Surgical Technique 2 (Baerveldt Glaucoma Implant)
George Baerveldt, Anthony Leoncavallo
![]()
Introduction
The Baerveldt glaucoma implant (Advanced Medical Optics, Inc., Santa Ana, CA) was first introduced in 1990. Its design is a modification of the earlier Molteno implant, with a nonvalved silicone tube (0.63 mm external diameter, 0.30 mm bore) attached to a medical-grade silicone plate. The implant is currently available in two sizes, 250 mm and 350 mm (Fig. 112-1A and B). The pars plana version is based on the 350 mm size plate, with the silicone tube attached to a small episcleral plate with an angled cannula to be inserted through a sclerotomy (Fig. 112-1C). In all designs, pressure reduction is achieved by redirecting aqueous fluid from within the eye through the silicone tube to an encapsulated space surrounding the plate, where it can diffuse into the fibrous walls of the bleb.
The glaucoma implant plate is barium-impregnated, which allows radiographic identification, as well as gamma-irradiated. It is also tumble polished to produce a low wetting angle. There is an anterior flange with two large suture holes and a 1-mm-high ridge with the tube opening on the posterior aspect of the ridge. The implant profile is 0.84 mm, a design that allows for a smooth, thin plate that maintains the flexibility and rigidity necessary for implantation. The plate itself is fenestrated with four holes to allow fibrous tissue growth between the anterior, conjunctival, and posterior scleral walls of the resulting fibrous capsule after implantation. This is thought to reduce the height of the bleb and thereby limit problems with ocular motility.
Indications for Use
The Baerveldt implant is useful in patients with medically uncontrolled glaucoma who are poor candidates for standard trabeculectomy surgery. This includes patients with neovascular glaucoma, penetrating keratoplasty with glaucoma, retinal detachment surgery with glaucoma, iridocorneal endothelial (ICE) syndrome, traumatic glaucoma, uveitic glaucoma, previous failed trabeculectomy, epithelial downgrowth, refractory infantile glaucoma, and contact lens wearers who need glaucoma filtration surgery.
Preoperative Considerations
The most difficult decision can be whether or not to proceed with the surgery, depending on the patient's overall health situation, potential for useful vision after surgery, and the likelihood of vision-threatening postoperative complications.
Having ascertained that proceeding with the Baerveldt implant surgery would be prudent, a thorough preoperative examination will aid in surgical planning and should be directed at identifying characteristics that would impede the success of the surgery. For instance, a very high preoperative intraocular pressure (IOP) increases the possibility of a delayed suprachoroidal hemorrhage, which may be mitigated by a stepwise decrease in IOP during the surgery. The condition of the conjunctiva and previous scarring will direct which quadrant to choose for the surgery. A sufficiently clear peripheral cornea, not obscured with arcus senilis, scarring, or sutures, will allow easier confirmation of intraocular tube position. A shallow chamber or one with vitreous strands may require a vitrectomy and pars plana implant.
The most appropriate site of implantation is the superotemporal quadrant, as this area offers the largest potential space between the globe and orbital wall, allowing excellent exposure and reducing the chance of postoperative motility problems. It also allows for the best cosmesis, as the graft is covered completely by the upper lid. In cases of a second implant or extenuating circumstances, the usual order for quadrant selection after superotemporal is superonasal, then inferonasal, then inferotemporal.
Anesthetic Considerations
A retrobulbar block is the method of choice for anesthesia of the globe. Intravenous sedation is often administered as an adjuvant. Topical or intracameral methods are inadequate for pain control, especially during manipulation of the rectus muscles. Some surgeons prefer a parabulbar block performed after topical anesthesia. A conjunctiva and Tenon's incision is made at the desired location as described below. Before extensive dissection is carried out, a blunt-tipped cannula is inserted under Tenon's and threaded posteriorly into the muscle cone, where the usual anesthetic mixture is injected. General anesthesia is only necessary when the patient is unable to comply with direction due to age, cognitive status, or history of inadequate pain control with local anesthesia.
Operative Techniques and Potential Modifications
See Box 112-1 for a list of necessary instrumentation and supplies.
After the appropriate anesthesia is administered, the eye is prepared in the normal sterile fashion (Video 112-1![]() ). An operating microscope is routinely used. A lightweight lid speculum should be considered to avoid excessive positive pressure and to avoid overstretching the eyelids. Corneal traction may be utilized to aid in quadrant exposure. This is done with 6/0 Vicryl on an S-29 needle passed through the superior peripheral cornea at about 50% thickness. The suture ends can then be clipped inferiorly to the drape with a small mosquito clamp. The first incision is made with scissors, opening the conjunctiva and Tenon's about 4–5 mm posterior to the limbus for approximately 14 mm, or roughly four clock-hours of the superotemporal quadrant. The incision needs to be wide enough to expose the borders of the superior and lateral rectus muscles. Posterior dissection between the muscles is carried out with scissors under the Tenon's, while anteriorly, a limbus-based flap of conjunctiva and Tenon's is created with blunt dissection. This can be accomplished by grasping a Weck-cel sponge bimanually with both a forceps at the tip and grasping the stem and dragging it across the scleral surface anteriorly, bluntly dissecting the conjunctiva and Tenon's in one step. Light cautery may be needed to maintain hemostasis and visibility.
). An operating microscope is routinely used. A lightweight lid speculum should be considered to avoid excessive positive pressure and to avoid overstretching the eyelids. Corneal traction may be utilized to aid in quadrant exposure. This is done with 6/0 Vicryl on an S-29 needle passed through the superior peripheral cornea at about 50% thickness. The suture ends can then be clipped inferiorly to the drape with a small mosquito clamp. The first incision is made with scissors, opening the conjunctiva and Tenon's about 4–5 mm posterior to the limbus for approximately 14 mm, or roughly four clock-hours of the superotemporal quadrant. The incision needs to be wide enough to expose the borders of the superior and lateral rectus muscles. Posterior dissection between the muscles is carried out with scissors under the Tenon's, while anteriorly, a limbus-based flap of conjunctiva and Tenon's is created with blunt dissection. This can be accomplished by grasping a Weck-cel sponge bimanually with both a forceps at the tip and grasping the stem and dragging it across the scleral surface anteriorly, bluntly dissecting the conjunctiva and Tenon's in one step. Light cautery may be needed to maintain hemostasis and visibility.
Muscle hooks are used to identify and isolate both superior and lateral rectus muscles. The Baerveldt implant is then placed on the field and rinsed with saline. The superior rectus is isolated and retracted from the globe with a muscle hook. A second muscle hook is inserted under the belly of the muscle more posteriorly and used to aid in elevation and retraction. A large, nontoothed forceps, such as the Bonnacolto, is used to hold the implant longitudinally, and the superior wing of the implant is inserted between the superior rectus muscle and the sclera. The implant is pushed superonasally until about three-fourths of it is under the muscle. It is important to maintain visualization and purchase of the plate during this insertion so as to be able to pull the implant forward in case it slides too far posterior. While maintaining the position of the implant under the superior rectus, a similar maneuver is performed to insert the other wing of the implant under the lateral rectus muscle, with the nontoothed forceps and muscle hook. After both wings of the implant are secure under the respective rectus muscle, the implant should be fairly mobile in all directions in its pocket, except anteriorly, where the insertion of the muscles should prevent anterior movement towards the limbus. The implant is then positioned such that the flange and tube lie between the two muscle insertions.
Calipers are used to confirm that the anterior edge of the implant is 10–12 mm posterior to the limbus. The implant is then sutured to the sclera with 7/0 or 8/0 nonabsorbable suture, such as Prolene, through the suture holes. Care must be taken to make the needle passes partial thickness, and not full thickness, as retinal or choroidal damage may result. The author prefers to use 7/0 Prolene on a BVI needle with a large needle holder, which allows controlled insertion into the sclera. The suture knots should be rotated into the suture hole eyelets.
Attention is then turned to insertion of the drainage tube. The anterior conjunctival and Tenon's flap should be extended to the limbus. Light cautery may be necessary. The tube is then draped over the limbus at the proposed entry site into the anterior chamber. Scissors are used to cut the tube to allow roughly a 1–2 mm length inside the eye, and an upward-facing bevel is created by cutting the tube at an angle. The tube is then ligated within a few millimeters of the implant flange with 7/0 or 8/0 absorbable suture, such as Vicryl. The tube should be checked for complete occlusion by inserting a 30-gauge cannula at the cut end and attempting to flush saline. If it is still patent, a second suture should be placed and checked. A 7/0 Vicryl ligature will usually rupture after 4–6 weeks, and an 8/0 after 2–4 weeks (Fig. 112-2).
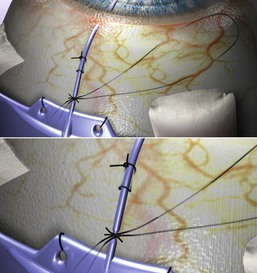
A clear corneal paracentesis is made into the anterior chamber. Intraocular pressure may be adjusted with balanced salt solution, or the chamber deepened to allow for easier insertion of the tube.
The most critical maneuver is creating the needle track through which the tube will be inserted into the anterior chamber. Usually, a 23-gauge needle is used, as this will assure a tight fit around the tube with minimal leakage. A 22-gauge needle will allow a small amount of fluid to flow around the tube, which may be useful to control intraocular pressure for the initial 2–3 weeks. A 21-gauge needle is only recommended in patients with florid rubeosis. A pilot entry into the eye with a 27-gauge needle may be used to provide for easier subsequent entry of the 23-gauge needle and to allow for preliminary visualization of the tube track position.
The needle track is started 0.5–0.75 mm posterior to the limbus, bevel down, and parallel to the iris plane. A more corneal approach may be required in the phakic patient. The needle is advanced until it is clearly visible in the anterior chamber. If it is too close to the cornea, the needle should be removed from the eye, repositioned just posterior to the initial track, and re-inserted. The presence of the tube in the second track will occlude the first and prevent leakage.
The tube is then inserted through the needle track, usually with the aid of straight and curved tying forceps or with specially designed tube insertion forceps. The tube should lie 1–2 mm into the anterior chamber, just anterior to the iris, parallel with the iris plane and away from the cornea. The bevel should face upward toward the cornea to minimize the chance of iris incarceration. If it is too long, it should be removed, cut again with a bevel, and reinserted into the anterior chamber. Once it is properly positioned, the tube is loosely sutured to the episclera with an absorbable suture. Early intraocular pressure relief may also be aided by piercing the tube close to its insertion into the limbus, anterior to the ligature. This is done with a double perforating cut with the spatulated needle on the Vicryl suture. One or two perforations are made in this fashion.
A connective tissue graft, such as a donor sclera, dura mater, fascia lata, pericardium or corneal tissue, is used to cover the anterior portion of the tube (Video 112-2![]() ). The graft is reconstituted and cut to the appropriate shape and size freehand, about 4 × 6 mm. This is sutured to the sclera with interrupted absorbable suture, such as 7/0 or 8/0 polyglactin. The graft's purpose is to cover any staphylomas from previous surgeries as well as to cover the drainage tube to help prevent extrusion through the conjunctiva.
). The graft is reconstituted and cut to the appropriate shape and size freehand, about 4 × 6 mm. This is sutured to the sclera with interrupted absorbable suture, such as 7/0 or 8/0 polyglactin. The graft's purpose is to cover any staphylomas from previous surgeries as well as to cover the drainage tube to help prevent extrusion through the conjunctiva.
A double-layer closure is usually preferred, with the Tenon's closed first with a running 7/0 or 8/0 absorbable suture. If the anterior Tenon's is too thin, the posterior edge of the capsule can be sutured to the patch graft itself or the episclera, thereby protecting the implant plate. The conjunctiva is then closed with a running 7/0 or 8/0 absorbable suture as well. A watertight closure is desired to help prevent postoperative hypotony and epithelial ingrowth.
Subconjunctival injection of antibiotics and steroids is recommended. The eye is then patched and shielded overnight.
Pars Plana Insertion
A pars plana insertion of the drainage tube may be necessary during certain conditions, such as a shallow anterior chamber, aphakia, penetrating keratoplasty, vitreous in the anterior chamber, concomitant need for retinal surgery due to diabetic retinopathy or an extensive anterior staphyloma with scarring. A vitrectomy with adequate trimming of the vitreous base is required.
Two options are available for pars plana insertion, depending on the circumstances of the case. If the patient's eye is having a pars plana vitrectomy just prior to insertion of the implant, the Baerveldt Pars Plana implant is used. The implant's plate is secured as described above. The angled cannula of the Hoffman elbow is inserted through the MVR sclerostomy following vitrectomy and gas–fluid exchange. The Hoffman cannula is preferred, as a watertight closure cannot always be achieved if the standard implant's tube is inserted into the sclerostomy. The episcleral plate is then sutured to the sclera with nonabsorbable suture, a biograft secured, and the remainder of the case finished as described above.
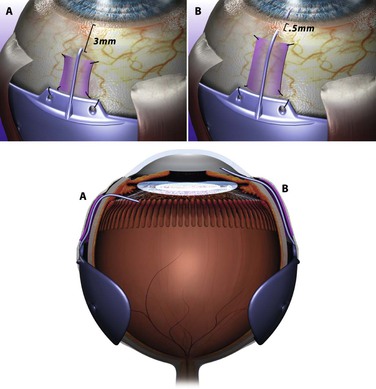
If a pars plana tube insertion is considered on an eye that already has had a vitrectomy, a standard 350-mm implant can be used. The plate is secured as described above. A 21-gauge needle is inserted through the sclera at an angle parallel to the iris plane 3 mm posterior to the limbus (Fig. 112-3). This is used as a needle track to insert the tube. The tube needs to remain 4–5 mm long so as to allow visualization through the pupil to assure proper positioning. A large-gauge needle is used to pierce both the sclera and the inner pars plana adequately to avoid tunneling of the tube into the choroid. The tube is ligated, secured to the sclera, and the remainder of the case can be completed as described above.
Postoperative Management and Interventions
The patient is seen the day after surgery and started on topical antibiotics and steroids, the latter being dosed every 1–3 hours depending on the amount of inflammation. Patients should be advised of the standard precautions after intraocular surgery, such as the avoidance of Valsalva maneuvers. An IOP spike may be treated with leaking of fluid through the paracentesis, and possibly restarting glaucoma medications. The main consideration is to modulate eye pressure while an encapsulating bleb forms around the implant and the absorbable suture ligating the tube ruptures. Topical antibiotics are typically continued for 7–10 days, but topical steroids are kept on longer and tapered slowly. Follow-up examinations should be focused on anterior chamber reaction and depth, tube position, and conjunctival healing. Depending on the size of ligating suture used, the tube should become patent between 3 and 6 weeks postoperatively. Patients should be counseled that a sudden drop in IOP can be expected, with some discomfort and drop in vision when this occurs. An inflammatory reaction can occur, along with debris or even heme in the anterior chamber, all of which resolve with judicious topical steroid use.
A hypertensive phase is often described in patients about 2 months after surgery as IOP rises. This is thought to be caused by inflammation and fibrosis of the capsule surrounding the implant, making the walls impermeable to aqueous. Topical glaucoma medications should be reinstated to blunt the rise in intraocular pressure until this phase resolves.
The Use of Adjuvants
The use of intraoperative and postoperative adjuvant treatments has not been shown to increase the success rates of the surgery. Although there have been no prospective, randomized trials on the Baerveldt implant and adjuvants, inferences can be made from the few trials done with other implants, as well as from some retrospective studies. Two randomized trials were conducted to study the effectiveness of adjunctive mitomycin C use intraoperatively in the insertion of the Molteno and Ahmed valves.1,2 Both trials found no evidence of its benefits in terms of mean postoperative IOP, logMAR visual acuity scores, or postoperative antiglaucoma medications at 12 months' follow-up. Trible et al.3 showed in a retrospective analysis that the use of antimetabolites did not result in lower IOP or less medication needed for any group at any interval studied. Thus, the intraoperative use of mitomycin C is not recommended for the Baerveldt glaucoma implant.
Systemic steroid therapy after surgery has been considered to help reduced bleb fibrosis. Valimaki et al.4 showed that routine use of oral corticosteroid medication after Molteno surgery does not appear to be useful in controlling bleb fibrosis or the postoperative hypertensive stage. Systemic steroid treatment is not recommended following Baerveldt implant surgery.
Outcomes and Comparison with Other Techniques
Comparison of the safety and efficacy of the Baerveldt glaucoma implant to other surgical approaches is underway in a variety of studies. The Tube Versus Trabeculectomy (TVT) Study, by Gedde et al.,5 is a multicenter randomized clinical trial designed to compare nonvalved tube shunt surgery to trabeculectomy with mitomycin C. In this study, a total of 212 eyes with medically uncontrolled glaucoma and a history of previous trabeculectomy, cataract surgery, or both were randomized to surgical treatment with either Baerveldt 350 mm glaucoma implant or trabeculectomy with mitomycin C. The 5-year results demonstrated similar IOP reduction and medication use between the two groups. However, the postoperative complication rate was higher in the trabeculectomy group, as was the cumulative rate of failure. Failure in this study was defined by IOP greater than 21 mmHg or less than 5 mmHg, IOP not reduced by 20% postoperatively, the need for reoperation for glaucoma, or the loss of light perception. The trabeculectomy group had a failure rate after 5 years of 46.9%, while in the tube group it was just 29.8%. The need for reoperation to achieve adequate IOP control was 29% in the trabeculectomy group and 9% in the tube group.
The Ahmed Baerveldt Comparison (ABC) Study by Budenz et al.6 is a multicenter, randomized, controlled clinical trial which seeks to compare efficacy and rates of complication between the two devices for a 5-year follow-up period. During the first year of the study, fewer serious complications were seen in the early postoperative period in the Ahmed group (20%) than the Baerveldt group (34%). The average IOP, however, was noted to be slightly lower in the Baerveldt group at one year and at 3 years with a lower risk of re-operation for glaucoma in the Baerveldt group.7 Similarly, the 1-year results of the Ahmed Versus Baerveldt (AVB) Study by Christakis et al.8 showed lower IOP in the Baerveldt group (13.6 ± 4.8) in comparison to the Ahmed patients (16.5 ± 5.3mmHg). In this first year of follow-up, the authors found a higher rate of subsequent intervention in the Baerveldt group than in the Ahmed group although at 3 years this did not reach statistical significance.9
In a Cochrane review of glaucoma implants, Minckler et al.10 concluded in a meta-analysis that no specific aqueous shunt stands out as clearly clinically superior with regard to either safety or efficacy.
Complications and How to Avoid Them
Please refer also to Chapter 116.
Hypotony
There are a variety of factors that could cause hypotony. Early in the postoperative period, hypotony is usually due to incomplete occlusion of the implant tube. Other reasons include larger than desired tube fenestrations or leakage of aqueous around the tube. Hypotonous eyes can be observed for spontaneous elevation of pressure, provided that the anterior chamber is relatively maintained. If the implant tube is in contact with corneal endothelium or lens epithelium, the chamber needs to be reformed immediately, such as with a viscoelastic through a paracentesis track.
Tube-Related Complications
As mentioned earlier, the most important step during implantation is making the needle track through which the tube is passed into the anterior chamber. Proper orientation of the needle will best help prevent malpositioning of the tube. Tubes that are too close to the cornea may cause decreased endothelial cell counts, which may lead to graft failure in a setting of penetrating keratoplasty. Tubes that are too close to the iris may cause inflammation and hemorrhage, while those that are too close to the lens may cause cataract formation. If malposition occurs during surgery, the tube should be removed, the needle track closed with 10/0 nylon suture, and a second track then made. If the tube is cut too short, it can be repaired with the Teflon sheath of an angiocath, or with available repair kits.
The opening of the tube in the anterior chamber may become obstructed with fibrin, iris, heme, or vitreous. If the culprit is a small amount of iris or inflammatory tissue, an Nd:YAG laser can be used to try to disrupt the tissues from the tip of the tube. Larger amounts of tissue or vitreous strands usually require surgical revision and/or a vitrectomy.
Over the course of extended follow-up visits, the conjunctiva overlying the tube and graft should be examined carefully. When the vasculature in the overlying tissue disappears, or thinning is noticed, this is an indication for a surgical revision to prevent extrusion or endophthalmitis. If there is a clear reason for extrusion, such as an anteriorly placed graft with constant interaction with the eyelid, the entire graft may need to be removed and replaced. If no clear etiology is seen, a lesser revision of the superficial tissues may be adequate. The conjunctiva and Tenon's are undermined in the area surrounding the defect to allow mobility and sufficient tissue to cover the defect. A tissue graft, such as donor sclera, is placed on top of the tube or plate underneath the defect, and the superficial tissues are then closed with sutures that incorporate the graft for additional strength.
Strabismus
Diplopia is usually secondary to the mass effect created by the implant and its surrounding bleb. Other possible causes include entering into the orbital fat during surgery and causing fat fibrosis syndrome, injury to the rectus muscles during isolation or retrobulbar injection, and entrapment of the superior oblique tendon. The mass effect often produces a concomitant strabismus, typically an exotropia with hypotropia where the implant is placed superotemporally. A pseudo-Brown's syndrome can be seen with the implant in the superonasal position. Strabismus following implant surgery is difficult to treat. Prism correction is tried first, and strabismus surgery may be considered. At times, the only option may be removal of the implant, with possible replacement with a smaller implant. Fortunately, the incidence of strabismus with the Baerveldt glaucoma implant has been reduced by incorporating fenestrations into the implant plate. As mentioned above, this allows fibrous tissue growth between the anterior and posterior walls of the resulting fibrous capsule after implantation. This is thought to reduce the height of the bleb and thereby limit problems with ocular motility.
