Anesthesia
• Perioperative Considerations of the Pregnant Patient
Administration of anesthesia remains an important part of office-based oral and maxillofacial surgery. The most important step in delivering safe and effective anesthesia is preparation. Preparation begins with a thorough knowledge and understanding of the anatomy, physiology, and pharmacology relevant to anesthesia. From this point, safe anesthetic techniques are developed and used based on the preoperative patient evaluation, practitioner's preference, and individual clinical situations. Preparation also includes measures to prevent and manage emergencies. Despite a thorough preoperative patient evaluation, use of safe and proven anesthetic techniques, and vigilant monitoring, emergency situations may arise. For these reasons, it is essential that all practitioners continually hone their skills in the recognition and management of life-threatening emergencies in the office or operating room. Our excellent safety record is evidence of oral and maxillofacial surgeons' training and preparation in the delivery of safe anesthesia.
Each of the following teaching cases deals with the management of specific clinical scenarios. We also include a new case discussing trigeminal neuralgia/facial pain. The sections are structured to emphasize the key points in the preoperative evaluation and recognition of impending emergencies. Strategies for reducing the risk of emergent situations—and for their management when they do arise—are discussed. The highlighted clinical pearls in the preoperative patient evaluation should be incorporated into all practitioners' routine preoperative assessment.
The intent of this section is to familiarize readers with the risk factors and clinical signs associated with disastrous outcomes involving anesthesia (local, sedation, or general).
Laryngospasm
CC
A 12-year-old female is scheduled for extraction of four bicuspids under intravenous general anesthesia. (Laryngospasm may occur more often in children because of the frequency of upper respiratory tract infections in this patient population.)
HPI
Preoperative evaluation of the patient revealed no recent respiratory tract infections. The lungs were clear to auscultation. After ECG, blood pressure, pulse oximeter, and capnography monitors were applied, the patient was administered 4 L of oxygen and 2 L of nitrous oxide via nasal hood. Sedation was achieved using 4 mg of midazolam and 50 µg of fentanyl titrated to effect. Prior to administration of local anesthesia, 40 mg of propofol was infused. During the first extraction, respiratory stridor (a high-pitched, inspiratory “crowing” sound) was noted. A noisy, harsh sound was heard on inspiration through the precordial stethoscope, and the patient's oxygen saturation decreased from 99% to 65%. Capnography indicated no ventilation. At this point, the respiratory noises ceased. Tracheal tug and paradoxical chest wall motion were observed (signs of upper airway obstruction), and the patient began to appear cyanotic.
PMHX/PDHX/Medications/Allergies/SH/FH
Noncontributory. A recent history of upper respiratory tract infection (URI) may indicate an increased risk of perioperative respiratory complications, especially laryngospasm. In the event of a recent URI, it may be prudent to reschedule surgery after a 2-week symptom-free period. Patients with reactive airway disease may be more prone to experience laryngospasm.
Examination
General. A harsh inspiratory noise, or crowing, is audible on inspiration, which is best heard through the precordial stethoscope. The patient's skin color is assessed for signs of cyanosis, which is seen with severe hypoxemia. In pediatric patients, hypoxemia is often a late finding of decreased ventilation or apnea. End tidal CO2 monitoring and use of the precordial stethoscope indicate hypoventilation or apnea prior to changes in pulse oximetry.
Oropharynx. The throat pack is removed, and there is no evidence of foreign bodies. Copious amounts of mucous secretions are observed. (Blood and mucus are common stimuli for airway irritation.)
Neck and chest. There is evidence of tracheal tug and paradoxical chest wall motion (despite chin-lift and jaw-thrust maneuvers). This phenomenon is the result of forced inspiration against a closed glottis.
Vital signs. The patient's heart rate is 160 bpm, blood pressure 145/78 mm Hg, Etco2 0, and respirations 0 breaths per minute.
Oxygen saturation. Oxygen saturation decreased from 99% to 65% with the onset of laryngospasm. (Continued decline in the oxygen saturation can result in respiratory acidosis.)
ECG. The patient is in sinus tachycardia. (This is a common finding, but hypoxia can trigger more life-threatening cardiac arrhythmias. Hypoxemia in children may result in bradycardia.)
Imaging
Imaging is not relevant in the acute management of laryngospasm. This is an anesthetic emergency and is diagnosed based on the clinical presentation. Chest films can be ordered if there is suspicion of foreign body aspiration or to aid in the diagnosis of negative pressure/post obstructive pulmonary edema after the acute management of the airway.
Treatment
Prompt recognition and treatment of laryngospasm usually result in a good outcome. Upon diagnosis, the airway should be suctioned clear of noxious stimuli and the surgical site should be packed. Any foreign bodies are removed from the oral cavity and 100% oxygen is administered. Positive pressure ventilation should be attempted, ideally with a two-person technique and jaw-thrust maneuver; this often “breaks” the laryngospasm (jaw thrust and pressure at the angle of the mandible may also assist in breaking laryngospasms).
A technique described by Dr. N.P. Guadagni also has been found to effective at “breaking” laryngospasm. This involves placing the middle finger of each hand anterior to the mastoid and posterior to the condyle. The fingers then press inward while at the same time positioning the mandible forward. If the patient cannot be ventilated, the plane of anesthesia may be deepened with a short-acting intravenous general anesthetic; this often obviates the need for a skeletal muscle relaxant.
In rare situations these methods are unsuccessful, and it is necessary to administer succinylcholine, a short- and fast-acting depolarizing neuromuscular blocking agent. If intravenous access is not available, succinylcholine may be administered intramuscularly at a dose of 4 mg/kg. A dose of 20 mg intravenously is usually sufficient to break the spasm (pediatric dose, 0.25 mg/kg). However, up to 60 mg can be administered if laryngospasm persists. Rapacurium, rocuronium, and mivacurium can be used for patients in whom succinylcholine is contraindicated. The longer half-life of these nondepolarizing muscle relaxants may require continuous bag-mask ventilation until spontaneous respiration resumes.
Bradycardia is not uncommon after administration of succinylcholine. This usually occurs in children and in adults after repeated doses. Atropine may be administered in an effort to prevent this. Intravenous lidocaine 2 mg/kg administered before extubation was found to be effective in preventing postextubation laryngospasm in patients undergoing tonsillectomy. Other studies have found the prophylactic use of intravenous lidocaine to be ineffective.
Complications
Laryngospasm may produce partial or complete respiratory obstruction. Fortunately, early recognition and management allow for rapid resolution and minimal morbidity. However, with prolonged hypoxemia, the complications can be devastating. Laryngospasm may result in an acid-base disturbance, such as respiratory acidosis. Rare complications of laryngospasm include cardiac arrhythmias, anoxic brain injury, negative pressure pulmonary edema, and death.
If succinylcholine is administered, the patient may complain of general postoperative myalgia secondary to the rapid muscle depolarization. Other potential complications of succinylcholine include masticator muscle rigidity, malignant hyperthermia, and hyperkalemic cardiac arrest (secondary to the transient hyperkalemia), which can be seen in patients with undiagnosed myopathies (e.g., Duchenne's and Becker's muscular dystrophies).
Discussion
Laryngospasm results in tight approximation of the true vocal cords (Figure 3-1). It is a protective reflex that is most commonly caused by a noxious stimulus to the airway during a light plane of anesthesia. The structural and functional bases of the laryngospasm reflex were described by Rex. Secretions, vomitus, blood, pungent volatile anesthetics, painful stimuli, and oral and nasal airways may elicit this protective reflex. Mediated by the vagus nerve, this reflex is designed to prevent foreign materials from entering the tracheobronchial tree. During laryngospasm, the false vocal cords and supraglottic tissues act as a ball valve and obstruct the laryngeal inlet during inspiration. Laryngospasm has a reported occurrence of 8.7 per 1,000 patients receiving general anesthesia. It is 19 times more frequent than bronchospasm.
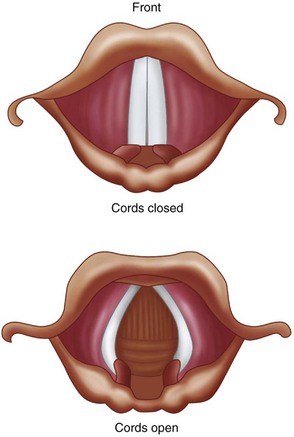
Figure 3-1 Tight approximation of the true vocal cords as seen during laryngospasm. (From Malamed SF: Sedation: a guide to patient management, ed 5, St Louis, 2010, Mosby.)
Laryngospasm accounts for more than 50% of the cases of negative pressure/post obstructive pulmonary edema. With the use of general endotracheal intubation, laryngospasm classically occurs during extubation in a light plane of anesthesia (stage II). Children and patients who have had a recent upper respiratory tract infection are predisposed to developing laryngospasm during anesthesia.
Efforts to prevent laryngospasm include postponing surgery in patients who have had recent upper respiratory infections, maintaining a dry surgical field, and using anticholinergics and avoiding extubation during stage II of anesthesia. Laryngospasm is not uncommon in outpatient and inpatient oral and maxillofacial surgery. Recognition and early intervention are essential in preventing morbidity and mortality.
Baraka, A. Intravenous lidocaine controls extubation laryngospasm in children. Anesth Analg. 1978; 57:506–507.
Ciavarro, C, Kelly, JP. Postobstructive pulmonary edema in an obese child after an oral surgery procedure under general anesthesia: a case report. J Oral Maxillofac Surg. 2002; 60(12):1503–1505.
Hartley, M, Vaughan, RS. Problems associated with tracheal extubation. Br J Anaesth. 1993; 71:561–568.
Hurford, WE, Bailin, MT, Davison, JK, et al. Clinical procedures of the Massachusetts General Hospital, ed 5. Philadelphia: Lippincott-Raven; 1998.
Larson, CP. Laryngospasm–the best treatment. Journal of the American Society of Anesthesiologists. 1998; 89(5):1293–1294.
Leicht, P, Wisborg, T, Chraemmer-Joorgensen, B. Does intravenous lidocaine prevent laryngospasm after extubation in children? Anesth Analg. 1985; 64:1193–1196.
Louis, PJ, Fernandes, R. Negative pressure pulmonary edema. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 93(1):4–6.
Rex, MAE. A review of the structural and functional basis of laryngospasm and a discussion of the nerve pathways involved in the reflex and its clinical significance in man and animals. Br J Anaesth. 1970; 42:891–898.
Stoelting, RK, Miller, RD. Basics of anesthesia, ed 3. New York: Churchill Livingstone; 1994.
Perioperative Considerations for the Pregnant Patient
CC
A 29-year-old primigravida (first pregnancy) with twins at 25 weeks of gestation presents to your office for evaluation and management of an odontogenic abscess.
HPI
The patient presents complaining of a 2-week history of dental pain in the right mandible and a 2-day history of progressive swelling of her right face. She is being followed by her obstetrician, and she states that her pregnancy is progressing without complications. Recently she has noted good fetal movements. She denies having any vaginal bleeding or leakage of vaginal fluid (a sign that amniotic fluid may be leaking from ruptured membranes). She started having pelvic cramping just recently (cramping described by pregnant patients may actually be contractions). She has been taking acetaminophen (the analgesic of choice during pregnancy) for pain and has not been able to eat adequately for the past 3 days (pregnant patients have a higher nutritional and fluid requirement). There is no history of dysphagia (difficulty swallowing), odynophagia (painful swallowing), dyspnea (difficulty breathing), or subjective fevers.
PMHX/PDHX/Medications/Allergies/SH/FH
POBHx. This is the patient's first pregnancy. Her only high-risk diagnosis is carrying twins. They are diamniotic/dichorionic (two separate sacs). The twins have had concordant growth on serial ultrasounds, and her cervical length was 4 cm (good length) at 24 weeks. (A normal cervical length indicates a reduced risk for an incompetent cervix and preterm labor and delivery. Twins are at higher risk compared to singleton pregnancies).
Examination
Vital signs. The patient's blood pressure was 110/55 mm Hg, heart rate 110 to 140 bpm (tachycardic), respirations 20 per minute, and temperature 39°C (febrile).
General. She is well developed and well nourished and in no apparent distress.
Maxillofacial. There is a large right-sided facial swelling. The swelling is above the inferior border of the mandible (rules out submandibular space involvement) and below the zygomatic arch (buccal space abscess). It extends from the masseter (submasseteric space involvement) to the oral commissure. The swelling is tense, erythematous, warm, and tender to palpation. The submandibular and submental regions are normal. She has limited opening (trismus due to involvement of the lateral masticator space) secondary to pain, which limits the intraoral examination. The right mandibular first molar is grossly decayed, with adjacent vestibular swelling and purulence extravasating from the gingival sulcus. The oropharynx is not completely visible due to limited mouth opening. The floor of the mouth is nonelevated (rules out sublingual space involvement). She is in no respiratory distress and is tolerating her secretions well.
Cardiovascular. The patient is tachycardic with an II/VI systolic ejection murmur. (Early systolic ejection murmur is very common in pregnant women due to the high volume of flow; it is accentuated by acute tachycardia secondary to the elevated temperature.)
Abdominal. Examination reveals a gravid uterus (pregnant uterus) appearing larger than the stated gestational age (due to twins), nontender abdomen, and fetal heart rates of 190s for twin A (the lower presenting fetus in the uterus) and 180s for twin B (the heart rates are elevated above normal due to maternal temperature).
Extremities. The extremities are nontender with 1+ pitting edema at the ankles bilaterally (common during pregnancy), no cords, and 2+ equal pulses. She has a negative Homans sign (calf pain upon dorsiflexion of the foot, suggestive of deep vein thrombosis [DVT]).
Imaging
A panoramic radiograph is the initial diagnostic study of choice. When the oropharynx cannot be adequately visualized due to trismus or when a deep neck space abscess is suspected, computed tomography (CT) scanning of the head and neck is necessary to rule out involvement of the parapharyngeal spaces (lateral pharyngeal and retropharyngeal spaces). The use of CT with intravenous contrast material is generally considered safe during pregnancy; however, special attention must be paid to the gestational age of the fetus and the amount of ionizing radiation that would be absorbed. In this particular case, the approximate exposure rate is less than 0.05 rad per examination, which is significantly less than the 5 rad cumulative upper limit for exposure during pregnancy (Dollard). The use of iodinated IV contrast does not produce any radiation exposure. The benefit of using IV contrast outweighs any theoretical risk of transient neonatal thyroid suppression; therefore, it can be used as needed, as long as there is no other contraindication to using the contrast material.
Despite the potential theoretical effects of radiation exposure to the fetus, all necessary plain film and CT studies for diagnosing and managing head and neck infections can be safely performed as needed. The radiation exposure to the developing fetus is minimal, especially when imaging the head and neck, and is further reduced by using shielding devices. Furthermore, the benefits outweigh the risks of exposure when dealing with acute head and neck infections. Nonionization techniques, such as ultrasound scans and magnetic resonance imaging (MRI) of the head and neck, are also considered safe during pregnancy and can aid in imaging soft tissue pathology. The use of gadolinium contrast with an MRI is currently not recommended during pregnancy, although there have not been any reported adverse fetal outcomes. Gadolinium does cross the placenta and can accumulate in the amniotic fluid of the fetus. The risk of nephrogenic systemic fibrosis with gadolinium is rare but warrants special attention in patients with renal insufficiency.
In this particular patient, the panoramic radiograph showed a grossly decayed right mandibular first molar with a large periradicular radiolucent lesion. CT was not indicated, because the clinical suspicion of parapharyngeal space involvement was low.
Labs
A complete blood count (CBC) with platelets and differential is the baseline study.
This patient's hemoglobin and hematocrit were 11 g/dl and 32.6%, respectively. (Anemia is commonly seen during pregnancy secondary to hemodilution, because the increase in plasma volume is relatively larger than the increase in red blood cells; however, pregnant patients are also at risk for iron deficiency anemia.) The patient's white blood cell (WBC) count was 18,000/mm3 with a 15% bandemia. (Although a slight, nonspecific elevation in the WBC count can be seen during pregnancy, this patient's elevated WBC count, in conjunction with bandemia and fever, is indicative of an acute infection until proven otherwise.)
Assessment
A 29-year-old primigravida with twins at 25 weeks of gestation with a large right-sided buccal and submasseteric space abscess secondary to a necrotic right mandibular first molar, complicated by dehydration and potential early onset of sepsis.
Treatment
The ideal time to perform elective or semielective oral and maxillofacial surgical procedures is postpartum; otherwise, the second trimester is considered the safest period for performing nonelective surgery. However, urgent or emergent surgery should not be delayed at any gestation of pregnancy, especially if procedures can be carried out under local anesthesia. Local anesthesia is the preferred method for simple procedures that can be performed in an office setting (there are no contraindications to vasoconstrictors, but aspiration to avoid intravascular injection is important). If the need arises, intravenous sedation and general anesthesia (in a hospital setting, when appropriate) can be safely performed without significant risk to the mother or fetus in an uncomplicated pregnancy (this is discussed later in the chapter).
There should be a lower threshold for hospital admission in the pregnant patient with a maxillofacial infection. Fever, dehydration, inability to tolerate oral intake, and potential airway compromise are all indications for hospital admission and initiation of supportive measures. (The risk of airway and pharyngeal edema is higher during pregnancy, especially when parapharyngeal spaces are involved.) Other obstetric concerns include risk factors for preterm contractions and preterm labor (onset of labor before 37 weeks of gestation): twins, dehydration, infection, and potential early sepsis.
When a pregnant woman is hospitalized, an obstetric consultation should be obtained. Fluid resuscitation, intravenous antibiotics, fetal monitoring, and nutritional support are extremely important. Caution should be exercised to avoid excessive fluid overload that can lead to pulmonary edema, because pregnancy and sepsis both can lead to third spacing due to increased capillary permeability. Usually, a 500-ml crystalloid bolus, followed by a maintenance level of 100 to 150 ml/hr until the patient is tolerating liquids, is appropriate for the average patient. Intravenous antibiotics should also be initiated (the penicillin and cephalosporin families are considered safe first-line antibiotics during pregnancy). Oral and maxillofacial infections should be aggressively treated, because untreated infections and abscesses have been associated with preterm labor and maternal sepsis.
If a pregnant woman is admitted to the hospital, she and the fetus should be monitored for contractions and fetal well-being. Pain management with a patient-controlled analgesia pump until the patient is tolerating oral medications is appropriate. Intravenous morphine, meperidine (Demerol), and fentanyl or orally administered hydrocodone, oxycodone, or codeine with acetaminophen combinations are all considered safe during pregnancy for necessary pain control. Routine use of nonsteroidal antiinflammatory drugs (NSAIDs), such as ibuprofen and aspirin, for postoperative pain control during pregnancy is generally not recommended; however, these drugs can be used short term in the second trimester only.
The current patient was admitted to the hospital, and an obstetric consultation was obtained. Intravenous fluids and piperacillin/tazobactam 3.375 g every 6 hours were administered. The twins were evaluated for heart tones and contractions. No significant abnormalities were noted. The fetal heart rates were slightly higher than normal due to the maternal temperature but were otherwise reassuring. There were no contractions noted. The pelvic examination was unremarkable, with no evidence of cervical effacement (thinning and shortening of the cervix associated with labor) or of cervical dilatation (also a sign of labor).
The patient was taken to the operating room for incision and drainage of the submasseteric and buccal space abscess and extraction of the right mandibular first molar. Ideally, pregnant patients should be NPO (nothing to eat or drink, including chewing gum) for at least 8 hours before surgery. In addition to the slower gastric emptying time and the enlarged uterus (which takes up more abdominal space, thus leaving less room for the gastrointestinal organs), pregnancy causes relaxation of the esophageal sphincter. The combination of these conditions increases the risk of aspiration associated with general anesthesia. Therefore, preoperatively, this patient received an oral antacid (to increase the pH of gastric contents), an H2 antagonist (to decrease gastric acid production), and metoclopramide (to accelerate gastric emptying). Due to the gestational age of the patient, the uterus was enlarged (especially with twins). Therefore, a roll was placed under her right back and hip, and she was slightly tilted to the left. (A left lateral tilt of 15 to 30 degrees displaces the uterus off the aorta and inferior vena cava and prevents supine hypotensive syndrome, which is due to prolonged compression of the great vessels, leading to decreased venous return and cardiac output.)
After the patient had been positioned, she was intubated via an awake nasal fiberoptic intubation, due to her trismus (pregnant patients have edematous nasal mucosa and a potential for epistaxis, especially during a traumatic nasal intubation). The procedure was completed without complications. A thorough intraoperative examination of the oropharynx revealed no parapharyngeal involvement or edema. Her mouth opening increased to normal range after decompression of the lateral masticator (submasseteric) space. Her airway was deemed stable, without risk for postoperative upper airway obstruction, and she was successfully extubated. She remained in the hospital for a total of 4 days. She received daily antenatal testing to check for fetal well-being and contractions.
The patient was discharged home on postoperative day 4 after she had been afebrile for longer than 48 hours and was tolerating full liquids and a regular diet. She received intravenous antibiotics postoperatively until she was able to tolerate medications by mouth; she was then switched to oral antibiotics. Upon discharge, the patient's facial swelling had decreased significantly, there was no oral purulent discharge, the WBC count had normalized and there was no bandemia, and pain control was adequate with opioid analgesics.
Complications
Complications associated with surgery under general anesthesia during pregnancy include the risk of DVT, pulmonary embolism (PE), aspiration (decreased esophageal sphincter tone, decreased gastric emptying, increased gastric pressures, and hyperemesis increase the risk of regurgitation and aspiration), pulmonary edema, acute respiratory distress syndrome (ARDS), spontaneous abortion during the first trimester, and preterm labor. These are weighed against the risks of an untreated oral or maxillofacial infection, which pose a greater direct danger to both the fetus and the mother; these risks include preterm labor and delivery with complications of a premature neonate, fetal death, maternal sepsis, and septic shock. All elective surgical procedures should be avoided during pregnancy, but necessary surgical interventions should not be delayed.
For the pregnant female undergoing general anesthesia, surgery should be performed in a setting where an obstetrician is available for consultation and where anesthesiologists are familiar with the physiologic changes associated with pregnancy. Teratogenic and abortive agents should be avoided (this is most important during the first trimester). Specific modifications may be needed as the gestational age of the fetus advances (discussed previously). A collaborative, multidisciplinary approach involving obstetricians, anesthesiologists, and oral and maxillofacial surgeons provides the most appropriate management and treatment plan for the pregnant patient.
A potential complication risk that is increased in pregnancy is the risk of deep vein thrombosis and pulmonary embolism due to the prothrombotic state of a pregnant woman. Physiologic changes that occur during pregnancy, such as increased clotting factors, increased plasma volume, increased venous stasis, decreased blood flow velocity, and decreased fibrinolytic activity, increase the risk of DVT (signs include leg pain, tenderness, edema, discoloration, palpable cord, and positive Homans sign) and subsequent PE (signs include shortness of breath, tachypnea, hypoxemia, and respiratory distress) by twofold to fourfold during pregnancy and early postpartum (Cromwell). Other exogenous factors, such as tobacco use, obesity, and immobility, add even further risks. Venous thromboembolic events are still a leading cause of maternal morbidity and mortality. Early detection of DVT (using duplex Doppler ultrasound) and initiation of heparin therapy have significantly reduced maternal mortality. Chest CT or a V/Q scan are options for evaluation if a pulmonary embolus is suspected. DVT/PE prophylaxis should be initiated upon the patient's admission, with the use of support stockings, compression devices, and/or subcutaneous heparin.
Discussion
Several other anesthetic considerations in pregnancy are noteworthy. Propofol, thiopental, etomidate, and ketamine are examples of generally safe induction agents for the pregnant patient. The use of a 50 : 50 mixture of nitrous oxide and oxygen, and of halogenated agents (desflurane, isoflurane, sevoflurane) in low concentrations, is also considered safe. Careful examination of the patient's oropharyngeal and neck structures, noting range of movement and any obstructing lesions, is critical. Preoxygenation, intravenous fluid resuscitation, and left lateral tilt are all important steps in preventing hypoperfusion in both the mother and the fetus. Opiates, including fentanyl and morphine, are also considered safe to administer.
The use of local anesthesia is safe during pregnancy and is well tolerated by most pregnant patients undergoing minor oral surgical procedures. Although there is a theoretical concern that epinephrine-induced vasoconstriction could lead to decreased placental blood flow, epinephrine in local anesthetics is generally considered safe. Local anesthesia without epinephrine is an alternative. Despite concerns about potential teratogenic effects of benzodiazepines in the first trimester, they can be safely administered when the usual and appropriate doses are used.
For nursing mothers, it has been historically recommended that the patient “pump and dump” after receiving a general anesthetic. With current medications, nursing mothers can pump and discard breast milk for 8 to 24 hours after receiving an intravenous sedative or general anesthetic, to err on the side of caution. However, most agents have a very short half-life and very minimal crossover into breast milk that would affect the baby. Postoperative analgesics (hydrocodone, oxycodone, morphine, ketorolac [Toradol], NSAIDs) are safe to use without pumping and discarding breast milk. Perioperative intravenous steroids to help reduce postoperative swelling can be used in pregnancy and in breast-feeding mothers if necessary. Antinausea medications, as needed, also are generally safe to use.
Briggs, GG, Freeman, RK, Yaffe, SJ. Drugs in pregnancy and lactation, ed 5. Philadelphia: Williams & Wilkins; 2005.
Cromwell, C. Hematologic changes in pregnancy. In Hoffman R, Benz EJ, Jr., Silberstein L, et al, eds. : Hoffman hematology: basic principles and practice, ed 6, St Louis: Saunders, 2012.
Cumminham, FG, Leveno, KJ, Bloom, SL, et al. Williams obstetrics, ed 21. New York: McGraw-Hill Professional; 2005.
Dollard, DF. Radiation in pregnancy and clinical issues of radiocontrast agents. In Roberts JR, Hedges JR, eds. : Clinical procedures in emergency medicine, ed 5, St Louis: Saunders, 2009.
Hawkins, JL, Bucklin, BA. Obstetrical anesthesia. In Gabbe SG, Niebyl JR, Galan HL, et al, eds. : Obstetrics: Normal and problem pregnancies, ed 6, St Louis: Saunders, 2012.
Lawrenz, DR, Whitley, BD, Helfrick, JF. Considerations in the management of maxillofacial infections in the pregnant patient. J Oral Maxillofac Surg. 1996; 54:474–485.
Mozurkewich, EL, Pearlman, MD. Trauma and related surgery in pregnancy. In Gabbe SG, Niebyl JR, Galan HL, et al, eds. : Obstetrics: Normal and problem pregnancies, ed 6, St Louis: Saunders, 2012.
Schwartz, N, Adamczak, J, Ludmir, J. Surgery during pregnancy. In Gabbe SG, Niebyl JR, Galan HL, et al, eds. : Obstetrics: Normal and problem pregnancies, ed 6, St Louis: Saunders, 2012.
Turner, M, Aziz, SR. Management of the pregnant oral and maxillofacial surgery patient. J Oral Maxillofac Surg. 2002; 60:1470–1488.
Respiratory Depression Secondary to Oversedation
HPI
The patient is an otherwise healthy woman for whom treatment was planned for bilateral upper and lower eyelid blepharoplasties with intravenous sedation. After the incision lines had been marked in the usual manner, ECG, blood pressure, pulse oximeter and a sidestream capnograph monitors were applied. The patient was administered 4 L of oxygen and 2 L of nitrous oxide via nasal hood (nitrous oxide decreases the amount of intravenous sedatives needed). Sedation was achieved using 5 mg of midazolam, 100 µg of fentanyl, and a propofol drip titrated to effect. Verrill's sign (50% upper eyelid ptosis, indicating adequate sedation) was observed. Prior to administration of local anesthesia, 40 mg of propofol was administered as a bolus (propofol may cause a 20% to 25% drop in systolic blood pressure when given as a bolus). Upon administration of local anesthesia, loss of the capnogram, with no chest wall movement, was observed. (This indicates the presence of central apnea. Capnography is considered to be more sensitive than clinical assessment of ventilation in the detection of apnea. In a study by Soto and colleagues (2004), 10 of 39 patients (26%) experienced 20-second periods of apnea during procedural sedation and analgesia. All 10 episodes of apnea were detected by capnography but not by the anesthesia providers.) The apnea was attributed to the propofol bolus (combined with the respiratory depressant effects of fentanyl), which was anticipated to resolve shortly. However, the patient continued to be apneic, and her oxygen saturation decreased from 99% to 80% (pulse oximeter readings are about 30 seconds behind the real-time oxygen saturation). Tracheal tug and paradoxical chest wall motion were not observed (these would be signs of upper airway obstruction and inspiratory efforts). The patient began to appear cyanotic (bluish hue to facial skin and lips due to prolonged hypoxemia).
PMHX/PDHX/Medications/Allergies/SH/FH
A thorough medical history is important during the preoperative evaluation of any patient undergoing intravenous sedation or general anesthesia to identify potential risk factors of intraoperative or postoperative anesthetic complications.
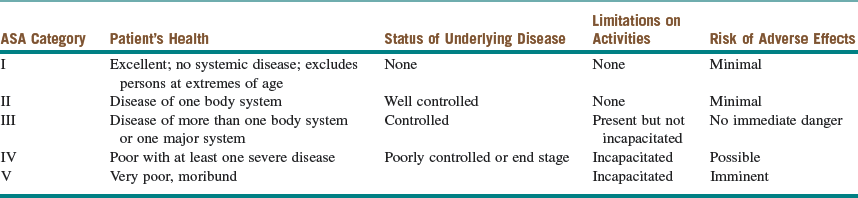
The past medical and surgical histories are noncontributory. This patient is categorized as American Society of Anesthesiologist (ASA) Class I (Table 3-1). She does not use any medications and has no known drug allergies. She denies previous problems with local anesthetics (e.g., methemoglobinemia), intravenous sedation, or general anesthetics (problems with previous anesthesia or adverse drug reactions should alert clinicians to possible complications that may require modification of anesthetic techniques). There is no family history of complications with general anesthetics (e.g., malignant hyperthermia). She denies a history of drug or alcohol use (patients with a previous drug history or alcohol abuse may require higher doses of sedative-hypnotic drugs), and she does not smoke (smoking decreases oxyhemoglobin concentrations and increases pulmonary secretions).
Examination
Preoperative. A thorough preoperative evaluation is important to identify potential risk factors for negative anesthetic outcomes, with an emphasis on airway anatomy.
General. The patient is a well-developed and well-nourished woman in no apparent distress who weighs 60 kg.
Airway. Maximal interincisal opening (MIO) is within normal limits (difficult intubation occurs with decreased MIO). Her oropharynx is Mallampati class I (soft palate, tonsillar pillars, and uvula completely visualized), and the thyromental distance (TMD) is three finger-breadths (intubation is more difficult with retrognathia, a short TMD, and/or a higher Mallampati classification). The cervical spine has a full range of motion.
Cardiovascular. Heart is regular rate and rhythm without murmurs, rubs, or gallops.
Pulmonary. Lung fields are clear to auscultation bilaterally (preoperative wheezing may increase the risk of intraoperative bronchospasm).
Intraoperative. During the course of intravenous sedation (conscious sedation, deep sedation, or general anesthesia), it is important to continuously monitor the patient's level of sedation and anesthesia (to prevent oversedation and respiratory depression) and to survey the ABCs (airway, breathing, and circulation [Box 3-1]).
General. The patient is sedated/unconscious and unresponsive to painful stimulus (a state of general anesthesia).
Imaging
Preoperative and serial postoperative photoimaging is mandatory for cosmetic procedures. A preoperative chest radiograph has a limited role in healthy individuals and is not warranted unless dictated by other medical factors.
Labs
Routine laboratory tests are not indicated in healthy patients undergoing cosmetic blepharoplasty with intravenous sedation. Females of childbearing age who are sexually active and/or have missed their last menstrual period may require a urine pregnancy test (UPT).
Assessment
Central apnea secondary to oversedation during intravenous sedation for cosmetic upper and lower eyelid blepharoplasties.
Treatment
Before the diagnosis of respiratory depression (apnea or hypopnea) as the cause of hypoxemia, possible causes of upper airway obstruction need to be rapidly ruled out by evaluating the airway, jaw position, and possibility of foreign body aspiration. Subsequently, the procedure should be stopped, any open or bleeding wounds packed, and necessary assistance should be elicited. Attempts to arouse the patient with verbal command and painful stimulus should be made. Unresponsiveness to painful stimulus is considered to be a state of general anesthesia.
Respiratory depression secondary to oversedation is a self-limiting process that requires adequate supportive measures or pharmacologic interventions until spontaneous respirations resume. Respiratory depression causes a reduction in alveolar ventilation through a decrease in the respiratory rate or tidal volume, which in turn is caused by a decrease in respiratory drive. All sedatives, opioids, and potent inhalation general anesthesia agents have the potential to depress central hypercapnic and/or peripheral hypoxemic drives. Opioids primarily depress the central chemosensitive area (i.e., hypercapnic drive), whereas inhalation anesthetics and benzodiazepines exert greater influence on the chemoreceptors in the carotid and aortic bodies (i.e., hypoxemic drive). At high doses, all classes can depress both these mechanisms
Nitrous oxide is not a respiratory depressant; however, when it is combined with sedatives or opioids that depress ventilation, a more pronounced and clinically important depression may result. Therefore, it should be discontinued to allow more rapid arousal from anesthesia and delivery of 100% oxygen, with subsequent resolution of spontaneous respirations. Any anesthetic intravenous drips should be discontinued immediately. Jaw-thrust maneuvers and/or tugging on the tongue anteriorly will improve the opening of the airway for more effective oxygen delivery. The anesthesia circuit should be flushed to evacuate residual nitrous oxide and to deliver a higher flow of oxygen. If these measures fail, the patient's breathing can be assisted with positive pressure ventilation (PPV), at one breath every 5 seconds (coordinated with any apparent shallow breathing). If oxygenation proves to be successful with PPV, continued ventilatory support is maintained until the sedation lightens and respiratory depression resolves. However, if ventilation is not achieved, rapid re-evaluation for other etiologies (laryngospasm, bronchospasm, foreign body aspiration, chest wall rigidity) should be considered. The airway is reassessed, and chin lift/jaw thrust maneuvers should be optimized. Oral and/or nasal airways can be inserted if there is continued difficulty with PPV. If laryngospasm or bronchospasm is diagnosed, it should be treated promptly (see Laryngospasm earlier in this chapter). If these measures fail to re-establish ventilation, more advanced airway interventions may be necessary. These include the use of a laryngeal mask airway, endotracheal intubation, or establishment of a surgical airway (cricothyrotomy). Despite the infrequency of the latter scenario, the clinician should be prepared to establish an airway as soon as possible (see Emergent Surgical Airway later in this chapter). Once the oxygen saturation returns above 95%, the clinician can decide whether to cautiously continue with the procedure and intermittently apply PPV as needed or to abort the procedure for further evaluation.
If prolonged respiratory depression occurs, the sedative effects of some agents can be pharmacologically reversed. Flumazenil (Romazicon) reverses the sedative effects of benzodiazepines. It is given at 0.2 mg intravenously (or 0.01 to 0.02 mg/kg in small children) every minute up to five doses (maximum total dose of 1 mg) until reversal of sedation is accomplished. It may be repeated every 20 minutes for resedation. Naloxone (Narcan) is an opioid antagonist that reverses the sedative, respiratory depressant, and analgesic effects of opiates. Low doses are recommended (to prevent adverse effects of reversal) at 0.04 mg intravenously (or 0.001 mg/kg) every 2 to 3 minutes until reversal is accomplished (a higher dosing schedule is used in narcotic overdose). Once sedation has been reversed, the patient needs to be monitored for resedation, because the half-lives of naloxone and flumazenil are shorter than those of their sedative counterparts, potentially requiring redosing of the reversal agent(s). There are no reversal agents for barbiturates or propofol. Reversal of sedation from these agents relies on rapid redistribution of the drugs. It is important to remember that hypoxemia and hypercarbia can further contribute to central nervous system (CNS) depression.
In the current patient, supportive measures included 100% oxygen delivered via PPV with a bag-valve-mask device. PPV was easily accomplished, and the patient's oxygen saturation steadily increased to 99%. After sufficient ventilation and oxygenation, the surgery was resumed. The propofol intravenous drip was discontinued during the apnea/hypopnea episode and was subsequently titrated down as the procedure was completed. The patient began to have spontaneous respirations and maintained a normal capnogram and an adequate oxygen saturation, and she arose from sedation shortly after completion of the procedure. Reversal agents were not required.
Complications
Oversedation and respiratory depression can have devastating outcomes if not promptly treated as outlined here. In most circumstances, the patient's airway and breathing can be easily supported. However, it is important to identify those patients at higher risk of difficult mask ventilation and endotracheal intubation (see Emergent Surgical Airway later in this chapter) before administering deep sedation. The loss of the patient's airway (cannot intubate and cannot ventilate scenario) can lead to prolonged hypoxemia, which can in turn lead to cardiovascular collapse, cerebral anoxia, and death if not managed promptly.
Precipitous reversal of sedation and respiratory depression with opioid antagonists is not without adverse side effects. Naloxone (Narcan) may cause cardiac arrhythmias, pulmonary edema, severe hypotension, and cardiac arrest when given at higher doses. The analgesic effects are also reversed, which may cause the patient to experience profound surgical pain, accompanied by hypertension and tachycardia. Patients with acute or chronic opioid dependence can experience acute withdrawal symptoms. Naloxone and flumazenil have short half-lives and may require redosing every 20 to 30 minutes if resedation occurs; therefore, close patient observation is paramount.
Discussion
Various levels of intravenous sedation can be administered by oral and maxillofacial surgeons. Conscious sedation is defined as “a controlled, pharmacologically induced, minimally depressed level of consciousness that retains the patient's ability to maintain a patent airway independently and continuously, with the ability to respond appropriately to physical stimulation and/or verbal command.” Deep sedation is defined as “a controlled, pharmacologically induced state of depressed consciousness from which the patient is not easily aroused, and which may be accompanied by a partial loss of protective reflexes, including the ability to maintain a patent airway independently and/or respond purposefully to physical stimulation or verbal command.” General anesthesia is defined as “an induced state of unconsciousness accompanied by partial or complete loss of protective reflexes, including the ability to independently maintain an airway and respond purposefully to physical stimulation or verbal command.”
Respiratory depression from oversedation can occur during the course of a procedure or in the recovery period; however, it is relatively uncommon when sedation is administered by an experienced oral and maxillofacial surgeon (short half-lives and lack of active metabolites are ideal properties of intravenous anesthetic agents). The short duration of action of modern intravenous anesthetics relies on rapid redistribution (alpha half-life) and/or rapid metabolism. However, repeated doses of opioids, benzodiazepines, or barbiturates for longer procedures may cause accumulation in inactive tissues (especially adipose tissue), which is later released into circulation to cause delayed emergence (beta half-life), thereby on occasion requiring a reversal agent. Naloxone is an opioid antagonist that competitively binds to mu receptors, effectively reversing the sedative, analgesic, and respiratory-depressant effects of any given opioid (e.g., fentanyl, morphine, sufentanil, alfentanil, remifentanil, meperidine). Flumazenil is a competitive antagonist to benzodiazepines (e.g., midazolam, lorazepam, diazepam) at the central benzodiazepine receptor (alpha subunits of the GABA receptor), and it reverses all effects of benzodiazepines (e.g., sedation, respiratory depression, anxiolysis). The respiratory-depressant effects of midazolam (Versed, the most commonly used benzodiazepine) are minimal compared to those of propofol and narcotics.
Inadequate local anesthesia or insufficient time allocation for its onset may make the sedated patient appear uncooperative or undersedated. The clinician may decide to deepen the sedation to control the uncooperative patient and overcome the effects of inadequate local anesthesia. Once the painful stimulus is gone or the local anesthesia has set in, the patient may return to a deeper level of sedation or may become oversedated with respiratory depression. Risk of oversedation and respiratory depression can be minimized by using local anesthesia effectively.
Some additional precautions should be noted when administering anesthesia to pediatric and elderly patients. Small doses of benzodiazepines and opioids can cause significant respiratory depression in the elderly patient. The changes in physiology and medical comorbidities associated with aging are beyond the scope of this section, but a general precaution used by clinicians is “go low and go slow.” It is important to remember that children have a lower functional residual capacity (FRC) and do not tolerate hypoventilation and hypoxemia well, which is evidenced by a more rapid drop in oxygen saturation. Differences in the pediatric airway (larger tongue, lymphoid hypertrophy, more rostrally positioned larynx, long and floppy epiglottis, narrowest at cricoid cartilage, more compliant tracheal walls, more caudal anterior cord attachment, underdeveloped accessory muscles) are important to recognize.
Capnography is the noninvasive measurement of the partial pressure of CO2 in the exhaled breath. The relationship of CO2 concentration to time is graphically represented by the CO2 waveform or capnogram (Figure 3-2). (Time capnograms are more commonly used than volume capnograms, on which CO2 is plotted against expired volume.) Pulse oximetry provides real-time information about arterial oxygenation, whereas capnography provides breath-to-breath information about ventilation (how effectively CO2 is being eliminated by the pulmonary system), perfusion (how effectively CO2 is being transported through the vascular system), and metabolism (how effectively CO2 is being produced).
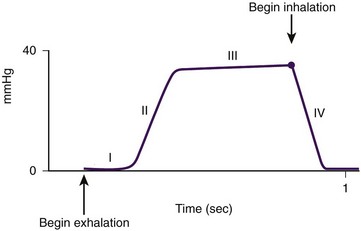
Figure 3-2 The relationship of CO2 concentration to time, represented by a capnograph. (From Krauss B, Hess DR: Capnography for procedural sedation and analgesia in the emergency department, Ann Emerg Med 50:172, 2007.)
Capnography refers to monitors that display a continuous waveform reflecting inspiration and expiration. Although capnometers and capnographs both display numeric values for the end-tidal carbon dioxide (Etco2) level and respiratory rate, capnography is preferred because visualization of the waveform allows continuous assessment of the depth and frequency of each ventilatory cycle (see Figure 3-2).
CO2 monitors measure the gas concentration, or partial pressure, using one of two configurations, depending on the location of the sensor: mainstream or sidestream. Mainstream devices measure CO2 directly from the airway, with the sensor located directly on the endotracheal tube. Sidestream devices measure CO2 by sampling from the exhaled breath and analyzing via a sensor located inside the monitor.
The capnogram, corresponding to a single tidal breath (see Figure 3-2), consists of four phases. Phase I represents the beginning of exhalation. Phase II (ascending phase) represents the increase in CO2 concentration in the breath stream as the CO2 reaches the upper airway. Phase III (alveolar plateau) represents the CO2 concentration reaching a uniform level in the entire breath stream (from alveolus to nose) and the point of maximum CO2 concentration (Etco2); this is the value displayed on the monitor. Phase IV represents the inspiratory cycle, in which the CO2 concentration drops to zero.
In a normal capnogram, for patients of all ages, the CO2 concentration starts at zero and returns to zero (there is no rebreathing of CO2); a maximum CO2 concentration is reached with each breath (Etco2); the amplitude is determined by the Etco2 concentration; the width is determined by the expiratory time; and a characteristic shape is seen for normal lung function.
Capnography has been shown to be one of the earliest indicators of airway or respiratory compromise; it registers changes well before pulse oximetry registers a decreasing oxyhemoglobin saturation, especially in individuals receiving supplemental oxygen.
Both central and obstructive apnea can readily be detected by capnography (Table 3-2). Central apnea is confirmed with the loss of the capnogram, in conjunction with no chest wall movement and no breath sounds on auscultation. Obstructive apnea is characterized by loss of the capnogram, chest wall movement, and absent breath sounds. Two types of drug-induced hypoventilation occur during procedural sedation and analgesia (see Table 3-2): bradypneic hypoventilation (type 1), which is commonly seen with opioids, and hypopneic hypoventilation (type 2), which is commonly seen with sedative-hypnotic drugs.
Table 3-2
Capnographic Airway Assessment for Procedural Sedation and Analgesia
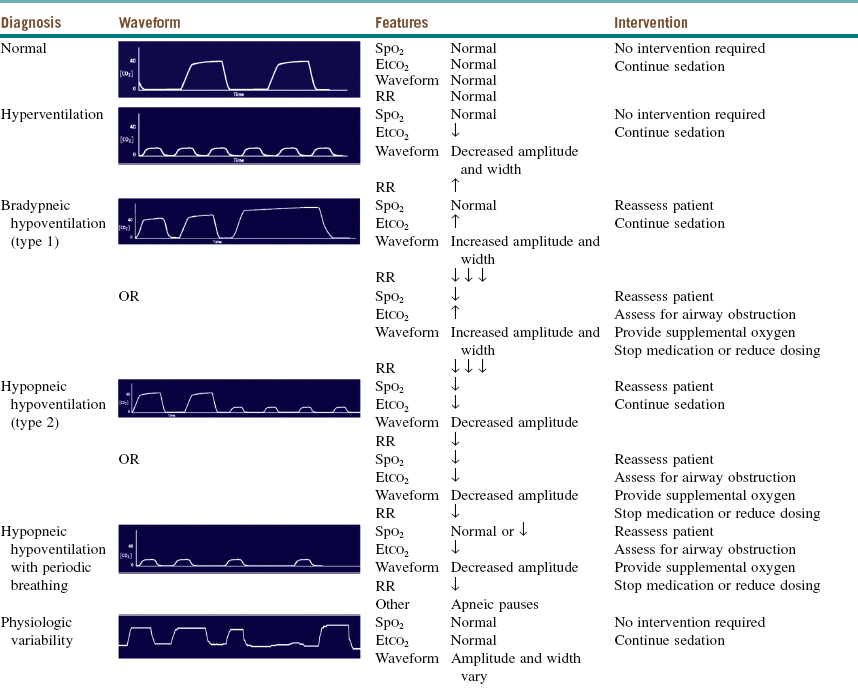
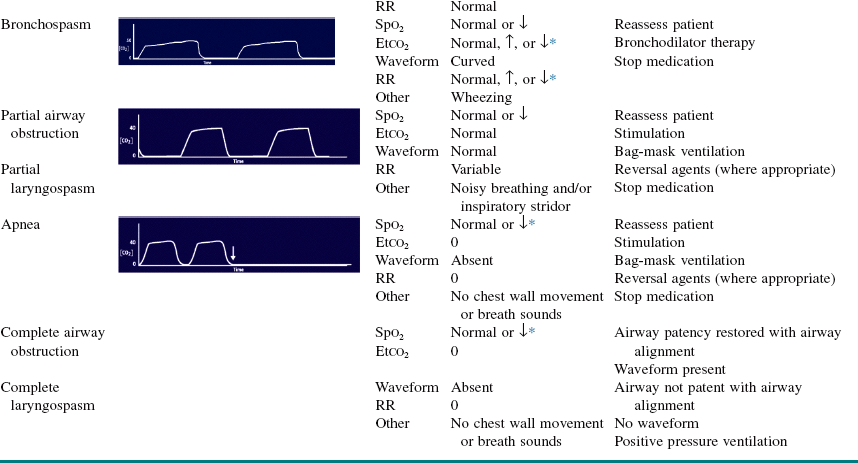
Etco2, End-tidal carbon dioxide; RR, respiratory rate; Spo2, oxygen saturation; ↓ decreased; ↑ increased; ↓ ↓ ↓.
*Depending on duration and severity of bronchospasm.
From Krauss B, Hess DR: Capnography for procedural sedation and analgesia in the emergency department, Ann Emerg Med 50:172, 2007.
The Etco2 may initially be high (bradypneic hypoventilation) or low (hypopneic hypoventilation) without significant changes in oxygenation, especially with the use of supplemental oxygen. Therefore, drug-induced changes in the Etco2 do not necessarily lead to oxygen desaturation and thus may not require intervention.
Hypopneic hypoventilation can remain stable, with low tidal volume breathing resolving over time as CNS drug levels decrease with redistribution; or, it may lead to periodic breathing, with intermittent apneic pauses (which may resolve spontaneously or progress to central apnea) or progress directly to central apnea.
Capnography to monitor ventilation has been shown to provide the earliest indicator of airway or respiratory compromise. It is the only single monitoring modality that provides airway, breathing, and circulation assessment. The presence of a normal waveform denotes that the airway is patent and that the patient is breathing. A normal Etco2 value (35 to 45 mm Hg), in the absence of obstructive lung disease, reflects adequate perfusion. Unlike pulse oximetry, the capnogram remains stable during patient motion and is reliable in low-perfusion states. Capnography has been shown to trigger early intervention and decrease the incidence of oxygen desaturation. Capnography can forewarn of impending hypoxia by about 5 to 240 seconds. Administration of supplemental oxygen delays the onset of desaturation after apnea; therefore, relying on pulse oximetry alone delays that intervention. Starting in January, 2014, capnography will be required by law for procedural sedation in oral surgery offices.
Becker, DE, Haas, DA. Recognition and management of complications during moderate and deep sedation. Part 1. Respiratory considerations. Anesth Prog. 2011; 58(2):82–92.
Becker, DE, Rosenberg, M. Nitrous oxide and the inhalation anesthetics. Anesth Prog. 2008; 55(4):124–131.
Bennet, J. Intravenous anesthesia for oral and maxillofacial office practice. Oral Maxillofac Surg Clin North Am. 1999; 11(4):601–610.
Burton, JH, Harrah, JD, Germann, CA, et al. Does end-tidal carbon dioxide monitoring detect respiratory events prior to current sedation monitoring practices? Acad Emerg Med. 2006; 13:500–504.
D'Eramo, EM, Bookless, SJ, Howard, JB. Adverse events with outpatient anesthesia in Massachusetts. J Oral Maxillofac Surg. 2003; 61:793–800.
Dripps RD, Eckenhoff JE, Vandam LD, eds. Introduction to anesthesia: the principles of safe practice, ed 7, Philadelphia: Saunders, 1988.
Krauss, B, Hess, DR. Capnography for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007; 50:172.
Perrott, DH, Yuen, JP, Andresen, RV, et al. Office-based ambulatory anesthesia: outcomes of clinical practice of oral and maxillofacial surgeons. J Oral Maxillofac Surg. 2003; 61:983–995.
Roberts, JR, Hedges, JR. Clinical procedures in emergency medicine, ed 5. St Louis: Saunders; 2010.
Rodgers, SF. Safety of intravenous sedation administered by the operating oral surgeon: the first 7 years of office practice. J Oral Maxillofac Surg. 2005; 63:1478–1483.
Soto, RG, Fues, ES, Miguel, RV. Capnography accurately detects apnea during monitored anesthesia care. Anesth Analg. 2004; 99(2):379–382.
Vezeau, PJ. Anesthetic and medical management of the elderly oral and maxillofacial surgery patient. Oral Maxillofac Surg Clin North Am. 1999; 11(4):549–559.
Winikoff, SI, Rosenblum, M. Anesthetic management of the pediatric patient for ambulatory surgery. Oral Maxillofac Surg Clin North Am. 1999; 11(4):505–517.
Inadequate Local Anesthesia
CC
A 38-year-old man presents with left facial swelling and pain secondary to a carious left maxillary cuspid (acute infection decreases the efficacy of local anesthetics).
HPI
The patient reports a 2-month period of a toothache localized to the upper left quadrant. Two days ago, he experienced an acute exacerbation of his pain along with the development of progressively enlarging left facial swelling. He was subsequently seen by his general dental practitioner, who attempted to extract the tooth but was unsuccessful due to persistent pain despite several local anesthetic injections.
PMHX/PDHX/Medications/Allergies/SH/FH
Noncontributory. The patient has had multiple dental restorations under local anesthesia without any complications. He has no known drug allergies.
Although occasionally patients report an “allergy” to epinephrine, this is a naturally occurring catecholamine present in all individuals that plays a critical role in homeostasis and is not a source of allergic reactions. The transient tachycardia that may be seen with local anesthetic injections that contain epinephrine (especially with intravascular injection) is simply an adrenergic response to the epinephrine.
Examination
Maxillofacial. There is a fluctuant and tender swelling extending from the midline of the upper lip to the left cheek, consistent with left canine space abscess.
Imaging
Panoramic radiograph reveals a carious left maxillary cuspid with an associated 1.5-cm periapical radiolucent lesion.
Labs
No routine laboratory tests are indicated unless dictated by the medical history. A WBC count may be obtained to evaluate the degree of leukocytosis during an infection.
Assessment
Left canine space infection complicated by failure to extract the left maxillary cuspid secondary to inadequate local anesthesia.
Treatment
Management of failure to achieve adequate local anesthesia should involve a thorough examination for the cause of the failure. Anatomic variation, accessory innervation, poor technique, inadequate volume or concentration of local anesthetic, the presence of infection, or an excessively anxious patient may all contribute to the failure to achieve adequate local anesthesia. Upon identification of potential causes, the clinician should develop a stepwise plan to address the problem. A reasonable approach may use supplemental anesthetic injections, regional block anesthesia (rather than infiltration), intraligamentary injections, intraosseous injections, intrapulpal injections, or addition of other medications, such as nitrous oxide, for anxiolysis and analgesia. With failure of all the above measures, consideration must be given to either deep sedation or general anesthesia.
In the current patient, the infraorbital rim (the infraorbital foramen is approximately 8 mm inferior to the rim) was palpated, and the overlying tissues was cleansed with an alcohol wipe. A total of 5 ml of 2% lidocaine with 1 : 100,000 epinephrine was injected after the tip of the hypodermic needle was placed through the skin against the foramen. The tissue was gently massaged to facilitate diffusion of the local anesthetic into the foramen to anesthetize the infraorbital and anterior superior alveolar nerves. The nasopalatine and greater palatine nerves were anesthetized in the usual manner. After 10 minutes, the left maxillary cuspid was extracted without any subjective pain. No purulent drainage was obtained through the socket; therefore, a horizontal vestibular incision was made, allowing the release of approximately 10 ml of purulent drainage. A sample of the pus was sent for Gram stain, aerobic and anaerobic cultures, and antibiotic sensitivity. A drain was placed, and the wound was left open to facilitate continued drainage. The patient was prescribed a 10-day course of penicillin and instructed to rinse with warm saltwater five times a day. An opioid with acetaminophen was prescribed for postoperative pain control. The patient was seen at 2 and 7 days for drain removal and follow-up, with subsequent resolution of the infection.
Complications
Complications related to failure to achieve local anesthesia are related to the psychological impact on and frustration of the patient and clinician, the added cost and time to complete a necessary procedure using a different modality, and possible toxic effects of local anesthetic due to repeat injections. The negative impact on a patient's perception of the dental profession should not be underestimated.
Complications may also be related to progression of the disease process (e.g., the spread of infection) that could not be treated due to the inadequacy of local anesthesia to permit extraction. Infection must be treated as soon as possible to reduce the likelihood of the patient becoming septic (disseminated infection). Furthermore, infections in close proximity to vital structures (e.g., the eye) may rapidly spread, resulting in dissemination of the infection into distant areas. This includes spread of infection into the orbit or, via communicating veins, into the cavernous sinus, with the development of cavernous sinus thrombosis. The presence of significant infraorbital swelling and tenderness is a relative contraindication to the use of the extraoral infraorbital anesthetic block due to concern for the development of cavernous sinus thrombosis.
There are several strategies to overcome the inadequacy of local anesthesia in the presence of infection. A larger volume or a higher concentration of the local anesthetic solution may be used. However, care must be taken to avoid toxicity from an excessive volume of local anesthetic. Toxicity initially presents as central nervous system (CNS) excitation, but with increasing doses, CNS depression (including respiratory depression) can be seen. Epinephrine substantially reduces toxicity by decreasing tissue absorption. However, epinephrine can result in tachyarrhythmias and increased blood pressure, particularly with inadvertent intravascular injection. Also, attention must be paid to the concentration of local anesthetic used, because anesthetics with higher concentration, such as 4% articaine and 4% prilocaine, have been found to be associated with an increased incidence of neurotoxicity. For this reason, it may be wise to limit the use of these anesthetics to local infiltrations rather than direct nerve blocks. Care must be taken when infiltrating the area of the mandibular premolars with these anesthetics, because the mental nerve is in close proximity and could be affected by the higher concentration anesthetics.
The initial excitement and agitation seen with local anesthetic toxicity are due to suppression of the inhibitory cortical neurons, whereas the more common somnolence and decreased consciousness are secondary to inhibition of excitatory cortical neurons. Treatment is purely supportive, with attention to the airway, breathing, and circulation (ABCs)(see Box 3-1). An increase in heart rate (β1-receptor stimulation) and blood pressure (α1-receptor stimulation) may also follow inadvertent intravascular administration of a local anesthetic containing epinephrine. This should be considered carefully when patients with cardiac conditions are treated, although local anesthetics containing epinephrine are more efficacious due to the localized vasoconstriction that reduces systemic absorption of the catecholamine. However, the pain and anxiety generated with inadequate local anesthesia may be more harmful than the administration of a local anesthetic containing epinephrine due to the release of endogenous catecholamines. Aspiration after placement of the local anesthetic needle in the tissues and prior to injection should always be performed to reduce the likelihood of intravascular injection. The maximum doses of the most commonly used anesthetics are listed in Table 3-3.
Table 3-3
Commonly Used Local Anesthetics in Oral and Maxillofacial Surgery

*With or without epinephrine.
Discussion
Failure to achieve local anesthesia can be due to multiple factors, including the technique, anatomy, infection, and patient selection. The latter can often be predicted from the initial patient consultation and can potentially be avoided by alternate or modified surgical planning (sedation, regional block, preoperative anxiolytics). The appropriate choice of local anesthetic agent and adequate volume, and of infiltration versus regional block anesthesia, must be considered carefully. Anatomic variations may result in unusual neural innervation of mandibular and maxillary structures. A stepwise progression from more distal infiltration to more proximal regional block anesthesia often overcomes this difficulty. Anesthesia of maxillary structures may necessitate extraoral infraorbital anesthesia (as in the current case) or a maxillary (V2) block anesthesia via the greater palatine foramen or pterygomaxillary fissure. Anesthesia of the mandibular structures may necessitate consideration of accessory neural pathways, such as the nerve to the mylohyoid. The use of the closed mouth (Akinosi) technique for patients with trismus should be considered. The Gow-Gates technique may also be helpful when anesthesia of the mandibular trunk is desired or in cases of failed attempts at inferior alveolar nerve block using standard technique.
The presence of infection is generally considered to reduce the effectiveness of local anesthetics due to several mechanisms. The primary mechanism is due to the altered pH of the tissue. Different local anesthetics have different dissociation constants (pKa), which define the concentration of ionized and nonionized local anesthetic at a given pH. The nonionized form of the local anesthetic is responsible for penetrating nerve membranes and subsequently dissociating to give the ionized form, which blocks sodium channels. In the presence of infection (and hence a lower tissue pH), lidocaine, with a pKa of 7.9, exists predominantly in the ionized form, which is unable to diffuse through membranes. Although giving additional anesthetic is reasonable, a better choice would be to increase the concentration (percent) of the agent or to use a different agent with a lower pKa so that more of the nonionized form is available. Articaine is an amide local anesthetic with a lower pKa of 7.8 (more rapid onset), which when combined with high lipid solubility (depth of anesthesia) may be of benefit when infection is present.
Additionally, the increased tissue perfusion secondary to inflammation may increase removal of the local anesthetic from the site of administration, although this plays a minor role. A more proximal regional block anesthetic injection may also suffice in the presence of infection, because the local anesthetic is delivered into the uninfected tissue, and therefore problems related to a decreased tissue pH are avoided. It should be remembered, however, that chronic pain may result in both peripheral and CNS sensitization, which may reduce the efficacy of the local anesthetic, even when it is given as a regional block away from the source of infection or pain.
In the management of postoperative pain and discomfort, long-acting local anesthetics are often used when significant pain is expected, such as after multiple or third molar extractions. Bupivacaine and etidocaine are long-acting anesthetics with a duration of action approximately two to three times that of lidocaine or mepivacaine. The effectiveness of bupivacaine in obtaining profound anesthesia has been shown to be comparable to that of lidocaine, although the former drug has been shown to have a slower onset of action. Etidocaine has a slightly faster onset than bupivacaine and provides equally profound mandibular anesthesia when given as a regional block. However, the depth of anesthesia obtained when it is given as maxillary infiltrates has been shown to be somewhat less. A long-acting anesthetic is typically used in conjunction with another, shorter acting anesthetic for profound anesthesia during the procedure and extended pain control postoperatively. The benefit of prolonged anesthesia must be weighed against the risks, such as self-inflicted trauma and patient concern over prolonged numbness. If the decision is made to use a long-acting anesthetic, the postoperative expectations should be discussed thoroughly with the patient; delay in recovery of sensation beyond 10 hours is not uncommon.
Bouloux, GF, Punnia-Moorthy, A. Bupivacaine versus lignocaine for third molar surgery: a double blind randomized crossover study. J Oral Maxillofac Surg. 1999; 57:510–514.
Feldman, H. Toxicology of local anesthetic agents. In: Rice S, Fish K, eds. Anesthetic toxicity. New York: Raven Press; 1994:107–133.
Gaffen, AS, Haas, DA. Retrospective review of voluntary reports of nonsurgical paresthesia in dentistry. J Can Dent Assoc. 2009; 75(8):579.
Garisto, GA, Gaffen, GA, Lawrence, HP, et al. Occurrence of paresthesia after dental local anesthetic administration in the United States. J Am Dent Assoc. 2010; 141(7):836–844.
Hawkins, JM, Moore, PA. Local anesthesia: advances in agents and techniques. Dent Clin North Am. 2002; 46(4):719–732.
Tofoli, GR, Ramacciato, JC, De Oliveira, PC, et al. Comparison of effectiveness of 4% articaine associated with 1:100,000 or 1:200,000 epinephrine in inferior alveolar nerve block. Anesth Prog. 2003; 50:164–168.
Trigeminal Neuralgia
CC
A 52-year-old Caucasian female is referred by her physician for evaluation of an 11-month history of intermittent severe, stabbing pain and dull throbbing pain in the right maxillary zygomatic buttress area. (Trigeminal neuralgia [TN] is more common in women than in men by a ratio of 3 : 2. The condition usually affects the middle aged or older; however, young adults and children can also be affected.)
HPI
The patient reports the pain over her right check area as stabbing and at times shocklike, superimposed on a dull background pain of varying duration (95% of the time TN is located in the lower face and/or malar region). She rates her pain severity as a 9 on a 0 to 10 visual analog scale (most patients with TN rate their pain as 9 or 10 on a VAS). These episodes last about 10 to 35 seconds and are triggered by chewing, washing her face, or brushing her teeth. (Triggering stimuli may include talking [76%], chewing [74%], touch [65%], cold temperature [48%], wind, applying makeup and shaving. Intraoral TN triggers are associated with the gingiva). Between attacks the individual has periods of temporary remission, called refractory periods, during which it is impossible or extremely difficult to trigger pain (trigger zones characteristic of TN are not clinically identifiable in 40% to 50% of cases). The pain does not awake her from sleep unless she has slept on her right side (pain occurs on the right side over the left by a ratio of 3 : 2; it is typically unilateral, with bilateral pain reported in 1% to 4% of cases).
PMHX/PDHX/Medications/Allergies/SH/FH
The patient's medical history is unremarkable (the presence of hypertension increases the risk of TN 2.1 times in females and 1.5 times in males; multiple sclerosis increases the risk by a factor of 20). Her dental history indicates that she saw a dentist shortly after her symptoms began. She had received two root canals on her upper right first and second molars. Her symptoms did not resolve, and both teeth were subsequently extracted (due to its location and paroxysmal nature, TN has often been confused with dental pathology, leading to unnecessary dental treatment in 33% to 65% of cases). She still experiences bouts of pain that are triggered by eating, and she was treated for a temporomandibular disorder (pain with chewing is consistent with TMD) with oral appliance therapy. She is anxious, depressed, and fearful of recurring attacks (quality of life is severely impaired with TN; depression is common, and suicides have been reported). She is married and has two young children. Presently she does not work because of her symptoms (talking provokes attacks in 74% of patients).
Examination
The patient is anxious; she appears well developed and well nourished. (Some patients limit their diet and thus exhibit signs of undernourishment.) There is no extraoral swelling or asymmetry. On palpation there is tenderness of the temporalis and masseter on her right side. She is very resistant to any palpation over her zygomatic area and to opening her mouth, for fear of eliciting sharp, shooting pain. Her opening is 42 mm with lateral excursions of 11 mm bilaterally and protrusive movement of 6 mm. Cranial nerve examination is noncontributory, and sensory testing is normal (this potentially differentiates between symptomatic and idiopathic TN). Oral hygiene is poor, with significant plaque buildup on the buccal surfaces of her right premolar area. No evidence of dental caries noted. Percussion and palpation over her premolars were negative. Gingival tissue is inflamed, primarily due to plaque buildup.
The physical examination in patients with TN is generally normal. Diagnosis of trigeminal neuralgia is largely based on an accurate clinical history (sudden onset of severe, unilateral facial pain lasting seconds) and necessary imaging (MRI with contrast or CT scan) to differentiate between symptomatic and idiopathic TN, regardless of age. Ruling out ear, mucosal, sinus, teeth, and temporomandibular joint (TMJ) pathologies is necessary, because problems in these areas may cause facial pain (see Table 3-6 for differential diagnoses).
Imaging
Obtaining a panoramic radiograph (and, when indicated, periapical radiographs) is prudent to rule out the presence of dental pathology.
Radiologic investigations are important to distinguish between symptomatic and idiopathic TN. An MRI scan with gadolinium enhancement can demonstrate arterial compression of the nerve or rule out tumor or demyelination, as is seen in multiple sclerosis. (Compression lesions, such as vestibular schwannomas, meningiomas, epidermoid cysts, and other tumors, can cause symptomatic TN.) Magnetic resonance angiography (MRA) is useful diagnostically and can aid surgical treatment of the offending blood vessel. (Compression of the fifth or the ninth nerve root by a blood vessel, usually a tortuous artery at the root entry zone into the brainstem, is the most common source of neuropathic pain in idiopathic TN). If neither MRI nor MRA is available, contrast-enhanced CT scanning is effective in ruling out neoplastic causes.
In the current patient, the panoramic radiograph revealed multiple missing teeth. No osseous pathology was noted, and the results were otherwise unremarkable. The MRI scan of the head was within normal limits (WNL).
Labs
There are no specific laboratory tests required for diagnosis of TN. However, because TN is a diagnosis of exclusion, specific tests can be ordered to rule out other infectious or inflammatory conditions.
Assessment
Idiopathic TN predominantly involving the second division of the right trigeminal nerve (V2).
Treatment
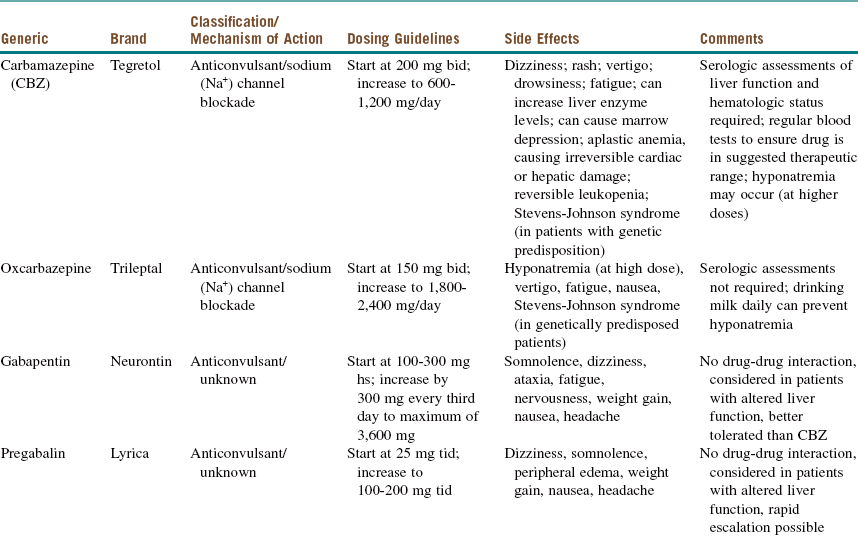
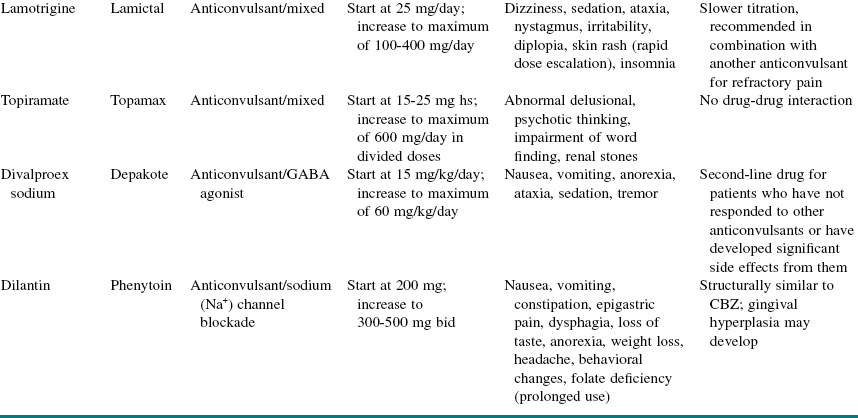
For newly diagnosed TN, medical management is the first-line therapy (it reduces or eliminates pain in approximately 75% of patients). Medications used in the medical management of TN can be divided into antiepileptic drugs (AEDs) and non-AEDs (Tables 3-4 and 3-5).
Table 3-5
Non-Antiepileptic Drugs Most Commonly Used for Trigeminal Neuralgia
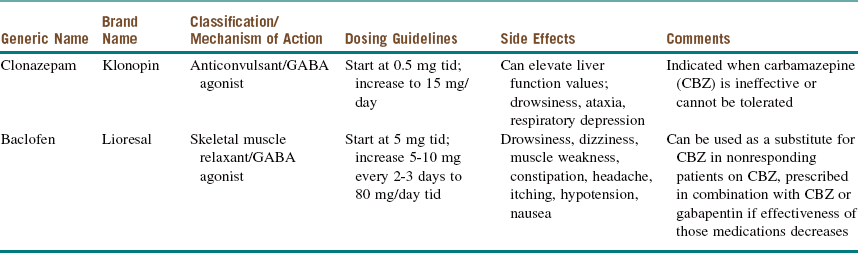
Modified from Reisner L, Pettengill CA: The use of anticonvulsants in orofacial pain, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91:2-7, 2001.
Patients with TN do not respond to conventional analgesic drugs. However, almost all these patients (80% to 90%) respond to carbamazepine (Tegretol), and some clinicians use this response as a diagnostic criterion. Although the initial response is good, the long-term response is not as favorable, and a significant number of patients may become refractory as the symptoms increase. The dose of carbamazepine should be slowly increased until the pain disappears or the patient experiences side effects. After the pain has been adequately controlled for 6 to 8 weeks, it is advisable to titrate the dosage of carbamazepine to the lowest level that controls the pain. To avoid the side effects of carbamazepine, oxcarbazepine (Trileptal, a keto analogue of carbamazepine) can be used instead. Oxcarbazepine appears to achieve similar levels of pain control with a less distressing side effect profile and without the need for monitoring of hematologic profiles.
Over time many patients with TN on carbamazepine experience “breakthrough” pain attacks and require increased dosages for pain control. This is also true for other AEDs and may reflect progression of the disease process (see Table 3-4 for AED medications and dosing).
Other strategies worth considering for acute management of TN pain attacks are peripheral local anesthetic block (injecting the trigger zone), intravenous lidocaine (100 mg infused at 20 mg/min) and intravenous AED administration. Several case studies have reported on the use of botulinum toxin A (50 U), which is injected into trigger zones, in patients who are drug refractory. This approach was shown to improve the pain threshold, and a stronger stimuli was necessary to provoke pain. In one open label trial, topical capsaicin (Zostrix), applied locally to the trigger zone, was helpful for TN pain.
Surgical treatment options are divided into procedures that directly decompress the trigeminal nerve (involving a posterior fossa craniectomy and translocation of the offending structures); procedures that partially injure the nerve (percutaneous thermal radiofrequency ablation, percutaneous glycerol rhizotomy, mechanical balloon compression, trigeminal rhizotomy, and stereotactic radiosurgery); and palliative procedures (deep pain stimulation). Factors such as the patient's age, the location of the pain, and any associated comorbidities all are considered before a decision is made on which procedure to perform.
The current patient was started on carbamazepine 200 mg two times a day, which relieved her attacks for a month only. For her recurrent episodes, the carbamazepine dosage was increased to 1,000 mg a day. The patient's liver function test (LFT) results and CBC were monitored prior to medication initiation and at 3 months, with no significant changes seen while the patient was taking the medication. After 3 months the patient was experiencing some attacks, although not as frequently as before. Baclofen 10 mg three times a day was added to her regimen, which helped control her pain. After she had been pain free for 3 months, her medications were tapered over a 1-month period (if mild pain remains, maintenance at a low dose of an effective drug is preferable).
Complications
Medication management comes with its own risks and benefits. Carbamazepine, although an effective drug, produces several adverse effects that need to be considered and monitored. Carbamazepine is metabolized by the liver cytochrome P450 enzyme 3A4; in addition, it induces the several cytochrome P450 enzyme systems, thus altering the circulation levels of other medications. It is necessary to conduct serologic assessments of the patient's liver function and hematologic status; also, to determine whether the drug is in the suggested therapeutic range, the patient must have regular blood tests (these are repeated monthly for 3 months and then once every 3 to 6 months). The administration of carbamazepine during pregnancy has been associated with various birth defects, ranging from neural tube defects to congenital heart disease. (See Table 3-4 for additional information.)
Surgical procedures that produce destructive lesions in the trigeminal system usually provide effective pain control. However, these procedures do not treat the cause of the TN, which leads to the recurrence of pain over time. In addition, these procedures are more likely to produce other sensory disturbances involving the trigeminal nerve; these can vary from minor dysesthesias to more severe symptoms, such as analgesia dolorosa or anesthesia dolorosa.
Discussion
Patients with TN report that they have severe head pain that lasts for hours to days; however, they may not specify that the individual unit of pain is brief (lasting seconds to 1 to 2 minutes) but occurs repetitively over the duration of the attack. Generally the first attack is totally unexpected, and most patients describe it as by far the worst pain they have ever experienced. The details of the first attack generally are remembered forever. The pain is described as lancinating, resembling an electric shock, feeling as if glass is grinding into the face, or much worse (Table 3-6). Patients may comment that attacks can be precipitated by various stimuli to the face. No neurologic deficits are present except when a tumor exists. Pain is confined to the distribution of the trigeminal nerve and is almost always unilateral. It is estimated that in 5% to 8% of cases, TN is precipitated by trauma, most commonly an acute flexion-extension injury. The pain is more commonly located in the V3>V2>V1 nerve distribution.
Table 3-6
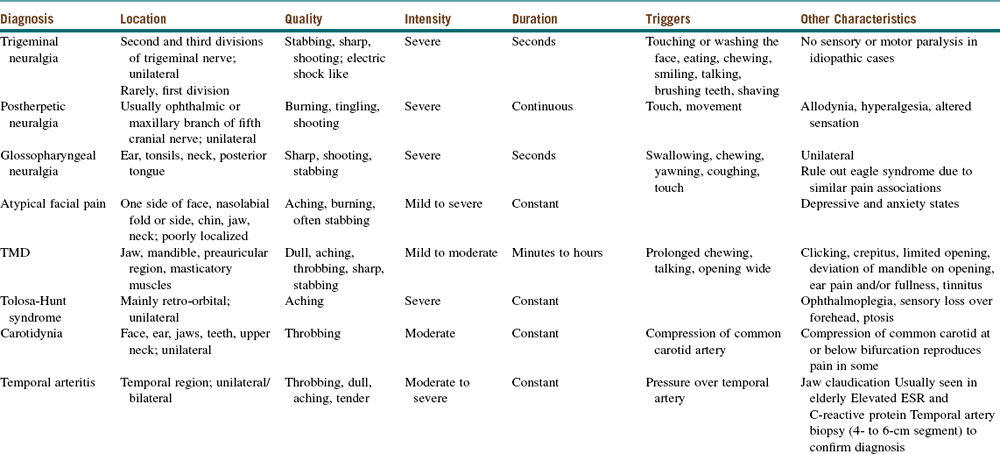
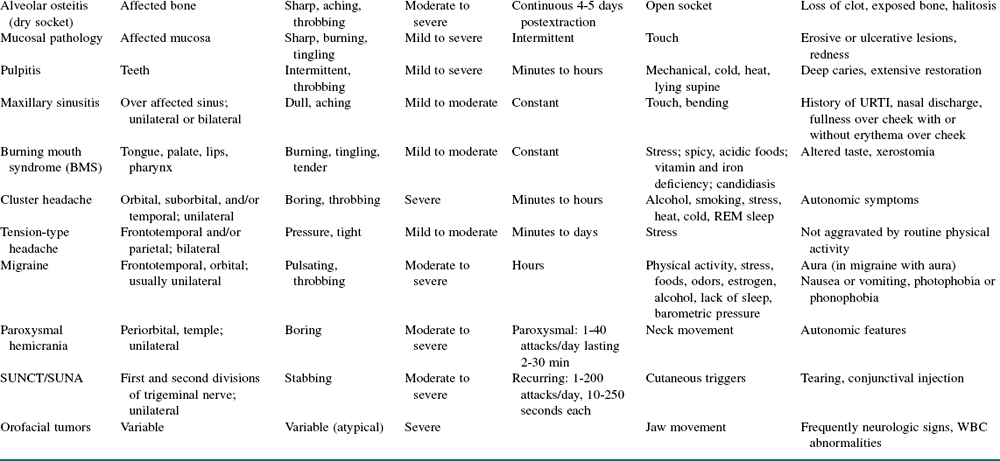
ESR, Erythrocyte sedimentation rate; SUNA, short-lasting unilateral autonomic headaches; SUNCT, severe unilateral neuralgiform headache with conjunctival injection and tearing; TMD, temporomandibular disorder; URTI, upper respiratory tract infection.
Modified from Agostoni E, Frigerio R, Santoro P: Atypical facial pain: clinical considerations and differential diagnosis, Neurol Sci 26:s71-s74, 2005.
In deciding on the treatment options for a patient with TN, the clinician must take into account various clinical factors. The International Headache Society has classified TN into two categories: classic (idiopathic) and secondary (symptomatic). The two categories present with similar symptoms, but they differ with respect to causality. Classic TN includes neuralgia that is idiopathic or caused by compression of the trigeminal nerve by a nearby blood vessel (arterial loop of the basilar artery, most commonly anterior inferior cerebellar or superior cerebellar), and it is by far more common (90%) than the secondary type. Secondary TN accounts for cases triggered by structural abnormalities (tumors, vascular malformation or demyelinating diseases). Rarely TN results from bony compression of the nerve (e.g., due to an osteoma or deformity resulting from osteogenesis imperfecta).
TN rarely occurs bilaterally; when it does, it is usually secondary to multiple sclerosis. TN never crosses the midline but on rare occasions may switch to the opposite side in different attack periods. Typically the time pattern of the pain is episodic, and the pain lasts for a few weeks to approximately a month. This can be followed by a remission period of several weeks, months, or years. Over time, there is a tendency to exacerbations and remissions, with an overall progressive increase in the frequency, duration, and severity of the attacks until the symptoms become continuous without remission. The character of the pain may also change, with an aching or burning sensation accompanying the shooting pains.
TN was originally called tic douloureux (painful tic), because the patient, when experiencing the pain, grimaces, especially on the ipsilateral side. Before the onset of TN, some patients have a prodrome of discomfort or moderate pain in the tooth, face, or jaw. In some cases this may precede an actual TN attack by weeks to months. During this period, patients usually present to a dentist and in many cases have teeth extracted, root canal procedures, and sometimes oral appliance therapy. A timely, accurate diagnosis of TN is particularly important, because a variety of specific treatments can greatly reduce or eliminate TN pain symptoms in many patients.
Bagheri, SC, Farhidvash, F, Perciaccante, VJ. Recognition and management of trigeminal neuralgia. J Am Dent Assoc. 2004; 135:1713–1717.
Bohluli, B, Motamedi, MHK, Bagheri, SC, et al. Use of botulinum toxin A for drug refractory trigeminal neuralgia: preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 111:47–50.
Clark, GT, Teruel, A. Anticonvulsant agents used for neuropathic pain including trigeminal neuralgia. In: Clark GT, Dionne RA, eds. Orofacial pain: a guide to medications and management. West Sussex, UK: Wiley-Blackwell; 2012:95–114.
Cohen, J. Current medical therapy. In: Jannetta PJ, ed. Trigeminal neuralgia. New York: Oxford University Press; 2011:56–73.
Elias, WJ, Burchiel, KJ. Trigeminal neuralgia and other neuropathic pain syndromes of the head and face. Curr Pain Headache Rep. 2002; 6:115–124.
International Headache Society, Headache Classification subcommittee. The international classification of headache disorders, second edition. Cephalgia. 24(Suppl 1), 2004.
Jannetta, PJ. Typical and atypical symptoms. In: Jannetta PJ, ed. Trigeminal neuralgia. New York: Oxford University Press; 2011:41–45.
Jannetta, PJ, Hadeed, GJ. Medical therapy: the dentist's perspective. In: Jannetta PJ, ed. Trigeminal neuralgia. New York: Oxford University Press; 2011:46–50.
Krafft, RM. Trigeminal neuralgia. Am Fam Physician. 2008; 77(9):1291–1296.
Linskey, ME, Jannetta, PJ. Differential diagnosis: look-alike diseases, atypical trigeminal neuralgia. In: Jannetta PJ, ed. Trigeminal neuralgia. New York: Oxford University Press; 2011:74–86.
Love, S, Coakham, HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001; 24:2347–2360.
Pawl, RP. Trigeminal neuralgia and atypical facial pain. Curr Pain Headache Rep. 1997; 1:175–181.
Reisner, L, Pettengill, CA. The use of anticonvulsants in orofacial pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001; 91:2–7.
Scrivani, SJ, Mathews, ES, Maciewicz, RJ. Trigeminal neuralgia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 100:527–538.
Scrivani, SJ, Keith, DA, Bassiur, JP, et al. Nonsurgical management of facial pain. In: Bagheri SC, Bell RB, Khan HA, eds. Current therapy in oral and maxillofacial surgery. Philadelphia: Elsevier/Mosby; 2011:247–264.
Scrivani, SJ, Keith, DA, Mathews, ES, Kaban, LB. Percutaneous stereotactic differential radiofrequency thermal rhizotomy for the treatment of trigeminal neuralgia. J Oral Maxillofac Surg. 1999; 57(2):104–111.
Zakrzewska, JM, Linksey, ME. Trigeminal neuralgia. In: Zakrzewska JM, ed. Orofacial pain. New York: Oxford University Press; 2009:119–133.
Zakrzewska, JM, McMillan, R. Trigeminal neuralgia: the diagnosis and management of this excruciating and poorly understood facial pain. Postgrad Med J. 2011; 87:410–416.
Malignant Hyperthermia
CC
A 15-year-old boy (incidence of malignant hyperthermia [MH] is highest in children and 75% in males) is undergoing an open reduction with internal fixation (ORIF) of a left mandibular angle fracture in the operating room. He had presented to the emergency department complaining of pain, swelling, and malocclusion. He has previously had ear tubes and a tonsillectomy under general anesthesia without anesthesia complication.
HPI
While playing ball, the patient sustained an accidental blow to the left side of the jaw from an opponent's elbow. He was diagnosed with a fractured mandible and subsequently was admitted to the hospital for treatment of his injury under general anesthesia. The patient was induced with propofol, given succinylcholine, and nasotracheally intubated without difficulty. He was maintained on sevoflurane (halogenated inhaled anesthetic) and intravenous agents. The patient had a smooth anesthetic course for the first 20 minutes of the procedure before the onset of unexplained tachycardia and elevation in his end-tidal CO2 (earliest signs of MH). The diagnosis of MH was considered.
PMHX/PDHX/Medications/Allergies/SH/FH
The patient underwent tonsillectomy and adenoidectomy at age 6 under general anesthesia without any surgical or anesthetic complications (50% of MH cases occur in patients with two or more prior uneventful experiences with anesthetics). His family history is negative for MH. (MH is an autosomal dominant inherited disorder. However, many patients present with MH without any prior documented family history.)
Examination
MH is a life-threatening, pharmacogenetic hypermetabolic state that can be recognized by a variety of signs and symptoms, most commonly while the patient is under general anesthesia.
General. Muscle rigidity (commonly seen in the masseter, including masseter spasm) and skin mottling are present.
Vital signs. Heart rate is 130 bpm (unexplained tachycardia is one of the early signs), temperature 39.2°C (hyperthermia is regarded as a hallmark, but it may be a late finding, and the likelihood of complication increases 2.9 times per 2°C increase in maximum temperature), and respirations 34 per minute (tachypnea).
Adjunctive monitors. End-tidal CO2 is 57 mm Hg and rising (hypercapnia). (The end-tidal CO2 can rise to two to three times normal at a constant minute ventilation.)
ECG. Sinus tachycardia at 130 bpm (supraventricular and ventricular arrhythmias, including cardiac arrest, can be observed).
Imaging
No imaging studies are indicated in the acute management of MH. A chest radiograph is obtained after the initial treatment to evaluate the position of the endotracheal tube if the patient remains intubated and for evaluation of the lung fields.
Labs
Upon diagnosis of MH, a full set of serum electrolytes, liver function tests, urinalysis, and arterial blood gases should be ordered to aid in the correction and diagnosis of electrolyte and acid-base disturbances.
The laboratory findings would characteristically reflect the following metabolic conditions:
• Acidemia (elevated Pco2 and metabolic acidosis) (of cases seen between 1987 and 2006, 78.6% presented with both muscular abnormalities and respiratory acidosis; only 26% had metabolic acidosis).
• Hyperkalemia (secondary to acidosis)
• Hypercalcemia (secondary to reduced uptake of calcium from the sarcoplasmic reticulum of skeletal muscles)
• Elevated serum transaminases and creatinine kinase (CK) and subsequent rhabdomyolysis, causing myoglobinuria (secondary to hypermetabolic skeletal muscle activity)
The standard for diagnostic testing for suspected susceptibility to malignant hyperthermia is the caffeine-halothane contracture test (CHCT), which is performed on muscle biopsy specimens at specialized centers.
ECG changes and dysrhythmias can occur; these are late findings. They are due to elevated potassium levels from muscle breakdown. They can occur more rapidly in muscular patients.
The presence of premature ventricular contractions may indicate a life-threatening hyperkalemia and is an ominous sign, because this condition may degrade into ventricular tachycardia or ventricular fibrillation.
Treatment
Successful treatment of MH consists of early administration of dantrolene (the likelihood of complication increases 1.6 times per 30-minute delay in dantrolene administration) and removal of the triggering agent. Box 3-2 details the steps that should be followed in treating MH.
Arrhythmias usually respond to correction of acidosis and hyperkalemia. Antiarrhythmic agents, excluding calcium channel blockers, may be used for arrhythmias that do not respond to correction of acidosis and hyperkalemia.
Complications
The mortality rate for untreated MH has been reported to be as high as nearly 70%. Approximately 25% of patients who experience MH relapse within the first 24 hours (recrudescence). After an MH episode, the patient should be transferred to an intensive care unit until all vital signs have returned to normal. Dantrolene treatment should be continued during this period; the usual dose is 1 mg/kg intravenously every 4 to 6 hours.
Discussion
The incidence of MH in children may be as high as 1 in 5,000 to 1 in 65,000. In a genetically predisposed patient, triggering agents, such as succinylcholine and volatile anesthetic agents, release calcium from the sarcoplasmic reticulum, leading to elevated concentrations of calcium in the muscle cells. This increased metabolism causes the muscles to contract and become rigid. The increased metabolism leads to the elevated end-tidal CO2 and acidosis. Rhabdomyolysis leads to hyperkalemia and potential arrhythmias, in addition to myoglobinuria and potential renal failure.
The routine use of succinylcholine has fallen out of favor. Many anesthesiologists use nondepolarizing agents, such as rapacuronium or rocuronium, when possible. However, none of these drugs have replaced succinylcholine for rapid sequence intubation or the reversal of laryngospasm. Dantrolene sodium should be available in all facilities at which any triggering agents are routinely used.
In preparation for an anesthetic procedure on a known MH-susceptible patient, anesthetic vaporizers are removed or taped in the OFF position. The carbon dioxide absorbent (soda lime or baralyme) is changed. Oxygen at 10 L/min is flushed through the circuit via the ventilator for at least 20 minutes. During this time, a disposable, unused breathing bag should be attached to the Y-piece of the circle system and the ventilator set to inflate the bag periodically. The use of a new or disposable breathing circuit is recommended.
Allen, GC, Larach, MG, Kunselman, AR. The sensitivity and specificity of the caffeine-halothane contracture test: a report from the North American Malignant Hyperthermia Registry. Anesthesiology. 1998; 88:579–588.
Ball, SP, Johnson, KJ. The genetics of malignant hyperthermia. J Med Genet. 1993; 30:89.
Collins, CP, Beirne, OR. Concepts in the prevention and management of malignant hyperthermia. J Oral Maxillofac Surg. 2010; 61(11):1340–1345.
Davison, JK, Eckhardt, WF, Perese, DA. Clinical anesthesia procedures of the Massachusetts General Hospital. Boston: Little, Brown; 2003.
Larach, MG, Gronert, GA, Allen, GC, et al. MH presentation, treatment, and complications, 1987-2006. Anesth Analg. 2010; 110(2):498–507.
Malignant Hyperthermia Association of the United States Web site. www. mhaus. org.
Patil, PM. Malignant hyperthermia in the oral and maxillofacial surgery patient: an update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 112:e1–e7.
Rosenberg, H, Fletcher, JE. An update on the malignant hyperthermia syndrome. Ann Acad Med Singapore. 1994; 23:84.
Emergent Surgical Airway
CC
A 48-year-old male presents to the emergency department (ED) complaining of difficulty breathing and the inability to swallow.
HPI
The patient has a 2-month history of intermittent swelling of his right mandible associated with caries affecting tooth #30. For the past 3 days he has developed rapidly increasing swelling of the right floor of the mouth (sublingual space) and submandibular triangle (submandibular space) that has spread to the contralateral side, consistent with Ludwig's angina (see Ludwig's angina in Chapter 4). For the past 24 hours, he has been sitting upright in the sniffing position (unable to lie supine because of a choking sensation), unable to control his secretions, having difficulty swallowing (dysphagia), and unable to open his mouth (trismus). His tongue protrudes (glossoptosis), and he experiences difficulty talking (dysphonia). His level of anxiety has increased with the onset of progressive dyspnea (difficulty breathing), and he is now unable to clear his airway. He makes gurgling noises with a faint, high-pitched, crowing sound (stridor); these are signs of upper airway obstruction.
PMHX/PDHX/Medications/Allergies/SH/FH
The patient's past medical history includes type II diabetes (well controlled) and morbid obesity.
Examination
General. Well-developed, moderately obese male in severe respiratory distress (obesity increases the difficulty of endotracheal intubation).
Vitals signs. Blood pressure is 168/94 mm Hg (hypertension), heart rate 120 bpm (tachycardia), respirations 25 per minute (tachypnea), temperature 39.4°C, and oxygen saturation 96% on 5 L oxygen via nasal cannula.
Maxillofacial exam. Bilateral submandibular and submental brawny cellulitis that is tender to palpation, warm to the touch, and erythematous. The neck is of moderate size; however, the trachea and larynx are easily palpable. The trachea is midline (a deviated trachea increases the difficulty of intubation). The cervical spine has full range of motion (neck extension facilitates intubation or securing a surgical airway).
Intraoral exam. Oral exam is limited due to decreased mouth opening. The patient is unable to control his secretions (dysphagia, evidenced by drooling of saliva). The floor of the mouth (FOM) is elevated, tender, and edematous. The tongue is large and protruding. The uvula and soft palate are not visible (Mallampati class IV). Tooth #30 is grossly carious. Further airway evaluation can be performed with fiberoptic nasopharyngoscopy (visualizing the hypopharynx, base of the tongue, pharyngeal walls, epiglottis, and vocal cords) to determine airway patency and the extent of airway edema. Figure 3-3 shows the surgical landmarks related to the airway; these are the thyroid notch, cricothyroid membrane, cricoid cartilage, and suprasternal notch.
Imaging
In the setting of impending airway embarrassment, any attempt to obtain imaging studies should be delayed until a secure airway has been established. Loss of an airway in the radiology suite, where adequately trained personnel are not immediately available, can be devastating. For this reason, upon evaluation of the patient, the surgeon must quickly decide either to proceed to the operating room (OR), where optimal personnel and equipment for advanced airway intervention are available or, in a sudden emergency, proceed with immediate placement of a surgical airway in the ED. Immediate intervention should be considered only when the circumstances allow for other options.
If the patient is deemed stable, with no immediate threat to airway obstruction, a panoramic radiograph (to evaluate possible odontogenic sources of infection), and a computed tomography (CT) scan with contrast (to localize loculated areas of abscess formation and to assist in airway evaluation) can be obtained. A lateral cephalometric radiograph provides a reliable study that can be obtained at bedside. It can provide important information regarding the posterior airway space (prevertebral soft tissue should be less than 7 mm at the level of C3 and 20 mm at the level of C7). However, with the recent advent of fiberoptic nasopharyngoscopy and the use of CT, lateral cephalographs are rarely used today.
In the current patient, no radiographic studies were ordered due to airway instability; the patient was taken directly to the operating room.
Labs
Laboratory studies should not delay the establishment of a secure airway. When possible, a complete blood count (CBC) with differential and a basic metabolic panel (BMP) are indicated for evaluation of the systemic response and metabolic derangements associated with severe odontogenic fascial space infections. Arterial blood gas analysis can be used to determine the adequacy of ventilation. Other laboratory values are ordered based on pertinent medical information.
In the current patient, a STAT laboratory study obtained by the ED physician demonstrated a white blood cell (WBC) count of 18,000 cells/mm3 and a blood glucose level of 310 mg/dl.
Treatment
Upon suspicion or diagnosis of impending airway embarrassment, the anesthesia team and OR/ED staff must be notified immediately. The surgeon and anesthesiologist (if available) must decide upon the safest means of rapidly obtaining a secure airway. Risk factors that predispose patients to difficult mask ventilation and intubation should be identified in anticipation of using advanced airway intervention techniques. Such risk factors include obesity, a short neck, a rigid neck, a small mouth opening, retrognathia, Mallampati class III or IV, and prominent upper incisors. An awareness of these factors by the surgeon contributes greatly to successful management of a compromised airway.
Patients deemed difficult to intubate are potential candidates for awake fiberoptic nasal intubation or an elective awake tracheotomy in a controlled OR setting. However, it is possible for a “routine” intubation procedure to develop into a difficult airway scenario. During the course of a difficult laryngoscopy, the anesthetist or anesthesiologist may not be able to intubate or adequately ventilate the patient, requiring an emergent surgical airway (fortunately, this is uncommon).
For emergent intervention for a comprised airway in the adult patient, a cricothyroidotomy is the procedure of choice. Tracheostomy is used for emergency surgical airways in pediatric patients younger than 10 to 12 years. The small size of the cricothyroid membrane (3 mm) and the poorly defined anatomic landmarks make performing a cricothyroidotomy extremely difficult in children. There is also an increased risk of laryngeal and vocal cord injury with cricothyroidotomy in this age group.
Needle cricothyroidotomy with jet insufflation can also be performed by skilled anesthesia personnel (providing temporary oxygenation, but not ventilation). The patient can be oxygenated while the surgeon establishes a definitive surgical airway (tracheotomy or cricothyroidotomy). If a surgical cricothyroidotomy is performed, conversion into a formal tracheotomy should be considered, primarily depending on the anticipated duration of the surgical airway (patients requiring prolonged ventilatory support should be converted to a tracheotomy).
Needle cricothyroidotomy with jet insufflation. Ideally the patient should be supine or semisupine with a shoulder bolster to hyperextend the neck. The cricothyroid membrane (the slight depression between the thyroid cartilage and cricoid cartilage) is palpated, and the larynx is stabilized using the thumb and forefinger. In a thin neck with prominent landmarks, an incision is not usually needed, and direct puncture through the skin and cricothyroid membrane can be accomplished. Otherwise, it may be necessary to make a small incision through the skin over the identified region of the cricothyroid membrane. A 3-cc syringe is then attached to a 14-gauge angiocatheter, and the catheter and needle are inserted through the cricothyroid membrane at a 45-degree angle caudally. Negative pressure is applied by withdrawing the plunger of the syringe while the needle is advanced (aspiration of air indicates entry into the tracheal lumen). The 14-gauge needle is removed from the angiocatheter, leaving the angiocatheter in the trachea. Once in place, the catheter can be insufflated with oxygen to provide aeration of the lungs. The intent is to provide oxygen to the lungs and also to allow passive exhalation. Various “kits” are commercially available that accomplish this; however, the effect can be obtained by cutting a small hole in the oxygen tubing near the attachment to the 3-cc syringe (can also be attached to a 7.5 endotracheal tube connector). The hole is occluded for 1 second and left open for 4 seconds, forcing oxygen into the trachea and allowing for some passive exhalation (if any). Adequate oxygenation can be maintained for 30 to 45 minutes, but hypercarbia results from inadequate ventilation. Therefore, preparations should be made to convert the airway to a tracheotomy to secure a patent, reliable airway.
Surgical cricothyroidotomy. The nondominant hand is used to stabilize the laryngeal cartilage, and a vertical skin incision is made using a #15 or #11 scalpel blade over the cricothyroid membrane (this provides the option of superior-inferior extension of the incision, if needed). The incision is carried through the skin and superficial fat layer, immediately over the cricothyroid membrane, which is also vertically incised with the blade. The scalpel handle is inserted into the incision site and rotated 90 degrees to provide access into the tracheal lumen. The lumen is further dilated with finger dissection or with the use of a Trousseau dilator. A small, cuffed endotracheal tube or a tracheotomy tube is inserted, and the patient is ventilated. A positive return of carbon dioxide is the best means of confirming correct tube placement. The tube is secured, and the chest is auscultated for bilateral breath sounds.
In the current patient, an emergent surgical cricothyroidotomy was performed after the airway was lost during an unsuccessful attempt at an awake fiberoptic nasal intubation. Due to the severity of the infection, the difficult airway, and the anticipation of prolonged cannulation, the cricothyroidotomy was subsequently converted to a formal tracheotomy.
Complications
The most feared complication of a needle cricothyroidotomy procedure is inadequate oxygenation and ventilation, leading to anoxic brain injury and cardiovascular collapse. Proper placement of the insufflating needle is paramount for a successful outcome. Placement and manipulation of the 14-gauge needle under emergency circumstances is also a grave concern. Laceration of adjacent structures, including the thyroid, posterior tracheal wall, and the esophagus, can occur, leading to severe hemorrhage that can cause embarrassment of the already compromised airway. Hematoma formation and aspiration of blood can contribute to a negative outcome. Improper placement of the needle can also result in subcutaneous or mediastinal emphysema.
Complications associated with surgical cricothyroidotomy include all the acute events discussed previously with the needle cricothyroidotomy procedure, in addition to chronic complications associated with the surgical intervention. Creation of a false passage into the surrounding connective tissue and damage to the larynx and vocal cords can result from improper or forced introduction of the endotracheal tube. Subsequent laryngeal stenosis or vocal cord paralysis may occur, resulting in permanent damage.
Discussion
The possible etiologies of airway embarrassment (loss of airway) secondary to upper airway obstruction include severe maxillofacial trauma, infection, tumors, congenital or developmental deformities, laryngospasm, foreign body obstruction, and edema. Determination of the exact cause of airway embarrassment is based on physical and radiographic findings, in addition to the chronologic progression. A true loss of the airway is defined as the inability to ventilate (with a bag-valve-mask airway, a laryngeal mask airway, or a Combitube) and the inability to intubate. Although airway loss is usually progressive, it may have a sudden onset and occur prior to arrival in the operating room, during attempted intubation, after extubation, or during intravenous sedation.
It is important to differentiate loss of the airway from loss of protective airway reflexes, in which the patient has an upper airway obstruction that can be alleviated with chin lift/jaw thrust maneuvers, placement of an oral or a nasal airway, or positive pressure mask ventilation (see respiratory Depression Secondary to Oversedation early in this chapter).
There are numerous situations in which an oral and maxillofacial surgeon may encounter patients with difficult airways and/or acute airway embarrassment. The surgeon and anesthesia team should communicate to anticipate the likelihood of a difficult intubation (requiring awake fiberoptic nasal intubation) or possible loss of the airway (requiring emergent surgical intervention). The surgeon should take charge once the airway has been lost (cannot intubate, cannot ventilate scenario) and rapidly secure a surgical airway, as described previously. The operating room staff should be prepared to assist in an emergent cricothyroidotomy (in adults) or a tracheotomy (in pediatric patients).
Recognition of a compromised airway is the most difficult and crucial step in airway management. A compromised airway may be classified as sudden and complete, insidious and partial, or progressive. Assessment and frequent reassessment of airway patency and adequacy of ventilation are crucial. A patient's refusal to lie down may indicate that the patient is unable to maintain the airway or handle the secretions. Tachypnea is generally related to pain and anxiety, but it may also be an early indicator of airway or ventilatory collapse. Compromised ventilatory efforts may occur in unconscious patients suffering from alcohol and drug abuse, neck or thoracic injury, or intracranial damage. These patients often require endotracheal intubation to establish and maintain a definitive airway and to protect against pulmonary aspiration.
Objective signs of airway obstruction may be revealed with visual assessment to determine whether the patient is agitated or obtunded. Agitation may suggest hypoxia, whereas an obtunded patient may have hypercarbia. The abusive patient may be hypoxic and should not be presumed to be intoxicated. Cyanosis related to hypoxemia is manifested by the presence of a blue hue in the perioral tissues and nail beds. Visualization of chest retractions and the use of accessory muscles provides further evidence of airway compromise. The chest should be evaluated by auscultation. Noisy breath sounds, snoring, gurgling, and crowing sounds (stridor) may be associated with partial upper airway obstruction. Hoarseness (dysphonia) may indicate laryngeal obstruction or edema. The trachea should be palpated to determine its position.
A definitive airway comprises an endotracheal tube, connected to an oxygen source, that is positioned in the trachea. The three forms of definitive airways are (1) an orotracheal tube, (2) a nasotracheal tube, and (3) a surgical airway (cricothyroidotomy or tracheotomy).
Initial attempts should be directed toward placement of an orotracheal or a nasotracheal tube. If edema or severe oropharyngeal hemorrhage obstructs the airway, preventing endotracheal tube placement, then a surgical airway must be created. Depending on the experience of the surgeon, a surgical cricothyroidotomy is preferred to a tracheotomy in the emergent setting, because it is easier to perform; it is associated with less bleeding; and it requires less time. An awake tracheotomy is more appropriate in the difficult airway without acute loss of the airway.
Altman, KW, Waltonen, JD, Kern, RC. Urgent airway intervention: a 3-year county hospital experience. Laryngoscope. 2005; 115:2101–2104.
American College of Surgeons Committee on Trauma, Advanced Trauma Life Support for Doctors. 2004.
Bernard, AC, Kenady, DE. Conventional surgical tracheostomy as the preferred method of airway management. J Oral Maxillofac Surg. 1999; 57(3):310–315.
Bobek, S, Bell, RB, Dierks, EJ, et al. Tracheotomy in the unprotected airway. J Oral Maxillofacial Surg. 2011; 69:2198–2203.
Dierks, EJ. Tracheotomy: elective and emergent. Oral Maxillofacial Surg Clin North Am. 2008; 20:513–520.
Haspel, AC, Coviello, VF, Stevens, M. Retrospective study of tracheostomy indications and perioperative complications on oral and maxillofacial surgery service. J Oral Maxillofac Surg. 2012; 70:890–895.
Lewis, RJ. Tracheostomies: indications, timing and complications. Clin Chest Med. 1992; 13(1):137–149.
Paw, HG, Sharma, S. Cricothyroidotomy: a short-term measure for elective ventilation in a patient with challenging neck anatomy. Anaesth Intensive Care. 2006; 34(3):384–387.
Salvino, CK, Dries, D, Gamelli, R, et al. Emergency cricothyroidotomy in trauma victims. J Trauma. 1993; 34(4):503–505.
Spitalnic, SJ, Sucov, A. Ludwig's angina: a case report and review. J Emerg Med. 1995; 13:499.
Standley, TD, Smith, HL. Emergency tracheal catheterization for jet ventilation: a role for the ENT surgeon. J Laryngotology Otol. 2005; 119(3):235–236.
Stauffer, JL, Olsen, DE, Petty, TL. Complications and consequences of endotracheal intubation and tracheostomy. Am J Med. 1981; 70:65.
Stock, CR. What is past is prologue: a short history of the development of tracheostomy. Throat. 1987; 66(4):166–169.
Taicher, S, Givol, N, Peleg, M, et al. Changing indications for tracheostomy in maxillofacial trauma. J Oral Maxillofac Surg. 1996; 54(3):292–295.
Walts, PA, Murphy, SC, DeCamp, NM. Techniques of surgical tracheostomy. Clin Chest Med. 2003; 24(3):413–427.
Wood, DE. Tracheostomy. Chest Surg Clin North Am. 1996; 6(4):749–764.