Musculoskeletal Disorders
Roanne Preston MD, FRCPC
Chapter Outline
Scoliosis Associated with Neuromuscular Disease
CHRONIC INFLAMMATORY ARTHRITIDES
Tethered Cord and Arnold-Chiari Malformation
Pregnancy commonly results in musculoskeletal complaints. Although they typically are benign and self-limited, symptoms may be disabling in some women. In addition, preexisting musculoskeletal disorders interact with pregnancy to a variable extent. These interactions range from an ameliorating effect of pregnancy on the course of the disease (e.g., rheumatoid arthritis) to the potential for a significant and possibly life-threatening deterioration in maternal condition (e.g., uncorrected severe thoracic scoliosis). The purpose of this chapter is to discuss the most common musculoskeletal disorders encountered in pregnant women and their implications for obstetric and anesthesia providers.
Lumbopelvic Pain of Pregnancy
Lumbopelvic pain is the most common musculoskeletal complaint during pregnancy; it comprises two distinct areas of discomfort: (1) the lumbar spine area (low back pain) and (2) the posterior pelvic girdle area (from the sacroiliac joints radiating down into the posterior thighs), which has been termed pelvic girdle pain.1-3 Lumbopelvic pain of pregnancy occurs at some time during gestation in more than 50% of pregnant women and impairs at least one normal activity of daily life, including sleep. Although originally it seemed to be a more significant problem in Scandinavian countries than elsewhere, it is now recognized as a universal issue, with the prevalence ranging from 25% to 70%. It is the most common reason for sick leave during pregnancy.2 Women with mainly pelvic girdle pain report more disability during pregnancy than those with lumbar pain alone.3,4 Differentiation between pelvic girdle pain and low back pain is important because the management differs and the disability of pelvic girdle pain is more likely to extend into the postpartum period for up to 1 to 2 years.2 Risk factors for lumbopelvic pain of pregnancy include a history of low back pain, young age, hypermobile joints, low socioeconomic class, multiparity, and spondylolisthesis; however, the strongest factors are prior history of lumbopelvic pain of pregnancy, previous non–pregnancy-related low back pain, and strenuous work.3-7 Unfortunately, women who suffer from lumbopelvic pain in one pregnancy have a very high risk for experiencing it during subsequent pregnancies.
The etiology includes hormonal and mechanical factors. The corpus luteum synthesizes and releases relaxin, and maternal blood concentrations of this peptide hormone increase 10-fold during gestation. Relaxin induces ligamentous softening and peripheral and pelvic joint laxity, which cause instability of the symphysis pubis and sacroiliac joints; the extent of instability and disability may be related to the maternal concentration of relaxin. There is a correlation between mean serum levels of relaxin and the occurrence of back pain during pregnancy, and women with incapacitating symptoms have the greatest serum concentrations of relaxin.8
Mechanical changes have a later onset than hormonal changes. Women with pelvic girdle pain have increased pelvic joint motion, which increases sheer forces across the joints and likely results in pain.2 In all pregnant women uterine enlargement results in a forward rotation of the sacrum and an increase in the lumbar lordotic curve, which tends to close the lumbar interlaminar space (Figure 48-1). This change exaggerates the mechanical load borne by both the facet joints and the posterior aspect of the intervertebral discs. These mechanical changes also may compromise nerve root foramina. Sciatica occurs in 1% of pregnant women, and most cases occur late in pregnancy.9 Sciatica is distinguished from pelvic girdle pain by its extension to the ankle or involvement of the foot, and it may be associated with neurologic changes.9 Disc herniation is rare in pregnancy but does occur.10 Incapacitating pain that radiates below the knee, typically accompanied by progressive neurologic deficits or bowel and bladder dysfunction, distinguishes disc herniation from the more common and benign lumbopelvic pain of pregnancy.3,10
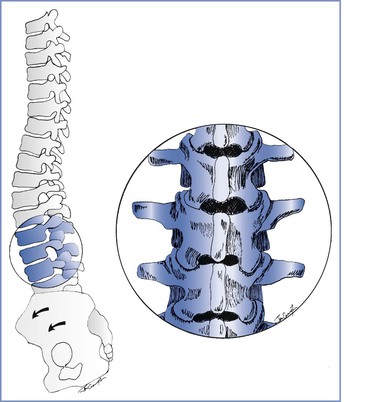
FIGURE 48-1 Musculoskeletal changes of pregnancy. Forward rotation of the pelvis and greater lumbar lordosis increase the load borne by the posterior vertebral elements and tend to close the lumbar interlaminar spaces. Inset, Lumbar vertebrae L2 to L4.
In summary, hormonal changes cause sacroiliac joint dysfunction, which is responsible for the lumbopelvic pain that occurs early in pregnancy. Mechanical changes are primarily responsible for the pain that manifests during late gestation, although symphysis pubis and sacroiliac joint instability may also continue to cause pain. Disc herniation is uncommon and is characterized by the presence of neurologic findings.
Obstetric Management
Treatment is conservative in the absence of neurologic compromise. Structured exercise programs, acetaminophen, transcutaneous electrical nerve stimulation (TENS), and acupuncture have been shown to be beneficial for women suffering from lumbopelvic pain during pregnancy.11,12 Bed rest is reserved for patients with neurologic symptoms or disability secondary to pelvic instability. Patients with severe neurologic signs or symptoms of disc herniation should be assessed by a consultant neurosurgeon who can provide recommendations for intrapartum and postpartum care. Surgical intervention may be required in women with incapacitating pain or progressive neurologic deficits.13 In a woman with severe symptoms, the obstetrician may choose to perform elective instrumental vaginal delivery to decrease maternal work and back stress during the second stage of labor. Because the disability associated with lumbopelvic pain of pregnancy, especially pelvic girdle pain, can impair the woman's ability to function postpartum, it is important to diagnose lumbopelvic pain of pregnancy and treat it appropriately.2
Anesthetic Management
No evidence suggests that epidural or spinal anesthesia is contraindicated in patients with lumbopelvic pain. The anesthesia provider may administer neuraxial anesthesia, even to patients with sciatica. However, neurologic signs and symptoms should be first identified, delineated, and recorded. It seems prudent to administer a dilute solution of local anesthetic, with or without an opioid, to minimize motor block associated with epidural analgesia during labor to reduce any further stress on relaxed sacroiliac joints. Women with lumbopelvic pain of pregnancy may be reluctant to have neuraxial anesthesia because of concern that it may aggravate symptoms. The literature does not support this fear, and reassurance may be required.
All members of the obstetric care team must pay careful attention to the positioning of the patient with back complaints. The patient must not be placed in a position that she could not tolerate before the administration of neuraxial anesthesia. The lithotomy position puts significant stress on the lower back and should be avoided whenever possible. If it is used, care must be taken to raise and lower both legs simultaneously to prevent injury to the lumbar spine and to avoid extremes when positioning the legs. Finally, caregivers should avoid rotational movements of the spine during transfer of the patient between the bed and the operating table.2
Chronic Low Back Pain
Approximately 50% of pregnant women with a previous history of back pain or those with chronic low back pain experience a recurrence or exacerbation of their symptoms during pregnancy.6 Neuraxial anesthesia is more likely to fail in patients with chronic low back pain and in those who have had back surgery.14-16 Benzon et al.14 reported a delayed onset of epidural anesthesia in patients with back pain or sciatica; the affected roots were blocked 10 to 70 minutes later than the contralateral roots at the same level. The delay in block onset most likely results from the inability of the local anesthetic agent to diffuse into the area of the injured root. Luyendijk and van Voorthuisen17 evaluated 600 epidurograms and confirmed that contrast material failed to reach the nerve root in 33% of patients with uncomplicated disc prolapse and did not move beyond the affected disc space in 5% of cases. This may be due to epidural scarring and adhesions that may develop during healing after disc injury. During epidurography, they noted that contrast material did not diffuse past the level of an injured disc and exited through the foramina below the abnormal disc. Prolapse of an intervertebral disc may result in relative or total obstruction of the flow of local anesthetic agent within the epidural space. The unblocked area includes the affected segment but also may include all segments (either ipsilateral or bilateral) distal to the affected level.
Sharrock et al.15 reported a high rate (91%) of successful epidural anesthesia in patients with a history of limited spinal surgery. However, the success rate was lower than that achieved by the same group of anesthesiologists in a population with no history of back surgery (98.7%). They attributed the greater rate of failure to the distortion of surface anatomy and the tethering of the dura to the ligamentum flavum by scar formation, which rendered the epidural space discontinuous or obliterated it entirely. Support for this hypothesis is provided by LaRocca and MacNab's18 description of the post-laminectomy membrane. They noted the post-laminectomy formation of organized fibrous tissue surrounding the dura and, at times, binding of the nerves to the posterior aspect of the disc and adjacent vertebral body. The fibrous response was proportional to the extent of surgical trauma and was more marked with greater operative exposures. Consequently, a local anesthetic agent injected into the epidural space may not diffuse beyond the area of scarring and an inadequate or unexpectedly high block may result.16 Post-laminectomy spinal stenosis also may lead to attenuation or obliteration of the epidural space, and the most common site of obstructive stenosis is immediately above the fusion mass.19
Obstetric Management
It is not uncommon for obstetricians to offer pregnant women who have had persistent chronic low back pain the option of cesarean delivery to decrease the potential for further back injury during labor. There are no data to either encourage or discourage this option.
Anesthetic Management
The anesthesia provider may offer epidural or spinal anesthesia to patients with previous lumbar spine pathology or surgery after an appropriate history and screening examination to identify any neurologic deficits.10 A decreased incidence of successful epidural anesthesia may be expected, especially in patients who have had extensive surgery. Nonetheless, the experienced anesthesia provider will likely administer epidural anesthesia successfully in the majority of patients. Sharrock et al.15 recommended administration of epidural anesthesia one or two interspaces above the operated segment to improve the likelihood of a successful block. Subarachnoid anesthesia is likely to be more reliable than epidural anesthesia in this patient population.
Postpartum Backache
Postpartum backache is a common complaint worldwide, occurring in at least 25% of women, with 5% to 7% of women seeking medical help.3 MacArthur et al.,20,21 citing data obtained from a postal survey of 11,701 women who had delivered 1 to 9 years previously, reported that postpartum backache, starting within 3 months of delivery and persisting for 6 weeks or longer, occurred in 23% of women. Approximately 25% of these women had experienced backache before delivery, but 14% reported new-onset backache. In many women, the pain was persistent; 70% had experienced it for more than 2 years, and 65% had pain at the time of questioning 1 to 9 years later. Back pain was more common in women who delivered vaginally with epidural analgesia than in those who did not have epidural analgesia (18.9% versus 10.5%, respectively). Women who had epidural analgesia also were more likely to have had induced labor, an abnormal fetal position, a multiple pregnancy, a prolonged first or second stage of labor, forceps delivery, episiotomy, cesarean delivery, postpartum hemorrhage, or a large infant.
MacLeod et al.22 also performed a postal survey of 2065 patients 1 year postpartum and reported a 26.2% incidence of postpartum backache in women who had received epidural analgesia, compared with a 1.7% incidence in those who had not; the latter incidence of postpartum backache (1.7%) is the lowest, by far, reported by any investigator in a postpartum population in the first year after delivery. Orlikowski et al.,23 who examined data from 992 women as a secondary analysis of a prospective randomized study on epidural analgesia versus continuous midwifery support, found no relationship between back pain at 6 months postpartum and the use of epidural analgesia. Mogren24 sent a questionnaire to 639 women who had completed an earlier postpartum survey in which they indicated that they had suffered from lumbopelvic pain during pregnancy. Mogren explored the relationship between persistent lumbopelvic pain at 6 months postpartum, mode of delivery, and the use of epidural or spinal anesthesia. She concluded that use of epidural or spinal anesthesia was not associated with persistent lumbopelvic pain.
A number of investigators have carried out prospective evaluations to eliminate the potential for reporting bias that may confound retrospective surveys. Breen et al.25 assessed 1042 women at 6 months postpartum. Although 44% of women experienced postpartum backache, there was no difference between those who had received epidural analgesia and those who had not. The most significant predictor of postpartum backache was antenatal back pain. Weight gain was greater in patients with postpartum and new-onset back pain.
Macarthur et al.26 also prospectively studied the association between epidural analgesia and early, new-onset postpartum backache in 329 women. In patients who labored without epidural analgesia, the incidence of postpartum backache was 43% at 1 day, 23% at 7 days, and 7% at 6 weeks. The incidence of symptoms in patients who had received epidural analgesia was greater on the first postpartum day (53%), but this increase was not persistent. At 1 year postpartum, 12% of the patients had back pain (9.9% in the epidural group and 13.8% in the control group). Howell et al.27 performed a randomized controlled trial comparing epidural with nonepidural analgesia during labor in 369 nulliparous women. There was no difference in the incidence or characteristics of postpartum backache at 3 and 12 months postpartum. In a follow-up study, there was no difference between the two groups in the incidence of back pain, disability, or movement restriction more than 2 years after delivery.28
The type of epidural analgesia provided has also been reviewed to determine whether alteration of the technique affects postpartum backache. Wilson et al.29 reported the incidence of postpartum backache in 1054 nulliparous women enrolled in the Comparative Obstetric Mobile Epidural Trial (COMET). The women had received either high-dose labor epidural analgesia (bupivacaine 0.25% administered with intermittent bolus injections) or low-dose mobile analgesia (either combined spinal-epidural [CSE] analgesia followed by intermittent epidural bolus injections of 0.1% bupivacaine with fentanyl or a low-dose epidural infusion of 0.1% bupivacaine with fentanyl). The incidence of backache that started within 3 months and lasted for at least 6 weeks did not differ between the three groups: 46.9% (high dose), 41.7% (CSE), and 45.8% (low-dose infusion). These findings are similar to results of previous studies of this issue.
Both transient and more persistent postpartum backaches are common, but there is little evidence that they are related to the provision of epidural analgesia during labor. Similarly, no evidence suggests that denying a parturient epidural analgesia results in a lower incidence of back problems during the postpartum period. Factors associated with more persistent postpartum backache include the presence of back pain before pregnancy, the presence of lumbopelvic pain of pregnancy, cesarean delivery, and performance of physically demanding work.3,24
Scoliosis
Scoliosis is a lateral deviation in the vertical axis of the spine. The severity of scoliosis is determined by measurement of the angle of the spinal curve, the Cobb angle, which is expressed in degrees (Figure 48-2). The incidence of minor curves is 4 per 1000 in the North American population; larger curves occur less frequently, predominantly in females. Severe scoliosis is relatively rare in pregnant women, occurring in 0.03% of pregnancies.30 Although women with moderate to severe scoliosis constitute a small population of obstetric patients, pregnancy within this population is common.31 Most cases of scoliosis are idiopathic, although some are associated with other conditions, most commonly neuromuscular disorders (Box 48-1).
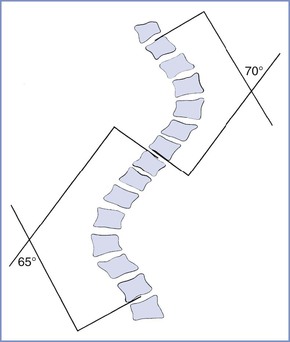
FIGURE 48-2 Schematic representation of the Cobb angle. A line is drawn parallel to the superior cortical plate of the proximal end vertebrae and another line parallel to the inferior cortical plate of the distal end vertebrae. A perpendicular line is drawn to each of these lines. The angle of intersection is the Cobb angle of the curve.
Scoliotic curves can be divided into structural and nonstructural varieties. Nonstructural curves are those seen with postural scoliosis, sciatica, and leg-length discrepancies. They do not affect the mobility of the spine and are nonprogressive. Structural curves are seen in patients with idiopathic scoliosis and with scoliosis resulting from the conditions listed in Box 48-1. Structural curves lead to reduced spinal mobility, and affected patients typically have a fixed prominence (rib hump) on the convex side of the curve. There is also a rotatory component associated with the structural scoliotic curve. The axial rotation of the vertebral body is such that the spinous processes rotate away from the convexity of the curve and back toward the midline of the patient (Figures 48-3 and 48-4).32 Deformation of the vertebral bodies results in shorter, thinner pedicles and laminae and a more narrow vertebral canal on the concave side. Vertebral deformation is unusual in patients with a Cobb angle less than 40 degrees.
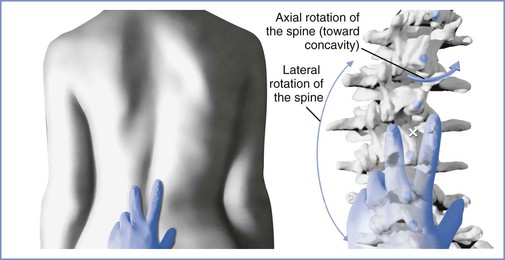
FIGURE 48-3 Spinal rotation with scoliosis. Left, View of the lumbar spine in a patient with a scoliotic curve to the left demonstrating surface landmark palpation. Right, Skeletal anatomy at the same level in the same patient. There is a reduction in the dimensions of the interlaminar space on the concave side of the curve (to the right) and an expansion on the convex side. These changes are enhanced with greater severity of the curve. As the curve increases, the spinous processes rotate into the concavity of the curve, further altering the local anatomy. Surface landmark palpation from the view at left superimposed on the skeleton reveals how the palpated midline (indicated by the white X) is to the right of the true midline. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
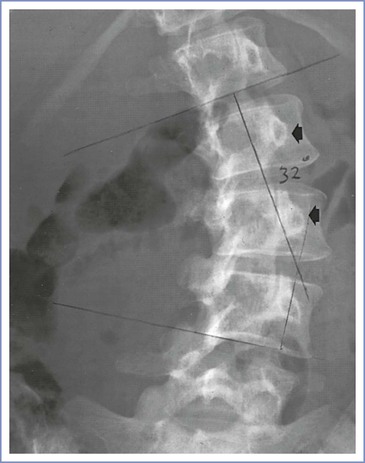
FIGURE 48-4 Radiographic study of the lumbar spine in a 26-year-old woman with idiopathic scoliosis. The spinous processes and pedicles (arrows) are rotated away from the convexity and into the concavity of the curve. (The epidural space was entered easily with direction of the needle approximately 15 degrees off the perpendicular at the skin level toward the convexity of the curve.)
Scoliosis interferes with the formation, growth, and development of the lungs; the occurrence of scoliosis before lung maturity may reduce the number of alveoli that ultimately form. The pulmonary vasculature develops in parallel with the alveoli; early-onset scoliosis and severe scoliosis may result in greater pulmonary vascular resistance and eventually lead to pulmonary hypertension. Musculoskeletal deformities also affect the mechanical function of the lungs; anatomic findings in scoliosis that are most commonly associated with respiratory compromise include the presence of a thoracic curve, thoracic lordosis, and a rib cage deformity. The most common pulmonary function abnormality is a restrictive pattern with decreases in vital capacity, total lung capacity, and lung compliance. This pattern occurs in all patients with a thoracic curve greater than 65 degrees. The functional residual capacity (FRC) is reduced, and airways may close during normal tidal breathing. If the FRC is reduced to the extent that it falls below the closing capacity, atelectasis may occur in basal alveoli. The most common blood gas abnormality is an increased alveolar-to-arterial oxygen gradient, with reduced PaO2 and a normal PaCO2. It results from both venoarterial shunting and altered regional perfusion. Venous admixture may lead to arterial hypoxemia. The natural history of severe, progressive scoliosis includes early death from cardiopulmonary failure.33
Permanent changes of the pulmonary vasculature are common in patients with a curve greater than 65 degrees. Pulmonary hypertension (a resting mean pulmonary artery pressure exceeding 25 mm Hg) occurs in many patients with severe deformity long before the onset of right-sided heart failure and is largely attributable to increases in vascular resistance resulting from chronic alveolar hypoxia, hypoxic pulmonary vasoconstriction, and anatomic changes in the vascular bed. Fixed pulmonary hypertension carries a grave prognosis in pregnancy and may prompt a recommendation to avoid or terminate pregnancy.34
Scoliosis Associated with Neuromuscular Disease
When scoliosis develops secondary to a neurologic or myopathic disorder, abnormal respiratory function results not only from the skeletal deformity but also from abnormalities in the central control of respiration and the supraspinal innervation of the respiratory muscles, as well as from the loss of muscle function caused by the underlying disorder. Respiratory function may be further compromised by (1) impairment of the defense mechanisms of the airways due to loss of control of the pharynx and the larynx, (2) an ineffective cough mechanism, and (3) infrequent or reduced large breaths. Recurrent aspiration pneumonitis may result from compromised protective airway reflexes. In general, the prognosis of scoliosis due to neuromuscular disease is worse than that of idiopathic scoliosis and is determined predominantly by progression of the primary disorder. Affected patients typically develop irreversible respiratory failure at a younger age, and pulmonary hypertension is common; pregnancy is uncommon in this population.
Interaction with Pregnancy
Pregnancy may exacerbate both the severity of the spinal curvature and cardiopulmonary abnormalities in women with uncorrected scoliosis. Progression of a curve, defined as an increase in the Cobb angle of 5 degrees or more over subsequent assessments, most likely occurs during periods of rapid growth and in patients with larger curves at the time of diagnosis. Curves that are less than 25 degrees or curves that have been stable before pregnancy typically do not progress during pregnancy.35,36 In contrast, more severe curves and those that have not stabilized may worsen. Some investigators have described a correlation between the severity of the curve and maternal morbidity and mortality. However, it is likely that the severity of functional cardiopulmonary impairment before pregnancy is a better predictor of maternal outcome than the severity of the curve.37 Patients with a severe curve (i.e., Cobb angle > 60 degrees) but good cardiopulmonary function tolerate pregnancy well, whereas in those with significant cardiopulmonary compromise, and especially in those with pulmonary hypertension, maternal mortality is high.38,39
The physiologic changes of pregnancy include decreases in both functional residual and closing capacities and increases in minute ventilation and oxygen demand. The thoracic cage normally increases in circumference during pregnancy as a result of increases in both anteroposterior and transverse diameters. If the chest cage is relatively fixed by scoliosis, the diaphragm is responsible for all increments in minute ventilation. As the enlarging uterus causes elevation of the diaphragm, diaphragmatic activity is restricted and further decreases in residual and closing capacities may occur, which may result in both greater ventilation-perfusion mismatch and decreased arterial oxygen content. The antepartum onset of new symptoms of respiratory compromise or the exacerbation of preexisting symptomatology is associated with higher maternal morbidity and a greater likelihood that assisted ventilation will be required after cesarean delivery.37
Minute ventilation typically increases by 45% during pregnancy. In normal pregnancy, the increase is primarily a result of increased tidal volume. In the scoliotic patient with restrictive lung disease, a larger tidal volume may not be possible, and the increased minute ventilation is achieved by means of a higher respiratory rate and increased work of breathing. Peak increases in pulmonary activity are reached by the middle of the third trimester, but the uterus continues to grow until term, and it may further encroach on the noncompliant thorax, causing late gestational deterioration despite stabilization of respiratory demand.
Dyspnea on exertion is uncommon in patients with scoliosis who have curves less than 70 degrees, but it becomes more common as the deformity exceeds 100 degrees. In younger patients with a curve less than 70 degrees, exercise capacity is more likely to be impaired because of the lack of regular aerobic exercise and subsequent deconditioning rather than intrinsic ventilatory impairment.40 Dyspnea is common in many pregnant women, typically begins in the first or second trimester, and is most prevalent at term. Two features help distinguish physiologic from pathologic dyspnea.41 Physiologic dyspnea tends to occur earlier in pregnancy and often plateaus or even improves as term approaches. The pathologic dyspnea of cardiopulmonary decompensation more often begins in the second half of pregnancy and is progressive, often becoming most severe as gestation advances and the physiologic loading is maximal. Second, physiologic dyspnea is rarely extreme, and patients can maintain most daily activities. Dyspnea that is extreme or has a limiting effect on normal activity may signal maternal cardiorespiratory decompensation. Dyspnea at rest is also rare in the absence of cardiopulmonary dysfunction, as is dyspnea that is acute in onset or progressive and intractable.
Minute ventilation of the unmedicated parturient increases by a further 75% to 150% in the first stage of labor and by 150% to 300% in the second stage. Oxygen consumption increases above prelabor values by 40% in the first stage and 75% in the second stage. These levels may be unattainable by the scoliotic parturient with restrictive lung disease, and respiratory failure and hypoxemia may result during labor.
Pregnant women with pulmonary hypertension have a limited ability to increase cardiac output. During normal pregnancy, cardiac output increases 40% to 50% above nonpregnant measurements; during labor and delivery, even greater increases are observed. These increases are achieved with both larger stroke volume and a higher heart rate. These demands may put an excessive burden on the cardiovascular system in parturients who had marginal cardiac reserve before pregnancy. If the right ventricle fails in the presence of pulmonary hypertension, left ventricular filling will decrease and low-output failure and sudden death may occur.34
Surgical Management
During spinal fusion and instrumentation, the spinal musculature is reflected off the vertebrae over the course of the curve and the spinous processes and interspinous ligaments are removed. The spine is subsequently extended, correcting the curve. The vertebrae are decorticated throughout the extent of the planned fusion, instrumentation is placed, and bone graft material from the ileum is placed over the decorticated vertebrae. A number of techniques for fusion have been described, but all involve both spinal instrumentation and extensive bone grafting in the axial spine (Figure 48-5).
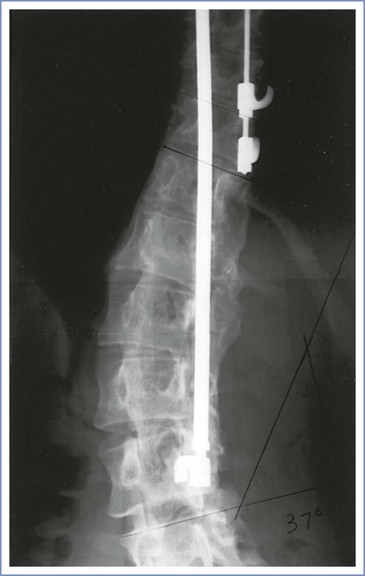
FIGURE 48-5 Harrington rod instrumentation. Radiographic study of the lumbar spine in a 31-year-old woman with thoracolumbar scoliosis corrected with spinal instrumentation. There is rotation of the vertebrae into the curve (toward the rod), and extensive bone grafting is evident adjacent to the rod. Two lumbar interspaces (L4 to L5 and L5 to S1) are not involved in the fusion.
Obstetric Management
Pregnant women with corrected scoliosis tolerate pregnancy, labor, and delivery well. In the absence of major lumbosacral deformity, there is little alteration of the pelvic cavity and malpresentation is not more common than in women without scoliosis. Uterine function is normal, and labor is not prolonged. Spontaneous vaginal delivery is anticipated, and cesarean delivery should be reserved for obstetric indications.
One uncontrolled study suggested no difference in the requirement for cesarean delivery in patients with previous Harrington rod instrumentation for correction of idiopathic scoliosis31; a second and similar study reported a higher incidence of operative delivery.42 The difference in outcomes may be influenced by the severity and etiology of the scoliosis in the populations reviewed and/or differences in the local practice patterns for managing atypical patients. Pelvic abnormalities are more common when scoliosis is associated with neuromuscular disorders and in patients with a severe, uncorrected curve.43 In addition, abdominal and pelvic muscle weakness predisposes parturients to problems with expulsion of the infant during the second stage of labor and may necessitate instrumental vaginal delivery. The need for instrumental or cesarean delivery seems to be related to the severity of skeletal deformity, the resulting maternal compromise, and cephalopelvic disproportion.
In the second stage of labor, the diaphragm has a nonrespiratory function. With expulsive efforts, maximal isometric contractions may be sustained for 20 seconds or more, and diaphragmatic fatigue has been demonstrated even in normal, laboring women. In parturients whose diaphragmatic function is compromised by neuromuscular disease or severe scoliosis, the potential for fatigue and failure is greater; expulsive forces are decreased, the second stage may be prolonged, and a trial of labor may fail, necessitating instrumental or cesarean delivery. In addition, women with severe cardiopulmonary disease (especially those with gestational decompensation) may require urgent or emergency cesarean delivery because of maternal compromise or nonreassuring fetal status.
Anesthetic Management
Pregnant women who have thoracolumbar scoliosis with a Cobb angle greater than 30 degrees or who have undergone spinal instrumentation and fusion for scoliosis should be referred to an anesthesiologist for antepartum consultation. The anesthesiologist should (1) determine the etiology of the scoliosis, as well as the severity and stability of the curve; (2) obtain a history of maternal musculoskeletal and cardiopulmonary symptoms; and (3) review prior obstetric and anesthetic experiences. For patients with scoliosis secondary to neuromuscular disorders, the anesthesiologist should also become familiar with anesthetic considerations specific to those underlying disorders.
Women with suspected or evident pulmonary compromise should undergo evaluation by a pulmonologist, and pulmonary function studies and arterial blood gas measurements should be obtained. These patients must be reevaluated periodically to ensure that they are tolerating the increasing physiologic demands of pregnancy. Echocardiography is useful to assess right-sided heart function in patients with one or more of the following: (1) a curve of 60 degrees or more, (2) hypoxemia on arterial blood gas measurement, (3) moderate or greater reductions in predicted lung volumes or flows, and/or (4) pulmonary hypertension. Radiographic studies performed before pregnancy and operative notes describing spinal surgical procedures should be reviewed before neuraxial anesthesia is given to any patient with significant scoliosis or previous spinal surgery. The anesthesiologist should also examine the spine and note the surface landmarks and interspaces that are least affected by the deformity. Modes of analgesia and anesthesia for labor and delivery can be discussed during antepartum consultation.
Invasive hemodynamic monitoring is rarely indicated during labor and delivery. Pulmonary function studies that suggest significant respiratory compromise or clinical evidence of impending respiratory failure warrant placement of an arterial catheter and serial assessment of blood gas measurements. Echocardiographic demonstration of significant right-sided heart dysfunction may warrant central venous pressure monitoring. The use of echocardiography may allow for detailed anatomic and physiologic assessment in severely ill mothers with advanced and decompensated cardiopulmonary disease.
The anesthesiologist may offer epidural analgesia for labor and delivery to patients with severe thoracolumbar scoliosis. Identification of the epidural space is more difficult in such patients, and the anesthesiologist should anticipate a greater incidence of complications. It is useful to remember the presence of the vertebral rotation during the performance of neuraxial anesthesia in a patient with a significant lumbar curve, which results in the spinous processes (which often may be structurally deformed) rotating into the concavity of the curve. Therefore, the midline of the epidural space is deviated toward the convexity of the curve relative to the spinous process palpable at the skin level (see Figures 48-3 and 48-4). The extent of lateral deviation is determined largely by the severity of the deformity.44 One method of placing an epidural or spinal needle is to direct the needle from a palpated spinous process toward the convexity of the curve, often at a significant angle. The experienced anesthesiologist can track the resistance of both the interspinous ligament and the ligamentum flavum to maintain the correct course into the epidural space. The extent of the local anatomic distortion is the limiting factor, and the selection of spaces that are least involved with the curve is advised (Figure 48-6). More recently, Huang45 suggested a modified paramedian approach based on Boon's46 work in cadavers, in which the needle is placed lateral to the spinous process on the convex side of the curve (taking advantage of the wider interlaminar spaces on that side) and then aimed directly perpendicular to the skin. The intent is to find lamina with the needle tip and then “walk up or down” the lamina to enter the epidural space (Figure 48-7).
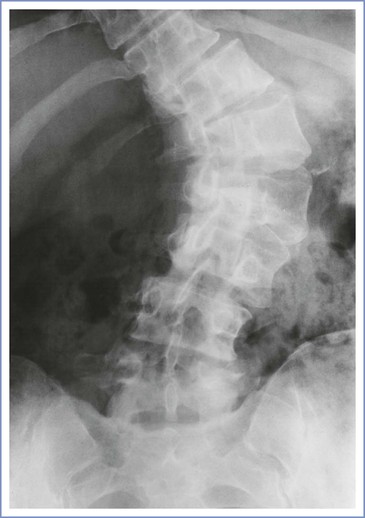
FIGURE 48-6 Radiograph of the lower thoracolumbar spine in a young woman with scoliosis predominantly affecting the lumbar spine and markedly distorting her local anatomy. She received lumbar epidural analgesia for labor during her first pregnancy, after a catheter was placed with some difficulty. She received patient-controlled intravenous nalbuphine for labor analgesia during her second pregnancy after persistent, unsuccessful attempts to insert an epidural catheter. For her third pregnancy, this radiograph directed the practitioner toward the lower lumbar spaces, where anatomic distortion is less pronounced.
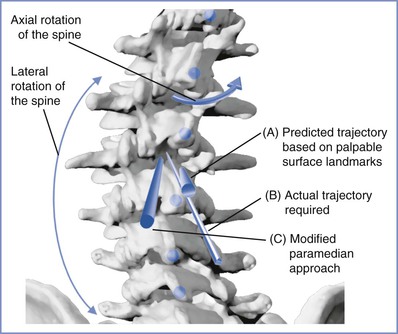
FIGURE 48-7 Image of the lumbosacral vertebrae affected by a scoliotic curve to the left. Note the axial and lateral rotation of the vertebral bodies, and the rotation of the affected spinous processes (blue dots) into the concavity of the curve; these spinous processes are frequently anatomically abnormal. The apparent midline (trajectory A) suggested by palpated surface landmarks is to the right (toward the concavity of the curve) of the true midline. The figure demonstrates two approaches to enter the epidural/subarachnoid space: one where the needle is directed along the line of a spinous process toward the convexity of the curve (trajectory B), and the other a modified paramedian approach where needle entry starts on the convex side of the curve and is directed perpendicular to the skin (trajectory C). (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
In patients with scoliosis resulting from myopathic or neurologic disease, distortion of spinal anatomy may be significant enough to prohibit the administration of neuraxial anesthesia. Ultrasonography is becoming a useful technique to facilitate administration of neuraxial anesthesia in parturients with challenging spinal anatomy, including those with scoliosis.47,48 Asymmetry of the spine is not difficult to appreciate; learning how to use the information and determine the optimal space and needle direction requires more experience.49 The paramedian view may provide the best information with respect to the widest interlaminar space in a woman with lumbar scoliosis.46 There is limited published experience with use of real-time ultrasonography during neuraxial needle placement. One report described its use in two patients; one patient had scoliosis, for whom standard preprocedural ultrasonographic imaging did not provide adequate information.50
Local anesthetic dose requirements for epidural and spinal anesthesia in the patient with scoliosis are variable. Moreover, during administration of spinal anesthesia in a patient with a severe scoliotic curve, hyperbaric local anesthetic solution may pool in dependent portions of the spine, resulting in an inadequate block.51 Thus, it may be preferable to use a continuous technique in those women with severe scoliosis so that the dose of local anesthetic agent can be titrated to the desired segmental level of anesthesia.
When offering neuraxial anesthesia to patients with a history of corrective surgery, the anesthesiologist must consider the following potential problems:
• Persistent back pain occurs in many patients with corrected scoliosis and correlates with both the extent of fusion and the time since surgery.52,53
• Degenerative changes occur in the spine below the area of fusion, and there is a higher incidence of both retrolisthesis and spondylolisthesis.53
• Twenty percent of patients undergo fusion to the lowest lumbar levels, limiting the potential for neuraxial anesthesia.42,54
• Insertion of an epidural needle in the fused area may not be possible because of the presence of instrumentation, scar tissue, and bone graft material.
• Intraoperative trauma to the ligamentum flavum may result in adhesions in or obliteration of the epidural space, and these changes may interfere with the spread of injected local anesthetic.18
• Obliteration of the epidural space may increase the incidence of unintentional dural puncture.
• These patients often manifest a high level of anxiety about their backs and may be reluctant to have neuraxial anesthesia.
A recent qualitative literature review by Ko and Leffert55 on neuraxial anesthesia in the parturient with scoliosis provides insight into the challenges of neuraxial anesthesia administration in these women. There were 117 attempted neuraxial procedures, the majority (93) in surgically corrected patients. Overall, 71% had a successful neuraxial block; however, the corrected patients proved to be more challenging, with a 69% success rate versus 79% for the uncorrected group. The challenges in corrected patients included (1) inability to place the needle (22%), (2) multiple attempts (13%), (3) patchy analgesia (10%), (4) excessive local anesthetic dose requirements (9%), (5) unintentional dural puncture (4%), and (6) inadequate analgesia (4%). In the uncorrected group, the issues included (1) patchy, asymmetric, or unilateral blocks (8% of each) and (2) multiple attempts or failed placement (4% of each). There were also two cases of persistent low back pain of unknown etiology. Complications seem to occur more frequently in patients with fusion that extends to the lower lumbar and lumbosacral interspaces than in those with fusion that ends in the upper lumbar spine.
Both the anesthesiologist and the patient should anticipate the possibility that blind attempts to identify the epidural space may fail, and consideration should be given to the use of ultrasonography to guide the performance of a neuraxial block.47 Although there is limited published experience to date with this specific population, Costello and Balki56 reported the use of ultrasonography to facilitate the successful administration of neuraxial anesthesia in a patient with Harrington rod instrumentation. Alternative modes of intrapartum analgesia include administration of intraspinal opioids, caudal anesthesia, patient-controlled intravenous opioid analgesia, and inhalation analgesia.55 Continuous spinal analgesia or anesthesia is a reasonable alternative to epidural analgesia or anesthesia for labor or cesarean delivery in parturients who have undergone major spinal surgery with instrumentation. Even making a dural puncture with a small-gauge spinal needle to confirm needle location and enhance spread of epidural local anesthetic is not unreasonable in this patient population.55 It is possible to make an intentional dural puncture with a small-gauge needle either through or adjacent to a fusion mass in some patients in whom epidural space identification was previously unsuccessful and then consider performing repeat subarachnoid injections as needed for labor analgesia.
Chronic Inflammatory Arthritides
This group of diseases includes rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and undifferentiated arthritis. Of these, rheumatoid arthritis is most affected by pregnancy and has been studied most extensively with respect to the immunologic relationship between mother and fetus. The most common arthritides seen in pregnancy are rheumatoid arthritis and ankylosing spondylitis and are discussed here in more detail.
Rheumatoid Arthritis
Rheumatoid arthritis is a chronic systemic disorder characterized by synovial proliferation that leads to joint destruction and subsequent deformity. It is becoming increasingly a disease of older people. Since 1985, the overall prevalence in the United States has fallen from 1.07% to 0.6%; the greatest decline in prevalence has occurred among people younger than age 55 years.57 The disease occurs approximately two times more frequently in women than in men.57
The lumbosacral spine is affected in only 5% of patients with rheumatoid arthritis. In contrast, the cervical spine is commonly involved, and atlantoaxial subluxation occurs in 30% to 45% of patients with rheumatoid arthritis.58,59 Although atlantoaxial subluxation may occur early in the course of the disease, it most often occurs in patients with a history of 5 years or more of highly active and erosive disease.60 Atlantoaxial subluxation occurs as a result of an attenuation or disruption of the transverse ligament, which allows anterior movement of C1 on C2 during neck flexion. Radiographically, atlantoaxial subluxation is marked by an increase in the atlas-dens interval, which is best demonstrated on the lateral cervical spine radiograph with the neck flexed (Figure 48-8).
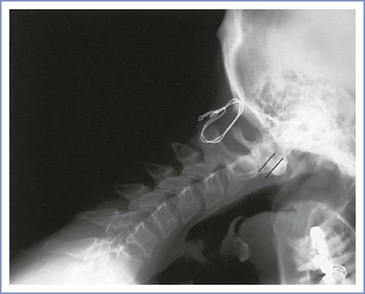
FIGURE 48-8 Lateral radiographic study of the cervical spine (in flexion) in a 32-year-old woman with rheumatoid arthritis. There is isolated atlantoaxial subluxation (6 mm) in the absence of other radiologic changes of rheumatoid arthritis. She presented with neck pain, and a wire was placed between the occiput and the spinous process of C2 to limit the subluxation.
Vertical subluxation of the odontoid process occurs primarily in older patients with severe and long-standing disease. A scoliotic deformity of the trachea and larynx has been reported in patients with vertical subluxation.61 The deformity is complex, involving both rotation and deviation of the larynx from the midline, and it may make laryngoscopy and tracheal intubation difficult. However, vertical subluxations occur primarily in older patients with severe, long-standing disease and are unlikely to be seen in women of childbearing age. Other airway issues such as cricoarytenoiditis and temporomandibular joint (TMJ) dysfunction may occur earlier in the disease and complicate airway management of the parturient.62
Extra-articular features are common in patients with rheumatoid arthritis. Anesthesiologists have a special interest in abnormalities that affect the airway and the cardiovascular and respiratory systems (Box 48-2). Although these complications occur more typically in patients with long-standing disease, it is prudent to inquire about those with more significant anesthetic implications. Cardiovascular mortality is especially high in patients with rheumatoid arthritis, similar to that of patients with type 2 diabetes, and often presents atypically.62
Interaction with Pregnancy
Rheumatoid arthritis is associated with a slightly higher incidence of preterm birth, small for gestational age infants, and elective cesarean delivery, as compared with a reference population of women as shown in large databases from Norway and the United States.63,64 In the absence of vasculitis, fetal outcome is good, although as a group the chronic inflammatory arthritides are associated with a higher perinatal mortality rate.63 Since the 1930s, evidence has consistently shown that pregnancy has a beneficial, ameliorating effect on the activity of rheumatoid arthritis.65 Approximately 75% of women note improvement in symptoms during pregnancy, which is typically evident by the end of the first trimester, with a gradual resolution of pain, swelling, and stiffness. The clinical improvement usually continues throughout pregnancy and recurs in future pregnancies. This remission often occurs despite the discontinuation of effective but teratogenic disease-modifying antirheumatic drugs and a substantial reduction in the dosage of safe drugs (e.g., corticosteroids). Relapse occurs postpartum in approximately 60% to 90% of women,66 beginning as early as the second week after delivery. It appears that most women return to a disease status comparable to their prepregnant state.
Medical Management
Drug therapy for rheumatoid arthritis is divided into (1) symptom-modifying drugs, (2) disease-modifying antirheumatic drugs, and (3) biologic drugs.
Symptom-modifying therapies include nonsteroidal anti-inflammatory drugs (NSAIDs), including cyclooxygenase-2 (COX-2) inhibitors, and corticosteroids. NSAIDs and corticosteroids have been the mainstay of therapy in rheumatoid arthritis during pregnancy for many years.67 No evidence suggests that these drugs are teratogenic. However, there is limited experience with COX-2 inhibitors; hence, they are best avoided in pregnancy.66 NSAIDs are classified as U.S. Food and Drug Administration (FDA) Category B medications in the first part of pregnancy, even though they are associated with an increased incidence of early pregnancy loss.68 When used later in pregnancy, NSAIDs are associated with premature closure of the ductus arteriosus and increased risk for neonatal bleeding and are therefore classified as FDA Category C medications after 30 to 32 weeks' gestation. All NSAIDs except low-dose aspirin should be discontinued at 32 weeks' gestation.67
Corticosteroids are considered safe in doses up to 15 mg/day of prednisone equivalent. Larger doses increase risk for maternal infection and neonatal prematurity.66
Disease-modifying antirheumatic drugs reduce flares, prevent joint erosions, and have proven efficacy in decreasing morbidity and mortality from rheumatoid arthritis. This category of drugs for anti–rheumatoid arthritis treatment includes sulfasalazine, azathioprine, methotrexate, leflunomide, gold salts, and antimalarial agents. Methotrexate is considered a first-line treatment for rheumatoid arthritis and is typically started at diagnosis, but unfortunately it and leflunomide are highly teratogenic and must be stopped several months before conception.66 Sulfasalazine inhibits folate synthesis, and therefore additional folate supplementation is required during pregnancy. Azathioprine is an FDA Category D drug, and although it may be used during pregnancy at doses less than 2 mg/kg/day, other options are preferable.
The biologic drugs, which are proving as effective as methotrexate in preventing erosions and reducing long-term disability, include the anti–tumor necrosis factor-alpha (TNF-α) therapies (e.g., etanercept), which are classified as FDA Category B in pregnancy and lactation. The other biologic drugs (e.g., abatacept, rituximab, tocilizumab) are Category C drugs, and there is very limited experience with their use in pregnancy; thus, the recommendation is to discontinue them 10 weeks before conception.66
An attempt is made to reduce the dose of all antirheumatic agents during pregnancy and resume antepartum therapy after delivery before disease activity flares.
Obstetric Management
Vaginal delivery is preferred for parturients with rheumatoid arthritis, and cesarean delivery should be reserved for obstetric indications. A major concern is maternal positioning during labor. Rheumatoid joints are unstable because of ligament loosening associated with chronic swelling and because of the destruction of ligaments and cartilage. It is important to determine the permissible range of motion and activity for affected joints. Special emphasis should be given to the hips, knees, and neck. Physicians and nurses should be aware of the potential risks of forcing motion beyond the disease-imposed limits.
Anesthetic Management
The preanesthetic evaluation should include a careful evaluation of the airway. Patients with rheumatoid arthritis may have a small mandible, TMJ dysfunction, cricoarytenoid arthritis, and laryngeal deviation, all of which may complicate direct laryngoscopy.62 In particular, these findings may be present in parturients with juvenile rheumatoid arthritis. Cervical spine involvement is not common in young patients but may occur in patients with disease of long duration and in those with severe, deforming disease—typically, patients with juvenile rheumatoid arthritis. Although there is no guideline or consensus on the need to obtain cervical spine radiographs in patients with rheumatoid arthritis, it would be reasonable even in the pregnant woman if her rheumatoid arthritis includes severe erosive disease, neck symptoms, or a history of disease of 10 or more years' duration.62 The cardiac and pulmonary features of rheumatoid arthritis are not common in young patients, but signs and symptoms of pleural and pericardial effusions and pulmonary parenchymal involvement should be sought.
No evidence contraindicates the administration of spinal or epidural anesthesia in patients with rheumatoid arthritis. However, recent evidence in the nonpregnant population has indicated that spinal blocks rise higher than expected in patients with rheumatoid arthritis, independent of body mass index.69,70 Care should be taken to avoid excessive manipulation of the neck during administration of general anesthesia. Finally, joints should be padded and protected appropriately during anesthesia.
Ankylosing Spondylitis
Ankylosing spondylitis is a chronic inflammatory arthropathy characterized by infiltration of granulation tissue into the bony insertions of ligaments and joint capsules, with subsequent fibrosis, ossification, and ankylosis. Ankylosing spondylitis is a major subtype of an interrelated group of rheumatic disease called the spondylarthritides, which are linked by the major histocompatibility complex (MHC) Class 1 tissue antigen HLA B27.71 Ankylosing spondylitis primarily affects the sacroiliac, facet, and costovertebral joints; sacroiliitis is pathognomonic. There is progressive flexion and fusion of the spine and fixation of the rib cage; however, the clinical spectrum is wide, and only a small proportion of patients progress to total spinal ankylosis.
The prevalence of ankylosing spondylitis is 0.1% to 1.4%.71 Onset is common during the second and third decades of life, a period of peak childbearing potential. The disease is milder in women than in men, but women are more likely to have peripheral arthritis and involvement of the cervical spine and symphysis pubis.72 Although clinically significant lesions of the cervical spine may occur early in the course of the disease, they are far more common in patients with long-standing ankylosing spondylitis (Figure 48-9).61,73 Ultimately, 21% of patients with ankylosing spondylitis develop clinically significant atlantoaxial subluxation. TMJ involvement causes limited mouth opening in 10% of patients early in the disease, increasing to 30% to 40%.72 A slower development of radiologic changes of the dorsolumbar spine occurs in women, and spinal rigidity or deformity and extra-articular manifestations are rare in young patients (Box 48-3).62,71,74
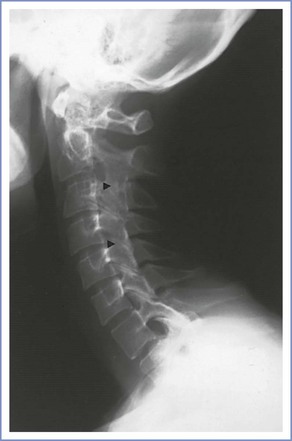
FIGURE 48-9 Lateral radiographic study of the cervical spine in a 31-year-old woman with ankylosing spondylitis. There is evidence of facet joint ankylosis (arrowheads), although the lordotic curve remains well preserved. The ligaments of the thoracic spine are undergoing calcification with the spine in flexion, and there is a compensatory increase in the lumbar lordosis to maintain erect posture. (Flexion of the lumbar spine proved difficult, and a paramedian approach was used to enter the epidural space.)
The mainstays of therapy for ankylosing spondylitis are NSAIDs and exercise programs. Disease-modifying antirheumatic drugs have not been proven beneficial, but for those patients not controlled on NSAIDs, anti–TNF-α therapies have proven quite effective.71
Interaction with Pregnancy
In contrast to rheumatoid arthritis, pregnancy does not seem to reduce the symptoms or slow the progression of disease in patients with ankylosing spondylitis, and many patients experience an aggravation of morning stiffness and back pain.75 However, a recent retrospective review of 35 case-control matched pregnancies revealed improvement in pain and stiffness in 51% of women in early pregnancy, although unfortunately there was some return of pain as pregnancy progressed.76 Pregnancy may ameliorate the extra-articular features of this disease (e.g., psoriasis, inflammatory bowel disease, small joint arthritis), but it appears that women with ankylosing spondylitis are more likely than women with rheumatoid arthritis to enter pregnancy with active disease and, hence, to have higher levels of pain at the beginning of pregnancy.75 Ankylosing spondylitis does not adversely affect pregnancy, labor, or delivery; in the absence of pelvic joint ankylosis and/or hip joint involvement, an uncomplicated vaginal delivery at term should be anticipated in most patients. As in rheumatoid arthritis, NSAID therapy should be withdrawn by 32 weeks' gestation.
Anesthetic Management
An anesthesiologist should review the patient's history with respect to the duration of the disease, the presence of extra-articular features, and the recent use of analgesics. Temporomandibular joint disorders, cervical spine involvement, and cardiopulmonary complications are rare early in the disease course; however, difficult tracheal intubation has been reported in parturients.77 Severity of back symptoms is often out of proportion to the radiographic appearance of the spine, and calcification of the spinal ligaments is typically not advanced in young patients. Neuraxial anesthesia is acceptable in parturients with ankylosing spondylitis; however, even in young patients it may be technically challenging. Calcification of the interspinous ligaments and osteophyte formation may limit the parturient's ability to flex forward, making midline needle placement difficult.72 A paramedian approach can be considered in this instance.78 Additionally, the epidural space becomes narrowed in patients with ankylosing spondylitis, and there have been reports of unexpectedly high blocks,79 as well as failed blocks despite confidence that the catheter was in the epidural space. After multiple failed attempts to provide epidural analgesia in a parturient with advanced ankylosing spondylitis, Hoffman et al.80 suggested that a highly calcified posterior longitudinal ligament may limit rostral spread of local anesthetic in the epidural space. Preprocedural ultrasonography may be helpful, either to identify the best interlaminar space, or to indicate that administration of neuraxial anesthesia may be impossible.81
Spina Bifida
Spina bifida results from the failure of the developing spine to completely enclose the neural elements in a bony canal. There is a wide spectrum with respect to the severity of the deformity and its implications. Spina bifida occulta is defined as failed fusion of the neural arch without herniation of the meninges or neural elements. A defect limited to a single vertebra, typically L5 or S1, is so common (occurring in 5% to 36% of the population) that it can be considered a normal variant.82 Superficial signs of spina bifida occulta include a tuft of hair, cutaneous angioma, lipoma, or a skin dimple, but such signs are not common in patients with isolated vertebral arch anomalies and an underlying normal cord. Patients with spina bifida occulta rarely have symptoms related to this anomaly, although they may have a higher incidence of posterior disc herniation.82
Spina bifida cystica is defined as failed closure of the neural arch with herniation of the meninges (i.e., meningocele) or the meninges and neural elements (i.e., myelomeningocele) through the vertebral defect. These conditions are relatively uncommon, occurring in 1 to 3 per 1000 births.83 Neurologic deficits involving the lower extremities and sphincters occur in almost all patients, and these deficits vary primarily in severity. Hydrocephalus is present in many patients, and shunting of the ventricular system is common, with revisions often required during childhood. By puberty as many as 50% of patients who have received shunts have little or no requirement for them.84 Early and aggressive surgical treatment of spina bifida cystica has improved survival from 45% in the early 1970s to 70% to 90% by the mid-1980s. Obstetric and anesthesia providers can expect to encounter a growing number of pregnant women with spina bifida cystica.85 Unfortunately, many surviving patients have significant residual neurologic impairment and ongoing orthopedic and genitourinary complications. Myelomeningocele is a progressive neurologic disease that eventually produces orthopedic, neurologic, and genitourinary complications. Kyphoscoliosis, which is common in patients with a thoracic lesion, occurs in 20% of patients with a lumbosacral defect.86 Paralytic scoliosis is the most common type and results from an imbalance of paravertebral muscle tone.
Occult spinal dysraphism is an intermediate group of conditions in which the bony defect is associated with one or more anomalies of the spinal cord, including intraspinal lipomas (the most common), dermal sinus tracts, dermoid cysts, fibrous bands, and diastematomyelia (split cord). These lesions are differentiated from the more benign occulta lesions described previously.87,88 Affected patients may have no neurologic symptoms or may have minor sensory, motor, and functional deficits of the lower limbs, bowel, and bladder89; they also may have orthopedic issues, such as scoliosis, limb pain, and lower extremity abnormalities.90 Patients with cord abnormalities have cutaneous stigmata in 50% of cases, and 70% have a tethered spinal cord, which has implications for neuraxial anesthesia.91
Tethered Cord and Arnold-Chiari Malformation
Tethered cord syndrome (TCS) is characterized by neurologic deterioration secondary to traction on the conus medullaris, which typically, but not invariably, is low lying (L2 to L3).87,91 Congenital abnormalities of the spinal cord such as lipoma, tight filum terminale, dermal sinus, meningocele manqué, and diastematomyelia are found in more than 50% of patients with adult-onset TCS.
A new classification of TCS in adults has been proposed to differentiate tethered cord occurring secondary to spina bifida cystica from the adult-onset neurologic syndrome associated with spinal dysraphism.92 Magnetic resonance imaging studies suggest that tethering is present in virtually all patients with spina bifida cystica and myelomeningocele, and it is also common in patients with occult spinal dysraphism.88 Although many of the latter patients do not have obvious neurologic impairment secondary to the tethering, adults with TCS often have a long history of minor neurologic or orthopedic issues.87,92 Others present with acute symptoms after a precipitating event that stretched the spinal cord, such as heavy lifting or placement in the lithotomy position.93 Of note, more than 50% of adults with TCS present only with a history of low back and leg pain before a precipitating event that leads to the diagnosis.87,88
Low-lying spinal cords and the possibility of undiagnosed TCS have come to the attention of obstetric anesthesiologists with the publication of several case reports of neurologic injury after spinal or epidural anesthesia for delivery in women subsequently diagnosed with occult spinal dysraphism.89,90 An important feature of adult TCS is that the low-lying cord is located more posteriorly than a normal cord, increasing the likelihood of direct needle trauma during administration of spinal or epidural anesthesia (Figure 48-10).94 As noted previously, the isolated finding of a defective laminar arch (spina bifida occulta) is not associated with a low-lying or tethered cord.87
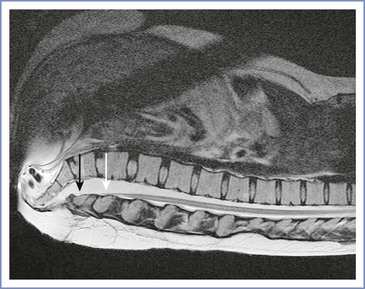
FIGURE 48-10 Magnetic resonance image of the spine in a 27-year-old woman with history of a lumbar myelomeningocele excised as a neonate, with residual bladder dysfunction. A tethered spinal cord is present with a typical posterior low-lying position. The white arrow indicates the termination of the conus medullaris at L4 to L5, and the black arrow indicates the filum terminale located at L5 to S1. (This patient had a vaginal delivery at term, requiring only nitrous oxide for analgesia.)
More severe forms of spina bifida may also be associated with Arnold-Chiari malformation, which is characterized by cerebellar herniation through the foramen magnum and descent of the pons and medulla. Tethered cord from a tight filum terminale has also been deemed causal in the development of syringomyelia and subsequent Arnold-Chiari syndrome.95 Symptoms are more common if the cerebellar herniation exceeds 12 mm or if syringomyelia is present.96,97
Obstetric Management
Pregnancy is not complicated by the presence of a spina bifida occulta lesion. However, women with known TCS should avoid both the squatting position and a prolonged lithotomy position for delivery. Recurrent urinary tract infection is the most common antenatal complication in patients with spina bifida cystica and is associated with preterm labor.85 Intestinal and urinary tract obstruction, as well as problems related to ileal conduits and stomas, are common during pregnancy, as are pressure sores resulting from greater immobility.85 Uterine enlargement may compromise pulmonary function, especially in patients with kyphoscoliosis. Vaginal delivery is more common in women who are independently mobile and is less common in wheelchair-dependent patients.85 Pelvic and lower limb anomalies and contractures may obstruct the pelvic outlet and warrant cesarean delivery. The obstetrician should evaluate the adequacy of the pelvis to determine whether a trial of labor is appropriate. Cesarean delivery is reserved for obstetric indications, and its incidence is increased and proportionate to the severity of the underlying defect and its consequences. Cesarean delivery is complicated by the presence of stomas and conduits; postoperative complications and prolonged hospital stays are common.85
Anesthetic Management
The epidural space may be incomplete or discontinuous across the level of spina bifida occulta lesions because of absence of the lamina and variable formation of the ligamentum flavum at this site. An attempt to identify the epidural space at the site of this lesion will likely result in unintentional dural puncture, although successful epidural analgesia has been reported with the catheter placed within the zone of the lesion. Fortunately, for two reasons, spina bifida occulta rarely causes issues for administration of neuraxial anesthesia. First, the lesion typically occurs at the L5 to S1 segments, below the level at which most epidural and spinal anesthetics are administered. Second, the most common anomaly is a simple midline split in the lamina, and this defect rarely seems to interfere with either the performance or the development of spinal or epidural anesthesia.
Occult spinal dysraphism is of more concern because of the potential for a low-lying, posteriorly located, and tethered spinal cord. A neurologic history should be taken and a screening neurologic examination should be performed in all women with a known defective laminar arch, preferably antenatally, to determine whether magnetic resonance imaging for spinal dysraphism is necessary.90,98 The presence of skin dimpling or hair tufts should raise suspicion that an underlying cord abnormality exists. In my judgment, in women with a known low-lying cord, epidural anesthesia performed by an experienced anesthesiologist is safer than spinal anesthesia. If spinal dysraphism is suspected but no imaging studies are available, it would be prudent to avoid neuraxial anesthesia. Women with TCS may prefer to avoid neuraxial anesthesia because of the greater potential for direct neural trauma.
In the patient with a spina bifida cystica lesion, the anesthesiologist should determine the level of the lesion and whether the patient has residual spinal cord function below it. Patients with a complete lesion at or above T11 are likely to experience painless labor. However, the risk for autonomic hyperreflexia should be evaluated in patients with thoracic lesions, and prophylaxis should be provided if the patient is deemed to be at risk; this issue is especially important if the lesion is between T5 and T8. If the patient has undergone ventricular shunt placement, the current status of the shunt should be determined. Neurosurgical consultation should be obtained if questions remain about the requirement for—or function of—the shunt. Pulmonary function should be assessed, especially in patients with scoliosis. Baseline renal function also should be determined.
There are published reports of the use of epidural and spinal anesthesia in patients with spina bifida cystica.83,98-100 Unfortunately the experience is limited, and most published series of pregnant women with spina bifida cystica report neither the type of anesthesia provided nor the complications experienced. Tidmarsh and May99 reported management of intrapartum analgesia in 16 patients with spina bifida, 8 of whom had spina bifida cystica (with meningomyelocele). Five of the eight patients with spina bifida cystica received epidural analgesia for labor and/or delivery. Three patients had a “normal” block, one patient had a somewhat high block (sensory block level of T3 after administration of 10 mL of 0.25% bupivacaine), and one patient had poor sacral analgesia. In the U.K. Registry of High-Risk Obstetric Anesthesia, the period from 1997 through 2002 included 23 cases of parturients with spina bifida among 102 cases of neurologic conditions.101 Of those, the extent of spina bifida was defined further in only 10 cases, and 8 had spina bifida occulta. Neuraxial anesthesia was provided in 8 cases, although 17 patients had planned to receive neuraxial anesthesia if needed. Only epidural anesthesia was used owing to concern about a low-lying or tethered cord; only 5 patients had magnetic resonance imaging performed before delivery.
Limited data exist on the obstetric anesthesia experience in parturients with Arnold-Chiari malformations. The largest series is that of Chantigian et al.,96 who described their experience with 12 parturients who delivered a total of 30 infants. Nine deliveries were accomplished with neuraxial anesthesia, including six vaginal deliveries with epidural analgesia, two cesarean deliveries performed with single-shot spinal anesthesia, and one cesarean delivery performed with a spinal catheter. No patient experienced postprocedural neurologic sequelae related to the use of neuraxial anesthesia.
Administration of epidural or spinal anesthesia may be considered in women with various forms of spina bifida and stable neurologic function. Patients should be informed that there is limited published information on the administration of neuraxial anesthesia (and the risk for neurologic injury) in patients with neural tube defects, and these patients should be actively involved in decision-making. In patients with either surgically corrected spina bifida cystica or occult spinal dysraphism, the anesthesiologist should be aware that the terminal portion of the spinal cord typically lies at a vertebral level lower than normal. Imaging studies provide valuable information on neural anatomy and facilitate anesthetic management. In women with spinal dysraphism, a neurologic examination, as well as a full discussion of the risks and benefits of neuraxial anesthesia, should be performed and documented before administration of neuraxial anesthesia. A recent report by Asakura demonstrated that ultrasonography can be used to detect spina bifida occulta102; however, ultrasonographic imaging of the terminal portion of the cord would be much more difficult, although it has been shown to be feasible in children.103 Spinal anesthesia should be performed below the known level of the conus or avoided in favor of epidural anesthesia. In women with spina bifida or occult spinal dysraphism, the epidural space is often abnormal, which increases the likelihood of inadequate epidural anesthesia. In our judgment, spinal anesthesia is not contraindicated in women with negligible function of the lower extremities and sphincters, given that the concern for direct neural trauma to a low-lying spinal cord is not clinically relevant.
Achondroplasia
Achondroplasia, an inherited disorder of bone metabolism, is the most common cause of disproportionate dwarfism, with a prevalence of 1 in 26,000 live births. Although it is inherited in an autosomal dominant mode, most cases arise from spontaneous mutation.104 The range of cervical motion may be decreased, lumbar lordosis and thoracic kyphosis are increased, and thoracic kyphoscoliosis occurs.105 The vertebral pedicles are short, and reduced length of the neural arch leads to shortened anteroposterior and transverse diameters of the vertebral canal, resulting in foramen magnum and spinal stenosis.106,107 Although it may occur earlier, symptomatic spinal stenosis often does not present until the fourth or fifth decade, when kyphosis, scoliosis, osteophytes, and herniated discs typically cause further narrowing of the spinal canal. There is considerable interindividual variation in the clinical and radiographic characteristics, and skeletal abnormalities often show more variation than consistency.106,108
Obstetric Management
The uterus is an abdominal organ in the achondroplastic patient.109 With advancing pregnancy it may encroach on the small thoracic cage and lead to decreases in the functional residual and closing capacities; severe dyspnea may occur with advancing gestational age. Back discomfort is common during pregnancy, and the reported incidence of sciatica is greater than in normal pregnant women,110 most likely owing to the underlying spinal abnormalities. Typically, an inadequate maternal pelvis combined with a normal-sized (nonachondroplastic) fetus results in cephalopelvic disproportion. Imaging techniques may be used to confirm this situation, and the obstetrician should anticipate the need to deliver most patients with achondroplasia by cesarean delivery.105,106
Anesthetic Management
The short, obese limbs of the patient with achondroplasia may make it difficult to obtain measurements of blood pressure with a noninvasive cuff, and an intra-arterial catheter may be necessary. Prominent paraspinal muscles and marked lumbar lordosis may complicate attempts to palpate landmarks during the administration of spinal or epidural anesthesia; the use of ultrasonography may help identify landmarks.105 Scoliosis of the spine also may cause technical difficulties with neuraxial anesthesia attempts. The small stature and spinal stenosis reduce the dose of local anesthetic required for major neuraxial anesthesia.105,108,109,111 It is difficult to estimate the appropriate dose of local anesthetic for single-shot spinal anesthesia. Continuous epidural anesthesia or CSE anesthesia is preferable because it allows the anesthesia provider to titrate the dose of local anesthetic to the desired level of anesthesia. Local anesthetic dose requirements are typically smaller than those in parturients of normal stature112; this is not always the case, however, supporting the use of a neuraxial anesthetic technique that may be titrated to the desired effect.110,113,114
Difficult tracheal intubation has been reported in achondroplastic patients and should be anticipated. A case report noted that use of a video laryngoscope did not allow visualization of the vocal cords, and flexible fiberoptic bronchoscopy was required to intubate the trachea in an achondroplastic dwarf undergoing cesarean delivery.115
Osteogenesis Imperfecta
Osteogenesis imperfecta is an inherited condition that occurs with an incidence between 1 in 21,000 and 1 in 60,000.116,117 The genetic defect is within the genome that encodes for type I collagen, the major collagen in tissues that require structural strength. The disease is a generalized connective tissue disorder, and expression ranges from mild osteoporosis to the classical clinical stigmata characterized by multiple bone fractures and skeletal deformities, blue sclera, and middle ear deafness (otosclerosis). Four types may be distinguished clinically, and a system of classification (types I through IV) has been proposed.118 Type I is the prototype disease. It is inherited as an autosomal dominant trait and is the most common and mildest form of this disease. It typically manifests in childhood as multiple fractures after minor trauma.118 Types II and III are inherited as autosomal recessive traits and are characterized by extreme bone fragility. Type II is uniformly lethal, and stillbirth or early neonatal death is common; death in utero is caused by skeletal collapse, and early neonatal death typically results from chest wall failure and respiratory insufficiency. Infants with type III disease may have fractures at birth and may have progressive skeletal deformities during the first two decades of life. Type IV, also autosomal dominant, is much less common and is variable in expressivity.
The majority of pregnant women with osteogenesis imperfecta have type I disease, although pregnancy has been reported in more severe forms of the disease.117,119 There is considerable variability among affected patients as to the age at onset and frequency of fractures. Dwarfism is typical, and kyphoscoliosis is common, as are other chest wall abnormalities. These chest and spinal abnormalities result in restrictive lung disorders (Figure 48-11). Other abnormalities include a decrease in the range of motion of the shortened cervical spine, micrognathia, and malformed, brittle teeth.119 Poor platelet adhesion may cause platelet dysfunction and a modest bleeding tendency. Hyperthyroidism occurs in 40% of patients with osteogenesis imperfecta; an elevated concentration of thyroxine leads to increases in both oxygen consumption and heat production.118,119 Association with malignant hyperthermia is very weak; there is only one published case of a patient with osteogenesis imperfecta who developed malignant hyperthermia.120 Intraoperative hyperthermia has often been reported; however, a recent retrospective cohort study of 49 patients with osteogenesis imperfecta undergoing noncardiac surgery revealed no difference in intraoperative temperature changes as compared with matched controls.121 This recent information should allay ongoing concerns about risk for developing malignant hyperthermia.

FIGURE 48-11 A chest radiograph of a 30-year-old woman with osteogenesis imperfecta type I. Generalized osteoporosis, corrected thoracic kyphoscoliosis, a restricted thoracic cage, and multiple old fractures are demonstrated. (General anesthesia was provided for cesarean delivery and tubal ligation.)
Obstetric Management
Pregnancy results in transfer of calcium from the mother to the fetus, which in the patient with osteogenesis imperfecta can lead to increased fracture risk. In one published case, quantitative ultrasonometry of the phalanges was used to follow a woman with type I osteogenesis imperfecta who had increasing joint arthralgias to assess her fracture risk.122 By 33 weeks' gestation, the ultrasonometry data and increased pain level guided the decision for early elective cesarean delivery. Platelet dysfunction in osteogenesis imperfecta may result in a higher incidence of intrapartum and postpartum hemorrhage, although this finding is uncommon. Labor and vaginal delivery is associated with an increased risk for uterine rupture and pelvic fracture123; maternal pelvic fracture during pregnancy is an indication for cesarean delivery. These complications are also uncommon and most likely are influenced primarily by the severity of the underlying disease. Cephalopelvic disproportion typically mandates cesarean delivery in severely affected parturients.
Anesthetic Management
The anesthesiologist must be aware of the fragility of the bones, the potential for difficult tracheal intubation, and the presence and severity of restrictive lung disease. Transfers, positioning, and any invasive intervention must be accomplished with extreme care. Blood pressure cuffs and tourniquets to facilitate placement of intravenous catheters should be applied gently to prevent fractures. Difficult tracheal intubation may occur because of a short neck with limited range of motion and should be anticipated.119 The anesthesiologist should take care not to hyperextend the neck, and laryngoscopy should be gentle to avoid fractures. Alternatives to direct laryngoscopy may be considered to reduce the applied forces necessary. If succinylcholine is used, a defasciculating dose of a nondepolarizing muscle relaxant should be employed in an attempt to prevent fasciculations.124 An alternative to succinylcholine (e.g., rocuronium) may also be administered to prevent fasciculations.
Before administration of neuraxial anesthesia, the anesthesiologist should consider the technical difficulties inherent in performing neuraxial anesthesia in patients with spinal deformities. The anesthesiologist also should be aware of the platelet dysfunction in these patients. This risk is best evaluated by obtaining a thorough history before the administration of neuraxial anesthesia. In the setting of a reassuring history, neuraxial anesthesia need not be withheld. A hematology consult to better assess platelet function can be requested, if there is any doubt. Small stature and spinal abnormalities reduce the local anesthetic dose requirements and increase the risk for both misplaced injection and local anesthetic toxicity. It may be difficult to estimate the appropriate dose for single-shot spinal anesthesia in these patients. Thus, continuous epidural, continuous spinal, or CSE anesthesia is the neuraxial anesthetic technique of choice, barring other contraindications.125 Yeo and Paech126 reported the successful use of both epidural and subarachnoid blocks for cesarean delivery on five occasions over 9 years in a single patient with type I osteogenesis imperfecta. The use of ultrasonography may facilitate the administration of neuraxial anesthesia.
Spondylolisthesis
Isthmic spondylolisthesis is a condition in which a defect in the pars interarticularis of a vertebra allows anterior slippage of the subjacent portion of the spine. As the body slips forward, it does not carry the neural arch with it; thus, there is little tendency toward spinal canal stenosis. Isthmic spondylolisthesis typically occurs at the lumbosacral junction, although it is not uncommon in the lumbar spine. Approximately 38% of cases occur in women.127 The onset is common in the second and third decades, a period of peak childbearing potential.
Back pain is the presenting feature. Strenuous physical activity often precipitates the onset of pain. Pregnancy increases back symptoms in approximately half of patients with isthmic spondylolisthesis, but obstetric complications are unusual.127,128 This disorder is not affected by pregnancy.129
Degenerative spondylolisthesis occurs when there is a forward slip of one vertebra on the subjacent level with no break in the neural arch.130 It is four times more common in women than in men and occurs most frequently at the L4 to L5 level. It is not usually diagnosed before the onset of the fourth decade of life and seems to be twice as common in parous women. It is suggested that rotational and sheer stresses on the L4 to L5 joint during gestation may be responsible for the higher incidence of degenerative spondylolisthesis in women who have borne children.
There is no published case of neuraxial anesthesia during labor in a parturient with severe spondylolisthesis (e.g., lumbosacral subluxation). Ideally, an anesthesiologist will see the patient during pregnancy to assess her symptoms and lumbar spinal anatomy. In the absence of evidence of a neurologic deficit, it seems appropriate to administer either epidural or spinal anesthesia. There is one note of caution: Horduna and Legaye131 have shown that the presence of spondylolisthesis affects the relative position of the palpated intercristine line. As a result, in patients with high-grade spondylolisthesis, the actual lumbar interspace is one to three segments higher than is normally expected when using the palpated intercristine line as a guide for neuraxial anesthesia needle placement. It would be reasonable to use preprocedural ultrasonography to identify the appropriate lumbar interspace before needle insertion in parturients with known spondylolisthesis.
References
1. Vleeming A, Albert HB, Ostgaard HC, et al. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17:794–819.
2. Vermani E, Mittal R, Weeks A. Pelvic girdle pain and low back pain in pregnancy: a review. Pain Pract. 2010;10:60–71.
3. Wu WH, Meijer OG, Uegaki K, et al. Pregnancy-related pelvic girdle pain (PPP), I: Terminology, clinical presentation, and prevalence. Eur Spine J. 2004;13:575–589.
4. Gutke A, Ostgaard HC, Oberg B. Pelvic girdle pain and lumbar pain in pregnancy: a cohort study of the consequences in terms of health and functioning. Spine. 2006;31:E149–E155.
5. Mogren IM. BMI, pain and hyper-mobility are determinants of long-term outcome for women with low back pain and pelvic pain during pregnancy. Eur Spine J. 2006;15:1093–1102.
6. To WW, Wong MW. Factors associated with back pain symptoms in pregnancy and the persistence of pain 2 years after pregnancy. Acta Obstet Gynecol Scand. 2003;82:1086–1091.
7. Bastiaanssen JM, de Bie RA, Bastiaenen CH, et al. A historical perspective on pregnancy-related low back and/or pelvic girdle pain. Eur J Obstet Gynecol Reprod Biol. 2005;120:3–14.
8. Aldabe D, Ribeiro DC, Milosavljevic S, Dawn Bussey M. Pregnancy-related pelvic girdle pain and its relationship with relaxin levels during pregnancy: a systematic review. European Spine Journal. 2012;21:1769–1776.
9. Ostgaard HC, Zetherstrom G, Roos-Hansson E, Svanberg B. Reduction of back and posterior pelvic pain in pregnancy. Spine. 1994;19:894–900.
10. Chow J, Chen K, Sen R, et al. Cauda equina syndrome post-caesarean section. Australian N Z J Obstet Gynaecol. 2008;48:218–220.
11. Pennick VE, Young G. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev. 2007.
12. Keskin EA, Onur O, Keskin HL, et al. Transcutaneous Electrical Nerve Stimulation Improves Low Back Pain during Pregnancy. Gynecol Obstet Invest. 2012;74:76–83.
13. Abou-Shameh MA, Dosani D, Gopal S, McLaren AG. Lumbar discectomy in pregnancy. Int J Gynaecol Obstet. 2006;92:167–169.
14. Benzon HT, Braunschweig R, Molloy RE. Delayed onset of epidural anesthesia in patients with back pain. Anesth Analg. 1981;60:874–877.
15. Sharrock NE, Urquhart B, Mineo R. Extradural anaesthesia in patients with previous lumbar spine surgery. Br J Anaesth. 1990;65:237–239.
16. Calleja MA. Extradural analgesia and previous spinal surgery. A radiological appraisal. Anaesthesia. 1991;46:946–947.
17. Luyendijk W, van Voorthuisen AE. Contrast examination of the spinal epidural space. Acta Radiol Diagn (Stockh). 1966;5:1051–1066.
18. LaRocca H, Macnab I. The laminectomy membrane. Studies in its evolution, characteristics, effects and prophylaxis in dogs. J Bone Joint Surg Br. 1974;56B:545–550.
19. Brodsky AE. Post-laminectomy and post-fusion stenosis of the lumbar spine. Clin Orthop Relat Res. 1976;130–139.
20. MacArthur C, Lewis M, Knox EG, Crawford JS. Epidural anaesthesia and long term backache after childbirth. BMJ. 1990;301:9–12.
21. MacArthur C, Lewis M, Knox EG. Investigation of long term problems after obstetric epidural anaesthesia. BMJ. 1992;304:1279–1282.
22. MacLeod J, MacIntyre C, McClure JH, Whitfield A. Backache and epidural analgesia: a retrospective survey of mothers 1 year after childbirth. Int J Obstet Anesth. 1995;4:21–25.
23. Orlikowski CE, Dickinson JE, Paech MJ, et al. Intrapartum analgesia and its association with post-partum back pain and headache in nulliparous women. Aust N Z J Obstet Gynaecol. 2006;46:395–401.
24. Mogren IM. Does caesarean section negatively influence the post-partum prognosis of low back pain and pelvic pain during pregnancy? Eur Spine J. 2007;16:115–121.
25. Breen TW, Ransil BJ, Groves PA, Oriol NE. Factors associated with back pain after childbirth. Anesthesiology. 1994;81:29–34.
26. Macarthur A, Macarthur C, Weeks S. Epidural anaesthesia and low back pain after delivery: a prospective cohort study. BMJ. 1995;311:1336–1339.
27. Howell CJ, Kidd C, Roberts W, et al. A randomised controlled trial of epidural compared with non-epidural analgesia in labour. BJOG. 2001;108:27–33.
28. Howell CJ, Dean T, Lucking L, et al. Randomised study of long term outcome after epidural versus non-epidural analgesia during labour. BMJ. 2002;325:357.
29. Wilson MJ, Moore PA, Shennan A, et al. Long-term effects of epidural analgesia in labor: a randomized controlled trial comparing high dose with two mobile techniques. Birth. 2011;38:105–110.
30. To WW, Wong MW. Kyphoscoliosis complicating pregnancy. Int J Gynaecol Obstet. 1996;55:123–128.
31. Danielsson AJ, Nachemson AL. Childbearing, curve progression, and sexual function in women 22 years after treatment for adolescent idiopathic scoliosis: a case-control study. Spine. 2001;26:1449–1456.
32. White AA, Panjabi MM. Practical biomechanics of scoliosis and kyphosis. White AA, Panjabi MM. Clinical Biomechanics of the Spine. 2nd edition. JB Lippincott: Philadelphia; 1990:127–168.
33. Pehrsson K, Larsson S, Oden A, Nachemson A. Long-term follow-up of patients with untreated scoliosis. A study of mortality, causes of death, and symptoms. Spine. 1992;17:1091–1096.
34. Katsuragi S, Yamanaka K, Neki R, et al. Maternal outcome in pregnancy complicated with pulmonary arterial hypertension. Circ J. 2012;76:2249–2254.
35. Berman AT, Cohen DL, Schwentker EP. The effects of pregnancy on idiopathic scoliosis. A preliminary report on eight cases and a review of the literature. Spine. 1982;7:76–77.
36. Blount WP, Mellencamp D. The effect of pregnancy on idiopathic scoliosis. J Bone Joint Surg Am. 1980;62:1083–1087.
37. Sawicka EH, Spencer GT, Branthwaite MA. Management of respiratory failure complicating pregnancy in severe kyphoscoliosis: a new use for an old technique? Br J Dis Chest. 1986;80:191–196.
38. Bonnin M, Mercier FJ, Sitbon O, et al. Severe pulmonary hypertension during pregnancy: mode of delivery and anesthetic management of 15 consecutive cases. Anesthesiology. 2005;102:1133–1137.
39. Warnes CA. Pregnancy and pulmonary hypertension. Int J Cardiol. 2004;97(Suppl 1):11–13.
40. Kesten S, Garfinkel SK, Wright T, Rebuck AS. Impaired exercise capacity in adults with moderate scoliosis. Chest. 1991;99:663–666.
41. Zeldis SM. Dyspnea during pregnancy. Distinguishing cardiac from pulmonary causes. Clin Chest Med. 1992;13:567–585.
42. Crosby ET, Halpern SH. Obstetric epidural anaesthesia in patients with Harrington instrumentation. Can J Anaesth. 1989;36:693–696.
43. Shneerson JM. Pregnancy in neuromuscular and skeletal disorders. Monaldi Arch Chest Dis. 1994;49:227–230.
44. Liljenqvist UR, Allkemper T, Hackenberg L, et al. Analysis of vertebral morphology in idiopathic scoliosis with use of magnetic resonance imaging and multiplanar reconstruction. J Bone Joint Surg Am. 2002;84-A:359–368.
45. Huang J. Paramedian approach for neuroaxial anesthesia in parturients with scoliosis. Anesth Analg. 2010;111:821–822.
46. Boon JM, Prinsloo E, Raath RP. A paramedian approach for epidural block: an anatomic and radiologic description. Reg Anesth Pain Med. 2003;28:221–227.
47. Carvalho JC. Ultrasound-facilitated epidurals and spinals in obstetrics. Anesthesiol Clin. 2008;26:145–158.
48. Schnabel A, Schuster F, Ermert T, et al. Ultrasound Guidance for Neuraxial Analgesia and Anesthesia in Obstetrics: a Quantitative Systematic Review. Ultraschall Med. 2010.
49. Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115:94–101.
50. Chin KJ, Chan VW, Ramlogan R, Perlas A. Real-time ultrasound-guided spinal anesthesia in patients with a challenging spinal anatomy: two case reports. Acta Anaesthesiol Scand. 2010;54:252–255.
51. Moran DH, Johnson MD. Continuous spinal anesthesia with combined hyperbaric and isobaric bupivacaine in a patient with scoliosis. Anesth Analg. 1990;70:445–447.
52. Cochran T, Irstam L, Nachemson A. Long-term anatomic and functional changes in patients with adolescent idiopathic scoliosis treated by Harrington rod fusion. Spine. 1983;8:576–584.
53. Sponseller PD, Cohen MS, Nachemson AL, et al. Results of surgical treatment of adults with idiopathic scoliosis. J Bone Joint Surg Am. 1987;69:667–675.
54. Aaro S, Ohlen G. The effect of Harrington instrumentation on the sagittal configuration and mobility of the spine in scoliosis. Spine. 1983;8:570–575.
55. Ko JY, Leffert LR. Clinical implications of neuraxial anesthesia in the parturient with scoliosis. Anesth Analg. 2009;109:1930–1934.
56. Costello JF, Balki M. Cesarean delivery under ultrasound-guided spinal anesthesia [corrected] in a parturient with poliomyelitis and Harrington instrumentation. Can J Anaesth. 2008;55:606–611.
57. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25.
58. Yurube T, Sumi M, Nishida K, et al. Incidence and Aggravation of Cervical Spine Instabilities in Rheumatoid Arthritis: A Prospective Minimum 5-Year Follow-Up Study of Patients Initially without Cervical Involvement. Spine. 2012;37:2136–2144.
59. Younes M, Belghali S, Kriaa S, et al. Compared imaging of the rheumatoid cervical spine: prevalence study and associated factors. Joint Bone Spine. 2009;76:361–368.
60. Shen FH, Samartzis D, Jenis LG, An HS. Rheumatoid arthritis: evaluation and surgical management of the cervical spine. Spine J. 2004;4:689–700.
61. Keenan MA, Stiles CM, Kaufman RL. Acquired laryngeal deviation associated with cervical spine disease in erosive polyarticular arthritis. Use of the fiberoptic bronchoscope in rheumatoid disease. Anesthesiology. 1983;58:441–449.
62. Samanta R, Shoukrey K, Griffiths R. Rheumatoid arthritis and anaesthesia. Anaesthesia. 2011;66:1146–1159.
63. Wallenius M, Skomsvoll JF, Irgens LM, et al. Pregnancy and delivery in women with chronic inflammatory arthritides with a specific focus on first birth. Arthritis Rheum. 2011;63:1534–1542.
64. Chakravarty EF, Nelson L, Krishnan E. Obstetric hospitalizations in the United States for women with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:899–907.
65. Marker-Hermann E, Fischer-Betz R. Rheumatic diseases and pregnancy. Curr Opin Obstet & Gynecol. 2010;22:458–465.
66. Hazes JM, Coulie PG, Geenen V, et al. Rheumatoid arthritis and pregnancy: evolution of disease activity and pathophysiological considerations for drug use. Rheumatology. 2011;50:1955–1968.
67. Ostensen M, Forger F. Management of RA medications in pregnant patients. Nat Rev Rheumatol. 2009;5:382–390.
68. Li DK, Liu L, Odouli R. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: population based cohort study. BMJ. 2003;327:368.
69. Leino KA, Kuusniemi KS, Palve HK, et al. Spread of spinal block in patients with rheumatoid arthritis. Acta Anaesthesiol Scand. 2010;54:65–69.
70. Leino KA, Kuusniemi KS, Palve HK, et al. The effect of body mass index on the spread of spinal block in patients with rheumatoid arthritis. J Anesth. 2011;25:213–218.
71. Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–1390.
72. Woodward LJ, Kam PC. Ankylosing spondylitis: recent developments and anaesthetic implications. Anaesthesia. 2009;64:540–548.
73. Sorin S, Askari A, Moskowitz RW. Atlantoaxial subluxation as a complication of early ankylosing spondylitis. Two cases reports and a review of the literature. Arthritis Rheum. 1979;22:273–276.
74. Neva MH, Isomaki P, Hannonen P, et al. Early and extensive erosiveness in peripheral joints predicts atlantoaxial subluxations in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:1808–1813.
75. Ostensen M, Fuhrer L, Mathieu R, et al. A prospective study of pregnant patients with rheumatoid arthritis and ankylosing spondylitis using validated clinical instruments. Ann Rheum Dis. 2004;63:1212–1217.
76. Lui NL, Haroon N, Carty A, et al. Effect of pregnancy on ankylosing spondylitis: a case-control study. J Rheumatol. 2011;38:2442–2444.
77. Broomhead CJ, Davies W, Higgins D. Awake oral fibreoptic intubation for caesarean section. Int J Obstet Anesth. 1995;4:172–174.
78. Kumar CM, Mehta M. Ankylosing spondylitis: lateral approach to spinal anaesthesia for lower limb surgery. Can J Anaesth. 1995;42:73–76.
79. Batra YK, Sharma A, Rajeev S. Total spinal anaesthesia following epidural test dose in an ankylosing spondylitic patient with anticipated difficult airway undergoing total hip replacement. Eur J Anaesthesiol. 2006;23:897–898.
80. Hoffman SL, Zaphiratos V, Girard MA, et al. Failed epidural analgesia in a parturient with advanced ankylosing spondylitis: a novel explanation. Can J Anaesth. 2012.
81. Chin KJ, Chan V. Ultrasonography as a preoperative assessment tool: predicting the feasibility of central neuraxial blockade. Anesth Analg. 2010;110:252–253.
82. Avrahami E, Frishman E, Fridman Z, Azor M. Spina bifida occulta of S1 is not an innocent finding. Spine. 1994;19:12–15.
83. Altamimi Y, Pavy TJ. Epidural analgesia for labour in a patient with a neural tube defect. Anaesth Intensive Care. 2006;34:816–819.
84. Farine D, Jackson U, Portale A, et al. Pregnancy complicated by maternal spina bifida. A report of two cases. J Reprod Med. 1988;33:323–326.
85. Jackson AB, Mott PK. Reproductive health care for women with spina bifida. Scientific World Journal. 2007;7:1875–1883.
86. Muller EB, Nordwall A. Prevalence of scoliosis in children with myelomeningocele in western Sweden. Spine. 1992;17:1097–1102.
87. Warder DE. Tethered cord syndrome and occult spinal dysraphism. Neurosurg Focus. 2001;10:1–9.
88. Hertzler DA 2nd, DePowell JJ, Stevenson CB, Mangano FT. Tethered cord syndrome: a review of the literature from embryology to adult presentation. Neurosurg Focus. 2010;29:E1.
89. Wenger M, Hauswirth CB, Brodhage RP. Undiagnosed adult diastematomyelia associated with neurological symptoms following spinal anaesthesia. Anaesthesia. 2001;56:764–767.
90. Ahmad FU, Pandey P, Sharma BS, Garg A. Foot drop after spinal anesthesia in a patient with a low-lying cord. Int J Obstet Anesth. 2006;15:233–236.
91. Warder DE, Oakes WJ. Tethered cord syndrome: the low-lying and normally positioned conus. Neurosurgery. 1994;34:597–600.
92. Yamada S, Won DJ, Pezeshkpour G, et al. Pathophysiology of tethered cord syndrome and similar complex disorders. Neurosurg Focus. 2007;23:E6.
93. Huttmann S, Krauss J, Collmann H, et al. Surgical management of tethered spinal cord in adults: report of 54 cases. J Neurosurg. 2001;95:173–178.
94. Yamada S, Won DJ, Yamada SM, et al. Adult tethered cord syndrome: relative to spinal cord length and filum thickness. Neurol Res. 2004;26:732–734.
95. Royo-Salvador MB, Sole-Llenas J, Domenech JM, Gonzalez-Adrio R. Results of the section of the filum terminale in 20 patients with syringomyelia, scoliosis and Chiari malformation. Acta Neurochir (Wien). 2005;147:515–523.
96. Chantigian RC, Koehn MA, Ramin KD, Warner MA. Chiari I malformation in parturients. J Clin Anesth. 2002;14:201–205.
97. Landau R, Giraud R, Delrue V, Kern C. Spinal anesthesia for cesarean delivery in a woman with a surgically corrected type I Arnold Chiari malformation. Anesth Analg. 2003;97:253–255.
98. Wood GG, Jacka MJ. Spinal hematoma following spinal anesthesia in a patient with spina bifida occulta. Anesthesiology. 1997;87:983–984.
99. Tidmarsh MD, May AE. Epidural anaesthesia and neural tube defects. Int J Obstet Anesth. 1998;7:111–114.
100. Kreeger RN, Hilvano A. Anesthetic options for the parturient with a neural tube defect. Int Anesthesiol Clin. 2005;43:65–80.
101. May AE, Fombon FN, Francis S. UK registry of high-risk obstetric anaesthesia: report on neurological disease. Int J Obstet Anesth. 2008;17:31–36.
102. Asakura Y, Kandatsu N, Hashimoto A, et al. Ultrasound-guided neuroaxial anesthesia: accurate diagnosis of spina bifida occulta by ultrasonography. J Anesth. 2009;23:312–313.
103. Kim JE, Hong JY, Han SW, Kil HJ. Occult spinal dysraphism: detection during ultrasound for epidural blockade in children. Anaesthesia. 2009;64:1026–1028.
104. Berkowitz ID, Raja SN, Bender KS, Kopits SE. Dwarfs: pathophysiology and anesthetic implications. Anesthesiology. 1990;73:739–759.
105. Carstoniu J, Yee I, Halpern S. Epidural anaesthesia for caesarean section in an achondroplastic dwarf. Can J Anaesth. 1992;39:708–711.
106. Wynne-Davies R, Walsh WK, Gormley J. Achondroplasia and hypochondroplasia. Clinical variation and spinal stenosis. J Bone Joint Surg Br. 1981;63B:508–515.
107. Lutter LD, Longstein JE, Winter RB, Langer LO. Anatomy of the achondroplastic lumbar canal. Clin Orthop Relat Res. 1977;139–142.
108. Wardall GJ, Frame WT. Extradural anaesthesia for caesarean section in achondroplasia. Br J Anaesth. 1990;64:367–370.
109. Brimacombe JR, Caunt JA. Anaesthesia in a gravid achondroplastic dwarf. Anaesthesia. 1990;45:132–134.
110. Beilin Y, Leibowitz AB. Anesthesia for an achondroplastic dwarf presenting for urgent cesarean section. Int J Obstet Anesth. 1993;2:96–97.
111. Palomero MA, Vargas MC, Pelaez EM, et al. Spinal anaesthesia for emergency Caesarean section in an achondroplastic patient. Eur J Anaesthesiol. 2007;24:981–982.
112. Smiley R. Test-dosing through the Tuohy and the standard of care: the devil is in the details. Int J Obstet Anesth. 2009;18:92.
113. Morrow MJ, Black IH. Epidural anaesthesia for caesarean section in an achondroplastic dwarf. Br J Anaesth. 1998;81:619–621.
114. DeRenzo JS, Vallejo MC, Ramanathan S. Failed regional anesthesia with reduced spinal bupivacaine dosage in a parturient with achondroplasia presenting for urgent cesarean section. Int J Obstet Anesth. 2005;14:175–178.
115. Huang J, Babins N. Anesthesia for cesarean delivery in an achondroplastic dwarf: a case report. AANA J. 2008;76:435–436.
116. Cunningham AJ, Donnelly M, Comerford J. Osteogenesis imperfecta: anesthetic management of a patient for cesarean section: a case report. Anesthesiology. 1984;61:91–93.
117. Fiegel MJ. Cesarean delivery and colon resection in a patient with type III osteogenesis imperfecta. Semin Cardiothorac Vasc Anesth. 2011;15:98–101.
118. Byers PH, Steiner RD. Osteogenesis imperfecta. Annu Rev Med. 1992;43:269–282.
119. Vogel TM, Ratner EF, Thomas RC Jr, Chitkara U. Pregnancy complicated by severe osteogenesis imperfecta: a report of two cases. Anesth Analg. 2002;94:1315–1317.
120. Benca J, Hogan K. Malignant hyperthermia, coexisting disorders, and enzymopathies: risks and management options. Anesth Analg. 2009;109:1049–1053.
121. Bojanic K, Kivela JE, Gurrieri C, et al. Perioperative course and intraoperative temperatures in patients with osteogenesis imperfecta. Eur J Anaesthesiol. 2011;28:370–375.
122. Anderer G, Hellmeyer L, Hadji P. Clinical management of a pregnant patient with type I osteogenesis imperfecta using quantitative ultrasonometry—a case report. Ultraschall Med. 2008;29:201–204.
123. Krishnamoorthy U, Vausse S, Donnai P. Management of pregnancy complicated by maternal osteogenesis imperfecta. Report of a case with uterine rupture. J Obstet Gynaecol. 2002;22:316.
124. Cho E, Dayan SS, Marx GF. Anaesthesia in a parturient with osteogenesis imperfecta. Br J Anaesth. 1992;68:422–423.
125. Murray S, Shamsuddin W, Russell R. Sequential combined spinal-epidural for caesarean delivery in osteogenesis imperfecta. Int J Obstet Anesth. 2010;19:127–128.
126. Yeo ST, Paech MJ. Regional anaesthesia for multiple caesarean sections in a parturient with osteogenesis imperfecta. Int J Obstet Anesth. 1999;8:284–287.
127. Vebostad A. Spondylolisthesis. A review of 71 patients. Acta Orthop Scand. 1974;45:711–723.
128. Dandy DJ, Shannon MJ. Lumbo-sacral subluxation. (Group 1 spondylolisthesis). J Bone Joint Surg Br. 1971;53:578–595.
129. Saraste H. Spondylolysis and pregnancy–a risk analysis. Acta Obstet Gynecol Scand. 1986;65:727–729.
130. Sanderson PL, Fraser RD. The influence of pregnancy on the development of degenerative spondylolisthesis. J Bone Joint Surg Br. 1996;78:951–954.
131. Horduna M, Legaye J. Influence of the sagittal anatomy of the pelvis on the intercrestal line position. Eur J Anaesthesiol. 2008;25:200–205.