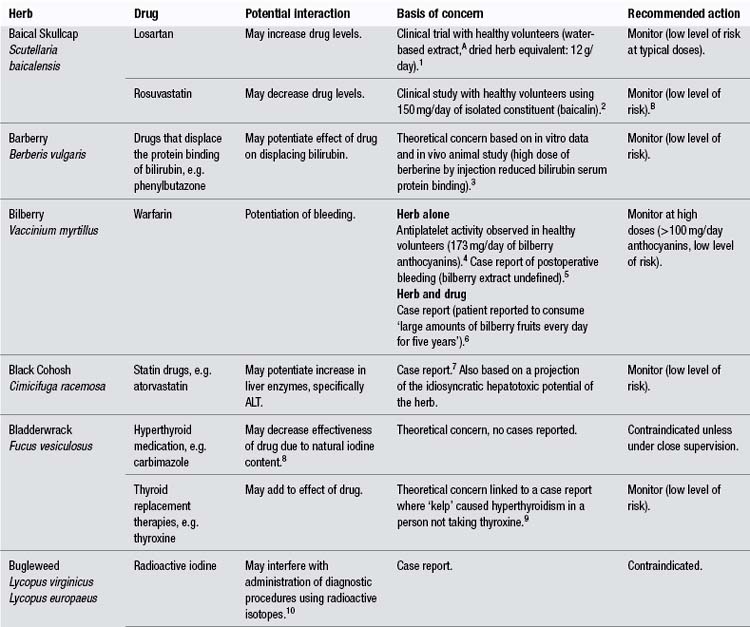
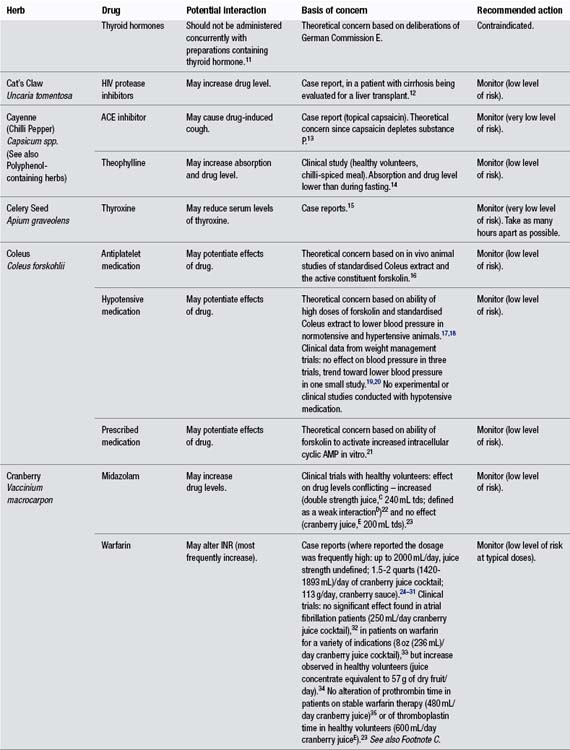
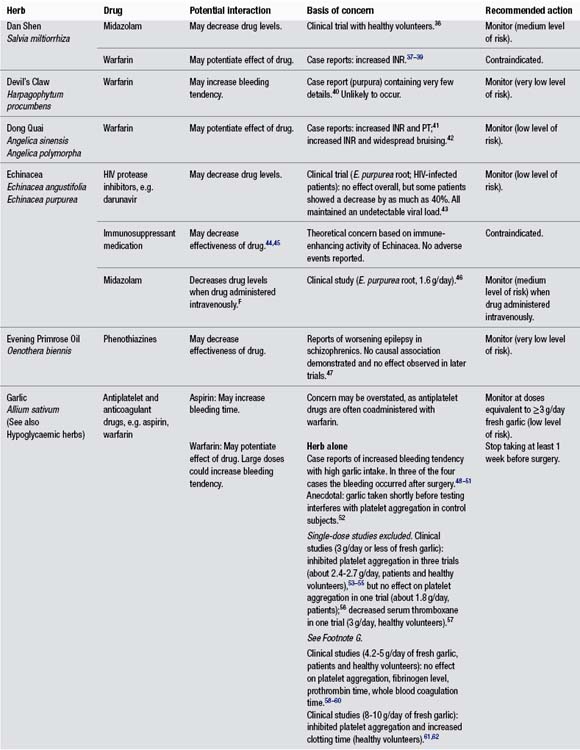
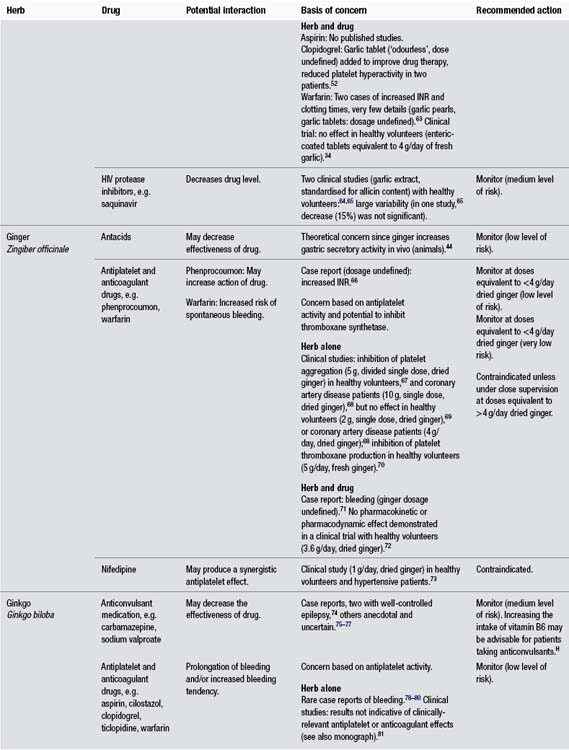
Appendix C Potential herb–drug interactions for commonly used herbs
General prescribing guidelines
• Exercise great caution when prescribing herbs for patients taking drugs with a narrow therapeutic window. These drugs may become dangerously toxic or ineffective with only relatively small changes in their blood concentrations. Examples include digoxin, warfarin, antirejection (immunosuppressive) drugs, many anti–HIV drugs, theophylline, phenytoin and phenobarbital. These patients need to be monitored on a frequent, regular basis.
• Exercise great caution when prescribing herbs for patients taking drugs:
These patients need to be monitored on a frequent, regular basis.
• Care should be exercised with patients who exhibit long-term use of laxative herbs or potassium-losing diuretics.
• Critical drugs should be taken at different times of the day from herbs (and food) to reduce chemical or pharmacokinetic interactions. They should be separated by at least 1 h, preferably more.
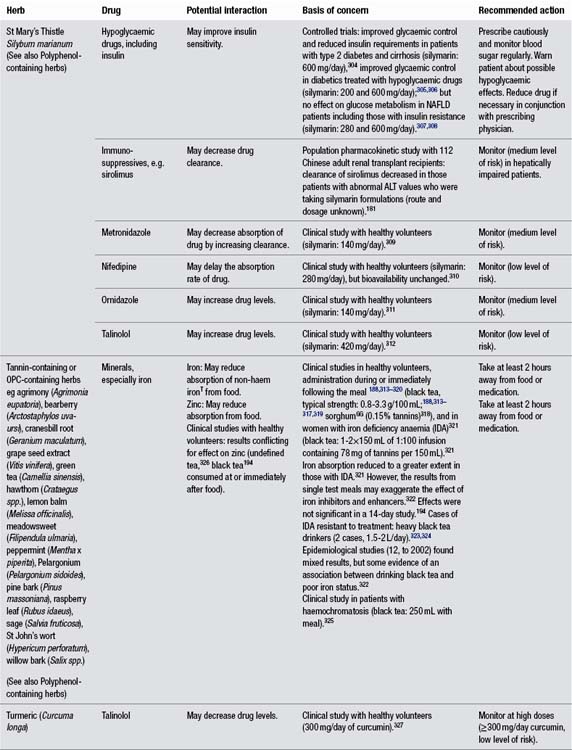
• Stop all herbs approximately 1 week before surgery. St Mary’s thistle may help reduce the toxic after-effects of anaesthetic drugs, so it can be taken up to the day before, and then again after surgery (it will not interact with the anaesthesia – see monograph).
• Carefully monitor the effects of drugs such as antihypertensives and antidiabetic drugs when combining with herbal remedies. The herbs may make them more or less effective. In the ideal situation the dose of the drug could be adjusted.
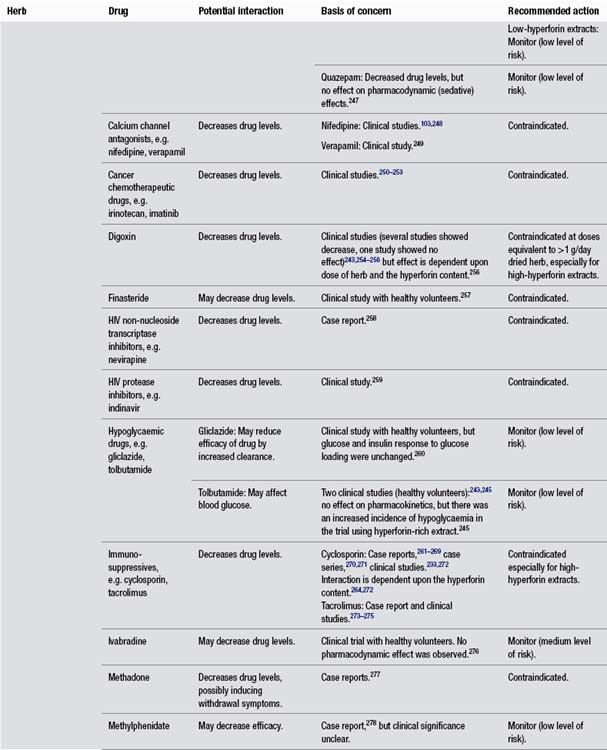
• Interactions may be dose related for the herb and the drug, for example, St John’s wort and digoxin (see monograph).
Assessment of risk and recommended action
The best information about HDIs comes from case observations (detailed and validated if possible) and clinical studies. Assessment of the risk of an adverse effect from a potential herb–drug interaction (as done for the table in this appendix) considers several factors:1,2
• The quality of the evidence, such as probable causality from case reports (probable/possible/unlikely) and confirmation and ideally repeated results from clinical studies with clinically relevant endpoints.A
 A well-documented case report (especially with a positive rechallenge) does not always constitute a lower level of evidence than a negative result from a controlled trial. This is provided that all other possible causes have been considered and adequately dealt with
A well-documented case report (especially with a positive rechallenge) does not always constitute a lower level of evidence than a negative result from a controlled trial. This is provided that all other possible causes have been considered and adequately dealt with The quality of the publication should also be considered – a poster from a scientific meeting is regarded as a lower level of evidence than well-documented case reports and controlled trials (due to lack of peer review). Is the pharmacokinetic study placebo-controlled?B
The quality of the publication should also be considered – a poster from a scientific meeting is regarded as a lower level of evidence than well-documented case reports and controlled trials (due to lack of peer review). Is the pharmacokinetic study placebo-controlled?B Theoretical concerns based on pharmacological activity are considered the lowest quality of evidence and are often speculative at best.
Theoretical concerns based on pharmacological activity are considered the lowest quality of evidence and are often speculative at best.• The incidence of the interaction (what is the chance that the interaction occurs? How many well–documented cases are reported compared to the extent of use of the herb?)
• The seriousness of the potential adverse reaction, for example in order of increasing importance:
 An insignificant clinical effect from an increased drug level without clinical symptoms or an increase in INR (international normalised ratio)Cup to 4 in the case of warfarin
An insignificant clinical effect from an increased drug level without clinical symptoms or an increase in INR (international normalised ratio)Cup to 4 in the case of warfarinBy considering these factors and the totality of evidence, the risk of a herb–drug interaction would range from very low (such as where evidence is poor or lacking and the effect is clinically irrelevant) to contraindicated (such as where the evidence consists of controlled, published interaction studies with a clinically relevant endpoint, the adverse outcome is clinically very relevant, including decreasing plasma levels of drugs being prescribed for serious conditions). An altered plasma drug level in healthy volunteers or even patients without a substantial clinical effect would be considered low or medium risk.
Probe drugs
Studies using probe drugs (which assess individual cytochrome P450 enzyme activity, and hence potential interactions for drugs that utilise that enzyme) are only included in the chart where the drug is currently used clinically. For example, midazolam (a benzodiazepine, used clinically as a sedative and frequently in anaesthesia) is metabolised by CYP3A4 and can be used to assess the interaction of other drugs and herbs with this enzyme (e.g. another drug or herb may inhibit or induce CYP3A4 resulting in increased or decreased plasma drug levels, respectively). A number of drugs are used as probes for CYP3A4 activity such as nifedipine and alprazolam. Other examples of probe drugs included in the chart due to their therapeutic activity include tolbutamide (for CYP2C9 activity), omeprazole (CYP2C19) and talinolol (P-glycoprotein). (P-glycoprotein helps transport molecules across biological membranes and hence can affect the absorption and elimination of a drug.) Although there are a large number of cytochrome P450 enzymes, more than 90% of the metabolism of drugs is due to the activity of CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4. For a comprehensive review of the effect of herbs on probe drugs in clinical trials the reader is referred to the following systematic review: Kennedy DA, Seely D. Expert Opin Drug Saf 9(1): 79-124, 2010.
Midazolam is used clinically as a sedative and frequently in anaesthesia. Such studies ideally need to have administered the herb for at least 7 days and investigated drug clearance by using the area under the serum concentration–time curve (AUC).
The US Food and Drug Administration has defined the clinical significance of an interaction that causes an increased plasma level of oral midazolam. The interaction is weak (hence low risk) if the increase in the AUC is between 1.25–-and 2-fold; AUC changes ranging from 2- to 5-fold are moderate (hence medium risk) and greater than a 5-fold increase is a strong interaction and may result in contraindication.3 An increase below 1.25-fold, in other words an increase in the plasma level of midazolam by up to 25%, means no interaction has occurred (i.e. it is clinically insignificant).
HDI chart examples: how the recommended action is determined
Contraindicated
St John’s Wort and Digoxin
• Three clinical studies (two controlled with placebo) found St John’s wort extract high in hyperforin sharply decreased drug levels. The decrease in drug levels increased with increasing doses of St John’s wort.
The evidence is considered to be strong and the adverse outcome is potentially serious, in addition to the drug having a narrow therapeutic window (small changes in blood levels may have considerable pharmacological effect).
Dan Shen and Warfarin
• Three case reports of bleeding complications and elevated INR values (above 5.5) were observed. INR returned to normal target range after cessation of herb. Dan shen was the only substance introduced after the INR was initially stabilised.
Reasonably strong case report evidence and adverse effects considered serious.
Monitor (medium level of risk)
St John’s Wort and Amitriptyline
Evidence considered moderate, given the wide use of the herb. Assigned a medium rather than a low risk due to seriousness of potential adverse outcome.
Garlic and HIV Protease Inhibitors
• Clinical study (2000; not controlled with placebo) with healthy volunteers found an allicin-producing garlic tablet caused significant decrease (from baseline) in plasma drug concentration.
• A probe drug study (2010) also with healthy volunteers taking an allicin-producing garlic tablet, found a non–significant decrease overall in AUC (15%) with large variability (AUC increased in several volunteers).
Evidence considered moderate, as there is an absence of case reports despite the fact that garlic is widely consumed in the diet. Assigned medium rather than low risk due to potential adverse outcome.
Ginkgo and HIV Non–nucleoside Transcriptase Inhibitors
This is the first case reported for this interaction. The decrease in drug levels coincided approximately when ‘the patient appeared to be using Ginkgo’. The long history of stable drug levels prior to the ingestion of Ginkgo, and the patient not taking medications other than antiretroviral therapy are suggestive of a causal effect. Patient ‘successfully switched to alternative antiretroviral therapy’. Despite the lack of strong causality, assigned medium due to potential adverse outcome.
Monitor (low level of risk)
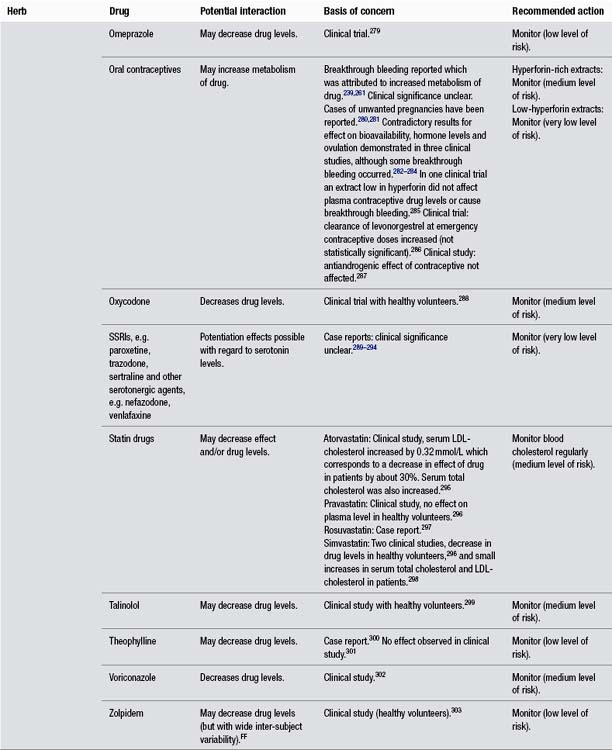
St John’s Wort and Omeprazole
• Clinical study with healthy volunteers found that St John’s wort extract caused significant decrease in plasma drug concentration.
Evidence considered preliminary or moderate at best. Assigned low due to a lack of seriousness of effects from a potential interaction.
St John’s Wort and Anticonvulsants
• Theoretical concern raised in 2000 by regulators on the basis that St John’s wort may induce cytochrome P450, the pathway by which some drugs including anticonvulsants are metabolised, thereby potentially increasing their breakdown and reducing their blood concentrations.
• No effect on carbamazepine pharmacokinetics found in a 2000 clinical study with healthy volunteers (not controlled with placebo).
• One case reported with few details in 2007 in which an increase in the frequency and severity of seizures was reported in a patient taking several anticonvulsants, two of which are not metabolised by cytochrome P450.
• A probe drug study (2004) found increased excretion of a mephenytoin metabolite in some volunteers – those with a CYP2C19 wild-type genotype (extensive metabolisers). In poor metabolisers (mutant genotype; having a deficiency of CYP2C19 activity) there was no significant alteration. (Mephenytoin is almost exclusively metabolised by CYP2C19.) The clinical significance is unknown, as plasma drug levels of mephenytoin were not measured.
Evidence considered low, with a clinical study supporting a lack of interaction despite the theoretical concern. Due to the recognised ability of St John’s wort to interact with some drugs metabolised via cytochrome P450 (particularly CYP3A4), and the importance of maintaining stable blood levels of anticonvulsants this interaction was assigned low rather than very low risk. The 2008 case report does not support the theoretical concern (different metabolism). Additional and well-documented case reports would be required to alter the risk assessment.
Ginkgo and Tolbutamide
• Clinical study with healthy volunteers found a high dose of Ginkgo (50:1 extract: 360 mg/day, equivalent to 18 g/day of dried leaf) decreased the area under concentration versus time curve by 16% (statistically significant, but being less than a 20% decrease this is not regarded as clinically significant). No statistically significant differences found for other pharmacokinetic parameters.
• To assess the effect on the pharmacodynamics of tolbutamide, volunteers were also given a 75 g oral dose of glucose. When combined with Ginkgo the blood glucose lowering effect of tolbutamide was less than with tolbutamide alone, but the difference was not even statistically significant.
The decrease in exposure to tolbutamide caused by a high dose of Ginkgo did not have a significant effect on the ability of tolbutamide to lower glucose in healthy volunteers. Assigned low risk until information in diabetic patients becomes available.
Monitor (very low level of risk)
Siberian Ginseng and Digoxin
• One case report: possibly increased plasma concentration of drug but ECG (electrocardiogram) unchanged. The possibility that the herb may have interfered with the digoxin measurement was also raised, and later supported.
• No effect on plasma concentration of drug in later controlled clinical trial.
The case report provides minimal evidence, with a lack of clinical relevance (ECG results) and the possibility of testing interference. The clinical trial results reduce the strength of evidence.
General reference
Braun L. Herb Drug Interaction Guide for Pharmacists. FH Faulding, August 2000; Fugh-Berman A. Lancet. 2000 355(9198):134–138.
References
1. Yi SJ, Cho JY, Lim KS, et al. Basic Clin Pharmacol Toxicol. 2009;105(4):249–256.
2. Fan L, Zhang W, Guo D, et al. Clin Pharmacol Ther. 2008;83(3):471–476.
3. Chan E. Biol Neonate. 1993;63(4):201–208.
4. Pulliero G, Montin S, Bettini V, et al. Fitoterapia. 1989;60(1):69–75.
5. Duterte M, Waugh S, Thanawala R. Am J Gastroenterol. 2007;102(Suppl 2):S350.
6. Aktas C, Senkal V, Sarikaya S, et al. Turk J Geriatr. 2011;14(1):79–81.
7. Patel NM, Derkits RM. J Pharm Pract. 2007;20(4):341–346.
8. de SmetP.A.G.M., Keller K, Hansel R, et al, Adverse Effects of Herbal Drugs, Berlin, Springer-Verlag, 1997;vol. 3.
9. Miller LG. Arch Intern Med. 1998;158(20):2200–2211.
10. de SmetP.A.G.M., Keller K, Hansel R, et al, Adverse Effects of Herbal Drugs, Berlin, Springer-Verlag, 1993;vol. 2.
11. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Austin: American Botanical Council, 1998.
12. Lopez Galera RM, Ribera Pascuet E, Esteban Mur JI, et al. Eur J Clin Pharmacol. 2008;64(12):1235–1236.
13. Hakas JF. Ann Allergy. 1990;65(4):322–323.
14. Bouraoui A, Toum A, Bouchoucha S, et al. Therapie. 1986;41(6):467–471.
15. Moses G. Australian Prescriber. 2001;24(1):6.
16. de Souza NJ. J Ethnopharmacol. 1993;38(2–3):177–180.
17. de Souza NJ, Dohadwalla AN, Reden J. Med Res Rev. 1983;3(2):201–219.
18. Dubey MP, Srimal RC, Nityanand S, et al. J Ethnopharmacol. 1981;3(1):1–13.
19. Sabinsa Corporation. ForsLean® Product Information. Available from <www.forslean.com>; Accessed November 2004.
20. Henderson S, Magu B, Rasmussen C, et al. J Int Soc Sports Nutr. 2005;2(2):54–62.
21. Seamon KB, Daly JW. J Cyclic Nucleotide Res. 1981;7(4):201–224.
22. Ngo N, Yan Z, Graf TN, et al. Drug Metab Dispos. 2009;37(3):514–522.
23. Lilja JJ, Backman JT, Neuvonen PJ. Clin Pharmacol Ther. 2007;81(6):833–839.
24. Medicines and Healthcare Products Regulatory Agency, Committee on Safety of Medicines. Current Problems in Pharmacovigilance. vol. 30. October 2004, p. 10.
25. Rindone JP, Murphy TW. Am J Ther. 2006;13(3):283–284.
26. Sylvan L, Justice NP. Am Fam Physician. 2005;72(6):1000.
27. Paeng CH, Sprague M, Jackevicius CA. Clin Ther. 2007;29(8):1730–1735.
28. Welch JM, Forster K. J Pharm Technol. 2007;23(2):104–107.
29. Mergenhagen KA, Sherman O. Am J Health Syst Pharm. 2008;65(22):2113–2116.
30. Griffiths AP, Beddall A, Pegler S. J R Soc Promot Health. 2008;128(6):324–326.
31. Hamann GL, Campbell JD, George CM. Ann Pharmacother. 2011;45(3):e17.
32. Li Z, Seeram NP, Carpenter CL, et al. J Am Diet Assoc. 2006;106(12):2057–2061.
33. Ansell J, McDonough M, Zhao Y, et al. J Clin Pharmacol. 2009;49(7):824–830.
34. Mohammed Abdul MI, Jiang X, Williams KM, et al. Br J Pharmacol. 2008;154(8):1691–1700.
35. Mellen CK, Ford M, Rindone JP. Br J Clin Pharmacol. 2010;70(1):139–142.
36. Qiu F, Wang J, Zhang R, et al. Brit J Clin Pharmacol. 2010;69(6):656–662.
37. Tam LS, Chan TYK, Leung WK, et al. Aust NZ J Med. 1995;25(3):258.
38. Yu CM, Chan JCN, Sanderson JE. J Intern Med. 1997;241(4):337–339.
39. Izzat MB, Yim APC, El-Zufari MH. Ann Thorac Surg. 1998;66(3):941–942.
40. Shaw D, Leon C, Kolev S, et al. Drug Saf. 1997;17(5):342–356.
41. Page RL, Lawrence JD. Pharmacotherapy. 1999;19(7):870–876.
42. Ellis GR, Stephens MR. BMJ. 1999;319(7210):650.
43. Moltó J, Valle M, Miranda C, et al. Antimicrob Agents Chemother. 2011;55(1):326–330.
44. Mills S, Bone K. Principles and Practice of Phytotherapy: Modern Herbal Medicine. Edinburgh: Churchill Livingstone, 2000.
45. Newall CA, Anderson LA, Phillipson JD. Herbal Medicines – A Guide for Health–Care Professionals. London: Pharmaceutical Press, 1996.
46. Gorski JC, Huang SM, Pinto A, et al. Clin Pharmacol Ther. 2004;75(1):89–100.
47. Mills S, Bone K. The Essential Guide to Herbal Safety. USA: Churchill Livingstone, 2005.
48. Rose KD, Croissant PD, Parliament CF, et al. Neurosurgery. 1990;26(5):880–882.
49. Burnham BE. Plast Reconstr Surg. 1995;95(1):213.
50. German K, Kumar U, Blackford HN. Br J Urol. 1995;76(4):518.
51. Carden SM, Good WV, Carden PA, et al. Clin Experiment Ophthalmol. 2002;30(4):303–304.
52. Manoharan A, Gemmell R, Hartwell T. Am J Hematol. 2006;81(9):676–683.
53. Legnani C, Frascaro M, Guazzaloca G, et al. Arzneim Forsch. 1993;43(2):119–122.
54. Kiesewetter H, Jung F, Jung EM, et al. Eur J Clin Pharmacol. 1993;45(4):333–336.
55. Kiesewetter H, Jung F, Jung EM, et al. Clin Investig. 1993;71(5):383–386.
56. Harenberg J, Giese C, Zimmermann R. Atherosclerosis. 1988;74(3):247–249.
57. Ali M, Thomson M. Prostaglandins Leukot Essent Fatty Acids. 1995;53(3):211–212.
58. Luley C, Lehmann-Leo W, Moller B, et al. Arzneim Forsch. 1986;36(4):766–768.
59. Scharbert G, Kalb ML, Duris M, et al. Anesth Analg. 2007;105(5):1214–1218.
60. Jain RC. Am J Clin Nutr. 1977;30(9):1380–1381.
61. Lawson LD. FASEB J. 2007;21(6):A1126.
62. Gadkari JV, Joshi VD. J Postgrad Med. 1991;37(3):128–131.
63. Sunter W. Pharm J. 1991;246:722.
64. Piscitelli SC, Burstein AH, Welden N, et al. Clin Infect Dis. 2002;34(2):234–238.
65. Hajda J, Rentsch KM, Gubler C, et al. Eur J Pharm Sci. 2010;41(5):729–735.
66. Kruth P, Brosi E, Fux R, et al. Ann Pharmacother. 2004;38(2):257–260.
67. Verma SK, Singh J, Khamesra R, et al. Indian J Med Res. 1993;98:240–242.
68. Bordia A, Verma SK, Srivastava KC. Prostaglandins Leukot Essent Fatty Acids. 1997;56(5):379–384.
69. Lumb AB. Thromb Haemost. 1994;71(1):110–111.
70. Srivastava KC. Prostaglandins Leukot Essent Fatty Acids. 1989;35(3):183–185.
71. Lesho EP, Saullo L, Udvari-Nagy S. Cleve Clin J Med. 2004;71(8):651–656.
72. Jiang X, Williams KM, Liauw WS, et al. Br J Clin Pharmacol. 2005;59(4):425–432.
73. Young HY, Liao JC, Chang YS, et al. Am J Chin Med. 2006;34(4):545–551.
74. Granger AS. Age Ageing. 2001;30(6):523–525.
75. Gregory PJ. Ann Intern Med. 2001;134(4):344.
76. Kupiec T, Raj V. J Anal Toxicol. 2005;29(7):755–758.
77. Bruhn JG. Phytomedicine. 2003;10(4):358.
78. Bent S, Goldberg H, Padula A, et al. J Gen Intern Med. 2005;20(7):657–661.
79. Griffiths J, Jordon S, Pilon K. Canadian Adverse Reaction Newsletter. 2004;14(1):2–3.
80. Pedroso JL, Henriques Aquino CC, Escórcio Bezerra ML, et al. Neurologist. 2011;17(2):89–90.
81. Bone KM. Mol Nutr Food Res. 2008;52(7):764–771.
82. Chan AL, Leung HW, Wu JW, et al. J Altern Complement Med. 2011;17(6):513–517.
83. DeLoughery TG, Kaye JA, Morris CD, et al. Blood. 2002;11(100): [Abstract #3809]
84. Gardner CD, Zehnder JL, Rigby AJ, et al. Blood Coagul Fibrinolysis. 2007;18(8):787–793.
85. Wolf HR. Drugs R D. 2006;7(3):163–172.
86. Aruna D, Naidu MU. Br J Clin Pharmacol. 2007;63(3):333–338.
87. Yeo C, Cho H, Park S, et al. Clin Pharm Ther. 2010;87:S43.
88. Kim BH, Kim KP, Lim KS, et al. Clin Ther. 2010;32(2):380–390.
89. Lu WJ, Huang JD, Lai ML. J Clin Pharmacol. 2006;46(6):628–634.
90. Engelsen J, Nielsen JD, Winther K. Thromb Haemost. 2002;87(6):1075–1076.
91. Lai CF, Chang CC, Fu CH, et al. Pharmacotherapy. 2002;22(10):1326.
92. Zhang XY, Zhou DF, Su JM, et al. J Clin Psychopharmacol. 2001;21(1):85–88.
93. Zhang XY, Zhou DF, Zhang PY, et al. J Clin Psychiatry. 2001;62(11):878–883.
94. Atmaca M, Tezcan E, Kuloglu M, et al. Psychiatry Clin Neurosci. 2005;59(6):652–656.
95. Doruk A, Uzun O, Ozsahin A. Int Clin Psychopharmacol. 2008;23(4):223–237.
96. Zuo XC, Zhang BK, Jia SJ, et al. Eur J Clin Pharmacol. 2010;66(5):503–509.
97. Uchida S, Yamada H, Li XD, et al. J Clin Pharmacol. 2006;46(11):1290–1298.
98. Robertson SM, Davey RT, Voell J, et al. Curr Med Res Opin. 2008;24(2):591–599.
99. Wiegman DJ, Brinkman K, Franssen EJ. AIDS. 2009;23(9):1184–1185.
100. Kudolo GB, Wang W, Javors M, et al. Clin Nutr. 2006;25(4):606–616.
101. Personal communication from trial author. Kudolo GB. 29 February 2008.
102. Wang W, Javors M, Blodgett J, et al. Diabetes. 2007;56(Suppl 1):A560.
103. Smith M, Lin KM, Zheng MD. Clin Pharmacol Ther. 2001;69(2):P86. [Abstract #PIII–89]
104. Yoshioka M, Ohnishi N, Koishi T, et al. Biol Pharm Bull. 2004;27(12):2006–2009.
105. Yin OQ, Tomlinson B, Waye MM, et al. Pharmacogenetics. 2004;14(12):841–850.
106. Fan L, Mao XQ, Tao GY, et al. Xenobiotica. 2009;39(3):249–254.
107. Gurley BJ, Swain A, Hubbard MA, et al. Clin Pharmacol Ther. 2008;83(1):61–69.
108. Golden EB, Lam PY, Kardosh A, et al. Blood. 2009;113(23):5927–5937.
109. Alemdaroglu NC, Dietz U, Wolffram S, et al. Biopharm Drug Dispos. 2008;29(6):335–348.
110. Werba JP, Giroli M, Cavalca V, et al. Ann Intern Med. 2008;149(4):286–287.
111. Taylor JR, Wilt VM. Ann Pharmacother. 1999;33(4):426–428.
112. Wolkerstorfer H. MMW. 1966;108(8):438–441.
113. Jaursch U, Landers E, Schmidt R, et al. Med Welt. 1969;27:1547–1552.
114. Tankanow R, Tamer HR, Streetman DS, et al. J Clin Pharmacol. 2003;43(6):637–642.
115. Iwamoto M, Ishizaki T, Sato T. Planta Med. 1981;42(1):1–16.
116. Leuchtgens H. Fortschr Med. 1993;111(20–21):352–354.
117. Walker AF, Marakis G, Simpson E. Br J Gen Pract. 2006;56(527):437–443.
118. Shanmugasundaram ER, Rajeswari G, Baskaran K, et al. J Ethnopharmacol. 1990;30(3):281–294.
119. Baskaran K, Kizar Ahamath B, Radha Shanmugasundaram K, et al. J Ethnopharmacol. 1990;30(3):295–300.
120. Sharma RD, Raghuram TC, Rao NS. Eur J Clin Nutr. 1990;44(4):301–306.
121. Sharma RD, Raghuram TC. Nutr Res. 1990;10(7):731–739.
122. Sharma RD, Sarkar A, Hazra DK, et al. Nutr Res. 1996;16(8):1331–1339.
123. Madar Z, Abel R, Samish S, et al. Eur J Clin Nutr. 1988;42(1):51–54.
124. Raghuram TC, Sharma RD, Sivakumar B, et al. Phytother Res. 1994;8:83–86.
125. Kassaian N, Azadbakht L, Forghani B, et al. Int J Vitam Nutr Res. 2009;79(1):34–39.
126. Ziai SA, Larijani B, Akhoondzadeh S, et al. J Ethnopharmacol. 2005;102(2):202–207.
127. Sartore G, Reitano R, Barison A, et al. Eur J Clin Nutr. 2009;63(10):1269–1271.
128. Anderson JW, Allgood LD, Turner J, et al. Am J Clin Nutr. 1999;70(4):466–473.
129. Rodriguez-Moran M, Guerrero-Romero F, Lazcano-Burciaga G. J Diabetes Complications. 1998;12(5):273–278.
130. Florholmen J, Arvidsson-Lenner R, Jorde R, et al. Acta Med Scand. 1982;212(4):237–239.
131. Scott AR, Attenborough Y, Peacock I, et al. BMJ. 1988;297(6650):707–710.
132. Sobenin IA, Nedosugova LV, Filatova LV, et al. Acta Diabetol. 2008;45(1):1–6.
133. Almeida JC, Grimsley EW. Ann Intern Med. 1996;125(11):940–941.
134. Cartledge A, Rutherford J. Rapid response (electronic letter), BMJ 12 Feb 2001. Available from bmj.com/cgi/eletters/322/7279/139#12643, downloaded 21/2/02.
135. Herberg KW, Winter U. 2nd International Congress on Phytomedicine. Munich: September 11–14, Abstract P-77, 1996.
136. Herberg KW. Blutalkohol. 1993;30(2):96–105.
137. Schelosky L, Raffauf C, Jendroska K, et al. J Neurol Neurosurg Psychiatry. 1995;58(5):639–640.
138. Yamamoto M, Tamura Y, Kuashima K, et al, [Cited in: Han KH, Choe SC, Kim HS, et al.]. Am J Chin Med, 1998;2(26):199–209.
139. Han KH, Choe SC, Kim HS, et al. Am J Chin Med. 1998;26(2):199–209.
140. Chung IM, Lim JW, Pyun WB, et al. J Ginseng Res. 2010;34(3):212–218.
141. Rhee MY, Kim YS, Bae JH, et al. J Altern Complement Med. 2011;17(1):45–49.
142. Lee JH, Park HJ. J Ginseng Res. 1998;22(3):173–180.
143. Lee JH, Kim SH. Korean J Nutr. 1995;28(9):862–871.
144. Shin KS, Lee JJ, Kim YI, et al. J Ginseng Res. 2007;31(2):109–116.
145. Janetzky K, Morreale AP. Am J Health Syst Pharm. 1997;54(6):692–693.
146. Jiang X, Williams KM, Liauw WS, et al. Br J Clin Pharmacol. 2004;57(5):592–599.
147. Lee SH, Ahn YM, Ahn SY, et al. J Altern Complement Med. 2008;14(6):715–721.
148. Lee YH, Lee BK, Choi YJ, et al. Int J Cardiol. 2010;145(2):275–276.
149. Sotaniemi EA, Haapakoski E, Rautio A. Diabetes Care. 1995;18(10):1373–1375.
150. Reeds DN, Patterson BW, Okunade A, et al. Diabetes Care. 2011;34(5):1071–1076.
151. Okuda H, Yoshida R. Proceedings of the Third International Ginseng Symposium. Seoul, Korea: Korea Ginseng Research Institute, 1980. pp. 53–57
152. Ma SW, Benzie IF, Chu TT, et al. Diabetes Obes Metab. 2008;10(11):1125–1127.
153. Vuksan V, Sung MK, Sievenpiper JL, et al. Nutr Metab Cardiovasc Dis. 2008;18(1):46–56.
154. Tetsutani T, Yamamura M, Yamaguchi T, et al. Ginseng Rev. 2000;28:44–47.
155. Jones BD, Runikis AM. J Clin Psychopharmacol. 1987;7(3):201–202.
156. Shader RI, Greenblatt DJ. J Clin Psychopharmacol. 1988;8(4):235.
157. Gillis CN. Biochem Pharmacol. 1997;54(1):1–8.
158. Kim HJ, Woo DS, Lee G, et al. Br J Urol. 1998;82(5):744–748.
159. ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products, 2nd ed. Exeter, ESCOP, European Scientific Cooperative on Phytotherapy, 2003.
160. Stormer FC, Reistad R, Alexander J. Food Chem Toxicol. 1993;31(4):303–312.
161. Sigurjonsdottir HA, Franzson L, Manhem K, et al. J Hum Hypertens. 2001;15(8):549–552.
162. Sigurjonsdottir HA, Manhem K, Axelson M, et al. J Hum Hypertens. 2003;17(2):125–131.
163. Sigurjonsdottir HA, Ragnarsson J, Franzson L, et al. J Hum Hypertens. 1995;9(5):345–348.
164. Sobieszczyk P, Borlaug BA, Gornik HL, et al. Clin Sci. 2010;119(10):437–442.
165. Brouwers AJ, van der Meulen J. Ned Tijdschr Geneeskd. 2001;145(15):744–747.
166. Iida R, Otsuka Y, Matsumoto K, et al. Clin Exp Nephrol. 2006;10(2):131–135.
167. Maeda Y, Inaba N, Aoyagi M, et al. Intern Med. 2008;47(14):1345–1348.
168. Shintani S, Murase H, Tsukagoshi H, et al. Eur Neurol. 1992;32(1):44–51.
169. Bernardi M, d–Intimo PE, Trevisani F, et al. Life Sci. 1994;55(11):863–872.
170. Harada T, Ohtaki E, Misu K, et al. Cardiology. 2002;98(4):218.
171. Armanini D, Castello R, Scaroni C, et al. Eur J Obstet Gynecol Reprod Biol. 2007;131(1):61–67.
172. Kurisu S, Inoue I, Kawagoe T, et al. J Am Geriatr Soc. 2008;56(8):1579–1581.
173. Heidemann HT, Kreuzfelder E. Klin Wochenschr. 1983;61(6):303–305.
174. Chataway SJ, Mumford CJ, Ironside JW. Postgrad Med J. 1997;73(863):593–594.
175. Folkersen L, Knudsen NA, Teglbjaerg PS. Ugeskr Laeger. 1996;158(51):7420–7421.
176. Famularo G, Corsi FM, Giacanelli M. Acad Emerg Med. 1999;6(9):960–964.
177. Nielsen I, Pedersen RS. Lancet. 1984;323(8389):1305.
178. Conn JW, Rovner DR, Cohen EL. JAMA. 1968;205(7):492–496.
179. Sontia B, Mooney J, Gaudet L, et al. J Clin Hypertens. 2008;10(2):153–157.
180. Hukkanen J, Ukkola O, Savolainen MJ. Blood Press. 2009;18(4):192–195.
181. Jiao Z, Shi XJ, Li ZD, et al. Br J Clin Pharmacol. 2009;68(1):47–60.
182. Tu JH, He YJ, Chen Y, et al. Eur J Clin Pharmacol. 2010;66(8):805–810.
183. Tu JH, Hu DL, Dai LL, et al. Xenobiotica. 2010;40(6):393–399.
184. Chen MF, Shimada F, Kato H, et al. Endocrinol Jpn. 1991;38(2):167–174.
185. Conti M, Frey FJ, Escher G, et al. Nephrol Dial Transplant. 1994;9(11):1622–1628.
186. Liapina LA, Koval’chuk GA. Izv Akad Nauk Ser Biol. 1993;4:625–628.
187. Nowack R, Nowak B. Nephrol Dial Transplant. 2005;20(11):2554–2556.
188. Hurrell RF, Reddy M, Cook JD. Br J Nutr. 1999;81(4):289–295.
189. Samman S, Sandstrom B, Toft MB, et al. Am J Clin Nutr. 2001;73(3):607–612.
190. Tuntipopipat S, Judprasong K, Zeder C, et al. J Nutr. 2006;136(12):2970–2974.
191. Olivares M, Pizarro F, Hertrampf E, et al. Nutrition. 2007;23(4):296–300.
192. Kubota K, Sakurai T, Nakazato K, et al. Nippon Ronen Igakkai Zasshi. 1990;27(5):555–558.
193. Mitamura T, Kitazono M, Yoshimura O, et al. Nippon Sanka Fujinka Gakkaai Zasshi. 1989;41(6):688–694.
194. Prystai EA, Kies CV, Driskell JA. Nutr Res. 1999;19(2):167–177.
195. Mennen L, Hirvonen T, Arnault N, et al. Eur J Clin Nutr. 2007;61(10):1174–1179.
196. Imai K, Nakachi K. BMJ. 1995;310(6981):693–696.
197. Ullmann U, Haller J, Bakker GC, et al. Phytomedicine. 2005;12(6–7):410–415.
198. Hutchinson C, Bomford A, Geissler CA. Eur J Clin Nutr. 2010;64(10):1239–1241.
199. Etman MA. Drug Dev Indust Pharm. 1995;21(16):1901–1906.
200. Ettinger AB, Shinnar S, Sinnett MJ, et al. J Epilepsy. 1992;5(3):191–193.
201. Brown DD, Juhl RP, Warner SL. Am J Cardiol. 1977;39(2):297.
202. Nordstrom M, Melander A, Robertsson E, et al. Drug Nutr Interact. 1987;5(2):67–69.
203. Walan A, Bergdahl B, Skoog M–L. Scand J Gastroenterol. 1977;12(Supp 45):111.
204. Reissell P, Manninen V. Acta Med Scand Suppl. 1982;668:88–90.
205. Rossander L. Scand J Gastroenterol Suppl. 1987;129:68–72.
206. Sierra M, García JJ, Fernández N, et al. Eur J Clin Nutr. 2002;56(9):830–842.
207. Dennison BA, Levine DM. J Pediatr. 1993;123(1):24–29.
208. Burton R, Manninen V. Acta Med Scand Suppl. 1982;668:91–94.
209. Kawatra A, Bhat CM, Arora A. Eur J Clin Nutr. 1993;47(4):297–300.
210. Enzi G, Inelmen EM, Crepaldi G. Pharmatherapeutica. 1980;2(7):421–428.
211. Perlman BB. Lancet. 1990;335(8686):416.
212. Toutoungi M, Schulz P, Widmer J, et al. Therapie. 1990;45(4):358–360.
213. Robinson DS, Benjamin DM, McCormack JJ. Clin Pharmacol Ther. 1971;12(3):491–495.
214. Cavaliere H, Floriano I, Medeiros-Neto G. Int J Obes Relat Metab Disord. 2001;25(7):1095–1099.
215. Liel Y, Harman-Boehm I, Shany S. J Clin Endocrinol Metab. 1996;81(2):857–859.
216. Chiu AC, Sherman SI. Thyroid. 1998;8(8):667–671.
217. Xin HW, Wu XC, Li Q, et al. Br J Clin Pharmacol. 2007;64(4):469–475.
218. Jiang W, Wang X, Xu X, et al. Int J Clin Pharmacol Ther. 2010;48(3):224–229.
219. Jiang W, Wang X, Kong L. Immunopharmacol Immunotoxicol. 2010;32(1):177–178.
220. Xin HW, Wu XC, Li Q, et al. Br J Clin Pharmacol. 2009;67(5):541–546.
221. Ko KM, Ip SP, Poon MK, et al. Planta Med. 1995;61(2):134–137.
222. Lu H, Liu GT. Zhongguo Yao Li Xue Bao. 1990;11(4):331–335.
223. McRae S. Can Med Assoc J. 1996;155(3):293–295.
224. Cicero AF, Derosa G, Brillante R, et al. Arch Gerontol Geriatr Suppl. 2004;9:69–73.
225. Johne A, Schmider J, Brockmoller J, et al. J Clin Psychopharmacol. 2002;22(1):46–54.
226. Australian Therapeutic Goods Administration. Media Release, March 2000.
227. Breckenridge A. Message from Committee on Safety of Medicines, 29 February. London, 2000, Medicines Control Agency.
228. Henney JE. JAMA. 2000;283(13):1679.
229. Burstein AH, Horton RL, Dunn T, et al. Clin Pharmacol Ther. 2000;68(6):605–612.
230. Drug Safety Update. November 2007;1(4):p 7. Available from <www.mhra.gov.uk/Publications/Safetyguidance/DrugSafetyUpdate/index.htm> Accessed 18.04.08.
231. Wang LS, Zhu B, Abd El-Aty AM, et al. J Clin Pharmacol. 2004;44(6):577–581.
232. Wang Z, Hamman MA, Huang SM, et al. Clin Pharmacol Ther. 2002;71(6):414–420.
233. Dresser GK, Schwarz UI, Wilkinson GR, et al. Clin Pharmacol Ther. 2003;73(1):41–50.
234. Lau WC, Gurbel PA, Carville DG, et al. J Am Coll Cardiol. 2007;49(9, Suppl 1):343A–344A.
235. Lau WC, Welch TD, Shields TA, et al. J Am Coll Cardiol. 2010;55(10, Suppl 1):A171. [E1600]
236. Lau WC, Welch TD, Shields T, et al. J Cardiovasc Pharmacol. 2011;57(1):86–93.
237. Fitzgerald DJ, Maree A. Hematology Am Soc Hematol Educ Program. 2007;1:114–120.
238. Maurer A, Johne A, Bauer S, et al. Eur J Clin Pharmacol. 1999;55(3):A22.
239. Yue QY, Bergquist C, Gerden B. Lancet. 2000;355(9203):576–577.
240. Barnes J, Anderson LA, Phillipson JD. J Pharm Pharmacol. 2001;53(5):583–600.
241. Uygur Bayramıçlı O, Kalkay MN, Oskay Bozkaya E, et al. Turk J Gastroenterol. 2011;22(1):115.
242. Mueller SC, Majcher-Peszynska J, Uehleke B, et al. Eur J Clin Pharmacol. 2006;62(1):29–36.
243. Arold G, Donath F, Maurer A, et al. Planta Med. 2005;71(4):331–337.
244. Markowitz JS, Donovan JL, DeVane CL, et al. JAMA. 2003;290(11):1500–1504.
245. Wang Z, Gorski JC, Hamman MA, et al. Clin Pharmacol Ther. 2001;70(4):317–326.
246. Mueller SC, Majcher–Peszynska J, Mundkowski RG, et al. Eur J Clin Pharmacol. 2009;65(1):81–87.
247. Kawaguchi A, Ohmori M, Tsuruoka S, et al. Br J Clin Pharmacol. 2004;58(4):403–410.
248. Wang XD, Li JL, Lu Y, et al. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852(1–2):534–544.
249. Tannergren C, Engman H, Knutson L, et al. Clin Pharmacol Ther. 2004;75(4):298–309.
250. Mathijssen RH, Verweij J, de Bruijn P, et al. J Natl Cancer Inst. 2002;94(16):1247–1249.
251. Mansky PJ, Straus SE. J Natl Cancer Inst. 2002;94(16):1187–1188.
252. Smith PF, Bullock JM, Booker BM, et al. Blood. 2004;104(4):1229–1230.
253. Frye RF, Fitzgerald SM, Lagattuta TF, et al. Clin Pharmacol Ther. 2004;76(4):323–329.
254. Johne A, Brockmoller J, Bauer S, et al. Clin Pharmacol Ther. 1999;66(4):338–345.
255. Durr D, Stieger B, Kullak-Ublick GA, et al. Clin Pharmacol Ther. 2000;68(6):598–604.
256. Mueller SC, Uehleke B, Woehling H, et al. Clin Pharmacol Ther. 2004;75(6):546–557.
257. Lundahl A, Hedeland M, Bondesson U, et al. Eur J Pharm Sci. 2009;36(4–5):433–443.
258. de Maat MMR, Hoetelmans RMW, Mathot RAA, et al. AIDS. 2001;15(3):420–421.
259. Piscitelli SC, Burstein AH, Chaitt D, et al. Lancet. 2000;355(9203):547–548.
260. Xu H, Williams KM, Liauw WS, et al. Br J Pharmacol. 2008;153(7):1579–1586.
261. Bon S, Hartmann K, Kuhn M. Schweiz Apoth. 1999;16:535–536.
262. Ahmed SM, Banner NR, Dubrey SW. J Heart Lung Transplant. 2001;20(7):795.
263. Ruschitzka F, Meier PJ, Turina M, et al. Lancet. 2000;355(9203):548–549.
264. Mai I, Kruger H, Budde K, et al. Int J Clin Pharmacol Ther. 2000;38(10):500–502.
265. Karliova M, Treichel U, Malago M, et al. J Hepatol. 2000;33(5):853–855.
266. Rey JM, Walter G. Med J Aust. 1998;169(11–12):583–586.
267. Barone GW, Gurley BJ, Ketel BL, et al. Transplantation. 2001;71(2):239–241.
268. Barone GW, Gurley BJ, Ketel BL, et al. Ann Pharmacother. 2000;34(9):1013–1016.
269. Moschella C, Jaber BL. Am J Kidney Dis. 2001;38(5):1105–1107.
270. Beer AM, Ostermann T. Med Klin. 2001;96(8):480–483.
271. Breidenbach T, Kliem V, Burg M, et al. Transplantation. 2000;69(10):2229–2230.
272. Mai I, Bauer S, Perloff ES, et al. Clin Pharmacol Ther. 2004;76(4):330–340.
273. Bolley R, Zulke C, Kammerl M, et al. Transplantation. 2002;73(6):1009.
274. Mai I, Stormer E, Bauer S, et al. Nephrol Dial Transplant. 2003;18(4):819–822.
275. Hebert MF, Park JM, Chen YL, et al. J Clin Pharmacol. 2004;44(1):89–94.
276. Portoles A, Terleira A, Calvo A, et al. J Clin Pharmacol. 2006;46(10):1188–1194.
277. Eich-Hochli D, Oppliger R, Golay KP, et al. Pharmacopsychiatry. 2003;36(1):35–37.
278. Niederhofer H. Med Hypotheses. 2007;68(5):1189.
279. Wang LS, Zhou G, Zhu B, et al. Clin Pharmacol Ther. 2004;75(3):191–197.
280. Information from the MPA (Medical Products Agency, Sweden) and the MCA (Medicines Control Agency, UK), 2000–2002.
281. Schwarz UI, Buschel B, Kirch W. Br J Clin Pharmacol. 2003;55(1):112–113.
282. Murphy PA, Kern SE, Stanczyk FZ, et al. Contraception. 2005;71(6):402–408.
283. Hall SD, Wang Z, Huang SM, et al. Clin Pharmacol Ther. 2003;74(6):525–535.
284. Pfrunder A, Schiesser M, Gerber S, et al. Br J Clin Pharmacol. 2003;56(6):683–690.
285. Will-Shahab L, Bauer S, Kunter U, et al. Eur J Clin Pharmacol. 2009;65(3):287–294.
286. Murphy P, Bellows B, Kern S. Contraception. 2010;82(2):191.
287. Fogle RH, Murphy PA, Westhoff CL, et al. Contraception. 2006;74(3):245–248.
288. Nieminen TH, Hagelberg NM, Saari TI, et al. Eur J Pain. 2010;14(8):854–859.
289. Gordon JB. Am Fam Phys. 1998;57(5):950–953.
290. Dermott K. Clinical Psychiatry News. 1998;26(3):28.
291. Barbenel DM, Yusuf B, O’Shea D, et al. J Psychopharmacol. 2000;14(1):84–86.
292. Lantz MS, Buchalter E, Giambanco V. J Geriatr Psychiatry Neurol. 1999;12(1):7–10.
293. Prost N, Tichadou L, Rodor F, et al. Presse Med. 2000;29(23):1285–1286.
294. Waksman JC, Heard K, Jolliff H, et al. Clin Toxicol. 2000;38(5):521.
295. Andren L, Andreasson A, Eggertsen R. Eur J Clin Pharmacol. 2007;63(10):913–916.
296. Sugimoto K, Ohmori M, Tsuruoka S, et al. Clin Pharmacol Ther. 2001;70(6):518–524.
297. Gordon RY, Becker DJ, Rader DJ. Am J Med. 2009;122(2):e1–e2.
298. Eggertsen R, Andreasson A, Andren L. Scand J Prim Health Care. 2007;25(3):154–159.
299. Schwarz UI, Hanso H, Oertel R, et al. Clin Pharmacol Ther. 2007;81(5):669–678.
300. Nebel A, Schneider BJ, Baker RK, et al. Ann Pharmacother. 1999;33(4):502.
301. Morimoto T, Kotegawa T, Tsutsumi K, et al. J Clin Pharmacol. 2004;44(1):95–101.
302. Rengelshausen J, Banfield M, Riedel KD, et al. Clin Pharmacol Ther. 2005;78(1):25–33.
303. Hojo Y, Echizenya M, Ohkubo T, et al. J Clin Pharm Ther. 2011;36(6):711–715.
304. Velussi M, Cernigoi AM, de Monte A, et al. J Hepatol. 1997;26(4):871–879.
305. Hussain SA. J Med Food. 2007;10(3):543–547.
306. Huseini HF, Larijani B, Heshmat R, et al. Phytother Res. 2006;20(12):1036–1039.
307. Hashemi SJ, Hajiani E, Sardabi EH. Hep Mon. 2009;9(4):265–270.
308. Deng YQ, Fan XF, Li JP. Chin J Integr Med. 2005;11(2):117–122.
309. Rajnarayana K, Reddy MS, Vidyasagar J, et al. Arzneim Forsch. 2004;54(2):109–113.
310. Fuhr U, Beckmann-Knopp S, Jetter A, et al. Planta Med. 2007;73(14):1429–1435.
311. Repalle SS, Yamsani SK, Gannu R, et al. Acta Pharm Sci. 2009;51(1):15–20.
312. Han Y, Guo D, Chen Y, et al. Xenobiotica. 2009;39(9):694–699.
313. Rossander L, Hallberg L, Bjorn-Rasmussen E. Am J Clin Nutr. 1979;32(12):2484–2489.
314. Disler PB, Lynch SR, Charlton RW, et al. Gut. 1975;16(3):193–200.
315. Brune M, Rossander L, Hallberg L. Eur J Clin Nutr. 1989;43(8):547–557.
316. Derman D, Sayers M, Lynch SR, et al. Br J Nutr. 1977;38(2):261–269.
317. Hallberg L, Rossander L. Hum Nutr Appl Nutr. 1982;36(2):116–123.
318. Chung KT, Wong TY, Wei CI, et al. Crit Rev Food Sci Nutr. 1998;38(6):421–464.
319. Morck TA, Lynch SR, Cook JD. Am J Clin Nutr. 1983;37(3):416–420.
320. Layrisse M, García-Casal MN, Solano L, et al. J Nutr. 2000;130(9):2159–2195. [Erratum in: J Nutr 130(12): 3106, 2000]
321. Thankachan P, Walczyk T, Muthayya S, et al. Am J Clin Nutr. 2008;87(4):881–886.
322. Nelson M, Poulter J. J Hum Nutr Diet. 2004;17(1):43–54.
323. Gabrielli GB, De Sandre G. Haematologica. 1995;80(6):518–520.
324. Mahlknecht U, Weidmann E, Seipelt G. Haematologica. 2001;86(5):559.
325. Kaltwasser JP, Werner E, Schalk K, et al. Gut. 1998;43(5):699–704.
326. Ganji V, Kies CV. Plant Foods Hum Nutr. 1994;46(3):267–276.
327. Juan H, Terhaag B, Cong Z, et al. Eur J Clin Pharmacol. 2007;63(7):663–668.
328. U.S.P. Drug Information, US Pharmacopeia Patient Leaflet, Valerian (Oral). Rockville: The United States Pharmacopeial Convention; 1998.
329. Herberg KW. Therapiewoche. 1994;44(12):704–713.
330. Carrasco MC, Vallejo JR, Pardo-de-Santayana M, et al. Phytother Res. 2009;23(12):1795–1796.
331. Krivoy N, Pavlotzky E, Chrubasik S, et al. Planta Med. 2001;67(3):209–212.
332. Personal communication from trial author Yu KS. 2 February 2010.
333. Makino T, Hishida A, Goda Y, et al. Nat Med. 2008;62(3):294–299.
334. Product information for Cranberry Classic juice drink. Available from <www.oceanspray.com.au> Accessed November 2009.
335. Warshafsky S, Kamer RS, Sivak SL. Ann Intern Med. 1993;119(7 Pt 1):599–605.
336. Lawson LD, Wang ZJ, Papadimitriou D. Planta Med. 2001;67(1):13–18.
337. Leistner E, Drewke C. J Nat Prod. 2010;73(1):86–92.
338. Kajiyama Y, Fujii K, Takeuchi H, et al. Pediatrics. 2002;109(2):325–327.
339. Hasegawa S, Oda Y, Ichiyama T, et al. Pediatr Neurol. 2006;35(4):275–276.
340. Arenz A, Klein M, Fiehe K, et al. Planta Med. 1996;62(6):548–551.
341. Kuenick C. Dtsch Apoth Ztg. 2010;150(5):60–61.
342. Gaus W, Westendorf J, Diebow R, et al. Methods Inf Med. 2005;44(5):697–703.
343. DeKosky ST, Williamson JD, Fitzpatrick AL, et al. JAMA. 2008;300(19):2253–2262.
344. Kim TE, Kim BH, Kim J, et al. Clin Ther. 2009;31(10):2249–2257.
345. Henning SM, Niu Y, Liu Y, et al. J Nutr Biochem. 2005;16(10):610–616.
346. Mattarello MJ, Benedini S, Fiore C, et al. Steroids. 2006;71(5):403–408.
347. Kageyama Y, Suzuki H, Saruta T. Endocrinol Jpn. 1991;38(1):103–108.
348. Haslam E, Lilley TH. Crit Rev Food Sci Nutr. 1988;27(1):1–40.
349. Price ML, Butler LG. J Agric Food Chem. 1977;25(6):1268–1273.
350. Ewerth S, Ahlberg J, Holmstrom B, et al. Acta Chir Scand Suppl. 1980;500:49–50.
351. Moreyra AE, Wilson AC, Koraym A. Arch Intern Med. 2005;165(10):1161–1166.
352. Agrawal AR, Tandon M, Sharma PL. Int J Clin Pract. 2007;61(11):1812–1818.
353. Halstead CW, Lee S, Khoo CS, et al. J Pharm Biomed Anal. 2007;45(1):30–37.
354. Zhu M, Chen XS, Wang KX. Chromatographia. 2007;66(1–2):125–128.
355. Dasgupta A, Wu S, Actor J, et al. Am J Clin Pathol. 2003;119(2):298–303.
356. Ang CYW, Hu L, Heinze TM, et al. J Agric Food Chem. 2004;52(20):6156–6164.
357. MediHerb Research Laboratories, 2004.
358. Tomlinson B, Hu M, Lee VW. Mol Nutr Food Res. 2008;52(7):799–809.
359. Lynch SR. Nutr Rev. 1997;55(4):102–110.
360. Gillooly M, Bothwell TH, Charlton RW, et al. Br J Nutr. 1984;51(1):37–46.
References
1. Mills S, Bone K, eds. The Essential Guide to Herbal Safety. USA: Churchill Livingstone, 2005. 50–88
2. De Smet PA. Br J Clin Pharmacol. 2007;63(3):258–267.
3. Guidance for industry: drug interaction studies: study design, data analysis and implications for dosing and labeling. Draft published September 2006. Available from <http://www.fda.gov/ohrms/dockets/dockets/06d0344/06d–0344–c000008–01–vol1.pdf> Accessed November 2011.
4. Fuhr U. Eur J Clin Pharmacol. 2007;63(10):897–899.
5. Greenblatt DJ. Cardiovasc Ther. 2009;27(4):226–229.
6. Mueller SC, Majcher-Peszynska J, Mundkowski RG, et al. Eur J Clin Pharmacol. 2009;65(1):81–87.
7. Butterweck V, Derendorf H. Clin Pharmacokinet. 2008;47(6):383–397.