Nervous Regulation of the Circulation and Rapid Control of Arterial Pressure
Nervous Regulation of the Circulation
As discussed in Chapter 17, adjustment of blood flow in the tissues and organs of the body is mainly the function of local tissue control mechanisms. In this chapter we discuss how nervous control of the circulation has more global functions, such as redistributing blood flow to different areas of the body, increasing or decreasing pumping activity by the heart, and providing rapid control of systemic arterial pressure.
The nervous system controls the circulation almost entirely through the autonomic nervous system. The total function of this system is presented in Chapter 61, and this subject was also introduced in Chapter 17. In this chapter we consider additional specific anatomical and functional characteristics.
Autonomic Nervous System
By far the most important part of the autonomic nervous system for regulating the circulation is the sympathetic nervous system. The parasympathetic nervous system, however, contributes importantly to regulation of heart function, as described later in the chapter.
Sympathetic Nervous System.
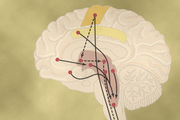
Figure 18-1 shows the anatomy of sympathetic nervous control of the circulation. Sympathetic vasomotor nerve fibers leave the spinal cord through all the thoracic spinal nerves and through the first one or two lumbar spinal nerves. They then pass immediately into a sympathetic chain, one of which lies on each side of the vertebral column. Next, they pass by two routes to the circulation: (1) through specific sympathetic nerves that innervate mainly the vasculature of the internal viscera and the heart, as shown on the right side of Figure 18-1, and (2) almost immediately into peripheral portions of the spinal nerves distributed to the vasculature of the peripheral areas. The precise pathways of these fibers in the spinal cord and in the sympathetic chains are discussed in Chapter 61.
Sympathetic Innervation of the Blood Vessels.
Figure 18-2 shows distribution of sympathetic nerve fibers to the blood vessels, demonstrating that in most tissues all the vessels except the capillaries are innervated. Precapillary sphincters and metarterioles are innervated in some tissues, such as the mesenteric blood vessels, although their sympathetic innervation is usually not as dense as in the small arteries, arterioles, and veins.
The innervation of the small arteries and arterioles allows sympathetic stimulation to increase resistance to blood flow and thereby decrease the rate of blood flow through the tissues.
The innervation of the large vessels, particularly of the veins, makes it possible for sympathetic stimulation to decrease the volume of these vessels. This decrease in volume can push blood into the heart and thereby plays a major role in regulation of heart pumping, as we explain later in this and subsequent chapters.
Sympathetic Stimulation Increases Heart Rate and Contractility.
Sympathetic fibers also go directly to the heart, as shown in Figure 18-1 and as discussed in Chapter 9. It should be recalled that sympathetic stimulation markedly increases the activity of the heart, both increasing the heart rate and enhancing its strength and volume of pumping.
Parasympathetic Stimulation Decreases Heart Rate and Contractility.
Although the parasympathetic nervous system is exceedingly important for many other autonomic functions of the body, such as control of multiple gastrointestinal actions, it plays only a minor role in regulating vascular function in most tissues. Its most important circulatory effect is to control heart rate by way of parasympathetic nerve fibers to the heart in the vagus nerves, shown in Figure 18-1 by the dashed red line from the brain medulla directly to the heart.
The effects of parasympathetic stimulation on heart function were discussed in detail in Chapter 9. Principally, parasympathetic stimulation causes a marked decrease in heart rate and a slight decrease in heart muscle contractility.
Sympathetic Vasoconstrictor System and Its Control by the Central Nervous System
The sympathetic nerves carry tremendous numbers of vasoconstrictor nerve fibers and only a few vasodilator fibers. The vasoconstrictor fibers are distributed to essentially all segments of the circulation, but more to some tissues than to others. This sympathetic vasoconstrictor effect is especially powerful in the kidneys, intestines, spleen, and skin but is much less potent in skeletal muscle and the brain.
Vasomotor Center in the Brain and Its Control of the Vasoconstrictor System.
Located bilaterally mainly in the reticular substance of the medulla and of the lower third of the pons is an area called the vasomotor center, shown in Figures 18-1 and 18-3. This center transmits parasympathetic impulses through the vagus nerves to the heart and sympathetic impulses through the spinal cord and peripheral sympathetic nerves to virtually all arteries, arterioles, and veins of the body.
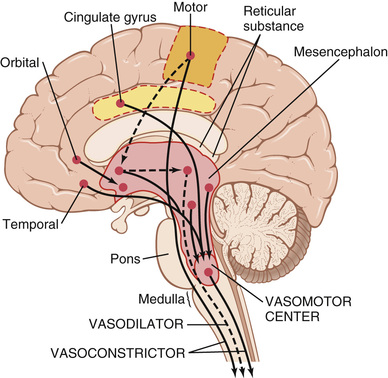
Although the total organization of the vasomotor center is still unclear, experiments have made it possible to identify certain important areas in this center:
1. A vasoconstrictor area located bilaterally in the anterolateral portions of the upper medulla. The neurons originating in this area distribute their fibers to all levels of the spinal cord, where they excite preganglionic vasoconstrictor neurons of the sympathetic nervous system.
2. A vasodilator area located bilaterally in the anterolateral portions of the lower half of the medulla. The fibers from these neurons project upward to the vasoconstrictor area just described; they inhibit the vasoconstrictor activity of this area, thus causing vasodilation.
3. A sensory area located bilaterally in the nucleus tractus solitarius in the posterolateral portions of the medulla and lower pons. The neurons of this area receive sensory nerve signals from the circulatory system mainly through the vagus and glossopharyngeal nerves, and output signals from this sensory area then help to control activities of both the vasoconstrictor and vasodilator areas of the vasomotor center, thus providing “reflex” control of many circulatory functions. An example is the baroreceptor reflex for controlling arterial pressure, described later in this chapter.
Continuous Partial Constriction of the Blood Vessels Is Normally Caused by Sympathetic Vasoconstrictor Tone.
Under normal conditions, the vasoconstrictor area of the vasomotor center transmits signals continuously to the sympathetic vasoconstrictor nerve fibers over the entire body, causing slow firing of these fibers at a rate of about one half to two impulses per second. This continual firing is called sympathetic vasoconstrictor tone. These impulses normally maintain a partial state of contraction in the blood vessels, called vasomotor tone.
Figure 18-4 demonstrates the significance of vasoconstrictor tone. In the experiment of this figure, a total spinal anesthetic was administered to an animal. This anesthetic blocked all transmission of sympathetic nerve impulses from the spinal cord to the periphery. As a result, the arterial pressure fell from 100 to 50 mm Hg, demonstrating the effect of the loss of vasoconstrictor tone throughout the body. A few minutes later, a small amount of the hormone norepinephrine was injected into the blood (norepinephrine is the principal vasoconstrictor hormonal substance secreted at the endings of the sympathetic vasoconstrictor nerve fibers). As this injected hormone was transported in the blood to blood vessels, the vessels once again became constricted and the arterial pressure rose to a level even greater than normal for 1 to 3 minutes, until the norepinephrine was destroyed.
Control of Heart Activity by the Vasomotor Center.
At the same time that the vasomotor center regulates the amount of vascular constriction, it also controls heart activity. The lateral portions of the vasomotor center transmit excitatory impulses through the sympathetic nerve fibers to the heart when there is a need to increase heart rate and contractility. Conversely, when there is a need to decrease heart pumping, the medial portion of the vasomotor center sends signals to the adjacent dorsal motor nuclei of the vagus nerves, which then transmit parasympathetic impulses through the vagus nerves to the heart to decrease heart rate and heart contractility. Therefore, the vasomotor center can either increase or decrease heart activity. Heart rate and strength of heart contraction ordinarily increase when vasoconstriction occurs and ordinarily decrease when vasoconstriction is inhibited.
Control of the Vasomotor Center by Higher Nervous Centers.
Large numbers of small neurons located throughout the reticular substance of the pons, mesencephalon, and diencephalon can either excite or inhibit the vasomotor center. This reticular substance is shown in Figure 18-3. In general, the neurons in the more lateral and superior portions of the reticular substance cause excitation, whereas the more medial and inferior portions cause inhibition.
The hypothalamus plays a special role in controlling the vasoconstrictor system because it can exert powerful excitatory or inhibitory effects on the vasomotor center. The posterolateral portions of the hypothalamus cause mainly excitation, whereas the anterior portion can cause either mild excitation or inhibition, depending on the precise part of the anterior hypothalamus that is stimulated.
Many parts of the cerebral cortex can also excite or inhibit the vasomotor center. Stimulation of the motor cortex, for instance, excites the vasomotor center because of impulses transmitted downward into the hypothalamus and then to the vasomotor center. Also, stimulation of the anterior temporal lobe, the orbital areas of the frontal cortex, the anterior part of the cingulate gyrus, the amygdala, the septum, and the hippocampus can all either excite or inhibit the vasomotor center, depending on the precise portions of these areas that are stimulated and the intensity of the stimulus. Thus, widespread basal areas of the brain can have profound effects on cardiovascular function.
Norepinephrine Is the Sympathetic Vasoconstrictor Neurotransmitter.
The substance secreted at the endings of the vasoconstrictor nerves is almost entirely norepinephrine, which acts directly on the alpha adrenergic receptors of the vascular smooth muscle to cause vasoconstriction, as discussed in Chapter 61.
Adrenal Medullae and Their Relation to the Sympathetic Vasoconstrictor System.
Sympathetic impulses are transmitted to the adrenal medullae at the same time that they are transmitted to the blood vessels. These impulses cause the medullae to secrete both epinephrine and norepinephrine into the circulating blood. These two hormones are carried in the blood stream to all parts of the body, where they act directly on all blood vessels, usually to cause vasoconstriction. In a few tissues epinephrine causes vasodilation because it also has a beta-adrenergic receptor stimulatory effect, which dilates rather than constricts certain vessels, as discussed in Chapter 61.
 Sympathetic Vasodilator System and Its Control by the Central Nervous System.
Sympathetic Vasodilator System and Its Control by the Central Nervous System.
The sympathetic nerves to skeletal muscles carry sympathetic vasodilator fibers, as well as constrictor fibers. In some animals such as the cat, these dilator fibers release acetylcholine, not norepinephrine, at their endings, although in primates, the vasodilator effect is believed to be caused by epinephrine exciting specific beta-adrenergic receptors in the muscle vasculature.
The pathway for central nervous system (CNS) control of the vasodilator system is shown by the dashed lines in Figure 18-3. The principal area of the brain controlling this system is the anterior hypothalamus.
 Possible Role of the Sympathetic Vasodilator System.
Possible Role of the Sympathetic Vasodilator System.
The sympathetic vasodilator system does not appear to play a major role in the control of the circulation in humans because complete block of the sympathetic nerves to the muscles hardly affects the ability of these muscles to control their own blood flow in many physiological conditions. Yet, some experiments suggest that at the onset of exercise, the sympathetic system might cause initial vasodilation in skeletal muscles to allow an anticipatory increase in blood flow even before the muscles require increased nutrients. There is evidence in humans that this “sympathetic” vasodilator response in skeletal muscles may be mediated by circulating epinephrine, which stimulates beta-adrenergic receptors, or by nitric oxide released from the vascular endothelium in response to stimulation by acetylcholine.
 Emotional Fainting—Vasovagal Syncope.
Emotional Fainting—Vasovagal Syncope.
An interesting vasodilatory reaction occurs in people who experience intense emotional disturbances that cause fainting. In this case, the muscle vasodilator system becomes activated and, at the same time, the vagal cardioinhibitory center transmits strong signals to the heart to slow the heart rate markedly. The arterial pressure falls rapidly, which reduces blood flow to the brain and causes the person to lose consciousness. This overall effect is called vasovagal syncope. Emotional fainting begins with disturbing thoughts in the cerebral cortex. The pathway probably then goes to the vasodilatory center of the anterior hypothalamus next to the vagal centers of the medulla, to the heart through the vagus nerves, and also through the spinal cord to the sympathetic vasodilator nerves of the muscles. ![]()
Role of the Nervous System in Rapid Control of Arterial Pressure
One of the most important functions of nervous control of the circulation is its capability to cause rapid increases in arterial pressure. For this purpose, the entire vasoconstrictor and cardioaccelerator functions of the sympathetic nervous system are stimulated together. At the same time, there is reciprocal inhibition of parasympathetic vagal inhibitory signals to the heart. Thus, the following three major changes occur simultaneously, each of which helps to increase arterial pressure:
1. Most arterioles of the systemic circulation are constricted, which greatly increases the total peripheral resistance, thereby increasing the arterial pressure.
2. The veins especially (but the other large vessels of the circulation as well) are strongly constricted. This constriction displaces blood out of the large peripheral blood vessels toward the heart, thus increasing the volume of blood in the heart chambers. The stretch of the heart then causes the heart to beat with far greater force and therefore to pump increased quantities of blood. This also increases the arterial pressure.
3. Finally, the heart is directly stimulated by the autonomic nervous system, further enhancing cardiac pumping. Much of this enhanced cardiac pumping is caused by an increase in the heart rate, which sometimes increases to as much as three times normal. In addition, sympathetic nervous signals have a significant direct effect to increase contractile force of the heart muscle, increasing the capability of the heart to pump larger volumes of blood. During strong sympathetic stimulation, the heart can pump about two times as much blood as under normal conditions, which contributes still more to the acute rise in arterial pressure.
Nervous Control of Arterial Pressure Is Rapid.
An especially important characteristic of nervous control of arterial pressure is its rapidity of response, beginning within seconds and often increasing the pressure to two times normal within 5 to 10 seconds. Conversely, sudden inhibition of nervous cardiovascular stimulation can decrease the arterial pressure to as little as one-half normal within 10 to 40 seconds. Therefore, nervous control is by far the most rapid mechanism for arterial pressure regulation.
Increases in Arterial Pressure during Muscle Exercise and Other Types of Stress
An important example of the nervous system's ability to increase arterial pressure is the rise in pressure that occurs during muscle exercise. During heavy exercise, the muscles require greatly increased blood flow. Part of this increase results from local vasodilation of the muscle vasculature caused by increased metabolism of the muscle cells, as explained in Chapter 17. An additional increase results from simultaneous elevation of arterial pressure caused by sympathetic stimulation of the overall circulation during exercise. In heavy exercise, the arterial pressure rises about 30 to 40 percent, which increases blood flow almost an additional twofold.
The increase in arterial pressure during exercise results mainly from effects of the nervous system. At the same time that the motor areas of the brain become activated to cause exercise, most of the reticular activating system of the brain stem is also activated, which includes greatly increased stimulation of the vasoconstrictor and cardioacceleratory areas of the vasomotor center. These effects increase the arterial pressure instantaneously to keep pace with the increase in muscle activity.
In many other types of stress besides muscle exercise, a similar rise in pressure can also occur. For instance, during extreme fright, the arterial pressure sometimes rises by as much as 75 to 100 mm Hg within a few seconds. This response is called the alarm reaction, and it provides an excess of arterial pressure that can immediately supply blood to the muscles of the body that might need to respond instantly to enable flight from danger.
Reflex Mechanisms for Maintaining Normal Arterial Pressure
Aside from the exercise and stress functions of the autonomic nervous system to increase arterial pressure, multiple subconscious special nervous control mechanisms operate all the time to maintain the arterial pressure at or near normal. Almost all of these are negative feedback reflex mechanisms, which we describe in the following sections.
Baroreceptor Arterial Pressure Control System—Baroreceptor Reflexes
By far the best known of the nervous mechanisms for arterial pressure control is the baroreceptor reflex. Basically, this reflex is initiated by stretch receptors, called either baroreceptors or pressoreceptors, located at specific points in the walls of several large systemic arteries. A rise in arterial pressure stretches the baroreceptors and causes them to transmit signals into the CNS. “Feedback” signals are then sent back through the autonomic nervous system to the circulation to reduce arterial pressure downward toward the normal level.
Physiologic Anatomy of the Baroreceptors and Their Innervation.
Baroreceptors are spray-type nerve endings that lie in the walls of the arteries and are stimulated when stretched. A few baroreceptors are located in the wall of almost every large artery of the thoracic and neck regions, but as shown in Figure 18-5, baroreceptors are extremely abundant in (1) the wall of each internal carotid artery slightly above the carotid bifurcation, an area known as the carotid sinus, and (2) the wall of the aortic arch.
Figure 18-5 shows that signals from the “carotid baroreceptors” are transmitted through small Hering's nerves to the glossopharyngeal nerves in the high neck, and then to the nucleus tractus solitarius in the medullary area of the brain stem. Signals from the “aortic baroreceptors” in the arch of the aorta are transmitted through the vagus nerves to the same nucleus tractus solitarius of the medulla.
Response of the Baroreceptors to Arterial Pressure.
Figure 18-6 shows the effect of different arterial pressure levels on the rate of impulse transmission in a Hering's carotid sinus nerve. Note that the carotid sinus baroreceptors are not stimulated at all by pressures between 0 and 50 to 60 mm Hg, but above these levels, they respond progressively more rapidly and reach a maximum at about 180 mm Hg. The responses of the aortic baroreceptors are similar to those of the carotid receptors except that they operate, in general, at arterial pressure levels about 30 mm Hg higher.
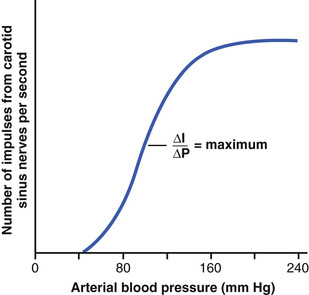
Note especially that in the normal operating range of arterial pressure, around 100 mm Hg, even a slight change in pressure causes a strong change in the baroreflex signal to readjust arterial pressure back toward normal. Thus, the baroreceptor feedback mechanism functions most effectively in the pressure range where it is most needed.
The baroreceptors respond rapidly to changes in arterial pressure; in fact, the rate of impulse firing increases in the fraction of a second during each systole and decreases again during diastole. Furthermore, the baroreceptors respond much more to a rapidly changing pressure than to a stationary pressure. That is, if the mean arterial pressure is 150 mm Hg but at that moment is rising rapidly, the rate of impulse transmission may be as much as twice that when the pressure is stationary at 150 mm Hg.
Circulatory Reflex Initiated by the Baroreceptors.
After the baroreceptor signals have entered the nucleus tractus solitarius of the medulla, secondary signals inhibit the vasoconstrictor center of the medulla and excite the vagal parasympathetic center. The net effects are (1) vasodilation of the veins and arterioles throughout the peripheral circulatory system and (2) decreased heart rate and strength of heart contraction. Therefore, excitation of the baroreceptors by high pressure in the arteries reflexly causes the arterial pressure to decrease because of both a decrease in peripheral resistance and a decrease in cardiac output. Conversely, low pressure has opposite effects, reflexly causing the pressure to rise back toward normal.
Figure 18-7 shows a typical reflex change in arterial pressure caused by occluding the two common carotid arteries. This reduces the carotid sinus pressure; as a result, signals from the baroreceptors decrease and cause less inhibitory effect on the vasomotor center. The vasomotor center then becomes much more active than usual, causing the aortic arterial pressure to rise and remain elevated during the 10 minutes that the carotids are occluded. Removal of the occlusion allows the pressure in the carotid sinuses to rise, and the carotid sinus reflex now causes the aortic pressure to fall immediately to slightly below normal as a momentary overcompensation and then return to normal in another minute.
The Baroreceptors Attenuate Blood Pressure Changes During Changes in Body Posture.
The ability of the baroreceptors to maintain relatively constant arterial pressure in the upper body is important when a person stands up after having been lying down. Immediately on standing, the arterial pressure in the head and upper part of the body tends to fall, and marked reduction of this pressure could cause loss of consciousness. However, the falling pressure at the baroreceptors elicits an immediate reflex, resulting in strong sympathetic discharge throughout the body that minimizes the decrease in pressure in the head and upper body.
Pressure “Buffer” Function of the Baroreceptor Control System.
Because the baroreceptor system opposes either increases or decreases in arterial pressure, it is called a pressure buffer system, and the nerves from the baroreceptors are called buffer nerves.
Figure 18-8 shows the importance of this buffer function of the baroreceptors. The upper record in this figure shows an arterial pressure recording for 2 hours from a normal dog, and the lower record shows an arterial pressure recording from a dog whose baroreceptor nerves from both the carotid sinuses and the aorta had been removed. Note the extreme variability of pressure in the denervated dog caused by simple events of the day, such as lying down, standing, excitement, eating, defecation, and noises.
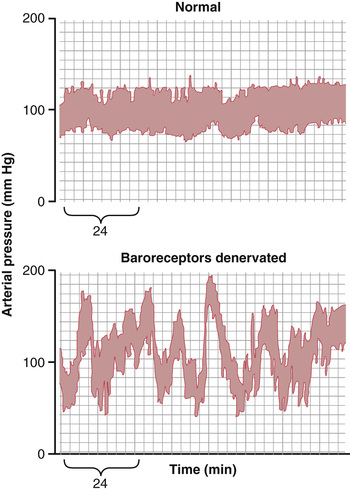
Figure 18-9 shows the frequency distributions of the mean arterial pressures recorded for a 24-hour day in both the normal dog and the denervated dog. Note that when the baroreceptors were functioning normally the mean arterial pressure remained within a narrow range of between 85 and 115 mm Hg throughout the day, and for most of the day it remained at about 100 mm Hg. After denervation of the baroreceptors, however, the frequency distribution curve became the broad, low curve of the figure, showing that the pressure range increased 2.5-fold, frequently falling to as low as 50 mm Hg or rising to more than 160 mm Hg. Thus, one can see the extreme variability of pressure in the absence of the arterial baroreceptor system.
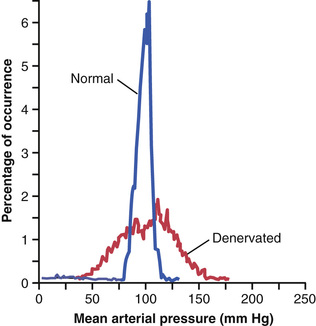
Thus, a primary purpose of the arterial baroreceptor system is to reduce the minute-by-minute variation in arterial pressure to about one third that which would occur if the baroreceptor system was not present.
Are the Baroreceptors Important in Long-Term Regulation of Arterial Pressure?
Although the arterial baroreceptors provide powerful moment-to-moment control of arterial pressure, their importance in long-term blood pressure regulation has been controversial. One reason that the baroreceptors have been considered by some physiologists to be relatively unimportant in chronic regulation of arterial pressure is that they tend to reset in 1 to 2 days to the pressure level to which they are exposed. That is, if the arterial pressure rises from the normal value of 100 mm Hg to 160 mm Hg, a very high rate of baroreceptor impulses are at first transmitted. During the next few minutes, the rate of firing diminishes considerably; then it diminishes much more slowly during the next 1 to 2 days, at the end of which time the rate of firing will have returned to nearly normal despite the fact that the mean arterial pressure still remains at 160 mm Hg. Conversely, when the arterial pressure falls to a very low level, the baroreceptors at first transmit no impulses, but gradually, over 1 to 2 days, the rate of baroreceptor firing returns toward the control level.
This “resetting” of the baroreceptors may attenuate their potency as a control system for correcting disturbances that tend to change arterial pressure for longer than a few days at a time. Experimental studies, however, have suggested that the baroreceptors do not completely reset and may therefore contribute to long-term blood pressure regulation, especially by influencing sympathetic nerve activity of the kidneys. For example, with prolonged increases in arterial pressure, the baroreceptor reflexes may mediate decreases in renal sympathetic nerve activity that promote increased excretion of sodium and water by the kidneys. This action, in turn, causes a gradual decrease in blood volume, which helps to restore arterial pressure toward normal. Thus, long-term regulation of mean arterial pressure by the baroreceptors requires interaction with additional systems, principally the renal–body fluid–pressure control system (along with its associated nervous and hormonal mechanisms), discussed in Chapters 19 and 30.
Control of Arterial Pressure by the Carotid and Aortic Chemoreceptors—Effect of Low Oxygen on Arterial Pressure.
Closely associated with the baroreceptor pressure control system is a chemoreceptor reflex that operates in much the same way as the baroreceptor reflex except that chemoreceptors, instead of stretch receptors, initiate the response.
The chemoreceptors are chemosensitive cells sensitive to low oxygen, carbon dioxide excess, and hydrogen ion excess. They are located in several small chemoreceptor organs about 2 millimeters in size (two carotid bodies, one of which lies in the bifurcation of each common carotid artery, and usually one to three aortic bodies adjacent to the aorta). The chemoreceptors excite nerve fibers that, along with the baroreceptor fibers, pass through Hering's nerves and the vagus nerves into the vasomotor center of the brain stem.
Each carotid or aortic body is supplied with an abundant blood flow through a small nutrient artery, so the chemoreceptors are always in close contact with arterial blood. Whenever the arterial pressure falls below a critical level, the chemoreceptors become stimulated because diminished blood flow causes decreased oxygen, as well as excess buildup of carbon dioxide and hydrogen ions that are not removed by the slowly flowing blood.
The signals transmitted from the chemoreceptors excite the vasomotor center, and this response elevates the arterial pressure back toward normal. However, this chemoreceptor reflex is not a powerful arterial pressure controller until the arterial pressure falls below 80 mm Hg. Therefore, it is at the lower pressures that this reflex becomes important to help prevent further decreases in arterial pressure.
The chemoreceptors are discussed in much more detail in Chapter 42 in relation to respiratory control, in which they play a far more important role than in blood pressure control.
Atrial and Pulmonary Artery Reflexes Regulate Arterial Pressure.
Both the atria and the pulmonary arteries have in their walls stretch receptors called low-pressure receptors. Low-pressure receptors are similar to the baroreceptor stretch receptors of the large systemic arteries. These low-pressure receptors play an important role, especially in minimizing arterial pressure changes in response to changes in blood volume. For example, if 300 milliliters of blood suddenly are infused into a dog with all receptors intact, the arterial pressure rises only about 15 mm Hg. With the arterial baroreceptors denervated, the pressure rises about 40 mm Hg. If the low-pressure receptors also are denervated, the arterial pressure rises about 100 mm Hg.
Thus, one can see that even though the low-pressure receptors in the pulmonary artery and in the atria cannot detect the systemic arterial pressure, they do detect simultaneous increases in pressure in the low-pressure areas of the circulation caused by increase in volume, and they elicit reflexes parallel to the baroreceptor reflexes to make the total reflex system more potent for control of arterial pressure.
Atrial Reflexes That Activate the Kidneys—The “Volume Reflex.”
Stretch of the atria also causes significant reflex dilation of the afferent arterioles in the kidneys. Signals are also transmitted simultaneously from the atria to the hypothalamus to decrease secretion of antidiuretic hormone (ADH). The decreased afferent arteriolar resistance in the kidneys causes the glomerular capillary pressure to rise, with resultant increase in filtration of fluid into the kidney tubules. The diminution of ADH diminishes the reabsorption of water from the tubules. The combination of these two effects—an increase in glomerular filtration and a decrease in reabsorption of the fluid—increases fluid loss by the kidneys and reduces an increased blood volume back toward normal. (We will also see in Chapter 19 that atrial stretch caused by increased blood volume also elicits a hormonal effect on the kidneys—release of atrial natriuretic peptide—that adds still further to the excretion of fluid in the urine and return of blood volume toward normal.)
All these mechanisms that tend to return the blood volume back toward normal after a volume overload act indirectly as pressure controllers, as well as blood volume controllers, because excess volume drives the heart to greater cardiac output and leads to greater arterial pressure. This volume reflex mechanism is discussed again in Chapter 30, along with other mechanisms of blood volume control.
Atrial Reflex Control of Heart Rate (the Bainbridge Reflex).
An increase in atrial pressure also causes an increase in heart rate, sometimes increasing the heart rate as much as 75 percent. A small part of this increase is caused by a direct effect of the increased atrial volume to stretch the sinus node; it was pointed out in Chapter 10 that such direct stretch can increase the heart rate as much as 15 percent. An additional 40 to 60 percent increase in rate is caused by a nervous reflex called the Bainbridge reflex. The stretch receptors of the atria that elicit the Bainbridge reflex transmit their afferent signals through the vagus nerves to the medulla of the brain. Then efferent signals are transmitted back through vagal and sympathetic nerves to increase heart rate and strength of heart contraction. Thus, this reflex helps prevent damming of blood in the veins, atria, and pulmonary circulation.
CNS Ischemic Response—Control of Arterial Pressure by the Brain'S Vasomotor Center in Response to Diminished Brain Blood Flow
Most nervous control of blood pressure is achieved by reflexes that originate in the baroreceptors, the chemoreceptors, and the low-pressure receptors, all of which are located in the peripheral circulation outside the brain. However, when blood flow to the vasomotor center in the lower brain stem becomes decreased severely enough to cause nutritional deficiency—that is, to cause cerebral ischemia—the vasoconstrictor and cardioaccelerator neurons in the vasomotor center respond directly to the ischemia and become strongly excited. When this excitation occurs, the systemic arterial pressure often rises to a level as high as the heart can possibly pump. This effect is believed to be caused by failure of the slowly flowing blood to carry carbon dioxide away from the brain stem vasomotor center. At low levels of blood flow to the vasomotor center, the local concentration of carbon dioxide increases greatly and has an extremely potent effect in stimulating the sympathetic vasomotor nervous control areas in the brain's medulla.
It is possible that other factors, such as buildup of lactic acid and other acidic substances in the vasomotor center, also contribute to the marked stimulation and elevation in arterial pressure. This arterial pressure elevation in response to cerebral ischemia is known as the CNS ischemic response.
The ischemic effect on vasomotor activity can elevate the mean arterial pressure dramatically, sometimes to as high as 250 mm Hg for as long as 10 minutes. The degree of sympathetic vasoconstriction caused by intense cerebral ischemia is often so great that some of the peripheral vessels become totally or almost totally occluded. The kidneys, for instance, often entirely cease their production of urine because of renal arteriolar constriction in response to the sympathetic discharge. Therefore, the CNS ischemic response is one of the most powerful of all the activators of the sympathetic vasoconstrictor system.
Importance of the CNS Ischemic Response as a Regulator of Arterial Pressure.
Despite the powerful nature of the CNS ischemic response, it does not become significant until the arterial pressure falls far below normal, down to 60 mm Hg and below, reaching its greatest degree of stimulation at a pressure of 15 to 20 mm Hg. Therefore, the CNS ischemic response is not one of the normal mechanisms for regulating arterial pressure. Instead, it operates principally as an emergency pressure control system that acts rapidly and powerfully to prevent further decrease in arterial pressure whenever blood flow to the brain decreases dangerously close to the lethal level. It is sometimes called the “last-ditch stand” pressure control mechanism.
Cushing Reaction to Increased Pressure Around the Brain.
The so-called Cushing reaction is a special type of CNS ischemic response that results from increased pressure of the cerebrospinal fluid around the brain in the cranial vault. For instance, when the cerebrospinal fluid pressure rises to equal the arterial pressure, it compresses the whole brain, as well as the arteries in the brain, and cuts off the blood supply to the brain. This action initiates a CNS ischemic response that causes the arterial pressure to rise. When the arterial pressure has risen to a level higher than the cerebrospinal fluid pressure, blood will flow once again into the vessels of the brain to relieve the brain ischemia. Ordinarily, the blood pressure comes to a new equilibrium level slightly higher than the cerebrospinal fluid pressure, thus allowing blood to begin to flow through the brain again. The Cushing reaction helps protect vital centers of the brain from loss of nutrition if the cerebrospinal fluid pressure ever rises high enough to compress the cerebral arteries.
Special Features of Nervous Control of Arterial Pressure
Role of the Skeletal Nerves and Skeletal Muscles in Increasing Cardiac Output and Arterial Pressure
Although most rapidly acting nervous control of the circulation is effected through the autonomic nervous system, at least two conditions exist in which the skeletal nerves and muscles also play major roles in circulatory responses.
Abdominal Compression Reflex.
When a baroreceptor or chemoreceptor reflex is elicited, nerve signals are transmitted simultaneously through skeletal nerves to skeletal muscles of the body, particularly to the abdominal muscles. Muscle contraction then compresses all the venous reservoirs of the abdomen, helping to translocate blood out of the abdominal vascular reservoirs toward the heart. As a result, increased quantities of blood are made available for the heart to pump. This overall response is called the abdominal compression reflex. The resulting effect on the circulation is the same as that caused by sympathetic vasoconstrictor impulses when they constrict the veins: an increase in both cardiac output and arterial pressure. The abdominal compression reflex is probably much more important than has been realized in the past because it is well known that people whose skeletal muscles have been paralyzed are considerably more prone to hypotensive episodes than are people with normal skeletal muscles.
Increased Cardiac Output and Arterial Pressure Caused by Skeletal Muscle Contraction During Exercise.
When the skeletal muscles contract during exercise, they compress blood vessels throughout the body. Even anticipation of exercise tightens the muscles, thereby compressing the vessels in the muscles and in the abdomen. This compression translocates blood from the peripheral vessels into the heart and lungs and, therefore, increases cardiac output. This effect is essential in helping to cause the fivefold to sevenfold increase in cardiac output that sometimes occurs during heavy exercise. The rise in cardiac output in turn is an essential ingredient in increasing the arterial pressure during exercise, an increase usually from a normal mean of 100 mm Hg up to 130 to 160 mm Hg.
Respiratory Waves in the Arterial Pressure
With each cycle of respiration, the arterial pressure usually rises and falls 4 to 6 mm Hg in a wavelike manner, causing respiratory waves in the arterial pressure. The waves result from several different effects, some of which are reflex in nature, as follows:
1. Many of the “breathing signals” that arise in the respiratory center of the medulla “spill over” into the vasomotor center with each respiratory cycle.
2. Every time a person inspires, the pressure in the thoracic cavity becomes more negative than usual, causing the blood vessels in the chest to expand. This reduces the quantity of blood returning to the left side of the heart and thereby momentarily decreases the cardiac output and arterial pressure.
3. The pressure changes caused in the thoracic vessels by respiration can excite vascular and atrial stretch receptors.
Although it is difficult to analyze the exact relations of all these factors in causing the respiratory pressure waves, the net result during normal respiration is usually an increase in arterial pressure during the early part of expiration and a decrease in pressure during the remainder of the respiratory cycle. During deep respiration, the blood pressure can rise and fall as much as 20 mm Hg with each respiratory cycle.
Arterial Pressure “Vasomotor” Waves—Oscillation of Pressure Reflex Control Systems
Often while recording arterial pressure, in addition to the small pressure waves caused by respiration, some much larger waves are also noted—as great as 10 to 40 mm Hg at times—that rise and fall more slowly than the respiratory waves. The duration of each cycle varies from 26 seconds in the anesthetized dog to 7 to 10 seconds in the unanesthetized human. These waves are called vasomotor waves or Mayer waves. Such records are demonstrated in Figure 18-10, showing the cyclical rise and fall in arterial pressure.
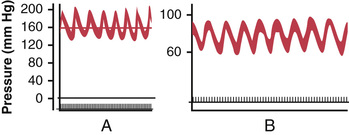
The cause of vasomotor waves is “reflex oscillation” of one or more nervous pressure control mechanisms, some of which are the following.
Oscillation of the Baroreceptor and Chemoreceptor Reflexes.
The vasomotor waves of Figure 18-10B are often seen in experimental pressure recordings, although they are usually much less intense than shown in the figure. They are caused mainly by oscillation of the baroreceptor reflex. That is, a high pressure excites the baroreceptors, which then inhibits the sympathetic nervous system and lowers the pressure a few seconds later. The decreased pressure in turn reduces the baroreceptor stimulation and allows the vasomotor center to become active once again, elevating the pressure to a high value. The response is not instantaneous, and it is delayed until a few seconds later. This high pressure then initiates another cycle, and the oscillation continues on and on.
The chemoreceptor reflex can also oscillate to give the same type of waves. This reflex usually oscillates simultaneously with the baroreceptor reflex. It probably plays the major role in causing vasomotor waves when the arterial pressure is in the range of 40 to 80 mm Hg because in this low range, chemoreceptor control of the circulation becomes powerful, whereas baroreceptor control becomes weaker.
Oscillation of the CNS Ischemic Response.
The record in Figure 18-10A resulted from oscillation of the CNS ischemic pressure control mechanism. In this experiment, the cerebrospinal fluid pressure increased to 160 mm Hg, which compressed the cerebral vessels and initiated a CNS ischemic pressure response up to 200 mm Hg. When the arterial pressure rose to such a high value, the brain ischemia was relieved and the sympathetic nervous system became inactive. As a result, the arterial pressure fell rapidly back to a much lower value, causing brain ischemia once again. The ischemia then initiated another rise in pressure. Again the ischemia was relieved and again the pressure fell. This response repeated itself cyclically as long as the cerebrospinal fluid pressure remained elevated.
Thus, any reflex pressure control mechanism can oscillate if the intensity of “feedback” is strong enough and if there is a delay between excitation of the pressure receptor and the subsequent pressure response. The vasomotor waves illustrate that the nervous reflexes that control arterial pressure obey the same principles as those applicable to mechanical and electrical control systems. For instance, if the feedback “gain” is too great in the guiding mechanism of an automatic pilot for an airplane and there is also delay in response time of the guiding mechanism, the plane will oscillate from side to side instead of following a straight course.




