Pediatric Polysomnography
Pediatric Polysomnography: General Considerations
As polysomnography (PSG) has become more widely available and used for the assessment of sleep in children, it has become evident that the methods and facilities used for the study of adult patients do not always meet the needs of young children. There are several strategies by which the likelihood of successful pediatric studies can be maximized.
It is essential for sleep laboratories that study young children regularly to prepare a child-friendly environment. Appropriately decorated pediatric rooms containing an additional bed for a parent are optimal. The laboratory should provide age-appropriate distractions and activities that can be used during study setup, such as nonstimulating videos. Stuffed animals, toys, and stickers are useful for both distraction and reward for younger children. Snacks such as crackers, milk, or juice should also be available.
Proper preparation of the laboratory technical staff is also essential. Pediatric studies should always be performed by staff who are comfortable with and oriented toward children. Younger children, children with behavioral or developmental disabilities, and children with significant medical comorbidities may require additional staff for study setup and higher staffing levels during the night. Finally, the laboratory should schedule pediatric studies to coincide as closely as possible with the child’s typical sleep schedule, particularly for younger children with early bedtimes.
Advance preparation of families for the study is also quite important. Children should told what to expect during the study at a level appropriate for age. An advance tour of the sleep laboratory often helps acclimate youngsters to the equipment that will be used. Any potentially uncomfortable aspects of the study such as esophageal pressure (Pes) monitoring should be discussed with parents well in advance of the study.
Standard and Custom Polysomnographic Montages
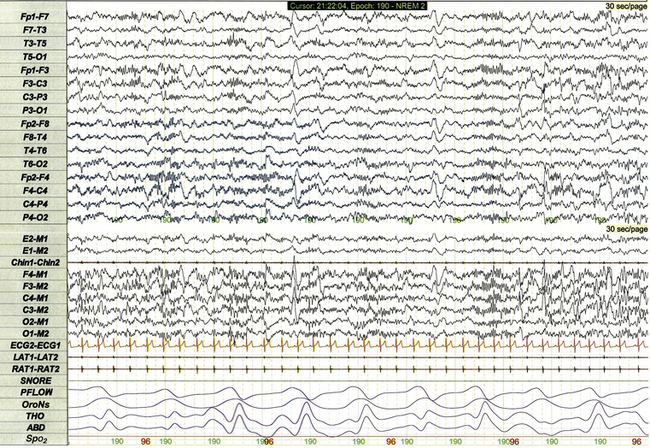
Figure 17.1 demonstrates a standard recording montage for children that conforms to the recommended specifications of the American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events. Electroencephalography (EEG) recording includes frontal (F4, F3), central (C4, C3), and occipital (O2, O1) leads referenced to the contralateral mastoid (M1, M2). Bilateral electro-oculogram (EOG) leads (E2, E1) permit detection of the slow eye movements of light sleep and the rapid movements associated with stage R. Surface electromyography (EMG) over the chin (Chin1-Chin2) permits detection of increased muscle tone with arousals or atonia associated with stage R sleep. Additional EMG leads over the left and right anterior tibialis muscles (LAT1-LAT2, RAT1-RAT2) permit recording of periodic limb movements and other limb movements associated with arousals or sleep-related movement disorders. Electrocardiogram (ECG) channels provide concurrent data reflecting cardiac rate and rhythm.
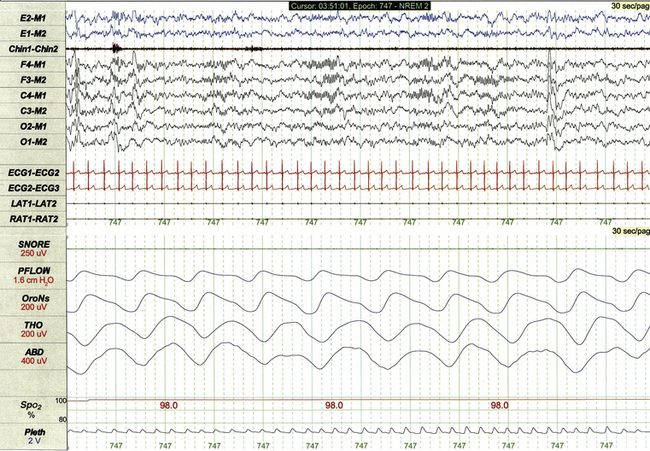
FIGURE 17.1 Standard pediatric recording montage demonstrating stage N2 sleep in a healthy 5-year-old (30-second epoch).
Standard respiratory monitoring for pediatric PSG includes a snore channel (SNORE), nasal pressure transducer (PFLOW), oronasal airflow (OroNs), thoracic effort (THO), and abdominal effort (ABD). Pulse oximetry (SpO2) is used for monitoring of baseline oxygen saturation and for detection of desaturations associated with sleep-disordered breathing.
The PSG recording montage for children can be customized depending on the nature of the sleep disorder being investigated. End-tidal carbon dioxide (ETCO2) or transcutaneous carbon dioxide (TcCO2) monitoring (Fig. 17.2) during PSG is appropriate for children with neuromuscular disorders, morbid obesity, Chiari I malformation, and other medical conditions associated with increased risk for nocturnal hypoventilation.
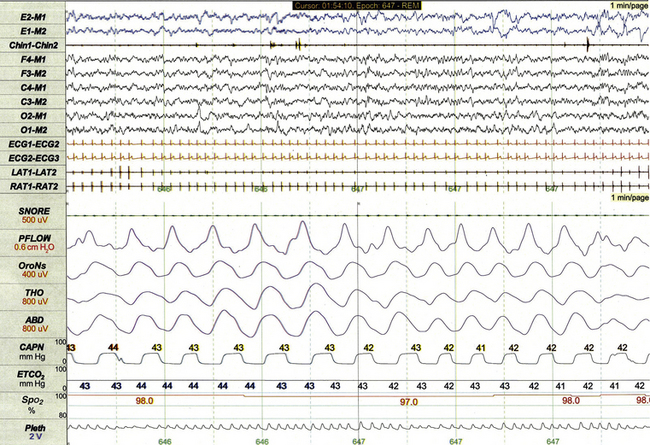
FIGURE 17.2 Pediatric polysomnographic montage with supplemental end-tidal CO2 (ETCO2) monitoring demonstrating normal CO2 levels during stage R sleep in a 9-year-old boy (1-minute epoch). ETCO2 is calculated by measuring peak CO2 levels at the end of each breath as displayed by the capnogram (CAPN). The channel labeled ETCO2 displays a rolling average of the peak levels recorded on the capnogram.
Use of Pes monitoring improves the sensitivity of PSG for detection of chronic partial airway obstruction and other findings associated with upper airway resistance syndrome (UARS). The technique uses a small water- or air-filled esophageal catheter attached to a pressure transducer for direct quantitative assessment of Pes fluctuations during each respiratory cycle (Fig. 17.3). Although minimally invasive, Pes monitoring can detect varieties of sleep-related airway obstruction that are often not evident on standard PSG. The technique is well tolerated by most school-age children and has negligible impact on sleep architecture.
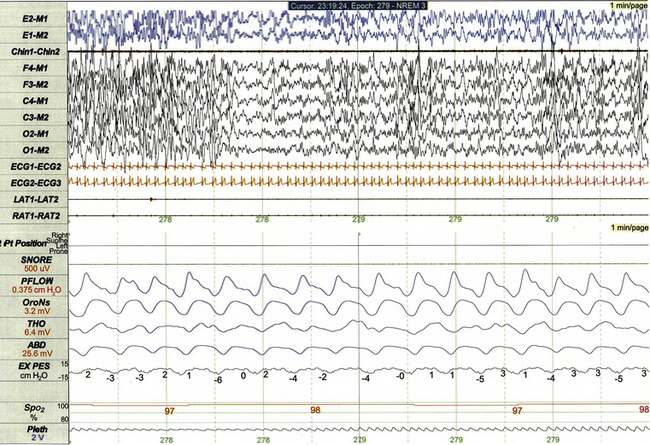
FIGURE 17.3 Pediatric polysomnographic montage with supplemental esophageal pressure (Pes) monitoring demonstrating normal Pes fluctuations during stage N3 sleep in a 10-year-old boy (1-minute epoch). The esophageal pressure tracing (labeled EX PES) demonstrates normal fluctuations (0 to −10 cm H2O, measured from peak to trough) with inspiration.
Sixteen-lead EEG can be performed during pediatric PSG (Fig. 17.4) when sleep-related seizures represent a clinical consideration. Technically satisfactory video recording is particularly important during expanded EEG studies to verify the presence or absence of any clinical symptoms coinciding with EEG findings. Although technically straightforward to perform, interpretation of expanded EEG studies is time consuming and requires that the polysomnographer be experienced in the interpretation of normal and abnormal EEG findings for the pediatric age-group.
Normal Sleep in Infants
The EEG characteristics of sleep for term newborns and younger infants are immature and poorly differentiated compared to the sleep of older children and adults. As a result, sleep staging for this age-group is initially restricted to stage N (undifferentiated non–rapid eye movement [non-REM] sleep, historically called quiet sleep) and stage R (infant REM sleep, historically called active sleep). For healthy term newborns, sleep spindles, slow wave activity, and other markers that distinguish lighter and deeper stages of non-REM sleep become evident beginning at 2 to 3 months postterm, eventually permitting more detailed scoring of non-REM sleep as stages N1, N2, or N3.
Infants often enter sleep with a period of stage R sleep, which constitutes almost half of total sleep time in term infants (Fig. 17.5). In Figure 17.6 the EEG background during stage R sleep for this 2-month-old infant is characterized by mixed frequencies of somewhat higher amplitude than those typical of REM sleep for older children. The rapid eye movements demonstrated in this epoch are not always apparent in younger infants, whereas the reduced EMG activity and irregular respiration demonstrated represent somewhat more consistent findings.

FIGURE 17.5 Hypnogram illustrating sleep cycling for a 5-month-old boy.
Typical characteristics of infant sleep include a sleep-onset rapid eye movement period, a high proportion of stage R sleep (49.8% in this study), and shorter sleep cycling time than the 90 minutes typical for older children.
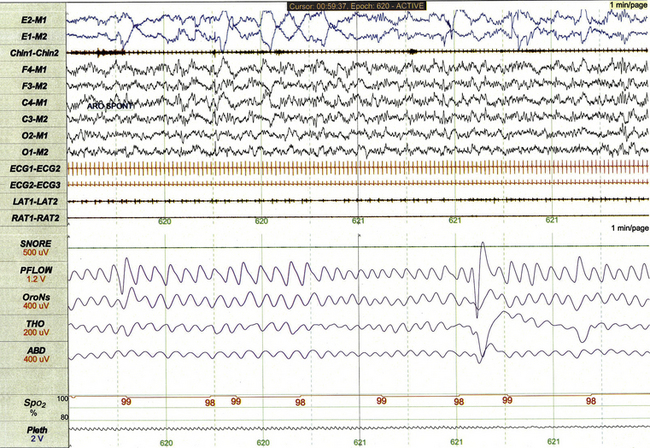
FIGURE 17.6 Infant rapid eye movement (REM) sleep (active sleep, stage R).
This 1-minute polysomnographic epoch for a 2-month-old girl with Prader-Willi syndrome demonstrates clearly evident rapid eye movements, muscle atonia with phasic twitches on chin electromyogram, and mixed-frequency electroencephalographic background. The irregular respiratory rate and effort in this epoch are also typical for infant REM sleep.
Stage N sleep during infancy is characterized primarily by high-amplitude delta and theta frequencies associated with deep, regular respiration and less-frequent limb and body movements compared to infant REM sleep (Fig. 17.7, demonstrating findings for the same 2-month-old infant shown in Fig. 17.6).
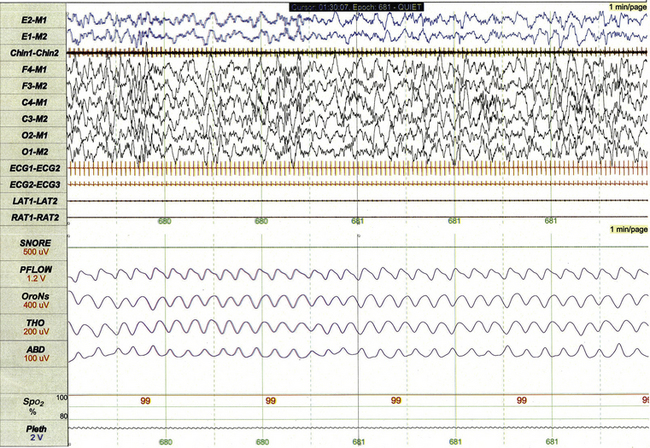
FIGURE 17.7 Infant non–rapid eye movement sleep (quiet sleep, stage N).
This 1-minute polysomnographic epoch for a 2-month-old-girl with Prader-Willi syndrome demonstrates typical findings of quiet sleep, including higher baseline chin electromyographic tone compared to active sleep, deep and regular respiration, and high-amplitude delta and theta frequency electroencephalographic (EEG) background. Immature sleep spindles are apparent in the central EEG channels.
Periodic breathing is a respiratory pattern characterized by cyclical periods of normal breathing interrupted at regular intervals by brief pauses in respiration (Fig. 17.8). This pattern is observed most commonly in premature infants but is occasionally observed in younger term infants as well. Scoring rules established by the 2007 AASM Manual for the Scoring of Sleep and Associated Events specify that periodic breathing may be scored in the presence of more than three episodes of central apnea separated by no more than 20 seconds of normal breathing.
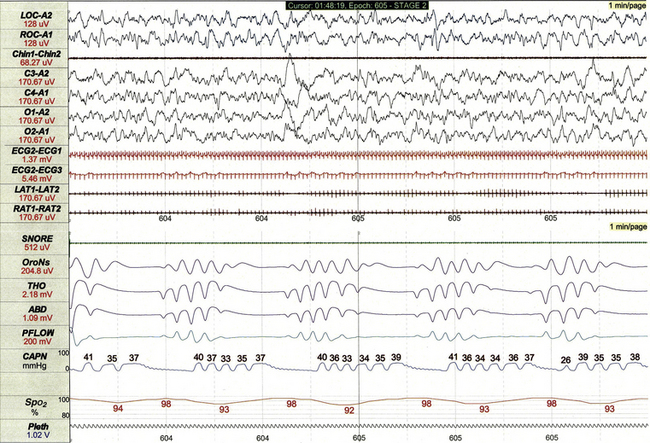
FIGURE 17.8 Periodic breathing.
This 1-minute polysomnographic epoch demonstrates periodic breathing in an otherwise healthy 2-month-old infant born at term. Although the central apneas are associated with desaturations of SpO2 of 5% to 6% below baseline, nadir levels of SpO2 are only 92% to 94%. Note that flow on the capnogram (CAPN) channel is delayed compared to other effort and airflow channels, reflecting the cannula transit time of exhaled air before CO2 levels can be measured.
Normal Sleep in Children
After infancy most aspects of sleep staging for children are comparable to those for adults, although PSG findings reflect overall maturation of sleep architecture as healthy children grow older. Stage R sleep declines from almost 50% of sleep time for newborns to about 20% by age 5 years. Sleep cycle duration gradually increases from 50 to 60 minutes during infancy to 90 to 100 minutes in later childhood.
Figures 17.1, 17.9, and 17.10 demonstrate normal non-REM and REM sleep in the same healthy 5-year-old girl.
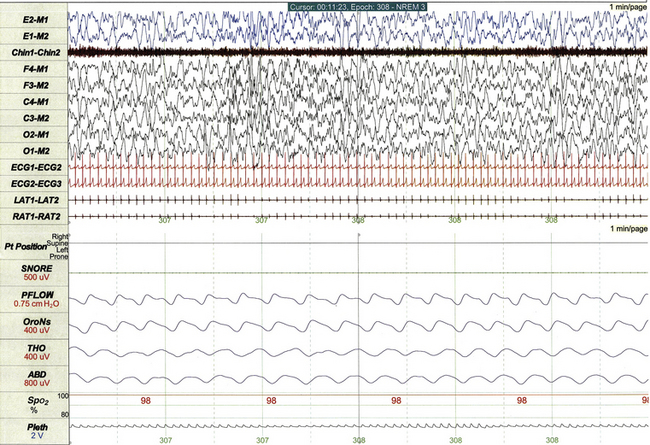
FIGURE 17.9 Normal non–rapid eye movement sleep in a 5-year-old.
This 1-minute epoch demonstrates stage N3 sleep, characterized by high-amplitude slow wave activity on electroencephalogram, deep regular respiration, and relatively high muscle tone on chin electromyogram.
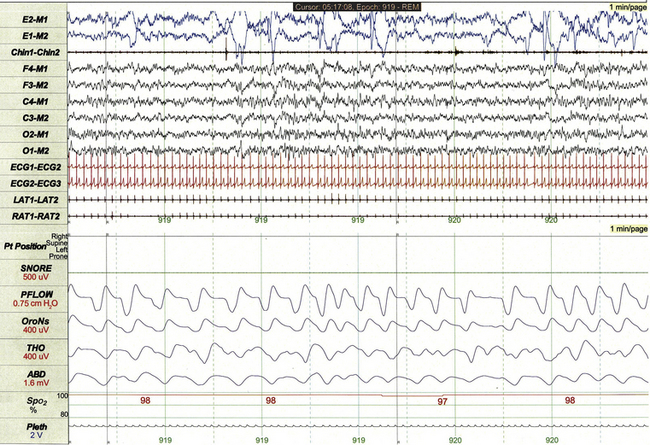
FIGURE 17.10 Normal rapid eye movement (REM) sleep in a 5-year-old.
This 1-minute epoch demonstrates stage R sleep, characterized by low-amplitude faster frequencies on electroencephalogram and less regular respiratory rate and effort compared to non-REM sleep. Rapid eye movements are clearly apparent on electro-oculographic leads. Note the absence of muscle tone on chin electromyogram compared to non-REM sleep.
Abnormal Polysomnographic Findings in Children
Sleep-Disordered Breathing
The 2007 AASM scoring manual respiratory rules for children require that obstructive apneas (Fig. 17.11) last at least the duration of two breaths, be associated with at least a 90% decrement in air flow from baseline, and be associated with continued or increased respiratory effort for the duration of the event. Arousals or desaturation of SpO2 levels are not required for scoring of obstructive apneas.
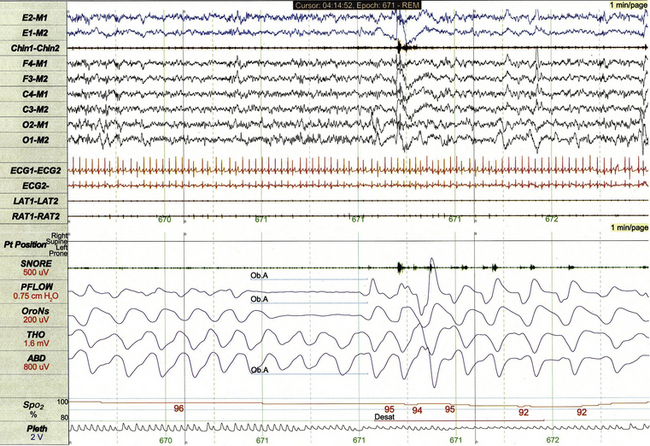
FIGURE 17.11 Obstructive apnea.
This 1-minute polysomnographic epoch demonstrates a brief obstructive apnea arising from rapid eye movement sleep in a 4-year-old girl. The event is associated with mild desaturation of SpO2, subtle arousal from sleep, and subsequent mild tachycardia.
These scoring rules require that hypopneas (Fig. 17.12) last at least the duration of two breaths, be associated with at least a 50% decrement in nasal pressure or alternative flow signal from baseline, and be associated with arousal, awakening, or at least a 3% desaturation of SpO2. Pes monitoring (Fig. 17.13) often demonstrates crescendo increases in respiratory effort when hypopneas are obstructive in nature.
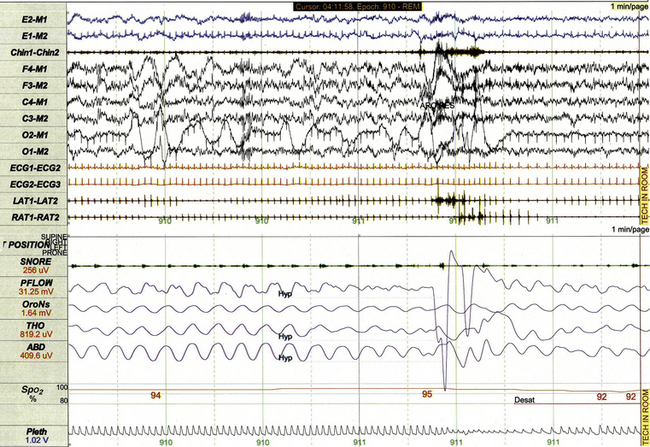
FIGURE 17.12 Hypopnea.
This 1-minute polysomnographic epoch demonstrates a hypopnea arising from REM sleep in a 12-year-old boy. The event is associated with brisk arousal from sleep, transient increase in electromyographic tone, and modest desaturation of SpO2 from baseline.
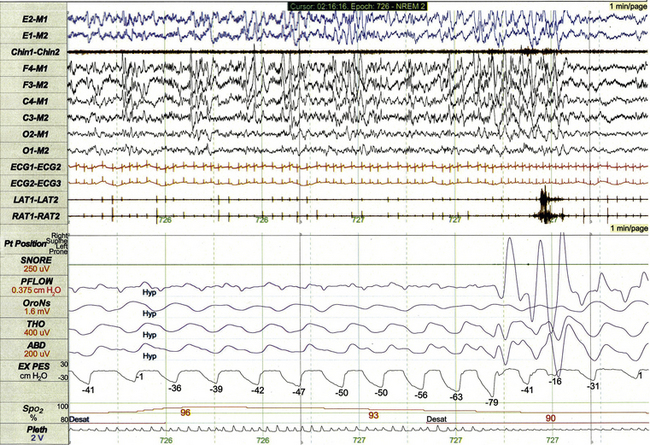
FIGURE 17.13 Obstructive hypopnea with esophageal pressure (Pes) crescendo.
This 1-minute polysomnographic epoch demonstrates an obstructive hypopnea arising from non–rapid eye movement sleep in a 16-year-old. The event is characterized by substantial decrement in nasal pressure flow (PFLOW) and subtle progressive decrement in oronasal flow (OroNs) associated with desaturation and electroencephalographic state change. The Pes tracing (labeled EX PES) demonstrates excessively negative fluctuations (measured from peak to trough) with inspiration, which become progressively more severe before partially normalizing upon termination of the event.
Central apneas (Fig. 17.14) may be scored on pediatric PSGs whenever respiratory effort is absent for at least 20 seconds or whenever respiratory pauses lasting at least the duration of two breaths are associated with arousal, awakening, or at least a 3% desaturation of SpO2. Polysomnographers should take care to distinguish when frequent central apneas meet criteria for the diagnosis of periodic breathing (Fig. 17.15) or Cheyne-Stokes respiration.
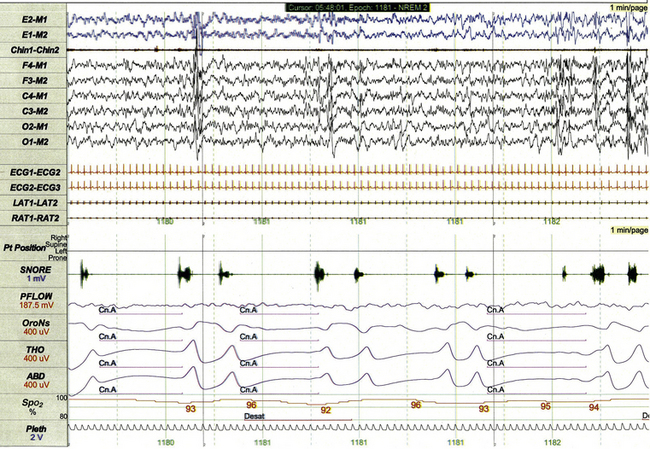
FIGURE 17.14 Central apneas.
This 1-minute polysomnographic epoch demonstrates several brief central apneas in a 5-year-old girl with familial dysautonomia and concurrent obstructive sleep apnea. The central apneas at the beginning of the epoch are associated with 3% to 4% desaturation of SpO2 from baseline, whereas the event at the end of epoch is associated with subtle arousal (partially shown). Poor nasal pressure flow in this tracing is secondary to chronic mouth breathing.
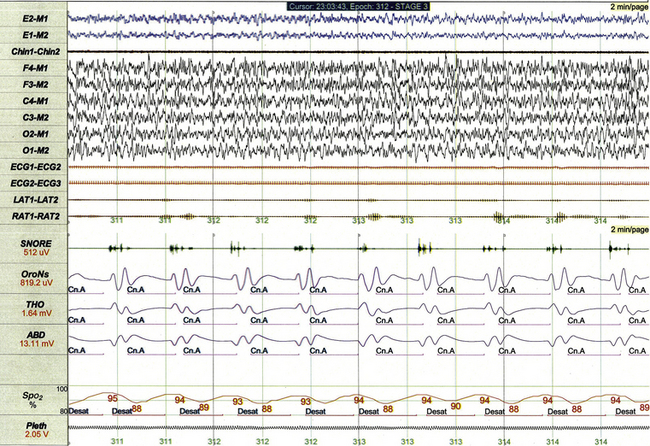
FIGURE 17.15 Recurrent central apneas meeting criteria for periodic breathing.
This 2-minute polysomnographic epoch demonstrates recurrent central apneas meeting criteria for classification of periodic breathing in a 3-month-old with a poorly characterized encephalopathy. Although periodic breathing can represent a mild and spontaneously remitting condition primarily affecting premature infants, it may also occur as a persistent variety of sleep-disordered breathing in infants and children with underlying neurological disorders.
Sleep-related hypoventilation (Fig. 17.16) is scored when at least 25% of total sleep time is spent with ETCO2 or TcCO2 levels exceeding 50 mm Hg. CO2 monitoring is not standard in many sleep laboratories but should be considered when performing PSG in children with neuromuscular disorders, Chiari malformation, obesity, or other medical conditions associated with increased risk for nocturnal hypoventilation.
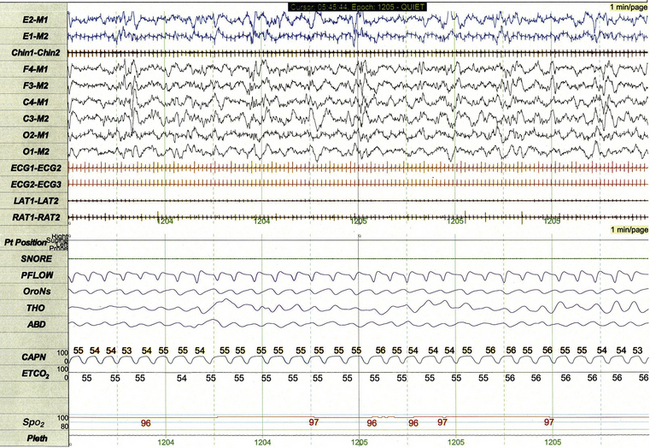
FIGURE 17.16 Sleep-related hypoventilation.
This 1-minute polysomnographic epoch demonstrates persistent hypercapnia during sleep in a 7-week-old girl with Down's syndrome, ventricular septal defect, and persistent pulmonary hypertension. ETCO2 is calculated by measuring peak CO2 levels at the end of each breath as displayed by the capnogram (CAPN). The channel labeled ETCO2 displays a running average of the peak levels recorded on the capnogram. Although SpO2 levels are normal in this epoch, more severe forms of hypoventilation may be associated with concurrent hypoxemia.
Increased resistance of the upper airway (Fig. 17.17) is the characteristic PSG feature of UARS, a condition in which chronic partial airway obstruction and increased work of breathing disturb sleep in the absence of scorable respiratory events and gas exchange abnormalities. Although flow limitation is occasionally apparent using standard PSG methods in patients with UARS, Pes monitoring represents a more sensitive measure of partial airway obstruction in these patients.
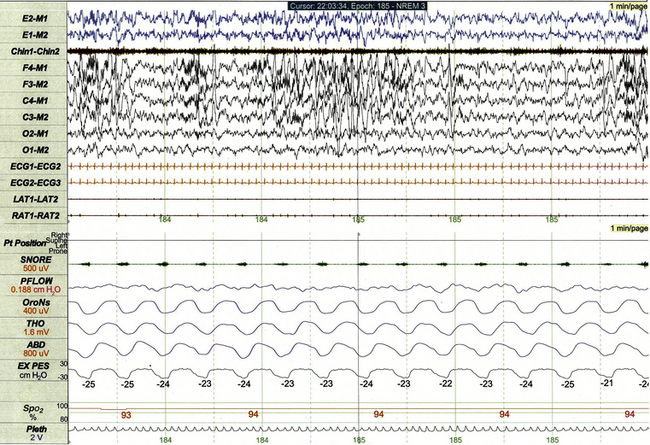
FIGURE 17.17 Increased resistance of the upper airway.
This 1-minute polysomnographic epoch demonstrates chronic partial airway obstruction during non–rapid eye movement sleep in a 9-year-old girl with Apert’s syndrome, cleft palate, and upper airway resistance syndrome. The esophageal pressure channel (labeled EX PES) demonstrates excessively negative pressure fluctuations with inspiration (measured from peak to trough, with normal being 0 to 10 cm H2O). Note that standard respiratory channels do not clearly demonstrate the presence or severity of underlying obstruction. The nasal pressure channel was unrevealing on this study because of mouth breathing.
It is important to recognize that PSG findings in children with sleep-disordered breathing must occasionally be correlated to a broader medical context. For example, the strikingly regular hypopneas illustrated in Figure 17.18 correlated exactly to the activation cycle of the vagus nerve stimulator used to treat this child’s epilepsy.
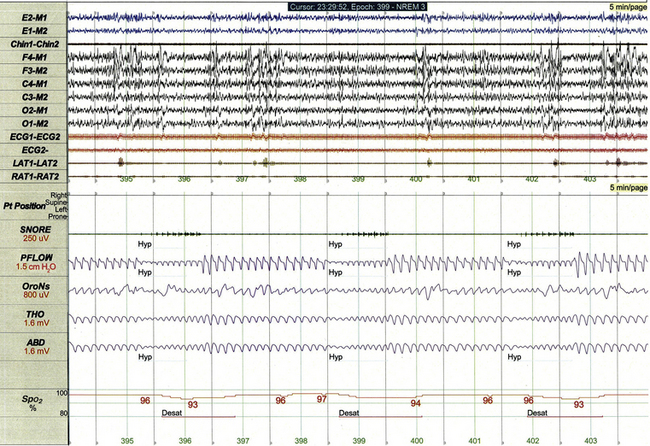
FIGURE 17.18 Hypopneas correlating with vagus nerve stimulation.
This 5-minute polysomnographic epoch demonstrates several 30-second hypopneas occurring at regular intervals in a 5-year-old girl with Down’s syndrome and medically refractory symptomatic generalized epilepsy. The pattern of hypopneas coincided exactly with the activation cycle of the child’s vagus nerve stimulator: 30 seconds on followed by 60 seconds off.
Parasomnias and Sleep-Related Movement Disorders
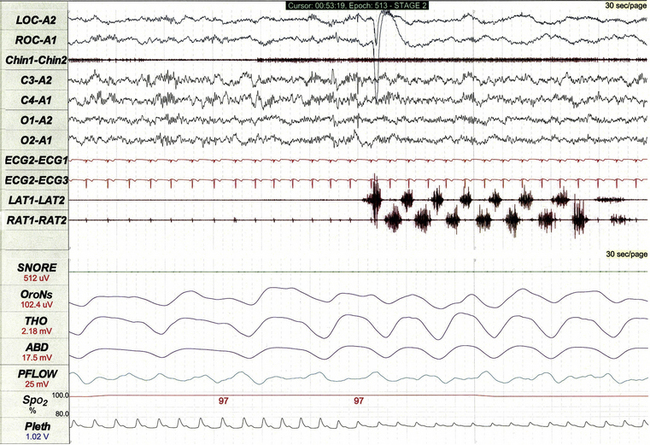
Non-REM arousal parasomnias such as night terrors (Fig. 17.19) and sleepwalking are common in children and occasionally recorded during PSG. These events are polysomnographically characterized by abrupt partial arousal from deep non-REM sleep, often associated with movement and/or muscle artifact. Sixteen-lead EEG (when available) and digital video recording help distinguish atypical varieties of parasomnia from seizure.
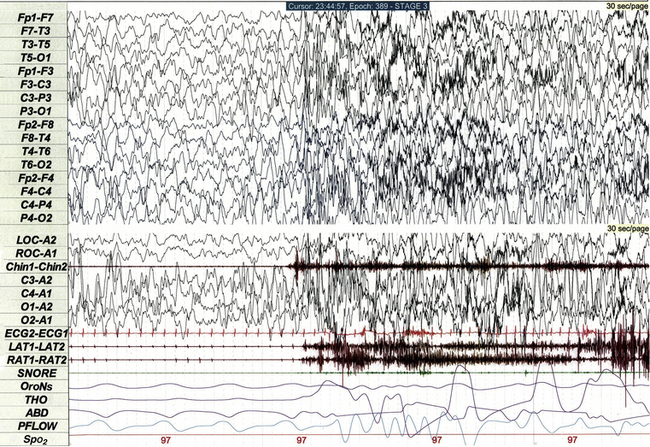
FIGURE 17.19 Night terror arising from slow wave sleep.
This 30-second polysomnographic epoch demonstrates a night terror arising from stage N3 sleep in a 4-year-old boy. The event is characterized electrographically by electroencephalographic (EEG) evidence of partial arousal from slow wave sleep. Although the EEG tracing is affected by movement and muscle artifact, the 16-lead EEG does not demonstrate any epileptiform activity. The event was clinically characterized by agitation and crying.
Sleep related rhythmical movement disorder is more common in children than in adults. The condition is clinically characterized by episodes of stereotyped limb and body movements that are often accompanied by synchronous vocalizations. Episodes may arise from wakefulness, during sustained sleep, or following arousal from sleep, lasting from seconds to minutes. Events recorded during PSG are associated with rhythmical high-amplitude movement artifact at typical frequencies of 0.5 to 2 Hz. Common varieties of rhythmical movement disorder include body rolling (Fig. 17.20), body rocking, leg banging (Fig. 17.21), and head banging (jactatio capitis nocturna).
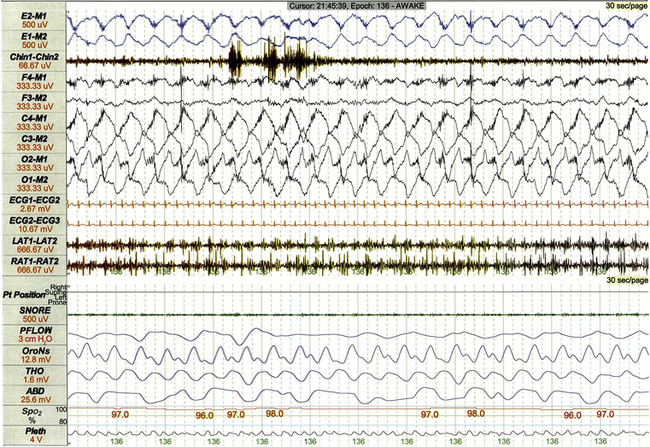
FIGURE 17.20 Sleep-related rhythmical movement disorder: body rolling.
This 30-second polysomnographic epoch demonstrates body rolling during wakefulness in a 9-year-old boy with rhythmical movement disorder. High-amplitude movement artifact at a frequency of 0.75 Hz is most apparent in central and occipital electroencephalographic leads.
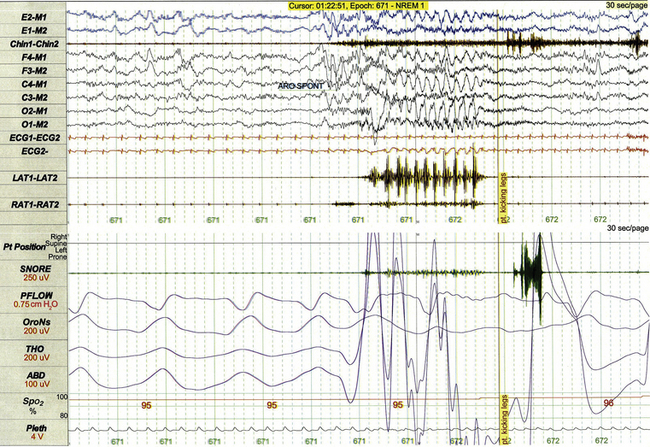
FIGURE 17.21 Sleep-related rhythmic movement disorder: leg banging.
This 30-second polysomnographic epoch demonstrates unilateral leg banging following arousal from sleep in a 12-year-old boy. Movement of the left leg (LAT1-LAT2) follows cortical arousal and coincides with high-amplitude rhythmical movement artifact in multiple electroencephalographic leads.
PSG features of periodic limb movement disorder in children are comparable to those of adults. Other sleep-related movement disorders occasionally observed in children include benign sleep myoclonus of infancy and alternating leg muscle activation (Fig. 17.22).
Electroencephalographic Abnormalities
Because epilepsy affects about 1% of children and because sleep often activates epileptiform EEG discharges, it is relatively common to identify spikes (20 to 70 msec in duration) and sharp waves (70 to 200 msec) during pediatric PSG studies. Although focal or generalized discharges (Figs. 17.23 and 17.24) are sometimes clearly demonstrable on standard PSG montages, supplemental 16-lead EEG allows more precise characterization and localization of epileptiform discharges (Fig. 17.25).
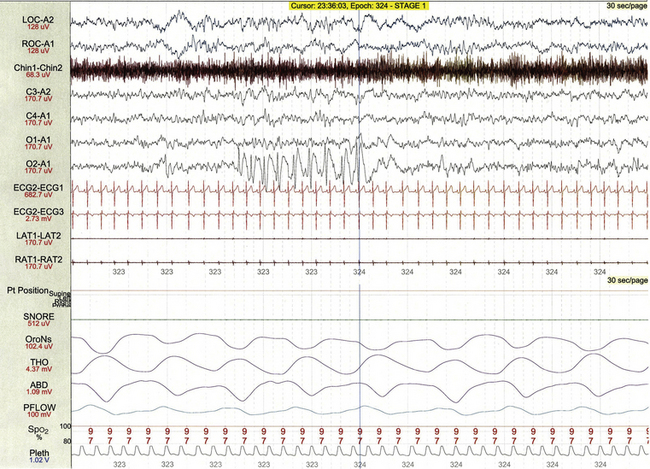
FIGURE 17.23 Occipital spikes.
This 30-second polysomnographic epoch demonstrates a brief run of 2-Hz spike and wave discharges localized to the right occipital region (O2-A1) in 9-year-old boy with obstructive sleep apnea but no known history of seizures.
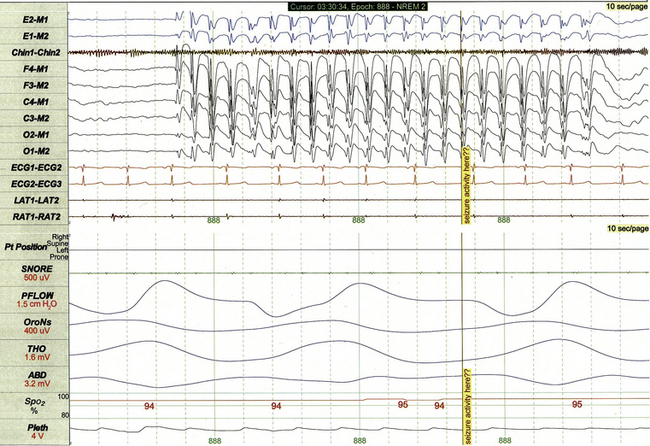
FIGURE 17.24 Generalized spike and wave discharges.
This 10-second polysomnographic epoch demonstrates a run of generalized 3-Hz spike and wave discharges in an 8-year-old girl with refractory juvenile absence epilepsy.
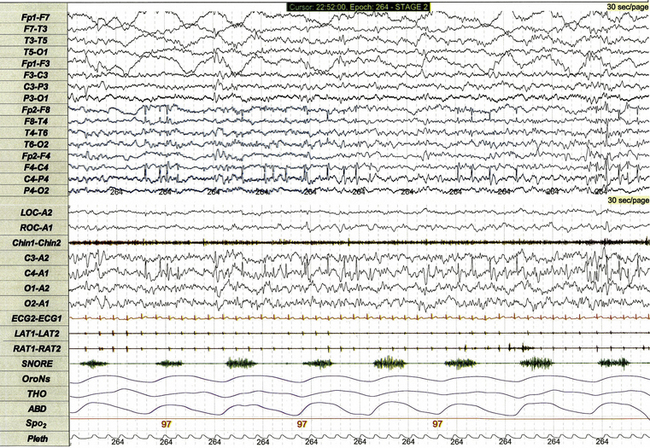
FIGURE 17.25 Sharp waves on standard and expanded electroencephalographic (EEG) montages.
This 30-second polysomnographic epoch demonstrates intermittent sharp waves arising from non–rapid eye movement sleep in a 4-year-old girl with a history of a single localization-related seizure arising from sleep. Although the central head lead used in the standard recording montage (C4-A1) demonstrates multiple sharp waves, the 16-lead EEG demonstrates more precise localization of the discharges to the right central, frontal, and temporal leads.
On occasion, clinical or electrographic seizures may be recorded for a child during PSG. Although tonic-clonic and other generalized seizures are often obvious to parents and technical staff attending the study, seizures characterized by subtle clinical manifestations are sometimes recognized only when the polysomnographer interpreting the study detects evolving rhythmical EEG activity (Fig. 17.26, A) that appears epileptiform in nature upon closer inspection (Fig. 17.26, B).
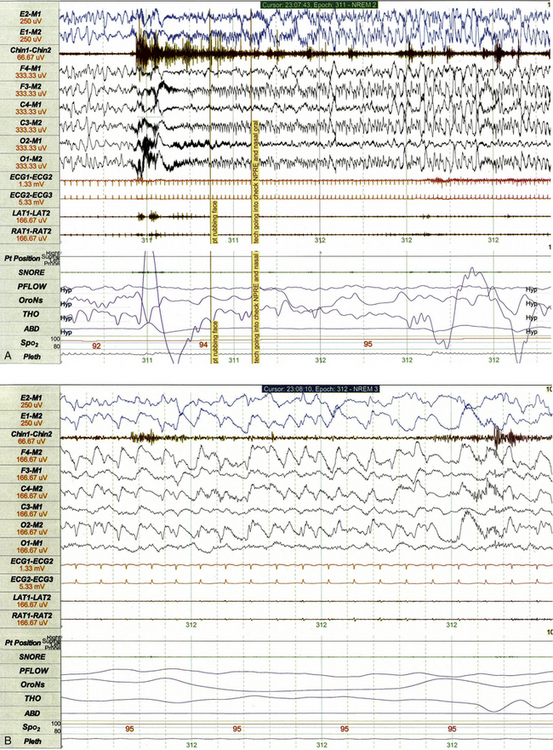
FIGURE 17.26 A, Subtle clinical seizure recorded on standard polysomnographic (PSG) montage. This 1-minute PSG epoch demonstrates findings recorded during a subtle clinical seizure for a 13-year-old girl with a metabolic disorder and refractory epilepsy. The event is electrographically characterized by abrupt arousal from slow wave sleep followed by evolving rhythmical activity best appreciated in the electroencephalographic leads referenced to the M2 electrode. The event was clinically characterized by face rubbing and stereotypical chewing movements, which were clearly evident upon review of the concurrent video recording. B, Subtle clinical seizure recorded on standard PSG montage. Closer inspection of the same event illustrated in A using a 10-second epoch length confirms that rhythmical slowing represents an ictal event. Electroencephalographic leads have been re-referenced to the ipsilateral rather than contralateral mastoid to permit more accurate localization and demonstration of rhythmical spike and wave activity in the right posterior head region (O2-M2).
Nocturnal frontal lobe epilepsy can affect children as a dominantly inherited genetic disorder or occur as a sporadic condition. When nocturnal frontal lobe seizures are recorded during PSG, EEG frequently does not demonstrate pathognomonic spike and wave activity (Fig. 17.27), so diagnosis is usually established on the basis of the medical history, event symptoms, or genetic testing.
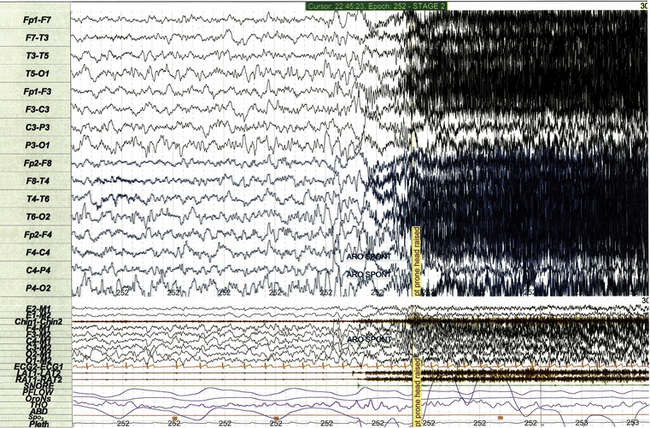
FIGURE 17.27 Nocturnal frontal lobe seizure on standard and expanded electroencephalographic montage.
This 30-second polysomnographic epoch demonstrates one of the numerous nocturnal seizures recorded in a 7-year-old boy with autosomal dominant nocturnal frontal lobe epilepsy (CHRNA4 sequence variant). The event is electrographically characterized by rhythmical fast activity in the frontal leads (Fp1-F3, Fp2-F4), which is subsequently obscured by movement and electromyographic artifact. Classic spike and wave discharges were not observed during any recorded seizure, even upon close inspection. Seizures were clinically characterized by groaning and extreme extension/arching of the neck and trunk.
Artifacts Unique to Pediatric Polysomnography
Several varieties of artifact are unique to pediatric PSG. Bottle-sucking artifact (Fig. 17.28) is characterized by increased muscle tone on chin EMG with or without associated movement artifact that is recorded when an infant or toddler is sucking and swallowing while awake. Rocking artifact (Fig. 17.29) results from movement when an infant or younger child is rocked by a parent.
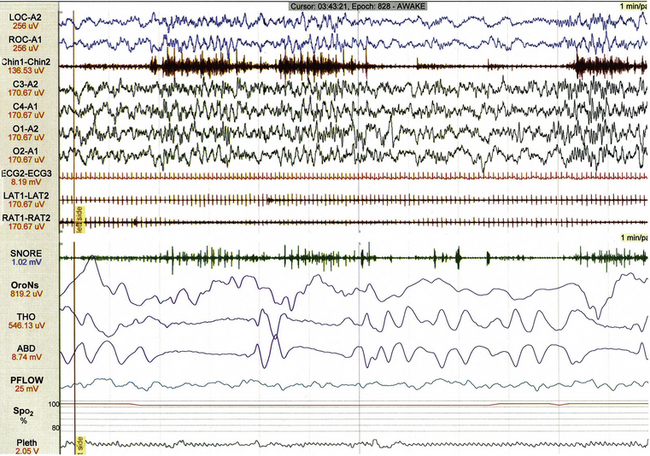
FIGURE 17.28 Bottle-sucking artifact.
This 1-minute polysomnographic epoch demonstrates sucking artifact in a 2-year-old girl. Increased muscle tone on chin electromyogram associated with high-amplitude movement artifact (best seen in ROC-A1) becomes evident 10 seconds following the beginning of the epoch.
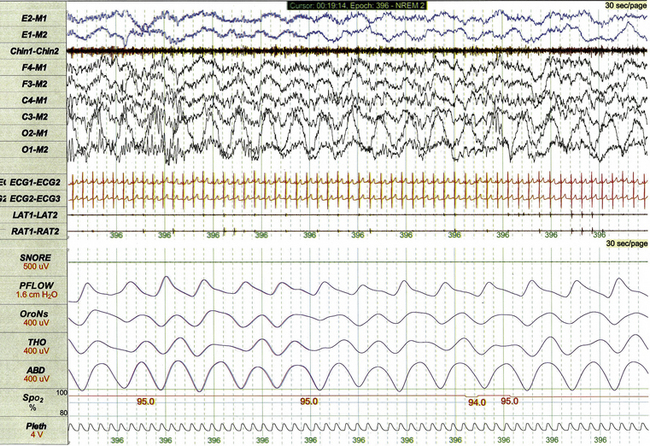
FIGURE 17.29 Rocking artifact.
This 30-second polysomnographic epoch demonstrates rocking artifact observed during the study of a 2-year-old. The artifact is most apparent in the O2-M1 derivation as rhythmical high-amplitude slowing. The child was being held by her mother, who was seated in a rocking chair.
Anders, T. F., Emde, R. N., Parmelee, A. H. A Manual of Standardized Terminology, Techniques, and Criteria for Scoring of States of Sleep and Wakefulness in Newborn Infants. Los Angeles: UCLA Brain Information Service, NINDS Neurological Information Network; 1971.
Chervin, R. D., Aldrich, M. S. Effects of esophageal pressure monitoring on sleep architecture. Am J Respir Crit Care Med. 1997; 156(3 Pt 1):881–885.
Chervin, R. D., Consens, F. B., Kutluay, E. Alternating leg muscle activation during sleep and arousals: a new sleep-related motor phenomenon? Mov Disord. 2003; 18(5):551–559.
Chervin, R. D., Ruzicka, D. L., Wiebelhaus, J. L., et al. Tolerance of esophageal pressure monitoring in children. Sleep. 2003; 26(8):1022–1026.
Grigg-Damberger, M., Gozal, D., Marcus, C. L., et al. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med. 2007; 3(2):201–240.
Guilleminault, C., Pelayo, R., Leger, D., Clerk, A., Bocian, R. C. Recognition of sleep-disordered breathing in children. Pediatrics. 1996; 98(5):871–882.
Hoban, T. F. Sleep disorders in children. Ann N Y Acad Sci. 2010; 1184:1–14.
Hoban, T. F. Sleep related rhythmic movement disorder. In: Thorpy M., Plazzi G., eds. The Parasomnias and Other Sleep-Related Movement Disorders. New York: Cambridge University Press, 2010.
Iber, C., Ancoli-Israel, S., Chesson, A. L., Jr., Quan, S. F. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, Ill: American Academy of Sleep Medicine; 2007.
Marzec, M., Edwards, J., Sagher, O., et al. Effects of vagus nerve stimulation on sleep-related breathing in epilepsy patients. Epilepsia. 2003; 44(7):930–935.
Provini, F., Plazzi, G., Tinuper, P., et al. Nocturnal frontal lobe epilepsy: a clinical and polygraphic overview of 100 consecutive cases. Brain. 1999; 122:1017–1031.