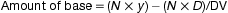Suppositories and pessaries
 Suppository moulds and mould calibration
Suppository moulds and mould calibration
 Methods of preparation of suppositories and pessaries
Methods of preparation of suppositories and pessaries
 Containers, labelling and patient advice for suppositories and pessaries
Containers, labelling and patient advice for suppositories and pessaries
Introduction
Drug administration by the rectum can be used for local or systemic action. Dosage forms used include suppositories, rectal tablets, capsules, ointments and enemas. Vaginal administration can be for both local and systemic action, using pessaries and vaginal formulations of tablets, capsules, solutions, sprays, creams, ointments and foams. This chapter gives details of how suppositories and pessaries are prepared extemporaneously, the substances and equipment used in their preparation, the calculations involved and patient advice.
Suppositories and pessaries are drug delivery systems where the drug is incorporated into an inert vehicle; base. Suppositories are formed by melting the base, incorporating the drug and then allowing them to set in a suitable metal or plastic mould.
Suppository bases
A number of criteria can be identified as desirable in an ideal base, including:
 Melt at, or just below, body temperature or dissolve in body fluids
Melt at, or just below, body temperature or dissolve in body fluids
 Solidify quickly after melting
Solidify quickly after melting
 Easily moulded and removed from the mould
Easily moulded and removed from the mould
 Chemically stable even when molten
Chemically stable even when molten
No base meets all these requirements, so a compromise is required. There are two groups of materials, the fatty bases and the water-soluble or water-miscible bases.
The fatty bases
Theobroma oil
Theobroma oil, a naturally occurring oil, has a melting point range of 30–36°C and so readily melts in the body. It liquefies easily on heating but sets rapidly when cooled. It is also bland, therefore no irritation occurs. The main technical difficulty is the ease with which lower melting point polymorphic forms of theobroma oil are formed. The stable β-form has a melting point of 34.5°C and forms after melting at 36°C and slowly cooling. If it is overheated, the unstable α-form (melting point 23°C) and γ-form (melting point 19°C) are produced. These forms will eventually return to the stable form but this may take several days. The melting point is a problem in hot climates and can be reduced further by the addition of a soluble drug. The latter effect can be counteracted by adding beeswax (up to 10%), but care must be taken not to raise the melting point too high, as the suppository would not melt in the rectum. In addition, theobroma oil is prone to oxidation. Theobroma oil shrinks only slightly on cooling and therefore tends to stick to the suppository mould; thus requiring a mould lubricant.
Synthetic fats
These are hydrogenated vegetable oils. Synthetic fatty bases have many of the advantages but there are a few potential problems:
 The viscosity of the melted fats is lower than that of theobroma oil. As a result there is a greater risk of drug particles sedimenting during preparation leading to a lack of uniform drug distribution. This problem is partly compensated for in that these bases set very quickly
The viscosity of the melted fats is lower than that of theobroma oil. As a result there is a greater risk of drug particles sedimenting during preparation leading to a lack of uniform drug distribution. This problem is partly compensated for in that these bases set very quickly
 These bases become brittle if cooled too rapidly, so should not be refrigerated during preparation
These bases become brittle if cooled too rapidly, so should not be refrigerated during preparation
 These bases are produced in series of grades, each with different hardness and melting point ranges. These can be used to compensate for melting point reduction by soluble drugs. However, release and absorption of the drug in the body may vary depending on the base being used.
These bases are produced in series of grades, each with different hardness and melting point ranges. These can be used to compensate for melting point reduction by soluble drugs. However, release and absorption of the drug in the body may vary depending on the base being used.
Further information on these bases can be found in the Pharmaceutical Codex (1994).
Water-soluble and water-miscible bases
Glycerol-gelatin bases
These bases are a mixture of glycerol and water stiffened with gelatin. The commonest is Glycerol Suppositories Base BP, which has 14% weight in weight (w/w) gelatin, and 70% w/w glycerol. In hot climates, the gelatin content can be increased to 18% w/w. Pharmaceutical grade gelatin is a pathogen-free, purified protein produced by the hydrolysis of the collagenous tissue, such as skins and bones, of animals. Some people may have ethical problems with the use of such a product.
Two types of gelatin are used for pharmaceutical purposes: Type A, which is prepared by acid hydrolysis and is cationic, and Type B, which is prepared by alkaline hydrolysis and is anionic. Type A is compatible with substances such as boric acid and lactic acid, while Type B is compatible with substances like ichthammol and zinc oxide. The ‘jelly strength’ or ‘bloom strength’ of gelatin is important, particularly when it is used in the preparation of suppositories or pessaries.
Glycerol-gelatin bases have a physiological effect which can cause rectal irritation because of the small amount of liquid present. As they dissolve in the mucous secretions of the rectum, osmosis occurs producing a laxative effect. The solution time depends on the content, quality of the gelatin and the age of the suppository. Because of the water content, microbial contamination is more likely than with the fatty bases. Preservatives may be added to the product, but can lead to problems of incompatibility. In addition, glycol-gelatin bases are hygroscopic and therefore require careful storage.
Macrogols
These polyethylene glycols can be blended together to produce suppository bases with varying melting points, dissolution rates and physical characteristics. Drug release depends on the base dissolving rather than melting (the melting point is often around 50°C). Higher proportions of high molecular weight polymers produce preparations which release the drug slowly and are also brittle. Less brittle products which release the drug more readily can be prepared by mixing high polymers with medium and low polymers. Details of combinations which are used are found in the Pharmaceutical Codex (1994). Macrogols have several properties which make them useful as suppository bases including the absence of a physiological effect, are not prone to microbial contamination and have a high water-absorbing capacity. As they dissolve, a viscous solution is produced which means there is less likelihood of leakage from the body.
The macrogol bases have a number of disadvantages. They are hygroscopic, which means they must be carefully stored, and this could lead to irritation of the rectal mucosa. This latter disadvantage can be alleviated by dipping the suppository in water prior to insertion. They become brittle if cooled too quickly and also may become brittle on storage. Incompatibility with several drugs and packaging materials, e.g. benzocaine and plastic, may limit their use. In addition, crystal growth occurs, with some drugs causing irritation to the rectal mucosa and may prolong dissolution times.
Preparation of suppositories
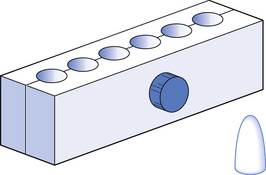
Suppositories are made using a metal or plastic suppository mould. Traditional metal moulds (Fig. 37.1) are in two halves which are clamped together with a screw. The internal surface is normally plated to ensure that the suppositories have a smooth surface.
Before use the mould should be completely cleaned by washing carefully in warm, soapy water and thoroughly dried, taking care not to scratch the internal surface. The exact shape can vary slightly from one mould to another.
Preparation of suppositories containing an active ingredient which is insoluble in the base
The bases used, most commonly, for extemporaneous preparation of suppositories and pessaries are the synthetic fats and glycerol-gelatin base.
1. When calculating the quantity of ingredients it is necessary to prepare excess due to unavoidable wastage. Usually, an excess of two should be calculated for, e.g. to prepare 12 suppositories, calculate for 14.
2. The mould should be carefully washed and dried.
3. Ensure that the two halves fit together correctly. This is necessary to ensure that there is no leakage of material. They usually have code letters and/or numbers which should match.
4. For some bases the mould will need to be lubricated. The lubricants are given in Table 37.1.
Table 37.1
Lubricants for use with suppository bases
| Base | Lubricant |
| Theobroma oil | Soap spirit |
| Glycerol-gelatin base | Almond oil, liquid paraffin |
| Synthetic fats | No lubricant required |
| Macrogols | No lubricant required |
5. If a lubricant is necessary, apply it carefully to the two halves of the mould using gauze or other non-fibrous material. Do not use cotton wool as fibres may be left on the mould surface and become incorporated into the suppositories.
6. Invert the mould to allow any excess lubricant to drain off.
7. Accurately weigh the required amount of base. If large lumps are present the material should be grated.
8. Place in a porcelain basin and warm gently using a water bath or hot plate. Allow approximately two-thirds of the base to melt and remove from the heat. The residual heat will be sufficient to melt the rest of the base.
9. Reduce the particle size of the active ingredient, if necessary. Either grinding in a mortar and pestle or sieving (see Ch. 30).
10. Weigh the correct amount of medicament and place on a glass tile (ointment slab).
11. Add about half of the molten base to the powdered drug and rub together with a spatula.
12. Scrape the dispersion off the tile using the spatula and place it back in the basin.
13. If necessary, put the basin back over the water bath to remelt the ingredients.
14. Remove from the heat and stir constantly until almost on the point of setting. If the mixture is not stirred at this stage the active ingredient will sediment and uniform distribution of the drug will not be achieved.
15. Quickly pour into the mould, slightly overfilling each cavity to allow for contraction on cooling. Do not start pouring the suppositories while the mixture is still very molten. If this is done, a suspended drug will sediment to the bottom of the mould and the base shrinks excessively so that the tops become concave.
16. Leave the mould and its contents to cool for about 5 min and then, using a spatula, trim the tops of the suppositories. Do not leave the suppositories too long before trimming, as they will be too hard and trimming becomes very difficult.
17. Allow cooling for another 10–15 min until the suppositories are completely firm and set. Do not try to speed up the cooling process by putting the mould in a refrigerator. Synthetic fats in particular are inclined to become brittle and break if cooled too quickly.
18. Unscrew the mould and remove the suppositories.
19. Each perfect suppository should then be wrapped in greaseproof paper and packed in an appropriate container and labelled.
When preparing suppositories where the active ingredient is either a semi-solid, is soluble in the base or is a liquid which is miscible with the base, the melting point of the base will be lowered. In these situations, a base with a higher than normal melting point should be used if available. The base is melted as normal and the active ingredient is added directly to the base and incorporated by stirring.
Moulds are made in four sizes: 1 g, 2 g, 4 g and 8 g. Unless otherwise stated, the 1 g size is used for suppositories. The same moulds are used to prepare pessaries, when the two larger sizes are generally used. A suppository mould is filled by volume, but the suppository is formulated by weight. The capacity of a suppository mould is nominal and each mould will have minor variations. Therefore, the weight of material contained in different moulds may be different and will also depend on the base being used. It is therefore essential that each mould be calibrated for each different base.
Mould calibration
The capacity of the mould is confirmed by filling the mould with the chosen base. The total weight of the perfect suppositories is taken and a mean weight calculated. This value is the calibration value of the mould for that particular base (see Example 37.1).
Displacement values
The volume of a suppository from a particular mould is uniform but its weight can vary when a drug is present because the density of the drug may be different from that of the base. For example, a drug which has twice the density of the base will occupy half the volume which the same weight of base occupies, and a drug whose density is four times that of the base will occupy a quarter the volume which the same weight of base occupies. Allowance must be made for this by using displacement values (DV).
The displacement value of a drug is the number of parts by weight of drug which displaces 1 part by weight of the base.
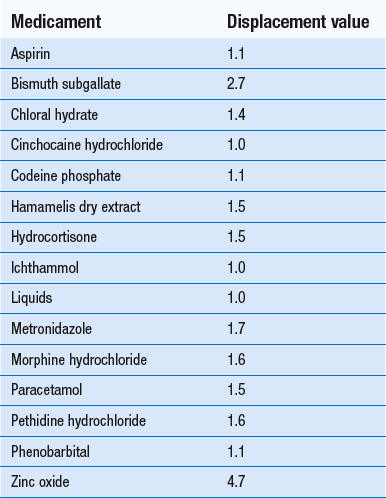
Displacement values for a variety of medicaments are given in Table 37.2. Other reference sources such as the Pharmaceutical Handbook (Wade 1980) and the Pharmaceutical Codex also give information on displacement values. Minor variations may occur in the values quoted so it is always advisable to indicate the source of your information (see Example 37.2).
Displacement values in the literature normally refer to values for theobroma oil. These values can also be used for other fatty bases. With glycerol-gelatin suppository base, approximately 1.2 g occupies the same volume as 1 g of theobroma oil. Using this information, the relevant displacement values can be calculated.
There may be occasions when information on the DV of a drug is not available. In these situations the DV must be determined (see Example 37.3).
Calculation of quantities when the active ingredient is stated as a percentage
A displacement value is not required when calculating quantities stated as percentages (see Example 37.4).
When there is more than one active ingredient present the quantity of each medicament is calculated and the amount of base is calculated using the displacement value for each ingredient (see Example 37.5).
Preparation of suppositories using a glycerol-gelatin base
The formula for Glycerol Suppository Base BP is:
| Gelatin | 14% |
| Glycerol | 70% |
| Water | to 100% |
1. The gelatin strip is cut into small pieces, approximately 1 cm square, trimming off any hard outer edges.
2. The required amount of gelatin is weighed and placed in a previously weighed porcelain evaporating basin.
3. Sufficient water to just cover the gelatin is added and the contents left for about 5 min.
4. When the gelatin has softened (hydrated), any excess water is drained off. This step is not necessary if powdered gelatin is being used.
5. The exact amount of glycerol is then weighed into the basin.
6. The basin is heated gently on a water bath or hot plate and the mixture gently stirred until the gelatin has dissolved. Do not stir vigorously as this will create air bubbles which are very difficult to remove. At this stage the base may need to be heat treated as noted below.
7. When the gelatin is dissolved, the basin is removed from the heat and weighed. If the weight is less than the required total (basin plus ingredients), water is added to give the correct weight. If the contents of the basin are too heavy, it must be heated further to evaporate the excess water.
8. When the correct weight is achieved, the active ingredient is added, with careful stirring.
9. The mixture is then poured into the prepared mould and lubricated with an oil such as almond oil or liquid paraffin. The mould must not be overfilled because glycerol-gelatin base cannot be trimmed.
10. The preparation is left to set. After removing from the mould, each suppository should be smeared with liquid paraffin before being wrapped in greaseproof paper.
Note: Pharmaceutical gelatin should not contain any pathogens, but as a precaution, the base may be heat treated. This is done by heating the base for 1 h at 100°C in an electric steamer. This should be done before the base is adjusted to weight (at Stage 7 above).
This base is commonly used for the preparation of pessaries, as described in Example 37.6.
Containers for suppositories
Glass or plastic screw-topped jars are the best choice of container for extemporaneously prepared suppositories and pessaries. Cardboard cartons may be used but these offer little protection from moisture or heat for hygroscopic materials.
Shelf-life
Suppositories and pessaries are relatively stable preparations, if well packaged and stored at a low temperature. Unless other information is available, an expiry date of 1 month is appropriate.
Labelling for suppositories
Adequate information should appear on the label so that the patient knows how to use the product. In addition, the following information should appear: ‘Store in a cool place’ and ‘For rectal use only’ or ‘For vaginal use only’, whichever is appropriate.
Patient advice
In addition to what appears on the label, patients should be told to unwrap the suppository or pessary (this may appear to be unnecessary advice but there is sufficient evidence to show that it is not always done) and insert it as high as possible into the rectum or vagina. It may be helpful to provide the patient with a diagram and instruction leaflet, such as that produced by the National Pharmaceutical Association. When suppositories are for children it is likely that an adult will have to carry out the insertion.
Key Points
 Both rectal and vaginal administration can be used for local or systemic drug action
Both rectal and vaginal administration can be used for local or systemic drug action
 Bases may be fatty or water miscible
Bases may be fatty or water miscible
 Synthetic fatty bases are easier to use than theobroma oil
Synthetic fatty bases are easier to use than theobroma oil
 Glycerol-gelatin base produces a laxative effect
Glycerol-gelatin base produces a laxative effect
 Type A (anionic) or Type B (cationic) gelatin can be used to avoid incompatibilities
Type A (anionic) or Type B (cationic) gelatin can be used to avoid incompatibilities
 Macrogol bases are blends of high and low molecular weight polymers which dissolve in rectal contents
Macrogol bases are blends of high and low molecular weight polymers which dissolve in rectal contents
 Suppository moulds have nominal capacities of 1, 2, 4 and 8 g and must be calibrated with the base to be used
Suppository moulds have nominal capacities of 1, 2, 4 and 8 g and must be calibrated with the base to be used
 When using theobroma oil and glycerol-gelatin base, the mould has to be lubricated
When using theobroma oil and glycerol-gelatin base, the mould has to be lubricated
 To allow for contraction on cooling, overfilling with oily bases is required
To allow for contraction on cooling, overfilling with oily bases is required
 Each mould should be calibrated for each base
Each mould should be calibrated for each base
 Because glycerol-gelatin base has a higher density than fatty bases, moulds hold approximately 1.2 times the nominal weight
Because glycerol-gelatin base has a higher density than fatty bases, moulds hold approximately 1.2 times the nominal weight
 The displacement value is the number of parts by weight of drug which displaces one part by weight of base
The displacement value is the number of parts by weight of drug which displaces one part by weight of base
 Unless the density of the drug and base are the same, a displacement value is required to calculate the amount of base displaced by the drug
Unless the density of the drug and base are the same, a displacement value is required to calculate the amount of base displaced by the drug
 Labels should include either ‘For rectal use only’ or ‘For vaginal use only’, and ‘Store in a cool place’
Labels should include either ‘For rectal use only’ or ‘For vaginal use only’, and ‘Store in a cool place’