavonoid glycoside bioavailable in the small intestine.
Once absorbed, the flavonoid aglycones are subject to three main types of conjugation: methylation, sulphation and glucuronidation.543 The extent of conjugation is high and only a small percentage of free flavonoid aglycones is present in the plasma.543,544 The presence of conjugated metabolites in the portal blood of rats suggests that conjugation first occurs in the enterocytes before further metabolism in the liver.543,545
The issue of the bioavailability of the flavonoid aglycone quercetin is controversial. Many nutritional supplement companies market products containing quercetin. The rationale for including quercetin in such products is often largely based on in vitro research or in vivo models following dosage by injection (although there are also clinical trials demonstrating positive effects from high doses - see previous). The consensus of studies is that the bioavailability of quercetin (as quercetin) is poor. This is due to its propensity to undergo C-ring fission. Following the Zutphen Elderly Study and the Netherlands Cohort Study where flavonol and flavone dietary intakes were inversely associated with mortality from coronary heart disease and risk of stroke, Dutch researchers once again studied quercetin bioavailability.546 Conclusions from their study on ileostomy patients were uncertain because they did not measure quercetin in the bloodstream.547 It was assumed that, because of the ileostomy, any unrecovered quercetin was absorbed. But significant microbial degradation could also have occurred. This is supported by the minimal level of quercetin (0.5% of original dose) found in subjects’ urine. Nonetheless, this study has been misinterpreted by many authors as unequivocally demonstrating a high oral bioavailability for quercetin. More recent studies tend to confirm the relatively low and variable bioavailability of intact quercetin.230,544 Gut flora metabolism might account for a large part of this variability.
One study of the C-ring fission products of quercetin found that 3,4-dihydroxyphenylacetic acid possessed significant antioxidant activity.548 In vitro antiplatelet activity is also suggested in another.549 Pharmacodynamic research on flavonoids should include more studies on their C-ring fission products, which in many instances are probably the main bioavailable and active form of this important group of archetypal plant constituents.550
Isoflavones
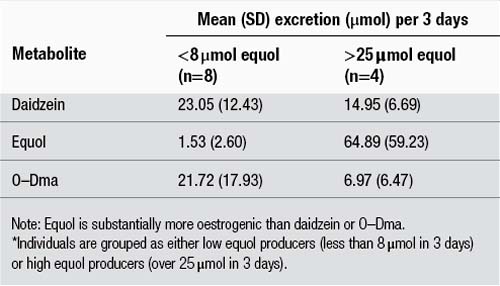
As noted earlier, the isoflavones are a group of archetypal plant constituents attracting increasing attention because of their affinity for oestrogen receptors. The principal isoflavones are the glycosides genistin and daidzin and their aglycones genistein and daidzein, from the soya plant, and the aglycones formononetin and biochanin A from red clover (see Fig. 2.11). Formononetin can be converted to daidzein, which in turn can be metabolised to equol by bowel flora. This is a significant reaction pathway from a pharmacodynamic perspective, because equol has substantially more oestrogenic activity than its precursors. However, equol appears to be produced to different degrees in different people. Table 2.6551 illustrates that individuals can be grouped into high and low equol producers. The high equol producers are likely to experience significantly greater oestrogenic effects from the consumption of soya or red clover.
Table 2.6 Comparison of the urinary excretion rates of daidzein and two daidzein551 metabolites in individuals over a 3-day period following soya challenge*
Early studies also found the following:
• Soya isoflavones are 85% degraded in the intestine552
• Differences in faecal flora account for the differing metabolism of soya isoflavones553
• Faecal flora could completely degrade genistein and daidzein553
• Differences in faecal excretion of isoflavones profoundly altered isoflavone bioavailability: higher faecal excretion was correlated with higher bioavailability. Such people may have fewer bacteria that degrade isoflavones, leaving more intact for absorption.548 Bioavailability varied from 13% to 35% depending on the individual gut microflora553
• Soya protein (containing isoflavone glycosides) increased follicular phase length in women, while miso (containing isoflavone aglycones) did not.554 This suggests that the glycosidic group delays the degradation of isoflavones, resulting in higher bioavailability of their aglycones or equol.
These earlier studies served to emphasise the importance of bowel flora in determining the bioavailability and hence pharmacodynamic activity of isoflavones.
Recent reviews of the pharmacokinetics of isoflavones have noted similar mechanisms to flavonoids. LPH on the enterocyte cell membrane is thought to play a key role in the removal of sugar from isoflavone glycosides. However, in contrast to the flavonoid glycosides, there is no evidence that isoflavone glycosides are transported by SGLT-1 (or other transmembrane transporters) into the enterocyte cytoplasm.555 Any glycoside not remaining after passage through the small intestine is converted to the aglycone by colonic bacteria.
Contradictory results have been recorded for the differences in bioavailability between isoflavone aglycones and glycosides.556 However, a review of 16 human pharmacokinetic studies determined that genistin (the glycoside) was about 60% more bioavailable than genistein (the aglycone). Similarly, daidzin was around 80% more bioavailable than daidzein.557 These reviewers stated that some comparative studies used different food sources to compare aglycone versus glycoside bioavailability (for example, comparing tempeh with soya bean pieces), yielding contradictory results that were confounded by food matrix effects. Both reviews confirmed that isoflavones possess substantially higher relative bioavailabilities than flavonoids.556,557
Equol production was significantly higher after ingestion of daidzin than daidzein, but this has not been confirmed in every study, again possibly because of food matrix effects. Genistin and daidzin exhibit relatively similar bioavailabilities, as do genistein and daidzein. It was thought that the greater urinary excretion of daidzein metabolites reflected its greater bioavailability compared with genistein. However, the explanation for this finding is that a greater fraction of genistein is eliminated via bile.556,557
The nature of isoflavone metabolites in plasma is the same after glycoside or aglycone ingestion. Glycosides are not found. Unconjugated aglycones represent about 5% of the total level in plasma, together with their main metabolites the 7-O-glucuronides and 4′-O-glucuronides, with smaller proportions of sulphate esters. Additional metabolites include equol, dihydroequol and dihydrogenistein. Equol itself possesses good bioavailability and is excreted as the glucuronide.555,556
Saponins
If a saponin exhibits good fat solubility it can be absorbed unchanged in significant quantities in the small intestine. This is the case for many cardiac glycosides, a related chemical group. If saponins are not absorbed, they will pass to the large intestine where the gut flora will convert them to the sapogenin (aglycone). The sapogenin usually has better lipid solubility and will be absorbed to some extent. Hence, in these cases the saponin acts as a pro-drug. Since the bioactivity of saponins may be due to their aglycone, the extrapolation of in vitro results for saponins is potentially unreliable.
Glycyrrhizin (GL) is a triterpenoid saponin with hepatoprotective activity in vitro and by injection. It also has antiviral activity, even against HIV-1. This has led to suggestions that oral doses of licorice might be used as a systemic antiviral treatment or for hepatoprotective activity. Early pharmacokinetic experiments with licorice or GL demonstrated that the aglycone glycyrrhetinic acid (GA) was the predominant form absorbed into the bloodstream after oral doses.558,559 GL is converted to GA by human intestinal flora.560 Some GL may be absorbed, although this could also be recycled GA-glucuronide being misread as GL on chromatograms.558,561 So licorice may possess antiviral activity after oral doses, but only if the GA-glucuronide or GA itself possesses this activity. GA has been shown to be more hepatoprotective than GL,562 so licorice may also prove to be a valuable hepatoprotective herb (this awaits clinical confirmation for oral doses). (For a further discussion of the pharmacokinetics of glycyrrhizin, with a focus on the human data, see the licorice monograph.)
Other triterpenoid saponins appear to follow a similar pathway, for example the ginsenosides.563 The bacterial metabolism and subsequent pharmacokinetics of ginsenosides are complex, because these saponins typically contain several sugars bonded at various positions on the triterpenoid structure. Hence, metabolism by the gut flora usually occurs in several stages as sugars are progressively stripped from the molecule. (For a discussion of the key issues pertaining to the pharmacokinetics of the ginsenosides, see the ginseng monograph.) Some studies on the bioavailability of steroidal saponins have also been published. Ruscus extract (1 g), containing 60 mg degluconeoruscin and other steroidal saponins, was orally administered to three human volunteers.564 Plasma analysis showed significant absorption: degluconeoruscin concentrations peaked after about 90 minutes. Hence, some steroidal saponins may therefore show good bioavailability (this work does need to be repeated). However, others probably follow the same pattern as glycyrrhizin; the aglycone formed after colonic bacterial metabolism is the absorbed form. This is supported by extensive historical work on cardioactive glycosides showing that some glycosides, such as digitoxin, are quantitatively absorbed (the oral dose of digitoxin is the same as the intravenous dose). In contrast, ouabain (from Strophanthus) exhibited poor and erratic oral absorption and was only given by injection.
Tannins and catechins
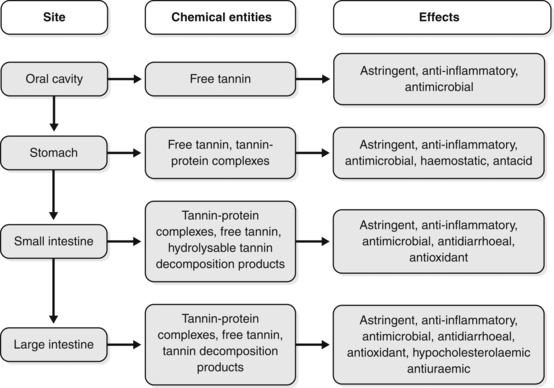
Because of their large size, high affinity to bond with proteins and poor lipid solubility, tannins have negligible bioavailability as such. Hence, the activity of tannins (and the herbs that contain them) should be explained in terms of local effects. This poor bioavailability of intact tannins is important, since hydrolysable tannins absorbed into the bloodstream can cause hepatotoxicity. Many common herbs would be poisonous if tannins were highly bioavailable. Also tannins injected subcutaneously cause cancers, so it is also fortunate that they do not penetrate the skin. Breakdown products of tannins produced in the colon by gut flora (and perhaps spontaneously in the small intestine in the case of hydrolysable tannins) are absorbed and probably explain many of the modern uses of tannin and OPC-containing herbs (see previous).
Early in vitro experiments found that ellagic acid was liberated from condensed tannins at pH 7 to 8 (not at pH 2) and also by microflora when in contact with caecum contents (ellagic acid has antioxidant and anticancer properties).565 About 95% of orally administered tannic acid is decomposed (as assessed by faecal excretion).566 Tannic acid is a hydrolysable tannin that probably releases gallic acid and other compounds on decomposition. Condensed tannins, OPCs and green tea polyphenols show more complex decomposition patterns, but microflora again produce smaller bioavailable phenolic compounds (see below).567
The probable behaviour of tannins as they pass through the digestive tract is summarised in Figure 2.12. Apart from antioxidant activity and other systemic effects from the decomposition products of tannins, all the other possible effects are due to local activity at the designated site. The remote astringency and antihaemorrhagic properties sometimes attributed to oral doses of tannin-containing herbs must be in doubt (in other words, tannins given orally will not, for example, astringe lung tissue or staunch uterine bleeding, unless such effects are due to their smaller breakdown products).
Because of their widespread occurrence in many foods and beverages (for example berries, nuts and wine), the bioavailability and metabolism of ellagitannins has been the subject of several studies and a 2010 review.568 The review noted the following:
• In vitro digestion studies have shown that in general ellagitannins are stable under stimulated stomach conditions
• Under the physiological conditions of the small intestine (neutral to mild alkaline pH) there is hydrolysis and release of free ellagic acid
• Microbial metabolites of ellagic acid are then formed, mainly urolithins (A to D), which are largely absorbed and glucuronidated by enterocytes and in the liver (in both humans and rodents)
• Low levels of intact ellagitannin and ellagic acid may also be absorbed, although results are inconsistent
• In human studies large interindividual variability has been observed in levels of urolithins (probably related to gut flora variability)
• The urolithins undergo enterohepatic circulation and their conjugates are the main metabolites detected in plasma and urine.
As stated earlier, OPCs are a part of the archetypal group known as condensed tannins. Products high in OPCs, such as pine bark and grapeseed extracts, enjoy enormous popularity as antioxidants and even cure-alls. This popularity was originally based on the work of the French scientist Masquelier, although there is now an impressive body of accumulated clinical data (see previous).
One feature of the early work is the reputed high bioavailability of OPCs, which were even said to cross the blood-brain barrier. Masquelier conducted pharmacokinetic studies on OPCs using radioactively labelled compounds. On the basis of these studies, he concluded that OPCs have good bioavailability and cross the blood-brain barrier. However, the bioavailability of OPCs observed by Masquelier was probably that of their smaller decomposition products, since his study only measured radioactivity, not the chemical entity carrying that radioactivity.
This assertion was certainly supported by a 2005 review556 which concluded that polymeric procyanidins (condensed tannins) are not absorbed as such and even the absorption of OPC dimers was very minor, about 100-fold lower than the flavonol monomers (catechin and epicatechin).556 The review pointed out that commercial extracts such as those from grape seed and pine bark contain a substantial percentage of monomers that could be largely responsible for the observed therapeutic effects. In addition, gut flora metabolites of OPCs might also deliver biological effects. Similar to flavonoids, OPCs are degraded by the microflora into various aromatic acids such as m-hydroxyphenylpropionic acid, m-hydroxyphenylacetic acid and their p-hydroxy isomers.
A rat study found that the extent of degradation into aromatic acids decreased as the degree of polymerisation increased. In other words, tannins will tend to resist microbial degradation (presumably because they tend to inactivate the very enzymes that might decompose them).
An in vitro study published since the review found that procyanidin B dimers are metabolised by human gut bacteria to yield 2-(3,4-dihydroxyphenyl) acetic acid and 5-(3,4-dihydroxyphenyl)-gamma-valerolactone (DHPV) as the major metabolites.569 This finding was corroborated by an investigation in 11 healthy volunteers given a single dose of 300 mg of pine bark extract.570 Catechin, taxifolin, caffeic acid, ferulic acid and DHPV were the major compounds present in plasma, together with 10 unknown compounds. Analysis of steady state plasma samples after chronic dosing revealed significant phase II metabolism.
Green tea is rich in polyphenols such as epigallocatechin (EGC) and epigallocatechin gallate (EGCG). Black tea is fermented green tea. During fermentation, the simple polyphenols undergo polymerisation leading to more complex molecules, such as theaflavins and therarubigens (MW 500 to 3000). Green and black tea have marked antioxidant activity in vitro, but green tea is about five times more potent than black. Adding milk has no effect. Early human experiments using oral doses demonstrated that:571
• green tea and black tea cause a significant increase in the antioxidant activity of plasma
• green tea is only about 50% stronger than black tea in vivo
• the effect is rapid, peaking at about 30 minutes after consumption for green tea and 50 minutes for black tea
• in contrast to the in vitro study, adding milk completely destroys this effect in people.
One possible explanation is that the tea polyphenols undergo spontaneous decomposition in the gut and the smaller antioxidant molecules are then absorbed. This would explain the similar activities of green and black tea in vivo. Adding milk causes protein binding, which would inhibit this decomposition. This implies that only tea without milk will render significant antioxidant activity in the bloodstream. However, this study did not monitor plasma antioxidant activity after more than a few hours. It is possible that the tannin-protein complexes formed after milk is added are decomposed further down in the gut and bacterial action on the liberated tannins (or just spontaneous breakdown) leads to absorption of antioxidant phenolics from the colon into the bloodstream.
Another possible explanation is that the observed antioxidant effects in plasma were due to absorption of unchanged tea phenolics. Both EGC and EGCG have been detected in the plasma of healthy human volunteers 90 minutes after they consumed capsules containing green tea extract.572 However, as might be expected for such large polar molecules, levels detected corresponded to only 0.2% to 2.0% of the ingested amount.
The 2005 review quoted above reflected that bioavailability differs markedly among catechins.556 Galloylation of catechins reduces their absorption. Epigallocatechin (EGC) is methylated on absorption, with 4′-O-methyl-epigallocatechin accounting for 30% to 40% of the total metabolites. EGCG is also methylated, as is catechin itself. EGCG is the only green tea polyphenol present in plasma mainly as the free form, although its overall bioavailability is low (about one-third of catechin and one-tenth of EGC). The other catechins are highly conjugated with glucuronic acid and/or sulphate. Microbial metabolites similar to those found after ingestion of herbal OPC extracts, namely various valerolactone derivatives (mostly in conjugated forms) were identified in the plasma and urine of human volunteers after ingestion of green tea. These metabolites accounted for 6% to 39% of ingested EGC and epicatechin and were 8 to 25 times the levels measured for the unchanged compounds. Galloylated catechins are only eliminated via the bile. These findings were corroborated in a later 3-month study in human volunteers.573
Polysaccharides
In some modern herbal literature, great emphasis is placed on the role of polysaccharides as immune-enhancing agents, particularly in the context of herbs such as Echinacea and the medicinal mushrooms. However, the main evidence for this is in vitro research. Polysaccharides are polymers based on sugars and uronic acids. They are found in all plants, especially as a component of the cell wall. However, some plants particularly accumulate polysaccharides. Any herbal extract prepared in 50% ethanol or stronger will not contain significant quantities of polysaccharides because of their insolubility in ethanol. Since they are large water-soluble molecules, which may even carry an ionic charge, polysaccharides will have low (but not zero) bioavailability. Pharmacokinetic considerations therefore dictate that if a herb is to be used as a source of polysaccharides it must be rich in these compounds, be prepared in a way to preserve or extract the polysaccharides and be administered in sufficient doses to compensate for the poor bioavailability. Such considerations will only apply in special cases and probably do not apply for oral doses of any Echinacea preparation (examples might include high doses of aqueous extracts of medicinal mushrooms and Astragalus). Unabsorbed polysaccharides will pass into the large intestine where they are broken down by bowel flora (and may have an effect on flora balance).
Whole-leaf Aloe vera preparations appear to be one good source of active polysaccharides, provided they are prepared to contain high levels of acemannan (about 1%). Acemannan is a polysaccharide found under the skin in Aloe vera leaves and is often not present in Aloe gel or juice preparations, because the outer leaf is not incorporated or enzymes used during manufacture have destroyed the acemannan. Doses of 50 to 100 mL/day of this concentrate can provide significant doses of acemannan. In a little-known, open, uncontrolled clinical study, 29 AIDS patients received Aloe vera whole-leaf juice, essential fatty acids and nutrients.574 The Aloe dose was the equivalent of 1200 mg/day of acemannan. Karnofsky scores improved in 100% of these patients after 180 days. Although this study had many design flaws, it does suggest that this type of preparation as a source of polysaccharides is worthy of further study.
While medicinal mushroom polysaccharide preparations such as PSK from Coriolus versicolor show activity after oral doses,575 others such as lentinan from Lentinus edodes are clinically administered by injection, presumably because of poor oral bioavailability.576 However, an oral formulation of lentinan (superfine dispersed lentinan) has recently become available.577
Optimising efficacy
Hopefully the above discussion demonstrates that knowledge of factors influencing bioavailability can lead to the more effective use of herbal medicines. In particular, the bowel flora characteristics that can optimise the efficacy of many herbal treatments need to be better understood, as highlighted in two recent reviews.578,579 This factor probably underlines the importance of a wholesome diet and adequate fibre intake, which will lead to healthy bowel flora. Other issues to be considered in the context of optimising efficacy include the following:
 Lipophilic components will probably be better absorbed with a high fat meal and polar (hydrophilic) compounds will probably be better absorbed with a low-fat meal
Lipophilic components will probably be better absorbed with a high fat meal and polar (hydrophilic) compounds will probably be better absorbed with a low-fat meal• saponins can be used to improve absorption
• some foods can be used to inhibit gut biotransformation, for example grapefruit juice
• the frequency of dosage should be based on bioavailability and metabolism.
References
1. Munson PL, Muller RA, Beese GR. Principles of Pharmacology. Basic Concepts and Clinical Applications. New York: Chapman and Hall, 1995. pp. 1–5
2. Currie HA, Perry CC. Chemical evidence for intrinsic ‘Si’ within Equisetum cell walls. Phytochemistry. 2009;70(17–18):2089–2095.
3. Vyas S, Collin SM, Bertin E, et al. Leaf concentrate as an alternative to iron and folic acid supplements for anaemic adolescent girls: a randomised controlled trial in India. Public Health Nutr. 2009;13(3):418–423.
4. Upton R. Classical bontanical pharmacognosy: from Dioscorides to modern herbal medicines. J Am Herbalists Guild. 2010;9(2):47–52.
5. Ganora L. Herbal Constituents: Foundations of Phytochemistry. Colorado: Herbalchem Press, 2008.
6. Pengelly A. The Constituents of Medicinal Plants: An Introduction to the Chemistry and Therapeutics of Herbal Medicine, 2nd ed. Australia: Allen & Unwin, 2004.
7. Baker ME. Endocrine activity of plant–derived compounds: an evolutionary perspective. Proc Soc Exp Biol Med. 1995;208:131–138.
8. Croteau R, Kutchan TM, Lewis NG. Natural products (secondary metabolites). In: Buchanan BB, Gruissem W, Jones RL, eds. Biochemistry and Molecular Biology of Plants. USA: American Society of Plant Physiologists, 2000.
9. Firn RD, Jones CG. A Darwinian view of metabolism: molecular properties determine fitness. J Exp Bot. 2009;60(3):719–726.
10. Macías, Galindo JCG, Molinillo JMG, eds. Allelopathy: Chemistry and Mode of Action of Allelochemicals. USA: CRC Press, 2004.
11. Efferth T, Koch E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr Drug Targets. 2011;12(1):122–132.
12. Laks PE. Wood preservation as trees do it. Scottish Forestry. 1991;45(4):275–284.
13. Wink M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry. 2003;64(1):3–19.
14. Castells E, Penuelas J. Towards a global theory of chemical defense: the case of alkaloids (French). Orsis. 1997;12:141–161.
15. Oleszek W. Glucosinolates: occurrence and ecological significance (Polish). Wiad Botaniczne. 1995;39(1–2):49–58.
16. Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23(5):283–333.
17. Bennett RN, Wallsgrove RM. Tansley review no. 72: Secondary metabolites in plant defence mechanisms. New Phytol. 1994;127(4):617–633.
18. Dixon RA, Lamb CJ, Masoud S, et al. Metabolic engineering: prospects for crop improvement through the genetic manipulation of phenylpropanoid biosynthesis and defense responses: a review. Gene. 1996;179(1):61–71.
19. Inderijt. Plant phenolics in allelopathy. Bot Rev. 1996;62(2):186–202.
20. Langenheim HJ. Higher plant terpenoids: a phytocentric overview of their ecological roles. J Chem Ecol. 1994;20(6):1223–1280.
21. Macías, Galindo JCG, Molinillo JMG, eds. Allelopathy: Chemistry and Mode of Action of Allelochemicals. USA: CRC Press, 2004.
22. Callaway R. Positive Interactions and Interdependence in Plant Communities. The Netherlands: Springer, 2007.
23. Sharma HM. Phytochemical synergism: beyond the active ingredient model. Altern Ther Clin Pract. 1997;4:91–96.
24. Heinrich M, Barnes J, Gibbons S, et al. Fundamentals of Pharmacognosy and Phytotherapy. London: Elsevier, 2004. p. 161
25. Berenbaum MC. What is synergy? Pharmacol Rev. 1989;41(2):93–141.
26. Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8(5):401–409.
27. Houghton P. Synergy and polyvalence: paradigms to explain the activity of herbal products. In Houghton P, Mukherjee PK, eds.: Evaluation of Herbal Medicinal Products. Perspectives on Quality, Safety and Efficacy, 1st ed, London: Pharmaceutical Press, 2009.
28. Kisa K, Sasaki K, Yamauchi K, et al. Potentiating effect of sennoside C on purgative activity of sennoside A in mice. Planta Med. 1981;42(3):302–303.
29. Nho CW, Jeffery E. The synergistic upregulation of phase II detoxification enzymes by glucosinolate breakdown products in cruciferous vegetables. Toxicol Appl Pharmacol. 2001;174(2):146–152.
30. Kiuchi F, Goto Y, Sugimoto N, et al. Nematocidal activity of turmeric: synergistic action of curcuminoiods. Chem Pharm Bull. 1993;41(9):1640–1643.
31. Onawunmi GO, Yisak W, Ogunlana EO. Antibacterial constituents in the essential oil of Cymbopogon citratus (DC.) Stapf. J Ethnopharmacol. 1984;12:279–286.
32. Ye XL, Huang WW, Chen Z, et al. Synergetic effect and structure–activity relationship of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors from Crataegus pinnatifida Bge. J Agric Food Chem. 2010;58(5):3132–3138.
33. Gao JL, He TC, Li YB, et al. A traditional Chinese medicine formulation consisting of Rhizoma corydalis and Rhizoma curcumae exerts synergistic anti-tumor activity. Oncol Rep. 2009;22(5):1077–1083.
34. Wang XN, Han X, Xu LN, et al. Enhancement of apoptosis of human hepatocellular carcinoma SMMC-7721 cells through synergy of berberine and evodiamine. Phytomedicine. 2008;15(12):1062–1068.
35. Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16(1):97–110.
36. Savelev SU, Okello EJ, Perry EK. Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother Res. 2004;18(4):315–324.
37. Greay SJ, Carson C, Beilharz et al. Anticancer activity of tea tree oil. RIRDC Publication No. 10/060 2010.
38. Eder M, Mehnert W. Bedeutung pflanzlicher Begleitstoffe in Extrakten. Pharmazie. 1998;53(5):285–293.
39. Keung W, Lazo O, Kunze L, et al. Potentiation of the bioavailability of daidzin by an extract of Radix puerariae. Proc Natl Acad Sci USA. 1996;93:4284–4288.
40. Vinson JA, Bose PB. Comparative bioavailability to humans of ascorbic acid alone or in a citrus extract. Am J Clin Nutr. 1988;48:601–604.
41. Vissers MC, Bozonet SM, Pearson JF, et al. Dietary ascorbate intake affects steady state tissue concentrations in vitamin C-deficient mice: tissue deficiency after suboptimal intake and superior bioavailability from a food source (kiwifruit). Am J Clin Nutr. 2011;93(2):292–301.
42. Zuo F, Zhou ZM, Zhang Q, et al. Pharmacokinetic study on the multi–constituents of Huangqin–Tang decoction in rats. Biol Pharm Bull. 2003;26(7):911–919.
43. Butterweck V, Petereit F, Winterhoff H, et al. Solubilized hypericin and pseudohyerpicin from Hypericum perforatum exert antidepressant activity in the forced swimming test. Planta Med. 1998;64(4):291–294.
44. Butterweck V, Jurgenliemk G, Nahrstedt A, et al. Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med. 2000;66(1):3–6.
45. Calapai G, Crupi A, Firenzuoli F, et al. Effects of Hypericum perforatum on levels of 5-hydroxytryptamine, noradrenaline and dopamine in the cortex, diencephalon and brainstem of the rat. J Pharm Pharmacol. 1999;51(6):723–728.
46. Butterweck V, Christoffel V, Nahrstedt A, et al. Step by step removal of hyperforin and hypericin: activity profile of different Hypericum preparations in behavioral models. Life Sci. 2003;73(5):627–639.
47. Capasso A, Sorrentino L. Pharmacological studies on the sedative and hypnotic effect of Kava kava and Passiflora extracts combination. Phytomedicine. 2005;12(1):39–45.
48. Gertsch J. Botanical drugs, synergy, and network pharmacology: forth and back to intelligent mixtures. Planta Med. 2011;77(11):1086–1098.
49. Hopkins AL. Network pharmacology. Nat Biotechnol. 2007;25(10):1110–1111.
50. Ulrich–Merzenich G, Panek D, Zeitler H, et al. New perspectives for synergy research with the ‘omic’-technologies. Phytomedicine. 2009;16(6–7):495–508.
51. Kolodziej H. Fascinating metabolic pools of Pelargonium sidoides and Pelargonium reniforme, traditional and phytomedicinal sources of the herbal medicine Umckaloabo. Phytomedicine. 2007;14(suppl 6):9–17.
52. Agbabiaka TB, Guo R, Ernst E. Pelargonium sidoides for acute bronchitis: a systematic review and meta-analysis. Phytomedicine. 2008;15(5):378–385.
53. Matthys H, Eisebitt R, Seith B, et al. Efficacy and safety of an extract of Pelargonium sidoides (EPs 7630) in adults with acute bronchitis. A randomised, double blind, placebo-controlled trial. Phytomedicine. 2003;10(suppl 4):7–17.
54. Patroni C. Aspirin as an antiplatelet drug. NEJM. 1994;330(18):1287–1294.
55. Lamaison JL, Petitjean–Freyet C, Carnat A. Lamiacées médicinales à propriétés antioxydantes, sources potentielles d’acide rosmarinique. Pharma Acta Helv. 1991;66(7):185–188.
56. Auf’mkolk M, Amir SM, Kubota K, et al. The active principles of plant extracts with antithyrotropic activity: oxidation products of derivatives of 3,4-dihydroxycinnamic acid. Endocrinology. 1985;116(5):1677.
57. Chlabicz J, Galasinski W. The components of Melissa officinalis L. that influence protein biosynthesis in-vitro. J Pharm Pharmacol. 1986;38(11):791–794.
58. Csiszar A. Anti-inflammatory effects of resveratrol: possible role in prevention of age–related cardiovascular disease. Ann N Y Acad Sci. 2011;1215:117–122.
59. Richard T, Pawlus AD, Iglésias ML, et al. Neuroprotective properties of resveratrol and derivatives. Ann N Y Acad Sci. 2011;1215:103–108.
60. Szkudelski T, Szkudelska K. Anti-diabetic effects of resveratrol. Ann N Y Acad Sci. 2011;1215:34–39.
61. Pezzuto JM. The phenomenon of resveratrol: redefining the virtues of promiscuity. Ann N Y Acad Sci. 2011;1215:123–130.
62. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506.
63. Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009;53(1):115–128.
64. Agarwal B, Baur JA. Resveratrol and life extension. Ann N Y Acad Sci. 2011;1215:138–143.
65. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342.
66. la Porte C, Voduc N, Zhang G, et al. Steady-state pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin Pharmacokinet. 2010;49(7):449–454.
67. Lechtenberg M, Nahrstedt A. Cyanogenic glycosides. In: Ikan R, ed. Naturally Occurring Glycosides. UK: John Wiley, 1999. Chapter 5
68. Miller KW, Anderson JL, Stoewsand GS. Amygdalin metabolism and effect on reproduction of rats fed apricot kernels. J Toxicol Environ Health. 1981;7(3–4):457–467.
69. Moertel CG, Ames MM, Kovach JS, et al. A pharmacologic and toxicological study of amygdalin. JAMA. 1981;245(6):591–594.
70. Adewusi SR, Oke OL. On the metabolism of amygdalin. 1. The LD50 and biochemical changes in rats. Can J Physiol Pharmacol. 1985;63(9):1080–1083.
71. Vetter J. Plant cyanogenic glycoides. Toxicon. 2000;38(1):11–36.
72. Fenselau C, Pallante S, Batzinger RP, et al. Mandelonitrile beta-glucuronide: synthesis and characterization. Science. 1977;198(4317):625–627.
73. Dorr RT, Paxinos J. The current status of laetrile. Ann Intern Med. 1978;89(3):389–397.
74. Hill GJ, II., Shine TE, Hill HZ, et al. Failure of amygdalin to arrest B16 melanoma and BW5147 AKR leukemia. Cancer Res. 1976;36(6):2102–2107.
75. Moertel CG, Fleming TR, Rubin J, et al. A clinical trial of amygdalin (Laetrile) in the treatment of human cancer. NEJM. 1982;306(4):201–206.
76. Hill HZ, Backer R, Hill GJ, II. Blood cyanide levels in mice after administration of amygdalin. Biopharm Drug Dispos. 1980;1(4):211–220.
77. Syrigos KN, Rowlinson–Busza G, Epenetos AA. In vitro cytotoxicity following specific activation of amygdalin by beta-glucosidase conjugated to a bladder cancer-associated monoclonal antibody. Int J Cancer. 1998;78(6):712–719.
78. Cliff J, Muquingue H, Nhassico D, et al. Konzo and continuing cyanide intoxication from cassava in Mozambique. Food Chem Toxicol. 2011;49(3):631–635.
79. Elli M, Cattivelli D, Soldi S, et al. Evaluation of prebiotic potential of refined psyllium (Plantago ovata) fiber in healthy women. J Clin Gastroenterol. 2008;42(suppl 3 Pt 2):S174–S176.
80. Fujimori S, Tatsuguchi A, Gudis K, et al. High dose probiotic and prebiotic cotherapy for remission induction of active Crohn’s disease. J Gastroenterol Hepatol. 2007;22(8):1199–1204.
81. Rodríguez-Cabezas ME, Gálvez J, Camuesco D, et al. Intestinal anti-inflammatory activity of dietary fiber (Plantago ovata seeds) in HLA-B27 transgenic rats. Clin Nutr. 2003;22(5):463–471.
82. Obolentseva GV, Khadzhai YI, Vidyukova AI, et al. Effect of some natural substances on ulceration of the rat stomach caused by acetylsalicylic acid. Bull Exp Biol Med. 1974;77:256–257.
83. Rafatullah S, Al-Yahya MA, Al-Said MS, et al. Gastric anti-ulcer and cytoprotective effects of Cyamopsis tetragonolaba (‘Guar’) in rats. Int J Pharmacog. 1994;32(2):163–170.
84. Blackburn NA, Johnson IT. The influence of guar gum on the movements of insulin, glucose and fluid in rat intestine during perfusion in vivo. Pflügers Archiv. 1983;397:144–148.
85. Schmidgall J, Schnetz E, Hensel A. Evidence for bioadhesive effects of polysaccharides and polysaccharide-containing herbs in an ex vivo bioadhesion assay on buccal membranes. Planta Med. 2000;66(1):48–53.
86. Deters A, Zippel J, Hellenbrand N, et al. Aqueous extracts and polysaccharides from Marshmallow roots (Althea officinalis L.): cellular internalisation and stimulation of cell physiology of human epithelial cells in vitro. J Ethnopharmacol. 2010;127(1):62–69.
87. Morcos SR, El-Baradie AA. Fenugreek mucilage and its relation to the reputed laxative action of this seed. Egypt J Chem. 1959;2(1):163–168.
88. Stevens J, Levitsky DA, Van Soest PJ, et al. Effect of psyllium gum and wheat bran on spontaneous energy intake. Am J Clin Nutr. 1987;46:812–817.
89. Voinchet O, Mouchet A. Obstruction de l’oesophage par mucilage. Nouvelle Presse Médicale. 1974;3(19):1223–1225.
90. [No authors listed]. Health Canada Advises Canadians that Natural Health Products containing Glucomannan May Cause Serious Choking if Used with Insufficient Fluid. Advisory No. 2010–16, January 29, 2010.
91. Nosalova G, Strapkova A, Kardosova A, et al. Antitussive Wirkung des Extraktes und der Polysaccharide aus Eibisch (Althaea officinalis L., var. robusta). Pharmazie. 1992;47:224–226.
92. Sutovska M, Nosalova G, Franova S, et al. The antitussive activity of polysaccharides from Althaea officinalis l., var. Robusta, Arctium lappa L., var. Herkules, and Prunus persica L., Batsch. Bratisl Lek Listy. 2007;108(2):93–99.
93. Sutovská M, Nosálová G, Sutovský J, et al. Possible mechanisms of dose-dependent cough suppressive effect of Althaea officinalis rhamnogalacturonan in guinea pigs test system. Int J Biol Macromol. 2009;45(1):27–32.
94. Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung. 2010;188(suppl 1):S81–S86.
95. Pauwels A, Blondeau K, Dupont L, et al. Cough and gastroesophageal reflux: from the gastroenterologist end. Pulm Pharmacol Ther. 2009;22(2):135–138.
96. Thakkar K, Boatright RO, Gilger MA, et al. Gastroesophageal reflux and asthma in children: a systematic review. Pediatrics. 2010;125(4):e925–e930.
97. Barberio G, Ruggeri C, Pajno GB, et al. [Gastro-esophageal reflux and asthma. Clinical experience]. Minerva Pediatr. 1989;41(7):363–366.
98. Giudicelli R, Dupin B, Surpas P, et al. [Gastroesophageal reflux and respiratory manifestations: diagnostic approach, therapeutic indications and results]. Ann Chir. 1990;44(7):552–554.
99. Larrain A, Carrasco E, Galleguillos F, et al. Medical and surgical treatment of nonallergic asthma associated with gastroesophageal reflux. Chest. 1991;99(6):1330–1335.
100. Morice AH. Is reflux cough due to gastroesophageal reflux disease or laryngopharyngeal reflux? Lung. 2008;186(suppl 1):S103–S106.
101. Avidan B, Sonnenberg A, Schnell TG, et al. Temporal associations between coughing or wheezing and acid reflux in asthmatics. Gut. 2001;49(6):767–772.
102. Kollarik M, Brozmanova M. Cough and gastroesophageal reflux: insights from animal models. Pulm Pharmacol Ther. 2009;22(2):130–134.
103. Undem BJ, Carr MJ. Targeting primary afferent nerves for novel antitussive therapy. Chest. 2010;137(1):177–184.
104. Roberts DCK, Truswell AS, Bencke A, et al. The cholesterol-lowering effect of a breakfast cereal containing psyllium fibre. Med J Aust. 1994;161:660–664.
105. Yu LL, Lutterodt H, Cheng Z. Beneficial health properties of psyllium and approaches to improve its functionalities. Adv Food Nutr Res. 2009;55:193–220.
106. Leeds AR. Psyllium – a superior source of soluble dietary fibre. Food Aust. 1995;47(suppl 2):S2–S4.
107. Frati-Munari AC, Flores-Garduno MA, Ariza-Andraca R, et al. Effect of different doses of Plantago psyllium mucilage on the glucose tolerance test. Arch Invest Med (Mexico). 1989;20(2):147–152.
108. Lis-Balchin M, Deans SG, Eaglesham E. Relationship between bioactivity and chemical composition of commercial essential oils. Flavour Fragrance J. 1998;13:98–104.
109. Römmelt H, Zuber A, Dirnagl K, et al. Zur Resorption von Terpenen aus Badezusätzen. MMW Fortschr Med. 1974;116(11):537–540.
110. Woronuk G, Demissie Z, Rheault M, et al. Biosynthesis and therapeutic properties of Lavandula essential oil constituents. Planta Med. 2011;77(1):7–15.
111. Janssen AM, Scheffer JJC, Baerheim Svendsen A. Antimicrobial activity of essential oils: a 1976–1986 literature review. Aspects of the test methods. Planta Med. 1987;53:395–398.
112. Bakkali F, Averbeck S, Averbeck D, et al. Biological effects of essential oils – a review. Food Chem Toxicol. 2008;46(2):446–475.
113. Janssen AM, Chin NLJ, Scheffer JJC, et al. Screening for antimicrobial activity of some essential oils by the agar overlay technique. Pharm Weekbl Sci. 1986;8:289–292.
114. Baser KH. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr Pharm Des. 2008;14(29):3106–3119.
115. Soderberg TA, Johansson A, Gref R. Toxic effects of some conifer resin acids and tea tree oil on human epithelial and fibroblast cells. Toxicology. 1996;107(2):99–109.
116. Cox SD, Gustafson JE, Mann CM, et al. Tea tree oil causes K+ leakage and inhibits respiration in Escherichia coli. Lett Appl Microbiol. 1998;26(5):355–358.
117. Moleyar V, Narasimham P. Antibacterial activity of essential oil components. Int J Food Microbiol. 1992;16(4):337–342.
118. Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 2007;21(4):308–323.
119. Pattnaik S, Subramanyam VR, Bapaji M, et al. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios. 1997;89(358):39–46.
120. Carson CF, Riley TV. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J Appl Bacteriol. 1995;78(3):264–269.
121. Williams LR, Home VN. Factors determining the quality of tea tree oil in formulations for clinical use. Cosmet Aerosol Toiletries Aust. 1995;9(2):14–18.
122. Reichling J, Schnitzler P, Suschke U, et al. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties – an overview. Forsch Komplementmed. 2009;16(2):79–90.
123. Tong MM, Altman PM, Barnetson RS. Tea tree oil in the treatment of tinea pedis. Aust J Dermatol. 1992;33(3):145–149.
124. Bassett IB, Pannowitz DL, Barnetson RS. A comparative study of tea-tree oil versus benzoylperoxide in the treatment of acne. MJA. 1990;153(8):455–458.
125. Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19(1):50–62.
126. Enshaieh S, Jooya A, Siadat AH, et al. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: a randomized, double blind placebo-controlled study. Indian J Dermatol Venereol Leprol. 2007;73(1):22–25.
127. Carson CF, Ashton L, Dry L, et al. Melaleuca alternifolia (tea tree) oil gel (6%) for the treatment of recurrent herpes labialis. J Antimicrob Chemother. 2001;48(3):450–451.
128. Barker SC, Altman PM. A randomised, assessor blind, parallel group comparative efficacy trial of three products for the treatment of head lice in children – melaleuca oil and lavender oil, pyrethrins and piperonyl butoxide, and a ‘suffocation’ product. BMC Dermatol. 2010;10:6.
129. Heukelbach J, Canyon DV, Oliveira FA, et al. In vitro efficacy of over-the-counter botanical pediculicides against the head louse Pediculus humanus var capitis based on a stringent standard for mortality assessment. Med Vet Entomol. 2008;22(3):264–272.
130. Force M, Sparks WS, Ronzio RA. Inhibition of enteric parasites by emulsified oil of oregano in vivo. Phytother Res. 2000;14(3):213–214.
131. Kliks MM. Studies on the traditional herbal anthelmintic Chenopodium ambrosioides L.: ethnopharmacological evaluation and clinical field trials. Soc Sci Med. 1985;21(8):879–886.
132. Pisseri F, Bertoli A, Pistelli L. Essential oils in medicine: principles of therapy. Parassitologia. 2008;50(1–2):89–91.
133. Reiter M, Brandt W. Relaxant effects on tracheal and ileal smooth muscles of the guinea pig. ArzneimittelForschung. 1985;35(1A):408–414.
134. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68(812):841–843.
135. Giachetti D, Taddei E, Taddei I. Pharmacological activity of essential oils on Oddi’s sphincter. Planta Med. 1988;54:389–392.
136. Creamer B. Oesophageal reflux and the action of carminatives. Lancet. 1955;1:590–592.
137. Stanic G, Samarzija I, Blazevic N. Time-dependent diuretic response in rats treated with juniper berry preparations. Phytotherapy Res. 1998;12:494–497.
138. Schilcher H, Leuschner F. Studies of potential nephrotoxic effects of essential juniper oil (German). Arzneimittel-Forschung. 1997;47(7):855–858.
139. Al-Mosawi AJ. Essential oil terpenes: adjunctive role in the management of childhood urolithiasis. J Med Food. 2010;13(2):247–250.
140. Dorow P, Weiss TH, Felix R, et al. Effect of a secretolytic and a combination of pinene, limonene and cineole on mucociliary clearance in patients with chronic pulmonary obstruction. ArzneimittelForschung. 1987;37(12):1378–1381.
141. Boyd EM. Expectorants and respiratory tract fluid. Pharma Rev. 1954;6:521–542.
142. Boyd EM, Pearson GL. On the expectorant action of volatile oils. Am J Med Sci. 1946;211:602–610.
143. Boyd EM, Palmer G, Pearson GL. Is there any advantage in combining several expectorant drugs in a compound cough mixture? Can Med Assoc J. 1946;54(3):216–220.
144. Sherry E, Warnke PH. Successful use of an inhalational phytochemical to treat pulmonary tuberculosis: a case report. Phytomedicine. 2004;11(2–3):95–97.
145. Guillemain J, Rousseau A, Delaveau P. Effets neurodépresseurs de l’huile essentielle de Lavandula angustifolia Mill. Annales de Pharmaceutiques Françaises. 1989;47(6):337–343.
146. Kasper S, Gastpar M, Müller WE, et al. Efficacy and safety of silexan, a new, orally administered lavender oil preparation, in subthreshold anxiety disorder – evidence from clinical trials. Wien Med Wochenschr. 2010;160(21–22):547–556.
147. Kovar KA, Gropper D, Friess D, et al. Blood levels of 1,8-cineole and locomotor activity of mice after inhalation and oral administration of rosemary oil. Planta Med. 1987;53:315–318.
148. Haines DJ, Jr. Sassafras tea and diaphoresis. Postgrad Med. 1991;90(4):75–76.
149. de Sousa DP. Analgesic-like activity of essential oils constituents. Molecules. 2011;16(3):2233–2252.
150. Ghelardini C, Galeotti N, Salvatore G, et al. Local anaesthetic activity of the essential oil of Lavandula angustifolia. Planta Med. 1999;65(8):700–703.
151. Edris AE. Anti-cancer properties of Nigella spp. essential oils and their major constituents, thymoquinone and beta-elemene. Curr Clin Pharmacol. 2009;4(1):43–46.
152. Tisserand R, Balacs T. Essential Oil Safety: A Guide for Health Care Professionals. Edinburgh: Churchill Livingstone, 1995.
153. Macht DI. The action of the so-called emmenagogue oils on the isolated uterine strip. J Pharmacol Exp Ther. 1912;4:547–552.
154. Arnold WN. Absinthe. Sci Am. 1989;260:86–91.
155. Albert-Puleo M. Van Gogh’s vision: thujone intoxication. JAMA. 1981;246(1):42.
156. Lachenmeier DW. [Absinthe – history of dependence to thujone or to alcohol?]. Fortschr Neurol Psychiatr. 2007;75(5):306–308.
157. Rutherford T, Nixon R, Tam M, et al. Allergy to tea tree oil: retrospective review of 41 cases with positive patch tests over 4.5 years. Australas J Dermatol. 2007;48(2):83–87.
158. Pulverer G. Benzylsenföl: ein Breitbandantibiotikum aus der Kapuzinerkresse. Dtsch Med Wochenschr. 1968;93:1642–1649.
159. Sehrawat A, Singh SV. Benzyl isothiocyanate inhibits epithelial–mesenchymal transition in cultured and xenografted human breast cancer cells. Cancer Prev Res (Phila). 2011;4(7):1107–1117.
160. Langer P, Stolc V. Goitrogenic activity of allylisothiocyanate – a widespread natural mustard oil. Endocrinol. 1965;76:151–155.
161. Higdon JV, Delage B, Williams DE, et al. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–236.
162. Hayes JD, Kelleher MO, Eggleston IM. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr. 2008;47(suppl 2):73–88.
163. Herr I, Büchler MW. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev. 2010;36(5):377–383.
164. Verhoeven DT, Goldbohm RA, van Poppel G, et al. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5(9):733–748.
165. Nestle M. Broccoli sprouts as inducers of carcinogen-detoxifying enzyme systems: clinical, dietary, and policy implications. Proc Natl Acad Sci USA. 1997;94(21):11149–11151.
166. Shapiro TA, Fahey JW, Dinkova-Kostova AT, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55(1):53–62.
167. Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94(19):10367–10372.
168. Zhang Y, Talalay P, Cho CG, et al. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89(6):2399–2403.
169. Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64(9):1105–1127.
170. Kensler TW, Chen JG, Egner PA, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin–DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2605–2613.
171. Riedl MA, Saxon A, Diaz–Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009;130(3):244–251.
172. Janssen K, Mensink RP, Cox FJ, et al. Effects of the flavonoids quercetin and apigenin on hemostasis in healthy volunteers: results from an in vitro and a dietary supplement study. Am J Clin Nutr. 1998;67(2):255–262.
173. Hughes RE, Wilson HK. Flavonoids: some physiological and nutritional considerations. Prog Med Chem. 1977;14:285–301.
174. Amiel M, Barbe R. Study of the pharmacodynamic activity of daflon 500 mg. Ann Cardiol Angiol. 1998;47(3):185–188.
175. Le Devehat C, Khodabandehlou T, Vimeux M, et al. Evaluation of haemorheological and microcirculatory disturbances in chronic venous insufficiency: activity of Daflon 500 mg. Int J Microcirc Clin Exp. 1997;17(suppl 1):27–33.
176. Guilhou JJ, Fevrier F, Debure C, et al. Benefit of a 2–month treatment with a micronized, purified flavonoidic fraction on venous ulcer healing. A randomized, double blind, controlled versus placebo trial. Int J Microcirc Clin Exp. 1997;17(suppl 1):21–26.
177. Cospite M. Double blind, placebo-controlled evaluation of clinical activity and safety of Daflon 500 mg in the treatment of acute hemorrhoids. Angiology. 1994;45(6 pt 2):566–573.
178. Gohel MS, Davies AH. Pharmacological treatment in patients with C4, C5 and C6 venous disease. Phlebology. 2010;25(suppl 1):35–41.
179. Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, et al. Meta-analysis of flavonoids for the treatment of haemorrhoids. Br J Surg. 2006;93(8):909–920.
180. Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96(2–3):67–202.
181. Cazarolli LH, Zanatta L, Alberton EH, et al. Flavonoids: prospective drug candidates. Mini Rev Med Chem. 2008;8(13):1429–1440.
182. Ferrali M, Signorini C, Caciotti B, et al. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997;416(2):123–129.
183. Aviram M, Fuhrman B. Polyphenolic flavonoids inhibit macrophage-mediated oxidation of LDL and attenuate atherogenesis. Atherosclerosis. 1998;137(suppl):S45–S50.
184. Robak J, Gryglewski RJ. Bioactivity of flavonoids. Polish J Pharmacol. 1996;48(6):555–564.
185. Saiija A, Scalese M, Lanza M, et al. Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic Biol Med. 1995;19(4):481–486.
186. Van Acker SA, Tromp MN, Haenen GR, et al. Flavonoids as scavengers of nitric oxide radical. Biochem Biophys Res Commun. 1995;214(3):755–759.
187. Hu JP, Calomme M, Lasure A, et al. Structure–activity relationship of flavonoids with superoxide scavenging activity. Biol Trace Elem Res. 1995;47(1–3):327–331.
188. Tournaire C, Croux S, Maurette MT, et al. Antioxidant activity of flavonoids: efficiency of singlet oxygen (1 delta g) quenching. J Photochem Photobiol B Biol. 1993;19(3):205–215.
189. Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behaviour of flavonoids: structure–activity relationships. Free Radic Biol Med. 1997;22(5):749–760.
190. Alcaraz MJ, Ferrandiz ML. Modifications of arachidonic metabolism by flavonoids. J Ethnopharmacol. 1987;21:209–229.
191. Okuda J, Miwa I, Inagaki K, et al. Inhibition of aldose reductases from rat and bovine lenses by flavonoids. Biochem Pharmacol. 1982;31(23):3807–3822.
192. Chang WS, Lee YJ, Chiang HC. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993;13(6A):2165–2170.
193. Ruckstuhl M, Beretz A, Anton R, et al. Flavonoids are selective cyclic GMP phosphodiesterase inhibitors. Biochem Pharmacol. 1979;28:535–538.
194. Petkov E, Nikolov N, Uzunov P. Inhibitory effect of some flavonoids and flavonoid mixtures on cyclic AMP phospho diesterase activity of rat heart. Planta Med. 1981;43:183–186.
195. Pathak D, Pathak K, Singla AK. Flavonoids as medicinal agents – recent advances. Fitoterapia. 1991;62(5):371–389.
196. Pelissero C, Lenczowski MJ, Chinzi D, et al. Effects of flavonoids on aromatase activity, an in vitro study. J Steroid Biochem Mol Biol. 1996;57(3–4):215–223.
197. Divi RL, Doerge DR. Inhibition of thyroid peroxidase by dietary flavonoids. Chem Res Toxicol. 1996;9(1):16–23.
198. Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 2010;69(3):273–278.
199. Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26(5):343–356.
200. Amella M, Bronner C, Briancon F, et al. Inhibition of mast cell histamine release by flavonoids and biflavonoids. Planta Med. 1985;51:16–20.
201. Kawai M, Hirano T, Higa S, et al. Flavonoids and related compounds as anti-allergic substances. Allergol Int. 2007;56(2):113–123.
202. El Haouari M, Rosado JA. Modulation of platelet function and signaling by flavonoids. Mini Rev Med Chem. 2011;11(2):131–142.
203. Hammad HM, Abdalla SS. Pharmacological effects of selected flavonoids on rat isolated ileum: structure–activity relationship. Gen Pharmacol. 1997;28(5):767–771.
204. Ko WC, Liu PY, Chen JL, et al. Relaxant effects of flavonoids in isolated guinea pig trachea and their structure–activity relationships. Planta Med. 2003;69(12):1086–1090.
205. Kitaoka M, Kadokawa H, Sugano M, et al. Prenylflavonoids: a new class of non-steroidal phytooestrogen (Part 1). Isolation of 8-isopentenylnaringenin and an initial study on its structure–activity relationship. Planta Med. 1998;64(6):511–515.
206. Böttner M, Christoffel J, Wuttke W. Effects of long–term treatment with 8-prenylnaringenin and oral estradiol on the GH–IGF-1 axis and lipid metabolism in rats. J Endocrinol. 2008;198(2):395–401.
207. Erkkola R, Vervarcke S, Vansteelandt S, et al. A randomized, double blind, placebo-controlled, cross-over pilot study on the use of a standardized hop extract to alleviate menopausal discomforts. Phytomedicine. 2010;17(6):389–396.
208. Fremont L, Gozzelino MT, Franchi MP, et al. Dietary flavonoids reduce lipid peroxidation in rats fed polyunsaturated or monounsaturated fat diets. J Nutr. 1998;128(9):1495–1502.
209. Mota KS, Dias GE, Pinto ME, et al. Flavonoids with gastroprotective activity. Molecules. 2009;14(3):979–1012.
210. Pelzer LE, Guardia T, Osvaldo JA, et al. Acute and chronic anti-inflammatory effects of plant flavonoids. Farmaco. 1998;53(6):421–424.
211. Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 2010;69(3):273–278.
212. Galvez J, Zarzuelo A, Crespo ME, et al. Antidiarrhoeic activity of Euphorbia hirta extract and isolation of an active flavonoid constituent. Planta Med. 1993;59:333–336.
213. Elangovan V, Sekar N, Govindasamy S. Chemopreventive potential of dietary bioflavonoids against 20-methylcholanthrene-induced tumorigenesis. Cancer Lett. 1994;87:107–113.
214. Yasukawa K, Takido M, Takeuchi M, et al. Inhibitory effects of flavonol glycosides on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion. Chem Pharm Bull (Tokyo). 1990;38(3):774–776.
215. Prasad S, Phromnoi K, Yadav VR, et al. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010;76(11):1044–1063.
216. Salgueiro JB, Ardenghi P, Dias M, et al. Anxiolytic natural and synthetic flavonoid ligands of the central benzodiazepine receptor have no effect on memory tasks in rats. Pharmacol Biochem Behav. 1997;58(4):887–891.
217. Medina JH, Viola H, Wolfman C, et al. Overview – flavonoids: a new family of benzodiazepine receptor ligands. Neurochem Res. 1997;22(4):419–425.
218. Keli SO, Hertog MG, Feskens EJ. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen Study. Arch Intern Med. 1996;156(6):637–642.
219. Knekt P, Jarvinen R, Reunanen A, et al. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996;312(7029):478–481.
220. Rimm EB, Katan MB, Ascherio A, et al. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann Intern Med. 1996;125(5):384–389.
221. Arts IC, Hollman PC, Feskens EJ, et al. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74(2):227–232.
222. Sesso HD, Gaziano JM, Liu S, et al. Flavonoid intake and the risk of cardiovascular disease in women. Am J Clin Nutr. 2003;77(6):1400–1408.
223. Song Y, Manson JE, Buring JE, et al. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr. 2005;24(5):376–384.
224. Wang L, Lee IM, Zhang SM, et al. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle–aged and older women. Am J Clin Nutr. 2009;89(3):905–912.
225. Knekt P, Kumpulainen J, Järvinen R, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76(3):560–568.
226. Mollace V, Sacco I, Janda E, et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: from animal models to human studies. Fitoterapia. 2011;82(3):309–316.
227. Levy RM, Khokhlov A, Kopenkin S, et al. Efficacy and safety of flavocoxid, a novel therapeutic, compared with naproxen: a randomized multicenter controlled trial in subjects with osteoarthritis of the knee. Adv Ther. 2010;27(10):731–742.
228. Morand C, Dubray C, Milenkovic D, et al. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr. 2011;93(1):73–80.
229. Lin TS, Blum KA, Fischer DB, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol. 2010;28(3):418–423.
230. Jin F, Nieman DC, Shanely RA, et al. The variable plasma quercetin response to 12-week quercetin supplementation in humans. Eur J Clin Nutr. 2010;64(7):692–697.
231. Davis JM, Carlstedt CJ, Chen S, et al. The dietary flavonoid quercetin increases VO(2max) and endurance capacity. Int J Sport Nutr Exerc Metab. 2010;20(1):56–62.
232. Abbey EL, Rankin JW. Effect of quercetin supplementation on repeated-sprint performance, xanthine oxidase activity, and inflammation. Int J Sport Nutr Exerc Metab. 2011;21(2):91–96.
233. Bigelman KA, Fan EH, Chapman DP, et al. Effects of six weeks of quercetin supplementation on physical performance in ROTC cadets. Mil Med. 2010;175(10):791–798.
234. Cureton KJ, Tomporowski PD, Singhal A, et al. Dietary quercetin supplementation is not ergogenic in untrained men. J Appl Physiol. 2009;107(4):1095–1104.
235. Egert S, Bosy-Westphal A, Seiberl J, et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double blinded, placebo-controlled cross-over study. Br J Nutr. 2009;102(7):1065–1074.
236. Nagao M, Naokata M, Yahagi T, et al. Mutagenicities of 61 flavonoids and 11 related compounds. Environ Mutagen. 1981;3:401–419.
237. Czeczot H, Tudek B, Kusztelak J, et al. Isolation and studies of the mutagenic activity in the Ames test of flavonoids naturally occurring in medical herbs. Mutat Res. 1990;240(3):209–216.
238. Connolly JA, Morgan IM, Jackson ME, et al. The BPV-4 co-carcinogen quercetin induces cell cycle arrest and up-regulates transcription from the LCR of BPV-4. Oncogene. 1998;16(21):2739–2746.
239. Barotto NN, Lopez CB, Enyard AR, et al. Quercetin enhances pretumorous lesions in the NMU model of rat pancreatic carcinogenesis. Cancer Lett. 1998;129(1):1–6.
240. Chaumontet C, Suschetet M, Honikman-Leban E, et al. Lack of tumor-promoting effects of flavonoids: studies on rat liver preneoplastic foci and on in vivo and in vitro gap junctional intercellular communication. Nutr Cancer. 1996;26(3):251–263.
241. Wall ME, Wani MC, Manikumar G, et al. Plant antimutagenic agents, 2. Flavonoids. J Nat Prod. 1988;51(6):1084–1091.
242. Duthie SJ, Collins AR, Duthie GG, et al. Quercetin and myricetin protect against hydrogen peroxide-induced DNA damage (strand breaks and oxidised pyrimidines) in human lymphocytes. Mutat Res. 1997;393(3):223–231.
243. Zhu BT, Ezell EL, Leihr JG. Catechol-O-methyltransferase-catalyzed rapid O-methylation of mutagenic flavonoids. J Biol Chem. 1994;269(1):292–299.
244. Harwood M, Danielewska-Nikiel B, Borzelleca JF, et al. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45(11):2179–2205.
245. Nonaka GI, Sakai R, Nishioka T. Hydrolysable tannins and proanthocyanidins from green tea. Phytochemistry. 1984;23(8):1753–1755.
246. Hör M, Rimpler H, Heinrich M. Inhibition of intestinal chloride secretion by proanthocyanidins from Guazuma ulmifolia. Planta Med. 1995;61:208–212.
247. Wongsamitkul N, Sirianant L, Muanprasat C, et al. A plant-derived hydrolysable tannin inhibits CFTR chloride channel: a potential treatment of diarrhea. Pharm Res. 2010;27(3):490–497.
248. Tomczyk M, Latté KP. Potentilla – a review of its phytochemical and pharmacological profile. J Ethnopharmacol. 2009;122(2):184–204.
249. Subbotina MD, Timchenko VN, Vorobyov MM, et al. Effect of oral administration of tormentil root extract (Potentilla tormentilla) on rotavirus diarrhea in children: a randomized, double blind, controlled trial. Pediatr Infect Dis J. 2003;22(8):706–711.
250. Maity S, Vedasiromoni JR, Ganguly DK. Anti-ulcer effect of the hot water extract of black tea (Camellia sinensis). J Ethnopharmacol. 1995;46:167–174.
251. Ezaki N, Kato M, Takizawa N, et al. Pharmacological studies on Linderae umbellatae Ramus, IV. Effects of condensed tannin related compounds on peptic activity and stress-induced gastric lesions in mice. Planta Med. 1985;51(1):34–38.
252. Murakami S, Isobe Y, Kijima H, et al. Inhibition of gastric H+, K+-ATPase and acid secretion by ellagic acid. Planta Med. 1991;57:305–308.
253. Alam MS, Alam MA, Ahmad S, et al. Protective effects of Punica granatum in experimentally-induced gastric ulcers. Toxicol Mech Methods. 2010;20(9):572–578.
254. Terada A, Hara H, Nakajyo S, et al. Effect of supplements of tea polyphenols on the caecal flora and caecal metabolites of chicks. Microb Ecol Health Dis. 1993;6:3–9.
255. Yokozawa T, Fujioka, Oura H, et al. Confirmation that tannin-containing crude drugs have a uraemic toxin-decreasing action. Phytother Res. 1995;9:1–5.
256. Akah PA. Haemostatic activity of aqueous leaf extract of Ageratum conyzoides L. Int J Crude Drug Res. 1988;26(2):97–101.
257. Root-Bernstein RS. Tannic acid, semipermeable membranes and burn treatment. Lancet. 1982;2:1168.
258. Haslam E. Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J Nat Prod. 1996;9:205–215.
259. Okuda T, Yoshida T, Hatano T. Chemistry and biological activity of tannins in medicinal plants. In: In: Wagner N, Farnsworth NR, eds. Economic and Medicinal Plant Research, vol. 5. London: Academic Press; 1991:130–165.
260. Koleckar V, Kubikova K, Rehakova Z, et al. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev Med Chem. 2008;8(5):436–447.
261. Coimbra S, Castro E, Rocha-Pereira P, et al. The effect of green tea in oxidative stress. Clin Nutr. 2006;25(5):790–796.
262. Gomikawa S, Ishikawa Y, Hayase W, et al. Effect of ground green tea drinking for 2 weeks on the susceptibility of plasma and LDL to the oxidation ex vivo in healthy volunteers. Kobe J Med Sci. 2008;54(1):E62–E72.
263. Tinahones FJ, Rubio MA, Garrido-Sánchez L, et al. Green tea reduces LDL oxidability and improves vascular function. J Am Coll Nutr. 2008;27(2):209–213.
264. Erba D, Riso P, Bordoni A, et al. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J Nutr Biochem. 2005;16(3):144–149.
265. Rossi R, Porta S, Canovi B. Overview on cranberry and urinary tract infections in females. J Clin Gastroenterol. 2010;44(suppl 1):S61–S62.
266. Pappas E, Schaich KM. Phytochemicals of cranberries and cranberry products: characterization, potential health effects, and processing stability. Crit Rev Food Sci Nutr. 2009;49(9):741–781.
267. Howell AB, Botto H, Combescure C, et al. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect Dis. 2010;10:94.
268. Adhami VM, Khan N, Mukhtar H. Cancer chemoprevention by pomegranate: laboratory and clinical evidence. Nutr Cancer. 2009;61(6):811–815.
269. Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008;269(2):262–268.
270. Murthy KN, Reddy VK, Veigas JM, et al. Study on wound healing activity of Punica granatum peel. J Med Food. 2004;7(2):256–259.
271. Ducrey B, Marston A, Göhring S, et al. Inhibition of 5α-reductase and aromatase by the ellagitannins oenothein A and oenothein B from Epilobium species. Planta Med. 1997;63:111–114.
272. Mizuno T, Uchino K, Toukairin T, et al. Inhibitory effect of tannic acid sulfate and related sulfates on infectivity, cytopathic effect, and giant cell formation of human immunodeficiency virus. Planta Med. 1992;58:535–539.
273. Lamaison JL, Carnat A, Petitjean-Freytet C. Teneur en tanins et activité inhibitrice de l’élastase chez les Rosaceae. Ann Pharm Fr. 1990;48(6):335–340.
274. Kashiwada Y, Nonaka G, Nishioka I, et al. Antitumor agents, 129. Tannins and related compounds as selective cytotoxic agents. J Nat Prod. 1992;55(8):1033–1043.
275. Schwitters B, Masquelier J. OPC in Practice: Bioflavanols and Their Application, 2nd ed. Rome: Alfa Omega, 1993.
276. Maimoona A, Naeem I, Saddiqe Z, et al. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. J Ethnopharmacol. 2011;133(2):261–277.
277. Ahmed AE, Smithard R, Ellis M. Activities of enzymes of the pancreas, and the lum-en and mucosa of the small intestine in growing broiler cockerels fed on tannin-containing diets. B J Nutr. 1991;65:189–197.
278. South PK, House WA, Miller DD. Tea consumption does not affect iron absorption in rats unless tea and iron are consumed together. Nutr Res. 1997;17(8):1303–1310.
279. Ruenwongsa P, Pattanavibag S. Effect of tea consumption on the levels of alpha-ketoglutarate and pyruvate dehydrogenase in rat brain. Experientia. 1982;38:787–788.
280. Eshchar J, Friedman G. Acute hepatotoxicity of tannic acid added to barium enemas. Dig Dis. 1974;19(9):825–829.
281. Paton A. Tannic acid in barium enemas. Lancet. 1964;1:934.
282. Gloro R, Hourmand-Ollivier I, Mosquet B, et al. Fulminant hepatitis during self-medication with hydroalcoholic extract of green tea. Eur J Gastroenterol Hepatol. 2005;17(10):1135–1137.
283. Kapadia GJ, Chung EB, Ghosh B, et al. Carcinogenicity of some folk medicinal herbs in rats. J Natl Cancer Inst. 1978;60(3):683–686.
284. Morton JF. Further associations of plant tannins and human cancer. Q J Crude Drug Res. 1972;12:1829–1841.
285. Chung KT, Wong TY, Wei CI, et al. Tannins and human health: a review. Crit Rev Food Sci Nutr. 1998;38(6):421–464.
286. Al-Habbal MJ, Al-Habbal Z, Huwezi FU. A double blind controlled clinical trial of mastic and placebo in the treatment of duodenal ulcer. Clin Exp Pharmacol Physiol. 1984;11:541–544.
287. Al-Said MA, Ageel AM, Parmar NS, et al. Evaluation of mastic a crude drug obtained from Pistacia lentiscus for gastric and duodenal anti-ulcer activity. J Ethnopharmacol. 1986;15:271–278.
288. Loughlin MF, Ala’Aldeen DA, Jenks PJ. Monotherapy with mastic does not eradicate Helicobacter pylori infection from mice. J Antimicrob Chemother. 2003;51(2):367–371.
289. Dabos KJ, Sfika E, Vlatta LJ, et al. Is Chios mastic gum effective in the treatment of functional dyspepsia? A prospective randomised double blind placebo controlled trial. J Ethnopharmacol. 2010;127(2):205–209.
290. Lee TY, Lam TH. Allergic contact dermatitis due to a Chinese orthopaedic solution tieh ta yao gin. Contact Dermatitis. 1993;28(2):89–90.
291. Meyerhof W. Elucidation of mammalian bitter taste. Rev Physiol Biochem Pharmacol. 2005;154:37–72.
292. Behrens M, Meyerhof W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol Behav. 2011;105(1):4–13.
293. Behrens M, Meyerhof W. Oral and extraoral bitter taste receptors. Results Probl Cell Differ. 2010;52:87–99.
294. Krasteva G, Canning BJ, Hartmann P, et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA. 2011;108(23):9478–9483.
295. Deshpande DA, Wang WC, McIlmoyle EL, et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16(11):1299–1304.
296. Meyerhof W, Born S, Brockhoff A, et al. Molecular biology of mammalian bitter taste receptors. A review. Flavor Fragrance J. 2011;26(4):260–268.
297. Ogeto JO, Maitai CK. The scientific basis for the use of Strychnos henningsii (Gilg) plant material to stimulate appetite. East Afr Med J. 1983;60:603–607.
298. Carlson AJ, Torchiani B, Hallock R. Contributions to the physiology of the stomach. XXI. The supposed actions of the bitter tonic on the secretion of gastric juice in man and dog. JAMA. 1915;64(1):15–17.
299. Moorhead LD. Contributions to the physiology of the stomach. XXVIII. Further studies on the action of the bitter tonic on the secretion of gastric juice. J Pharmacol Exp Ther. 1915;7:577–589.
300. Glatzel H, Hackenberg K. [Röntgenologische Untersuchungen der Wirkungen von Bittermitteln auf die Verdauungsorgane.]. Planta Med. 1967;3:223–232.
301. Baumann IC, Glatzel H, Muth HW. [Untersuchungen der Wirkungen von Wermut (Artemisia absinthium L.) auf die Gallen–und Pankreassaft–Sekretion des Menschen.]. Z Allgemeinmed. 1975;51(17):784–791.
302. Herman JH, Nolan DS. A bitter cure. NEJM. 1981;305:1654.
303. Wolf S, Mack M. Experimental study of the action of bitters on the stomach of a fistulous human subject. Drug Stand. 1956;24(3):98–101.
304. Gebhardt R. Stimulation of acid secretion by extracts of Gentiana lutea L. in cultured cells from rat gastric mucosa. Pharm Pharmacol Lett. 1997;7(2–3):106–108.
305. Wegener T. [Anwendung eines Trockenextraktes aus Gentianae luteae radix bei dyspeptischem Symptomkomplex.]. Z Phytother. 1998;19:163–164.
306. Ralph A, Giannella MD, Selwyn A, et al. Influence of gastric acidity on bacterial and parasitic enteric infections. Ann Intern Med. 1973;78(2):271–276.
307. Werbach MR. Nutritional Influences on Illness: A Sourcebook of Clinical Research. California: Third Line Press, 1987.
308. Gonzalez H, Ahmed T. Suppression of gastric H2-receptor mediated function in patients with bronchial asthma and ragweed allergy. Chest. 1986;89(4):491–496.
309. Kemp DR, Herrera F, Isaza J, et al. On the critical nature of blood sugar levels in the vagal stimulation of gastric acid secretion in normal and diabetic dogs. Surgery. 1968;64(5):958–966.
310. Mukherjee B, Mukherjee SK. Blood sugar lowering activity of Swertia chirata (Buch–Ham) extract. Int J Crude Drug Res. 1987;25(2):97–102.
311. Saltzman MB, McCallum RW. Diabetes and the stomach. Yale J Biol Med. 1983;56:179–187.
312. Niwa Y, Miyachi Y, Ishimoto K, et al. Why are natural plant medicinal products effective in some patients and not in others with the same disease? Planta Med. 1991;57(4):299–304.
313. Krebs S, Omer TN, Omer B. Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn’s disease – A controlled clinical trial. Phytomedicine. 2010;17(5):305–309.
314. Omer B, Krebs S, Omer H, et al. Steroid-sparing effect of wormwood (Artemisia absinthium) in Crohn’s disease: a double blind placebo-controlled study. Phytomedicine. 2007;14(2–3):87–95.
315. Weiss RF. Amara in current therapy (German). Planta Med. 1966;14(suppl):128–132.
316. Carrai M, Steinke V, Vodicka P, et al. Association between TAS2R38 gene polymorphisms and colorectal cancer risk: a case–control study in two independent populations of Caucasian origin. PLoS One. 2011;6(6):e20464.
317. Bartoshuk LM, Duffy VB, Reed D, et al. Supertasting, earaches and head injury: genetics and pathology alter our taste worlds. Neurosci Behaviour Rev. 1996;20(1):79–87.
318. Wang JC, Hinrichs AL, Bertelsen S, et al. Functional variants in TAS2R38 and TAS2R16 influence alcohol consumption in high risk families of African–American origin. Alcohol Clin Exp Res. 2007;31(2):209–215.
319. Tepper BJ, Koelliker Y, Zhao L, et al. Variation in the bitter-taste receptor gene TAS2R38, and adiposity in a genetically isolated population in Southern Italy. Obesity (Silver Spring). 2008;16(10):2289–2295.
320. Dotson CD, Shaw HL, Mitchell BD, et al. Variation in the gene TAS2R38 is associated with the eating behavior disinhibition in Old Order Amish women. Appetite. 2010;54(1):93–99.
321. Feeney E, O’Brien S, Scannell A, et al. Genetic variation in taste perception: does it have a role in healthy eating? Proc Nutr Soc. 2011;70(1):135–143.
322. Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11(8):1359–1371.
323. Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther. 2010;125(2):181–195.
324. Geppetti P, Patacchini R, Nassini R, et al. Cough: the emerging role of the TRPA1 channel. Lung. 2010;188(suppl 1):S63–S68.
325. Okumura Y, Narukawa M, Iwasaki Y, et al. Activation of TRPV1 and TRPA1 by black pepper components. Biosci Biotechnol Biochem. 2010;74(5):1068–1072.
326. Dedov VN, Tran VH, Duke CC, et al. Gingerols: a novel class of vanilloid receptor (VR1) agonists. Br J Pharmacol. 2002;137(6):793–798.
327. Peng J, Li YJ. The vanilloid receptor TRPV1: role in cardiovascular and gastrointestinal protection. Eur J Pharmacol. 2010;627(1–3):1–7.
328. Biro T, Acs G, Acs P, et al. Recent advances in understanding of vanilloid receptors: a therapeutic target for treatment of pain and inflammation in skin. J Invest Dermatol Symp Proc. 1997;2(1):56–60.
329. Towheen TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the knee, with an emphasis on trial methodology. Semin Arthritis Rheum. 1997;26(5):755–770.
330. Kingery WS. A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain. 1997;73(2):123–139.
331. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7(4):317–328.
332. Hautkappe M, Roizen MF, Toledano A, et al. Review of the effectiveness of capsaicin for painful cutaneous disorders and neural dysfunction. Clin J Pain. 1998;14(2):97–106.
333. Baron R, Wasner G, Lindner V. Optimal treatment of phantom limb pain in the elderly. Drugs Aging. 1998;12(5):361–376.
334. Sanico A, Togias A. Noninfectious, nonallergic rhinitis (NINAR): considerations on possible mechanisms. Am J Rhinol. 1998;12(1):65–72.
335. Gooding SM, Canter PH, Coelho HF, et al. Systematic review of topical capsaicin in the treatment of pruritus. Int J Dermatol. 2010;49(8):858–865.
336. Derry S, Lloyd R, Moore RA, et al. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 4, 2009. CD007393
337. Cheng J, Yang XN, Liu X, et al. Capsaicin for allergic rhinitis in adults. Cochrane Database Syst Rev. (2):, 2006. CD004460
338. Kosuwon W, Sirichatiwapee W, Wisanuyotin T, et al. Efficacy of symptomatic control of knee osteoarthritis with 0.0125% of capsaicin versus placebo. J Med Assoc Thai. 2010;93(10):1188–1195.
339. Altman R, Barkin RL. Topical therapy for osteoarthritis: clinical and pharmacologic perspectives. Postgrad Med. 2009;121(2):139–147.
340. Cameron M, Gagnier JJ, Little CV, et al. Evidence of effectiveness of herbal medicinal products in the treatment of arthritis. Part I: osteoarthritis. Phytother Res. 2009;23(11):1497–1515.
341. Visudhiphan S, Poolsuppasit S, Piboonnukarintr O, et al. The relationship between high fibrinolytic activity and daily capsicum ingestion in Thais. Am J Clin Nutr. 1982;35:1452–1458.
342. Desai HG, Venugopalan K, Philipose M, et al. Effect of red chilli powder on gastric mucosal barrier and acid secretion. Indian J Med Res. 1977;66(3):440–448.
343. Abdel-Salam OM, Szolcsanyi J, Mozsik G. Capsaicin and the stomach. A review of experimental and clinical data. J Physiol Paris. 1997;91(3–5):151–171.
344. Allameh A, Sexena M, Biswas G, et al. Piperine, a plant alkaloid of the piper species, enhances the bioavailability of aflatoxin B1 in rat tissues. Cancer Lett. 1992;61(3):195–199.
345. Bano G, Raina RK, Zutshi U, et al. Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. Eur J Clin Pharmacol. 1991;41(6):615–617.
346. Shoba G, Joy D, Joseph T, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–356.
347. Atal CK, Zutshi U, Rao PG. Scientific evidence on the role of Ayurvedic herbals on bioavailability of drugs. J Ethnopharmacol. 1981;4(2):229–232.
348. Atal CK, Dubey RK, Singh J. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. J Pharmacol Exp Ther. 1985;232(1):258–262.
349. Johri RK, Thusi N, Khajuria A, et al. Piperine-mediated changes in the permeability of rat intestinal epithelial cells. The status of gamma-glutamyl transpeptidase activity, uptake of amino acids and lipid peroxidation. Biochem Pharmacol. 1992;43(7):1401–1407.
350. Khajuria A, Zutshi U, Bendi KL. Permeability characteristics of piperine on oral absorption – an active alkaloid from peppers and a bioavailability enhancer. Indian J Exp Biol. 1998;36(1):46–50.
351. Srinivasan K. Black pepper and its pungent principle – piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr. 2007;47(8):735–748.
352. Pei YQ. A review of pharmacology and clinical use of piperine and its derivatives. Epilepsia. 1983;24(2):177–182.
353. Kawada T, Sakabe S, Watanabe T, et al. Some pungent principles of spices cause the adrenal medulla to secrete catecholamine in anesthetized rats. Proc Soc Exp Biol Med. 1988;188(2):229–233.
354. Doucet E, Tremblay A. Food intake, energy balance and body weight control. Eur J Clin Nutr. 1997;51(12):846–855.
355. Leung FW. Capsaicin-sensitive intestinal mucosal afferent mechanism and body fat distribution. Life Sci. 2008;83(1–2):1–5.
356. Surh YJ, Lee SS. Capsaicin in hot chili pepper: carcinogen, co-carcinogen or anticarcinogen? Food Chem Toxicol. 1996;34(3):313–316.
357. Surh YJ, Lee SS. Capsaicin, a double-edged sword: toxicity, metabolism, and chemopreventive potential. Life Sci. 1995;56(22):1845–1855.
358. Karekar VR, Mujumdar AM, Joshi SS, et al. Assessment of genotoxic effect of piperine using Salmonella typhimurium and somatic and germ cells of Swiss albino mice. ArzneimittelForschung. 1996;46(10):972–975.
359. [No authors listed]. Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin. Int J Toxicol. 2007;26(suppl 1):3–106.
360. Segal R, Shatkovsky P, Milo-Goldzweig I. On the mechanisms of saponin hemolysis–I. Hydrolysis of the glycosidic bond. Biochem Pharmacol. 1974;23:973–981.
361. Segal R, Milo-Goldzweig I. On the mechanism of saponin hemolysis–II. Inhibition of hemolysis by aldonolactones. Biochem Pharmacol. 1975;24:77–81.
362. Boyd EM, Palmer ME. The effect of quillaia, senega, squill, grindelia, sanguinaria, chionanthus and dioscorea upon the output of respiratory tract fluid. Acta Pharmacol. 1946;2:235–246.
363. Walthelm U, Dittrich K, Gelbrich G, et al. Effects of saponins on the water solubility of different model compounds. Planta Med. 2001;67(1):49–54.
364. Bangham AD, Horne RW. Action of saponin on biological cell membranes. Nature. 1962;196:952–953.
365. Gee JM, Johnson IT. Interactions between hemolytic saponins, bile salts and small intestinal mucosa in the rat. J Nutr. 1988;118:1391–1397.
366. Yamasaki K. Effect of some saponins on glucose transport system. In: Waller GR, Yamasaki K, eds. Saponins Used in Traditional and Modern Medicine. New York: Plenum Press; 1996:195–206.
367. Yoshikawa M, Yamahara J. Inhibitory effect of oleanene-type triterpene oligoglycosides on ethanol absorption: the structure–activity relationships. In: Waller GR, Yamasaki K, eds. Saponins Used in Traditional and Modern Medicine. New York: Plenum Press; 1996:207–218.
368. Sapra B, Jain S, Tiwary AK. Effect of Asparagus racemosus extract on transdermal delivery of carvedilol: a mechanistic study. AAPS PharmSciTech. 2009;10(1):199–210.
369. Cayen MN, Dvornik D. Effect of diosgenin on lipid metabolism in rats. J Lipid Res. 1979;20(2):162–174.
370. Oakenfull D, Sidhu GS. Could saponins be a useful treatment for hypercholesterolaemia? Eur J Clin Nutr. 1990;44:79–88.
371. Sears C. How to survive the world’s worst diet. New Scientist. 18, February 10, 1995.
372. Patra AK, Saxena J. The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr Res Rev. 2009;22(2):204–219.
373. Milanov S, Maleeva E, Taskov M. Tribestan: effect on the concentration of some hormones in the serum of healthy volunteers. Medicobiol Info. 1985;4:27–29.
374. Zava DT, Dollbaum CM, Blen M. Estrogen and progestin bioactivity of foods, herbs and spices. Proc Soc Exp Biol Med. 1998;217:369–378.
375. Aradhana, Rao AR, Kale RK. Diosgenin – a growth stimulator of mammary gland of ovariectomized mouse. Ind J Exp Biol. 1992;30(5):367–370.
376. Dinda B, Debnath S, Mohanta BC, et al. Naturally occurring triterpenoid saponins. Chem Biodivers. 2010;7(10):2327–2580.
377. Marston A, Hostettmann K. Plant molluscicides. Phytochemistry. 1985;24(4):639–652.
378. Oakenfull D. Saponins in food – a review. Food Chem. 1981;6:19–40.
379. Van Tonder EM, Basson PA, Van Rensburg IBJ. Geeldikkop: experimental induction by feeding the plant Tribulus terrestris L (Zygophyllaceae). J S Afr Vet Assoc. 1972;43(4):363–375.
380. Weisse AB. A fond farewell to the foxglove? The decline in the use of digitalis. J Card Fail. 2010;16(1):45–48.
381. Weiss RF. Herbal Medicine. Beaconsfield: Beaconsfield Publishers, 1988.
382. Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008;7(11):926–935.
383. Newman RA, Yang P, Pawlus AD, et al. Cardiac glycosides as novel cancer therapeutic agents. Mol Interv. 2008;8(1):36–49.
384. Mascolo N, Capasso R, Capasso F. Senna: a safe and effective drug. Phytother Res. 1998;12:S143–S145.
385. Nijs G, De Witte P, Geboes K, et al. In vitro demonstration of a positive effect of rhein anthrone on peristaltic reflex of guinea pig ileum. Pharmacology. 1993;47(suppl 1):40–48.
386. Leng–Peschlow E. Sennoside-induced secretion is not caused by changes in mucosal permeability or Na(+), K(+)-ATPase activity. J Pharm Pharmacol. 1993;45(11):951–954.
387. Yagi T, Miyawaki Y, Nishikawa A, et al. Suppression of the purgative action of rhein anthrone, the active metabolite of sennosides A and B, by indomethacin in rats. J Pharm Pharmacol. 1991;43(5):307–310.
388. Capasso F, Izzo AA, Mascolo N, et al. Effect of senna is not mediated by platelet-activating factor. Pharmacology. 1993;47(suppl 1):58–63.
389. Anton R, Haag-Berrurier M. Therapeutic use of natural anthraquinone for other than laxative actions. Pharmacology. 1980;20(suppl 1):104–112.
390. van de Kerkhof PC, Vissers WH. The topical treatment of psoriasis. Skin Pharmacol Appl Skin Physiol. 2003;16(2):69–83.
391. Rossi–Soffar G. Psoriasis. Dermatologica. 1965;130:53–79.
392. Ashton RE, Andre P, Lowe NJ, et al. Anthralin: historical and current perspectives. J Am Acad Dermatol. 1983;9(2):173–192.
393. Muller K, Leukel P, Ziereis K, et al. Antipsoriatic anthrones with modulated redox properties. 2. Novel derivatives of chrysarobin and isochrysarobin – antiproliferative activity and 5-lipoxygenase. J Med Chem. 1994;37(11):1660–1669.
394. Wang XJ, Warren BS, Rupp T, et al. Loss of mouse epidermal protein kinase C isozyme activities following treatment with phorbol ester and non-phorbol ester tumor promotors. Carcinogenesis. 1994;15(12):2795–2803.
395. Barnard DL, Huffman JH, Morris JL, et al. Evaluation of the antiviral activity of anthraquinones, anthrones and anthraquinone derivatives against human cytomegalovirus. Antiviral Res. 1992;17(1):63–77.
396. Spencer CM, Wilde MI. Diacerein. Drugs. 1997;53(1):98–106.
397. Fidelix TS, Soares BG, Trevisani VF. Diacerein for osteoarthritis. Cochrane Database Syst Rev. (1):, 2006. CD005117
398. Blomeke B, Poginsky B, Schmutte C, et al. Formation of genotoxic metabolites from anthraquinone glycosides present in Rubia tinctorum L. Mutat Res. 1992;265(2):263–272.
399. Ino N, Tanaka T, Okumura A, et al. Acute and subacute toxicity tests of madder root, natural colorant extracted from madder (Rubia tinctorum), in (C57BL/6 X C3H)F1 in mice. Toxicol Ind Health. 1995;11(4):449–458.
400. Inoue K, Yoshida M, Takahashi M, et al. Induction of kidney and liver cancers by the natural food additive madder color in a two-year rat carcinogenicity study. Food Chem Toxicol. 2009;47(1):184–191.
401. Leng-Peschlow E. Senna and its rational use. Pharmacology. 1992;44(suppl 1):26–29.
402. Leng-Peschlow E. Senna and its rational use. Pharmacology. 1992;44(suppl 1):30–32.
403. Howard LRC, Hughes-Roberts HE. The treatment of constipation in mental hospitals. Gut. 1962;3:89–90.
404. Leng-Peschlow E. Senna and its rational use. Pharmacology. 1992;44(suppl 1):36–40.
405. Campbell WL. Cathartic colon: reversibility of roentgen changes. Dis Colon Rectum. 1983;26:445–448.
406. Leng-Peschlow E. Senna and its rational use. Pharmacology. 1992;44(suppl 1):20–22.
407. Leng-Peschlow E. Senna and its rational use. Pharmacology. 1992;44(suppl 1):23–25.
408. Leng-Peschlow E. Senna and its rational use. Pharmacology. 1992;44(suppl 1):33–35.
409. Harris A, Buchanan GN. Melanosis coli is reversible. Colorectal Dis. 2009;11(7):788–789.
410. Brown JP. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutat Res. 1980;75:243–277.
411. Heidemann A, Miltenburger HG, Mengs U. The genotoxicity status of senna. Pharmacology. 1993;47(suppl):178–186.
412. Brusick D, Mengs U. Assessment of the genotoxic risk from laxative senna products. Environ Mol Mutagen. 1997;29:1–19.
413. Siegers CP. Anthranoid laxatives and colorectal cancer. Trends Pharmacol Sci. 1992;13:229–231.
414. Morales MA, Hernández D, Bustamante S, et al. Is senna laxative use associated to cathartic colon, genotoxicity, or carcinogenicity? J Toxicol. 2009. 2009:287247
415. Marles RJ, Compadre CM, Farnsworth NR. Coumarin in vanilla extracts: its detection and significance. Econ Bot. 1987;41(1):41–47.
416. Stohr H, Herrmann K. On the phenolic acids of vegetables. III Hydroxycinnamic acids and hydroxybenzoic acids of root vegetables (German). Z Lebensmittel Untersuchung Forschung. 1975;159(4):218–224.
417. Arora RB, Mathur CN. Relationship between structure and anticoagulant activity of coumarin derivatives. Br J Pharmacol. 1963;20:29–35.
418. Hörhammer L, Wagner H, Reinhardt H. On new constituents from the barks of Viburnum prunifolium L. (American Snowball) and Virbunum opulus (Common Snowball). Z Naturforschung. 1967;22b:768–776.
419. Jarboe CH, Zirvi KA, Nicholson JA, et al. Scopoletin, an antispasmodic component of Viburnum opulus and prunifolium. J Med Chem. 1967;10:488–489.
420. Ojewole JAO, Adesina SK. Mechanism of the hypotensive effect of scopoletin isolated from fruit of Tetrapleura tetraptera. Planta Med. 1983;49(1):45–50.
421. Chen YF, Tsai HY, Wu TS. Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med. 1995;61(1):2–8.
422. Kimura Y, Okuda H, Arichi S, et al. Inhibition of the formation of 5-hydroxy-6, 8,11,14-eicosatetraenoic acid from arachidonic acid in polymorphonuclear leukocytes by various coumarins. Biochim Biophys Acta. 1985;834(2):224–229.
423. Chang WS, Chiang HC. Structure–activity relationship of coumarins in xanthine oxidase inhibition. Anticancer Res. 1995;15(5B):1969–1973.
424. Lin HC, Tsai SH, Chen CS, et al. Structure–activity relationship of coumarin derivatives on xanthine oxidase-inhibiting and free radical-scavenging activities. Biochem Pharmacol. 2008;75(6):1416–1425.
425. Kong L, Zhou J, Wen Y, et al. Aesculin possesses potent hypouricemic action in rodents but is devoid of xanthine oxidase/dehydrogenase inhibitory activity. Planta Med. 2002;68(2):175–178.
426. Ding Z, Dai Y, Wang Z. Hypouricemic action of scopoletin arising from xanthine oxidase inhibition and uricosuric activity. Planta Med. 2005;71(2):183–185.
427. Ohta T, Watanabe K, Moriya M, et al. Anti-mutagenic effects of coumarin and umbelliferone on mutagenesis induced by 4-nitroquinoline 1-oxide or UV irradiation in E. coli. Mutat Res. 1983;117:135–138.
428. Romert L, Jansson T, Curvall M, et al. Screening for agents inhibiting the mutagenicity of extracts and constituents of tobacco products. Mutat Res. 1994;322(2):97–110.
429. Wattenberg LW, Lam LKT, Fladmoe AV. Inhibition of chemical carcinogen-induced neoplasia by coumarins and a-angelicalactone. Cancer Res. 1979;39:1651–1654.
430. Kolodziej H, Kayser O, Woerdenbag HJ, et al. Structure–cytotoxicity relationships of a series of natural and semi-synthetic simple coumarins as assessed in two human tumour cell lines. Z Naturforschung. 1997;52(3/4):240–244.
431. Weber US, Steffen B, Siegers CP. Antitumor-activities of coumarin, 7-hydroxy-coumarin and its glucuronide in several human tumor cell lines. Res Commun Mol Pathol Pharmacol. 1998;99(2):193–206.
432. Lacy A, O’Kennedy R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr Pharm Des. 2004;10(30):3797–3811.
433. Musa MA, Cooperwood JS, Khan MO. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr Med Chem. 2008;15(26):2664–2679.
434. Rios ER, Rocha NF, Venâncio ET, et al. Mechanisms involved in the gastroprotective activity of esculin on acute gastric lesions in mice. Chem Biol Interact. 2010;188(1):246–254.
435. Vasconcelos JF, Teixeira MM, Barbosa-Filho JM, et al. Effects of umbelliferone in a murine model of allergic airway inflammation. Eur J Pharmacol. 2009;609(1–3):126–131.
436. Panda S, Kar A. Evaluation of the antithyroid, antioxidative and antihyperglycemic activity of scopoletin from Aegle marmelos leaves in hyperthyroid rats. Phytother Res. 2006;20(12):1103–1105.
437. Ramesh B, Pugalendi KV. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J Med Food. 2006;9(4):562–566.
438. Pathak MA, Carbonare MD. Melanogenic potential of various furocoumarins in normal and vitiliginous skin. In: Fitzpatrick TB, Forlot P, Pathak MA, et al, eds. Psoralens: Past, Present and Future of Photochemoprotection and Other Biological Activities. Paris: John Libbey Eurotext; 1989:87–101.
439. Fitzpatrick TB. The psoralen story: photochemotherapy and photochemoprotection. In: Fitzpatrick TB, Forlot P, Pathak MA, et al, eds. Psoralens: Past, Present and Future of Photochemoprotection and Other Biological Activities. Paris: John Libbey Eurotext; 1989:5–10.
440. Kalis B, Sayag S, Forlot P. Photochemotherapy (PUVA) of psoriasis: a double blind comparative study of 5- and 8-methoxypsoralen. In: Fitzpatrick TB, Forlot P, Pathak MA, et al, eds. Psoralens: Past, Present and Future of Photochemoprotection and Other Biological Activities. Paris: John Libbey Eurotext; 1989:277–282.
441. Forlot P. Psoralen induced pigmentation in human skin: overview of clinical evidence, mechanisms of induction and future research. In: Fitzpatrick TB, Forlot P, Pathak MA, et al, eds. Psoralens: Past, Present and Future of Photochemoprotection and Other Biological Activities. Paris: John Libbey Eurotext; 1989:63–71.
442. Kligman AM, Forlot P. Comparative photoprotection in humans by tans induced either by solar stimulating radiation or after a psoralen–containing sunscreen. In: Fitzpatrick TB, Forlot P, Pathak MA, et al, eds. Psoralens, Past, Present and Future of Photochemoprotection and Other Biological Activities. Paris: John Libbey Eurotext; 1989:407–420.
443. Gasparro FP. The role of PUVA in the treatment of psoriasis. Photobiology issues related to skin cancer incidence. Am J Clin Dermatol. 2000;1(6):337–348.
444. Matz H. Phototherapy for psoriasis: what to choose and how to use: facts and controversies. Clin Dermatol. 2010;28(1):73–80.
445. Musajo L, Rodighiero G, Colombo G, et al. Photosensitizing furocoumarins: interaction with DNA and photoinactivation of DNA containing viruses. Experientia. 1965;21:22.
446. Oginsky EL, Green GS, Griffith DG, et al. Lethal photosensitization of bacteria with 8-methoxy-psoralen to long wave length ultra-violet radiation. J Bacteriol. 1959;78:821.
447. Rauwald HW, Brehm O, Odenthal KP. The involvement of Ca2+ channel blocking mode of action in the pharmacology of Ammi visnaga fruits. Planta Med. 1994;60:101–105.
448. Meyer U. From khellin to sodium cromoglycate – a tribute to the work of Dr. R.E.C. Altounyan (1922–1987). Pharmazie. 2002;57(1):62–69.
449. Hogan RP. Hemorrhagic diathesis caused by drinking an herbal tea. JAMA. 1983;249(19):2679–2680.
450. Ivie GW. The chemistry of plant furanocoumarins and their medical, toxicological, environmental, and coevolutionary significance. Rev Latinoam Quim. 1987;18(1):1–5.
451. Cassier-Chauvat C, Averbeck D. Photomutagenic effects induced by psoralen derivatives in the yeast Saccharomyces cerevisiae. In: Fitzpatrick TB, Forlot P, Pathak MA, et al, eds. Psoralens: Past, Present and Future of Photochemoprotection and Other Biological Activities. Paris: John Libbey Eurotext; 1989:329–335.
452. Marzin D, Olivier P. Study of the protective activity against photomutagenicity of 5-MOP. In: Fitzpatrick TB, Forlot P, Pathak MA, et al, eds. Psoralens: Past, Present and Future of Photochemoprotection and Other Biological Activities. Paris: John Libbey Eurotext; 1989:337–343.
453. Young AR, Walker SL. Experimental photocarcinogenesis of psoralens. In: Fitzpatrick TB, Forlot P, Pathak MA, et al, eds. Psoralens: Past, Present and Future of Photochemoprotection and Other Biological Activities. Paris: John Libbey Eurotext; 1989:357–366.
454. Stern RS. PUVA: its status in the United States, 1988. In: Fitzpatrick TB, Forlot P, Pathak MA, et al, eds. Psoralens: Past, Present and Future of Photochemoprotection and Other Biological Activities. Paris: John Libbey Eurotext; 1989:367–376.
455. Henseler T. Risk of non melanoma skin tumors in patients treated with long–term–photochemotherapy (PUVA). In: Fitzpatrick TB, Forlot P, Pathak MA, et al, eds. Psoralens: Past, Present and Future of Photochemoprotection and Other Biological Activities. Paris: John Libbey Eurotext; 1989:377–386.
456. Messina M. A brief historical overview of the past two decades of soy and isoflavone research. J Nutr. 2010;140(7):1350S–1354S.
457. Molteni A, Brizio-Molteni L, Persky V. In vitro hormonal effects of soybean isoflavones. J Nutr. 1995;125:751S–756S.
458. Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen receptor beta: an overview and update. Nucl Recept Signal. 2008;6:e003.
459. Koehler KF, Helguero LA, Haldosen LA, et al. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005;26(3):465–478.
460. Benassayag C, Perrot-Applanat M, Ferre F. Phytoestrogens as modulators of steroid action in target cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777(1–2):233–248.
461. Gustafsson JA. ERbeta scientific visions translate to clinical uses. Climacteric. 2006;9(3):156–160.
462. McCarty MF. Isoflavones made simple – genistein’s agonist activity for the beta-type estrogen receptor mediates their health benefits. Med Hypotheses. 2006;66(6):1093–1114.
463. Rissman EF. Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: beyond Yin/Yang. J Neuroendocrinol. 2008;20(6):873–879.
464. Turner JV, Agatonovic-Kustrin S, Glass BD. Molecular aspects of phytoestrogen selective binding at estrogen receptors. J Pharm Sci. 2007;96(8):1879–1885.
465. Oseni T, Patel R, Pyle J, et al. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008;74(13):1656–1665.
466. Jeng YJ, Kochukov MY, Watson CS. Membrane estrogen receptor-alpha-mediated nongenomic actions of phytoestrogens in GH3/B6/F10 pituitary tumor cells. J Mol Signal. 2009;4:2.
467. Wuttke W, Jarry H, Seidlova-Wuttke D. Isoflavones – safe food additives or dangerous drugs? Ageing Res Rev. 2007;6(2):150–188.
468. Casanova M, You L, Gaido KW, et al. Developmental effects of dietary phytoestrogens in Sprague–Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro. Toxicol Sci. 1999;51(2):236–244.
469. Murray MJ, Meyer WR, Lessey BA, et al. Soy protein isolate with isoflavones does not prevent estradiol-induced endometrial hyperplasia in postmenopausal women: a pilot trial. Menopause. 2003;10(5):456–464.
470. Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010;29(3):465–482.
471. Virk-Baker MK, Nagy TR, Barnes S. Role of phytoestrogens in cancer therapy. Planta Med. 2010;76(11):1132–1142.
472. Cano A, García-Pérez MA, Tarín JJ. Isoflavones and cardiovascular disease. Maturitas. 2010;67(3):219–226.
473. Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125(2):315–323.
474. Hilakivi-Clarke L, Andrade JE, Helferich W. Is soy consumption good or bad for the breast? J Nutr. 2010;140(12):2326S–2334S.
475. Lagari VS, Levis S. Phytoestrogens and bone health. Curr Opin Endocrinol Diabetes Obes. 2010;17(6):546–553.
476. Bolaños R, Del Castillo A, Francia J. Soy isoflavones versus placebo in the treatment of climacteric vasomotor symptoms: systematic review and meta-analysis. Menopause. 2010;17(3):660–666.
477. Kurzer MS. Soy consumption for reduction of menopausal symptoms. Inflammopharmacology. 2008;16(5):227–229.
478. de Cremoux P, This P, Leclercq G, et al. Controversies concerning the use of phytoestrogens in menopause management: bioavailability and metabolism. Maturitas. 2010;65(4):334–339.
479. Hooper L, Ryder JJ, Kurzer MS, et al. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15(4):423–440.
480. Kim J. Protective effects of Asian dietary items on cancers – soy and ginseng. Asian Pac J Cancer Prev. 2008;9(4):543–548.
481. Van Patten CL, de Boer JG, Tomlinson Guns ES. Diet and dietary supplement intervention trials for the prevention of prostate cancer recurrence: a review of the randomized controlled trial evidence. J Urol. 2008;180(6):2314–2321.
482. Pase MP, Grima NA, Sarris J. The effects of dietary and nutrient interventions on arterial stiffness: a systematic review. Am J Clin Nutr. 2011;93(2):446–454.
483. Taku K, Lin N, Cai D, et al. Effects of soy isoflavone extract supplements on blood pressure in adult humans: systematic review and meta-analysis of randomized placebo-controlled trials. J Hypertens. 2010;28(10):1971–1982.
484. Beavers DP, Beavers KM, Miller M, et al. Exposure to isoflavone-containing soy products and endothelial function: a Bayesian meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2012;22(3):182–191.
485. Vatanparast H, Chilibeck PD. Does the effect of soy phytoestrogens on bone in postmenopausal women depend on the equol-producing phenotype? Nutr Rev. 2007;65(6):294–299.
486. Rowland IR, Wiseman H, Sanders TAB. Interindividual variations in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36(1):27–32.
487. Tokunaga T. Novel physiological functions of fructooligosaccharides. Biofactors. 2004;21(1–4):89–94.
488. Setchell KDR, Lawson AM, Borriello SP, et al. Lignan formation in man – microbial involvement and possible roles in relation to cancer. Lancet. 1981;2:4–7.
489. Phipps WR, Martini MC, Lampe JW, et al. Effect of flax seed ingestion on the menstrual cycle. J Clin Endocrinol Metab. 1993;77(5):1215–1219.
490. Frische EJ, Hutchins AM, Martini MC, et al. Effect of flaxseed and wheat bran on serum hormones and lignan excretion in premenopausal women. J Am Coll Nutr. 2003;22(6):550–554.
491. Hutchins AM, Martini MC, Olson BA, et al. Flaxseed consumption influences endogenous hormone concentrations in postmenopausal women. Nutr Cancer. 2001;39(1):58–65.
492. Serraino M, Thompson LU. The effect of flaxseed supplementation on early risk markers for mammary carcinogenesis. Cancer Lett. 1991;60:135–142.
493. Adlercreutz H, Fotsis T, Heikkinen R, et al. Excretion of the lignans enterolactone and enterodiol and of equol in omnivorous and vegetarian postmenopausal women and in women with breast cancer. Lancet. 1982;2:1295–1299.
494. Sonestedt E, Wirfält E. Enterolactone and breast cancer: methodological issues may contribute to conflicting results in observational studies. Nutr Res. 2010;30(10):667–677.
495. Brooks JD, Ward WE, Lewis JE, et al. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am J Clin Nutr. 2004;79(2):318–325.
496. Haggans CJ, Travelli EJ, Thomas W, et al. The effect of flaxseed and wheat bran consumption on urinary estrogen metabolites in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;9(7):719–725.
497. Haggans CJ, Hutchins AM, Olson BA, et al. Effect of flaxseed consumption on urinary estrogen metabolites in postmenopausal women. Nutr Cancer. 1999;33(2):188–195.
498. Simbalista RL, Sauerbronn AV, Aldrighi JM, et al. Consumption of a flaxseed-rich food is not more effective than a placebo in alleviating the climacteric symptoms of postmenopausal women. J Nutr. 2010;140(2):293–297.
499. Irvine CHG, Fitzpatrick MG, Alexander SL. Phytoestrogens in soy-based infant foods: concentrations, daily intake, and possible biological effects. Proc Soc Exp Biol Med. 1998;217:247–253.
500. Messina M. Soybean isoflavone exposure does not have feminizing effects on men: a critical examination of the clinical evidence. Fertil Steril. 2010;93(7):2095–2104.
501. Hamilton-Reeves JM, Vazquez G, Duval SJ, et al. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: results of a meta–analysis. Fertil Steril. 2010;94(3):997–1007.
502. Hooper L, Madhavan G, Tice JA, et al. Effects of isoflavones on breast density in pre- and post-menopausal women: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2010;16(6):745–760.
503. Wu AH, Ursin G, Koh WP, et al. Green tea, soy, and mammographic density in Singapore Chinese women. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3358–3365.
504. Bandera EV, Williams MG, Sima C, et al. Phytoestrogen consumption and endometrial cancer risk: a population-based case-control study in New Jersey. Cancer Causes Control. 2009;20(7):1117–1127.
505. Lethaby AE, Brown J, Marjoribanks J, et al. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev. (4):2007. CD001395
506. Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16(3):249–258.
507. Rolland A, Fleurentin J, Lanahers MC, et al. Behavioural effects of the American traditional plant Eschscholzia californica: sedative and anxiolytic properties. Planta Med. 1991;57:212–216.
508. Schäfer HL, Schäfer H, Schneider W, et al. Sedative action of extract combinations of Eschscholtzia californica and Corydalis cava. ArzneimittelForschung. 1995;45(1):124–126.
509. Kleber E, Schneider W, Schäfer HL, et al. Modulation of key reactions of the catecholamine metabolism by extracts from Eschscholtzia californica and Corydalis cava. Arzneimittel-Forschung. 1995;45(1):127–131.
510. Lui LM, Sheu SJ, Chiou SH, et al. A comparative study on commercial samples of Ephedrae herba. Planta Med. 1993;59:376–378.
511. Thies PW. [Spartium und spartein.]. Pharm Unserer Zeit. 1986;6:172–176.
512. Northover BJ. Effect of pre-treating rat atria with potassium channel blocking drugs on the electrical and mechanical responses to phenylephrine. Biochem Pharmacol. 1994;47(12):2163–2169.
513. Eichelbaum M, Spannbrucker N, Steincke B, et al. Defective N-oxidation of sparteine in man: a new pharmacogenetic defect. Eur J Clin Pharm. 1979;16:183–187.
514. Vinks A, Inaba T, Otton SV, et al. Sparteine metabolism in Canadian Caucasians. Clin Pharmacol Ther. 1982;31(1):23–29.
515. Hempelmann FW, Heinz N, Flasch H. Lipophilicity–protein binding relationship in cardenolides. Arzneimittel-Forschung. 1978;28(12):2182–2185.
516. Tschanz C, Stargel WW, Thomas JA. Interactions between drugs and nutrients. Adv Pharmacol. 1996;35:1–26.
517. Sterk V, Büchele B, Simmet T. Effect of food intake on the bioavailability of boswellic acids from a herbal preparation in healthy volunteers. Planta Med. 2004;70(12):1155–1160.
518. Schubert W, Culberg G, Edgar B, et al. Inhibition of 17 beta-estradiol by grapefruit juice in ovariectomized women. Maturitas. 1995;20:155–163.
519. Bailey DG, Arnold JM, Spence JD. Grapefruit juice and drugs. How significant is the interaction? Clin Pharmacokinet. 1994;26(2):91–98.
520. Fuhr U, Klittich K, Staib AH. Inhibitory effect of grapefruit juice and its bitter principle, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br J Clin Pharmacol. 1993;35(4):431–436.
521. Miniscalco A, Lundahl J, Regardh CG, et al. Inhibition of dihydropyridine metabolism in rat and human liver microsomes by flavonoids found in grapefruit juice. J Pharmacol Exp Ther. 1992;261(3):1195–1199.
522. Min DI, Ku YM, Perry PJ, et al. Effect of grapefruit juice on cyclosporine pharmacokinetics in renal transplant patients. Transplant. 1996;62(1):123–125.
523. Hukkinen SK, Varhe A, Olkkola KT, et al. Plasma concentrations of triazolam are increased by concomitant ingestion of grapefruit juice. Clin Pharmacol Ther. 1995;58(2):127–131.
524. Lundahl JUE, Regårdh CG, Edgar B, et al. The interaction effect of grapefruit juice is maximal after the first glass. Eur J Clin Pharm. 1998;54:75–81.
525. Lee YS, Lorenzo BJ, Koufis T, et al. Grapefruit juice and its flavonoids inhibit 11 beta-hydroxysteroid dehydrogenase. Clin Pharmacol Ther. 1996;59(1):62–71.
526. Edwards DJ, Bernier SM. Naringin and naringenin are not the primary CYP3A inhibitors in grapefruit juice. Life Sci. 1996;59(13):1025–1030.
527. Runkel M, Bourian M, Tegtmeier M, et al. The character of inhibition of the metabolism of 1,2-benzopyrone (coumarin) by grapefruit juice in humans. Eur J Clin Pharm. 1997;53:265–269.
528. He K, Iyer KR, Hayes RN, et al. Inactivation of cytochrome P450 3A4 by bergamottin, a component of grapefruit juice. Chem Res Toxicol. 1998;11(4):252–259.
529. Woo WS, Shin KH, Lee CK. Effect of naturally occurring coumarins on the activity of drug metabolizing enzymes. Biochem Pharmacol. 1983;32(11):1800–1803.
530. Diaconu CH, Cuciureanu M, Vlase L, et al. Food–drug interactions: grapefruit juice. Rev Med Chir Soc Med Nat Iasi. 2011;115(1):245–250.
531. Steinegger E, Hövel H. Analytic and biologic studies on Salicaceae substances, especially on salicin. II. Biological study. Pharm Acta Helv. 1972;47:222–234.
532. Fotsch G, Pfeifer S, Bartoszek M, et al. Biotransformation of phenolglycosides leiocarposide and salicin. Pharmazie. 1989;44(8):555–558.
533. Akao T, Yoshino T, Kobashi K, et al. Evaluation of salicin as an antipyretic prodrug that does not cause gastric injury. Planta Med. 2002;68(8):714–718.
534. Leng–Peschlow E. Senna and its rational use. Pharmacology. 1992;44(suppl):10–15.
535. Soulimani R, Younos C, Mortier F, et al. The role of stomachal digestion on the pharmacological activity of plant extracts, using as an example extracts of Harpagophytum procumbens. Can J Physiol Pharmacol. 1994;72:1532–1536.
536. Wagner H, Jurcic K, Schaette R. Comparative studies on the sedative action of Valeriana extracts, valepotriates and their degradation products. Planta Med. 1980;39:358–365.
537. Valdes LJ, III. Salvia divinorum and the unique diterpene hallucinogen Salvinorin (Divinorin) A. J Psychoactive Drugs. 1994;26(3):277–283.
538. Griffiths LA, Barlow A. The fate of orally and parenterally administered flavonoids in the mammal. The significance of biliary excretion. Angiology. 1972;9(3–6):162–174.
539. Kim DH. Herbal medicines are activated by intestinal microflora. Nat Prod Sci. 2002;8(2):35–43.
540. Ishimi Y, Arai N, Wang X, et al. Difference in effective dosage of genistein on bone and uterus in ovariectomized mice. Biochem Biophys Res Commun. 2000;274(3):697–701.
541. Pino AM, Valladares LE, Palma MA, et al. Dietary isoflavones affect sex hormone-binding globulin levels in postmenopausal women. J Clin Endocrinol Metab. 2000;85(8):2797–2800.
542. Graefe EU, Wittig J, Mueller S, et al. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J Clin Pharmacol. 2001;41(5):492–499.
543. Jäger AK, Saaby L. Flavonoids and the CNS. Molecules. 2011;16(2):1471–1485.
544. Moon YJ, Wang L, DiCenzo R, et al. Quercetin pharmacokinetics in humans. Biopharm Drug Dispos. 2008;29(4):205–217.
545. Zhang L, Zuo Z, Lin G. Intestinal and hepatic glucuronidation of flavonoids. Mol Pharm. 2007;4(6):833–845.
546. Hertog MGL, Hollman PCH. Potential health effects of the dietary flavonol quercetin. Eur J Clin Nutr. 1996;50(2):63–71.
547. Hollman PC, De Vries JH, Van Leeuwen SD, et al. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr. 1995;62(6):1276–1282.
548. Merfort I, Heilmann J, Weiss M, et al. Radical scavenger activity of three flavonoid metabolites studied by inhibition of chemiluminescence in human PMNs. Planta Med. 1996;62(4):289–292.
549. Rechner AR, Kroner C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb Res. 2005;116(4):327–334.
550. Rechner AR, Kuhnle G, Bremner P, et al. The metabolic fate of dietary polyphenols in humans. Free Radic Biol Med. 2002;33(2):220–235.
551. Kelly GE, Joannou GE, Reeder AY, et al. The variable metabolic response to dietary isoflavones in humans. Proc Soc Exp Biol Med. 1995;208(1):40–43.
552. Xu X, Wang HJ, Murphy PA, et al. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr. 1994;124(6):825–832.
553. Xu X, Harris KS, Wang HJ, et al. Bioavailability of soybean isoflavones depends upon gut microflora in women. J Nutr. 1995;125(9):2307–2315.
554. Cassidy A, Bingham S, Setchell K. Biological effects of isoflavones in young women: importance of the chemical composition of soyabean products. Br J Nutr. 1995;74(4):587–601.
555. Larkin T, Price WE, Astheimer L. The key importance of soy isoflavone bioavailability to understanding health benefits. Crit Rev Food Sci Nutr. 2008;48(6):538–552.
556. Manach C, Williamson G, Morand C, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 suppl):230S–242S.
557. Nielsen IL, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer. 2007;57(1):1–10.
558. Wang Z, Kurosaki Y, Nakayama T, et al. Mechanism of gastrointestinal absorption of glycyrrhizin in rats. Biol Pharm Bull. 1994;17(10):1399–1403.
559. Krahenbuhl S, Hasler F, Krapf R. Analysis and pharmacokinetics of glycyrrhizic acid and glycyrrhetinic acid in humans and experimental animals. Steroids. 1994;59(2):121–126.
560. Hattori M, Sakamoto T, Kobashi K, et al. Metabolism of glycyrrhizin by human intestinal flora. Planta Med. 1983;48(1):38–42.
561. Sakiya Y, Akada Y, Kawano S, et al. Rapid estimation of glycyrrhizin and glycyrrhetinic acid in plasma by high-speed liquid chromatography. Chem Pharm Bull. 1979;27(5):1125–1129.
562. Nose M, Ito M, Kamimura K, et al. A comparison of the antihepatotoxic activity between glycyrrhizin and glycyrrhetinic acid. Planta Med. 1994;60(2):136–139.
563. Hasegawa H, Sung JH, Matsumiya S, et al. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996;62(5):453–457.
564. Rauwald HW, Grunwild J. Ruscus aculeatus extract: unambiguous proof of the absorption of spirostanol glycosides in human plasma after oral administration. Planta Med. 1991;57(suppl 2):A75–A76.
565. Daniel EM, Ratnayake S, Kinstle T, et al. The effects of pH and rat intestinal contents on the liberation of ellagic acid from purified and crude ellagitannins. J Nat Prod. 1991;54(4):946–952.
566. Bravo L, Abia R, Eastwood MA, et al. Degradation of polyphenols (catechin and tannic acid) in the rat intestinal tract. Effect on colonic fermentation and faecal output. Br J Nutr. 1994;71(6):933–946.
567. Groenewoud G, Hundt HKL. The microbial metabolism of condensed (+)-catechins by rat-caecal microflora. Xenobiotica. 1986;16(2):99–107.
568. Larrosa M, García-Conesa MT, Espín JC, et al. Ellagitannins, ellagic acid and vascular health. Mol Aspects Med. 2010;31(6):513–539.
569. Appeldoorn MM, Vincken JP, Aura AM, et al. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-gamma-valerolactone as the major metabolites. J Agric Food Chem. 2009;57(3):1084–1092.
570. Grimm T, Skrabala R, Chovanová Z, et al. Single and multiple dose pharmacokinetics of maritime pine bark extract (pycnogenol) after oral administration to healthy volunteers. BMC Clin Pharmacol. 2006;6:4.
571. Serafini M, Ghiselli A, Ferro-Luzzi A. In vivo antioxidant effect of green and black tea in man. Eur J Clin Nutr. 1996;50(1):28–32.
572. Nakagawa K, Okuda S, Miyazawa T. Dose-dependent incorporation of tea catechins, (−)-epigallocatechin-3-gallate and (−)-epigallocatechin, into human plasma. Biosci Biotechnol Biochem. 1997;61(12):1981–1985.
573. Wang JS, Luo H, Wang P, et al. Validation of green tea polyphenol biomarkers in a phase II human intervention trial. Food Chem Toxicol. 2008;46(1):232–240.
574. Pulse TL, Uhlig E. A significant improvement in a clinical pilot study utilizing nutritional supplements, essential fatty acids and stabilized aloe vera juice in 29 HIV seropositive ARC and AIDS patients. J Adv Med. 1990;3(4):209–230.
575. Sullivan R, Smith JE, Rowan NJ. Medicinal mushrooms and cancer therapy: translating a traditional practice into Western medicine. Perspect Biol Med. 2006;49(2):159–170.
576. Yajima Y, Satoh J, Fukuda I, et al. Quantitative assay of lentinan in human blood with the limulus colorimetric test. Tohoku J Exp Med. 1989;157(2):145–151.
577. Yoshino S, Watanabe S, Imano M, et al. Improvement of QOL and prognosis by treatment of superfine dispersed lentinan in patients with advanced gastric cancer. Hepatogastroenterology. 2010;57(97):172–177.
578. van Duynhoven J, Vaughan EE, Jacobs DM, et al. Metabolic fate of polyphenols in the human superorganism. Proc Natl Acad Sci USA. 2011;108(suppl 1):4531–4538.
579. Possemiers S, Bolca S, Verstraete W, et al. The intestinal microbiome: a separate organ inside the body with the metabolic potential to influence the bioactivity of botanicals. Fitoterapia. 2011;82(1):53–66.
580. Boyd EM, Sheppard EP. The effect of steam inhalation of volatile oils on the output and composition of respiratory tract fluid. J Pharmacol Exp Ther. 1968;163(1):250–256.
581. Boyd EM, Sheppard EP. A bronchomucotropic action in rabbits from inhaled menthol and thymol. Arch Int Pharmacodyn. 1969;182(1):206–214.
582. Sheppard EP, Boyd EM. Lemon oil as an expectorant inhalant. Pharmacol Res Commun. 1970;2(1):1–16.
583. Boyd EM, Sheppard EP. Nutmeg oil and camphene as inhaled expectorants. Arch Otolaryngol. 1970;92(4):372–378.
584. Boyd EM, Sheppard EP. The effect of inhalation of citral and geraniol on the output and composition of respiratory tract fluid. Arch Int Pharmacodyn. 1970;188(1):5–13.