The Pain of Childbirth and Its Effect on the Mother and the Fetus
Peter H. Pan MD, James C. Eisenach MD
Chapter Outline
MEASUREMENT AND SEVERITY OF LABOR PAIN
PERSONAL SIGNIFICANCE AND MEANING
Cardiac, Respiratory, and Gastrointestinal Effects
The gate control theory of pain, described more than 40 years ago by Melzack and Wall,1 has revolutionized the understanding of the mechanisms responsible for pain and analgesia. Originally explained as the regulation of pain signals from the peripheral nerve to the spinal cord by the activity of other peripheral nerves, interneurons in the spinal cord, and central supraspinal centers (Figure 20-1), the theory has been refined with the concept of a neuromatrix, a remarkably dynamic system capable of undergoing rapid change.2 Neural circuits and intraneural mechanisms regulate sensitivity at peripheral afferent terminals; along the conducting axons of peripheral nerves; in the spinal cord, pons, medulla, and thalamus; and at cortical sites of pain transmission and projection. For example, the peripheral application of capsaicin to the skin alters spinal gating mechanisms within 10 minutes, resulting in a light touch signal's being interpreted as burning pain.3
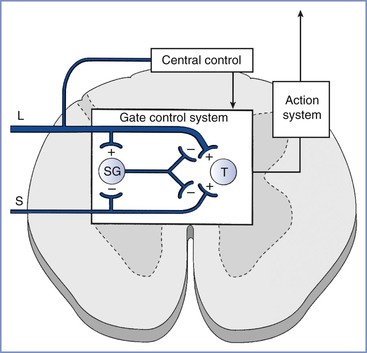
FIGURE 20-1 Gate control theory of pain. Activity in small-diameter afferents (S) stimulates transmission cells in the spinal cord (T ), which send signals supraspinally and results in the perception of pain. Small-diameter afferents also inhibit cells in the spinal cord substantia gelatinosa (SG), the activity of which reduces excitatory input to T cells. Activity in large-diameter afferents (L) also stimulates T cells in a manner that is perceived as nonpainful and excites SG cells to “close the gate” and reduce small-diameter afferent activation of T cells. The gate mechanism is under regulation by central sites. (From Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965; 150:971-5.)
Despite extensive research (initiated by the gate control theory) into the mechanisms and treatments for chronic pain, virtually no research on the neurophysiologic basis or therapies for labor pain has been performed. This discrepancy in focus has led to vastly different approaches to the treatment of patients with chronic versus obstetric pain. A patient with chronic pain typically undergoes a sophisticated physical assessment of sensory function; is offered therapies, on the basis of the assessment, from nearly a dozen different classes of analgesics; and can benefit from the enormous resources expended by the pharmaceutical industry to introduce agents that act on novel receptors or enzymes. By contrast, a laboring woman receives no physical assessment of sensory function and is offered only a handful of systemic drugs that act primarily through the anatomic blockade of neural traffic.
In this chapter, this paradox in the approach to labor pain is examined and the basis for current therapy (anatomy), the basis for future therapy (neurophysiology), and the effects of labor pain on the mother and the infant are reviewed.
Measurement and Severity of LABOR Pain
The recognition and acceptance of chronic pain, which frequently lacks an obvious outward cause, contrasts to the recurrent denial of labor pain, which is accompanied by visible tissue injury. Dick-Read4 suggested that labor is a natural process not considered painful by women in primitive cultures that should be handled with education and preparation rather than through pain medications. Lamaze5 popularized psychoprophylaxis as a method of birth preparation; this method now forms the basis for prepared childbirth training in the developed world. Although childbirth training acknowledges the existence of pain during labor, some scientific-thought leaders still consider labor pain to be minor.
The severity of labor pain has been recognized previously. Melzack,6 using a questionnaire developed to assess the intensity and emotional impact of pain, observed that nulliparous women with no prepared childbirth training rated labor pain to be as painful as a digit amputation without anesthesia (Figure 20-2).6 More than 30 years before Melzack's quantification of pain, Javert and Hardy7,8 trained subjects to reproduce the intensity of labor pain with the sensation of noxious heat applied to the skin from a radiant heat source. In these experiments, several women achieved “ceiling pain”—resulting in second-degree burns to the skin—when they attempted to match the intensity of uterine contraction pain.7 Individual women also reported a close positive correlation between cervical dilation and pain intensity. Logistic regression analysis of the investigators' original data7 indicates a high likelihood of severe pain as labor progresses, with a time course closely associated with cervical dilation (Figure 20-3). Other investigators have noted that uterine pressure during contractions accounts for more than 90% of the variability in labor pain intensity.9 These observations are consistent with the conclusion that cervical distention is the primary cause of pain during the first stage of labor.
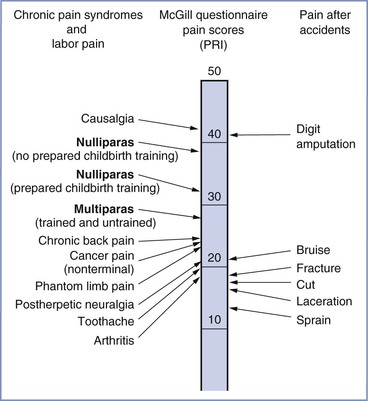
FIGURE 20-2 A comparison of pain scores obtained through the McGill Pain Questionnaire. Scores were collected from women in labor, patients in a general hospital clinic, and patients in the emergency department after accidents involving traumatic injury. Note the modest difference in pain scores between nulliparous women with and without prepared childbirth training. PRI, Pain rating index, which represents the sum of the rank values of all the words chosen from 20 sets of pain descriptors. (Modified from Melzack R. The myth of painless childbirth [The John J. Bonica Lecture]. Pain 1984; 19:321-37.)
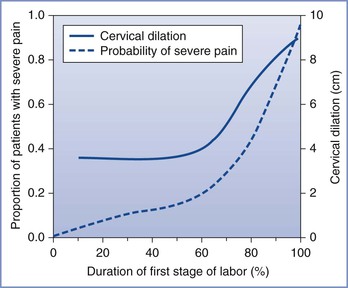
FIGURE 20-3 Likelihood of severe pain during labor. A significant minority of women (approximately one third) have severe pain in early labor, and the proportion of women with severe pain increases to nearly 90% later in labor, in close relationship with cervical dilation. (Data adapted from Hardy JD, Javert CT. Studies on pain: measurements of pain intensity in childbirth. J Clin Invest 1949; 28:153-62.)
There is considerable variability in the rated intensity of pain during labor. Nulliparous women rate labor pain as more severe than parous women; however, the differences are small and of questionable clinical relevance.10 There is a correlation between the intensity of menses and labor pain, especially back discomfort,10 although the reason for this relationship is unknown. It is possible that the rated intensity of labor pain reflects individual differences in the perception of all types of pain. In a study of factors affecting labor pain, 10 of 97 subjects reported that they had never experienced pain before childbirth; these women reported significantly less pain during labor and delivery compared with women who had previously experienced pain.11 In other studies, the variability of pain after cesarean delivery could be predicted with preoperative quantitative sensory testing (such as rating the intensity of pain with a standardized noxious thermal stimulus), psychologic constructs, and their combinations.12,13
The mechanism by which people perceive different levels of pain from the same stimulus remains unclear. A study involving brain imaging and a fixed acute noxious heat stimulus showed a strong correlation between verbal pain assessment and the level of activation of various cortical brain regions, especially the contralateral somatosensory cortex and anterior cingulate cortex.14 The investigators also found that the degree of activation of the thalamus was essentially identical in all subjects, suggesting that differences in perceived pain resulted from modulation at suprathalamic levels rather than in the peripheral nerves or spinal cord. The situation in labor may be more complex. For example, a large genetic polymorphism regulates cytokine production and function as well as pregnancy outcome.15 It is possible that interindividual differences in labor pain may partially reflect genetic differences in cytokine production or response.
In evaluating and studying labor pain and its treatment, most studies have tended to assess labor pain by using a set of discrete pain scores. However, labor pain is a complex, subjective, multidimensional, and dynamically changing experience with both sensory and affective components that are influenced by many factors. As a result, there are substantial individual differences in labor pain. Therefore, better identification of the covariates that affect labor progress and its associated pain is needed. Recently, Conell-Price et al.16 developed and validated a dynamic model to account for labor progress in the assessment of labor pain. Subsequently, Debiec et al.,17 at the same institution, combined a biexponential model that describes labor progress with a sigmoidal labor pain model to assess the influence of patient covariates on labor pain.17 Both studies used retrospective patient data to develop and test their models. In the former study,16 the prediction error for the pain scores was large, but the purpose of the model was to identify and remove variability associated with labor progress so that other factors (e.g., genetic polymorphisms) can be quantitatively studied. In this study,16 cervical dilation accounted for only 16% to 20% of the variability in reported pain. In the latter study,17 the covariate of ethnicity was found to have a statistically significant but clinically trivial effect on labor progress. The modeling described by these investigators provides a useful quantitative tool for future studies to identify and assess the effect—or the lack of effect—of patient and/or environmental covariates on labor progress, labor pain, and therapeutic responses. Better understanding of underlying causes of interindividual variability in labor progress, labor pain, and therapeutic responses is likely to lead to more tailored therapy.
In summary, although significant variability exists in the rated intensity of pain during labor and delivery, the majority of women experience more than minimal pain. The close correlation between cervical dilation and the rated severity of pain implies the existence of a causal relationship and increases the likelihood that a parturient will request analgesia as labor progresses.
Personal Significance and Meaning
The International Association for the Study of Pain (IASP) has defined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”18 Clearly, this reflects an intensity-discriminatory component and an emotional-cognitive component, with powerful interactions between the two. The focus of current interventions is heavily weighted toward the first component and assumes that labor pain is severe and in need of pharmacologic treatment. Largely ignored are coping strategies and the personal meaning of labor pain, which varies considerably among women.19
Although many women rate the pain of labor and delivery as severe, the terms used to more fully describe this pain reflect an emotional meaning. In a pioneering study of the quantification of pain from experimental dilation of the cervix, Bajaj et al.20 compared pain descriptors in women who were in labor, had experimental cervical dilation, were undergoing spontaneous abortion, or who had dysmenorrhea (Table 20-1). Women with dysmenorrhea used words that indicate suffering, such as “punishing” and “wretched,” whereas those in labor did not. Some researchers have drawn parallels between the pain derived from mountain climbing, which is associated with a sense of euphoria, and the pain of labor.19 As noted by one woman, “You mature and become a stronger personality when you've had a baby and have gone through the pain. I think that is the purpose of it, what the meaning of life is … to protect our children, to be stronger.”21 However, other women have found no deeper meaning to the pain of labor or reasons why it should not be treated. Many conditions that involve pain (e.g., trauma, severe dental disease, cancer) are considered a “normal” part of human life without a spiritual meaning, thereby making labor pain unique.
TABLE 20-1
Word Descriptors from the McGill Pain Questionnaire Used to Describe Pain from the Uterus and Cervix
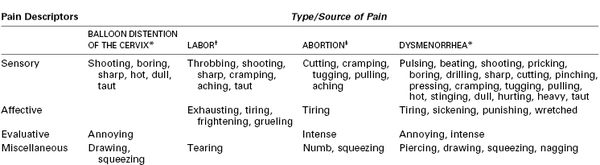
* Data from Bajaj P, Drewes AM, Gregersen H, et al. Controlled dilatation of the uterine cervix: an experimental visceral pain model. Pain 2002; 99:433-42.
† Data from Niven C, Gijsbers K. A study of labour pain using the McGill Pain Questionnaire. Soc Sci Med 1984; 19:1347-51.
‡ Data from Wells N. Pain and distress during abortion. Health Care Women Int 1991; 12:293-302.
In summary, there are large interindividual differences in how women experience the personal significance or meaning of labor pain. These different perceptions can lead to a long-term sense of failure and guilt when pharmacologic pain relief is accepted or emotional trauma when it is withheld.
Anatomic Basis
First Stage of Labor
Several lines of evidence suggest that the pain experienced during the first stage of labor is transduced by afferents with peripheral terminals in the cervix and lower uterine segment rather than the uterine body, as is often depicted (Figure 20-4). Uterine body afferents fire in response to distention, but in the absence of inflammation, uterine body distention has no or minimal effect on the behavior of laboratory animals.22,23 These observations suggest that uterine body afferents may be an important site of chronic inflammatory disease and chronic pelvic pain but are much less relevant to acute obstetric and uterine cervical pain. In addition, afferents to the uterine body regress during normal pregnancy, whereas those to the cervix and lower uterine segment do not.24 This denervation of the myometrium may protect against preterm labor by limiting α1-adrenergic receptor stimulation by locally released norepinephrine. Hardy and Javert8 reproduced the pain of uterine contractions in women during labor by manual distention of the cervix. Bonica and Chadwick25 later confirmed that women undergoing cesarean delivery under a local anesthetic field block experience pain from cervical distention (which mimics that of labor pain) but do not experience pain from uterine distention.25
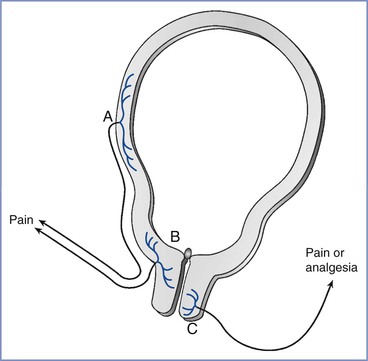
FIGURE 20-4 Uterocervical afferents activated during the first stage of labor. Uterine body afferents (A) partially regress during pregnancy and may contribute to the pain of the first stage of labor. However, the major input is from afferents in the lower uterine segment and endocervix (B). By contrast, at least in animals, the activation of afferents that innervate the vaginal surface of the cervix (C) result in analgesia, not pain, and they enter the spinal cord in sacral areas rather than at the site of referred pain in labor.
The uterine cervix has dual innervation; afferents innervating the endocervix and lower uterine segment have cell bodies in thoracolumbar dorsal root ganglia (DRG), whereas afferents innervating the vaginal surface of the cervix and upper vagina have cell bodies in sacral DRG.26 These two innervations result in different sensory input and referral of pain. Pelvic afferents that innervate the vaginal surface of the cervix are almost exclusively C fibers, with the majority containing the peptides substance P and calcitonin gene–related peptide (CGRP). These afferents express alpha and beta estrogen receptors and have an innervation pattern that is not affected by pregnancy.27-29 Stimulation of the vaginal surface of the cervix in rats results in antinociception, lordosis, ovulation, and a hormonal state of pseudopregnancy, all of which are related to mating behaviors in this species.30 In rats, these vaginal afferent terminals are activated only during delivery and not during labor, which suggests that they are not relevant to the pain of the first stage of labor.31 By contrast, dilation of the endocervix in rats results in the activation of afferents entering the lower thoracic spinal cord and nociception rather than antinociception. These afferents, which are mostly or exclusively C fibers,32 are activated during the first stage of labor, suggesting that they are relevant to pain during this period.
More than 80 years ago, experiments in dogs allowed Cleland33 to identify T11 to T12 as the segmental level of entry into the spinal cord of afferents that transmit the pain of the first stage of labor. Because dysmenorrhea could be treated through the destruction of the superior or inferior hypogastric plexus,34 Cleland reasoned that the sensory afferents and sympathetic efferents were likely intermingled; he subsequently demonstrated that the bilateral blockade of the lumbar paravertebral sympathetic chain could produce analgesia during the first stage of labor.33 First-stage labor pain is transmitted by afferents that have cell bodies in T10 to L1 DRG and pass through the paracervical region, the hypogastric nerve and plexus, and the lumbar sympathetic chain.
Classical teaching states that pain-transmitting C and A-delta nerve fibers enter the spinal cord through the dorsal roots and terminate in a dense network of synapses in the ipsilateral superficial laminae (I and II) of the dorsal horn, with minimal rostrocaudal extension of fibers. Whereas this characterization is true for somatic afferents, visceral C fiber afferents enter the cord primarily—but not exclusively—through the dorsal roots and terminate in a loose network of synapses in the superficial and deep dorsal horn and the ventral horn. These afferents also cross to the contralateral dorsal horn, with extensive rostrocaudal extension of fibers. This anatomic distinction underlies the precise localization of somatic pain and the diffuse localization of visceral pain, which may cross the midline; it may also determine the potency or efficacy of drugs that must reach afferent terminals, such as intrathecal opioids.
Pain-transmitting neurons in the spinal cord dorsal horn send axons to the contralateral ventral spinothalamic tract (stimulating thalamic neurons) with further projections to the somatosensory cortex, where pain is perceived. These spinal neurons also send axons through the spinoreticular and spinomesencephalic tracts to provide signals to the areas of vigilance (locus coeruleus, reticular formation), cardiorespiratory regulation (nucleus tractus solitarius, caudal medulla), and reflex descending inhibition (periaqueductal gray, locus coeruleus and subcoeruleus, nucleus raphe magnus, rostral medial medulla, cerebellum). Thalamic activation from painful stimuli results in the activation not only of the somatosensory cortex but also areas of memory (prefrontal cortex), motor response (M1 motor cortex), and emotional response (insular cortex, anterior cingulate cortex). Supraspinal pain pathways activated by pain of the first stage of labor can be briefly described sequentially, starting with the ascending pathways projecting to the pons and the medulla, thereby activating centers of cardiorespiratory control and descending pathways as well as the thalamus, which in turn sends projections to the anterior cingulate, motor, somatosensory, and limbic regions.
The anatomic basis for pain of the first stage of labor implies that amelioration of pain should occur after blockade of peripheral afferents (by paracervical, paravertebral, lumbar sympathetic, or epidural [T10 to L1 dermatome] block) or after blockade of spinal cord transmission (by intrathecal injection of local anesthetic and/or opioid) (Figure 20-5). In addition, the widespread distribution of visceral synapses in the spinal cord implies that intrathecally administered drugs (e.g., opioids) must have physicochemical properties that facilitate deep penetration into the cord to reach the terminals responsible for pain transmission.
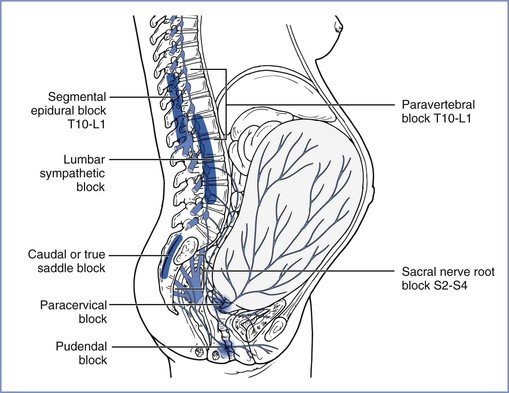
FIGURE 20-5 Transmission of labor pain. Labor pain has a visceral component and a somatic component. Uterine contractions may result in myometrial ischemia, which causes the release of potassium, bradykinin, histamine, and serotonin. In addition, stretching and distention of the lower segments of the uterus and the cervix stimulate mechanoreceptors. These noxious impulses follow the sensory nerve fibers accompanying sympathetic nerve endings, travel through the paracervical region and the pelvic and hypogastric plexus, and enter the lumbar sympathetic chain. Through the white rami communicantes of the T10, T11, T12, and L1 spinal nerves, they enter the dorsal horn of the spinal cord. These pathways could be mapped successfully by demonstration that blockade at different levels along this path (sacral nerve root block of S2-4, pudendal block, paracervical block, low caudal or true saddle block, lumbar sympathetic block, segmental epidural block of T10-L1, and paravertebral block T10-L1) can alleviate the visceral component of labor pain. (From Eltzschig HK, Lieberman ES, Camann WR. Regional anesthesia and analgesia for labor and delivery. N Engl J Med 2003; 348:319-32.)
Second Stage of Labor
Pain during the second stage of labor is transmitted by the same afferents activated during the first stage of labor but with additional afferents that innervate the cervix (vaginal surface), vagina, and perineum. These additional afferents course through the pudendal nerve DRG at S2 to S4 and are somatic. Thus, the pain specific to the second stage of labor is precisely localized to the vagina and perineum and reflects distention, ischemia, and frank injury, either by stretching to the point of disruption or by surgical incision. Studies in nonpregnant women indicate a minor analgesic effect of mechanical self-stimulation of the vaginal surface of the cervix35; this effect may result from the stimulation of C fibers, because in women with a high oral intake of capsaicin, the activity of such fibers is reduced.36 The relevance of this minor effect in reducing the pain of the second stage of labor is questionable and has not been examined; however, it does appear to provide evidence that noxious input during labor may activate endogenous analgesia (see later discussion).
The anatomic basis for pain of the second stage of labor implies that analgesia can be obtained through a combination of methods used to treat the pain of the first stage with a pudendal nerve block or extension of the epidural blockade from T10 to S4 (see Figure 20-5).
Neurophysiologic Basis
Peripheral Afferent Terminals
Visceral nociceptors, such as those that transduce the pain of the first stage of labor, are activated by stretching and distention. However, unlike somatic afferents, they are not activated by cutting. With each uterine contraction, pressure is transmitted to distort and stretch the uterine cervix, thereby leading to the activation of these nerve terminals. How mechanical distention results in the depolarization of the nerve terminal and the generation of an action potential is not entirely known, but the following three mechanisms are likely:
1. A variety of ion channels respond to the distortion of the cell membrane, and one of them—brain sodium channel-1 (BNC-1) or acid-sensing ion channel-2 (ASIC-2)—is exclusively expressed in sensory afferents and might directly depolarize the nerve terminal by opening its channel when the membrane is distorted (Figure 20-6).37
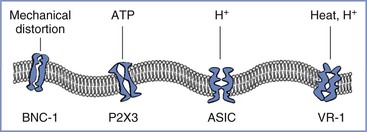
FIGURE 20-6 Afferent nerve endings contain multiple excitatory ligand-gated ion channels, including those that respond to mechanical distortion: BNC-1, brain sodium channel-1; ATP, adenosine triphosphate; P2X3, purinergic receptor; H +, hydrogen ion; ASIC, acid-sensing ion channel; VR-1, vanilloid receptor type 1.
2. Mechanical distortion may result in the acute release of a short-acting neurotransmitter that directly but transiently stimulates ion channel receptors on nerve terminals. Although this process has not yet been examined in the uterine cervix, studies have observed that stretching the bladder urothelium releases adenosine triphosphate, which directly stimulates a type of ligand-gated ion channel—P2X3—on sensory afferents in the bladder wall.38 Because P2X3 receptors are widely expressed in C fibers,39 this mechanism might be responsible for the pain that results from the acute distention of the uterine cervix.
3. Local ischemia during contractions may result in gated or spontaneous activity of other ion channels. Some of these ion channels—the ASIC family—respond directly to the low pH that occurs during ischemia,40 whereas other classes of ion channels may be activated to open spontaneously. For example, the vanilloid receptor type 1 (VR-1) can be stimulated by capsaicin. It is likely that VR-1 receptors (which also respond to noxious heat) are expressed on visceral afferent terminals, given that the application of capsaicin or heat to the distal esophagus in humans results in pain.41 VR-1 receptor–gated ion channels are not normally open in the absence of high temperature or capsaicin-like ligands; however, in the presence of low pH, the temperature response of these receptors shifts so that their channels open at body temperature.42
Uterine cervical afferents (including the C fibers that innervate the vaginal surface of the cervix) contain substance P, CGRP, and the enzyme nitric oxide synthase.43 C fibers can be divided into two groups: (1) those that contain substance P and CGRP and respond to nerve growth factor through actions on tyrosine kinase A receptors and (2) those that contain somatostatin, instead of substance P and CGRP, and respond to glially derived growth factor through actions on a c-ret complex.44 Some overlap exists between these rough classifications, and further definition of C fiber subtypes will likely occur as more markers and neuropeptides are examined. Other compounds commonly contained in C fiber terminals include glutamate, vasoactive intestinal peptide, and neuropeptide Y. The variable role of C fiber subtypes in the transmission of pain is also unclear. Given that somatostatin typically inhibits substance P release and pain transmission,45,46 the net transmission of nociception at the spinal cord level may reflect a complex interaction between excitatory and inhibitory C fiber subtypes.
The peripheral afferent neurophysiology of pain during the first stage of labor suggests that the largely unexplored multiple ion channels that transduce the mechanical signal of cervical stretching to an electrical signal generating the perception of pain may represent important new targets for local or systemic analgesic drug delivery. In addition, the understanding of the classification, function, and relevance to pain of different C fiber subtypes remains in its infancy. Research involving endocervical C fiber subtypes may identify new targets for the treatment of labor pain.
Role of Sensitization
Peripheral afferent terminals, like other parts of the sensory system, can change their properties in response to various conditions. Afferent terminals can be directly stimulated by the low pH associated with inflammation (Figure 20-7), and selective ligand-gated ion channels on these terminals can be stimulated by the release of bradykinin.47 In addition, peripheral inflammation sensitizes afferent terminals by changing their properties; this process can result, over a short time, in a change in gene expression by these nerve fibers, thereby leading to a large amplification of pain signaling.
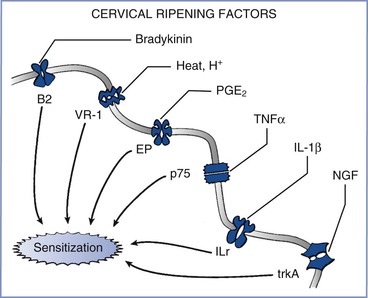
FIGURE 20-7 Effects of inflammation from cervical ripening on afferent terminals. A variety of factors—including bradykinin, heat and hydrogen ions, prostaglandins (including PGE2), tumor necrosis factor-α (TNFα), interleukin-1 beta (IL-1β), and nerve growth factor (NGF)—act on their cognate receptors to sensitize nerve endings and amplify the perception and severity of pain from nerve stimulation. B2, bradykinin-2 receptor; EP, prostaglandin E receptor; ILr, interleukin-1 receptor; p75, p75 tumor necrosis factor-α receptor; trkA, tyrosine kinase A; VR-1, vanilloid receptor type 1.
Although peripheral inflammation is most commonly associated with the pain that results from acute postoperative and chronic arthritic conditions, it may also play an essential role in labor pain. The cervical ripening process and labor itself both result from local synthesis and release of a variety of inflammatory products. The clinical implications of these inflammatory pathways include the application of inflammatory mediators (e.g., prostaglandin E2 [PGE2]) to prepare the cervix for labor induction and the administration of inflammatory mediator inhibitors (e.g., indomethacin) to stop preterm labor.
PGE2 is an especially important sensitizing agent for uterine cervical afferents. In most species, the onset of labor is triggered by a sudden decrease in circulating estrogen concentration. This decrease removes a tonic block on the expression of cyclooxygenase, leading to an increase in local production of prostaglandins, especially PGE2.48 PGE2 is central to a variety of processes that are activated to allow ripening and dilation of the uterine cervix. During the 24 to 72 hours preceding the onset of labor, collagen in the cervix becomes disorganized owing to the activation of prostaglandin receptors and the activity of inflammatory cytokines (mostly interleukin-1-beta [IL-1β] and tumor necrosis factor-alpha [TNF-α]) and matrix metalloproteinases (especially types 2 and 9).49,50 A series of studies in the rat paw have demonstrated that PGE2 induces peripheral sensitization in a sex-independent manner by activation of protein kinase A51 and nitric oxide synthase.52
Cytokines and growth factors are also released into the uterine cervix immediately before and during labor. The cytokine IL-1β enhances cyclooxygenase activity and substance P release in the DRG and spinal cord.53,54 TNF-α increases the spontaneous activity of afferent fibers55 and enhances CGRP release and VR-1 receptor expression in DRG cells in culture.56 Nerve growth factor also induces mechanical hypersensitivity.57 These sensitizing substances (prostaglandins, cytokines, and growth factors) signal peripheral nerves in a manner that results in a host of changes in DRG cell number, peptide expression and release, receptor and ion channel expression, and biophysical properties. For example, inflammatory mediators alter the expression of sodium (Na+) channel subtypes,58,59 thereby resulting in more rapid, repetitive firing capability60 and spontaneous afferent activity.61
Estrogen receptor signaling can dramatically affect the structure of the uterine cervix and possibly modulate pain responses. Long-term estrogen exposure sensitizes a subset of mechanosensitive afferents innervating the uterine cervix. The hypogastric afferents that innervate the uterine cervix are polymodal and contain high-threshold (HT) and low-threshold (LT) fibers. Long-term estrogen exposure increases the spontaneous activities of both HT and LT fibers, but only HT fibers show greater responses to uterine cervical distention.62 Long-term estrogen exposure also increases the proportion of hypogastric afferents innervating the uterine cervix, which express transient receptor potential vanilloid type 1 (TRPV-1). Capsazepine, a TRPV-1 channel antagonist, reduces the hypogastric afferent responses to cervical distention in estrogen-treated animals but not in ovariectomized animals without estrogen replacement.63,64 These data suggest that the TRPV-1 receptor is important for estrogen-induced sensitization and amplification of pain responses to uterine cervical distention, and that it may therefore represent a potential new target for preventing or treating such enhanced pain.
Implications of the peripheral sensitization of cervical afferents during labor are as follows:
1. Braxton-Hicks contractions, prior to the onset of this inflammatory process, may be as powerful as labor contractions but are painless.
2. Pain may increase with the progress of labor as a result of sensitization.
3. Inflammatory mediators may provide new targets to treat labor pain.
Inhibitory Receptors
Given the multiplicity of direct excitatory and sensitizing mechanisms on peripheral terminals, more plausible targets for peripheral pain treatment are the endogenous inhibitory receptors expressed on the afferent terminals (Figure 20-8). Opioid receptors have achieved the widest attention. Although µ-opioid receptors are expressed in some afferents in the setting of inflammation,65 the efficacy of morphine provided by local instillation has proved disappointing,66 with the exception of an intra-articular injection.67 Similarly, µ-opioid receptor agonists produce antinociception to uterine cervical distention through actions in the central nervous system but not in the periphery.68
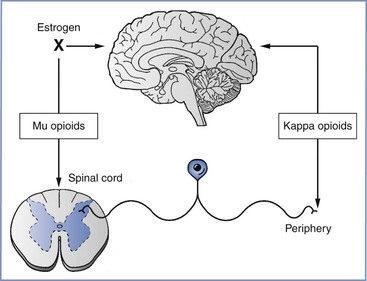
FIGURE 20-8 κ-Opioid receptor agonists act primarily at visceral afferent terminals in the periphery and in the supraspinal central nervous system to provide analgesia during the first stage of labor, whereas µ-opioid receptor agonists act in the spinal cord and the supraspinal central nervous system. Estrogens block the effect of µ-opioid receptor agonists at supraspinal sites.
κ-Opioid receptor agonists may effectively treat visceral pain owing to the presence of these receptors in visceral, but not somatic afferents, at least in the gastrointestinal tract.69 κ-Opioid receptor agonists can also produce antinociception in response to uterine cervical distention through actions in the peripheral nervous system.31,68 Pharmaceutical firms are developing drugs of this class that are restricted to the periphery, have few central side effects,70,71 and presumably express little potential for placental transfer; potentially, such agents would be useful for labor analgesia. One of these new agents has been observed to effectively treat chronic visceral pain from pancreatitis in patients receiving poor analgesia from µ-opioid receptor agonists.72
Estrogen and progesterone can alter the analgesic response to opioids. In most cases involving somatic stimulation, tonic estrogen treatment reduces the efficacy of µ-opioid but not κ-opioid receptor agonists.73 Further, κ-opioid receptor agonists have greater analgesic efficacy in women than in men.74 In animals, tonic estrogen exposure reduces the inhibitory responses to uterine cervical distention by morphine but not to the κ-opioid receptor agonist U-50488.75 In contrast, the inhibitory action of intrathecal morphine against responses to uterine cervical distention is unaffected by tonic estrogen exposure,76 which is consistent with the observation that intrathecal opioids relieve the pain of the first, but not second, stage of labor.
Implications of inhibitory receptors on afferent terminals are that κ- but not µ-opioid receptor agonists may produce pain relief through their actions in the periphery. Selective, peripherally restricted drugs are under development for the systemic treatment of visceral pain. In addition, estrogen-dependent inhibition of the supraspinal (but not the spinal) analgesic action of µ-opioid receptor agonists may underlie the limited analgesic effect produced by systemic opioids,77 a finding that is in contrast to the efficacy of intrathecal opioids78 in relieving the pain of the first stage of labor.
Peripheral Nerve Axons
The current approach to labor analgesia relies primarily on an understanding of the afferent axons and their level of entry into the spinal cord and on the administration of local anesthetics to block afferent traffic conduction. Traditionally, axons have been considered conduits that allow for the propagation of action potentials by the transitory opening of sodium channels. More recent investigations have confirmed the existence of a variety of sodium channel subtypes and axons that modulate transmission through other ion channels.
Although a number of voltage-gated sodium channel subtypes exist, most studies have focused on three specific subtypes that are expressed in sensory afferents.79 Two of these, NaV1.8 and NaV1.9, are relatively resistant to blockade by tetrodotoxin (TTX-R); NaV1.9 is often referred to as “persistent,” owing to its very slow inactivation kinetics.80 Inflammation and injury to nerves decrease the TTX-R current density in afferent cell bodies.81 Some investigators have suggested that NaV1.8 is selectively trafficked to the periphery after injury and inflammation81 and that a reduction of its expression reduces hypersensitivity.82 Other investigators, using sucrose gap measurements of compound action potentials, have demonstrated a shortened refractory period and a decrease in delayed depolarization after nerve injury83,84 that are consistent with the greater expression of rapidly repriming tetrodotoxin-sensitive (TTX-S) channels and the decreased expression of kinetically slow TTX-R channels. To date, these studies have focused primarily on peripheral nerve injury models of chronic pain, and neither the subtypes nor their change during the cervical inflammation of labor has been studied.
Several pharmaceutical firms have discovery programs to produce sodium channel subtype-selective blockers that could improve both the safety and efficacy of the treatment of labor pain, because such agents would not interact with sodium channels in the brain, heart, or motor nerve fibers. Some investigators have observed that injection of the antidepressant amitriptyline, an agent known to block the NaV1.8 sodium channel, around the peripheral nerves provides a neural blockade twofold to fivefold longer than that provided by injection of long-acting local anesthetics.85,86
Another subject of current research is the extension of the duration of selective antinociception with no motor or sympathetic block by manipulation of the TRPV-1 receptor, which is a nonselective ligand-gated cation channel. TRPV-1 receptors are expressed in peripheral primary afferent neurons, the nociceptive C and A-delta fibers, and the dorsal root ganglia, as well as the structures involving the endogenous antinociceptive descending pathway. TRPV-1 receptors can be activated by capsaicin, heat, and endovanilloids, leading to release of substance P, which in turn excites inhibitory neurons in laminae I, III, and IV. Further, activation of the TRPV-1 receptors causes opening of the small TRPV-1 channels on the neurons and allows entry of co-administered charged molecules of certain sizes. Permanently charged local anesthetic, when applied alone, would not be able to cross the nerve membrane to exert its effect on sodium channels in small sensory neurons, but when applied in the presence of capsaicin or other TRPV-1 agonists, the permanently charged local anesthetic would become permeant to exert its local anesthetic effect. Binshtok et al.87 reported the inhibition of nociceptors by TRPV-1–mediated entry of impermeant sodium channel blockers. However, stimulation of TRPV-1 receptors, such as with capsaicin alone, will also result in the induction of receptor-mediated acute pain. Therefore, these investigators subsequently performed another study in rodents, and they observed that the co-application of lidocaine and its quaternary permanently charged derivative QX-314 produces a prolonged, predominantly nociceptor-selective block by allowing QX314 entry through the TRPV-1 channels without the nocifensive behavior associated with capsaicin when lidocaine is used instead to activate the TRPV-1 receptors.88 The issues of pain elicited with administration of TRPV-1 agonists such as capsaicin and, more importantly, the neurotoxicity of permanently charged Na+ channel blockers remain to be overcome and require further research. Additional investigations of the exploitation of new targets may allow provision of safer and prolonged selective antinociception. Should future research prove the absence of toxicity, it is conceivable that amitriptyline or other agents that interact with Na+ channel subtypes and/or TPRV-1 receptors could be considered for single-injection techniques to produce prolonged and selective analgesia for labor pain and postoperative pain relief.
Interactions within the large number of ion channels expressed on axons can alter neural conduction. An example is the transient refractory period caused by the membrane hyperpolarization that follows a short burst of nerve firing. This phenomenon results from the activation of the Na+/K+ exchange pump and dampens high-frequency nerve activity. The Na+/K+ exchange pump activity, in turn, can be reduced by a hyperpolarization-induced current termed Ih. Drugs that block the Ih current enhance the hyperpolarization caused by the Na+/K+ exchange pump and ultimately serve to reduce nerve traffic89 and provide prolonged analgesia.90 A second example is the desensitization of VR-1 receptors present on the axons of C fibers. The perineural injection of drugs that desensitize these receptors without first stimulating them avoids the induction of receptor-mediated acute pain and instead produces very long periods of selective sensory analgesia without motor effects.91 The mechanism by which VR-1 receptor desensitization alters the transmission of action potentials is under investigation.
Implications of the neurophysiology of axonal transmission of labor pain are that sodium channel subtype-selective agents—or those that affect other ion channels expressed on axons—may provide safer and more selective tools for regional analgesic techniques.
Spinal Cord
When action potentials invade the central terminals of C and A-delta fiber afferents in the spinal cord, voltage-gated calcium channels open and cause intracellular calcium concentration to increase; this increase triggers a multistep process of neurotransmitter docking and fusion with the plasma membrane, which results in neurotransmitter release.92 Inhibition of these calcium channels produces analgesia. Studies in animals suggest that at least one agent that affects the calcium channels, gabapentin, produces antinociception to visceral stimulation.93
A nociceptive stimulus can result in the release of multiple excitatory neurotransmitters, including amino acids (glutamate, aspartate) and peptides (especially substance P, CGRP, and neurokinin A) that interact with specific receptors on spinal cord neurons. Although the stimulation of neurokinin receptors is necessary for the perception of moderate to severe pain,94 a complex and poorly understood interplay exists among these released neurotransmitters.
Neurotransmitter release at sensory afferent terminals is controlled by presynaptic receptors that act primarily by altering the flux of intracellular calcium when an action potential arrives. Some of these neurotransmitters are excitatory; for example, the action of acetylcholine on nicotinic acetylcholine receptors amplifies further neurotransmitter release.95 Gamma-aminobutyric acid (GABA) is the key endogenous inhibitory neurotransmitter in the nervous system, and stimulation of GABA receptors significantly reduces the afferent terminal release of other neurotransmitters.96 Multiple compounds produce analgesia by enhancing the release of GABA at afferent terminals in the spinal cord. The existence of excitatory and inhibitory systems can make the response to a neurotransmitter or an exogenously-administered agent (such as a local anesthetic drug given intrathecally) difficult to predict. For example, acetylcholine can enhance or reduce the afferent terminal release of neurotransmitters by actions on nicotinic and muscarinic receptors, respectively.97,98 The net effect of acetylcholine appears to be inhibitory, which is indicated by the analgesic effect of intrathecal administration of the cholinesterase inhibitor neostigmine.99
The primary mechanism of action of the neurotransmitter enkephalin, which is released by spinal cord interneurons, and of norepinephrine, which is released by axons descending from pontine centers, is the inhibition of neurotransmitter release from primary afferent terminals. These substances act on µ-opioid and α2-adrenergic receptors, respectively,100,101 and may produce some of the similar effects observed after the intrathecal administration of opioids and α2-adrenergic agonists for the treatment of labor pain.
Amino acids and peptides released from sensory afferents stimulate a heterogeneous group of spinal cord neurons, including neurons that project to supraspinal structures, interneurons that modulate transmission at the afferent terminal itself (the “gate” of the control theory), and interneurons that stimulate motor and sympathetic nervous system reflexes. Large and sustained glutamate release from an intensely noxious stimulus can activate N-methyl-D-aspartate (NMDA) receptors, resulting in sustained depolarization and enhanced excitability of projection neurons (Figure 20-9).102 Although the intrathecal injection of NMDA receptor antagonists (e.g., ketamine) has been restricted because of neurotoxicity concerns,103 systemic infusion of magnesium sulfate has been observed to produce postoperative analgesia.104 Magnesium is an endogenous inhibitory modulator of NMDA receptors, and it is conceivable that magnesium sulfate administered systemically for obstetric indications may have a minor effect on labor pain.
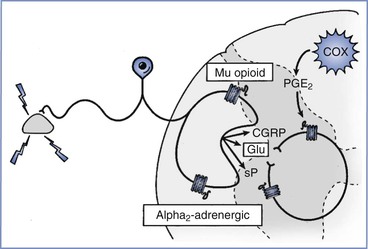
FIGURE 20-9 Pain transmission in the spinal cord. Excitatory transmission occurs directly by release of amino acids such as glutamate (Glu) and peptides (sP [substance P], CGRP [calcitonin gene–related peptide]) and indirectly via activation of enzymes such as cyclooxygenase (COX) in nearby glia, which synthesize prostaglandins, including prostaglandin E2 (PGE2). Inhibitory mechanisms are primarily presynaptic, with µ-opioid and α2-adrenergic receptors being the most common (or at least the most studied).
Prolonged and intense nociceptive stimuli can produce sensitization and amplification of pain signaling at the spinal cord level like the peripheral sensitization that occurs as a result of inflammation. Some of these processes are a direct consequence of receptors (e.g., NMDA receptors) that are activated only with highly intense and prolonged stimulation or by the long-term release of neurotransmitters that simultaneously activate the glutamate and substance P receptors on the same cell. Others reflect the synthesis and release of classic “inflammatory” substances by the spinal cord glial cells in response to prolonged afferent stimulation from nitric oxide and prostaglandins, especially PGE2. Some non-opioid analgesic drugs produce analgesia by actions exclusively (e.g., acetaminophen) or primarily (e.g., aspirin) in the central nervous system (especially the spinal cord).105
Spinal sensitization processes represent a novel target for the treatment of labor pain. More than 80 years ago, Cleland33 noted the presence of hypersensitivity to light touch on the skin of dermatomes T11 and T12 in laboring women, which likely represents the enhanced sensitivity of spinal cord neurons receiving both visceral input from the cervix and skin input at those levels.106 When the visceral stimulation to these dermatomes was blocked by a paravertebral local anesthetic injection, Cleland33 observed that the hypersensitivity was ablated; this observation is consistent with the later finding that ongoing C fiber input is required for hypersensitivity to occur.107 Uterine cervical distention (UCD) results in a pattern of spinal cord neuronal activation similar to that witnessed during labor and delivery. In a study in rats reported by Tong et al.,108 UCD significantly increased c-fos immunoreactivity in the spinal cord from T12 to L2, with most of the c-fos expression occurring in the deep dorsal horn and central canal regions. UCD-evoked c-fos expression was prevented by prior infiltration of lidocaine into the cervix or by intrathecal administration of ketorolac (a cyclooxygenase [COX] inhibitor) in a dose-dependent manner.108 Intrathecal administration of indomethacin (a nonspecific COX inhibitor) and the selective COX-2 inhibitor SC-58238 effectively ablated UCD-induced electromyographic activity without altering the hemodynamic response to UCD. By contrast, the selective COX-1 inhibitor SC-58360 was ineffective in ablating UCD-induced electromyographic activity, as was ketorolac, an agent with higher affinity for COX-1 than COX-2.109 Together, these data suggest that targeting COX-2 is necessary to treat the acute visceral pain often associated with brief infrequent contractions in late pregnancy; therefore, intrathecal ketorolac would be predicted to be ineffective. However, in the setting of sustained, frequent, and repetitive contractions for a prolonged period (as occurs during active labor), intrathecal ketorolac might be effective. The intrathecal injection of ketorolac has been introduced into experimental human trials110 and warrants examination as a potential modality for selective treatment of labor pain.
The neurophysiologic basis for labor pain in the spinal cord implies that purely inhibitory mechanisms (e.g., opioid and α2-adrenergic receptors) can be mimicked by the intrathecal injection of agonists to these receptors. However, the administration of other agents (e.g., acetylcholine) in this location has less predictable results. Central sensitization mechanisms in the spinal cord most certainly occur during labor, and future treatments may target these mechanisms.
Ascending Projections
Spinal cord neurons project to multiple brainstem sites as well as the thalamus. More than 30 years ago, it was noted that descending systems—activated primarily by stimulation of the nucleus raphe magnus, the periaqueductal gray, and the locus coeruleus—could reduce pain transmission as described in the gate control theory.111 Activation of descending pathways results in the spinal release of endogenous ligands for serotonergic, opioid, and α2-adrenergic receptor–mediated analgesia. Spillover of neurotransmitters into the cerebrospinal fluid has been used as a measure of activation of these systems, and studies measuring these substances have shown no increase in enkephalin, but an increase in norepinephrine, in laboring women.112 These descending systems can be activated by psychoprophylactic methods,113 and agents that prolong or intensify the action of these ligands, such as enkephalinase inhibitors and blockers of monoamine reuptake, might further enhance analgesia.114
Brainstem activation by the pain of labor leads to other reflexes, such as increases in sympathetic nervous system activity and respiratory drive and, with prolonged activation, stimulation of descending pathways that amplify rather than reduce pain transmission at the spinal cord.115,116 The circuitry and pharmacology of such pain-enhancing systems in the brainstem and their potential applications for treatment are under current investigation.
Our understanding of the areas of the brain activated during labor pain is limited, although studies of other types of experimental nociception in healthy volunteers indicate that visceral pain is considered more unpleasant than somatic pain; this difference reflects, in part, the greater activation of centers for negative emotions, including fear. Although distraction methods do not alter the thalamic activation from noxious stimulation, a reduction in cortical activation and the report of pain have been observed,117 supporting a suprathalamic mechanism of psychoprophylaxis in the reduction of pain.
The neurophysiologic basis of labor pain and ascending projections suggests the activation of multiple supraspinal sites. Some of these sites stimulate potentially detrimental cardiorespiratory reflexes. Other sites, which send descending projections that either reduce or enhance pain transmission in the spinal cord, may be targeted for the provision of analgesia. In addition, suprathalamic modulation of pain signals appears to account for the interindividual differences in pain perception and for the relative efficacy of psychoprophylaxis in reducing the intensity of reported pain.
Effect on the Mother
Obstetric Course
Several aspects of labor pain can affect the course of labor and delivery (Figure 20-10). Pain-induced increases in the activity of the sympathetic nervous system lead to higher plasma concentrations of catecholamines, especially epinephrine. The provision of labor analgesia reduces the plasma concentration of epinephrine and its associated beta-adrenergic tocolytic effects on the myometrium. This process may underlie the observations by some investigators who have noted, either anecdotally or under controlled conditions, a shift from dysfunctional to normal labor patterns in some women when analgesia is achieved with paravertebral33 or epidural118 blocks or with systemic meperidine analgesia.119 The abrupt reduction in plasma epinephrine concentration that follows the rapid onset of intrathecal opioid analgesia may result in an acute reduction of beta-adrenergic tocolysis and a transient period of uterine hyperstimulation; in some cases, these changes may lead to transient fetal stress and fetal heart rate abnormalities.120,121
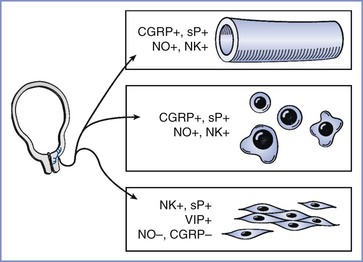
FIGURE 20-10 Aspects of pain that may affect the course of labor. In addition to indirect effects (e.g., beta-adrenergic tocolysis from increased secretion of epinephrine, greater release of oxytocin via Ferguson's reflex), depolarization of afferent terminals in the lower uterine segment and cervix can directly alter aspects of labor. Substances released by nerve terminals include those that increase local blood flow (CGRP [calcitonin gene–related peptide], sP [substance P], NO [nitric oxide], NK [neurokinin]), those that stimulate immune cell function, and those that stimulate (+) or inhibit (−) myometrial smooth muscle activity, including vasoactive intestinal peptide (VIP).
Ferguson's reflex involves neural input from ascending spinal tracts (especially from sacral sensory input) to the midbrain, thereby leading to enhanced oxytocin release. Although spontaneous labor and delivery occur in women with spinal cord injury (which disrupts this tract122), some investigators have argued that neuraxial analgesia can inhibit this reflex and prolong labor, especially the second stage. However, strong evidence for this does not exist. Some studies have noted a reduction in plasma oxytocin concentration with epidural local anesthetic123 or intrathecal opioid124 analgesia, whereas others have not noted such a reduction.125
Papka and Shew24 suggested that afferent terminals in the lower uterine segment and cervix might have an important secretory (efferent) function in the regulation of labor. Afferent terminals contain many substances that can stimulate (substance P, glutamate, vasoactive intestinal peptide) or inhibit (CGRP, nitric oxide) myometrial activity, and these substances can be released locally into the cervix and lower uterine segment when terminals are depolarized by contraction-related tissue distortion. In addition, depolarization of the afferent terminal can result in an action potential that, upon reaching a site of nerve branching, invades adjacent branches and travels distally to depolarize distant terminals of the same nerve. This axon reflex has long been recognized to occur in somatic nerves; owing to the more extensive arborizations believed to exist in visceral nerves, local stimulation should result in more widespread release of these transmitters. Therefore, it is tempting to speculate that these axon reflexes are more profoundly affected when local anesthetic is administered closer to the terminals associated with cervical dilation and labor (e.g., as occurs with paracervical and paravertebral blocks) than occurs when local anesthetic is administered farther away from the terminals (e.g., with epidural block). This speculation would imply that the net effect of afferent terminal–released substances inhibits rather than accelerates labor.
In summary, neural stimulation through pain pathways leads to the release of substances that either increase (oxytocin) or inhibit (epinephrine) uterine activity and cervical dilation. Therefore, the effect of analgesia on the course of labor can vary between and within individuals. In addition, axon reflexes can result in the release of neurotransmitters from afferents into the lower uterine segment and cervix. It is hoped that future investigation will determine whether the proximity of local anesthetic deposition affects the response of cervical dilation and labor.
Cardiac, Respiratory, and Gastrointestinal Effects
Labor exerts stresses on the cardiovascular and respiratory systems. The elevated plasma catecholamine concentrations observed during labor pain can increase maternal cardiac output and peripheral vascular resistance and decrease uteroplacental perfusion. Even transient stress is associated with dramatic increases in plasma concentration of norepinephrine and subsequent decreases in uterine blood flow (Figure 20-11). Plasma epinephrine concentrations in women with painful labor are similar to those observed after intravenous administration of a bolus of epinephrine 15 µg126; intravenous bolus injection of 10 to 20 µg of epinephrine resulted in a significant (albeit transient) reduction in uterine blood flow in gravid ewes.127 Effective neuraxial analgesia, provided by epidural local anesthetic128 or intrathecal opioid administration,129 significantly reduces (50%) maternal catecholamine concentrations. By contrast, neonatal catecholamine concentrations do not appear to be altered by maternal neuraxial anesthetic techniques; this relative independence of neonatal catecholamine responses may be important for the neonatal adaptation to extrauterine life.130
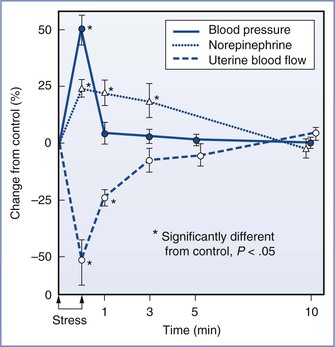
FIGURE 20-11 Effect of a painful stimulus on the hind leg on maternal blood pressure, norepinephrine concentrations, and uterine blood flow in gravid ewes. The increase in blood pressure was transient, but plasma norepinephrine concentrations remained elevated for several minutes; the elevation is reflected in the slow return of uterine blood flow to normal. (From Shnider SM, Wright RG, Levinson G, et al. Uterine blood flow and plasma norepinephrine changes during maternal stress in the pregnant ewe. Anesthesiology 1979; 50:524-7.)
The intermittent pain of uterine contractions also stimulates the respiratory system and leads to periods of intermittent hyperventilation. In the absence of supplemental oxygen administration, compensatory periods of hypoventilation between contractions result in transient episodes of maternal, and even fetal, hypoxemia (Figure 20-12). Treatment of labor pain with epidural analgesia minimizes the increase in net minute ventilation and the accompanying increase in oxygen consumption.131 In general, the cardiovascular and respiratory system changes induced by labor pain are well tolerated by healthy parturients (with normal uteroplacental perfusion) and their fetuses. Some authors have concluded that these changes are of no concern or relevance in uncomplicated labor.19 However, when maternal or fetal disease or compromise is observed, significant cardiopulmonary alterations may lead to maternal or fetal decompensation; effective analgesia may be especially important in such cases.
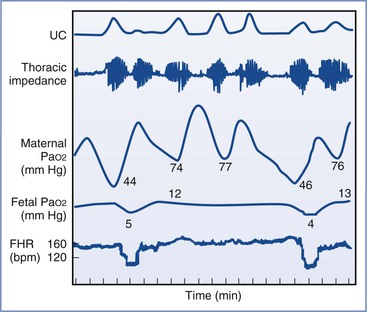
FIGURE 20-12 Maternal and fetal hypoxemia during hypoventilation between uterine contractions (UC), which are associated with maternal hyperventilation. FHR, fetal heart rate. (From Bonica JJ. Labour pain. In Wall PD, Melzack R, editors. Textbook of Pain. Edinburgh, Churchill Livingstone, 1984, as redrawn from Huch A, Huch R, Schneider H, Rooth GL. Continuous transcutaneous monitoring of fetal oxygen tension during labour. Br J Obstet Gynaecol 1977; 84(Suppl):1-39.)
Labor pain, anxiety, and emotional stress increase gastrin release and inhibit the segmental and suprasegmental reflexes of gastrointestinal and urinary motility. This in turn results in an increase in gastric acidity and volume and a delay in bladder emptying.132 These changes are further aggravated by the recumbent position, opioids, and other depressant medications (e.g., barbiturates), putting laboring parturients at risk for pulmonary aspiration of gastric contents, especially during emergency induction of general anesthesia for cesarean delivery.
In summary, pain-induced activation of the sympathetic nervous system during labor is associated with cardiovascular, respiratory, and gastrointestinal effects that may alter maternal and fetal well-being. The provision of effective neuraxial analgesia may mitigate many of these cardiopulmonary effects.
Psychological Effects
The meaning of labor pain is greatly influenced by psychosocial and environmental factors (as previously discussed) and varies considerably among women. Although the acceptance of labor analgesia has a minor overall effect on maternal satisfaction with the labor and delivery process,133 individual women can be profoundly influenced. It has been suggested that women who understand the origin of their pain and view the labor and delivery process as positive and nonthreatening may undergo pain without suffering.19 Billewicz-Driemel and Milne134 reported that a small proportion (< 5%) of women who requested and received epidural labor analgesia described a sense of deprivation from having missed the natural labor experience in its entirety; some of these women may subsequently seek psychiatric counseling.135
By contrast, unrelieved severe labor pain can have psychological and physical consequences, including depression and negative thoughts about sexual relationships.6,10 In a 5-year study in Sweden, 43 women requested elective cesarean delivery owing to a fear of labor and vaginal delivery.136 Some countries (e.g., Brazil) have an extremely high elective cesarean delivery rate (> 80%) among upper-class women because of their concerns about reduced sexual function after vaginal delivery. Frank psychotic reactions resembling post-traumatic stress disorder can occur after childbirth, although the incidence is rare (< 1%).137
Psychological effects of labor pain can occur in a small proportion of women. Psychological harm can be experienced through the provision or withholding of labor analgesia, underscoring the tremendous variability in the meaning of labor pain for different women.
Pain after Delivery
Many women undergo delivery without negative sequelae, but some may experience significant persistent postpartum pain and even depression. Studies of acute and chronic postpartum pain have shown a 7% incidence of perineal pain at 8 weeks after vaginal delivery138 and a 43% incidence of hyperalgesia at 48 hours and a 23% incidence of residual pain at 6 months after cesarean delivery.139 In a multicenter, prospective, longitudinal cohort study of 1288 parturients delivering either by cesarean or vaginal delivery, Eisenach et al.140 tested whether the mode of delivery had an independent role in persistent pain and depression at 8 weeks postpartum. The impact of mode of delivery on acute postpartum pain, persistent pain, depressive symptoms, and their interrelationships was assessed using regression analysis and propensity adjustment. They reported a 10.9% prevalence of severe acute pain within 36 hours postpartum, whereas the prevalence of persistent pain and depression at 8 weeks postpartum was 9.8% and 11.2%, respectively. The severity of acute postpartum pain, but not the mode of delivery, was independently related to risk for persistent pain and depression at 8 weeks postpartum, both of which also resulted in negative effects on activities of daily living and on sleep. Those women with severe acute postpartum pain had a 2.5- and 3.0-fold increased risk for persistent pain and depression, respectively, when compared with those with mild acute postpartum pain. These findings suggest these morbidities may not be related to degrees of physical tissue trauma but rather may be related to an individual's pain response to that injury.
Although there is significant interindividual variability with regard to acute postpartum pain,141 the severity of acute postoperative pain in nonobstetric surgical patients has been correlated with the occurrence of chronic pain.142 Whether the presence and severity of labor pain or the presence and severity of acute postpartum pain after either vaginal or cesarean delivery predicts the occurrence of chronic pain is under investigation. Studies in animals suggest that acute intervention at the time of tissue injury reduces the likelihood of developing chronic pain.143 It is likely that severity of the acute pain is not just a marker of chronic pain but rather an active participatory component in the pathophysiology of transitioning from acute to chronic pain.140 Therefore, more careful attention to pain treatment and follow-up in days after childbirth may potentially reduce long-term morbidities and improve overall outcomes.
Reports on the incidence of chronic pain after delivery vary widely in part because of the difference in the definition and the inclusion or exclusion of types of chronic pain and not critically separating new pain after delivery from preexisting pain. Long-term follow up of postpartum patients showed that the incidence of chronic pain (defined as new pain that began at the time of labor and delivery) at 6 months and 1 year was remarkably low at 3% and 0.1%, respectively, compared with nonobstetric surgeries with similar tissue injury.144 By using a sciatic nerve injury–induced neuropathic pain model in rats, it has been observed that the birthing process plus the nursing of the pups in combination, but not individually, may be protective of the development of surgical nerve injury–induced hypersensitivity to pain. This protection is likely mediated by spinal oxytocin because the protective effect is abolished by administration of spinal atosiban, an oxytocin antagonist.145
Further research is needed to better define protective and/or predictive factors in patients who are at risk for developing severe acute and/or chronic postpartum pain. Persistent or chronic pain may be particularly difficult for postpartum patients owing to the multiple stresses (e.g., care of the neonate) and sequelae encountered. An association between pain and depression exists, and depression is the most common complication after delivery, affecting approximately 13% of postpartum women.146 Postpartum patients with depression are among those who frequently do not disclose depression even though they desire assistance.147,148 Immediate and effective postpartum pain management (after both vaginal and cesarean deliveries) with adequate long-term follow-up may potentially prevent long-term morbidity and improve overall outcomes.
Effect on the Fetus
Because of the absence of direct neural connections from the mother to the fetus, maternal labor pain has no direct effects on the fetus. However, maternal labor pain can affect a number of systems that determine uteroplacental perfusion, as follows: (1) uterine contraction frequency and intensity, by the effect of pain on the release of oxytocin and epinephrine; (2) uterine artery vasoconstriction, by the effect of pain on the release of norepinephrine and epinephrine; and (3) maternal oxyhemoglobin desaturation, which may result from intermittent hyperventilation followed by hypoventilation, as discussed earlier. Although these effects are well tolerated in normal circumstances and are effectively blocked by analgesia, fetal well-being may be affected in situations of limited uteroplacental reserve.
Summary
Pain during the first stage of labor results from the stimulation of visceral afferents that innervate the lower uterine segment and cervix, intermingle with sympathetic efferents, and enter the spinal cord at the T10 to L1 segments. Pain during the second stage of labor results from the additional stimulation of somatic afferents that innervate the vagina and perineum, travel within the pudendal nerve, and enter the spinal cord at the S2 to S4 segments. These pain signals are processed in the spinal cord and are transmitted to brainstem, midbrain, and thalamic sites, the last with projections to the cortex, resulting in the sensory-emotional experience of pain. Current obstetric anesthesia practice relies nearly exclusively on the blocking of pain transmission by deposition of local anesthetic—with or without adjuncts—along the afferent nerves from sites near the peripheral afferent terminals to sites near their central terminals.
The neurophysiology of visceral pain, especially in relation to labor pain, is currently under investigation, with considerable academic and pharmaceutical targeting of (1) the normal ionic transduction mechanisms and processes of sensitization in peripheral afferent terminals, (2) the mechanisms of inhibition available in the spinal cord and brainstem, and (3) the processes by which conscious distraction methods can be amplified and can relieve pain. Labor pain is an intensely variable and personal experience, and it is essential that the anesthesia provider play a flexible role within this context.
References
1. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–975.
2. Melzack R. From the gate to the neuromatrix. Pain. 1999;82(Suppl 6):S121–S126.
3. Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107.
4. Dick-Read GP. Childbirth Without Fear: The Principles and Practice of Natural Childbirth. Harper: New York; 1953.
5. Lamaze F. Qu’est-ce que l’Accouchement sans Douleur par la Méthode Psychoprophylactique? Ses Principes, sa Réalisation, ses Résultats. Savouret Connaitre: Paris; 1956.
6. Melzack R. The myth of painless childbirth (the John J. Bonica lecture). Pain. 1984;19:321–327.
7. Javert CT, Hardy JD. Influence of analgesics on pain intensity during labor (with a note on “natural childbirth”). Anesthesiology. 1951;12:189–215.
8. Hardy JD, Javert CT. Studies on pain: measurements of pain intensity in childbirth. J Clin Invest. 1949;28:153–162.
9. Algom D, Lubel S. Psychophysics in the field: perception and memory for labor pain. Percept Psychophys. 1994;55:133–141.
10. Melzack R, Taenzer P, Feldman P, Kinch RA. Labour is still painful after prepared childbirth training. Can Med Assoc J. 1981;125:357–363.
11. Niven CA, Gijsbers KJ. Do low levels of labour pain reflect low sensitivity to noxious stimulation? Soc Sci Med. 1989;29:585–588.
12. Granot M, Lowenstein L, Yarnitsky D, et al. Postcesarean section pain prediction by preoperative experimental pain assessment. Anesthesiology. 2003;98:1422–1426.
13. Pan PH, Coghill R, Houle TT, et al. Multifactorial preoperative predictors for postcesarean section pain and analgesic requirement. Anesthesiology. 2006;104:417–425.
14. Coghill RC, McHaffie JG, Yen Y-F. Neural correlates of inter-individual differences in the subjective experience of pain. Proc Natl Acad Sci U S A. 2003;100:8538–8542.
15. Reid JG, Simpson NA, Walker RG, et al. The carriage of pro-inflammatory cytokine gene polymorphisms in recurrent pregnancy loss. Am J Reprod Immunol. 2001;45:35–40.
16. Conell-Price J, Evans JB, Hong D, et al. The development and validation of a dynamic model to account for the progress of labor in the assessment of pain. Anesth Analg. 2008;106:1509–1515.
17. Debiec J, Conell-Price J, Evansmith J, et al. Mathematical modeling of the pain and progress of the first stage of nulliparous labor. Anesthesiology. 2009;111:1093–1110.
18. Merskey H. Pain terms: a list with definitions and a note on usage. Recommended by the International Association for the Study of Pain (IASP) Subcommittee on Taxonomy. Pain. 1979;6:249–252.
19. Lowe NK. The nature of labor pain. Am J Obstet Gynecol. 2002;186:S16–S24.
20. Bajaj P, Drewes AM, Gregersen H, et al. Controlled dilatation of the uterine cervix: an experimental visceral pain model. Pain. 2002;99:433–442.
21. Lundgren I, Dahlberg K. Women's experience of pain during childbirth. Midwifery. 1998;14:105–110.
22. Robbins A, Sato Y, Hotta H, Berkley KJ. Responses of hypogastric nerve afferent fibers to uterine distention in estrous or metestrus rats. Neurosci Lett. 1990;110:82–85.
23. Bradshaw HB, Temple JL, Wood E, Berkley KJ. Estrous variations in behavioral responses to vaginal and uterine distention in the rat. Pain. 1999;82:187–197.
24. Papka RE, Shew RL. Neural input to the uterus and influence on uterine contractility. Garfield RE, Tabb TN. Control of Uterine Contractility. CRC Press: London; 1993:375–399.
25. Bonica JJ, Chadwick HS. Labour pain. Wall PD, Melzack R. Textbook of Pain. 2nd edition. Churchill Livingstone: New York; 1989:482–499.
26. Berkley KJ, Robbins A, Sato Y. Functional differences between afferent fibers in the hypogastric and pelvic nerves innervating female reproductive organs in the rat. J Neurophysiol. 1993;69:533–544.
27. Papka RE, Storey-Workley M, Shughrue PJ, et al. Estrogen receptor-α and -β immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001;304:193–214.
28. Papka RE, Storey-Workley M. Estrogen receptor-α and -β coexist in a subpopulation of sensory neurons of female rat dorsal root ganglia. Neurosci Lett. 2002;319:71–74.
29. Pokabla MJ, Dickerson IM, Papka RE. Calcitonin gene–related peptide-receptor component protein expression in the uterine cervix, lumbosacral spinal cord, and dorsal root ganglia. Peptides. 2002;23:507–514.
30. Komisaruk BR, Wallman J. Antinociceptive effects of vaginal stimulation in rats: neurophysiological and behavioral studies. Brain Res. 1977;137:85–107.
31. Papka RE, Hafemeister J, Puder BA, et al. Estrogen receptor-α and neural circuits to the spinal cord during pregnancy. J Neurosci Res. 2002;70:808–816.
32. Sandner-Kiesling A, Pan HL, Chen SR, et al. Effect of kappa opioid agonists on visceral nociception induced by uterine cervical distention in rats. Pain. 2002;96:13–22.
33. Cleland JG. Paravertebral anaesthesia in obstetrics. Surg Gynecol Obstet. 1933;57:51–62.
34. Cotte G. Sur le traitement des dysmenorrhées rébelles par la sympathectomie hypogastrique périarterielle ou la section du nerf présacre. Lyon Med. 1925;LVI:153.
35. Whipple B, Komisaruk BR. Elevation of pain threshold by vaginal stimulation in women. Pain. 1985;21:357–367.
36. Whipple B, Martinez-Gomez M, Oliva-Zarate L, et al. Inverse relationship between intensity of vaginal self-stimulation-produced analgesia and level of chronic intake of a dietary source of capsaicin. Physiol Behav. 1989;46:247–252.
37. Lingueglia E, de Weille JR, Bassilana F, et al. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783.
38. Cockayne DA, Hamilton SG, Zhu QM, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015.
39. Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84:476–488.
40. Waldmann R, Champigny G, Lingueglia E, et al. H(+)-gated cation channels. Ann N Y Acad Sci. 1999;868:67–76.
41. Drewes AM, Schipper KP, Dimcevski G, et al. Multimodal assessment of pain in the esophagus: a new experimental model. Am J Physiol Gastrointest Liver Physiol. 2002;283:G95–103.
42. Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210.
43. Papka RE, McNeill DL, Thompson D, Schmidt HH. Nitric oxide nerves in the uterus are parasympathetic, sensory, and contain neuropeptides. Cell Tissue Res. 1995;279:339–349.
44. Bennett DL, Michael GJ, Ramachandran N, et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072.
45. Kim SJ, Chung WH, Rhim H, et al. Postsynaptic action mechanism of somatostatin on the membrane excitability in spinal substantia gelatinosa neurons of juvenile rats. Neuroscience. 2002;114:1139–1148.
46. Carlton SM, Du JH, Zhou ST, Coggeshall RE. Tonic control of peripheral cutaneous nociceptors by somatostatin receptors. J Neurosci. 2001;21:4042–4049.
47. Linhart O, Obreja O, Kress M. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience. 2003;118:69–74.
48. Sato T, Michizu H, Hashizume K, Ito A. Hormonal regulation of PGE2 and COX-2 production in rabbit uterine cervical fibroblasts. J Appl Physiol. 2001;90:1227–1231.
49. Lyons CA, Beharry KD, Nishihara KC, et al. Regulation of matrix metalloproteinases (type IV collagenases) and their inhibitors in the virgin, timed pregnant, and postpartum rat uterus and cervix by prostaglandin E2-cyclic adenosine monophosphate. Am J Obstet Gynecol. 2002;187:202–208.
50. Stygar D, Wang H, Vladic VS, et al. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol Reprod. 2002;67:889–894.
51. Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186.
52. Aley KO, McCarter G, Levine JD. Nitric oxide signaling in pain and nociceptor sensitization in the rat. J Neurosci. 1998;18:7008–7014.
53. Samad TA, Moore KA, Sapirstein A, et al. Interleukin-1beta-mediated induction of COX-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475.
54. Inoue A, Ikoma K, Morioka N, et al. Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem. 1999;73:2206–2213.
55. Leem J-G, Bove GM. Mid-axonal tumor necrosis factor-alpha induces ectopic activity in a subset of slowly conducting cutaneous and deep afferent neurons. J Pain. 2002;3:45–49.
56. Winston J, Toma H, Shenoy M, Pasricha PJ. Nerve growth factor regulates VR-1 mRNA levels in cultures of adult dorsal root ganglion neurons. Pain. 2001;89:181–186.
57. Rueff A, Dawson AJ, Mendell LM. Characteristics of nerve growth factor induced hyperalgesia in adult rats: dependence on enhanced bradykinin-1 receptor activity but not neurokinin-1 receptor activation. Pain. 1996;66:359–372.
58. Waxman SG, Kocsis JD, Black JA. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons and is reexpressed following axotomy. J Neurophysiol. 1994;72:466–470.
59. Kim CH, Oh Y, Chung JM, Chung K. The changes in expression of three subtypes of TTX sensitive sodium channels in sensory neurons after spinal nerve ligation. Mol Brain Res. 2001;95:153–161.
60. Black JA, Cummins TR, Plumpton C, et al. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J Neurophysiol. 1999;82:2776–2885.
61. Liu CN, Wall PD, Ben Dor E, et al. Tactile allodynia in the absence of C-fiber activation: Altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85:503–521.
62. Baogang L, Eisenach JC, Tong C. Chronic estrogen sensitizes a subset of mechanosensitive afferents innervating the uterine cervix. J Neurophysiol. 2005;93:2167–2173.
63. Yan T, Liu B, Du D, et al. Estrogen amplifies pain responses to uterine cervical distension in rats by altering transient receptor potential-1 function. Anesth Analg. 2007;104:1246–1250.
64. Tong C, Conklin D, Clyne BB, et al. Uterine cervical afferents in thoracolumbar dorsal root ganglia express transient receptor potential vanilloid type 1 channel and calcitonin gene-related peptide, but not P2X3 receptor and somatostatin. Anesthesiology. 2006;104:651–657.
65. Mousa SA, Zhang Q, Sitte N, et al. β-Endorphin-containing memory-cells and µ-opioid receptors undergo transport to peripheral inflamed tissue. J Neuroimmunol. 2001;115:71–78.
66. Picard PR, Tramèr MR, McQuay HJ, Moore RA. Analgesic efficacy of peripheral opioids (all except intra-articular): a qualitative systematic review of randomised controlled trials. Pain. 1997;72:309–318.
67. Kalso E, Tramèr MR, Carroll D, et al. Pain relief from intra-articular morphine after knee surgery: a qualitative systematic review. Pain. 1997;71:127–134.
68. Sandner-Kiesling A, Eisenach JC. Pharmacology of opioid inhibition to noxious uterine cervical distention. Anesthesiology. 2002;97:966–971.
69. Sengupta JN, Su X, Gebhart GF. Kappa, but not µ or δ, opioids attenuate responses to distention of afferent fibers innervating the rat colon. Gastroenterology. 1996;111:968–980.
70. Gebhart GF, Su X, Joshi S, et al. Peripheral opioid modulation of visceral pain. Ann N Y Acad Sci. 2000;909:41–50.
71. Binder W, Walker JS. Effect of the peripherally selective kappa-opioid agonist, asimadoline, on adjuvant arthritis. Br J Pharmacol. 1998;124:647–654.
72. Eisenach JC, Carpenter R, Curry R. Analgesia from a peripherally active kappa-opioid receptor agonist in patients with chronic pancreatitis. Pain. 2003;101:89–95.
73. Cicero TJ, Nock B, O’Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther. 2002;300:695–701.
74. Gear RW, Miaskowski C, Gordon NC, et al. Kappa-opioids produce significantly greater analgesia in women than in men. Nature Med. 1996;2:1248–1250.
75. Sandner-Kiesling A, Eisenach JC. Estrogen reduces efficacy of µ- but not κ-opioid agonist inhibition in response to uterine cervical distention. Anesthesiology. 2002;96:375–379.
76. Shin SW, Eisenach JC. Intrathecal morphine reduces the visceromotor response to acute uterine cervical distention in an estrogen-independent manner. Anesthesiology. 2003;98:1467–1471.
77. Olofsson C, Ekblom A, Ekman-Ordeberg G, et al. Lack of analgesic effect of systemically administered morphine or pethidine on labour pain. Br J Obstet Gynaecol. 1996;103:968–972.
78. Leighton BL, DeSimone CA, Norris MC, Ben-David B. Intrathecal narcotics for labor revisited: the combination of fentanyl and morphine intrathecally provides rapid onset of profound, prolonged analgesia. Anesth Analg. 1989;69:122–125.
79. Goldin AL, Barchi RL, Caldwell JH, et al. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–368.
80. Renganathan M, Cummins TR, Waxman SG. Nitric oxide blocks fast, slow, and persistent Na+ channels in C-type DRG neurons by S-nitrosylation. J Neurophysiol. 2002;87:761–775.
81. Gold MS, Weinreich D, Kim CS, et al. NaV1.8 mediates neuropathic pain via redistribution in the axons of uninjured afferents. Soc Neurosci. 2001;55:6.
82. Lai J, Gold MS, Kim CS, et al. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95:143–152.
83. Nonaka T, Honmou O, Sakai J, et al. Excitability changes of dorsal root axons following nerve injury: implications for injury-induced changes in axonal Na(+) channels. Brain Res. 2000;859:280–285.
84. Sakai J, Honmou O, Kocsis JD, Hashi K. The delayed depolarization in rat cutaneous afferent axons is reduced following nerve transection and ligation, but not crush: implications for injury-induced axonal Na+ channel reorganization. Muscle Nerve. 1998;21:1040–1047.
85. Gerner P, Mujtaba M, Sinnott CJ, Wang GK. Amitriptyline versus bupivacaine in rat sciatic nerve blockade. Anesthesiology. 2001;94:661–667.
86. Gerner P, Mujtaba M, Khan M, et al. N-Phenylethyl amitriptyline in rat sciatic nerve blockade. Anesthesiology. 2002;96:1435–1442.
87. Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610.
88. Binshtok AM, Gerner P, Oh SB, et al. Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long lasting nociceptive blockade in rodents. Anesthesiology. 2009;111:127–137.
89. Dalle C, Schneider M, Clergue F, et al. Inhibition of the Ih current in isolated peripheral nerve: a novel mode of peripheral antinociception? Muscle Nerve. 2001;24:254–261.
90. Chaplan SR, Guo HQ, Lee DH, et al. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169–1178.
91. Kissin I, Bright CA, Bradley EL. Selective and long-lasting neural blockade with resiniferatoxin prevents inflammatory pain hypersensitivity. Anesth Analg. 2002;94:1253–1258.
92. Ludwig M, Sabatier N, Bull PM, et al. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89.
93. Feng Y, Cui ML, Willis WD. Gabapentin markedly reduces acetic acid-induced visceral nociception. Anesthesiology. 2003;98:729–733.
94. Cao YQ, Mantyh PW, Carlson EJ, et al. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394.
95. Khan IM, Marsala M, Printz MP, et al. Intrathecal nicotinic agonist-elicited release of excitatory amino acids as measured by in vivo spinal microdialysis in rats. J Pharmacol Exp Ther. 1996;278:97–106.
96. Riley RC, Trafton JA, Chi SI, Basbaum AI. Presynaptic regulation of spinal cord tachykinin signaling via GABA-B but not GABA-A receptor activation. Neuroscience. 2001;103:725–737.
97. Li DP, Chen SR, Pan YZ, et al. Role of presynaptic muscarinic and GABA-B receptors in spinal glutamate release and cholinergic analgesia in rats. J Physiol. 2002;543:807–818.
98. Baba H, Kohno T, Okamoto M, et al. Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn. J Physiol. 1998;508:83–93.
99. Lauretti GR, Hood DD, Eisenach JC, Pfeifer BL. A multi-center study of intrathecal neostigmine for analgesia following vaginal hysterectomy. Anesthesiology. 1998;89:913–918.
100. Lombard M-C, Besson J-M. Attempts to gauge the relative importance of pre- and postsynaptic effects of morphine on the transmission of noxious messages in the dorsal horn of the rat spinal cord. Pain. 1989;37:335–345.
101. Kuraishi Y, Hirota N, Sato Y, et al. Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res. 1985;359:177–182.
102. Headley PM, Grillner S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. Trends Pharmacol Sci. 1990;11:205–211.
103. Karpinski N, Dunn J, Hansen L, Masliah E. Subpial vacuolar myelopathy after intrathecal ketamine: report of a case. Pain. 1997;73:103–105.
104. Wilder-Smith CH, Knöpfli R, Wilder-Smith OHG. Perioperative magnesium infusion and postoperative pain. Acta Anaesthesiol Scand. 1997;41:1023–1027.
105. Svensson CI, Yaksh TL. The spinal phospholipase-cyclooxygenase-prostanoid cascade in nociceptive processing. Annu Rev Pharmacol Toxicol. 2002;42:553–583.
106. Roza C, Laird JM, Cervero F. Spinal mechanisms underlying persistent pain and referred hyperalgesia in rats with an experimental ureteric stone. J Neurophysiol. 1998;79:1603–1612.
107. Ossipov MH, Lopez Y, Nichols ML, et al. The loss of antinociceptive efficacy of spinal morphine in rats with nerve ligation injury is prevented by reducing spinal afferent drive. Neurosci Lett. 1995;199:87–90.
108. Tong C, Ma W, Shin S, James RL, Eisenach JC. Uterine cervical distention induces cFos expression in deep dorsal horn neurons of the rat spinal cord. Anesthesiology. 2003;99:205–211.
109. Du D, Eisenach JC, Ririe DG, Tong C. The antinociceptive effects of spinal cyclooxygenase inhibitors on uterine cervical distention. Brain Res. 2004;1024:130–136.
110. Eisenach JC, Curry R, Hood DD, Yaksh TL. Phase I safety assessment of intrathecal ketorolac. Pain. 2002;99:599–604.
111. Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–462.
112. Eisenach JC, Dobson CE, Inturrisi CE, et al. Effect of pregnancy and pain on cerebrospinal fluid immunoreactive enkephalins and norepinephrine in healthy humans. Pain. 1990;43:149–154.
113. Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J Neurosci. 1999;19:3639–3648.
114. Millan MJ. The role of descending noradrenergic and serotoninergic pathways in the modulation of nociception: Focus on receptor multiplicity. Dickenson A, Besson JM. Handbook of Experimental Pharmacology: The Pharmacology of Pain. Springer-Verlag: Berlin; 1997:385–446.
115. Zhuo M, Sengupta JN, Gebhart GF. Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophysiol. 2002;87:2225–2236.
116. Al Chaer ED, Traub RJ. Biological basis of visceral pain: recent developments. Pain. 2002;96:221–225.
117. Jones AK, Kulkarni B, Derbyshire SW. Pain mechanisms and their disorders. Br Med Bull. 2003;65:83–93.
118. Moir DD, Willocks J. Management of incoordinate uterine action under continuous epidural analgesia. BMJ. 1967;3:396–400.
119. Riffel HD, Nochimson DJ, Paul RH, Hon EH. Effects of meperidine and promethazine during labor. Obstet Gynecol. 1973;42:738–745.
120. Abrão KC, Francisco RP Miyadahira S, et al. Elevation of uterine basal tone and fetal heart rate abnormalities after labor analgesia: a randomized controlled trial. Obstet Gynecol. 2009;113:41–47.
121. Mardirosoff C, Dumont L, Boulvain M, Tramèr MR. Fetal bradycardia due to intrathecal opioids for labor analgesia: a systematic review. BJOG. 2002;109:274–281.
122. Hingson RA, Hellman LM. Anatomic and physiologic considerations. Hingson RA, Hellman LM. Anesthesia for Obstetrics. JB Lippincott: Philadelphia; 1956:74.
123. Rahm VA, Hallgren A, Hogberg H, et al. Plasma oxytocin levels in women during labor with or without epidural analgesia: a prospective study. Acta Obstet Gynecol Scand. 2002;81:1033–1039.
124. Stocche RM, Klamt JG, Antunes-Rodrigues J, et al. Effects of intrathecal sufentanil on plasma oxytocin and cortisol concentrations in women during the first stage of labor. Reg Anesth Pain Med. 2001;26:545–550.
125. Scull TJ, Hemmings GT, Carli F, et al. Epidural analgesia in early labour blocks the stress response but uterine contractions remain unchanged. Can J Anaesth. 1998;45:626–630.
126. Leighton BL, Norris MC, Sosis M, et al. Limitations of epinephrine as a marker of intravascular injection in laboring women. Anesthesiology. 1987;66:688–691.
127. Hood DD, Dewan DM, James FM. Maternal and fetal effects of epinephrine in gravid ewes. Anesthesiology. 1986;64:610–613.
128. Shnider SM, Abboud TK, Artal R, et al. Maternal catecholamines decrease during labor after lumbar epidural anesthesia. Am J Obstet Gynecol. 1983;147:13–15.
129. Cascio M, Pygon B, Bernett C, Ramanathan S. Labour analgesia with intrathecal fentanyl decreases maternal stress. Can J Anaesth. 1997;44:605–619.
130. Jouppila R, Puolakka J, Kauppila A, Vuori J. Maternal and umbilical cord plasma noradrenaline concentrations during labour with and without segmental extradural analgesia, and during caesarean section. Br J Anaesth. 1984;56:251–254.
131. Hagerdal M, Morgan CW, Sumner AE, Gutsche BB. Minute ventilation during and oxygen consumption during labor with epidural analgesia. Anesthesiology. 1983;59:425–457.
132. Buchan AS, Sharwood-Smith GH. Physiological changes in pregnancy. Buchas AS, Sharwood-Smith GH. The Simpson Handbook of Obstetric Anaesthesia. on behalf of the Royal College of Surgeons of Edinburgh: Edinburgh, Albamedia; 1999.
133. Hodnett ED. Pain and women's satisfaction with the experience of childbirth: a systematic review. Am J Obstet Gynecol. 2002;186:S160–S172.
134. Billewicz-Driemel AM, Milne MD. Long-term assessment of extradural analgesia for pain relief in labour. II. Sense of deprivation after extradural analgesia in labour: relevant or not? Br J Anaesth. 1976;48:139–144.
135. Stewart DE. Psychiatric symptoms following attempted natural childbirth. Can Med Assoc J. 1982;127:713–716.
136. Ryding E. Psychosocial indications for cesarean section. Acta Obstet Gynecol Scand. 1991;70:47–49.
137. Ballard CG, Stanley AK. Post-traumatic stress disorder after childbirth. Br J Psych. 1995;166:525–528.
138. Macarthur AJ, Macarthur C. Incidence, severity, and determinants of perineal pain after vaginal delivery: a prospective cohort study. Am J Obstet Gynecol. 2004;191:1199–1204.
139. Lavand’homme PM, Roelants F, Waterloos H, De Kock MF. Postoperative analgesic effects of continuous wound infiltration with diclofenac after cesarean delivery. Anesthesiology. 2007;106:1220–1225.
140. Eisenach JC, Pan PH, Smiley RM, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87–94.
141. Pan PH, Coghill R, Houle TT, et al. Multifactorial preoperative predictors for post–cesarean section pain and analgesic requirement. Anesthesiology. 2006;104:417–425.
142. Kehlet H, Jensen TS, Woolf C. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625.
143. Hefferan MP, O’Rielly DD, Loomis CW. Inhibition of spinal prostaglandin synthesis early after L5/L6 nerve ligation prevents the development of prostaglandin-dependent and prostaglandin-independent allodynia in the rat. Anesthesiology. 2003;99:1180–1188.
144. Eisenach JC, Pan PH, Smiley RM, et al. Resolution of pain after childbirth. Anesthesiology. 2013;118:143–151.
145. Gutierrez S, Baogang L, Hayashida K, et al. Reversal of peripheral nerve injury–induced hypersensitivity in the postpartum period: role of spinal oxytocin. Anesthesiology. 2013;118:152–159.
146. Wisner KL, Parry BL, Piontek CM. Clinical practice: Postpartum depression. N Engl J Med. 2002;337:194–199.
147. Brown S, Lumley J. Maternal health after childbirth: results of an Australian population based survey. Br J Obstet Gynaecol. 1998;105:156–161.
148. Lydon-Rochelle MT, Holt VL, Martin DP. Delivery method and self-reported postpartum general health status among primiparous women. Paediatr Perinat Epidemiol. 2001;15:232–240.