Systemic Analgesia
Parenteral and Inhalational Agents
Niveen El-Wahab MBBCh, MRCP, FRCA, Roshan Fernando MD, FRCA
Chapter Outline
Systemic drugs have been used to decrease the pain of childbirth since 1847, when James Young Simpson used diethyl ether to anesthetize a parturient with a deformed pelvis. Since that time, the provision of labor analgesia has advanced significantly owing to a heightened awareness of the neonatal effects of heavy sedation or general anesthesia administered during vaginal delivery and a greater desire of women to actively participate in childbirth.
Neuraxial (i.e., epidural, spinal, combined spinal-epidural [CSE]) analgesic techniques have replaced systemic drug administration as the preferred method for intrapartum analgesia in both the United States and Canada (Table 22-1).1,2 By contrast, in the United Kingdom, fewer than one third of parturients received a neuraxial analgesic technique during labor and vaginal delivery in 2011.3
TABLE 22-1
Types of Labor Analgesia at Hospitals Providing Obstetric Care by Number of Births
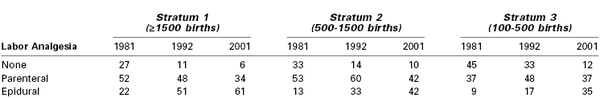
Note: Data are presented as percentages.
Modified from Bucklin BA, Hawkins JL, Anderson JR, Ulrich FA. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology 2005; 103:645-53.
Despite the increased use of neuraxial analgesia for labor, the use of systemic analgesia remains a common practice in many institutions worldwide for several reasons. Many women labor and deliver in an environment where the provision of safe neuraxial analgesia is not available. Some parturients decline neuraxial analgesia or choose to receive systemic analgesia during early labor. Finally, some women may have a medical condition that contraindicates a neuraxial procedure (e.g., coagulopathy) or presents technical challenges (e.g., severe scoliosis, the presence of spinal hardware).
Parenteral Opioid Analgesia
Opioids are the most widely used systemic medications for labor analgesia. These compounds are agonists at opioid receptors (Table 22-2). Their popularity lies in their low cost, ease of use, and the lack of need for specialized equipment and personnel. Although these drugs provide moderate pain relief, parturients commonly report dissociation from the reality of pain rather than complete analgesia. Since neuraxial labor analgesia has become more accessible, systemic opioids have become less popular, owing to the frequency of maternal side effects (e.g., nausea, vomiting, delayed gastric emptying, dysphoria, drowsiness, hypoventilation) and the potential for adverse neonatal effects. However, a renewed interest in opioid administration during labor has occurred owing to the growing use of patient-controlled delivery systems.
TABLE 22-2
Classification of Opioid Receptors
| Current Classification | Previous Classification | Effects |
| µ or MOP | OP3 | Analgesia, meiosis, euphoria, respiratory depression, bradycardia |
| κ or KOP | OP2 | Analgesia, sedation, meiosis |
| δ or DOP | OP1 | Analgesia, respiratory depression |
| Nociception or NOP | OP4 | Inhibition of opioid analgesia* May cause hyperalgesia* |
* Modified from the International Union of Basic and Clinical Pharmacology (IUPHAR) database. Available at http://www.iuphar-db.org. Accessed March 9, 2013.
OP, opioid peptide.
Although systemic opioids have long been used for labor analgesia, there is little scientific evidence to suggest that one drug is superior to another; most often, drug selection is based on local policy or personal preference (Table 22-3). The efficacy of systemic opioid analgesia and the incidence of side effects are largely dose dependent rather than drug dependent.
TABLE 22-3
Systemic Opioids for Labor Analgesia
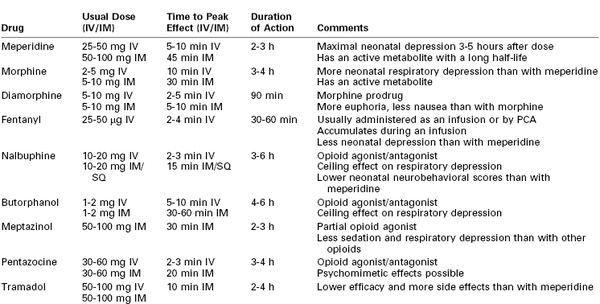
IM, Intramuscular; IV, intravenous; PCA, patient-controlled analgesia; SQ, subcutaneous.
As a result of their high lipid solubility and low molecular weight (< 500 Da), all opioids readily cross the placenta by diffusion and are associated with the risks of neonatal respiratory depression and neurobehavioral changes. Opioids may also affect the fetus in utero. The fetus and neonate are particularly susceptible to opioid-induced side effects for several reasons. The metabolism and elimination of these drugs are prolonged compared with adults, and the blood-brain barrier is less well developed, allowing for greater central effects. Opioids may result in decreased variability of the fetal heart rate (FHR), although this change usually does not reflect a worsening of fetal oxygenation or acid-base status. The likelihood of neonatal respiratory depression depends on the dose and timing of opioid administration. Even in the absence of obvious neonatal depression at birth, there may be subtle changes in neonatal behavior for several days. Reynolds et al.4 performed a meta-analysis of studies that compared epidural analgesia with systemic opioid analgesia using meperidine, butorphanol, or fentanyl. The authors concluded that lumbar epidural analgesia was associated with improved neonatal acid-base status at delivery. Similarly, in a multicenter randomized trial, Halpern et al.5 compared patient-controlled epidural analgesia using a local anesthetic combined with an opioid to patient-controlled analgesia (PCA) using a parenteral opioid; the investigators demonstrated an increased need for active neonatal resuscitation in the parenteral opioid group (52% versus 31%).
Opioids may be administered as intermittent bolus doses or by PCA. Bolus doses are used more commonly, but the newer synthetic opioids are increasingly used with patient-controlled devices. Because the mode of delivery influences a drug's pharmacologic profile, the opioids will be discussed by route of administration.
Intermittent Bolus Parenteral Opioid Analgesia
Opioids may be given intermittently by subcutaneous, intramuscular, or intravenous injection. The route and timing of administration influence maternal uptake and placental transfer to the fetus. Subcutaneous and intramuscular routes have the advantage of ease of administration but are painful. Absorption varies with the site of injection and depends on local and regional blood flow; consequently, the onset, quality, and duration of analgesia are highly variable.
Intravenous administration offers several advantages. The onset of analgesia is faster, and the timing and magnitude of the peak plasma concentration of drug are more predictable. It is also possible to titrate dose to effect. For these reasons the intravenous route is generally preferred when available.
Meperidine
In 1947, meperidine (pethidine) became the first synthetic opioid to be used for intrapartum analgesia. More extensively studied than newer drugs, meperidine is the most common opioid given for labor analgesia in the United Kingdom.6 The usual dose is 50 to 100 mg intramuscularly, which can be repeated every 4 hours. The onset of analgesia occurs in 10 to 15 minutes, but 45 minutes may be required to reach peak effect. The duration of action is typically 2 to 3 hours.
Meperidine is highly lipid soluble, readily crosses the placenta, and equilibrates between the maternal and fetal compartments within 6 minutes. Meperidine is metabolized in the liver to produce normeperidine, a pharmacologically active metabolite that is a potent respiratory depressant. Normeperidine also crosses the placenta and is largely responsible for the neonatal side effects encountered with meperidine use.7
Maternal administration of meperidine may reduce fetal aortic blood flow, fetal muscle activity, and FHR variability.7 Sosa et al.8 demonstrated that intravenous meperidine 100 mg, given during the first stage of labor, resulted in an increased incidence of umbilical cord arterial blood acidemia at delivery when compared with a placebo. Normeperidine may result in neonatal respiratory depression. After maternal intramuscular administration of meperidine, the greatest risk for neonatal respiratory depression occurs if meperidine is given to the mother 3 to 5 hours before delivery, whereas the risk is least if it is given within 1 hour before delivery.7 Normeperidine accumulation is associated with altered neonatal behavior, manifesting as reduced duration of wakefulness and attentiveness and impaired breast-feeding.9 Meperidine administration is associated with lower Apgar scores and muscle tone in the neonate. Maternal side effects are of less clinical concern, although there is a high incidence of nausea, vomiting, and dysphoria.
The maternal half-life of meperidine is 2.5 to 3 hours, whereas that of normeperidine is 14 to 21 hours.10 The half-life of both compounds is increased by up to three times in the neonate as a result of reduced clearance.10 Consequently, the adverse effects may be seen in the neonate up to 72 hours after delivery. The action of meperidine is reversed by naloxone; however, the action of normeperidine is not. This is important because antagonism with naloxone may exacerbate normeperidine-induced seizures owing to suppression of the anticonvulsant effect of meperidine.
The quality of labor analgesia with meperidine has been questioned, with some reports indicating that less than 20% of laboring women obtain satisfactory pain relief with its use. The analgesic benefit of intravenous meperidine 50 mg has been observed to be comparable with that of intravenous acetaminophen 1000 mg, but with a greater incidence of adverse effects (64% versus none).11
A randomized, double blind, placebo-controlled trial of intramuscular meperidine 100 mg versus 0.9% normal saline for labor analgesia was terminated after an interim analysis of 50 patients, which revealed a significantly greater reduction in pain scores with meperidine.12 However, the analgesic effect of meperidine was modest, with a median change in visual analog pain scores (VAPS) of 11 mm at 30 minutes; 68% of women required additional analgesia during labor.
The effect of meperidine on the progress of labor is unclear. Historically, meperidine has been given to decrease the length of the first stage of labor in cases of dystocia; however, recent studies have not demonstrated such an effect, and investigators have concluded that meperidine should not be given for this purpose.8
Despite the concerns just highlighted, meperidine continues to be the most common opioid given for labor analgesia worldwide; this is most likely the result of its familiarity, ease of administration, availability, and low cost.
Morphine
Several decades ago, morphine was administered in combination with scopolamine to provide “twilight sleep” during labor and delivery. Analgesia was obtained at the expense of excessive maternal sedation and neonatal depression. Morphine is infrequently used during labor, but it can be given every 4 hours intravenously (0.05 to 0.1 mg/kg) or intramuscularly (0.1 to 0.2 mg/kg), with a peak effect observed in 10 and 30 minutes, respectively. The duration of action when given intravenously or intramuscularly is 3 to 4 hours.10
Morphine is principally metabolized by conjugation in the liver, with up to 70% being transformed into the largely inactive morphine-3-glucuronide. The remainder is transformed into the active metabolite morphine-6-glucuronide, which is 13 times more potent than morphine and has significant analgesic properties.10 Both metabolites are excreted in the urine and have elimination half-lives of up to 4.5 hours in the presence of normal renal function.13 Morphine rapidly crosses the placenta, and a fetal-to-maternal blood concentration ratio of 0.96 is observed at 5 minutes. The elimination half-life of morphine is longer in neonates than in adults.
Maternal side effects include respiratory depression and histamine release, which may result in a rash and pruritus. Like many opioids, morphine is emetogenic and is associated with sedation and dysphoria with increasing doses.10
The greatest neonatal concern is that of respiratory depression. Way et al.14 observed that intramuscular morphine given to newborns caused greater respiratory depression than an equipotent dose of meperidine when response to carbon dioxide was measured. This finding was attributed to an increased permeability of the neonatal brain to morphine.
Pregnancy alters the pharmacokinetics of morphine. Greater plasma clearance, shorter elimination half-life, and earlier peak metabolite levels occur in pregnant women than in nonpregnant women. In theory, these characteristics should reduce fetal exposure. One study observed no cases of neonatal depression after morphine administration during labor, prompting the researchers to suggest that morphine use in labor should be reevaluated.15 Subsequently, Oloffson et. al.16 assessed the analgesic efficacy of intravenous morphine during labor (0.05 mg/kg every third contraction, to a maximum dose of 0.2 mg/kg) and observed clinically insignificant reductions in pain intensity. These investigators also compared intravenous morphine (up to 0.15 mg/kg) with intravenous meperidine (up to 1.5 mg/kg) and found that high pain scores were maintained in both groups despite high levels of maternal sedation.17
Diamorphine
Diamorphine (3,6-diacetylmorphine, heroin) is a synthetic morphine derivative in common use in the United Kingdom, with 34% of obstetric units reporting its use for labor analgesia.6 Diamorphine is twice as potent as morphine. As a prodrug, diamorphine has no direct affinity for opioid receptors, but it is rapidly hydrolyzed by plasma esterases to active metabolites, which are responsible for its clinical effect.10 The metabolite 6-monoacetylmorphine is responsible for a significant proportion of analgesic activity, and it is further metabolized to morphine.13
Typical parenteral doses (intravenous or intramuscular) are 5 to 10 mg. The most common route of administration is intramuscular and results in labor analgesia with a duration of approximately 90 minutes. Both diamorphine and its active metabolite 6-monoacetylmorphine are more lipid soluble than morphine, resulting in a faster onset of analgesia with more euphoria but less nausea and vomiting. These pharmacokinetic properties may also predispose to maternal respiratory depression. Neonatal respiratory depression may also occur due to rapid placental transfer, although this has mainly been reported with high doses.18
Rawal et al.18 investigated the relationship between the dose-delivery interval (following intramuscular administration of a single dose of diamorphine 7.5 mg) and the concentration of free morphine in umbilical cord blood and neonatal outcome. The negative correlation between the dose-delivery interval and umbilical cord blood morphine levels was significant. The correlation between higher free morphine concentrations and lower 1-minute Apgar scores (and the need for neonatal resuscitation) was nonsignificant. Their findings suggest that infants born shortly after interval diamorphine administration are at greater risk for respiratory depression.
Fairlie et al.19 randomized 133 pregnant women to receive intramuscular meperidine 150 mg or diamorphine 7.5 mg and found that significantly more women in the meperidine group reported poor or no pain relief at 60 minutes. However, approximately 40% of women in the two groups requested second-line analgesia, suggesting that both drugs had poor analgesic efficacy. The incidence of maternal sedation was comparable, but vomiting occurred much less frequently and neonatal Apgar scores were higher at 1 minute in the diamorphine group. The trial was small, but the results suggested that at the administered doses, diamorphine conferred some benefit over meperidine with regard to maternal side effects and initial neonatal condition. Currently, a much larger trial of the two drugs, at the same dose and with the same route of administration, is underway in the United Kingdom.
Fentanyl
Fentanyl is a highly lipid-soluble, highly protein-bound synthetic opioid that is highly selective for the µ-opioid receptor, resulting in an analgesic potency 100 times that of morphine and 800 times that of meperidine. Its rapid onset (peak effect, 2 to 4 minutes), short duration of action (30 to 60 minutes), and lack of active metabolites make it attractive for labor analgesia. Although it can be administered intramuscularly, fentanyl is most commonly given intravenously and is titrated to effect; frequently it is administered with a patient-controlled device.
Although small doses of fentanyl undergo rapid redistribution, large or repeated doses may accumulate.10 Importantly, clearance of fentanyl by elimination represents only 20% of that occurring by redistribution, resulting in a rapid increase in context sensitive half-life with an increased duration of infusion.10 Fentanyl has a longer elimination half-life than morphine, but it is metabolized to inactive metabolites in the liver that are excreted in the urine.
Fentanyl readily crosses the placenta; however, the average umbilical vein/maternal vein ratio remains low, most likely owing to a significant degree of maternal protein binding and drug redistribution. In a chronically instrumented sheep model, Craft et al.20 detected fentanyl in fetal plasma as early as 1 minute after maternal administration; however, maternal plasma levels were approximately 2.5 times greater than fetal plasma levels.
Rayburn et al.21 compared responses in women who received intravenous fentanyl (50 to 100 µg as often as once per hour at maternal request) with the experience of women who did not receive analgesia. The mean dose of fentanyl administered was 140 µg (range, 50 to 600 µg). All patients who received fentanyl experienced brief analgesia (mean duration, 45 minutes), sedation, and a transient reduction in FHR variability (30 minutes). There was no difference between groups in neonatal Apgar scores, respiratory status, or Neurologic and Adaptive Capacity Scores (NACS). Rayburn et al.22 also compared intravenous fentanyl (50 to 100 µg every hour) with an equi-analgesic dose of meperidine (25 to 50 mg every 2 to 3 hours). The researchers observed less sedation, vomiting, and neonatal naloxone administration with fentanyl, but they observed no difference between groups in NACS. The two groups had similarly high pain scores, suggesting that both drugs have poor analgesic efficacy.
Nalbuphine
Nalbuphine is a mixed agonist-antagonist opioid analgesic with agonist activity at κ-opioid receptors, thereby producing analgesia, and partial agonist activity at µ-opioid receptors, thus resulting in less respiratory depression.13 A partial agonist is a drug that has receptor affinity but produces a submaximal effect compared with a full agonist, even when given at very high doses.10
Nalbuphine can be administered by intramuscular, intravenous, or subcutaneous injection, with a usual dose of 10 to 20 mg every 4 to 6 hours. The onset of analgesia occurs within 2 to 3 minutes of intravenous administration and within 15 minutes of intramuscular or subcutaneous administration. The drug is metabolized in the liver to inactive compounds that are then secreted into bile and excreted in feces.13
Nalbuphine and morphine are of equal analgesic potency and result in sedation and respiratory depression at similar doses. However, because of its mixed receptor affinity, nalbuphine demonstrates a ceiling effect for respiratory depression at a dose of 0.5 mg/kg.13 Nalbuphine causes less nausea, vomiting, and dysphoria than morphine. Concerns that it may have an antianalgesic effect, particularly in men, led to the withdrawal of nalbuphine in the United Kingdom in 2003.13
Wilson et al.23 performed a randomized, double-blind comparison of intramuscular nalbuphine 20 mg and meperidine 100 mg for labor analgesia. Nalbuphine was associated with less nausea and vomiting but more maternal sedation. Analgesia was comparable between the groups. Neonatal neurobehavioral scores were lower in the nalbuphine group at 2 to 4 hours, but there was no difference between groups at 24 hours. The umbilical vein-to-maternal vein concentration ratio was higher with nalbuphine (mean ± SEM, 0.78 ± 0.03) than with meperidine (0.61 ± 0.02). A subsequent study failed to demonstrate an analgesic advantage with either drug but again reported transient neonatal neurologic depression with nalbuphine.24
Amin et al.25 compared the neonatal outcome for women who received either nalbuphine or saline-control before elective cesarean delivery. They found lower 1-minute Apgar scores and a significantly longer time to sustained respiration in the nalbuphine group. However, 5-minute Apgar scores and umbilical cord blood gas measurements were similar between groups.
Nicolle et al.26 evaluated the transplacental transfer and neonatal pharmacokinetics of nalbuphine in 28 women who received the drug either intramuscularly or intravenously during labor. The investigators found a high umbilical vein-to-maternal vein concentration ratio of 0.74, which did not correlate with the administered dose. The estimated neonatal half-life was 4.1 hours, which is greater than the adult half-life and, more importantly, longer than the half-life of naloxone. There was a transient reduction in FHR variability in 54% of the fetuses, which was not associated with the plasma concentration of nalbuphine. Analgesia was rated as effective by 54% of parturients.
Giannina et al.27 compared the effects of intravenous nalbuphine and meperidine on intrapartum FHR tracings. Nalbuphine significantly reduced both the number of FHR accelerations and FHR variability, whereas meperidine had little effect.
A recent prospective pilot study of 302 nulliparous parturients (57 women who received nalbuphine, and a control group of 245 women who received neither nalbuphine nor epidural analgesia) reported a marked reduction in duration of the active phase of the first stage of labor in the nalbuphine group (75 minutes versus 160 minutes in the control group); this effect appeared to be independent of oxytocin use.28 Additional investigations are needed to verify this finding.
Butorphanol
Butorphanol is an opioid with agonist-antagonist properties that resemble those of nalbuphine. It is 5 times as potent as morphine and 40 times more potent than meperidine.29 The typical dose during labor is 1 to 2 mg intravenously or intramuscularly. Butorphanol is 95% metabolized in the liver to inactive metabolites. Excretion is primarily renal. A plateau effect for respiratory depression is noted, where butorphanol 2 mg produces respiratory depression similar to that of morphine 10 mg or meperidine 70 mg. However, butorphanol 4 mg results in less respiratory depression than morphine 20 mg or meperidine 140 mg.29
Maduska and Hajghassemali30 compared intramuscular butorphanol (1 to 2 mg) with meperidine (40 to 80 mg) and found similar efficacy of labor analgesia. Butorphanol and meperidine exhibited rapid placental transfer with similar umbilical vein-to-maternal vein concentration ratios (0.84 and 0.89, respectively) and no differences in FHR tracings, Apgar scores, time to sustained respiration, or umbilical cord blood gas measurements at delivery.
Hodgkinson et al.31 performed a similar study comparing the intravenous administration of butorphanol (1 or 2 mg) and meperidine (40 or 80 mg) for labor analgesia. Maternal pain relief was found to be adequate and comparable, but there were fewer maternal side effects (e.g., nausea, vomiting, dizziness) in the women who received butorphanol. There was no difference between groups in neonatal Apgar or neurobehavioral scores.
Conversely, in a double-blind comparison of intravenous butorphanol (1 or 2 mg) and meperidine (40 or 80 mg) during labor, Quilligan et al.32 noted lower pain scores at 30 minutes and 1 hour after the administration of butorphanol. There was no significant difference in Apgar scores between the two groups of infants; however, the mean FHR was noted to be higher among those fetuses whose mothers received butorphanol.
Nelson and Eisenach33 investigated the possible synergistic effect of giving both intravenous butorphanol and meperidine; they compared the administration of both drugs with the administration of either drug alone. Women received intravenous butorphanol 1 mg, meperidine 50 mg, or butorphanol 0.5 mg with meperidine 25 mg. All three groups reported a similar reduction in pain intensity; however, only 29% of the women achieved clinically significant pain relief. There was no difference among groups in maternal side effects or neonatal Apgar scores. The investigators concluded that there was no therapeutic benefit to combining the two drugs.
Atkinson et al.34 performed a double-blind trial of intravenous butorphanol (1 to 2 mg) and fentanyl (50 to 100 µg) administered hourly on maternal request. The investigators found that butorphanol provided better analgesia initially, with fewer requests for additional drug doses or progression to epidural analgesia. There was no difference in adverse maternal or neonatal effects between the two groups.
Meptazinol
Meptazinol is a partial opioid agonist specific to µ-opioid receptors with a rapid onset of action (i.e., 15 minutes after intramuscular administration). The intramuscular dose (50 to 100 mg) and duration of action for labor analgesia are similar to those for meperidine. Its partial agonist activity is thought to result in less sedation, respiratory depression, and risk for dependence than occurs with other opioid agonists.
Meptazinol is metabolized by glucuronidation in the liver and then excreted in the urine. This process is more mature in the neonate than is the metabolic pathway of meperidine. The adult half-life is 2.2 hours, and the neonatal half-life is 3.4 hours.35
Theoretically, this rapid elimination should confer a lower incidence of adverse neonatal effects than occurs with meperidine. In a single-blind study, Jackson and Robson36 compared intramuscular meptazinol with meperidine at the same doses (100 mg if maternal weight was ≤ 60 kg, 125 mg if 61 to 70 kg, and 150 mg if ≥ 70 kg). Meptazinol provided significantly better analgesia than meperidine but resulted in a similar frequency of maternal side effects.
Nicholas and Robson37 subsequently compared intramuscular meptazinol 100 mg with meperidine 100 mg in a randomized, double-blind trial in 358 parturients. Meptazinol provided significantly better pain relief at 45 and 60 minutes, but the two drugs provided a similar duration of analgesia, and there was no significant difference between groups in maternal side effects. Neonatal outcomes were similar between groups, except significantly more infants whose mothers had received meptazinol had an Apgar score of 8 or higher at 1 minute.
Other investigators have reported little difference in analgesic efficacy, maternal side effects, or neonatal outcomes between meptazinol and meperidine. In a study of 1100 patients, Morrison et al.38 found that neither drug given at equal doses (150 mg in patients weighing > 70 kg, 100 mg in those weighing ≤ 70 kg) was effective at relieving pain. Maternal drowsiness was significantly less pronounced with meptazinol, but the incidence of vomiting was higher. FHR changes and neonatal outcomes, including Apgar scores, need for resuscitation, and suckling ability, were comparable. The overall use of naloxone was similar in the two groups, but if the dose-delivery interval exceeded 180 minutes, significantly more neonates in the meperidine group required naloxone.
De Boer et al.39 assessed neonatal blood gas and acid-base measurements after maternal intramuscular administration of meptazinol (1.5 mg/kg) or meperidine (1.5 mg/kg) during labor. Capillary blood gas measurements at 10 minutes of life showed a significantly lower pH and a higher PaCO2 in the meperidine group, although this difference resolved by 60 minutes. These findings suggest that meptazinol causes less neonatal respiratory depression.
Meptazinol may confer some benefits over meperidine in early neonatal outcome, but it is not widely used. A recent survey indicated that it is the intramuscular labor analgesic of choice in only 14% of obstetric units in the United Kingdom.6 The cost of meptazinol is considerably higher than that of meperidine. Meptazinol is not available in the United States.
Pentazocine
Pentazocine is a selective κ-opioid receptor agonist with some weak antagonist activity at µ-opioid receptors.13 It may be given orally or systemically by intramuscular or intravenous injection. The typical parenteral adult dose is 30 to 60 mg, which is equivalent to morphine 10 mg. Onset of action occurs within 2 minutes when given intravenously and within 20 minutes if given by the intramuscular route. Metabolism occurs in the liver by oxidation and glucuronidation; metabolites are then excreted in the urine.
Pentazocine causes similar respiratory depression to that seen with equipotent doses of morphine and meperidine, but it exhibits a ceiling effect with doses in excess of 60 mg. Psychomimetic effects (e.g., dysphoria, hallucinations) may complicate its use, particularly with increasing doses.
In a double-blind study of 94 laboring women who received intramuscular administration of pentazocine (up to 60 mg) and meperidine (up to 150 mg), Mowat and Garrey40 observed equivalent and adequate analgesia for approximately 40% of women in each group. The incidence of sedation was comparable between groups, and fewer women in the pentazocine group complained of nausea and vomiting.
In a randomized study comparing intramuscular administration of pentazocine 30 mg with tramadol 100 mg in 100 laboring women, Kuti et al.41 observed greater analgesia in the pentazocine group at 1 hour, with a longer time to subsequent request for additional analgesia (181 minutes versus 113 minutes, P < .05). The overall analgesic effect of both drugs was modest, with only 30% to 50% of women reporting satisfactory pain relief. More women in the pentazocine group were drowsy, but the result did not achieve statistical significance. There were no cases of maternal respiratory depression, and there was no difference between groups in neonatal outcomes. The investigators concluded that pentazocine provides better labor analgesia than tramadol.
Tramadol
Tramadol is an atypical, weak, synthetic opioid that has affinity for all opioid receptors, but particularly the µ-opioid subtype. Tramadol also inhibits neuronal reuptake of norepinephrine and serotonin, and it directly stimulates presynaptic serotonin release, which may account for some of its analgesic effects.13 Tramadol can be administered orally or by intramuscular or intravenous injection at a dose of 50 to 100 mg every 4 to 6 hours in adults. Although the initial bioavailability after oral administration is only 70% owing to a significant first-pass effect, this increases to almost 100% with repeated doses.10,13
The analgesic potency of tramadol is equal to that of meperidine and one fifth to one tenth that of morphine. In equi-analgesic doses, tramadol causes less respiratory depression than morphine; at usual doses, no clinically significant respiratory depression occurs. The onset of analgesia is within 10 minutes of intramuscular administration, with an effective duration of 2 to 4 hours. Tramadol is metabolized by demethylation and glucuronidation in the liver to several metabolites, one of which has independent analgesic activity (M1). The metabolites are almost entirely excreted in the urine. The elimination half-life is 5 to 6 hours, whereas that of the active metabolite is 9 hours.
Tramadol readily crosses the placenta, and an umbilical vein-to-maternal vein ratio of 0.94 has been observed at delivery.42 Neonates possess complete hepatic capacity for metabolism of tramadol to its active metabolite M1. The elimination profile of M1 suggests a terminal half-life of 85 hours because of its requirement for renal elimination, which is an immature process in neonates. Claahsen-van der Grinten et al.42 reported that intrapartum tramadol (initial dose of 100 mg, then subsequent doses of 50 to 100 mg, up to a maximum dose of 250 mg) resulted in normal Apgar scores and NACS, with no correlation to tramadol or M1 concentrations. However, the single neonate who required naloxone had the highest plasma concentration of tramadol.
The analgesic efficacy of tramadol in labor has been questioned. Keskin et al.43 compared intramuscular tramadol 100 mg and meperidine 100 mg for labor analgesia; they observed greater pain relief and a lower incidence of nausea and fatigue with meperidine. There was no significant difference between groups in neonatal outcome, but more infants in the tramadol group required supplemental oxygen for respiratory distress and hypoxemia. The investigators concluded that meperidine provided superior analgesia and was associated with a better side-effect profile.
By contrast, Viegas et al.44 conducted a randomized, double-blind trial to compare intramuscular administration of tramadol 50 mg, tramadol 100 mg, and meperidine 75 mg during labor. Tramadol 100 mg and meperidine 75 mg provided similar labor analgesia; however, a higher incidence of maternal and neonatal adverse effects was observed with meperidine.
Kooshideh and Shahriari45 evaluated the intramuscular administration of tramadol 100 mg or meperidine 50 mg on labor duration and analgesic efficacy in 160 parturients. The investigators observed that tramadol was associated with a reduced duration of both the first stage (140 versus 190 minutes, P < .001) and the second stage of labor (25 versus 33 minutes, P = .001). There was no difference in median and maximum pain scores between groups 1 hour after drug administration; however, lower pain scores were observed during the second stage of labor in the meperidine group. Nausea, vomiting, and drowsiness occurred less frequently in the tramadol group.
Patient-Controlled Analgesia
Patient-controlled analgesia has been used to control postoperative pain for several decades and for the provision of labor analgesia in more recent years. First described in women with thrombocytopenia who were unable to undergo a neuraxial analgesia procedure, its use has grown in availability and popularity. A 2007 survey demonstrated that 49% of obstetric units in the United Kingdom offered PCA for labor analgesia.46 Purported advantages of PCA include (1) superior pain relief with lower doses of drug, (2) less risk for maternal respiratory depression compared with bolus intravenous administration, (3) less placental transfer of drug, (4) less need for antiemetic agents, and (5) greater patient satisfaction.47 The smaller, more frequent dosing used with this mode of analgesia may result in a more stable plasma drug concentration and a more consistent analgesic effect when compared with that of intermittent bolus administration regimens.47
PCA represents an alternative method of labor analgesia when neuraxial analgesia is not requested or is unavailable, contraindicated, or unsuccessful. The parturient can tailor the administration of analgesia according to her individual needs, and with some regimens the bolus dose can be altered to allow further titration of analgesia as labor progresses.
However, PCA for labor is not without limitations. Despite the frequency of dose administration, the coordination of peak opioid concentrations with uterine contractions can be difficult and result in suboptimal analgesia. In addition, the relatively small doses of opioid may be less effective at controlling pain as labor progresses. Finally, a number of maternal and fetal side effects have been described (see later discussion). A variety of drugs, doses, and regimens have been studied, including comparisons of PCA with and without a continuous intravenous infusion (Table 22-4).
TABLE 22-4
Opioids Used for Intravenous Patient-Controlled Analgesia in Labor
| Drug | Bolus Dose | Lockout Interval (min) |
| Meperidine | 5-15 mg | 10-20 |
| Nalbuphine | 1-3 mg | 6-10 |
| Fentanyl | 10-25 µg | 5-12 |
| Alfentanil | 200 µg (+ 200 µg/h infusion) | 5 |
| Remifentanil (bolus only) | 0.2-0.8 µg/kg (low dose initially, then titrated to effect) | 2-3 |
| Remifentanil (background infusion with bolus dose) | Infusion rate: 0.025-0.1 µg/kg/min Bolus dose: 0.25 µg/kg | 2-3 |
Meperidine
Meperidine was the first opioid to be used for PCA during labor. Isenor and Penny-McGillivray48 compared PCA meperidine (background infusion 60 mg/h with bolus doses of 25 mg, up to a maximum dose of 200 mg) with intermittent intramuscular meperidine (50 to 100 mg every 2 hours). Women in the PCA group reported lower pain scores than women in the intramuscular group, even when adjustment was made for the increased total amount of meperidine used.48 There was no difference in maternal side effects, FHR abnormalities, or neonatal Apgar scores between groups.
More recent studies have indicated that when administered by PCA, meperidine appears to be less effective than the shorter-acting opioids. Douma et al.49 randomized parturients in labor to receive either meperidine (49.5 mg loading dose, 5 mg bolus dose with 10 min lockout, maximum total dose 200 mg), fentanyl (50 µg loading dose, 20 µg bolus dose with 5 min lockout, maximum dose 240 µg/h), or remifentanil (40 µg loading dose, 40 µg bolus dose with 2 min lockout, maximum dose 1200 µg/h). Meperidine provided the least effective analgesia, with no change in pain scores from baseline at 2 hours after administration and the highest rate of conversion to epidural analgesia.
Morphine and Diamorphine
When administered by PCA, morphine and diamorphine are rarely used for labor analgesia in parturients with a live fetus, but they represent an option for women with intrauterine fetal demise.46 The accumulation of the active metabolite morphine-6-glucuronide, which is a potent respiratory depressant, is a concern in mothers with a live fetus. No studies have compared the analgesic efficacy of morphine administered by PCA versus intermittent bolus administration during labor. In a single study of diamorphine, administered by either PCA or intermittent intramuscular bolus doses, less effective analgesia and lower satisfaction scores were observed in the PCA group.50
Fentanyl
The pharmacokinetic profile for fentanyl (i.e., rapid onset, high potency, short duration of action, absence of active metabolites) has resulted in its selection as one of the most commonly used opioids for PCA during labor and delivery. In the United Kingdom, it is used in 26% of the units that offer PCA during labor.46
Nikkola et al.51 observed that fentanyl PCA (50 µg loading dose, 20 µg bolus, 5 minute lockout) provided a moderate reduction in labor pain in 50% of the parturients receiving this mode of analgesia; however, less overall pain relief was experienced when compared with a group that received epidural analgesia. The use of fentanyl was also associated with a higher incidence of maternal dizziness and sedation.
Rayburn et al.52 compared fentanyl PCA (bolus 10 µg, lockout interval 12 minutes) to intermittent intravenous nurse-administered fentanyl boluses (50 to 100 µg every hour, on demand). The degree of analgesia, adverse maternal effects, and neonatal outcomes (e.g., neonatal Apgar scores, naloxone requirements, neurobehavioral scores) were similar between the two groups. The two groups used a similar total amount of fentanyl, had comparable umbilical serum concentrations of fentanyl, and had incomplete analgesia during late labor.
Morley-Forster and Weberpals53 observed a 44% incidence of moderate neonatal depression (1 minute Apgar < 6) in a retrospective review of 32 neonates whose mothers had received fentanyl PCA (at various initial doses, basal infusion rates, and lockout intervals) during labor. A total of 9.4% of the neonates required naloxone; the total dose of fentanyl was significantly higher in the mothers of neonates who required naloxone than in those who did not (mean ± SD, 770 ± 233 µg versus 298 ± 287 µg, respectively). By contrast, in a retrospective evaluation of fentanyl PCA (loading 50 µg, bolus 20 µg, lockout interval 5 minutes) compared with no analgesia during labor, Hosokawa et al.54 observed lower mean umbilical arterial blood pH measurements, but comparable Apgar scores and no requirement for naloxone or bag-and-mask ventilation in the 129 neonates whose mothers received fentanyl.
Alfentanil
Alfentanil is a highly selective µ-opioid receptor agonist that is administered by the intravenous route only.13 Although infrequently used during labor, it is typically administered by PCA. A fentanyl derivative, it is approximately 10 times less potent than fentanyl. It is less lipophilic and more protein bound than its parent compound, resulting in a smaller volume of distribution. Its low volume of distribution and low pKa result in a rapid onset (within 1 minute), a short duration of action, and rapid clearance (elimination half-life of 90 minutes). Furthermore, its context-sensitive half-life is shorter than that of fentanyl. Metabolism of alfentanil occurs by demethylation in the liver to noralfentanil, which is then conjugated and excreted in the urine. Importantly, alfentanil is a potent respiratory depressant, and consequently there are concerns regarding potential adverse neonatal effects.
Morley-Forster et al.55 compared alfentanil PCA (bolus 200 µg, lockout interval 5 minutes, background infusion 200 µg/h) to fentanyl PCA (bolus 20 µg, lockout interval 5 minutes, background infusion 20 µg/h). The two drugs appeared equally effective in early labor, up to a cervical dilation of 6 cm. Subsequently, fentanyl was associated with a greater reduction in pain scores compared with alfentanil. There were no significant differences in maternal side effects or neonatal outcomes.
Pentazocine
The use of pentazocine is uncommon in the western world, and there has been little evaluation of its use via PCA. One study in South Africa compared pentazocine PCA with meperidine PCA and reported acceptable maternal analgesia and neonatal outcomes with both, but a higher incidence of maternal nausea and sedation with meperidine.56
Tramadol
Tramadol is not commonly used via PCA. Long and Yue57 compared tramadol PCA with CSE analgesia and found that both forms of pain relief resulted in a significant decrease in pain scores compared with a third group not receiving analgesia; however, the CSE technique provided the best analgesia. The tramadol group experienced a higher incidence of adverse maternal events (including one case of cardiovascular collapse) and neonatal depression.
Nalbuphine
Few studies have evaluated nalbuphine PCA in labor. In one study, maternal satisfaction was higher with nalbuphine PCA (bolus 1 mg, lockout interval 6 to 10 minutes) compared with intermittent intravenous administration (bolus 10 to 20 mg every 4 to 6 hours).58 Analgesia and Apgar scores were similar between groups, and no neonates required naloxone.
Frank et al.59 concluded that nalbuphine PCA (bolus 3 mg, lockout interval 10 minutes) provided better analgesia in nulliparous women than meperidine PCA (bolus 15 mg, lockout interval 10 minutes). Maternal sedation scores were similar, and there was no difference in neonatal outcome as assessed by Apgar scores, time to sustained respiration, or neurobehavioral assessment at 6 to 10 hours after delivery.
Remifentanil
Remifentanil is a synthetic anilidopiperidine derivative with selective activity at the µ-opioid receptor, low lipid solubility, and a low volume of distribution (0.39 L/kg). Functional brain magnetic resonance imaging reveals an onset time of 20 to 30 seconds, peak concentration within 80 to 90 seconds at the cortical loci, and a blood-brain equilibration time of 1.2 to 1.4 minutes.60 Remifentanil undergoes rapid hydrolysis by nonspecific plasma and tissue esterases to an inactive metabolite, resulting in a short elimination half-life of approximately 9.5 minutes.61 The context sensitive half-life is 3.5 minutes, irrespective of duration of infusion. The effective analgesic half-life is 6 minutes, thus allowing effective analgesia for consecutive uterine contractions.61 Plasma concentrations of remifentanil in pregnant patients are approximately half those found in nonpregnant patients.62 This difference may be due to the greater volume of distribution (increased blood volume and reduced protein binding), greater clearance (increased cardiac output and renal perfusion), and higher esterase activity during pregnancy.
Remifentanil readily crosses the placenta, resulting in a fetal-to-maternal blood ratio of 0.88; however, the lower umbilical artery-to-vein concentration ratio of 0.29 demonstrates that the drug is either extensively redistributed or metabolized by the fetus.62 These pharmacokinetic properties are ideal for labor analgesia63; moreover, remifentanil is rapidly titratable, allowing dose adjustments with labor progress or in response to side effects. For example, termination of a continuous remifentanil infusion results in a 50% recovery in minute ventilation within 5.4 minutes. The rapid elimination of remifentanil also reduces the propensity for neonatal respiratory depression compared to that with longer-acting opioids. Kan et al.62 found no adverse neonatal effects after a remifentanil infusion during cesarean delivery.
Comparison with Other Forms of Labor Analgesia
The efficacy of remifentanil PCA has been compared with that of other labor analgesic agents and regimens (Table 22-5).
TABLE 22-5
Trials Comparing Remifentanil Patient-Controlled Analgesia with Alternative Labor Analgesia
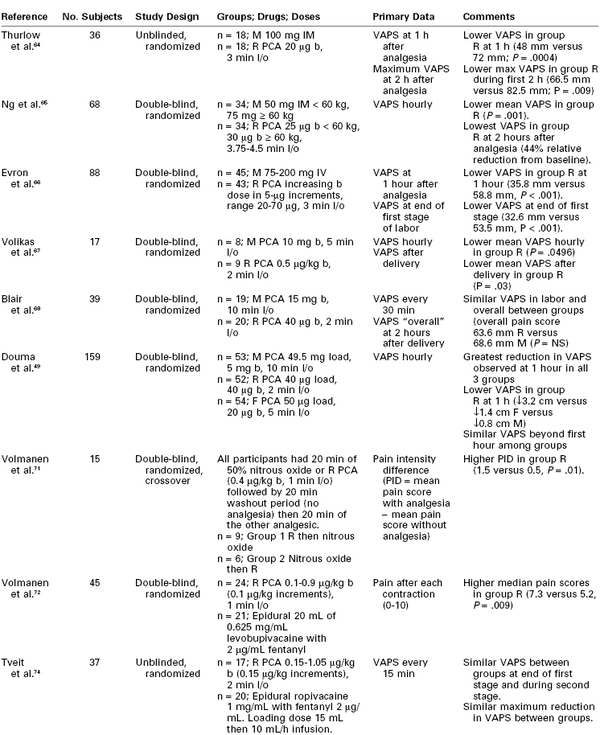
VAPS, Visual analog pain score (0-100 mm); M, meperidine; R, remifentanil; F, fentanyl; b, bolus; l/o, lockout; NS, nonsignificant.
Remifentanil versus Meperidine.
Thurlow et al.64 conducted a randomized unblinded study comparing remifentanil PCA (bolus 20 µg, lockout interval 3 minutes) with intramuscular meperidine 100 mg. The remifentanil group experienced significantly lower pain scores (median maximum pain score 66.5/100 versus 82.5/100, P = .009) within the first 2 hours of commencing analgesia. Parturients in the remifentanil group experienced more sedation and episodes of SaO2 less than 94% but less nausea and vomiting. No significant difference in neonatal Apgar scores was found. Patient and midwife satisfaction were both higher in the remifentanil group.
Ng et al.65 conducted a randomized, double-blind study in which all 69 patients used a PCA device containing either remifentanil (PCA group) or 0.9% saline (meperidine group), and also received an intramuscular injection of either saline (PCA group) or meperidine (meperidine group). The doses administered depended on patient weight; women weighing less than 60 kg or 60 kg or more were given a bolus dose of remifentanil of 25 µg or 30 µg, respectively. Similarly, the doses of meperidine were either 50 mg or 75 mg. The PCA lockout interval was 3.75 to 4.5 minutes, and a background infusion was not used. Maternal analgesia was greater (particularly in the first 2 hours after initiation), the median time to first rescue analgesic request was longer (8.0 hours versus 4.9 hours), and maternal satisfaction scores were higher in the remifentanil PCA group than in the meperidine group. There were no differences between groups in maternal sedation, nausea, or SaO2. Neonatal outcomes were also similar.
Evron et al.66 compared increasing doses of remifentanil PCA (0.27 to 0.93 µg/kg) with intravenous meperidine. All women receiving remifentanil started with a bolus dose of 20 µg (lockout interval 3 minutes), which was increased in 5-µg increments until effective analgesia was obtained. The meperidine group received an infusion of 75 mg over 30 minutes, followed by an additional 50 mg on request up to a maximum dose of 200 mg. Remifentanil was associated with significantly lower pain scores and higher satisfaction scores than meperidine. There was also a lower incidence of maternal sedation and oxyhemoglobin desaturation with remifentanil.
Several studies have compared the efficacy of remifentanil PCA versus meperidine PCA. In a study that compared remifentanil PCA (bolus 0.5 µg/kg, lockout interval 2 minutes) and meperidine PCA (bolus 10 mg, lockout interval 5 minutes), Volikas et al.67 terminated the study after enrollment of 17 subjects, owing to significantly lower Apgar scores in the meperidine group. The limited data indicated that women who received remifentanil had better analgesia than those who received meperidine. Blair et al.68 also observed that the use of remifentanil PCA (bolus 40 µg, lockout interval 2 minutes) resulted in higher maternal satisfaction scores, despite similar pain scores, when compared with meperidine PCA (bolus 15 mg, lockout interval 10 minutes). Despite similar Apgar scores, neonatal NACS at 30 minutes after delivery were significantly lower in the meperidine group.
Douma et al.49 compared remifentanil PCA (bolus 40 µg, lockout interval 2 minutes), fentanyl PCA (bolus 20 µg, lockout interval 5 minutes), and meperidine PCA (bolus 5 mg, lockout interval 10 minutes). All women received an initial bolus of their allocated drug before commencing PCA. The remifentanil group experienced the greatest decrease in pain scores; however, the effect was transient and pain scores returned toward baseline within 3 hours. Sedation and pruritus, as well as overall satisfaction, were highest in the remifentanil group. The parturients receiving meperidine had the highest crossover rate to epidural analgesia. There were no differences in neonatal outcome among groups.
In a systematic review of seven randomized controlled trials (n = 349 parturients), Leong et al.69 evaluated the administration of remifentanil versus meperidine provided through a variety of drug delivery methods (e.g., PCA, continuous infusion, intramuscular) for labor analgesia. Remifentanil was noted to reduce mean VAPS by 25 mm more than meperidine in the first hour. Conversion rates to epidural analgesia were less than 10% when using remifentanil. The evaluated studies were too small to make definitive conclusions regarding maternal side effects; however, the incidence of SaO2 less than 95% and bradypnea was similar between the two drugs. None of the studies demonstrated adverse neonatal outcomes with remifentanil. A meta-analysis concluded that women who received remifentanil PCA had lower mean pain scores after 1 hour, a lower crossover rate to epidural analgesia, and higher satisfaction scores than women who received meperidine.70
Remifentanil versus Nitrous Oxide.
Volmanen et al.71 performed a double-blind crossover trial comparing remifentanil (bolus 0.4 µg/kg, lockout interval 1 minute) with 50% nitrous oxide during the first stage of labor. The 20 patients used both analgesics in a random order for 20 minutes, with an intervening washout period of 20 minutes. Pain relief (although modest), maternal sedation, and patient satisfaction were greater in the remifentanil group. No difference in the incidence of FHR changes was observed between the two groups.
Remifentanil versus Epidural Analgesia.
In a randomized, double-blind trial, Volmanen et al.72 compared titrated remifentanil PCA (mean effective bolus 0.5 µg/kg [range 0.3 to 0.7 µg/kg], lockout interval 1 minute) with lumbar epidural analgesia (20 mL of levobupivacaine 0.0625% with fentanyl 2 µg/mL). All patients received both an epidural technique and PCA, using a saline infusion as the control. Mean cervical dilation at initiation of analgesia was 4 cm, and the study was conducted over 1 hour. Parturients receiving epidural analgesia had a more significant and rapid reduction in pain scores than those receiving remifentanil (10 minutes versus 40 minutes to reach the individual effective dose). Median pain scores were lower in the epidural group, but median “pain relief” scores were similar between the two groups. Sedation and low SaO2 were observed more often during remifentanil infusion. Women in the remifentanil group were given supplemental oxygen more often than those who received epidural analgesia; the need for supplemental oxygen was related to bolus doses of remifentanil of 0.5 µg/kg or greater. There was no difference between groups in neonatal outcomes. Although the investigators concluded that epidural analgesia is superior to remifentanil PCA, they also postulated that high maternal satisfaction with intravenous PCA may be the result of factors other than the degree of analgesia produced. Other studies appear to corroborate this suggestion.71,73,74
Tveit et al.74 conducted a similar study with remifentanil PCA (titrated bolus dose 0.3 to 1.05 µg/kg, lockout interval 2 minutes) compared with lumbar epidural analgesia (10 mL of ropivacaine 0.1% with fentanyl 2 µg/mL). Both treatments provided effective analgesia, and the two groups had similar pain scores at the end of the first stage and during the second stage of labor. Maternal satisfaction was similar, although the incidence of sedation, oxyhemoglobin desaturation (SaO2 < 92%), and the need for supplemental oxygen were higher with remifentanil.
Efficacy and Optimal Regimen
Remifentanil can be given as a PCA bolus, as a continuous infusion, or as a combination of the two. Although a number of studies have compared remifentanil to other opioids using fixed, nontitratable PCA doses, Volmanen et al.75 attempted to determine the minimum effective dose of remifentanil for labor analgesia by titration to effect. Using a starting bolus dose of 0.2 µg/kg, and dose increases of 0.2 µg/kg (lockout interval 1 minute) over a 1-hour study period, the median effective bolus dose was observed to be 0.4 µg/kg (range, 0.2 to 0.8 µg/kg). However, frequent episodes of SaO2 below 94% (in 10 of 17 subjects), maternal sedation, and reduced FHR variability were observed.
Starting with a remifentanil PCA bolus dose of 0.25 µg/kg (lockout interval 2 minutes) without a background infusion, Blair et al.76 titrated the bolus and infusion doses to a maximum of 1 µg/kg and 0.05 µg/kg/min, respectively. In 17 of 21 participants, satisfactory analgesia was achieved with a PCA bolus dose of 0.25 µg/kg or 0.5 µg/kg with no background infusion; adding a background infusion resulted in no further improvement of analgesia but an increased incidence of adverse effects.
D'Onofrio et al.77 conducted an observational study of 205 parturients in whom a continuous infusion of remifentanil was titrated (initial to maximum dose range, 0.025 to 0.15 µg/kg/min) with a goal of achieving pain scores less than or equal to 4 during contractions. Adequate analgesia was achieved within 30 minutes but required a median remifentanil infusion dose of 0.075 µg/kg/min. The SaO2 remained above 95% in all patients without supplemental oxygen, and there were no reported neonatal side effects.
Balki et al.78 compared the effect of a fixed remifentanil bolus dose with a titratable background infusion versus a fixed background infusion with a titratable bolus dose. Both groups started with a remifentanil bolus dose of 0.25 µg/kg (lockout interval 2 minutes) and a background infusion rate of 0.025 µg/kg/min. If analgesia was inadequate, either the background infusion or bolus dose was increased in a stepwise manner to a maximum of 0.1 µg/kg/min or 1 µg/kg, respectively. The mean pain scores, satisfaction scores, and cumulative remifentanil doses were similar in the two groups; only one patient eventually requested epidural analgesia. The incidence of maternal side effects was higher in the escalating bolus dose group, including drowsiness (100% versus 30%) and frequency of SaO2 less than 95% (60% versus 40%). There was no difference in the incidence of adverse neonatal effects. The investigators advocated the use of a titrated background infusion (range, 0.025 to 0.1 µg/kg/min) with a constant PCA bolus dose (0.25 µg/kg, lockout interval 2 minutes).
Balcioglu et al.79 compared two remifentanil background infusion rates (0.1 and 0.15 µg/kg/min) with a constant PCA bolus dose (15 µg, lockout interval 5 minutes). During the study period of 90 minutes, pain scores were significantly lower in the group receiving the higher infusion dose, but there was no difference between groups in patient satisfaction, FHR abnormalities, or neonatal Apgar scores.
Altogether, these studies suggest that fixed-dose remifentanil PCA protocols are less effective than titratable regimens, with the potential for low and high doses resulting in poor analgesia or adverse effects, respectively. Evidence is conflicting as to whether the use of a background infusion confers additional benefits, particularly given the greater risk for maternal sedation and respiratory depression; one parturient became apneic within 3 minutes of increasing a background infusion from 0.05 to 0.1 µg/kg/min.80 Furthermore, a continuous remifentanil infusion regimen may not take advantage of the drug's intrinsic onset and offset characteristics in targeting the episodic nature of uterine contraction pain.73
The analgesic benefit of remifentanil may be optimized by training parturients to press the PCA button with the first perception of a contraction, given that the peak analgesic effect occurs within 1 to 3 minutes.13 A novel delivery system (preemptive remifentanil analgesia modality [PRAM]) is in development; this system uses a mathematical analysis of the previous three contractions to deliver a bolus dose 45 seconds before the next predicted contraction to coordinate the peak action of remifentanil with uterine contractions.81
Side Effects
Remifentanil can cause significant respiratory depression through reductions in the ventilatory rate and tidal volume.13 Although the safety profile of remifentanil PCA in labor has been specifically evaluated, the data are conflicting.77,82 Volikas et al.82 investigated the maternal and neonatal effects of remifentanil PCA (bolus dose 0.5 µg/kg, lockout interval 2 minutes) in 50 women. Effective analgesia was reported in 86% of study participants, and 44% experienced slight drowsiness (but were arousable to voice and maintained SaO2 > 93%). Mild itching and FHR changes occurred in the first 20 minutes of remifentanil PCA but did not require treatment. Umbilical cord blood gas measurements and neonatal Apgar scores and neurologic examinations were all within normal limits.
Several theories have attempted to explain the relatively high incidence of sedation and respiratory depression with the use of remifentanil PCA in laboring women. Despite the rapid onset of remifentanil, administration of a bolus dose of remifentanil may result in an onset of analgesia after the cessation of uterine contractions (which have an average duration of 60 to 70 seconds). Thurlow et al.64 have encouraged parturients to request a bolus dose at the very first detection of a contraction. Some investigators have suggested that administration of a PCA bolus between contractions might reduce the risk for sedation and improve the efficacy of analgesia; however, a report by Volmanen et al.83 does not support this hypothesis. After administration of a remifentanil bolus, the onset of electroencephalographic depression occurs before the onset of respiratory depression and the peak respiratory depression occurs approximately 2.5 minutes after bolus injection.84
Studies of remifentanil PCA during labor have reported a wide range in the incidence of nausea (0% to 60%).77,78 Pruritus occurs in approximately 16% of parturients.49 Nausea and emesis can occur through an opioid-induced increase in vagal activity, which may result in a decrease in mean arterial pressure and heart rate; however, this has not been reported in laboring women receiving remifentanil PCA, perhaps reflecting the doses administered and/or the high maternal sympathetic activity during labor.
Maternal administration of remifentanil PCA during labor appears to have minimal effect on FHR abnormalities, umbilical cord blood gas measurements, and Apgar scores.73 Comparison studies have reported a lower incidence of FHR abnormalities with remifentanil than with meperidine.68 Neonatal depression has been observed after general anesthesia that includes remifentanil; however, any contribution from remifentanil is likely minor given its rapid neonatal metabolism.73 This last characteristic has led to the use of remifentanil for sedation in neonatal intensive care units.
Remifentanil is the most commonly used opioid for PCA during labor in the United Kingdom.46 However, it is not specifically approved for this use in the United Kingdom or the United States, and strict adherence to local guidelines is required for safe practice (Box 22-1). Labor nurses and midwives should undergo a period of training and supervised practice with remifentanil PCA until they are deemed competent. Women must not have received other opioids in the previous 4 hours and should be fully informed of the potential side effects, including the possibility of requiring supplemental oxygen and needing increased levels of monitoring. Continuous pulse oximetry is advocated, and supplemental oxygen should be readily available and administered if SaO2 consistently falls below 95% (see Box 22-1). The PCA infusion should be given via a dedicated intravenous catheter and infusion set with anti-syphon valves. Sedation scores and respiratory rate should be recorded every 30 minutes, and orders should specify clear triggers for contacting the anesthesia service. Continuous FHR monitoring is essential.
In summary, the analgesic efficacy of remifentanil, particularly in early labor, has been demonstrated. Optimal labor analgesia may require titration of remifentanil PCA with labor progression; however, clinicians should remain vigilant for the common side effects (i.e., maternal sedation, respiratory depression) and comply with local protocols to ensure safe drug use. To date, the studies of remifentanil administration for labor analgesia have included only healthy parturients with low-risk singleton pregnancies. Therefore, the information may not be applicable to all laboring women, and analgesia must be administered on an individual patient basis.
Opioid Antagonists
Naloxone is a pure opioid antagonist at the µ-, κ-, and δ-opioid receptors, although it has the greatest affinity for the µ-opioid receptor.10,13 It is the drug of choice to treat adverse opioid effects in both the mother and the newborn, and it may be given intravenously, subcutaneously, or intramuscularly. The onset of action after an intravenous dose (1 to 4 µg/kg) is 2 minutes, with a duration of action of 30 to 40 minutes; this duration may be less than that of the opioid whose action it antagonizes, and repeated doses or an infusion may be necessary.
The administration of naloxone during labor or before delivery may reverse the quality of analgesia and confer only a limited reduction in maternal side effects; however, some neonatal benefit may be obtained. Hodgkinson et al.85 reported significantly higher neurobehavioral scores in neonates born to mothers who had received an intrapartum combination of meperidine and naloxone compared with meperidine alone. However, this difference did not persist beyond 2 hours after birth. Clark et al.86 reported minimal differences in the neurologic and acid-base status of neonates born to mothers who had received both meperidine and naloxone compared with no-analgesia controls, although there was some evidence that high-dose naloxone may have resulted in beneficial neonatal effects.86 When neonatal depression is anticipated due to maternal opioid administration, it is best to administer naloxone directly to the infant. Naloxone reverses neonatal respiratory depression by increasing both minute ventilation and the gradient of the CO2 response curve.
Studies have evaluated prophylactic neonatal naloxone administration immediately after delivery of infants whose mothers received opioids during labor. When compared with saline administration, Weiner et al.87 observed that intravenous naloxone resulted in a short-lived (30 minutes) improvement in neurobehavioral scores, whereas intramuscular naloxone resulted in similar improvements for the duration of the study period (48 hours).
The recommended neonatal dose of naloxone is 0.1 μg/kg (1 μg/mL solution). Administration of naloxone is not recommended during the primary steps of neonatal resuscitation; however, it may be given after positive-pressure ventilation has restored normal heart rate and SaO2, if maternal opioid administration occurred during the 4 hours before delivery.88 The preferred route of naloxone administration is intravenous; intramuscular administration is acceptable, although absorption may be delayed with this route. Endotracheal administration of naloxone is not recommended. Naloxone should not be given to the neonate of a mother who is opioid dependent or on methadone maintenance therapy; this action may result in withdrawal activity and seizures.88
Opioid Adjuncts and Sedatives
Historically, many drugs have been used as adjuncts to parenteral opioid analgesia. Most of them cause maternal sedation and neonatal depression and are now used infrequently, particularly because neuraxial and opioid PCA techniques achieve satisfactory analgesia more safely.
Barbiturates are sedative agents with no analgesic effect. They are lipid soluble, rapidly cross the placenta, are detectable in fetal blood, and can result in neonatal depression, especially if combined with systemic opioid administration.
Phenothiazines (e.g., chlorpromazine, promethazine, propiomazine) are dopamine antagonists that have sedative, antiemetic, and antipsychotic properties. They rapidly cross the placenta and reduce FHR variability. Neurobehavioral outcomes after the maternal administration of these agents have not been studied carefully, but there is no evidence that they cause neonatal respiratory depression. Phenothiazines (particularly chlorpromazine) may cause hypotension from alpha-adrenergic receptor blockade, and they may produce unwanted extrapyramidal movements.13 Parenterally administered promethazine (25 to 50 mg) has an onset of 15 minutes and a duration of action of up to 20 hours; it rapidly crosses the placenta, resulting in detectable fetal levels within 1 to 2 minutes of maternal intravenous administration.13 Propiomazine is a mild respiratory depressant that may further depress maternal ventilation when co-administered with opioids. It has a faster onset and shorter duration of action than promethazine.
Metoclopramide is a procainamide derivative that can increase gastric motility and reduce nausea and vomiting. As an antagonist at central dopamine receptors, it can also cause drowsiness.13 After meperidine administration for labor analgesia, Vella et al.89 found metoclopramide as effective as promethazine for reducing the incidence of nausea and vomiting. Reduced pain scores and nitrous oxide use were observed in women who received metoclopramide compared with those who received promethazine or placebo; this may reflect either an antianalgesic effect of promethazine or a possible analgesic effect of metoclopramide.
Benzodiazepines (e.g., diazepam, lorazepam, midazolam) have been used for sedation in labor but are associated with significant side effects. Diazepam rapidly crosses the placenta and accumulates in the fetus at concentrations that may exceed maternal concentrations. The elimination half-life of the parent drug is 24 to 48 hours, but active metabolites may persist for up to 120 hours. Diazepam may cause maternal and neonatal respiratory depression, as well as neonatal hypotonicity, impaired thermoregulation, and an abnormal stress response. These effects may be dose related. Lorazepam has a half-life of 12 hours and is metabolized to an inactive glucuronide. McAuley et al.90 gave lorazepam 2 mg or placebo prior to the intramuscular administration of meperidine 100 mg for labor analgesia. Analgesia was better in the lorazepam group, but lorazepam administration was associated with a nonsignificant increase in neonatal respiratory depression. Neonatal neurobehavioral scores were similar in the two groups. Amnesia was common with lorazepam.
Midazolam has a rapid onset of action and an elimination half-life of 1 to 4 hours.13 It is metabolized in the liver to one major and several minor pharmacologically active compounds, which may persist in patients with critical illnesses accompanied by hepatic and/or renal impairment. Midazolam readily crosses the placenta and when used at high doses (e.g., induction of general anesthesia) can result in neonatal hypotonia. Midazolam causes potent anterograde amnesia, a characteristic that may be undesirable for the childbirth experience.
Ketamine is a phencyclidine derivative that acts as a noncompetitive antagonist at the NMDA receptor and, at high doses, as an agonist at µ-opioid receptors. Most commonly given by intravenous or intramuscular injection, ketamine in small doses (0.2 to 0.5 mg/kg intravenously) can provide dissociative analgesia whereas larger doses (1 to 2 mg/kg intravenously, 5 to 10 mg/kg intramuscularly) can be used to induce general anesthesia.
When given intravenously, ketamine has an onset within 30 seconds and a duration of action of 5 to 10 minutes; intramuscular administration has an onset of 2 to 8 minutes with a duration of 10 to 20 minutes.13 Ketamine is hepatically metabolized to active metabolites, which are excreted in the urine.
Ketamine's sympathomimetic properties cause an increase in heart rate, systolic pressure, and cardiac output, which should be avoided in preeclamptic and hypertensive patients; however, these effects may be valuable during the induction of anesthesia in hypovolemic patients. In addition, given its bronchodilatory effects, ketamine has long been considered the intravenous induction agent of choice for asthmatic subjects.
Ketamine may be used for labor analgesia. Joselyn et al.91 reported acceptable labor analgesia with an intravenous infusion of ketamine (bolus 0.1 mg/kg with an infusion of 0.2 mg/kg/h, titrated to effect). The average ketamine infusion rate was 0.17 mg/kg/h, yielding an average total dose of 57 mg (range, 18 to 160 mg). No unpleasant hallucinations were experienced; however, with the initial dose, emesis and transient light-headedness and nystagmus occurred. All neonates had a 5-minute Apgar score of 9 or 10.
Ketamine may also provide effective analgesia just before vaginal delivery in parturients without neuraxial anesthesia, or it may be used as an adjunctive agent in parturients with unsatisfactory neuraxial analgesia/anesthesia. Using incremental doses of intravenous ketamine (0.2 to 0.4 mg/kg, up to a maximum dose of 100 mg) immediately before delivery, Akamatsu et al.92 reported that 78 of 80 women experienced complete analgesia with no adverse maternal or neonatal effects. The occurrence of amnesia and a dreamlike state was high, but only one woman found this unpleasant.
Administration of small doses of ketamine (10- to 20-mg doses, repeated at intervals of 2 to 5 minutes, while not exceeding a total dose of 1 mg/kg during a 30-minute period) is associated with a low incidence of maternal hallucinations; however, amnesia is common. In these settings, the anesthesia provider must maintain continual verbal contact with the patient and must ensure that the patient remains sufficiently awake to maintain adequate ventilation and protect her airway.
Inhalational Analgesia
The use of inhalational analgesia for labor varies by country. Although many of the anesthetic agents used in surgery have been administered for pain relief during childbirth, only nitrous oxide has achieved wide clinical use.
Nitrous Oxide
Globally, nitrous oxide is the most common inhalational agent used for labor analgesia.93 Typically it is administered as 50% nitrous oxide in oxygen using a blender device (e.g., Nitronox in the United States) or premixed in a single cylinder (e.g., Entonox in the United Kingdom). The availability and use of nitrous oxide for labor analgesia varies greatly between countries; when provided alone or in combination with other forms of analgesia, the incidence of its use during labor is 1% (or less) in the United States, 43% in Canada, and 62% in the United Kingdom.93
The mechanism of action of nitrous oxide is not fully understood, although it is believed to enhance the release of endogenous opioid peptides in the midbrain and modulate descending spinal pain pathways.94 Because of its low solubility, it has a very rapid onset and offset, and it undergoes minimal metabolism. Although nitrous oxide has limited cardiovascular effects, it can cause depression of ventilation through a reduction in tidal volume; partial compensation of this ventilatory effect is achieved by an increase in respiratory rate. Nitrous oxide is nonirritating to the airway and does not interfere with uterine activity.
Nitrous oxide readily crosses the placenta, and a fetal-to-maternal concentration ratio of 0.8 occurs within 15 minutes; however, no apparent detrimental effects on FHR, Apgar scores, or umbilical cord blood gas measurements have been reported.94 Even when used immediately prior to delivery, there is no evidence that nitrous oxide causes neonatal respiratory depression or altered neurobehavioral scores. The neonate rapidly eliminates nitrous oxide by respiration, resulting in a half-life of less than 3 minutes.94
A systematic review of 16 studies concluded that nitrous oxide is not a potent labor analgesic, but it appears to confer benefit and high levels of satisfaction for some women.94 In one observational study in laboring women, nitrous oxide was rated as being more effective than systemic meperidine, although it was less effective than epidural analgesia.95
To achieve optimal analgesia from nitrous oxide, inhalation should ideally begin in anticipation of the next contraction; however, the timing of uterine contractions is not always predictable. Therefore the patient is encouraged to breathe the mixture of nitrous oxide in oxygen from the very beginning of the contraction until the end of the contraction.
Although a low incidence of serious adverse events (3 per 10,000 administrations of 50% nitrous oxide in oxygen) has been reported, suitable equipment must be available to ensure the safe administration of nitrous oxide. An apparatus that limits the concentration of nitrous oxide (e.g., a nitrous oxide/oxygen blender or a premixed 1 : 1 cylinder) is required and should be checked periodically for correct delivery concentrations. The constituent gases separate at approximately 7° C (19.4 F), which is not usually of practical concern.13 Inhalation may occur through a mask or mouthpiece containing a one-way demand valve, which opens only when negative inspiratory pressure is applied. This is a safety feature that limits gas delivery in a drowsy patient, and it also helps limit pollution of the environment with unscavenged gases.
Environmental pollution from unscavenged gases may be significant, and it remains unclear whether regular occupational exposure to subanesthetic concentrations of nitrous oxide results in significant health risks for health care workers. Overall, epidemiologic data do not suggest the presence of higher reproductive risks in health care workers exposed to nitrous oxide in the work environment (see Chapter 17).
The most common side effects of nitrous oxide are nausea and vomiting (occurring in up to 33% of parturients), drowsiness, dizziness, and the presence of paresthesias, which may be related to maternal hyperventilation during contractions.94 There is a potential risk for oxyhemoglobin desaturation due to diffusion hypoxia, although this appears to occur very rarely. It is unclear whether the incidence of intrapartum maternal hypoxemia differs among women who use nitrous oxide when compared with women who receive no analgesia during labor. However, the risk for maternal hypoxemia with nitrous oxide may be more common with the concomitant administration of opioids or other sedatives96; the entire obstetric care team should be aware of this possibility.
Although the intrapartum use of nitrous oxide has plateaued or slightly declined in the United Kingdom, some anesthesiologists, obstetricians, and midwives in the United States have expressed a renewed interest in the use of nitrous oxide for intrapartum analgesia.93,97 Nitrous oxide clearly does not provide analgesia comparable with that provided by neuraxial techniques, but it has other benefits, including its lower cost and “less invasive nature.”97 In 2012, the United States Agency for Healthcare Research and Quality (AHRQ)97 published a review of the effectiveness of nitrous oxide for analgesia during labor. The report included a systematic review of 58 publications. The authors concluded that inhalation of nitrous oxide provides less effective intrapartum pain relief than epidural analgesia, but the quality of the published studies was predominantly poor. Apgar scores in newborn infants whose mothers used nitrous oxide were similar to those of newborns whose mothers used other methods of analgesia or received no analgesia. The report concluded that additional research is needed to address all of the following key questions: efficacy, patient satisfaction, effect on the route of delivery, adverse effects, and health system factors affecting the use of nitrous oxide.97
Volatile Halogenated Agents
All volatile halogenated agents cause dose-dependent relaxation of uterine smooth muscle. Yoo et al.98 observed that the minimum alveolar concentration (MAC) of volatile agents required to decrease the spontaneous myometrial contractility of isolated uterine muscle from pregnant women by 50% (ED50) was similar for halothane, sevoflurane, and desflurane (1.72, 1.44, and 1.66 MAC, respectively). Other in vitro studies demonstrated that the ED50 for sevoflurane varied from 0.8 to 1.0 MAC and that the ED50 for desflurane varied from 0.9 to 1.4 MAC.99,100 In contrast, Yoo et al.98 observed that the ED50 of isoflurane was significantly higher (2.35 MAC) than that for sevoflurane, desflurane, and halothane; an additional unique feature observed with isoflurane was its ability to modulate KATP channels.98 Whether these findings suggest that isoflurane is less likely to be associated with uterine atony in clinical practice requires further study. In general, when uterine tone is desirable (e.g., after delivery), volatile anesthetic concentrations higher than 0.5 MAC are not recommended and intravenous oxytocin should be administered concurrently.
A number of studies have examined the use of volatile agents for labor analgesia; most of these investigations used special breathing equipment and did not fully address the issue of unscavenged gases, which may limit their clinical application.
Enflurane
Abboud et al.101 compared the analgesic effects of 0.25% to 1.25% enflurane with the administration of 30% to 60% nitrous oxide, both given in oxygen during the second stage of labor in 105 women. Satisfactory pain relief was reported in approximately 89% of women in the enflurane group and 76% of women in the nitrous oxide group; this outcome was achieved most frequently with the use of 0.5% enflurane and 40% nitrous oxide, respectively. Amnesia rates were less than 10% in both groups, and there were no differences observed in maternal blood loss, Apgar scores, or umbilical cord blood gas measurements. The mean umbilical cord concentrations of fluoride ions in the enflurane group were below that associated with nephrotoxicity. A subsequent study that compared administration of 1% enflurane in air to 50% nitrous oxide in oxygen during the first stage of labor observed lower pain scores, but higher levels of drowsiness, with enflurane.102
Isoflurane
McLeod et al.103 observed improved labor analgesia with the self-administration of 0.75% isoflurane in oxygen compared with Entonox. Subsequent studies observed satisfactory pain relief with minimal levels of drowsiness with various concentrations of an isoflurane-Entonox mixture.104,105 Ross et al.106 evaluated the use of a 0.25% isoflurane-Entonox mixture in 221 parturients in whom Entonox alone provided inadequate analgesia. No mothers became unduly sedated or lost consciousness, and there was no adverse effect on Apgar scores, neonatal respiratory status, or maternal blood loss. The requirement for neonatal resuscitation was greater in mothers who had received systemic opioids within 5 hours of birth in addition to the inhaled mixture. The same investigators verified clinically acceptable performance and safety of premixed isoflurane-Entonox cylinders.107
Desflurane
Because of its low blood-gas partition coefficient, desflurane has a rapid onset and offset; however, it is also highly irritating to the airway, making it a less attractive option for inhalation analgesia. Abboud et al.108 compared inhalation of 1% to 4.5% desflurane versus 30% to 60% nitrous oxide, both in oxygen, and found similar analgesia scores and neonatal outcomes between groups. The incidence of amnesia was significantly greater with desflurane than with nitrous oxide (23% versus 0%).
Sevoflurane
Sevoflurane is the volatile halogenated agent most commonly used for inhalation induction of general anesthesia. It has a short onset and offset of action, but it is less irritating and has a less unpleasant odor than the other volatile agents.
Toscano et al.109 conducted a safety and feasibility pilot study of 50 parturients breathing 2% to 3% sevoflurane in an oxygen/air mixture via a compact anesthesia delivery system. They aimed for an end-tidal sevoflurane concentration of 1.2% to 1.4% (0.8 to 0.9 MAC during pregnancy) at the peak of uterine contractions. The mean (± SD) pain score was significantly lower during the times when parturients inhaled sevoflurane compared with times when they did not inhale sevoflurane (3.3 ± 1.5 versus 8.7 ± 1.0, respectively, on a scale of 0 to 10). Four women became drowsy, but there were no episodes of oxyhemoglobin desaturation or loss of consciousness, and there were no unexpected increases in blood loss. In addition, there were no reported adverse effects on FHR or neonatal Apgar scores.
In an escalating dose study, Yeo et al.110 determined that 0.8% sevoflurane was the optimal concentration for labor analgesia, beyond which an increased level of sedation with no additional analgesic benefit was observed. The same investigators subsequently compared 0.8% sevoflurane with Entonox in 32 parturients, using a double-crossover study design.111 Median with interquartile range (IQR) pain relief scores (100 mm scale) were significantly higher for sevoflurane (67 mm, 55 to 74 mm IQR) than Entonox (51 mm, 41 to 70 mm IQR). There was an increased incidence of nausea and vomiting in the Entonox group, while sedation was increased in the sevoflurane group. The investigators observed no other adverse events, such as SaO2 less than 98%, apnea, or changes in end-tidal CO2, although two subjects could not tolerate the odor of sevoflurane. Five parturients requested epidural analgesia during the Entonox phase. All 29 women who completed the study preferred sevoflurane. The investigators concluded that self-administered sevoflurane can provide useful labor analgesia and is superior to Entonox.
Routine use of inhalational labor analgesia may be limited by the need for specialized equipment, concern for environmental pollution, and the potential for maternal amnesia and the loss of protective airway reflexes. Although sedation occurs during intermittent inhalation of volatile anesthetic agents, profound sedation to the extent that airway reflexes are jeopardized has not been reported. Further research and refinement of inhalational labor analgesia may allow the use of this technique for women in whom neuraxial anesthesia is contraindicated.
References
1. Bucklin BA, Hawkins JL, Anderson JR, Ullrich FA. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005;103:645–653.
2. Canadian Institute of Health Information. Highlights of 2010-11: Selected indicators describing the birthing process in Canada. [Available at] http://www.cihi.ca [Accessed March 2013] .
3. NHS Maternity Statistics. The Information Centre for Health and Social Care. www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=1815; 2011 [Available at http://www.hscic.gov.uk/pubs/maternity1011. Accessed March 2013] .
4. Reynolds F, Sharma SK, Seed PT. Analgesia in labour and fetal acid-base balance: a meta-analysis comparing epidural with systemic opioid analgesia. BJOG. 2002;109:1344–1353.
5. Halpern SH, Muir H, Breen TW, et al. A multicenter randomized controlled trial comparing patient-controlled epidural with intravenous analgesia for pain relief in labor. Anesth Analg. 2004;99:1532–1538.
6. Tuckey JP, Prout RE, Wee MY. Prescribing intramuscular opioids for labour analgesia in consultant-led maternity units: a survey of UK practice. Int J Obstet Anesth. 2008;17:3–8.
7. Reynolds F. Labour analgesia and the baby: good news is no news. Int J Obstet Anesth. 2011;20:38–50.
8. Sosa CG, Balaguer E, Alonso JG, et al. Meperidine for dystocia during the first stage of labor: a randomized controlled trial. Am J Obstet Gynecol. 2004;191:1212–1218.
9. Nissen E, Widstrom AM, Lilja G, et al. Effects of routinely given pethidine during labour on infants’ developing breastfeeding behaviour: effects of dose-delivery time interval and various concentrations of pethidine/norpethidine in cord plasma. Acta Paediatr. 1997;86:201–208.
10. Peck TE, Hill SA, Williams M. Pharmacology for Anaesthesia and Intensive Care. 3rd edition. Cambridge University Press: Cambridge, UK; 2008.
11. Elbohoty AE, Abd-Elrazek H, Abd-El-Gawad M, et al. Intravenous infusion of paracetamol versus intravenous pethidine as an intrapartum analgesic in the first stage of labor. Int J Gynaecol Obstet. 2012;118:7–10.
12. Tsui MH, Ngan Kee WD, Ng FF, Lau TK. A double blinded randomised placebo-controlled study of intramuscular pethidine for pain relief in the first stage of labour. Br J Obstet Gynaecol. 2004;111:648–655.
13. Sasada M, Smith S. Drugs in Anaesthesia & Intensive Care. 3rd edition. Oxford University Press: Oxford, UK; 2003.
14. Way WL, Costley EC, Leongway E. Respiratory sensitivity of the newborn infant to meperidine and morphine. Clin Pharmacol Ther. 1965;6:454–461.
15. Gerdin E, Salmonson T, Lindberg B, Rane A. Maternal kinetics of morphine during labour. J Perinat Med. 1990;18:479–487.
16. Olofsson C, Ekblom A, Ekman-Ordeberg G, et al. Analgesic efficacy of intravenous morphine in labour pain: a reappraisal. Int J Obstet Anesth. 1996;5:176–180.
17. Olofsson C, Ekblom A, Ekman-Ordeberg G, et al. Lack of analgesic effect of systemically administered morphine or pethidine on labour pain. Br J Obstet Gynaecol. 1996;103:968–972.
18. Rawal N, Tomlinson AJ, Gibson GJ, Sheehan TM. Umbilical cord plasma concentrations of free morphine following single-dose diamorphine analgesia and their relationship to dose-delivery time interval, Apgar scores and neonatal respiration. Eur J Obstet Gynecol Reprod Biol. 2007;133:30–33.
19. Fairlie FM, Marshall L, Walker JJ, Elbourne D. Intramuscular opioids for maternal pain relief in labour: a randomised controlled trial comparing pethidine with diamorphine. Br J Obstet Gynaecol. 1999;106:1181–1187.
20. Craft JB, Coaldrake LA, Bolan JC, et al. Placental passage and uterine effects of fentanyl. Anesth Analg. 1983;62:894–898.
21. Rayburn W, Rathke A, Leuschen MP, et al. Fentanyl citrate analgesia during labor. Am J Obstet Gynecol. 1989;161:202–206.
22. Rayburn WF, Smith CV, Parriott JE, Woods RE. Randomized comparison of meperidine and fentanyl during labor. Obstet Gynecol. 1989;74:604–606.
23. Wilson CM, McClean E, Moore J, Dundee JW. A double-blind comparison of intramuscular pethidine and nalbuphine in labour. Anaesthesia. 1986;41:1207–1213.
24. Dan U, Rabinovici Y, Barkai G, et al. Intravenous pethidine and nalbuphine during labor: a prospective double-blind comparative study. Gynecol Obstet Invest. 1991;32:39–43.
25. Amin SM, Amr YM, Fathy SM, Alzeftawy AE. Maternal and neonatal effects of nalbuphine given immediately before induction of general anesthesia for elective cesarean section. Saudi J Anaesth. 2011;5:371–375.
26. Nicolle E, Devillier P, Delanoy B, et al. Therapeutic monitoring of nalbuphine: transplacental transfer and estimated pharmacokinetics in the neonate. Eur J Clin Pharmacol. 1996;49:485–489.
27. Giannina G, Guzman ER, Lai YL, et al. Comparison of the effects of meperidine and nalbuphine on intrapartum fetal heart rate tracings. Obstet Gynecol. 1995;86:441–445.
28. Kim TH, Kim JM, Lee HH, et al. Effect of nalbuphine hydrochloride on the active phase during first stage of labour: a pilot study. J Obstet Gynaecol. 2011;31:724–727.
29. Nagashima H, Karamanian A, Malovany R, et al. Respiratory and circulatory effects of intravenous butorphanol and morphine. Clin Pharmacol Ther. 1976;19:738–745.
30. Maduska AL, Hajghassemali M. A double-blind comparison of butorphanol and meperidine in labour: maternal pain relief and effect on the newborn. Can Anaesth Soc J. 1978;25:398–404.
31. Hodgkinson R, Huff RW, Hayashi RH, Husain FJ. Double-blind comparison of maternal analgesia and neonatal neurobehaviour following intravenous butorphanol and meperidine. J Int Med Res. 1979;7:224–230.
32. Quilligan EJ, Keegan KA, Donahue MJ. Double-blind comparison of intravenously injected butorphanol and meperidine in parturients. Int J Gynaecol Obstet. 1980;18:363–367.
33. Nelson KE, Eisenach JC. Intravenous butorphanol, meperidine, and their combination relieve pain and distress in women in labor. Anesthesiology. 2005;102:1008–1013.
34. Atkinson BD, Truitt LJ, Rayburn WF, et al. Double-blind comparison of intravenous butorphanol (Stadol) and fentanyl (Sublimaze) for analgesia during labor. Am J Obstet Gynecol. 1994;171:993–998.
35. Franklin RA, Frost T, Robson PJ, Jackson MB. Preliminary studies on the disposition of meptazinol in the neonate. Br J Clin Pharmacol. 1981;12:88–90.
36. Jackson MB, Robson PJ. Preliminary experience of the use of meptazinol as an obstetric analgesic. Br J Obstet Gynaecol. 1980;87:296–301.
37. Nicholas AD, Robson PJ. Double-blind comparison of meptazinol and pethidine in labour. Br J Obstet Gynaecol. 1982;89:318–322.
38. Morrison CE, Dutton D, Howie H, Gilmour H. Pethidine compared with meptazinol during labour: a prospective randomised double-blind study in 1100 patients. Anaesthesia. 1987;42:7–14.
39. de Boer FC, Shortland D, Simpson RL, et al. A comparison of the effects of maternally administered meptazinol and pethidine on neonatal acid-base status. Br J Obstet Gynaecol. 1987;94:256–261.
40. Mowat J, Garrey MM. Comparison of pentazocine and pethidine in labour. Br Med J. 1970;2:757–759.
41. Kuti O, Faponle AF, Adeyemi AB, Owolabi AT. Pain Relief in labour: a randomized controlled trial comparing pentazocine with Tramadol. Nep J Obstet Gynaecol. 2008;3:14–18.
42. Claahsen-van der Grinten HL, Verbruggen I, van den Berg PP, et al. Different pharmacokinetics of tramadol in mothers treated for labour pain and in their neonates. Eur J Clin Pharmacol. 2005;61:523–529.
43. Keskin HL, Keskin EA, Avsar AF, et al. Pethidine versus tramadol for pain relief during labor. Int J Gynaecol Obstet. 2003;82:11–16.
44. Viegas OA, Khaw B, Ratnam SS. Tramadol in labour pain in primiparous patients: a prospective comparative clinical trial. Eur J Obstet Gynecol Reprod Biol. 1993;49:131–135.
45. Khooshideh M, Shahriari A. A comparison of tramadol and pethidine analgesia on the duration of labour: a randomised clinical trial. Aust N Z J Obstet Gynaecol. 2009;49:59–63.
46. Saravanakumar K, Garstang JS, Hasan K. Intravenous patient-controlled analgesia for labour: a survey of UK practice. Int J Obstet Anesth. 2007;16:221–225.
47. McIntosh DG, Rayburn WF. Patient-controlled analgesia in obstetrics and gynecology. Obstet Gynecol. 1991;78:1129–1135.
48. Isenor L, Penny-MacGillivray T. Intravenous meperidine infusion for obstetric analgesia. J Obstet Gynecol Neonatal Nurs. 1993;22:349–356.
49. Douma MR, Verwey RA, Kam-Endtz CE, et al. Obstetric analgesia: a comparison of patient-controlled meperidine, remifentanil, and fentanyl in labour. Br J Anaesth. 2010;104:209–215.
50. McInnes RJ, Hillan E, Clark D, Gilmour H. Diamorphine for pain relief in labour: a randomised controlled trial comparing intramuscular injection and patient-controlled analgesia. Br J Obstet Gynaecol. 2004;111:1081–1089.
51. Nikkola EM, Ekblad UU, Kero PO, et al. Intravenous fentanyl PCA during labour. Can J Anaesth. 1997;44:1248–1255.
52. Rayburn WF, Smith CV, Leuschen MP, et al. Comparison of patient-controlled and nurse-administered analgesia using intravenous fentanyl during labor. Anesthesiol Rev. 1991;18:31–36.
53. Morley-Forster PK, Weberpals J. Neonatal effects of patient-controlled analgesia using fentanyl in labor. Int J Obstet Anesth. 1998;7:103–107.
54. Hosokawa Y, Morisaki H, Nakatsuka I, et al. Retrospective evaluation of intravenous fentanyl patient-controlled analgesia during labor. J Anesth. 2012;26:219–224.
55. Morley-Forster PK, Reid DW, Vandeberghe H. A comparison of patient-controlled analgesia fentanyl and alfentanil for labour analgesia. Can J Anaesth. 2000;47:113–119.
56. Erskine WA, Dick A, Morrell DF, et al. Self-administered intravenous analgesia during labour: a comparison between pentazocine and pethidine. S Afr Med J. 1985;67:764–767.
57. Long J, Yue Y. Patient controlled intravenous analgesia with tramadol for labor pain relief. Chinese Med J. 2003;116:1752–1755.
58. Podlas J, Breland BD. Patient-controlled analgesia with nalbuphine during labor. Obstet Gynecol. 1987;70:202–204.
59. Frank M, McAteer EJ, Cattermole R, et al. Nalbuphine for obstetric analgesia: a comparison of nalbuphine with pethidine for pain relief in labour when administered by patient-controlled analgesia (PCA). Anaesthesia. 1987;42:697–703.
60. Leppa M, Korvenoja A, Carlson S, et al. Acute opioid effects on human brain as revealed by functional magnetic resonance imaging. Neuroimage. 2006;31:661–669.
61. Glass SA, Hardman D, Kamiyama Y, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil (GI87084B). Anesth Analg. 1993;77:1031–1040.
62. Kan RE, Hughes SC, Rosen MA, et al. Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology. 1998;88:1467–1474.
63. Hinova A, Fernando R. Systemic remifentanil for labor analgesia. Anesth Analg.. 2009;109:1925–1929.
64. Thurlow JA, Laxton CH, Dick A, et al. Remifentanil by patient-controlled analgesia compared with intramuscular meperidine for pain relief in labour. Br J Anaesth. 2002;88:374–378.
65. Ng TK, Cheng BC, Chan WS, et al. A double-blind randomised comparison of intravenous patient-controlled remifentanil with intramuscular pethidine for labour analgesia. Anaesthesia. 2011;66:796–801.
66. Evron S, Glezerman M, Sadan O, et al. Remifentanil: a novel systemic analgesic for labor pain. Anesth Analg. 2005;100:233–238.
67. Volikas I, Male D. A comparison of pethidine and remifentanil patient-controlled analgesia in labour. Int J Obstet Anesth. 2001;10:86–90.
68. Blair JM, Dobson GT, Hill DA, et al. Patient controlled analgesia for labour: a comparison of remifentanil with pethidine. Anaesthesia. 2005;60:22–27.
69. Leong WL, Sng BL, Sia AT. A comparison between remifentanil and meperidine for labor analgesia: a systematic review. Anesth Analg. 2011;113:818–825.
70. Schnabel A, Hahn N, Jens Broscheit J, et al. Remifentanil for labour analgesia: a meta-analysis of randomised controlled trials. Eur J Anaesthesiol. 2012;29:177–185.
71. Volmanen P, Akural E, Raudaskoski T, et al. Comparison of remifentanil and nitrous oxide in labour analgesia. Acta Anaesthesiol Scand. 2005;49:453–458.
72. Volmanen P, Sarvela J, Akural EI, et al. Intravenous remifentanil vs. epidural levobupivacaine with fentanyl for pain relief in early labour: a randomised, controlled, double-blinded study. Acta Anaesthesiol Scand. 2008;52:249–255.
73. Hill D. Remifentanil in obstetrics. Curr Opin Anaesthesiol. 2008;21:270–274.
74. Tveit TO, Seiler S, Halvorsen A, Rosland JH. Labour analgesia: a randomised, controlled trial comparing intravenous remifentanil and epidural analgesia with ropivacaine and fentanyl. Eur J Anaesthesiol. 2012;29:129–136.
75. Volmanen P, Akural EI, Raudaskoski T, Alahuhta S. Remifentanil in obstetric analgesia: a dose-finding study. Anesth Analg. 2002;94:913–917.
76. Blair JM, Hill DA, Fee JP. Patient-controlled analgesia for labour using remifentanil: a feasibility study. Br J Anaesth. 2001;87:415–420.
77. D’Onofrio P, Novelli AM, Mecacci F, Scarselli G. The efficacy and safety of continuous intravenous administration of remifentanil for birth pain relief: an open study of 205 parturients. Anesth Analg. 2009;109:1922–1924.
78. Balki M, Kasodekar S, Dhumne S, et al. Remifentanil patient-controlled analgesia for labour: optimizing drug delivery regimens. Can J Anaesth. 2007;54:626–633.
79. Balcioglu O, Akin S, Demir S, Aribogan A. Patient-controlled intravenous analgesia with remifentanil in nulliparous subjects in labor. Expert Opin Pharmacother. 2007;8:3089–3096.
80. Waring J, Mahboobi SK, Tyagaraj K, et al. Use of remifentanil for labor analgesia: the good and the bad. Anesth Analg. 2007;104:1616–1617.
81. Birnbach DJ, et al. Preemptive remifentanil analgesia modality (PRAM): a new paradigm for intravenous labor analgesia. ASA abstract. October 2009.
82. Volikas I, Butwick A, Wilkinson C, et al. Maternal and neonatal side-effects of remifentanil patient-controlled analgesia in labour. Br J Anaesth. 2005;95:504–509.
83. Volmanen PV, Akural EI, Raudaskoski T, et al. Timing of intravenous patient-controlled remifentanil bolus during early labour. Acta Anaesthesiol Scand. 2011;55:486–494.
84. Babenco HD, Conard PF, Gross JB. The pharmacodynamic effect of a remifentanil bolus on ventilatory control. Anesthesiology. 2000;92:393–398.
85. Hodgkinson R, Bhatt M, Grewal G, Marx GF. Neonatal neurobehavior in the first 48 hours of life: effect of the administration of meperidine with and without naloxone in the mother. Pediatrics. 1978;62:294–298.
86. Clark RB, Beard AG, Greifenstein FE, Barclay DL. Naloxone in the parturient and her infant. South Med J. 1976;69:570–575.
87. Wiener PC, Hogg MI, Rosen M. Effects of naloxone on pethidine-induced neonatal depression. I. Intravenous naloxone. Br Med J. 1977;23:228–229.
88. American Academy of Pediatrics and American Heart Association. Neonatal Resuscitation. 6th edition. American Academy of Pediatrics: Elk Grove Village, IL; 2011.
89. Vella L, Francis D, Houlton P, Reynolds F. Comparison of the antiemetics metoclopramide and promethazine in labour. Br Med J (Clin Res Ed). 1985;290:1173–1175.
90. McAuley DM, O’Neill MP, Moore J, Dundee JW. Lorazepam premedication for labour. Br J Obstet Gynaecol. 1982;89:149–154.
91. Joselyn AS, Cherian VT, Joel S. Ketamine for labour analgesia. Int J Obstet Anesth. 2010;19:122–123.
92. Akamatsu TJ, Bonica JJ, Rehmet R, et al. Experiences with the use of ketamine for parturition. I. Primary anesthetic for vaginal delivery. Anesth Analg. 1974;53:284–287.
93. Rooks J. Nitrous oxide for pain in labor—why not in the United States? Birth. 2007;34:1.
94. Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol.. 2002;186:S110–S126.
95. Harrison RF, Shore M, Woods T, et al. A comparative study of transcutaneous electrical nerve stimulation (TENS), Entonox, pethidine + promazine and lumbar epidural for pain relief in labor. Acta Obstet Gynecol Scand. 1987;66:9–14.
96. Zelcer J, Owers H, Paull JD. A controlled oximetric evaluation of inhalational, opioid and epidural analgesia in labour. Anaesth Intensive Care. 1989;17:418–421.
97. Agency for Healthcare Research and Quality. Nitrous oxide for the management of labor pain. AHRQ Comparative Effectiveness Review No. 67. [AHRQ Publication No. 12-EHC071-EF, August, 2012. Available at] http://www.effectivehealthcare.ahrq.gov/ehc/products/260/1175/CER67_NitrousOxideLaborPain_FinalReport_20120817.pdf [Accessed March 12, 2013] .
98. Yoo KY, Lee JC, Yoon MH, et al. The effects of volatile anesthetics on spontaneous contractility of isolated human pregnant uterine muscle: a comparison among sevoflurane, desflurane, isoflurane, and halothane. Anesth Analg. 2006;103:443–447.
99. Yildiz K, Dogru K, Dalgic H, et al. Inhibitory effects of desflurane and sevoflurane on oxytocin-induced contractions of isolated pregnant human myometrium. Acta Anaesthesiol Scand. 2005;49:1355–1359.
100. Turner RJ, Lambros M, Kenway L, Gatt SP. The in-vitro effects of sevoflurane and desflurane on the contractility of pregnant human uterine muscle. Int J Obstet Anesth. 2002;11:246–251.
101. Abboud TK, Shnider SM, Wright RG, et al. Enflurane analgesia in obstetrics. Anesth Analg. 1981;60:133–137.
102. McGuinness C, Rosen M. Enflurane as an analgesic in labour. Anaesthesia. 1984;39:24–26.
103. McLeod DD, Ramayya GP, Tunstall ME. Self-administered isoflurane in labour: a comparative study with Entonox. Anaesthesia. 1985;40:424–426.
104. Arora S, Tunstall M, Ross J. Self-administered mixture of Entonox and isoflurane in labour. Int J Obstet Anesth. 1992;1:199–202.
105. Wee MY, Hasan MA, Thomas TA. Isoflurane in labour. Anaesthesia. 1993;48:369–372.
106. Ross JA, Tunstall ME, Campbell DM, Lemon JS. The use of 0.25% isoflurane premixed in 50% nitrous oxide and oxygen for pain relief in labour. Anaesthesia. 1999;54:1166–1172.
107. Ross JA, Tunstall ME. Simulated use of premixed 0.25% isoflurane in 50% nitrous oxide and 50% oxygen. Br J Anaesth. 2002;89:820–824.
108. Abboud TK, Swart F, Zhu J, et al. Desflurane analgesia for vaginal delivery. Acta Anaesthesiol Scand. 1995;39:259–261.
109. Toscano A, Pancaro C, Giovannoni S, et al. Sevoflurane analgesia in obstetrics: a pilot study. Int J Obstet Anesth. 2003;12:79–82.
110. Yeo ST, Holdcroft A, Yentis SM, Stewart A. Analgesia with sevoflurane during labour. I. Determination of the optimum concentration. Br J Anaesth. 2007;98:105–109.
111. Yeo ST, Holdcroft A, Yentis SM, et al. Analgesia with sevoflurane during labour. II. Sevoflurane compared with Entonox for labour analgesia. Br J Anaesth. 2007;98:110–115.