Sleep-Disordered Breathing and Scoring
The diagnosis and treatment of sleep-disordered breathing remains the driving force behind clinical sleep medicine. As understanding and measurement techniques have evolved, so have respiratory event scoring criteria and principles of positive airway pressure titration. A polysomnogram (PSG) is a treasure trove of physiological data. The electroencephalographic (EEG), respiratory, electro-oculographic (EOG), and electromyographic (EMG) patterns are complex, varying, and dynamic and interact in infinite ways, forming complex but recognizable patterns in health and disease. Other configurations that include less and sometimes more signals add to the complexity of analysis. Accurate scoring of sleep is central to sleep medicine and research and will undoubtedly evolve continuously for the decades ahead.
Scoring of abnormal respiration is the component that is most controversial. This section of the atlas provides a number of snapshots (Figs. 4.1-4.30) to illustrate some of the patterns and challenges. Scoring has evolved through time, and most recently, recognizing and mapping dynamic patterns have added new dimensions. New treatment modalities such as adaptive ventilation fundamentally alter waveform characteristics and challenge the current scoring guidelines.
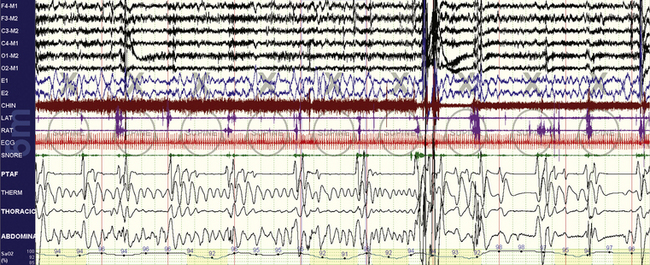
FIGURE 4.1 Obstructive sleep apnea.
A 10-minute compression is shown in a 47-year-old man. Overt and repetitive obstructive respiratory events with associated oxygen desaturations are seen. Following a larger arousal, postarousal central apneas and respiratory instability are seen. Periodic tibialis motor activations occur associated with respiratory arousals. However, note that many of the events, especially the fourth to seventh events from the left, have a nearly identical timing of 30 seconds. Such timing characteristics (short, metronomical) can reflect strong respiratory chemoreflex modulation of sleep breathing, but by any criteria the events shown here need to be scored as obstructive hypopneas.
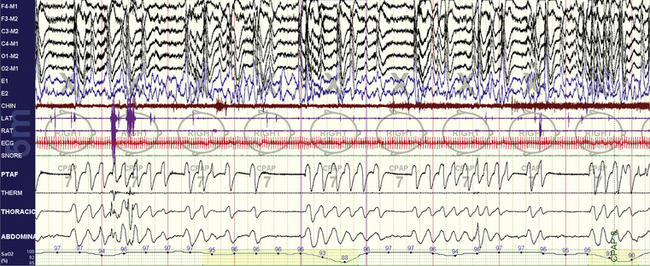
FIGURE 4.2 Complex sleep apnea.
The same subject as in Figure 4.1 while on continuous positive airway pressure (CPAP). A 10-minute compression is shown. Note the emergence of central apneas at low to moderate CPAP pressures. This pattern persisted through much of non–rapid eye movement sleep. In this snapshot the patient is nonsupine.
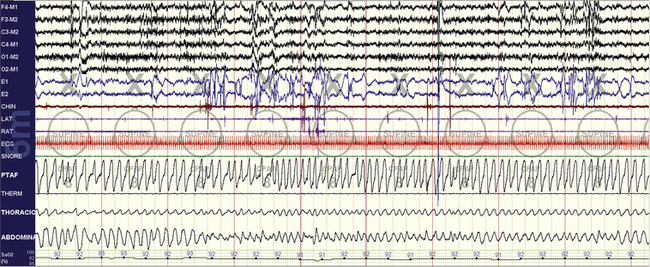
FIGURE 4.3 Stabilization effects of rapid eye movement (REM) sleep on chemoreflex-modulated sleep apnea.
The same subject as in Figures 4.1 and 4.2. A 10-minute compression is shown. Note the excellent response to therapy. The body position is supine, and thus positional effects cannot explain the non-REM (NREM) versus REM sleep differences. The NREM-dominant sleep apnea phenotype is readily recognizable and confers a high risk for conversion to the “complex” phenotype.
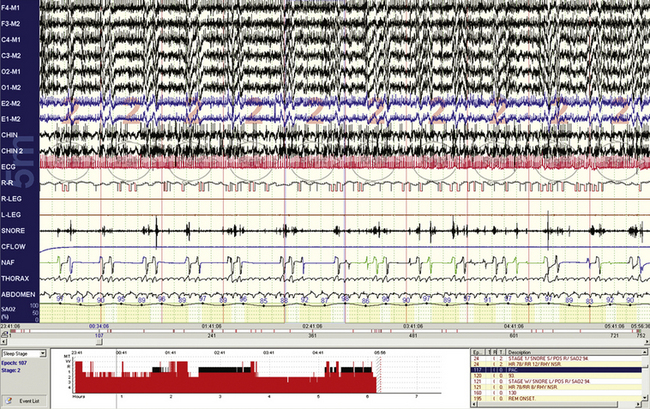
FIGURE 4.4 Short cycle obstructive apneas in non–rapid eye movement sleep.
A 10-minute compression is shown in a 32-year-old woman. The events are clearly obstructive yet have few recovery breaths after individual events. The return of oxygen saturation levels to the baseline after arousals suggests that there is no hypoventilation, but end-tidal CO2 monitoring would be required. Note the robust cardiac accelerations and decelerations, but these do not exactly match the timing of arousal/recovery breaths.
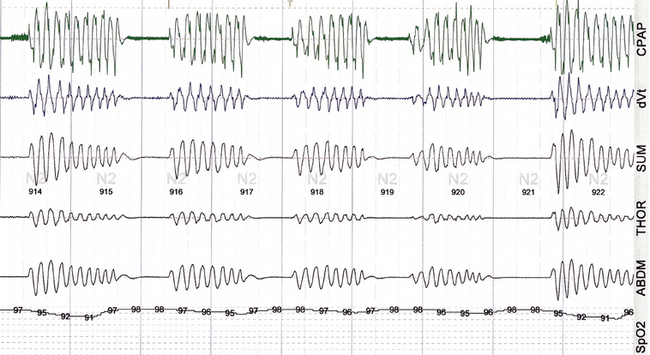
FIGURE 4.5 Classic periodic breathing/Cheyne-Stokes pattern during continuous positive airway pressure titration.
A 59-year-old man with clinical symptoms consistent with sleep apnea, with no other comorbidities. The vertical lines are 30 seconds. The pattern during the diagnostic assessment was identical. This readily recognizable pattern reflects unstable and exaggerated respiratory chemoreflex influences.
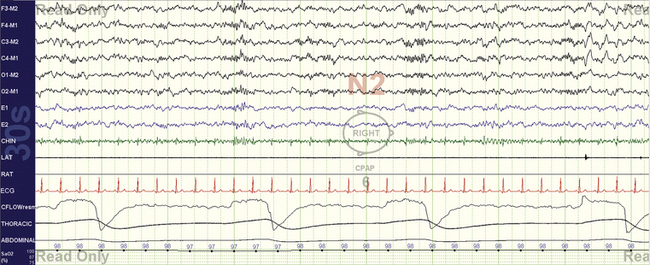
FIGURE 4.6 Bradypnea.
A 30-second epoch snapshot from a 22-year-old with mixed sleep apnea. Respiratory rate is about 8 breaths/min, with prolonged expiration phases that show some variability. The rate while awake was 14 breaths/min, and this pattern was seen during continuous positive airway pressure application. Unexplained sleep bradypnea (which will be designated central apneas if the rate slows down enough) or ataxic respiration in the absence of opiate use in a young individual could raise the possibility of a craniovertebral junction abnormality.
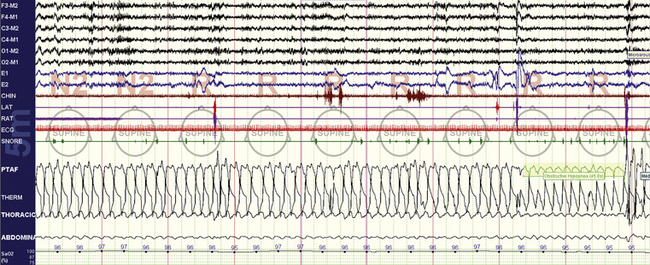
FIGURE 4.7 Increased upper airway resistance with consequences.
A 26-year-old woman, 10-minute screen compression. The stage is rapid eye movement sleep, with variable degrees of flow limitation (PTAF channel) and arousals characterized by transient chin electromyographic tone increases and recovery breaths. There is no oxygen desaturation.
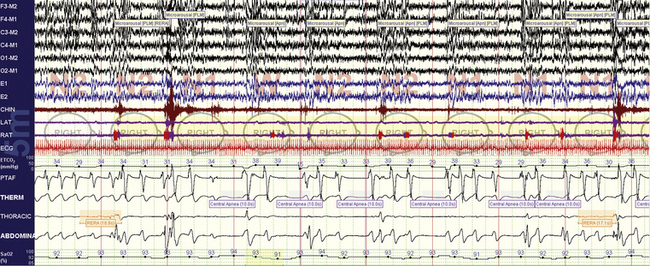
FIGURE 4.8 Ataxic respiration and opiates.
A 77-year-old woman with spinal stenosis and methadone use. Note the unpredictable variability of the expiratory phase.
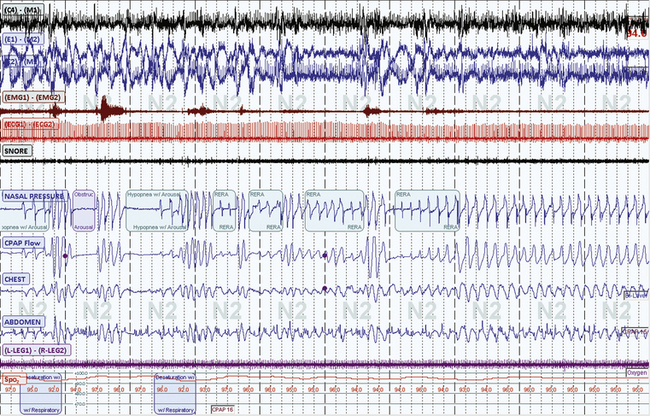
FIGURE 4.9 Dynamism in sleep-breathing disorders.
A 10-minute screen compression from a 66-year-old male patient with long-standing sleep apnea and a new diagnosis of myasthenia gravis. Repetitive obstructive respiratory events and respiratory instability occur and lead relatively abruptly to a switch to stable non–rapid eye movement (NREM) sleep. Such periods of stability are intrinsic to NREM sleep and in this case occur in stage N2.
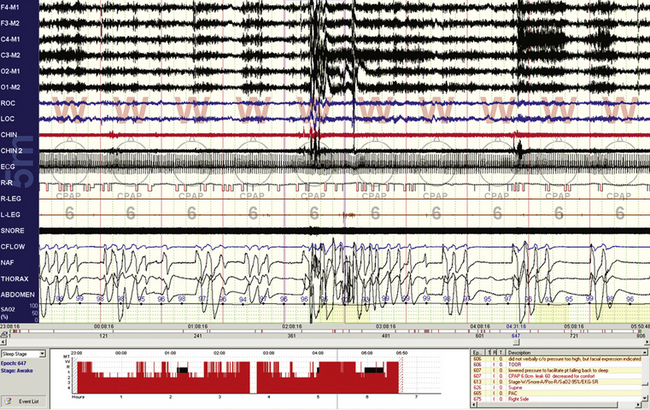
FIGURE 4.10 Respiratory events while awake.
A 10-minute compression from a 60-year-old man with stable congestive heart failure. Note that even though the epochs are scored “wake” by conventional criteria, there are repetitive central apneas on continuous positive airway pressure and fluctuating electroencephalographic (EEG) amplitudes and frequency (see EEG channels) associated with these events. The problem reflects limitations of the current epoch-based scoring system. These events may be preventing a proper transition to sleep.
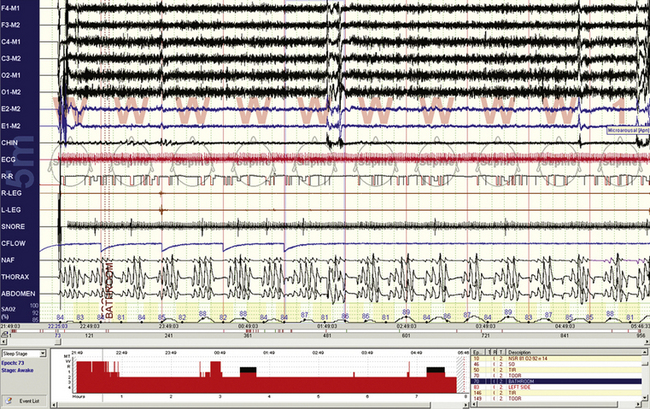
FIGURE 4.11 Respiratory events while awake.
A 10-minute compression from a 56-year-old man with chronic obstructive pulmonary disease. Note that even though the epochs are scored “wake” by conventional criteria, there are repetitive central apneas during this diagnostic assessment. The electroencephalogram less clearly shows state transitions than in Figure 4.10, there are no slow eye movements of drowsiness, but the oxygenation fluctuations and respiratory cycling suggest that these events are pathological and related to sleep state or at least the transition to it. Note that the oxygen saturation levels remain low during recovery phases, likely because of reduced lung functional reserve.
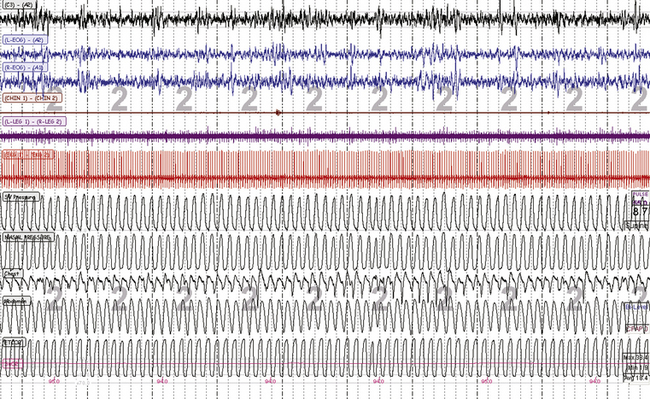
FIGURE 4.12 Stable non–rapid eye movement sleep during adaptive ventilation.
A 10-minute screen compression during titration of adaptive ventilation and dead space use in a 58-year-old male patient with congestive heart failure and Cheyne-Stokes respiration. Note overall stability of sleep and respiration. The respiratory signals from top are servo ventilation (SV) pressure (pressure output from the ResMed VPAP Adapt SV), nasal pressure (mask pressure), chest and abdominal effort, and mainstream end-tidal CO2 (maximum is 38.4 mm Hg).
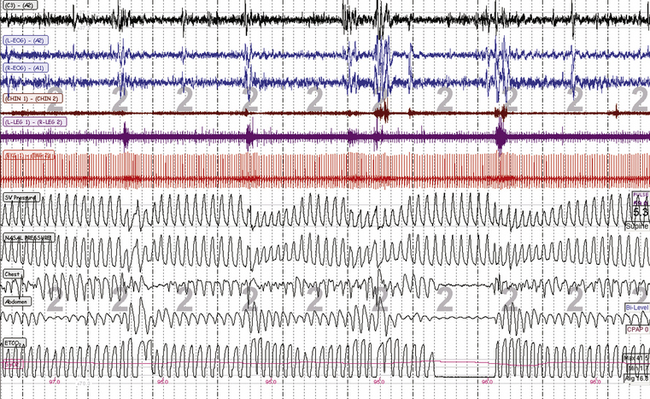
FIGURE 4.13 Unstable non–rapid eye movement sleep during adaptive ventilation.
A 10-minute screen compression during titration of adaptive ventilation and dead space in the same patient as in Figure 4.12. The respiratory signals from top are spontaneous ventilation (SV) pressure (pressure output from the ResMed VPAP Adapt SV), nasal pressure (mask pressure), chest and abdominal effort, and mainstream end-tidal CO2 (maximum is 38.4 mm Hg). Note the “pressure cycling” profile of the servo ventilation (SV) pressure that signifies adaptive responses to ongoing periodic breathing. Conventional scoring can be challenging during adaptive ventilation: effort signals reflect machine plus patient, and the flow signals can be continuously undulating—with a reduction in pressure during recovery breaths, exactly the opposite relative to continuous positive airway pressure titration. However, the ongoing arousals suggest that these events need to be scored as hypopneas with arousals.
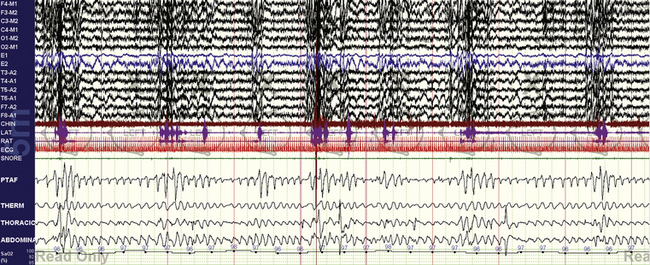
FIGURE 4.14 Sleep apnea and parasomnias.
A 25-year-old man presents with what could be nocturnal seizures or non–rapid eye movement (NREM) parasomnias. There is no snoring or daytime sleepiness. The diagnostic polysomnogram shows repetitive nonhypoxic respiratory events, no clear epileptiform activity, and vigorous arousals. Sleep apnea may be asymptomatic (no sleepiness) in those with NREM parasomnias, whereas treatment of sleep apnea can stop clinical expression of NREM parasomnias.
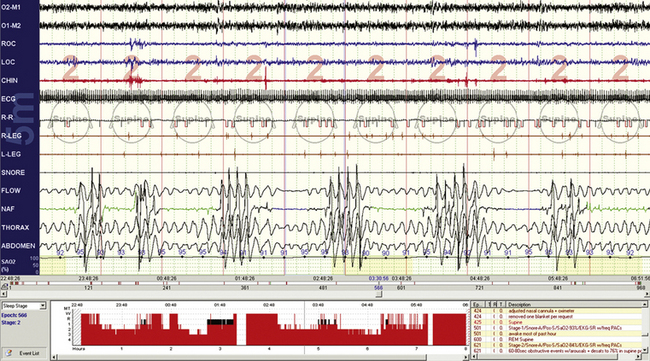
FIGURE 4.15 Obstructive sleep apnea—but where are the arousals?
This 10-minute compression shows typical obstructive sleep apnea—maintained effort, variable-duration respiratory events, recovery breaths, electromyographic tone fluctuations—but there is a paucity of electroencephalographic (EEG) arousals. This 26-year-old is using clonazepam for anxiety disorder; benzodiazepines can inhibit the full expression of EEG arousals. In this instance the 30-second compression (not shown) did show brief alpha intrusions, but these did not reach the threshold of conventional arousal scoring.
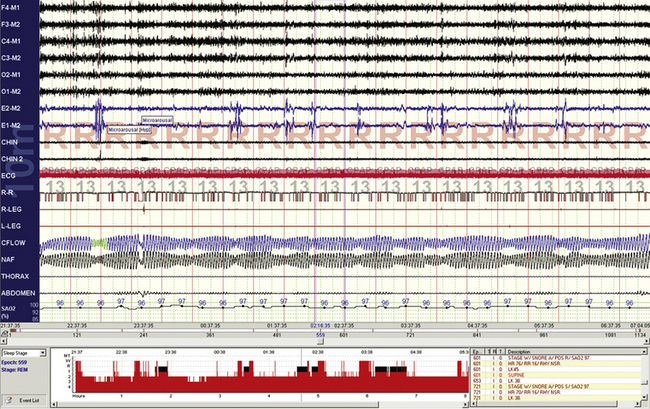
FIGURE 4.16 Pseudoperiodic breathing during rapid eye movement (REM) sleep.
Periodic breathing (at sea level or high altitude) is rare or absent during REM sleep. Phasic REM events can inhibit respiration, and well-timed phasic REM phenomena can cause sufficient respiratory inhibition to cause this oscillatory pattern. In this sample from a continuous positive airway pressure titration study, the “abnormality” seems to be associated with no significant pathophysiological consequences such as arousals or oxygen desaturations.
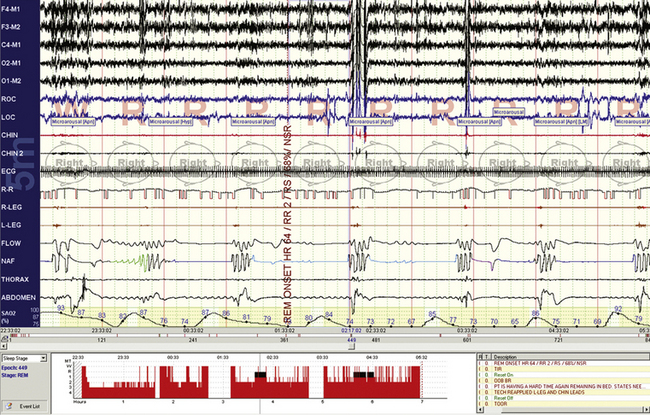
FIGURE 4.17 Rapid eye movement–dominant obstructive sleep apnea.
A 10-minute screen compression from a 33-year-old obese woman. Repetitive respiratory events and severe oxygen desaturation are noted. In this case, contrary to the patient in Figure 4.15, the electroencephalographic responses are vigorous but chin tone elevations are minimal to absent.
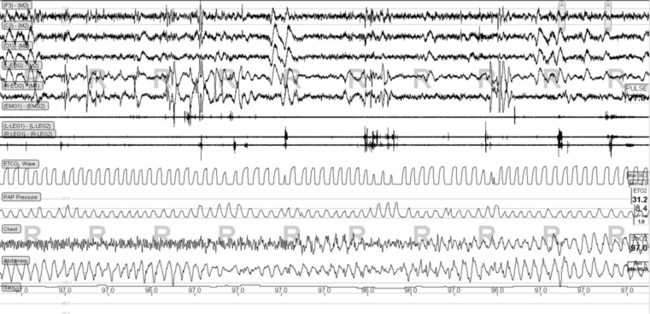
FIGURE 4.18 Rapid eye movement (REM)-related pressure cycling during adaptive ventilation.
Phasic REM sleep-related tidal volume fluctuations can trigger the adaptive algorithms in the ResMed VPAP Adapt SV and “cause” periodic breathing during REM sleep. In this snapshot note that the PAP Pressure channel, which is the ventilator pressure output in this study, shows increases shortly after a cluster of phasic eye movements. No intervention is required. Some increase in phasic motor activity is also seen in the tibialis electromyographic channels (R and L Legs) time synchronized with phasic REM bursts.
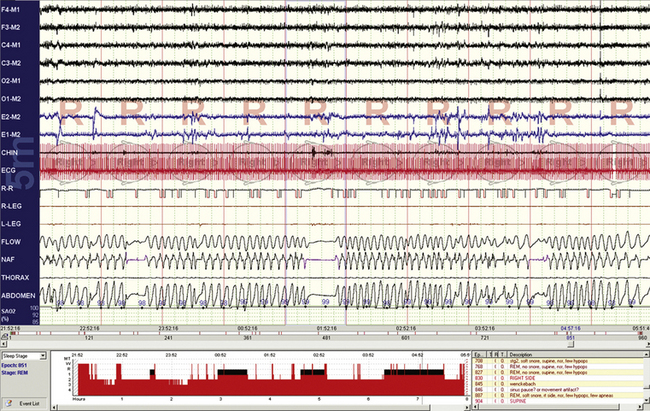
FIGURE 4.19 Central apneas during phasic rapid eye movement sleep.
A normal phenomenon, along with other features of respiratory variability and instability.
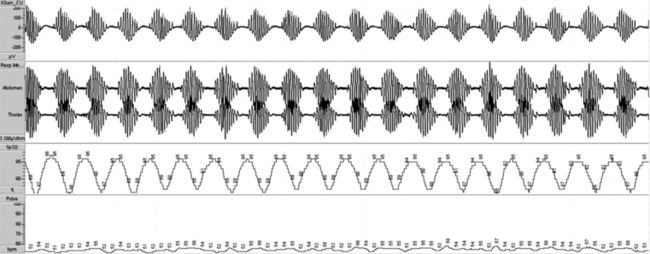
FIGURE 4.20 Ambulatory recording of periodic breathing.
This metronomical pattern can be characteristic enough on an ambulatory monitoring study that the patient can be triaged to sleep laboratory management and not initiated on auto–continuous positive airway pressure.
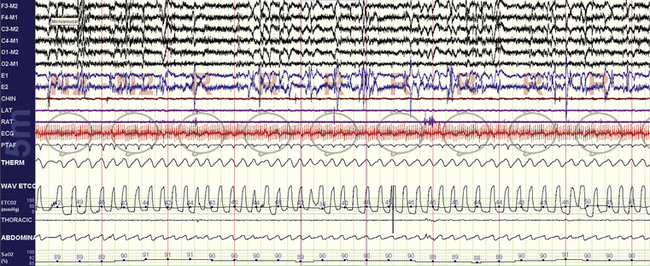
FIGURE 4.21 Rapid eye movement (REM) sleep effects on opiate-induced sleep apnea.
Opiate-induced sleep apnea has several features that in aggregate form a relatively unique polysomnographic picture: ataxic respiration and central apneas during non-REM sleep, mild hypercapnia, and relative respiratory stability during REM sleep. This 10-minute compression from the same patient as in Figure 4.8 shows stable breathing during REM sleep and end-tidal CO2 levels that go as high as 50 mm Hg.
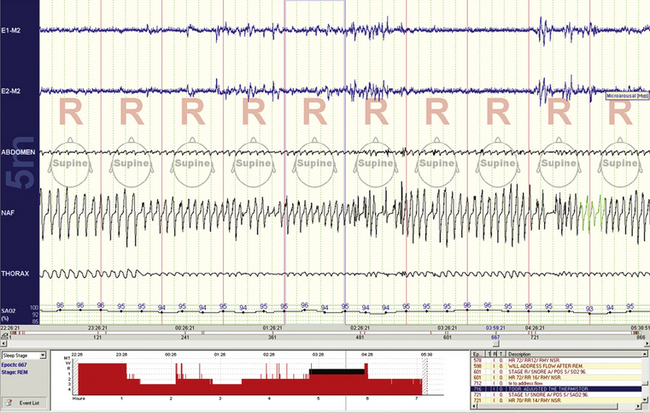
FIGURE 4.22 Normal respiratory variability during rapid eye movement sleep.
This 10-minute compression shows prominent but normal tidal volume and respiratory rate variability. There are no consequences to the reductions in airflow (i.e., no arousals or oxygen desaturations), and these events therefore cannot be scored.
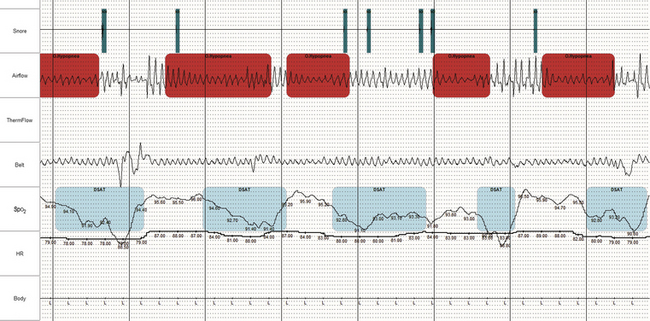
FIGURE 4.23 Obstructive sleep apnea during ambulatory recording.
Information on airflow, effort, and oximetry are typically considered sufficient to confirm sleep apnea in subjects with high clinical probability. In selected instances, oximetry and airflow alone may be sufficient (even if not reimbursable). When the signal quality is acceptable and the abnormality is obvious, as in this figure, the diagnosis is straightforward.
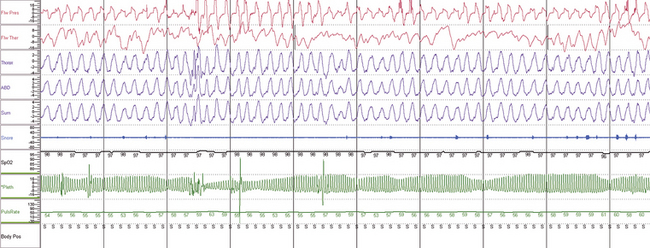
FIGURE 4.24 Obstructive sleep apnea during ambulatory recording.
A 10-minute compression showing, from top, nasal pressure, thermistor, thoracic and abdominal effort, sum of effort, snoring, pulse oximetry, finger pulse plethysmogram, and derived pulse rate. Does this patient have sleep apnea? By many sets of criteria, no (absent oxygen desaturation). However, the flow limitation and recovery breaths associated with plethysmographic amplitude reductions are suggestive of respiratory effort–related arousals. Note that the heart rate change with presumptive arousal is small in this patient.
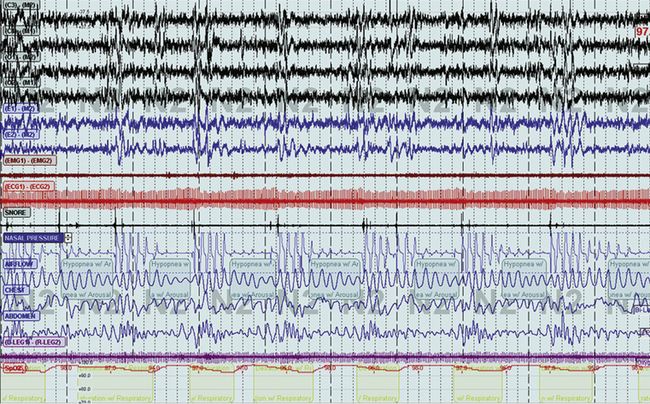
FIGURE 4.25 Non–rapid eye movement (NREM)-dominant obstructive sleep apnea during NREM sleep.
A 10-minute compression from a 37-year-old man with otherwise typical symptoms of sleep apnea. Repetitive obstructive hypopneas with arousal and relatively minor oxygen desaturation. The events are not particularly short cycle (not less than 30 seconds) and are of variable length, with well-maintained effort. The body position is supine.
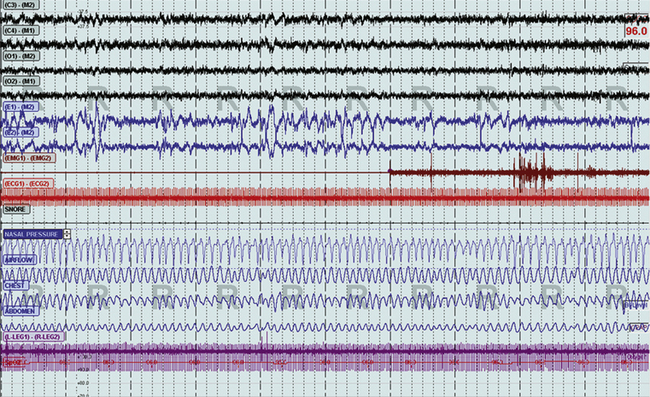
FIGURE 4.26 Non–rapid eye movement (NREM)-dominant obstructive sleep apnea changes during rapid eye movement (REM) sleep.
The same patient as in Figure 4.25, still supine, now during REM sleep. Note the complete absence of abnormal respiration.
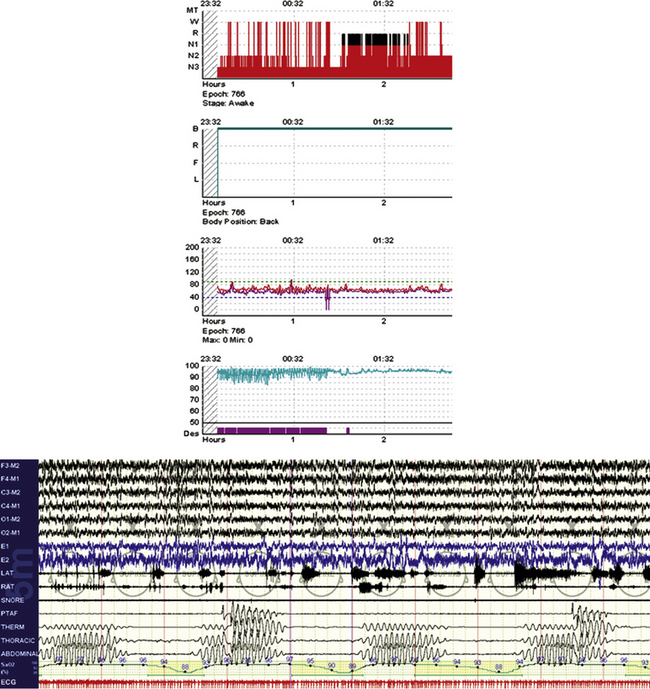
FIGURE 4.27 “Banding” of oximetry and nonobstructive sleep apnea.
An 84-year-old woman with atrial fibrillation and congestive heart failure. The integrated snapshot shows part of the hypnogram, with oxygen desaturations that are nearly identical and the associated periodic breathing/Cheyne-Stokes pattern.
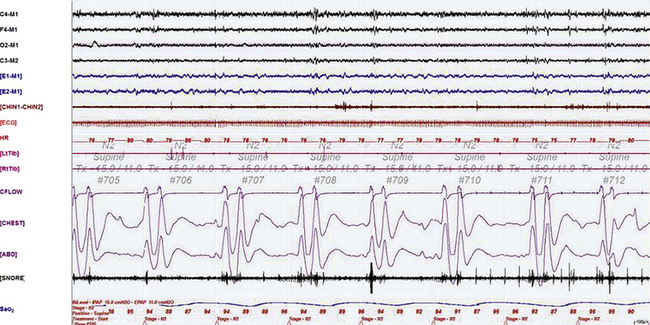
FIGURE 4.28 Ataxic breathing caused by narcotics.
A 240-second excerpt taken from the bilevel titration study in a 53-year-old woman on large doses of oxycodone (OxyContin, 260 mg daily) for chronic severe lower back pain associated with lumbosacral disk disease. This epoch shows several episodes of Biot’s breathing (a type of ataxic breathing characterized by recurrent central apneas followed by two to three breaths) accompanied by oxygen desaturation in stage N2. This is a characteristic finding that may be noted in patients on chronic narcotics ingestion, as well as in those with brainstem lesions destabilizing the central respiratory controllers. Top four channels: electroencephalograms (international nomenclature); E1-M1 and E2-M1, right and left electro-oculograms; CHIN1-CHIN2, mentalis muscle electromyogram (EMG); ECG, electrocardiogram; HR, heart rate; Tib, tibialis anterior muscle EMGs; Lt, left; Rt, right; CFLOW and effort, channels 12 to 14; Sao2, arterial oxygen saturation by finger oximetry. Also included is a snore channel.
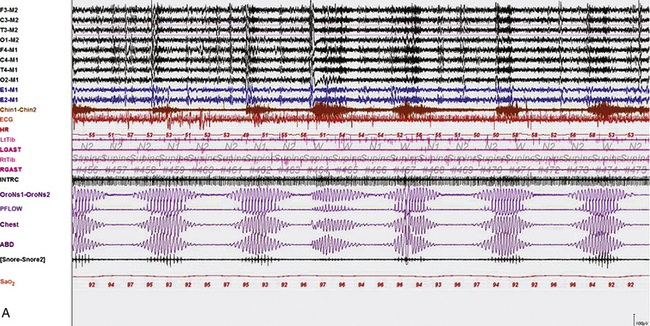
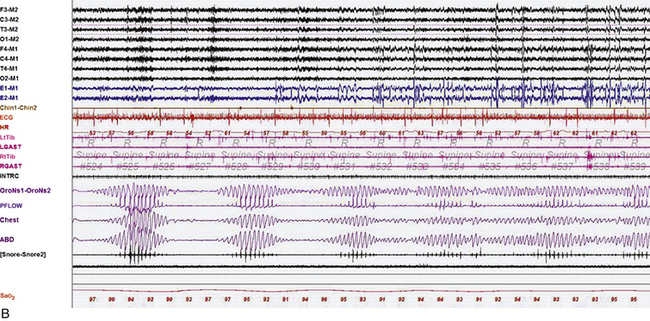
FIGURE 4.29 The effect of stage on Cheyne-Stokes respiration.
Epochs of 600 seconds from the overnight polysomnogram (PSG) of an 82-year-old man with a history of atrial fibrillation and stroke. His PSG showed evidence of central sleep apnea with Cheyne-Stokes respirations (apnea-hypopnea index, 40.1/hr, and arterial oxygen saturation [SaO2], nadir of 89%), characterized by crescendo-decrescendo pattern of hyperventilation and central apneas, with longer hyperpneic than apneic phase and prolonged circulation time (measured from the termination of apnea to the lowest SaO2 toward the end of the hyperpneic phase). A, An epoch of stage N2 sleep. Cheyne-Stokes respiration with the characteristics described earlier persisted throughout non–rapid eye movement sleep. B, Note improvement of Cheyne-Stokes respiration as the patient transitions to rapid eye movement sleep. Top eight channels: electroencephalograms (international nomenclature); E1-M1 and E2-M1, right and left electro-oculograms; Chin1-Chin2, mentalis muscle electromyogram (EMG); ECG, electrocardiogram; HR, heart rate; LtTib, RtTib, left and right tibialis anterior EMG; LGAST, RGAST, left and right gastrocnemius EMG; INTRC, intercostal EMG (right 8th space); airflow and effort, channels 19 to 22; Sao2, arterial oxygen saturation by finger oximetry.
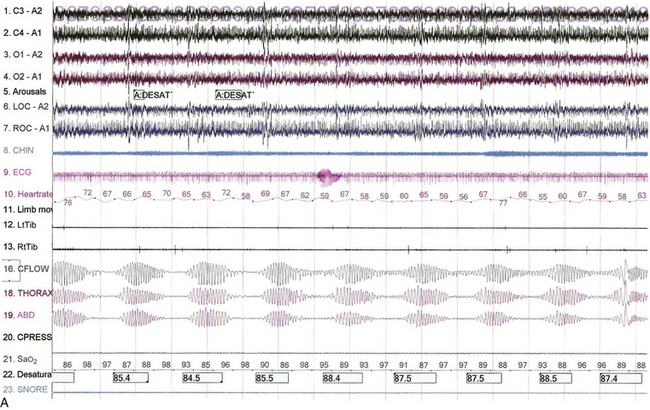
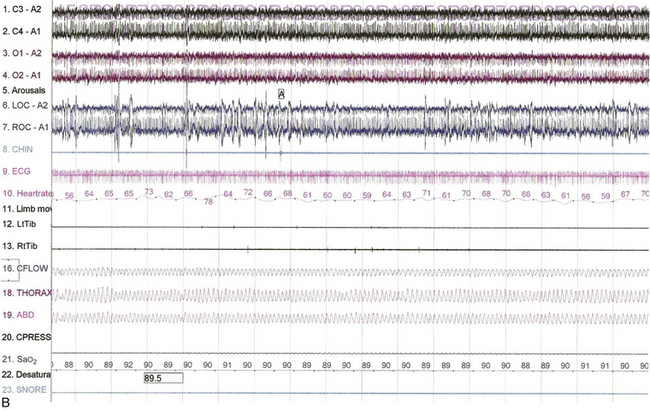
FIGURE 4.30 The effect of position on Cheyne-Stokes respiration.
Epochs of 480 seconds from the bilevel titration of a 68-year-old man with a history of myocardial infarction and congestive heart failure. His spouse reported irregular breathing and witnessed apneas in sleep, and he had excessive daytime sleepiness. He also had hypertension and asthma. A previously performed polysomnogram showed severe central sleep apnea (apnea-hypopnea index, 48/hr) with a mean arterial oxygen saturation (SaO2) of 84% and an O2 saturation nadir of 74%. A, Note the presence of Cheyne-Stokes respirations in stage N2 sleep with the patient in the supine position The patient is on a pressure of 12/9 cm. B, Improvement of Cheyne-Stokes respirations when the patient assumes the lateral position. He remains in stage N2 sleep at the same bilevel pressure. Top four channels, electroencephalograms (international nomenclature); LOC-A2, ROC-A1, elecro-oculogram; CHIN, chin electromyogram (EMG); LtTib, RtTib, right and left lower limb EMGs; CFLOW, CPAP airflow channel; THORAX, ABD, thoracic and abdominal respiratory belts; Sao2, arterial oxygen saturation by finger pulse oximetry. Also included are channels for ECG, heart rate, and snoring.
In the 1970s to early 1980s only apneas were scored. Later, varying percent reductions in signal were included in scoring. The fundamental problem with this was the use of a nonquantitative signal (thermistor) that does not correlate with true flow. The American Academy of Sleep Medicine (AASM) research criteria (Chicago criteria) (1999) included any clear reduction in flow or effort signal associated with an oxygen desaturation or an arousal. The AASM clinical criteria (2001) included a 30% reduction in airflow or thoracoabdominal movement associated with a 4% oxygen desaturation. The 2007 AASM scoring rules offer more than one option, from hypopneas associated with a 4% desaturation to respiratory effort–related arousals; these guidelines are due for an update. There are currently no scoring rules for ambulatory studies, in which arousal equivalents may be derived from non-EEG signals. The practitioner should appreciate the richness of physiological and pathological human variability and incorporate the best evidence into clinical practice. Automated computer-based methods will provide complementary, perhaps non–epoch-based, methods of quantifying disease.
Current Considerations in the Scoring of Respiratory Events
1. Degree of oxygen desaturation required to score a hypopnea. The smallest saturation change that can be visually detected is 2% to 3%. Although a 3% to 4% change may be linked epidemiologically to adverse outcomes, in clinical practice nondesaturating hypopneas have clinical consequences. Integrating arousals into the core of hypopnea-scoring rules seems essential.
2. Sleep state modulates respiration. Airflow patterns and arousal thresholds are modulated by sleep macrostructure (rapid eye movement [REM], non-REM [NREM] sleep, stages of NREM sleep), sleep microstructure (cyclic alternating pattern [CAP] and non-CAP), sleep deprivation, medication, and age. There are periods of stability intrinsic to NREM sleep that determine the instantaneous presence or absence of sleep apnea.
3. Using nonhypoxic associations to score respiratory events. The recovery breath or breath sequence (more than one large breath) draws attention to a possible event termination. To increase confidence that this event or sequence reflects a transient perturbation of sleep state, associated cues are useful. Other than the arousal, desaturation, and autonomic (heart rate increases, pulse plethysmographic amplitude reductions) associations, the following may also be useful: limb movement, brief bursts of slow or rapid eye movements, reversal of paradox on effort channels, and burst of bruxism activity.
4. The spectrum of arousal EEG. Application of the AASM 3-second arousal rule applies constraints that are not biologically based. In the specific context of respiratory event termination, shorter degrees of alpha/beta intrusion (less than 3 seconds) or K-complex and delta bursts may be considered arousal equivalents. However, current AASM guidelines require 3-second arousals for scoring and tabulating arousals. Thus arousal scoring implies classic AASM arousals, whereas arousal detection could imply using a broader spectrum of EEG transients to bias differentiation of significant versus nonsignificant respiratory events.
5. Apneas are usually scored. Obstructive apneas should always be scored. Nonobstructive apneas (especially during REM sleep and not leading to an arousal or associated with oxygen desaturation), isolated postarousal apneas, and apneas during sleep-wake transition periods should not be scored if desaturations and arousals are not consequences or associations. Prolonged sleep-wake transition respiratory events could be scored if it appears that sleep onset is being interfered with. Short (less than 10 seconds) apneas with consequences should reasonably be scored—there is no hard biological basis for a 10-second rule.
6. Scoring central hypopneas is a challenge with no ready solution. Flow limitation itself does not exclude a centrally mediated hypopnea, which is an event dominantly driven by respiratory chemoreflex mechanisms. The availability of treatments for central forms of sleep apnea (adaptive ventilation, CO2-based treatments) increases the importance of scoring central hypopneas. The following clues may be useful to scoring events that are less than the 10-minute minimum often recommended to tag as a periodic breathing sequence: NREM sleep only, continuous six cycles or 3 minutes of symmetrical and concordant waxing/waning of effort and flow, bandlike oxygen desaturation pattern, short cycle time (less than 30 seconds), and nearly identical timing of consecutive events.
The 2012 update of the AASM scoring guidelines (available online at www.aasmnet.org) recommends scoring a hypopnea with a 3% desaturation or arousal association. Events with lesser degrees of signal amplitude reduction, typically associated with flow limitation that terminates with an EEG arousal and abrupt rounded recovery breath are “respiratory effort–related arousals” (RERAs). However, RERA scoring has been left optional, which is a debatable decision.
The update also proposes guidelines for scoring central hypopneas and Cheyne-Stokes respiration/periodic breathing: “If electing to score central hypopneas, score a hypopnea as central if none of the following criteria are met: 1. Snoring during the event. 2. Increased inspiratory flattening of the nasal pressure or positive airway pressure (PAP) device flow signal compared to baseline breathing. 3. Associated thoracoabdominal paradox occurs during the event but not during pre-event breathing.” As noted earlier, events that are clearly associated with respiratory chemoreflex activation (e.g., heart failure periodic breathing, high altitude) may not fit these criteria exactly.
The 2012 guidelines recommend to “score a respiratory event as Cheyne-Stokes breathing if both of the following are met: 1. There are episodes of at least 3 consecutive central apneas and/or central hypopneas separated by a crescendo and decrescendo change in breathing amplitude with a cycle length of at least 40 seconds (typically 45 to 90 seconds). 2. There are 5 or more central apneas and/or central hypopneas per hour associated with the crescendo/decrescendo breathing pattern recorded over a minimum of 2 hours of monitoring.” As noted earlier, short-cycle events occur in those without heart failure and often are similar to high-altitude periodic breathing. The 10-minute minimum seems to have been dropped.
7. Hypoventilation. This is a state characterized by a sustained reduction in alveolar ventilation and hypoxemia not associated with phasic breathing events such as apneas and hypopneas. According to the 2012 AASM manual, “if electing to score hypoventilation, score hypoventilation during sleep if either of the below occur: 1. There is an increase in the arterial PaCO2 (or surrogate) to a value >55 mm Hg for ≥10 minutes. 2. There is ≥10 mm Hg increase in PaCO2 (or surrogate) during sleep (in comparison to an awake supine value) to a value exceeding 50 mm Hg for ≥10 minutes. Surrogates include end-tidal PaCO2 or transcutaneous PaCO2 for diagnostic study or transcutaneous PaCO2 for PAP titration study.”
8. Scoring hypopneas on ambulatory cardiopulmonary studies. The lack of EEG limits the scoring of conventional arousals, but using all the surrogate information such as flow reversal/recovery breaths, heart rate increases, and pulse plethysmographic amplitude changes can provide arousal equivalents.
9. Adaptive ventilation and respiratory signals. The pressure output of these ventilators is equal and opposite to detected periodic breathing/central apneas. Thus as long as there is a “cycling of pressure,” it signifies ongoing central respiratory abnormality. Oxygen desaturation may not occur during such periods. If arousals occur, these pressure cycling events should reasonably be recognized as hypopneas.
Arzt, M., Wensel, R., Montalvan, S., et al. Effects of dynamic bilevel positive airway pressure support on central sleep apnea in men with heart failure. Chest. 2008; 134:61–66.
Berry, R. C., Budhiraja, R., Gottlieb, D. J., et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012; 8:597–619.
Carnevale, C., Georges, M., Rabec, C., Tamisier, R., Levy, P., Pépin, J. L. Effectiveness of adaptive servo ventilation in the treatment of hypocapnic central sleep apnea of various etiologies. Sleep Med. 2011; 12:952–958.
Loewen, A., Ostrowski, M., Laprairie, J., et al. Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired? Sleep. 2009; 32:1355–1365.
Younes, M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol. 2008; 105:1389–1405.