Ventricular preexcitation syndromes (e.g., Wolff-Parkinson-White syndrome) are rarely encountered in pregnancy; these patients are usually identified at a younger age and have undergone highly effective electrophysiologic treatment. A few case reports suggest that preexcitation syndromes may be associated with an increased rate of supraventricular arrhythmias during pregnancy.
Ventricular Arrhythmias
Ventricular arrhythmias are commonly associated with underlying structural heart disease. The diagnostic evaluation warrants a baseline ECG and echocardiography. During pregnancy, peripartum cardiomyopathy as cause of ventricular arrhythmias needs to be ruled out.
Idiopathic ventricular tachycardia is most commonly monomorphic, originating from the right ventricular outflow tract. New-onset idiopathic ventricular tachycardia during pregnancy has been reported in a very small series of patients. These reported arrhythmias were catecholamine sensitive and responsive to beta-adrenergic receptor antagonist therapy.149 Idiopathic ventricular tachycardia may also be sensitive to treatment with verapamil150 or isoproterenol.151 Polymorphic ventricular tachycardia152 and electrical storm with Brugada syndrome153 have also been reported during pregnancy.
Sudden cardiac death due to idiopathic ventricular tachycardia has been described in pregnant women with hypertrophic cardiomyopathy.154-156 Sustained idiopathic ventricular tachycardia, successfully treated with lidocaine infusion, has been described in patients with repaired tetralogy of Fallot.
Congenital Long QT Syndrome
The congenital long QT syndrome is caused by mutations in cardiac ion channels resulting in prolongation of ventricular repolarization. The clinical spectrum ranges from a lack of symptoms to arrhythmia-associated syncope and sudden cardiac death. Risk for cardiac events (syncope, arrhythmias, or death) is decreased in pregnant women with long QT syndrome, due at least in part to the increase in heart rate that occurs during pregnancy.157 Compared with the 40-week prepregnancy period, the 40-week postpartum period has been associated with an increased risk for ventricular tachycardia in women with long QT syndrome.158 Different long QT syndrome genotypes may have different risks associated with pregnancy. The LQT2 genotype is associated with a higher rate of cardiac events in the 9-month postpartum period than the LQT1 and LQT3 genotypes.157,159 Prophylactic treatment with a beta-adrenergic receptor antagonist is recommended during pregnancy and postpartum in patients with long QT syndrome.157,160
Antiarrhythmic Drugs
The risk for adverse fetal effects of antiarrhythmic drugs should be assessed on an individual basis (see Chapter 14). Beta-adrenergic receptor antagonists, amiodarone, and sotalol are effective in preventing idiopathic ventricular tachycardia during pregnancy. Fetal exposure to amiodarone (pregnancy category D) has been associated with hypothyroidism and, possibly, fetal growth restriction. Sotalol is classified as pregnancy category B and appears safe. Because sotalol is a beta-adrenergic receptor antagonist, its use may be associated with neonatal bradycardia and hypoglycemia.
Electric Cardioversion
Life-threatening or hemodynamically unstable arrhythmias should be terminated by electric cardioversion.146,160 Defibrillation refers to administration of electric energy to terminate ventricular fibrillation. By contrast, synchronized cardioversion is delivery of an electric shock synchronized to the QRS complex. Synchronized cardioversion is administered for supraventricular rhythms (i.e., atrial fibrillation, atrial flutter, atrial tachycardia) as well as for monomorphic ventricular tachycardia with a pulse.146 Pulseless ventricular tachycardia and polymorphic ventricular tachycardia should be treated with unsynchronized cardioversion.
Electric cardioversion can be performed safely throughout pregnancy without apparent adverse effects on fetal hemodynamic function.161,162 Nonetheless, it is prudent to monitor the fetal heart rate (FHR) during cardioversion.163 Current guidelines recommend the use of a biphasic defibrillator. Ventricular fibrillation can be successfully terminated with biphasic devices that use lower energy than is required with monophasic devices. Biphasic automatic external defibrillators (AEDs) are more effective in terminating ventricular fibrillation with lower energy than older monophasic devices.
If hemodynamically significant or severely symptomatic arrhythmias develop during pregnancy, electrophysiologic interventional management may be performed. Successful radiofrequency catheter-based ablation has been reported in pregnant women with no or minimal ionizing radiation exposure.
Patients usually require anesthesia care for cardioversion. The risk for pulmonary aspiration of gastric contents associated with sedation (with an unprotected airway) should be weighed against the risks associated with general anesthesia and tracheal intubation. The judicious use of sedation rather than general anesthesia is usually preferred. A benzodiazepine or propofol can provide satisfactory sedation and amnesia. Regardless of whether sedation or general anesthesia is selected, a nonparticulate oral antacid should be administered; administration of a histamine-2 (H2)-receptor antagonist to increase gastric pH should also be considered. The use of metoclopramide in these patients is controversial owing to its possible association with tachyarrhythmias.
Maintenance of Sinus Rhythm
Development of atrial fibrillation may cause significant hemodynamic compromise, particularly in pregnant women with stenotic valvular lesions (aortic stenosis or mitral stenosis) or hypertrophic cardiomyopathy with its associated diastolic dysfunction. Hemodynamic compromise results from the diminished diastolic filling time and loss of the atrial contraction contribution to ventricular filling. Maintenance of sinus rhythm and strict rate control have not been shown to be beneficial in older (nonpregnant) patients with paroxysmal or permanent atrial fibrillation. However, published studies are largely not applicable to pregnant women, and it seems reasonable to maintain sinus rhythm and control heart rate in this population. Rate control is particularly important in patients with stenotic valvular lesions and hypertrophic cardiomyopathy because the hemodynamic condition of these patients may quickly deteriorate in the presence of tachycardia.
Myocardial Infarction
The term myocardial infarction signifies myocardial cell death caused by ischemia.25 Myocardial infarction is diagnosed by clinical signs and symptoms, ECG patterns, elevation of biomarkers (CK-MB fraction, troponin), and various imaging modalities (echocardiography, radionuclide imaging, CMR imaging, CT). The current Universal Classification of Myocardial Infarction recognizes five types of myocardial infarction (Box 42-9).25
Myocardial infarction during pregnancy is rare; the estimated incidence is 2.8 to 6.2 per 100,000 deliveries.164,165 It occurs most commonly in the third trimester or the immediate postpartum period. Myocardial infarction during pregnancy is associated with maternal use of tobacco, dyslipidemia, family history of myocardial infarction, hypertension, African race, Hispanic ethnicity, and diabetes. Contemporary evidence suggests that the maternal mortality rate from myocardial infarction during pregnancy is approximately 5%.165
Acute coronary syndrome is an encompassing term used to describe unstable angina, non–ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI). Unstable angina is differentiated from NSTEMI by lack of elevation of cardiac biomarkers. Compared with unstable angina or NSTEMI, STEMI is an emergency and requires early reperfusion; a “door-to-balloon” interval of less than 90 minutes is optimal.
Only 40% of pregnant patients with myocardial infarction have evidence of coronary artery atherosclerosis (type I myocardial infarction); spontaneous coronary artery dissection (type II myocardial infarction) is observed in as many as 27% of patients. Angiographically normal coronary arteries are seen in 13% of patients166; in these cases, myocardial infarction likely results from coronary artery spasm or embolism. Spasm can be spontaneous, or it can be due to cocaine or ergot alkaloids. Septic and metastatic neoplastic coronary embolism after abortion have been described. Atherosclerosis is more commonly the cause of myocardial infarction in the antepartum period than in the peripartum or postpartum period, whereas coronary artery dissection is observed more frequently in the peripartum and postpartum periods.166 It has been hypothesized that the high rate of spontaneous coronary artery dissection is related to hormonal changes of pregnancy.
Cesarean delivery is commonly associated with ischemic-appearing ST-segment depression.14,167-170 These changes have been attributed, at least in part, to oxytocin administration (see earlier discussion).12,13 ST-segment elevation is not a normal occurrence during labor and delivery or with any type of intrapartum anesthesia. Hence, ST-segment elevation should always be considered abnormal, and ST-segment depression should be carefully evaluated.
CK-MB fraction may be elevated during normal pregnancy and labor (see Figure 47-1); thus, measurement of this enzyme is less useful for the diagnosis of myocardial infarction in pregnant women than in nonpregnant women.28 An elevated troponin level is much more specific, although troponin may be elevated in patients with gestational hypertension and preeclampsia/eclampsia.26,27 Both markers are quite sensitive for the diagnosis of myocardial infarction.
Percutaneous Coronary Intervention
STEMI during pregnancy should be treated with primary percutaneous coronary intervention. Although successfully used in anecdotal reports, use of thrombolytic therapy for STEMI in pregnant women should be reserved for rare instances. Radial arterial access for the percutaneous coronary intervention procedure is preferable because it has fewer bleeding complications and a lower mortality rate than femoral arterial access. Systemic anticoagulation with unfractionated heparin appears most reasonable owing to its short half-life, the availability of activated clotting time (ACT) monitoring, and the ability to rapidly reverse its anticoagulant effect. Importantly, larger doses of heparin are needed to achieve the desired level of anticoagulation in pregnant women than in nonpregnant patients. Anticoagulation for percutaneous coronary intervention can be achieved with low-molecular weight heparin, although inability to rapidly assess its anticoagulant effect and its altered pharmacokinetics during pregnancy are major disadvantages.
Percutaneous coronary interventions for treatment of myocardial infarction include conventional “plain old balloon angioplasty” and stent placement. An advantage of conventional balloon angioplasty is that it does not require dual antiplatelet therapy; however, placement of a stent is associated with a lower risk for abrupt vessel closure in the short term and a lower long-term risk for restenosis.
Dual antiplatelet therapy is mandatory after placement of a coronary artery stent to prevent stent thrombosis (Table 42-3). This therapy consists of both aspirin (81 to 325 mg daily) and clopidogrel. Clopidogrel can be substituted with a newer agent (e.g., prasugrel, ticagrelor) in specific nonpregnant patients. However, the safety and efficacy of these newer antiplatelet agents in pregnancy are unknown.
TABLE 42-3
Dual Antiplatelet Therapy Recommendations

* Acute coronary syndrome includes unstable angina, non–ST-segment elevation myocardial infarction, ST-segment elevation myocardial infarction.
† If necessary for the safe care of the obstetric patient, dual antiplatelet therapy can be discontinued 1 month after placement of a bare-metal stent.
Stent Type Choice
In patients with a nonacute coronary syndrome (e.g., chronic stable angina), bare-metal stents require a minimum of 1 month of dual antiplatelet therapy, whereas drug-eluting stents require a minimum of 12 months. When either bare-metal stents or drug-eluting stents are placed in a patient with any acute coronary syndrome (e.g., unstable angina, NSTEMI, STEMI), 12 months of dual antiplatelet therapy is recommended. The advantage of drug-eluting stents is a decreased risk for in-stent restenosis than occurs with bare-metal stents. Drug-eluting stents elute an antiproliferative agent (everolimus, sirolimus, paclitaxel) into the arterial wall and reduce the neointimal proliferation that causes in-stent restenosis. However, the stent strut endothelialization process is slower with drug-eluting stents than with bare-metal stents; thus, a longer duration of dual antiplatelet therapy is required to prevent stent thrombosis. Overall rates of death and myocardial infarction are similar with drug-eluting and bare-metal stents. The risk for stent thrombosis may be lower with contemporary drug-eluting stents than with bare-metal stents. Newer-generation drug-eluting stents may allow for earlier discontinuation of dual antiplatelet therapy.
Dual Antiplatelet Therapy
Bare-metal stents allow for discontinuation of dual antiplatelet therapy 1 month after percutaneous coronary intervention, if necessary, even in the setting of acute coronary syndrome (see Table 42-3). This may be advantageous in pregnant women who are at risk for intrapartum and postpartum hemorrhage. Therefore, most interventional cardiologists will place a bare-metal stent in a pregnant woman with acute coronary syndrome. Although it appears reasonable to continue dual antiplatelet therapy as long as feasible in a pregnant woman with acute coronary syndrome, there are little data on the optimal duration of dual antiplatelet therapy in this unique clinical setting. Continuing research is being performed to determine the optimal duration of dual antiplatelet therapy after percutaneous coronary intervention in the nonpregnant population.
It is not advisable to perform neuraxial anesthesia in patients receiving dual antiplatelet therapy.43 After clopidogrel is discontinued, aspirin should be continued indefinitely in patients with any type of stent. Both spinal and epidural anesthesia can be performed safely in patients receiving aspirin. Clopidogrel should be discontinued 7 days before performance of neuraxial anesthesia.43
Aspirin crosses the placenta. A 2002 meta-analysis found no overall increase in the risk for congenital malformations when aspirin was administered in the first trimester; however, a twofold increase in the risk for gastroschisis was observed.171 Another meta-analysis that evaluated the use of aspirin for prevention of preeclampsia found no evidence of increased risk for fetal growth restriction, pregnancy loss, or neonatal hemorrhage.172 Most evidence suggests that aspirin use is safe during pregnancy.173
Coronary Artery Anomalies
Coronary artery anomalies occur in approximately 1% of the general population. Coronary artery anomalies are frequently associated with congenital heart disease such as d-transposition of the great arteries and tetralogy of Fallot.
There are numerous types of coronary anomalies, and most are benign. The two most common clinically significant anomalies are (1) a left main coronary artery that originates from the right coronary cusp and (2) a right coronary artery that originates from the left coronary cusp. External mechanical compression of those anomalous coronary vessels may cause myocardial ischemia and eventually lead to arrhythmias. The resulting ischemia and arrhythmias have been associated with sudden cardiac death. The effect of the physiologic changes of pregnancy in women with coronary artery anomalies has not been well studied.
Valvular Heart Disease
Pregnancy and its associated changes in cardiovascular physiology present a unique clinical challenge to women with underlying valvular heart disease. The general management principles for pregnant women with valvular heart disease are directed toward specific hemodynamic goals and the need for anticoagulation in patients with a mechanical valve.
Aortic Stenosis
The most common cause of aortic stenosis in pregnant women is a congenital bicuspid aortic valve. Less common causes/types of aortic stenosis include rheumatic, supravalvular, and subvalvular aortic stenosis.174 Rheumatic aortic stenosis is invariably associated with some degree of mitral valve involvement. The hemodynamic implications of the various causes of aortic stenosis are similar. Calcific aortic stenosis of an anatomically normal tricuspid aortic valve occurs much later in life and is unlikely to be encountered in women of childbearing age.
Bicuspid aortic valve, the most common congenital heart defect, occurs in 0.5% to 2% of the population; women are affected four times less commonly than men. It is heritable; therefore, first-degree relatives of patients with a bicuspid aortic valve should be screened.54 Bicuspid aortic valve is associated with accelerated and premature valve stenosis as well as aortic valve regurgitation. Symptoms of aortic stenosis (dyspnea on exertion, chest pain, syncope) generally present in the third and fourth decades of life. Importantly, patients with a bicuspid aortic valve have underlying aortic root pathology in the media that is associated with aortic root dilation, thus predisposing to ascending root dissection.49
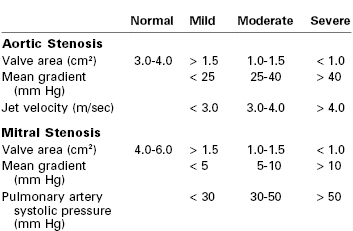
The normal aortic valve area is 3.0 to 4.0 cm2 (Table 42-4). There is no pressure gradient across a normal aortic valve with normal resting cardiac output. Patients generally develop symptoms when the valve area is less than 1.0 cm2 and the pressure gradient across the valve is greater than 40 mm Hg. The published definition of severe aortic stenosis varies. An aortic valve area less than 1.0 to 1.5 cm2 and a mean valve gradient of 25 to 50 mm Hg generally define the group of patients with a high risk for cardiovascular complications due to aortic stenosis.35,36,38,60
TABLE 42-4
Severity Classification of Valvular Lesions
Modified from Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 52:e1-142.
Classically, the aortic valve area is estimated invasively in the cardiac catheterization laboratory using the Gorlin equation. Forward cardiac output is determined by thermodilution and/or Fick methods, and left ventricular and aortic pressures are simultaneously recorded. Doppler velocity measurements during echocardiography allow noninvasive estimation of aortic valve area using the continuity equation.
Using both these methods, the estimated transvalvular gradient increases during pregnancy because of the physiologic increase in cardiac output. The calculated valve area remains unchanged, however, because both the Gorlin equation and the continuity equation take cardiac output into account. The echocardiographic dimensionless valve index is useful in pregnant women as well as other patients; it takes into account both left ventricular outflow velocity and the aortic velocity. Because both velocities are increased during pregnancy, the dimensionless index and estimated valve area remain unchanged during pregnancy.
The severity of aortic stenosis affects maternal risk during pregnancy. Mild and moderate aortic stenosis are associated with favorable pregnancy outcomes.174-176 Women with severe aortic stenosis experience frequent cardiac complications during pregnancy (e.g., worsening NYHA functional class, pulmonary edema, congestive heart failure, arrhythmias, hospitalization). The reported rates of these complications during pregnancy vary widely (e.g., heart failure and pulmonary edema 4% to 78%, arrhythmias 2% to 33%, hospitalization rate as high as 78%). Mortality is rare, however.174,175,177 Additionally, women with severe disease are more likely to require a cardiac intervention (balloon valvuloplasty, valve replacement) during pregnancy or immediately postpartum. Percutaneous balloon valvuloplasty of the aortic valve is a palliative procedure that allows completion of pregnancy before definitive repair.
Obstetric and Anesthetic Management
Labor and assisted vaginal delivery are preferred. Cesarean delivery is reserved for obstetric indications.
Whether general or neuraxial anesthesia is more appropriate for parturients with aortic stenosis has been a matter of debate.178-180 Historically, neuraxial anesthesia has been thought to be relatively contraindicated in patients with aortic stenosis; the simultaneous decrease in preload and afterload associated with neuraxial anesthesia may be particularly hazardous in these patients. However, case reports have documented successful administration of neuraxial anesthesia (spinal,181 continuous spinal,182 combined spinal-epidural,180,183 epidural184) and general anesthesia185,186 with favorable maternal and neonatal outcomes.
The choice between neuraxial and general anesthesia should not be made based on the aortic valve gradient or aortic valve area alone. The preanesthetic assessment should include physical examination, symptom evaluation, and comprehensive assessment of the right and left ventricular structure and function, as well as the structure and function of other cardiac valves. The presence or absence of pulmonary hypertension should also be determined.
Echocardiography offers indispensable information regarding left and right ventricular ejection fraction, left ventricular wall thickness, pulmonary artery pressure, presence of aortic insufficiency, mitral valvular structure and function, and aortic root size. Serial assessment of the aortic valve area before and during pregnancy is very helpful in managing these patients. Patients with normal right and left ventricular function are more likely to tolerate fluid shifts and the depressant effects of general anesthetic agents. If left ventricular dysfunction develops in the presence of aortic stenosis, a condition referred to as low-output, low-gradient aortic stenosis may be present.
The presence of pulmonary hypertension, right ventricular dysfunction, or mitral regurgitation is associated with greater dependence on preload and may unfavorably affect the hemodynamic response to neuraxial anesthesia. Patients with a dilated aortic root will likely benefit from gradual blood pressure changes, because aortic root dilation has been associated with aortic dissection. Left ventricular hypertrophy, frequently present with aortic stenosis, is associated with significant diastolic dysfunction and may impede left ventricular filling. The presence of left ventricular hypertrophy will render these patients more sensitive to the adverse effects of decreased preload, tachycardia, and the development of congestive heart failure, especially in the setting of acute-onset atrial fibrillation. Aortic regurgitation is quite common in patients with a bicuspid aortic value. The ventricles are exposed to both pressure and volume overload, further complicating hemodynamic management and response to anesthesia.
The goals of anesthetic management are (1) maintenance of a normal heart rate, sinus rhythm, and adequate systemic vascular resistance; (2) maintenance of intravascular volume and venous return; (3) avoidance of aortocaval compression; and (4) avoidance of myocardial depression during general anesthesia (see Table 42-2). In the absence of prospective randomized trials in this patient population, current clinical evidence suggests that either neuraxial analgesia/anesthesia or general anesthesia is safe for patients with mild or moderate aortic stenosis with normal right and left ventricular ejection fraction and the absence of other significant valvular lesions or pulmonary hypertension. Neuraxial anesthetic techniques that allow gradual titration of anesthesia seem advantageous in these patients.
In patients with severe aortic stenosis, general anesthesia remains the gold standard. Although published reports have described successful administration of neuraxial anesthesia for labor and vaginal and cesarean delivery in women with severe aortic stenosis, the influence of publication bias in these reports cannot be excluded. In contemporary obstetric anesthesiology practice, an opioid-based neuraxial labor analgesia technique, along with alternative forms of analgesia (e.g., pudendal nerve block, intravenous opioids) is well tolerated in parturients with severe aortic stenosis. A slowly dosed, low-concentration local anesthetic epidural technique, chosen to mitigate the untoward effects of sympathectomy, may be safely performed with vigilant blood pressure monitoring. The anesthesiologist may choose to avoid use of an epinephrine-containing local anesthetic solution because the unintentional intravenous injection of epinephrine can precipitate tachycardia; further, systemic absorption of epinephrine can diminish SVR and reduce venous return. General anesthesia may be the best choice for cesarean delivery in patients with severe aortic stenosis and other significant valvular lesions, pulmonary hypertension, and/or left ventricular dysfunction. Induction of anesthesia with a combination of etomidate and a moderate dose of a lipid-soluble opioid may be preferable to agents that cause myocardial depression and vasodilation (propofol, thiopental) and tachycardia (ketamine). Anesthesia can be maintained with an opioid and a low-dose volatile anesthetic technique.
Peripartum invasive arterial blood pressure monitoring is recommended for parturients with moderate and severe aortic stenosis. Pulmonary artery catheterization is unlikely to provide much clinical benefit in this patient population. Development of atrial fibrillation with rapid ventricular response is deleterious in these patients because it decreases diastolic filling time and eliminates the atrial component of left ventricular filling. If new-onset atrial fibrillation results in hypotension or pulmonary edema, sinus rhythm should be promptly restored.
Aortic Regurgitation
The most common etiology of chronic aortic regurgitation in pregnant women is a degenerated bicuspid aortic value; rheumatic aortic regurgitation occurs less frequently. Dilation of the ascending aorta and the resulting aortic leaflet separation may also result in aortic regurgitation. Most commonly, aortic root dilation results from cystic medial necrosis associated with Marfan syndrome, or it occurs in association with a bicuspid aortic value. Chronic aortic regurgitation is generally well tolerated during pregnancy, especially in patients with preserved left ventricular ejection fraction.187,188 The physiologic changes of pregnancy (increased heart rate resulting in a shorter duration of diastole, as well as reduced SVR) contribute to an overall reduction in regurgitant aortic flow.
Although patients with chronic aortic regurgitation can compensate for the hemodynamic stress over time, patients with acute aortic regurgitation are frequently very ill and may require surgery. Endocarditis is the most common etiology of acute aortic regurgitation during pregnancy. Ascending aortic dissection may also result in severe acute aortic regurgitation. Affected patients may require valve replacement during pregnancy.
Anesthetic Management
The goals of anesthetic management are (1) maintenance of a normal to slightly elevated heart rate, (2) prevention of an increase in SVR, (3) avoidance of aortocaval compression, and (4) avoidance of myocardial depression during general anesthesia (see Table 42-2).
Antepartum echocardiography may guide decisions regarding the choice of anesthesia and monitoring. Patients with chronic, compensated aortic insufficiency and normal ejection fraction tolerate the hemodynamic changes of pregnancy well; however, patients with left ventricular dysfunction will likely require careful assessment of volume status and pulmonary artery pressures during labor and delivery. Traditionally, a pulmonary artery catheter has been used for this purpose. In the future, transthoracic echocardiography will likely be used for this purpose.
The degree of aortic insufficiency, concomitant involvement of the mitral valve, and the size of the aortic root help define hemodynamic goals. Patients with a bicuspid aortic valve may have simultaneous aortic stenosis and aortic regurgitation. Both neuraxial analgesia/anesthesia and general anesthesia can be safely performed in patients with aortic regurgitation and preserved left ventricular ejection fraction. Severe aortic insufficiency with left ventricular dysfunction is not a contraindication for neuraxial anesthesia. Initiation of neuraxial analgesia during early labor may mitigate pain-associated increases in SVR that can be deleterious in patients with aortic regurgitation. Intra-aortic balloon pump placement is contraindicated in patients with aortic regurgitation because its use increases regurgitant flow.
Mitral Stenosis
Mitral stenosis frequently becomes symptomatic during pregnancy owing to the increase in maternal blood volume and heart rate. Increased blood volume with decreased diastolic filling time can result in pulmonary edema. Additionally, mitral stenosis predisposes patients to development of atrial tachyarrhythmias (atrial fibrillation, atrial flutter) as well as thromboembolic complications, with or without atrial arrhythmias. The underlying hypercoagulable state of pregnancy also increases the risk for thromboembolic complications in patients with mitral stenosis. Therefore, systemic anticoagulation is recommended for the duration of pregnancy and postpartum.
The etiology of mitral stenosis is almost invariably rheumatic.175,189 Severe mitral stenosis is defined as a valve area less than 1.0 cm2 (see Table 42-4). In addition to valve area, a mean value gradient of greater than 10 mm Hg and a mean PAP greater than 50 mm Hg are used to define severe mitral stenosis. Transmitral valve gradients measured by echocardiography increase during pregnancy.190
Poor functional status NYHA (e.g., III to IV) portends a greater risk for adverse outcomes.60,191,192 When possible, preconception treatment of symptomatic moderate or severe mitral stenosis is preferred.38,60 The procedure of choice is percutaneous balloon mitral valvuloplasty. Suitability for percutaneous balloon valvuloplasty is determined by an echocardiography-based scoring system, which takes into account valve calcification and mobility as well as valvular and subvalvular thickening.193
For patients who require percutaneous valvuloplasty during pregnancy, the procedure is ideally performed after 12 to 14 weeks' gestation to minimize fetal radiation exposure during the period of organogenesis. If the patient can be stabilized with medical management, delaying the procedure to 26 to 30 weeks' gestation will reduce the risk for preterm birth. Open surgical mitral valve commissurotomy is associated with higher rates of fetal mortality than percutaneous valvuloplasty (38% versus 5%).194
Standard medical therapy during pregnancy includes a reduction in activity level (e.g., bed rest), beta-adrenergic receptor antagonist and diuretic therapy, and anticoagulation.
Obstetric and Anesthetic Management
Cesarean delivery is typically reserved for obstetric indications. Vaginal delivery is usually assisted, because the Valsalva maneuver during the second stage of labor may result in a sudden increase in central venous pressure. Regardless of the method of delivery, patients are at risk for hemodynamic compromise and pulmonary edema immediately after delivery because uterine contraction results in autotransfusion.195 Therefore, these patients usually require postpartum intensive care.
Neuraxial analgesia/anesthesia for labor and vaginal or cesarean delivery can be safely performed in patients with mitral stenosis.189,196 The important anesthetic goals are (1) maintenance of a low-normal heart rate and preservation of sinus rhythm; (2) aggressive treatment of atrial fibrillation, if present; (3) avoidance of aortocaval compression; (4) maintenance of venous return; (5) maintenance of adequate SVR; and (6) prevention of pain, hypoxemia, hypercarbia, and acidosis, which may increase pulmonary vascular resistance (see Table 42-2).
Parturients with mitral stenosis are at risk for both intrapartum and postpartum hemodynamic compromise and pulmonary edema. Invasive hemodynamic monitoring is often helpful, and close peripartum monitoring of filling pressures is important. Echocardiographic assessment of right-sided pressures appears to somewhat overestimate true pressures; therefore, in selected patients with severe mitral stenosis, a pulmonary artery catheter may help guide fluid management. The decision to place a pulmonary artery catheter should be guided by assessment of NYHA functional status, right ventricular function, pulmonary artery pressure, and severity of symptoms related to mitral stenosis before pregnancy.
Adequate analgesia during the first stage of labor is essential. Intrathecal administration of a lipid-soluble opioid during the first stage of labor provides excellent analgesia without causing sympathetic blockade. Neuraxial administration of an opioid with a small dose of local anesthetic may provide satisfactory analgesia for the second stage of labor. Hypotension should be treated with a direct-acting vasoconstrictor (phenylephrine).
Neuraxial anesthesia for cesarean delivery is best administered with a titratable technique (epidural anesthesia, sequential combined spinal-epidural anesthesia), judicious intravenous fluid administration, and titration of phenylephrine to maintain hemodynamic stability. During induction and maintenance of general anesthesia, tachycardia should be prevented by administration of a beta-adrenergic receptor antagonist and/or an opioid. After delivery, care should be taken with the administration of the uterotonic agent 15-methyl prostaglandin-F2a, because it may increase pulmonary vascular resistance.
Mitral Regurgitation
Mitral regurgitation is generally well tolerated during pregnancy. Nonetheless, some evidence suggests that the volume overload associated with pregnancy may induce unfavorable structural alterations in women with mitral regurgitation.197 Both neuraxial and general anesthesia are well tolerated. Chronic mitral regurgitation may be associated with left ventricular dysfunction; thus, echocardiographic assessment of left ventricular function helps guide anesthetic and fluid management. The goals of anesthetic management in patients with mitral regurgitation include (1) prevention of an increase in SVR, (2) maintenance of a normal to slightly increased heart rate, (3) maintenance of sinus rhythm, (4) aggressive treatment of acute atrial fibrillation, (5) avoidance of aortocaval compression, (6) maintenance of venous return, (7) prevention of an increase in central venous volume, (8) avoidance of myocardial depression during general anesthesia, and (9) prevention of pain, hypoxemia, hypercarbia, and acidosis, which may increase pulmonary vascular resistance (see Table 42-2).
Mitral Valve Prolapse Syndrome
Historically, prior to the contemporary understanding of the complex three-dimensional echocardiographic anatomy of the mitral valve, mitral valve prolapse tended to be overdiagnosed. Currently, the term mitral valve prolapse should be restricted to conditions in which the free margin of the anterior, posterior, or both leaflets of the mitral valve is displaced superior to the annular plane of the mitral valve.198 With these diagnostic criteria, the prevalence of mitral valve prolapse in the general population is 2% to 3%, with no female preponderance.199 Mitral valve prolapse is a heterogeneous disorder with various causes and associations, including familial and degenerative or myxomatous disorders, Marfan syndrome, Ehlers-Danlos syndrome, a redundant papillary chordal apparatus, and many other diseases.200 Varying degrees of mitral regurgitation can be associated with this condition. There is some overlap between the strictly defined mitral valve prolapse syndrome and mitral regurgitation. Therefore, along with history and physical examination, echocardiography plays a central role in the diagnosis, treatment, and ongoing assessment of these patients.
The overall clinical course in patients with mitral valve prolapse is excellent.201 Pregnancy and vaginal or cesarean delivery with either neuraxial or general anesthesia are well tolerated. Patients with moderate or severe mitral regurgitation and/or depressed left ventricular function are at increased risk for morbidity and mortality.202 The recommendations for anesthetic management of parturients with mitral regurgitation and/or left ventricular dysfunction also apply to patients with these conditions associated with mitral value prolapse. Antibiotic endocarditis prophylaxis is not recommended.127
Tricuspid Stenosis and Regurgitation
Rheumatic tricuspid stenosis is rarely encountered in pregnancy; most frequently, it accompanies rheumatic mitral stenosis. Clinically, it is associated with dyspnea, pulmonary hypertension, and congestive heart failure. Successful treatment with balloon valvuloplasty has been described.
Tricuspid regurgitation is rarely found in isolation during pregnancy. Functional tricuspid regurgitation is often observed in normal pregnancy with little clinical consequence. Severe tricuspid regurgitation is often associated with congenital heart disease such as Ebstein's anomaly. Overall, pregnancy is well tolerated in the presence of tricuspid regurgitation. Affected patients may be susceptible to hypotension with a decrease in preload.60
Pulmonic Stenosis and Regurgitation
Pregnancy is well tolerated in patients with pulmonic stenosis, and isolated pulmonic stenosis has not been found to affect maternal and fetal outcomes203 (see Table 42-2). In a small series of cases, affected patients had normal right ventricular function and only mild symptoms on initial presentation.203 Nonetheless, valvuloplasty is recommended in asymptomatic nonpregnant patients with pulmonic stenosis and a transpulmonic valve gradient greater than 40 mm Hg and in symptomatic nonpregnant patients with a gradient greater than 30 mm Hg.60
Pulmonic regurgitation occurs infrequently as an isolated valvular lesion; it is most commonly associated with congenital heart disease, such as repaired tetralogy of Fallot100 or repaired pulmonic stenosis. Physiologic pulmonic regurgitation is commonly seen during pregnancy.204 Overall, pregnancy and labor are well tolerated in the presence of mild or moderate pulmonic regurgitation; however, severe pulmonic regurgitation is highly associated with maternal cardiac events during pregnancy.205,206 Right-sided heart failure due to pulmonic regurgitation occurs more commonly in patients with multiple gestation, pulmonary artery stenosis distal to the left and right pulmonary arteries, preexisting right ventricular systolic dysfunction, and/or right ventricular hypertrophy.206
Prosthetic Heart Valves
Bioprosthetic Valves
The advantage of bioprosthetic valves is that long-term systemic anticoagulation is not required. It is unclear whether pregnancy, with its associated cardiovascular changes, accelerates bioprosthetic valve structural degeneration. Several studies observed complications related to valve deterioration, most of which required reoperation during pregnancy or shortly after delivery.207-211 Other studies reported little or no impact of pregnancy on valve structural deterioration.212-216 Hence, although a bioprosthetic valve simplifies the required anticoagulant regimen during pregnancy, it may expose the mother to a high risk for reoperation and associated operative mortality (ranging from 3.8%213 to 8.7%217). The 10-year incidence of valve replacement or valve-related death ranges from 50% to 80% in young women of childbearing age.213,215,217 The risk for valve failure appears higher in the mitral position than in the aortic position.215 Bioprosthetic homografts appear to be more durable than heterografts. Autografts have a better hemodynamic profile than heterografts.
Anticoagulation for Patients with a Mechanical Valve
All patients with a mechanical valve require systemic anticoagulation. Mechanical valves have a higher risk for thromboembolism than bioprosthetic valves. Valves in the mitral position have a higher risk for thromboembolism than valves in the aortic position. A higher risk for mechanical valve thromboembolic complications has been observed during pregnancy.218 No large prospective randomized trials have examined the use of various anticoagulation strategies for pregnant women with a mechanical valve. Additionally, newer prosthetic valve designs may have a lower risk for thromboembolism than older models. The current anticoagulation recommendations are based on expert consensus guidelines from three major professional organizations: the ACC/AHA,60 the American College of Chest Physicians (ACCP),173,219 and the European Society of Cardiology (ESC).38 Differences among these guidelines are summarized in Table 42-5.
TABLE 42-5
Major Professional Society Guidelines for Anticoagulation in Pregnant Patients with Mechanical Valve(s)
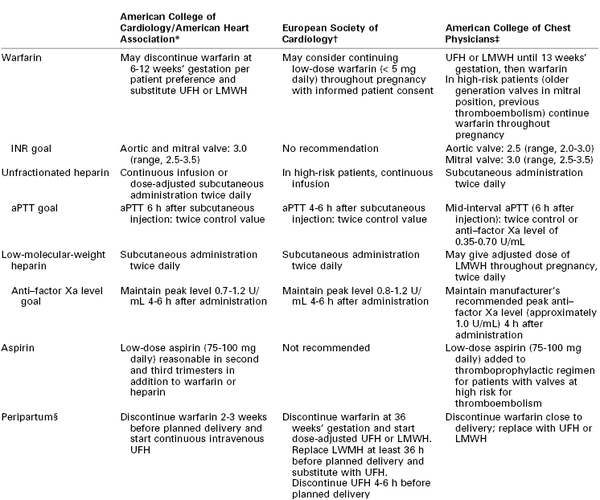
* Modified from Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines J Am Coll Cardiol 2008; 52:e1-142.
† Modified from Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011; 32:3147-97.
‡ Modified from Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141:e691S-736S and Whitlock RP, Sun JC, Fremes SE, et al. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141:e576S-600S.
§ For resumption of anticoagulation in the postoperative period, the 2010 American Society of Regional Anesthesia and Pain Medicine Practice Advisory43 recommends the following: (1) Warfarin: The indwelling neuraxial catheter should be removed when the INR is less than 1.5. (2) UFH: The indwelling neuraxial catheter should be removed 2 to 4 hours after the last dose of UFH, after checking the patient's coagulation status (aPTT). The next dose of UFH should not be given until at least 1 hour has elapsed after removal of the catheter. (3) LMWH: The first therapeutic dose should not be administered until 24 hours after the initiation of the neuraxial procedure and until at least 2 hours after removal of the indwelling neuraxial catheter. The U.S. Food and Drug Administration recommends waiting at least 4 hours after removal of a neuraxial catheter before administering a postprocedure dose of LMWH. (U.S. Food and Drug Administration. Low Molecular Weight Heparins: Drug Safety Communication—Recommendations to Decrease Risk of Spinal Column Bleeding and Paralysis. November 6, 2013. Available at http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm373918.htm. Accessed November 2013.)
aPTT, activated partial thromboplastin time; INR, international normalized ratio; LMWH, low-molecular-weight heparin; UFH, unfractionated heparin.
Warfarin.
In patients with a mechanical valve, anticoagulation with warfarin is associated with lower rates of thromboembolic complications and maternal death than is heparin.220 Warfarin crosses the placenta. Use of warfarin between 6 and 12 weeks' gestation has been associated with embryopathy; therefore, consensus guidelines have recommended substitution with unfractionated heparin or LMWH during this period.220 Additionally, the use of warfarin at any time during pregnancy has been associated with fetal wasting, central nervous system abnormalities, and fetal hemorrhagic complications.173
Warfarin anticoagulation effect is monitored by periodic assessment of the international normalized ratio (INR). Low-dose aspirin may be safely administered in addition to warfarin. Warfarin should be discontinued before delivery; in patients with a mechanical heart valve, heparin must be substituted.
Warfarin anticoagulation should be stopped for 4 to 5 days and the INR measured immediately before administration of a neuraxial block (see Chapter 44). Concurrent administration of other anticoagulants or antiplatelet agents increases the risk for bleeding complications. After delivery, patients with a mechanical valve typically require bridging to warfarin; the type of anesthesia and timing of neuraxial catheter removal may significantly affect the timing of anticoagulation after delivery.43,44
Unfractionated Heparin.
Unfractionated heparin does not cross the placenta and therefore is safer for the fetus than warfarin. However, use of unfractionated heparin in the first trimester and close to term has been associated with an increased risk for valve thrombosis.220 Unfractionated heparin can be administered subcutaneously or as a continuous intravenous infusion. Its therapeutic efficacy is monitored with the aPTT. Heparin requirements are higher during pregnancy due to underlying physiologic changes (e.g., increased levels of heparin-binding proteins, factor VIII, fibrinogen). The incidence of heparin-induced thrombocytopenia is three times higher in women than in men.
Intravenous unfractionated heparin should be discontinued 4 to 6 hours before administration of a neuraxial anesthetic technique or anticipated delivery (see Chapter 44).43 The aPTT should be determined to verify normalization of coagulation function, and a platelet count should be checked to rule out heparin-induced thrombocytopenia. An indwelling neuraxial catheter can be removed 2 to 4 hours after the last dose of unfractionated heparin. Unfractionated heparin can be restarted 1 hour after removal of a neuraxial catheter.
Low-Molecular-Weight Heparin.
LMWH does not cross the placenta and is administered subcutaneously for most indications. The pharmacokinetics of LMWH are altered in pregnancy. Use of LMWH for thromboprophylaxis during pregnancy has been associated with mechanical valve thrombosis.221-224 The efficacy of LMWH can be monitored with anti–factor Xa levels. Peak anti–factor Xa levels should be checked 4 to 6 hours after LMWH administration in pregnant women with a mechanical valve. Importantly, in pregnant women with a mechanical valve, the required doses of LMWH are considered therapeutic and are therefore higher than those required for deep vein thrombosis prophylaxis. Additional monitoring of trough anti–factor Xa levels may allow further refinement of management. In the absence of anti–factor Xa monitoring, therapeutic use of LMWH in pregnant women with a mechanical valve is not advisable.38
A neuraxial block should not be performed until at least 24 hours have elapsed after the last dose of LMWH. Anti–factor Xa levels do not predict the risk for bleeding. Other anticoagulants or antiplatelet agents coadministered with LMWH increase the risk for bleeding complications. After delivery, LMWH should not be administered until at least 24 hours have elapsed after performance of the neuraxial block procedure (see Chapter 44).43 After removal of a neuraxial catheter, at least 4 hours should elapse before administration of the next dose of LMWH (see Table 42-5).
New Anticoagulants.
There are no published reports of the use of new oral anticoagulants in pregnancy. Therefore, use of oral direct thrombin inhibitors (dabigatran) or oral anti–factor Xa inhibitors (apixiban, rivaroxaban) cannot be recommended during pregnancy.
There are no published reports of the administration of bivalirudin during pregnancy; the use of argatroban during pregnancy has been described.225 The current ASRA guidelines recommend against neuraxial anesthesia administration in patients receiving direct thrombin inhibitors.43
Neuraxial Anesthesia in Patients with a Mechanical Valve.
No published observational or prospective randomized trials have investigated the use of neuraxial anesthetic techniques in patients with a mechanical heart valve who require systemic anticoagulation. Therefore, current clinical practice is guided by consensus guidelines.43,44,226,227 Systemic anticoagulation may affect the choice and timing of anesthesia, and the neuraxial anesthetic technique and timing of catheter removal may influence the choice and timing of resumption of systemic anticoagulation after vaginal or cesarean delivery. Therapeutic anticoagulation is usually not resumed for at least 12 hours after delivery. The choice and timing of the anesthetic technique should be developed in discussion with the patient, the cardiologist, and the obstetrician, with consideration of consensus guidelines (see Table 39-4).
Cardiomyopathies
Heart Failure Nomenclature
Accurate description of patients with heart failure is important for diagnostic, therapeutic, and prognostic purposes. It is helpful to broadly describe symptoms of heart failure as new-onset (acute) or chronic. Acute-on-chronic heart failure describes patients with worsening symptoms after an earlier diagnosis of heart failure.
Heart failure can be separated into left sided (predominantly pulmonary congestion and pulmonary edema) and right sided (predominantly peripheral edema). Biventricular heart failure describes symptoms that result from both right and left ventricular involvement.
Assessment of ventricular function allows for differentiation between systolic and diastolic heart dysfunction. Arbitrarily, left ventricular dysfunction is defined by a left ventricular ejection fraction less than 45% to 50%.
Left ventricular systolic dysfunction (decreased ejection fraction) is not synonymous with left-sided heart failure because patients may be largely asymptomatic, even with left ventricular ejection fraction less than 30%. Similarly, diastolic dysfunction is not synonymous with diastolic heart failure. Diastolic heart failure is more accurately described as heart failure with preserved ejection fraction (HFpEF).
The NYHA classification describes patients' functional status (see Box 42-1), and the functional status can change over time. In contrast, the staging classification of heart failure recognizes that heart failure is a progressive condition and may manifest as variable symptoms over time.34,228 Both classifications remain highly useful in clinical practice.
Peripartum Cardiomyopathy
Peripartum cardiomyopathy is a unique cardiomyopathy of unknown cause that occurs during pregnancy or the postpartum period.229-231 The timing of development of heart failure is important to help exclude other causes of heart failure. To make the diagnosis of peripartum cardiomyopathy, the development of heart failure should occur in the last month of pregnancy or within 5 months of delivery (Box 42-10). Other identifiable causes of heart failure should be excluded, and the absence of other recognizable cardiac disease prior to the last month of pregnancy should be verified. Echocardiographic diagnostic criteria include (1) left ventricular ejection fraction less than 45% (and/or M-mode fractional shortening < 30%) and (2) end-diastolic left ventricular dimension greater than 27 mm/m2 body surface area.232 Some women develop cardiomyopathy before the last month of pregnancy. This condition is referred to as pregnancy-associated cardiomyopathy. Its clinical signs, symptoms, and outcomes are similar to those for peripartum cardiomyopathy.233
The incidence of peripartum cardiomyopathy varies around the world and has been reported to be as high as 1 in 300 live births in Haiti234 and 1 per 3000 to 4000 live births in the United States.229,235 The cause(s) for such regional variation are unclear. Risk factors for peripartum cardiomyopathy include African race, multiparity, multiple gestation, preeclampsia, gestational hypertension, use of tocolytic agents, cocaine abuse, and age older than 30 years.
The etiology of this relatively rare condition remains unclear; several possible causes have been proposed, including underlying myocarditis, apoptosis, inflammation, pathologic maternal immune response to fetal antigens, effects of prolactin, viral triggers, and hereditary/familial factors.
Patients with peripartum cardiomyopathy have typical signs and symptoms of systolic heart failure. On clinical examination, the apical impulse is displaced laterally and an S3 gallop is appreciated. Because of left ventricular enlargement (Figure 42-6) and changes in ventricular cavity geometry, functional mitral regurgitation is frequently seen. Peripartum cardiomyopathy is primarily a diagnosis of exclusion, and the symptoms may be disguised as physiologic changes of pregnancy; thus, the use of echocardiography helps confirm the diagnosis. Right-sided heart pressures can almost always be assessed by echocardiography; hence, right-sided heart catheterization is rarely necessary. Left-sided heart catheterization may be necessary to assess the coronary arteries. However, given that the risk for ischemic cardiomyopathy in pregnancy is very low, and because coronary angiography results in fetal radiation exposure, left-sided heart catheterization is infrequently used to make the diagnosis. Ischemic cardiomyopathy is typically accompanied by regional wall motion abnormalities, whereas peripartum or nonischemic cardiomyopathy typically results in a global decrease in contractility.
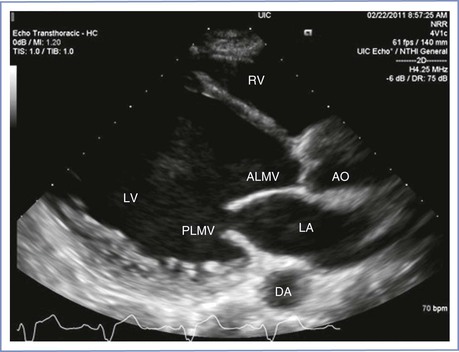
FIGURE 42-6 Echocardiographic image of dilated cardiomyopathy. RV, right ventricle; LA, left atrium; LV, left ventricle; ALMV, anterior leaflet of the mitral valve; PLMV, posterior leaflet of the mitral valve; AO, aorta; DA, descending aorta. (Courtesy Dr. Mayank Kansal, University of Illinois at Chicago, Chicago, IL.)
Medical management of peripartum cardiomyopathy rests on basic treatment paradigms for congestive heart failure and dilated cardiomyopathy (see later discussion). Compared with other forms of nonischemic cardiomyopathy (e.g., infiltrative or HIV-related), peripartum cardiomyopathy has a better prognosis, with a 94% 5-year survival rate in the United States.236 Recovery of left ventricular function occurs in slightly more than half of the affected women.233 Black women are 6.4 times more likely to die of peripartum cardiomyopathy than white women. In contrast, in Haiti the 2-year mortality is 15.4% and only a fourth of the patients experience normalization of left ventricular function.234
The risk for subsequent pregnancy is substantially higher in women who do not recover normal left ventricular function.237 In a series of 44 women with peripartum cardiomyopathy, the maternal mortality rate in subsequent pregnancies was 19% in women with persistent left ventricular dysfunction compared with 0% in women without residual dysfunction.237 Therefore, subsequent pregnancy should be discouraged if left ventricular function has not recovered. Even in patients with normalized left ventricular function, careful counseling is advised owing to a significant risk for recurrence of left ventricular dysfunction in a subsequent pregnancy.38
Obstetric and Anesthetic Management
Obstetric management involves expedient delivery after stabilization of the mother; in most cases, cesarean delivery is reserved for obstetric indications. Continuous spinal anesthesia238 and combined spinal-epidural anesthesia239,240 have been safely administered in patients with severe peripartum cardiomyopathy. Given the intravascular fluid shifts associated with labor, delivery, and the immediate postpartum period, invasive blood pressure and central venous pressure monitoring are recommended. Neuraxial anesthesia appears ideally suited for these patients because it results in a beneficial decrease in both preload and afterload. Particular attention should be paid to the immediate postpartum period when autotransfusion combined with the regression of neuraxial anesthesia may cause worsening of heart failure.
Other Nonischemic Cardiomyopathies
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy is relatively common, with a prevalence estimated at 1 in 500. It is an autosomal dominant disorder associated with various forms of left ventricular hypertrophy; the risk that an affected patient will transmit the disease to offspring is 50%. Additionally, hypertrophic cardiomyopathy mutations are highly penetrant. Preconception genetic counseling is recommended.154
The complications that result from hypertrophic cardiomyopathy can be separated into two categories: mechanical and electrophysiologic. The mechanical consequences relate to left ventricular outflow tract obstruction, mitral regurgitation, diastolic dysfunction, and development of heart failure. The electrophysiologic complications include atrial and ventricular arrhythmias and, most important, the risk for sudden cardiac death. The myocardial ischemia observed in patients with hypertrophic cardiomyopathy, often at ages younger than expected, is caused by supply-demand mismatch rather than coronary atherosclerosis. Hypertrophic cardiomyopathy is associated with disorganized myocardial architecture, myocardial disarray, and fibrosis (Figure 42-7).
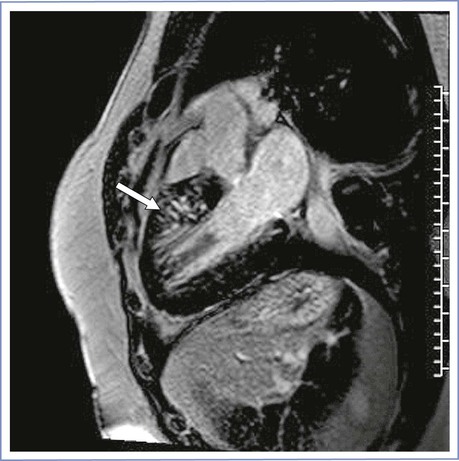
FIGURE 42-7 Cardiac magnetic resonance image of hypertrophic cardiomyopathy with myocardial scar. Arrow shows scar with delayed enhancement with gadolinium in the hypertrophied myocardium. (Courtesy Dr. Afshin Farzaneh-Far, University of Illinois at Chicago, Chicago, IL.)
One of the hallmarks of hypertrophic cardiomyopathy is the dynamic left ventricular outflow tract obstruction. The obstruction gradient typically increases after a premature ventricular contraction. One third of patients have left ventricular outflow tract obstruction at rest, one third have a physiologically provocable gradient, and one third have no gradient (nonobstructive form of hypertrophic cardiomyopathy). A gradient of 30 mm Hg or more is clinically significant.241
Pregnant women with hypertrophic cardiomyopathy may have dyspnea, fatigue, angina, palpitations, and/or syncope. Symptoms of congestive heart failure are rarely seen in patients in sinus rhythm; these symptoms are more frequently encountered when atrial fibrillation is present.
On physical examination the classic obstructive systolic murmur is heard at the apex (grade 3 to 4/6), and it radiates to the left sternal border. Although most patients will have a displaced and forceful left ventricular impulse, the presence of a murmur depends on the degree and type of obstruction. The intensity of a hypertrophic cardiomyopathy murmur increases with a Valsalva maneuver or standing (decreased preload or afterload causes more obstruction), and it decreases with squatting (increased afterload causes less obstruction). Invasive arterial pressure monitoring allows recognition of a bifid arterial pulse waveform.
The ECG is abnormal in the vast majority of patients and demonstrates an increase in voltage, T-wave inversions, and pathologic Q waves. ECG abnormalities correlate poorly with the severity of hypertrophic cardiomyopathy.155 Transthoracic echocardiography is indispensable for making the diagnosis, determining prognosis, and guiding management decisions.154 Diagnostic criteria include a septal thickness greater than 15 mm and an increased septal-to-posterior wall thickness ratio (1.3). Systolic anterior motion of the mitral valve has a specificity of 98% for hypertrophic cardiomyopathy. Left ventricular outflow tract obstruction is apparent during Doppler interrogation; the continuous wave tracing has a classic “dagger-shaped” contour (Figure 42-8). In addition, echocardiography allows identification of various morphologic variants and assessment of the extent of hypertrophy. A wall thickness of 30 mm or greater is associated with a high risk for sudden cardiac death.242 Patients with a gradient of 50 mm Hg or greater are at highest risk for complications.154
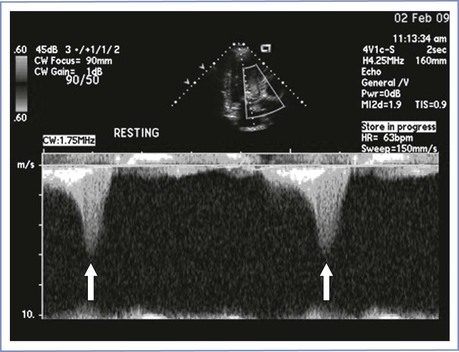
FIGURE 42-8 Dynamic left ventricular outflow obstruction in hypertrophic cardiomyopathy. Continuous wave Doppler tracing in left ventricular outflow tract displays typical late-peaking (“dagger-shaped”) contour of dynamic outflow obstruction (arrows). (Courtesy Dr. Mayank Kansal, University of Illinois at Chicago, Chicago, IL.)
Fortuitously, the increase in blood volume allows most patients with hypertrophic cardiomyopathy to tolerate pregnancy well.156,243,244 Both asymptomatic and symptomatic women with hypertrophic cardiomyopathy should continue taking a beta-adrenergic receptor antagonist throughout pregnancy and the peripartum period.154
Patients with hypertrophic cardiomyopathy are at increased risk for developing atrial fibrillation. Treatment of atrial fibrillation rests on rate and rhythm control as well as systemic anticoagulation to prevent thromboembolic stroke. Rate control can alleviate symptoms; beta-adrenergic receptor antagonists and nondihydropyridine calcium entry−blocking agents (e.g., verapamil, diltiazem) provide best results. Digoxin is less effective in controlling rate and may be harmful in patients with hypertrophic cardiomyopathy in the absence of atrial fibrillation.154 Amiodarone can be successfully used for rate control in patients with hypertrophic cardiomyopathy. Rhythm control can be attempted pharmacologically with amiodarone and sotalol. In patients with a rapid ventricular response and significant hemodynamic compromise, electric cardioversion should be performed.
Obstetric and Anesthetic Management.
Traditionally, general anesthesia has been considered the anesthetic technique of choice for parturients with hypertrophic cardiomyopathy.245-249 It avoids a precipitous decrease in preload, and the negative inotropic effect of inhalation anesthetic agents may help reduce the degree of dynamic obstruction (see Table 42-2). No prospective controlled trials have compared general anesthesia with neuraxial anesthesia in parturients with hypertrophic cardiomyopathy; numerous case reports have described successful use of neuraxial anesthesia in these patients.250-257 Given that hypertrophic cardiomyopathy is a heterogeneous condition, and given the high likelihood of selection and publication bias in these case reports, it is difficult to make generalized recommendations for these patients. In deciding whether to administer general or neuraxial anesthesia, it is important to recognize the degree of left ventricular outflow tract obstruction. For cesarean delivery, it appears reasonable to recommend general anesthesia for patients with a gradient of 50 mm Hg or greater or for those with symptoms of heart failure during pregnancy. In asymptomatic parturients with a lower gradient, slowly titrated neuraxial analgesia/anesthesia appears safe.
Because a decrease in preload is expected to increase the degree of left ventricular outflow tract obstruction, central venous pressure monitoring seems reasonable. Similarly, invasive arterial blood pressure monitoring may be helpful. Transthoracic echocardiography facilitates assessment of intravascular volume. Beta-adrenergic receptor antagonists decrease myocardial contractility and decrease the severity of left ventricular outflow tract obstruction. Phenylephrine is the drug of choice for the treatment of hypotension. Inotropic agents (e.g., dopamine, dobutamine) may be harmful in these patients.154 Rapid administration of intravenous oxytocin has been associated with hypotension; slow administration is therefore recommended.
Stress-Induced Cardiomyopathy
Stress-induced cardiomyopathy, also known as takotsubo cardiomyopathy, broken heart syndrome, or apical ballooning syndrome, is a transient cardiomyopathy with typical left ventricular systolic dysfunction of the apical and mid-cavity segments.258 The basal ventricular segments are frequently hyperkinetic and may cause left ventricular outflow tract obstruction. Affected patients typically do not have underlying coronary artery disease. Stress-induced cardiomyopathy occurs predominantly in postmenopausal women in the seventh and eight decades of life and is frequently associated with emotional and physical stressors. It has also been described during pregnancy in the setting of emotional and physical triggers, postpartum depression, and administration of pharmacologic triggers such as ergot alkaloids. It has been reported in the absence of apparent triggers259 during otherwise uncomplicated spinal anesthesia for cesarean delivery.260,261
Clinically, patients have chest pain, dyspnea, and symptoms of left ventricular systolic dysfunction and heart failure. ECG abnormalities are frequently observed in left precordial leads and may mimic STEMI due to plaque rupture. Coronary angiography is required to rule out STEMI; ventriculography or echocardiography allows recognition and characterization of wall motion abnormalities. The most critical differential diagnoses are STEMI and peripartum cardiomyopathy. When treating patients who develop shock, it is critically important to determine the degree of left ventricular outflow tract obstruction. Shock without left ventricular outflow tract obstruction can be treated in standard fashion with an inotropic agent. Patients with left ventricular outflow tract obstruction require optimization of intravascular volume and administration of a beta-adrenergic receptor antagonist; use of an intra-aortic balloon pump may be necessary to relieve the obstruction.
Dilated and Other Cardiomyopathies
In women with idiopathic or anthracycline-induced cardiomyopathy, pregnancy is associated with a high risk for adverse maternal, fetal, and neonatal outcomes. Pregnancy appears to unfavorably affect the short-term course of these cardiomyopathies. The NYHA functional status and ejection fraction may predict outcomes in this population.262 Other causes of cardiomyopathy during pregnancy include cocaine abuse and hemochromatosis; the latter results in an infiltrative cardiomyopathy.
Rapid atrial or ventricular rates due to arrhythmia can cause tachycardia-induced cardiomyopathy. This cardiomyopathy is a potentially reversible condition that has been described in pregnancy263 and successfully treated with radiofrequency ablation.264,265 Differentiation from other forms of cardiomyopathy may be difficult, but it is important from a prognostic standpoint.
Medical Management of Heart Failure
Principles of heart failure management for pregnant patients are similar to those for nonpregnant patients with two notable exceptions: angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blocking agents and aldosterone antagonists (spironolactone, eplerenone) should not be used in pregnant women. A beta-adrenergic receptor antagonist should be administered, and hydralazine can be substituted for ACE inhibitors/angiotensin receptor blocking agents. Digoxin use is safe in pregnancy. Loop diuretics and sodium restriction are indicated to prevent or treat volume overload. In patients with decompensated heart failure, treatment with intravenous nitroglycerin and dopamine or dobutamine is indicated.
Ventricular Assist Devices
Anecdotal reports have described ventricular assist device use during pregnancy. A successful completion of pregnancy with cesarean delivery in a patient with a ventricular assist device has been reported.266 In another patient with myocardial infarction and subsequent severe left ventricular dysfunction, a ventricular assist device was placed, although pregnancy was not recognized at the time of placement; fetal death subsequently ensued.267 The preconception presence of a ventricular assist device remains a contraindication to pregnancy.
Pericardial Disease
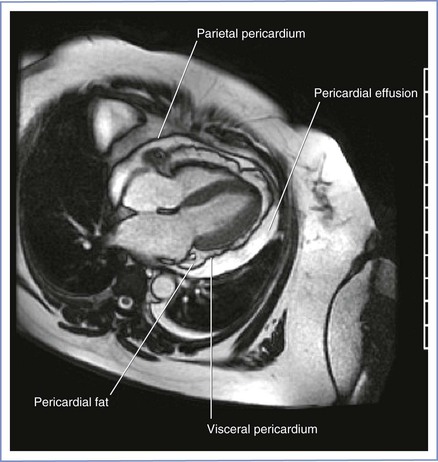
Pericardial Effusion
Asymptomatic pericardial effusions are frequently found in otherwise healthy pregnant women (Figure 42-9). A pericardial effusion may be seen in 15% to 20% of pregnancies in the first and second trimester; this rate increases to approximately 40% in the third trimester. The effusion is seen more often in nulliparous women than in parous women.268 These effusions are transudative and disappear within 2 months after delivery. Treatment is rarely required. Physical signs of tamponade or pulsus paradoxus are rarely seen. Similarly, electrical alternans is not seen on the ECG.
Acute Pericarditis
Pericarditis remains a clinical diagnosis confirmed by ECG and echocardiography. Patients typically have precordial pain that improves with sitting and learning forward. A pericardial friction rub may be appreciated on physical examination; this finding is frequently evanescent, and repeat examinations may be required. ECG findings consist of diffuse concave ST-segment elevations. Diffuse PR interval depression is frequently seen except in lead aVR, which demonstrates near-pathognomonic PR-interval elevation. Echocardiography may demonstrate various degrees of pericardial effusion. Importantly, the presence of a pericardial effusion is not synonymous with pericarditis because pericarditis remains a clinical diagnosis.
The etiology of acute pericarditis during pregnancy is most likely similar to that in the general population. A viral etiology is most common. Less common causes include tuberculosis, connective tissue disease (e.g., systemic lupus erythematosus), and neoplasm.
Treatment of pericarditis in the general population includes nonsteroidal anti-inflammatory drugs, glucocorticoids, colchicine, and, rarely, immunosuppressant drugs. During pregnancy, the maternal and fetal risks and benefits of these agents should be weighed carefully.
Cardiac Tamponade
Therapeutic and diagnostic pericardiocentesis or a surgical pericardial window can be safely performed during pregnancy. However, echocardiographic rather than fluoroscopic guidance is preferred because it avoids fetal radiation exposure.
Constrictive Pericarditis
Constrictive pericarditis is a condition in which a noncompliant pericardium prevents filling of the right- and left-sided heart chambers; specific hemodynamic features include equalization and elevation of both left and right ventricular diastolic pressures, preserved systolic function, and a “dip and plateau” pattern of the ventricular pressure tracing. Potential causes of constrictive pericarditis during pregnancy include previous irradiation, recurrent pericarditis due to rheumatoid arthritis, tuberculosis, and neoplasm. Patients may be asymptomatic before pregnancy; the physiologic changes of pregnancy may exacerbate the condition.
Anesthetic Management
Constrictive physiology and cardiac tamponade share some common pathophysiologic features that are important for the conduct of anesthesia (see Table 42-2). Impaired filling of the right side of the heart results from either a noncompliant pericardium (constrictive physiology) or pericardial fluid (tamponade physiology). Cardiac output is reduced by any intervention that reduces preload (e.g., aortocaval compression, sympathectomy with neuraxial anesthesia, positive-pressure ventilation with general anesthesia). Therefore, maintenance of preload is critically important; this goal can be achieved with central venous pressure monitoring, slowly titrated neuraxial anesthesia, and avoidance of positive-pressure ventilation. Invasive blood pressure monitoring is recommended.
Cardiopulmonary Resuscitation during Pregnancy
Cardiac arrest during pregnancy is rare; it occurs in 1 in 20,000 to 30,000 pregnancies. Overall survival rates are poorer than expected compared with other clinical arrest scenarios.269,270 The reversible causes of cardiac arrest during pregnancy are similar to those in nonpregnant patients. Additional causes specific to pregnancy include amniotic fluid embolism, eclampsia, placental abruption, and uterine atony (see Box 55-5).
Standard Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) principles apply to these patients. However, anatomic and physiologic changes of pregnancy require several specific modifications to the resuscitation protocol (Box 42-11; see also Box 55-5).269 The hands performing chest compressions should be placed slightly higher on the sternum because the abdominal contents and the diaphragm are displaced cephalad during the third trimester of pregnancy. Intravenous access should be obtained above the diaphragm. In pregnant patients, aortocaval compression reduces the cardiac output that results from chest compressions. Although it is typically advised to tilt the patient 15 to 30 degrees to facilitate left uterine displacement and optimize venous return and cardiac output, such a maneuver may impede the effectiveness of chest compressions. Therefore, current guidelines advocate manual left uterine displacement rather than the usual whole-body tilt (see Figure 55-2). If this technique is not successful, a firm wedge may be placed under a resuscitation board to tilt the patient approximately 30 degrees. In the field, the responder may use his or her knees to tilt the patient.
If spontaneous circulation does not return within 4 minutes of cardiac arrest, hysterotomy or cesarean delivery should be performed, with the goal of achieving delivery within 5 minutes of cardiac arrest. The primary purpose of cesarean delivery is to improve the chance of maternal survival, but timely delivery also improves the chances of infant survival.
Defibrillation should be performed based on current ACLS protocols. Transthoracic impedance is not changed during pregnancy, and the standard recommended electric energies should be used. Both cardioversion and defibrillation are considered safe at all stages of pregnancy. External and internal fetal monitoring probes and leads should be removed before defibrillation or cardioversion.
An intra-aortic balloon pump, a percutaneous left ventricular assist device, cardiopulmonary bypass, and extracorporeal membrane oxygenation have been successfully used in pregnant women with cardiac arrest; favorable outcomes have been reported. Therapeutic hypothermia has also been used after cardiac arrest during pregnancy, with successful delivery and favorable neonatal outcome.
Pregnancy after Heart Transplantation
The first successful pregnancy in a heart transplant recipient was described in 1988. Subsequently, several reports have documented the feasibility and relative safety of pregnancy and delivery in female heart transplant recipients.271,272 Successful patient management requires an interdisciplinary team approach.
The transplanted heart is denervated and the increase in cardiac output with pregnancy primarily results from an increase in stroke volume rather than heart rate. Because the risk for acute rejection is not increased in pregnant patients with a heart transplant, the immunosuppressant medications should be maintained at the lowest possible dose. The risk for infection is greater in patients receiving immunosuppression therapy. The AHA considers the presence of valvulopathy in heart transplant recipients a high-risk lesion for the development of bacterial endocarditis. Thus, given the unfavorable outcomes in heart transplant recipients who develop endocarditis, it appears reasonable to recommend antibiotic endocarditis prophylaxis at the time of membrane rupture for these parturients undergoing labor and vaginal delivery (see Box 42-7).127
Cardiac transplant vasculopathy is a disease specific to transplanted hearts; it consists of concentric and longitudinal intimal hyperplasia in the coronary arteries. In contrast, native coronary atherosclerosis manifests as focal, noncircumferential lesions. After malignancy, cardiac transplant vasculopathy constitutes the second most common cause of death 1 year after heart transplantation. Therefore, monitoring for ischemia appears prudent in these patients, especially because they may not present with classic anginal symptoms owing to denervation of the transplanted heart.
Baseline echocardiography allows assessment of ejection fraction and the presence of transplant valvulopathy. Most parturients with a heart transplant with a normal ejection fraction and normal right-sided pressures tolerate labor and delivery well; both neuraxial and general anesthesia are acceptable. Peripartum immunosuppression therapy should be managed by a multidisciplinary team.
Cardiopulmonary Bypass during Pregnancy
In the current era, maternal mortality associated with elective cardiopulmonary bypass during pregnancy is comparable to that for nonpregnant women.273 Fetal mortality remains high (16% to 33%),274-276 although some series report excellent fetal outcomes.277 The optimal timing of cardiopulmonary bypass appears to be the second trimester of pregnancy. Procedures performed immediately after delivery and on an emergency basis appear to confer added risk for maternal mortality. The degree of hypothermia is associated with poor fetal outcome. There is no evidence that anesthesia per se contributes to adverse maternal or fetal outcomes in this setting.273 The need for intraoperative FHR monitoring is universally accepted. Measures to lower fetal mortality include normothermic cardiopulmonary bypass with flow rates in excess of 2.4 L/min/m2 while maintaining mean arterial blood pressure above 70 to 75 mm Hg. Left uterine displacement and maintenance of hematocrit greater than 28% are recommended.
References
1. Sheifer SE, Canos MR, Weinfurt KP, et al. Sex differences in coronary artery size assessed by intravascular ultrasound. Am Heart J. 2000;139:649–653.
2. D’Antono B, Dupuis G, Fortin C, et al. Angina symptoms in men and women with stable coronary artery disease and evidence of exercise-induced myocardial perfusion defects. Am Heart J. 2006;151:813–819.
3. Canto JG, Rogers WJ, Goldberg RJ, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307:813–822.
4. Blomkalns AL, Chen AY, Hochman JS, et al. Gender disparities in the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: large-scale observations from the CRUSADE (Can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the American College of Cardiology/American Heart Association Guidelines?) National Quality Improvement Initiative. J Am Coll Cardiol. 2005;45:832–837.
5. Singh M, Rihal CS, Gersh BJ, et al. Mortality differences between men and women after percutaneous coronary interventions: a 25-year, single-center experience. J Am Coll Cardiol. 2008;51:2313–2320.
6. Lawlor DA, Emberson JR, Ebrahim S, et al. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the British Women's Heart and Health Study and the British Regional Heart Study. Circulation. 2003;107:1260–1264.
7. Skilton MR, Bonnet F, Begg LM, et al. Childbearing, child-rearing, cardiovascular risk factors, and progression of carotid intima-media thickness: the Cardiovascular Risk in Young Finns study. Stroke. 2010;41:1332–1337.
8. McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–930.
9. Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125:1367–1380.
10. Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–1309.
11. Oram S, Holt M. Innocent depression of the S-T segment and flattening of the T-wave during pregnancy. J Obstet Gynaecol Br Emp. 1961;68:765–770.
12. Jonsson M, Hanson U, Lidell C, Norden-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose: a randomised controlled trial. BJOG. 2010;117:76–83.
13. Svanstrom MC, Biber B, Hanes M, et al. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during Caesarean section. Br J Anaesth. 2008;100:683–689.
14. Moran C, Ni Bhuinneain M, Geary M, et al. Myocardial ischaemia in normal patients undergoing elective Caesarean section: a peripartum assessment. Anaesthesia. 2001;56:1051–1058.
15. Kametas NA, McAuliffe F, Hancock J, et al. Maternal left ventricular mass and diastolic function during pregnancy. Ultrasound Obstet Gynecol. 2001;18:460–466.
16. Desai DK, Moodley J, Naidoo DP. Echocardiographic assessment of cardiovascular hemodynamics in normal pregnancy. Obstet Gynecol. 2004;104:20–29.
17. Kametas NA, McAuliffe F, Krampl E, et al. Maternal cardiac function in twin pregnancy. Obstet Gynecol. 2003;102:806–815.
18. Borghi C, Esposti DD, Immordino V, et al. Relationship of systemic hemodynamics, left ventricular structure and function, and plasma natriuretic peptide concentrations during pregnancy complicated by preeclampsia. Am J Obstet Gynecol. 2000;183:140–147.
19. Lippi G, Albiero A, Montagnana M, et al. Lipid and lipoprotein profile in physiological pregnancy. Clin Lab. 2007;53:173–177.
20. Resnik JL, Hong C, Resnik R, et al. Evaluation of B-type natriuretic peptide (BNP) levels in normal and preeclamptic women. Am J Obstet Gynecol. 2005;193:450–454.
21. Tanous D, Siu SC, Mason J, et al. B-type natriuretic peptide in pregnant women with heart disease. J Am Coll Cardiol. 2010;56:1247–1253.
22. Grunewald C, Nisell H, Carlstrom K, et al. Acute volume expansion in normal pregnancy and preeclampsia: effects on plasma atrial natriuretic peptide (ANP) and cyclic guanosine monophosphate (cGMP) concentrations and feto-maternal circulation. Acta Obstet Gynecol Scand. 1994;73:294–299.
23. Ozcan T, Senoz S, Sahin N, et al. Change in atrial natriuretic peptide concentration after acute plasma volume expansion in normal pregnancy and preeclampsia. Gynecol Obstet Invest. 1995;39:229–233.
24. Eindhoven JA, van den Bosch AE, Jansen PR, et al. The usefulness of brain natriuretic peptide in complex congenital heart disease: a systematic review. J Am Coll Cardiol. 2012.
25. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;60:1581–1598.
26. Fleming SM, O’Gorman T, Finn J, et al. Cardiac troponin I in pre-eclampsia and gestational hypertension. BJOG. 2000;107:1417–1420.
27. Shivvers SA, Wians FH Jr, Keffer JH, Ramin SM. Maternal cardiac troponin I levels during normal labor and delivery. Am J Obstet Gynecol. 1999;180:122.
28. Abramov Y, Abramov D, Abrahamov A, et al. Elevation of serum creatine phosphokinase and its MB isoenzyme during normal labor and early puerperium. Acta Obstet Gynecol Scand. 1996;75:255–260.
29. Nabatian S, Quinn P, Brookfield L, Lakier J. Acute coronary syndrome and preeclampsia. Obstet Gynecol. 2005;106:1204–1206.
30. Goldberg LM, Uhland H. Heart murmurs in pregnancy: a phonocardiographic study of their development, progression and regression. Dis Chest. 1967;52:381–386.
31. Cutforth R, MacDonald CB. Heart sounds and murmurs in pregnancy. Am Heart J. 1966;71:741–747.
32. Marcus FI, Ewy GA, O’Rourke RA, et al. The effect of pregnancy on the murmurs of mitral and aortic regurgitation. Circulation. 1970;41:795–805.
33. New York Heart Association. Diseases of the Heart and Blood Vessels: Nomenclature and Criteria for Diagnosis. 6th edition. Brown and Co.: Boston; 1964.
34. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:e391–e479.
35. Siu SC, Sermer M, Colman JM, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104:515–521.
36. Drenthen W, Boersma E, Balci A, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010;31:2124–2132.
37. Thorne S, MacGregor A, Nelson-Piercy C. Risks of contraception and pregnancy in heart disease. Heart. 2006;92:1520–1525.
38. Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:3147–3197.
39. Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol. 2007;49:2303–2311.
40. Savu O, Jurcut R, Giusca S, et al. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging. 2012;5:289–297.
41. Melchiorre K, Sutherland GR, Baltabaeva A, et al. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 2011;57:85–93.
42. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. A Reference Guide to Fetal and Neonatal Risk. 8th edition. Lippincott Williams & Wilkins: Philadelphia; 2008:2144.
43. Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med. 2010;35:64–101.
44. Gogarten W, Vandermeulen E, Van Aken H, et al. Regional anaesthesia and antithrombotic agents: recommendations of the European Society of Anaesthesiology. Eur J Anaesthesiol. 2010;27:999–1015.
45. Williams GM, Gott VL, Brawley RK, et al. Aortic disease associated with pregnancy. J Vasc Surg. 1988;8:470–475.
46. Anderson RA, Fineron PW. Aortic dissection in pregnancy: importance of pregnancy-induced changes in the vessel wall and bicuspid aortic valve in pathogenesis. Br J Obstet Gynaecol. 1994;101:1085–1088.
47. Hiratzka L, Bakris G, Beckman J, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation. 2010;121:e266–e369.
48. Weissmann-Brenner A, Schoen R, Divon MY. Aortic dissection in pregnancy. Obstet Gynecol. 2004;103:1110–1113.
49. Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg. 2003;76:309–314.
50. Pacini L, Digne F, Boumendil A, et al. Maternal complication of pregnancy in Marfan syndrome. Int J Cardiol. 2009;136:156–161.
51. Meijboom LJ, Vos FE, Timmermans J, et al. Pregnancy and aortic root growth in the Marfan syndrome: a prospective study. Eur Heart J. 2005;26:914–920.
52. Rossiter JP, Repke JT, Morales AJ, et al. A prospective longitudinal evaluation of pregnancy in the Marfan syndrome. Am J Obstet Gynecol. 1995;173:1599–1606.
53. Donnelly RT, Pinto NM, Kocolas I, Yetman AT. The immediate and long-term impact of pregnancy on aortic growth rate and mortality in women with Marfan syndrome. J Am Coll Cardiol. 2012;60:224–229.
54. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008;118:e714–e833.
55. Lind J, Wallenburg HC. Pregnancy and the Ehlers-Danlos syndrome: a retrospective study in a Dutch population. Acta Obstet Gynecol Scand. 2002;81:293–300.
56. Karnis MF, Zimon AE, Lalwani SI, et al. Risk of death in pregnancy achieved through oocyte donation in patients with Turner syndrome: a national survey. Fertil Steril. 2003;80:498–501.
57. Carlson M, Silberbach M. Dissection of the aorta in Turner syndrome: two cases and review of 85 cases in the literature. J Med Genet. 2007;44:745–749.
58. Cabanes L, Chalas C, Christin-Maitre S, et al. Turner syndrome and pregnancy: clinical practice. Recommendations for the management of patients with Turner syndrome before and during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2010;152:18–24.
59. Milewicz DM, Dietz HC, Miller DC. Treatment of aortic disease in patients with Marfan syndrome. Circulation. 2005;111:e150–e157.
60. Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;52:e1–142.
61. Buser RT, Mordecai MM, Brull SJ. Combined spinal-epidural analgesia for labor in a patient with Marfan's syndrome. Int J Obstet Anesth. 2007;16:274–276.
62. Gordon CF 3rd, Johnson MD. Anesthetic management of the pregnant patient with Marfan syndrome. J Clin Anesth. 1993;5:248–251.
63. Singh SI, Brooks C, Dobkowski W. General anesthesia using remifentanil for Cesarean delivery in a parturient with Marfan's syndrome. Can J Anaesth. 2008;55:526–531.
64. Lacassie HJ, Millar S, Leithe LG, et al. Dural ectasia: a likely cause of inadequate spinal anaesthesia in two parturients with Marfan's syndrome. Br J Anaesth. 2005;94:500–504.
65. Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010;31:2915–2957.
66. Actis Dato GM, Rinaudo P, Revelli A, et al. Atrial septal defect and pregnancy: a retrospective analysis of obstetrical outcome before and after surgical correction. Minerva Cardioangiol. 1998;46:63–68.
67. Yap SC, Drenthen W, Meijboom FJ, et al. Comparison of pregnancy outcomes in women with repaired versus unrepaired atrial septal defect. BJOG. 2009;116:1593–1601.
68. Jaffe RA, Siegel LC, Schnittger I, et al. Epidural air injection assessed by transesophageal echocardiography. Reg Anesth. 1995;20:152–155.
69. Yap SC, Drenthen W, Pieper PG, et al. Pregnancy outcome in women with repaired versus unrepaired isolated ventricular septal defect. BJOG. 2010;117:683–689.
70. Green NJ, Rollason TP. Pulmonary artery rupture in pregnancy complicating patent ductus arteriosus. Br Heart J. 1992;68:616–618.
71. Beauchesne LM, Connolly HM, Ammash NM, Warnes CA. Coarctation of the aorta: outcome of pregnancy. J Am Coll Cardiol. 2001;38:1728–1733.
72. Vriend JW, van Montfrans GA, van der Post JA, et al. An unusual cause of hypertension in pregnancy. Hypertens Pregnancy. 2004;23:13–17.
73. Vriend JW, Drenthen W, Pieper PG, et al. Outcome of pregnancy in patients after repair of aortic coarctation. Eur Heart J. 2005;26:2173–2178.
74. Venning S, Freeman LJ, Stanley K. Two cases of pregnancy with coarctation of the aorta. J R Soc Med. 2003;96:234–236.
75. Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation. 2006;114:1873–1882.
76. Walker E, Malins AF. Anaesthetic management of aortic coarctation in pregnancy. Int J Obstet Anesth. 2004;13:266–270.
77. Manullang TR, Chun K, Egan TD. The use of remifentanil for Cesarean section in a parturient with recurrent aortic coarctation. Can J Anaesth. 2000;47:454–459.
78. Stephenson EA, Lu M, Berul CI, et al. Arrhythmias in a contemporary Fontan cohort: prevalence and clinical associations in a multicenter cross-sectional study. J Am Coll Cardiol. 2010;56:890–896.
79. Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Pregnancy and delivery in women after Fontan palliation. Heart. 2006;92:1290–1294.
80. Carp H, Jayaram A, Vadhera R, et al. Epidural anesthesia for cesarean delivery and vaginal birth after maternal Fontan repair: report of two cases. Anesth Analg. 1994;78:1190–1192.
81. Ioscovich A, Briskin A, Fadeev A, et al. Emergency cesarean section in a patient with Fontan circulation using an indwelling epidural catheter. J Clin Anesth. 2006;18:631–634.
82. Eid L, Ginosar Y, Elchalal U, et al. Caesarean section following the Fontan procedure: two different deliveries and different anaesthetic choices in the same patient. Anaesthesia. 2005;60:1137–1140.
83. Warnes CA. Transposition of the great arteries. Circulation. 2006;114:2699–2709.
84. Drenthen W, Pieper PG, Ploeg M, et al. Risk of complications during pregnancy after Senning or Mustard (atrial) repair of complete transposition of the great arteries. Eur Heart J. 2005;26:2588–2595.
85. Clarkson PM, Wilson NJ, Neutze JM, et al. Outcome of pregnancy after the Mustard operation for transposition of the great arteries with intact ventricular septum. J Am Coll Cardiol. 1994;24:190–193.
86. Genoni M, Jenni R, Hoerstrup SP, et al. Pregnancy after atrial repair for transposition of the great arteries. Heart. 1999;81:276–277.
87. Canobbio MM, Morris CD, Graham TP, Landzberg MJ. Pregnancy outcomes after atrial repair for transposition of the great arteries. Am J Cardiol. 2006;98:668–672.
88. Guedes A, Mercier LA, Leduc L, et al. Impact of pregnancy on the systemic right ventricle after a Mustard operation for transposition of the great arteries. J Am Coll Cardiol. 2004;44:433–437.
89. Tobler D, Fernandes SM, Wald RM, et al. Pregnancy outcomes in women with transposition of the great arteries and arterial switch operation. Am J Cardiol. 2010;106:417–420.
90. Connolly HM, Grogan M, Warnes CA. Pregnancy among women with congenitally corrected transposition of great arteries. J Am Coll Cardiol. 1999;33:1692–1695.
91. Cordone M, Wolfson A, Wolfson N, Penning D. Anesthetic management of labor in a patient with congenitally corrected transposition of the great arteries. Int J Obstet Anesth. 2008;17:57–60.
92. Schabel JE, Jasiewicz RC. Anesthetic management of a pregnant patient with congenitally corrected transposition of the great arteries for labor and vaginal delivery. J Clin Anesth. 2001;13:517–520.
93. Ploeg M, Drenthen W, van Dijk A, Pieper PG. Successful pregnancy after an arterial switch procedure for complete transposition of the great arteries. BJOG. 2006;113:243–244.
94. Connolly HM, Warnes CA. Ebstein's anomaly: outcome of pregnancy. J Am Coll Cardiol. 1994;23:1194–1198.
95. Donnelly JE, Brown JM, Radford DJ. Pregnancy outcome and Ebstein's anomaly. Br Heart J. 1991;66:368–371.
96. Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981.
97. Singh H, Bolton PJ, Oakley CM. Pregnancy after surgical correction of tetralogy of Fallot. Br Med J (Clin Res Ed). 1982;285:168–170.
98. Nissenkorn A, Friedman S, Schonfeld A, Ovadia J. Fetomaternal outcome in pregnancies after total correction of the tetralogy of Fallot. Int Surg. 1984;69:125–128.
99. Meijer JM, Pieper PG, Drenthen W, et al. Pregnancy, fertility, and recurrence risk in corrected tetralogy of Fallot. Heart. 2005;91:801–805.
100. Veldtman GR, Connolly HM, Grogan M, et al. Outcomes of pregnancy in women with tetralogy of Fallot. J Am Coll Cardiol. 2004;44:174–180.
101. Arendt KW, Fernandes SM, Khairy P, et al. A case series of the anesthetic management of parturients with surgically repaired tetralogy of Fallot. Anesth Analg. 2011;113:307–317.
102. Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54.
103. McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association. J Am Coll Cardiol. 2009;53:1573–1619.
104. Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Heart J. 2009;30:2493–2537.
105. Weiss BM, Zemp L, Seifert B, Hess OM. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol. 1998;31:1650–1657.
106. Bédard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J. 2009;30:256–265.
107. Robinson JN, Banerjee R, Landzberg MJ, Thiet MP. Inhaled nitric oxide therapy in pregnancy complicated by pulmonary hypertension. Am J Obstet Gynecol. 1999;180:1045–1046.
108. Lam GK, Stafford RE, Thorp J, et al. Inhaled nitric oxide for primary pulmonary hypertension in pregnancy. Obstet Gynecol. 2001;98:895–898.
109. Goodwin TM, Gherman RB, Hameed A, Elkayam U. Favorable response of Eisenmenger syndrome to inhaled nitric oxide during pregnancy. Am J Obstet Gynecol. 1999;180:64–67.
110. Bendayan D, Hod M, Oron G, et al. Pregnancy outcome in patients with pulmonary arterial hypertension receiving prostacyclin therapy. Obstet Gynecol. 2005;106:1206–1210.
111. Avila WS, Grinberg M, Snitcowsky R, et al. Maternal and fetal outcome in pregnant women with Eisenmenger's syndrome. Eur Heart J. 1995;16:460–464.
112. Geohas C, McLaughlin VV. Successful management of pregnancy in a patient with Eisenmenger syndrome with epoprostenol. Chest. 2003;124:1170–1173.
113. O’Hare R, McLoughlin C, Milligan K, et al. Anaesthesia for caesarean section in the presence of severe primary pulmonary hypertension. Br J Anaesth. 1998;81:790–792.
114. Goland S, Tsai F, Habib M, et al. Favorable outcome of pregnancy with an elective use of epoprostenol and sildenafil in women with severe pulmonary hypertension. Cardiology. 2010;115:205–208.
115. Ng WP, Yip WL. Successful maternal-foetal outcome using nitric oxide and sildenafil in pulmonary hypertension with atrial septal defect and HIV infection. Singapore Med J. 2012;53:e3–e5.
116. Monnery L, Nanson J, Charlton G. Primary pulmonary hypertension in pregnancy; a role for novel vasodilators. Br J Anaesth. 2001;87:295–298.
117. Olofsson C, Bremme K, Forssell G, Ohqvist G. Cesarean section under epidural ropivacaine 0.75% in a parturient with severe pulmonary hypertension. Acta Anaesthesiol Scand. 2001;45:258–260.
118. Martin JT, Tautz TJ, Antognini JF. Safety of regional anesthesia in Eisenmenger's syndrome. Reg Anesth Pain Med. 2002;27:509–513.
119. Blaise G, Langleben D, Hubert B. Pulmonary arterial hypertension: pathophysiology and anesthetic approach. Anesthesiology. 2003;99:1415–1432.
120. Kiely DG, Condliffe R, Webster V, et al. Improved survival in pregnancy and pulmonary hypertension using a multiprofessional approach. BJOG. 2010;117:565–574.
121. Bonnin M, Mercier FJ, Sitbon O, et al. Severe pulmonary hypertension during pregnancy: mode of delivery and anesthetic management of 15 consecutive cases. Anesthesiology. 2005;102:1133–1137.
122. Decoene C, Bourzoufi K, Moreau D, et al. Use of inhaled nitric oxide for emergency Cesarean section in a woman with unexpected primary pulmonary hypertension. Can J Anaesth. 2001;48:584–587.
123. Weiss BM, Maggiorini M, Jenni R, et al. Pregnant patient with primary pulmonary hypertension: inhaled pulmonary vasodilators and epidural anesthesia for cesarean delivery. Anesthesiology. 2000;92:1191–1194.
124. Elliot CA, Stewart P, Webster VJ, et al. The use of iloprost in early pregnancy in patients with pulmonary arterial hypertension. Eur Respir J. 2005;26:168–173.
125. Campuzano K, Roque H, Bolnick A, et al. Bacterial endocarditis complicating pregnancy: case report and systematic review of the literature. Arch Gynecol Obstet. 2003;268:251–255.
126. American College of Obstetricians and Gynecologists. Use of prophylactic antibiotics in labor and delivery. [ACOG Practice Bulletin No. 120. Washington, DC, June 2011] Obstet Gynecol. 2011;117:1472–1483.
127. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation. 2007;116:1736–1754.
128. Nishimura RA, Carabello BA, Faxon DP, et al. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008;118:887–896.
129. Wedel DJ, Horlocker TT. Regional anesthesia in the febrile or infected patient. Reg Anesth Pain Med. 2006;31:324–333.
130. Kane RE. Neurologic deficits following epidural or spinal anesthesia. Anesth Analg. 1981;60:150–161.
131. Auroy Y, Narchi P, Messiah A, et al. Serious complications related to regional anesthesia: results of a prospective survey in France. Anesthesiology. 1997;87:479–486.
132. Thaman R, Curtis S, Faganello G, et al. Cardiac outcome of pregnancy in women with a pacemaker and women with untreated atrioventricular conduction block. Europace. 2011;13:859–863.
133. Jaffe R, Gruber A, Fejgin M, et al. Pregnancy with an artificial pacemaker. Obstet Gynecol Surv. 1987;42:137–139.
134. Cevik B, Colakoglu S, Ilham C, Orskiran A. Anesthetic management of cesarean delivery in pregnant women with a temporary pacemaker. Anesth Analg. 2006;103:500–501.
135. Hidaka N, Chiba Y, Kurita T, et al. Is intrapartum temporary pacing required for women with complete atrioventricular block? An analysis of seven cases. BJOG. 2006;113:605–607.
136. Dhiman N, Sarda N, Arora R. Management of complete heart block during pregnancy. J Obstet Gynaecol Res. 2012.
137. Suri V, Keepanasseril A, Aggarwal N, et al. Maternal complete heart block in pregnancy: analysis of four cases and review of management. J Obstet Gynaecol Res. 2009;35:434–437.
138. Adekanye O, Srinivas K, Collis RE. Bradyarrhythmias in pregnancy: a case report and review of management. Int J Obstet Anesth. 2007;16:165–170.
139. Natale A, Davidson T, Geiger MJ, Newby K. Implantable cardioverter-defibrillators and pregnancy: a safe combination? Circulation. 1997;96:2808–2812.
140. Bonini W, Botto GL, Broffoni T, Dondina C. Pregnancy with an ICD and a documented ICD discharge. Europace. 2000;2:87–90.
141. Shotan A, Ostrzega E, Mehra A, et al. Incidence of arrhythmias in normal pregnancy and relation to palpitations, dizziness, and syncope. Am J Cardiol. 1997;79:1061–1064.
142. Silversides CK, Harris L, Haberer K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tachyarrhythmia and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206–1212.
143. Li JM, Nguyen C, Joglar JA, et al. Frequency and outcome of arrhythmias complicating admission during pregnancy: experience from a high-volume and ethnically-diverse obstetric service. Clin Cardiol. 2008;31:538–541.
144. Lee SH, Chen SA, Wu TJ, et al. Effects of pregnancy on first onset and symptoms of paroxysmal supraventricular tachycardia. Am J Cardiol. 1995;76:675–678.
145. Tawam M, Levine J, Mendelson M, et al. Effect of pregnancy on paroxysmal supraventricular tachycardia. Am J Cardiol. 1993;72:838–840.
146. Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:223–242.
147. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870.
148. Burkart TA, Kron J, Miles WM, et al. Successful termination of atrial flutter by ibutilide during pregnancy. Pacing Clin Electrophysiol. 2007;30:283–286.
149. Brodsky M, Doria R, Allen B, et al. New-onset ventricular tachycardia during pregnancy. Am Heart J. 1992;123:933–941.
150. Cleary-Goldman J, Salva CR, Infeld JI, Robinson JN. Verapamil-sensitive idiopathic left ventricular tachycardia in pregnancy. J Matern Fetal Neonatal Med. 2003;14:132–135.
151. Mittadodla PS, Salen PN, Traub DM. Isoproterenol as an adjunct for treatment of idiopathic ventricular fibrillation storm in a pregnant woman. Am J Emerg Med. 2012;30:251 e3–5.
152. Burrows K, Fox J, Biblo LA, Roth JA. Pregnancy and short-coupled torsades de pointes. Pacing Clin Electrophysiol. 2013;36:e77–e79.
153. Sharif-Kazemi MB, Emkanjoo Z, Tavoosi A, et al. Electrical storm in Brugada syndrome during pregnancy. Pacing Clin Electrophysiol. 2011;34:e18–e21.
154. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:e212–e260.
155. Maron BJ. The electrocardiogram as a diagnostic tool for hypertrophic cardiomyopathy: revisited. Ann Noninvasive Electrocardiol. 2001;6:277–279.
156. Autore C, Conte MR, Piccininno M, et al. Risk associated with pregnancy in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:1864–1869.
157. Seth R, Moss AJ, McNitt S, et al. Long QT syndrome and pregnancy. J Am Coll Cardiol. 2007;49:1092–1098.
158. Rashba EJ, Zareba W, Moss AJ, et al. Influence of pregnancy on the risk for cardiac events in patients with hereditary long QT syndrome. LQTS Investigators. Circulation. 1998;97:451–456.
159. Khositseth A, Tester DJ, Will ML, et al. Identification of a common genetic substrate underlying postpartum cardiac events in congenital long QT syndrome. Heart Rhythm. 2004;1:60–64.
160. Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines. Circulation. 2006;114:e385–e484.
161. Wang YC, Chen CH, Su HY, Yu MH. The impact of maternal cardioversion on fetal haemodynamics. Eur J Obstet Gynecol Reprod Biol. 2006;126:268–269.
162. Parasuraman R, Gandhi MM, Liversedge NH. Nifedipine tocolysis associated atrial fibrillation responds to DC cardioversion. BJOG. 2006;113:844–845.
163. Barnes EJ, Eben F, Patterson D. Direct current cardioversion during pregnancy should be performed with facilities available for fetal monitoring and emergency caesarean section. BJOG. 2002;109:1406–1407.
164. Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet Gynecol. 2005;105:480–484.
165. James AH, Jamison MG, Biswas MS, et al. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006;113:1564–1571.
166. Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol. 2008;52:171–180.
167. Mathew JP, Fleisher LA, Rinehouse JA, et al. ST segment depression during labor and delivery. Anesthesiology. 1992;77:635–641.
168. Palmer CM, Norris MC, Giudici MC, et al. Incidence of electrocardiographic changes during cesarean delivery under regional anesthesia. Anesth Analg. 1990;70:36–43.
169. Zakowski MI, Ramanathan S, Baratta JB, et al. Electrocardiographic changes during cesarean section: a cause for concern? Anesth Analg. 1993;76:162–167.
170. McLintic AJ, Pringle SD, Lilley S, et al. Electrocardiographic changes during cesarean section under regional anesthesia. Anesth Analg. 1992;74:51–56.
171. Kozer E, Nikfar S, Costei A, et al. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: a meta-analysis. Am J Obstet Gynecol. 2002;187:1623–1630.
172. Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791–1798.
173. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e691S–736S.
174. Silversides CK, Colman JM, Sermer M, et al. Early and intermediate-term outcomes of pregnancy with congenital aortic stenosis. Am J Cardiol. 2003;91:1386–1389.
175. Hameed A, Karaalp IS, Tummala PP, et al. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol. 2001;37:893–899.
176. Yap SC, Drenthen W, Pieper PG, et al. Risk of complications during pregnancy in women with congenital aortic stenosis. Int J Cardiol. 2008;126:240–246.
177. Tzemos N, Silversides CK, Colman JM, et al. Late cardiac outcomes after pregnancy in women with congenital aortic stenosis. Am Heart J. 2009;157:474–480.
178. Whitfield A, Holdcroft A. Anaesthesia for caesarean section in patients with aortic stenosis: the case for general anaesthesia. Anaesthesia. 1998;53:109–112.
179. Pittard A, Vucevic M. Regional anaesthesia with a subarachnoid microcatheter for caesarean section in a parturient with aortic stenosis. Anaesthesia. 1998;53:169–173.
180. Ioscovich AM, Goldszmidt E, Fadeev AV, et al. Peripartum anesthetic management of patients with aortic valve stenosis: a retrospective study and literature review. Int J Obstet Anesth. 2009;18:379–386.
181. Tihtonen K, Koobi T, Yli-Hankala A, Uotila J. Maternal hemodynamics during cesarean delivery assessed by whole-body impedance cardiography. Acta Obstet Gynecol Scand. 2005;84:355–361.
182. Van de Velde M, Budts W, Vandermeersch E, Spitz B. Continuous spinal analgesia for labor pain in a parturient with aortic stenosis. Int J Obstet Anesth. 2003;12:51–54.
183. Hamlyn EL, Douglass CA, Plaat F, et al. Low-dose sequential combined spinal-epidural: an anaesthetic technique for caesarean section in patients with significant cardiac disease. Int J Obstet Anesth. 2005;14:355–361.
184. Suntharalingam G, Dob D, Yentis SM. Obstetric epidural analgesia in aortic stenosis: a low-dose technique for labour and instrumental delivery. Int J Obstet Anesth. 2001;10:129–134.
185. Redfern N, Bower S, Bullock RE, Hull CJ. Alfentanil for Caesarean section complicated by severe aortic stenosis: a case report. Br J Anaesth. 1987;59:1309–1312.
186. Orme RM, Grange CS, Ainsworth QP, Grebenik CR. General anaesthesia using remifentanil for caesarean section in parturients with critical aortic stenosis: a series of four cases. Int J Obstet Anesth. 2004;13:183–187.
187. Sheikh F, Rangwala S, DeSimone C, et al. Management of the parturient with severe aortic incompetence. J Cardiothorac Vasc Anesth. 1995;9:575–577.
188. Erturk E, Bostan H, Eroglu A. Epidural analgesia and vaginal delivery in a patient with aortic stenosis and insufficiency. Med Princ Pract. 2011;20:574–576.
189. Silversides CK, Colman JM, Sermer M, Siu SC. Cardiac risk in pregnant women with rheumatic mitral stenosis. Am J Cardiol. 2003;91:1382–1385.
190. Bryg RJ, Gordon PR, Kudesia VS, Bhatia RK. Effect of pregnancy on pressure gradient in mitral stenosis. Am J Cardiol. 1989;63:384–386.
191. Madazli R, Sal V, Cift T, et al. Pregnancy outcomes in women with heart disease. Arch Gynecol Obstet. 2010;281:29–34.
192. Lesniak-Sobelga A, Tracz W, KostKiewicz M, et al. Clinical and echocardiographic assessment of pregnant women with valvular heart diseases—maternal and fetal outcome. Int J Cardiol. 2004;94:15–23.
193. Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60:299–308.
194. de Souza JA, Martinez EE Jr, Ambrose JA, et al. Percutaneous balloon mitral valvuloplasty in comparison with open mitral valve commissurotomy for mitral stenosis during pregnancy. J Am Coll Cardiol. 2001;37:900–903.
195. Clark SL, Phelan JP, Greenspoon J, et al. Labor and delivery in the presence of mitral stenosis: central hemodynamic observations. Am J Obstet Gynecol. 1985;152:984–988.
196. Hemmings GT, Whalley DG, O’Connor PJ, et al. Invasive monitoring and anaesthetic management of a parturient with mitral stenosis. Can J Anaesth. 1987;34:182–185.
197. Borges VT, Matsubara BB, Magalhaes CG, et al. Effect of physiological overload on pregnancy in women with mitral regurgitation. Clinics (Sao Paulo). 2011;66:47–50.
198. Shah PM. Current concepts in mitral valve prolapse—diagnosis and management. J Cardiol. 2010;56:125–133.
199. Freed LA, Levy D, Levine RA, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7.
200. Hayek E, Gring CN, Griffin BP. Mitral valve prolapse. Lancet. 2005;365:507–518.
201. Freed LA, Benjamin EJ, Levy D, et al. Mitral valve prolapse in the general population: the benign nature of echocardiographic features in the Framingham Heart Study. J Am Coll Cardiol. 2002;40:1298–1304.
202. Avierinos JF, Gersh BJ, Melton LJ 3rd, et al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation. 2002;106:1355–1361.
203. Hameed AB, Goodwin TM, Elkayam U. Effect of pulmonary stenosis on pregnancy outcomes–a case-control study. Am Heart J. 2007;154:852–854.
204. Campos O, Andrade JL, Bocanegra J, et al. Physiologic multivalvular regurgitation during pregnancy: a longitudinal Doppler echocardiographic study. Int J Cardiol. 1993;40:265–272.
205. Khairy P, Ouyang DW, Fernandes SM, et al. Pregnancy outcomes in women with congenital heart disease. Circulation. 2006;113:517–524.
206. Greutmann M, Von Klemperer K, Brooks R, et al. Pregnancy outcome in women with congenital heart disease and residual haemodynamic lesions of the right ventricular outflow tract. Eur Heart J. 2010;31:1764–1770.
207. Sbarouni E, Oakley CM. Outcome of pregnancy in women with valve prostheses. Br Heart J. 1994;71:196–201.
208. Salazar E, Zajarias A, Gutierrez N, Iturbe I. The problem of cardiac valve prostheses, anticoagulants, and pregnancy. Circulation. 1984;70:I169–I177.
209. Born D, Martinez EE, Almeida PA, et al. Pregnancy in patients with prosthetic heart valves: the effects of anticoagulation on mother, fetus, and neonate. Am Heart J. 1992;124:413–417.
210. Hanania G, Thomas D, Michel PL, et al. Pregnancy in patients with heart valve prosthesis: a French retrospective cooperative study (155 cases). Arch Mal Coeur Vaiss. 1994;87:429–437.
211. Bortolotti U, Milano A, Mazzucco A, et al. Pregnancy in patients with a porcine valve bioprosthesis. Am J Cardiol. 1982;50:1051–1054.
212. Salazar E, Espinola N, Roman L, Casanova JM. Effect of pregnancy on the duration of bovine pericardial bioprostheses. Am Heart J. 1999;137:714–720.
213. Jamieson WR, Miller DC, Akins CW, et al. Pregnancy and bioprostheses: influence on structural valve deterioration. Ann Thorac Surg. 1995;60:S282–S286.
214. Avila WS, Rossi EG, Grinberg M, Ramires JA. Influence of pregnancy after bioprosthetic valve replacement in young women: a prospective five-year study. J Heart Valve Dis. 2002;11:864–869.
215. North RA, Sadler L, Stewart AW, et al. Long-term survival and valve-related complications in young women with cardiac valve replacements. Circulation. 1999;99:2669–2676.
216. Arabkhani B, Heuvelman HJ, Bogers AJ, et al. Does pregnancy influence the durability of human aortic valve substitutes? J Am Coll Cardiol. 2012;60:1991–1992.
217. Badduke BR, Jamieson WR, Miyagishima RT, et al. Pregnancy and childbearing in a population with biologic valvular prostheses. J Thorac Cardiovasc Surg. 1991;102:179–186.
218. Elkayam U, Singh H, Irani A, Akhter MW. Anticoagulation in pregnant women with prosthetic heart valves. J Cardiovasc Pharmacol Ther. 2004;9:107–115.
219. Whitlock RP, Sun JC, Fremes SE, et al. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e576S–600S.
220. Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med. 2000;160:191–196.
221. Yinon Y, Siu SC, Warshafsky C, et al. Use of low molecular weight heparin in pregnant women with mechanical heart valves. Am J Cardiol. 2009;104:1259–1263.
222. Abildgaard U, Sandset PM, Hammerstrom J, et al. Management of pregnant women with mechanical heart valve prosthesis: thromboprophylaxis with low molecular weight heparin. Thromb Res. 2009;124:262–267.
223. McLintock C, McCowan LM, North RA. Maternal complications and pregnancy outcome in women with mechanical prosthetic heart valves treated with enoxaparin. BJOG. 2009;116:1585–1592.
224. Quinn J, Von Klemperer K, Brooks R, et al. Use of high intensity adjusted dose low molecular weight heparin in women with mechanical heart valves during pregnancy: a single-center experience. Haematologica. 2009;94:1608–1612.
225. Ekbatani A, Asaro LR, Malinow AM. Anticoagulation with argatroban in a parturient with heparin-induced thrombocytopenia. Int J Obstet Anesth. 2010;19:82–87.
226. Vandermeulen E, Singelyn F, Vercauteren M, et al. Belgian guidelines concerning central neural blockade in patients with drug-induced alteration of coagulation: an update. Acta Anaesthesiol Belg. 2005;56:139–146.
227. Kozek-Langenecker SA, Fries D, Gutl M, et al. [Locoregional anesthesia and coagulation inhibitors. Recommendations of the Task Force on Perioperative Coagulation of the Austrian Society for Anesthesiology and Intensive Care Medicine]. Anaesthesist. 2005;54:476–484.
228. Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Eur J Heart Fail. 2008;10:933–989.
229. Pearson GD, Veille JC, Rahimtoola S, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283:1183–1188.
230. Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet 2006; 368:687-93.
231. Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767–778.
232. Hibbard JU, Lindheimer M, Lang RM. A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet Gynecol. 1999;94:311–316.
233. Elkayam U, Akhter MW, Singh H, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111:2050–2055.
234. Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 2005;80:1602–1606.
235. Mielniczuk LM, Williams K, Davis DR, et al. Frequency of peripartum cardiomyopathy. Am J Cardiol. 2006;97:1765–1768.
236. Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084.
237. Elkayam U, Tummala PP, Rao K, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344:1567–1571.
238. Velickovic IA, Leicht CH. Continuous spinal anesthesia for cesarean section in a parturient with severe recurrent peripartum cardiomyopathy. Int J Obstet Anesth. 2004;13:40–43.
239. Shnaider R, Ezri T, Szmuk P, et al. Combined spinal-epidural anesthesia for Cesarean section in a patient with peripartum dilated cardiomyopathy. Can J Anaesth. 2001;48:681–683.
240. Pirlet M, Baird M, Pryn S, et al. Low dose combined spinal-epidural anaesthesia for caesarean section in a patient with peripartum cardiomyopathy. Int J Obstet Anesth. 2000;9:189–192.
241. Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239.
242. Spirito P, Bellone P, Harris KM, et al. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785.
243. Oakley GD, McGarry K, Limb DG, Oakley CM. Management of pregnancy in patients with hypertrophic cardiomyopathy. Br Med J. 1979;1:1749–1750.
244. Turner GM, Oakley CM, Dixon HG. Management of pregnancy complicated by hypertrophic obstructive cardiomyopathy. Br Med J. 1968;4:281–284.
245. Thompson RC, Liberthson RR, Lowenstein E. Perioperative anesthetic risk of noncardiac surgery in hypertrophic obstructive cardiomyopathy. JAMA. 1985;254:2419–2421.
246. Ferguson EA, Paech MJ, Veltman MG. Hypertrophic cardiomyopathy and caesarean section: intraoperative use of transthoracic echocardiography. Int J Obstet Anesth. 2006;15:311–316.
247. Loubser P, Suh K, Cohen S. Adverse effects of spinal anesthesia in a patient with idiopathic hypertrophic subaortic stenosis. Anesthesiology. 1984;60:228–230.
248. Stergiopoulos K, Shiang E, Bench T. Pregnancy in patients with pre-existing cardiomyopathies. J Am Coll Cardiol. 2011;58:337–350.
249. Boccio RV, Chung JH, Harrison DM. Anesthetic management of cesarean section in a patient with idiopathic hypertrophic subaortic stenosis. Anesthesiology. 1986;65:663–665.
250. Autore C, Brauneis S, Apponi F, et al. Epidural anesthesia for cesarean section in patients with hypertrophic cardiomyopathy: a report of three cases. Anesthesiology. 1999;90:1205–1207.
251. Dolbeau JB, Hebert T, Espitalier F, et al. Obstructive hypertrophic cardiomyopathy and caesarean section under combined spinal and epidural anaesthesia with prophylactic vascular ligation: regarding a case. Ann Fr Anesth Reanim. 2011;30:64–66.
252. Pryn A, Bryden F, Reeve W, et al. Cardiomyopathy in pregnancy and caesarean section: four case reports. Int J Obstet Anesth. 2007;16:68–73.
253. Deiml R, Hess W, Bahlmann E. [Primary cesarean section: use of phenylephrine during anesthesia in a patient with hypertrophic obstructive cardiomyopathy]. Anaesthesist. 2000;49:527–531.
254. Thaman R, Varnava A, Hamid MS, et al. Pregnancy related complications in women with hypertrophic cardiomyopathy. Heart. 2003;89:752–756.
255. Ho KM, Ngan Kee WD, Poon MC. Combined spinal and epidural anesthesia in a parturient with idiopathic hypertrophic subaortic stenosis. Anesthesiology. 1997;87:168–169.
256. Okutomi T, Kikuchi S, Amano K, et al. Continuous spinal analgesia for labor and delivery in a parturient with hypertrophic obstructive cardiomyopathy. Acta Anaesthesiol Scand. 2002;46:329–331.
257. Ishiyama T, Oguchi T, Iijima T, et al. Combined spinal and epidural anesthesia for cesarean section in a patient with hypertrophic obstructive cardiomyopathy. Anesth Analg. 2003;96:629–630.
258. Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118:2754–2762.
259. Brezina P, Isler CM. Takotsubo cardiomyopathy in pregnancy. Obstet Gynecol. 2008;112:450–452.
260. Zdanowicz JA, Utz AC, Bernasconi I, et al. “Broken heart” after cesarean delivery: case report and review of literature. Arch Gynecol Obstet. 2011;283:687–694.
261. Crimi E, Baggish A, Leffert L, et al. Images in cardiovascular medicine: acute reversible stress-induced cardiomyopathy associated with cesarean delivery under spinal anesthesia. Circulation. 2008;117:3052–3053.
262. Grewal J, Siu SC, Ross HJ, et al. Pregnancy outcomes in women with dilated cardiomyopathy. J Am Coll Cardiol. 2009;55:45–52.
263. Hasdemir C, Ulucan C, Yavuzgil O, et al. Tachycardia-induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: the incidence, clinical and electrophysiologic characteristics, and the predictors. J Cardiovasc Electrophysiol. 2011;22:663–668.
264. Ferguson JD, Helms A, Mangrum JM, DiMarco JP. Ablation of incessant left atrial tachycardia without fluoroscopy in a pregnant woman. J Cardiovasc Electrophysiol. 2011;22:346–349.
265. Pagad SV, Barmade AB, Toal SC, et al. ‘Rescue’ radiofrequency ablation for atrial tachycardia presenting as cardiomyopathy in pregnancy. Indian Heart J. 2004;56:245–247.
266. LaRue S, Shanks A, Wang IW, et al. Left ventricular assist device in pregnancy. Obstet Gynecol. 2011;118:426–428.
267. Etz C, Welp H, Scheld HH, Schmid C. Near fatal infection of a patient with a left ventricular assist device due to unrecognized fetal death. Eur J Cardiothorac Surg. 2005;27:722–723.
268. Abduljabbar HS, Marzouki KM, Zawawi TH, Khan AS. Pericardial effusion in normal pregnant women. Acta Obstet Gynecol Scand. 1991;70:291–294.
269. Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S829–S861.
270. Dijkman A, Huisman CM, Smit M, et al. Cardiac arrest in pregnancy: increasing use of perimortem caesarean section due to emergency skills training? BJOG. 2010;117:282–287.
271. Morini A, Spina V, Aleandri V, et al. Pregnancy after heart transplant: update and case report. Hum Reprod. 1998;13:749–757.
272. Armenti VT, Radomski JS, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl. 2004;103–114.
273. John AS, Gurley F, Schaff HV, et al. Cardiopulmonary bypass during pregnancy. Ann Thorac Surg. 2011;91:1191–1196.
274. Becker RM. Intracardiac surgery in pregnant women. Ann Thorac Surg. 1983;36:453–458.
275. Chambers CE, Clark SL. Cardiac surgery during pregnancy. Clin Obstet Gynecol. 1994;37:316–323.
276. Bernal JM, Miralles PJ. Cardiac surgery with cardiopulmonary bypass during pregnancy. Obstet Gynecol Surv. 1986;41:1–6.
277. Iscan ZH, Mavioglu L, Vural KM, et al. Cardiac surgery during pregnancy. J Heart Valve Dis. 2006;15:686–690.
* Wood unit, unit of measure of vascular resistance (mm Hg • min/L); multiply by 80 to convert to dyne•s•cm−5.