General Principles of Gastrointestinal Function—Motility, Nervous Control, and Blood Circulation

The alimentary tract provides the body with a continual supply of water, electrolytes, vitamins, and nutrients, which requires (1) movement of food through the alimentary tract; (2) secretion of digestive juices and digestion of the food; (3) absorption of water, various electrolytes, vitamins, and digestive products; (4) circulation of blood through the gastrointestinal organs to carry away the absorbed substances; and (5) control of all these functions by local, nervous, and hormonal systems.
Figure 63-1 shows the entire alimentary tract. Each part is adapted to its specific functions: some parts to simple passage of food, such as the esophagus; others to temporary storage of food, such as the stomach; and others to digestion and absorption, such as the small intestine. In this chapter we discuss the basic principles of function in the entire alimentary tract, and in subsequent chapters the specific functions of different segments of the tract will be addressed.
General Principles of Gastrointestinal Motility
Physiological Anatomy of the Gastrointestinal Wall
Figure 63-2 shows a typical cross section of the intestinal wall, including the following layers from the outer surface inward: (1) the serosa, (2) a longitudinal smooth muscle layer, (3) a circular smooth muscle layer, (4) the submucosa, and (5) the mucosa. In addition, sparse bundles of smooth muscle fibers, the mucosal muscle, lie in the deeper layers of the mucosa. The motor functions of the gut are performed by the different layers of smooth muscle.
The general characteristics of smooth muscle and its function are discussed in Chapter 8, which should be reviewed as a background for the following sections of this chapter.
Gastrointestinal Smooth Muscle Functions as a Syncytium.
The individual smooth muscle fibers in the gastrointestinal tract are 200 to 500 micrometers in length and 2 to 10 micrometers in diameter, and they are arranged in bundles of as many as 1000 parallel fibers. In the longitudinal muscle layer, the bundles extend longitudinally down the intestinal tract; in the circular muscle layer, they extend around the gut.
Within each bundle, the muscle fibers are electrically connected with one another through large numbers of gap junctions that allow low-resistance movement of ions from one muscle cell to the next. Therefore, electrical signals that initiate muscle contractions can travel readily from one fiber to the next within each bundle but more rapidly along the length of the bundle than sideways.
Each bundle of smooth muscle fibers is partly separated from the next by loose connective tissue, but the muscle bundles fuse with one another at many points, so in reality each muscle layer represents a branching latticework of smooth muscle bundles. Therefore, each muscle layer functions as a syncytium; that is, when an action potential is elicited anywhere within the muscle mass, it generally travels in all directions in the muscle. The distance that it travels depends on the excitability of the muscle; sometimes it stops after only a few millimeters, and at other times it travels many centimeters or even the entire length and breadth of the intestinal tract.
Also, because a few connections exist between the longitudinal and circular muscle layers, excitation of one of these layers often excites the other as well.
Electrical Activity of Gastrointestinal Smooth Muscle
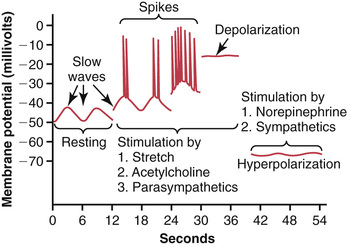
The smooth muscle of the gastrointestinal tract is excited by almost continual slow, intrinsic electrical activity along the membranes of the muscle fibers. This activity has two basic types of electrical waves: (1) slow waves and (2) spikes, both of which are shown in Figure 63-3. In addition, the voltage of the resting membrane potential of the gastrointestinal smooth muscle can change to different levels, which can also have important effects in controlling motor activity of the gastrointestinal tract.
Slow Waves.
Most gastrointestinal contractions occur rhythmically, and this rhythm is determined mainly by the frequency of so-called “slow waves” of smooth muscle membrane potential. These waves, shown in Figure 63-3, are not action potentials. Instead, they are slow, undulating changes in the resting membrane potential. Their intensity usually varies between 5 and 15 millivolts, and their frequency ranges in different parts of the human gastrointestinal tract from 3 to 12 per minute—about 3 in the body of the stomach, as much as 12 in the duodenum, and about 8 or 9 in the terminal ileum. Therefore, the rhythm of contraction of the body of the stomach, the duodenum, and the ileum is usually about 3 per minute, about 12 per minute, and 8 to 9 per minute, respectively.
The precise cause of the slow waves is not completely understood, although they appear to be caused by complex interactions among the smooth muscle cells and specialized cells, called the interstitial cells of Cajal, which are believed to act as electrical pacemakers for smooth muscle cells. These interstitial cells form a network with each other and are interposed between the smooth muscle layers, with synaptic-like contacts to smooth muscle cells. The interstitial cells of Cajal undergo cyclic changes in membrane potential due to unique ion channels that periodically open and produce inward (pacemaker) currents that may generate slow wave activity.
The slow waves usually do not by themselves cause muscle contraction in most parts of the gastrointestinal tract, except perhaps in the stomach. Instead, they mainly excite the appearance of intermittent spike potentials, and the spike potentials in turn actually excite the muscle contraction.
Spike Potentials.
The spike potentials are true action potentials. They occur automatically when the resting membrane potential of the gastrointestinal smooth muscle becomes more positive than about −40 millivolts (the normal resting membrane potential in the smooth muscle fibers of the gut is between −50 and −60 millivolts). Note in Figure 63-3 that each time the peaks of the slow waves temporarily become more positive than −40 millivolts, spike potentials appear on these peaks. The higher the slow wave potential rises, the greater the frequency of the spike potentials, usually ranging between 1 and 10 spikes per second. The spike potentials last 10 to 40 times as long in gastrointestinal muscle as the action potentials in large nerve fibers, with each gastrointestinal spike lasting as long as 10 to 20 milliseconds.
Another important difference between the action potentials of the gastrointestinal smooth muscle and those of nerve fibers is the manner in which they are generated. In nerve fibers, the action potentials are caused almost entirely by rapid entry of sodium ions through sodium channels to the interior of the fibers. In gastrointestinal smooth muscle fibers, the channels responsible for the action potentials are somewhat different; they allow especially large numbers of calcium ions to enter along with smaller numbers of sodium ions and therefore are called calcium-sodium channels. These channels are much slower to open and close than are the rapid sodium channels of large nerve fibers. The slowness of opening and closing of the calcium-sodium channels accounts for the long duration of the action potentials. Also, the movement of large amounts of calcium ions to the interior of the muscle fiber during the action potential plays a special role in causing the intestinal muscle fibers to contract, as we discuss shortly.
Changes in Voltage of the Resting Membrane Potential.
In addition to the slow waves and spike potentials, the baseline voltage level of the smooth muscle resting membrane potential can also change. Under normal conditions, the resting membrane potential averages about −56 millivolts, but multiple factors can change this level. When the potential becomes less negative, which is called depolarization of the membrane, the muscle fibers become more excitable. When the potential becomes more negative, which is called hyperpolarization, the fibers become less excitable.
Factors that depolarize the membrane—that is, make it more excitable—are (1) stretching of the muscle, (2) stimulation by acetylcholine released from the endings of parasympathetic nerves, and (3) stimulation by several specific gastrointestinal hormones.
Important factors that make the membrane potential more negative—that is, that hyperpolarize the membrane and make the muscle fibers less excitable—are (1) the effect of norepinephrine or epinephrine on the fiber membrane and (2) stimulation of the sympathetic nerves that secrete mainly norepinephrine at their endings.
Entry of Calcium Ions Causes Smooth Muscle Contraction.
Smooth muscle contraction occurs in response to entry of calcium ions into the muscle fiber. As explained in Chapter 8, calcium ions, acting through a calmodulin control mechanism, activate the myosin filaments in the fiber, causing attractive forces to develop between the myosin filaments and the actin filaments, thereby causing the muscle to contract.
The slow waves do not cause calcium ions to enter the smooth muscle fiber (they only cause entry of sodium ions). Therefore, the slow waves by themselves usually do not cause muscle contraction. Instead, it is during the spike potentials, generated at the peaks of the slow waves, that significant quantities of calcium ions enter the fibers and cause most of the contraction.
Tonic Contraction of Some Gastrointestinal Smooth Muscle.
Some smooth muscle of the gastrointestinal tract exhibits tonic contraction as well as, or instead of, rhythmical contractions. Tonic contraction is continuous; it is not associated with the basic electrical rhythm of the slow waves but often lasts several minutes or even hours. The tonic contraction often increases or decreases in intensity but continues.
Tonic contraction is sometimes caused by continuous repetitive spike potentials—the greater the frequency, the greater the degree of contraction. At other times, tonic contraction is caused by hormones or other factors that bring about continuous partial depolarization of the smooth muscle membrane without causing action potentials. A third cause of tonic contraction is continuous entry of calcium ions into the interior of the cell brought about in ways not associated with changes in membrane potential. The details of these mechanisms are still unclear.
Neural Control of Gastrointestinal Function—Enteric Nervous System
The gastrointestinal tract has a nervous system all its own called the enteric nervous system. It lies entirely in the wall of the gut, beginning in the esophagus and extending all the way to the anus. The number of neurons in this enteric system is about 100 million, nearly equal to the number in the entire spinal cord. This highly developed enteric nervous system is especially important in controlling gastrointestinal movements and secretion.
The enteric nervous system is composed mainly of two plexuses, shown in Figure 63-4: (1) an outer plexus lying between the longitudinal and circular muscle layers, called the myenteric plexus or Auerbach's plexus, and (2) an inner plexus, called the submucosal plexus or Meissner's plexus, which lies in the submucosa. The nervous connections within and between these two plexuses are also shown in Figure 63-4.
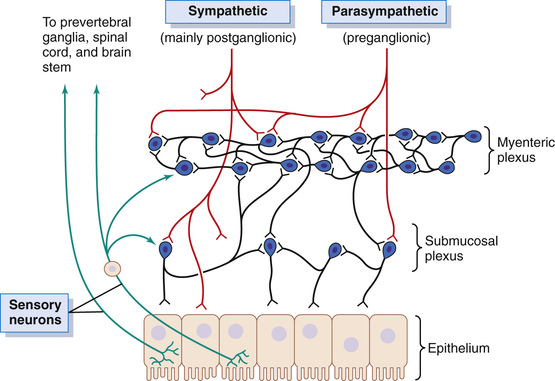
The myenteric plexus controls mainly the gastrointestinal movements, and the submucosal plexus controls mainly gastrointestinal secretion and local blood flow.
In Figure 63-4, note especially the extrinsic sympathetic and parasympathetic fibers that connect to both the myenteric and submucosal plexuses. Although the enteric nervous system can function independently of these extrinsic nerves, stimulation by the parasympathetic and sympathetic systems can greatly enhance or inhibit gastrointestinal functions, as we discuss later.
Also shown in Figure 63-4 are sensory nerve endings that originate in the gastrointestinal epithelium or gut wall and send afferent fibers to both plexuses of the enteric system, as well as (1) to the prevertebral ganglia of the sympathetic nervous system, (2) to the spinal cord, and (3) in the vagus nerves all the way to the brain stem. These sensory nerves can elicit local reflexes within the gut wall itself and still other reflexes that are relayed to the gut from either the prevertebral ganglia or the basal regions of the brain.
Differences between the Myenteric and Submucosal Plexuses
The myenteric plexus consists mostly of a linear chain of many interconnecting neurons that extends the entire length of the gastrointestinal tract. A section of this chain is shown in Figure 63-4.
Because the myenteric plexus extends all the way along the intestinal wall and lies between the longitudinal and circular layers of intestinal smooth muscle, it is concerned mainly with controlling muscle activity along the length of the gut. When this plexus is stimulated, its principal effects are (1) increased tonic contraction, or “tone,” of the gut wall; (2) increased intensity of the rhythmical contractions; (3) slightly increased rate of the rhythm of contraction; and (4) increased velocity of conduction of excitatory waves along the gut wall, causing more rapid movement of the gut peristaltic waves.
The myenteric plexus should not be considered entirely excitatory because some of its neurons are inhibitory; their fiber endings secrete an inhibitory transmitter, possibly vasoactive intestinal polypeptide or some other inhibitory peptide. The resulting inhibitory signals are especially useful for inhibiting some of the intestinal sphincter muscles that impede movement of food along successive segments of the gastrointestinal tract, such as the pyloric sphincter, which controls emptying of the stomach into the duodenum, and the sphincter of the ileocecal valve, which controls emptying from the small intestine into the cecum.
The submucosal plexus, in contrast to the myenteric plexus, is mainly concerned with controlling function within the inner wall of each minute segment of the intestine. For instance, many sensory signals originate from the gastrointestinal epithelium and are then integrated in the submucosal plexus to help control local intestinal secretion, local absorption, and local contraction of the submucosal muscle that causes various degrees of infolding of the gastrointestinal mucosa.
Types of Neurotransmitters Secreted by Enteric Neurons
In an attempt to understand better the multiple functions of the gastrointestinal enteric nervous system, researchers have identified a dozen or more different neurotransmitter substances that are released by the nerve endings of different types of enteric neurons, including: (1) acetylcholine, (2) norepinephrine, (3) adenosine triphosphate, (4) serotonin, (5) dopamine, (6) cholecystokinin, (7) substance P, (8) vasoactive intestinal polypeptide, (9) somatostatin, (10) leu-enkephalin, (11) met-enkephalin, and (12) bombesin. The specific functions of many of these substances are not known well enough to justify discussion here, other than to point out the following characteristics.
Acetylcholine most often excites gastrointestinal activity. Norepinephrine almost always inhibits gastrointestinal activity, as does epinephrine, which reaches the gastrointestinal tract mainly by way of the blood after it is secreted by the adrenal medullae into the circulation. The other aforementioned transmitter substances are a mixture of excitatory and inhibitory agents, some of which we will discuss in Chapter 64.
Autonomic Control of the Gastrointestinal Tract
Parasympathetic Stimulation Increases Activity of the Enteric Nervous System.
The parasympathetic supply to the gut is divided into cranial and sacral divisions, which were discussed in Chapter 61.
Except for a few parasympathetic fibers to the mouth and pharyngeal regions of the alimentary tract, the cranial parasympathetic nerve fibers are almost entirely in the vagus nerves. These fibers provide extensive innervation to the esophagus, stomach, and pancreas and somewhat less to the intestines down through the first half of the large intestine.
The sacral parasympathetics originate in the second, third, and fourth sacral segments of the spinal cord and pass through the pelvic nerves to the distal half of the large intestine and all the way to the anus. The sigmoidal, rectal, and anal regions are considerably better supplied with parasympathetic fibers than are the other intestinal areas. These fibers function especially to execute the defecation reflexes, discussed in Chapter 64.
The postganglionic neurons of the gastrointestinal parasympathetic system are located mainly in the myenteric and submucosal plexuses. Stimulation of these parasympathetic nerves causes general increase in activity of the entire enteric nervous system, which in turn enhances activity of most gastrointestinal functions.
Sympathetic Stimulation Usually Inhibits Gastrointestinal Tract Activity.
The sympathetic fibers to the gastrointestinal tract originate in the spinal cord between segments T5 and L2. Most of the preganglionic fibers that innervate the gut, after leaving the cord, enter the sympathetic chains that lie lateral to the spinal column, and many of these fibers then pass on through the chains to outlying ganglia such as to the celiac ganglion and various mesenteric ganglia. Most of the postganglionic sympathetic neuron bodies are in these ganglia, and postganglionic fibers then spread through postganglionic sympathetic nerves to all parts of the gut. The sympathetics innervate essentially all of the gastrointestinal tract, rather than being more extensive nearest the oral cavity and anus, as is true of the parasympathetics. The sympathetic nerve endings secrete mainly norepinephrine.
In general, stimulation of the sympathetic nervous system inhibits activity of the gastrointestinal tract, causing many effects opposite to those of the parasympathetic system. It exerts its effects in two ways: (1) to a slight extent by direct effect of secreted norepinephrine to inhibit intestinal tract smooth muscle (except the mucosal muscle, which it excites) and (2) to a major extent by an inhibitory effect of norepinephrine on the neurons of the entire enteric nervous system.
Strong stimulation of the sympathetic system can inhibit motor movements of the gut so greatly that this can literally block movement of food through the gastrointestinal tract.
Afferent Sensory Nerve Fibers From the Gut
Many afferent sensory nerve fibers innervate the gut. Some of the nerve fibers have their cell bodies in the enteric nervous system and some have them in the dorsal root ganglia of the spinal cord. These sensory nerves can be stimulated by (1) irritation of the gut mucosa, (2) excessive distention of the gut, or (3) the presence of specific chemical substances in the gut. Signals transmitted through the fibers can then cause excitation or, under other conditions, inhibition of intestinal movements or intestinal secretion.
In addition, other sensory signals from the gut go all the way to multiple areas of the spinal cord and even to the brain stem. For example, 80 percent of the nerve fibers in the vagus nerves are afferent rather than efferent. These afferent fibers transmit sensory signals from the gastrointestinal tract into the brain medulla which, in turn, initiates vagal reflex signals that return to the gastrointestinal tract to control many of its functions.
Gastrointestinal Reflexes
The anatomical arrangement of the enteric nervous system and its connections with the sympathetic and parasympathetic systems support three types of gastrointestinal reflexes that are essential to gastrointestinal control:
1. Reflexes that are integrated entirely within the gut wall enteric nervous system. These reflexes include those that control much gastrointestinal secretion, peristalsis, mixing contractions, local inhibitory effects, and so forth.
2. Reflexes from the gut to the prevertebral sympathetic ganglia and then back to the gastrointestinal tract. These reflexes transmit signals long distances to other areas of the gastrointestinal tract, such as signals from the stomach to cause evacuation of the colon (the gastrocolic reflex), signals from the colon and small intestine to inhibit stomach motility and stomach secretion (the enterogastric reflexes), and reflexes from the colon to inhibit emptying of ileal contents into the colon (the colonoileal reflex).
3. Reflexes from the gut to the spinal cord or brain stem and then back to the gastrointestinal tract. These reflexes include especially (1) reflexes from the stomach and duodenum to the brain stem and back to the stomach—by way of the vagus nerves—to control gastric motor and secretory activity; (2) pain reflexes that cause general inhibition of the entire gastrointestinal tract; and (3) defecation reflexes that travel from the colon and rectum to the spinal cord and back again to produce the powerful colonic, rectal, and abdominal contractions required for defecation (the defecation reflexes).
Hormonal Control of Gastrointestinal Motility
The gastrointestinal hormones are released into the portal circulation and exert physiological actions on target cells with specific receptors for the hormone. The effects of the hormones persist even after all nervous connections between the site of release and the site of action have been severed. Table 63-1 outlines the actions of each gastrointestinal hormone, as well as the stimuli for secretion and sites at which secretion takes place.
Table 63-1
Gastrointestinal Hormone Actions, Stimuli for Secretion, and Site of Secretion
| Hormone | Stimuli for Secretion | Site of Secretion | Actions |
| Gastrin | G cells of the antrum, duodenum, and jejunum | ||
| Cholecystokinin | I cells of the duodenum, jejunum, and ileum | ||
| Secretin | S cells of the duodenum, jejunum, and ileum | ||
| Gastric inhibitory peptide | K cells of the duodenum and jejunum | ||
| Motilin | M cells of the duodenum and jejunum |

In Chapter 65, we discuss the extreme importance of several hormones for controlling gastrointestinal secretion. Most of these same hormones also affect motility in some parts of the gastrointestinal tract. Although the motility effects are usually less important than the secretory effects of the hormones, some of the more important motility effects are described in the following paragraphs.
Gastrin is secreted by the “G” cells of the antrum of the stomach in response to stimuli associated with ingestion of a meal, such as distention of the stomach, the products of proteins, and gastrin-releasing peptide, which is released by the nerves of the gastric mucosa during vagal stimulation. The primary actions of gastrin are (1) stimulation of gastric acid secretion and (2) stimulation of growth of the gastric mucosa.
Cholecystokinin (CCK) is secreted by “I” cells in the mucosa of the duodenum and jejunum mainly in response to digestive products of fat, fatty acids, and monoglycerides in the intestinal contents. This hormone strongly contracts the gallbladder, expelling bile into the small intestine, where the bile, in turn, plays important roles in emulsifying fatty substances and allowing them to be digested and absorbed. CCK also inhibits stomach contraction moderately. Therefore, at the same time that this hormone causes emptying of the gallbladder, it also slows the emptying of food from the stomach to give adequate time for digestion of the fats in the upper intestinal tract. CCK also inhibits appetite to prevent overeating during meals by stimulating sensory afferent nerve fibers in the duodenum; these fibers, in turn, send signals by way of the vagus nerve to inhibit feeding centers in the brain as discussed in Chapter 72.
Secretin, the first gastrointestinal hormone discovered, is secreted by the “S” cells in the mucosa of the duodenum in response to acidic gastric juice emptying into the duodenum from the pylorus of the stomach. Secretin has a mild effect on motility of the gastrointestinal tract and acts to promote pancreatic secretion of bicarbonate, which in turn helps to neutralize the acid in the small intestine.
Glucose-dependent insulinotropic peptide (also called gastric inhibitory peptide [GIP]) is secreted by the mucosa of the upper small intestine, mainly in response to fatty acids and amino acids but to a lesser extent in response to carbohydrate. It has a mild effect in decreasing motor activity of the stomach and therefore slows emptying of gastric contents into the duodenum when the upper small intestine is already overloaded with food products. Glucose-dependent insulinotropic peptide, at blood levels even lower than those needed to inhibit gastric motility, also stimulates insulin secretion.
Motilin is secreted by the stomach and upper duodenum during fasting, and the only known function of this hormone is to increase gastrointestinal motility. Motilin is released cyclically and stimulates waves of gastrointestinal motility called interdigestive myoelectric complexes that move through the stomach and small intestine every 90 minutes in a person who has fasted. Motilin secretion is inhibited after ingestion of food by mechanisms that are not fully understood.
Functional Types of Movements in the Gastrointestinal Tract
Two types of movements occur in the gastrointestinal tract: (1) propulsive movements, which cause food to move forward along the tract at an appropriate rate to accommodate digestion and absorption, and (2) mixing movements, which keep the intestinal contents thoroughly mixed at all times.
Propulsive Movements—Peristalsis
The basic propulsive movement of the gastrointestinal tract is peristalsis, which is illustrated in Figure 63-5. A contractile ring appears around the gut and then moves forward; this mechanism is analogous to putting one's fingers around a thin distended tube, then constricting the fingers and sliding them forward along the tube. Any material in front of the contractile ring is moved forward.
Peristalsis is an inherent property of many syncytial smooth muscle tubes; stimulation at any point in the gut can cause a contractile ring to appear in the circular muscle, and this ring then spreads along the gut tube. (Peristalsis also occurs in the bile ducts, glandular ducts, ureters, and many other smooth muscle tubes of the body.)
The usual stimulus for intestinal peristalsis is distention of the gut. That is, if a large amount of food collects at any point in the gut, the stretching of the gut wall stimulates the enteric nervous system to contract the gut wall 2 to 3 centimeters behind this point, and a contractile ring appears that initiates a peristaltic movement. Other stimuli that can initiate peristalsis include chemical or physical irritation of the epithelial lining in the gut. Also, strong parasympathetic nervous signals to the gut will elicit strong peristalsis.
Function of the Myenteric Plexus in Peristalsis.
Peristalsis occurs only weakly or not at all in any portion of the gastrointestinal tract that has congenital absence of the myenteric plexus. Also, it is greatly depressed or completely blocked in the entire gut when a person is treated with atropine to paralyze the cholinergic nerve endings of the myenteric plexus. Therefore, effectual peristalsis requires an active myenteric plexus.
Peristaltic Waves Move Toward the Anus With Downstream Receptive Relaxation—“Law of the Gut.”
Peristalsis, theoretically, can occur in either direction from a stimulated point, but it normally dies out rapidly in the orad (toward the mouth) direction while continuing for a considerable distance toward the anus. The exact cause of this directional transmission of peristalsis has never been ascertained, although it probably results mainly from the fact that the myenteric plexus is “polarized” in the anal direction, which can be explained as follows.
When a segment of the intestinal tract is excited by distention and thereby initiates peristalsis, the contractile ring causing the peristalsis normally begins on the orad side of the distended segment and moves toward the distended segment, pushing the intestinal contents in the anal direction for 5 to 10 centimeters before dying out. At the same time, the gut sometimes relaxes several centimeters downstream toward the anus, which is called “receptive relaxation,” thus allowing the food to be propelled more easily toward the anus than toward the mouth.
This complex pattern does not occur in the absence of the myenteric plexus. Therefore, the complex is called the myenteric reflex or the peristaltic reflex. The peristaltic reflex plus the anal direction of movement of the peristalsis is called the “law of the gut.”
Mixing Movements
Mixing movements differ in different parts of the alimentary tract. In some areas, the peristaltic contractions cause most of the mixing. This is especially true when forward progression of the intestinal contents is blocked by a sphincter so that a peristaltic wave can then only churn the intestinal contents, rather than propelling them forward. At other times, local intermittent constrictive contractions occur every few centimeters in the gut wall. These constrictions usually last only 5 to 30 seconds; new constrictions then occur at other points in the gut, thus “chopping” and “shearing” the contents first here and then there. These peristaltic and constrictive movements are modified in different parts of the gastrointestinal tract for proper propulsion and mixing, as discussed for each portion of the tract in Chapter 64.
Gastrointestinal Blood Flow—Splanchnic Circulation
The blood vessels of the gastrointestinal system are part of a more extensive system called the splanchnic circulation, shown in Figure 63-6. It includes the blood flow through the gut plus blood flows through the spleen, pancreas, and liver. The design of this system is such that all the blood that courses through the gut, spleen, and pancreas then flows immediately into the liver by way of the portal vein. In the liver, the blood passes through millions of minute liver sinusoids and finally leaves the liver by way of hepatic veins that empty into the vena cava of the general circulation. This flow of blood through the liver, before it empties into the vena cava, allows the reticuloendothelial cells that line the liver sinusoids to remove bacteria and other particulate matter that might enter the blood from the gastrointestinal tract, thus preventing direct transport of potentially harmful agents into the remainder of the body.
The nonfat, water-soluble nutrients absorbed from the gut (such as carbohydrates and proteins) are transported in the portal venous blood to the same liver sinusoids. Here, both the reticuloendothelial cells and the principal parenchymal cells of the liver, the hepatic cells, absorb and store temporarily from one half to three quarters of the nutrients. Also, much chemical intermediary processing of these nutrients occurs in the liver cells. These nutritional functions of the liver are discussed in Chapters 68 through 72. Almost all of the fats absorbed from the intestinal tract are not carried in the portal blood but instead are absorbed into the intestinal lymphatics and then conducted to the systemic circulating blood by way of the thoracic duct, bypassing the liver.
Anatomy of the Gastrointestinal Blood Supply
Figure 63-7 shows the general features of the arterial blood supply to the gut, including the superior mesenteric and inferior mesenteric arteries supplying the walls of the small and large intestines by way of an arching arterial system. Not shown in the figure is the celiac artery, which provides a similar blood supply to the stomach.
Upon entering the wall of the gut, the arteries branch and send smaller arteries circling in both directions around the gut, with the tips of these arteries meeting on the side of the gut wall opposite the mesenteric attachment. From the circling arteries, still much smaller arteries penetrate into the intestinal wall and spread (1) along the muscle bundles, (2) into the intestinal villi, and (3) into submucosal vessels beneath the epithelium to serve the secretory and absorptive functions of the gut.
Figure 63-8 shows the special organization of the blood flow through an intestinal villus, including a small arteriole and venule that interconnect with a system of multiple looping capillaries. The walls of the arterioles are highly muscular and highly active in controlling villus blood flow.
Effect of Gut Activity and Metabolic Factors on Gastrointestinal Blood Flow
Under normal conditions, the blood flow in each area of the gastrointestinal tract, as well as in each layer of the gut wall, is directly related to the level of local activity. For instance, during active absorption of nutrients, blood flow in the villi and adjacent regions of the submucosa increases as much as eightfold. Likewise, blood flow in the muscle layers of the intestinal wall increases with increased motor activity in the gut. For instance, after a meal, the motor activity, secretory activity, and absorptive activity all increase; likewise, the blood flow increases greatly but then decreases back to the resting level over another 2 to 4 hours.
Possible Causes of the Increased Blood Flow During Gastrointestinal Activity.
Although the precise causes of the increased blood flow during increased gastrointestinal activity are still unclear, some facts are known.
First, several vasodilator substances are released from the mucosa of the intestinal tract during the digestive process. Most of these substances are peptide hormones, including cholecystokinin, vasoactive intestinal peptide, gastrin, and secretin. These same hormones control specific motor and secretory activities of the gut, as discussed in Chapters 64 and 65.
Second, some of the gastrointestinal glands also release into the gut wall two kinins, kallidin and bradykinin, at the same time that they secrete other substances into the lumen. These kinins are powerful vasodilators that are believed to cause much of the increased mucosal vasodilation that occurs along with secretion.
Third, decreased oxygen concentration in the gut wall can increase intestinal blood flow at least 50 to 100 percent; therefore, the increased mucosal and gut wall metabolic rate during gut activity probably lowers the oxygen concentration enough to cause much of the vasodilation. The decrease in oxygen can also lead to as much as a fourfold increase of adenosine, a well-known vasodilator that could be responsible for much of the increased flow.
Thus, the increased blood flow during increased gastrointestinal activity is probably a combination of many of the aforementioned factors plus still others yet undiscovered.
“Countercurrent” Blood Flow in the Villi.
Note in Figure 63-8 that the arterial flow into the villus and the venous flow out of the villus are in directions opposite to each other and that the vessels lie in close apposition to each other. Because of this vascular arrangement, much of the blood oxygen diffuses out of the arterioles directly into the adjacent venules without ever being carried in the blood to the tips of the villi. As much as 80 percent of the oxygen may take this short-circuit route and is therefore not available for local metabolic functions of the villi. The reader will recognize that this type of countercurrent mechanism in the villi is analogous to the countercurrent mechanism in the vasa recta of the kidney medulla, which was discussed in detail in Chapter 29.
Under normal conditions, this shunting of oxygen from the arterioles to the venules is not harmful to the villi, but in disease conditions in which blood flow to the gut becomes greatly curtailed, such as in circulatory shock, the oxygen deficit in the tips of the villi can become so great that the villus tip or even the whole villus undergoes ischemic death and disintegrates. For this reason and other reasons, in many gastrointestinal diseases the villi become seriously blunted, leading to greatly diminished intestinal absorptive capacity.
Nervous Control of Gastrointestinal Blood Flow
Stimulation of the parasympathetic nerves going to the stomach and lower colon increases local blood flow at the same time that it increases glandular secretion. This increased flow probably results secondarily from the increased glandular activity, not as a direct effect of the nervous stimulation.
Sympathetic stimulation, by contrast, has a direct effect on essentially all the gastrointestinal tract to cause intense vasoconstriction of the arterioles with greatly decreased blood flow. After a few minutes of this vasoconstriction, the flow often returns to near normal by means of a mechanism called “autoregulatory escape.” That is, the local metabolic vasodilator mechanisms that are elicited by ischemia override the sympathetic vasoconstriction, returning toward normal the necessary nutrient blood flow to the gastrointestinal glands and muscle.
Importance of Nervous Depression of Gastrointestinal Blood Flow When Other Parts of the Body Need Extra Blood Flow.
A major value of sympathetic vasoconstriction in the gut is that it allows shutoff of gastrointestinal and other splanchnic blood flow for short periods during heavy exercise, when the skeletal muscle and heart need increased flow. Also, in circulatory shock, when all the body's vital tissues are in danger of cellular death for lack of blood flow—especially the brain and the heart—sympathetic stimulation can decrease splanchnic blood flow to very little for many hours.
Sympathetic stimulation also causes strong vasoconstriction of the large-volume intestinal and mesenteric veins. This vasoconstriction decreases the volume of these veins, thereby displacing large amounts of blood into other parts of the circulation. In persons experiencing hemorrhagic shock or other states of low blood volume, this mechanism can provide as much as 200 to 400 milliliters of extra blood to sustain the general circulation.





