Positive Pressure Titration
Overview
Positive airway pressure therapy is a core skill in sleep medicine and laboratory practice. This area has seen significant changes since the first edition of the Atlas, including the following: (1) availability of adaptive ventilation and average volume assured pressure ventilation, (2) the recognition of complex sleep apnea, (3) demonstrated investigative and clinical efficacy of low-concentration carbon dioxide (CO2) as a positive airway pressure adjunct, (4) changes in the pressure profile delivered by clinical devices that attempt to maximize comfort and improve synchrony between the patient and the ventilator, (5) formal guidelines for titration of positive airway pressure and noninvasive ventilation from the American Academy of Sleep Medicine (AASM), and (6) formal recommendations for polysomnography in noninvasive ventilation practice from the SomnoNIV group. This chapter will aim to provide complementary information using informative snapshots, with a focus on clinical management of abnormal respiratory chemoreflexes and hypoventilation syndromes. Bilevel ventilation will be discussed in greater depth than continuous positive airway pressure (CPAP). Slightly edited summaries of the AASM guidelines are provided below as a starting point.
Positive airway pressure (PAP) devices treat patients with sleep-related breathing disorders, including obstructive sleep apnea (OSA). Continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BPAP) represent the two forms of PAP that are manually titrated during polysomnography (PSG) to determine the single fixed pressure of CPAP or the fixed inspiratory and expiratory positive airway pressures (IPAP and EPAP, respectively) of BPAP for subsequent nightly use. A PAP Titration Task Force of the AASM reviewed the available literature.
Major Recommendations for Positive Airway Pressure Titration
1. All potential PAP titration candidates should receive adequate PAP education, hands-on demonstration, careful mask fitting, and acclimatization before titration.
2. CPAP (IPAP and EPAP for patients on BPAP) should be increased until the following obstructive respiratory events are eliminated (no specific order) or the recommended maximum CPAP (IPAP for patients on BPAP) is reached: apneas, hypopneas, respiratory effort–related arousals (RERAs), oxygen desaturations, and snoring.
3. The recommended minimum starting CPAP should be 4 cm H2O for pediatric and adult patients, and the recommended minimum starting IPAP and EPAP should be 8 cm H2O and 4 cm H2O, respectively, for pediatric and adult patients on BPAP.
4. The recommended maximum CPAP should be 15 cm H2O (or recommended maximum IPAP of 20 cm H2O if on BPAP) for patients younger than 12 years, and maximum CPAP of 20 cm H2O (or recommended maximum IPAP of 30 cm H2O if on BPAP) for patients 12 years or older.
5. The recommended minimum IPAP-EPAP differential is 4 cm H2O, and the recommended maximum IPAP-EPAP differential is 10 cm H2O.
6. CPAP (IPAP or EPAP for patients on BPAP depending on the type of event) should be increased by at least 1 cm H2O with an interval no shorter than 5 minutes, with the goal of eliminating obstructive respiratory events.
7. CPAP (IPAP and EPAP for patients on BPAP) should be increased from any CPAP (or IPAP) level if at least one obstructive apnea is observed for patients younger than 12 years, or if at least two obstructive apneas are observed for patients 12 years or older.
8. CPAP (IPAP for patients on BPAP) should be increased from any CPAP (or IPAP) level if at least one hypopnea is observed for patients younger than 12 years, or if at least three hypopneas are observed for patients 12 years or older.
9. CPAP (IPAP for patients on BPAP) should be increased from any CPAP (or IPAP) level if at least three RERAs are observed for patients younger than 12 years, or if at least five RERAs are observed for patients 12 years or older.
10. CPAP (IPAP for patients on BPAP) may be increased from any CPAP (or IPAP) level, if at least 1 minute of loud or unambiguous snoring is observed for patients younger than 12 years, or if at least 3 minutes of loud or unambiguous snoring is observed for patients 12 years or older.
11. The titration algorithm for split-night CPAP or BPAP titration studies should be identical to that of full-night CPAP or BPAP titration studies, respectively.
12. If the patient is uncomfortable or intolerant of high pressures on CPAP, the patient may be tried on BPAP. If there are continued obstructive respiratory events at 15 cm H2O of CPAP during the titration study, the patient may be switched to BPAP.
13. The pressure of CPAP or BPAP selected for patient use following the titration study should reflect control of the patient’s obstructive respiration by a low (preferably <5/hr) respiratory disturbance index (RDI) at the selected pressure, a minimum sea level oxygen saturation measured by pulse oximetry (SpO2) above 90% at the pressure, and with a leak within acceptable parameters at the pressure.
14. An optimal titration reduces RDI to less than 5 for at least a 15- minute duration and should include supine rapid eye movement (REM) sleep at the selected pressure that is not continually interrupted by spontaneous arousals or awakenings.
15. A good titration reduces RDI to 10 or less or by 50% if the baseline RDI is less than 15 and should include supine REM sleep that is not continually interrupted by spontaneous arousals or awakenings at the selected pressure.
16. An adequate titration does not reduce the RDI to 10 or less but reduces the RDI by 75% from baseline (especially in severe OSA patients), or is one in which the titration grading criteria for optimal or good are met with the exception that supine REM sleep did not occur at the selected pressure.
17. An unacceptable titration is one that does not meet any one of the above grades.
18. A repeat PAP titration study should be considered if the initial titration does not achieve a grade of optimal or good and, if it is a split-night PSG study, it fails to meet AASM criteria (i.e., titration duration should be longer than 3 hours).
Noninvasive positive pressure ventilation (NPPV) devices are used during sleep to treat patients with diurnal chronic alveolar hypoventilation (CAH). BPAP using a mask interface is the most commonly used method of providing ventilatory support in these patients. BPAP devices deliver separately adjustable IPAP and EPAP. The IPAP and EPAP levels are adjusted to maintain upper airway patency, and the pressure support (PS; PS = IPAP − EPAP) augments ventilation. NPPV devices can be used in the spontaneous mode (the patient cycles the device from EPAP to IPAP), the spontaneous-timed (ST) mode (a backup rate is available to deliver IPAP for the set inspiratory time if the patient does not trigger an IPAP/EPAP cycle within a set time window), and the timed mode (inspiratory time and respiratory rate are fixed). During NPPV titration with PSG the pressure settings, backup rate, and inspiratory time (if applicable) are adjusted to maintain upper airway patency and support ventilation. However, there are no widely available guidelines for the titration of NPPV in the sleep center. An NPPV Titration Task Force of the AASM reviewed the available literature and developed recommendations based on consensus and published evidence when available.
General Recommendations for Noninvasive Positive Pressure Ventilation Titration
1. The indications, goals of treatment, and side effects of NPPV treatment should be discussed in detail with the patient before the NPPV titration study.
2. Careful mask fitting and a period of acclimatization to low pressure before the titration should be included as part of the NPPV protocol.
3. NPPV titration with PSG is the recommended method to determine an effective level of nocturnal ventilatory support in patients with CAH. In circumstances in which NPPV treatment is initiated and adjusted empirically in the outpatient setting based on clinical judgment, a PSG should be used if possible to confirm that the final NPPV settings are effective or to make adjustments as necessary.
4. NPPV treatment goals should be individualized but typically include prevention of worsening of hypoventilation during sleep, improvement in sleep quality, relief of nocturnal dyspnea, and providing respiratory muscle rest.
5. When OSA coexists with CAH, pressure settings for treatment of OSA may be determined during attended NPPV titration PSG following AASM Clinical Guidelines for the Manual Titration of Positive Airway Pressure in Patients with Obstructive Sleep Apnea.
6. Attended NPPV titration with PSG is the recommended method to identify optimal treatment pressure settings for patients with the obesity hypoventilation syndrome, CAH caused by restrictive chest wall disease, and acquired or central CAH syndromes in whom NPPV treatment is indicated.
7. Attended NPPV titration with PSG allows definitive identification of an adequate level of ventilatory support for patients with neuromuscular disease in whom NPPV treatment is planned.
Recommendations for Noninvasive Positive Pressure Ventilation Titration Equipment
1. The NPPV device used for titration should have the capability of operating in the spontaneous, ST, and timed mode.
2. The airflow, tidal volume, leak, and delivered pressure signals from the NPPV device should be monitored and recorded if possible. The airflow signal should be used to detect apnea and hypopnea, whereas the tidal volume signal and respiratory rate are used to assess ventilation.
3. Transcutaneous or end-tidal partial pressure of carbon dioxide (PCO2) may be used to adjust NPPV settings if adequately calibrated and ideally validated with arterial blood gas testing.
4. An adequate assortment of masks (nasal, oral, and oronasal) in both adult and pediatric sizes (if children are being titrated), a source of supplemental oxygen, and heated humidification should be available.
Recommendations for Limits of Inspiratory Positive Airway Pressure, Expiratory Positive Airway Pressure, and Pressure Support Settings
1. The recommended minimum starting IPAP and EPAP should be 8 cm H2O and 4 cm H2O, respectively.
2. The recommended maximum IPAP should be 30 cm H2O for patients 12 years or older and 20 cm H2O for patients younger than 12 years.
3. The recommended minimum and maximum levels of PS are 4 cm H2O and 20 cm H2O, respectively.
4. The minimum and maximum incremental changes in PS should be 1 and 2 cm H2O, respectively.
Recommendations for Adjustment of Inspiratory Positive Airway Pressure, Expiratory Positive Airway Pressure, and Pressure Support
1. IPAP and EPAP should be increased as described in the AASM “Clinical Guidelines for the Manual Titration of Positive Airway Pressure in Patients With Obstructive Sleep Apnea” until the following obstructive respiratory events are eliminated (no specific order): apneas, hypopneas, RERAs, oxygen (O2) desaturation, and snoring.
2. The PS should be increased every 5 minutes if the tidal volume is low (<6 to 8 mL/kg)
3. The PS should be increased if the arterial PCO2 remains 10 mm Hg or more above the PCO2 goal at the current settings for 10 minutes or more. An acceptable goal for PCO is a value less than or equal to the awake PCO2.
4. The PS may be increased if respiratory muscle rest has not been achieved by NPPV treatment at the current settings for 10 minutes or more.
5. The PS may be increased if the SpO2 remains below 90% for 5 minutes or more and tidal volume is low (<6 to 8 mL/kg).
Recommendations for Use and Adjustment of the Backup Rate/Respiratory Rate
1. A backup rate (i.e., ST mode) should be used in all patients with central hypoventilation, those with a significant number of central apneas or an inappropriately low respiratory rate, and those who unreliably trigger IPAP/EPAP cycles because of muscle weakness.
2. The ST mode may be used if adequate ventilation or adequate respiratory muscle rest is not achieved with the maximum (or maximum tolerated) PS in the spontaneous mode.
3. The starting backup rate should be equal to or slightly less than the spontaneous sleeping respiratory rate (minimum of 10 breaths/min).
4. The backup rate should be increased in increments of 1 to 2 breaths/min every 10 minutes if the desired goal of the backup rate has not been attained.
5. The IPAP time (inspiratory time) should be set based on the respiratory rate to provide an IPAP time between 30% and 40% of the cycle time (60/respiratory rate in breaths per minute).
6. If the ST mode is not successful at meeting titration goals, then the timed mode can be tried.
Recommendations Concerning Supplemental Oxygen
1. Supplemental oxygen may be added in patients with an awake SpO2 that is less than 88% or when the PS and respiratory rate have been optimized but the SpO2 remains less than 90% for 5 minutes or more.
2. The minimum starting supplemental oxygen rate should be 1 L/min and increased in increments of 1 L/min about every 5 minutes until an adequate SpO2 is attained (>90%).
Recommendations to Improve Patient Comfort and Synchrony Between the Patient and the Noninvasive Positive Pressure Ventilation Device
1. If the patient awakens and complains that the IPAP, EPAP or both is too high, pressure should be lowered to a level comfortable enough to allow return to sleep.
2. NPPV device parameters (when available) such as pressure relief, rise time, and maximum and minimum IPAP durations should be adjusted for patient comfort and to optimize synchrony between the patient and the NPPV device.
3. During the NPPV titration, mask refit or adjustment or change in mask type should be performed whenever any significant unintentional leak is observed or the patient complains of mask discomfort. If mouth leak is present and is causing significant symptoms (e.g., arousals), use of an oronasal mask or chin strap may be tried. Chin straps are frequently ineffective. One caution with the use of an oronasal mask is that if it is too tight, the jaw can be pushed back and thus worsen obstruction. Heated humidification should be added if the patient complains of dryness or significant nasal congestion or can be used as the default approach; current positive pressure devices offer advanced humidity control and heated tubing to reduce condensation.
Recognition of Strong Chemoreflex Influences on Sleep Respiration
An important clinical challenge is the recognition of strong effects of the respiratory chemoreflex on sleep breathing outside central apneas and classic periodic breathing/Cheyne-Stokes pattern. Table 15.1 offers practical tips. Often the dynamic pattern rather than individual events need to be assessed. In its simplest form, non-REM (NREM)-dominant obstructive sleep apnea with short-cycle (≤30 seconds) event cycle length associated with oximetry banding suggests strong chemoreflex influences. The REM versus NREM differences may be the most important differentiator.
Table 15.1
Recognition of Strong Chemoreflex Modulation of Sleep Breathing
| Polysomnographic Characteristic | Relatively Pure Obstructive Sleep Apnea | Chemoreflex-Modulated Sleep Apnea |
| Obstructive apneas | Dominant. | Less common. |
| Central apneas | Rare. | Dominant form. |
| Progressive flow limitation | Typical; long variable-length segments of limited breaths are typical. | Unusual in purest form (e.g., in classic Cheyne-Stokes respiration), but quite common otherwise. |
| Periodic breathing/Cheyne-Stokes pattern | Rare; may be seen at sleep onset or around sleep-wake transitions. | Typical. |
| Relative severity in unstable-NREM versus REM sleep | Greater severity in REM sleep. | Minimal severity in REM sleep; this may be best evident during CPAP titrations. |
| Break point during titration (induction of complex apnea before elimination of flow limitation) | Rare. | Typical. |
| Respiratory event cycle durations | Variable. | Short cycle (25-35 seconds) is highly suggestive, but long cycle forms also occur. |
| Respiratory event symmetry in unstable/CAP-NREM sleep including a mirror-imaging pattern of events | Minimal symmetry. | Very symmetrical, and mirror imaging is typical (one half of an event is the mirror image of the other). |
| Effort signal morphology | Maintained during obstructed breath. | Complete or partial loss between recovery breaths clusters. This contributes to the “concordant waxing and waning of flow and effort” feature that is at the core of recognizing central hypopneas/periodic breathing pattern. |
| Flow-effort relationships | Discordant: flow is reduced disproportionately to reduction in effort signals. | Concordant: flow and effort follow each other in amplitude. |
| Arousal timing | Early part of event termination, often first recovery breath related. | “Crests” event, often in the center of a sequence of recovery breaths that progressively increase and decrease in amplitude. |
| Oxygen desaturation profile | Irregular, progressive drops, sharp contour. | Smooth, symmetrical, progressive drops rare. Rarely < 80%. |
| Nonadaptive bilevel PAP–induced instability | Less common, usually with clearly excessive pressure support only. | Typical. |
CAP, Cyclic alternating pattern; CPAP, continuous positive airway pressure; NREM, non–rapid eye movement; PAP, positive airway pressure; REM, rapid eye movement.
One of the features of chemoreflex-modulated obstructive sleep apnea is “nonlinearity” in the response to increasing positive pressure. This means that after a progressive improvement in flow with progressive increases in pressures (all else being equal—sleep stage, state, type, body position, and leak) there is no further improvement with several centimeters increase in pressure. This could mean an anatomical problem (nasal obstruction, tracheomalacia, oropharyngeal soft tissue, or severe retrognathia) or reflect periodic breathing induced by hypocapnia. A “break point” may be seen—this differs from nonlinearity itself, in that periodic breathing and central apneas are induced before eliminating flow limitation.
Recognition of the Role of Rapid Eye Movement Sleep in Positive Pressure Titration
Some variability of respiration during REM sleep is normal, especially in phasic REM. Hypopneas should not be “chased” unless they are associated with arousals or oxygen desaturation. Brief central apneas may occur normally during REM, especially phasic REM. Flow limitation remains a valid indicator of increased upper airway resistance during REM sleep, but mild intermittent degrees of abnormality that is not associated with arousals or desaturations can be tolerated. Obviously, NREM-dominant sleep apnea will be relatively easy to treat during REM sleep.
If oxygen desaturation (e.g., 3% to 4%, or saturation drops to < 90%) occurs and persists during REM sleep in spite of what seems to be reasonable control of obstructive events, the first step would be a small (2 to 3 cm) increase in CPAP. If this does not resolve the problem, the options include doing nothing (if the changes are minor), adding oxygen, or (better) switching to nonadaptive BPAP. Also check for oximetry artifacts; reapply if in doubt.
Recognition of the Role of Non–Rapid Eye Movement Sleep States in Titration
Most patients, including older individuals or those on benzodiazepines/sedative drugs, exhibit periods of sleep that are not scored as N3 by conventional criteria (or any modification thereof) but clearly have excessive slow wave frequency waves. Respiration is usually quite stable during this period (functionally, think of it as slow wave sleep), and if recognized, the same precautions as during conventionally scored slow wave sleep apply. Those who study the cyclic alternating pattern (CAP) will recognize this type of “stable” NREM sleep as predominantly non-CAP. In fact, for the purpose of titration, dividing sleep into stable and unstable NREM sleep (or non-CAP/CAP) and REM works well, better than N1 to N3 and REM sleep. It may be easier to recognize stable and unstable NREM sleep from nonelectroencephalographic signals, such as respiration, heart rate variability, and respiratory amplitude modulation of the electrocardiogram.
The stability of this non-CAP or slow wave–like (frequency but not amplitude) sleep can cause a false sense of security (of treatment efficacy); it is also important not to change pressures if this state is recognized (unless obstructions and flow limitation are quite overt). Flow can look absolutely normal in this state, and then rather abruptly (often within a minute) a switch to being quite abnormal may occur. Such switches of stable/unstable state are spontaneous, and disease or treatment needs to deal with this dynamic.
Responding to Periodic Motor Activation
Periodic motor activation, usually lower limb movements captured as typical periodic limb movements in sleep (PLMS) seen in individuals with sleep-disordered breathing in the absence of restless legs syndrome, is usually related to the respiratory events. Persistence of PLMS on what looks like reasonably good control of sleep apnea may mean residual subtle hypopneas, especially if they occur during REM sleep or during the latter half of the night. If this is the case, a small increase in CPAP (or IPAP of the BPAP) may be considered, but not greater than 3 to 4 cm H2O beyond the level needed to abolish flow limitation or until central apneas/periodic breathing appear.
Common Errors During Bilevel Titration
1. Late recognition of the lack or inadequate efficacy of CPAP.
2. Inadequate EPAP—this is a “fatal error” (100% failure is expected). Besides the fact that apneas cannot be eliminated by increase in IPAP alone, expiratory airway instability in general is a more recently recognized and clinically important phenomenon.
3. Inadequate (typically <3 to 4 cm) IPAP-EPAP difference, especially if hypoventilation is the main indication (the closer, the more like CPAP it is).
4. Trying to use a fixed “formula” for titration.
5. Simultaneously increasing IPAP and EPAP, especially if this is done repeatedly across the whole night.
6. Not recognizing significant degrees of mouth leak.
7. Inadequate humidification with resultant increase in nasal resistance.
8. Worsening of inspiratory airflow caused by possible glottic narrowing while using the timed mode; hypocapnia is usually associated.
9. Too high a starting pressure (always taking off from the “end” of CPAP)
Bilevel Ventilation Practice Points
1. It is best to increase pressure in increments of 1 cm H2O. With experience, it will be possible to judge when greater increases are required at the individual steps (e.g., IPAP increases for hypoventilation).
2. It is typical to raise either the IPAP or the EPAP and not both (beyond that required to maintain an IPAP-EPAP difference 3 to 4 cm H2O). For example, moving from 10/6 to 11/7 is reasonable, not 10/6 to 13/7.
3. It can be more difficult to recognize hypopneas and flow limitation while on BPAP (compared to CPAP), but use all data you have (arousals, snoring, pressure, effort channels).
4. Do not increase IPAP more than 20 cm H2O (even if available on some of the newer machines) without consulting the physician or technical backup, unless requested in the order. Inadequate control at high pressures is rarely fixed by just more pressure.
5. The final IPAP will usually be at least close to (or even higher than) the CPAP required (if known) to eliminate most, if not all, hypopneas and flow limitation. The ideal end point of titration is to normalize sleep, prevent desaturations and overt airflow obstructions (apneas, hypopneas), and eliminate flow limitation.
6. There is increasing experimental and clinical (human) evidence that expiratory airway narrowing is an important component of upper airway pathophysiology in patients with obstructive sleep-disordered breathing. Real-time video recording of the upper airway and pressure profile measurements have often shown narrowing of the airway to be maximal (or very close to that) in end-expiration. This can vary from patient to patient. What this means in a practical sense is that if EPAP is inadequate, it may be manifested as inspiratory flow limitation or otherwise unstable breathing and arousals. If reasonable IPAP increases (IPAP-EPAP difference of 4 to 5) do not seem to be improving the polysomnographic patterns, increase the EPAP.
7. It is a common error not to choose an adequate EPAP when switching to BPAP from CPAP. Technicians may fall into a pattern of using a “standard” starting BPAP levels, such as 8/5. This can be completely inappropriate if the pressure requirements are higher, and this is best judged based on response to previous CPAP. Use guidelines and examples given later.
8. It is advantageous to simultaneously monitor pneumotachograph (independent inline or PAP machine) flow and the pressure-time profile (mask pressure). The information obtained is complementary, and the implications of the patterns of the pressure profile are well described in the mechanical ventilation literature (e.g., inadequate triggering, overlap of neural expiration and machine inspiration).
9. In is advisable to accurately identify the inspiratory signal early, such as during biocalibrations (this may not be as easy as it may seem once the study is running). A good intercostal electromyogram is useful in this discrimination but may not be available, especially in overweight individuals. The inspiratory part of the curve is usually of lesser duration. Have this part of the flow profile above the baseline, because it is easier, from a visuospatial basis, to appreciate flow limitation (like a “slice cut from the top of a mountain”). With a good pressure trace, flattening and “wobbling” during inspiratory flow limitation, somewhat similar to that seen during CPAP, should be visible.
10. The upper airway may be more unstable on BPAP than on CPAP in individual patients. This phenomenon is characterized by the requirement of an EPAP of very close to or greater than CPAP required to normalize flow, repetitive obstructive apneas in spite of increasing EPAP, and generally increased arousals or patient’s perceived discomfort.
11. The newer bilevel ventilators have settings that can be manipulated to increase patient comfort, especially in those with obstructive and restrictive lung disease. The process does have a trial-and-error component. For example, “wide cycle sensitivity” is the breath detection sensitivity in Philips Respironics BiPAP devices to cycle down to EPAP; there are five cycle sensitivities, at 50%, 35%, 25%, 15%, and 8% of peak flows. The “wide trigger sensitivity” is the breath detection sensitivity to initiate IPAP; there are five trigger sensitivities, at 2.4, 4, 6, 10, and 15 L/min. ResMed has an “easy breathe” shark fin–like profile of inspiratory flow that is more comfortable while awake and perhaps during sleep.
Adaptive Ventilation and Volume-Assured Ventilation
The ResMed Adapt SV provides a baseline degree of ventilatory support. The subject’s ventilation is servo controlled with a high-gain integral controller (0.3 cm H2O per L/min per second, clipped to 4 to 10 cm H2O) to equal a moving target ventilation of 90% of the long-term average ventilation (time constant 3 minutes). If the subject suddenly ceases all central respiratory effort, machine support (i.e., pressure swing amplitude) will increase from the minimum of 4 cm H2O up to whatever is required to maintain ventilation at 90% of the long-term average (up to a maximum of 10 cm H2O, reached in approximately 12 seconds). In the CPAP mode the pressure can be set at 4 to 20 cm H2O. In the ASV and ASV Auto mode EPAP: 4 to 15 cm H2O; pressure support: 0 to 20 cm H2O, but the pressure will not exceed 30 cm H2O. The Auto mode is an auto EPAP. The previous restrictions to PS, such as a minimum support of 3 cm, have been removed with the most recent versions of the software.
The Philips Respironics BiPAP autoSV Advanced (flow-targeted dynamic bilevel positive pressure ventilator) provides PAP support to sustain upper-airway patency. The current version is the “Advanced”, which has auto-CPAP and thus an EPAP minimum and maximum setting. The maximum inspiratory support level is up to 30 cm H2O minus the expiratory pressure. The expiratory pressure automatically adjusts within the available (or prescribed) range (4 to 25 cm H2O). Expiratory pressures can also be set at fixed levels if desired. The algorithm’s automatic backup rate is based on calculations performed on a moving window of the last 12 spontaneous breaths. The flow-targeted dynamic BPAP device modulates the maximum and minimum PS above the EPAP as required to maintain a target peak inspiratory airflow: when the device detects normal breathing, flow-targeted dynamic BPAP operates like conventional CPAP by providing the minimal PS; when the patient does not maintain the target peak inspiratory airflow, the device increases the PS up to a maximum IPAP, which can be set by the user. Peak flow is captured on a breath-by-breath basis and is monitored over a moving 4-minute window; as one breath is added, the initial breath falls off. At every point within this 4-minute period an average peak flow is calculated, and the peak flow target is established around that average and is based on the patient’s needs. The device also provides an automatic backup rate should sustained apnea be detected. To avoid hyperventilating the patient and to promote spontaneous breathing, the target inspiratory flow is set to below the mean inspiratory flow during spontaneous breathing by the patient, and the timing of the backup rate begins with time delay and is set to a slower rate than the average respiratory rate of the patient.
The Weinmann SOMNOvent CR is available in Europe and combines auto and adaptive pressure. The pressure is measured using a pressure sensor, snoring is calculated based on the high-frequency variations of the pressure signal, and the flow is determined based on pressure and blower parameters. The minute ventilation is compared with the average minute ventilation in a moving window and is focused by 50% on the last 2 minutes and by 50% on the previous time. The treatment mode is called anticyclic modulated ventilation. Apneas are defined by a cessation of breathing for more than 10 seconds. Hypopneas are detected if the minute ventilation is reduced by 40% for at least two breaths as compared to the average minute ventilation or if the peak flow is reduced by 50% as compared to the average peak flow. Obstructive and central events are discriminated based on the recognition of flattening of the flow curve and on the reaction on additional PS: obstructions are detected by an increased need for respiratory support with flattening or snoring or by time-cycled breath insufficient to generate flow. The device applies three pressure levels: the IPAP, which represents the pressure level during inspiration; the EPAP, which represents the pressure level in the early expiration; and the end-expiratory positive airway pressure (EEPAP). The EEPAP regulation is based on the cumulative sum of obstructions within a 2-minute epoch, not on single events. During periodic hypopneas the algorithm increases the difference between IPAP and EPAP continuously to raise the tidal volume. During hyperventilation the difference between IPAP and EPAP is reduced and can reach CPAP level (zero difference between IPAP and EPAP). During apneas, mandatory breaths are applied automatically. The frequency of these breaths depends on the baseline respiratory frequency of the patients. The physician can choose a manually fixed instead of automatic backup frequency. In addition, the device uses pressure relief during expiration in the course of normal breathing.
The Philips Respironics AVAPS (average volume assured positive pressure support) has the additional flexibility of determining a target tidal volume. This device is specifically designed for hypoventilation management. The pressures can be constrained as with the BiPAP AutoSV, but within those constraints the device will attempt to reach the target tidal volume. If this target is excessive, the pressure fluctuations can cause arousals and patient-ventilator synchrony difficulty. If the target is insufficient, hypoventilation will persist. Tracking transcutaneous and possibly end-tidal carbon dioxide concentration (ETCO2) using a nonvented mask is necessary to know the effect on ventilation. The device allows manipulation of inspiratory time (usually 1 to 1.2 seconds), inspiratory/expiratory time ratios (inspiration is typically 30% of the cycle), rise time (in 100-msec increments). Using these options requires substantial hands-on experience and careful attention to patient comfort, oxygenation/ventilation/sleep quality, and signs of abnormal synchrony. The manufacturer’s starting recommendations are to set the target tidal volume to 8 mL/kg of ideal weight; IPAP limits, maximum: 25 cm H2O depending on patient pathological condition and minimum: EPAP + 4 cm H2O; set respiratory rate 2 to 3 breaths/min below resting respiratory rate, and set inspiratory time and rise time to patient comfort (e.g., 1 second and 300 msec respectively). These recommendations are approximates at best and need real-time adjustments based on oxygenation, ventilation, sleep quality, and obstructive features.
The ResMed iVAPS (intelligent volume-assured pressure support), like the AVAPS, is designed to treat hypoventilation. This device has some differences from the AVAPS, and it would be reasonable to evaluate both volume target pressure ventilators in a given patient. The iVAPS offers a range of controls similar to the AVAPS but also some different options, described in Table 15.2. For example, the target patient rate (TPR) is set to match the patient’s average spontaneous rate. During spontaneous ventilation the intelligent backup rate (iBR) adjusts to remain in the background, at two thirds of the TPR. This “background” backup rate is lower than a traditional spontaneous timed rate, so it gives the patient maximum opportunity to spontaneously trigger. When spontaneous triggering ceases (e.g., at the onset of an apnea/hypopnea), the iBR adjusts from its background frequency to its TPR. It will adjust most quickly (within four to five breaths) when ventilation is below the target ventilation. A single spontaneous triggered breath resets the iBR to its background rate (two thirds of TBR). There is no evidence or data presented by the manufacturer or independent investigators that this type of backup rate setting has any advantage over other approaches.
Table 15.2
Use of Adaptive and Volume-Assured Ventilation
| Device | Setting | Practical Points |
| ResMed VPAP Adapt SV | EPAP | The majority of patients seem to need 6-8 cm H2O (maximum: 15 cm H2O). If there is overt obstruction (apneas) at these settings, the obstructive component is probably too severe for the device to provide unique adaptive benefit. |
| ResMed VPAP Adapt SV | Minimum pressure support | The minimum PS used to be 3 cm H2O (zero is possible now). I rarely find increasing above 3 cm H2O of benefit at the start of titration. If there is clear evidence of inspiratory flow limitation, increases can be made. |
| ResMed VPAP Adapt SV | Maximum pressure support | The minimum-maximum PS used to be 8 cm H2O and the maximum PS 15 cm H2O (now 20 H2O). I prefer to use a low level of pressure support and have found a maximum PS of 8 cm H2O works well in most instances. Moreover, keeping the support at the lower end reduces the risk for excessive pressure cycling with this device. If CO2 is tracked and there is a concern for hypoventilation, such as in opiate-induced ataxic respiration, increases in minimum or maximum PS could have beneficial effects, with the risk for inducing excessive pressure cycling. |
| ResMed VPAP Adapt SV | Backup rate | When activated, fixed at 15 cm H2O, not user modifiable. |
| Philips Respironics BiPAP autoSV Advanced | EPAP Min and Max | Allows for autotitration of expiratory pressures. I do not use this as a routine, but those who have significant additional obstruction during REM sleep may benefit. |
| Philips Respironics BiPAP autoSV Advanced | EPAP min = EPAP max and PS min = zero | In this mode the expiratory pressure minimum and maximum are equalized, and the pressure support minimum is kept at zero. There is this no bilevel support if no periodic breathing or central apneas are detected. The PS max delivers bilevel ventilation above what is essentially CPAP if the algorithm detects periodic breathing. |
| Philips Respironics BiPAP autoSV Advanced | PS min is greater than zero | This is the minimum bilevel pressure the patient will receive. Adaptive ventilation will occur between PS min and PS max. |
| Philips Respironics BiPAP autoSV Advanced | Maximum IPAP is constrained | Inspiratory pressure will not exceed this set point regardless of other settings. |
| Philips Respironics BiPAP autoSV Advanced | Backup rate | Options for on/off, automatic, or user selected. |
| SOMNOvent CR | IPAP | Suggested start 16-20 cm H2O. |
| SOMNOvent CR | EPAP | Algorithm determined. |
| SOMNOvent CR | EEPAP | Suggested start 6 cm H2O. |
| Philips Respironics AVAPS | Tidal volume | Cannot be delivered in the Auto SV mode. The pressure differential between IPAP-min and IPAP-max is used to target the set tidal volume. The “adaptation” is to a tidal volume target. |
| Philips Respironics AVAPS | IPAP-min and IPAP-max | The difference between these settings is the “space” that is allowed to attain the target. The smaller the difference, the greater the constraints applied to the ventilator. |
| Philips Respironics AVAPS | Rate | Backup rate can be manipulated to increase ventilation, but if clearly faster or slower than spontaneous respiration, there is a risk for inducing patient-ventilator desynchrony. |
| ResMed iVAPS | Height | Used to compute anatomical dead space and thus make an estimate of target alveolar ventilation. |
| ResMed iVAPS | Target alveolar ventilation | Alveolar ventilation cannot be measured directly, so iVAPS estimates it using a height-approximated value of anatomical deadspace. |
| ResMed iVAPS | Target patient rate | This metric defines the upper boundary for an “intelligent backup rate (iBR).” |
| ResMed iVAPS | Inspiratory and expiratory pressures, minimum and maximum inspiratory time, trigger and cycle sensitivity | Other options that can be set in the iVAPS. These are similar to options available in ventilators used in the hospital setting and pose a challenge to the average sleep laboratory in training and appropriate oversight. |
CPAP, Continuous positive airway pressure; EPAP, expiratory positive airway pressure; IPAP, inspiratory positive airway pressure; PS, pressure support; REM, rapid eye movement.
Titration With the Adaptive Ventilators
The individual machines are sufficiently different that until they are actually evaluated, efficacy cannot be predicted. One device may be effective when another is not (see Table 15.2).
1. These are powerful and complicated devices trying to overcome disorders of respiratory control. The more predictable the respiratory abnormality, regardless of severity (relatively pure central apnea syndromes and severe periodic breathing in heart failure patients can be highly metronomic), the more readily the adaptive ventilator algorithms will control disease.
2. The adaptive ventilators react to longer-range fluctuations than traditional positive pressure devices. Thus waiting at a given setting for longer periods (such as 20 minutes) can be reasonable, particularly if there seem to be improvements.
3. Sleep stage and state are important modifiers of ventilator effects and efficacy. Stable NREM sleep has essentially no respiratory abnormality, and the adaptive ventilators should show minimal “reactive” patterns. The normal tidal volume fluctuations on REM sleep can trigger adaptive responses. The REM-related tidal volume changes have a far shorter time scale than the algorithms of the adaptive ventilators, so it is important not to chase these as long as there are no obstructive apneas, arousals, or oxygen desaturations. One characteristic feature is pressure cycling seen during phasic REM sleep when using the ResMed Adapt SV.
4. There are major tidal volume and flow-profile fluctuations during wake and sleep-wake transitions. Although this may be less of a problem with traditional continuous or bilevel ventilation, the adaptive ventilators will respond to these fluctuations. This response may feel uncomfortable and in the worst case prevent sleep onset. Thus it is important to initiate this mode around typical sleep-onset times. Using a sedative is one medical strategy to enable smoothness of this transition.
5. The adaptive ventilators alter polysomnogram respiratory signals. One common theme is that the effort signals may not be an accurate estimate of residual abnormality because what is seen is the composite of the patient and the machine (cannot discriminate an 80/20 or 20/80 relative contribution, for example).
6. Maneuvers that reduce the demand on the adaptive ventilators can have positive adjunctive effects. These include nonsupine positioning, adding supplemental oxygen or minimizing hypocapnia with a nonvented mask approach, lowering the NREM sleep carbon dioxide (CO2) apneic threshold with acetazolamide, or inducing stable NREM sleep with sedatives.
7. The larger the allowed PS, the greater the reliance on the adaptive algorithms. The ResMed device offers less capability for operator adjustments. One approach is to open the machines wide and progressively constrain; the alternative approach is to constrain and then open progressively. Initial constraint seems from personal experience to be more effective.
8. A reasonable starting strategy is to use an EPAP minimum/maximum of 5 and 8 cm H2O, minimum/maximum PS of 3 and 8 cm, an “Automatic” backup rate, and an IPAP maximum of 16 to 18 cm H2O. Note that some of the settings can constrain the others; for example, the maximum allowable IPAP may not allow the full PS to express itself.
9. If the physician orders Adapt SV without prescribed settings, I prefer to use the following: set EEP at 5 cm H2O. Set the PS minimum to 3 cm H2O and PS maximum to 8 cm H2O. The maximum IPAP possible is 25 cm H2O. The ResMed Adapt SV has a default backup rate of 15; some patients may note it makes them feel as though their breathing is quicker.
10. Increasing the minimum EPAP or EEP should be done in steps of 1-cm H2O for overt obstructive apneas. These changes can be made relatively quickly (a few minutes) if the nature of the apneas is obvious.
11. Persistent hypopneas can be caused by both an excessive machine response and inadequate responsiveness to periodic breathing. Depending on the initial approach, support constraint should be reduced or increased. The general principles of responding to respiratory abnormality noted earlier remain valid, the difference is that the target is periodic breathing.
12. More can be less in those with strong chemoreflex effects on sleep breathing, and lowering the support is an equally important option.
13. The utility of the auto EPAP function in the adaptive ventilators is not clear. If it is not functioning well, there will be an impact on the adaptive component. In instances where upper airway obstruction is low, as in nearly pure periodic breathing or central sleep apnea, a low fixed EPAP may be better. In a patient with very clear obstruction (especially in REM sleep), the auto function could be of benefit. More experience is needed with this mode.
Carbon Dioxide Therapeutic Modulation: Positive Airway Pressure Gas Modulator and Enhanced Expiratory Rebreathing Space
The positive airway pressure gas modulator is an investigational device that adds and maintains a low concentration of CO2 to the air in a positive pressure circuit.
Enhanced expiratory rebreathing space (EERS) is the dead space concept applied to pressure ventilation. Positive airway pressure therapy usually induces mild relative (to pretreatment) hypocapnia. Central apneas and periodic breathing can be generated when the arterial PCO2 level falls below that required to stimulate respiration. This level is referred to as the apnea threshold. Hypocapnia at or near the apnea or CO2 control threshold destabilizes sleep-breathing control, resulting in periodic breathing patterns of various severities and morphological characteristics. Preventing hypocapnia is a powerful stabilizing influence on sleep-breathing control, regardless of the presence of hypoxia. Atmospheric CO2 levels are near zero, therefore the CO2 levels in the blood are the result of the balance between metabolism in the patient and blow-off during breathing.
Addition of a closed volume (space) to exhale increases rebreathing of the exhaled air and results in a quick increase in CO2 levels and an increased tidal volume and respiratory rate. This is similar to breathing into a paper bag when trying to treat anxiety-induced hyperventilation. Increased amounts (>300 mL) manifestly feel uncomfortable from both CO2 retention and volume effects. The concept has been used in mechanical ventilation to reduce hypocapnia for several years and more recently has been successfully used to treat central sleep apnea and Cheyne-Stokes respiration in heart failure. None of these uses combine it with positive airway pressure.
There is strictly no true dead space when this concept is used with pressure ventilation. Mixing, turbulence, and leaks ensure variable degrees of effective rebreathing (thus the suggested term enhanced expiratory rebreathing space). There is, however, a reduction in ventilatory blow-off, with remarkable clinical effectiveness. There is no/minimal increase in inspiratory CO2 because of the positive pressure–induced washout. One important practical effect of using positive airway pressure with enhanced expiratory rebreathing space is that the respiratory rate seems unchanged, and the subject does not notice any significant discomfort. This is not surprising, given the known effects of positive pressure support on relieving shortness of breath in mechanically ventilated patients. Such symptoms minimization is obviously important when considering use in patients who are already short of breath, such as in heart failure.
The physiological target for titrations with enhanced expiratory rebreathing space is to maintain ETCO2 at the low normal range for sleep. Normal sleep results in an increase of 2 to 8 mm Hg in ETCO2. With this technique the target is to keep the ETCO2 in the high 30s to low 40s, the lower end of normal. Those with central and mixed sleep-disordered breathing show a prominent tendency to hypocapnia during sleep. The primary monitoring is that of ETCO2 with a mainstream CO2 sensor at the mask.
Transcutaneous CO2 may usefully complement end-tidal measures but is not critical for treatment of hypocapnic central/complex sleep apnea. Transcutaneous measurements can have a role in hypercapnic central apnea management. A biocalibration of ETCO2 is done before sleep study (1 to 2 minutes rested steady breathing, average total of 10 breaths), with a nonvented (NV) mask and 50, 100, and 150 mL added dead space. The biocalibration is used to determine the starting EERS volume and is summarized in Table 15.3. The CO2 targets are to keep the ETCO2 below 50 mm Hg during sleep and not greater than 5 mm Hg increase from wake. If transcutaneous CO2 is monitored, keep at less than 50 mm Hg or limit increases to 5 mm Hg from baseline. The latter approach is somewhat approximate, because sleep baseline in the patient will probably not be known and it is unclear how much time is required for equilibration after sleep onset.
Table 15.3
Determination of Starting Enhanced Expiratory Rebreathing Space Volume
| Starting Dead Space Condition | Baseline “Wake” ETCO2∗ | Suggested Starting EERS |
| ETCO2 with NV mask only∗ and ≥ 45 mm Hg with 150 mL EERS | ≤40 | 50 mL |
| ETCO2 with NV mask only∗ and ≥ 45 mm Hg with 100 mL EERS | ≤40 | 0 or 50 mL |
| ETCO2 with NV mask only∗ and ≥ 45 mm Hg with 50 mL EERS | ≤40 | NV mask only |
| ETCO2 with NV mask only∗ and ≥ 50 mm Hg with 50 mL EERS | 40-45 | NV mask only |
| ETCO2 with NV mask only∗ and ≤ 50 mm Hg with 50 mL EERS | 40-45 | NV mask only |
| ETCO2 with NV mask only∗ and ≤ 50 mm Hg with 100 mL EERS | 40-45 | 0 or 50 mL |
| ETCO2 with NV mask only∗ and ≤ 50 mm Hg with 150 mL EERS | 40-45 | 0 or 50 mL |
| ETCO2 with NV mask only∗ | ≥45 | Physician approval |
EERS, Enhanced expiratory rebreathing space; ET, end-tidal; NV, nonvented.
The mask-fitting clinic at the Beth Israel Deaconess Medical Center has modified a large number of masks. Specific masks, methods to convert to NV, and appropriate connectors are available on request (contact Dr. Thomas at rthomas1@bidmc.harvard.edu) and are tabulated with mask-specific PowerPoint files. The nonvented configuration, including the safety valve, adds 60 to 100 mL of dead space (including the intramask space), and this “NV alone” usually means about 60 to 100 mL of dead space/EERS compared to a vented mask. What this means in practical terms is that adding further EERS is often not necessary any more.
There are two approaches to titration of EERS, “forward” and “backward.” In the forward approach, incremental options are considered with positive airway pressure first with a vented mask, then converting to a nonvented mask, then adding rebreathing space (e.g., 50 mL, then 100 mL, etc), O2. In the backward approach, maximal stabilization is provided at the outset, starting at 50 mL “dead space” (which seem to be adequate for most) and 2 L/min O2. After obtaining control in REM/stable and unstable NREM sleep, the O2 and CO2 modulations can be progressively withdrawn to assess the minimum requirement. Typically with successful dead space, O2 is not critical but can be a useful backup, because consistent good seals at home are hard to maintain. Obtaining a plateau on the ETCO2 signal ensures effectiveness (that the seal is adequate) and accuracy of the reading. An alternate approach is to simply start with an NV mask and no added EERS and titrate as required.
Nasal Resistance Valves and Internally Generated Expiratory Positive Pressure Therapy
Provent (Ventus Medical) is a Food and Drug Administration (FDA)-approved treatment for obstructive sleep apnea. These disposable valves are stuck onto the nares and offer resistance during the expiratory but not inspiratory phase of respiration. The resistance is fixed, one size for all; a range of expiratory resistance levels could be of benefit and may be available in the future. The published data show highly variable efficacy in those with mild to more severe disease—nearly none to great. This variability is in distinct contrast to continuous positive airway pressure, which invariably benefits obstructive sleep apnea. Suggested mechanisms, based on physiological experiments, include increased tracheal tug from higher lung volumes, expiratory airway support, and increased CO2 from mild hypoventilation. The latter effect could be theoretically beneficial for those prone to CO2-related ventilatory instabilities by raising the CO2 further away from the NREM CO2 apnea threshold.
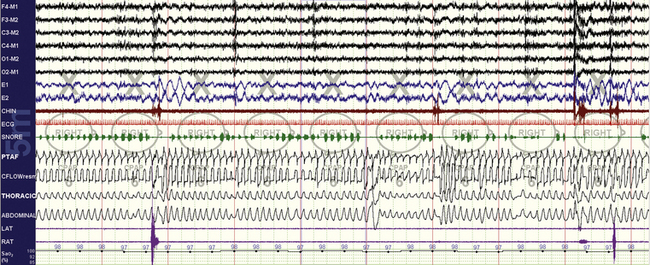
FIGURE 15.1 Continuous positive airway pressure (CPAP) titration in progress.
A 55-year-old man, 10-minute screen compression, non–rapid eye movement (NREM) sleep. CPAP titration has just been initiated, yet already oxygenation is much improved and essentially normal. Flow is clearly abnormal, with severe flow limitation and snoring and associated arousals. PTAF is nasal pressure; CFLOW is flow from the laboratory titrating unit. It is often far easier to normalize oxygenation relative to sleep quality and airflow.
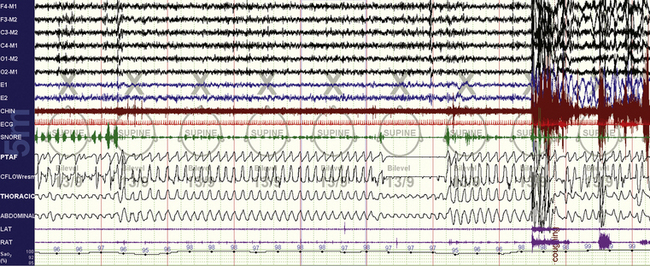
FIGURE 15.7 Bilevel with inadequate expiratory positive airway pressure (EPAP).
The same patient as in Figure 15.6. An obstructive apnea is seen followed by an arousal. This requires an increase in EPAP unless the loss of control was caused by leak. It may also signal a shift to rapid eye movement (REM) sleep, in which respiratory effects may occur before recognizable surface REM sleep.
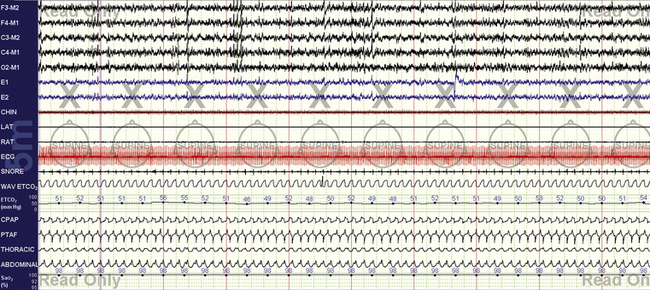
FIGURE 15.13 Chronic obstructive pulmonary disease with mixed sleep apnea.
The same patient as in Figure 15.12, at optimal settings. Continuous positive airway pressure (CPAP) is 17 cm H2O, 2 L of supplemental oxygen, and 50 mL of enhanced expiratory rebreathing space (EERS). The end-tidal CO2 is high 40s to low 50s, and the transcutaneous CO2 (not shown) was similar. Further increases in CPAP did not improve airflow (seen limited in the figure) and thus was dropped back to 17 cm H2O. Local tissue factors may prevent normalization of flow, but tracheobronchomalacia is another possibility. Use of EERS in this instance prevented positive pressure–induced respiratory instability even though the patient started out mildly hypercapnic. Over time, some healing of the respiratory system can be expected, and on retitration it would be prudent to assess the continued need for EERS.
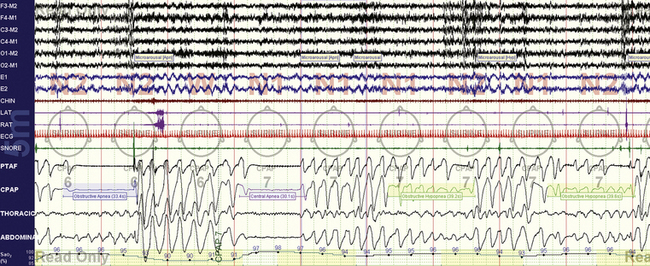
FIGURE 15.15 Positive pressure–induced bradypnea—resolution.
The same patient as in Figure 15.14. There is no resolution of the respiratory instability causing central apneas and obstructive hypopneas as he continues into sleep, but respiratory rate is about 12 breaths/min. In this instance the severe bradypnea seen during drowsiness is absent.
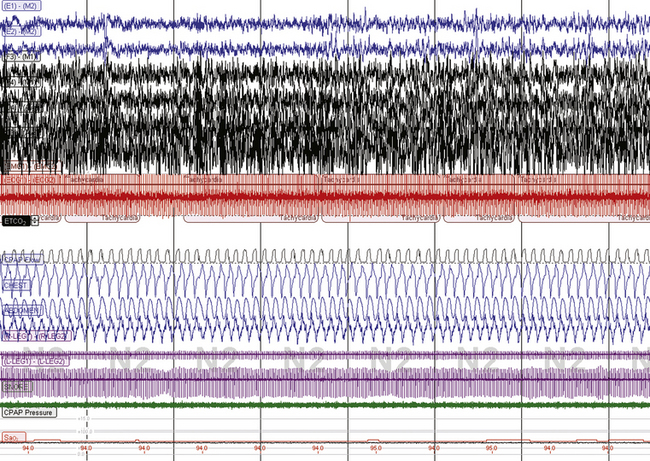
FIGURE 15.17 Effect of CO2 on respiratory instability and CO2 sensitivity.
The same patient as in Figure 15.16. With the use of a nonvented mask, end tidal CO2 is now 36 mm Hg and sleep respiration is stable. However, note that the heart rate is now 120 beats/min, respiratory rate about 20 breaths/min. This patient appears to be unusually sensitive to effects of CO2. The patient could not tolerate a nonvented mask during home CPAP use and ultimately settled upon using supplemental O2 to prevent nocturnal oxygen desaturations if subject sleep quality remained poor.
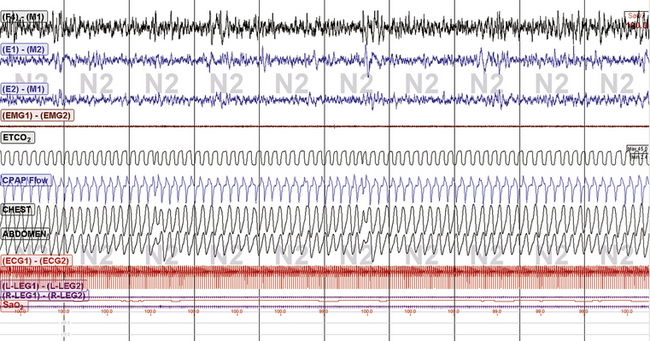
FIGURE 15.20 Subarachnoid hemorrhage and periodic breathing: continuous positive airway pressure: Philips Respironics BiPAP autoSV Advanced. The same patient as in Figures 15.18 and 15.19. Excellent treatment response to the BiPAP SV Auto plus 50 mL enhanced expiratory rebreathing space and 2 L supplemental O2. End-tidal CO2 is in the 44- to 45-mm range. The patient is tolerating this treatment well at home.
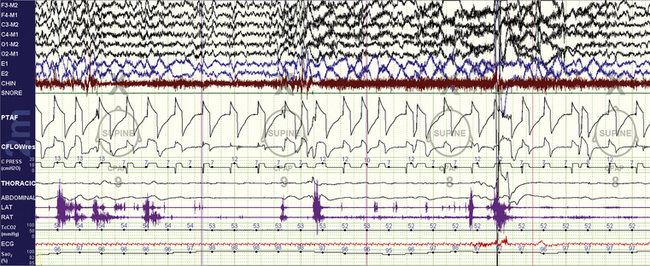
FIGURE 15.22 Neuromuscular disorder and Philips Respironics AVAPS.
The same patient as in Figure 15.21. A switch to the AVAPS mode is evaluated to try and treat hypoventilation. At any reasonable tidal volume target (such as 300 mL ), there was immediate induction of sleep fragmentation that resolved with a switch back to the BiPAP Auto SV. This is a problem with AVAPS: although the targets of ventilation are improved, there is a tendency to cause sleep fragmentation. The ResMed VPAP Adapt SV is not appropriate for hypoventilation syndromes.
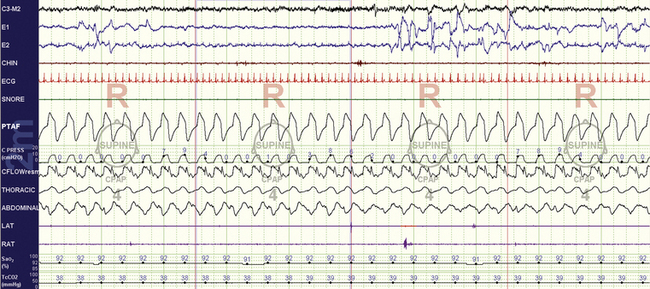
FIGURE 15.24 Tracheostomy volume ventilation for residual central sleep apnea: rapid eye movement (REM) sleep.
The same patient as in Figure 15.23, during REM sleep. There is no snoring present, perhaps because of REM-induced atonia of the soft palate and thus reduced vibrations.
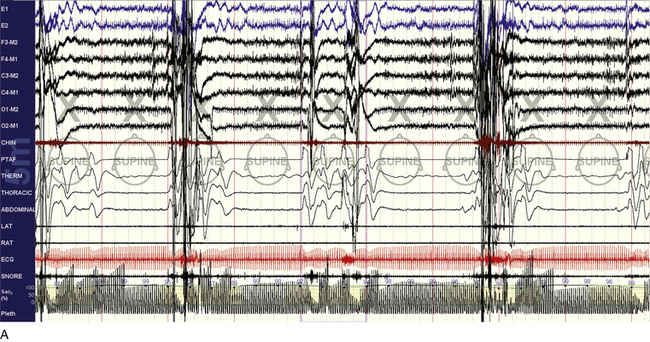
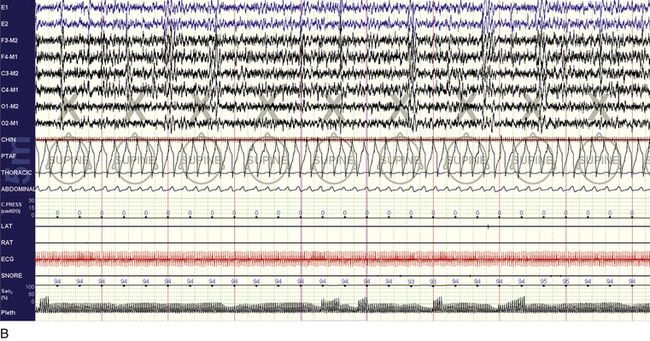
FIGURE 15.29 Efficacy of Provent.
A 55-year-old man with idiopathic central sleep apnea (central apnea index 27/hr, entirely during non–rapid eye movement sleep). A desire to evaluate non–positive airway pressure treatments for travel convenience led to titration with continuous positive airway pressure plus dead space and Provent. The snapshots show before (A) and after (B) examples of abnormality and treatment efficacy, respectively. It is possible that besides positive pressure and increased lung volumes and thus tracheal tug, the CO2-retentive effects of Provent were important in this instance.
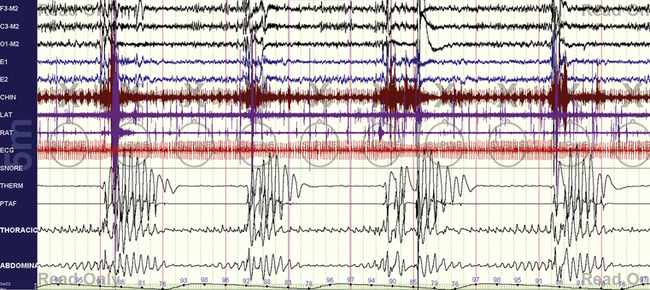
FIGURE 15.2 Typical split-night study—diagnostic.
Figures 15. 2 to 15.5 are from the same patient. Straightforward obstructive sleep apnea with all the typical features—severe obstruction, oxygen desaturations, arousals, and heart rate acceleration and deceleration. Tibialis electromyogram shows excessive activity, but not periodic limb movements. The mentalis muscle seems recruited for inspiratory effort.
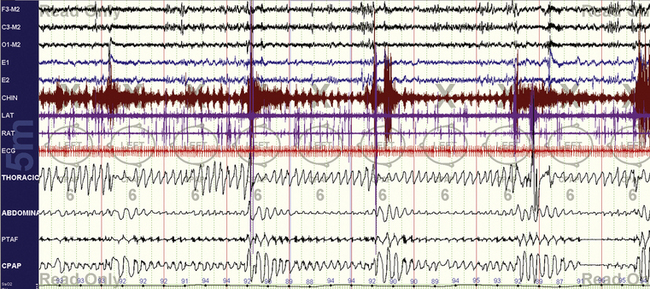
FIGURE 15.3 Typical split-night study—early titration.
Continuous positive airway pressure is 6 cm H2O, clearly inadequate. Oxygenation is already improving markedly, but all other abnormalities persist.
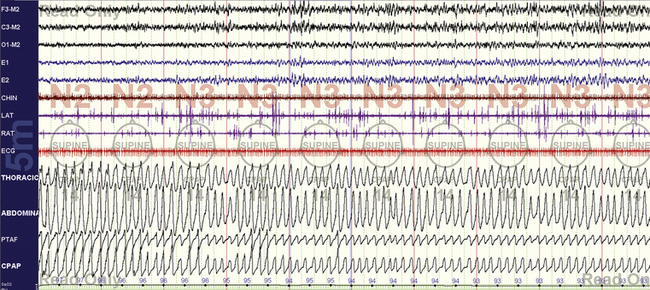
FIGURE 15.4 Typical split-night study—optimal non–rapid eye movement (NREM).
Further along in the titration, pressure is 14 cm H2O, and oxygenation, sleep, and airflow are normal during NREM sleep.
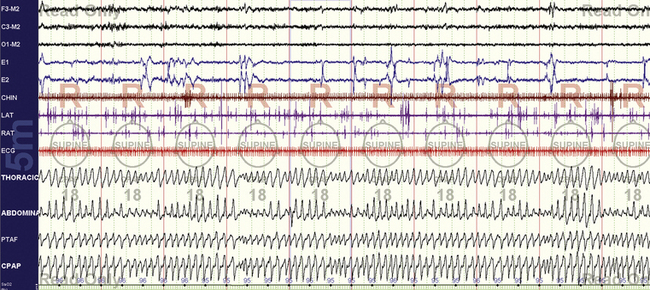
FIGURE 15.5 Typical split-night study—optimal rapid eye movement (REM).
With the optimal pressure being determined during REM sleep (18 cm H2O), in the supine position, the rest of the night can be used for a bilevel pressure evaluation or lowering the pressure gradually to determine the minimum effective pressure. Switching to an auto-CPAP mode is also an option to objectively test the response characteristics of the laboratory titration units. It is also perfectly reasonable to just leave the patient alone.
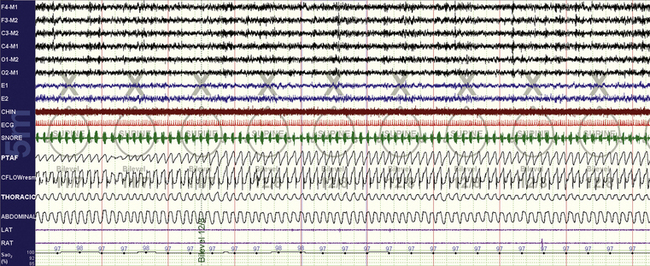
FIGURE 15.6 Bilevel titration.
A 44-year-old woman who is intolerant to continuous positive airway pressure; a 10-minute screen compression in non–rapid eye movement sleep during bilevel positive pressure ventilation titration. There is inspiratory snoring, and the technician increases the pressure from 11/7 to 12/8 cm H2O. Ideally only the inspiratory positive airway pressure should have been increased first.
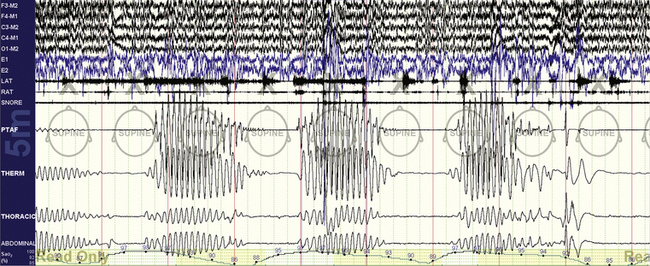
FIGURE 15.8 Cheyne-Stokes respiration pretreatment.
Figures 15.8 to 15.11 show treatment challenges in patients with classic Cheyne-Stokes pattern/periodic breathing; 10-minute screen compression. Note the long-cycle periodic breathing (nearly a minute) that is typical of those with congestive heart failure, as in this instance. End-tidal CO2 while awake was 32 mm Hg.
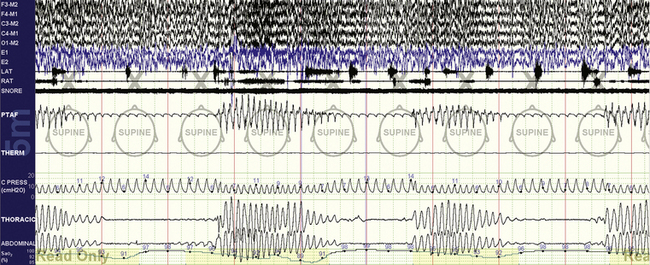
FIGURE 15.9 Cheyne-Stokes respiration treated with ResMed VPAP Adapt SV.
The same patient as in Figure 15.8. Note the inability of this adaptive ventilator in this case to convert the unstable respiratory pattern to a stable one devoid of periodic breathing. The ventilator appropriately increases pressure during periods of apnea, but increased pressure support was not effective. The sawtooth appearance is a characteristic of the ResMed adaptive ventilator.
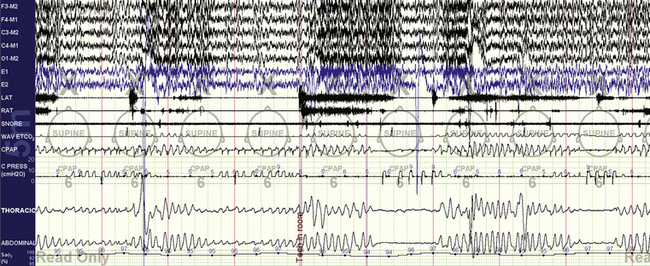
FIGURE 15.10 Cheyne-Stokes respiration treated with Philips Respironics BiPAP autoSV.
The same patient as in Figure 15.8. Note the inability of this adaptive ventilator in this case to convert the unstable respiratory pattern to a stable one devoid of periodic breathing. The ventilator appropriately increases pressure (the CPRESS channel) during periods of apnea, but increased pressure support was not effective. During the first two cycles the patient provides the trigger, but in the following one the ventilator provides machine-triggered breaths during the apnea. Note the difference in morphological characteristics and timing of patient- versus machine-triggered breaths.
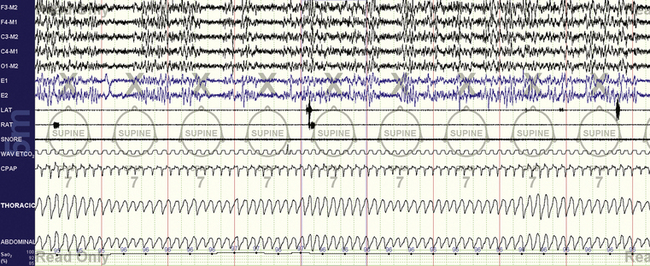
FIGURE 15.11 Cheyne-Stokes respiration treated with continuous positive airway pressure (CPAP) and enhanced expiratory rebreathing space (EERS). The same patient as in Figure 15.8.Note stability of respiration and sleep with the use of 50 mL of EERS; the end-tidal CO2 was increased to 36 mm Hg. There is still seen a gentle cycling of respiration. The CPAP channel shows flow limitation—now that the periodic breathing is stabilized, the obstructive component can be treated with pressure increases. The “plateau” in the end-tidal CO2 signal signifies a maintained seal; leak would result in loss of this signal.
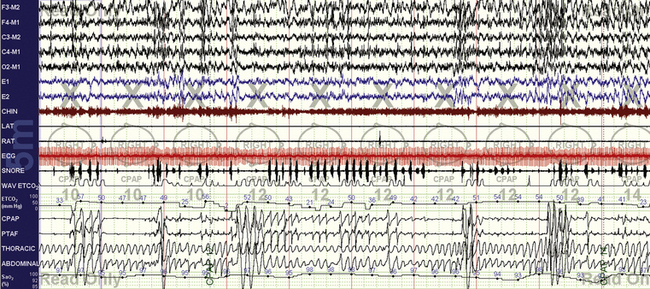
FIGURE 15.12 Chronic obstructive pulmonary disease (COPD) with mixed sleep apnea.
A 56-year-old obese man with “uncontrollable” mixed sleep apnea with CPAP up to 20 cm H2O and bilevel ventilation up to 25/20 cm H2O and 4 L of additional oxygen. The COPD–sleep apnea combination can be one of the most challenging ones—because upper airway, sleep, O2, and CO2 are all simultaneous targets. Transcutaneous CO2 monitoring is useful, and end-tidal CO2 (ETCO2) monitoring can provide complementary information, especially if the intent is to also minimize the reduction in CO2 that will occur with improved ventilation in these patients. Early during titration (12 cm H2O CPAP, nonvented mask, 2 L of supplemental oxygen) the following can be seen: oxygen desaturations, upper airway obstructions, hypercapnia resulting from the hypopneas (note ETCO2 in the low 50s associated with the arousals), and periodic breathing–like effort fluctuations.
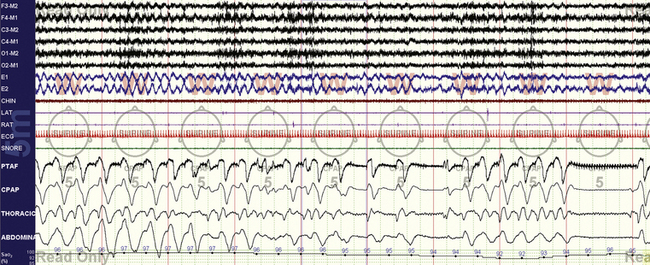
FIGURE 15.14 Positive pressure–induced bradypnea—sleep onset.
Patients with posterior fossa lesions can demonstrate unusual respiratory patterns. This 30-year-old man with remote resected cerebellar astrocytoma demonstrates respiratory rates of 6 breaths/min and slower and ultimately meets criteria for central apneas during this period of drowsiness leading to N1 sleep. The arousal speeds up the rate to nearly 8 breaths/min, but then the (likely) mild hypocapnia leads to a central apnea.
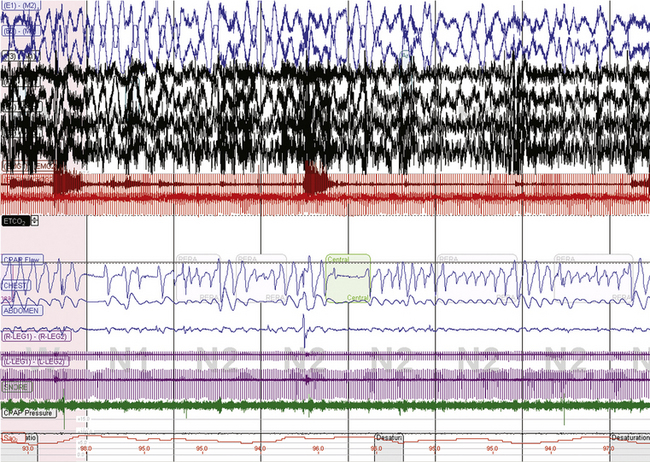
FIGURE 15.16 CO2 dependence of sleep-related respiratory instability in medically ill patients.
Several attempts to treat central sleep apnea (continuous positive airway pressure [CPAP], supplemental oxygen, adaptive ventilation) in a 63-year-old with past encephalitis, bronchiectasis, and asthma have failed. A new diagnostic assessment, a sample of which is in this figure at a 10-minute screen compression, shows unstable respiratory patterns. Application of CPAP resulted in immediate emergence of central apneas. Note that the heart rate is about 110 beats/min, respiratory rate about 16 breaths/min. The resting wake end-tidal CO2 was 30 to 32 mm Hg.
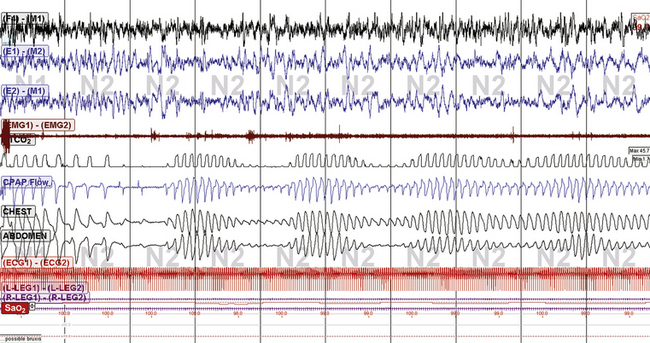
FIGURE 15.18 Subarachnoid hemorrhage and periodic breathing: continuous positive airway pressure (CPAP).
A 77-year-old man with amyloid angiopathy and recurrent intracerebral hemorrhage with subarachnoid extension. This classic Cheyne-Stokes pattern/periodic breathing was seen during the diagnostic sleep study, CPAP, and nonadaptive bilevel ventilation titrations with or without supplemental oxygen and enhanced expiratory rebreathing space. Adding a backup rate was uncomfortable.
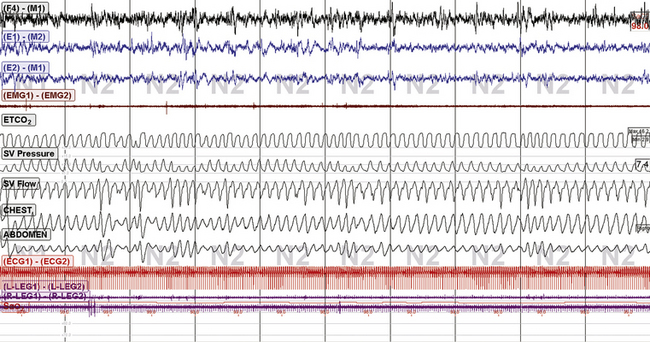
FIGURE 15.19 Subarachnoid hemorrhage and periodic breathing: continuous positive airway pressure: ResMed VPAP Adapt SV. The same patient as in Figure 15.18. Marked improvement of sleep and sleep respiration with the use of 50-mL enhanced expiratory rebreathing space, 2 L supplemental O2, and VPAP Adapt SV. Note the mild ongoing pressure cycling on the SV pressure channel, but there appears to be no adverse consequence on sleep. The end-tidal CO2 was 40 mm Hg wake baseline and 44 to 46 mm Hg during adaptive ventilation. Thus adaptive ventilation and CO2 minimization strategies are synergistic, or additive.
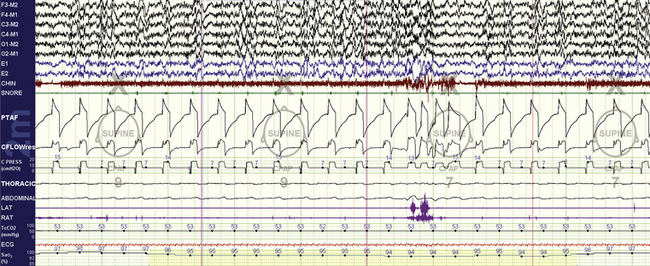
FIGURE 15.21 Neuromuscular disorder and adaptive ventilation.
A 48-year-old with nocturnal respiratory failure from a myopathy, showing the efficacy of BiPAP autoSV set at EPAP Min and Max of 7 cm H2O, minimum/maximum pressure support 7/10 cm H2O. On the previous titration there was an abundance of central apneas that were helped somewhat by the superadded adaptive features of this ventilator (the patient thus received a fixed bilevel support of 14/7 cm H2O at least, with a backup rate of 12 beats/min). Note: The majority of breaths are machine triggered (except around the arousal), the PTAF (nasal/mask pressure) shows the pressure delivered to the patient, the CFLOW the flow volume profile, and the CPRESS channel the machine pressure output. The “CPAP tags” are erroneous. The transcutaneous CO2 is 53 mm Hg—thus there is hypoventilation.
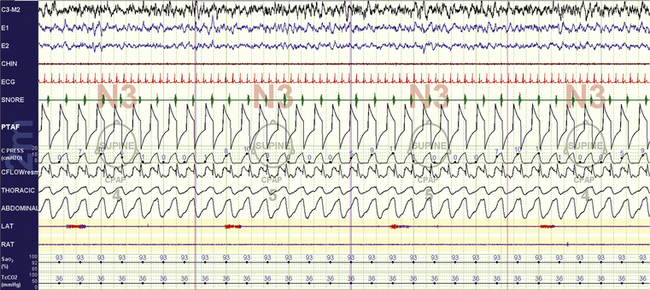
FIGURE 15.23 Tracheostomy volume ventilation for residual central sleep apnea: non–rapid eye movement sleep.
A 59-year-old man uses volume-based nocturnal ventilation for longstanding posttracheostomy central sleep apnea. He cannot tolerate an inflated tracheostomy tube cuff, and after a number of manipulations under polysomnographic guidance, the “right” volume, inspiratory time, and rise time settings have been achieved. This 10-minute compression screen shot shows the escape of a small amount of air (“upwards”) through the upper airway, inducing soft-palate vibrations. Note that flow reduces momentarily when this occurs (CFLOW channel). The pressure profile is captured, but the ventilation here is volume based. Though unconventional, this configuration works for this patient.
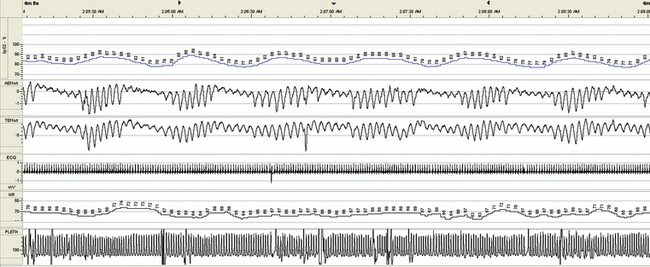
FIGURE 15.25 Hypoventilation, chronic obstructive pulmonary disease (COPD), respiratory failure, tracheostomy, and residual central sleep apnea—diagnostic. Figures 15.25 to 15.28 are from the same patient. A 70-year-old man with recurrent hospitalizations for severe obstructive sleep apnea and respiratory failure despite attempts to use bilevel ventilation at home. COPD is moderate, but CO2 retention is 90 to 100 mm Hg during acute admissions, which responds readily to intubation and ventilation. A tracheostomy markedly improves his status and keeps him out of the hospital for about 3 years, with then a return to the old pattern. This 4-minute screen shot from a portable monitor recording during hospitalization shows his residual sleep apnea (severe periodic breathing); end-tidal CO2 (not in the graphs) ranges from 50 to 60 mm Hg.
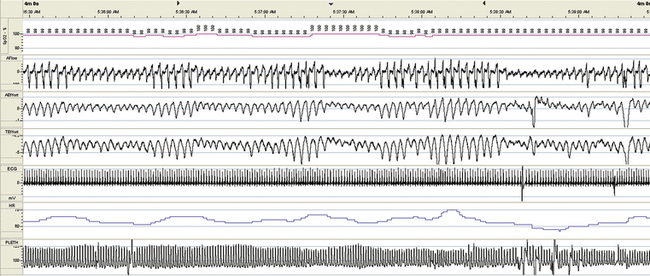
FIGURE 15.26 Hypoventilation, chronic obstructive pulmonary disease, respiratory failure, tracheostomy, and residual central sleep apnea: mask ventilation with tracheostomy closed. An attempt is made to deliver bilevel positive pressure ventilation with the tracheostomy closed. The patient had a problem of mucous plugging of the tracheostomy, and the treating team wondered if bilevel ventilation could be effective as a bridge treatment. Note the persistent periodic breathing.
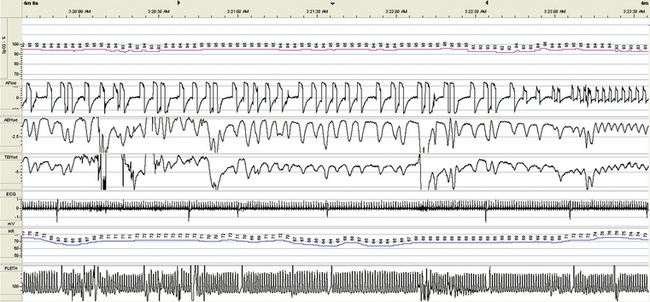
FIGURE 15.27 Hypoventilation, chronic obstructive pulmonary disease, respiratory failure, tracheostomy, and residual central sleep apnea: pressure support ventilation. Pressure support ventilation is attempted through the tracheostomy. Although oxygenation improved, respiratory dysrhythmia ensued and the patient experienced discomfort from ventilatory desynchrony. The end of this 4-minute screen shot shows a switch to intermittent mandatory volume ventilation.
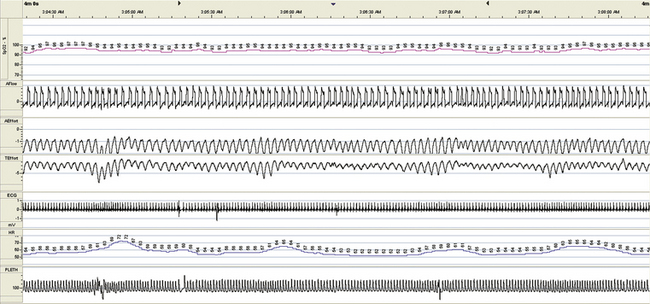
FIGURE 15.28 Hypoventilation, chronic obstructive pulmonary disease, respiratory failure, tracheostomy, and residual central sleep apnea: intermittent mandatory ventilation. Intermittent mandatory ventilation through the tracheostomy results in reasonable stabilization of respiration, the patient could tolerate this treatment, oxygenation was adequate, and end-tidal CO2 was brought down to about 50 mm Hg. Figures 15.25 to 15.28 demonstrate some of the complexities encountered in ventilatory management when subjective sleep quality and patient comfort are important, as in the home environment.
Arzt, M., Wensel, R., Montalvan, S., et al. Effects of dynamic bilevel positive airway pressure support on central sleep apnea in men with heart failure. Chest. 2008; 134:61–66.
Berry, R. B., Chediak, A., Brown, L. K., et al. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med. 2010; 6:491–509.
Berry, R. B., Kryger, M. H., Massie, C. A. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep. 2011; 34:479–485.
Braga, C. W., Chen, Q., Burschtin, O. E., Rapoport, D. M., Ayappa, I. Changes in lung volume and upper airway using MRI during application of nasal expiratory positive airway pressure in patients with sleep-disordered breathing. J Appl Physiol. 2011; 111:1400–1409.
Gilmartin, G. S., Daly, R. W., Thomas, R. J. Recognition and management of complex sleep-disordered breathing. Curr Opin Pulm Med. 2005; 11:485–493.
Gilmartin, G., McGeehan, B., Vigneault, K., et al. Treatment of positive airway pressure treatment-associated respiratory instability with enhanced expiratory rebreathing space (EERS). J Clin Sleep Med. 2010; 6:529–538.
Gonzalez-Bermejo, J., Perrin, C., Janssens, J. P., et al. Proposal for a systematic analysis of polygraphy or polysomnography for identifying and scoring abnormal events occurring during non-invasive ventilation. Thorax. 2012; 67:546–552.
Janssens, J. P., Borel, J. C., Pépin, J. L., SomnoNIV Group. Nocturnal monitoring of home non-invasive ventilation: the contribution of simple tools such as pulse oximetry, capnography, built-in ventilator software and autonomic markers of sleep fragmentation. Thorax. 2011; 66:438–445.
Javaheri, S., Goetting, M. G., Khayat, R., Wylie, P. E., Goodwin, J. L., Parthasarathy, S. The performance of two automatic servo-ventilation devices in the treatment of central sleep apnea. Sleep. 2011; 34:1693–1698.
Kushida, C. A., Chediak, A., Berry, R. B., et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008; 4:157–171.
Rabec, C., Rodenstein, D., Leger, P., et al. Ventilator modes and settings during non-invasive ventilation: effects on respiratory events and implications for their identification. Thorax. 2011; 66:170–178.
Teschler, H., Döhring, J., Wang, Y. M., Berthon-Jones, M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001; 164:614–619.
Thomas, R. J., Daly, R. W., Weiss, J. W. Low-concentration carbon dioxide is an effective adjunct to positive airway pressure in the treatment of refractory mixed central and obstructive sleep-disordered breathing. Sleep. 2005; 28:69–77.