Fatigue and debility
Scope
Apart from their use to provide non-specific support for recuperation and repair, specific phytotherapeutic strategies include the following.
• chronic fatigue syndrome and related conditions such as fibromyalgia
• fatigue and debility after illness, prolonged stress, injury or trauma (convalescence).
Because of the use of secondary plant products, caution is necessary in applying phytotherapy in cases of:
Orientation
A debilitating symptom
Phytotherapists increasingly find that a major indication for treatment is a degenerative or debilitating illness. Unlike their forebears, for whom acute diseases were the norm and recuperative support of debility was usually convalescent aftercare, the modern practitioner will be less often involved in first-line treatment. Patients will more often report for help after years of ill health or when conventional medicine has run out of options.
There are many diseases that can lead to such signs of debility as tiredness, inability to rest, weakness, depression, wasting and anorexia. Indeed, any illness of sufficient duration or severity can lead to such symptoms; chronic low-grade infections, especially viral infections, are particular precursors in modern times. In some cases severe or traumatic diseases from the distant past can lead to a legacy of weaknesses of this type. A few are constitutionally enfeebled and are prone to debilitating responses to a range of stressors. A good practitioner will obviously seek to address current problems as far as possible. However, one of the prominent elements of a debilitating condition is that the weakness imposes its own limitations on any treatment. It is often impractical to embark upon the usual treatment strategy while the patient is at a low ebb, as even the gentlest remedies can provoke uncomfortable responses.
Finding a regime of treatment that simply addresses the debility with little consideration of the causes or background factors might be the only strategy feasible if the condition is especially severe. The principles involved in such approaches can best be reviewed for a classic modern syndrome of debility – chronic fatigue syndrome.
Chronic fatigue syndrome
Although the name might be relatively new, chronic fatigue syndrome (CFS) is not a new disorder. While the affliction, described as ‘neurasthenia’ in Victorian times, does not necessarily represent an early forerunner, the ‘bed cases’ or ‘sofa cases’ reported among middle-class women in the period from 1860 to 1910 probably were CFS and, by the time of World War I, a syndrome resembling CFS was a common complaint in Europe and North America.1 CFS is also known as postviral fatigue syndrome or myalgic encephalomyelitis (ME). Although the medical profession was reluctant at first to recognise CFS as a physical disorder rather than a variant of depression or neurosis, this opinion is changing. Nonetheless, treatment of CFS as a psychiatric problem is still relatively widespread, with a conventional treatment preference for antidepressants. More enlightened thinking is to see the disturbance in biopsychosocial terms, as a complex disruption of a psychoneuroimmunoendocrine information network which, when dysregulated, leads to both psychological and somatic symptoms.2 The basic principles applied here in the discussion of phytotherapy of CFS will be relevant to the management of patients suffering from most types of fatigue.
CFS was formally defined in 1988 as disabling fatigue of at least 6 months duration of uncertain aetiology. Additional symptoms can include mild fever, sore throat, painful lymph nodes, weight gain, exertional malaise, muscle weakness, muscle and joint pain, headaches, depression, light-headedness, anxiety, visual and cognitive impairment and disturbed sleep patterns. It usually has a relatively definite onset that resembles influenza. Six of these additional symptoms must be present, plus two or more of the following signs: low-grade fever, non-exudative pharyngitis and palpable or tender lymph nodes.3
This 1988 definition was amended in 1994 by the United States Centers for Disease Control and Prevention (CDC) to the following:4
1. Having severe chronic fatigue for at least 6 months or longer with other known medical conditions (that can cause fatigue) excluded by clinical diagnosis.
2. Concurrently having four or more of the following symptoms:
3. The symptoms must have persisted or recurred during 6 or more consecutive months of illness and must not have predated the fatigue.
Currently there is no accepted biochemical test for the condition. Another problem is that the definitions are somewhat restrictive. Many patients with chronic, unexplained fatigue and typical symptoms of CFS may not exactly fulfil the above criteria.
Possible causes of chronic fatigue syndrome
Viruses
The fact that CFS can occur in epidemics has always pointed to an infectious origin. However, despite the fact that various researchers have implicated a number of viruses, a clear association with a single viral infection has not been established. Originally, Epstein-Barr (EBV) virus was thought to be the cause, since CFS can follow glandular fever. Human herpesvirus-6 (HHV-6) is another virus that has been plausibly lined to CFS outbreaks and prevalence.5
The link between CFS and some kind of enterovirus, possibly a Coxsackie B virus, is particularly interesting. Coxsackie B viruses are related to the polio virus, which can infect and weaken muscle tissue. There was evidence from British research that enteroviral RNA occurs in the muscle tissue of CFS patients6 and this may lead to mitochondrial injury.7 However, a Spanish investigation found only minor changes in muscle tissue, which did not support the hypothesis that viral infection is a cause of muscle fatigue.8 Also the British research group appears to have abandoned their stance on enteroviruses, concluding it was unlikely that a persistent enterovirus plays a pathogenic role in CFS.9 Nonetheless, an effect in initiating the disease process should not be excluded,9 and other groups have certainly pursued the enterovirus connection.10,11 More recently, the human parvovirus (HPV)-B19 has been the most reported CFS-associated virus,12 and a new infectious human gamma-retrovirus, xenotropic murine leukaemia virus-related virus, has also been detected at high levels in CFS patients.13,14
The only sense which can be made of this research is that:
1. Either a number of viruses are capable of triggering CFS, in which case CFS is not an infection in the strict meaning of the term because there is no single causative agent
2. CFS may involve the reactivation of the immune response to previous viral infections. In other words, the immune system may be fighting the ghosts of past viral infections.
On the latter point it is interesting to note that several investigators have reported increased 2′,5′-oligoadenylate synthetase activity in the mononuclear cells of patients with CFS, with levels correlating with disease severity. This protein is induced by interferons and is an important defence against viral proliferation.10 It has been suggested that stress-induced EBV reactivation may represent the initial event that leads to a disturbance of immune memory, which in turn leads to a prolongation and accentuation of viral symptoms.
It is worthwhile to examine the implications of CFS epidemics. The Royal Free Hospital epidemic in London was a famous epidemic where a polio-like illness struck down many people. The illness also affected cranial nerves, which is not a feature of CFS. The majority recovered in a matter of weeks to months, but a significant number went on to develop CFS. These susceptible people among the staff at the Royal Free Hospital were left with CFS, and the original epidemic was probably a Coxsackie viral infection.15 Hence the CFS was probably caused by this viral trigger. The viral infection occurred in an epidemic and created a related epidemic of CFS.15 It is likely that the same conclusion could be drawn from studying other CFS epidemics.
Other microorganisms
Other microorganisms have also been linked to the incidence of CFS. Polymerase chain reaction (PCR) techniques have established a connection between possible mycoplasmal blood infections and CFS in 50% to 60% of patients.16–18 However, until these organisms are isolated and cultured from the blood of CFS sufferers, such a link must be regarded as tenuous. Mycoplasmal DNA was not detected in the plasma of 34 sufferers of CFS.19
Infection with coagulase negative Staphylococcus has been described as another indirect connection. The pattern of muscle catabolism seen in CFS corresponds to that produced by this organism.20 Also CFS and fibromyalgia patients treated with staphylococcus toxoid vaccine do show some clinical improvement.21
Infection with yeast, possibly Candida albicans, has been hypothesised.22 Since there is a high prevalence in CFS sufferers of non-allergic sinusitis, and this condition is associated with fungal infection and fungal allergy, it was suggested that the upper respiratory tract could harbour a chronic yeast infection in CFS.23
Chronic Lyme disease due to past or current infection with Borrelia burgdorferi has been described as a possible variant of CFS, although this connection is controversial.24
Inflammatory disease and immune abnormalities
Chronically elevated levels of proinflammatory cytokines are associated with inflammatory diseases and psychological symptoms of depression and tiredness. There is now evidence that fatigue correlates closely with inflammatory symptoms (relating to allergy, gastrointestinal upset and to pain) in otherwise healthy populations. It has been suggested that immune dysregulation may explain the existence and covariation of psychological and physical symptoms in the healthy population, including people with medically unexplained symptoms.25 The immune abnormalities that occur in CFS are, however, inconsistent, perhaps because different viral triggers might cause different malfunctions. One important study found no difference between CFS patients and controls for any white blood cell counts, save the CD8 T-lymphocytes.26 These cells were activated as in a viral infection and the cytotoxic cell subset was increased. These differences were significant (p=0.01) in patients with major symptoms of CFS. The study was noteworthy because of the large number of patients involved and also because the degree of these changes corresponded to the severity of the CFS. The authors concluded that immune activation in CFS leads to increased secretion of cytokines causing the observed symptoms. Their findings were consistent with chronic stimulation of the immune system, perhaps by a virus. If this is correct, the feeling of malaise experienced in the early stages of influenza, when cytokine output is increased, is similar to the way CFS sufferers must feel most of the time. Reviews have supported findings that proinflammatory cytokines such as IL-1, IL-6 and TNF-alpha are raised in CFS, with impaired natural killer (NK) cell function as well. Cancer patients treated with the cytokine IL-2 to boost immunity experience side effects remarkably similar to CFS. Serum levels of some cytokines are often raised in CFS. For example, levels of IL-1 alpha,27 TNF-alpha10 and TNF-beta28 were significantly more often increased in CFS patients. A recent review concluded that, despite the heterogeneity in CFS, there is growing evidence that immune dysfunction plays an important role, with cytokine dysregulation a key feature.10
Reduced NK cell activity has been reported in several studies. For example, a correlation between low levels of NK cell activity and severity of CFS was found in 20 CFS patients.29 Also, a marked decrease in NK cell activity was found in almost all patients with CFS, as compared with healthy individuals.30 However, a Danish study found that NK cell activity in CFS patients was no different from healthy controls.31 Another relatively common, but inconsistent, finding is the reduced response of lymphocytes to stimulation by mitogens.32,33
An increased occurrence of autoantibodies such as rheumatoid factor, thyroid antibodies and antinuclear antibodies (ANA) can be found in CFS patients.34 This, together with an observed high incidence of circulating immune complexes, led a German research team to conclude that CFS is associated with, or is the beginning of, manifest autoimmune disease.35 These findings were somewhat supported by a large study on 579 patients from Boston and Seattle that found levels of immune complexes were abnormal in 35% of CFS patients compared with 2% of controls (p=0.0001), and ANA was abnormally high in 15% of CFS patients compared with 0% of controls (p=0.003).36 The same study found that serum cholesterol and IgG levels were also significantly raised in CFS. Immunodeficiency and disturbed immunological memory have also been explored as contributors to the pathophysiology of CFS.10
Gut dysfunction
In any condition marked by immunological disturbances, the digestive system is likely to be implicated. There is increasing evidence of this. CFS is marked by lower levels in the bowel of Bifidobacteria and higher levels of aerobic bacteria and higher prevalence and median values for serum IgA against enterobacteria lipopolysaccharides (pointing also to increased leakiness of the gut wall).37 These findings resonate with clinical experience that symptoms of gut dysbiosis, irritable bowel and food intolerances are widely encountered in chronically fatigued patients.
Circulatory abnormalities
A study of 24 CFS patients who were 50 years or younger found that 100% had slightly abnormal ECG readings, compared with only 22.4% of controls (p<0.01).38 Mild left ventricular dysfunction was found in 8 of 60 patients with CFS, and gross dysfunction occurred with increasing workloads.38 Some studies suggest that CFS patients have a low cardiac output due to a small heart39,40 or perhaps a comorbid hypovolaemic condition.41 Lower blood pressure and abnormal diurnal blood pressure can be associated with CFS.42
A significant breakthrough came with the study by Peckerman and co-workers published in 2003.43 Impedance cardiography and symptom data were collected from 38 patients with CFS grouped into cases with severe (n=18) and less severe (n=20) illness and compared with those from 27 matched, sedentary control subjects. The patients with severe CFS had significantly lower stroke volume and cardiac output than the controls and less ill patients. Post-exertional fatigue and flu-like symptoms of infection differentiated the patients with severe CFS from those with less severe CFS (88.5% concordance) and were predictive (p<0.0002) of lower cardiac output.
Simpson and co-workers found that subjects complaining of chronic fatigue were more likely to have abnormally shaped (nondiscocytic) red blood cells.44 They concluded that this association between increased nondiscocytes and impaired muscle function could indicate a cause and effect relationship, which would be in agreement with the physiological concept of fatigue resulting from inadequate oxygen delivery.44 Simpson advocated the use of evening primrose oil and fish oil to decrease nondiscocytes, and given the favourable influence of Ginkgo biloba on red blood cell fragility and blood rheology, it might also be indicated.
Regional cerebral blood flows to the cortex and basal ganglia were significantly reduced in a majority of CFS patients.45,46 This finding of reduced regional cerebral blood flow in CFS is supported by a study in older patients, which found that the abnormal blood flow in CFS was different to that observed in depression.47
Delayed orthostatic hypotension caused by excessive venous pooling (and also linked to a subnormal circulating erythrocyte volume) is a frequent finding in CFS that appears to be linked to fatigue.48–51 This can be associated with orthostatic hypocapnia.52
An autonomic imbalance with a sympathetic dominance expressed as dysregulated cardiac function has been observed in several studies. For example, heart rate during sleep and mean arterial blood pressure were significantly higher in CFS patients,53 and another study also observed a higher heart rate together with reduced heart rate variability (a sign of sympathetic dominance) during sleep.54 Reduced heart rate variability also predicts poor sleep quality in CFS55 and is associated with orthostatic stress in CFS patients.56
Brain and cognitive abnormalities
Magnetic resonance imaging (MRI) scans of the brains of CFS sufferers found a high incidence of inflammation (oedema and demyelination) in association with serological evidence of active HHV-6 infection.57 This controversial finding of brain abnormalities in CFS has been somewhat supported by a study which observed that CFS patients had significantly more abnormal scans than controls: 27% versus 2%.58 However, the authors felt that this might instead indicate that some patients labelled with CFS could actually be suffering from other medical conditions. Abnormal MRI and single-photon emission computed tomography (SPECT) scans were found with far greater frequency in CFS patients compared to normal controls.59 SPECT abnormalities were present in 81% of CFS patients versus 21% of control subjects (p<0.01).59
The presence of brain abnormalities in CFS, as assessed by MRI, was related to subjective reports of poor physical function60 and mental fatigue.61 A strong correlation in CFS between brainstem grey matter volume and pulse pressure suggested impaired cerebrovascular autoregulation.62 However, abnormal MRI findings have not always been observed in CFS.63
It should also be stressed that modern techniques of brain imaging are highly sensitive, and these findings do not necessarily indicate gross organic brain defects. They are probably more indicative of chronic encephalitis, which is possibly either viral or immunological in origin.
A number of objective tests have revealed memory deterioration in CFS patients compared with healthy controls, but findings between studies have not been consistent. Short-term memory,64 general memory,65 retrieval from semantic memory66 and memory requiring cognitive effort67 have been found to be impaired. Attention can also be impaired.68 However, other studies have found that memory was not affected,69 or was only mildly impacted.70 It has been suggested that impaired information processing, rather than a primary memory dysfunction, may underlie the cognitive problems that afflict so many patients with CFS.71 A 2010 meta-analysis that included 50 eligible studies concluded that patients with CFS demonstrate moderate to large impairments in simple and complex information processing speed and in tasks requiring working memory over a sustained period of time.72
Pituitary and hypothalamic abnormalities
Patients with CFS have a mild central adrenal insufficiency secondary to either a deficiency of corticotropin-releasing hormone or some other central stimulus to the pituitary-adrenal axis.73 This leads to a decreased response of the adrenal cortex. Abnormalities in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis are also a well-recognised feature of endogenous depression. It has been suggested that, since cytokines potently influence the HPA axis, their activation may underlie many of the features found in CFS and depression.74
A comprehensive 2007 review of this topic concluded that there is distinct evidence for a hypofunction of the HPA axis in a proportion of patients with CFS, despite negative studies and methodological difficulties.75 About half the reviewed studies indicated this finding; the others found no significant changes. The following mechanisms underlying the hypocortisolism were discussed:
• Reduced biosynthesis of releasing factors (CRH, ACTH)
• Downregulation of central receptors
• Increased negative feedback sensitivity to endogenous glucocorticoids
• Decreased availability of free cortisol
• Reduced effects of cortisol on target tissues (relative cortisol resistance).
The above findings suggest that a focus on regulating and restoring normal HPA function in CFS should be a priority for herbal clinicians, one that they are well equipped to deal with. Most patients with CFS were found to have sleep disorders that are likely to contribute to the daytime fatigue and may also be important in the aetiology of the syndrome.76,77 CFS patients exhibited significant elevations in fatigue, subjective sleep disturbance and objective sleep disorders compared to MS patients and a healthy control group.78
A 2008 study confirmed these observations, finding that CFS patients had significant differences in polysomnographic recordings compared with healthy controls and felt sleepier and more fatigued than controls after a night’s sleep.79 CFS patients also had less total sleep time, lower sleep efficiency and less rapid eye movement (REM) sleep than controls.
Biochemical abnormalities
It has been hypothesised that the imbalances in immune function, the HPA axis and the sympathetic nervous system in CFS can be explained by changes in essential fatty acid (EFA) metabolism. Dietary EFA modulation afforded substantial improvement in a majority of cases.80 A Japanese study did find that serum concentrations of EFAs were depleted in CFS sufferers81 and controlled clinical trials of evening primrose oil82 and fish oil demonstrated significant symptom reduction.83 Japanese scientists have found lower levels of serum acylcarnitine in CFS, which they proposed might explain the fatigue and muscle weakness.84 Also, the concentration of serum acylcarnitine tended to increase to normal with recovery from fatigue in CFS.65 However, an open clinical study in 20 CFS patients found no improvement after 3 months of L-carnitine therapy.8
Studies on the magnesium status of CFS patients have not resolved this issue. A report on one patient found considerable improvement after 6 weeks of therapy with intravenous magnesium sulphate,85 but a study of 89 patients with CFS found no evidence of magnesium deficiency in any patient.86
When serum folate levels of 60 patients with CFS were assayed it was found that 50% had values below 3.0 µg/L.87 The authors concluded that some CFS patients are deficient in folic acid.
Clinical trials
There have been a few studies of herbal interventions for CFS or chronic fatigue (not necessarily CFS) in randomised, controlled trials. A randomised, double blind, placebo-controlled clinical study of a standardised Lycium barbarum juice product was conducted (by the manufacturer) with 60 older healthy adults (55 to 72 years old). Participants either took 120 mL/day of the juice, equivalent to at least 150 g of fresh fruit, or placebo for 30 days. The Lycium group showed significantly improved measures of fatigue and sleep, and a statistically significant increase in the number of lymphocytes and levels of IL-2 and immunoglobulin G compared to the placebo group. The number of CD4, CD8 and NK cells and levels of IL-4 and immunoglobulin A were not significantly altered.88
Consuming high-cocoa liquor/polyphenol-rich chocolate 45 g/day for 8 weeks was beneficial in improving fatigue and residual function in CFS, compared with the consumption of simulated isocaloric low polyphenol chocolate.89 In a separate study, flavanol-rich cocoa in drinks at 520 mg and 994 mg were compared to matched controls in a randomised, controlled, double blinded, three period crossover trial in 30 healthy adults undergoing the sort of sustained mental demands often leading to fatigue. Various assessments, psychomotor tests and measures of cognitive performance showed the highest dose of cocoa flavanols had the clearest benefit on mood and psychomotor performance in these circumstances.90 While the mechanisms underlying the effects are unknown, they are thought to be related to known effects of cocoa flavanols on endothelial function and blood flow, a further link between inflammatory processes and the aetiology of fatigue that picks up discussions earlier in this chapter.
A randomised, double blind, controlled trial found that a combination of Astragalus and Salvia miltiorrhiza ameliorated chronic fatigue.91 (See the Astragalus monograph for more details.) There is also the trial assessing Rhodiola in chronic fatigue mentioned later in this chapter.
Phytotherapeutics
Clinical impressions of fatigue
CFS appears to involve a complex interaction between emotional, infectious and environmental stressors leading to subtle immune dysfunction. The extreme debility sometimes encountered has the unfortunate effect of blocking many treatment approaches: rest may be disrupted, exercise may be debilitating and even the simplest foods may seem to be too demanding. Many otherwise useful remedies may be too stimulating or unsettling.
Fatigue may take different forms and arise from different stresses. There may be a deficiency condition, there may be an obstruction to normal functions (such as the effects of clinical depression) or fatigue may follow excessive activity, perhaps marked by anxiety and nervous stress. In other cases it may predispose to recurrent infections, creating a vicious cycle. The therapeutic approach in each case will be different. In the first instance, nutritional and supportive therapies will dominate. In the second, there may be the need to embark upon substantial constitutional and metabolic strategies. Where tension is the predominant factor then repair will be difficult if there is not some relaxant or even sedative relief. If poor immune function is evident, then emphasis needs to be placed here.
The majority of CFS patients were probably devitalised before they contracted the disorder. This might have been due to emotional pressures, work pressures, family pressures, ambition, toxins, pregnancy, or even a bad diet, but the end result is the same. This observation is supported by the finding that stress is a significant predisposing factor in CFS.92 Any stressor, be it chemical, physical, biological or emotional, then acts to aggravate this condition. This reduced capacity to cope with stress is a key factor in creating the vicious cycle that perpetuates the syndrome.
The devitalisation leads to weakened immunity and finally to an abnormal immune response to a viral infection. A stalemate is reached where the resultant hyperimmune state causes autotoxicity, but is not sufficiently focused to resolve a viral presence, or any other cause, and restore health. It is a curious state where some compartments of the immune system are overactive, but other compartments are deficient.93Figure 8.1 summarises the interplay between psychosocial, immune and viral factors in the initiation and perpetuation of CFS.10
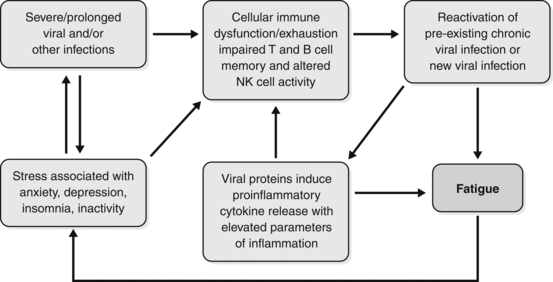
Figure 8.1 The inter-relationship between psychosocial, immune and viral factors in the initiation and perpetuation of chronic fatigue.10
Reproduced from Bansal AS, Bradley AS, Bishop KN, et al. Chronic fatigue syndrome, the immune system and viral infection. Brain Behav Immun 2011, with permission from Elsevier.
Sometimes benefits will follow a focus on what could be exacerbating or even causative factors. These might include:
• intestinal dysbiosis, endotoxaemia or similar syndromes, other syndromes involving autotoxicity, chronic inflammation
• allergies or food intolerances
Above all else, it is important to ensure that sleep and rest are adequate and much useful treatment effort can be directed to this end as a first priority. Whatever the initiating disturbance, the treatment of fatigue must be marked by extreme gentleness and patience.
Convalescence
With any fatigue syndrome the fundamental principle of treatment is to set up an appropriate recuperative regime. Remedies should be set against a wider programme of convalescence. Convalescence as a strategy is outlined in Chapter 3. Extensive and appropriate rest is essential and may need to be supported by treatment to help with sleep and relaxation: exercise, even if minimal, will help engage adrenosympathetic disturbances; the diet should be based on the most easily assimilable foods possible. Only when such a regime is in place can herbal treatment have a chance of facilitating further improvements.
Herbal remedies useful in CFS include the following.
Tonic and adaptogenic herbs
Tonics help revitalise the patient and adaptogens improve the response to stress. Tonics were traditionally used to help build strength after illness and trauma and may also build immune function.
Major herbs in this group are:
• Astragalus membranaceus (Astragalus): tonic and immune enhancing (see monograph)
• Eleutherococcus senticosus (Siberian ginseng): adaptogenic, stimulates T-lymphocyte function (see monograph)
• Lycium spp. (goji): a tonic remedy in China with some evidence as an adaptogen
• Panax ginseng (Ginseng): tonic, adaptogenic, stimulates hypothalamic output and ACTH and hence adrenal cortex function, increases stamina, spares muscle use of carbohydrate (see monograph)
• Rhodiola rosea: a herb with adaptogenic and ergogenic reputations
• Schisandra chinensis: a herb with adaptogenic and hepatoprotective reputations94
• Withania somnifera (Withania): a tonic herb which is not stimulating and facilitates sleep (as its name implies), hence is ideal in situations of sympathetic dominance (see monograph).
Of this group, most interest in recent years has been in Rhodiola, with a wide range of clinical studies reported. These have been unsympathetically reviewed95 and defended.96 However, in one case a substantial positive study has been reported: 60 men and women with stress-related fatigue were randomised into two equal groups, one received four tablets daily of standardised Rhodiola extract (576 mg/day), and the other placebo. After comparing results for various psychomotor and quality of life tests and serum cortisol levels, it was concluded that, compared with placebo, repeated administration of this particular extract exerts an anti-fatigue effect with increased mental performance, ability to concentrate and decreased cortisol response to awakening stress.97 This probably also has relevance to the use of this herb in CFS. Rhodiola has the additional advantage that its efficacy in depression has been demonstrated in a randomised, placebo-controlled trial and also has considerable pharmacological support.98
Adrenal supportive herbs
The main herbs for this purpose are Glycyrrhiza (licorice) and Rehmannia glutinosa (see also the relevant monographs). There is one case report of recovery from CFS with the use of licorice that speculates a role in adrenocortical function.99 Another case report from Japan observed that a chronic fatigue patient went into remission when she developed hyperaldosteronism due to an adrenal tumour. When the adrenal tumour was removed the fatigue returned.100 Licorice in high doses can cause pseudoaldosteronism due to its aldosterone-like action, but obviously should not be used to this level. A high-potassium, low-sodium diet, as in the Gerson therapy, can also raise plasma aldosterone.
Immune-enhancing herbs
Although these may seem contraindicated, immune-enhancing herbs are often needed to help prevent the recurrent viral infections that can plague patients with CFS. In cases of infection, treatment with tonic herbs may need to be discontinued so that defensive measures can be applied. Echinacea angustifolia and E. purpurea root are safe to use since, on current knowledge, they mainly enhance innate immunity. This can improve antigen recognition, which leads to better immune responsiveness. Picrorrhiza kurroa should be used with caution as it is a potent promoter of all aspects of immune function, but it may be indicated for patients who have frequent viral infections. Andrographis and Astragalus also have a role, especially the latter where there is chronic immune depletion.
Antiviral herbs
Although the viral association is not always clear, these herbs can be useful in many cases. Hypericum perforatum (St John’s wort) may contribute to antiviral and antidepressant activity. Hypericum is probably active against enveloped viruses such as EBV and HHV-6. Thuja occidentalis is also active against enveloped viruses as well as naked viruses, such as the wart virus and enteroviruses.
Immune-depressing and anti-inflammatory herbs
There are a few non-toxic herbs that can depress immune function and may be useful at some stages of treatment. The safest to use is the Indian herb Hemidesmus indicus (Indian sarsaparilla). Rehmannia, Bupleurum and Boswellia can be useful anti-inflammatories and may downregulate cytokine production and responses (see monographs).
Others
Ginkgo biloba, Salvia miltiorrhiza and Zanthoxylum (prickly ash) can improve blood flow. Ginkgo decreases erythrocyte fragility and improves blood rheology and short-term memory. Valeriana (valerian), Passiflora incarnata (passionflower), Piper methysticum (kava) and other such herbs will help the disordered sleep pattern and sympathetic dominance. Crataegus (hawthorn) may help any cardiac and circulatory abnormalities and the sympathetic dominance. Butcher’s broom (Ruscus aculeatus) and horsechestnut (Aesculus hippocastanum), being venous toning, should be considered for orthostatic symptoms (see monographs). EFA therapy with evening primrose oil and fish oil is also recommended.
Notes about treatment
Deep-seated devitalisation is a major part of CFS, so results must be measured in months not weeks. If results are slow to come about the patient should be encouraged, since improvement usually does occur with consistent use of herbs. Patients must be instructed not to overexert themselves when they begin to feel improvement, as this can cause a relapse. They should only partake in mild exercise and not exercise beyond the point of fatigue. Sleep helps to restore the immune system and CFS patients will not improve unless they have adequate sleep, which may be more than they needed previously. They must avoid stress and emotional crises as much as possible. The importance of stress as a cause and sustaining factor of CFS should not be underestimated and appropriate lifestyle measures must be incorporated into the treatment plan.
Case History
‘William’, aged 48, sought treatment for CFS, from which he had suffered for 3 years after exposure to Ross River virus. Symptoms included aches and pains, especially in the first 6 months after the infection, malaise, mild fever, physical weakness, lack of stamina and poor sleep. He worked as a manager at a cemetery and was still able to manage (barely) his job, despite the CFS. He was previously very active. Current medication was salbutamol for asthma and the occasional use of a benzodiazepine to help his sleep
He was placed on the following herbal treatment:
| Echinacea angustifolia and E. purpurea (root) | 1:2 | 35 mL |
| Glycyrrhiza glabra (high in glycyrrhizin) | 1:1 | 15 mL |
| Astragalus membranaceus | 1:2 | 20 mL |
| Hypericum perforatum (high in hypericin) | 1:2 | 20 mL |
| Withania somnifera | 1:1 | 20 mL |
| TOTAL | 110 mL |
Dose: 8 mL with water twice a day.
B. Withania (600 mg) and Korean ginseng (125 mg) tablets 3/day
There was not much improvement over the first 8 to 12 weeks, but after that he felt the herbs were helping, especially the feeling of malaise and low energy. He then changed to a more stressful job, but was able to cope well with this, relying on the support of the herbal treatment. After another 8 weeks he reported a noticeable improvement all round and was able to undertake a regular programme of exercise.
Case History
‘Kylie’, aged 17, had glandular fever 6 years previously. She had suffered from fatigue ever since, but after beginning Year 12 at school her fatigue was particularly severe. Often catching colds and influenza, her attendance was less than 50%. Even on ‘well’ days, she often did not have the energy to attend school. Herbal treatment consisted of the following basic formula (written for 1 week):
| Astragalus membranaceus | 1:2 | 30 mL |
| Panax ginseng (standardised extract) | 1:2 | 15 mL |
| Ginkgo biloba (standardised extract) | 2:1 | 20 mL |
| Echinacea angustifolia root | 1:2 | 35 mL |
| Total: | 100 mL |
Dose: 5 mL with water three times a day.
In addition, Withania 1:2 5 mL with water once a day was prescribed.
If a cold came on, the above treatment was stopped for 3 to 4 days and the following formula was taken:
| Zingiber officinale | 1:2 | 10 mL |
| Echinacea angustifolia root | 1:2 | 40 mL |
| Euphrasia officinalis | 1:2 | 20 mL |
| Achillea millefolium | 1:2 | 30 mL |
| Total: | 100 mL |
Dose: 5 mL with warm water up to five times a day.
Initially, Kylie had difficulty taking the herbal formula because it was too stimulating. So the dose was reduced to half and gradually increased. There was only slight improvement in her condition for 3 months, but then gradual and steady progress was made. While she received herbal treatment from time to time, she was free of CFS for more than 2 years.
Case History
‘John’, was 35 and had not worked for 3 years. By the time he sought herbal treatment he complained that he was getting sicker and sicker. He experienced constant headaches and had suffered from chronic sinusitis for about 10 years. His history showed a previous high exposure to insecticides and years of overwork due to family pressures. His wife could not cope with his not working and his marriage was strained. Various formulas were given but the treatment settled at the following (for 1 week):
| Panax ginseng (standardised extract) | 1:2 | 15 mL |
| Astragalus membranaceus | 1:2 | 30 mL |
| Crataegus spp. fol. | 1:2 | 20 mL |
| Ginkgo biloba (standardised extract) | 20 mL | |
| Picrorrhiza kurroa | 1:2 | 15 mL |
| Glycyrrhiza glabra (high in glycyrrhizin) | 1:1 | 20 mL |
| Scutellaria baicalensis | 1:2 | 30 mL |
| Total: | 150 mL |
Dose: 8 mL with water three times a day.
In addition, Echinacea angustifolia root 1:2 5 mL once a day was prescribed from time to time. Also an ‘acute formula’, similar to the one for ‘Kylie’, was taken during colds and influenza instead of the basic formula. The Crataegus was for the headaches and circulation and the Baical skullcap for the sinusitis. ‘John’ actually worsened in the first 3 months of treatment, probably because of the natural progression of the disorder. However, after 5 months he thanked the friend who recommended herbal treatment because it was ‘the best thing I could have done’. He gradually improved over a period of several more months.
Tonics
Plant remedies traditionally used as tonics
Cautions in the use of tonics
The use of tonic herbs may be difficult in the following circumstances:
Application
The state of digestion is the main determinant of dosage times. Tonics may be best taken with or after meals if the stomach and digestive system are weakened; in severe cases, they may need to be taken with fluid nourishment. Dosage should be rather more than less frequent: ‘little and often’ might be a useful axiom. Tonics taken immediately before bed can help when sleep is non-restorative. Withania is ideal for this.
Long-term therapy with tonics is generally indicated and often advisable.
Chinese tonics
In Chinese medicine, tonic remedies are generally divided into four groups, depending on whether they are seen to particularly support qi, yang, xue or yin. The first two groups tend to be warming, the last two cooling. They are often more dynamic than the tonics listed above and may thus be more likely to generate adverse reactions. It is more important, therefore, to be careful in their prescription and to take close account of the interpretation of debilitated conditions that Chinese practitioners use. However, they reflect a perspective on debility and its treatment that is not well articulated in the Western traditions. This works both ways: unfortunately methodological limitations have also undermined the evidence for efficacy in the West so far published.101
In the following review, Chinese terms will therefore be used. They are briefly introduced in the summary of Chinese herbal medicine in this text (see p. 5) but any practitioner wishing to apply them should be well trained in that tradition. Nevertheless, there is some overlap with Western remedies and some useful insights are possible for a Western phytotherapist.
Qi tonics
These support active energies; they are used for depletion of qi, particularly in the Spleen and Lungs.
In the first case, possibly as a result of prolonged illness or constitutional weakness, debility may affect the functions of assimilation and distribution and be associated with such symptoms as fatigue and depression with depressed digestion, diarrhoea, abdominal pain or tension, visceral prolapse, pale yellow complexion with a tinge of red or purple, pale tongue with white coating and/or languid, frail or indistinct pulses. This may lead in turn to a ‘damp’ condition developing.
In the second case, extreme or prolonged stress or disease, or chronic pulmonary disease, leads to depletion or cold in the Lungs, with easy fatigue and prostration associated with disturbances of regulation, shortness of breath or shallow breathing, rapid, slow or little speech, spontaneous perspiration, pallid complexion, dry skin, pale tongue with thin white coating, weak and depleted pulses.
Yang tonics
These remedies support the active energies, particularly those of the Kidneys (but also Heart and Spleen).
Deficient Kidney yang leads to listlessness with a feeling of cold and cold extremities, back and loins; there may be weak legs, poor reproductive function, frequency of micturition, nocturia, diarrhoea (especially early in the morning), pale complexion and submerged weak pulses.
Deficiency affecting the Heart involves poor performance and coordination associated with profuse cold sweating, asthmatic states, thoracic or anginal pain on exertion, palpitations and fear attacks, cyanosis, white tongue coating and/or diminished, hesitant or intermittent pulses.
Xue tonics
These are remedies that support more substantial energies, those manifesting in substantial disturbances or pathologies. By definition, such disturbances are serious and profound and treatment will need to be prolonged. Symptoms of depletion of xue may include cyanosis, pallor, vertigo or tinnitus, palpitations, loss of memory, insomnia or menstrual problems.
Yin tonics
These are remedies for replenishing the body fluids and essence, supplying condensed energies and nourishment, for the most depleted conditions. Areas in most need of support are the Kidneys, Liver, Lungs and Stomach.
Deficient Kidney yin often follows very serious debilitating disease or, alternatively, extended sexual or alcohol abuse or overwhelming nervous stress. It may manifest as a deficient Fire condition, marked by a pale complexion with red cheeks, red lips, dry mouth, dry but deeply red tongue, dry throat, hot palms and soles, palpitations, vertigo or tinnitus, pains in the loins, night sweats, nocturnal emissions, nightmares, urinary retention, constipation, accelerated though weak pulses.
Deficient Liver yin, usually following the above, is often associated with dry eyes, poor vision and vertigo or tinnitus, deafness, muscle twitching, sleeplessness, hot flushed face with red cheeks, red dry tongue with little coating, diminished, stringy and accelerated pulses.
Deficient Stomach yin is marked by anorexia, regurgitation, thirst, abdominal rumbling, red lips and red tongue with no coating.
Deficient Lung yin, often following prolonged exposure to dryness or chronic pulmonary disease, is marked by dry cough, haemoptysis, hoarseness and loss of voice, strong thirst and/or restlessness and insomnia.
References
1. Shorter E. Chronic fatigue in historical perspective. Ciba Found Symp. 1993;173:6–16.
2. Hyland ME. Network origins of anxiety and depression. Behav Brain Sci. 2010;33(2–3):161–162.
3. Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1988;108(3):387–389.
4. Centers for Disease Control and Prevention. CFS Case Definition. <http://www.cdc.gov/cfs/general/case_definition/index.html>. Accessed 28.08.2011.
5. Komaroff AL. Is human herpesvirus-6 a trigger for chronic fatigue syndrome? J Clin Virol. 2006;37(suppl 1):S39–S46.
6. Gow JW, Behan WM, Clements GB, et al. Enteroviral RNA sequences detected by polymerase chain reaction in muscle of patients with postviral fatigue syndrome. BMJ. 1991;302(6778):692–696.
7. Gow JW, Behan WM. Amplification and identification of enteroviral sequences in the postviral fatigue syndrome. Br Med Bull. 1991;47(4):872–885.
8. Grau JM, Casademont J, Pedrol E, et al. Chronic fatigue syndrome: studies on skeletal muscle. Clin Neuropathol. 1992;11(6):329–332.
9. Gow JW, Behan WM, Simpson K, et al. Studies on enterovirus in patients with chronic fatigue syndrome. Clin Infect Dis. 1994;18(suppl 1):S126–S129.
10. Bansal AS, Bradley AS, Bishop KN, et al. Chronic fatigue syndrome, the immune system and viral infection. Brain Behav Immun. 2012;26(1):24–31.
11. Chia JK. The role of enterovirus in chronic fatigue syndrome. J Clin Pathol. 2005;58(11):1126–1132.
12. Frémont M, Metzger K, Rady H. Detection of herpes viruses and parvovirus B19 in gastric and intestinal mucosa of chronic fatigue syndrome patients. In Vivo. 2009;23(2):209–213.
13. Lombardi VC, Ruscetti FW, Das Gupta J, et al. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science. 2009;326(5952):585–589.
14. Coffin JM, Stoye JP. Virology. A new virus for old diseases? Science. 2009;326(5952):530–531.
15. The Medical Staff of the Royal Free Hospital. An outbreak of encephalomyelitis in the Royal Free Hospital group London, in 1955. BMJ. 1957;2:895–904.
16. Nasralla M, Haier J, Nicolson GL. Multiple mycoplasmal infections detected in blood of patients with chronic fatigue syndrome and/or fibromyalgia syndrome. Eur J Clin Microbiol Infect Dis. 1999;18(12):859–865.
17. Vojdani A, Choppa PC, Tagle C, et al. Detection of mycoplasma genus and Mycoplasma fermentans by PCR in patients with chronic fatigue syndrome. FEMS Immunol Med Microbiol. 1998;22(4):355–365.
18. Choppa PC, Vojdani A, Tagle C, et al. Multiplex PCR for the detection of Mycoplasma fermentans, M. hominis and M. penetrans in cell cultures and blood samples of patients with chronic fatigue syndrome. Mol Cell Probes. 1998;12(5):301–308.
19. Vernon SD, Shukla SK, Reeves WC. Absence of Mycoplasma species DNA in chronic fatigue syndrome. J Med Microbiol. 2003;52(Pt 11):1027–1028.
20. Dunstan RH, McGregor NR, Roberts TK, et al. The development of laboratory-based tests in chronic pain and fatigue: (1) Muscle catabolism and coagulase negative staphylococci which produce membrane damaging toxins. JCFS. 2000;7:23–28.
21. Andersson M, Bagby JR, Dyrehag L, et al. Effects of staphylococcus toxoid vaccine on pain and fatigue in patients with fibromyalgia/chronic fatigue syndrome. Eur J Pain. 1998;2(2):133–142.
22. Levin AM. Chronic fatigue syndrome: the yeast concept. JCFS. 2001;8(2):71–76.
23. Chester AC. Yeast and chronic fatigue syndrome. JCFS. 2000;7(3):87–88.
24. Marques A. Chronic Lyme disease: a review. Infect Dis Clin North Am. 2008;22(2):341–360.
25. Whalley B, Jacobs PA, Hyland ME. Correlation of psychological and physical symptoms with chronically elevated cytokine levels associated with a common immune dysregulation. Ann Allergy Asthma Immunol. 2007;99(4):345–348.
26. Landay AL, Jessop C, Lennette ET, et al. Chronic fatigue syndrome: clinical condition associated with immune activation. Lancet. 1991;338(8769):707–712.
27. Linde A, Andersson B, Svenson SB, et al. Serum levels of lymphokines and soluble cellular receptors in primary Epstein-Barr virus infection and in patients with chronic fatigue syndrome. J Infect Dis. 1992;165(6):994–1000.
28. Patarca R, Klimas NG, Lugtendorf S, et al. Dysregulated expression of tumor necrosis factor in chronic fatigue syndrome: interrelations with cellular sources and patterns of soluble immune mediator expression. Clin Infect Dis. 1994;18(1):147–153.
29. Ojo-Amaize EA, Conley EJ, Peter JB. Decreased natural killer cell activity is associated with severity of chronic fatigue immune dysfunction syndrome. Clin Infect Dis. 1994;18(suppl 1):S157–S159.
30. Barker E, Fujimura SF, Fadem MB, et al. Immunologic abnormalities associated with chronic fatigue syndrome. Clin Infect Dis. 1994;18(suppl 1):S136–S141.
31. Rasmussen AK, Nielsen H, Andersen V, et al. Chronic fatigue syndrome–a controlled cross sectional study. J Rheumatol. 1994;21(8):1527–1531.
32. Straus SE, Fritz S, Dale JK, et al. Lymphocyte phenotype and function in the chronic fatigue syndrome. J Clin Immunol. 1993;13(1):30–40.
33. Lloyd A, Hickie I, Hickie C, et al. Cell-mediated immunity in patients with chronic fatigue syndrome, healthy control subjects and patients with major depression. Clin Exp Immunol. 1992;87(1):76–79.
34. Hashimoto N, Kuraishi Y, Yokose T, et al. Chronic fatigue syndrome-51 cases in the Jikei University School of Medicine. Nippon Rinsho. 1992;50(11):2653–2664.
35. Hilgers A, Frank J. Chronic fatigue syndrome: immune dysfunction, role of pathogens and toxic agents and neurological and cardial changes. Wien Med Wochenschr. 1994;144(16):399–406.
36. Bates DW, Buchwald D, Lee J, et al. Clinical laboratory test findings in patients with chronic fatigue syndrome. Arch Intern Med. 1995;155(1):97–103.
37. Lakhan SE, Kirchgessner A. Gut inflammation in chronic fatigue syndrome. Nutr Metab (Lond). 2010;7:79.
38. Lerner AM, Lawrie C, Dworkin HS. Repetitively negative changing T waves at 24-h electrocardiographic monitors in patients with the chronic fatigue syndrome. Left ventricular dysfunction in a cohort. Chest. 1993;104(5):1417–1421.
39. Miwa K, Fujita M. Cardiovascular dysfunction with low cardiac output due to a small heart in patients with chronic fatigue syndrome. Intern Med. 2009;48(21):1849–1854.
40. Miwa K, Fujita M. Cardiac function fluctuates during exacerbation and remission in young adults with chronic fatigue syndrome and ‘small heart’. J Cardiol. 2009;54(1):29–35.
41. Hurwitz BE, Coryell VT, Parker M, et al. Chronic fatigue syndrome: illness severity, sedentary lifestyle, blood volume and evidence of diminished cardiac function. Clin Sci (Lond). 2009;118(2):125–135.
42. Newton JL, Sheth A, Shin J, et al. Lower ambulatory blood pressure in chronic fatigue syndrome. Psychosom Med. 2009;71(3):361–365.
43. Peckerman A, Lamanca JJ, Dahl KA, et al. Abnormal impedance cardiography predicts symptom severity in chronic fatigue syndrome. Am J Med Sci. 2003;326(2):55–60.
44. Simpson LO, Murdoch JC, Herbison GP. Red cell shape changes following trigger finger fatigue in subjects with chronic tiredness and healthy controls. N Z Med J. 1993;106(952):104–107.
45. Ichise M, Salit IE, Abbey SE, et al. Assessment of regional cerebral perfusion by 99 Tcm–HMPAO SECT in chronic fatigue syndrome. Nucl Med Commun. 1992;10:767–772.
46. Biswal B, Kunwar P, Natelson BH. Cerebral blood flow is reduced in chronic fatigue syndrome as assessed by arterial spin labeling. J Neurol Sci. 2011;301(1–2):9–11.
47. Goldstein JA, Mena I, Jouanne E, et al. The assessment of vascular abnormalities in late life chronic fatigue syndrome by brain SPECT: comparison with late life major depressive disorder. JCFS. 1995;1(1):55–79.
48. Streeten DH, Thomas D, Bell DS. The roles of orthostatic hypotension, orthostatic tachycardia, and subnormal erythrocyte volume in the pathogenesis of the chronic fatigue syndrome. Am J Med Sci. 2000;320(1):1–8.
49. Schondorf R, Freeman R. The importance of orthostatic intolerance in the chronic fatigue syndrome. Am J Med Sci. 1999;317(2):117–123.
50. Streeten DH, Anderson GH, Jr. The role of delayed orthostatic hypotension in the pathogenesis of chronic fatigue. Clin Auton Res. 1998;8(2):119–124.
51. Wilke WS, Fouad-Tarazi FM, Cash JM, et al. The connection between chronic fatigue syndrome and neurally mediated hypotension. Cleve Clin J Med. 1998;65(5):261–266.
52. Natelson BH, Intriligator R, Cherniack NS, et al. Hypocapnia is a biological marker for orthostatic intolerance in some patients with chronic fatigue syndrome. Dyn Med. 2007;30(6):2.
53. Hurum H, Sulheim D, Thaulow E, et al. Elevated nocturnal blood pressure and heart rate in adolescent chronic fatigue syndrome. Acta Paediatr. 2011;100(2):289–292.
54. Boneva RS, Decker MJ, Maloney EM, et al. Higher heart rate and reduced heart rate variability persist during sleep in chronic fatigue syndrome: a population-based study. Auton Neurosci. 2007;137(1–2):94–101.
55. Burton AR, Rahman K, Kadota Y, et al. Reduced heart rate variability predicts poor sleep quality in a case-control study of chronic fatigue syndrome. Exp Brain Res. 2010;204(1):71–78.
56. Wyller VB, Barbieri R, Thaulow E, et al. Enhanced vagal withdrawal during mild orthostatic stress in adolescents with chronic fatigue. Ann Noninvasive Electrocardiol. 2008;13(1):67–73.
57. Buchwald D, Cheney PR, Peterson DL, et al. A chronic illness characterized by fatigue, neurologic and immunologic disorders, and active human herpesvirus type 6 infection. Ann Intern Med. 1992;116(2):103–113.
58. Natelson BH, Cohen JM, Brassloff I, et al. A controlled study of brain magnetic resonance imaging in patients with the chronic fatigue syndrome. J Neurol Sci. 1993;120(2):213–217.
59. Schwartz RB, Garada BM, Komaroff AL, et al. Detection of intracranial abnormalities in patients with chronic fatigue syndrome: comparison of MR imaging and SPECT. Am J Roentgenol. 1994;162(4):935–941.
60. Cook DB, Lange G, DeLuca J, et al. Relationship of brain MRI abnormalities and physical functional status in chronic fatigue syndrome. Int J Neurosci. 2001;107(1–2):1–6.
61. Cook DB, O’Connor PJ, Lange G, et al. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage. 2007;36(1):108–122.
62. Barnden LR, Crouch B, Kwiatek R, et al. A brain MRI study of chronic fatigue syndrome: evidence of brainstem dysfunction and altered homeostasis. NMR Biomed. 2011;24(10):1302–1312.
63. Perrin R, Embleton K, Pentreath VW, et al. Longitudinal MRI shows no cerebral abnormality in chronic fatigue syndrome. Br J Radiol. 2010;83(989):419–423.
64. Riccio M, Thompson C, Wilson B, et al. Neuropsychological and psychiatric abnormalities in myalgic encephalomyelitis: a preliminary report. Br J Clin Psychol. 1992;31(Part 1):111–120.
65. Sandman CA, Barron JL, Nackoul K, et al. Memory deficits associated with chronic fatigue immune dysfunction syndrome. Biol Psychiatry. 1993;33(8–9):618–623.
66. Smith AP, Behan PO, Bell W, et al. Behavioural problems associated with the chronic fatigue syndrome. Br J Psychol. 1993;84(Pt 3):411–423.
67. McDonald E, Cope H, David A. Cognitive impairment in patients with chronic fatigue: a preliminary study. J Neurol Neurosurg Psychiatry. 1993;56(7):812–815.
68. Constant EL, Adam S, Gillain B, et al. Cognitive deficits in patients with chronic fatigue syndrome compared to those with major depressive disorder and healthy controls. Clin Neurol Neurosurg. 2011;113(4):295–302.
69. Scheffers MK, Johnson R, Jr., Grafman J, et al. Attention and short-term memory in chronic fatigue syndrome patients: an event-related potential analysis. Neurology. 1992;42(9):1667–1675.
70. Grafman J, Schwartz V, Dale JK, et al. Analysis of neuropsychological functioning in patients with chronic fatigue syndrome. J Neurol Neurosurg Psychiatry. 1993;56(6):684–689.
71. Johnson SK, DeLuca J, Fiedler N, et al. Cognitive functioning of patients with chronic fatigue syndrome. Clin Infect Dis. 1994;18(suppl 1):S84–S85.
72. Cockshell SJ, Mathias JL. Cognitive functioning in chronic fatigue syndrome: a meta-analysis. Psychol Med. 2010;40(8):1253–1267.
73. Demitrack MA, Dale JK, Straus SE, et al. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. 1991;73(6):1224–1234.
74. Ur E, White PD, Grossman A. Hypothesis: cytokines may be activated to cause depressive illness and chronic fatigue syndrome. Eur Arch Psychiatry Clin Neurosci. 1992;241(5):317–322.
75. Van Den Eede F, Moorkens G, Van Houdenhove B, et al. Hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome. Neuropsychobiology. 2007;55(2):112–120.
76. Morriss R, Sharpe M, Sharpley AL, et al. Abnormalities of sleep in patients with the chronic fatigue syndrome. BMJ. 1993;306(6886):1161–1164.
77. McCluskey DR. Pharmacological approaches to the therapy of chronic fatigue syndrome. Ciba Found Symp. 1993;173:280–287.
78. Krupp LB, Jandorf L, Coyle PK, et al. Sleep disturbance in chronic fatigue syndrome. J Psychosom Res. 1993;37(4):325–331.
79. Togo F, Natelson BH, Cherniack NS, et al. Sleep structure and sleepiness in chronic fatigue syndrome with or without coexisting fibromyalgia. Arthritis Res Ther. 2008;10(3):R56.
80. Gray JB, Martinovic AM, Horrobin D. Eicosanoids and essential fatty acid modulation in chronic disease and the chronic fatigue syndrome. Med Hypotheses. 1994;43(1):31–42.
81. Ogawa R, Toyama S, Matsumoto H. Chronic fatigue syndrome – cases in the Kanebo Memorial Hospital. Nippon Rinsho. 1992;50(11):2648–2652.
82. Nicolson GL. Lipid replacement as an adjunct to therapy for chronic fatigue, anti-aging and restoration of mitochondrial function. JANA. 2003;6(3):22–28.
83. Behan PO, Behan WM, Horrobin D. Effect of high doses of essential fatty acids on the postviral fatigue syndrome. Acta Neurol Scand. 1990;82(3):209–216.
84. Kuratsune H, Yamaguti K, Takahashi M, et al. Acylcarnitine deficiency in chronic fatigue syndrome. Clin Infect Dis. 1994;18(suppl 1):S62–S67.
85. Takahashi H, Imai K, Katanuma A, et al. A case of chronic fatigue syndrome who showed a beneficial effect by intravenous administration of magnesium sulphate. Aerugi. 1992;41(11):1605–1610.
86. Hinds G, Bell NP, McMaster D, et al. Normal red cell magnesium concentrations and magnesium loading tests in patients with chronic fatigue syndrome. Ann Clin Biochem. 1994;31(Pt 5):459–461.
87. Jacobson W, Saich T, Borysiewicz LK, et al. Serum folate and chronic fatigue syndrome. Neurology. 1993;43(12):2645–2647.
88. Amagase H, Sun B, Nance DM. Immunomodulatory effects of a standardized Lycium barbarum fruit juice in Chinese older healthy human subjects. J Med Food. 2009;12(5):1159–1165.
89. Sathyapalan T, Beckett S, Rigby AS, et al. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J. 2010;9:55.
90. Scholey AB, French SJ, Morris PJ, et al. Consumption of cocoa flavonols results in acute improvements in mood and cognitive performance during sustained mental effort. J Psychopharmacol. 2010;24(10):1505–1514.
91. Cho JH, Cho CK, Shin JW, et al. Myelophil, an extract mix of Astragali Radix and Salviae Radix, ameliorates chronic fatigue: a randomised, double blind, controlled pilot study. Complement Ther Med. 2009;17(3):141–146.
92. Stricklin A, Sewell M, Austad C. Objective measurement of personality variables in epidemic neuromyasthenia patients. S Afr Med J. 1990;77(1):31–34.
93. Patarca-Montero R, Antoni M, Fletcher MA, et al. Cytokine and other immunologic markers in chronic fatigue syndrome and their relation to neuropsychological factors. Appl Neuropsychol. 2001;8(1):51–64.
94. Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail. An overview of Russian research and uses in medicine. J Ethnopharmacol. 2008;118(2):183–212.
95. Blomkvist J, Taube A, Larhammar D. Perspective on roseroot (Rhodiola rosea) studies. Planta Med. 2009;75(11):1187–1190.
96. Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17(7):481–493.
97. Olsson EM, von Schéele B, Panossian AG. A randomised, double blind, placebo-controlled, parallel-group study of the standardised extract shr-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Med. 2009;75(2):105–112.
98. Sarris J, Panossian A, Schweitzer I, et al. Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol. 2011;21(12):841–860.
99. Baschetti R. Chronic fatigue syndrome and licorice. N Z Med J. 1995;108(998):156–157.
100. Kato Y, Kamijima S, Kashiwagi A, et al. Chronic fatigue syndrome, a case of high anti-HHV-6 antibody titer and one associated with primary hyperaldosteronism. Nippon Rinsho. 1992;50(11):2673–2678.
101. Adams D, Wu T, Yang X, et al. Traditional Chinese medicinal herbs for the treatment of idiopathic chronic fatigue and chronic fatigue syndrome. Cochrane Database Syst Rev. 2009;4:CD006348.
