Peppermint
Synonyms
Pfefferminze, Katzenkraut (Ger), menthe anglaise, menthe poivrée, feuilles de menthe (Fr), menta prima (Ital), pebermynte (Dan).
What is it?
The mints, including peppermint, are amongst the oldest European herbs used for both culinary and medicinal purposes. The Greeks and Romans crowned themselves with peppermint at their feasts and adorned their tables with its sprays. Their cooks flavoured both their sauces and their wines with its essence. Peppermint was cultivated by the Egyptians and is mentioned in the Icelandic pharmacopoeias of the 13th century, but only came into general use in the medicine of western Europe in the 18th century. Mints are used in both home remedies and pharmaceutical preparations to relieve the stomach of intestinal gas associated with the consumption of certain foods; hence the many different varieties of after-dinner mints. Menthol, a major compound in peppermint, has been used as an inhalant for upper respiratory ailments and as an ingredient in many liniments and rubs for sore muscles. Recently, peppermint oil has been established as an evidence-based treatment for irritable bowel syndrome (IBS) symptoms.
Effects
Gastrointestinal spasmolytic; carminative; increases bile production; reduces cough frequency; local anaesthetic; antimicrobial; activates the TRPM8 ion channel in cold-sensitive sensory neurons; regulates body temperature during fever.
Traditional view
Peppermint was used to treat flatulent colic, digestive pain, cramps and spasms of the stomach, dyspepsia, nausea and vomiting, morning sickness and dysmenorrhoea. As an inhalant it was used to relieve the cough of bronchitis and pneumonia and to induce perspiration in the early phase of a cold. The bruised fresh herb was applied over the bowel to allay a sick stomach and the same kind of application was also used to relieve headaches.1,2 An infusion of peppermint in combination with wood betony and caraway was used in the treatment of nervous disorders and hysteria and in combination with elder flowers, yarrow or boneset for the treatment of colds and mild cases of influenza.3
Summary actions
Spasmolytic, carminative, cholagogue, antiemetic, antitussive, antimicrobial, diaphoretic. Locally: antiseptic, analgesic, antipruritic.
Can be used for
Indications supported by clinical trials
Indications supported by trials using menthol: to reduce airway hyper-responsiveness in asthma (by inhalation).
Indications supported by trials using peppermint oil: symptoms of IBS (good evidence); postoperative nausea; bacterial lung infection (by inhalation); topically as an analgesic for headaches and postherpetic neuralgia.
Indications supported by trials using a combination of peppermint oil and caraway oil: non-ulcer dyspepsia.
Indications supported by trials using peppermint leaf in combination with other herbs: for the treatment of dyspepsia.
May also be used for
Extrapolations from pharmacological studies
Reduction in pain sensitivity by activating the endogenous opiate system and TRPM8 ion channels; countering increased bronchial secretion and inhibition of cough; reduction of dental plaque by topical application; antiviral effects by topical application; acceleration of gastric emptying time.
Preparations
As with all essential oil-containing herbs, use of the fresh plant or carefully dried herb is advised. Keep covered if infusing the herb to retain the essential oil.
Dried leaf as an infusion, liquid extract, tincture or essential oil for internal use. The essential oil dissolved in alcohol works well for topical use.
Summary assessment of safety
No significant adverse effects from the ingestion of peppermint leaf are expected, but higher doses of the essential oil can produce a variety of adverse reactions including skin rashes, headaches, bradycardia, muscle tremor, heartburn and ataxia. The oil and herb can rarely cause contact dermatitis and large quantities of the oil in the stomach will predispose to gastric reflux and heartburn (as might be expected from its carminative properties).
Technical data
Botany
Mentha × piperita, a member of the Labiatae (Lamiaceae, mint) family, is a perennial plant approximately 50 cm in height with quadrangular stems terminated with a flower spike consisting of numerous congested whorls. The leaves have very short petioles, are opposed, ovate-lanceolate from a wedge shape to an almost heart-shaped base. They have a venation that gives them a rough-textured appearance, are dark green on the upper surface and slightly paler on the lower. The pinkish mauve flowers are tubular with four lobes, one of which is normally larger than the others, contained within a calyx with five pointed lobes. The fruits are dark, four-sectioned, glossy ovoid cremocarps. The plant is always sterile and has a pungent peppermint scent.6,7 Peppermint is a hybrid species from two parents: Mentha spicata (spearmint) and Mentha aquatica (water mint).8
Adulteration
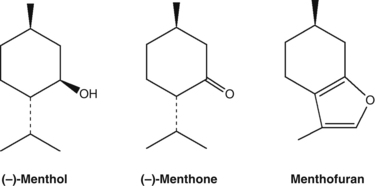
Although many Mentha species are also used, notably Mentha arvensis and M. spicata, the cultivation of most peppermint makes confusion rare. Peppermint oil is liable to augmentation with extra menthol, synthetic or natural menthofuran and menthyl acetate.
Adulteration with Mentha pulegium (pennyroyal) may occur from wildcrafting.9
Key constituents
• Essential oil (0.5% to 4%), consisting predominantly of menthol (35% to 45%) and (−)-menthone (10% to 30%)10
• Flavonoids, tannins (6% to 12%), triterpenes and bitter substances.11
The European Pharmacopoeia recommends that whole peppermint leaf contain not less than 12 mL/kg and the cut leaf not less than 9 mL/kg of essential oil.12 Peppermint oil is obtained by steam distillation from the fresh aerial parts of flowering Mentha × piperita.13
Pharmacodynamics
Gastrointestinal effects
The in vitro effects of peppermint oil on the gastrointestinal smooth muscle of guinea pigs and rabbits resemble those of calcium antagonist drugs. Peppermint oil markedly attenuated contractile responses in guinea pig taenia coli to acetylcholine, histamine, serotonin and substance P. It also reduced contractions evoked by potassium depolarisation and inhibited potential-dependent calcium currents in rabbit jejunum smooth muscle cells in a dose-dependent manner.14 Intravenous administration of an aqueous solution of peppermint oil reduced morphine-induced spasm in Oddi’s sphincter in guinea pigs.15
Peppermint leaf extract demonstrated spasmolytic activity on acetylcholine-, carbachol- and histamine-induced contractions in isolated guinea pig ileum.16,17 An aqueous solution of flavonoids isolated from peppermint inhibited barium chloride-induced contractions in a similar model.18
The intraluminal (topical) administration of peppermint oil to the sigmoid colon of five normal people produced increased intraluminal pressure, abdominal cramps and the urge to defecate and micturate, which suggested a widespread stimulation of smooth muscle.19 This might reflect a local irritant effect of the peppermint oil, since in other studies examining the impact of peppermint oil on colonic spasm during colonoscopy the opposite effect was observed. For example, peppermint oil injected along the biopsy channel of the colonoscope in 20 patients relieved colonic spasm within 30 s, allowing easier passage of the instrument or assisting in polypectomy. Due to the potential irritant action of peppermint oil, a diluted suspension is often used with equally good effects.20 The direct administration of 15 drops of peppermint oil in 30 mL of water into the stomachs of 27 volunteers caused relaxation of the lower oesophageal sphincter and equalisation of intragastric and intra-oesophageal pressures (carminative activity). Reflux occurred in 25 out of 27 patients within 1 to 7 min of administration. The sphincter relaxation lasted approximately 30 s and was terminated by an oesophageal peristaltic wave.21
Since the above research, several clinical trials have demonstrated that intraluminal administration of peppermint oil reduces gastric and colonic spasm, and is safe and useful for upper gastrointestinal endoscopy, colonoscopy and double contrast barium enema examination (DCBE). A prospective, case-controlled study evaluated the efficacy of orally administered peppermint oil (10 mL of a 1.6% emulsified solution) as an antispasmodic for DCBE. Oral peppermint oil emulsification reduced spasm of the oesophagus, lower stomach and duodenal bulb and improved the diagnostic quality of the procedure, without requiring injection of an antispasmodic drug.22 The efficacy of topical peppermint oil in producing duodenal relaxation has also been demonstrated in endoscopic retrograde cholangiopancreatography.23 Clinical studies indicate that the duration of the spasmolytic action of peppermint oil is limited to approximately 20 min.24
The above effects were confirmed in a large study where 409 patients received about 200 mL of an oil-in-water emulsion containing 8 mL/L of peppermint oil and 0.2 mL/L of Tween 80 via a colonoscope using a hand pump.25 A spasmolytic action was seen in 88.5% of treated patients versus 33.3% of 36 controls (p<0.0001). Onset was in seconds and the spasmolytic effect lasted for at least 20 min, although the efficacy was significantly lower in patients with IBS.
Results from a randomised, double blind, controlled trial in 100 patients found that intraluminal administration of a peppermint oil solution had superior efficacy and fewer side effects than injection of hyoscine-N-butylbromide during upper endoscopy, in terms of reducing hyperperistalsis in the stomach.26 A pilot study in 10 healthy men found that peppermint oil (0.64 mL) combined with a test meal accelerated only the early stages of gastric emptying.27 An earlier study found peppermint oil accelerated gastric emptying rate in both dyspeptic patients and controls. The gastric emptying rate of dyspeptic patients became comparable with age-matched controls after administration of 0.2 mL peppermint oil in 25 mL of water (p<0.001).28
Development of symptoms in functional gastrointestinal disorders is frequently preceded by acute gastrointestinal infections and linked to visceral hyperalgesia. Administration of a peppermint and caraway oil preparation reduced experimentally induced visceral hyperalgesia in rats.29 The effects of enteric-coated and non-enteric-coated preparations each containing 90 mg peppermint oil and 50 mg caraway oil were studied on gastroduodenal motility in six healthy volunteers.30 Both preparations caused smooth muscle relaxation, with the effect of the enteric-coated preparation being relatively delayed. Each oil, tested separately by intraduodenal application to healthy volunteers, was found to contribute to this activity.31 Further testing in healthy volunteers (90 mg peppermint oil, 50 mg caraway oil) found that both oils relaxed the gallbladder, but only peppermint oil slowed orocaecal transit time.32 Neither oil in these quantities influenced gastric emptying time.
Peppermint aqueous extract (infusion) and isolated flavonoids given to rats by injection increased bile acid production.18 A single oral dose of peppermint oil (0.83 mL/kg) to rats resulted in a 70% increase in bile flow.33
Peppermint oil in the intestinal lumen at concentrations varying from 1 to 5 mg/mL inhibited enterocyte glucose uptake via a direct action at the brush border membrane in vitro. This was thought to be due to changes in the charge on tight junctions between cells and to an inhibition of sodium-linked active transport. The standard bolus dose of peppermint oil for humans is about 400 mg and this could achieve a local concentration within the tested range in the intestinal lumen during the fasting state.34 An ethanolic extract of peppermint leaf demonstrated antiulcerogenic activity after oral doses when given to rats pre-treated with indomethacin.35
Antimicrobial and antiparasitic activity
Peppermint oil has shown significant antibacterial and antifungal effects in several studies.36–38 Samples of 18 different commercial peppermint oils were tested for antibacterial activity against 25 different species of bacteria and 20 different strains of Listeria monocytogenes isolated from different food sources. Antifungal activity was also assessed against Aspergillus niger, Aspergillus ochraceus and Fusarium culmorum. The chemical composition of the oils varied, with menthone ranging from 16.7% to 31.4%, menthol 32.1% to 49% and menthofuran 5.1% to 12%. Growth of most of the species of bacteria tested, with the exception of Acaligenes faecalis, Flavobacterium suaveolens, Leuconostoc cremoris, Pseudomonas aeruginosa and Streptococcus faecalis, was inhibited by some of the peppermint oils, with nine species being inhibited by all the oils. All strains of Listeria monocytogenes were inhibited by some peppermint oils, nine strains by all of the oils and one strain by only three of the oils. The three filamentous fungi were inhibited by all peppermint oils, but three oils showed a low activity against Fusarium culmorum. The oils showing the most potent antibacterial activity were amongst the most ineffectual oils against one of the fungal species, and there appeared to be an inverse relationship between antibacterial and antifungal activity.36 Another study found that peppermint oil (0.1%) was more inhibitory towards Gram-negative than Gram-positive bacteria.39
Peppermint oil has demonstrated moderate antimicrobial activity towards Candida albicans in vitro. Moderate activity was defined as a MIC (minimum inhibitory concentration) value of between 0.6 and 1.5 mg/mL. (MIC is the lowest concentration of substance needed to prevent the growth of a bacterial or fungal suspension.) The MIC of essential oil of peppermint distilled from fresh leaf in Brazil was measured at 0.6 mg/mL. The ethanol extract was inactive (defined as having a MIC higher than 2 mg/mL). By comparison, the antibiotic drug nystatin had value of 0.05 mg/mL.40 In Yugoslavia, similar results were obtained (MIC 0.8 mg/mL), which was stronger than the antifungal drug bifonazole (2 mg/mL). Peppermint oil was also active against other pathogenic fungi, especially Trichophyton tonsurans (0.4 mg/mL).41
In Turkey, a research team tested four peppermint oils (one from Turkey, two from the USA, one from India) for in vitro antimicrobial activity against pathogenic bacteria. Variation in activity was observed between the oils. The best activity was against Listeria monocytogenes with MIC values for three of the oils ranging from 0.16 to 0.6 mg/mL. Staphylococcus aureus was inhibited by two oils (MIC 0.6 mg/mL). The activity towards Candida albicans was similar to that reported above (MIC 0.3 to 0.6 mg/mL for the four oils), and was weaker than the antifungal drug ketoconazole (MIC 0.1 mg/mL). In terms of components, menthol demonstrated stronger antimicrobial activity than menthone.42 Of several Mexican traditional herbs tested, an aqueous extract of peppermint effected relatively good inhibition of the growth of Helicobacter pylori.43
Peppermint oil inhibited the replication of a plasmid of Escherichia coli by 37.5% in vitro. (Plasmids are DNA molecules that are self-replicating and transferable from one organism to another.) Menthol also had antiplasmid activity, with a concentration of 0.325 mg/mL approximating 100% plasmid elimination. An additive antibacterial activity against the E. coli strain was observed from the combination of either peppermint oil or menthol with oxytetracycline.44
Peppermint essential oil showed better activity than chlorhexidine against the cariogenic organisms Streptococcus mutans and S. pyogenes in vitro using antibacterial and biofilm models.45 A peppermint essential oil toothpaste was more effective than chlorhexidine in controlling supragingival dental plaque.
Of a range of extracts and fractions of peppermint, the dichloromethane fraction showed the best in vitro inhibition of Giardia lamblia.46 However, peppermint essential oil was less active in vitro against Giardia than several other oils containing phenolic compounds.47
Peppermint oil demonstrated a virucidal activity on herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. The effect was similar to that previously documented for tea tree oil. The oil affected the virus before its adsorption onto the cell, but not after penetration, indicating that it had a direct virucidal action. It was also active against an acyclovir-resistant strain of HSV-1.48 Aqueous extracts from peppermint, sage and lemon balm displayed strong anti-HIV activity in vitro and ex vivo, acting directly on the virion before entry into the cell.49 These observed antiviral activities would be most relevant to topical use.
Respiratory effects
Volatile aromatics such as menthol exhibit a surfactant-like effect in vitro. In vivo, menthol decreased the surface tension between water and air and therefore improved lung compliance values.50 Oral administration of a fraction obtained from an aqueous ethanolic extract of peppermint inhibited nasal symptoms, sneezing and nasal rubbing induced by antigen challenge in sensitised rats in an experimental model of allergic rhinitis.51 Menthol inhalation produced a significant reduction in cough frequency and an increase in cough latency in guinea pigs challenged with aerosolised citric acid for 2 min, demonstrating the efficacy of menthol as an antitussive in chemically induced cough.52
In studies on healthy volunteers (inhalation of menthol vapour)53,54 and those with nasal congestion associated with common cold infection (oral administration of a menthol lozenge),55 menthol brought about a change in the nasal sensation of airflow, with a subjective sensation of nasal decongestion. However, it had no effect on the nasal resistance to airflow. This finding is due to a significant pharmacological action on nasal sensory nerve endings and is unrelated to the peppermint smell.56 These findings were confirmed in a study involving 18 healthy volunteers who inhaled menthol vapour and rated their subjective impression of nasal potency.57 Objective measurements included the septal mucosal temperature within the nasal valve area and nasal airflow. While 16 of the 18 volunteers reported a subjective improvement of nasal breathing after menthol inhalation, there were no significant changes in measured nasal airflow and mucosal temperature. These results support the hypothesis that menthol leads to a direct stimulation of mucosal cold receptors creating a subjective feeling of clear and wide nasal passages, but without any objective change in nasal airflow.
The transient receptor potential (TRP) channel melastatin 8 (TRPM8) is a non-selective cation channel on primary afferent nerve fibres activated by noxious and innocuous cool temperatures.58 TRPM8 is the predominant thermoceptor for cellular and behavioural responses to cold temperatures. It is now recognised that TRPM8 is also activated by compounds evoking cooling sensations such as menthol. The above effects on nasal sensations are probably mediated by TRPM8 stimulation by menthol. See also below.
The spasmolytic and secretolytic effects of an ointment containing menthol, camphor and essential oils were tested in animals. Acetylcholine-induced bronchospasm was reduced by 50% when the ointment was insufflated through the respiratory tract, whereas the epicutaneous application of the ointment produced only a slight reduction. Significant secretolytic effects were demonstrated after insufflation and topical administration.59 Using magnetic resonance imaging, the secretory response to essential oil inhalation was assessed in vivo in rats.60 Scotch pine and rosemary oils increased tracheal respiratory secretions, but peppermint oil had no effect. Peppermint oil (100 and 300 µg/mL) exhibited spasmolytic activity on rat trachea in vitro via mechanisms involving prostaglandins and nitric oxide synthase.61
Analgesic effects
An ethanolic extract of peppermint produced dose-dependent central and peripheral analgesic effects in vivo when administered orally or via injection at relatively high doses (200 to 400 mg/kg) to mice.62
Topical application of menthol (1% to 30% concentration in ethanol) showed a major antinociceptive activity in the early phase of the pain response using the formalin test in mice. Menthol-induced analgesia was blocked by naloxone and potentiated by bestatin. Menthol also produced antinociceptive effects in the hot plate test of mice and hind paw pressure test in rats, but did not inhibit carrageenan-induced paw oedema in rats and synthesis of prostaglandin E2 in vitro. These results suggest that menthol produces antinociceptive effects by activation of the endogenous opioid system and/or partially by local anaesthetic actions, but without anti-inflammatory effects.63 (However, an anti-inflammatory effect was seen in one study; see below.)
The long-lasting cooling effect produced by the topical application of peppermint oil is caused by a steric alteration of the calcium channels of cold receptors.64,65 In a double blind, crossover study in 15 healthy volunteers, the analgesic activity of peppermint oil was differentiated from the physical effect resulting from heat of evaporation and appeared to be based on central inhibitory effects mediated by cold-sensitive A delta nerve fibres.66 Further experimental studies indicate that this central analgesic activity of menthol could occur via activation of the kappa opioid system.67
A role for TRPM8 in nocioceptive pathways has been described. This led a team of researchers to investigate the role of this ion channel in colonic sensory pathways as a possible explanation for the value of peppermint oil in IBS.68 TRPM8 was present on a select population of colonic high threshold sensory neurons, which may also co-express pain-sensing (TRPV1) and mechanosensory (TRPA1) receptors. TRPM8 activation couples to these to inhibit their downstream actions, thereby potentially relieving sensations of pain and fullness in IBS patients.
Radioprotective activity
Aqueous extract of peppermint leaf (1 g/kg) given orally to mice prior to exposure to gamma radiation provided a protective effect. In comparison with control animals, administration of peppermint increased spleen weight, improved haematological parameters, protected intestinal mucosa, provided antioxidant activity and protected against chromosomal damage in bone marrow.69–71 Enhanced survival and improved haematological parameters were also observed in separate experiments after oral administration of peppermint essential oil (0.04 mL per animal).72 Peppermint leaf extract (1 g/kg/day for 3 days, oral) before radiation exposure protected against testicular damage in mice.73 These and other studies were the subject of a 2010 review of the radioprotective properties of peppermint.74
Other effects
Peppermint oil induced a significant increase in the skin blood flow of capillaries of the forehead in healthy subjects and migraine patients after local application, as measured by laser Doppler flowmetry.75
A methanolic extract of peppermint exhibited neuroprotective and MAO-A (monoamine oxidase A) inhibiting activities in vitro.76
A dried aqueous extract of peppermint containing approximately 3.3% flavonoids, 18.4% tannins and 1.2% essential oil produced an initial excitatory effect followed by a mild sedative action on mice at a dose of 1000 mg/kg. The initial excitation was thought to be due to a stimulation of the sensorial system. The same extract also showed a mild diuretic activity.77
An ethanolic extract of peppermint demonstrated anti-inflammatory activity in rodent models of acute and chronic inflammation. The extract was administered by injection in the former model and by oral route in the latter at doses of 200 to 400 mg/kg.62
Daily consumption of peppermint tea (20 g/L) instead of drinking water decreased total testosterone levels and spermatogenesis in male rats, compared with a control group. Follicle stimulating hormone and luteinising hormone levels were increased.78
Antitumour and antigenotoxic activity was demonstrated for oral administration of peppermint (water extract, 1 g/kg) given subsequent to an initiating dose of benzo(a)pyrene in newborn mice. Antioxidant effects may have contributed to this demonstrated activity.79 Dietary additions of menthol and limonene resulted in a significant inhibition of DMBA-initiated rat mammary tumours.80
The action of peppermint tea (2%, w/v) as drinking water for 4 weeks on hepatic drug metabolising enzymes was investigated in rats. The activities of cytochrome P450 1A2 and 2E were significantly decreased.81 Single oral doses of menthol (468 mg/kg) or cineole (262 mg/kg) inhibited HMG-CoA reductase activity in rats by up to 70%. The effect was specific and not due to generalised hepatotoxicity.82,83
The addition of dried peppermint leaf at 5% to feed did not significantly affect dry matter intake, nutrient digestibility, ruminal fermentation or milk production in early lactating dairy cows. Compared with cows on a control feed, there was no difference in milk composition, except for the milk fat content. There was a tendency for the milk fat content to be lower in the cows receiving peppermint.84 Peppermint ingestion by late lactating cows led to decreased nutrient digestibility, which may have been due to a difference in the passage rate of the feed. (The passage rate of feed in early lactating cows is higher than that in late lactating cows.)84,85
Pharmacokinetics
Peppermint oil was relatively rapidly absorbed after oral administration to rats and eliminated mainly via the bile. The major biliary metabolite is menthol glucuronide, which undergoes enterohepatic circulation. Urinary metabolites included a series of mono- and dihydroxymenthols and carboxylic acids, some of which were excreted in part as glucuronic acid conjugates.86 Pharmacokinetic studies in healthy volunteers demonstrate that peppermint oil in normal capsules gives higher peak excretion levels of menthol (as glucuronide) than enteric-coated capsules. Peppermint oil is mainly absorbed in the upper gastrointestinal tract unless enterically coated, and hence should be taken in this form for effects in the lower gastrointestinal tract.24,87
Clinical trials
Gallstones
In a controlled, double blind trial, the addition of menthol to ursodeoxycholic acid significantly reduced the size of gallstones by assisting in their dissolution, and lowered the incidence of stone calcification.88 A proprietary choleretic product containing menthol 32%, pinene 17%, menthone 6%, borneol 5%, camphene 5% and cineole 2% dissolved in olive oil (hence with a similar profile to peppermint oil) significantly lowered the cholesterol saturation index of human bile. Twenty-four patients with radiolucent gallstones were given two capsules three times daily for periods in excess of 6 months in an uncontrolled study. At 6 months the gallstones had disappeared in two patients and were significantly fewer or smaller in a further three patients. The stones in the remaining 19 patients were unchanged, but one of these showed evidence of a reduction in size after 1 year.89
Irritable bowel syndrome
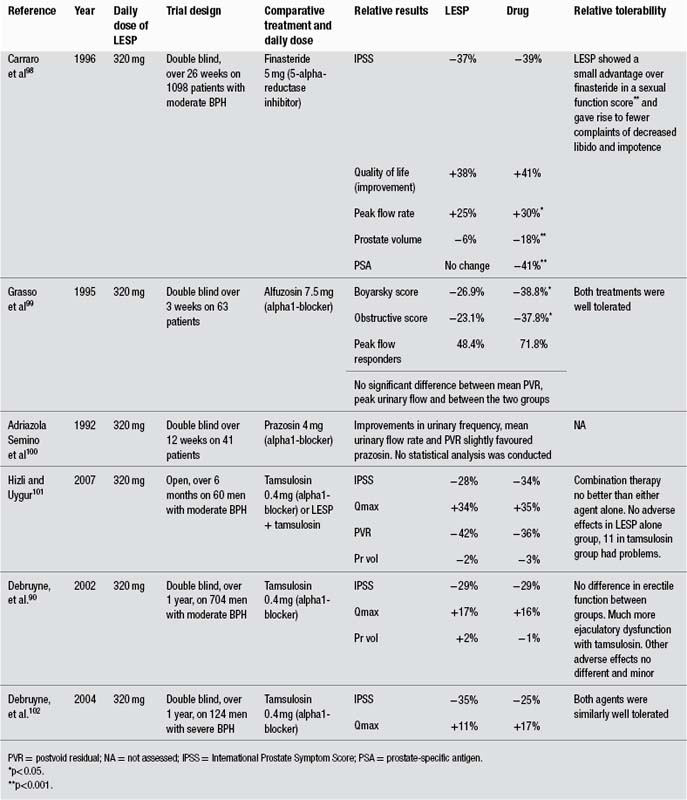
There is high level clinical evidence that enteric-coated peppermint oil can alleviate symptoms of IBS. A review published in 2005 found 16 clinical trials dating from 1979 to 1997.90 All but two were randomised and double blind in design (the others were open label studies). Of the randomised, double blind trials, nine were also crossover. Twelve trials were placebo-controlled, and three utilised anticholinergic drugs for comparison. One trial investigated recurrent abdominal pain in children, and was included in the review due to the spasmodic nature of this condition. In total, 651 patients were enrolled. Eight of the 12 placebo-controlled trials showed statistically significant effects for administration of peppermint oil. Overall, the results indicate that peppermint oil administered orally in an enteric-coated form is a safe and efficacious symptomatic short-term treatment. Peppermint oil reduced global symptoms and pain. In 11 of the 16 studies the efficacy was assessed by a daily patient rating of a set of or selected symptoms (e.g. abdominal pain, distension, flatulence, stool frequency, stool quality, urgency, bloating, frequency of attacks and severity of attacks). To allow for comparison of results, ‘overall success’ (overall benefit, global improvement, overall assessment) was calculated where possible in the review process. Such average response rates were 58% for peppermint oil and 29% for placebo. No differences were observed in the three comparative trials against smooth muscle relaxant drugs, suggesting a similar efficacy between peppermint oil and the anticholinergics. Thirteen of the 16 trials used a defined peppermint oil preparation with enteric coating. (Peppermint oil capsules are usually enteric coated to prevent the side-effect of gastric reflux and to deliver the dose further down the gastrointestinal tract.) Dosage was one to two capsules, three times a day, with each capsule containing between 182 and 200 mg of peppermint oil. Treatment duration was usually 2 to 4 weeks. Mild and transient adverse effects were observed: heartburn, anal burning or discomfort for peppermint oil, dry mouth and blurred vision for the anticholinergics.90 An earlier meta-analysis that assessed five of these trials (1979 to 1991) found a significant (p<0.001) global improvement of IBS symptoms in patients treated with peppermint oil compared with placebo, but did note methodological flaws.91
Trials of peppermint oil in IBS published since 1997 add further weight to the positive findings of the 2005 review. In a randomised, double blind trial, 42 children with IBS (aged 8 to 17 years) received enteric-coated peppermint oil capsules (270 to 540 mg/day) or placebo.92 After 2 weeks, 75% of the children in the peppermint oil group experienced reduced pain severity compared with 43% for placebo. An Italian study assessed the impact of enteric-coated peppermint oil (1590 mg/day) or placebo for 3 months in 178 patients with IBS.93 Using a double blind, placebo-controlled design there was a significant advantage observed for peppermint oil over placebo in terms of overall symptoms, with 80% improved versus 36%, respectively (p<0.02). Another Italian study over 4 weeks used a similar design in a trial involving 57 patients with IBS.94 Symptoms evaluated included abdominal bloating, abdominal pain, diarrhoea, constipation and passage of gas or mucus. By the end of the trial, 75% of patients taking peppermint oil achieved a >50% reduction in symptom score, compared with 39% in the placebo group (p<0.009). Only the herbal group exhibited a significant overall reduction in symptom score compared with baseline (p<0.01). A randomised, double blind Iranian trial over 8 weeks assessed 540 mg/day of enteric-coated peppermint oil or placebo in 90 patients with IBS.95 There was a significant difference favouring peppermint oil in terms of the number of pain-free patients (p<0.001) by the end of the trial.
A systematic review and meta-analysis identified and combined four high-quality trials of peppermint oil (average Jadad score 4.25) and found that it was more effective than fibre or conventional antispasmodic drugs in relieving symptoms of IBS.96 Compared with placebo, the relative risk of persistent symptoms after peppermint oil use was 0.43 (confidence interval 0.32 to 0.59). This meta-analysis included the two Italian trials93,94 discussed above and two earlier trials.
Treatment with enteric-coated peppermint oil (540 mg/day for 20 days) is said to have reduced small-intestinal overgrowth in one patient with IBS and improved symptoms,97 although the method used to assess efficacy (breath hydrogen excretion) has been questioned.98
The spasmolytic activity of peppermint oil on the gastrointestinal tract has been demonstrated in a number of studies in patients undergoing diagnostic procedures (see Gastrointestinal effects under Pharmacodynamics for more details).
Other gastrointestinal disorders
Fifty-four patients with non-ulcer dyspepsia were given one enteric-coated capsule containing 90 mg of peppermint oil and 50 mg of caraway oil three times a day in a double blind, placebo-controlled, multicentre trial. After 4 weeks of treatment the intensity of pain (p=0.015) and global clinical impression score (p=0.008) were significantly improved for the active group compared with the placebo group. Before treatment commenced, all active patients reported moderate to severe pain, but by the end of the study 63.2% were pain free and 26.3% reported a reduction of their pain.99 The same preparation at the same dose was found to have comparable efficacy to the drug cisapride in a randomised, double blind trial conducted over 4 weeks in 118 patients with functional dyspepsia.100 Another trial in 223 patients with non-ulcer dyspepsia established the superiority of the enteric-coated capsule delivery over a normal acid-soluble capsule in terms of reduced side effects, although both preparations exhibited somewhat comparable efficacy when the differing dosage used is taken into account.101 The enteric-coated peppermint and caraway oil combination was tested against placebo at two capsules per day for 28 days in 96 patients with functional dyspepsia in a randomised, double blind trial.102 There were significant reductions in a variety of typical symptoms for the active group compared with placebo. The herbal treatment was well tolerated.
In two randomised, double blind trials, a tablet containing fennel fruit, peppermint, caraway and gentian was evaluated in patients with idiopathic dyspepsia. The tablets reduced both acute and chronic symptoms.103 A liquid herbal formula (25 drops three times daily) containing, in increasing proportions, wormwood, caraway, fennel and peppermint was found to be superior to the spasmolytic drug metoclopramide in terms of relief of symptoms such as pain, nausea, belching and heartburn in a randomised, double blind clinical trial of the treatment of dyspepsia (p=0.02).104 In another placebo-controlled, randomised, double blind clinical trial, 70 patients with marked chronic digestive problems such as flatulence or bloating were treated with either a herbal formula containing caraway, fennel, peppermint and gentian in tablet form or a placebo over a 14-day period. Analysis of the trial results established a significant improvement in the gastrointestinal complaint scores of the group receiving herbal tablets compared with the placebo group (p<0.05). Ultrasound results evaluating the amount of gas present in the bowel also demonstrated a significant benefit from the herbal formula (p<0.05).105
A proprietary formulation containing Iberis amara, chamomile, peppermint, caraway, licorice and other herbs was assessed in the treatment of functional dyspepsia using a randomised, double blind, placebo-controlled design involving 120 patients.106 After 8 weeks 43.3% of patients on the herbal treatment versus 3.3% on placebo reported complete relief of symptoms (p<0.001). There have been other positive trials in functional dyspepsia for this and a similar formulation.107,108
Diffuse oesophageal spasm (DOS) is a rare condition that results in simultaneous oesophageal contractions, leading to symptoms of chest pain and dysphagia. Diagnosis can be controversial. In an open label pilot study in eight patients with DOS, five drops of peppermint oil in 10 mL of water completely eliminated simultaneous oesophageal contractions in all patients (p<0.01).109 The number of multiphasic, spontaneous and missed contractions also improved. Two of the eight patients had chest pain that resolved after the peppermint oil.
Headache
The topical application of peppermint oil in an ethanol solution has proven to be a well-tolerated and cost-effective treatment for tension headache. In a randomised, placebo-controlled, double blind, crossover study, 10% of peppermint oil (in 90% ethanol solution) was compared with paracetamol (1 g) and placebo in the treatment of 164 chronic tension headaches in 41 patients of both sexes. Headache episodes were treated with the following: placebo capsule and peppermint oil, paracetamol (acetaminophen) and placebo solution, paracetamol and peppermint oil, or placebo capsule and placebo solution. Peppermint oil solution was spread across the forehead and temples and the application was repeated after 15 and 30 min. The oil solution significantly reduced headache intensity after 15 min compared with placebo (p<0.01). Paracetamol was effective relative to placebo (p<0.01), but did not differ significantly from treatment with peppermint oil. The simultaneous administration of the peppermint oil solution with paracetamol produced an additive effect (p<0.001). The authors concluded that peppermint oil was an acceptable and cost-effective alternative to oral analgesics in the treatment of tension headache.110 This peppermint oil preparation was also evaluated against 1 g of aspirin (acetylsalicylic acid). Forty-four patients with episodic tension-type headache treated four headache attacks each in a randomised, double blind study with a double-dummy design: peppermint oil + placebo, peppermint oil + aspirin, placebo oil + aspirin or placebo oil + placebo. Application of peppermint oil resulted in a highly significant reduction in pain intensity compared with placebo and with similar efficacy to aspirin. The combination of peppermint oil and aspirin was significantly superior to either single preparation and a significant reduction in headache-induced general incapacity was observed only for the combination.111
In a randomised, double blind, placebo-controlled crossover study, the effects of topical application of peppermint and/or eucalyptus oil preparations on headache parameters were investigated in 32 healthy men. The combination of peppermint oil, eucalyptus oil and ethanol increased cognitive performance while exerting muscle-relaxing and mentally relaxing effects, but had no significant influence on pain sensitivity. In contrast, the peppermint oil and ethanol preparation produced a significant analgesic effect, with reduction in sensitivity to headache (p<0.01 for experimental ischaemia, p<0.001 for experimental heat stimuli). These pharmacological and clinical results indicate that peppermint oil has both central and peripheral activity.112,113
The topical application of a 10% menthol solution in ethanol as an abortive treatment of migraine headache without aura was studied in a randomised, double blind, placebo-controlled, crossover study.114 The intention-to-treat population consisted of 35 patients (28 women, seven men) with 118 migraine attacks. Menthol solution applied to the forehead and temples was statistically superior to the placebo on 2-h pain free (p=0.001), 2-h pain relief (p<0.001) and sustained pain free and pain relief endpoints (p=0.008). It was also superior in terms of relief of nausea and/or vomiting and phonophobia and/or photophobia (p=0.02). No significant difference was seen between adverse effects in the treatment and placebo groups (p=0.13).
Respiratory conditions
Peppermint oil in the form of an inhalation (20-minute heat evaporation into the patient’s room daily for a period of 2 months) was used to supplement multidrug therapy for pulmonary tuberculosis in Russia. Positive results were observed: reductions of bacterial infection by 26.8% and 58.5% occurred with doses of 0.01 mL/m3 and 0.005 mL/m3, respectively. This was followed by earlier onset of positive X-ray changes in the lung.115
The impact of inhaled menthol on asthma was studied in a placebo-controlled trial involving 23 patients. The menthol vapour did not produce acute bronchodilatory effects, but long-term inhalation over 4 weeks produced an improvement of airway hyper-responsiveness without altering the magnitude of airflow limitation. There was decreased diurnal variation in peak expiratory flow rate (p<0.05), a parameter that reflects airway hyper-responsiveness, but no significant effects on the forced expiratory volume in 1 sec. The number of metered dose inhalations of bronchodilator drugs were also significantly reduced in the menthol group (p<0.01).116
Other conditions
Topical application of peppermint oil (containing 10% menthol) provided an analgesic effect in a woman with postherpetic neuralgia whose pain had been resistant to standard therapies.117
The incidence of postoperative nausea in gynaecological patients was significantly reduced (p=0.02) in the group that inhaled peppermint oil in a placebo-controlled trial involving 18 patients.118 However, a comparison between inhalation of peppermint oil, isopropyl alcohol and a saline placebo in 33 patients with postoperative nausea found good and equal efficacy for all three interventions.119
A study in 196 mothers was conducted to assess the efficacy of peppermint water in the prevention of nipple cracks during breastfeeding compared with the application of expressed breast milk.120 The peppermint water was significantly more effective at preventing nipple pain and damage (p<0.01). A follow-up study compared a peppermint gel, lanolin ointment and a placebo gel in a 14-day randomised, double blind trial involving 216 primiparous, breastfeeding mothers.121 The peppermint gel was superior to both the lanolin and placebo in terms of preventing nipple crack.
Inhalation of peppermint during acute exercise had no significant influence on pulmonary function and physical performance in a randomised, controlled clinical trial involving 36 women soccer players.122 However, when 144 volunteers were randomly assigned to inhalation of peppermint, ylang-ylang or no aroma, peppermint was found to enhance memory and alertness, whereas ylang-ylang decreased these parameters.123 Other clinical studies have also shown that inhalation of peppermint aroma can improve cognitive function and alertness.124
Toxicology and other safety data
Toxicology
Oral administration of a 4.2:1 peppermint concentrate (4 g/kg) did not result in any macroscopic signs of toxicity or death in mice over a 7-day period.77 Peppermint infusion (20 g/L) provided as drinking water for 30 days did not produce nephrotoxicity in rats. Only minimal hepatic degeneration was observed.125,126
Chronic oral administration of peppermint oil (83 µL/kg/day for 28 days) to rats resulted in a 45% increase in alkaline phosphatase. No other change in liver enzyme activity was found.33 The oral LD50 of peppermint oil has been measured at 2.4 g/kg in mice and 4.4 g/kg in rats.127 Histopathological changes, consisting of cyst-like spaces scattered in the white matter of the cerebellum and nephropathy were seen in male rats given a daily oral dose of 100 mg/kg of peppermint oil for 90 days. No other signs of encephalopathy were observed. Nephropathy was also seen in the male rats in the highest dose group. No adverse effects were seen at doses below 40 mg/kg.128 Peppermint oil containing 1% to 2% pulegone was administered to rats (20 to 500 mg/kg/day) for 5 weeks. The rats exhibited no adverse effects on general health, behaviour nor body weight, and haematological and urinary parameters were normal. Histological examination revealed no specific pathological lesions.129 Repeated intradermal dosing with peppermint oil produced moderate and severe reactions in rabbits, although peppermint oil did not appear to be phototoxic.127
The acute oral LD50 of menthol was reported to be 3.3 g/kg in the rat and 0.8 to 1 g/kg in the cat.127,130 The estimated lethal dose for menthol in humans may be as low as 2 g but there are reports of individuals surviving doses as high as 9 g.131
Menthone at dose levels in rats of 200, 400 and 800 mg/kg/day for 28 days led to a dose-dependent decrease in creatinine and increases in alkaline phosphatase and bilirubin. The no-effect level for menthone in this study was lower than 200 mg/kg/day.132
Peppermint infusion did not show significant genotoxicity in the somatic mutation and recombination test in Drosophila melanogaster.133 However, peppermint oil induced mutations in a dose-independent manner in this test.134
Contraindications
Patients with oesophageal reflux symptoms should eliminate high doses of agents that decrease lower oesophageal sphincter pressure, including peppermint.135
The Commission E suggests that peppermint oil is contraindicated for internal use in occlusion of the gallbladder passages, cholecystitis and severe liver disease.
Peppermint oil should not be applied to the facial areas and chest of babies and small children, and especially not around the nose.136
Special warnings and precautions
Use with care in patients with salicylate sensitivity and aspirin-induced asthma. Care should be taken in patients with gallstones.11 Oral intake of peppermint oil should be used with caution in patients with pre-existing heartburn. Enteric-coated capsules may produce anal burning in patients with diarrhoea, due to excreted peppermint oil.130
Interactions
Peppermint tea reduced the absorption of iron by 84% from a bread meal (compared with a water control) in adult volunteers. The inhibition was dose-dependent and related to its tannin content. Inhibition by black tea was 79% to 94%.137 This indicates a potential interaction for concomitant administration of peppermint during iron intake. In anaemia and cases where iron supplementation is required, peppermint should not be taken simultaneously with meals or iron supplements.
There is evidence that topical use of menthol could enhance penetration of other agents. This could affect the use of other topical ingredients that have a safety assessment based on their relative lack of absorption.127 Peppermint oil also slows intestinal transit in healthy volunteers;32,138 this may slow the absorption rate or increase the total absorption of other drugs.
Peppermint oil (600 mg) increased the oral bioavailability of the calcium channel blocking drug felodipine and simvastatin in healthy volunteers. However, the increase was not as great as that produced by administration of grapefruit juice.139 A case of suspected interaction between the high intake of menthol cough drops and warfarin (reduced activity) has been described.140
Use in pregnancy and lactation
Category B2 – no increase in frequency of malformation or other harmful effects on the fetus from limited use in women. Animal studies are largely lacking.
A tea consisting of peppermint, Urtica dioica, Glycyrrhiza glabra, Helichrysum arenarium and a species of Rosa did not affect postnatal development or demonstrate embryotoxicity or teratogenicity when administered to rats.141
Teratogenic effects were not observed in mice, rats, hamsters and rabbits for menthol tested at maximum oral doses of 190, 220, 400 and 430 mg/kg/day, respectively.142
Peppermint is compatible with breastfeeding but caution should be used. While the leaf probably is compatible with breastfeeding, use of the oil should be discouraged. Caution should be exercised because there is the view that use of peppermint may dry up milk secretions. However, this was not observed in lactating dairy cows (see earlier in this monograph).
Side effects
Allergic reactions to peppermint leaf appear to be rare or of a relatively minor nature. In fact, most adverse reactions relate to the use of the oil or pure menthol. Reports of gastrointestinal irritation or aggravation of gastrointestinal complaints including stomatitis, severe oesophagitis, gastritis, unexplained diarrhoea and pancreatitis have been associated with the use of peppermint preparations, including confectionery.131 It has been reported from hospitals in a particular area of Turkey that daily consumption of four cups of tea made from peppermint or Mentha spicata has resulted in reduced libido in men.78
Peppermint oil use has been associated with mucosal ulcerations.143,144 These are consistent with the development of a buccal contact sensitivity reaction to peppermint or menthol.145,146 Three constituents of peppermint oil, alpha-pinene, limonene and phellandrene, also found in turpentine oil, are thought to be the primary sensitising agents.147 Cases of allergic contact sensitivity have been reported for peppermint oil and the tea.148,149
Skin rashes, headache, bradycardia, muscle tremor, heartburn and ataxia are rarely reported side effects associated with enteric-coated capsules of peppermint oil.131,150 The use of non-enteric-coated oil preparations occasionally causes heartburn, especially in persons suffering from reflux oesophagitis.87,150 Enteric-coated capsules may produce anal burning in patients with diarrhoea due to excreted peppermint oil.
Mild dermatological reactions on the skin and mucous membranes have been described, and neat peppermint oil can produce chemical burns.131
Menthol can cause jaundice in newborn babies. This has been linked to glucose-6-phosphate dehydrogenase deficiency in some cases.151 A case of exacerbation of urticaria and asthma after ingestion of menthol-containing lozenges has been reported.152
Menthol inhalations can also cause breathlessness and laryngeal spasm in susceptible individuals.153 Nasal preparations containing menthol may cause spasm of the glottis in young children. Bradycardia has been reported in a patient addicted to menthol cigarettes, and fibrillation has been associated with the excessive consumption of peppermint-flavoured confectionery.131
High doses of tannins can lead to excessive astringency on mucous membranes, which has an irritating effect.
Overdosage
Bradycardia has been reported in a patient addicted to menthol cigarettes154 and fibrillation has been associated with the excessive consumption of peppermint-flavoured confectionery (up to 225 g/day).155
Excessive inhalation of mentholated products has caused reversible, undesirable effects, such as nausea, anorexia, cardiac problems, ataxia and other CNS problems, probably due to the presence of volatile menthol.
Regulatory status in selected countries
Both peppermint leaf and peppermint oil are official in the European Pharmacopoeia (2011), the British Pharmacopoeia (2011) and the United States Pharmacopeia-National Formulary (USP 34-NF 29 2011).
Peppermint leaf and peppermint oil are covered by positive Commission E monographs and can be used for the following applications.
• Internal: spastic discomfort of the upper gastrointestinal tract and bile ducts, irritable colon, catarrh of the respiratory tract, inflammation of the oral mucosa
Peppermint is on the UK General Sale List. A peppermint oil product in aqueous solution has achieved Traditional Herbal Registration in the UK with the traditional indication of symptomatic relief of minor digestive complaints such as dyspepsia, flatulence and stomach cramps.
Peppermint, peppermint oil and menthol have GRAS status. Peppermint is also freely available as a ‘dietary supplement’ in the USA under DSHEA legislation (1994 Dietary Supplement Health and Education Act). Peppermint has been present in OTC (over-the-counter) digestive aid drug products. Peppermint oil has been present in OTC nasal decongestant drug products (mouthwash), expectorant drug products, digestive aid drug products, insect bite and sting drug products and astringent drug products. The FDA, however, advises that: ‘based on evidence currently available, there is inadequate data to establish general recognition of the safety and effectiveness of these ingredients for the specified uses’.
Peppermint and peppermint oil are not included in Part 4 of Schedule 4 of the Therapeutic Goods Act Regulations of Australia and are freely available for sale.
References
1. Felter HW, Lloyd JU. King’s American Dispensatory. 18th ed. rev 3, vol. 1. 1905. Portland: reprinted by Eclectic Medical Publications; 1983. pp. 1254–1255.
2. British Herbal Medicine Association Scientific Committee. British Herbal Pharmacopoeia. Cowling: BHMA, 1983. p. 142
3. Grieve M, A Modern Herbal, New York, Dover Publications, 1971;vol. 2. pp. 537–543
4. Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics, 2nd ed. New York: John Wiley, 1996. pp. 368–372
5. Robson NJ. Anaesthesia. 1987;42(7):776–777.
6. Chiej R. The Macdonald Encyclopedia of Medicinal Plants. London: Macdonald, 1984. Entry no. 195
7. Launert EL. The Hamlyn Guide to Edible and Medicinal Plants of Britain and Northern Europe. London: Hamlyn, 1981. p. 156
8. Evans WC. Trease and Evans’ Pharmacognosy, 14th ed. London: WB Saunders, 1996. pp. 259–261
9. Blaschek W, Ebel S, Hackenthal E, et al. HagerROM 2002: Hagers Handbuch Der Drogen und Arzneistoffe. Heidelberg: Springer, 2002.
10. Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed. Berlin: Springer-Verlag, 1996. p. 156
11. Bisset NG, ed. Herbal Drugs and Phytopharmaceuticals. Stuttgart: Medpharm Scientific, 1994. pp. 336–338
12. European Pharmacopoeia, 3rd ed. Strasbourg: European Department for the Quality of Medicines within the Council of Europe; 1996. p. 1298.
13. European Pharmacopoeia, 3rd ed. Strasbourg: European Department for the Quality of Medicines within the Council of Europe; 1996. p. 1299.
14. Hills JM, Aaronson PI. Gastroenterology. 1991;101(1):55–65.
15. Giachetti D, Taddei E, Taddei I. Planta Med. 1988;54(5):389–392.
16. Forster HB, Niklas H, Lutz S. Planta Med. 1980;40(4):309–319.
17. Forster H. Z Allg Med. 1983;59(24):1327–1333.
18. Lallement-Guilbert N, Bezanger-Bearuquesne L. Plant Med Phytother. 1970;4:92–107.
19. Rogers J, Tay HH, Misiewicz JJ. Lancet. 1988;2(8602):99.
20. Leicester RJ, Hunt RH. Lancet. 1982;2(8305):989.
21. Sigmund CJ, McNally EF. Gastroenterology. 1969;56(1):13–18.
22. Mizuno S, Kato K, Ono Y, et al. J Gastroenterol Hepatol. 2006;21(8):1297–1301.
23. Yamamoto N, Nakai Y, Sasahira N, et al. J Gastroenterol Hepatol. 2006;21(9):1394–1398.
24. Grigoleit HG, Grigoleit P. Phytomedicine. 2005;12(8):607–611.
25. Asao T, Mochiki E, Suzuki H, et al. Gastrointest Endosc. 2001;53(2):172–177.
26. Hiki N, Kurosaka H, Tatsutomi Y, et al. Gastrointest Endosc. 2003;57(4):475–482.
27. Inamori M, Akiyama T, Akimoto K, et al. J Gastroenterol. 2007;42(7):539–542.
28. Dalvi SS, Nadkarni PM, Pardesi R, et al. Indian J Physiol Pharmacol. 1991;35(3):212–214.
29. Holtmann G, Adam B, Liebregts T, et al. Gastroenterology. 2004;126(4 suppl 2):A640.
30. Micklefield GH, Creving I, May B. Phytother Res. 2000;14(1):20–23.
31. Micklefield G, Jung O, Greving I, et al. Phytother Res. 2003;17(2):135–140.
32. Goerg KJ, Spilker T. Aliment Pharmacol Ther. 2003;17(3):445–451.
33. Vo LT, Chan D, King RG. Clin Exp Pharmacol Physiol. 2003;30(10):799–804.
34. Beesley A, Hardcastle J, Hardcastle PT, et al. Gut. 1996;39(2):214–219.
35. Kayyal MT, El-Ghazaly MA, Kenawy SA, et al. Arzneimittelforschung/Drug Res. 2001;51:545–553.
36. Lis-Balchin M, Deans SG, Hart S. Med Sci Res. 1997;25:151–152.
37. El-Naghy MA, Maghazy SN, Fadl-Allah EM, et al. Zentralbl Mikrobiol. 1992;147(3–4):214–220.
38. Maiti D, Kole CR, Sen C. Pfl Krankh. 1985;92(1):64–68.
39. Shapiro S, Meier A, Guggenheim B. Oral Microbiol Immunol. 1994;9:202–208.
40. Duarte MC, Figueira GM, Sartoratto A, et al. J Ethnopharmacol. 2005;97(2):305–311.
41. Mimica-Dukic N, Bozin B, Sokovic M, et al. Planta Med. 2003;69(5):413–419.
42. Iscan G, Kirimer N, Kurkcuoglu M, et al. J Agric Food Chem. 2002;50(14):3943–3946.
43. Castillo-Juárez I, González V, Jaime-Aguilar H, et al. J Ethnopharmacol. 2009;122(2):402–405.
44. Schelz Z, Molnar J, Hohmann J. Fitoterapia. 2006;77(4):279–285.
45. Shayegh S, Rasooli I, Taghizadeh M, et al. Nat Prod Res. 2008;22(5):428–439.
46. Vidal F, Vidal JC, Gadelha AP, et al. Exp Parasitol. 2007;115(1):25–31.
47. Machado M, Sousa C, Salgueiro L, et al. Nat Prod Commun. 2010;5(1):137–141.
48. Schuhmacher A, Reichling J, Schnitzler P. Phytomedicine. 2003;10(6–7):504–510.
49. Geuenich S, Goffinet C, Venzke S, et al. Retrovirology. 2008;5:27.
50. Zanker KS, Tolle W, Blumel G, et al. Respiration. 1980;39(3):150–157.
51. Inoue T, Sugimoto Y, Masuda H, et al. Biol Pharm Bull. 2001;24(1):92–95.
52. Laude EA, Morice AH, Grattan TJ. Pulmon Pharmacol. 1994;7(3):179–184.
53. Eccles R, Jones AS. J Laryngol Otol. 1983;97(8):705–709.
54. Burrow A, Eccles R, Jones AS. Acta Otolaryngol. 1983;96(1–2):157–161.
55. Eccles R, Jawad MS, Morris S. J Pharm Pharmacol. 1990;42(9):652–654.
56. Eccles R, Griffiths DH, Newton CG, et al. Clin Otolaryngol. 1988;13(1):25–29.
57. Lindemann J, Tsakiropoulou E, Scheithauer MO, et al. Am J Rhinol. 2008;22(4):402–405.
58. Knowlton WM, McKemy DD. Curr Pharm Biotechnol. 2011;12(1):68–77.
59. Rai MK, Upadhyay S. Arzneimittelforschung. 1981;31(1):82–86.
60. Nicolato E, Boschi F, Marzola P, et al. J Ethnopharmacol. 2009;124(3):630–634.
61. de Sousa AA, Soares PM, de Almeida AN, et al. J Ethnopharmacol. 2010;130(2):433–436.
62. Atta AH, Alkofahi A. J Ethnopharmacol. 1998;60(2):117–124.
63. Taniguchi Y, Deguchi Y, Saita M, et al. Nippon Yakurigaku Zasshi. 1994;104(6):433–446.
64. Watson HR, Hems R, Rowsell DG, et al. J Soc Cosmet Chem. 1978;29(4):185–200.
65. Eccles R. J Pharm Pharmacol. 1994;46:618–630.
66. Bromm B, Scharein E, Darsow U, et al. Neurosci Lett. 1995;187:157–160.
67. Galeotti N, Di Cesare Mannelli L, Mazzanti G, et al. Neurosci Lett. 2002;322(3):145–148.
68. Harrington AM, Hughes PA, Martin CM, et al. Pain. 2011;152(7):1459–1468.
69. Samarth RM, Kumar A. J Radiat Res. 2003;44(2):101–109.
70. Samarth RM, Kumar A. Indian J Exp Biol. 2003;41(3):229–237.
71. Samarth RM, Saini MR, Maharwal J, et al. Indian J Exp Biol. 2002;40(11):1245–1249.
72. Samarth RM, Goyal PK, Kumar A. Phytother Res. 2004;18(7):546–550.
73. Samarth RM, Samarth M. Basic Clin Pharmacol Toxicol. 2009;104(4):329–334.
74. Baliga MS, Rao S. J Cancer Res Ther. 2010;6(3):255–262.
75. Gobel H, Dworschak M, Ardabili S, et al. The 7th International Headache Congress.Toronto; September 16–20, 1995.
76. Lopez V, Martin S, Gomez-Serranillos MP, et al. Phytother Res. 2010;24(6):869–874.
77. Della Loggia R, Tubaro A. Fitoterapia. 1990;61(3):215–221.
78. Akdogan M, Ozguner M, Kocak A, et al. Urology. 2004;64(2):394–398.
79. Samarth RM, Panwar M, Kumar A. Environ Mol Mutagen. 2006;47(3):192–198.
80. Russin WA, Hoesly JD, Elson CE, et al. Carcinogenesis. 1989;10(11):2161–2164.
81. Maliakal PP, Wanwimolruk S. J Pharm Pharmacol. 2001;53(10):1323–1329.
82. Clegg RJ, Middleton B, Bell GD, et al. J Biol Chem. 1982;257(5):2294–2299.
83. Clegg RJ, Middleton B, Bell GD, et al. Biochem Pharmacol. 1980;29(15):2125–2128.
84. Hosoda K, Matsuyama H, Park WY, et al. Anim Sci J. 2006;77(5):503–509.
85. Hosoda K, Nishida T, Park WY, et al. Asian Australas J Anim Sci. 2005;18(12):1721–1726.
86. Grigoleit HG, Grigoleit P. Phytomedicine. 2005;12(8):612–616.
87. Somerville KW, Richmond CR, Bell GD. Br J Clin Pharmacol. 1984;18:638–640.
88. Leuschner M, Leuschner U, Lazarovici D, et al. Gut. 1988;29(4):428–432.
89. Bell CD, Doran J, Middleton A, et al. Br J Clin Pharmacol. 1978;6(5):454P.
90. Grigoleit HG, Grigoleit P. Phytomedicine. 2005;12(8):601–606.
91. Pittler MH, Ernst E. Am J Gastroenterol. 1998;93(7):1131–1135.
92. Kline RM, Kline JJ, Di Palma J, et al. J Pediatr. 2001;138(1):125–128.
93. Capanni M, Surrenti E, Biagini MR, et al. Gazz Med Ital Arch Sci Med. 2005;164:119–126.
94. Cappello G, Spezzaferro M, Grossi L, et al. Dig Liver Dis. 2007;39(6):530–536.
95. Merat S, Khalili S, Mostajabi P, et al. Dig Dis Sci. 2010;55(5):1385–1390.
96. Ford AC, Talley NJ, Spiegel BMR, et al. BMJ. 2008;337:a2313.
97. Logan AC, Beaulne TM. Altern Med Rev. 2002;7(5):410–417.
98. Gaby AR. Altern Med Rev. 2003;8(1):3.
99. May B, Kuntz HD, Kieser M, et al. Arzneimittelforschung. 1996;46(12):1149–1153.
100. Madisch A, Heydenreich C–J, Wieland V, et al. Arzneimittelforschung. 1999;49(11):925–932.
101. Freise J, Kohler S. Pharmazie. 1999;54(3):210–215.
102. May B, Kohler S, Schneider B. Aliment Pharmacol Ther. 2000;14(12):1671–1677.
103. Uehleke B, Silberhorn H, Wohling H. Fortschr Med. 2002;144(27/28):695.
104. Westphal J, Hörning M, Leonhardt K. Phytomedicine. 1996;2(4):285–291.
105. Silberhorn H, Landgrebe N, Wohling D, et al. 6th Phytotherapy Conference. Berlin: October 5–7, 1995.
106. Madisch A, Holtmann G, Mayr G, et al. Digestion. 2004;69(1):45–52.
107. Raedsch R, Hanisch J, Bock P, et al. Z Gastroenterol. 2007;45(10):1041–1048.
108. von Arnim U, Peitz U, Vinson B, et al. Am J Gastroenterol. 2007;102(6):1268–1275.
109. Pimentel M, Bonorris GG, Chow EJ, Lin HC. J Clin Gastroenterol. 2001;33(1):27–31.
110. Gobel H, Fresenius J, Heinze A, et al. Nervenarzt. 1996;67(8):672–681.
111. Gobel H, Heinze A, Wagner S, et al. Cephalalgia. 1999;19(4):452. Abstract VI–G4–2
112. Gobel H, Schmidt G, Soyka D. Cephalalgia. 1994;14(3):228–234.
113. Gobel H, Schmidt G, Dworschak M, et al. Phytomedicine. 1995;2(2):93–102.
114. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Int J Clin Pract. 2010;64(4):451–456.
115. Shkurupii VA, Kazarinova NV, Ogirenko AP, et al. Probl Tuberk. 2002;4:36–39.
116. Tamaoki J, Chiyotani A, Sakai A, et al. Respir Med. 1995;89(7):503–504.
117. Davies SJ, Harding LM, Baranowski AP. Clin J Pain. 2002;18(3):200–202.
118. Tate S. J Adv Nurs. 1997;26(3):543–549.
119. Anderson LA, Gross JB. J Perianesth Nurs. 2004;19(1):29–35.
120. Sayyah MM, Rashidi MR, Delazar A, et al. Int Breastfeed J. 2007;2:7.
121. Melli MS, Rashidi MR, Nokhoodchi A, et al. Med Sci Monit. 2007;13(9):CR406–CR411.
122. Pournemati P, Azarbayjani MA, Rezaee MB, et al. Bratisl Lek Listy. 2009;110(12):782–787.
123. Moss M, Hewitt S, Moss L, et al. Int J Neurosci. 2008;118(1):59–77.
124. Blumenthal M. The ABC Clinical Guide to Herbs. USA: American Botanical Council, 2003. pp. 300–308
125. Akdogan M, Kilinc I, Oncu M, et al. Hum Exp Toxicol. 2003;22(4):213–219.
126. Akdogan M, Ozguner M, Aydin G, et al. Hum Exp Toxicol. 2004;23(1):21–28.
127. Nair B. Int J Toxicol. 2001;20(suppl 3):61–73.
128. Spindler P, Madsen C. Toxicol Lett. 1992;62(2–3):215–220.
129. Mengs U, Stotzem CD. Med Sci Res. 1989;17:499–500.
130. Opdyke DLJ. Food Cosmet Toxicol. 1976;14:471–472.
131. De Smet PAGM, Keller K, Hansel R, et al, eds., Adverse Effects of Herbal Drugs, Berlin, Springer-Verlag, 1992;vol. 1. pp. 171–176
132. Madsen C, Wurtzen G, Carstensen J. Toxicol Lett. 1986;32(1–2):147–152.
133. Romero-Jimenez M, Campos-Sanchez J, Analla M, et al. Mutat Res. 2005;585(1–2):147–155.
134. Lazutka JR, Mierauskiene J, Slapsyte G, et al. Food Chem Toxicol. 2001;39(5):485–492.
135. Friedman G. Gastroenterol Clin North Am. 1991;20(2):313–324.
136. German Federal Minister of Justice. German Commission E for Human Medicine Monograph.Bundes-Anzeiger (German Federal Gazette); no. 50, dated 13.03.1986.
137. Hurrell RF, Reddy M, Cook JD. Br J Nutr. 1999;81(4):289–295.
138. Holtmann G, Haag S, Adam B, et al. Phytomedicine. 2003;10(suppl 4):56–57.
139. Dresser GK, Wacher V, Ramtoola Z, et al. Clin Pharmacol Ther. 2002;71:P67. Abstract TPII–95
140. Coderre K, Faria C, Dyer E. Pharmacotherapy. 2010;30(1):50e–52e.
141. Ubasheev IO, Lonshakova KS, Matkhanov EI, et al. Khim Farm Zh. 1988;22(4):445–450.
142. Fifty-first Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Safety Evaluation of Certain Food Additives. WHO Food Additives Series: 42. Geneva: WHO; 1999.
143. Moghadam BK, Gier R, Thurlow T. Cutis. 1999;64(2):131–134.
144. Rogers SN, Pahor AL. Dent Update. 1995;22(1):36–37.
145. Morton CA, Garioch J, Todd P, et al. Contact Dermatitis. 1995;32(5):281–284.
146. Sainio EL, Kanerva L. Contact Dermatitis. 1995;33(2):100–105.
147. Dooms-Goossens A, Degreef H, Holvoet C, et al. Contact Dermatitis. 1977;3(6):304–308.
148. Tran A, Pratt M, DeKoven J. Dermatitis. 2010;21(2):111–115.
149. Vermaat H, van Meurs T, Rustemeyer T, et al. Contact Dermatitis. 2008;58(6):364–365.
150. Nash P, Gould SR, Barnardo DE. Br J Clin Pract. 1986;40(7):292–293.
151. Owa JA. Acta Paediatr Scand. 1989;78:848–852.
152. Marlowe KF. Am J Health Syst Pharm. 2003;60(16):1657–1659.
153. Lässig W, Graupner I, Leonhardt H, et al. Z Klin Med. 1990;45:969–971.
Poke root
Synonyms
Phytolacca americana L. (botanical synonym), poke weed (Engl), Phytolaccae radix (Lat), Kermesbeere (Ger), herbe de la laque (Fr), fitolacca (Ital), kermesbær (Dan).
What is it?
Phytolacca decandra is a striking plant with large leaves, clusters of purple berries often on the same branch with green unripe fruit and flowers still in bloom. It is indigenous to the United States of America with the following common names: poke root, poke weed. The common name derives from the indigenous word pocon meaning a plant with red or yellow dye (referring to the juice of the ripe berries). The genus name Phytolacca is from the Greek phuton meaning plant and from the Latin lacca meaning milky lac. Many parts have been used medicinally, including the berries, leaves and roots. This monograph focuses on the therapeutic use of the dried root, which is toxic in overdose.
The term ‘poke weed’ occurs extensively in medical literature due to the use of poke weed mitogens (PWM) to investigate cellular immune responses and a poke weed antiviral protein that inhibits viral protein synthesis. These entities are unlikely to be significantly absorbed into the bloodstream after oral doses, except when the gastrointestinal tract is damaged.
Effects
An anti-inflammatory remedy with action on the lymphatic and respiratory systems. Potentially immune stimulating, but caution is required with dosage.
Traditional view
Poke root has been used traditionally for the treatment of inflammatory conditions of the upper respiratory tract (such as laryngitis, tonsillitis), lymphadenitis, mumps and chronic rheumatism. Topically it has been used for the treatment of skin and glandular disorders, such as scabies, tinea, acne, mastitis and mammary abscess.1 The Eclectic physicians favoured poke root to act upon the skin and the glandular structures, particularly of the mouth, throat or reproductive tract (tonsillitis, ulceration, ovaritis, glandular swellings) and to act markedly on the mammary glands. It was used as an emetic and depurative.2 Traditional texts record its application in breast cancer (oral use) and topically for uterine cancer.3
Radix Phytolaccae, or shang lu in traditional Chinese medicine, refers to Phytolacca acinosa or P. americana, which has been used to treat oedema, oliguria and ascites and externally for trauma, haemorrhage and pyogenic infections of the skin.4
Can be used for
Traditional therapeutic uses
As a depurative for skin conditions acting primarily via the lymphatic system; for treatment of inflammatory conditions or infections, especially of the respiratory tract and reproductive systems. Topically for treatment of skin irritation/infection/infestation and female reproductive system disorders (mastitis, mammary abscess, possibly uterine cancer). This is a valuable herb which must be treated with respect.
Preparations
Only the dried root should be used for making decoctions (the fresh root should not be used). Tincture can also be used for internal or external use.
Dosage
Avoid the use of stronger liquid extracts and fresh plant tinctures because of potential toxic effects.
Duration of use
In light of the potential risks, medium-term use of poke root up to 6 months is advised.
Summary assessment of safety
Poke root tinctures may be safely prescribed if the recommended dosage is not exceeded and the contraindications below are observed. Liquid extracts and fresh plant tinctures have the potential to cause poisoning because they are more active and may contain higher levels of PWM.
Technical data
Botany
Poke root, a member of the Phytolaccaceae family, is a herbaceous perennial that grows up to 3 m. The stem divides into two, with the alternate leaves borne on a very short petiole. The flowers, carried on short pedicles, have a bract and no petals but five greenish-white tepals (combined calyx and corolla). The fruit consists of dark, fleshy berries with raised ribs on the surface.5 The large root is tuberous, with an outer colouring of yellowish-, reddish- or greyish-brown.6 The plant is striking as its large leaves and beautiful clusters of purple berries often mingle upon the same branch with the green unripe fruit and flowers.7
Key constituents
• Triterpenoid saponins (phytolac(c)osides, esculentosides and phytolaccasponins) with the main aglycone being phytolaccagenin.8,9 The nomenclature is inconsistent, with some saponins having two or more names, eg phytolac(c)-oside E = esculentoside A = phytolaccasaponin E
• Sterols; mitogens and antiviral proteins as noted above.8
Pharmacodynamics
The immunological activity of poke root is probably due to the presence of traces of lectins such as PWM which, although too large to be substantially absorbed through the gut wall, may interact with gut-associated lymphoid tissue and might even be absorbed in small quantities. In situations of overdosage the saponins may facilitate the bioavailability of the lectins via their detergent activity and their irritating effect on the gastrointestinal mucosa.
Immunological activity
Poke weed mitogen (PWM) is a lectin possessing three distinct biological activities: haemagglutination, leucagglutination and mitogenicity (stimulation of the replication of lymphocytes in vitro).10 The studies on or using the mitogenic activity of PWM are extensive. Peripheral blood plasmacytosis (increased plasma cells) occurred in children after systemic exposure to PWM from P. decandra berries. Such exposure occurred through oral ingestion of large amounts of berries or by contact of fresh cuts and abrasions with the berry juice.11
Lymphocyte-stimulating factors (LSFs) were isolated from cultures of murine spleen or thymus cells to which PWM was added. LSFs induced cultured lymphocytes to differentiate into IgM-secreting cells and to proliferate without the addition of mitogen. LSF also stimulated polyclonal B-cell differentiation into IgM-secreting cells.12 Extracts of P. americana ripe and unripe berries, seeds, pulp, stem, leaf and root demonstrated mitogenic effects in human peripheral blood cells in vitro. While some of the extracts showed mitogenic activity up to dilution of 1:15 000, the most potent root extract was mitogenic at a dilution of 1:1 000 000.13
Poke root antiviral protein (PAP, isolated from the leaves and seeds) is a ribosome-inactivating protein that acts on eukaryotic and prokaryotic ribosomes.14 PAP has potent antiviral activity against many plant and animal viruses in vitro, including HIV,15 and has demonstrated immunological activity in vivo by injection.16 Due to its potent antiviral activity and lack of spermicidal effects, PAP is under consideration as a topical anti-HIV agent. Topical administration of a gel containing 0.01% to 1% PAP resulted in moderate-to-marked vaginal irritation in one-third of animals treated.17 However, it should be noted that poke root probably does not contain significant quantities of PAP.
Toxicology and other safety data
Toxicology
Saline suspensions of poke root extract produced high intraperitoneal lethality in mice, rats and guinea pigs. Large oral doses of liquid extracts markedly impaired liver function, but not kidney function, in rabbits.20
An acidic steroidal saponin obtained from poke root had an LD50 of 0.065 mg/kg by the intraperitoneal route in mice, indicative of a high toxicity by this route.21 The following LD50 values of a saponin extract of P. americana root have been recorded: 181 mg/kg (ip, mice) and 208 mg/kg (ip, rats).18
A number of sources state that the root is considered the most toxic part of the plant, although all parts are noted as toxic. Toxicity is said to increase as the plant matures, with the only exception being that the green berries are more toxic than the mature berries. Primary references are not provided.22–24 However, early animal studies with the berry extract reported that it had milder toxic effects than the root. Poisonings after consumption of the leaves, berries or root have been reported in livestock.25 Two sheep receiving 20 and 25 g/kg of fresh green leaves of P. decandra died 6 h after feeding. The remaining nine became mildly sick but recovered.26
Parts of the plant are commonly assumed to be safe to eat when they are prepared properly: that is, when the berries have been cooked or when the young green shoots or leaves have been boiled using two changes of water.22–24 However, poisonings have still occurred when these precautions have been taken.22,27,28
From the literature, it is apparent that there is considerable variability in the toxicity of various Phytolacca preparations. The main toxic components are the saponins, which act as gastrointestinal irritants and probably account for the severe nausea, vomiting and diarrhoea that accompany an overdose.21,29 The immunological changes that usually accompany poisoning are probably due to the lectins.21,22 The saponins are not considered to be cardiotoxic.22 Cardiac effects may be secondary to the increased vagal tone seen with severe gastrointestinal irritation.30 To date, there have been no studies that correlate toxic effects with levels of particular saponins.
Contraindications
Pregnancy, lactation, lymphocytic leukaemia and gastrointestinal irritation. Poke root should not be used in children.
Special warnings and precautions
The recommended dosage of poke root has been exceeded in some cases (see section on Side effects) due to variation in the potency of the root. Fresh plant tinctures are potentially more unsafe and should not be used. Accurate measurement of a tincture dose is vital to ensure the safe dosage is not exceeded.
Use in pregnancy and lactation
Category D – has caused or is associated with a substantial risk of causing fetal malformation or irreversible damage.
Poke root is contraindicated in pregnancy due to its potential toxicity. Mid-term abortifacient activity has been reported for the seeds (10 mg/kg), roots (20 mg/kg) and leaves (40 mg/kg) of P. acinosa (a species used in traditional Chinese medicine) after intraperitoneal administration to pregnant mice.31 Abortion in cows has been described as a result of toxicity from the berries.23 Use of the root as an abortifacient has been reported.25
Poke root is used topically in traditional Western medicine to treat mastitis.1 Breastfeeding infants should not be exposed to poke root applied topically to the breasts, so application to the nipple should be avoided. Otherwise poke root is contraindicated during breastfeeding.
Side effects
As with all herbs rich in saponins, oral use may cause irritation of the gastric mucous membranes and reflux. Individual responses to the ingestion of poke root plant parts appear to vary greatly and can be independent of the quantity of the plant part consumed.27 More severe adverse reactions (possibly from mild overdose) include nausea, abdominal pain, haematemesis, diarrhoea, hypotension and tachycardia. A number of adverse events related to the use of poke root have occurred in Australia. These have all been caused by excessive intake.
Topical application of preparations derived from the green plant and root have produced inflammation of the skin.32 Reddening and irritation of the conjunctivae occurred after instillation of saline suspension of poke root extract into rabbit eyes.20 Topical application of poke root should be restricted to tinctures and contact with the eyes should be avoided.
Overdosage
Toxic effects will typically result from overdose with poke root. Medical advice should be sought immediately. Intoxication with poke root usually involves an initial burning sensation in the mouth and throat followed a few hours later by nausea, repeated vomiting, salivation, profuse sweating, severe abdominal cramps and watery or bloody diarrhoea. Other symptoms include generalised weakness, headaches, dizziness, hypotension and tachycardia. Urinary incontinence, confusion, unconsciousness and tremors may also occur. The cardiac effects of poke root may be secondary to the increased vagal tone that accompanies the usually severe gastrointestinal colic. The onset of symptoms usually occurs 2 to 4 h after ingestion. Non-fatal cases usually recover within 24 to 48 h with medical treatment.22–24,33
Poisonings were widespread in North America during the 19th century, due to overdose of tincture or ingestion of berries or roots mistaken for other vegetables. Fatalities were reported.25,34 Poisonings have continued into the 20th and 21st centuries from ingestion of the root (sometimes mistakenly) or leaves (fresh and/or cooked) and from drinking tea prepared from the leaf and stem. In one case of poisoning caused by chewing the fresh root, the patient’s lymphocyte count increased nearly 4-fold within 1 week of intoxication. A 43-year-old woman experienced overdosage symptoms after she drank one cup of powdered poke root tea, which was prepared as per the label directions (about 1 g/cup of boiling water).22,30,32,35–38
As noted earlier, peripheral blood plasmacytosis occurred in children exposed through the oral ingestion of large amounts of berries or by the exposure of fresh cuts and abrasions to berry juice.11 Large immature basophilic lymphocytes appeared in the peripheral blood of two adults shortly after accidental exposure to a root extract (one through the conjunctiva and the other through a subcutaneous puncture wound).39 Although described as a ‘root extract’ in the research paper39 the latter effect was likely to have been due to exposure to concentrated ‘root-derived poke weed mitogen’.40
Safety in children
There are no reports of harm in children from consuming poke root as a remedy. However, given the variation in product quality and the risk of overdosage, poke root should not be used in children.
Preschool children are more likely to be poisoned by ingestion of the berries rather than by leaf or root.24 Up to 10 raw berries can be considered harmless for adults and older children, but may lead to serious poisoning in infants.41 A few fatal cases of poisoning in children from eating the fruit have been recorded, but it is not clear whether death was caused by the seed or the pulp.25 Another source suggests that reports of poisoning of children by the berries are not conclusive.42
Regulatory status in selected countries
Poke root (P. acinosa) is official in the Pharmacopoeia of the People’s Republic of China (English edition, 2000). It is not covered by a Commission E monograph but it is on the UK General Sale List.
Poke root does not have GRAS status. However, it is freely available as a ‘dietary supplement’ in the USA under DSHEA legislation (1994 Dietary Supplement Health and Education Act).
Poke root is included in Part 4 of Schedule 4 of the Therapeutic Goods Act Regulations of Australia. This means that OTC (over-the-counter) products containing poke root need to undergo a full evaluation by a committee for quality, safety and efficacy. This restriction regulates the activity of suppliers of OTC products, but does not directly affect practitioners of herbal medicine, who use the tincture as a starting material for individual prescriptions.
References
1. British Herbal Medicine Association Scientific Committee. British Herbal Pharmacopoeia. Cowling: BHMA, 1983. pp. 156–157
2. Felter HW, Lloyd JU. King’s American Dispensatory, ed 18, 3rd rev, vol. 2, 1905. Portland: Reprinted by Eclectic Medical Publications; 1983. pp. 1471–1475.
3. Grieve M, A Modern Herbal, New York, Dover Publications, 1971;vol. 2. pp. 648–649
4. Chang HM, But PP, Pharmacology and Applications of Chinese Materia Medica, Singapore, World Scientific Publishing, 1987;vol. 2. pp. 1131–1134
5. Chiej R. The Macdonald Encyclopedia of Medicinal Plants. London: Macdonald, 1984. Entry no. 229
6. British Herbal Medicine Association Scientific Committee. British Herbal Pharmacopoeia, 4th ed. Bournemouth: BHMA, 1996. pp. 151–152
7. Osol A, Farrar GE, et al. The Dispensatory of the United States of America, 24th ed. Philadelphia: JB Lippincott, 1947. p. 1551
8. Tang W, Eisenbrand G. Chinese Drugs of Plant Origin. Berlin: Springer Verlag, 1992. pp. 763–775
9. Wang L, Bai L, Nagasawa T, et al. J Nat Prod. 2008;71:35–40.
10. Reisfeld RA, Börjeson J, Chessin LN, et al. Biochem. 1967;58:2020–2027.
11. Barker BE, Farnes P, LaMarche PH. Pediatrics. 1966;38(3):490–493.
12. Basham TY, Toyoshima S, Finkelman F, et al. Cell Immunol. 1981;63(1):118–133.
13. Farnes P, Barker BE, Brownhill LE, et al. Lancet. 1964;284(7369):1100–1101.
14. Cenini P, Bolognesi A, Stirpe F. J Protozool. 1988;35(3):384–387.
15. Tumer NE, Hwang DJ, Bonness M. Proc Natl Acad Sci USA. 1997;94(8):3866–3871.
16. Spreafico F, Malfiore C, Moras ML, et al. Int J Immunopharmacol. 1983;5(4):335–343.
17. D’Cruz OJ, Waurzyniak B, Uckun FM. Toxicol Pathol. 2004;32(2):212–221.
18. Woo WS, Shin KH, Kang SS. Soul Taehakkyo Saengyak Yonguso Opjukjip. 1976;15:103–106.
19. Woo WS, Shin KH. Soul Taehakkyo Saengyak Yonguso Opjukjip. 1976;15:90–96.
20. Macht DI. J Am Pharm Assoc Sci Ed. 1937;26:594–599.
21. Ahmed ZF, Zufall CJ, Jenkins GL. J Am Pharm Assoc. 1949;38:443–448.
22. Roberge R, Brader E, Martin ML, et al. Ann Emerg Med. 1986;15(4):470–473.
23. De Smet PAGN, Keller K, Hansel R, et al, eds., Adverse Effects of Herbal Drugs, Berlin, Springer-Verlag, 1993;vol. 2. pp. 253–261
24. Mack RB. North Carolina Med J. 1982;43(5):365.
25. Watt JM, Breyer-Brandwijk MG. The Medicinal and Poisonous Plants of Southern and Eastern Africa: Being an Account of Their Medicinal and Other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal, 2nd ed. Edinburgh: Livingstone, 1962. pp. 834–836
26. Ecco R, de Barros CSL, Irigoyen LF. Cienc Rural. 2001;31(2):319–322.
27. Edwards N, Rodgers GC. Vet Hum Toxicol. 1982;24(suppl):135–137.
28. [No authors listed]. MMWR Morb Mortal Wkly Rep. 1981;30(6):65–67.
29. Woo WS, Kang SS, Wagner H, et al. Planta Med. 1978;34:87–92.
30. Hamilton RJ, Shih RD, Hoffman RS. Vet Human Toxicol. 1995;37(1):66–67.
31. Yeung HW, Feng Z, Li WW, et al. J Ethnopharmacol. 1987;21(1):31–35.
32. Mitchell J, Rook A. Botanical Dermatology: Plants and Plant Products Injurious to the Skin. Vancouver: Greengrass, 1979. p. 513
33. Kell SO. Vet Hum Toxicol. 1982;24(suppl):138.
34. Lewis WH, Smith PR. JAMA. 1979;242(25):2759–2760.
35. Brooker J, Obar C, Courtemanche L. J Toxicol Clin Toxicol. 2001;39(5):549–550.
36. Jaeckle KA, Freemon FR. South Med J. 1981;74(5):639–640.
37. Stein ZLG. Am J Hosp Pharm. 1979;36:1303.
38. Lawrence RA. Vet Hum Toxicol. 1990;32(4):369.
39. Barker BE, Farnes P, Fanger H. Lancet. 1965;285(7377):170.
40. Wimer BM, Mann PL. Cancer Biother Radiopharm. 2002;17(5):569–597.
41. Frohne D, Pfander HJ. A Colour Atlas of Poisonous Plants: A Handbook for Pharmacists, Doctors, Toxicologists, and Biologists. Translated from the 2nd German edition by NG Bisset. London: Wolfe Publishing; 1984. pp. 166–167.
42. Kingsbury JM. Poisonous Plants for the United States and Canada. Englewood Cliffs: Prentice Hall, 1964. pp. 225–227
Rehmannia
Synonyms
Glutinous Rehmannia, Chinese foxglove (Engl), Rehmanniae radix (Lat), di huang (Chin), shojio (Jap), saengjihwang (Kor).
What is it?
The roots of Rehmannia glutinosa (Gaertn.) Libosch., R. glutinosa var. hueichingensis (Chao et Schih) Hsiae., R. glutinosa var. purpurea Makino or R. glutinosa var. lutea Makino have been used extensively in traditional Chinese medicine (TCM). Rehmannia can be used fresh, dried or after processing (curing), and has various Chinese names depending on its form: shen di huang (uncured), shu di hung (cured).
Effects
Anti-inflammatory in autoimmune disease and allergies and supports the adrenal cortex; may protect against the suppressive effects of corticosteroid therapy and chemotherapy.
Traditional view
Uncured Rehmannia is described as sweet, slightly bitter and cold; cured Rehmannia is sweet and slightly warm. The former clears Heat and cools the Blood (used in Warm-febrile diseases causing high fever, thirst and a scarlet tongue, haemorrhage due to Heat entering the Blood level), nourishes Yin and Blood and generates Fluids (used in some cases of dry mouth, low-grade fever, constipation), cools the upward blazing of Heart Fire (mouth sores, insomnia, low-grade fevers, constipation) and is used for Wasting and Thirsting syndrome (that probably included diabetes).1
Summary actions (uncured Rehmannia)
Antipyretic, adrenal trophorestorative, antihaemorrhagic, anti-inflammatory, mild laxative.
Can be used for
Indications supported by clinical trials
Although thorough clinical trial data on Rehmannia are lacking, the following conditions have been successfully treated in Chinese studies: rheumatoid arthritis, asthma, urticaria and chronic nephritis.
Traditional therapeutic use
• Uncured Rehmannia: antipyretic, haemostatic and removes latent heat from the blood; used for skin rashes, diabetes, low-grade fevers and bleeding.
• Cured Rehmannia: regulates menstruation and promotes blood production; used for anaemia, dizziness, weakness, tinnitus; amenorrhoea and metrorrhagia.
May also be used for
Extrapolations from pharmacological studies
To prevent the suppressive effects of corticosteroid drugs on endogenous levels of corticosteroids. Rehmannia gives support to adrenal function but, unlike licorice, is not hypertensive. A useful review paper of the pharmacological research has been published.2
Preparations
Dried or fresh root, decoction and liquid extract, tincture, tablet or capsule for internal use.
To prepare cured Rehmannia, fresh roots are washed in millet wine, steamed and dried with resteaming and redrying several times.
Technical data
Botany
Rehmannia glutinosa was classified as a member of the Scrophulariaceae (foxglove) family but is now provisionally assigned to the Orobanchaceae family. It is a hairy perennial herb growing to 40 cm. The plant bears light reddish-purple tubular flowers and has a thick orange tuberous root approximately 3 to 6 cm in diameter.3
Pharmacodynamics
Immune and adrenal cortex function
Uncured Rehmannia inhibited the metabolism of cortisol by hepatocytes in vitro. Simultaneous administration of exogenous adrenocortical hormones resulted in cortisol levels remaining close to normal. The authors believed the mechanism to be a competitive effect at the hepatocellular receptor that affected the uptake of corticosteroid hormone, thereby slowing the catabolism of cortisol.7
In a model designed for assessing effects in adrenal depletion, oral administration of uncured Rehmannia (3 g/kg) for 2 weeks to rabbits chronically treated with the glucocorticoid dexamethasone significantly raised serum corticosterone levels (p<0.001). Continuation of treatment resulted in further increases. Rehmannia treatment also prevented or reversed morphological changes in the pituitary and adrenal cortex, appearing to antagonise the suppressive effect of glucocorticoids on the hypothalamic-pituitary-adrenal (HPA) axis.8 Such inhibition of the negative feedback of glucocorticoids on the HPA axis by Rehmannia could explain a trophic effect on the adrenal cortex.9
Oral administration of Rehmannia enhanced experimentally induced alkaline phosphatase activity of splenocytes in thyroxine-treated mice. Juice of fresh Rehmannia was stronger than that of decoction of dried Rehmannia. This preparation also enhanced experimentally induced splenocyte mitogenesis.10
Oral administration (10 to 500 mg/kg) of several fractions from the ethanol extract of Rehmannia suppressed the induction of haemolytic plaque-forming cells in mice. Further fractionation yielded the following immunosuppressive phenolic glycosides: jionoside A1, jionoside B1, acetoside, isoacetoside, purpureaside C, cistanoside A and cistanoside F.6
Oral administration of cured Rehmannia to mice abolished the suppressive effects of cyclophosphamide and dexamethasone on immunity. Parameters measured included spleen and thymus indices, serum haemolysin, lymphocyte transformation rate, phagocytic activity of peritoneal macrophages and numbers of peritoneal T lymphocytes.11
Oral administration of a herbal preparation containing Rehmannia demonstrated protective effects on haematopoiesis, immunity, heart, liver and kidney functions during chemotherapy in tumour-bearing mice.12
Pretreatment with injection of a water extract of Rehmannia glutinosa var. purpurea inhibited fatal shock caused by an experimentally induced systemic allergic reaction. In this test model Rehmannia inhibited plasma histamine levels. By the same route, Rehmannia also inhibited an IgE-dependent skin (allergic) reaction, suggesting antiallergic activity.13
Neuroprotective activity
Among four herbs used in TCM for dementia, Rehmannia was found to induce the gene expression of glial cell line-derived neurotrophic factor (GDNF) in vitro.14 GDNF is a growth factor that promotes survival of various CNS neurons. Most of the interest in neuroprotective activity has focused on catalpol, isolated from the herb. Catalpol has demonstrated neuroprotective activity across a range of in vitro models including oxidative stress,15 ischaemia,16 various neurotoxins17–19 and even beta-amyloid.20 The neuroprotective activity of catalpol has also been reflected in vivo, albeit following injection of the iridoid glycoside.19,21
Antidiabetic activity
Uncured Rehmannia by oral route reduced the pathology of diabetic nephropathy in an animal model. Treatment with Rehmannia resulted in a decrease in the accumulation of advanced glycation endproducts, possibly due to a reduction in oxidative stress.22 A high dose of aqueous-ethanol extract of Rehmannia (by intragastric route) improved experimentally induced diabetic complications, including retinopathy23 and diabetic foot ulcers24 in rats.
The effect of Rehmannia individually and in combination with metformin was investigated in streptozotocin-induced diabetic rats.25 Rehmannia at 200 mg/kg (oral doses) did not reduce blood sugar, but plasma C-reactive protein was significantly lowered compared with diabetic controls (p<0.05) and the metformin-treated group (p<0.05), suggesting an anti-inflammatory activity for the herb in this context.
Other activity
Steamed Rehmannia was found in a dose dependent manner to reduce symptoms of unpredictable mild stress in mice, including aggravated gastric ulceration and altered liver enzymes and metabolites.26
An in vitro study has found that three compounds of Rehmannia (acetylacteoside, jionoside C and jionoside D) demonstrated aldose reductase inhibitory activity.27
Orally administered cured Rehmannia demonstrated improvement in haemorrheology in arthritic and thrombotic rats.28
Ethanol extract of cured Rehmannia increased erythrocyte deformability and erythrocyte ATP contents, reduced erythrocyte aggregation and promoted activity of the fibrinolytic system in vivo. Extracts of uncured Rehmannia had weak or no activity.29 Oral administration of Rehmannia inhibited aspirin-induced blood clotting in mice. The action of the juice of fresh Rehmannia was stronger than that of decoction of dried Rehmannia.10
In an animal model, cured Rehmannia (by oral route) prevented osteoporotic bone loss induced by ovariectomy. Rehmannia alleviated the decrease in the trabecular bone mineral density and increased cortical bone thickness and trabeculation of the bone marrow spaces.30
Oral administration of a water extract of cured Rehmannia reduced renal defects in rats with ischaemia-reperfusion induced acute renal failure.31 Reduced expression of angiotensin II and AT(1) receptor and regulation of tumour growth factor (TGF)-beta1 and type IV collagen expression has been suggested after similar studies.32
Uncured Rehmannia had a protective effect on experimentally induced cytotoxicity in cardiac muscle cells in vitro.33
Intraperitoneal administration of a polysaccharide isolated from Rehmannia to mice bearing sarcoma improved production of the suppressor T-lymphocyte subset.34 Two acidic polysaccharides isolated from raw Rehmannia showed remarkable reticuloendothelial system-potentiating activity in a carbon clearance test.35 A hot water extract of Rehmannia containing polysaccharides inhibited the proliferation of hepatocellular carcinoma cells in vitro. Rehmannia also stimulated apoptosis.36 Although polysaccharides may show considerable immune-enhancing activity in vitro or by injection, this activity may not be relevant to the oral use of Rehmannia.
Clinical trials
Uncured Rehmannia has produced therapeutic effects in uncontrolled trials involving patients with rheumatoid arthritis, asthma and urticaria.37
Oral administration of a herbal preparation including Rehmannia and Astragalus demonstrated therapeutic effects on chronic nephritis. Significant improvement was observed in 91% of patients in the treatment group, compared with 67% in the control group (p<0.001). The preparation also demonstrated antiallergic effects and promotion and modulation of immunity.38 The design of this clinical research was not rigorous and results should be interpreted with caution.
Toxicology and other safety data
Toxicology
The mutagenic potential of Rehmannia was tested with the Ames test and in vivo. Uncured Rehmannia showed no mutagenic activity, whereas cured Rehmannia was mutagenic in the in vivo mammalian (mice) assay when given by intraperitoneal injection (2 to 4 g/kg).39
Oral administration of Rehmannia tended to increase the levels of urea nitrogen, creatinine, methylguanidine and guanidinosuccinic acid in rats with renal failure.40
Intragastric administration of either Rehmannia decoction or an alcohol extract at a dose of 60 g/kg/day for 3 days did not cause adverse reactions in mice observed for 1 week. Rats were administered the same preparations by the same route at a dose of 18 g/kg and observed for 1.5 months. There were no significant changes in behaviour, body weight, serum non-protein nitrogen or hepatic or renal tissues.41
A Chinese herbal formula (Man-Shen-Ling) containing Rehmannia did not exhibit toxic, mutagenic, teratogenic or carcinogenic effects in acute and chronic toxicity tests in animal models.38
Special warnings and precautions
Cured Rehmannia may be unsuitable for gluten intolerant patients due to its treatment with millet wine.
Use in pregnancy and lactation
Category B3 – no increase in frequency of malformation or other harmful effects on the fetus from limited use in women. Evidence of increased fetal damage in animal studies exists, although the relevance to humans is unknown.
Subcutaneous administration of Rehmannia aqueous extract (0.1 to 0.4 mL/day) for 5 days decreased litter numbers in mice. This antifertility effect was not associated with systemic toxicity or interruption of the oestrus cycle.
In TCM, uncured Rehmannia is contraindicated in pregnant women with deficient blood, deficient spleen or deficient stomach.42
Side effects
In a small open trial involving patients with rheumatoid arthritis, intermittent treatment with Rehmannia decoction elicited mild oedema in a minority of patients.35
Based on clinical trials in China, a minority of patients may develop diarrhoea, abdominal pain, dizziness, fatigue and palpitations that disappear spontaneously within a few days.37 Excessive doses can cause diarrhoea.
Two cases of liver toxicity43,44 (one fatal) and two cases of elevated serum levels of a liver enzyme45 were reported after the ingestion of preparations containing Rehmannia used for the treatment of skin conditions. However, these formulations contained eight or more different Chinese herbs and the herb or herbs responsible have not been identified. The adverse reactions were not conclusively ascribed to the treatment with Chinese herbs in all cases.46
Regulatory status in selected countries
Rehmannia was official in the Pharmacopoeia of the People’s Republic of China (English edition, 1997).
Rehmannia is not covered by a Commission E monograph and is not on the UK General Sale List.
Rehmannia does not have GRAS status. However, it is freely available as a ‘dietary supplement’ in the USA under DSHEA legislation (1994 Dietary Supplement Health and Education Act).
Rehmannia is not included in Part 4 of Schedule 4 of the Therapeutic Goods Act Regulations of Australia and is freely available for sale.
References
1. Bensky D, Clavey S, Stoger E. Chinese Herbal Medicine Materia Medica, 3rd ed. Seattle: Eastland Press, 2004. pp. 120–123
2. Zhang RX, Li MX, Jia ZP. J Ethnopharmacol. 2008;117(2):199–214.
3. World Health Organization. Medicinal Plants in China. Manila: Regional Office for the Western Pacific, World Health Organization, 1989. p. 247
4. Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas. Berlin: Springer-Verlag, 1996. p. 76
5. Tang W, Eisenbrand G. Chinese Drugs of Plant Origin. Berlin: Springer Verlag, 1992. pp. 849–852
6. Sasaki H, Nishimura H, Morota T, et al. Planta Med. 1989;55:458–462.
7. Zhang LL. Acta Acad Med Primae Shanghai. 1980;7(1):37–42.
8. Zha LL, Shen ZY, Zhang XF, et al. Chin J Integr Trad West Med. 1988;8(2):95–97.
9. Chen JK, Chen TT, Crampton L. Chinese Medical Herbology and Pharmacology. California: Art of Medicine Press, 2004.
10. Liang A, Xue B, Wang J, et al. Zhongguo Zhong Yao Za Zhi. 1999;24(11):663–666. 702
11. Li P, Shi XH, Wang FL. Chin J Immunol. 1987;3(5):296–298. 320
12. Xu JP. Chung Kuo Chung Hsi I Chieh Ho Tsa Chih. 1992;12(12):734–737. 709–710
13. Kim H, Lee E, Lee S, et al. Int J Immunopharmacol. 1998;20(4–5):231–240.
14. Yu H, Oh-Hashi K, Tanaka T, et al. Pharmacol Res. 2006;54(1):39–45.
15. Bi J, Jiang B, Liu JH, et al. Neurosci Lett. 2008;442(3):224–227.
16. Li Y, Bao Y, Jiang B, et al. Int J Dev Neurosci. 2008;26(3–4):309–317.
17. Bi J, Wang XB, Chen L, et al. Toxicol In Vitro. 2008;22(8):1883–1889.
18. Jiang B, Zhang H, Bi J, et al. Neurol Res. 2008;30(6):639–644.
19. Zhang XL, Jiang B, Li ZB, et al. Pharmacol Biochem Behav. 2007;88(1):64–72.
20. Jiang B, Du J, Liu JH, et al. Brain Res. 2008;1188:139–147.
21. Zhang XL, An LJ, Bao YM, et al. Food Chem Toxicol. 2008;46(8):2888–28894.
22. Yokozawa T, Kim HY, Yamabe N. Am J Chin Med. 2004;32(6):829–839.
23. Zhang Y, Dai DZ. Drug Dev Res. 2005;66(3):238.
24. Lau TW, Lam FF, Lau KM, et al. J Ethnopharmacol. 2009;123(1):155–162.
25. Waisundara VY, Huang M, Hsu A, et al. Am J Chin Med. 2008;36(6):1083–1104.
26. Zhang D, Wen XS, Wang XY, et al. J Ethnopharmacol. 2009;123(1):55–60.
27. Nishimura H, Yamaguchi T, Sasaki H, et al. Planta Med. 1990;56:684.
28. Kubo M, Asano T, Shiomoto H, et al. Biol Pharm Bull. 1994;17(9):1282–1286.
29. Kubo M, Asano T, Matsuda H, et al. Yakugaku Zasshi. 1996;116(2):158–168.
30. Oh KO, Kim SW, Kim JY, et al. Clin Chim Acta. 2003;334(1–2):185–195.
31. Kang DG, Sohn EJ, Moon MK, et al. Biol Pharm Bull. 2005;28(9):1662–1667.
32. Lee BC, Choi JB, Cho HJ, Kim YS. J Ethnopharmacol. 2009;122(1):131–135.
33. Chae HJ, Kim HR, Kim DS, et al. Life Sci. 2005;76(18):2027–2042.
34. Chen LZ, Feng XW, Zhou JH. Chung Kuo Yao Li Hsueh Pao. 1995;16(4):337–340.
35. Tomoda MI, Miyamoto H, Shimizu N, et al. Biol Pharm Bull. 1994;17(11):1456–1459.
36. Chao JC, Chiang SW, Wang CC, et al. World J Gastroenterol. 2006;12(28):4478–4484.
37. Hu CS. Chin Med J. 1965;51:290.
38. Su ZZ, He YY, Chen G. Chung Kuo Chung Hsi I Chieh Ho Tsa Chih. 1993;13(5):259–260. 269–272
39. Yin XJ, Liu DX, Wang HC, et al. Mutat Res. 1991;260(1):73–82.
40. Yokozawa T, Fujioka K, Oura H, et al. Phytother Res. 1995;9(1):1–5.
41. Chang HM, But PP, Pharmacology and Applications of Chinese Materia Medica, Singapore, World Scientific, 1987;vol. 1. p. 465
42. Bensky D, Gamble A. Chinese Herbal Medicine Materia Medica. Seattle: Eastland Press, 1986. pp. 95–97
43. Perharic-Walton L, Murray V. Lancet. 1992;340(8820):674.
44. Kane JA, Kane SP, Jain S. Gut. 1995;36(1):146–147.
45. Sheehan MP, Atherton DJ. Br J Dermatol. 1994;130(4):488–493.
46. Rustin M, Atherton D. Lancet. 1992;340(8820):673–674.
47. Matsui AS, Rogers J, Woo YK, et al. Med Pharmacol Exp. 1967;16:414–424.
Saw palmetto
Synonyms
Serenoa serrulata (Michaux) Nutall ex Schultes, Sabal serrulata R. et Sch. (botanical synonyms), sabal (Engl), Sabal fructus (Lat), Zwegpalme, Sabalfrüchte (Ger), palmier de l’Amérique du Nord (Fr), savpalme (Dan).
What is it?
Saw palmetto has traditionally been associated with therapy for the prostate gland. Earlier last century, entries could be found in many pharmacopoeias testifying to its use for benign prostatic hyperplasia (BPH). The petiole has a sharp spiny edge that can cut the clothing or legs of those unfortunate enough to come in contact with it, hence the common name of saw palmetto (literally a small saw-like palm). The fruit, a one-seeded dark brown to black drupe (known as the berry), is the part used in medicine. In recent times a more sophisticated pharmaceutical form of saw palmetto has been developed, known as the liposterolic extract (containing lipids and sterols). This liposterolic extract (LESP) has been the subject of many clinical trials. Although these trials have resulted in increased use of the liposterolic extract, especially in Europe where it is widely prescribed by medical practitioners for BPH, the strong traditional use information suggests that the use of galenical forms of saw palmetto, such as extracts and tinctures, should not be discounted.
Traditional view
The dried berries, liquid extract or pressed oil of saw palmetto were used for respiratory complaints, particularly those accompanied by chronic catarrh, and conditions of the genitourinary tract, especially to reduce irritation (for all forms of cystitis) and for prostatic hyperplasia. It was considered to build tissues.1,2
The Eclectics used saw palmetto for upper and lower respiratory problems, atrophy of the breast, ovaries and testes and for BPH. It was described as the ‘old man’s friend’ and, with amazing accuracy, as ‘a remedy for prostatic irritation and relaxation of tissue (rather) than for a hypertrophied prostate’. It was also used for inflamed gonads in the male or female and as an aphrodisiac. One interesting application was uterine hypertrophy.3
Can be used for
Preparations
Decoction of dried berries, tablets, capsules or liquid extract for internal use.
The liposterolic extract is mentioned often in this monograph. According to the German Commission E, this can be prepared by extraction of dried saw palmetto berries with either hexane or 90% ethanol. Extracts prepared using supercritical carbon dioxide are also suitable. The hexane extract has probably been the subject of more clinical trials than the other preparations.
The liposterolic extract contains 85% to 95% fatty acids (including a relatively high percentage of free fatty acids, FFAs) and 0.2% to 0.4% total sterols (with 0.1% to 0.3% beta-sitosterol). Flavonoids are unlikely to be present, except in the extract prepared using 90% ethanol.
Dosage
The typically accepted dose is 160 mg twice a day or 320 mg once a day of the liposterolic extract. This extract is about an 8:1 to 10:1 concentrate compared to the original dried berries. Hence this dose corresponds to about 1.5 to 3 g of dried berries per day. Otherwise, around 2 to 5 mL/day of a 1:2 extract prepared using 45% to 90% ethanol can be used, or the equivalent in other dosage forms (for example, 5 to 12.5 mg/day of a 1:5 tincture). The higher ethanol percentages will better extract the liposterolic components. Saw palmetto combines well with pumpkin seed oil or extract, and with extract of nettle root (see the nettle monograph).
Duration of use
No restriction on long-term use in fact prolonged usage (months or years) is generally necessary for best clinical results.
Summary assessment of safety
No adverse effects are expected if used as recommended.
Technical data
Botany
Serenoa repens, a member of the Palmae (palm) family, is a small shrub native to the south-eastern region of North America.4,5 The leaves are palmate, without continuing rib, divided into lance-shaped linear-lanceolate leaflets, the petioles armed with spiny teeth. The inflorescence is many-branched, less than 1 m, with white flowers. The fruit is a prominent olive-like mesocarp, 16 to 25 mm long, with a single large oblong seed.5
Adulteration
No adulterants known for the dried herb. The liposterolic extract is susceptible to adulteration from other plant-derived fatty oils, for example palm or olive oil. Apparently such adulteration has occurred (see below under Key constituents).
Key constituents
• Lipid content: free fatty acids (including lauric, myristic, palmitic and oleic acids) together with triglycerides, diglycerides and monoglycerides, phytosterols (mainly beta-sitosterol) and fatty alcohols6
• A particularly active lipase which splits the triglycerides into free fatty acids during ripening and drying6
• Flavonoids and polysaccharides.6
The action of the lipase gives the fruit its characteristic rancid odour and taste due to the formation of free fatty acids. This ‘rancidity’ can cause digestive upsets in some people. Methyl and ethyl esters of fatty acids also form in the fruit and contribute to the characteristic aroma.6
A 2002 survey of six saw palmetto products on the Canadian market found that, based on expected fatty acid content, ‘actual’ dosages were within a range of −97% to 140% of stated dosages, with three products containing less than 20% of the stated dosages.7 However, these three ‘low’ products might have contained just the dried herb, as the authors based the ‘actual’ dose on the expected fatty acid content of the liposterolic extract, not the dried herb. This study highlights the often-encountered confusion over herbal dosage, where,for example, the statement ‘320 mg of saw palmetto’ on a label could mean 320 mg of the dried herb, 320 mg of the liposterolic extract, or even 320 mg of an undefined extract.
In a better-designed subsequent study, the chemical variation of various liposterolic extract of saw palmetto (LESP) brands was investigated using liquid and gas chromatography.8 Considerable differences in the free fatty acid (FFA) and glyceride contents were found. The FFA content varied from 81% to 41% and the glyceride content ranged from 7% to 52%. In general, there was an inverse relationship between the two, implying that the low FFA products might have been adulterated with other vegetable oils. The authors noted that such a wide variation in chemical content could impact significantly on clinical outcomes. However, this study analysed products, and in some cases the vegetable oil could have been intentionally added as a carrier during the soft gel capsule manufacturing process.
Pharmacodynamics
BPH is a slow, progressive enlargement of the fibromuscular and epithelial structures (due to the proliferation of stromal and epithelial cells) within the periurethral area of the prostate gland. This can eventually narrow the urethra, impeding the flow of urine. However, there is no direct correlation between histological and macroscopic BPH and lower urinary tract symptoms (LUTS). Histological evidence of BPH is found in more than 50% of men aged 60, and this increases to 90% at age 85. Yet of men with histological changes, only 50% will have clinical enlargement of the prostate or macroscopic changes, and of these individuals only 50% will develop LUTS. Symptoms can be due to obstruction or irritation or both. The clinical course of BPH is variable. Not every man with symptomatic BPH worsens clinically with time and symptom severity does not correlate well with prostate size or urinary outlet obstruction.9,10 Although BPH is a common problem, the pathogenesis of the disease is poorly understood. Many factors are thought to be involved including sex hormones, stem cells, growth factors, insulin and prolactin. Irritation and associated spasm of smooth muscle tissue, inflammation and oedema may also contribute to the development of symptoms.
The recent understanding of BPH downplays androgens, both testosterone and dihydrotestosterone, as aetiological agents.11 Their role is said to be permissive. However, a higher oestrogen/testosterone ratio could be a causative hormonal factor: increased peripheral conversion of testosterone to oestradiol by aromatase could be at play. Chronic inflammation is also a common finding. In fact, one theory has proposed that BPH is an immune-mediated inflammatory disease caused either by infection or autoimmunity (more likely the latter).12 There is a strong link between chronic prostatitis and BPH.13
Another theory proposes that higher circulating insulin stimulates prostate growth.10,14 Multiple experimental, clinical and epidemiological studies have demonstrated the link between either hyperinsulinaemia, elevated fasting blood glucose or type 2 diabetes and prostate enlargement and LUTS.14,15 An association with obesity has also been observed.16 The sympathetic overactivity linked to obesity, metabolic syndrome and hypertension may increase the risk of LUTS.17
In the context of these developments, attempts have been made to understand the pharmacological influence of saw palmetto extracts on some of the various pathogenic factors implicated in BPH.
Inhibition of 5-alpha-reductase
Testosterone is the major circulating androgen in humans. Most of the testosterone circulating in the bloodstream is bound to sex hormone-binding globulin (SHBG). The remaining unbound or free testosterone exerts the biological effects. Synthesis of SHBG is controlled by the ratio of oestradiol to testosterone. In many androgen target tissues, including the prostate, testosterone is converted by the enzyme 5-alpha-reductase into 5-alpha-dihydrotestosterone (DHT), which is about five times more potent than testosterone. There are two isoforms of 5-alpha-reductase (5-AR): type I and type II. Studies suggest that type 2 is mainly found in the prostate gland.
Inhibitors of 5-AR, such as the drug finasteride, block the conversion of testosterone to DHT and have been found to reduce the size of the prostate, leading to an increase in peak urinary flow rate and a reduction in LUTS.18 In various reviews, much is often made of the observation that saw palmetto extracts also appear to inhibit 5-AR. This, together with inhibition of androgen binding, is often given as an explanation for the pharmacological activity of saw palmetto in BPH.
In 1984 it was shown that LESP (see above in the Preparations section) inhibited the 5-AR-mediated intracellular conversion of testosterone to DHT in vitro using human foreskin fibroblasts.19 Inhibition of 5-AR was also demonstrated in vitro using genital fibroblasts for an alcoholic extract.20 Furthermore, in vitro studies using a eukaryotic (baculovirus-directed insect cell) expression system found that LESP inhibited the activity of both isoenzymes of 5-AR, whereas finasteride selectively inhibited type 2.21,22 Finasteride is a competitive inhibitor, whereas LESP displayed non-competitive inhibition of the type I isoenzyme and uncompetitive inhibition of the type II isoenzyme.23 These observations suggest that the lipid components of LESP might be responsible for the inhibitory effect by modulating the membrane environment of 5-AR. Subsequent in vitro studies using epithelial and stromal cells from human BPH confirmed that the inhibitory activity was mainly due to the FFAs found in LESP.24,25
However, in one study the relative in vitro inhibitory effects of various saw palmetto extracts on 5-AR activity were measured to be 5600 to 40 000 times weaker than finasteride.26 Since, LESP is usually administered at about 100 times the dose of finasteride, this suggests its clinical potency is about 60 times weaker in terms of 5-AR inhibition.
LESP at 10 μg/mL was incubated with cell cultures of fibroblast and epithelial cells from the prostate, epididymis testes, kidney, skin and breast.27 There were changes in the morphology of the prostate cells, including accumulation of lipid in the cytoplasm and damage to the nuclear mitochondrial membranes. No similar changes were observed in the other cells. LESP inhibited 5-AR types I and II in prostate cells, but the other cells exhibited no such inhibition of 5-AR. These results indicate a selectivity of LESP for prostate cells.
Several studies have further investigated the in vitro activities of fatty acids found in LESP on 5-AR. Using the eukaryotic (baculovirus-directed insect cell) expression system, IC50 values were determined at 4 μg/mL for myristic acid and 19 μg/mL for lauric acid on the type II isozymes of 5-AR.28 Long unsaturated fatty acids (oleic and linolenic) were much less active. In contrast, IC50 values for 5-AR inhibition in rat liver microsomes were 54 and 66 μg/mL for oleic and lauric acids, respectively.29 Using the same model, the IC50 ranged from 42 to 68 μg/mL for linoleic, oleic, myristic and lauric acids, and was 101 μg/mL for LESP.30
In a reflection of earlier work that compared different extracts, the testing of a range of commercial LESPs (including different batches of the same extract) found a wide variation in the inhibition of 5-AR activity using prostatic co-cultured epithelial and fibroblast cells.31 While extracts tested were able to inhibit both isoforms of 5-AR, the relative potencies varied by a factor of 24 for type I and 237 for type II inhibition. The lowest observed IC50 for type II inhibition was around 4 μg/mL of extract. The authors suggested this variability indicates the potential for a wide diversity of clinical activity for these different extracts of saw palmetto.
A novel LESP (extraction methodology not defined) demonstrated relatively good inhibition of 5-AR type II in transfected human embryonic kidney cells (IC50 2.9 μg/mL).32 However, while the authors state that this bioactivity ‘corresponds favourably … to the established prescription drug … finasteride’, in fact the IC50 in the same model for finasteride was found to be 3.2 nM (or about 0.0012 μg/mL), around 2000 times lower on a weight for weight basis. While this difference is not as high as the 5600 to 40 000 ratios observed in an earlier comparative study,26 it still indicates that saw palmetto is a relatively weak 5-AR inhibitor.
LESP at 10 μg/mL (the apparent calculated plasma concentration in a patient receiving the recommended therapeutic dosage; see later under Pharmacokinetics) inhibited both isozymes of 5-AR in vitro using the co-culture model of BPH, without influencing the secretion of prostate-specific antigen (PSA) by epithelial cells (even after stimulation with testosterone).33 This outcome was also demonstrated in a culture of human prostate cancer cells.34 The molecular mechanisms behind this novel finding were also elucidated.34 The authors of both studies noted that, unlike finasteride, LESP can inhibit 5-AR in vitro without inhibiting PSA secretion. The clinical extrapolation from this finding is that LESP does not lower PSA and hence interfere with its value as a cancer biomarker. However, while it has been observed that finasteride lowers PSA clinically and LESP does not (see later under Clinical trials), this observation could equally be explained by the lower clinical potency of LESP as a 5-AR inhibitor (since it does not reduce prostate size like finasteride).
In castrated rats stimulated with testosterone or DHT, finasteride but not LESP inhibited testosterone-stimulated prostate growth, while neither inhibited DHT-stimulated growth.26 LESP (300 mg/kg/day for 12 or 24 weeks, oral) reduced the concentration of DHT in the prostate in transgenic mice with prostate cancer.35 There was a significant increase in apoptosis and a decrease in tumour grade and incidence. A lower dose (50 mg/kg) was ineffective.
In one 7-day human trial, only finasteride (5 mg/day) and not LESP (320 mg/day) nor placebo decreased serum DHT in men.26 This finding was confirmed in a larger double blind, randomised study in 32 healthy male volunteers; normal doses of LESP (320 mg/day) had no effect above placebo on serum DHT levels.36 But there could be an effect within prostate tissue. A German group studied 18 patients in a randomised, placebo-controlled, double blind trial. Patients with BPH received six times the normal dose of LESP (eight cases) or placebo (10 cases) daily for 3 months. Following prostatectomy, prostatic epithelia and stroma were examined for enzyme activities. While these high doses of LESP caused some moderate biochemical changes, including a small reduction in 5-AR activity in prostatic epithelium, the authors volunteered that the significance of their results in understanding the effects of LESP in BPH were uncertain.37
In a 3-month open label, controlled trial, 25 symptomatic BPH patients were randomised to 320 mg/day LESP or no treatment.38 Following suprapubic prostatectomy, statistically significant reductions were observed for DHT (down 68%, p<0.001) and epidermal growth factor (down 67%, p<0.01) in the prostatic tissue from the LESP-treated men, mainly in the periurethral region. Testosterone was correspondingly increased (up 66%, p<0.001), but prostate size was largely unaffected.
Another trial used biopsy cores for in situ quantification of prostatic androgens and compared LESP with finasteride.39 Prostate levels of testosterone and DHT were measured in 5 to 10 mg biopsy specimens (18-gauge needle cores) in three groups of men with symptomatic BPH: 15 men receiving chronic finasteride therapy versus seven untreated controls; four men undergoing prostate adenomectomy to determine sampling variability (10 specimens each); and 40 men participating in a 6-month randomised trial of LESP (320 mg/day) versus placebo, before and after treatment. Prostatic tissue DHT levels were found to be several times higher than the levels of testosterone (5 versus 1.5 ng/g), that ratio becoming reversed (1.05 versus 3.63 ng/g) with chronic finasteride therapy. The finasteride effect was statistically significant for both androgens (p<0.01), and little overlap of individual values between finasteride-treated and control patients was seen. In the randomised trial, tissue DHT levels were reduced by 32% from 6.49 to 4.40 ng/g in the LESP group (p<0.005), with no significant change in the placebo group. Prostatic testosterone levels exhibited no significant change after LESP treatment.
Based on all the above findings, it can be concluded that while LESP is probably a significant inhibitor of the 5-AR type II isozymes in men with BPH, its clinical activity is relatively modest compared with finasteride, even in the best-case scenario for the most active forms of this extract. Hence, inhibition of 5-AR is likely to be only one factor behind the clinical effect of LESP in patients with BPH/LUTS, and certainly does not fully explain its therapeutic activity in this condition.
Spasmolytic activity
Antiadrenergic agents are used in BPH to decrease dynamically caused obstruction associated with increased smooth muscle tone in the bladder trigone and membranous urethra of the prostate. In particular, the selective alpha1-blockers are clinically preferred and include terazosin, doxazosin, tamsulosin and alfuzosin. Prazosin is also used. This has stimulated an interest among researchers as to whether saw palmetto extracts might act in a similar way.
An ethanolic LESP and a saponifiable extract produced a spasmolytic effect on isolated rat uterus, bladder and aorta in vitro.40 This effect appeared to be related to an inhibition of calcium influx and intracellular effects. Follow-up research suggested that cyclic AMP may be a possible mediator, together with the involvement of protein synthesis.41 Additional in vitro research on the above LESP found spasmolytic effects that were attributed to alpha-adrenoceptor and calcium channel blocking activities.42
Preparations of beta-sitosterol and extracts of nettle root, medicinal pumpkin seed and saw palmetto were tested for their in vitro ability to inhibit tamsulosin binding to human prostatic alpha1-adrenoceptors (A1A receptors) and prazosin binding to cloned human A1A and alpha1B-adrenoceptors.43 Only saw palmetto (several extracts) potently and non-competitively inhibited A1A in vitro. The in vitro binding affinities for A1A, muscarinic and purinergic receptors in the rat prostate and bladder were measured by radioligand binding assays for saw palmetto.44 LESP inhibited specific binding of prazosin and N-methylscopolamine (NMS) in the rat prostate and bladder. The binding activity of LESP for muscarinic receptors was four times greater than that for A1A receptors. This in vitro activity (including also binding to calcium channel antagonist receptors) was subsequently identified by the same research centre as due to the FFAs, specifically oleic, lauric, myristic and linoleic acids.29,30
In contrast, a different research group found that saw palmetto ethanolic extracts (45% and 70% ethanol) caused contraction of the rat prostate gland consistent with sympathomimetic activity and identified tyramine as the responsible agent.45 However, tyramine is likely to be inactivated by the gastrointestinal tract on oral ingestion, especially at the low doses involved (one saw palmetto 1:2 extract tested had a recommended dose of 2 to 4 mL/day and contained about 4 mg/mL tyramine.) Nonetheless, it does imply the theoretical possibility of an interaction of saw palmetto with monoamine oxidase inhibitor drugs, as noted by the authors.
LESP (12, 20 and 60 mg/day, intraduodenal) alleviated urodynamic symptoms in a model of hyperactive bladder in spontaneous hypertensive rats by increasing bladder capacity and subsequently prolonging the micturition interval.46 A repeated oral dose of 6 mg/kg in the same rat breed decreased voiding frequency under normal conditions. Rats given LESP (6 and 60 mg/kg/day, orally for 4 weeks) exhibited a significant increase in prostate binding sites for prazosin and a significant decrease in bladder binding sites for NMS.44 The exact meaning of these findings is not clear.
In a placebo-controlled, double blind, four-way, crossover study, 12 healthy young men received three different LESPs (320 mg/day) or placebo for 8 days each.47 Although the saw palmetto extracts caused minor reductions in supine blood pressure, they did not affect blood pressure during orthostatic stress testing nor influence plasma catecholamines. Plasma samples taken from the men did not demonstrate an influence on A1A receptors using radioligand binding assays. (This assay methodology does exhibit sensitivity to the drugs tamsulosin and terazosin.) The authors proposed the clinical effects of LESP are unlikely to result from A1A receptor antagonism. However, while this conclusion may be valid, animal experiments do suggest that LESP can influence bladder function in a way that would be beneficial in LUTS.
Inhibition of androgen binding and antiandrogenic activity
Androgens exert their effects by binding to an intracellular cytoplasmic receptor, forming a hormone-receptor complex that is transferred to the cell nucleus, binds to DNA and can activate and modulate protein transcription. Androgen potency is determined by their binding affinity to the receptor. Hence, another important component of the mechanism of LESP might be an inhibitory effect on the binding of DHT to androgen receptors in the cytosolic component of prostate cells.
This inhibitory effect was demonstrated for LESP prepared using n-hexane in two in vitro models,9,48 but not for an ethanolic LESP.10 Subsequent in vitro research found that LESP inhibited testosterone and DHT binding in several tissue specimens, including vaginal skin and prepuce.49 However, a later study found that LESP did not inhibit the binding of DHT to the rat prostatic androgen receptor at LESP concentrations up to 100 μg/mL.16
The effects of LESP on two prostatic cancer cell lines differing in androgen responsiveness were investigated.50 LESP had a proliferative effect on androgen-responsive cells at lower concentrations (≤10 μg/mL) and a cytotoxic effect at higher concentrations (≥25 μg/mL). At 25 μg/mL, LESP antagonised androgen-stimulated cell growth. In cells unresponsive to androgen stimulation, LESP had a concentration-dependent antiproliferative action. When these cells were co-transfected with wild-type androgen receptors, LESP inhibited androgen-induced effects. The authors concluded that their findings support an antiandrogenic action of LESP. This antiandrogenic activity was supported for LESP in several animal models.51,52 For example, a hypercritical carbon dioxide extract (300 mg/rat, oral) antagonised the effect of testosterone on prostate size in castrated rats.52
Other hormonal activity
Prolactin may stimulate prostate growth, although its role in BPH is controversial.53 Addition of LESP at concentrations ranging from 1 to 10 μg/mL to Chinese hamster ovary cells completely inhibited prolactin signal transduction pathways, implying that the extract may inhibit prolactin-induced prostate growth.54 The authors suggested that LESP might also be useful for other diseases implicating prolactin. (See also under ‘Activity in BPH or its experimental models’.)
Early in vivo experiments indicated that saw palmetto had oestrogenic activity.55 However, subsequent evidence implies that LESP in fact has anti-oestrogenic activity in patients with BPH.56 In a double blind, placebo-controlled study, 35 patients received either LESP at 320 mg/day (18 cases) or placebo (17 cases) for 3 months. Following prostatectomy, steroid receptors were evaluated in the nuclear and cytosolic fractions of prostate cells. Oestrogen receptors in the nuclear fraction were significantly lower in the group receiving LESP, as determined by three different tests. Single-point assay found that androgen receptors in the nuclear fraction were also reduced by treatment with LESP. These results indicate that LESP exerted an anti-oestrogenic effect, possibly by competitively blocking translocation of cytosolic oestrogen receptors to the nucleus. It may even be that the inactivation of androgen receptors is secondary to oestrogen blockade and this anti-oestrogenic effect may potentiate other actions of LESP.
Twenty men with BPH were treated with 320 mg/day of LESP for 30 days.57 No changes in plasma levels of testosterone, follicle-stimulating hormone and luteinizing hormone occurred as a result of treatment. A combination of LESP and astaxanthin demonstrated a significant dose-dependent reduction in serum oestrogen levels in an uncontrolled study in 42 healthy men.58
Basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) are two other mediators possibly involved in the pathogenesis of BPH. LESP did not affect basal prostate cell proliferation, with the exception of two prostate specimens, for which a significant inhibition of basal proliferation was observed with the highest concentration (30 μg/mL).59 In contrast, LESP inhibited bFGF-induced proliferation of human prostate cell cultures; this effect was significant at 30 μg/mL. Unsaturated FFAs from LESP markedly inhibited the bFGF-induced cell proliferation down to the basal value. Lupenone, hexacosanol and the unsaponified fraction of LESP also inhibited bFGF-induced cell proliferation, whereas a minimal effect on basal cell proliferation was noted. A less pronounced inhibition of EGF-induced proliferation was also observed for LESP. A clinical study cited previously found that LESP (320 mg/day) inhibited EGF in the prostate tissue of men with BPH.38
Anti-inflammatory activity
As a result of secretory stagnation, BPH is associated with congestion and non-infectious prostatitis, as evidenced by white cell infiltration of the prostate. For this reason, agents with anti-inflammatory and oedema-protective activities may improve the clinical picture of BPH.
French scientists have reported on the anti-oedematous activity of LESP.25,60 Various experimental models were used to test the influence of LESP on cutaneous permeability. Interestingly, an antagonistic effect of the extract was observed whenever histamine was involved, either directly through injection or indirectly via mast cell degranulation. An oral dose of 5 to 10 mL/kg produced a significant effect. However, this is a very high dose. LESP showed no action on serotonin- or bradykinin-induced weals in rats. Since the anti-oedematous effect was also demonstrable in adrenalectomised rats, the participation of glucocorticoids is definitely excluded. Adult male rats received either tocopherol (the LESP vehicle) or LESP (50 and 100 mg/kg orally every second day for 90 days).61 A dose-dependent, significant decrease in mast cell accumulation and a provocation of epithelial atrophy was observed within the central area of the rat ventral prostate. The authors noted that since prostatic mast cell degranulation can induce smooth muscle contraction in that gland, these findings could have implications for the activity of LESP in BPH.
A pronounced anti-oedematous effect was observed for oral doses of an alcoholic extract of saw palmetto in carrageenan-induced oedema of rat paw.62 A water-based preparation had no effect, and polysaccharides, beta-sitosterol derivatives and flavonoids were not responsible for the anti-inflammatory outcome. Another group of scientists reported on the isolation of an acidic polysaccharide with anti-inflammatory activity (after intravenous injection) from a water-based extract of saw palmetto.63,64 Since this polysaccharide could be expected to have poor oral bioavailability and would not occur in LESP, the clinical significance of this finding is doubtful.
The most important proinflammatory metabolites of arachidonic acid are prostaglandins and leukotrienes and their production is respectively mediated by the enzymes cyclo-oxygenase (COX) and 5-lipoxygenase (5-LOX). LESP prepared by supercritical liquid extraction with carbon dioxide was found in vitro to be a dual inhibitor of COX (IC50 28.1 μg/mL) and 5-LOX (IC50 18 μg/mL).65 Activity was found to reside in the acidic lipophilic fraction; fatty alcohols and sterols were inactive. It is possible that this inhibition of the arachidonic acid cascade causes the observed anti-oedematous effect of LESP. The potent in vitro inhibition of the production of 5-LOX metabolites (especially leukotriene B4) by LESP has been confirmed in another study using human neutrophils.66
After stimulation of peritoneal rat macrophages with lipopolysaccharide, an undefined saw palmetto extract inhibited the expression of the inflammatory enzymes COX, 5-LOX and inducible nitric oxide synthase.67 At an oral dose of 28.5 mg/kg it also inhibited prostate inflammation induced in rats by partial bladder outlet obstruction.
In an open clinical trial of 29 men with BPH, 12 took LESP (320 mg/day) for 3 months, while 17 controls received no treatment prior to surgical treatment for their condition.68 Assessment of surgically obtained samples revealed significantly lower levels of IL-1 and TNF-alpha in the prostate tissue of the men who took LESP compared with controls (p<0.006). This trial strongly supports a real-world reduction in inflammatory cytokines associated with the pathogenesis of BPH by the use of saw palmetto.
Activity in BPH or its experimental models
High oral doses of LESP (300 mg/day for 12 days) inhibited prostatic growth in castrated mice given testosterone, and 200 mg/day for 6 days achieved a similar outcome in a rat model.51 The increase of prostate weight induced by oestradiol/testosterone treatment in castrated rats was countered by oral administration of LESP at 50 mg/kg/day.69 This is a model that exhibits both prostatic inflammation and hyperplasia. In contrast, high oral doses of LESP (180 mg/day and 1800 mg/day) did not influence prostatic growth induced by testosterone and DHT in castrated rats in another study.26
One model of BPH uses transplants of human prostate tissue into hairless mice. Growth of this tissue is then stimulated by the administration of DHT and oestradiol. Using this model, high oral doses of LESP (6 mL/kg) were observed to reverse the hormonal stimulation of prostate growth.70
Using an in vivo model of rat prostate hyperplasia induced by hyperprolactinaemia, LESP (320 mg/kg, oral) but not finasteride (5 mg/kg, oral) countered the hyperplastic effect of prolactin in the lateral prostate in castrated rats treated with DHT.71
The rate of apoptosis and cell proliferation was compared in normal human prostate tissue (from organ donors) and tissue samples from patients with BPH either treated with LESP (320 mg/day) or not.72 There were 10 tissue samples in each group. No difference was observed between the mean proliferative index and the mean apoptotic index in the epithelium or stroma of the normal prostate samples. In contrast, proliferation exceeded apoptosis in the untreated BPH tissue samples, and the proliferative index was significantly higher than in the normal prostate. BPH tissue samples from the men treated with LESP reversed this apoptosis/proliferation ratio. In fact, a significantly higher apoptotic index and a significantly lower proliferative index were measured. The differences in these indexes resulting from LESP treatment were quite marked. The authors concluded that LESP induces apoptosis and inhibits cell proliferation at normal clinical doses in the prostates of men with BPH, and that these observations could explain its clinical benefit for this disorder. An increase in the apoptotic index in prostate tissue is also supported by in vitro work cited previously.27
In an open label pilot study, patients with BPH preceding surgery were randomised to be followed without any treatment for 3 weeks (control group) or received LESP (320 mg/day) for a 3-month period.73 Surgery was ultimately performed in 17 controls and 12 patients by using transurethral prostate resection or retropubic adenomectomy. The Bax/Bcl-2 ratio, which is an apoptotic index, was significantly increased in the prostatic tissue of treated patients, as was evidence of increased caspase 3 activity in the prostate.
Antitumour activity
Several studies have investigated the effect of saw palmetto extracts on various prostate cancer cell lines in vitro. Generally, increased apoptosis and growth inhibition have been observed, either for LESP74–78 or aqueous-ethanolic extracts.79–81 The ethanolic extract has also demonstrated STAT3 (signal transducer and activator of transcription 3) signalling inactivation,80 including in human multiple myeloma cell cultures.82 However, the 70% ethanolic extract was relatively weak at inhibiting the growth of three prostate cancer cell lines compared with other herbs,83 and one study found that LESP was not able to induce apoptosis or cell cycle arrest in two prostate cancer cell lines, although it did inhibit cell growth.84
Some in vivo studies have also been undertaken using either tumour xenograft or transgenic models. Saw palmetto 70% ethanolic extract (10 mg/kg, oral) inhibited the growth of xenografts in immunodeficient mice injected with prostate cancer cells.80 Serum levels of PSA were reduced by approximately 66%. As cited previously, LESP increased apoptosis and decreased tumour grade and incidence in transgenic mice with prostate cancer.35
Studies are ongoing and the clinical significance of the above work is not known. However, it does appear on current information that saw palmetto use will not prevent prostate cancer. To evaluate whether saw palmetto intake was associated with a reduced risk of prostate cancer, a prospective cohort study of 35 171 men aged 50 to 76 years in western Washington State, USA, was undertaken.85 Participants completed questionnaires between 2000 and 2002 on frequency of use of saw palmetto or saw palmetto-containing combination products over the previous 10 years, in addition to other information on supplement intake, medical history and demographics. Men were followed through to December 2003 (mean of 2.3 years of follow-up), during which time 580 developed prostate cancers. Ten per cent of the cohort used saw palmetto at least once per week for a year in the 10 years before baseline. No association was found between this level of use of saw palmetto and risk of prostate cancer development (hazard ratio 0.95; 95% CI 0.74 to 1.23) or with increasing frequency or duration of use. Certainly, the use of saw palmetto did not apparently increase the risk of prostate cancer. Whether LESP, which is probably more active than many of the products also included in the survey, will affect prostate cancer risk remains to be studied.
Other activity
A form of LESP stimulated macrophage phagocytosis in vitro, as well as human natural killer (NK) cell synthesis of interferon-gamma.86 However, relatively high concentrations were used (up to 1.28 mg/mL).
Saw palmetto (dosage and extract not defined) had no influence on platelet function after administration to 10 healthy volunteers for 2 weeks.87
Pharmacokinetics
A study carried out on rats given oral doses of LESP supplemented with 14C-labelled oleic or lauric acids or beta-sitosterol demonstrated that radioactivity uptake in prostatic tissue was highest after administration of LESP supplemented with 14C-labelled oleic acid.88 This uptake was clearly demonstrated in a rat with experimentally induced BPH. Interestingly, uptake of radioactivity in the prostate was greater than for other organs.
Human pharmacokinetic data on LESP can be gleaned from a bioequivalence study on two different dosage forms.89 Twelve healthy men each received a single oral dose of 320 mg of LESP. Since LESP is a complex mixture of several components, one component was chosen for the study. This component was not identified, but was defined by a validated HPLC method and referred to as ‘Serenoa repens second component’. Based on analysis of this component, a mean peak plasma concentration of 2.6 μg/mL was reached after 1.5 h. The mean elimination half-life was 1.9 h. Given this half-life, administration of LESP twice daily is probably preferable, although at least two clinical trials have found that 320 mg once daily appears to be as effective as 160 mg twice daily for BPH symptoms.90,91
Clinical trials
Saw palmetto has been used for the treatment of BPH for at least 100 years. The modern clinical evidence supporting the efficacy of its liposterolic extract for this disorder is reasonably good, especially in combination, despite the finding of a 2009 systematic review. While the evidence is controversial, it is considered sufficient to justify the use of this plant for the treatment of mild-to-moderate BPH in circumstances where conventional therapy is either not wanted or inadvisable. In particular, LESP appears to work well in combination with nettle root. The safety profile of this herbal preparation is very good.
Benign prostatic hyperplasia
The Fifth International Consultation on BPH in 2000 set the basic criteria for assessing the pharmacological treatment of BPH.92 All clinical trials were expected to be randomised, placebo-controlled and double blind and to include a follow-up period of at least 1 year. All trials should assess several independent parameters for treatment outcome and should include an analysis of the tolerability of the treatment. Several clinical trials of LESP in BPH have completely satisfied these criteria.
A 2009 systematic review and meta-analysis of clinical trials involving saw palmetto (and particularly various forms of LESP) in the treatment of BPH was published on behalf of the Cochrane Collaboration.93 Trials were eligible if they randomised men with symptomatic BPH to receive saw palmetto (alone or in combination) for at least 4 weeks in comparison with placebo or other interventions. Overall, 5222 patients from 30 randomised trials lasting from 4 to 60 weeks were assessed. Meta-analysis of nine trials for nocturia found saw palmetto (as the sole treatment) was significantly better than placebo, with an improvement of 0.78 fewer nocturnal visits (p<0.05). However, when the five larger trials (740 patients) were included in a meta-analysis a smaller improvement in nocturia was calculated that did not achieve significance. Using the subjective 8-question International Prostate Symptom Score (IPSS) as the outcome, meta-analysis of just two trials found saw palmetto was no better than placebo. Saw palmetto (as the sole treatment) was also not superior to placebo in terms of peak urine flow (meta-analysis of 10 trials) or reducing prostate size (results from three trials). However, it was superior to placebo on patient self-rating (meta-analysis of five trials, p=0.01) and clinician assessment (meta-analysis of three trials, p=0.015). Despite some of these positive outcomes, and the fact that the authors’ analysis of trial data concluded that saw palmetto as a sole treatment was equivalent to the standard BPH drugs finasteride (one trial) and tamsulosin (two trials), they concluded that saw palmetto was not more effective than placebo for the treatment of urinary symptoms consistent with BPH.
A large influence of this outcome was the US-government-funded trial of Bent and colleagues published in 2006 in the New England Journal of Medicine (NEJM).94 This was a well-designed and conducted double blind, randomised, placebo-controlled trial in 225 men with BPH that found LESP (160 mg twice a day) for 1 year was not superior to placebo in terms of changes in a US version of the IPSS, maximal urinary flow rate, prostate size, residual volume after voiding or PSA levels.
As noted in the accompanying editorial,95 a limitation of this study is that Bent and colleagues tested a specific preparation of saw palmetto, leaving open the possibility that a different preparation might have been effective. Specifically, they tested a US LESP product, whereas most of the positive earlier trials were on European products, especially a hexane extracted LESP. While they went to great lengths to authenticate the LESP used, this validation was based largely on the determination of total fatty acids. As noted above, the more active versions of LESP have higher levels FFAs, which will also reflect on their being free of adulteration with other plant-derived fatty oils.8 A lower level of FFAs in LESP will also reflect on less pharmacological activity, such as inhibition of 5-AR in vitro.31 It is clear from the information provided that FFAs were not determined in the LESP product used in the NEJM study.
A reviewer’s feedback commentary on the Cochrane analysis noted the same issue: that variability in the quality of the saw palmetto products used in clinical trials might have concealed a true benefit in BPH and undermined the authors’ conclusions.93
Earlier systematic reviews have drawn more positive conclusions regarding the efficacy of LESP in BPH. They have essentially drawn on the same data as the Cochrane review, apart from the NEJM trial. In 2004, an updated meta-analysis of 14 randomised clinical trials and three open label trials involving 4280 patients found a significant improvement in peak flow rate and a reduction in nocturia above placebo, together with a five-point reduction in the IPSS.96 Four of the randomised trials compared LESP with other drugs including finasteride and tamsulosin. The mean placebo effect on peak urinary flow rate was an increase of 1.20 mL/sec. The estimated effect of LESP was a further increase above placebo of 1.02 mL/sec (p=0.042), which means that the herbal treatment was associated with an overall increase in peak urinary flow of 2.22 mL/sec. This represents a clinically significant 15% to 20% increase. Effects on nocturia were less striking, due to the influence of one large study involving 396 patients that showed no difference to placebo treatment. Placebo was associated with a reduction in the mean number of nocturnal voids of 0.63 and there was a further reduction attributable to LESP of 0.38 (p<0.001). Hence the use of saw palmetto was associated with an average reduction from baseline of one event per night in terms of nocturnal voiding, which is clinically significant. Results were not significantly altered if the three open trials considered in this analysis were excluded. The authors included data from at least four unpublished trials to remove concerns about publication bias. It was concluded that LESP is extremely safe, with a very low rate of any adverse effects. To address concerns over product variability, this meta-analysis and the 2000 publication that it updated97 included only LESP from just one supplier (a hexane extract).
Apart from the NEJM study, the better conducted more recent trials on LESP have compared its efficacy in BPH with standard drug treatments, rather than placebo. These, together with some key earlier trials, are summarised in Table 1.
Although results from these trials were covered in the Cochrane review, their significance was not emphasised, as noted in the feedback reviewer’s comments.93 As shown in the table, results from these trials demonstrate that LESP is generally just as efficacious as standard drug treatments, and perhaps superior in some circumstances.
A significant comparative trial is the large-scale comparison with finasteride.98 Patients were recruited from a number of urology centres in nine European countries and the study was the largest international comparative trial for the treatment of BPH. The trial data clearly support the therapeutic value of LESP in BPH. However, this study did have some problems with its design. Most notable of these were the absence of a placebo group or placebo run-in period (which would have afforded a three-way comparison) and the insufficient duration of the trial (minimum 1 year). This latter point is particularly relevant to comparisons using finasteride, since this agent can show increasing efficacy up to 1 year after initiation of therapy.103
One important outcome of the study was the observation that LESP does not significantly influence prostate volume or serum PSA, whereas finasteride does. This is clear and significant clinical evidence that LESP does not act as a strong 5-AR inhibitor (although other evidence discussed above supports LESP as moderately active against type II intraprostatic 5-AR). Since PSA is used as a screening test for prostate cancer and 5-AR inhibitors can decrease PSA, there is a concern that these agents could mask the detection of prostate cancer. On the basis of the data provided by this large trial, it can be confidently concluded that this concern would not apply to LESP. This is particularly reassuring, since LESP is often self-prescribed and an investigating urologist may be unaware of its use by a particular patient.
The large comparison with tamsulosin was a French study known as the PERMAL study, published in 2002.90 Over 1 year in 704 patients the two treatments were found to be equivalent for BPH. A subset analysis published in 2004 examined results for patients with severe LUTS of BPH.102 Severe LUTS was defined as an IPSS greater than 19. Analysis was performed on 124 patients with severe LUTS (59 receiving LESP (320 mg/day) and 65 receiving tamsulosin). After 12 months, IPSS had decreased by 7.8 with saw palmetto and 5.8 with tamsulosin (p=0.051). The irritative symptoms subscore improved significantly more (p=0.049) with saw palmetto (−2.9 versus −1.9 with tamsulosin). The superiority of LESP in reducing irritative symptoms appeared at month 3 and was maintained up to month 12 (p=0.03).
One interesting study cited earlier compared two dosage forms of LESP in 132 patients with BPH over 1 year.91 The interest comes from the observation that both dosage forms strikingly and progressively reduced average IPSS from around 17 at the start of the trial to around 7 after 1 year. This would be a very unusual placebo effect if it was still actively reducing symptoms even after 9 months of use. Similar sustained and progressive IPSS improvement over 2 years was observed for a later uncontrolled study in 120 men with mild or moderate LUTS due to BPH taking LESP (320 mg/day).104
The TRIUMPH study recorded the treatment and outcomes of 2351 newly presenting LUTS/BPH patients in six European countries over a 1-year follow-up period.105 At each visit the clinician recorded the treatment, co-morbidities, complications and drugs prescribed, and the patient completed an IPSS questionnaire. The results were analysed using change in IPSS as the primary outcome measure. Over the study period 74.9% of patients were prescribed medication, the majority (83% of those medicated) were prescribed only a single treatment. Significant improvements were seen in 43% of patients on phytotherapy with saw palmetto or Pygeum africanum, compared with 57% of those on finasteride and 68% on alpha-blockers. All treatments showed some improvement over watchful waiting for most patients over the study period: the alpha-blockers were found to be the most effective. The average IPSS reductions were 1.4 points for watchful waiting, around three points for each herbal treatment and around six points for the alpha-blockers.
LESP may have a preventative role in BPH. A prospective, multicentre study was designed to determine the effect of treatment with LESP on the progression of mild symptoms of bladder outlet obstruction (defined as an IPSS of less than 8) secondary to BPH, compared with watchful waiting.106 Treatment with LESP reduced the incidence of clinical disease progression, and the effect was noticed as early as 6 months. At the end of 24 months, the rate of progression was 16% in the LESP group, compared to 24% in the watchful waiting group. This difference was significant (p<0.05). LESP also improved urinary symptoms, quality of life scores and urinary flow rates. It was administered for 2 years at a dose of 320 mg/day. The relevance of this study would have been improved by the inclusion of a treated or placebo control group, with appropriate randomisation.
Saw palmetto has demonstrated good clinical results in trials when used in combination with other agents. In particular, trials of LESP in conjunction with nettle root extract have been consistently positive. (These trials are reviewed in the nettle monograph).
Chronic prostatitis
Given the co-morbidity between BPH and chronic prostatitis (CP), it is not surprising that LESP has been investigated clinically for the latter. A study was undertaken to assess the efficacy of saw palmetto or finasteride in men with category III chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS).107 This category denotes non-bacterial prostatitis. Patients (n=64) were randomised to finasteride (5 mg/day) or LESP (320 mg/day) for 1 year using an open label study design. There were 61, 57 and 56 patients available for evaluation at 3, 6 and 12 months, respectively. At 1 year the mean total NIH Chronic Prostatitis Symptom Index (CPSI) score decreased from 23.9 to 18.1 in the finasteride group (p<0.003) and from only 24.7 to 24.6 in the saw palmetto group (p=0.41). The authors concluded that the patients treated with saw palmetto had no appreciable long-term improvement.
Other uncontrolled trials have demonstrated some clinical benefit for LESP in both bacterial and non-bacterial prostatitis, although the lack of a placebo group impairs the clinical relevance of such data.108,109 Saw palmetto as LESP has also been used as a part of a complex regime for bacterial and non-bacterial prostatitis including antibiotics and alpha-blockers, with positive results.110,111 However, it is difficult to ascertain what role the herbal extract played in patients’ recoveries.
In an interesting trial, 102 men with category IIIa CP/CPPS were randomised to receive LESP (320 mg/day) or LESP plus lycopene and selenium for 8 weeks.112 The mean CPSI score decreased significantly (p<0.001) in both groups, but was greater for the combination.
Prostate surgery
One intriguing application from clinical trials is the potential role of LESP in reducing complications following transurethral resection of the prostate (TURP).113 In an open label, controlled Italian study, patients were randomly assigned to receive either 320 mg/day of LESP or no additional treatment for at least 8 weeks before the TURP procedure. Out of 108 enrolled patients, 88 were evaluated at the end of the trial. In the treated group, perioperative bleeding was significantly lower than controls (124 mL versus 287 mL) and the need for transfusion was substantially reduced. In addition, for the saw palmetto group the duration of postoperative catheterisation was considerably lower (3 days versus 5 days for controls) and haematological findings (red cell count, haemoglobin and haematocrit) were more favourable. The authors concluded that pretreatment with saw palmetto before TURP improves the efficacy of the procedure and reduces the risk of complications.
A later Italian study of similar design included 144 patients who were candidates for either TURP or open prostatectomy.114 In the group receiving 320 mg/day of LESP, the duration of surgery was shorter than for the control group (59.8 min versus 77.6 min, p<0.001), no intraoperative complications were observed (0% versus 15%, p=0.001) and transfusion needs were remarkably lower (0% versus 38.3%, p<0.001). The postoperative course was more favourable after LESP pretreatment, with a shorter duration of catheterisation (65 h versus 92 h, p<0.001) and length of hospitalisation (5.9 days versus 7.9 days, p<0.001).
Another study compared the effects of the 5-AR inhibitor dutasteride (5 mg/day) with LESP (160 mg/day) in men about to undergo TURP.115 The treatments were given for 5 weeks and compared with an untreated control group. Neither treatment influenced blood loss during or after surgery. However, this was a Turkish study that used a local LESP product, so product quality might have been an issue, as per the discussions elsewhere in this monograph.
Androgenic alopecia and scalp problems
Given the influence of LESP on 5-AR activity, it follows that it might have value in the treatment of male pattern baldness (MPB). A group of scientists decided to test the effect of oral intake of LESP on MPB in a pilot study involving 19 men between the ages of 23 and 64 under double blind conditions.116 The product tested also contained beta-sitosterol (110 mg/day) and nutrients; the dose of LESP used was 400 mg/day. The blinded investigative staff rated 60% of the volunteers receiving active treatment as improved, compared to only 11% for the placebo group. Self-assessment by volunteers showed a similar but less striking trend.
Two studies involving topical use have only been published as conference proceedings and were summarised in a brief review.117 In a study involving 34 men and 28 women, saw palmetto applied topically for 3 months in a lotion and shampoo base led to a 35% increase in hair density and a 67% sebum reduction, as assessed using standard objective techniques. Addition of 0.5% saw palmetto (possibly LESP) and taurine to a 0.5% ketoconazole shampoo gave better results than ketoconazole alone (at 1.0%) in patients with dandruff and seborrhoeic dermatitis.
Toxicology and other safety data
Toxicology
Published toxicological data on saw palmetto and LESP is limited. Brine shrimp lethality directed fractionation of an ethanolic LESP led to the isolation of two monoacylglycerides.118 These compounds showed moderate biological activities in the brine shrimp lethality test and against renal and pancreatic human tumour cells in vitro; borderline cytotoxicity was exhibited against human prostatic cells.
The company Madaus has released toxicological data on its ethanolic LESP.119 The LD50 in the rat, mouse and guinea pig is greater than 10 g/kg. High doses given to rats over 6 weeks (360 times the human therapeutic dose of about 5 mg/kg) did not cause adverse haematological, histological or biochemical changes. A long-term study over 6 months in rats at 80 times the human dose again found no negative influences. The same dose administered to rats had no influence on fertility.
LESP as the hexane extract did not produce any indications of liver toxicity when given to rats for up to 4 weeks at oral doses of 9.14 or 22.86 mg/kg/day.120
A poorly characterised liposterolic extract fed to mice for 6 weeks failed to show any genotoxic effects.121
Special warnings and precautions
Prostate cancer should be excluded before patients receive saw palmetto treatment as the herb treatment may mask the symptoms of this disease (but will not alter PSA readings). Exercise caution for concurrent use with warfarin.
Interactions
LESP at relatively high concentrations inhibited CYP3A4, 2D6 and 2C9 in vitro.122 However, using alprazolam, midazolam, caffeine, chlorzoxazone, desbrisoquine and dextromorphan as probe drugs, two clinical studies found that LESP (320 mg/day) for 14 or 28 days exerted no significant influence on CYP3A4, CYP1A2, CYP2D6 and CYP2E1 in healthy volunteers.123,124
A 61-year-old man had long been treated with warfarin and simvastatin. His INR values had been stable at around 2.4. Due to micturition difficulties he started to take a saw palmetto, pumpkin seed and vitamin E preparation, five tablets daily. After 6 days’ treatment his INR increased to 3.4. The herbal product was discontinued and 1 week later the INR returned to its previous level.125
Use in pregnancy and lactation
Category B2 – no increase in frequency of malformation or other harmful effects on the fetus from limited use in women. Animal studies are lacking. However, saw palmetto treatment is unlikely to be indicated for a pregnant women.
Saw palmetto is probably compatible with breastfeeding.
Addition of liposterolic extract at concentrations ranging from 1 to 10 μg/mL to Chinese hamster ovary cells completely inhibited the effects of prolactin, suggesting that saw palmetto may inhibit prolactin-induced hormonal effects.54 However, the relevance of this research to normal human use is unclear.
Side effects
Saw palmetto is well tolerated by most patients and causes relatively few side effects. Most side effects are minor gastrointestinal problems such as nausea, which are usually resolved when the herb is taken with meals.
The large comparative study of Carraro and co-workers98 found that gastrointestinal complaints were the most frequently reported adverse events with both therapies and tended to occur more frequently with finasteride. As might be expected, decreased libido and impotence were also more common with finasteride treatment. Two deaths occurred during the trial (one in each group) and three serious adverse events occurred (two with saw palmetto, one with finasteride). None of these was deemed to be related to treatment. PSA was not changed by saw palmetto treatment, indicating that the herb is unlikely to interfere with the diagnostic value of this test.
The German Commission E lists stomach upsets as the only side-effect from treatment with saw palmetto liposterolic extract.126
A 2009 systematic review assessed all available human safety data for saw palmetto monopreparations up to early 2008.127 Systematic literature searches were conducted in five electronic databases, reference lists and departmental files were checked for further relevant publications. Information was requested from spontaneous reporting schemes of the WHO and national safety bodies. Twenty-four manufacturers/distributors of saw palmetto products and four herbalist organisations were contacted for additional information. Forty articles (26 randomised controlled trials, four non-randomised controlled trials, six uncontrolled trials and four case reports/series) were included. They suggest that adverse events associated with the use of saw palmetto are mild and similar to those with placebo. The most frequently reported adverse events were abdominal pain, diarrhoea, nausea, fatigue, headache, decreased libido and rhinitis. More serious adverse events, such as death and cerebral haemorrhage, are reported in isolated case reports and data from spontaneous reporting schemes, but causality was questionable.
A review of the data from three large clinical trials of the hexane extract of LESP that included 2511 patients found it had no negative impact on male sexual function.128
Intraoperative floppy-iris syndrome (IFIS) has been associated with many A1A receptor antagonist drugs including tamsulosin.129 Their probable mode of action in causing IFIS is a direct inhibition of the A1 receptors in the smooth muscle of the iris, which leads to a loss of iris tone. This can result in poor iris dilation and complications during cataract surgery (IFIS).
Two surgeons have reported IFIS in two patients taking saw palmetto for BPH who were undergoing cataract surgery.129 The first patient had used saw palmetto for 2 years and the other for 5 years, and neither patient reported taking conventional drugs for their BPH. Both patients experienced good outcomes following their surgery. No information regarding the saw palmetto dose and preparation was provided.
Analysis of 899 eyes of 660 patients undergoing routine cataract surgery found a strong association of IFIS with tamsulosin use (p<0.001).130 Saw palmetto showed a slight, non-significant trend, indicating that current or past use may be associated with IFIS.
As a precaution, patients taking saw palmetto should be advised to temporarily stop their herbal treatment 10 days before cataract surgery in order to minimise the potential for the mild complications described above.
One case of haemorrhage during surgery, which was associated with intake of saw palmetto extract, has been reported.131 A case has also been described of haematuria and coagulopathy (INR of 4.0) in a patient using saw palmetto.132 The 79-year-old man had been taking 320 mg/day LESP for 4 years, but had recently increased the dose to 1000 mg/day. He was also taking aspirin and clopidogrel. However, these reports contrast with clinical trials demonstrating reduced blood loss in BPH patients undergoing surgery (see above).
A case of protracted cholestatic hepatitis after the use of a herbal and nutritional preparation has been reported. The herbal preparation contained saw palmetto, hydrangea, Pygeum africanum, Panax ginseng, zinc picolinate, pyridoxine, alanine, glutamic acid, bee pollen and silica.133 The ingredient causing the adverse reaction was not identified. Use of saw palmetto (900 mg/day of a dried extract that was not LESP, together with 660 mg of berry powder) for a few days was associated with acute liver damage.134 The product was discontinued and all symptoms disappeared without residual harm to the patient. A causal association is unlikely, given the brief exposure to the product.
Two cases of acute pancreatitis associated with intake of LESP have been reported.135,136 One report suggested rechallenge with saw palmetto caused a recurrence of symptoms.136 The fatty nature of LESP could be a possible cause of this reaction, although the exact nature of the products involved was not provided.
A case of contact sensitivity following the topical use of saw palmetto was reported in a 24-year-old woman with androgenic alopecia.137
Exposure of human sperm samples to a relatively high concentration of LESP (900 μg/mL) in vitro had no impact on kinematic parameters.138 However, motility was impaired after 48 h of exposure to 9 mg/mL LESP. Even assuming a high bioavailability for LESP and efficient transport to the testes, this still represents an exposure of around 1000 times the normal human dose. Zona-free hamster oocytes incubated with LESP in vitro at the same high concentrations exhibited no change in their viability.139 Such studies are unlikely to have any clinical relevance (see also the discussion of this issue in Chapter 5).
Regulatory status in selected countries
Saw palmetto is official in the United States Pharmacopeia-National Formulary (USP34–NF 29 2011).
Saw palmetto is covered by a positive Commission E monograph and has the following application: urination problems in benign prostatic hyperplasia stages I and II.
Saw palmetto is on the UK General Sale List. Saw palmetto products have achieved Traditional Herbal Registration in the UK with the traditional indication of relief of symptoms of urinary discomfort in men who have BPH. Prior to treatment, other serious conditions should have been ruled out by a doctor.
Saw palmetto does not have GRAS status. However, it is freely available as a ‘dietary supplement’ in the USA under DSHEA legislation (1994 Dietary Supplement Health and Education Act). Saw palmetto has been present in OTC (over-the-counter) drug products to relieve the symptoms of BPH. The FDA, however, advises that: ‘based on evidence currently available, there is inadequate data to establish general recognition of the safety and effectiveness of these ingredients for the specified uses, and … there is no definitive evidence that any drug product offered for the relief of the symptoms of benign prostatic hypertrophy would alter the obstructive or inflammatory signs and symptoms of this condition’.
Saw palmetto is not included in Part 4 of Schedule 4 of the Therapeutic Goods Act Regulations of Australia and is freely available for sale.
References
1. Grieve M, A Modern Herbal, New York, Dover Publications, 1971;vol. 2. pp. 719–720
2. British Herbal Medicine Association Scientific Committee. British Herbal Pharmacopoeia. Cowling: BHMA, 1983. pp. 196–197
3. Felter HW, Lloyd JU. King’s American Dispensatory. ed18, rev 3, vol. 2, 1905. Portland: Reprinted by Eclectic Medical Publications; 1983 pp. 1750–1752.
4. Mabberley DJ. The Plant Book, 2nd ed. Cambridge: Cambridge University Press, 1997. p. 657
5. Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics, 2nd ed. New York: John Wiley, 1996. p. 467
6. Bombardelli E, Morazzoni P. Fitoterapia. 1997;68(2):99–113.
7. Feifer AH, Fleshner NE, Klotz L. J Urol. 2002;168(1):150–154.
8. Habib FK, Wyllie MG. Prostate Cancer Prostatic Dis. 2004;7(3):195–200.
9. Tenover JS. Endocrinol Metab Clin North Am. 1991;20(4):893–909.
10. Bushman W. Urol Clin North Am. 2009;36(4):403–415.
11. Roehrbornb CG. Int J Impot Res. 2008;20(suppl 3):S11–S18.
12. Kramer G, Mitteregger D, Marberger M. Eur Urol. 2007;51(5):1202–1216.
13. Sciarra A, Mariotti G, Salciccia S, et al. J Steroid Biochem Mol Biol. 2008;108(3–5):254–260.
14. Vikram A, Jena G, Ramarao P. Eur J Pharmacol. 2010;641(2–3):75–81.
15. Moul S, McVary KT. Curr Opin Urol. 2010;20(1):7–12.
16. Sarma AV, Parsons JK, McVary K, et al. J Urol. 2009;182(6 suppl):S32–S37.
17. Parsons JK, Sarma AV, McVary K, et al. J Urol. 2009;182(6 suppl):S27–S31.
18. Farmer A, Nobel J. Br Med J. 1997;314(7089):1215–1216.
19. Sultan C, Terraza A, Devillier C, et al. J Steroid Biochem. 1984;20(1):515–519.
20. Düker EM, Kopanski L, Schweikert HU. Planta Med. 1989;55:587.
21. Delos S, Iehle C, Martin P-M, et al. J Steroid Biochem Mol Biol. 1994;48(4):347–352.
22. Delos S, Carsol JL, Ghazararossian E, et al. J Steroid Biochem Mol Biol. 1995;55(34):375–383.
23. Iehle C, Delos S, Guirou O, et al. J Steroid Biochem Mol Biol. 1995;54(5–6):273–279.
24. Niederprüm HJ, Schweikert HU, Zanker KS. Phytomedicine. 1994;1:127–133.
25. Weisser H, Tunn S, Behnke B, et al. Prostate. 1996;28(5):300–306.
26. Rhodes L, Primka RL, Berman C, et al. Prostate. 1993;22(1):43–51.
27. Bayne CW, Ross M, Donnelly F, Habib FK. J Urol. 2000;164(3 Pt1):876–881.
28. Raynaud JP, Cousse H, Martin PM. J Steroid Biochem Mol Biol. 2002;82(2–3):233–239.
29. Abe M, Ito Y, Suzuki A, et al. Anal Sci. 2009;25(4):553–557.
30. Abe M, Ito Y, Oyunzul L, et al. Biol Pharm Bull. 2009;32(4):646–650.
31. Scaglione F, Lucini V, Pannacci M, et al. Pharmacology. 2008;82(4):270–275.
32. Pais P. Adv Ther. 2010;27(8):555–563.
33. Bayne CW, Donnelly F, Ross M, Habib FK. Prostate. 1999;40(4):232–241.
34. Habib FK, Ross M, Ho CK, et al. Int J Cancer. 2005;114(2):190–194.
35. Wadsworth TL, Worstell TR, Greenberg NM, Roselli CE. Prostate. 2007;67(6):661–673.
36. Strauch G, Perles P, Vergult G, et al. Eur Urol. 1994;26(3):247–252.
37. Weisser H, Behnke B, Helpap B, et al. Eur Urol. 1997;31(1):97–101.
38. Di Silverio F, Monti S, Sciarra A, et al. Prostate. 1998;37(2):77–83.
39. Marks LS, Hess DL, Dorey FJ, et al. Urology. 2001;57(5):999–1005.
40. Gutierrez M, Garcia de Boto MJ, Cantabrana B, et al. Gen Pharmacol. 1996;27(1):171–176.
41. Gutierrez M, Hidalgo A, Cantabrana B. Planta Med. 1996;62(6):507–511.
42. Odenthal KP. Phytother Res. 1996;10(suppl):S141–S143.
43. Goepel M, Hecker U, Krege S, et al. Prostate. 1999;38(3):208–215.
44. Suzuki M, Oki T, Sugiyama T, et al. Urology. 2007;69(6):1216–1220.
45. Chua T, Simpson JS, Ventura S. Prostate. 2011;71(1):71–80.
46. Oki T, Suzuki M, Nishioka Y, et al. J Urol. 2005;173(4):1395–1399.
47. Goepel M, Dinh L, Mitchell A, et al. Prostate. 2001;46(3):226–232.
48. Carilla E, Briley M, Fauran F, et al. J Steroid Biochem. 1984;20(1):521–523.
49. El Sheikh MM, Dakkak MR, Saddique A. Acta Obstet Gynecol Scand. 1988;67(5):397–399.
50. Ravenna L, Di Silverio F, Russo MA, et al. Prostate. 1996;29(4):219–230.
51. Stenger A, Tarayre JP, Carilla E, et al. Gaz Med Fr. 1982;89(17):2041–2048.
52. Cristoni A, Morazzoni P, Bombardelli E. Fitoterapia. 1997;68(4):355–358.
53. Buck AC. J Urol. 2004;172(5 Pt 1):1792–1799.
54. Vacher P, Prevarskaya N, Skryma R, et al. J Biomed Sci. 1995;2(4):357–365.
55. Elghamry MI, Hansel R. Experientia. 1969;25(8):828–829.
56. Di Silverio F, D’Eramo G, Lubrano C, et al. Eur Urol. 1992;21(4):309–314.
57. Casarosa C, Cosci di Coscio M, Fratta M. Clin Ther. 1988;10(5):585–588.
58. Angwafor F, III., Anderson ML. J Int Soc Sports Nutr. 2008;5:12.
59. Paubert–Braquet M, Cousse H, Raynaud JP, et al. Eur Urol. 1998;33(3):340–347.
60. Tarayre JP, Delhon A, Lauressergues H, et al. Ann Pharm Fr. 1983;41(6):559–570.
61. Mitropoulos D, Hyroudi A, Zervas A, et al. World J Urol. 2002;19(6):457–461.
62. Hiermann A. Arch Pharm. 1989;322(2):111–114.
63. Wagner H, Flachsbarth H. Planta Med. 1981;41(3):244–251.
64. Wagner H, Flachsbarth H, Vogel G. Planta Med. 1981;41(3):252–258.
65. Breu W, Hagenlocher M, Redl K, et al. Arzneimittelforschung. 1992;42(4):547–551.
66. Paubert-Braquet M, Mencia Huerta J-M, Cousse H, et al. Prostagland Leukot Essent Fatty Acids. 1997;57(3):299–304.
67. Bonvissuto G, Minutoli L, Morgia G, et al. Urology. 2011;77(1):248.e9–248.e16.
68. Vela Navarrete R, Garcia Cardoso JV, Barat A, et al. Eur Urol. 2003;44:549–555.
69. Paubert-Braquet M, Richardson FO, Servent-Saez N, et al. Pharmacol Res. 1996;34(3–4):171–179.
70. Otto U, Wagner B, Becker H, et al. Urol Int. 1992;48:167–170.
71. Van Coppenolle F, Le Bourhis X, Carpentier F, et al. Prostate. 2000;43(1):49–58.
72. Vacherot F, Azzouz M, Gil-Diez-De-Medina S, Colombel M, et al. Prostate. 2000;45(3):259–266.
73. Vela-Navarrete R, Escribano-Burgos M, Farré AL, et al. J Urol. 2005;173(2):507–510.
74. Ishii K, Usui S, Sugimura Y, et al. Biol Pharm Bull. 2001;24(2):188–190.
75. Iguchi K, Okumura N, Usui S, et al. Prostate. 2001;47(1):59–65.
76. Goldmann WH, Sharma AL, Currier SJ, et al. Cell Biol Int. 2001;25(11):1117–1124.
77. Petrangeli E, Lenti L, Buchetti B, et al. J Cell Physiol. 2009;219(1):69–76.
78. Baron A, Mancini M, Caldwell E, et al. BJU Int. 2009;103(9):1275–1283.
79. Hostanska K, Suter A, Melzer J, Saller R. Anticancer Res. 2007;27(2):873–881.
80. Yang Y, Ikezoe T, Zheng Z, et al. Int J Oncol. 2007;31(3):593–600.
81. Scholtysek C, Krukiewicz AA, Alonso JL, et al. Biochem Biophys Res Commun. 2009;379(3):795–798.
82. Che Y, Hou S, Kang Z, Lin Q. Oncol Rep. 2009;22(2):377–383.
83. Adams LS, Seeram NP, Hardy ML, et al. Evid Based Complement Altern Med. 2006;3(1):117–124.
84. Hill B, Kyprianou N. Prostate. 2004;61(1):73–80.
85. Bonnar-Pizzorno RM, Littman AJ, Kestin M, White E. Nutr Cancer. 2006;55(1):21–27.
86. Groom SN, Johns T, Oldfield PR. J Med Food. 2007;10(1):73–79.
87. Beckert BW, Concannon MJ, Henry SL, et al. Plast Reconstr Surg. 2007;120(7):2044–2050.
88. Chevalier G, Benard P, Cousse H, et al. Eur J Drug Metab Pharmacokinet. 1997;22(1):73–83.
89. De Bernardi Di Valserra M, Tripodi AS, Contos S, et al. Acta Toxicol Ther. 1994;15(1):21–39.
90. Debruyne F, Koch G, Boyle P, et al. Eur Urol. 2002;41:497–507.
91. Braeckman J, Bruhwyler J, Vandekerckhove K, Géczy J. Phytother Res. 1997;11:558–563.
92. 5th International Consultations of BPH. Paris: Recommendations of the International Scientific Committee; 2000.
93. Tacklind J, MacDonald R, Rutks I, Wilt TJ. Cochrane Database Syst Rev. 2009;2:CD001423.
94. Bent S, Kane C, Shinohara K, et al. N Engl J Med. 2006;354(6):557–566.
95. DiPaola RS, Morton RA. N Engl J Med. 2006;354(6):632–634.
96. Boyle P, Robertson C, Lowe F, et al. BJU Int. 2004;93(6):751–756.
97. Boyle P, Robertson C, Lowe F, Roehrborn C. Urology. 2000;55(4):533–539.
98. Carraro J-C, Raynaud J-P, Koch G, et al. Prostate. 1996;29(4):231–240.
99. Grasso M, Montesano A, Buonaguidi A, et al. Arch Esp Urol. 1995;48(1):97–103.
100. Adriazola Semino M, Lozano Ortega JL, Garcia Cobo E, et al. Arch Esp Urol. 1992;45(3):211–213.
101. Hizli F, Uygur MC. Int Urol Nephrol. 2007;39(3):879–886.
102. Debruyne F, Boyle P, Calais Da Silva F, et al. Eur Urol. 2004;45(6):773–780.
103. Denis LJ. Prostate. 1996;29:241–242.
104. Sinescu I, Geavlete P, Multescu R, et al. Urol Int. 2011;86(3):284–289.
105. Hutchison A, Farmer R, Verhamme K, et al. Eur Urol. 2007;51(1):207–216.
106. Djavan B, Fong YK, Chaudry A, et al. World J Urol. 2005;23(4):253–256.
107. Kaplan SA, Volpe MA, Te AE. J Urol. 2004;171(1):284–288.
108. Wu T, Zhang X, Wu R, Liu X. Zhonghua Nan Ke Xue. 2004;10(5):337–339. [Article in Chinese]
109. Lopatkin NA, Apolikhin OI, Sivkov AV, et al. Urologiia. 2007;5:3–7. [Article in Russian]
110. Magri V, Trinchieri A, Montanari E, et al. Arch Ital Urol Androl. 2007;79(2):84–92.
111. Magri V, Trinchieri A, Pozzi G, et al. Int J Antimicrob Agents. 2007;29(5):549–556.
112. Morgia G, Mucciardi G, Galì A, et al. Urol Int. 2010;84(4):400–406.
113. Pecoraro S, Annecchiarico A, Gambardella MC, et al. Minerva Urol Nefrol. 2004;56(1):73–78.
114. Anceschi R, Bisi M, Ghidini N, et al. Minerva Urol Nefrol. 2010;62(3):219–223.
115. Tuncel A, Ener K, Han O, et al. Scand J Urol Nephrol. 2009;43(5):377–382.
116. Prager N, Bickett R, French N, et al. J Altern Complement Med. 2002;8(2):143–152.
117. Murugusundram S. J Cutan Aesthet Surg. 2009;2(1):31–32.
118. Kondás J, Philipp V, Diószeghy G. Orv Hetil. 1997;138(7):419–421.
119. Prosta Urgenin® Uno, Zur Behandlung der BPH. Koln: Madaus AG; 1996. p. 33.
120. Singh YN, Devkota AK, Sneeden DC, et al. Phytomedicine. 2007;14(2–3):204–208.
121. Trinachartvanit W, Francis BM, Rayburn AL. Environ Toxicol Pharmacol. 2009;27:149–154.
122. Yale SH, Glurich I. J Altern Complement Med. 2005;11(3):433–439.
123. Markowitz JS, Donovan JL, Devane CL, et al. Clin Pharmacol Ther. 2003;74(6):536–542.
124. Gurley BJ, Gardner SF, Hubbard MA, et al. Clin Pharmacol Ther. 2004;76(5):428–440.
125. Yue Q-Y, Jansson K. J Am Geriatr Soc. 2001;310(4):838.
126. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Austin: American Botanical Council, 1998. p. 201
127. Agbabiaka TB, Pittler MH, Wider B, Ernst E. Drug Saf. 2009;32(8):637–647.
128. Zlotta AR, Teillac P, Raynaud JP, Schulman CC. Eur Urol. 2005;48(2):269–276.
129. Yeu E, Grostern R. J Cataract Refract Surg. 2007;33(5):927–928.
130. Neff KD, Sandoval HP, Fernández de Castro LE, et al. Ophthalmology. 2009;116(4):658–663.
131. Cheema P, El-Mefty O, Jazieh AR. J Intern Med. 2001;250(2):167–169.
132. Villanueva S, González J. Bol Asoc Med PR. 2009;101(3):48–50.
133. Hamid S, Bojter S, Vierling J. Ann Intern Med. 1997;127(2):169–170.
134. Lapi F, Gallo E, Giocaliere E, et al. Br J Clin Pharmacol. 2010;69(5):558–560.
135. Wargo KA, Allman E, Ibrahim F. South Med J. 2010;103(7):683–685.
136. Jibrin I, Erinle A, Saidi A, Aliyu ZY. South Med J. 2006;99(6):611–612.
137. Sinclair RD, Mallari RS, Tate B. Australas J Dermatol. 2002;43(4):311–312.
138. Ondrizek RR, Chan PJ, Patton WC, King A. J Assist Reprod Genet. 1999;16(2):87–91.
139. Ondrizek RR, Chan PJ, Patton WC, King A. Fertil Steril. 1999;71(3):517–522.