Powders and granules
Introduction
A powder may be defined as a solid material in a finely divided state. Granules are powders agglomerated to produce larger free-flowing particles. Powders and granules can be used to prepare other formulations, such as solutions, suspensions and tablets. A powdered drug on its own can be a dosage form for taking orally (called a simple powder), when they are usually mixed with water first, or for external application as a dusting powder. Alternatively, the drug may be blended with other ingredients (called a compound powder).
Powders for internal use
Powders for oral administration will comprise the active ingredients with excipients such as diluents, sweeteners and dispersing agents. These may be presented as undivided powders (bulk powders) or divided powders (individually wrapped doses).
Individually wrapped powders tend not to be official formulae and are rarely prescribed these days (see Examples 38.1 and 38.2). Magnesium Trisilicate Powder Compound BP (see Example 38.4) and Compound Kaolin Powder BP are examples of bulk powders for internal use. Proprietary powders and granules include Dioralyte®, Electrolade® (both oral rehydration salts), Normacol® (sterculia) and Fybogel® (ispaghula husk).
Bulk powders
Supplying as an undivided powder is useful for non-potent, bulky drugs with a large dose, e.g. antacids, or when the dry powder is more stable than its liquid-containing counterpart. A bulk powder can be supplied to the patient although this is rarely seen nowadays because the dosage form is inconvenient to carry and there are possible inaccuracies in measuring the dose.
Individually wrapped powders
Individually wrapped powders are used to supply some potent drugs, where accuracy of dose is important. Extemporaneously produced powders are wrapped separately in paper. They are convenient dosage forms for children’s doses of drugs which are not commercially available at the strength required, such as levothyroxine (thyroxine) or ibuprofen (see Example 38.2). Sealed sachets of powders are available commercially, e.g. Paramax® (paracetamol and metoclopramide) and oral rehydration salts. They are mixed with water prior to taking and are useful for patients who have difficulty swallowing or where rapid absorption of the drug is required.
Granules for internal use
Some preparations are supplied to the pharmacy as granules, for reconstitution immediately before dispensing, e.g. antibiotic suspensions. This protects drugs which are susceptible to hydrolysis or other degradation in the presence of water until the time of dispensing in order to give an adequate shelf-life.
Particle size
The particle size of a powder is described using standard descriptions given in the British Pharmacopoeia (BP). These refer to either the standardized sieve size that they are capable of passing through in a specified time under shaking, or to the microscopically determined particle size. Thus, powders for oral use would normally be a ‘moderately fine’ or a ‘fine’ powder. The former is able to pass through a sieve of nominal mesh aperture 355 μm and the latter one of 180 μm. Comminution is the process of particle size reduction. On a small scale, this can be achieved using a mortar and pestle when it is usually called trituration. This is a common first step in extemporaneous dispensing, after which the powder should be passed through the appropriate sieve before weighing.
Mixing the powder
Ingredients of powders should be mixed thoroughly, using the technique of ‘doubling-up’ (sometimes called geometric dilution) to ensure an even distribution. This process involves starting with the ingredient which has the smallest bulk. In Example 38.1, this is hyoscine hydrobromide. The other ingredient(s) are added progressively in approximately equal parts by volume. In this way the amount in the mortar is approximately doubled at each addition. Mixing in between additions continues until all the ingredients are incorporated. The powder can then be packed.
Preparing individually wrapped powders
The minimum weight of an individually wrapped powder is 120 mg. Dilution of a drug with a diluent, usually lactose, is often necessary to produce this weight.
Occasionally, manufactured tablets or capsules may be used to prepare oral powders (see Example 38.2). This involves either crushing the tablet in a mortar and pestle, or emptying the contents of the capsule and adding a suitable diluent. Lactose is the most commonly used diluent because it is colourless, odourless, soluble, and has good flow properties. Some patients may be unable to tolerate lactose and suitable inert alternative diluent include, light kaolin and starch.
Powder calculations
Quantities should be calculated to allow for loss of powder during manipulation. It is usual to allow for at least one extra powder. If the total amount of active ingredient required is less than the minimum weighable quantity, dilutions will be necessary. In this process, also called trituration, the minimum quantity of the active ingredient(s) is weighed and diluted, over several steps if necessary, in order to obtain the dose(s) required. Example 38.1 illustrates the process where two dilution steps are required (see also Ch. 19).
Folding papers
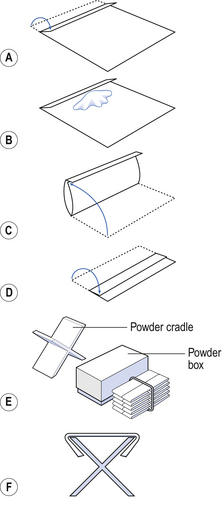
White glazed paper, called demy paper, is used for wrapping powders. A suitable size is 120 mm × 100 mm. The wrapping should be carried out on a clean tile or larger sheet of demy to protect the product. The papers should be folded with their long edges parallel to the front of the bench. Follow the steps illustrated in Figure 38.1 in order to fold the paper:
 The long edge, furthest away from the dispenser, should be turned over to about one-seventh of the paper width (step A)
The long edge, furthest away from the dispenser, should be turned over to about one-seventh of the paper width (step A)
 The powder should be weighed accurately and placed on the paper towards the folded edge of the centre of the paper (step B)
The powder should be weighed accurately and placed on the paper towards the folded edge of the centre of the paper (step B)
 The unfolded long edge (nearest the dispenser) should then be brought over the powder to meet the crease of the folded edge and the flap closed over it (step C)
The unfolded long edge (nearest the dispenser) should then be brought over the powder to meet the crease of the folded edge and the flap closed over it (step C)
 The folded edge should then be folded over (towards the dispenser) so that it covers about half the powder packet (step D)
The folded edge should then be folded over (towards the dispenser) so that it covers about half the powder packet (step D)
 The short edges of the powder packet should be folded over, using a powder cradle if available, so that the flaps are of equal lengths and the folded powder fits neatly into a box or jar (steps E and F). Before making these folds, ensure that there is no powder in the ends to be folded, otherwise it may fall out and be lost.
The short edges of the powder packet should be folded over, using a powder cradle if available, so that the flaps are of equal lengths and the folded powder fits neatly into a box or jar (steps E and F). Before making these folds, ensure that there is no powder in the ends to be folded, otherwise it may fall out and be lost.
The creases can be sharpened with a spatula, taking care not to tear the paper or use excessive pressure which would compress the powder inside the pack.
The powders can be packed in pairs, back to back, or in one bundle, with the final powder placed back to back. They should be held together with an elastic band. In a well-wrapped product, there will be no powder in the fold or flaps, so that all the powder is available for easy administration when unwrapped.
Manufactured powders are subject to a uniformity of weight test, or uniformity of content test if each dose contains<2 mg of active ingredient or the content of active ingredient represents<2% of the total weight.
Shelf-life and storage of internal powders
Extemporaneously prepared powders should have an expiry of between 2 and 4 weeks. Proprietary powders often have a longer shelf-life because of the protective packaging. Some powders may be hygroscopic, deliquescent or volatile and will need to be protected from decomposition. Storage for these powders should be moisture proof and airtight.
Containers for internal powders
Extemporaneously prepared individually wrapped powders are often dispensed in a paperboard box. However, it is preferable to use a screw-top glass or plastic container which provides an airtight seal and protection against moisture. Proprietary powders in individual sachets which are moisture proof may be dispensed in a paperboard box. Bulk powders are packed in an airtight glass or plastic jar. A 5 mL spoon should also be supplied with bulk powders.
Special labels and advice for internal powders
Powders are usually mixed with water or another suitable liquid before taking, depending on their solubility. Powders for babies or young children can be placed directly into the mouth on the back of the tongue, followed by a drink to wash down the powder. Bulk powders should be shaken and measured carefully before dissolving or dispersing in a little water and taking.
Formulation notes. A diluent is not required, since the weight of each powder will be above the minimum 120 mg required. Pure ibuprofen powder can be used. However, if it is not available, manufactured 200 mg ibuprofen tablets (not modified release) can be used to prepare these powders.
Method of preparation. Take 14 × 200 mg ibuprofen tablets (contain 2.8 g ibuprofen) and weigh them. This is necessary to allow for the weight of the tablet excipients. Grind to a fine powder in a mortar and pestle. Pass the resulting powder through a 250 μm sieve and lightly remix. Divide the original weight of tablets by 20, and weigh aliquots of the resulting amount of powder. Pack into individual powder papers. Fasten the 18 powders together with an elastic band and pack in an amber glass jar or plastic container with a screw cap.
Shelf-life and storage. Store in a cool, dry place. A shelf-life of 2–3 weeks is appropriate.
Powders for external use
Powders, with or without medicament, are frequently applied to the skin. Dusting powders contain one or more substances in fine powder and may be dispensed as single-dose or multidose preparations (see Example 38.3). They are used to treat a variety of skin conditions or to soothe skin. Examples are antifungal powders for athlete’s foot or talc dusting powder for the prevention of chafing and skin irritation. Zinc oxide and starch are added to formulations to absorb moisture and talc is used for lubricant properties. Talc, kaolin and other natural mineral materials are liable to contamination with bacteria such as Clostridium tetani, C. perfringens and Bacillus anthracis. These ingredients should be sterilized by dry heat or the final product should be sterilized. Dusting powders should be sterile if they are to be applied to large areas of open skin or wounds. They should not be used where there is a likelihood of large volumes of exudate, as hard crusts will form.
Preparing powders for external use
A sieve size of 180 μm should be used to obtain the finely divided powder. The constituents should be mixed using the doubling-up method, as described previously.
Shelf-life and storage for powders for external use
Dry powders should remain stable over a long period of time if packaged and protected from the atmosphere. For extemporaneously prepared products, an expiry of 4 weeks is appropriate.
Containers for powders for external use
Powders for external use may be packed in glass, metal or plastic containers with a sifter-type cap. Some are also available commercially in pressurized containers, containing other excipients such as a propellant and lubricants.
Special labels and advice for powders for external use
‘For external use only’ and ‘Store in a cool, dry place’.
Examples of official powders for external use include Zinc Oxide Dusting Powder Compound BPC, Chlorhexidine Dusting Powder BP and Talc Dusting Powder BP. There are now few proprietary examples of powders for external use one being Daktarin® (miconazole).
Method of preparation. Sieve the powders, using a 180 μm sieve, weigh and mix them by doubling-up in a mortar and pestle. Pack in an amber glass jar or plastic container with a screw cap (with a perforated, reclosable lid if possible).
Shelf-life and storage. Store in a dry place. An expiry date of 4 weeks is advisable.
Method of preparation. Sieve the powders, using a 250 μm sieve, weigh and mix them by doubling-up, using a mortar and pestle. Pack in an amber glass jar or plastic container with a screw cap.
Shelf-life and storage. Store in a dry place. A 4-week expiry date is reasonable if kept dry.
Key Points
 Powders may be prepared as bulk powders, divided powders or granules
Powders may be prepared as bulk powders, divided powders or granules
 Powders may be used internally or externally
Powders may be used internally or externally
 The particle size of a fine powder should be less than 180 μm
The particle size of a fine powder should be less than 180 μm
 The minimum weight of a divided powder is 120 mg
The minimum weight of a divided powder is 120 mg
 Lactose is a good diluent for internal powders
Lactose is a good diluent for internal powders
 Trituration is the process used to obtain small doses which are below the minimum weighable quantity
Trituration is the process used to obtain small doses which are below the minimum weighable quantity
 Ideally powders should be packed in a glass or plastic container
Ideally powders should be packed in a glass or plastic container
 A 5 mL spoon should be provided with bulk powders for oral use
A 5 mL spoon should be provided with bulk powders for oral use
 When dispensing divided powders, an excess of one or two should be prepared to allow for losses during processing
When dispensing divided powders, an excess of one or two should be prepared to allow for losses during processing