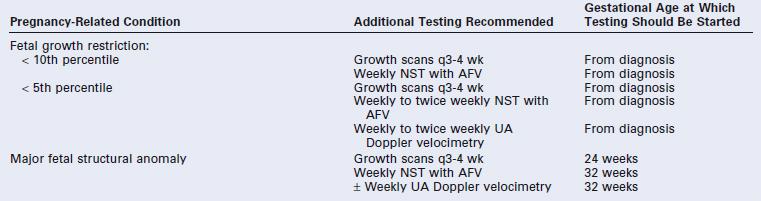
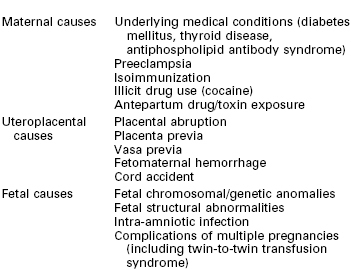
Antepartum Fetal Assessment and Therapy
Teresa Marino MD, Joong Shin Park MD, PhD, Errol R. Norwitz MD, PhD
Chapter Outline
PRENATAL CARE IN LOW-RISK PREGNANCIES
Determination of Gestational Age
Assessment of Fetal Well-Being
PRENATAL CARE IN HIGH-RISK PREGNANCIES
Goals of Antepartum Fetal Testing
SPECIAL TECHNIQUES FOR ANTEPARTUM SURVEILLANCE
Screening for Fetal Chromosomal Abnormalities
Definitive Diagnosis of Fetal Chromosomal Abnormalities
SPECIAL CIRCUMSTANCES REQUIRING ADDITIONAL FETAL SURVEILLANCE
Abnormal Serum Analyte and Nuchal Translucency Screening with Normal Fetal Karyotype
Obstetric care providers have two patients, the mother and the fetus. Although assessment of maternal health is relatively straightforward, assessment of fetal well-being is far more challenging. Several tests have been developed to assess the fetus during pregnancy, including some that are recommended for all pregnancies (e.g., ultrasonography for pregnancy dating) and others that are reserved only for women with pregnancy complications (e.g., middle cerebral artery Doppler velocimetry in pregnancies with isoimmunization). In addition, a limited number of fetal interventions are employed to improve fetal outcome, including some that are used frequently, such as maternal corticosteroid administration, and others that are used much more rarely, such as intrauterine fetal procedures. A review is presented here of the tests available to assess fetal well-being in both low- and high-risk pregnancies and of the fetal therapies used during the antepartum period.
Prenatal Care in Low-Risk Pregnancies
Determination of Gestational Age
The mean duration of a singleton pregnancy is 280 days (40 weeks) from the first day of the last normal menstrual period in women with regular 28-day menstrual cycles. Term, defined as the period from 37 weeks' (259 days') to 42 weeks' (294 days') gestation, is the optimal time for delivery. Both preterm births (defined as delivery before 37 weeks' gestation) and post-term births (delivery after 42 weeks' gestation) are associated with increased perinatal and neonatal morbidity and mortality. Evaluation of fetal growth, efficient use of screening and diagnostic tests, appropriate initiation of fetal surveillance, and optimal timing of delivery all depend on accurate dating of the pregnancy.
A number of clinical, biochemical, and radiologic tests are available to determine gestational age (Box 6-1).1,2 Determination of gestational age is most accurate in early pregnancy, and the estimated date of delivery (EDD) should be established at the first prenatal visit. Embryo transfer dating in women undergoing in vitro fertilization (IVF) is the most accurate clinical dating criterion. Among women with regular menstrual cycles who conceive spontaneously, if the first day of the last menstrual period (LMP) is known and if uterine size is consistent with dates by clinical examination, then Naegele's rule (subtract 3 months and add 7 days to the LMP) can be used to determine the EDD. Menstrual dating is known to be inaccurate in women taking oral contraceptives, in women who conceive in the immediate postpartum period, and in women who have irregular menstrual cycles or a history of intermenstrual bleeding. Moreover, clinical examination of uterine size can be inaccurate in women with a high body mass index (BMI), uterine fibroids, or a multifetal pregnancy. For these reasons, reliance on standard clinical criteria alone to determine the EDD will lead to an inaccurate diagnosis, with a tendency to overestimate gestational age.3-6 One study reported that reliance on LMP alone leads to a false diagnosis of preterm birth and post-term pregnancy in one fourth and one eighth of cases, respectively.7 Use of other historic factors (e.g., the date of the first positive pregnancy test result or the first perceived fetal movements [“quickening”]) and physical findings (e.g., the date when fetal heart sounds are first audible) may help obstetric providers determine the EDD more accurately (see Box 6-1).
Most early pregnancy tests involve the identification and quantification of human chorionic gonadotropin (hCG), a hormone produced by the syncytiotrophoblast of the fetoplacental unit.8,9 Levels in the maternal circulation increase exponentially to a peak of 80,000 to 100,000 mIU/mL at 8 to 10 weeks' gestation and then decrease to a level of 20,000 to 30,000 mIU/mL for the remainder of the pregnancy. Commercially available hCG test kits can detect concentrations as low as 25 to 50 mIU/mL in serum or urine, which are typically evident 8 to 9 days after conception.
Uncertainty in dating parameters should prompt ultrasonographic assessment of gestational age. Transabdominal ultrasonography can identify an intrauterine sac in 94% of eutopic (intrauterine) pregnancies once the serum hCG concentration is 6000 mIU/mL or higher.10 With the use of transvaginal ultrasonography, an intrauterine pregnancy can typically be confirmed at a serum hCG level of 1500 to 2000 mIU/mL.11 Failure to confirm an intrauterine sac at these hCG levels should raise concerns about an abnormal pregnancy (e.g., ectopic pregnancy, missed abortion) and requires further evaluation. A fetal pole and cardiac activity should be visible at a serum hCG concentration of approximately 1700 mIU/mL (5 to 6 weeks) and 5400 mIU/mL (6 to 8 weeks), respectively. The EDD derived from ultrasonographic evaluation should be used when there is a 5- to 7-day discrepancy from LMP dating in the first trimester.11
In the first trimester, the fetal crown-rump length (CRL) is the most accurate determinant of gestational age (± 3 to 5 days). In the second trimester, the biparietal diameter (BPD) and length of the long bones (especially femur length) are the ultrasonographic measurements used most often to determine gestational age. Of these, the BPD is the more accurate indicator with a variation of ± 7 to 10 days.12 Two large clinical studies of approximately 50,000 pregnancies demonstrated that a second-trimester BPD measurement, when used instead of menstrual dating to establish the EDD, resulted in a significant increase in the number of women who delivered within 7 days of their due dates and a 60% to 70% reduction in the number of pregnancies continuing post term.13,14 After 26 weeks' gestation, the variation in the BPD measurement is greater (± 14 to 21 days), thereby making it less valuable in estimating gestational age.12 Both femur length and humerus length correlate strongly with the BPD and gestational age and are sometimes used for additional confirmation.15 By contrast, because abdominal circumference (AC) reflects fetal nutritional status and growth, it is less accurate than either BPD or femur length. All fetal biometric measurements are subject to some degree of error, so a number of techniques have been used to predict gestational age more accurately. Serial determinations of gestational age at 2- to 3-week intervals may be more accurate than a single determination in the third trimester to confirm dating and eliminate the possibility of fetal growth restriction.
Routine Ultrasonography
Routine early ultrasonography significantly improves the accuracy of gestational age dating.3-6,16-19 Early ultrasonography can also detect pregnancy abnormalities (e.g., molar pregnancy), major fetal structural abnormalities (e.g., anencephaly), and multiple pregnancy. Although recommended in Europe, the practice of routine ultrasonography for pregnancy dating has not been recommended as a standard of prenatal care in the United States.20,21
The usefulness of routine second-trimester ultrasonography in all pregnant women remains a subject of debate. Early studies suggested an improvement in perinatal outcome with its use.17,22-25 For example, one prospective clinical trial in Helsinki, Finland, randomly assigned 9310 low-risk women either to a single screening ultrasonographic examination at 16 to 20 weeks' gestation or to ultrasonography for obstetric indications only; a significantly lower perinatal mortality rate was found in the screening ultrasonography group (4.6 versus 9.0 per 1000 births, respectively).18 This difference was due in part to earlier detection of major fetal malformations (which prompted elective abortion) and multiple pregnancies (which resulted in more appropriate antenatal care) with the screening examination. As expected, routine ultrasonography also led to improved pregnancy dating and a lower rate of induction of labor for post-term pregnancy.18
In contrast, a subsequent large multicenter randomized clinical trial involving 15,151 low-risk women in the United States (designated as the RADIUS study) concluded that screening ultrasonography did not improve perinatal outcomes and had no impact on the management of the anomalous fetus.19,26,27 Although this trial was adequately powered, it has been criticized for the highly selective entry criteria (by one estimate, less than 1% of pregnant women in the United States would have been eligible28) and the selection of primary outcomes (perinatal morbidity and mortality) that were inappropriate for the low-risk population studied. In addition, only 17% of major congenital anomalies were detected before 24 weeks' gestation in the routine ultrasonography group, so the rate of elective pregnancy termination for fetal anomalies was significantly lower than that in the Helsinki study. The skill and experience of the ultrasonographer is also an important variable in these studies.
Evaluation of Fetal Growth
Normal fetal growth is a critical component of a healthy pregnancy and the subsequent long-term health of the child. Maternal weight gain during pregnancy is at best an indirect measure of fetal growth, because much of the weight gain during pregnancy is the result of fluid (water) retention. Earlier recommendations for weight gain in pregnancy were based on the Institute of Medicine (IOM) guidelines published in 1990.29 In 2009, the IOM revised their 1990 recommendations to include an upper limit of weight gain for obese women (9 kg [20 lb]), and they altered the lower limit of weight gain from 6.8 kg (15 lb) to 5 kg (11 lb) (Table 6-1); they also recommended that all women try to be within the normal BMI range when they conceive.30
TABLE 6-1
Recommendations for Weight Gain in Pregnancy
| Mother's Body Mass Index | Recommended Weight Gain |
| 18.5 to 24.9 kg/m2 (normal weight) | 11.2 to 15.9 kg (25 to 35 lb) |
| 25 to 29.9 kg/m2 (overweight) | 6.8 to 11.2 kg (15 to 25 lb) |
| >30 kg/m2 (obesity) | 5.0 to 9.0 kg (11 to 20 lb) |
Data from the Institute of Medicine. Nutritional status and weight gain. In Nutrition during Pregnancy. Available at http://iom.edu/Reports/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines.aspx.
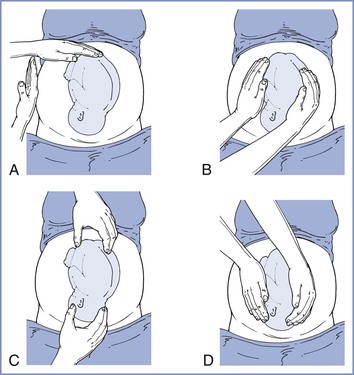
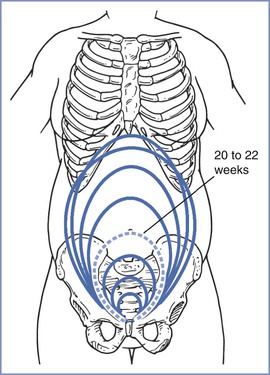
The size, presentation, and lie of the fetus can be assessed with abdominal palpation. A systematic method of examination of the gravid abdomen was first described by Leopold and Sporlin in 1894.31 Although the abdominal examination has several limitations (especially in the setting of a small fetus, maternal obesity, multiple pregnancy, uterine fibroids, or polyhydramnios), it is safe, is well tolerated, and may add valuable information to assist in antepartum management. Palpation is divided into four separate Leopold's maneuvers (Figure 6-1). Each maneuver is designed to identify specific fetal landmarks or to reveal a specific relationship between the fetus and mother. The first maneuver, for example, involves measurement of the fundal height. The uterus can be palpated above the pelvic brim at approximately 12 weeks' gestation. Thereafter, fundal height should increase by approximately 1 cm per week, reaching the level of the umbilicus at 20 to 22 weeks' gestation (Figure 6-2). Between 20 and 32 weeks' gestation, the fundal height (in centimeters) is approximately equal to the gestational age (in weeks) in healthy women of average weight with an appropriately growing fetus. However, there is a wide range of normal fundal height measurements. In one study, a 6-cm difference was noted between the 10th and 90th percentiles at each week of gestation after 20 weeks.32 Moreover, maximal fundal height occurs at approximately 36 weeks' gestation, after which time the fetus drops into the pelvis in preparation for labor. For all of these reasons, reliance on fundal height measurements alone fails to identify more than 50% of fetuses with fetal growth restriction (also known as intrauterine growth restriction).33 Serial fundal height measurements by an experienced obstetric care provider are more accurate than a single measurement and will lead to better diagnosis of fetal growth restriction, with reported sensitivities as high as 86%.34
If clinical findings are not consistent with the stated gestational age, ultrasonography is indicated to confirm gestational age and provide a more objective measure of fetal growth. Ultrasonography may also identify an alternative explanation for the discrepancy, such as multifetal pregnancy, polyhydramnios, fetal demise, and uterine fibroids. For many years, obstetric ultrasonography has used fetal biometry to define fetal size by weight estimations. This approach has a number of limitations. First, regression equations used to create weight estimation formulas are derived primarily from cross-sectional data for infants being delivered within an arbitrary period after the ultrasonographic examination. Second, these equations assume that body proportions (fat, muscle, bone) are the same for all fetuses.34-45 Finally, growth curves for “normal” infants between 24 and 37 weeks' gestation rely on data collected from pregnancies delivered preterm, which are abnormal and probably complicated by some element of uteroplacental insufficiency, regardless of whether the delivery was spontaneous or iatrogenic. Despite these limitations, if the gestational age is well validated, the prevailing data suggest that prenatal ultrasonography can be used to verify an alteration in fetal growth in 80% of cases and to exclude abnormal growth in 90% of cases.46
Ultrasonographic estimates of fetal weight are commonly derived from mathematical formulas that use a combination of fetal measurements, especially the BPD, AC, and femur length.47 The AC is the single most important measurement and is weighted more heavily in these formulas. Unfortunately, the AC is also the most difficult measurement to acquire, and small differences in the measured value result in large changes in the estimated fetal weight (EFW). The accuracy of the EFW depends on a number of variables, including gestational age (in absolute terms, EFW is more accurate in preterm or growth-restricted fetuses than in term or macrosomic fetuses), operator experience, maternal body habitus, and amniotic fluid volume (measurements are more difficult to acquire if the amniotic fluid volume is low). Objective ultrasonographic EFW estimations have an error of 15% to 20%, even in experienced hands.48 Indeed, an ultrasonographic EFW at term is no more accurate than a clinical estimate of fetal weight made by an experienced obstetric care provider or the mother's estimate of fetal weight if she has delivered before.49 Ultrasonographic estimates of fetal weight must therefore be evaluated within the context of the clinical situation and balanced against the clinical estimate. Serial ultrasonographic evaluations of fetal weight are more useful than a single measurement in diagnosing abnormal fetal growth. The ideal interval for fetal growth evaluations is every 3 to 4 weeks, because more frequent determinations may be misleading. Similarly, the use of population-specific growth curves, if available, improves the ability of the obstetric care provider to identify abnormal fetal growth. For example, growth curves derived from a population that lives at high altitude, where the fetus is exposed to lower oxygen tension, will be different from those derived from a population at sea level. Abnormal fetal growth can be classified as insufficient (fetal growth restriction) or excessive (fetal macrosomia).
Fetal Growth Restriction
The definition of fetal growth restriction has been a subject of long-standing debate. Distinguishing the healthy, constitutionally small-for-gestational-age (SGA) fetus, defined as having an EFW below the 10th percentile for a given week of gestation, from the nutritionally deprived, truly growth-restricted fetus has been particularly difficult. Fetuses with an EFW less than the 10th percentile are not necessarily pathologically growth restricted. Conversely, an EFW above the 10th percentile does not necessarily mean that an individual fetus has achieved its growth potential, and such a fetus may still be at risk for perinatal mortality and morbidity. Therefore, fetal growth restriction is best defined as either (1) an EFW less than the 5th percentile for gestational age in a well-dated pregnancy or (2) an EFW less than the 10th percentile for gestational age in a well-dated pregnancy with evidence of fetal compromise, such as oligohydramnios or abnormal umbilical artery Doppler velocimetry.
Fetal growth restriction has traditionally been classified as either asymmetric or symmetric fetal growth restriction. Asymmetric fetal growth restriction, characterized by normal head growth but suboptimal body growth, is seen most commonly in the third trimester. It is believed to result from a late pathologic event (e.g., chronic placental abruption leading to uteroplacental insufficiency) in an otherwise uncomplicated pregnancy and normal fetus. In cases of symmetric fetal growth restriction, both the fetal head size and body weight are reduced, indicating a global insult that likely occurred early in gestation. Symmetric fetal growth restriction may reflect an inherent fetal abnormality (e.g., fetal chromosomal anomaly, inherited metabolic disorder, early congenital infection) or long-standing severe placental insufficiency due to an underlying maternal disease (e.g., hypertension, pregestational diabetes mellitus, or collagen vascular disorder). In practice, the distinction between asymmetric and symmetric fetal growth restriction is not particularly useful.
Early and accurate diagnosis of fetal growth restriction coupled with appropriate intervention leads to an improvement in perinatal outcome. If fetal growth restriction is suspected clinically and on the basis of ultrasonography, a thorough evaluation of the mother and fetus is indicated. Referral to a maternal-fetal medicine specialist should be considered. Every effort should be made to identify the cause of the fetal growth restriction and to modify or eliminate contributing factors. Up to 20% of cases of severe fetal growth restriction are associated with fetal chromosomal abnormalities or congenital malformations, 25% to 30% are related to maternal conditions characterized by vascular disease, and a smaller proportion are the result of abnormal placentation. Other causes of fetal growth restriction include exposure to teratogens, alcohol, and substance abuse. In a substantial number of cases (>50% in some studies), the etiology of the fetal growth restriction remains unclear even after a thorough investigation.50
Fetal Macrosomia
Fetal macrosomia is defined as an EFW (not birth weight) of 4500 g or greater measured either clinically or by ultrasonography and is independent of gestational age, diabetic status, and actual birth weight.51 Fetal macrosomia should be differentiated from the large-for-gestational age (LGA) fetus, in whom the EFW is greater than the 90th percentile for gestational age. By definition, 10% of all fetuses are LGA at any given gestational age. Fetal macrosomia is associated with an increased risk for cesarean delivery, instrumental vaginal delivery, and birth injury to both the mother (including vaginal, perineal, and rectal trauma) and the infant (orthopedic and neurologic injury).52-56 Shoulder dystocia with resultant brachial plexus injury (Erb's palsy) is a serious consequence of fetal macrosomia; it is more likely in the setting of diabetes because of the larger diameters of the fetal upper thorax and neck.
Fetal macrosomia can be determined clinically (e.g., Leopold's maneuvers) or with ultrasonography, and these two techniques appear to be equally accurate.57 Estimated fetal weight measurements are less accurate in macrosomic fetuses than in normally grown fetuses, and factors such as low amniotic fluid volume, advancing gestational age, maternal obesity, and fetal position can compound these inaccuracies. Indeed, clinical examination has been shown to underestimate the birth weight by more than 0.5 kg in almost 80% of macrosomic fetuses.58 For all these reasons, prediction of fetal macrosomia is not particularly accurate, with a false-positive rate of 35% and a false-negative rate of 10%.57,58 A number of alternative ultrasonographic measurements have therefore been proposed in an attempt to better identify the macrosomic fetus, including fetal AC alone,59 umbilical cord circumference,60 cheek-to-cheek diameter,61 and subcutaneous fat in the mid humerus, thigh, abdominal wall, and shoulder.62 However, these measurements remain investigational.
Despite the inaccuracy in the prediction of fetal macrosomia, an EFW should be documented by either clinical estimation or ultrasonography in all high-risk women at approximately 38 weeks' gestation. Suspected fetal macrosomia is not an indication for induction of labor, because induction does not improve maternal or fetal outcomes and may increase the risk for cesarean delivery.51 The American College of Obstetricians and Gynecologists (ACOG) recommends performance of an elective cesarean delivery when the suspected birth weight exceeds 4500 g in a diabetic woman or 5000 g in a nondiabetic woman.51,52,63
Assessment of Fetal Well-Being
All pregnant women should receive regular antenatal care throughout their pregnancy, and fetal well-being should be evaluated at every visit. Fetal heart activity should be assessed and the fetal heart rate (FHR) estimated. A low FHR (<100 bpm) is associated with an increased risk for pregnancy loss, although congenital complete heart block should be excluded. In the latter half of pregnancy, physical examination of the abdomen should be performed to document fetal lie and presentation.
Fetal movements (“quickening”) are typically reported at 18 to 20 weeks' gestation by nulliparous women and at 16 to 18 weeks' gestation by parous women; the presence of fetal movements is strongly correlated with fetal health. Although the mother appreciates only 10% to 20% of total fetal movements,64-67 such movements are almost always present when she does report them.67 Factors associated with a diminution in perceived fetal movements include increasing gestational age, smoking, decreased amniotic fluid volume, anterior placentation, and antenatal corticosteroid therapy. Decreased fetal movements may also be a harbinger of an adverse pregnancy event (e.g., stillbirth) that can be averted if detected early. For these reasons, a subjective decrease in perceived fetal movements in the third trimester should prompt an immediate investigation.
Published studies support the value of fetal movement charts (“kick counts”) in the detection and prevention of fetal complications (including stillbirth) in both high- and low-risk populations.68-73 The normal fetus exhibits an average of 20 to 50 (range of 0 to 130) gross body movements per hour, with fewer movements during the day and increased activity between 9:00 PM and 1:00 AM.74 Several different schemes have been proposed to determine the baseline fetal activity pattern for an individual fetus after 28 weeks' gestation and to evaluate activity patterns that may represent fetal compromise. One commonly used scheme (“count-to-10”) instructs the mother to rest quietly on her left side once each day in the evening (between 7:00 PM and 11:00 PM) and to record the time interval required to feel 10 fetal movements. Most patients with a healthy fetus will feel 10 movements in approximately 20 minutes; 99.5% of women with a healthy fetus feel this amount of activity within 90 minutes.75 Under this scheme, failure to appreciate 10 fetal movements in 2 hours should prompt immediate fetal assessment. In one large clinical trial, institution of this fetal activity monitoring scheme resulted in a significant increase in hospital visits, labor induction, and cesarean deliveries, but also in a reduction in perinatal mortality from 44.5 to 10.3 per 1000 births.75 Taken together, these data suggest that daily or twice-daily fetal “kick counts” should be performed after 32 weeks' gestation in high-risk pregnancies. Currently there is insufficient evidence to recommend this practice in low-risk pregnancies.
Prenatal Care in High-Risk Pregnancies
Approximately 20% of all pregnancies should be regarded as high risk (Box 6-2). Because of the attendant risks to both the mother and fetus, additional efforts should be made to confirm fetal well-being throughout such pregnancies. In addition to the testing outlined previously, high-risk pregnancies should be monitored closely and regularly by a multidisciplinary team, including subspecialists in maternal-fetal medicine and neonatology, if indicated.
Goals of Antepartum Fetal Testing
The goal of antepartum fetal surveillance is the early identification of a fetus at risk for preventable neurologic injury or death. Numerous causes of neonatal cerebral injury exist, including congenital abnormalities, chromosomal abnormalities, intracerebral hemorrhage, hypoxia, infection, drugs, trauma, hypotension, and metabolic derangements (e.g., hypoglycemia, thyroid dysfunction). Antenatal fetal testing cannot reliably predict or detect all of these causes; however, those specifically associated with uteroplacental vascular insufficiency should be identified when possible. Antenatal fetal testing makes the following assumptions: (1) pregnancies may be complicated by progressive fetal asphyxia that can lead to fetal death or permanent neurologic handicap; (2) current antenatal tests can adequately discriminate between asphyxiated and nonasphyxiated fetuses; and (3) detection of asphyxia at an early stage can lead to an intervention that is capable of reducing the likelihood of an adverse perinatal outcome.
Of interest, it is not clear whether any of these assumptions are true, and nonreassuring fetal test results may reflect existing but not ongoing neurologic injury. At most, 15% of cases of cerebral palsy are thought to result from antepartum or intrapartum hypoxic-ischemic injury.75-78 Despite these limitations, a number of antepartum tests have been developed in an attempt to identify fetuses at risk. These include the nonstress test (NST), biophysical profile (BPP), and contraction stress test (CST). Such tests can be used either individually or in combination. There is no consensus as to which of these modalities is preferred, and no single method has been shown to be superior.1
Antepartum Fetal Tests
All antepartum fetal tests should be interpreted in relation to the gestational age, the presence or absence of congenital anomalies, and underlying clinical risk factors.79 For example, a nonreassuring NST in a pregnancy complicated by severe fetal growth restriction and heavy vaginal bleeding at 32 weeks' gestation has a much higher predictive value in identifying a fetus at risk for subsequent neurologic injury than an identical tracing in a well-grown fetus at 40 weeks, because of the higher prevalence of this condition in the former situation. It should be remembered that, in many cases, the efficacy of antenatal fetal testing in preventing long-term neurologic injury has not been validated by prospective randomized clinical trials. Indeed, because of ethical and medicolegal concerns, there are no studies of pregnancies at risk that include a nonmonitored control group, and it is highly unlikely that such trials will ever be performed.
Nonstress Test
The fetal nonstress test, also known as fetal cardiotocography, investigates changes in the FHR pattern with time and reflects the maturity of the fetal autonomic nervous system. For this reason, it is less useful in the extremely premature fetus (< 28 weeks) before the autonomic nervous system has matured sufficiently to influence the FHR. The NST is noninvasive, simple to perform, inexpensive, and readily available in all obstetric units. However, interpretation of the NST is largely subjective. Although a number of different criteria have been used to evaluate these tracings, most obstetric care providers have used the definitions for FHR interpretation established in 1997 by the National Institute of Child Health and Human Development (NICHD) Research Planning Workshop (Table 6-2).80 A 2008 NIH report summarized terminology and nomenclature used in contemporary clinical practice. This report described a three-tier system for FHR tracing interpretation: category I (normal), category II (indeterminate), and category III (abnormal).81
TABLE 6-2
Interpretation of Antepartum Nonstress Test Results

Data from the National Institute of Child Health and Human Development Research Planning Workshop. Electronic fetal heart rate monitoring: research guidelines for interpretation. Am J Obstet Gynecol 1997; 177:1385-90.
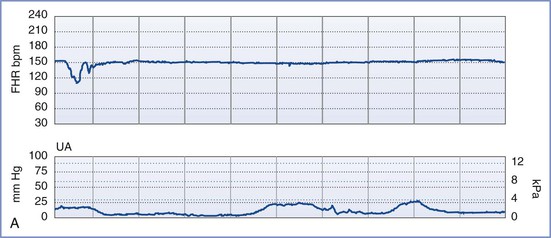
By definition, an NST is performed before the onset of labor and does not involve invasive (intrauterine) monitoring. The test is performed by recording the FHR for a period of 20 to 40 minutes; the recording is then evaluated for the presence of periodic changes. The FHR is determined externally with use of Doppler ultrasonography, in which sound waves emitted from the transducer are deflected by movements of the heart and heart valves. The shift in frequency of these deflected waves is detected by a sensor and converted into heart rate. The FHR is printed on a strip-chart recorder running at 3 cm/min. A single mark on the FHR tracing therefore represents the average rate in beats per minute (bpm) of 6 fetal heart beats. The presence or absence of uterine contractions is typically recorded at the same time with an external tonometer. This tonometer records myometrial tone and provides information about the timing and duration of contractions, but it does not measure intrauterine pressure or the intensity of the contractions. Results of the NST are interpreted as reactive or nonreactive. An FHR tracing is designated reactive if there are two or more accelerations of at least 15 bpm for 15 seconds in a 20-minute period (Figure 6-3).80-82 For preterm fetuses (<32 weeks' gestation), an FHR tracing is designated as reactive if there are two or more accelerations of at least 10 bpm for 10 seconds.
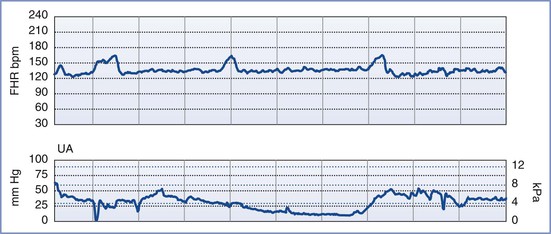
FIGURE 6-3 A normal (reactive) fetal heart rate (FHR) tracing. The baseline FHR is normal (between 110 and 160 bpm), there is moderate variability (defined as 6 to 25 bpm from peak to trough), there are no decelerations, and there are two or more accelerations (defined as an increase in FHR of ≥ 15 bpm above baseline lasting at least 15 seconds) in a 20-minute period.
An NST is performed when formal documentation of the fetal condition is necessary. Because most healthy fetuses move within a 75-minute period, the testing period for an NST should not exceed 80 minutes.83 The NST is most useful in cases of suspected uteroplacental insufficiency. A reactive NST is regarded as evidence of fetal health,84,85 but the interpretation of a nonreactive NST remains controversial. Determination of a nonreactive NST must consider the gestational age, the underlying clinical circumstance, and the results of previous FHR tracings. Only 65% of fetuses have a reactive NST by 28 weeks' gestation, whereas 95% do so by 32 weeks.79,86 However, once a reactive NST has been documented in a given pregnancy, the NST should remain reactive throughout the remainder of the pregnancy. A nonreactive NST at term is associated with poor perinatal outcome in only 20% of cases. The significance of such a result at term depends on the clinical endpoint under investigation. If the clinical endpoint of interest is a 5-minute Apgar score less than 7, a nonreactive NST at term has a sensitivity of 57%, a positive predictive value of 13%, and a negative predictive value of 98% (assuming a prevalence of 4%). If the clinical endpoint is permanent neurologic injury, a nonreactive NST at term has a 99.8% false-positive rate.87
Visual interpretation of the FHR tracing involves the following components: (1) baseline FHR, (2) baseline FHR variability, (3) presence of accelerations, (4) presence of periodic or episodic decelerations, and (5) changes of FHR pattern over time. The definitions of each of these variables are summarized in Table 6-2.80,81 The patterns are categorized as baseline, periodic (i.e., associated with uterine contractions), or episodic (i.e., not associated with uterine contractions). Periodic changes are described as abrupt or gradual (defined as onset-to-nadir time < 30 seconds or > 30 seconds, respectively). In contrast to earlier classifications, this classification makes no distinction between short-term and long-term variability, and certain characteristics (e.g., the definition of an acceleration) depend on gestational age (see Table 6-2).80,81
A normal FHR tracing is defined as having a normal baseline rate (110 to 160 bpm), normal baseline variability (i.e., moderate variability, defined as 6 to 25 bpm from peak to trough), presence of accelerations, and absence of decelerations. The FHR typically accelerates in response to fetal movement. Therefore, FHR accelerations usually indicate fetal health and adequate oxygenation.80-82 At-risk FHR patterns demonstrate recurrent late decelerations with absence of baseline variability, recurrent variable decelerations with absence of baseline variability, or substantial bradycardia with absence of baseline variability (Figure 6-4). Intermediate FHR patterns have characteristics between the two extremes of normal and at risk already described.80,81
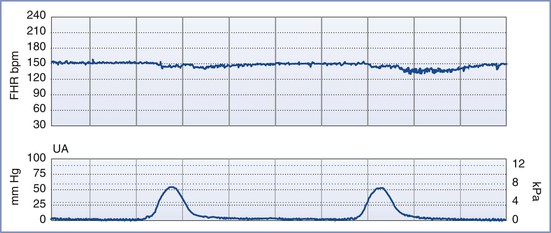
FIGURE 6-4 An “at risk” fetal heart rate (FHR) tracing. The baseline FHR is normal (between 110 and 160 bpm), but the following abnormalities can be seen: minimal baseline FHR variability (defined as 0 to 5 bpm from peak to trough), no accelerations, and decelerations that are late in character (start after the peak of the contraction) and repetitive (occur with more than half of the contractions).
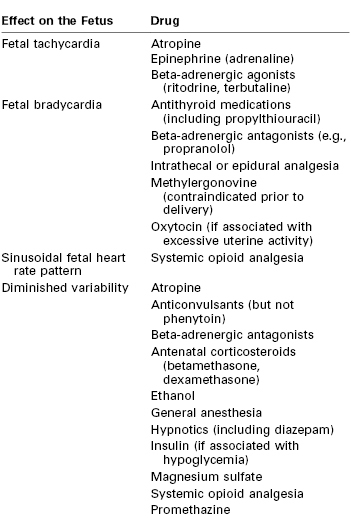
Persistent fetal tachycardia (defined as an FHR > 160 bpm) may be associated with fetal hypoxia, maternal fever, chorioamnionitis (intrauterine infection), administration of an anticholinergic or beta-adrenergic receptor agonist, fetal anemia, or tachyarrhythmia. Persistent fetal bradycardia (FHR < 110 bpm) may be a result of congenital heart block, administration of a beta-adrenergic receptor antagonist, hypoglycemia, or hypothermia (Table 6-3). However, it may also indicate fetal hypoxia.80,81 Both tachyarrhythmias and bradyarrhythmias require immediate evaluation.
Baseline FHR variability, perhaps the most important component of the NST, is determined on a beat-to-beat basis by the competing influences of the sympathetic and parasympathetic nervous systems on the fetal sinoatrial node. A variable FHR, characterized by fluctuations that are irregular in both amplitude and frequency,80,81 indicates that the autonomic nervous system is functioning and that the fetus has normal acid-base status. Variability is defined as absent, minimal, moderate, or marked (see Table 6-2) (Figure 6-5).80,81 The older terms short-term variability and long-term variability are no longer used.81 Normal (moderate) variability indicates the absence of cerebral hypoxia. With acute hypoxia, variability may be minimal or marked. Persistent or chronic hypoxia is typically associated with loss of variability. Reduced variability also may be the result of other factors, including maternal drug administration (see Table 6-3), fetal arrhythmia, and neurologic abnormality (e.g., anencephaly).1,80,81
Vibroacoustic Stimulation
Fetal vibroacoustic stimulation (VAS) refers to the response of the FHR to a vibroacoustic stimulus (82 to 95 dB) applied to the maternal abdomen for 1 to 2 seconds in the region of the fetal head. An FHR acceleration in response to VAS represents a positive result and is suggestive of fetal health. VAS is a useful adjunct to shorten the time needed to achieve a reactive NST and to decrease the proportion of nonreactive NSTs at term, thereby precluding the need for further testing. In one study of low-risk women at term, VAS reduced the proportion of nonreactive NSTs over a 30-minute period by 50% (from 14% to 9%) and shortened the time needed to achieve a reactive NST by an average of 4.5 minutes.88 VAS has no adverse effect on fetal hearing. The absence of an FHR acceleration in response to VAS at term is associated with an 18-fold higher risk for nonreassuring fetal testing in labor89 and a 6-fold higher risk for cesarean delivery.90
Biophysical Profile
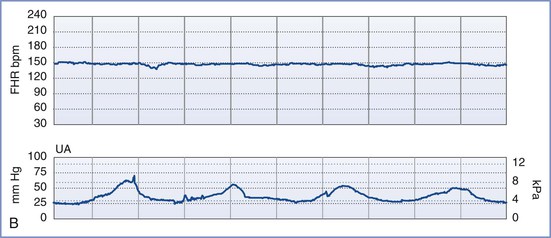
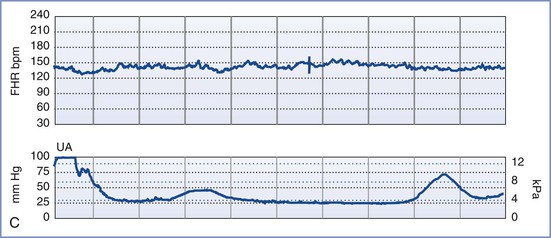
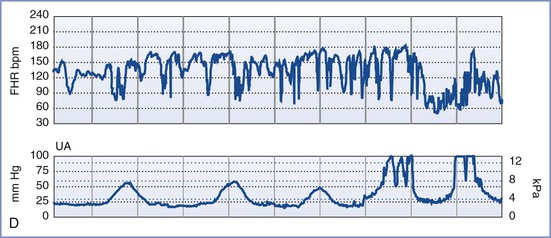
An NST alone may not be sufficient to confirm fetal well-being. In such cases, a biophysical profile (BPP) may be performed. The BPP is an ultrasonographic scoring system performed over a 30- to 40-minute period designed to assess fetal well-being. Initially described for testing of the post-term fetus, the BPP has since been validated for use in both term and preterm fetuses, but not during active labor.91-97 The five variables described in the original BPP were (1) gross fetal body movements, (2) fetal tone (i.e., flexion and extension of limbs), (3) amniotic fluid volume, (4) fetal breathing movements, and (5) the NST.97 More recently, the BPP has been interpreted without the NST (Table 6-4).
TABLE 6-4
Characteristics of the Biophysical Profile
| Biophysical Variable | Normal Score (Score = 2) | Abnormal Score (Score = 0) |
| Fetal breathing movements (FBMs) | At least one episode of FBM lasting at least 30 sec | Absence of FBM altogether or no episode of FBM lasting ≥ 30 sec |
| Gross body movements | At least three discrete body/limb movements in 30 min (episodes of active continuous movements should be regarded as a single movement) | Fewer than three episodes of body/limb movements over a 30-min period |
| Fetal tone | At least one episode of active extension with return to flexion of fetal limbs or trunk; opening and closing of hand are considered normal tone | Slow extension with return to partial flexion, movement of limb in full extension, or absence of fetal movements |
| Qualitative amniotic fluid (AF) volume | At least one pocket of AF that measures ≥ 1 cm in two perpendicular planes | No AF pockets or an AF pocket measuring < 1 cm in two perpendicular planes |
| Reactive nonstress test | At least two episodes of FHR acceleration of ≥ 15 bpm lasting ≥ 15 sec associated with fetal movements over 30 min of observation | Fewer than two episodes of FHR accelerations or accelerations of < 15 bpm over 30 min of observation |
Data from Manning FA. Fetal biophysical assessment by ultrasound. In Creasy RK, Resnik R, editors. Maternal-Fetal Medicine: Principles and Practice. 2nd edition. Philadelphia, WB Saunders, 1989:359.
The individual variables of the BPP become apparent in the normal fetus in a predictable sequence: fetal tone appears at 7.5 to 8.5 weeks' gestation, fetal movement at 9 weeks, fetal breathing at 20 to 22 weeks, and FHR reactivity at 24 to 28 weeks. In the setting of antepartum hypoxia, these characteristics typically disappear in the reverse order of their appearance (i.e., FHR reactivity is lost first, followed by fetal breathing, fetal movements, and finally fetal tone).93 The amniotic fluid volume, which is composed almost entirely of fetal urine in the second and third trimesters, is not influenced by acute fetal hypoxia or acute fetal central nervous system dysfunction. Rather, oligohydramnios (decreased amniotic fluid volume) in the latter half of pregnancy and in the absence of ruptured membranes is a reflection of chronic uteroplacental insufficiency and/or increased renal artery resistance leading to diminished urine output.98 It predisposes to umbilical cord compression, thus leading to intermittent fetal hypoxemia, meconium passage, or meconium aspiration. Adverse pregnancy outcome (including a nonreassuring FHR tracing, low Apgar scores, and/or admission to the neonatal intensive care unit) is more common when oligohydramnios is present.98-101 Weekly or twice-weekly screening of high-risk pregnancies for oligohydramnios is important because amniotic fluid can become drastically reduced within 24 to 48 hours.102
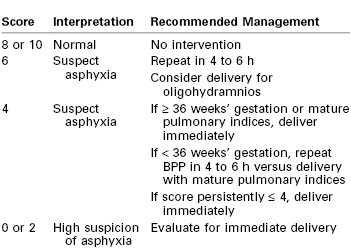
Although each of the five features of the BPP are scored equally (2 points if the variable is present or normal and 0 points if absent or abnormal, for a total of 10 points), they are not equally predictive of adverse pregnancy outcome. For example, amniotic fluid volume is the variable that correlates most strongly with adverse pregnancy events. The management recommended on the basis of the BPP score is summarized in Table 6-5.97 A score of 8 or 10 is regarded as reassuring; a score of 4 or 6 is suspicious and requires reevaluation; and a score of 0 or 2 suggests nonreassuring fetal status (previously referred to as “fetal distress”).91,92 Evidence of nonreassuring fetal status should prompt evaluation for immediate delivery.93,94
Contraction Stress Test
Also known as the oxytocin challenge test (OCT), the contraction stress test is an older test of uteroplacental function. It assesses the response of the FHR to uterine contractions induced by either intravenous oxytocin administration or nipple stimulation (which causes release of endogenous oxytocin from the maternal neurohypophysis). A minimum of three contractions of minimal-to-moderate strength in 10 minutes is required to interpret the test. A negative CST (no decelerations with contractions) is reassuring and suggestive of a healthy, well-oxygenated fetus. A positive CST (repetitive late or severe variable decelerations with contractions with at least 50% of the contractions) is suggestive of a fetus suffering from impaired maternal-to-fetal oxygen exchange during uterine contractions and is associated with adverse perinatal outcome in 35% to 40% of cases (Figure 6-6). The combination of a positive CST and absence of FHR variability is especially ominous. Consideration should be given to immediate and urgent delivery of a fetus with a positive CST, with or without FHR variability. It should be noted, however, that the false-positive rate of this test exceeds 50%.84 If the CST is uninterpretable or equivocal, the test should be repeated in 24 to 72 hours. Studies suggest that more than 80% of results of repeated tests are negative. The rate of antepartum intrauterine fetal demise within 1 week of a negative CST is 0.04%.84,97
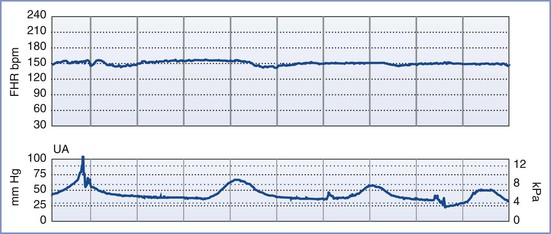
FIGURE 6-6 A positive contraction stress test (CST) result. There are at least three contractions in a 10-minute period. The baseline fetal heart rate (FHR) is 130 bpm, there is minimal baseline FHR variability (defined as 0 to 5 bpm from peak to trough), and there are decelerations that are late in character (start after the peak of the contraction) and repetitive (occur with more than half of the contractions).
Because this test is time consuming, requires skilled nursing care, and necessitates an inpatient setting owing to the possibility of precipitating fetal compromise requiring emergency cesarean delivery, the CST is reserved for specific clinical indications. Moreover, there are a number of contraindications to its use, including placenta previa, placental abruption, prior classic (high-vertical) cesarean delivery, and risk for preterm labor. Despite these limitations, the CST allows for indirect evaluation of fetal oxygenation during periods of uterine contractions and diminished uteroplacental perfusion and may therefore provide a better assessment of fetal well-being and fetal reserve than either the NST or the BPP (Table 6-6).1,84,95,103
TABLE 6-6
False-Positive and False-Negative Rates for the Nonstress Test, Biophysical Profile, and Contraction Stress Test
| Test | False-Positive Rate (%) | False-Negative Rate (per 1000 live births)* |
| Nonstress test (NST) | 58 | 1.4 to 6.2 |
| Biophysical profile (BPP): | 0.7 to 1.2 | |
| • Score 6/10 | 45 | |
| • Score 0/10 | 0 | |
| Contraction stress test (CST) | 30 | 0.4 to 0.6 |
* Data are presented as perinatal mortality rate within 1 wk of a reactive NST, a BPP score of 8 or 10, or a negative CST after adjustments for congenital anomalies and known causes.
Data from references 1, 84, 95, and 103.
Umbilical Artery Doppler Velocimetry
Doppler velocimetry shows the direction and characteristics of blood flow and can be used to examine the maternal, uteroplacental, or fetal circulation. The umbilical artery is one of the few arteries that normally has diastolic flow and consequently is one of the vessels most frequently evaluated during pregnancy. Umbilical artery Doppler velocimetry measurements reflect resistance to blood flow from the fetus to the placenta. Normally, umbilical artery resistance falls progressively throughout pregnancy, reflecting the increase in number of tertiary stem vessels. Factors that affect placental vascular resistance include gestational age, placental location, pregnancy complications (e.g., placental abruption, preeclampsia), and underlying maternal disease (chronic hypertension).
Doppler velocimetry of umbilical artery blood flow provides an indirect measure of fetal status. Decreased diastolic flow with a resultant increase in the systolic-to-diastolic (S/D) ratio suggests an increase in placental vascular resistance and fetal compromise. Severely abnormal umbilical artery Doppler velocimetry (defined as absence of or reversed diastolic flow) is an especially ominous observation and is associated with poor perinatal outcome in the setting of fetal growth restriction (Figure 6-7).104-108 The role of ductus venosus and/or middle cerebral artery (MCA) Doppler velocimetry in the management of fetal growth restriction pregnancies is not well defined. Preparation for delivery—including administration of corticosteroids for fetal lung maturity and transfer to a tertiary delivery center—should be considered when Doppler findings are severely abnormal in the setting of fetal growth restriction, regardless of gestational age. However, in the presence of a normally grown fetus, it is unclear how to interpret such findings. For these reasons, umbilical artery Doppler velocimetry should not be performed routinely in women at low risk for fetal abnormalities. Appropriate indications include fetal growth restriction, cord malformations, unexplained oligohydramnios, suspected or established preeclampsia, and, possibly, fetal cardiac anomalies.
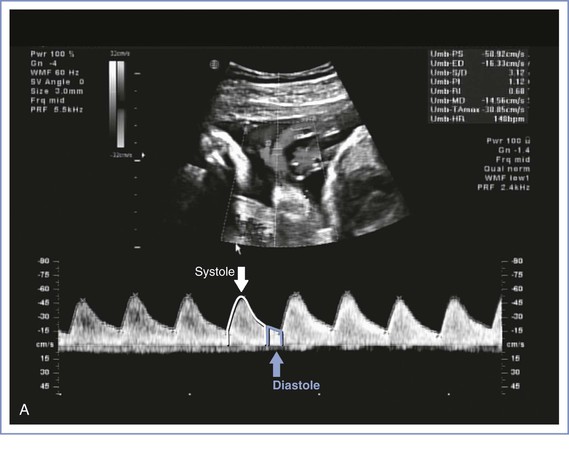
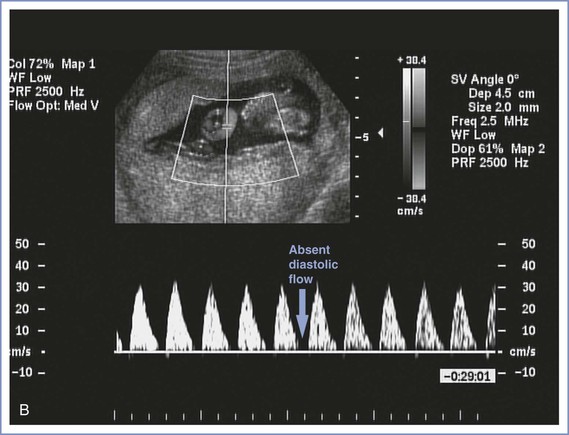
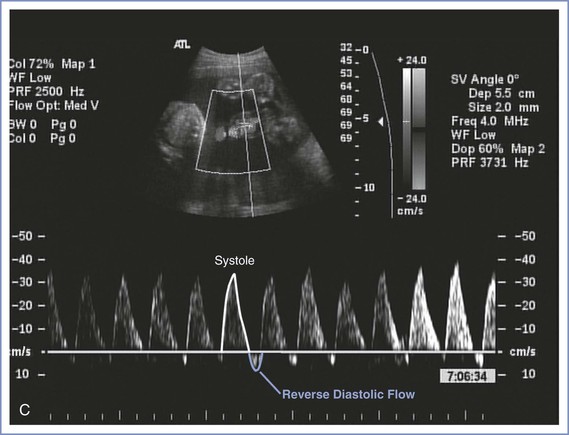
FIGURE 6-7 Umbilical artery Doppler velocimetry. A, Normal waveform in the umbilical artery as shown on Doppler velocimetry. Forward flow can be seen during both fetal systole and diastole. B, Absent end-diastolic flow. Forward flow can be seen during systole, but there is no flow during diastole. C, Reverse diastolic flow. Forward flow can be seen during systole, but there is reverse flow in the umbilical artery during diastole, which is suggestive of high resistance to blood flow in the placenta.
Umbilical artery Doppler velocimetry has not been shown to be useful in the evaluation of some high-risk pregnancies, including diabetic and post-term pregnancies, primarily owing to a high false-positive rate.2,109-112 Thus, in the absence of fetal growth restriction, obstetric management decisions are not usually made on the basis of Doppler velocimetry findings alone. New applications for Doppler technology include the use of MCA peak systolic velocity for the noninvasive evaluation of fetal anemia resulting from isoimmunization. When severe anemia develops in a fetus, blood is preferentially shunted to the vital organs, such as the brain, and the shunt can be demonstrated by an increase in MCA peak systolic flow velocity.113 This finding can help the perinatologist counsel affected patients about the need for cordocentesis and fetal blood transfusion. Doppler studies of other vessels (including the uterine artery, fetal aorta, ductus venosus, and fetal carotid arteries) have contributed to our knowledge of maternal-fetal physiology but as yet have resulted in few clinical applications.
Multiple Modalities to Assess Fetal Well-Being
All standard tests to assess antepartum fetal well-being (i.e., NST, BPP, CST) are evaluated according to their ability to predict the absence of fetal death during the 1-week period after the test. The false-negative rate (defined as a reassuring test result with a subsequent bad outcome) and false-positive rate (an abnormal result with a subsequent normal outcome) for each of these tests are listed in Table 6-6.1,84,95,103 The false-negative rates for all three tests are relatively low. Because the NST has a high false-positive rate, some authorities consider it a screening test to identify fetuses requiring further assessment with either a BPP or a CST. No method of fetal assessment is perfect, and clinical judgment plays a large role in any management decision.
Special Techniques for Antepartum Fetal Surveillance
Perinatal Ultrasonography
Ultrasonography uses high-frequency sound waves (3.5 to 5 MHz for transabdominal transducers and 5 to 7.5 MHz for transvaginal transducers) that are directed into the body by a transducer, reflected by maternal and fetal tissue, detected by a receiver, processed, and displayed on a screen. Increasing the wave frequency results in greater display resolution at the expense of diminished tissue penetration. Interpretation of images requires operator experience. Widespread clinical application of two-dimensional ultrasonography began in the 1960s after pioneering work by researchers in the United States and Great Britain.114 Although no deleterious biologic effects have been associated with obstetric ultrasonography, the rates of false-positive and false-negative diagnoses based on the images are a major limitation.
Perinatal ultrasonography can be classified broadly into three types of examinations: basic, targeted (comprehensive), and limited. The basic examination (level I) involves determination of fetal number, viability, position, gestational age, and gross malformations. Placental location, amniotic fluid volume, and the presence of abnormal maternal pelvic masses can be evaluated as well.20 Most pregnancies can be evaluated adequately with this type of examination alone. If the patient's history, physical findings, or basic ultrasonographic results suggest the presence of a fetal malformation, an ultrasonographer who is skilled in fetal evaluation should perform a targeted or comprehensive examination (level II). During a targeted ultrasonographic examination, which is best performed at 18 to 20 weeks' gestation, fetal structures are examined in detail to identify and characterize any fetal malformation. Ultrasonographic markers of fetal aneuploidy (see later discussion) can be evaluated as well. In some situations, a limited examination may be appropriate to answer a specific clinical question (e.g., fetal viability, amniotic fluid volume, fetal presentation, placental location, cervical length) or to provide ultrasonographic guidance for an invasive procedure (e.g., amniocentesis).
Current debate centers on identifying those patients who would benefit from an ultrasonographic evaluation and determining what type of evaluation would be optimal. Advocates of the universal application of ultrasonography cite the advantages of more accurate dating of pregnancy (see earlier discussion) and earlier and more accurate diagnosis of multiple gestation, structural malformations, and fetal aneuploidy (see later discussion). Opponents of routine ultrasonographic examination view it as an expensive screening test ($100 to $250 for a basic examination) that is not justified by published research, which suggests that routine ultrasonography does not change perinatal outcome significantly.19,26,27 Although routine ultrasonography for all low-risk pregnant women is controversial, few would disagree that the benefits far outweigh the costs for selected patients. The ACOG20 has recommended that the benefits and limitations of ultrasonography should be discussed with all pregnant women.
First-trimester ultrasonography is indicated to confirm an intrauterine pregnancy (i.e., exclude ectopic pregnancy), confirm fetal viability, document fetal number, estimate gestational age, and evaluate the maternal pelvis and ovaries.
Second-trimester ultrasonography is indicated in patients with an uncertain LMP date, uterine size larger or smaller than expected for the estimated gestational age, a medical disorder that can affect fetal growth and development (e.g., diabetes, hypertension, collagen vascular disorders), a family history of an inherited genetic abnormality, and suspected fetal malformation or growth disturbance.20 Most patients undergo a detailed fetal anatomic survey at 18 to 20 weeks' gestation to screen for structural defects. An understanding of normal fetal physiology is critical to the diagnosis of fetal structural anomalies. Placental location should be documented with the maternal bladder empty, because overdistention of the bladder or a lower uterine contraction can give a false impression of placenta previa. If placenta previa is identified at 18 to 22 weeks' gestation, serial ultrasonographic examinations should be performed to follow placental location. Only 5% of cases of placenta previa identified in the second trimester persist to term.115 The umbilical cord should also be imaged and the number of vessels, placental insertion, and fetal insertion should be noted. Evaluation of the amniotic fluid volume should also be done. In pregnancies at high risk for fetal cardiac anomalies or preterm birth, fetal echocardiography and cervical length measurements, respectively, should be performed.
The indications for third-trimester ultrasonography are similar to those for second-trimester ultrasonography. Fetal anatomic surveys and EFW become less accurate with greater gestational age, especially in obese women or pregnancies complicated by oligohydramnios. Fetal biometry and detailed anatomic surveys are still performed in late gestation, because certain fetal anomalies (e.g., achondroplasia, duodenal atresia) may become evident for the first time during this period. Transvaginal ultrasonographic measurement of cervical length (performed to identify women at risk for preterm birth) is of little use after 30 to 32 weeks' gestation.116
Screening for Fetal Chromosomal Abnormalities
Fetal chromosomal abnormalities are a major cause of perinatal morbidity and mortality, accounting for 50% of first-trimester spontaneous abortions, 6% to 12% of all stillbirths and neonatal deaths, and 10% to 15% of structural anomalies in live-born infants.117 The most common aneuploidy encountered during pregnancy (autosomal trisomy) results primarily from nondisjunction during meiosis I, an event that occurs with growing frequency in older women. Women of advanced maternal age (> 35 years or older at EDD) are at higher risk for having a pregnancy complicated by fetal aneuploidy and are routinely offered noninvasive prenatal screening as well as an invasive diagnostic procedure, either amniocentesis or chorionic villus sampling (CVS). However, because only 8% to 12% of all births occur in women age 35 and older, at most 20% to 25% of all cases of trisomy 21 (Down syndrome) would be identified if all women of advanced maternal age agreed to amniocentesis.118 Many older women are now opting for serum analyte screening for fetal aneuploidy, which is equally accurate in older women.119 All women, regardless of age, should be offered aneuploidy screening during early gestation.117
Second-Trimester Fetal Aneuploidy Screening
Methods have been developed to help identify women at high risk for fetal aneuploidy. The major focus of attention has been the detection of Down syndrome, because it is the most common chromosomal abnormality manifesting at term and because, unlike the less common disorders trisomy 13 and 18, its diagnosis can be very difficult to make with ultrasonography. In all of these screening tests, one or more serum analytes are used to adjust the a priori risk for fetal aneuploidy in a given pregnancy, which depends primarily on maternal age. The maternal serum analytes used most commonly in second-trimester aneuploidy screening protocols are maternal serum alpha-fetoprotein (MS-AFP), total or free β-subunit hCG (β-hCG), unconjugated estriol, and dimeric inhibin A (collectively known as the quadruple or “quad” screen). Screening results are reported as positive or negative. If the adjusted risk for fetal aneuploidy exceeds the age-related risk at age 35 or the rate of amniocentesis procedure-related pregnancy loss, which is currently defined as 1 in 300 to 500 (i.e., if the chance of finding a chromosomal abnormality on fetal karyotype is higher than the risk of the invasive procedure), then genetic amniocentesis is recommended.120 If all screen-positive women undergo amniocentesis and if the fetal karyotype analysis is successful in all cases, this protocol can identify 60% of all Down syndrome cases with a screen-positive (amniocentesis) rate of approximately 5%. Older women are more likely to be screen positive but also have higher detection rates. In women older than 35 years, this protocol identifies 75% of aneuploid fetuses with a screen-positive rate of approximately 25%.118,121,122
Second-Trimester Ultrasonographic Screening for Fetal Aneuploidy
Second-trimester ultrasonographic markers, such as intracardiac echogenic focus and echogenic bowel (Table 6-7), are not generally incorporated into standard algorithms to predict risk for fetal aneuploidy; however, a risk adjustment based on ultrasonographic markers can be made. Multiple major structural abnormalities, such as those often found in fetuses with trisomy 13 or 18, can be detected reliably by perinatal ultrasonography. Approximately 50% of fetuses with Down syndrome appear structurally normal on ultrasonography.123 Several major structural ultrasonographic abnormalities (e.g., endocardial cushion defect) may be associated with trisomy 21 in more than 30% of cases.123-125 The clinical significance of an isolated “soft” ultrasonographic marker for Down syndrome in a low-risk population is unclear.
TABLE 6-7
Accuracy Measurements of Second-Trimester Ultrasonographic “Soft Markers” for Trisomy 21 (Down Syndrome) When Identified as Isolated Anomalies
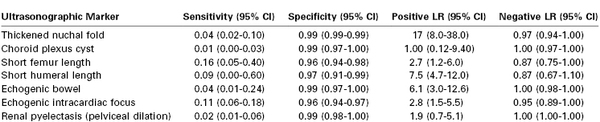
CI, confidence interval; LR, likelihood ratio.
Data from Smith-Bindman P, Hosmer W, Feldstein VA, et al. Second-trimester ultrasound to detect fetuses with Down syndrome: a meta-analysis. JAMA 2001; 285:1044-55; and Vintzeleos AM, Campbell WA, Rodis JF, et al. The use of second-trimester genetic sonogram in guiding clinical management of patients at increased risk for fetal trisomy 21. Obstet Gynecol 1996; 87:948-52.
First-Trimester Fetal Aneuploidy Screening
First-trimester fetal aneuploidy screening is a more recent development. The screening protocol involves the following three steps undertaken at 11 to 14 weeks' gestation: (1) maternal serum analyte screening for pregnancy-associated placental protein-A (PAPP-A) and total or free β-hCG, (2) ultrasonographic assessment of nuchal translucency, and (3) genetic counseling.126 The measurement of free rather than total β-hCG provides a small statistical advantage without apparent clinical benefit.127 First-trimester aneuploidy screening appears to be as good as second-trimester serum analyte screening in identifying fetuses with Down syndrome.128,129 The serum analytes in the first trimester associated with an increased risk for Down syndrome include a decrease in PAPP-A (< 0.4 multiples of the median [MoM]) and an increase in free hCG (> 1.8 MoM). Nuchal translucency is defined as the fluid-filled space between the back of the fetal neck and the overlying skin. Proper training and technique are needed to obtain this measurement. There is a correlation between an increased nuchal translucency measurement and a risk for Down syndrome.
The advantage of first-trimester aneuploidy screening is that it is performed early in pregnancy, allowing for more counseling, the option of CVS, and early pregnancy termination if desired. The screening test most commonly used in Europe for identifying pregnancies at risk for Down syndrome is the “integrated” test, which combines first-trimester aneuploidy screening with second-trimester serum analyte screening into a single adjusted risk in the mid to late second trimester. The integrated test can identify 85% to 90% of fetuses with Down syndrome with a false-positive rate of 2% (Table 6-8).129-135 However, the true application of the integrated screening test requires that the first-trimester test results, even if abnormal, be withheld from the patient until combined with the second-trimester test results; this practice of withholding information has generated controversy, particularly in the United States. To overcome this objection, sequential and contingent integrated screening tests have been developed, whereby the second-trimester test is performed after disclosure of the first-trimester screening result or if the first test result is abnormal, respectively. It remains unclear, however, whether the detection rates for these integrated tests are any better than those of the first-trimester screening test alone (see Table 6-8).129-132 Indeed, if the first-trimester aneuploidy screen result is negative (indicating low risk), the sensitivity of second-trimester serum analyte screening is reduced fivefold.136 For this reason, many authorities suggest that no further aneuploidy screening be done if the first-trimester screen result is negative, with the exception of the second-trimester fetal anatomic survey and possibly isolated MS-AFP serum screening for open neural tube defects at 15 to 20 weeks' gestation.
TABLE 6-8
Detection Rate of Down Syndrome Screening Tests
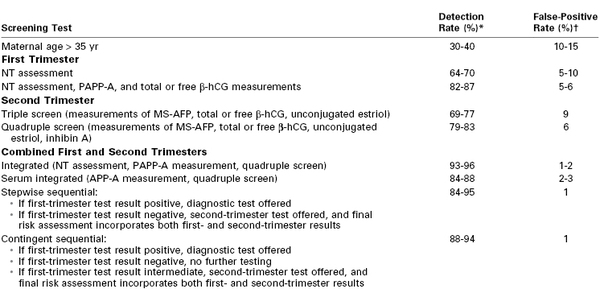
* Assuming a 5% false-positive rate.
† Assuming an 85% detection rate.
β-hCG, beta-human chorionic gonadotropin; MS-AFP, maternal serum level of alpha-fetoprotein; NT, nuchal translucency; PAPP-A, pregnancy-associated placental protein-A.
Data from references 128 and 130-132.
In addition to the nuchal translucency measurement, absence of the nasal bone on first-trimester ultrasonography has been correlated with Down syndrome. However, whether this ultrasonographic marker adds to the predictive value of first-trimester risk assessment in either low- or high-risk populations has been questioned.137,138 At this time, the presence or absence of the nasal bone is not included in the first-trimester screening test.
Risk assessment for Down syndrome can be performed in twin pregnancies using either first- or second-trimester serum analyte measurements but is less accurate than in singleton pregnancies.132 Such screening has not been validated for use in higher-order multiple pregnancies (triplets and up) or in multiple pregnancies with a nonviable fetus (either due to spontaneous demise or following a multifetal pregnancy reduction). In such cases, Down syndrome risk assessment can be achieved using first-trimester nuchal translucency measurements only, although this is not a particularly good screening test and has a lower sensitivity even than nuchal translucency alone in singleton pregnancies.132
Definitive Diagnosis of Fetal Chromosomal Abnormalities
Although an abnormal screening test result or the presence of ultrasonographic abnormalities may signal an increased risk for Down syndrome or other chromosomal abnormality, the majority of fetuses with such findings are chromosomally normal. To provide a definitive diagnosis, an invasive procedure is needed to obtain the fetal karyotype; generally amniocentesis or CVS is used, although in rare cases a cordocentesis is performed.
All invasive procedures are associated with risks to the pregnancy. Risks common to all invasive procedures include the chance of bleeding, isoimmunization (especially in women who are Rh negative), and infection. All women who are Rh negative should receive Rh0(D) immune globulin before or after the procedure. Although the risk for vertical transmission of viral infections (e.g., hepatitis B, hepatitis C, human immunodeficiency virus) with invasive procedures is believed to be low,139 every effort should be made to avoid invasive procedures in such patients, especially if there is a high viral load in the maternal circulation.
Amniocentesis
Amniotic fluid is composed of fetal urine, lung fluid, skin transudate, and water that is filtered across the amniotic membranes. It contains electrolytes, proteins, and desquamated fetal cells (amniocytes). Sampling of amniotic fluid (amniocentesis) can be used to measure various substances such as lecithin and sphingomyelin for assessing fetal lung maturity, to look for pathogenic bacteria for confirmation of an intra-amniotic infection, and to obtain fetal cells for determination of fetal karyotype or performance of specific genetic analyses.
Cell culture with karyotype analysis typically takes 10 to 14 days, although a small chance exists that the cells will fail to grow, resulting in an inconclusive result. Fluorescence in situ hybridization (FISH) does not require that the cells be cultured for any length of time, and its results can be obtained within a few days. This technique uses a series of chromosome-specific fluorescent probes to analyze the metaphase spread in fetal cells to determine fetal gender and detect common trisomies (21, 18, 13, X, and Y). It can also be used to identify chromosome deletions or duplications in pregnancies at risk for a specific genetic disorder because of a family history or suspicious ultrasonographic findings, such as the 22q11 deletion in DiGeorge's syndrome.140-142 Although FISH is highly sensitive (trisomy present on FISH testing is invariably present in the fetus), it is not particularly specific, with a false-negative rate of approximately 15%. For this reason, the American College of Medical Genetics (ACMG) and the American Society of Human Genetics (ASHG) recommend that all FISH results be confirmed by complete karyotype analysis.142
The most common indication for second-trimester amniocentesis is cytogenetic analysis of fetal cells, although on occasion it is performed to determine amniotic fluid AFP levels and acetylcholinesterase activity for the diagnosis of fetal open neural tube defects. Amniocentesis later in pregnancy is usually performed for nongenetic indications, such as (1) documentation of fetal pulmonary maturity prior to elective delivery before 39 weeks' gestation, (2) for amnioreduction in pregnancies complicated by severe polyhydramnios, (3) to confirm preterm premature rupture of membranes (PROM) (amniodye test), or (4) to exclude intra-amniotic infection.
Genetic amniocentesis typically involves the insertion of a 22-gauge spinal needle through the maternal abdominal wall and into the uterine cavity at 15 to 20 weeks' gestation. The procedure is now commonly performed under ultrasonographic guidance, which allows the operator to choose the safest site, preferably away from the fetal face and umbilical cord and when possible without passage of the needle through the placenta. The greatest risk of amniocentesis is spontaneous abortion; however, the procedure-related pregnancy loss rate for genetic amniocentesis appears to be only 1 in 300 to 500.120,143-145 Of interest, the pregnancy loss rate is not influenced by operator experience or needle placement through the placenta145,146 but is higher in the presence of first-trimester bleeding or recurrent miscarriage, ultrasonographic demonstration of chorioamniotic separation, discolored amniotic fluid at the time of the procedure, and an unexplained elevation in MS-AFP.144,147 Whether this risk is higher in twin pregnancies is not clear. Transient leakage of amniotic fluid can be seen in 1% to 2% of procedures. This leakage usually stops after 48 to 72 hours, infection is extremely rare (< 0.1% of cases), and the perinatal survival rate after mid-trimester fluid leakage may be as high as 90%.117,148-152
Compared with late second-trimester amniocentesis, early amniocentesis (before 15 weeks' gestation) is associated with significantly higher procedure-related pregnancy loss rates, ranging from 2.2% to 4.8%.148-151 This rate is fourfold higher than that of late amniocentesis and twice as high as that of CVS. Early amniocentesis has also been shown to be associated with higher rates of rupture of membranes, club foot, and amniocyte culture failures (2% to 5%) than late amniocentesis.148-153 For these reasons, amniocentesis before 15 weeks' gestation is not recommended.
If early karyotyping is desired, CVS is preferred over early amniocentesis (see later discussion). Amniocentesis in the third trimester is technically easier and is associated with fewer complications. If a late amniocentesis is being performed for any reason (e.g., to confirm fetal pulmonary maturity), consideration should be given to obtaining the karyotype if indicated, even though the pregnancy is too far along to be ended electively.
Amniocentesis in multiple gestations can be performed safely. Care must be taken to carefully map the fetal sacs so that the amniotic fluid for each fetus is sampled separately. A small amount of indigo carmine (3 to 5 mL) is typically inserted into the first sac after the fluid is sampled to ensure that the same sac is not sampled twice.
Chorionic Villus Sampling
Like that of amniocentesis, the goal of CVS is to provide fetal cells for genetic analysis, although in this case the cells are trophectoderm (placental) cells rather than amniocytes. The technique entails ultrasound-guided aspiration of chorionic villi by means of a 16-gauge catheter inserted transcervically or a 20-gauge spinal needle inserted transabdominally into the placenta. The 15 to 30 mg of villous material collected can be examined in two ways: (1) by direct cytogenetic analysis after an overnight incubation, which yields results in 2 to 3 days; and (2) by longer-term culture followed by cytogenetic analysis, which yields results in 6 to 8 days.154 To provide rapid and accurate results, many centers report the results of both methods. The main advantage of CVS over amniocentesis is that it allows for fetal karyotyping results in the first trimester, thereby allowing decisions about pregnancy termination to be made earlier if chromosomal abnormalities are detected. Moreover, although rare, certain genetic disorders (e.g., osteogenesis imperfecta) can be diagnosed antenatally only through analysis of placental tissue.
CVS is best performed between 10 and 12 weeks' gestation. CVS performed before 10 weeks' gestation has been associated with limb reduction defects,155,156 whereas no such association exists if the procedure is performed after 66 days' gestation.157 Transabdominal CVS can also be performed in the second or third trimester and is a reasonable alternative to cordocentesis for obtaining tissue for an urgent fetal karyotype.158
The most common complication of CVS is vaginal spotting, which occurs in 10% to 25% of patients within the first few days after the procedure. Fortunately, the bleeding is usually mild and resolves spontaneously with no long-term sequelae. The incidences of amnionitis (0.3%) and rupture of membranes (0.3%) after CVS do not differ significantly from those seen with late amniocentesis and are significantly lower than those reported after early amniocentesis.157 As with amniocentesis, the most serious complication of CVS is spontaneous abortion. CVS appears to be associated with a higher risk for pregnancy loss than late amniocentesis; the procedure-related loss rate in CVS is reported as 1.0% to 1.5%.157,159-164 This rate is significantly higher (0.6% to 0.8%) than that seen after late amniocentesis, with an adjusted odds ratio of 1.30 (95% confidence interval [CI], 1.17 to 1.52).163 Factors that increase the procedure-related loss rate are operator inexperience, number of needle passes, and a history of bleeding prior to the procedure.163 By contrast, the risk does not appear to be increased in twin gestations or with the anatomic approach used (i.e., transabdominal versus transcervical catheter placement).162,165 Some investigators have suggested that the apparently higher pregnancy loss related to CVS (compared with amniocentesis) is a function of the earlier gestational age at which the procedure is performed.120
One complication unique to CVS involves the interpretation of the genetic test results. Because the fetus and placenta both arise from the same cell, it is assumed that the genetic complements of these two tissues are identical, but this is not always the case. Confined placental mosaicism refers to the situation in which the karyotype of the chorionic villus is a mosaic (i.e., it contains two or more populations of cells with different karyotypes, usually one normal and one trisomic) but the karyotype of the fetus is normal. The incidence of confined placental mosaicism may be as high as 1% to 2% with the direct cytogenetic analysis method, but most cases are not confirmed by the long-term tissue-culture method,157,161 suggesting a methodologic error. For this reason, many centers report only the long-term culture results. On occasion, it may be necessary to repeat the fetal karyotype, either with a second CVS or with amniocentesis, to resolve the dilemma. The reverse situation, in which the CVS result is normal but the fetus has aneuploidy (a false-negative result), has also been reported166 but is rare. It may occur from contamination with maternal cells or from inadvertent sampling of a twin placenta.
Cordocentesis
In cases in which pregnancy complications or fetal abnormalities are discovered late in gestation, cordocentesis (also known as percutaneous umbilical blood sampling) is an option for rapid evaluation of the fetal karyotype. Cordocentesis involves the insertion of a 22-gauge spinal needle through the maternal abdominal and uterine walls and into the umbilical vein, preferably at the insertion site on the placenta, under direct ultrasonographic guidance. Considerable training and expertise are needed to perform this procedure. Karyotype analysis results can be obtained in 24 to 48 hours.
The first cordocentesis was reported in 1983.167 Although this procedure was originally considered superior to amniocentesis for a number of diagnostic indications, advances in laboratory analysis have allowed more information to be obtained through amniocentesis.168 For example, cordocentesis was commonly used to obtain a sample of fetal blood for rapid karyotyping when a major structural anomaly or severe fetal growth restriction was identified late in pregnancy; however, this sample can be obtained as rapidly from amniocentesis or CVS samples using FISH analysis. Similarly, DNA analysis of amniocytes can rapidly and accurately determine the fetal Rh status as well as the presence of other red cell and platelet antigens,169 which in the past was an absolute indication for cordocentesis. Now employed primarily for therapeutic indications, cordocentesis is most commonly used to transfuse fetuses with severe anemia from isoimmunization, parvovirus infection, or fetal-maternal hemorrhage (spilling of fetal blood cells into the maternal circulation). This intravascular route of fetal transfusion is preferred to the older technique of intraperitoneal transfusion.170 Other rare indications for cordocentesis are to measure drug concentrations in the fetal circulation, to document response to pharmacologic therapy, and to administer drugs directly to the fetus (e.g., adenosine to treat resistant fetal tachydysrhythmia).171
When skilled operators perform cordocentesis, complications are infrequent and similar to those encountered with amniocentesis. Specifically, there is risk for bleeding, cord hematoma, infection, and preterm PROM. The risk for pregnancy loss as a result of the procedure is estimated to be 1.2% to 4.9%,172 although fetuses with severe fetal growth restriction, hydrops or major structural anomalies may be at higher risk compared with well-grown, structurally normal fetuses. Operator experience is an important determinant of success, as are logistical issues (e.g., volume of amniotic fluid, placental position, location of the cord insertion site within the placenta). A transient fetal bradycardia may occur during the procedure, often resulting from unintentional placement of the needle into one of the umbilical arteries and leading to arterial vasospasm. Although this bradycardia invariably resolves, if the fetus is at a favorable gestational age (> 24 weeks), the procedure should be performed at a facility with the capacity to perform an emergency cesarean delivery. No consistent data or recommendations exist regarding the use of prophylactic antibiotics, tocolysis, and maternal sedation during cordocentesis.
Other Tests
Three-Dimensional Ultrasonography
Compared with standard two-dimensional ultrasonography, three-dimensional (3D) ultrasonography (or four-dimensional, if fetal movements are included) allows for concurrent visualization of fetal structures in all three dimensions for improved characterization of complex fetal structural anomalies. Unlike two-dimensional ultrasonographic images, 3D images are greatly influenced by fetal movements and are subject to more interference from structures such as fetal limbs, umbilical cord, and placental tissue. Because of movement interference, visualization of the fetal heart with 3D ultrasonography is suboptimal.
In addition to rapid acquisition of images that can be later reconstructed and manipulated, 3D ultrasonography has the following potential advantages:
1. The ability to provide clearer images of soft tissue structures through surface rendering. Such images may improve the diagnosis of certain fetal malformations, especially craniofacial anomalies (e.g., cleft lip and palate, micrognathia, ear anomaly, facial dysmorphism, intracranial lesions), club foot, finger and toe anomalies, spinal anomalies, ventral wall defects, and fetal tumors.
2. The ability to provide more accurate measurements of the gestational sac, yolk sac, and crown-rump length and to obtain a midsagittal view for measuring nuchal translucency.
3. The ability to measure tissue volume. Preliminary data suggest that assessment of cervical volume may identify women at risk for cervical insufficiency,173 and measurement of placental volume in the first trimester may determine fetuses at risk for fetal growth restriction.174
Despite these advantages, 3D ultrasonography has been used primarily as a complementary technique rather than the standard technique for ultrasonographic imaging. In the future, technical improvements should provide higher-quality images, perhaps similar to those offered by computed tomography and magnetic resonance imaging (MRI).
Complementary Radiographic Imaging
Ultrasonography remains the first-line imaging modality during pregnancy. In certain situations, however, enhanced imaging may be required to better define a particular fetal anomaly. For example, radiographic imaging is superior to ultrasonography in evaluating the fetal skeleton and may provide valuable information in the evaluation of a fetus with a suspected bony dystrophy. At least 25 different forms of skeletal dysplasias are identifiable at birth, 11 of which are lethal in the peripartum period.175 Although some of these forms can be identified from their unusual appearance on ultrasonography (e.g., cloverleaf skull and small thorax in thanatophoric dysplasia), the majority are difficult to identify. Timely radiographic imaging may allow an experienced pediatric radiologist to more thoroughly evaluate the fetal skeleton and determine the correct diagnosis. A simple maternal abdominal radiograph may be all that is required, because ossification is sufficient by 20 weeks' gestation to allow good visualization of the fetal bones.
Although computed tomography is best avoided in pregnancy because it exposes the fetus to ionizing radiation (albeit at small doses), MRI is regarded as safe. This latter technology relies on the interaction between an applied magnetic field and the inherent nuclear magnetism of atomic nuclei within the patient's tissues to generate a high-resolution anatomic image. Because MRI is particularly good at visualizing soft tissue rather than bony structures, it is uniquely suited to the evaluation of fetal intracranial defects and the soft tissues of the maternal pelvis (Figure 6-8).176,177 Although fetal motion artifact has previously been a major limitation in the use of MRI, new ultrafast technology allows for rapid image acquisition and has largely overcome this problem.
FIGURE 6-8 Magnetic resonance images of a fetus with holoprosencephaly. A, Sagittal view showing the proboscis (arrow). B, Coronal view showing the single ventricle and fused thalami (arrow). (Reprinted from Wenstrom KD, Williamson RA, Weiner CP, et al. Magnetic resonance imaging of fetuses with intracranial defects. Obstet Gynecol 1991; 77:529-32.)
Fetal Echocardiography
Cardiac anomalies are the most common major congenital defects encountered in the antepartum period. A four-chamber ultrasonographic view of the heart during the fetal anatomic survey at 18 to 20 weeks' gestation detects only 30% of congenital cardiac anomalies, although the detection rate can be increased to 60% to 70% if the outflow tracts are adequately visualized.178 Owing to the number of congenital cardiac anomalies that would be missed, however, fetal echocardiography should be performed by a skilled and experienced sonologist at 20 to 22 weeks' gestation in all pregnancies at high risk for a fetal cardiac anomaly. Indications for fetal echocardiography include (1) pregnancies complicated by pregestational diabetes mellitus, (2) a personal or family history of congenital cardiac disease (regardless of the nature of the lesion or whether it has been repaired), (3) maternal exposure to certain drugs (e.g., lithium, paroxetine),179 and (4) conception by in vitro fertilization (but not if the pregnancy was conceived through the use of clomiphene citrate or ovarian stimulation/intrauterine insemination alone).180
Fetal Cells or DNA in the Maternal Circulation
To minimize the risks associated with invasive prenatal diagnosis (amniocentesis and CVS), improved noninvasive tests are being developed for fetal aneuploidy genetic testing. Fetal cells are known to be present in the maternal circulation throughout pregnancy at a concentration of approximately 1 fetal cell for every 10,000 to 1 million maternal cells.181 However, we do not currently have the technology to isolate these cells with sufficient purity to develop a reliable prenatal test. Recent efforts have focused on genetic analysis of cell-free DNA in the maternal circulation. It is now apparent that fetal DNA (most of which comes from the placenta) accounts for 3% to 10% of all cell-free DNA in maternal serum, and it may account for as much as 20% of cell-free DNA in women with preeclampsia or after major fetal-maternal hemorrhage. Because of its relative abundance, purity, and short half-life (precluding contamination from a prior pregnancy), high-throughput sequencing of cell-free DNA provides new opportunities for noninvasive prenatal testing.182 Several commercial tests are now available that rely on analysis of cell-free DNA in the maternal circulation to screen for fetal aneuploidy as early as 10 weeks' gestation. Recent publications have shown that such noninvasive prenatal testing can increase the detection rate for trisomy 21 (Down syndrome) to approximately 99.8% with a 0.2% false-positive rate in high-risk pregnancies 183-186; these results have not yet been validated in low-risk pregnancies. Because of the small but finite false-positive rate, these tests should be considered screening and not diagnostic tests, and confirmatory CVS or amniocentesis is still recommended before acting on a positive test. The detection of trisomy 18 (98%) and trisomy 13 (65%) is also possible using this technology.184,186
An alternative approach under investigation for definitive genetic testing is the isolation of trophoblast cells from the cervicovaginal discharge of women in early pregnancy.187,188 Provisional studies have isolated these cells from the maternal cervix with cervical canal lavage at 7 to 10 weeks' gestation187 or with the use of a brush-type collection device at 5 to 12 weeks (the “genetic Pap smear”).188 With this technique, such cells have been isolated in 86% (195/227) of samples by immunocytochemistry with trophoblast-specific antibodies, and results agreed with those of placental tissue karyotyping via CVS in 95% (186/195) of cases.188 The ability to successfully collect trophoblast cells from the cervicovaginal discharge of women in early pregnancy may provide a simple, reliable, noninvasive, yet definitive genetic test for fetal aneuploidy in a singleton pregnancy with no risk to the mother or fetus.
Special Circumstances Requiring Additional Fetal Surveillance
Under certain circumstances, additional antenatal fetal surveillance may be required (see Box 6-2). If appropriate, early consultation with a specialist (e.g., a maternal-fetal medicine specialist, medical geneticist, pediatric surgeon, pediatric urologist, pediatric cardiologist, or infectious disease specialist) and delivery at a tertiary care center should be considered.
Abnormal Serum Analyte and Nuchal Translucency Screening with Normal Fetal Karyotype
Pregnancies with abnormal serum analyte screening in the first or second trimester are at increased risk for adverse outcomes, including preterm birth, preeclampsia, and stillbirth, even if the karyotype is normal (Tables 6-9 and 6-10).189-191 Such pregnancies therefore require more intensive fetal monitoring (Table 6-11), including serial growth evaluation and NST. Fetuses with a nuchal translucency measurement of 3.0 mm or more in the first trimester have a higher risk for congenital heart defects and other chest abnormalities, even with a negative aneuploidy screening test result and normal fetal chromosomes.132,192 Women with such pregnancies should be offered a fetal echocardiogram at 20 to 22 weeks' gestation in addition to a routine targeted fetal anatomic survey at 18 to 20 weeks.
TABLE 6-9
Relationship between First-Trimester PAPP-A Level at or below Fifth Percentile (0.42 MoM) and Risks of Adverse Pregnancy Outcomes
| Adverse Outcome | Adjusted Odds Ratio | 95% Confidence Interval |
| Spontaneous loss < 24 weeks | 2.50 | 1.76-3.56 |
| Fetal death ≥ 24 weeks | 2.15 | 1.11-4.15 |
| Preterm birth ≤ 37 weeks | 1.87 | 1.61-2.17 |
| Preterm birth ≤ 32 weeks | 2.10 | 1.59-2.76 |
| Preeclampsia | 1.54 | 1.16-2.03 |
| Gestational hypertension | 1.47 | 1.20-1.82 |
| Placental abruption | 1.80 | 1.15-2.84 |
| Fetal growth restriction | 3.22 | 2.38-4.36 |
MoM, multiple of the median; PAPP-A, pregnancy-associated placental protein-A test.
Data from Dugoff L, Hobbins JS, Malone FD, et al. First-trimester maternal serum PAPP-A and free beta-subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol 2004; 191:1446-51.
TABLE 6-10
Second-Trimester Serum Analyte (Marker) Screening and Adverse Pregnancy Outcome
| Adverse Outcome | Marker | Odds Ratio |
| Spontaneous loss < 24 weeks | MS-AFP | 7.8 |
| Fetal death ≥ 24 weeks | Inhibin A | 3.7 |
| Preterm birth ≤ 32 weeks | Inhibin A | 5.0 |
| Preterm premature rupture of membranes | MS-AFP | 1.9 |
| Preeclampsia | Inhibin A | 3.8 |
| Gestational hypertension | Inhibin A | 1.7 |
| Placental abruption | MS-AFP | 1.9 |
| Placenta previa (confirmed at delivery) | MS-AFP | 3.1 |
| Fetal growth restriction | Inhibin A | 3.0 |
| Birth weight ≤ 5th percentile | Inhibin A | 2.3 |
| • Delivery < 37 weeks | Inhibin A | 8.0 |
| • Delivery < 32 weeks | Inhibin A | 18.6 |
MS-AFP, maternal serum level of alpha-fetoprotein.
Data from Dugoff L, Hobbins JS, Malone FD, et al; FASTER Trial Research Consortium. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol 2005; 106:260-7.
Hydrops Fetalis
Hydrops fetalis (“edema of the fetus”) is a rare pathologic condition that complicates approximately 0.05% of all pregnancies. It is an ultrasonographic diagnosis requiring the presence of an abnormal accumulation of fluid in more than one fetal extravascular compartment, including ascites, pericardial effusion, pleural effusion, subcutaneous edema, and/or placental edema. Polyhydramnios is seen in 50% to 75% of cases. Although classically seen in fetuses with severe anemia resulting from Rh isoimmunization, the introduction of Rh0(D) immune globulin has led to a substantial decrease in the incidence of immune hydrops. Indeed, 90% of hydrops fetalis cases are due to nonimmune causes, such as maternal infection (e.g., with parvovirus B19, cytomegalovirus, syphilis), massive fetal-maternal hemorrhage, and fetal abnormalities (e.g., congenital cardiac defects, fetal thalassemia, twin-to-twin transfusion syndrome). Although the overall perinatal mortality rate in the setting of hydrops fetalis exceeds 50%, the prognosis depends on the underlying cause, severity, and gestational age.
Immune hydrops occurs when fetal erythrocytes express a protein that is not present on maternal erythrocytes. The maternal immune system can become sensitized and produce antibodies against these “foreign” proteins. These immunoglobulin (Ig) G antibodies can cross the placenta and destroy fetal erythrocytes, leading to fetal anemia and high-output cardiac failure. Immune hydrops is typically associated with a fetal hematocrit less than 15% (normal fetal hematocrit is 50%). The most antigenic protein on the surface of fetal erythrocytes is the D antigen of the Rhesus protein complex, also known as Rh(D). Other antigens that can cause severe immune hydrops are Kell (“Kell kills”), Rh(E), Rh(c), and Duffy (“Duffy dies”). Antigens causing less severe hydrops are ABO, Rh(e), Rh(C), Ce, k, and s. Lewis a and b (Lea, Leb) incompatibility can cause mild anemia but not hydrops, because this condition primarily results in production of IgM antibodies, which do not cross the placenta (“Lewis lives”). For identification of women at risk for isoimmunization, every pregnant woman should undergo blood type and antibody screening at the first prenatal visit and again in the third trimester.
Sixty percent of cases of immune hydrops result from ABO incompatibility; however, only Rh(D) isoimmunization can be prevented. The Rh(D) antigen is expressed only on primate erythrocytes and becomes evident by 38 days of intrauterine life. A mutation in the Rh(D) gene on chromosome 1 results in lack of expression of Rh(D) antigen on circulating erythrocytes (Rh[D] negative). This mutation arose in the Basque region of Spain, and the difference in prevalence of Rh(D)-negative individuals between the races likely reflects the amount of Spanish blood in their ancestry: Caucasian, 15%; African-American, 8%; African, 4%; Native American, 1%; and Asian, less than 1%.193 If the fetus of an Rh(D)-negative woman is Rh(D) negative, Rh(D) sensitization will not occur. However, 60% of Rh(D)-negative women have Rh(D)-positive fetuses, and exposure of these women to as little as 0.25 mL of Rh(D)-positive blood may induce an antibody response. Because the initial immune response is production of IgM, the index pregnancy is rarely affected. However, immunization in subsequent pregnancies triggers an IgG response that crosses the placenta and causes hemolysis. Risk factors for Rh(D) sensitization include a mismatched blood transfusion (95% sensitization rate), ectopic pregnancy (< 1%), abortion (3% to 6%), amniocentesis (1% to 3%), and pregnancy itself. Indeed, the sensitization rate is 16% to 18% after a normal pregnancy without Rh0(D) immune globulin administration, 1.3% with Rh0(D) immune globulin at delivery only, and 0.13% with anti-Rh0(D) immune globulin at 28 weeks and again after delivery.193,194 The risk for isoimmunization depends on the volume of fetal-maternal hemorrhage (Table 6-12). Passive immunization with Rh0(D) immune globulin can destroy fetal erythrocytes before they evoke a maternal immune response, thereby preventing sensitization. Therefore, Rh0(D) immune globulin should be given within 72 hours of potential exposure; 300 µg given intramuscularly is adequate for exposure to as much as 30 mL of fetal whole blood or 15 mL of fetal red blood cells.
TABLE 6-12
Fetal-Maternal Transfusion Volume and Risk for Rh(D) Isoimmunization in an Rh(D)-Negative Woman
| Transfusion Volume | Incidence at Delivery (%) | Risk for Isoimmunization (%)* |
| Unmeasurable | 50 | Minimal |
| < 0.1 mL | 45-50 | 3 |
| > 5.0 mL | 1 | 20-40 |
| > 30 mL | 0.25 | 60-80 |
* Without Rh0(D) immune globulin.
Data from American College of Obstetricians and Gynecologists. Prevention of Rh D alloimmunization. ACOG Practice Bulletin No. 4. Int J Gynaecol Obstet 1999; 66:63-70 (reaffirmed 2009); and Moise KJ. Red blood cell alloimmunization in pregnancy. Semin Hematol 2005; 42:169-78.
Once isoimmunization has occurred, passive immunoglobulin is not useful. Such pregnancies should be observed closely for evidence of fetal compromise. Fetal hemolysis results in release of bile pigment into the amniotic fluid, which can be quantified as a change in optical density measured at wavelength 450 nm. Traditionally, the extent of hemolysis had been measured with serial amniocenteses, with amniotic fluid optical density plotted against gestational age; increased density (upper 80% of zone 2 or zone 3 of the Liley curve) is associated with a poor prognosis,195,196 and prompt intervention is indicated. Measurements of peak systolic velocity in the fetal MCA by means of noninvasive Doppler velocimetry have now emerged as the best tool to accurately identify fetuses with severe anemia requiring urgent intervention, regardless of the cause of the anemia.113,194,197 The sensitivity of an elevated MCA peak systolic velocity (i.e., >1.5 MoM for a given gestational age) for predicting moderate to severe anemia approaches 100%.113 Depending on gestational age, these interventions may include immediate delivery or intrauterine blood transfusion.
Post-Term Pregnancy
Post-term (prolonged) pregnancy is defined as any pregnancy that continues to or beyond 42 weeks (294 days) from the first day of the last normal menstrual period or 14 days beyond the best obstetric estimate of the EDD.2,198 The prevalence of post-term pregnancy depends on the patient population (e.g., percentage of primigravidas, incidence of pregnancy complications, frequency of spontaneous preterm births) and the local practice patterns (e.g., use of ultrasonographic assessment of gestational age, cesarean delivery rates, use of labor induction). Approximately 10% (range, 3% to 14%) of all pregnancies continue beyond 42 weeks, and 4% (range, 2% to 7%) continue beyond 43 weeks in the absence of obstetric intervention.2 Compared with delivery at 40 weeks, post-term pregnancies pose significant risks to both the mother (including higher risk for cesarean delivery, severe perineal injury, and postpartum hemorrhage) and the fetus (including stillbirth, fetal macrosomia, birth injury, and meconium aspiration syndrome).2,198-201 The risks to the fetus can be largely prevented by routine induction of labor for all low-risk pregnancies at 40 to 41 weeks' gestation.2,199
Post-term pregnancy is a universally accepted indication for antenatal fetal surveillance,2 although the efficacy of this approach has not been validated by prospective randomized trials. Options for evaluating fetal well-being include NST with or without amniotic fluid volume assessment, BPP, CST, and a combination of these modalities. There is no consensus as to which of these modalities is preferred, and no single method has been shown to be superior.2 The ACOG has recommended that antepartum fetal surveillance be initiated by 42 weeks' gestation at the latest, without making a specific recommendation about the type of test or frequency.2 Many investigators would advise twice-weekly testing with some evaluation of amniotic fluid volume at least weekly. Doppler ultrasonography has no benefit in monitoring the post-term fetus and is not recommended for this indication.109,110 Although the data are inconsistent, there is a suggestion that antenatal testing at 40 to 42 weeks' gestation may be associated with improvements in perinatal outcome. In one retrospective study, women with routine antenatal testing beginning at 41 weeks had lower rates of cesarean delivery for nonreassuring fetal test results than women in whom testing was started at 42 weeks (2.3% versus 5.6%, respectively; P < .01).202 In addition, the group with delayed antenatal testing experienced three stillbirths and seven other neonatal major morbidity events, compared with none in the group who had antenatal testing from 41 weeks (P < .05).202
In the post-term period, evidence of fetal compromise (nonreassuring fetal test results) or oligohydramnios (e.g., low amniotic fluid volume) should prompt delivery.2 Oligohydramnios may result from uteroplacental insufficiency or increased renal artery resistance and may predispose to umbilical cord compression, thus leading to intermittent fetal hypoxemia, meconium passage, and meconium aspiration. A uniform definition for oligohydramnios has not been established; however, options are as follows: (1) a depth of less than 2 cm for the maximum vertical fluid pocket; (2) amniotic fluid index less than 5 cm (i.e., < 5 cm for the sum of the depths in cm of the largest vertical pocket in each of four uterine quadrants); and (3) product of length times width times depth of the largest pocket (in cm) less than 60. Adverse pregnancy outcomes (nonreassuring FHR tracing, low Apgar score, and neonatal intensive care unit admission) are more common when oligohydramnios is present. Frequent (twice-weekly) screening of post-term patients for oligohydramnios is important, because amniotic fluid can become dramatically reduced within 24 to 48 hours. One prospective double-blind cohort study of 1584 women after 40 weeks' gestation found that an amniotic fluid index less than 5 cm with no largest vertical fluid pocket depth greater than 2 cm was associated with birth asphyxia and meconium aspiration, although the sensitivity for adverse outcomes was low.203
Intrauterine Fetal Demise
Intrauterine fetal demise (IUFD), also known as stillbirth, is defined in the United States as demise of the fetus after 20 weeks' gestation and prior to delivery.204-206 In Europe, only fetuses more than 24 weeks' gestation are included. The stillbirth rate in the United States diminished from 15.8 per 1000 total births in 1960 to 7.5 per 1000 births in 1990.205,206 However, it remains a vastly underappreciated clinical problem, with antepartum stillbirths accounting for more perinatal deaths than either complications of prematurity or sudden infant death syndrome.207 Risk factors for stillbirth include extremes of maternal age, chromosomal disorders, congenital malformations, antenatal infection, multiple pregnancy, prior unexplained IUFD, post-term pregnancy, fetal macrosomia, male fetus, umbilical cord and placental abnormalities, and underlying maternal medical conditions (e.g., chronic hypertension, pregestational or gestational diabetes mellitus, autoimmune disorders, inherited or acquired thrombophilia).204,208,209
Although older studies observed that approximately 50% of cases of IUFD were unexplained, an aggressive approach may identify the cause in up to 80% to 90% of cases (Table 6-13).209-212 Pathologic examination of the fetus and the placenta/fetal membranes is the single most useful means of identifying a cause for the IUFD.210,211 Early detection and appropriate management of underlying maternal disorders (e.g., diabetes, preeclampsia) may also reduce the risk. Fetal karyotyping should be considered in all cases of fetal death to identify chromosomal abnormalities, particularly in cases with documented fetal structural abnormalities. Six to 10 percent of stillborn fetuses have an abnormal karyotype.212 On occasion, amniocentesis may be recommended to salvage viable amniocytes for cytogenetic analysis before delivery. Fetal-maternal hemorrhage occurs in all pregnancies but is usually minimal (< 0.1 mL total volume). In rare instances, this hemorrhage may be massive, leading to fetal demise. The Kleihauer-Betke (acid elution) test allows an estimate of the volume of fetal blood in the maternal circulation, and a maternal blood sample should be drawn within 6 to 8 hours of the purported bleeding episode because of rapid clearance of fetal cells from the maternal circulation.213 Intra-amniotic infection resulting in fetal death is usually evident on clinical examination. Placental membrane culture and autopsy examination of the fetus, placenta/fetal membranes, and umbilical cord may be useful. Fetal radiographic or MRI may sometimes be valuable if autopsy is declined.214,215
The inability to identify fetal heart activity or the absence of uterine growth may suggest the diagnosis. Ultrasonography is the “gold standard” for confirming IUFD by documenting the absence of fetal cardiac activity. Other ultrasonographic findings in late pregnancy include Spalding's sign (overlapping of the cranial sutures), scalp edema, and soft tissue maceration, although these usually take a few days to develop. Every effort should be made to avoid cesarean delivery in the setting of IUFD. Thus, in the absence of a contraindication, expectant management is often recommended. Latency (the period from fetal demise to delivery) varies according to the underlying cause and gestational age. In general, the earlier the gestational age, the longer the latency period. Overall, more than 90% of women go into spontaneous labor within 2 weeks of fetal death. However, many women find the prospect of carrying a dead fetus distressing and want the pregnancy terminated as soon as possible. Management options include surgical dilation and evacuation or induction of labor with cervical ripening, if indicated. Disseminated intravascular coagulation develops in 20% to 25% of women who retain a dead singleton fetus for longer than 3 weeks because of excessive consumption of clotting factors.216,217 Therefore, delivery should be effected within this period. Induction of labor with prostaglandins or oxytocin has been shown to be safe in the setting of an IUFD.
The death of one twin in a monochorionic twin gestation poses a particular challenge. In this setting, the surviving twin is at significant risk for major morbidity, including IUFD, neurologic injury, multiorgan system failure, thromboembolic events, placental abruption, and preterm birth.218-220 The prognosis for the surviving twin depends on the cause of death, gestational age, chorionicity, and the time between death of the first twin and delivery of the second. Dizygous twin pregnancies do not share a circulation, and death of one twin may have little impact on the surviving twin. The dead twin may be resorbed completely or may become compressed and incorporated into the membranes (fetus papyraceus). Disseminated intravascular coagulation in the surviving fetus and/or mother is rare.221 On the other hand, some level of shared circulation can be demonstrated in almost all monozygous twin pregnancies, and death of one fetus in this setting raises the risk for death of its co-twin owing to profound hypotension and/or purported transfer of thromboplastic proteins from the dead fetus to the live fetus.222 If it survives, the co-twin has a 20% risk for development of permanent neurologic injury (multicystic encephalomalacia), which may not be prevented by immediate delivery.223,224 Therefore, management of a surviving twin depends on chorionicity and gestational age. Regular fetal surveillance (kick counts, NST, BPP) should be instituted (see Table 6-11), and delivery considered in the setting of nonreassuring fetal test results or at a favorable gestational age.
Fetal Therapy
Continued assessment of the fetus throughout pregnancy is critical to optimizing pregnancy outcomes. In most cases, evidence of fetal compromise prompts delivery. However, in certain situations, treatment may be available to improve or even correct the underlying problem in utero. These interventions can be noninvasive (e.g., administration of digoxin to the mother to treat a fetal supraventricular tachycardia) or invasive (e.g., placement of a vesicoamniotic shunt) and are summarized in Tables 6-14 and 6-15, respectively.225-260 Some of these interventions have been subjected to rigorous clinical trials and have been shown to be effective, whereas others remain investigational. The intervention that has perhaps had the greatest effect on perinatal outcome is antenatal maternal administration of corticosteroids.
TABLE 6-14
Noninvasive Treatment Options to Improve Perinatal Outcome
| Clinical Condition | Treatment | Efficacy |
| Imminent risk for preterm birth < 34 weeks | Antenatal corticosteroids | Effective in decreasing respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis225 |
| Pregestational diabetes mellitus | Strict glycemic control | Effective in decreasing rate of stillbirths and birth defects226 |
| Phenylketonuria (autosomal recessive disorder due to phenylalanine hydroxylase deficiency) | Dietary manipulation (low-phenylalanine diet) | Effective in decreasing birth defects and brain damage in affected fetuses227 |
| Alloimmune thrombocytopenia | Maternal intravenous immunoglobulin ± corticosteroids | Data conflicting on effect of intravenous immunoglobulin on fetal platelets; steroids probably of no benefit228,229 |
| Fetal thyrotoxicosis | Maternal propylthiouracil | Effective in decreasing fetal growth restriction and subsequent neurodevelopmental defects230,231 |
| Congenital adrenal hyperplasia (due usually to 21-hydroxylase deficiency) | Maternal dexamethasone | Effective in preventing virilization of female fetus if given prior to 8 to 9 weeks' gestation232 |
| Fetal supraventricular tachycardia (SVT) | Maternal digoxin | Data conflicting on effect of digoxin to correct fetal SVT |
TABLE 6-15
Invasive Treatment Options to Improve Perinatal Outcome
| Clinical Condition | Treatment | Efficacy |
| Severe fetal anemia with or without hydrops fetalis | Intrauterine transfusion | Effective233,234 |
| Fetal supraventricular tachycardia | Digoxin given directly to fetus by intramuscular injection | Effective |
| Severe obstructive uropathy | Vesicoamniotic shunt | Effective in preventing renal injury and improving survival235 |
| Isolated fluid collection in the fetus (severe ascites, hydrothorax) | Fetoamniotic shunting | Effective236 |
| Severe valvular stenosis | Fetal surgery (in utero valvuloplasty) | Investigational237 |
| Fetal lung masses (congenital cystic adenomatous malformation, pulmonary sequestration) | Fetal surgery (in utero resection of lesion) | Investigational238,239 |
| Congenital hydrocephalus | Fetal surgery (in utero shunting) | Investigational240 |
| Congenital diaphragmatic hernia | Fetal surgery (in utero repair; tracheal occlusion) | Investigational241,242 |
| Fetal neural tube defect | Fetal surgery (in utero repair) | Investigational243-245 |
| Higher-order multiple pregnancy (≥ triplets) | Multifetal pregnancy reduction | Effective in improving perinatal outcomes with reduction to twins246-249 |
| Twin-to-twin transfusion syndrome (TTTS) | Serial amnioreduction versus septostomy versus fetal surgery (endoscopic laser ablation, cord ligation) | Effective Laser ablation appears to give the best chance of intact survival in severe TTTS250-255 |
| Preterm premature rupture of membranes | Serial amnioinfusion versus fetal surgery (laser coagulation, intra-amniotic amniopatch) | Investigational256-258 |
| Ex utero intrapartum therapy (EXIT) | To facilitate oxygenation at delivery prior to ligation of the umbilical cord when the infant's airway is obstructed; may facilitate transition to extracorporeal membrane oxygenation (ECMO) in infants with severe pulmonary or cardiac malformations | Case reports of success259,260 |
Antenatal Corticosteroids
Respiratory distress syndrome (RDS) refers to respiratory compromise presenting at or shortly after delivery due to a deficiency of pulmonary surfactant, an endogenous detergent that serves to decrease the surface tension within alveoli, thereby preventing alveolar collapse. Overall, neonatal RDS affects approximately 1% of live births, but not all infants are at equal risk. The pulmonary system is among the last of the fetal organ systems to become functionally mature. Thus, RDS is primarily, although not exclusively, a disease of preterm infants, with the incidence and severity highly dependent on gestational age. For example, RDS affects more than 80% of infants younger than 28 weeks' gestation and 10% to 15% of all infants weighing less than 2500 g.261,262 RDS remains a major cause of perinatal morbidity and mortality in extremely preterm infants. In addition to gestational age, a number of other factors influence the risk for RDS in a given fetus. For reasons that are not clear, African-American ethnicity, female gender, preeclampsia, and intrauterine exposure to cigarette smoke are protective against the development of RDS.
In 1972, Liggins and Howie263 demonstrated that the administration of a single course of two antenatal doses of a corticosteroid (betamethasone) reduced the incidence of RDS by 50%. This original observation has since been confirmed by a number of investigators.225,264-267 A meta-analysis of 12 randomized controlled trials with more than 3000 participants concluded that antenatal administration of corticosteroids to women in preterm labor reduced the incidence of neonatal RDS by 40% to 60% and resulted in an improvement in overall survival.225 In one study, a single course of antenatal corticosteroids resulted in a threefold rise in the chance of unaffected survival in neonates with a birth weight less than 1500 g.264 Certain steroids cross the placenta and induce cellular differentiation at the expense of growth. Type II pneumocytes in the lungs differentiate and begin making pulmonary surfactant, which accounts for the decrease in risk for RDS, and endothelial cells lining the vasculature undergo cellular maturation and stabilization, which explains the concomitant drop in incidence of bleeding into the brain (intraventricular hemorrhage) or gastrointestinal tract (necrotizing enterocolitis).265 Prednisone does not cross the placenta and therefore does not have a similar protective effect.
The National Institutes of Health and the ACOG have recommended that a single course of antenatal corticosteroids, defined as either betamethasone (12 mg intramuscularly q24 h × two doses) or dexamethasone (6 mg intramuscularly q12 h × four doses), be given after 23 to 24 weeks' gestation to any pregnant woman in whom delivery before 34 weeks' gestation is threatening.266,267 There is as yet no proven benefit to antenatal administration of corticosteroids after 34 weeks' gestation266,267 or between 32 to 34 weeks in the setting of preterm PROM,268 but this situation is largely due to the absence of data in these subgroups. Although the maximum benefit of antenatal corticosteroids is achieved 24 to 48 hours after the first injection, as little as 4 hours of treatment exerts some protective effect. This protective effect lasts for 7 days, after which further benefit is unclear. Multiple (three or more) courses of antenatal corticosteroids have been associated with fetal growth restriction, smaller head circumference, and (in animals) abnormal myelination of the optic nerves; consequently, multiple courses are not routinely recommended. If a threat of preterm delivery occurs more than 2 weeks after the initial course was completed, a rescue course of corticosteroids is recommended.269-273
Fetal Surgery
Fetal surgery has been proposed in selected cases to prevent progressive organ damage or to restore normal anatomy and fetal development (see Chapter 7). The ideal case for fetal surgery consists of a singleton pregnancy prior to fetal viability (i.e., before 23 to 24 weeks' gestation) in which the fetus has a normal karyotype and an isolated malformation that, if untreated, will result in fetal or neonatal demise. A detailed understanding of the natural history of the malformation is essential when one is considering whether to recommend surgery. Fetal surgery should not be attempted if the natural history of the disorder is unknown or if the chances of survival without in utero treatment are equal to or greater than the risks of the procedure. The only two randomized controlled trials published to date in fetal surgery—one on tracheal occlusion for the management of congenital diaphragmatic hernia274 and the other on prenatal versus postnatal repair of myelomeningocele275—found little significant benefit to in utero surgery. Repair of lesions that are not believed to be life threatening (e.g., cleft lip and palate) should be deferred until after delivery to minimize risks to the mother.
Before in utero surgery can be recommended, a thorough evaluation must be performed to (1) precisely characterize the defect, (2) exclude associated malformations, (3) perform a fetal karyotype analysis, and (4) eliminate the possibility that the condition can be treated using less aggressive technologies. Detailed counseling about the risks and benefits of the proposed procedure is required, and written informed consent is mandatory. Such a discussion must include a detailed review of the risks to both the fetus and the mother, including preterm PROM (28% to 100%), preterm labor and delivery (> 50%), maternal pulmonary edema (20% to 30%), placental abruption (5% to 10%), chorioamnionitis and sepsis (< 5%), and maternal death (rare).242,244 Specific examples of fetal surgical procedures are summarized in Table 6-15.
References
1. American College of Obstetricians and Gynecologists. Antepartum fetal surveillance. ACOG Practice Bulletin No. 9. [Washington, DC] 1999.
2. American College of Obstetricians and Gynecologists. Management of postterm pregnancy. ACOG Practice Bulletin No. 55. [Washington, DC] 2004.
3. Neilson JP. Ultrasound for fetal assessment in early pregnancy. Cochrane Database Syst Rev. 2000;(2).
4. Hertz RH, Sokol RJ, Knoke JD, et al. Clinical estimation of gestational age: rules for avoiding preterm delivery. Am J Obstet Gynecol. 1978;131:395–402.
5. Gardosi J, Vanner T, Francis A. Gestational age and induction of labor for prolonged pregnancy. Br J Obstet Gynaecol. 1997;104:792–797.
6. Taipale P, Hiilermaa V. Predicting delivery date by ultrasound and last menstrual period on early gestation. Obstet Gynecol. 2001;97:189–194.
7. Kramer MS, Mclean FH, Boyd ME, Usher RH. The validity of gestational age estimation by menstrual dating in term, preterm, and post-term gestations. JAMA. 1988;260:3306–3308.
8. Rasor JL, Farber S, Braunstein GD. An evaluation of 10 kits for determination of human chorionic gonadotropin in serum. Clin Chem. 1983;29:1828–1831.
9. American College of Obstetricians and Gynecologists. Medical management of ectopic pregnancy. ACOG Practice Bulletin No. 94. [Washington, DC] 2008.
10. Kadar N, DeVore G, Romero R. Discriminating hCG zone: its use in the sonographic evaluation for ectopic pregnancy. Obstet Gynecol. 1981;58:156–161.
11. Fossum GT, Davajan V, Kletzky OA. Early detection of pregnancy with transvaginal ultrasound. Fertil Steril. 1988;49:788–791.
12. Sabbaha RE, Hughey M. Standardization of sonar cephalometry and gestational age. Obstet Gynecol. 1978;52:402–406.
13. Mongelli M, Wilcox M, Gardosi J. Estimating the date of confinement: ultrasonographic biometry versus certain menstrual dates. Am J Obstet Gynecol. 1996;174:278–281.
14. Tunon K, Eik-Nes SH, Grottum P. A comparison between ultrasound and a reliable last menstrual period as predictors of the day of delivery in 15,000 examinations. Ultrasound Obstet Gynecol. 1996;8:178–185.
15. Seeds JW, Cefalo RC. Relationship of fetal limb lengths to both biparietal diameter and gestational age. Obstet Gynecol. 1982;60:680–685.
16. Bennett KA, Crane JM, O'Shea P, et al. First trimester ultrasound screening is effective in reducing postterm labor induction rates: a randomized controlled trial. Am J Obstet Gynecol. 2004;190:1077–1081.
17. Waldenström U, Axelsson O, Nilsson S, et al. Effects of routine one-stage ultrasound screening in pregnancy: a randomised controlled trial. Lancet. 1988;2:585–588.
18. Saari-Kemppainen A, Karjalainen O, Ylostalo P, Heinonen OP. Ultrasound screening and perinatal mortality: controlled trial of systematic one-stage screening in pregnancy. The Helsinki Ultrasound Trial. Lancet. 1990;336:387–391.
19. Ewigman BG, Crane JP, Frigoletto FD, et al. Effect of prenatal ultrasound screening on perinatal outcome. RADIUS Study Group. N Engl J Med. 1993;329:821–827.
20. American College of Obstetricians and Gynecologists. Ultrasonography in pregnancy. ACOG Practice Bulletin No. 101. [Washington, DC] 2009.
21. American Academy of Pediatrics and American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 6th edition. AAP and ACOG: Washington, DC; 2007.
22. Persson PH, Kullander S. Long-term experience of general ultrasound screening in pregnancy. Am J Obstet Gynecol. 1983;146:942–947.
23. Belfrage P, Fernström I, Hallenberg G. Routine or selective ultrasound examinations in early pregnancy. Obstet Gynecol. 1987;69:747–750.
24. Thacker SB. Quality of controlled clinical trials: the case of imaging ultrasound in obstetrics: a review. Br J Obstet Gynaecol. 1985;92:432–444.
25. Kieler H, Axelsson O, Nilsson S, Waldenström U. The length of human pregnancy as calculated by ultrasonographic measurement of the fetal biparietal diameter. Ultrasound Obstet Gynecol. 1995;6:353–357.
26. Crane JP, LeFevre ML, Winborn RC, et al. A randomized trial of prenatal ultrasonographic screening: impact on the detection, management, and outcome of anomalous fetuses. Am J Obstet Gynecol. 1994;171:392–399.
27. LeFevre ML, Bain RP, Ewigman BG. A randomized trial of prenatal ultrasonographic screening: impact on maternal management and outcome. Am J Obstet Gynecol. 1993;169:483–489.
28. Institute American. of Ultrasound in Medicine Bioeffects Committee. Review of the Radius Study. AIUM Reporter. 1994;10:2–4.
29. Institute of Medicine. Nutritional status and weight gain. In Nutrition During Pregnancy. National Academies Press: Washington, DC; 1990.
30. Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. May 28, 2009. [Consensus report. Available at] http://iom.edu/Reports/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines.aspx [Accessed November 18, 2012] .
31. Leopold G, Sporlin L. Conduct of normal births through external examination alone. Arch Gynaekol. 1894;45:337.
32. Belizan JM, Villar J, Nardin JC, et al. Diagnosis of intrauterine growth retardation by a simple clinical method: measurement of fundal height. Am J Obstet Gynecol. 1978;1313:643–646.
33. Gardosi J, Francis A. Controlled trial of fundal height measurement plotted on customised antenatal growth charts. Br J Obstet Gynaecol. 1999;106:309–317.
34. Warsof SL, Gohari P, Berkowitz RL, Hobbins JC. The estimation of fetal weight by computer-assisted analysis. Am J Obstet Gynecol. 1977;128:881–892.
35. Shepard MJ, Richards VA, Berkowitz RL. An evaluation of two equations for predicting fetal weight by ultrasound. Am J Obstet Gynecol. 1982;142:47–54.
36. Jeanty P, Cousaert E, Cantraine F, et al. A longitudinal study of fetal limb growth. Am J Perinatol. 1984;1:136–144.
37. Lockwood CJ, Weiner S. Assessment of fetal growth. Clin Perinatol. 1986;13:3–35.
38. Bahado-Singh RO, Dashe J, Deren O, et al. Prenatal prediction of neonatal outcome in the extremely low-birth-weight infant. Am J Obstet Gynecol. 1998;178:462–468.
39. Ehrenkranz RA. Estimated fetal weights versus birth weights: should the reference intrauterine growth curves based on birth weights be retired? Arch Dis Child Fetal Neonatal Ed.. 2007;92:161–162.
40. Deter RL, Hadlock FP, Harrist RB, Carpenter RJ. Evaluation of three methods for obtaining fetal weight estimates using dynamic image ultrasound. J Clin Ultrasound. 1981;9:421–425.
41. Deter RL, Rossavik IK, Harrist RB, Hadlock FP. Mathematic modeling of fetal growth: development of individual growth curve standards. Obstet Gynecol. 1986;68:156–161.
42. Rossavik IK, Deter RL. Mathematical modeling of fetal growth. I. Basic principles. J Clin Ultrasound. 1984;12:529–533.
43. Gardosi J. Customized fetal growth standards: rationale and clinical application. Semin Perinatol. 2004;28:33–40.
44. Nyberg DA, Abuhamad A, Ville Y. Ultrasound assessment of abnormal fetal growth. Semin Perinatol. 2004;28:3–22.
45. Deter RL. Individualized growth assessment: evaluation of growth using each fetus as its own control. Semin Perinatol. 2004;28:23–32.
46. Sabbagha RE. Intrauterine growth retardation. Sabbagha RE. Diagnostic Ultrasound Applied to Obstetrics and Gynecology. 2nd edition. JB Lippincott: Philadelphia; 1987:112.
47. Hadlock FP, Harrist RB, Carpenter RJ, et al. Sonographic estimation of fetal weight: the value of femur length in addition to head and abdomen measurements. Radiology. 1984;150:535–540.
48. Anderson NG, Jolley IJ, Wells JE. Sonographic estimation of fetal weight: comparison of bias, precision and consistency using 12 different formulae. Ultrasound Obstet Gynecol. 2007;30:173–179.
49. Chauhan SP, Lutton PM, Bailey KJ, et al. Intrapartum clinical, sonographic, and parous patients’ estimates of newborn birth weight. Obstet Gynecol. 1992;79:956–958.
50. Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–496.
51. American College of Obstetricians and Gynecologists. Fetal macrosomia. ACOG Practice Bulletin No. 22. [Washington, DC] 2000.
52. American College of Obstetricians and Gynecologists. Gestational diabetes. ACOG Practice Bulletin No. 30. [Washington, DC] 2001.
53. Magee MS, Walden CE, Benedetti TJ, Knopp RH. Influence of diagnostic criteria on the incidence of gestational diabetes and perinatal morbidity. JAMA. 1993;269:609–615.
54. O'Sullivan JB, Mahan CM, Charles D, Dandrow RV. Screening criteria for high-risk gestational diabetic patients. Am J Obstet Gynecol. 1973;116:895–900.
55. Kjos SL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med. 1999;341:1749–1756.
56. Widness JA, Cowett RM, Coustan DR, et al. Neonatal morbidities in infants of mothers with glucose intolerance in pregnancy. Diabetes. 1985;34:61–65.
57. Watson WJ, Soisson AP, Harlass FE. Estimated weight of the term fetus: accuracy of ultrasound vs clinical examination. J Reprod Med. 1988;33:369–371.
58. Niswander KR, Capraro VJ, Van Coevering RJ. Estimation of birth weight by quantified external uterine measurements. Obstet Gynecol. 1970;36:294–298.
59. Jazayeri A, Heffron JA, Phillips R, Spellacy WN. Macrosomia prediction using ultrasound fetal abdominal circumference of 35 centimeters or more. Obstet Gynecol. 1999;93:523–526.
60. Cromi A, Ghezzi F, Di Naro E, et al. Large cross-sectional area of the umbilical cord as a predictor of fetal macrosomia. Ultrasound Obstet Gynecol. 2007;30:861–866.
61. Abramowicz JS, Robischon K, Cox C. Incorporating sonographic cheek-to-cheek diameter, biparietal diameter and abdominal circumference improves weight estimation in the macrosomic fetus. Ultrasound Obstet Gynecol. 1997;9:409–413.
62. Sood AK, Yancey M, Richards D. Prediction of fetal macrosomia using humeral soft tissue thickness. Obstet Gynecol. 1995;95:937–940.
63. American College of Obstetricians and Gynecologists. Shoulder dystocia. ACOG Practice Bulletin No. 40, November 2002 (Reaffirmed 2008). Int J Gynaecol Obstet. 2003;80:887–892.
64. Sadovsky E, Polishuk WZ, Mahler Y, Malkin A. Correlation between electromagnetic recording and maternal assessment of fetal movement. Lancet. 1973;1:1141–1143.
65. Hertogs K, Roberts AB, Cooper D, et al. Maternal perception of fetal motor activity. Br Med J. 1979;2:1183–1185.
66. Schmidt W, Cseh I, Hara K, Kubli F. Maternal perception, tocodynamic findings and real-time ultrasound assessment of total fetal activity. Int J Gynaecol Obstet. 1984;22:85–90.
67. Rayburn WF. Clinical significance of perceptible fetal motion. Am J Obstet Gynecol. 1980;138:210–212.
68. Frøen JF. A kick from within—fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13–24.
69. Sadovsky E. Fetal movement in utero—a review. II. Fetal movements and fetal distress. Isr J. Obstet Gynecol. 1992;3:75–78.
70. Harper RG, Greenberg M, Farahani G, et al. Fetal movement, biochemical and biophysical parameters, and the outcome of pregnancy. Am J Obstet Gynecol. 1981;141:39–42.
71. Sadovsky E, Yaffe H, Plushuk W. Fetal movements in pregnancy and urinary estriol in prediction of impending fetal death in utero. Isr J Med Sci. 1974;10:1096–1099.
72. Moore T, Piacquadio K. A prospective evaluation of fetal movement screening to reduce the incidence of antepartum fetal death. Am J Obstet Gynecol. 1990;163:264–265.
73. Liston R, Cohen A, Mennuti M, Gabbe S. Antepartum fetal evaluation by maternal perception of fetal movement. Obstet Gynecol. 1982;60:424–426.
74. Patrick J, Campbell K, Carmichael L, et al. Patterns of gross fetal body movements over 24-hour observation intervals during the last 10 weeks of pregnancy. Am J Obstet Gynecol. 1982;142:363–371.
75. Moore TR, Piacquadio K. A prospective evaluation of fetal movement screening to reduce the incidence of antepartum fetal death. Am J Obstet Gynecol. 1989;160:1075–1080.
76. American College of Obstetricians and Gynecologists and American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. ACOG Task Force on Neonatal Encephalopathy and Cerebral Palsy. [Washington, DC] 2003.
77. Nelson KB. Can we prevent cerebral palsy? N Engl J Med. 2003;349:1765–1769.
78. Hankins GDV, Speer M. Defining the pathogenesis and pathophysiology of neonatal encephalopathy and cerebral palsy. Obstet Gynecol. 2003;102:628–636.
79. Leveno KJ, Cunningham FG, Nelson S, et al. A prospective comparison of selective and universal electronic fetal monitoring in 34,995 pregnancies. N Engl J Med. 1986;315:615–619.
80. National Institute of Child Health and Human Development Research Planning Workshop. Electronic fetal heart rate monitoring: research guidelines for interpretation. Am J Obstet Gynecol. 1997;177:1385–1390.
81. Macones GA, Hankins GD, Spong CY, et al. The 2008 National Institute of Child Health and Human Development workshop on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112:661–666.
82. Lee CY, DiLoreto PC, Logrand B. Fetal activity acceleration determination for the evaluation of fetal reserve. Obstet Gynecol. 1976;48:19–26.
83. Brown R, Patrick J. The nonstress test: how long is enough? Am J Obstet Gynecol. 1981;141:646–651.
84. Freeman RK, Anderson G, Dorchester W. A prospective multi-institutional study of antepartum fetal heart rate monitoring. I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol. 1982;143:771–777.
85. Boehm FH, Salyer S, Shah DM, Vaughn WK. Improved outcome of twice weekly nonstress testing. Obstet Gynecol. 1986;67:566–568.
86. Smith CV, Phelan JP, Paul RH. A prospective analysis of the influence of gestational age on the baseline fetal heart rate and reactivity in a low-risk population. Am J Obstet Gynecol. 1985;153:780–782.
87. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613–618.
88. Smith CV, Phelan JP, Platt LD, et al. Fetal acoustic stimulation testing. II. A randomized clinical comparison with the nonstress test. Am J Obstet Gynecol. 1986;155:131–134.
89. Ingemarsson I, Arulkumaran S, Paul RH, et al. Fetal acoustic stimulation in early labor in patients screened with the admission test. Am J Obstet Gynecol. 1988;158:70–74.
90. Sarno AP, Ahn MO, Phelan JP, Paul RH. Fetal acoustic stimulation in the early intrapartum period as a predictor of subsequent fetal condition. Am J Obstet Gynecol. 1990;162:762–767.
91. Manning FA, Baskett TF, Morrison I, Lange I. Fetal biophysical profile scoring: a prospective study in 1,184 high-risk patients. Am J Obstet Gynecol. 1981;140:289–294.
92. Manning FA, Morrison I, Harman CR, et al. Fetal assessment based on fetal biophysical profile scoring: experience in 19,221 referred high-risk pregnancies. Am J Obstet Gynecol. 1987;157:880–884.
93. Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. The use and misuse of the fetal biophysical profile. Am J Obstet Gynecol. 1987;156:527–533.
94. Vintzileos AM, Gaffney SE, Salinger LM, et al. The relationships among the fetal biophysical profile, umbilical cord pH, and Apgar scores. Am J Obstet Gynecol. 1987;157:627–631.
95. Manning FA, Morrison I, Lagne IR, et al. Fetal assessment based on fetal biophysical profile scoring: experience in 12,620 referred high-risk pregnancies. I. Perinatal mortality by frequency and etiology. Am J Obstet Gynecol. 1985;151:343–350.
96. Vintzileos AM, Campbell WA, Ingardia CJ, Nochimson DJ. The fetal biophysical profile and its predictive value. Obstet Gynecol. 1983;62:271–278.
97. Manning FA. Fetal biophysical assessment by ultrasound. Creasy RK, Resnik R. Maternal-Fetal Medicine: Principles and Practice. 2nd edition. WB Saunders: Philadelphia; 1989:359.
98. Oz AU, Holub B, Mendilcioglu I, et al. Renal artery Doppler investigation of the etiology of oligohydramnios in postterm pregnancy. Obstet Gynecol. 2002;100:715–718.
99. Tongsong T, Srisomboon J. Amniotic fluid volume as a predictor of fetal distress in postterm pregnancy. Int J Gynaecol Obstet. 1993;40:213–217.
100. Bochner CJ, Medearis AL, Davis J, et al. Antepartum predictors of fetal distress in postterm pregnancy. Am J Obstet Gynecol. 1987;157:353–358.
101. Morris JM, Thompson K, Smithey J, et al. The usefulness of ultrasound assessment of amniotic fluid in predicting adverse outcome in prolonged pregnancy: a prospective blinded observational study. Br J Obstet Gynaecol. 2003;110:989–994.
102. Clement D, Schifrin BS, Kates RB. Acute oligohydramnios in postdate pregnancy. Am J Obstet Gynecol. 1987;157:884–886.
103. Eden RD, Boehm FH. Assessment and Care of the Fetus; Physiological, Clinical, and Medicolegal Principles. Appleton & Lange: East Norwalk, CT; 1990:351–396.
104. McCallum WD, Williams CS, Nagel S, Daigle RE. Fetal blood velocity waveforms and intrauterine growth retardation. Am J Obstet Gynecol. 1978;132:425–429.
105. Rochelson B, Schulman H, Fleischer A, et al. The clinical significance of Doppler umbilical artery velocimetry in the small for gestational age fetus. Am J Obstet Gynecol. 1987;156:1223–1226.
106. Ducey J, Schulman H, Farmalcaides G, et al. A classification of hypertension in pregnancy based on Doppler velocimetry. Am J Obstet Gynecol. 1987;157:680–685.
107. Wenstrom KD, Weiner CP, Williamson RA. Diverse maternal and fetal pathology associated with absent diastolic flow in the umbilical artery of high risk fetuses. Obstet Gynecol. 1991;77:374–378.
108. Zelop CM, Richardson DK, Heffner LJ. Outcomes of severely abnormal umbilical artery Doppler velocimetry in structurally normal singleton fetuses. Obstet Gynecol. 1996;87:434–438.
109. Farmakides G, Schulman H, Ducey J, et al. Uterine and umbilical artery Doppler velocimetry in postterm pregnancy. J Reprod Med. 1988;33:259–261.
110. Stokes HJ, Roberts RV, Newnham JP. Doppler flow velocity waveform analysis in postdate pregnancies. Aust N Z J Obstet Gynaecol. 1991;31:27–30.
111. Baschat AA. Doppler application in the delivery timing of the preterm growth-restricted fetus: another step in the right direction. Ultrasound Obstet Gynecol. 2004;23:111–118.
112. Landon MB, Gable SG, Bruner JP, Ludmir J. Doppler umbilical artery velocimetry in pregnancy complicated by insulin-dependent diabetes mellitus. Obstet Gynecol. 1989;73:961–965.
113. Mari G, Adrignolo A, Abuhamad AZ, et al. Diagnosis of fetal anemia with Doppler ultrasound in the pregnancy complicated by maternal blood group immunization. Ultrasound Obstet Gynecol. 1995;5:400–405.
114. Donald I. On launching a new diagnostic science. Am J Obstet Gynecol. 1969;103:609–628.
115. Zelop CC, Bromley B, Frigoletto FD, Benacerraf BR. Second trimester sonographically diagnosed placenta previa: prediction of persistent previa at birth. Int J Gynaecol Obstet. 1994;44:207–210.
116. Berghella V, Roman A, Daskalakis C, et al. Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol. 2007;110:311–317.
117. American College of Obstetricians and Gynecologists. Invasive prenatal testing for aneuploidy. ACOG Practice Bulletin No. 88 (Reaffirmed 2009). Obstet Gynecol. 2007;110:1459–1467.
118. Haddow JE, Palomake GE, Knight GJ, et al. Prenatal screening for Down's syndrome with use of maternal serum markers. N Engl J Med. 1992;327:588–593.
119. Rose NC, Palomaki GE, Haddow JE, et al. Maternal serum alpha-fetoprotein screening for chromosomal abnormalities: a prospective study in women aged 35 and older. Am J Obstet Gynecol. 1994;170:1073–1078.
120. Eddleman KA, Malone FD, Sullivan L, et al. Pregnancy loss rates after midtrimester amniocentesis. Obstet Gynecol. 2006;108:1067–1072.
121. Canick JA, Palomaki GE, Osathanondh R. Prenatal screening for trisomy 18 in the second trimester. Prenat Diag. 1987;7:623–630.
122. Haddow JE, Palomaki GE, Knight GJ, et al. Reducing the need for amniocentesis in women 35 years of age or older with serum markers for screening. N Engl J Med. 1994;330:1114–1148.
123. Drugan A, Johnson MP, Reichler A, et al. Second trimester minor ultrasound abnormalities: impact on the risk of aneuploidy associated with advanced age. Obstet Gynecol. 1996;88:203–206.
124. Smith-Bindman P, Hosmer W, Feldstein VA, et al. Second-trimester ultrasound to detect fetuses with Down syndrome: a meta-analysis. JAMA. 2001;285:1044–1055.
125. Vintzeleos AM, Campbell WA, Rodis JF, et al. The use of second-trimester genetic sonogram in guiding clinical management of patients at increased risk for fetal trisomy 21. Obstet Gynecol. 1996;87:948–952.
126. Wapner R, Thom E, Simpson JL, et al. First Trimester Serum Biochemistry and Fetal Nuchal Translucency Screening (BUN) Study Group: First trimester screening for trisomies 21 and 18. N Engl J Med. 2003;349:1405–1413.
127. Canick JA, Lambert-Messerlian GM, Palomaki GE, et al. First- and Second-Trimester Evaluation of Risk (FASTER) Research Consortium. Comparison of serum markers in first-trimester Down syndrome screening. Obstet Gynecol. 2006;108:1192–1199.
128. Malone FD, Canick JA, Ball RH, et al. First- and Second-Trimester Evaluation of Risk (FASTER) Research Consortium. First-trimester or second-trimester screening, or both, for Down's syndrome. N Engl J Med. 2005;353:2001–2011.
129. American College of Obstetricians and Gynecologists. First-trimester screening for fetal aneuploidy. ACOG Committee Opinion No. 296, July 2004. Obstet Gynecol. 2004;104:215–217.
130. Evans MI, Cuckle HS. Biochemical screening for aneuploidy. Expert Rev. Obstet Gynecol. 2007;2:765–772.
131. Wald NJ, Rodeck DH, Hackshaw AK, et al. SURUSS Research Group. First and second trimester antenatal screening for Down syndrome: the results of the Serum, Urine, and Ultrasound Screening Study (SURUSS). Health Technol Assess. 2003;7:1–7.
132. American College of Obstetricians and Gynecologists. Screening for fetal chromosomal abnormalities. ACOG Practice Bulletin No. 77, January 2007. Obstet Gynecol. 2007;109:217–227.
133. Wald NJ, Hackshaw AK. Combining ultrasound and biochemistry in first-trimester screening for Down's syndrome. Prenat Diagn. 1997;17:821–829.
134. De Biasio P, Siccardi M, Volpe G, et al. First-trimester screening for Down syndrome using nuchal translucency measurement with free beta-hCG and PAPP-A between 10 and 13 weeks of pregnancy—the combined test. Prenat Diagn. 1999;19:360–366.
135. Krantz DA, Hallahan TW, Orlandi F, et al. First-trimester Down syndrome screening using dried blood biochemistry and nuchal translucency. Obstet Gynecol. 2000;96:207–213.
136. Ball RH, Caughey AB, Malone FD, et al. First- and Second-Trimester Evaluation of Risk (FASTER) Research Consortium. First- and second-trimester evaluation of risk for Down syndrome. Obstet Gynecol. 2007;110:10–17.
137. Malone FD, Ball RH, Nyberg DA, et al. FASTER Research Consortium. First-trimester nasal bone evaluation for aneuploidy in the general population. Obstet Gynecol. 2004;103:1222–1228.
138. Prefumo F, Sairam S, Bhide A, Thilaganathan B. First-trimester nuchal translucency, nasal bones, and trisomy 21 in selected and unselected populations. Am J Obstet Gynecol. 2006;194:828–833.
139. Towers CV, Asrat T, Rumney P. The presence of hepatitis B surface antigen and deoxyribonucleic acid in amniotic fluid and cord blood. Am J Obstet Gynecol. 2001;184:1514–1518.
140. D'Alton ME, Malone FD, Chelmow DM, et al. Defining the role of fluorescence in situ hybridization on uncultured amniocytes for prenatal diagnosis of aneuploidies. Am J Obstet Gynecol. 1997;176:769–776.
141. Oh DC, Min JY, Lee MH, et al. Prenatal diagnosis of tetralogy of Fallot associated with chromosome 22q11 deletion. J Korean Med Sci. 2002;17:125–128.
142. ACMG/ASHG Test and Technology Transfer Committee. Technical and clinical assessment of fluorescence in situ hybridization: an ACMG/ASHG position statement. I. Technical considerations. Genet Med. 2000;2:356–361.
143. Tabor A, Philip J, Madsen M, et al. Randomised controlled trial of genetic amniocentesis in 4606 low-risk women. Lancet. 1986;1:1287–1293.
144. Antsaklis A, Papantoniou N, Xygakis A, et al. Genetic amniocentesis in women 20-34 years old: associated risks. Prenat Diagn. 2000;20:247–250.
145. Daegan A, Johnson MP, Evans MI. Amniocentesis. Eden RD, Boehm FH. Assessment and Care of the Fetus: Physiological, Clinical, and Medicolegal Principles. Appleton & Lange: East Norwalk, CT; 1990:283–290.
146. Giorlandino C, Mobili L, Bilancioni E, et al. Transplacental amniocentesis: is it really a higher-risk procedure? Prenat Diagn. 1994;14:803–806.
147. Zorn EM, Hanson FW, Greve LC, et al. Analysis of the significance of discolored amniotic fluid detected at midtrimester amniocentesis. Am J Obstet Gynecol. 1986;154:1234–1240.
148. Alfirevic Z. Early amniocentesis versus transabdominal chorion villus sampling for prenatal diagnosis. Cochrane Database Syst Rev. 2000;(2).
149. Sundberg K, Bang J, Smidt-Jensen S, et al. Randomised study of risk of fetal loss related to early amniocentesis versus chorionic villus sampling. Lancet. 1997;350:697–703.
150. Tharmaratnam S, Sadek S, Steele EK, et al. Early amniocentesis: effect of removing a reduced volume of amniotic fluid on pregnancy outcome. Prenat Diagn. 1998;18:773–778.
151. Yoon G, Chernos J, Sibbald B, et al. Association between congenital foot anomalies and gestational age at amniocentesis. Prenat Diagn. 2001;21:1137–1141.
152. The Canadian Early and Mid-Trimester Amniocentesis Trial (CEMAT) Group. Randomized trial to assess safety and fetal outcome of early and midtrimester amniocentesis. Lancet. 1998;351:242–247.
153. Farrell SA, Summers AM, Dallaire L, et al. Club foot, an adverse outcome of early amniocentesis: disruption or deformation? J Med Genet. 1999;36:843–846.
154. Wapner RJ, Jackson L. Chorionic villus sampling. Clin Obstet Gynecol. 1988;31:328–344.
155. Firth HV, Boyd PA, Chamberlain P, et al. Severe limb abnormalities after chorion villus sampling at 56-66 days’ gestation. Lancet. 1991;337:762–763.
156. Burton BK, Schulz CJ, Burd LI. Limb anomalies associated with chorionic villus sampling. Obstet Gynecol. 1992;79:726–730.
157. Kuliev A, Jackson L, Froster U, et al. Chorionic villus sampling safety. Report of World Health Organization/EURO meeting in association with the Seventh International Conference on Early Prenatal Diagnosis of Genetic Diseases, Tel-Aviv, Israel, May 21, 1994. Am J Obstet Gynecol. 1996;174:807–811.
158. Carroll SGM, Davies T, Kyle PM, et al. Fetal karyotyping by chorionic villus sampling after the first trimester. Br J Obstet Gynaecol. 1999;106:1035–1040.
159. Multicentre randomised clinical trial of chorion villus sampling and amniocentesis. First report. [Canadian Collaborative CVS-Amniocentesis Clinical Trial Group] Lancet. 1989;1:1–6.
160. Rhoads GG, Jackson LG, Schlesselman SE, et al. The safety and efficacy of chorionic villus sampling for early prenatal diagnosis of cytogenetic abnormalities. N Engl J Med. 1989;320:609–617.
161. MRC Working Party on the Evaluation of Chorion Villus Sampling. Medical Research Council European trial of chorion villus sampling. Lancet. 1991;337:1491–1499.
162. Jackson LG, Zachary JM, Fowler SE, et al. A randomized comparison of transcervical and transabdominal chorionic-villus sampling. The U.S. National Institute of Child Health and Human Development Chorionic-Villus Sampling and Amniocentesis Study Group. N Engl J Med. 1992;327:594–598.
163. Alfirevic Z, Sundberg K, Brigham S. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database Syst Rev. 2003;(3).
164. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 27. Prenatal diagnosis of fetal chromosomal abnormalities. Obstet Gynecol. 2001;27:1–12.
165. Wapner RJ, Johnson A, Davis G, et al. Prenatal diagnosis in twin gestations: a comparison between second-trimester amniocentesis and first-trimester chorionic villus sampling. Obstet Gynecol. 1993;82:49–56.
166. Martin AO, Elias S, Rosinsky B, et al. False negative findings on chorion villus sampling. Lancet. 1986;2:391–392.
167. Daffos F, Capella-Bilovsky M, Forestier F. A new procedure for fetal blood sampling in utero: preliminary results of fifty three cases. Am J Obstet Gynecol. 1983;146:985–987.
168. Fisk N, Bower S. Fetal blood sampling in retreat. Br Med J. 1993;307:143–144.
169. Van den Veyver IB, Moise KJ. Fetal RhD typing by polymerase chain reaction in pregnancies complicated by rhesus alloimmunization. Obstet Gynecol. 1996;88:1061–1067.
170. Harman CR, Bowman JM, Manning FA, Menticoglou SM. Intrauterine transfusion—intraperitoneal versus intravascular approach: a case-control comparison. Am J Obstet Gynecol. 1990;162:1053–1059.
171. Weiner CP, Thompson MIB. Direct treatment of fetal supraventricular tachycardia after failed transplacental therapy. Am J Obstet Gynecol. 1988;158:570–573.
172. Ghidini A, Sepulveda W, Lockwood C, Romero R. Complications of fetal blood sampling. Am J Obstet Gynecol. 1993;168:1339–1344.
173. Rovas L, Sladkevicius P, Strobel E, Valentin L. Intraobserver and interobserver reproducibility of three-dimensional gray-scale and power Doppler ultrasound examinations of the cervix in pregnant women. Ultrasound Obstet Gynecol. 2005;26:132–137.
174. Schuchter K, Metzenbauer M, Hafner E, Philipp K. Uterine artery Doppler and placental volume in the first trimester in the prediction of pregnancy complications. Ultrasound Obstet Gynecol. 2001;18:590–592.
175. Rimoin DL, Lachman RS. The chondrodysplasias. Emergy AE, Rimoin DL. Principles and Practice of Medical Genetics. 2nd edition. Churchill Livingstone: New York; 1990:895–932.
176. Levine D, Barnes PD, Edelman RR. Obstetric MR imaging. Radiology. 1999;211:609–617.
177. Levine D. Fetal magnetic resonance imaging. J Matern Fetal Neonatal Med. 2004;15:85–94.
178. Kirk JS, Riggs TW, Comstock CH, et al. Prenatal screening for cardiac anomalies: the value of routine addition of the aortic root to the four-chamber view. Obstet Gynecol. 1994;84:427–431.
179. Bérard A, Ramos E, Rey E, et al. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res B Dev Reprod Toxicol. 2007;80:18–27.
180. Olson CK, Keppler-Noreuil KM, Romitti PA, et al. In vitro fertilization is associated with an increase in major birth defects. Fertil Steril. 2005;84:1308–1315.
181. Guetta E, Gutstein-Abo L, Barkai G. Trophoblasts isolated from the maternal circulation: in vitro expansion and potential application in non-invasive prenatal diagnosis. J Histochem Cytochem. 2005;53:337–339.
182. Lo YMD, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487.
183. Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study. Genet Med. 2011;30:913–920.
184. Bianchi DW, Platt LD, Goldberg JD, et al. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890–901.
185. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207:137.e1–137.e8.
186. Palomaki GE, Deciu C, Kloza EM, et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: an international collaborative study. Genet Med. 2012;14:296–305.
187. Bahado-Singh RO, Kliman H, Feng TY, et al. First-trimester endocervical irrigation: feasibility of obtaining trophoblast cells for prenatal diagnosis. Obstet Gynecol. 1995;85:461–464.
188. Fejgin MD, Diukman R, Cotton Y, et al. Fetal cells in the uterine cervix: a source for early non-invasive prenatal diagnosis. Prenat Diagn. 2001;21:619–621.
189. Dugoff L, Hobbins JS, Malone FD, et al. First-trimester maternal serum PAPP-A and free beta-subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol. 2004;191:1446–1451.
190. Dugoff L, Hobbins JS, Malone FD, et al. FASTER Trial Research Consortium. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106:260–267.
191. Duric K, Skrablin S, Lesin J, et al. Second trimester total human chorionic gonadotropin, alpha-fetoprotein and unconjugated estriol in predicting pregnancy complications other than fetal aneuploidy. Eur J Obstet Gynecol Reprod Biol. 2003;110:12–15.
192. Galindo A, Comas C, Martínez JM, et al. Cardiac defects in chromosomally normal fetuses with increased nuchal translucency at 10-14 weeks of gestation. J Matern Fetal Neonatal Med. 2003;13:163–170.
193. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 4. Prevention of Rh D alloimmunization. Int J Gynaecol Obstet. 1999;66:63–70.
194. Moise KJ. Red blood cell alloimmunization in pregnancy. Semin Hematol. 2005;42:169–178.
195. Spinnato JA, Clark AL, Ralston KK, et al. Hemolytic disease of the fetus: a comparison of the Queenan and extended Liley methods. Obstet Gynecol. 1998;92:441–445.
196. American College of Obstetricians and Gynecologists. Management of alloimmunization. ACOG Practice Bulletin No. 75, August 2006 (Reaffirmed 2008). Obstet Gynecol. 2006;108:457–464.
197. Bullock R, Martin WL, Coomarasamy A, Kilby MD. Prediction of fetal anemia in pregnancies with red-cell alloimmunization: comparison of middle cerebral artery peak systolic velocity and amniotic fluid OD450. Ultrasound Obstet Gynecol. 2005;24:331–334.
198. Eden RD, Seifert LS, Winegar A, Spellacy WN. Perinatal characteristics of uncomplicated postdate pregnancies. Obstet Gynecol. 1987;69:296–299.
199. Rand L, Robinson JN, Economy KE, Norwitz ER. Post-term induction of labor revisited. Obstet Gynecol. 2000;96:779–783.
200. Smith GC. Life-table analysis of the risk of perinatal death at term and post term in singleton pregnancies. Am J Obstet Gynecol. 2001;184:489–496.
201. Caughey AB, Bishop JT. Maternal complications of pregnancy increase beyond 40 weeks of gestation in low-risk women. J Perinatol. 2006;26:540–545.
202. Bochner CJ, Williams J, Castro L, et al. The efficacy of starting postterm antenatal testing at 41 weeks as compared with 42 weeks of gestational age. Am J Obstet Gynecol. 1988;159:550–554.
203. Morris JM, Thompson K, Smithey J, et al. The usefulness of ultrasound assessment of amniotic fluid in predicting adverse outcome in prolonged pregnancy: a prospective blinded observational study. Br J Obstet Gynaecol. 2003;110:989–994.
204. Pitkin RM. Fetal death: diagnosis and management. Am J Obstet Gynecol. 1987;157:583–589.
205. Spong CY, Erickson K, Willinger M, et al. Stillbirth in obstetric practice: report of survey findings. J Matern Fetal Neonatal Med. 2003;14:39–44.
206. MacDorman MF, Hoyert DL, Martin JA, et al. Fetal and perinatal mortality, United States, 2003. Natl Vital Stat Rep. 2007;55:1–17.
207. Cotzias CS, Paterson-Brown S, Fisk NM. Prospective risk of unexplained stillbirth in singleton pregnancies at term: population based analysis. Br Med J. 1999;319:287–288.
208. Huang DY, Usher RH, Kramer MS, et al. Determinants of unexplained antepartum fetal deaths. Obstet Gynecol. 2000;95:215–221.
209. Frøen JF, Arnestad M, Frey K, et al. Risk factors for sudden intrauterine unexplained death: epidemiologic characteristics of singleton cases in Oslo, Norway, 1986-1995. Am J Obstet Gynecol. 2001;184:694–702.
210. Faye-Petersen OM, Guinn DA, Wenstrom KD. The value of perinatal autopsy. Obstet Gynecol. 1999;96:915–920.
211. Craven CM, Demsey S, Carey JC, Kochenour NK. Evaluation of perinatal autopsy protocol: influence of the prenatal diagnosis conference team. Obstet Gynecol. 1990;76:684–688.
212. Wapner RJ, Lewis D. Genetics and metabolic causes of stillbirth. Semin Perinatol. 2002;26:70–74.
213. Kolialexi A, Tsangaris GT, Antsaklis A, Mavroua A. Rapid clearance of fetal cells from maternal circulation after delivery. Ann N Y Acad Sci. 2004;1022:113–118.
214. Brookes JS, Hagmann C. MRI in fetal necropsy. J Magn Reson Imaging. 2006;24:1221–1228.
215. Cernach MC, Patricio FR, Galera MF, et al. Evaluation of a protocol for postmortem examination of stillbirths and neonatal deaths with congenital anomalies. Pediatr Dev Pathol. 2004;7:335–341.
216. Reid DE, Weiner AE, Roby CC, Diamond LK. Maternal afibrinogenemia associated with long-standing intrauterine fetal death. Am J Obstet Gynecol. 1953;66:500–506.
217. Pritchard JA. Fetal death in utero. Obstet Gynecol. 1959;14:573–580.
218. Carlson NJ, Towers CV. Multiple gestation complicated by the death of one fetus. Obstet Gynecol. 1989;73:685–689.
219. D'Alton ME, Newton ER, Cetrulo CL. Intrauterine fetal demise in multiple gestation. Acta Genet Med Gemellol (Roma). 1984;33:43–49.
220. Bejar R, Vigliocco G, Gramajo H, et al. Antenatal origin of neurologic damage in newborn infants. II. Multiple gestations. Am J Obstet Gynecol. 1990;162:1230–1236.
221. Romero R, Duffy TP, Berkowitz RL, et al. Prolongation of a preterm pregnancy complicated by death of a single twin in utero and disseminated intravascular coagulation: effects of treatment with heparin. N Engl J Med. 1984;310:772–774.
222. Benirschke K. Intrauterine death of a twin: mechanisms, implications for surviving twin, and placental pathology. Semin Diagn Pathol. 1993;10:222–231.
223. Szymonowicz W, Preston H, Yu VY. The surviving monozygotic twin. Arch Dis Child. 1986;61:454–458.
224. Ben-Shlomo I, Alcalay M, Lipitz S, et al. Twin pregnancies complicated by the death of one fetus. J Reprod Med. 1995;40:458–462.
225. Crowley P, Chalmers I, Kierse MJ. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol. 1990;97:11–25.
226. Greene MF, Hare JW, Cloherty JP, et al. First-trimester hemoglobin A1 and risk for major malformation and spontaneous abortion in diabetic pregnancy. Teratology. 1989;39:225–231.
227. Leuke RR, Levy HL. Maternal phenylketonuria and hyperphenylalaninemia. N Engl J Med. 1980;303:1202–1208.
228. Bussel JB, Berkowitz RL, McFarland JG, et al. Antenatal treatment of neonatal alloimmune thrombocytopenia. N Engl J Med. 1988;319:1374–1378.
229. Bussel JB, Berkowitz RL, Lynch L, et al. Antenatal management of alloimmune thrombocytopenia with intravenous gammaglobulin: a randomized trial of the addition of low dose steroid to intravenous gammaglobulin. Am J Obstet Gynecol. 1996;174:1414–1423.
230. Bruinse HW, Vermeulen-Meiners C, Wit JM. Fetal treatment for thyrotoxicosis in non-thyrotoxic pregnant women. Fetal Ther. 1988;3:152–157.
231. Wenstrom KD, Weiner CP, Williamson RA, Grant SS. Prenatal diagnosis of fetal hyperthyroidism using funipuncture. Obstet Gynecol. 1990;76:513–517.
232. Pang S, Pollack MS, Marshall RN, Immken L. Prenatal treatment of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. 1990;322:111–115.
233. Weiner CP, Williamson RA, Wenstrom KD, et al. Management of hemolytic disease by cordocentesis. I. Prediction of fetal anemia. Am J Obstet Gynecol. 1991;165:546–553.
234. Weiner CP, Williamson RA, Wenstrom KD, et al. Management of hemolytic disease by cordocentesis. II. Outcome of treatment. Am J Obstet Gynecol. 1991;165:1303–1307.
235. Johnson MP, Bukowski TP, Reitleman C, et al. In utero surgical treatment of fetal obstructive uropathy: a new comprehensive approach to identify candidates for vesicoamniotic shunt therapy. Am J Obstet Gynecol. 1994;170:1770–1779.
236. Rodeck CH, Fisk NM, Fraser DI, Nicolini U. Long term in utero drainage of fetal hydrothorax. N Engl J Med. 1988;319:1135–1138.
237. Tworetzky W, Wilkins-Haug L, Jennings RW, et al. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome. Candidate selection, technique, and results of successful intervention. Circulation. 2004;110:2125–2131.
238. Romero R, Pilu G, Jeanty P, et al. Congenital cystic adenomatoid malformation of the lung. Prenatal Diagnosis of Congenital Anomalies. Appleton-Lange: Norwalk, CT; 1988.
239. Adzick NS, Harrison MR, Crombleholme TM, et al. Fetal lung lesions: management and outcome. Am J Obstet Gynecol. 1998;179:884–889.
240. Manning FA, Harrison MR, Rodeck CR, et al. Catheter shunts for fetal hydronephrosis and hydrocephalus: report of the International Fetal Surgery Registry. N Engl J Med. 1986;315:336–340.
241. Dommergues M, Louis-Sylvestre C, Mandelbrot L, et al. Congenital diaphragmatic hernia: can prenatal ultrasonography predict outcome? Am J Obstet Gynecol. 1996;174:1377–1381.
242. Harrison MR, Keller RL, Hawgood SB, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916–1924.
243. Bruner JP, Tulipan N, Paschall RL, et al. Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA. 1999;282:1819–1825.
244. Hirose S, Farmer DL, Albanese CT. Fetal surgery for meningomyelocele. Curr Opin Obstet Gynecol. 2001;13:215–222.
245. Adzick NS, Thom EA, Spong CY, et al. for the MOMS Investigators. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004.
246. American College of Obstetricians and Gynecologists. Multifetal pregnancy reduction and selective fetal termination. ACOG Committee Opinion No. 94, April 1991. Int J Gynaecol Obstet. 1992;38:140–142.
247. Boulot P, Hedon B, Pelliccia G, et al. Effects of selective reduction in triplet gestation: a comparative study of 80 cases managed with or without this procedure. Fertil Steril. 1993;60:497–503.
248. Berkowitz RL, Lynch L, Lapinski R, Bergh P. First-trimester transabdominal multifetal pregnancy reduction: a report of two hundred completed cases. Am J Obstet Gynecol. 1993;169:17–21.
249. Smith-Levitin M, Kowalik A, Birnholz J, et al. Selective reduction of multifetal pregnancies to twins improves outcome over nonreduced triplet gestations. Am J Obstet Gynecol. 1996;175:878–882.
250. Quintero RA, Dickinson JE, Morales WJ, et al. Stage-based treatment of twin-twin transfusion syndrome. Am J Obstet Gynecol. 2003;188:1333–1340.
251. Moise KJ, Dorman K, Lamvu G, et al. A randomized trial of amnioreduction versus septostomy in the treatment of twin-twin transfusion syndrome. Am J Obstet Gynecol. 2005;193:701–707.
252. Roberts D, Neilson JP, Weindling AM. Interventions for the treatment of twin-twin transfusion syndrome. Cochrane Database Syst Rev. 2001;(1).
253. Dickinson JE, Duncombe GJ, Evans SF, et al. The long term neurologic outcome of children from pregnancies complicated by twin-to-twin transfusion syndrome. Br J Obstet Gynaecol. 2005;112:63–68.
254. Senat MV, Deprest J, Boulvain M, et al. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med. 2004;351:136–144.
255. Foley MR, Clewell WH, Finberg HJ, Mills MD. Use of the Foley Cordostat grasping device for selective ligation of the umbilical cord of an acardiac twin: a case report. Am J Obstet Gynecol. 1995;172:212–214.
256. Quintero RA, Morales WJ, Kalter CS, et al. Transabdominal intra-amniotic endoscopic assessment of previable premature rupture of membranes. Am J Obstet Gynecol. 1998;179:71–76.
257. Quintero RA, Morales WJ, Allen M, et al. Treatment of iatrogenic previable premature rupture of membranes with intra-amniotic injection of platelets and cryoprecipitate (amniopatch): preliminary experience. Am J Obstet Gynecol. 1999;181:744–749.
258. Locatelli A, Vergani P, Di Pirro G, et al. Role of amnioinfusion in the management of premature rupture of the membranes at <26 weeks’ gestation. Am J Obstet Gynecol. 2000;183:878–892.
259. Oepkes D, Teunissen AK, Van De Velde M, et al. Congenital high airway obstruction syndrome successfully managed with ex-utero intrapartum treatment. Ultrasound Obstet Gynecol. 2003;22:437–439.
260. Kanamori Y, Kitano Y, Hashizume K, et al. A case of laryngeal atresia (congenital high airway obstruction syndrome) with chromosome 5p deletion syndrome rescued by ex utero intrapartum treatment. J Pediatr Surg. 2004;39:25–28.
261. American College of Obstetricians and Gynecologists. Fetal maturity assessment prior to elective repeat cesarean delivery. ACOG Committee Opinion No. 98. [Washington, DC] 1991.
262. American College of Obstetricians and Gynecologists. Fetal lung maturity. ACOG Practice Bulletin No. 97. [Washington, DC] 2008.
263. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525.
264. Rennie JM. Perinatal management at the lower end of viability. Arch Dis Child Fetal Neonatal Ed.. 1996;74:214–218.
265. Leviton A, Kuban KC, Pagano M, et al. Antenatal corticosteroids appear to reduce the risk of postnatal germinal matrix hemorrhage in intubated low birth weight newborns. Pediatrics. 1993;91:1083–1088.
266. NIH Consensus Development. Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273:413–418.
267. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Antenatal corticosteroid therapy for fetal maturation. ACOG Committee Opinion No. 402. [Washington, DC] 2008.
268. American College of Obstetricians and Gynecologists. Premature rupture of membranes. ACOG Practice Bulletin No. 1. [Washington, DC] 1998.
269. Vermillion ST, Bland ML, Soper DE. Effectiveness of a rescue dose of antenatal betamethasone after an initial single course. Am J Obstet Gynecol. 2001;185:1086–1089.
270. Guinn DA, Atkinson MW, Sullivan L, et al. Single vs weekly courses of antenatal corticosteroids for women at risk of preterm delivery. JAMA. 2001;286:1581–1587.
271. Wapner R, Sorokin Y, Thom EA, et al. National Institutes of Child Health and Human Development Maternal-Fetal Medicine Units Network. Single versus weekly courses of antenatal corticosteroids: evaluation of efficacy and safety. Am J Obstet Gynecol. 2006;195:633–642.
272. Crowther CA, Haslam RR, Hiller JE, et al. Australasian Collaborative Trial of Repeat Doses of Steroids (ACT ORDS) Study Group. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet. 2006;367:1913–1919.
273. Crowther CA, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for preventing neonatal respiratory disease. Cochrane Database Syst Rev. 2007;(3).
274. Harrison MR, Keller RL, Hawgood SB, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916–1924.
275. Adzick NS, Thom EA, Spong CY, et al. for the MOMS Investigators. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004.