Basic Circadian Rhythms and Circadian Sleep Disorders
The human sleep-wake cycle is regulated by the circadian system. Although circadian rhythms are endogenously generated, they are influenced by environmental cues such as exposure to light, dark, and social and physical activity, which synchronize the sleep-wake cycle to a stable 24-hour period. Circadian rhythm disorders result when there is a dysfunction of the central clock or when there is a misalignment between the endogenous rhythm and the environment, which can lead to disruption of sleep, excessive sleepiness, and impaired performance during scheduled wake times.
Circadian Rhythm Biology
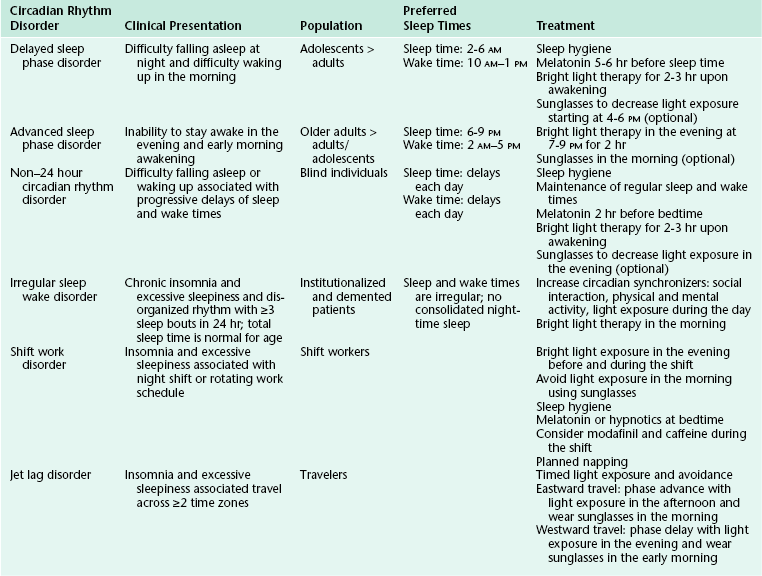
Circadian rhythms are seen in all living creatures. The underlying mechanisms regulate nearly all physiological functions in the body, including the sleep-wake cycle. The two-process model postulates that sleep and wake are regulated by a homeostatic drive (process S) and a circadian system (process C) (Fig. 5.1).1 The homeostatic drive for sleep increases during wakefulness and dissipates during sleep, whereas the circadian process increases its alerting signal during the day to promote alertness and diminishes during the night to help promote sleep. The master clock of the circadian system is the suprachiasmatic nucleus (SCN), which is located in the anterior hypothalamus. The SCN regulates the circadian timing of most physiological and behavioral functions, including sleep and wake, core body temperature, hormones, and mood.2 The human circadian oscillation or period is approximately 24.2 hours and is regulated at the molecular level. The genetic basis of the circadian system has recently been elucidated. The involved genes are highly conserved and use an autoregulatory feedback loop with translocation of protein products from the nucleus to the cytoplasm and back to the nucleus. The rate of transcription determines the period of the circadian rhythm.3
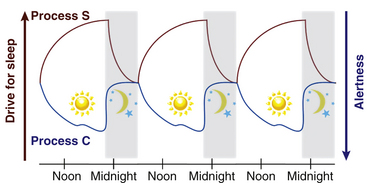
FIGURE 5.1 The two-process model of the sleep-wake cycle. Process S (red line) represents the homeostatic need for sleep that increases during wake and dissipates during sleep. Process C (circadian drive; blue line) increases alerting signals during the day to promote wake and decreases shortly before sleep onset.
Light is the strongest zeitgeber (German for time giver) that regulates circadian timing. The SCN receives input from light via specialized retinal photoreceptors containing melanopsin. Through this pathway, light input to the SCN inhibits pineal secretion of melatonin (Fig. 5.2); melatonin, once secreted, can also act as a zeitgeber and alter the phase of circadian rhythms.
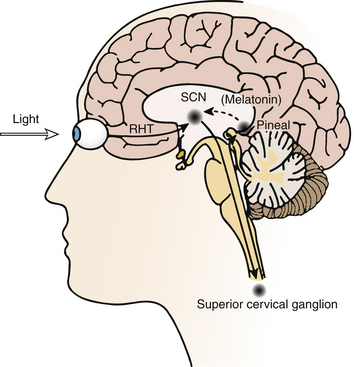
FIGURE 5.2 Schematic diagram of the neural pathways for light input to the circadian system. Light information from the melanopsin-containing retinal ganglion cells travels to the suprachiasmatic nucleus (SCN) via the retinohypothalamic tract (RHT). The SCN regulates the timing of nocturnal melatonin secretion from the pineal gland via the superior cervical ganglion. Exposure to bright light during the biological night suppresses melatonin production. (Modified with permission from Reid KJ, Zee PC. Circadian rhythm disorders. Semin Neurol. 2009;29[4]:393-405.)
Circadian timing can be modulated by controlling exposure to these zeitgebers, which can advance or delay the timing of circadian rhythms as predicted by the phase response curve (PRC) (Fig. 5.3).4,5 The direction of phase shift is dependent on the timing of zeitgeber exposure in relation to circadian phase. Biomarkers of circadian phase include the onset of melatonin secretion under dim light conditions (dim-light melatonin onset; DLMO) and core body temperature (CBT). Melatonin secretion begins about 2 hours before habitual bedtime, as the CBT begins to fall. Plasma melatonin levels peak during the CBT nadir and then fall as the CBT rises.6 For example, light exposure before the CBT nadir delays the timing of circadian rhythms, whereas exposure after the CBT nadir results in an advance. In contrast, exposure to melatonin before the CBT nadir advances and after the CBT nadir delays circadian rhythms.
FIGURE 5.3 Schematic representation of the phase response curves (PRCs) to light (solid line) and melatonin (dotted line). The upper x-axis shows clock time, and the lower x-axis shows the time relative to dim-light melatonin onset (DLMO), a circadian phase marker. The y-axis represents the degree of phase advance or delay. Typical clock times are shown for the DLMO (10 PM), core body temperature (CBT) nadir (5 AM), and sleep times (12 AM to 8 AM). DLMO typically occurs 2 hours before biological sleep-onset time. The timings of these markers for individuals with delayed sleep phase disorder or advanced sleep phase disorder should be adjusted accordingly. The light PRC shows that light exposure between the DLMO and CBT nadir causes phase delay; after the CBT nadir, light exposure results in phase advance. The magnitude of phase advance or delay is greatest close to the CBT nadir. The melatonin PRC (generated using melatonin 0.5 mg) shows that melatonin exposure results in phase advance before 2 AM and phase delay after 2 AM. The greatest phase delay occurs 3 hours before DLMO, or 5 hours before sleep onset. Rectangle, Sleep period; triangle, CBT nadir; upward arrow, DLMO. (Modified with permission from Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6[5]:407-420.)
Circadian Rhythm Sleep Disorders
Circadian rhythm sleep disorders result from dysfunction of the circadian timing system or from misalignment between biological circadian phase and behavioral sleep and wake rhythms. Disruption of circadian timing results in insomnia, excessive daytime sleepiness, and impaired performance. Symptoms may cause disruption in social, occupational, and other areas of function. A summary of these disorders is shown in Figure 5.4 and Table 5.1.
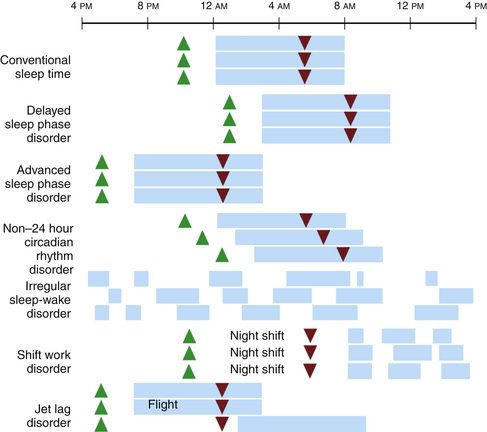
FIGURE 5.4 A schematic representation of circadian rhythm sleep disorders. Blue rectangles denote sleep time. Green triangles denote the dim-light melatonin onset, which occurs 2 hours before biological sleep-onset time. Red triangles denote the core body temperature nadir. Individuals with delayed sleep phase disorder have stable circadian rhythms that are delayed relative to conventional times, resulting in difficulty falling asleep and difficulty waking up in the morning. Those with advanced sleep phase disorder have stable endogenous rhythms that are early relative to conventional times, resulting in early evening sleepiness and early morning awakening. In non–24 hour circadian rhythm disorder, the rest/activity gradually delays each day, leading to difficulty falling asleep and daytime sleepiness, depending on when the circadian time for sleep falls on a particular day. Irregular sleep-wake rhythm disorder is characterized by a lack of consolidated sleep and at least three bouts of sleep each day; 24-hour total sleep time is normal for age. In shift work disorder, people working during the night shift work during the circadian time for sleep, which results in sleepiness and impaired performance. They sleep when circadian alerting signals are high, leading to disrupted and shorter sleep. Travelers flying two or more time zones may experience jet lag disorder. In the example given, westward travel results in the traveler’s circadian phase being advanced relative to the conventional sleep time in the destination, resulting in afternoon and evening sleepiness and early morning awakening.
Delayed Sleep Phase Disorder
Delayed sleep phase disorder (DSPD) is one of the most common circadian rhythm sleep disorders in which the circadian timing is delayed relative to the required sleep and wake times. This disorder is more common in adolescents and young adults. Symptoms include difficulty falling asleep at the desired bedtime and difficulty waking up at the desired wake time. When the person is allowed to sleep at the biologically correct time, sleep duration and architecture are typically normal. Sleep logs and actigraphy for 7 days and DLMO measurement can be helpful for distinguishing DSPD from other causes of insomnia or excessive daytime sleepiness (Fig. 5.5).7,8
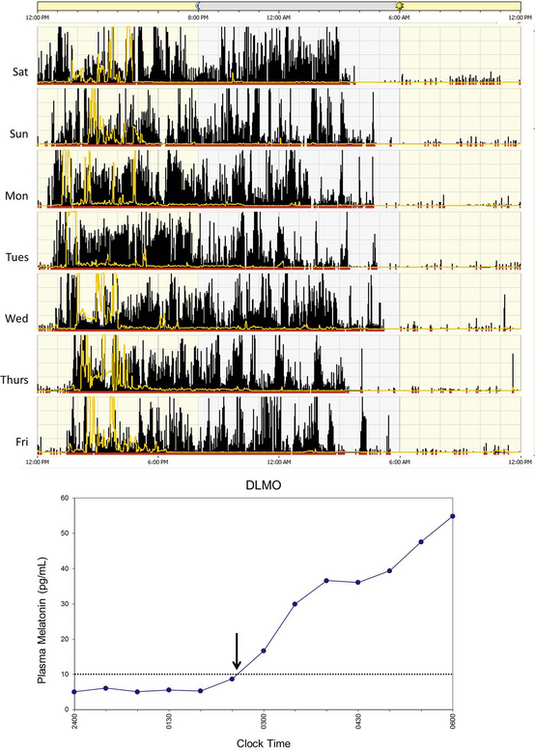
FIGURE 5.5 Above, An example of rest-activity cycle from a patient with delayed sleep phase disorder using wrist actigraphy monitoring. The black bars indicate activity level, and yellow lines indicate ambient light exposure at the nondominant wrist. This individual had a typical bedtime of 3 to 5 AM and wake time of 12 to 2 PM. Below, An example of plasma dim-light melatonin onset (DLMO) of the same individual. In plasma, DLMO is defined as the time at which the melatonin level reaches 10 pg/mL. The DLMO typically occurs about 2 hours before sleep onset. In this individual the DLMO was at about 2:30 AM (arrow) and sleep time was at 4:30 AM.
Treatment of DSPD involves advancing the endogenous circadian rhythm to the desired sleep and wake schedule. Bright light therapy shortly after the nadir of CBT (just before or at the biological wake time) will advance circadian phase and is a recommended treatment for DSPD. Exogenous melatonin administration (0.3 to 5 mg) 5 to 6 hours before DLMO or 6 to 8 hours before sleep onset time has the most consistent effect at advancing the timing of sleep and is a recommended treatment option, either alone or in conjunction with bright light.8,9
Advanced Sleep Phase Disorder
Advanced sleep phase disorder (ASPD) is a disorder of circadian timing being early relative to the desired and conventional bedtime. This disorder is more common among older adults. Symptoms include inability to stay awake until the desired bedtime and difficulty maintaining sleep until the desired wake time. When the person is allowed to sleep according to their endogenous sleep/wake rhythm propensity, sleep architecture and consolidation are normal, and the circadian rhythm is stably entrained to a 24-hour cycle. As with DSPD, sleep logs and actigraphy for 7 days and DLMO are helpful for establishing the diagnosis (Fig. 5.6).7,8
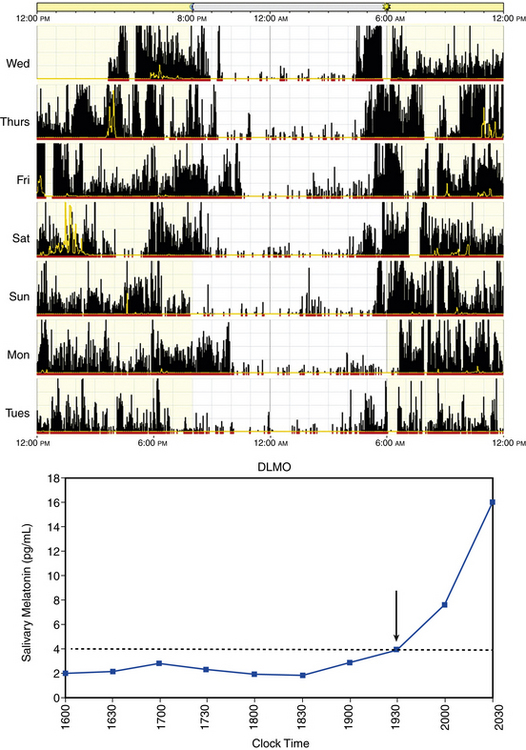
FIGURE 5.6 Above, An example of rest-activity cycle from a patient with advanced sleep phase disorder using wrist actigraphy monitoring. The black bars indicate activity level, and yellow lines indicate ambient light exposure at the nondominant wrist. This individual had a typical bedtime of 9 PM and wake time of 4 AM. Below, An example of salivary dim-light melatonin onset (DLMO). In saliva the DLMO time is determined when the melatonin level reaches 3-4 pg/mL, which corresponds to a plasma melatonin level of 10 pg/mL. In this case the DLMO is at approximately 7 PM (arrow), which corresponds to a circadian sleep onset of 9 PM.
Treatment of ASPD is based on delaying circadian timing to the desired sleep and wake schedule. Bright light exposure for approximately 2 hours in the evening (typically 7 to 9 PM) has been shown to be effective.8,10 Although the use of melatonin after the CBT nadir to delay circadian rhythm has been proposed, the clinical evidence is limited.8
Non–24 Hour Circadian Rhythm Disorder
Non–24 hour circadian rhythms are most commonly seen in blind individuals. This disorder is rare in sighted people, but it can be seen in individuals who are either isolated from or unable to entrain to normal time cues.11 Symptoms include difficulty falling asleep or waking up, progressive delays of sleep and wake times, and inability to maintain stable entrainment to a 24-hour rhythm for at least 6 weeks.7 Patients with this disorder have severely disrupted social function such as school absences, leaving school, and inability to maintain employment.11 Diagnosis can be established using sleep logs and actigraphy for at least 7 days, which show progressive delays of sleep onset and offset (Fig. 5.7).7 Measurement of circadian phase markers (melatonin rhythm and CBT) multiple times over several weeks may also be helpful in the diagnosis.8
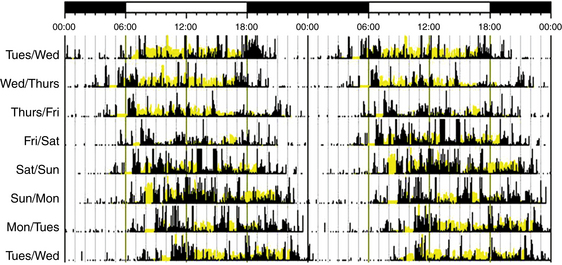
FIGURE 5.7 An example of rest-activity cycle from a patient with non–24 hour circadian rhythm disorder using wrist actigraphy monitoring. The black bars indicate activity level, and yellow lines indicate ambient light exposure at the nondominant wrist. There are progressive delays of sleep onset and offset by about 1 hour each day.
Treatment of non–24 hour circadian rhythm disorder involves establishing time cues to entrain the circadian rhythm. Treatment usually begins when the person’s sleep-wake cycle is at the desired time. In blind individuals, timed melatonin is recommended for entraining the endogenous rhythm. In sighted individuals, low dose melatonin at bedtime and bright light therapy in the morning are effective for entraining the endogenous rhythm. In addition, regular sleep and wake times should be maintained.8,12
Irregular Sleep-Wake Rhythm Disorder
Irregular sleep-wake rhythm disorder is characterized by the lack of a clearly defined major sleep period and multiple naps during the normal wake period. Patients complain of chronic insomnia, excessive sleepiness, or both. Actigraphy and sleep logs for at least 7 days show multiple (at least three) irregular sleep bouts over a 24-hour period (Fig. 5.8); the total sleep time over 24 hours is normal for age.7,8 Although the prevalence of this disorder is not known, irregular sleep-wake rhythm disorder is more commonly seen in institutionalized older adults, particularly in those with dementia. The irregularity may be caused by neurodegeneration in the SCN and a lack of time cues such as light, social stimulation, and physical activity.
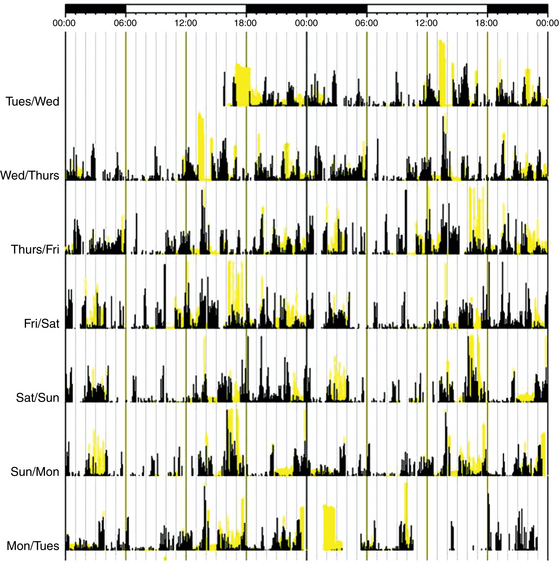
FIGURE 5.8 Rest-activity recorded with wrist actigraphy monitoring of an older adult with irregular sleep-wake disorder. The black bars indicate activity levels, and the yellow bars indicate the level of ambient light exposure recorded at the nondominant wrist. There is a lack of discernible circadian sleep-wake rhythm. Sleep is characterized by nocturnal fragmentation and multiple short periods of sleep and wake across the entire 24-hour day. (Modified with permission from Zee PC, Vitiello MV. Circadian rhythm sleep disorder: irregular sleep wake rhythm type. Sleep Med Clin. 2009; 4[2]:213-218.)
Therapy is aimed at increasing the amplitude of circadian rhythms to promote nocturnal sleep consolidation and daytime wakefulness. A multimodal approach, including establishing a regular physical activity and social schedule, avoidance of frequent napping during the day, ensuring a sleep-promoting environment (particularly in institutionalized persons), and increased daily bright light exposure is recommended.8,13
Shift Work Disorder
People involved in shift work (nonstandard work schedules) are at increased risk for shift work disorder (SWD). Approximately 10% of night and rotating shift workers meet criteria for SWD. Shift workers, particularly night and rotating workers, are working during their circadian time for sleep and trying to sleep when the circadian alerting signal is high. In patients with SWD, chronic misalignment of circadian rhythm with the required work and sleep schedules results in insomnia and excessive sleepiness. Thus shift workers are at increased risk for chronic medical comorbidities and accidents, including motor vehicle accidents.14 Diagnosis of SWD can be established using sleep logs and actigraphy monitoring for at least 7 days showing circadian and sleep-time misalignment.7,8
Treatment is aimed at realigning circadian rhythms to the appropriate work schedule, improving sleep quality, and improving alertness (Fig. 5.9). The key to shaping the desired light-dark cycle is to properly time light and dark exposure.8,14 For example, to accelerate entrainment, individuals working the night shift should increase bright light exposure during the work shift (coinciding with phase delay portion of the PRC) and avoid bright light exposure in the morning (during the phase advance portion of the PRC) such as by wearing sunglasses during the commute home.14 Good sleep hygiene is essential, including a quiet and dark sleep environment. Exogenous melatonin before daytime sleep has also been used and has been shown to improve sleep duration, but not alertness during night work. Hypnotic medications to improve daytime sleep may be considered, but the potential benefit should be balanced against the risk for carryover sedation into nighttime commute and work shift. Wake-promoting agents such as modafinil and armodafinil are approved by the Food and Drug Administration for the treatment of excessive sleepiness associated with SWD. Strategic use of caffeine has also been shown to reduce excessive sleepiness and improve performance during the night work shift. Finally, planned napping before and during the shift, alone or in conjunction with caffeine, can improve alertness and work performance.
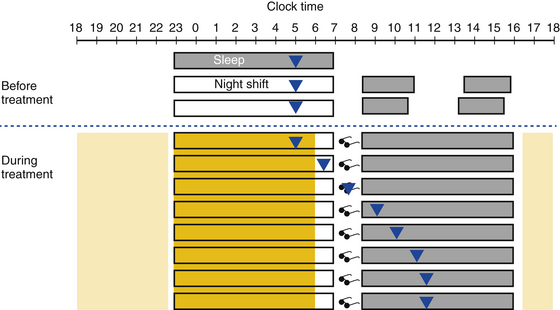
FIGURE 5.9 A schematic representation of a strategy using timed light exposure and avoidance to attain circadian realignment in shift work disorder. The triangles represent the core body temperature (CBT) nadir. Dark shaded boxes represent the sleep period; empty boxes represent work during the night shift. Before treatment, night shift workers work when the circadian alerting signals are low, resulting in excessive sleepiness during the shift; they sleep during the day when circadian alerting signals are high and have disturbed and shortened sleep during the day. Treatment is aimed at realignment of the circadian rhythm. During treatment, bright light therapy (dark yellow) during the night shift can delay the circadian phase and improve alertness. Bright light therapy should be stopped 1 to 2 hours before the end of the night shift. In the morning before sleep, such as during the commute home, light should be avoided (sunglasses) to prevent phase advance. When home and sleeping, the sleep environment should be kept dark. The CBT nadir will gradually shift to the daytime, which will improve daytime sleep. Upon awakening, individuals may be exposed to regular light (e.g., sunlight or ambient light, light yellow), which may help to prevent overshooting the intended phase delay.
Jet Lag Disorder
Jet lag disorder arises from circadian misalignment associated with travel across at least two time zones, which results in insomnia, daytime sleepiness, or both. The degree of misalignment and symptoms depends on the number of time zones crossed and the direction of travel. Diagnosis also requires daytime dysfunction, malaise, or somatic symptoms such as gastrointestinal complaints within 1 to 2 days after travel, and the symptoms cannot be explained by another disorder.7,8
Treatment of jet lag disorder is based on realignment of the circadian rhythm with the destination time if a person is staying in that time zone for at least 48 hours. Circadian phase shifting to the new time zone is accomplished by controlling the timing of bright light exposure with or without exogenous melatonin administration. If significant adjustment is required, phase shifting can begin before travel. Examples of eastward and westward travel are given in Figure 5.10. With eastward travel, adjustment requires advancing the timing of the circadian rhythm. Timed melatonin at the destination bedtime is recommended and may be used 3 days before departure and up to 5 days after arrival at the destination. At the new destination, avoidance of bright light (e.g., with use of sunglasses) in the early morning hours (which could coincide with the phase delay portion of the light PRC) and maximizing light exposure in the late morning and afternoon, after CBT nadir has passed, can accelerate the desired phase advancement. When possible, pretravel phase advancement can also be achieved with bright light delivered in the morning.8 Westward travel results in a CBT nadir occurring hours earlier than would be seen in an individual’s native time zone. Therefore staying awake and maximizing light exposure in the late afternoon to early evening (during the phase delay portion of the light PRC) is important.
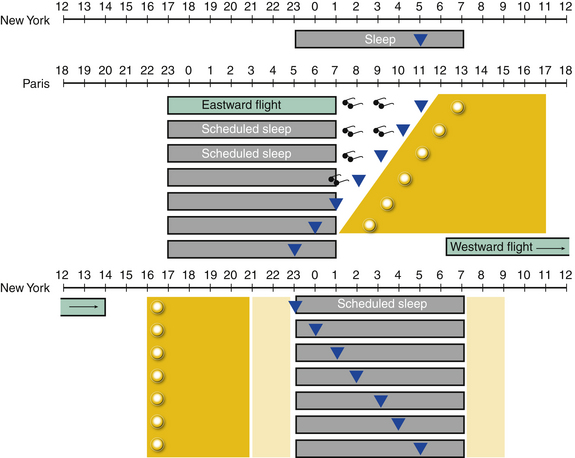
FIGURE 5.10 A schematic of circadian misalignment and entrainment that occurs following a trip from New York to Paris and back. The dark shaded rectangles represent sleep times. The triangles represent the nadir of the core body temperature (CBT). Following a flight from New York to Paris (eastward travel), the CBT nadir occurs at 11 AM in Paris. Reentrainment requires advance of the circadian phase. Light should be avoided in the early morning before the CBT nadir (sunglasses) for the first few days. Light exposure should be increased in the early afternoon, after the CBT nadir (dark yellow area and sun). Phase advance can occur about 1 hour per day, resulting in entrainment to the new sleep and wake times in about 5 to 6 days. Upon return from Paris (westward travel), the CBT nadir occurs at 11 PM in New York. Reentrainment now requires circadian phase delay. Light exposure should be increased in the late afternoon and early evening. Individuals may be exposed to ambient light (light yellow) at other times.
References
1. Borbely, A. A. A two process model of sleep regulation. Hum Neurobiol. 1982; 1(3):195–204.
2. Saper, C. B., Lu, J., Chou, T. C., Gooley, J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005; 28(3):152–157.
3. Reppert, S. M., Weaver, D. R. Coordination of circadian timing in mammals. Nature. 2002; 418(6901):935–941.
4. Czeisler, C. A., Allan, J. S., Strogatz, S. H., et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986; 233(4764):667–671.
5. Lewy, A. J., Bauer, V. K., Ahmed, S., et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998; 15(1):71–83.
6. Dijk, D. J., Cajochen, C. Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. J Biol Rhythms. 1997; 12(6):627–635.
7. American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic and coding manual, 2nd ed. Westchester, Ill: American Academy of Sleep Medicine; 2005.
8. Morgenthaler, T. I., Lee-Chiong, T., Alessi, C., et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007; 30(11):1445–1459.
9. Mundey, K., Benloucif, S., Harsanyi, K., Dubocovich, M. L., Zee, P. C. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005; 28(10):1271–1278.
10. Lack, L., Wright, H., Kemp, K., Gibbon, S. The treatment of early-morning awakening insomnia with 2 evenings of bright light. Sleep. 2005; 28(5):616–623.
11. Hayakawa, T., Uchiyama, M., Kamei, Y., et al. Clinical analyses of sighted patients with non-24-hour sleep-wake syndrome: a study of 57 consecutively diagnosed cases. Sleep. 2005; 28(8):945–952.
12. Lockley, S. W., Skene, D. J., James, K., Thapan, K., Wright, J., Arendt, J. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol. 2000; 164(1):R1–R6.
13. Okawa, M., Mishima, K., Hishikawa, Y., Hozumi, S., Hori, H., Takahashi, K. Circadian rhythm disorders in sleep-waking and body temperature in elderly patients with dementia and their treatment. Sleep. 1991; 14(6):478–485.
14. Revell, V. L., Eastman, C. I. How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms. 2005; 20(4):353–365.