Oral and Maxillofacial Infections
Odontogenic and nonodontogenic maxillofacial infections are among the oldest disease processes treated by oral and maxillofacial surgeons. They commonly present to the office, or in severe cases to the hospital emergency department. Although the majority of infections can be treated in a nonemergent fashion, early recognition and correct management of severe infections can be lifesaving. Knowledge of the surgical anatomy and path of spread of infections in the head and neck is fundamental to correct diagnosis and treatment. Severe infections of the sublingual, submandibular, and parapharyngeal spaces can cause airway compromise, cavernous sinus thrombosis, and possibly mediastinal spread of infection, resulting in significant mortality and morbidity, especially in the medically compromised patient who presents late in the disease process.
Despite the availability of a wide spectrum of antimicrobial agents and increasing knowledge of microbiology, the treatment of odontogenic infections remains primarily surgical. Removal of the source of infection and establishment of adequate drainage for elimination of the purulent material is the mainstay treatment. However, adequate antibiotic coverage is important and should not be overlooked.
The response to treatment can be monitored by several clinical (swelling, erythema, pain, interincisal opening) and laboratory (white blood cell [WBC] count, C-reactive protein) parameters. The measurement of temperature is an ancient method of monitoring the response to an infectious process. Fever occurs secondary to the production of endogenous pyrogens (cytokines, interleukins, tumor necrosis factor), which affect the hypothalamus and medulla to increase the temperature set point. The definition of fever is arbitrary, and there is considerable variability in “normal temperature” for a population of healthy adults. A range of definitions is acceptable (37.5° to 38.5°C), depending on how sensitive an indicator the surgeon wants to use. The lower the temperature used to define fever, the more sensitive the indicator is for detecting an infectious process, but the less specific it will be. Normal body temperature is generally considered to be 37°C and varies according to circadian rhythm and menstrual cycle. Many different variables can influence temperature such as exercise and environmental factors.
Chills occur in response to the elevation in temperature set point during the initiation of fever. This is often accompanied by the need for increased insulation and decreased exposure of skin. Shivering is also seen, contributing to the increase in temperature.
The differential is a ratio of the different types of white blood cells present (polymorphonuclear neutrophils [PMNs], lymphocytes, monocytes, eosinophils, and basophils). With a rise in the WBC count, as is seen during an acute infection, the predominant increase occurs in the PMNs. Chemotactic factors contribute to the recruitment of PMNs to the site of infection (or injury), with a subsequent increase in production of neutrophils by the bone marrow. Production of the precursor forms of the PMN (myelocytes and promyelocytes) increases, and these cells are released into the circulating blood. The movement toward circulation of immature forms is termed a shift to the left and is usually seen during acute infections.
In this chapter we present four teaching cases of infections that have an odontogenic etiology. We also present a case of osteomyelitis of the mandible.
Ludwig's Angina
Solon Kao, Jaspal Girn, Chris Jo and Shahrokh C. Bagheri*
CC
A 34-year-old, otherwise healthy man presents to the emergency department, stating, “My neck and tongue are swollen, and it all started with a toothache.” (Ludwig's angina is predominantly seen in young adults, with as much as a 3 : 1 male predilection.)
HPI
The patient presented to the emergency department with a 3-day history of progressive swelling and pain in his neck. He reports a 3-week history of severe, intermittent pain in his lower right third molar. Ten days ago, his general dentist prescribed him amoxicillin for a periapical abscess and pericoronitis associated with his right mandibular third molar, which was partially impacted and decayed. Despite compliance with antibiotics, he progressively developed persistent swelling and a foul-tasting drainage around the tooth. He began to have swelling under the right side of his tongue (right sublingual space), which spread to the contralateral side (there is no anatomic barrier between the right and left sublingual spaces). Simultaneous with the floor of the mouth swelling, the neck began to swell on the right side, and the swelling spread to the other side. The patient reports having subjective fevers and chills (signs of systemic inflammatory involvement), in addition to dysphagia (difficulty swallowing) and odynophagia (painful swallowing). He states that he has not been able to eat or drink in the past 48 hours (causing dehydration). He denies dysphonia (difficulty speaking, seen with edema of the vocal cords and upper airway) or any chest discomfort (seen with advanced mediastinal involvement).
PMHX/PDHX/Medications/Allergies/SH/FH
This patient is otherwise healthy. (Diabetes mellitus and other immunocompromised states are risk factors for poor outcome and death [see Complications]).
Examination
General. The patient is sitting upright, appears very restless, and is unable to tolerate his oral secretions (evidenced by constant use of a Yankauer suction and drooling of saliva). He appears to be in mild respiratory distress, but there is no evidence of stridor (a high-pitched, crowing noise due to partial upper airway obstruction).
Airway. The airway is stable on examination. The trachea is difficult to palpate due to edema but appears to be in the midline. Fiberoptic nasopharyngoscopy can be performed to further evaluate the patency of the upper airway and the amount of edema of the surrounding soft tissue (see Emergent Surgical Airway in Chapter 3). Alternatively, computed tomography (CT) scans of the neck can delineate neck and airway swelling.
Vital signs. The patient's blood pressure is 138/89 mm Hg, heart rate 110 bpm (tachycardia), respirations 28 per minute (tachypnea), temperature 40°C (febrile), and oxygen saturation 96% on room air.
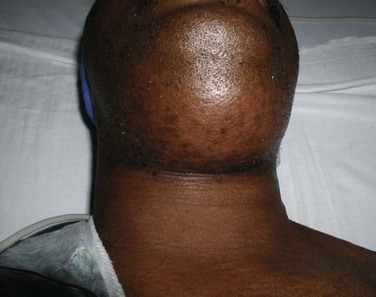
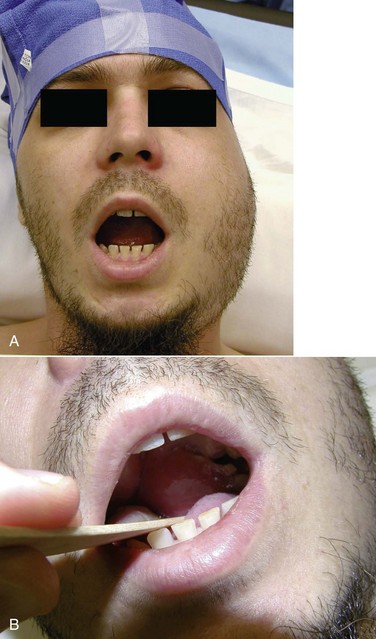
Maxillofacial. There is obvious moderate to severe facial swelling over the lower third of the face. Brawny and painful induration of the submandibular and submental spaces is noted bilaterally (Figure 4-1). There is erythema over the anterior neck extending down to the clavicles. However, subcutaneous crepitus (indicative of subcutaneous air from gas-producing organisms) is not present. No cervical lymphadenopathy or fluctuance was palpated (lymphadenopathy would be difficult to assess in the presence of neck edema or induration).
Intraoral. The patient's mouth opening is limited, with a maximal interincisal opening of 20 mm (trismus indicates masticator space involvement or guarding secondary to pain). The floor of the mouth and tongue are elevated and edematous (sublingual space). The oropharynx is not clearly visualized due to the limited mouth opening and elevated tongue (positive predictors of difficult laryngoscopy and endotracheal intubation).
Cardiovascular. The patient is tachycardic, without rubs, murmurs, or gallops (tachycardia and friction rubs can be indicative of mediastinitis). He is negative for Homan's sign (crepitus heard with a stethoscope during systole, indicative of mediastinitis).
Pulmonary. Lung fields are clear to auscultation bilaterally, without rales, bronchi, or wheezes (aspiration of saliva or exudates can be seen with advanced cases).
Imaging
Before obtaining any imaging studies, the surgeon needs to decide on the urgency of the infectious process compromising the airway. If the airway is deemed stable, imaging studies should be obtained to guide surgical treatment. However, any possibility of acute airway embarrassment should not delay direct transfer to the operating room for advanced airway interventions. Once the airway is stabilized, imaging studies can be safely obtained.
A panoramic radiograph is the initial screening study of choice. It provides an excellent overview of the dentition, identifying any odontogenic sources of the infection. CT scans of the neck with contrast material are indicated when dealing with deep neck space infections (chest CT should be included if there is a suspicion of descending mediastinitis). This study can help determine the anatomic spaces involved, localize any fluid collections (loculations of purulence), and determine whether the airway is deviated or compromised. CT is also helpful in surgical planning for incision and drainage. When a chest CT is deemed unnecessary, chest radiographs (posteroanterior and lateral views) can be an important screening tool to detect a widened mediastinum, which may be indicative of descending mediastinitis.
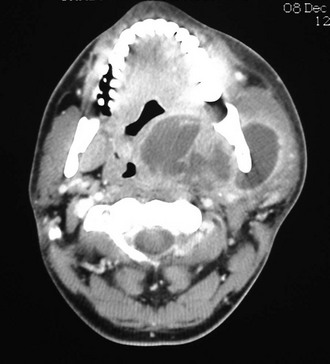
In the current patient, the panoramic radiograph revealed a carious right mandibular third molar with a large periapical radiolucent lesion. The CT scan of the patient's neck revealed a rim-enhancing fluid collection involving the bilateral submandibular, submental, and right sublingual spaces (Figure 4-2). In addition, there was diffuse soft tissue edema consistent with cellulitis in the involved spaces. No subcutaneous emphysema was seen in the cervical tissues (subcutaneous gas collection is considered a hallmark of cervical necrotizing fasciitis and is seen in up to 46% to 67% of cases). The patient's airway was patent and midline. A chest CT was ordered due to the erythema tracking down the anterior neck. No mediastinal involvement was observed.
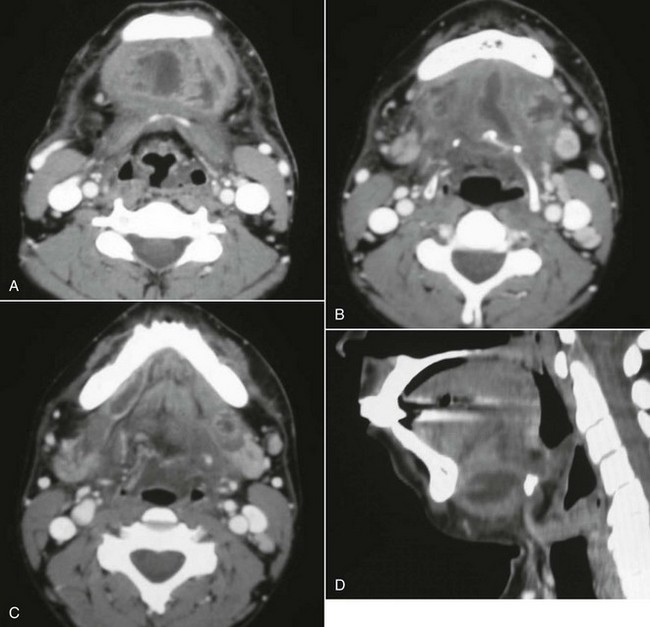
Figure 4-2 A, Axial view, soft tissue CT neck scan with contrast, showing an enhancing fluid collection in the submental space. B, Axial view, soft tissue CT neck scan with contrast, showing enhancing fluid collections in the submental and bilateral submandibular spaces. C, Axial view, soft tissue CT neck scan with contrast, showing an enhancing fluid collection in the right sublingual space. Note that Wharton's duct, seen on this view, confirms that this abscess is above the mylohyoid muscle. D, Sagittal reconstruction, soft tissue CT neck scan with contrast, showing a large submandibular space abscess extending from the inferior border of the anterior mandible to the hyoid bone.
Labs
A complete blood count (CBC) and complete metabolic panel (CMP) are indicated during the work-up of severe odontogenic infections. The presenting WBC count is a marker of the severity of infection, and this value should be followed during the course of treatment. C-reactive protein (CRP) is an acute-phase reactant that is released in response to inflammation, and it can be used to monitor the response to therapy. Studies have also suggested that a very high CRP level at the time of admission is a predictor of a complicated hospital course. Electrolyte disturbances (sodium, potassium, magnesium, calcium) are common among patients with severe head and neck infections, especially when the patient is not able to tolerate oral intake due to swelling or pain. Blood urea nitrogen (BUN) and creatinine levels are useful for evaluating for prerenal azotemia due to hypovolemia. Blood cultures are indicated in the patient with persistent fever. An electrocardiogram (ECG) should be obtained with suspicion of mediastinitis. Arterial blood gas (ABG) measurement is warranted in the critically ill patient presenting with septic shock.
The current patient presented with these lab values: WBC count 21,000 cells/mm3 with a 35% bandemia, BUN 30 mg/dl (normal range, 7 to 18 mg/dl), and creatinine 1.2 mg/dl (normal range, 0.6 to 1.2 mg/dl). The BUN/creatinine ratio was 25 (a ratio greater than 20 is indicative of prerenal azotemia). The remainder of his electrolyte values were within normal limits.
Assessment
Ludwig's angina secondary to carious right mandibular third molar (odontogenic source of infection accounts for 70% to 90% of cases, the vast majority arising from second or third molars).
Ludwig's angina was first described by Karl Friedrich Wilhelm von Ludwig in 1836 as a rapidly progressing, gangrenous cellulitis originating in the region of the submandibular area that extends without any tendency to form abscesses. Ludwig's angina is now known as an aggressively spreading cellulitis that simultaneously affects the bilateral submandibular, sublingual, and submental spaces. Although Ludwig's angina is classically described as a cellulitis, progression to abscess formation within the involved spaces is most often the case, and to this date, clinicians still use the term “Ludwig's angina” when describing a bilateral submandibular, sublingual, and submental space infection. Grodinsky and Holyoke's criteria for Ludwig's angina may no longer have any useful clinical application. The term “angina” is a misleading term, because any chest discomfort seen with this is from descending mediastinitis and is not related to ischemic heart disease.
Treatment
Treatment begins with evaluation of the patient's airway and appropriate management to prevent acute airway embarrassment (see Emergent Surgical Airway in Chapter 3). The airway is first evaluated by the general appearance of the patient (a distressed patient with stridorous respirations is assumed to have an airway compromise until proved otherwise). The oral cavity should be examined to evaluate the amount of tongue, floor of the mouth, soft palate, and pharyngeal wall edema (many times an oral examination is very limited due to the patient's inability to open). A fiberoptic nasopharyngoscopy can be performed in the emergency department to further assess the airway, including the vocal cords. Intravenous dexamethasone can be given to reduce the airway edema in patients with impending upper respiratory obstruction. An emergent cricothyroidotomy should be performed if the patient loses the airway before arrival in the operating room. An awake tracheotomy or an awake fiberoptic nasal intubation can be performed in the operating room if the situation is less acute (oral intubation by direct laryngoscopy may also be possible in less severe cases). There is support in the current literature for the assumption that a tracheotomy may be indicated in patients with Ludwig's angina (see Complications).
Supportive measures should be initiated while arrangements are made with the operating room. This should include fluid resuscitation and initiation of broad-spectrum empiric antibiotic therapy. Fluid resuscitation is commonly needed because patients present with hypovolemia due to lack of oral intake (insensible losses are accelerated by fever) and/or some degree of sepsis or septic shock. Adequacy of fluid resuscitation should be continuously monitored (heart rate, blood pressure, urine output, and BUN/creatinine). Vasopressive therapy may be indicated in patients presenting with septic shock. Tight glycemic control (blood glucose 90 to 110 mg/dl) is desirable, especially in the critically ill patient.
Empiric antimicrobial therapy should be promptly initiated to cover the mixed aerobic-anaerobic polymicrobial organisms (gram positive, gram negative, aerobic, and anaerobic) commonly involved in these infections. Penicillin G at an adult dose of 4 million to 30 million units per day, divided and given every 4 to 6 hours, in combination with metronidazole, is an appropriate regimen. Other recommendations include clindamycin 900 mg given intravenously every 8 hours; ticarcillin clavulanate 3.1 g given intravenously every 6 hours; ampicillin sulbactam 3 g given intravenously every 6 hours; and piperacillin tazobactam 3.375 g given intravenously every 6 hours. Chow in 1992 recommended high-dose intravenous penicillin G combined with clindamycin, metronidazole, or cefoxitin. When available, the antibiotic regimen should be guided by cultures and sensitivity studies. The CRP has been shown to be an excellent marker for the severity of the infection and the patient's response to surgical and antibiotic therapy.
Aggressive surgical drainage and debridement, along with elimination of the source of infection, are necessary for definitive treatment. Delay in taking the patient to the operating room for surgical treatment is associated with a worse outcome. Cultures should be taken either via aspiration techniques or with a culturette swab. Most cases of Ludwig's angina can be managed using small incisions in the submandibular and submental regions (larger cervical hockey-stick or apron incisions may be indicated when the condition is complicated by necrotizing fasciitis). Blunt dissection is carried out to explore all the involved spaces. Subperiosteal dissection and debridement are important in the area around the source of infection, and any offending teeth should be extracted. The intraoral and extraoral dissections can be dissected to freely communicate, allowing for dependent extraoral drainage. Therefore the abscess is decompressed, the necrotic debris is debrided, and the wounds are copiously irrigated. Red rubber catheters and/or Penrose drains can be used to facilitate postoperative wound irrigation and to allow dependent drainage. Drains can be slowly advanced out of the wound postoperatively or removed when purulent drainage ceases. Repeat drainage and lavage procedures in the operating room should be considered, especially in more severe infections that are refractory to treatment. Bouloux and associates evaluated the efficacy of irrigating surgical drains on postoperative odontogenic infections. They found that nonirrigating drains (Penrose drains) appear to be equally efficacious as irrigating drains (red rubber catheter).
The current patient was given 16 mg of dexamethasone intravenously in the emergency department; intravenous fluid resuscitation was initiated; and empiric intravenous antibiotics were administered. Antibiotic therapy consisted of ampicillin-sulbactam (Unasyn) 3 g every 6 hours and clindamycin 900 mg every 8 hours. The patient was urgently taken to the operating room for incision and drainage of the involved anatomic spaces of the neck and extraction of the right mandibular third molar. The patient was intubated successfully via an awake nasal fiberoptic endotracheal intubation. An 18-gauge needle was used to aspirate purulent exudate from the submandibular space, which was sent for Gram stain, aerobic and anaerobic cultures, and antibiotic sensitivity studies. The surgical drainage consisted of three incisions of 1.5 to 2 cm in length, 2 cm below the inferior border at the angle of the mandible bilaterally and anteriorly in the submental area. Consideration should be given to placement of the incisions to allow dependent drainage. Blunt dissection with a hemostat and a Kelly clamp was carried out to explore all involved spaces. Copious amounts of purulence and necrotic tissue were expressed from the surgical sites. The right mandibular third molar was elevated and extracted. The gingival cuff was elevated, and subperiosteal dissection was carried out along the lingual plate to enter the sublingual and submandibular spaces. All the incisions were connected to each other in the subplatysmal and subperiosteal planes. Irrigation drains were placed in the submandibular, sublingual, and submental spaces. All drains were irrigated with copious amounts of antibiotic irrigation and/or normal saline irrigation. The patient was left intubated for 3 days postoperatively due to surgical and airway edema. After significant resolution of the infection and edema, he had a positive cuff leak test and was extubated over an Eschmann tube, which was left in place for several hours. He did not experience any postextubation airway compromise and was transferred to the ward the following day.
Complications
The most feared complication associated with Ludwig's angina is death due to airway compromise. Loss of airway from upper airway obstruction can occur at any time during the perioperative period, before arrival at the operating room, during an attempted intubation, after an accidental or self-extubation in the intensive care unit (ICU), or after a planned extubation (see Emergent Surgical Airway in Chapter 3). Potter and colleagues in 2002 reported a 3% incidence of loss of airway for patients who received a tracheotomy versus 6% for patients maintained with endotracheal intubation. They reported two deaths (4% mortality rate) secondary to loss of airway, and both deaths occurred in the endotracheal intubation group (one occurred after a planned extubation and the other occurred after an unplanned extubation). The tracheotomy group had shorter ICU stay (1.1 versus 3.1 days) and shorter overall hospital stay (4.9 versus 5.9 days). Patients with Ludwig's angina or a retropharyngeal space abscess have a significant need for tracheotomy. Har-El et al's review of 110 patients showed that 4 of 8 patients meeting their criteria for severe infection who did not receive a tracheostomy developed upper airway obstruction necessitating an emergent surgical airway (50% incidence of airway loss in the endotracheal intubation group). They concluded that tracheotomy is indicated in patients with Ludwig's angina. In 1985, Loughnan and Allen reported successful endotracheal intubation in 9 of 10 patients with Ludwig's angina using an inhalational induction technique and direct laryngoscopy, but they did not report on the postoperative morbidity and mortality. If postoperative endotracheal intubation is planned, adequate sedation, four-point restraints, and a secured tube (taped around the head or wired to the teeth) are paramount to prevent unanticipated self or iatrogenic extubation. Upon extubation, a cuff leak test should be performed and an Eschmann tube should be left in place to facilitate reintubation if needed (postextubation laryngeal edema may cause loss of airway despite having a good cuff leak test result).
Before the advent of antibiotics, the mortality rate from Ludwig's angina was greater than 50%. Fortunately, the prevalence and mortality rates have significantly decreased due to better access to dental care and antibiotic therapy. When the condition is complicated by descending mediastinitis and thoracic empyema, the mortality rate remains as high as 38% to 60% despite antibiotic therapy (Figure 4-3). When the condition in complicated by cervical necrotizing fasciitis, the more recent reported mortality rate is 18% to 22% (any delay in surgical treatment increases mortality). Tung-Yiu and colleagues reported that an immunocompromised state (e.g., diabetes mellitus) increases the risk of an odontogenic infection developing into cervical necrotizing fasciitis. Of their series of 11 cases, seven patients were immunocompromised (four with diabetes mellitus), which accounted for all major complications, including two deaths. Others have reported a mortality rate as high as 67% with severe odontogenic infections associated with diabetes mellitus. Currently there is no evidence to suggest that HIV/AIDS status increases the risk of developing Ludwig's angina and its associated complications.
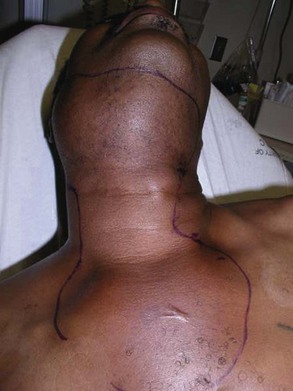
Figure 4-3 A patient with Ludwig's angina and descending mediastinitis via the anterior paratracheal spaces and bilateral carotid spaces. Note the soft tissue edema and erythema of the anterior neck tracking down to the sternum.
Other potential complications include aspiration, ventilator-acquired pneumonia, septic shock, and acute renal failure.
Discussion
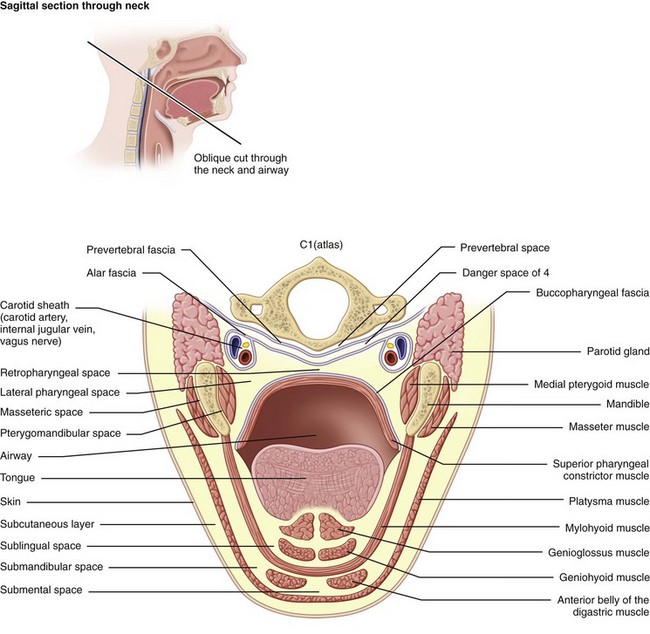
Ludwig's angina is defined by the involvement of specific anatomic spaces (bilateral submandibular, sublingual, and submental spaces). The sublingual spaces are bounded anteriorly and laterally by the mandible, superiorly by the floor of the mouth and tongue, and inferiorly by the mylohyoid muscle. There is no anatomic barrier between the left and right sublingual spaces. The submandibular space is separated from the sublingual space by the mylohyoid muscle, thus forming the roof of the submandibular space. The hyoglossus and styloglossus muscles form the medial border, and the body of the mandible forms the lateral border. The skin, superficial fascia, platysma, and superficial layer of the deep cervical fascia form the superficial boundary. The anterior bellies of the digastric muscles form the lateral borders of the submental space. The roof is formed by the mylohyoid muscle. The symphysis of the mandible and the hyoid bone form its anterior and posterior borders, respectively. The sublingual and submandibular spaces posteriorly communicate freely with each other and with the medial masticator and lateral pharyngeal spaces, which in turn is contiguous with the retropharyngeal space. Extension of the infection along the carotid sheath (contained within the posterior compartment of the lateral pharyngeal space [LPS]) or retropharyngeal space can lead to descent into the superior mediastinum. The alar fascia separates the retropharyngeal space from the “danger space” (space 4 of Grodinksy and Holyoke), which extends to the diaphragm and the posterior mediastinum. The anterior paratracheal spaces provide anterior access to the superior mediastinum (Figure 4-4).
Ludwig's angina most commonly has an odontogenic etiology (70% to 90%). A periapical abscess from the second or third mandibular molars is the most common cause. The roots of these teeth are commonly below the attachment level of the mylohyoid muscle to the internal oblique ridge. The periapical abscess perforates the lingual cortex, with spread into the sublingual (if the root is above the mylohyoid attachment) or submandibular (if the root is below the mylohyoid attachment) space. The infection can then rapidly spread to adjacent continuous or contiguous spaces. Other causes include peritonsillar or parapharyngeal abscesses, oral lacerations, mandibular fractures, and submandibular sialadenitis.
The bacteriologic profile of Ludwig's angina is usually polymicrobial and includes aerobes and anaerobes. The most common organisms are Streptococcus viridans, β-hemolytic streptococci, staphylococci, Klebsiella pneumoniae, anaerobic Bacteroides organisms, and Peptostreptococcus organisms. S. viridans is one of the most commonly isolated organisms. This is consistent with previous reports associating this organism with odontogenic infections. K. pneumoniae is another commonly isolated organism that has a higher incidence in patients with diabetes mellitus.
Allen, D, Loughnan, TE, Ord, RA. A re-evaluation of the role of tracheostomy in Ludwig's angina. J Oral Maxillofac Surg. 1985; 43:436–439.
Barsamian, JG, Scheffer, RB. Spontaneous pneumothorax: an unusual occurrence in a patient with Ludwig's angina. J Oral Maxillofac Surg. 1987; 45:161–168.
Bouloux, GF, Wallace, J, Xue, W. Irrigating drains for severe odontogenic infections do not improve outcome. J Oral Maxillofac Surg. 2013; 71:42–46.
Chidzonga, MM. Necrotizing fasciitis of the cervical region in an AIDS patient: report of a case. J Oral Maxillofac Surg. 2005; 63:855–859.
Chow, AW. Life-threatening infections of the head and neck. Clin Infect Dis. 1992; 14:991.
Dugan, MJ, Lazow, SK, Berger, JR. Thoracic empyema resulting from direct extension of Ludwig's angina: a case report. J Oral Maxillofac Surg. 1998; 56:968–971.
Fischmann, GE, Graham, BS. Ludwig's angina resulting from infection of an oral malignancy. J Oral Maxillofac Surg. 1985; 43:795–796.
Har-El, G, Aroesty, JH, Shana, A, et al. A retrospective study of 110 patients. Oral Surg Oral Med Oral Pathol. 1994; 77(5):446–450.
Loughnan, TE, Allen, DE. Ludwig's angina: the anaesthetic management of nine cases. Anaesthesia. 1985; 40:295–297.
Mihos, P, Potaris, K, Gakidis, I, et al. Management of descending necrotizing mediastinitis. J Oral Maxillofac Surg. 2004; 62:966–972.
Potter, JK, Herford, AS, Ellis, E. Tracheotomy versus endotracheal intubation for airway management in deep neck space infections. J Oral Maxillofac Surg. 2002; 60:349–354.
Steiner, M, Gau, MJ, Wilson, DL, et al. Odontogenic infection leading to cervical emphysema and fatal mediastinitis. J Oral Maxillofac Surg. 1982; 40:600–604.
Sugata, T, Fujita, Y, Myoken, Y, et al. Cervical cellulitis with mediastinitis from an odontogenic infection complicated by diabetes mellitus: report of a case. J Oral Maxillofac Surg. 1997; 55:864–869.
Tsuji, T, Shimono, M, Yamane, G, et al. Ludwig's angina as a complication of ameloblastoma of the mandible. J Oral Maxillofac Surg. 1984; 42:815–819.
Tsunoda, R, Suda, S, Fukaya, T, et al. Descending necrotizing mediastinitis caused by an odontogenic infection: a case report. J Oral Maxillofac Surg. 2000; 58:240–242.
Tung-Yiu, W, Jehn-Shyun, H, Ching-Hung, C, et al. Cervical necrotizing fasciitis of odontogenic origin: a report of 11 cases. J Oral Maxillofac Surg. 2000; 58:1347–1352.
Ylijoki, S, Suuronen, R, Jousimies-Somer, H, et al. Differences between patients with or without the need for intensive care due to severe odontogenic infections. J Oral Maxillofac Surg. 2001; 59:867–872.
Zachariades, N, Mezitis, M, Stavrinidis, P, et al. Mediastinitis, thoracic empyema, and pericarditis as complications of a dental abscess: report of a case. J Oral Maxillofac Surg. 1988; 46:493–495.
Buccal and Vestibular Space Abscess
CC
A 34-year-old woman presents to the urgent care clinic complaining of pain and progressively enlarging swelling of her right cheek within the past 4 days.
HPI
The patient has not received any dental care for the past several years (risk factor for odontogenic infections). Two weeks earlier, she noticed that a segment of a restoration broke off the right maxillary second molar. At the time, there was an acute, localized pain in the right posterior maxillary molar with subsequent development of swelling in the right buccal vestibule (buccal vestibular space infection). The patient decided to take over-the-counter medications, and her pain subsided but the swelling persisted. Two days before presentation, she developed acute onset of right-side swelling of the cheek associated with mild pain and discomfort that have progressively exacerbated. There is no history of trismus, visual changes, dysphagia, swelling of the floor of the mouth, or difficulty breathing (all of which are signs of more severe facial space involvement, such as masticator space infection, periorbital extension, and parapharyngeal or sublingual infections; these signs are not seen with pure buccal and vestibular space involvement).
PMHX/PDHX/Medications/Allergies/SH/FH
Noncontributory. Severe odontogenic infections are most commonly seen in patients with a history of dental neglect, although they are not exclusive to any group. When infections are seen in patients with compromised immunity (AIDS, diabetes, chemotherapy), the infectious process may prove more resistant to treatment.
Examination
General. The patient was a well-developed and well-nourished woman in mild distress (patients with buccal space infections are frequently very concerned due to the extent of swelling and pain).
Vital signs. Stable and WNL. Temperature of 98.8°F (an elevated temperature is not always seen, especially in well-localized infections, unless there is surrounding cellulitis or systemic dissemination of the infection).
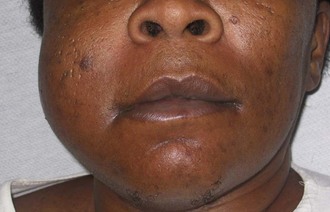
Maxillofacial. Significant right-side facial edema extending from the inferior border of the mandible superiorly to the level of the zygoma (Figure 4-5). The swelling is soft and fluctuant and with no apparent intraoral or extraoral drainage (untreated buccal space infections may spontaneously drain, providing some relief or spread into other fascial spaces). There is tender right submandibular lymphadenopathy (due to active infection).
Intraoral. The maximal interincisal opening is 35 mm (trismus is not seen with vestibular space or buccal space infections, because it does not involve the muscles of mastication, unless the infection has spread from buccal space to the submasseteric space posteriorly, pterygomandibular space inferiorly, and infratemporal space superiorly). Bimanual examination of the right posterior buccal vestibule and right cheek reveals fluctuance within the right maxillary vestibule extending to the depth of the mandibular vestibule. The right maxillary second molar (tooth #2) is grossly carious. The floor of the mouth is soft and not elevated (it is important to assess the airway for patency), the oropharynx is clear, and the uvula is midline (deviation is seen with lateral pharyngeal space [LPS] infections).
A key clinical finding of buccal space infections is the general lack of extension of swelling below the inferior border of the mandible. Infections can spread in a subcutaneous plane to the neck or the periorbital tissue, but extension beyond the buccal area implies tissue involvement beyond this space. The buccal space does not compromise the airway.
Imaging
The panoramic radiograph is the imaging study of choice for the evaluation of the odontogenic etiology of suspected vestibular and buccal space infections and is frequently the only imaging study that is necessary. It allows a general screening of the bony anatomy of the maxillofacial region, in addition to identification of potential odontogenic sources of infection (most commonly, the maxillary or mandibular first or second molars). A CT scan with intravenous contrast material can be obtained if there is clinical suspicion of orbital, parapharyngeal, or submandibular space involvement.
For the current patient, the panoramic radiograph demonstrated a severely carious right maxillary second molar with a well-demarcated periapical radiolucent lesion.
Labs
No routine laboratory tests are indicated for the evaluation and treatment of buccal space infections. A CBC can be obtained to assess for leukocytosis (elevated WBC count). The results of this test may be valuable in cases that are refractory to treatment or in the presence of other medical comorbidities. The WBC count may not be elevated with isolated vestibular or buccal space infections.
Routine use of culture and sensitivity studies for all vestibular and buccal space infections is not indicated. However, in patients with multiple comorbidities or infections that are resistant to conventional therapy, culture and sensitivity studies may be useful to guide antimicrobial therapy.
Assessment
Right vestibular space infection and subsequent spread to buccal space secondary to carious right maxillary second molar.
Treatment
Surgical establishment of drainage, along with removal of the source of infection, is the most important treatment for vestibular and buccal space infections. Antibiotic therapy is considered beneficial and should be initiated to aid resolution of the infection.
In cases of odontogenic etiology, tooth extraction or endodontic therapy eliminates the source of infection. Extraction, when possible, is more effective, because it also allows spontaneous drainage of the infection. Drainage allows removal of purulent material, increases tissue perfusion, and therefore enhances the delivery of both oxygen and antibiotics. Incision and drainage is one of the oldest and most effective surgical procedures. Ideally, abscesses should be drained when fluctuant, before spontaneous rupture and drainage.
Several basic principles should be applied when draining an infection:
• The incision is best placed in healthy mucosa or skin when possible.
• Camouflage of the incision can be achieved by placing the incision in an aesthetic area, such as inside the mouth or in the crease of the neck (for a buccal space infection).
• Anatomic placement of an incision allows drainage by gravity. Careful attention is given to the position of the mental nerve.
• A drain should be placed for open communication, with subsequent removal once drainage has ceased,
The incision is frequently placed intraorally through the mucosa in a transverse orientation (although transcutaneous incisions may be necessary in select cases). For a buccal space infection, the buccinator muscle is bluntly penetrated using a hemostat, entering the buccal space. Culture and sensitivity studies should be obtained when indicated (see earlier discussion). Frequent irrigation of the wound can be helpful. Other supportive measures, such as intravenous fluid therapy (hydration), good oral hygiene, and nutritional support, are prescribed as necessary.
In the current case, with the patient under intravenous sedation anesthesia, needle aspiration of the buccal space was accomplished, collecting 5 ml of brown, purulent material that was sent for culture and sensitivity studies. Subsequently, the second molar was extracted and the apex was curetted. Blunt dissection was carried to the buccal maxillary cortex, and an area of perforation adjacent to the tooth was identified. Next, a small transverse incision was placed intraorally about 1 cm superior to the depth of the mandibular vestibule, allowing drainage of another 8 ml of pus.
The vestibular and buccal spaces and the extraction socket were copiously irrigated, and a Penrose drain was placed in the buccal space and secured with a 2-0 silk suture. The patient was prescribed a 10-day course of penicillin (penicillin remains the empiric antibiotic of choice for outpatient odontogenic infections). The patient was seen in the office the next day and then 3 days later for removal of the drain because of progressive resolution of the infection.
Complications
Complications of vestibular and buccal space infections are related to:
• Delay in the diagnosis, leading to systemic or local spread of the infection (sepsis)
• Surgical interventions that result in inadequate drainage
• Compromised host immunity, leading to failure of therapy
• Antibiotic-resistant organisms or inadequate pharmacotherapy
Rapid recognition of these complications can improve the outcome.
The vestibular space primarily contains areolar connective tissue, but it is crossed by the parotid duct and the long buccal and mental nerves. Incisions should be made to avoid Stensen's duct in the posterior maxillary vestibule, the mental nerve in the apical region of the mandibular premolars, the infraorbital nerve in the apical region of the maxillary cuspids, and the greater palatine neurovascular bundle in cases of palatal infections. Vestibular infections can pass around the levator anguli oris muscle to enter the infraorbital space or between the buccinator and depressor anguli oris to enter the subcutaneous or buccal space.
Once in the buccal space, the infection can spread to the cavernous sinus via the transverse facial vein (uncommon), periorbital space through the subcutaneous plane, superficial to the submandibular space via inferior or posterior extensions, submasseteric space and pterygomandibular space superficially and inferiorly respectively and superficial temporal and infratemporal spaces via the buccal fat pad. Injury to adjacent structures is usually avoided by careful attention to the regional anatomy. The buccal fat pad, Stensen's (parotid) duct, and facial artery should be avoided. However, altered anatomy due to regional edema may alter the surgical anatomy.
In infections that do not appropriately respond to treatment, consideration should be given to inadequate drainage or resistant bacterial strains. Culture and sensitivity studies should then be obtained to guide antimicrobial therapy. By far the most common etiology of vestibular and buccal space infections is odontogenic. However, recurrent buccal space infections can be seen in patients with Crohn's disease (a chronic, granulomatous inflammatory bowel disorder of unknown etiology that can affect any part of the gastrointestinal tract with “skip lesions”). According to Mills, the recurrent buccal space infections in patients with Crohn's disease is due to soft tissue development of secondary infections within the deep mucosal fissures. Treatment of buccal space infections of odontogenic etiology in a patient with Crohn's disease or other granulomatous disease may prove to be more challenging.
Discussion
Carious exposure and subsequent bacterial invasion of the pulp lead to necrosis of the pulpal tissues. The inflammatory process then spreads to the surrounding periodontal ligament and bone. The first pathologic change in the area is apical periodontitis. This results in an inflammatory and immunologically mediated process that causes bone resorption and results in a localized abscess. Certain bacteria that produce enzymes that aid in the destruction of tissue (e.g., hyaluronidases produced by streptococci and collagenases produced by Bacteroides organisms) are more virulent (the relative pathogenicity or the relative ability to do damage to the host). Such bacteria more easily invade the potential spaces.
If allowed to continue, the inflammatory process spreads peripherally until cortical bone is destroyed and a subperiosteal abscess is formed. Eventually the periosteum is perforated as the infection spreads via the “path of least resistance.” The severity of the abscess depends on factors such as the virulence of the microorganism and the anatomic arrangement of adjacent muscles and fascia. Dense sheets of connective tissue, called fascia, encompass muscles, glands, and vascular and neural structures, facilitating movement during function. Bacterial infections that penetrate the fascial spaces can therefore spread via the anatomic confines of these “potential” spaces. Bacterial infections spread via hydrostatic pressure and follow the path of least resistance, which is the loose, areolar connective tissue that surrounds the muscles enclosed by the fascial layers.
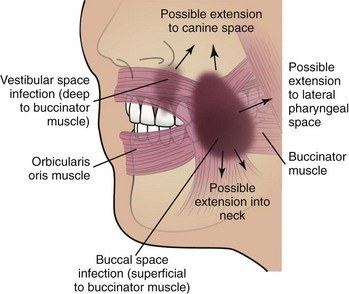
The vestibular space is the potential space between the vestibular mucosa and the underlying muscles of facial expression. The posterior boundary is bounded by the buccinator in either jaw, and the anterior boundary is made up by the intrinsic muscles of the lips and the orbicularis oris. In the anterior mandible, the abscess is confined to the vestibular space by the attachment of the mentalis muscle.
Vestibular space infections (Figure 4-6) are caused by perforation of the abscess through the buccal cortex superior to the attachment of the buccinator muscle in the mandible and inferior to the attachment of the buccinator muscle in the maxillary posterior region. The vestibular abscess is far more common than a palatal infection due to the thicker bone of the palate.
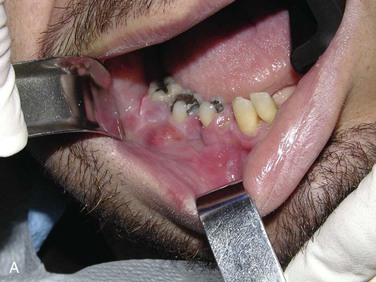
Figure 4-6 A, Right vestibular abscess secondary to carious right mandibular first molar. B, Extracted right mandibular first molar with placement of a Penrose drain in the right vestibule.
The buccal space (included in the primary fascial spaces) is confined anatomically by the subcutaneous skin layer superficially and medially by the buccinator muscle. Anteriorly, it ends at the modiolus (aponeurotic junction of the buccinator and orbicularis oris muscles just posterior to the oral commissure; Figure 4-7). Posteriorly and just medial to the ascending ramus, the buccinator muscle is attached to the superior pharyngeal constrictor muscle at the pterygomandibular raphe. This formation leads to important anatomic pathways for the spread of infection into other spaces. Laterally, it creates a communication with the masseteric space (the space between the masseter muscle and the lateral body of the ramus). Posteriorly and medially, the space communicates with the pterygomandibular, lateral pharyngeal, and infratemporal spaces superiorly. Extension of the buccal fat pad can allow buccal space infections to enter the superficial temporal space, extending via the transverse facial vein and pterygoid plexus into the infratemporal space. Rarely, it can erode into the transverse facial vein or pterygoid plexus and follow a posterior route to the cavernous sinus (the earliest sign of cavernous sinus thrombosis is congestion of the retinal veins of the eye and limitation in lateral movement of the eye due to pressure on the abducens nerve [CN VI]).
Infections that spread to the subcutaneous plane have no superficial anatomic barriers and can therefore spread to adjacent anatomic areas along this plane. Clinical inspection of the overlying skin can frequently aid in identification of subcutaneous spread of the infection. Demarcation of the areas of erythema with a skin-marking pen can be used to monitor the progression of the infectious process.
Odontogenic infections (e.g., buccal space infections) are mixed infections. A large proportion (more than half) are composed of anaerobes, mostly gram-negative rods (Fusobacterium, Bacteroides spp.). Gram-positive cocci (streptococci and peptostreptococci) are also seen in large numbers (more than 25%).
Noncomplicated buccal space infections frequently can be treated in the office using intravenous sedation and close outpatient follow-up. However, systemic involvement (fever, sepsis, leukocytosis), dehydration, medical comorbidities, noncompliant patients, compromised immune status (diabetes, AIDS, chemotherapy, malnutrition), or infections that involve other deep fascial spaces may warrant hospital admission for intravenous antibiotics and medical evaluation.
In 2006, Flynn and associates conducted a prospective study to predict the length of hospital stay and the failure to respond to penicillin in severe odontogenic infections. They reported a failure rate exceeding 20% with penicillin therapy. This finding suggests a correlation between the severity of an infection and penicillin resistance and is the basis for the recommendation of clindamycin or a β-lactam/β-lactamase inhibitor combination as the empiric antibiotic of choice in odontogenic infections serious enough to require hospitalization. For outpatient treatment, penicillin resistance has not been shown to be a significant problem; therefore, penicillin remains an acceptable antibiotic for the treatment of outpatient odontogenic infections. It has also been shown that amoxicillin may provide more rapid improvement in pain and swelling and that compliance with amoxicillin is better because of its longer dosage interval; therefore, the use of amoxicillin is gaining popularity as the antibiotic of choice for outpatient management of odontogenic infections. Additionally, in patients with orofacial odontogenic infections who received appropriate surgical treatment and/or extraction or endodontic therapy, studies have found no difference in the number of patients cured when patients were prescribed antibiotics for 3 to 4 or 7 days. Therefore, a 3- to 4-day regimen is usually adequate in otherwise healthy patients.
Flynn, RT, Halpern, RL. Antibiotic selection in head and neck infections. Oral Maxillofacial Surg Clin North Am. 2003; 15:17.
Flynn, RT, Shanti, MR, Levi, HM, et al. Severe odontogenic infections. Part 1. Prospective report. J Oral Maxillofac Surg. 2006; 64:1093.
Flynn, TR. Surgical management of orofacial infection. Atlas Oral Maxillofac Surg Clin. 2000; 8:77–99.
Flynn, TR. What are the antibiotics of choice for odontogenic infections, and how long should the treatment course last? Oral Maxillofacial Surg Clin North Am. 2011; 23:519–536.
Grodinsky, M, Holyoke, EA. The fasciae and fascial spaces of the head, neck, and adjacent regions. Am J Anat. 1938; 63:367.
Hollinshead, WH. Anatomy for surgeons: the head and neck, ed 2. Hagerstown, Md: Harper & Row; 1968.
Jones, JL, Candelaria, LM, Head and neck infections. Oral and maxillofacial surgery. Fonscea, RJ, eds. Oral and maxillofacial surgery; vol 5. Saunders, Philadelphia, 2000.
Laskin, DM. Anatomic considerations in diagnosis and treatment of odontogenic infections. J Am Dent Assoc. 1964; 69:308.
Matthews, DC, Sutherland, S, Basrani, B, et al. Emergency management of acute apical abscesses in the permanent dentition: a systematic review of the literature. J Can Dent Assoc. 2003; 69:660.
Mills, CC, Amin, M, Manisali, M. Salivary duct fistula and recurrent buccal space infection: a complication of Crohn's disease. J Oral Maxillofac Surg. 2003; 61:1085.
Mittal, N, Gupta, P. Management of extra oral sinus cases: a clinical dilemma. J Endod. 2004; 30:541–547.
Topazian, RG, Goldberg, MH, Hupp, JR. Oral and maxillofacial infections, ed 4. Philadelphia: Saunders; 2002.
Lateral Pharyngeal and Masticator Space Infection
CC
A 25-year-old man presents to the emergency department with the complaint that “my throat is swollen, and I cannot swallow.”
HPI
Approximately 1 week earlier, the patient began to experience acute pain localized to the posterior mandibular molars, with subsequent development of edema in his left posterior oropharynx 3 days later. He reports the onset of limited mouth opening, progressively worsening dysphagia (difficulty swallowing), and globus (sensation of a lump in the throat) that eventually prompted him to seek care. (Trismus and dysphagia have been shown to be significant indicators of severe odontogenic infection.) He has difficulty swallowing his secretions, either drooling or spitting them out (this is an important clinical note, because it denotes life-threatening oropharyngeal edema). He explains that he has had minimal oral intake with the onset of fever and chills. At this time he does not report any difficulty with breathing, but he feels more comfortable when sitting up (an important clinical sign of dangerous oropharyngeal edema). The patient has a muffled, “hot potato” voice (secondary to supraglottic edema).
Dental infections have become the most common etiology of deep neck infections in the Western world, involving the masticator, parapharyngeal, and submandibular spaces. More than 50% of patients presenting with infection involving these spaces have an odontogenic etiology, making oral and maxillofacial surgeons a preferred provider of surgical care for this group.
PMHX/PDHX/Medications/Allergies/SH/FH
The past medical and dental histories are unremarkable. The patient lives in a shelter and does not currently hold a job (although masticator space infections can be seen in individuals of all socioeconomic strata, the condition is far more predominant in the population with less access to health care, including frequent dental examinations).
Despite the lack of coexisting medical diseases in this patient, it is important to consider any conditions that impair the immune system, such as AIDS, diabetes mellitus, chronic corticosteroid therapy, or chemotherapy. Patients should be questioned about risk factors for HIV infection and appropriately tested as needed. Masticator space infections can have very aggressive behavior in the face of immunosuppression.
Examination
General. The patient is a thin and unkempt-appearing man with a noticeable pungent odor (indicative of neglect to health and hygiene). The patient is not in respiratory distress (it is important to assess the need for advanced airway intervention immediately upon examination). He appears anxious, sitting up holding an emesis basin to catch his secretions as they drool from his mouth (difficulty maintaining secretions).
Vital signs. His blood pressure is 104/68 mm Hg (hypotension secondary to dehydration), heart rate 116 bpm (tachycardia secondary to hypotension and fever), respirations 20 per minute, and temperature 39.2°C (febrile), with an oxygen saturation of 98% on room air.
Maxillofacial. There is significant swelling and induration of the left side extending from the level of the hyoid bone anterior to the sternocleidomastoid to the zygomatic arch. Cranial nerves II through XII are grossly intact. The pupils are equal, round, and reactive to light and accommodation (PERRLA), with no proptosis or ptosis of the eyelid (these would be suggestive of cavernous sinus involvement).
Intraoral. Maximal interincisal opening is 17 mm (trismus) (Figure 4-8, A). The floor of the mouth is soft (sublingual space not involved). The patient is able to protrude his tongue past the vermillion-cutaneous border of the upper lip (the ability to protrude the tongue past the vermilion border of the upper lip is a reliable sign that the sublingual space is not severely involved). There is significant fluctuant swelling of the left oropharynx toward the right tonsillar area, with the tip of the uvula touching the right pharyngeal wall (Figure 4-8, B). The operculum overlying the partially bony impacted left mandibular third molar is edematous, erythematous, and tender to palpation, with no obvious purulent discharge (mandibular third molars are a common cause of lateral pharyngeal infections). Mucous membranes of the buccal mucosa are dry (secondary to dehydration).
Imaging
Before any further diagnostic imaging, the treating surgeon must decide whether the patient (and the airway) is stable enough for obtaining further studies, or arrangements should be made to proceed directly to the operating room and establish a secure airway (endotracheal or nasotracheal intubation, tracheostomy, cricothyrotomy). Any possibility of acute respiratory obstruction should prompt the surgeon to proceed directly to the operating room. Imaging studies can be safely obtained to guide further treatment at a later time.
When available, a panoramic radiograph is an important imaging study for evaluation of suspected odontogenic infections. It provides an excellent overview of the mandible and maxilla and serves as a screening tool for evaluation of the dentition. Also, in patients with trismus, other dental radiographs may be difficult to obtain. Because mandibular third molars are the most common odontogenic cause of parapharyngeal space infections, this radiograph becomes necessary to evaluate the third molars. In addition, it delineates the relationship to adjacent structures, such as the inferior alveolar canal, and other possible bony pathology.
The combination of contrast-enhanced CT scans and clinical examination has the highest sensitivity and specificity in the diagnosis of deep neck infections. The use of contrast improves the ability to identify the hyperemic capsule of a longstanding abscess (abscesses are seen as discrete, hypodense areas that show an enhancing peripheral rim with use of intravenous contrast material). In general, most radiologists interpret hypodense areas without ring enhancement to represent cellulitis or edema. However, studies have shown that, when drained, approximately 45% of hypodense areas without ring enhancement yield pus. In a study by Miller and associates, a hypodense area of greater than 2 ml without ring enhancement yielded purulence at the time of surgery. In the same study, CT scans were able to correctly differentiate cellulitis from an abscess in 85% of deep neck space (lateral and retropharyngeal) infections.
CT also provides important information regarding the details of adjacent anatomic structures, such as the integrity of the airway, tracheal deviation, and the proximity of vascular structures (the carotid sheath). Airway deviation and the risk of rupture of the pharyngeal abscess during intubation are important factors in determining the choice of technique to secure the airway.
Magnetic resonance imaging (MRI) is also a useful imaging modality for soft tissue evaluation. Compared with CT, advantages of MRI include superior anatomic multiplanar display, high soft tissue contrast, fewer artifacts from dental amalgam, and lack of ionizing radiation. However, MRI is difficult and slower to perform on an emergency basis and is more costly, and claustrophobia may preclude examination in some patients. MRI, when possible, has been shown to be superior in the assessment of deep neck infections.
Ultrasonography has shown some benefit in differentiating cellulitis from an abscess in superficial locations, but the use of this modality as a sole imaging technique for deep neck infection is in its infancy. The ultrasound probe can be placed intraorally, although in the setting of an acute infection and trismus, this can be difficult. An abscess is seen as an echo-free cavity with an irregular, well-defined circumference.
In the current patient, the airway appeared clinically stable, and a panoramic radiograph demonstrated a carious and partially bony impacted left mandibular third molar. A CT scan with contrast demonstrated significant swelling of the lateral pharyngeal area and deviation of the airway (Figure 4-9). Large rim-enhancing hypodense areas consistent with pus are seen on the left lateral masticator and lateral pharyngeal (anterior compartment) spaces.
Labs
A CBC and a basic metabolic panel should be obtained during the initial evaluation of deep neck space infections. The WBC count is an indicator of the severity of the systemic response to the infection and can be obtained periodically to monitor the progression of infection (caution should be exercised in interpretation of this value in a patient who is at high risk for undiagnosed AIDS, because the WBC count may appear within the normal range secondary to the inability to mount an adequate immune response).
The serum creatinine and BUN levels should be obtained before contrast material is used for imaging. Contrast material has been known to cause contrast-associated nephropathy. The condition is defined as an increase in serum creatinine greater than 25% from baseline or a rise greater than 0.5 mg/dl within 48 hours of contrast exposure, in the absence of other causes. Risk factors for the development of contrast-associated nephropathy are summarized in Box 4-1. In the presence of risk factors, renal function should be carefully monitored, and a baseline serum creatinine obtained before and within 48 to 72 hours after the procedure.
The WBC count for the current patient was 18,500 cells/mm3; the differential included 80% polymorphonucleocytes with a shift to the left (indicative of an acute inflammatory process).
Serum chemistries showed a sodium level of 150 mEq/dl (hypovolemic hypernatremia due to dehydration), BUN of 48 mg/dl, and creatinine of 1.1 mg/dl (prerenal azotemia consistent with dehydration).
Assessment
Deep neck infection involving the anterior compartment of the left LPS with significant upper airway deviation and edema, and left medial and lateral masticator space infections secondary to an impacted mandibular third molar, complicated by dehydration and potential onset of sepsis.
Treatment
Successful treatment of fascial space infections should include the following:
• Surgical drainage of an abscess or, in select cases, drainage of cellulitis
• Identification and removal of the source of infection (the tooth, in cases of odontogenic etiology)
• Administration of antibiotics (guided by culture and sensitivity when possible)
Antimicrobial therapy can abort abscess formation if administered at an early stage of infection. However, once an abscess has formed, antimicrobial therapy is more effective in conjunction with adequate surgical drainage.
Impending airway obstruction may require immediate airway management (see Ludwig's Angina, earlier in this chapter, and Emergent Surgical Airway in Chapter 3). Maintaining spontaneous ventilation and airway patency is critical in patients with a compromised airway. Even a small dose of a respiratory depressant may change an apparently controlled situation into an emergent one, especially in the presence of a fatiguing patient. Morbidity or death due to the loss of an airway is still reported. Available options include endotracheal intubation versus establishment of a surgical airway. The advantages and disadvantages of these methods are summarized in Table 4-1. Consideration should be given to endotracheal intubation using an awake fiberoptic technique. This requires a skilled anesthesiologist and patient cooperation and can be time-consuming.
Table 4-1
Intubation Versus Surgical Airway
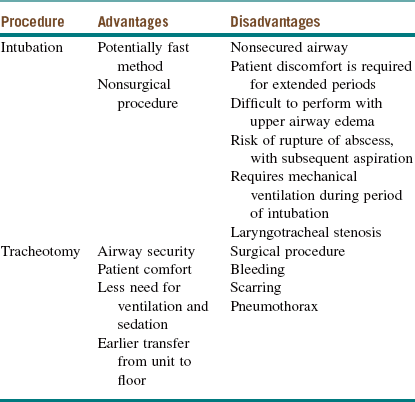
From Potter JK: Tracheotomy versus endotracheal intubation for airway management in deep neck space infections, J Oral Maxillofac Surg 60:349-354, 2002.
Regardless of the airway technique used, caution should be exercised to prevent rupture of the abscess during intubation, which can result in aspiration of purulent material and is associated with significant morbidity (aspiration pneumonitis, pneumonia, lung abscess, acute respiratory distress syndrome) and mortality. One useful technique is to aspirate the LPS before any intubation attempts. This can be done in the operating room under local anesthesia. The abscess can be decompressed significantly, thereby reducing the risk of aspiration during intubation.
The anterior compartment can be approached intraorally via an incision over the pterygomandibular raphe, with blunt dissection around the medial side to enter the LPS. The extraoral approach is accomplished by making a 1- to 2-cm incision approximately two finger-breadths inferior to the mandible; dissection is then carried through the platysma to the superficial layer of the deep cervical fascia. Sufficient fascia is exposed to identify the submandibular gland and the posterior belly of the digastric muscle. Dissection is then carried just posterior to the posterior belly of the digastric muscle in a superior, medial, and posterior direction into the LPS. If finger dissection is also used, the surgeon will be able to palpate the endotracheal tube medially and the carotid sheath posterolaterally. Through-and-through intraoral-extraoral drainage can be obtained by combining the intraoral approach with the extraoral approach. If old clots are found or if any signs of carotid sheath involvement are present, then vertical extension of the incision can be made along the anterior border of the sternocleidomastoid muscle. This extension allows the carotid artery to be pulled anteriorly and controlled as necessary.
The extraoral incision should parallel the lines of relaxed skin tension and lie in a cosmetically acceptable site whenever possible. The incision should also be supported by healthy underlying dermis and subcutaneous tissue. Placement of drains should allow for gravity-dependent drainage. A rigid drain should not be placed into the LPS because of the potential for erosion into the carotid sheath.
Supportive care to ensure adequate hydration, caloric intake, and analgesia is also important. It is reported that minimum daily fluid requirements increase by 300 ml per degree of fever (1°C) per day. Caloric requirements also increase by approximately 5% to 8% per degree of fever per day.
Studies have shown that gram-positive cocci and gram-negative rods have the greatest growth percentage in cultures from deep neck space infections of odontogenic origin. It should be noted that some microbiologists estimate that only 50% of the bacteria that comprise oral flora can be cultured in the laboratory. Additionally, it is believed that the majority of human infections are caused by bacteria in biofilms (a complex, usually multispecies, highly communicative community of bacteria that is surrounded by a polymer matrix). Bacteria present in biofilms are difficulty to culture with traditional methods. Future trends indicate that rather than relying on traditional culture and sensitivity testing, DNA analysis may be used for identification. Until strategies for the prevention of biofilm formation and disruption of existing biofilms are developed, surgical therapy is still necessary. Due to the rising incidence of penicillin resistance and failure of penicillin therapy, many clinicians advocate the empiric use of clindamycin (in a penicillin-allergic patient) or a combination of a β-lactam with penicillinase inhibitor (e.g., ampicillin/sulbactam) for deep neck space infections of odontogenic origin until an antibiogram is obtained. Clindamycin has the disadvantage of not covering Eikenella corrodens. If E. corrodens has been cultured, moxifloxacin is an excellent choice.
The current patient was given a bolus of normal saline and was taken urgently to the operating room. The anesthesiologist was informed about the parapharyngeal space involvement, and the anesthesiologist and surgeon agreed on a plan for airway management. Before any attempts at intubation, 6 ml of lidocaine was injected on the mucosa of the oropharynx superficially; subsequently, 35 ml of purulent material was evacuated, allowing decompression of the swelling (Figure 4-10). Subsequently, the patient was placed in the supine position, and anatomic landmarks were marked on the neck for a tracheotomy or cricothyroidotomy. The surgeon and operating room personnel were positioned and prepared for an emergent surgical airway, should the need arise. The anesthesiologist successfully intubated the patient using an awake fiberoptic nasal intubation technique. With a large-bore needle, the LPS was further aspirated and the material was sent for culture and sensitivity. The left mandibular third molar was extracted. The left medial and lateral masticator and the LPS were explored and drained via an intraoral and extraoral approach. A red rubber catheter was secured into the medial and lateral masticator spaces, and a Penrose drain was secured in the LPS. The patient was started on ampicillin/sulbactam 3 g intravenously every 6 hours. He remained intubated postoperatively and was transferred to the ICU. On the night of his surgery, he was weaned to minimal ventilator settings. He was awake and alert and in no apparent distress, with a Glasgow Coma Scale score of 11T.
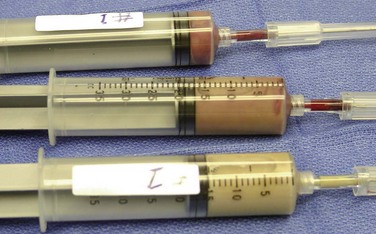
Figure 4-10 Samples of the aspirate to be sent for Gram stain and aerobic and anaerobic culture and sensitivity studies.
The wound care regimen included meticulous irrigation of the drains. On postoperative day 1, the patient's WBC count decreased to 13,000 cells/mm3 (it is not uncommon for the WBC count to increase immediately after surgery due to demargination), and there was a notable decrease in pharyngeal and facial edema (it is not uncommon for surgical edema and fluid resuscitation to worsen the preexisting edema). A Gram stain revealed the presence of gram-positive cocci in pairs and chains (Streptococcus species) and gram-negative rods (mixed infection). On the second postoperative day, the WBC count decreased to 10,200 cells/mm3 with a significant decrease in edema and return of the uvula to midline. All sedative medications were discontinued, and the patient was extubated after passing a cuff leak test. He was subsequently transferred to the ward and discharged to home care with oral antibiotics after 5 days of wound care and intravenous medications. At discharge there was no significant drainage, and all drains were removed. He was given instructions for jaw range-of-motion exercises and a follow-up appointment.
Complications
Complications of masticator space infections are partially dependent on the severity of the presenting infection, the status of the host immune system, the virulence and resistance patterns of the infecting bacteria, and the time of presentation. Complications can be major, ranging from unsightly scars from incisions for drainage or tracheostomy to death from airway embarrassment.
Infections that have gained entry into the LPS may erode into the carotid sheath or impair any of the nerves found in the posterior compartment. Signs that indicate possible carotid sheath involvement include the following:
• Ipsilateral Horner's syndrome (ptosis, miosis, anhidrosis)
• Unexplained palsies of cranial nerves IX through XII
• Recurrent small hemorrhages from the nose, mouth, or ear (herald bleeds)
• Hematoma in the surrounding tissue
• Persistent peritonsillar swelling despite adequate drainage
Any signs of carotid sheath involvement warrant immediate radiologic evaluation, CT, or CT angiography. Surgical exploration and control of the great vessels may be required.
Involvement of the cranial nerves (vagus and glossopharyngeal nerves) can result in sudden death from bradycardia, asystole, and cardiac arrhythmia. Involvement of the retropharyngeal space can lead to descending infection involving the mediastinum. Erythema over the upper chest is suggestive of descending infection and may require cardiothoracic consultation.
Of particular concern are infections that do not appropriately respond to treatment. Consideration should be given to inadequate drainage or resistant bacterial strains. Culture and sensitivity studies can be obtained on purulent aspirates to guide antimicrobial therapy.
Discussion
The LPS has the shape of an inverted pyramid or cone, the base of which is the sphenoid and the apex is the hyoid bone. The boundaries of this space are summarized in Table 4-2.
Table 4-2
Boundaries of the Lateral Pharyngeal Space
| Space | Boundary |
| Anterior | Pterygomandibular raphe (junction of buccinators and superior constrictor muscles) |
| Posterior | Prevertebral fascia that communicates with the retropharyngeal space |
| Medial | Buccopharyngeal fascia on lateral surface of the superior constrictor muscle |
| Lateral | Fascia over the medial masticator, the parotid gland, and mandible |
The LPS is divided by the styloid process and its muscles into an anterior and a posterior compartment. The anterior compartment contains only fat, muscle, connective tissue, and lymph nodes. The posterior compartment contains the glossopharyngeal, spinal accessory, and hypoglossal nerves. It also contains the carotid sheath (the carotid artery, internal jugular vein, and vagus nerve; the cervical sympathetic trunk lies posterior and medial to the carotid sheath). A strong fascial plane, the stylopharyngeal aponeurosis of Zuckerkandl and Testut, separates the anterior and posterior compartments. It is a barrier that helps prevent the spread of infection from the anterior to the posterior compartment.
Lateral pharyngeal infections can be caused by tonsillitis, otitis media, mastoiditis, or parotitis; most commonly they occur secondary to an odontogenic pathology. Involvement of the LPS can also occur via spread through lymphatic vessels and subsequent rupture of a node. Lymphatic drainage from the nose and paranasal sinuses, ear, or oral cavity can involve this area. Infection can also spread from retropharyngeal, sublingual, submandibular, or masticator space infections. Peritonsillar abscesses that rupture through the superior constrictor muscle can also cause entry and infection of the LPS directly.
Symptoms of LPS involvement vary according to whether the anterior or posterior compartment is involved. The four most common signs of involvement of the anterior compartment are:
2. Induration or swelling at the angle of the jaw
3. Pharyngeal bulging with or without deviation of the uvula
Deviation of the uvula with bulging of the pharyngeal wall can also be seen with peritonsillar abscesses; however, trismus is usually absent. With LPS infections, trismus is seen secondary to involvement of the adjacent medial pterygoid muscle. It can be difficult to differentiate a pterygomandibular space abscess from an LPS infection, but this distinction may be of academic interest only, because treatment would be similar. Involvement of the posterior compartment may show posterior tonsillar deviation and retropharyngeal bulging. In this scenario, palsies of cranial nerves IX through XII may be seen, in addition to ipsilateral Horner's syndrome (ipsilateral blepharoptosis, pupillary miosis, and facial anhidrosis). A common sign of LPS involvement is the presence of swelling of the lateral neck just above the hyoid and just anterior to the sternocleidomastoid muscle. This is the point at which the LPS is closest to the skin and where dependent edema or exudate is constrained by binding of the fascial layers to the hyoid bone.
Significant upper airway edema may require the patient to remain in an upright position, because assuming the supine position may lead to airway obstruction. Also, depending on the severity of the obstruction, patients may present with breathing with the mouth open or in the “sniffing position,” with extension of the neck, stridor, labored breathing, intercostal retractions, tracheal tug, sore throat, or globus. Changes in voice also provide a clue to the location of airway involvement. A muffled, or “hot potato,” voice usually signifies a supraglottic process, whereas hoarseness is a sign of vocal cord involvement.
Brook, I. Microbiology and management of peritonsillar, retropharyngeal and parapharyngeal abscesses. J Oral Maxillofac Surg. 2004; 62:1545–1550.
Dzyak, W, Zide, MF. Diagnosis and treatment of lateral pharyngeal space infections. J Oral Maxillofac Surg. 1984; 42:243–249.
Flynn, T. What are the antibiotics of choice for odontogenic infections and how long should the treatment course last? Oral Maxillofacial Surg Clin North Am. 2011; 23:519.
Flynn, TR. Surgical management of orofacial infection. Atlas Oral Maxillofac Surg Clin North Am. 2000; 8:77–99.
Flynn, TR, Shanti, RM, Levi, MH, et al. Severe odontogenic infections. Part 1. Prospective report. J Oral Maxillofac Surg. 2006; 64:1093–1103.
Hollinshead, W. Anatomy for surgeons. Philadelphia: Lippincott-Raven; 1982.
Miller, WD, Furst, IM, Sandor, GK, et al. A prospective blinded comparison of clinical examination and computed tomography in deep neck infections. Laryngoscope. 1999; 109:1873–1879.
Munoz, A. Acute neck infection: prospective comparison between CT and MRI in 47 patients. J Comput Assist Tomogr. 2001; 25:733–741.
O'Grady, NP, Barie, PS, Bartlett, JG, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008; 36(4):1330–1349.
Potter, JK. Tracheotomy versus endotracheal intubation for airway management in deep neck space infections. J Oral Maxillofac Surg. 2002; 60:349–354.
Ray, JM, Triplett, RG. What is the role of biofilms in severe head and neck infections? Oral Maxillofac Surg Clin North Am. 2011; 23:497.
Rega, AJ. Microbiology and antibiotic sensitivities of deep neck space infections. J Oral Maxillofac Surg. 2004; 62:25–26.
Soma, VR. Contrast-associated nephropathy. Heart Dis. 2002; 4:372–379.
Storoe, W. The changing face of odontogenic infections. J Oral Maxillofac Surg. 2001; 59:739–748.
Styer, TE. Peritonsillar abscess: diagnosis and treatment. Am Fam Physician. 2002; 65:95.
Osteomyelitis
CC
A 49-year-old man returns to your office 3 months after extraction of his mandibular third molars complaining that, “I still have some swelling and drainage from my mouth.” (Osteomyelitis is more common in the mandible than in the maxilla due to the relatively lower blood supply).
HPI
The patient presents with a history of persistent pain and swelling on the left side of his face, which has increased during the past month. He had four full bony impacted third molars removed 3 months ago without any acute complications. He returned 2 weeks after surgery for follow-up with a complaint of tenderness of the lower left extraction socket. The socket was noted to be filled with debris but without purulence and was irrigated clear. Instructions for follow-up were given, but the patient did not return for reevaluation. He now returns 3 months later due to the increasing pain, swelling, and some drainage from the left lower socket. He has not seen any other doctors and has not been on any antibiotics. He denies having fever or chills, difficulty swallowing (dysphagia), or difficulty talking (dysphonia).
PMHX/Medications/Allergies/SH/FH
Noncontributory. The patient has no risk factors for osteomyelitis.
Although osteomyelitis has a higher incidence in patients who are immunocompromised (diabetes, HIV/AIDS, chemotherapy), intravenous drug users, patients with compromised splenic function or splenectomy, patient undergoing radiation therapy, or patients who use tobacco, it can occur in patients with no risk factors.
The macrophages in the reticuloendothelial system of the spleen are involved in sequestration of encapsulated organisms (e.g., Haemophilus influenzae, Streptococcus pneumoniae, and Salmonella and Klebsiella species); therefore, an absent spleen or a compromised splenic function is a risk factor for osteomyelitis secondary to these organisms. Patients with sickle cell anemia are at risk because during a sickle cell crisis, the splenic reticuloendothelial system is overwhelmed and becomes “clogged” by sickled red blood cells, which can lead to splenic infraction or abscess requiring a splenectomy. Also, chronic splenomegaly in these patients can lead to splenic atrophy and eventual “autosplenectomy.” A history of radiation therapy or bisphosphonate use should also be noted, to rule out possible osteoradionecrosis (ORN) or bisphosphonate-related osteonecrosis of the jaws.
Examination
General. The patient is a well-developed and well-nourished man in no apparent distress.
Vital signs. His blood pressure is 132/81 mm Hg, heart rate 80 bpm, respirations 16 per minute, and temperature 37.1°C (afebrile).
Maxillofacial. There is mild left facial swelling, erythema, and tenderness at the inferior border of the mandible. No fistulous tract is present (Actinomyces israelii infections commonly cause cutaneous fistulas). The lymph nodes are palpable in the left submandibular area (secondary to chronic infection). The inferior alveolar nerve is intact bilaterally (altered sensation can be commonly seen in osteomyelitis of the mandible).
Intraoral. The maximal interincisal opening is 25 mm (decreased due to guarding secondary to pain). The left retromolar pad is tender to palpation, with moderate erythema and swelling. There is a small draining fistula distal to the left mandibular second molar in the attached gingiva. The oropharynx is clear, the uvula is midline, and the floor of the mouth is soft and nonelevated. The dentition is in good repair. The occlusion is stable and reproducible (occlusal change would be suggestive of a pathologic fracture).
Imaging
Several imaging modalities are available for the evaluation of suspected osteomyelitis. A panoramic radiograph is the initial diagnostic study of choice when dealing with postextraction complications (e.g., retained tooth fragments, local wound infections, mandibular fractures, foreign bodies, bony sequestra, and pathology of adjacent teeth) or osteomyelitis. The destructive process of osteomyelitis must extend at least 1 cm and demonstrate 30% to 50% bone demineralization before it becomes radiographically apparent. The radiograph would show a loss of definition and lytic changes within the trabecular patterns of the involved bone. Eventually, radiopaque sequestra (fragments of bone that have become devitalized) may become visible, demonstrating a “mottled” radiolucent appearance.
Two-dimensional CT with contrast material is useful for visualizing soft tissue abnormalities, such as the presence of an abscess, and for assessing bony integrity or cortical disruptions. MRI can be useful for the evaluation of osteomyelitis due to the superior imaging of soft tissue, defining the extent and location of osteomyelitis. However, MRI has limited ability to discriminate edema from outright infection and can result in nonspecific findings in posttraumatic and postsurgical patients. Nuclear medicine imaging modalities can detect osteomyelitis 10 to 14 days earlier than conventional radiographic imaging. Scintigraphy with technetium 99m scanning is very sensitive in detecting increased bone turnover, and its specificity in detecting osteomyelitis is improved with the use of gallium 67 or indium 111.
In the current patient, a panoramic radiograph (Figure 4-11) reveals a mottled radiolucent appearance in the area of the extracted left mandibular third molar that extends to the inferior border of the mandible. There is evidence of reactive extracortical bone formation at the inferior border of the mandible. The proximal segment is not rotated superiorly (a rotated proximal segment would indicate a pathologic fracture).
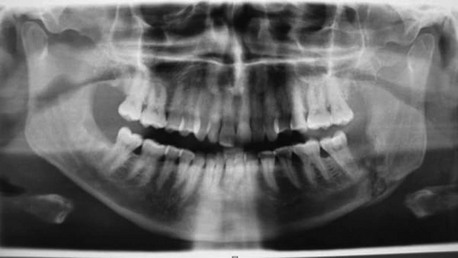
Figure 4-11 Panoramic radiograph showing mottled radiolucency at the extraction socket extending down to the inferior border. Extracortical reactive bone formation is seen at the inferior border. The proximal segment is not rotated or telescoped, suggesting that a pathologic fracture has not occurred.
Labs
A CBC is necessary to evaluate the initial WBC count and to monitor the response to surgical and antibiotic therapy (the WBC count may be elevated in acute or subacute osteomyelitis). The current patient had a WBC count of 15,000 cells/mm3 (elevated).
Assessment
Acute suppurative osteomyelitis of the left mandible.
Although a 3-month duration may indicate a chronic state, acute suppurative osteomyelitis is the corrected diagnosis based on the fact that this disease process has not yet been managed either medically or surgically. Suppuration indicates that the body has mounted an immune response to the infection.
Several classification systems have been proposed, but no one scheme has gained acceptance. In the simplest form, osteomyelitis can be classified as acute or chronic. It is further characterized as suppurative or nonsuppurative. Waldvogel and associates proposed the staging of osteomyelitis into three separate groups based on the etiology: osteomyelitis related to a hematogenous source, secondary to a contiguous focus of infection, or associated with vascular insufficiency. The Cierny-Mader staging system is the most commonly used classification system for long-bone osteomyelitis. It divides the disease into four stages of osseous involvement and then combines it with three physiologic host categories, resulting in 12 discrete clinical stages. In addition, chronic diffuse sclerosing osteomyelitis is a unique form of osteomyelitis that is described as a painful disease state that occurs only in the mandible and is more often seen in younger female patients.
Treatment
Osteomyelitis is treated with a combination of surgical and medical interventions. Nonsurgical management of true osteomyelitis is futile and only allows for exacerbation of the existing condition and delay of more extensive surgical interventions.
If feasible, empiric antibiotic therapy should not be initiated until cultures are taken (purulent exudate and/or bone cultures) to accurately identify the causative organism or organisms. A bone culture has higher bacterial counts than swabs or purulent drainage and is preferable for identification of the causative organism or organisms. Specimens should be sent to the laboratory as soon as possible, especially to prevent the loss of anaerobic species, which can happen in as little as 15 minutes. Osteomyelitis caused by a contiguous focus of infection is often polymicrobial; therefore, one or more empiric broad-spectrum antibiotics are initiated (more than 90% of cases of osteomyelitis of the jaws have a polymicrobial ethology). Once culture and sensitivity results are available, antibiotic regimens should be adjusted to target the causative organism or organisms. The course and route (intravenous or oral) of antibiotic therapy are debatable and are frequently determined by clinical judgment. It has been generally accepted that a 4-week course of intravenous antibiotics is necessary, especially when dealing with chronic or refractory osteomyelitis. However, earlier stages of osteomyelitis may require only a short course of intravenous antibiotics (if needed), followed by a 1- to 2-week course of oral antibiotics based on serial clinical examinations. Consultation with an infectious diseases specialist may be prudent to assist in effective antibiotic therapy.
Penicillin remains the empiric antibiotic of choice for osteomyelitis of tooth-bearing bone. However, many organisms responsible for osteomyelitis of the jaws are penicillin resistant, including Prevotella, Porphyromonas, Staphylococcus, and Fusobacterium spp. Therefore, it may be prudent to use a penicillin combined with a β-lactamase inhibitor (clavulanate) in combination with metronidazole (Flagyl) to improve anaerobic coverage. In patients who are allergic to β-lactam antibiotics, clindamycin is recommended due to its effectiveness against penicillinase-producing staphylococci, streptococci, and anaerobic bacteria. However, clindamycin is ineffective against Eikenella corrodens (a common organism in osteomyelitis and odontogenic infections), for which additional coverage is required. It is important to recognize that anaerobic organisms are difficult to culture, resulting in frequent false-negative anaerobic growth (therefore empiric anaerobic coverage may be prudent despite a negative anaerobic culture).
Surgical management of osteomyelitis should be planned in conjunction with medical treatment. In the early stages, surgical interventions should be limited to extraction of grossly loose teeth, debridement of fragments of bone, and incision and drainage of fluctuant areas. If the infection persists, further surgical procedures, such as sequestrectomy, saucerization, decortication, or resection, followed by reconstruction, should be considered.
Sequestra are devitalized pieces of bone, which act as a nidus of infection. Sequestra are eventually resorbed, removed, or spontaneously expelled through the mucosa or skin, but they may persist and propagate a host response. Due to the avascular nature of necrotic sequestra, they are poorly penetrated by antibiotics and should be removed with minimal trauma, intraorally and/or transcutaneously. Saucerization is the “unroofing” of bone to expose the medullary cavity. The buccal cortex of the mandible is removed until bleeding bone is encountered at all margins of the surgical defect, thus producing a saucerlike defect. The purpose of this is to decompress the bone to allow extrusion of pus, debris, and any bony sequestra. The defect can be packed with a medicated dressing, changed several times over subsequent days, and irrigated daily to ensure that no fragments of necrotic bone are left behind as the wound heals by secondary intention (or is simply left open for irrigation). The lingual aspect of the mandible rarely requires reduction, because it has a rich blood supply from the mylohyoid muscle. In the maxilla, saucerization is rarely needed, because the cortex is thin and formed sequestra can be easily removed. Decortication of the mandible refers to the removal of chronically infected cortical bone, which is usually extended 1 to 2 cm beyond the affected area. Corticocancellous bone grafting of osteomyelitis has traditionally not been advocated, but there is some recent evidence suggesting the feasibility of immediate bone grafting along with stabilization of the mandible.
Refractory cases or patients with a pathologic mandibular fracture (Figure 4-12) require resection of the diseased bone extending to a bleeding bone margin. Stabilization of the segments is accomplished with immediate placement of rigid internal fixation plates or, in rare cases, a Joe Hall Morris biphasic external fixator. After resolution of the infection, bony reconstruction can be completed with corticocancellous bone grafts or vascularized free flaps. Hyperbaric oxygen has been shown to be effective in treating osteomyelitis and should be considered in severe or refractory cases. Dental rehabilitation can be achieved after adequate bone and healing and reconstruction.
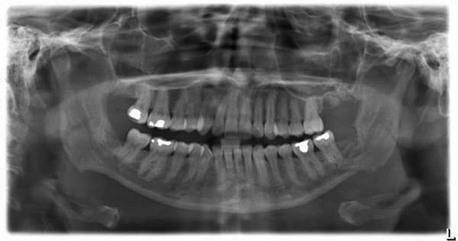
Figure 4-12 Panoramic radiograph of another patient with osteomyelitis of the left mandible after extraction of a third molar. Note that there is a pathologic fracture of the left mandible, with the proximal segment telescoped with the distal segment and rotated superiorly.
The current patient underwent a bone culture (antibiotic therapy was not initiated until cultures had been obtained) and local debridement via an intraoral approach. Intraoperatively, it was noted that the buccal and lingual cortices were intact. There were multiple loose fragments of devitalized bone (sequestra) and abundant granulation tissue tracking down to the inferior border of the mandible. After the wound had been debrided, a round burr was used to remove the surrounding affected bone until normal-appearing, bleeding bone was encountered. The patient was started on empiric intravenous antibiotics (ampicillin/sulbactam and metronidazole) and remained hospitalized until the initial culture and sensitivity results were available. Cultures demonstrated growth of Streptococcus anginosis that was pansensitive, with negative anaerobic cultures. The patient was discharged home on oral amoxicillin with clavulanic acid and metronidazole, in addition to chlorhexidine mouthwash. He was kept on a liquid diet until soft tissue healing occurred to prevent retention of food debris in the wound. He was subsequently switched to a soft diet for 8 weeks after surgery to prevent a postoperative pathologic fracture. Final cultures did not grow any additional organisms; therefore, the antibiotic regimen was not changed during the course of his treatment. He healed without any complications.
Complications
Osteomyelitis is a complication of various oral and maxillofacial surgical procedures, and its incidence is related to both host factors and the virulence of the infecting organisms. Treatment of osteomyelitis can itself be compounded by further complications. These include a persistent refractory infection of the bone, pathologic fractures (at presentation, intraoperatively, or postoperatively), the need for resection of chronically infected bone, disfigurement, neurosensory impairment, and systemic spread of the infection.
Antibiotic therapy is associated with side effects such as diarrhea and Clostridium difficile pseudomembranous colitis, in addition to the emergence of resistant microorganisms. Methicillin-resistant Staphylococcus aureus (MRSA) has become a very common community-acquired organism in many communities. MRSA osteomyelitis requires intravenous vancomycin until sensitivity profiles (antibiogram) are available. If the organism is sensitive to only vancomycin, a peripherally inserted central catheter (PICC) line can be used to allow delivery of intravenous antibiotics at home. Local antibiotic delivery systems have been used in an attempt to obtaining constant, higher local drug concentrations. The gentamycin-polymethylmethacrylate delivery system was introduced in the late 1970s. Despite some benefits, multiple shortcomings, including the need for additional surgery for removal, have limited its use. New research is focusing on delivery systems using reabsorbable carriers (polylactic acid, polyglycolic acid, and collagen sponges).
Uncontrolled diabetes mellitus presents a special challenge. Tight glycemic control (blood glucose 90 to 110 mg/dl) is paramount for recovery from any infection. Noncompliant patients may require extended hospitalization to achieve glycemic control and delivery of intravenous antibiotics. Some patients may require an insulin pump, and critically ill patients may require an insulin drip. Sliding scale insulin is generally not regarded as adequate treatment, because it is designed to treat hyperglycemia rather than to prevent it (therefore, it is always one step behind the hyperglycemia). Baseline insulin regimens need to be adjusted to prevent hyperglycemic episodes.
Pathologic fractures of the mandible (see Figure 4-12) occur with sufficient destruction of cortical bone, primarily due to osteolytic changes associated with osteomyelitis and/or the result of periosteal stripping during an aggressive debridement (at 2 to 3 weeks postdebridement, the mandible is at greatest risk of fracture due to the critical phase of remodeling/resorption). Maxillomandibular fixation (6 to 8 weeks) should be considered for patients at higher risk for postoperative pathologic fractures. A liquid or soft diet is prudent for those at moderate risk.
Discussion
Osteomyelitis is an inflammatory condition involving the medullary cavity of bone. It begins as a bacterial infection and can cause significant bony destruction. The microorganisms cause host tissue injury from direct cellular attack and through enzymatic degradation. The host responds by recruiting neutrophils to the area to digest the pathogens via release of enzymes and phagocytosis. When purulence (composed of necrotic tissue, dead bacteria, and WBCs) accumulates, it causes an increase in the intramedullary pressure, causing collapse of the vessels, venous stasis, and congestion. Vessels in the haversian system and Volkmann's canals of the cortical bone may undergo thrombosis or experience stasis and congestion; as a result, the surrounding bone becomes ischemic, thereby permitting the extension of osteomyelitis. If purulence continues to accumulate, the periosteum is penetrated and mucosal or cutaneous fistulas develop.
The establishment of an infection in bone is related to the compromise in the bone's vascular supply. However, other factors (e.g., the virulence of the organism and the integrity of the host's defenses, diabetes), blood dyscrasias (e.g., sickle cell disease), immunosuppression (e.g., HIV infection, posttransplant patients), and collagen vascular disorders or bone dysplasias (e.g., osteopetrosis) are also important.
Initially, S. aureus and Staphylococcus epidermidis accounted for 80% to 90% of cases of osteomyelitis of the jaws. However, the frequency of S. aureus involvement in osteomyelitis has decreased due to the improved culture methods used to identify organisms, especially anaerobes. Anaerobes are frequently associated with aerobic organisms in osteomyelitis. Currently, osteomyelitis is recognized as a disease commonly caused by streptococci (β-hemolytic) and oral anaerobes such as Peptostreptococcus, Fusobacterium, and Prevotella spp.
Adekeye, EO, Cornah, J. Osteomyelitis of the jaws: a review of 141 cases. Br J Oral Maxillofac Surg. 1985; 23:24–35.
Benson, PD, Marshall, MK, Engelstad, ME, et al. The use of immediate bone grafting in reconstruction of clinically infected mandibular fractures: bone grafts in the presence of pus. J Oral Maxillofac Surg. 2006; 64:122–126.
Cieny, G, Mader, JT, Pennick, H. A clinical staging system of adult osteomyelitis. Contemp Orthop. 1985; 10:17–37.
Cohen, MA, Embil, JM, Canosa, T. Osteomyelitis of the maxilla caused by methicillin-resistant Staphylococcus aureus. J Oral Maxillofac Surg. 2003; 61:387–390.
Flynn, TR. Anatomy and surgery of deep space infections of the head and neck. In: Knowledge updates. Rosemont, Ill: the Association; 1993.
Lew, DP, Waldvogel, FA. Osteomyelitis. N Engl J Med. 1997; 336:999–1007.
Marx, RE. Chronic osteomyelitis of the jaws. Oral Maxillofac Clin North Am. 1991; 3:367–381.
Marx, RE, Carlson, EC, Smith, BR, et al. Isolation of Actinomyces species and Eikenella corrodens from patients with chronic diffuse sclerosing osteomyelitis. J Oral Maxillofac Surg. 1994; 52:26–33.
Mercuri, LG. Acute osteomyelitis. Oral Maxillofac Surg Clin North Am. 1991; 3:355.
Paluska, S. Osteomyelitis. Clin Fam Pract. 2004; 6:127–156.
Peterson, LJ. Microbiology of head and neck infections. Oral Maxillofac Surg Clin North Am. 1991; 3:247.
Vibhagool, A, Calhoun, J, Mader, JP, et al. Therapy of bone and joint infections. Hosp Formul. 1993; 28:63.
Waldvogel, FA, Medoff, G, Swartz, MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970; 282:316–322.
*The authors and publisher wish to acknowledge Jaspal Girn, DMD, for his contributions to previous editions on this topic.