Salivary Glands
The salivary glands are a group of compound exocrine glands secreting saliva. Saliva is a complex fluid produced by the salivary glands. The saliva forms a film of fluid coating the teeth and mucosa thereby creating and regulating a healthy environment in the oral cavity.
The parenchymal elements are derived from the oral epithelium and consist of terminal secretory units leading into ducts that eventually open into the oral cavity. The connective tissue forms a capsule around the gland and extends into it, dividing groups of secretory units and ducts into lobes and lobules. The blood and lymph vessels and nerves that supply the gland are contained within the connective tissue.
The salivary glands are compound glands as they have more than one tubule entering the main duct. A duct is a passage that allows the glandular secretion emptied directly into an anatomic location where the secretion is to be used. The salivary glands have numerous ducts associated with them hence they are exocrine glands. The architectural arrangement of thesalivary glands is tubuloacinar, where acini are secretory units. These tubuloacinar units are merocrine as they release only the secretion of the cell from the secreting units.
Structure of terminal secretory units
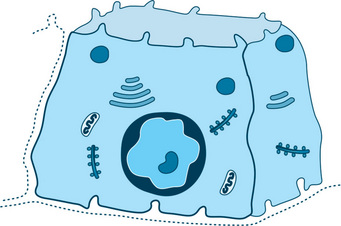
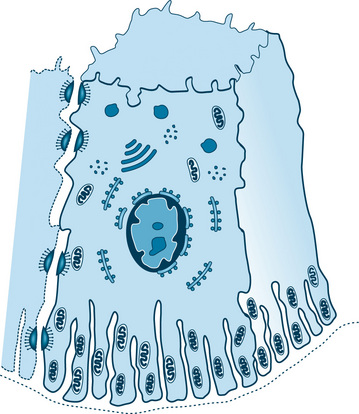
The basic functional unit of a salivary gland is the terminal secretory unit called acini. The terminal secretory unit irrespective of size and location is made up of epithelial secretory cells, namely serous and mucous cells. The serous, mucous along with myoepithelial cells are arranged in an acinus or acini (multiple) with a roughly spherical or tubular shape and a central lumen (Fig. 11.1).

Fig. 11.1 Schematic diagram of a typical salivary gland. (A) Serous acini, (A′) serous acini in cross-section. (B) Serous demilunes, (B′) serous demilunes in cross-section. (C) Mucous acini, (C′) mucous acini in cross-section. (D) Intercalated duct, (D′) intercalated duct in crosssection. (E) Striated duct, (E′) striated duct in cross-section. (F) Terminal excretory duct.
The cells in the acini rest on a basement membrane. They are arranged in a single layer. The intercellular spaces of the apical ends of the cells are separated from the lumen by junctional complexes which are tight (zonula occludens), intermediate junction (zonula adherens), and one or more desmosomes (maculae adherens). The junctional complexes hold the cells together in an acinus and regulate the permeability. Tight junctions seal the adjacent secretory cells, controlling paracellular ion influx. This helps in maintaining cell polarity and tissue homeostasis. The main tight junctional proteins are claudin, occludin and junctional adhesion molecules. The myoepithelial cells are located on the surface of the acini.
The central lumen of each acini may have a star-shaped morphology because of extension of lumen in between the cells called intercellular canaliculi. The central lumen of the acini continue via a fine series of ducts which constantly merge with each other and grow larger eventually to merge into the main excretory duct. These ducts comprise the ductal system.
The mucous acini have a larger lumen than serous acini (end piece).
The secretory terminal unit in serous acini is generally made of 8–12 serous acini surrounding a central lumen (Fig. 11.1).
Secretory end piece of mucous cells have a tubular configuration. The mucous cells are joined to each other by a variety of intracellular junctions but unlike the serous acini, they lack the presence of intercellular canaliculi. The intercellular canaliculi are said to be present only in acini with demilunes. Sometimes mucous acini have bonnet or crescent shaped covering which is made of serous cells. These are called demilunes (Fig. 11.1). The presence of demilunes is questioned. It has been shown thatdemilunes are as a result of artifact during tissue preparation. Recent methods like rapid freezing, freeze substitution and threedimensional reconstruction techniques have shown that serous cells align with mucous cells to surround a common lumen.
Serous cells
Serous secretory cells are pyramidal with a broad base on the basement membrane, the apex faces the lumen. The serous cells have a spherical nucleus placed at the basal region. The apical cytoplasm of these cells shows accumulation of secretory granules. The secretory granules are 1 μm in diameter with a distinct limiting membrane. In human beings the granules contain a dense core or a twisted skin like structure with a lighter matrix. They can be visualized in semi thin plastic embedded tissue section, stained with toluidine blue or specific cytochemical techniques. The granules are closely apposed to each other but retain their individuality (Fig. 11.2).
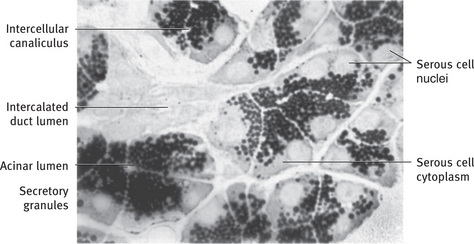
Fig. 11.2 Light micrograph of rat parotid gland illustrating general arrangement and cytologic features of serous cells. Gland was incubated in cytochemical medium to demonstrate the secretory enzyme peroxidase, resulting in unstained nuclei, lightly stained cytoplasm, and heavily stained secretory granules. Cells of intercalated duct are unreactive (1 μm; .990).
The granules are zymogen granules and are formed by glycolated proteins which are released into a vacuole. In electron microscopy the immature granules appear paler in density as compared to electron dense granules which are maturing and moving towards the luminal plasma membrane. The number of the secretory granules also vary with different levels of activity in an unstimulated or resting cell. There are numerous granules in the luminal portion of the cell, whereas in a stimulated cell the granules are few as they are depleted in huge numbers into the lumen by exocytosis.
The serous cells show acid phosphates, esterases, glucuronidase, glucosidase and galactoside activity.
The ultrastructural feature of a serous cell is typical of a protein secreting cell. A typical serous cell spends most of its synthetic capacity for producing the secretory protein. The basal cytoplasm is packed with parallelly stacked, with ribosome studded RER (rough endoplasmic reticulum). The RER is placed basal and lateral to the cell nucleus. A closed system of cisternae or membranous sacs constitutes RER (Fig. 11.3). The ribosomes consist of RNA and proteins.
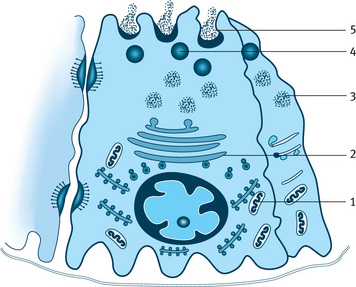
Fig. 11.3 Diagrammatic representation of serous cell and pathway of synthesis, storage and exocytosis of secretory protein. 1–Rough endoplasmic reticulum synthesizing protein, 2–Golgi complex transfer protein to transface, 3–Immature granules, 4–Mature granules with concentrated protein, 5–Exocytosis.
The nucleus of a cell by the way of m-RNA sends an encoded message which is translated by ribosomes. An appropriate amino acid with a specific sequence is synthesized. These proteins, or the preproteins have a NH2 terminal extension of 16–30 aminoacids called the signal sequence. As signal sequence is ready and emerges it is attached to the membrane of RER. RER recognizes them with the help of certain proteins and crosses the RER membrane along with the growing polypeptide chain. A proteolytic enzyme, signal peptidase removes the signal sequence and the protein newly synthesized reaches the cisternal space of RER. From here the protein is sent to the Golgi apparatus. The Golgi apparatus is a membranous cisternae of several stacks of 4 to 6 smooth surfaced saccules located apically and laterally to the cell nucleus. The Golgi apparatus is functionally connected to RER through budding vesicles at the end of RER. Each of the Golgi apparatus has a cis or convex face and a trans or concave face. The budding vesicles of RER enter the Golgi bodies from the cis face where the vesicles fuse with the Golgi saccules emptying its contents. The proteins migrate from the cis to trans face in the Golgi saccules where they are packed into vacuoles of variable density and size. These vacuoles are the forming secretory granules known by the name of condensing vacuoles, presecretory granules or immature granules. The immature granules are connected to the smooth membrane of the trans face. The limiting membrane of immature granules has irregularities which allow fusion of small vesicles. The immature granules which are pale increase in size and density to mature. This happens in a process of concentration gradient which continues during the transportation and packing of granules.
Following their synthesis, many secretory proteins undergo one or more covalent structural modifications prior to their secretion. The most common modification of salivary proteins is glycosylation (i.e., the addition of carbohydrate side chains to the amino acids asparagine, serine, and threonine in the protein). The carbohydrates of secretory glycoproteins include galactose, mannose, fructose, glucosamine, galactosamine, and sialic acid. Glycosylation is a multistep process that begins in the RER and is completed in the Golgi apparatus.
The mature granule stored at the apex of the cell is emptied into the lumen by exocytosis. This process involves the membrane of the granule to fuse with the plasma membrane of the cell at the lumen. This process prevents the loss of cell cytoplasm. Sometimes during rapid secretion a chain of granules may be released in the form of a string of pearls. This is called compound exocytosis. The serous cells devote 80% of its capacity in the production of zymogen granules but there are other activities also happening in the cell depicted by the other cell granules (Fig. 11.4).
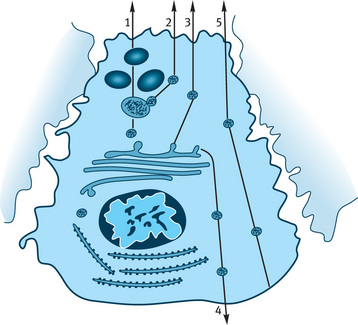
Fig. 11.4 Diagrammatic representation of possible vesicular protein secretory pathways in parotid acinar cells. 1–Storage granule pathway (main pathway), 2–Constitutive like pathway, 3– Constitutive pathway to the apical membrane, 4–Constitutive pathway to the basolateral membrane, 5–Transcytosis from basolateral to the apical membrane.
True or unattached ribosomes are seen which synthesize nonsecretory cellular proteins. A good number of mitochondria are seen in relation with RER and Golgi apparatus. They show the presence of enzymes of oxidative phosphoregulation, citric acid cycle and electron transport. In general they are power houses for numerous synthetic and transportation process. Lysosomes are seen with hydrolytic enzymes which help to destroy foreign material and worn out cell organelles.
Mucous cells
The mucous cell, like the serous cell, is specialized for the synthesis, storage, and secretion of a secretory product. However, its structure differs from that of the serous cell. In routine histologic preparations the apex of the cell appears empty except for thin strands of cytoplasm forming a trabecular network. The nucleus and a thin rim of cytoplasm are compressed against the base of the cell (Figs. 11.1 and 11.5).
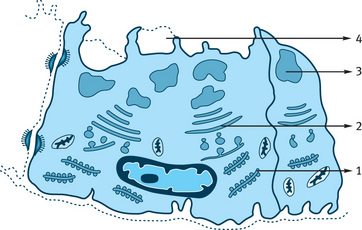
Fig. 11.5 Mucous cell and pathway of synthesis and exocytosis of mucous. 1–Rough endoplasmic reticulum synthesizing mucous protein, 2–Golgi complex transfer protein to transface, 3–Formation of mucous pool, 4–Exocytosis of mucous.
The mucous cell shows accumulations of large amounts of secretory product at the apical cytoplasm. The secretory product pushes the nucleus and endoplasmic reticulum against the basal cell membrane. The mucous secretion differs from secretion of serous in two important respects:
1. They have little or no enzymatic activity and probably serve mainly for lubrication and protection of the oral tissues.
2. The ratio of carbohydrate to protein is greater and larger amounts of sialic acid and occasionally sulfated sugars are present.
The differences in the carbohydrate content of a mucous cell and a serous cell can be demonstrated by histochemical staining techniques.
Most of the times the mucous secretion in a cell appears unstained in routine histologic section. However, when special stains like PAS or alcian blue are used they are strongly stained (Figs. 11.6 and 11.7).
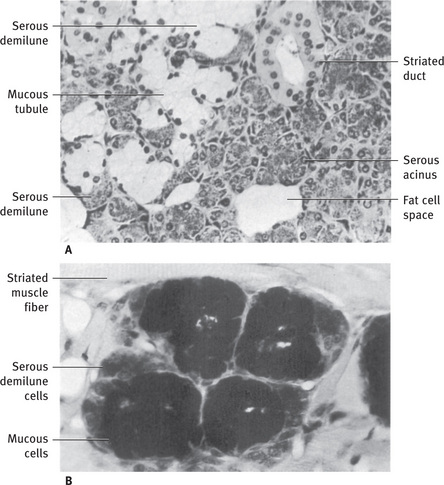
Fig. 11.6 (A) Light micrograph of human submandibular gland illustrating different appearance of mucous and serous cells. Mucous tubules are capped by serous demilunes. Two striated ducts are cut in cross-section. (B) Light micrograph of posterior lingual mucous gland of rat, stained with alcian blue and periodic acid-Schiff (PAS). Mucous secretory glycoprotein stains with both alcian blue and PAS, indicating acidic carbohydrate residues. Granules of serous demilune cells stain only with PAS, indicating neutral glycoproteins (A, .265; B, .420).
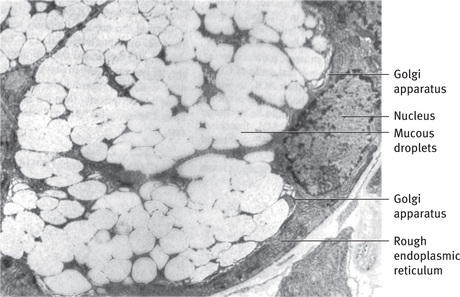
Fig. 11.7 Electron micrograph of mucous cell. Pale mucous droplets have flocculent content and tend to coalesce into larger masses. Golgi apparatus is well developed; rough endoplasmic reticulum and nucleus are compressed against base of cell (.7000).
The nucleus of the mucous cell is oval or flattened in shape and located just above the basal plasma membrane (Fig. 11.7). The RER is limited to a narrow band of cytoplasm along the base and lateral borders of the cell and to an occasional patch of cytoplasm between the mucous droplets. The mitochondria and other organelles are also primarily limited to this band of basal and lateral cytoplasm. The Golgi apparatus is large, consisting of several stacks of 10 to 12 saccules sandwiched between the basal RER and mucous droplets forming from the trans face. The Golgi apparatus plays an important role in these cells because of the large amount of carbohydrate that it adds to the secretory products.
The secretion of mucous droplets occurs by a somewhat different mechanism than the exocytotic process seen in the serous cells. When a single droplet is discharged, its limiting membrane fuses with the apical plasma membrane, resulting in a single membrane separating the droplet from the lumen. This separating membrane may then fragment, being lost with the discharge of mucus, or the droplet may be discharged with the membraneintact, surrounding it. During rapid droplet discharge, the apical cytoplasm may not seal itself off, and the entire mass of mucus may be spilled into the lumen (Fig. 11.5).
Myoepithelial cells
Myoepithelial (ME) cells are closely related to the secretory and intercalated duct cells.
They are stellate or spider-like, with a flattened nucleus, scanty perinuclear cytoplasm and long branching processes that embrace the secretory and duct cells (Fig. 11.8). In case of intercalated ducts the myoepithelial cells have a more fusiform shape and are elongated with a few short processes. The processes in the acini lie in the ‘gutters’, hence the outline of the acini appears smooth but in the intercalated duct the processes runs longitudinally on the surface creating a bulge. Their appearance is reminiscent of a basket cradling the secretory unit, hence the terms “basket cell”. ME cells are similar to smooth muscle cells but are derived from epithelium. These cells are located around the terminal secretory units and the first portion of the duct system. They lie between the basement membrane of the parenchyma cells and are attached to the cells by desmosomes.
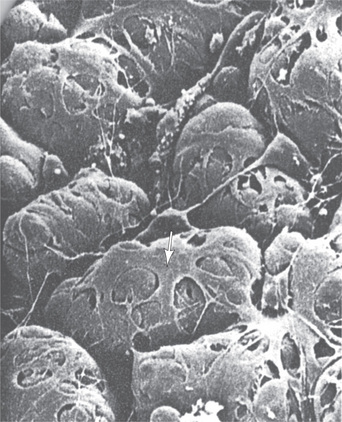
Fig. 11.8 Scanning electron micrograph of myoepithelial cells (arrow) and their processes covering the basal surfaces of the acinar cell. The basal lamina has been digested to reveal the basal surface of the acinar cells.
Myoepithelial cells are difficult to identify in routine histologic preparations, but their typical stellate shape can be observed in sections stained by special histochemical or immunofluorescent techniques (Fig. 11.9A).
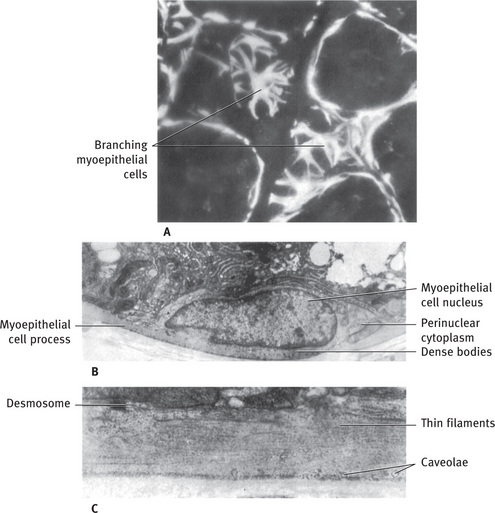
Fig. 11.9 (A) Fluorescent micrograph of rat sublingual gland treated with antibody to smooth muscle myosin, to localize myosin present in myoepithelial cells. Tangential sections of acini reveal branching nature of myoepithelial cells; myoepithelial cell processes cut in cross and longitudinal section surround adjacent acini. (B) Electron micrograph of myoepithelial cell body showing concentration of organelles in perinuclear cytoplasm and processes filled with fine filaments. The “dense bodies” are characteristic of myoepithelium and smooth muscle. Rat sublingual gland. (C) Higher magnification of myoepithelial cell process filled with longitudinally arranged thin filaments. Several caveolae are located along basal surface of cell, and a desmosome attaches the process to mucous cell (Rat sublingual gland; A, .700; B, .6000; C, .19,100) (A Courtesy D Drenckhahn, Kiel, Federal Republic of Germany).
ME cells contain cytokeratin intermediate filament and contractile actin filaments. These can be used to help identify them using immunocytochemistry. The presence of cytokeratin confirms the epithelial origin of myoepithelial cells.
The usual appearance of myoepithelial cells in electron micrographs is a section through one of their processes lying in a groove on the surface of a secretory or duct cell. The processes are filled with longitudinally oriented fine filaments about 6 nm (60 Å) thick (Fig. 11.9C). Small dense bodies are frequently present between the thin filaments; these are also present in smooth muscle cells, where they appear to form a cytoskeletal network in association with 10 nm (100 Å) diameter filaments. The usual cytoplasmic organelles are largely restricted to the perinuclear cytoplasm. The body of the cell, containing the nucleus, often lies in the space where the basal regions of two or three parenchymal cells come together (Fig. 11.9B). The plasma membrane of the myoepithelial cell closely parallels the basal membrane of the parenchymal cell, and the two are joined by occasional desmosomes. Numerous micropinocytotic vesicles, or caveolae, are located on the plasma membranes of the myoepithelial cells. The myoepithelial cells are innervated through the parasympathetic motor nerve.
The precise functional role of ME cells in salivary secretion is not very clear, however the following structural details clearly indicate its contractile function.
1. Structure of ME cells is similar to that of smooth muscles.
2. Immunofluorescent studies indicate presence of myosin, actin and related proteins.
3. After appropriate stimulation, the measurement of ductal pressure of ME cell indicates a contractile process.
4. Cinemicrography of individual secretory unit stimulated to secrete in vivo reveal a regular pulsatile movement of the entire unit.
The functions related to ME cells indicate clearly that ME cells actively can:
1. Accelerate the initial outflow of saliva from the acini.
2. Reduce luminal volume. The intercalated ducts may shorten and widen the ducts helping to maintain their patency.
3. Contribute to secretory pressure in the acini or duct.
4. Support the underlying parenchyma and reduce the back permeation of fluid.
5. Help salivary flow to overcome increase in peripheral resistance of the ducts.
Recent studies show ME cells are involved in signaling the secretory cells and protecting the salivary gland tissue. The ME cells provide signals to the acinar secretory cells that are needed for maintaining cell polarity and the structural organization of the acinus. There is evidence that ME cells also produce a number of proteins that have tumor suppressor activity, such as proteinase inhibitors and anti-angiogenesis factors, which act as barriers against invasive epithelial neoplasms.
Ducts
The ductal system of salivary glands consists of hollow tubes connected initially with the acinus and then with other ducts as the ducts progressively grow larger from the inner to the outer portion of the gland. Each type of duct is lined by different type of epithelium, depending on its location in the gland. In comparison each major salivary gland displays differences in the length or types of duct present.
The ductal system is not just a pipeline or conduit for the passageway for the saliva; it also actively participates in the production and modification of saliva.
In a salivary gland the smallest ducts are the intercalated ducts connecting the terminal secretory units to the next larger duct, the striated ducts. In the interlobar tissue the ducts continue to join one another increasing in size until the main excretory duct is formed.
Salivary glands have varying number of lobules depending on their size and each lobule is surrounded by connective tissue. The ductal system is sometimes also named according to its location. Some within the lobule, meaning intralobular ducts and some are interlobular ducts, which lie within the connective tissue within the lobules of the gland.
There are two types of intralobular ducts—the intercalated and striated ducts. The excretory ducts are interlobular.
Intercalated ducts
The intercalated ducts (Figs. 11.1 and 11.10) are lined by a single layer of low cuboid cells with relatively empty-appearing cytoplasm. They are often difficult to identify in the light microscope because they are compressed between secretory units. In electron micrographs the intercalated duct cells share several characteristics of serous cells (Fig. 11.10B). A small amount of RER is located in the basal cytoplasm, and a Golgi apparatus of moderate size is found apically. In proximally located cells (near the secretory units) a few small secretory granules may be found. The lateral membranes of adjacent cells are joined apically byjunctional complexes and several desmosomes. One or two areas of prominent interlocking folds of the lateral surface are located further basally (Fig. 11.11). At the periphery of the duct, processes and cell bodies of myoepithelial cells may be found, attached by desmosomes to the duct cells.
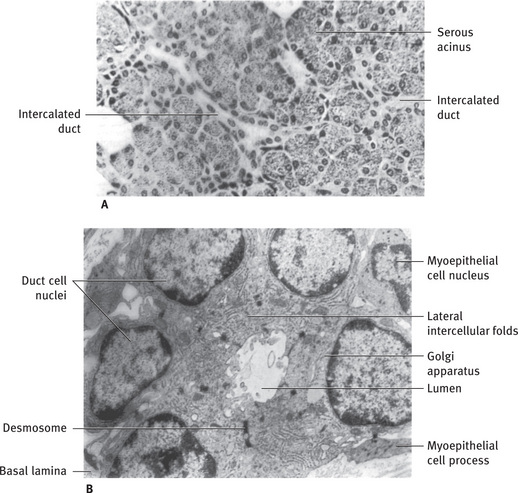
Fig. 11.10 (A) Light micrograph of human parotid gland showing long branching intercalated ducts between serous acini. (B) Electron micrograph of intercalated duct cut in cross-section. Duct cells contain a moderate amount of rough endoplasmic reticulum and a prominent Golgi apparatus but few or no secretory granules. Prominent desmosomes and interlocking folds are present between adjacent cells. Myoepithelial cell processes extend longitudinally along duct, inside basal lamina (Rat parotid gland A, .265; B, .9600).
The intercalated ducts, do not act as a simple conduit but modify the saliva through secretory and absorptive process. The intercalated ducts contribute to macromolecular components like lysozymes, lactoferrin and some unknown components to the saliva. These are stored in the secretory granules of the cells. It is believed that intercalated duct also house undifferentiated cells which can undergo differentiation to replace damaged or dying cells in the end piece or striated ducts.
Striated ducts
The striated ducts receive saliva from the intercalated ducts. They form the largest portion of the duct system constituting the intralobular component of the duct system.
The striated ducts are lined by a layer of tall columnar epithelial cells with large, spherical, centrally placed nuclei (Figs. 11.2 and 11.12). The cytoplasm is abundant and eosinophilic and shows prominent striations at the basal ends of the cells, perpendicular to the basal surface. An occasional basally located cell can be identified by the position of its nucleus, below the level of those of the other cells.
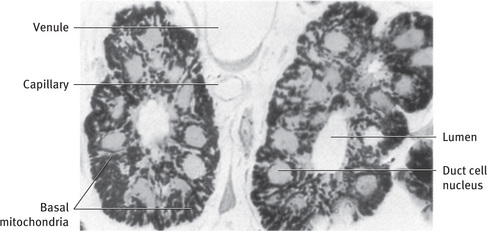
Fig. 11.12 Light micrograph of two striated ducts cut in cross-section. Large, primarily radially oriented mitochondria, stained for cytochrome oxidase activity, fill basal regions of duct cells. Unstained nuclei are centrally located, and small mitochondria are found in apical cytoplasm (Rat parotid gland; .990).
In electron micrographs the basal cytoplasm of the striated duct cells is partitioned by deep infoldings of the plasmamembrane, producing numerous sheet like folds that extend beyond the lateral boundaries of the cell and interdigitate with similar folds of adjacent cells (Fig. 11.13). Abundant large mitochondria, usually radially oriented, are located in portions of the cytoplasm between the membrane infoldings (Figs. 11.14 and 11.15). The combination of infoldings and mitochondria accounts for the striations seen in the light microscope.
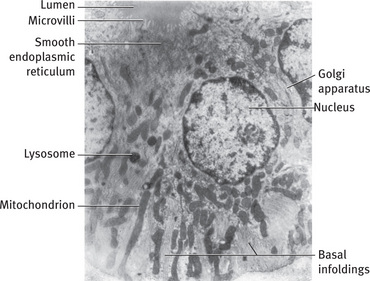
Fig. 11.13 Electron micrograph of striated duct cells of rat parotid gland. Numerous mitochondria are located between infoldings of basal plasma membrane. A few lysosomes and the Golgi apparatus are located in perinuclear region, and smooth endoplasmic reticulum is found in cell apices. Short microvilli project into lumen (.7400).
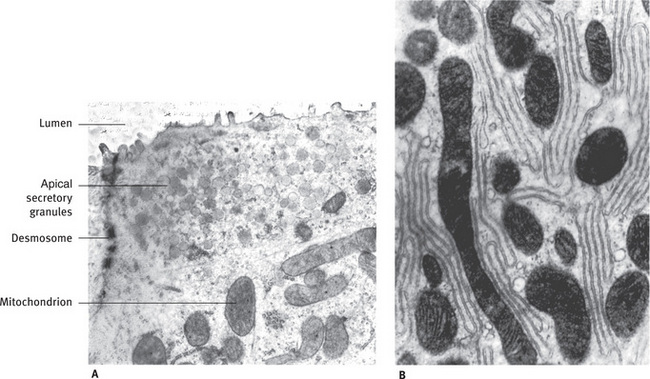
Fig. 11.14 (A) Electron micrograph of apical cytoplasm of striated duct cell. Small secretory granules are located near lumen. Mitochondria and smooth and rough endoplasmic reticulum are also present. (B) Basal region of striated duct cell, showing mitochondria and infolded plasma membranes (Rat sublingual gland; A, .22,200; B, .23,900).
The striations corresponding to multiple infolding of the plasma membrane of the cells packed with mitochondria occupying a large surface area with high levels of energy, clearly indicating that the cell is involved in active transport. The striated ducts are site of electrolyte reabsorption especially of sodium and chloride and secretion of potassium and bicarbonate. This reabsorption is against a concentration gradient, hence requires a substantial amount of energy. The luminal contents is converted from an isotonic or a slightly hypertonic fluid into a hypotonic fluid. The striated ducts also modify the organic content of the primary saliva. The duct cells synthesize and secrete glycoproteins such as kallikrein and epidermal growth factor. The cells are also capable of reabsorbing proteins from the luminal surface by endocytic mechanisms, this is evident by the presence of vesicles in the cytoplasm.
Essentially all of the water enters saliva at the level of the terminal secretory units; the striated and excretory ducts appear to be relatively impermeable to water. The ductal reabsorptionof Na+ and Cl– exceeds the secretion of K+ and HCO–3, leaving a hypotonic luminal fluid. Since active transport of water does not occur, the ducts cannot secrete water against the osmotic gradient to produce the final hypotonic saliva.
The single layered epithelium of striated ducts contains simple cytokeratin intermediate filaments 8 and 18.
Excretory ducts
The striated ducts join each other to form larger intralobular ducts. These ducts gradually increase in size and are surrounded by increasing layers of connective tissue. Progressively along the path, the duct becomes nonstriated and large, to become the excretory interlobular duct.
As the excretory duct enlarges it contains two layers: the mucosa and the outer connective tissue adventitia. The mucosal epithelium of the duct consists of pseudostratified columnar epithelium cells. In larger ducts occasional goblet cells and ciliated cells may be seen. The ductal epithelium slowly undergoes a transition to stratified epithelium, cuboidal epithelium and finally into stratified squamous epithelium when it merges with the epithelium of the oral cavity. When stratified, the duct epithelium contains keratin, the intermediate filament types are typical of stratified epithelium of oral cavity. The connective tissue on the external surface has collagen and elastic fibers which allow passive stretching of the duct to allow and accommodate varying volumes of saliva.
In the excretory ducts a small number of other types of cells are present. Tuft or brush cells with long stiff microvilli and apical vesicles are seen. They are thought to be receptor cells as they show nerve endings adjacent to the basal portion of the cell. Sometimes cells with pale cytoplasm and dense nuclear chromatin are seen towards the base of the duct epithelium. They appear to be lymphocytes and macrophages.
Dendritic cells or antigen presenting cells are seen as cells with long branching processes that extend between the epithelial cells. These are involved in processing and presentation of foreign antigens to T-lymphocytes and in immune surveillance.
Connective tissue elements
The cells found in the connective tissue of the salivary glands are the same as those in other connective tissues of the body and include fibroblasts, macrophages, mast cells, occasionalleukocytes, fat cells, and plasma cells. The cells, along with collagen and reticular fibers, are embedded in a ground substance composed of proteoglycans and glycoproteins.
Connective tissue of the salivary gland consists of a surrounding capsule that demarcates the gland from the adjacent structures. The extension of the connective tissue as septa inward from the capsule divide the gland into lobes and lobules. The septa contain blood vessels and nerves that supply the parenchymal components (glandular components) and excretory ducts.
The plasma cells produce immunoglobulins that are secreted into the saliva by transcytosis.
The main immunoglobulin in the saliva is the IgA, which is synthesized as a dimer complexed with an additional protein called J chain. The receptor bound IgA clinging with a portion of the receptor called secretory component is released at the luminal surface of the cell. Small amounts of IgG and IgM are also secreted into the saliva.
In the lobules of the salivary gland, finer partitions of connective tissue extend in between the adjacent end pieces and ducts. They carry the arterioles, capillaries and venule of the microcirculation and branches of the autonomic nerves that innervate the secretory and ductal cells.
Blood supply
The vascular supply to the gland is also embedded within the connective tissue, entering the glands along the excretory ducts and branching to follow them into the individual lobules.
Since the salivary secretion is predominantly 99% water it necessitates an extensive blood supply. The ducts, to the level of the intralobular striated ducts, are supplied with a dense capillary network: the capillary loops to the intercalated ducts and terminal secretory units are less extensive. Arteriovenous communications are seen around the larger interlobular ducts.
An extensive capillary plexus originates from separate arterioles that exists around the excretory ducts. The endothelium of the capillaries and postcapillary venules are fenestrated.
The venous return follows the arterial supply. In the arteriovenous anastomoses when the blood flow increases during increased secretion more blood is diverted through the anastomoses resulting in increased venous and capillary pressure, therefore increasing the fluid filtration rate across the capillary endothelium which takes care of the fluid necessary for maintaining the secretion.
Nerve supply and pattern of innervation
The main branches of the nerves supplying the glands follow the course of the vessels, breaking up into terminal plexuses in the connective tissue adjacent to the terminal portions of the parenchyma.
Both postganglionic nerve fibers of the sympathetic and parasympathetic divisions of the autonomic nervous system innervate the glands. The preganglionic parasympathetic fibers originate in the superior or the inferior salivatory nuclei in the brainstem and travel via the facial and glossopharyngeal nerves to the submandibular and otic ganglia. In the ganglia they synapse with the postganglionic neurons. The postganglionic fibers reach the gland through the lingual and auriculotemporal nerve. Preganglionic sympathetic nerves originate in the thoracic spinal cord, synapse with postganglionic neurons in the superior cervical ganglion. The postganglionic fibers reach the glands traveling along with the arterial blood supply.
In the gland lobules branches of nerve follow blood vessels finally forming a plexus of unmyelinated fibers. The axons of the nerve are invested by the cytoplasmic processes of Schwann cells and are distributed to secretory cells, myoepithelial cells, the smooth muscles of the arterioles and possibly intercalated nonstriated ducts.
The secretory cells receive their innervation by one of the two patterns. In the intraepithelial type (intraparenchymal), the axons split off from the nerve bundle and penetrate the basal lamina, lying adjacent to or between the secretory cells (Fig. 11.16). As the axons pass through the basal lamina, the Schwann cell covering is usually lost; occasionally it may be continued into the parenchyma and lie between the axons and the secretory cell. The site of innervation (neuroeffector site) is considered to be at varicosities of the axon, which contain small vesicles and mitochondria. The vesicles are believed to contain the chemical neurotransmitters norepinephrine and acetylcholine and presumably release them by an exocytosis like process. The membranes of the axon and secretory cell are separated by a space of only 10 to 20 nm (100 to 200 Å), but no specializations of the plasma membranes have been detected at these sites. A single axon may have several varicosities along its length, making contact with the same cell or with two or more cells.
The second type of innervation is subepithelial (extraparenchymal). Instead of penetrating the basal lamina, the axons remain associated with the nerve bundle in the connective tissue (Fig. 11.17). Where the nerve bundles approach the secretory cells, some of the axonal varicosities, which contain the small neurotransmitter vesicles, lose their covering of Schwann cell cytoplasm. Presumably, these bared axonal varicosities are the sites of transmitter release. The axons remain separated from the secretory cells by 100 to 200 nm (1000 to 2000 Å), andthe transmitters must diffuse across this space, which includes the basal lamina of the secretory cells and the nerve bundle.
The intraparenchymal and extraparenchymal innervations vary not only among the glands but also among different cells within the same single gland. Intraparenchymal innervation is seen occurring in the human submandibular gland and in the minor glands of the lip, whereas extraparenchymal innervation occurs in the parotid gland. The different types of innervation have no functional difference.
In some glands both sympathetic (adrenergic) and parasympathetic (cholinergic) terminals (distinguished by special fixation and cytochemical techniques) have been observed in proximity to the secretory cells. Similarly, physiologic studies indicate that the cells of some glands respond to both sympathetic and parasympathetic stimulation by changes in their membrane potential. However, the extent of participation by each division varies between glands and the composition of the saliva secreted in response to stimulation of each division is distinctly different. In general, a copious flow of watery saliva is secreted in response to parasympathetic stimulation, whereas that produced by sympathetic stimulation is thicker, higher in organic content, and comparatively less in quantity.
The innervation of duct cells is not clear. Intraepithelial terminals in ducts have been observed only rarely, but histochemical studies suggest that cholinergic and adrenergic nerves are found in the connective tissue around the ducts. Physiologic studies indicate that the ductal system is responsive to autonomic stimulation and membrane potential changes in the duct cells have been recorded, as well as changes in the transductal ion flux.
Classification and structure of human salivary glands
The salivary glands have been classified in a variety of ways, the most commonly used groupings are based on:
1. Size and location; namely major and minor gland and based on location as labial and lingual, etc.
2. Histochemical nature of secretory product; namely serous and mucous.
Though it has been seen that all serous secretions are not alike they may differ considerably in the type and the amount of enzymes and proteins they produce. The carbohydrate component shows a similar variability. This is seen in mucous cells also. Hence, histochemical characterization of the secretory products of the different salivary glands may be useful for the comparison with the other glands.
On basis of weight of salivary glands producing saliva, the volume exceeds that of other digestive organs by as much as 40 times.
Major salivary glands
The largest of the glands are the three bilaterally paired major salivary glands. They are all located extraorally, and their secretions reach the mouth by variably long ducts.
Parotid gland
The parotid is the largest major salivary gland. Its superficial portion is located subcutaneously lying in front of the external ear and its deeper portion lies behind the ramus of the mandible, filling the retromandibular fossae.
The parotid is 5.8 cm craniocaudally and 3.4 cm ventrodorsally. It weighs between 14 and 28 grams.
The main excretory duct, Stensen's duct crosses the masseter muscle and turns medially at the anterior edge penetrating the buccinator muscle to open at a papilla at the buccal mucosa opposite the maxillary 2nd molar. The duct measures 4–6 cm in length and 5 mm in diameter. A small portion of the parotid generally accompanies the duct forming an accessory gland, a few millimeter anterior to the superficial portion of the gland. The parotid gland receives its blood supply from the branches of the external carotid artery as they pass through the gland. The parasympathetic nerve supply is derived mainly from the ninth cranial nerve reaching the gland via the otic ganglion and the auriculotemporal nerve. The sympathetic innervation of all salivary glands is provided by the postganglionic fibers from the superior cervical ganglion and reaches the individual gland in association with their vascular supply.
The lymphatic drainage is via paraparotid and intraparotid lymph nodes into the superficial and deep cervical lymph nodes. The parotid gland is enclosed in a well defined connective tissue capsule which sends septa into the gland, separating it into lobes and lobules.
The parotid gland is a pure serous gland (Fig. 11.18A); all the acinar cells. The parotid gland is a pure serous gland (Fig. 11.18A); all the acinar cells are similar in structure to the serous cells described earlier. In the infant, however, a few mucous secretory units may be found. Electron microscopic studies indicate that the serous granules may have a dense central core. The intercalated ducts of the parotid are long and branching (Fig. 11.10), and the pale-staining striated ducts are numerous and stand out conspicuously against the more densely stained acini. The connective tissue septa in the parotid contain numerous fat cells, which increase in number with age and leave an empty space in histologic sections.
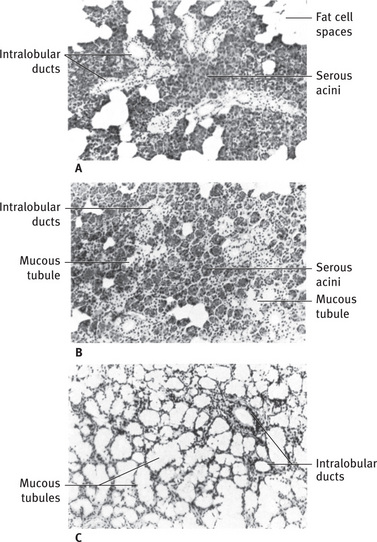
Fig. 11.18 (A) Light micrograph of human parotid gland, showing serous acini, several intralobular striated ducts, and numerous fat cell spaces. (B) Light micrograph of human submandibular gland. Serous acini predominate, but a few mucous secretory units are present. Several intralobular striated ducts are cut in crosssection. (C) Light micrograph of human sublingual gland showing large mucous secretory units with typical tubular structure. Serous demilunes are difficult to distinguish at low magnification. Intralobular ducts are poorly developed (A to C,. 90).
Submandibular gland
The submandibular gland is the second largest salivary gland, also called the submaxillary salivary gland. It weighs half the weight of parotid gland.
The submandibular gland is on the medial aspect of the body of the mandible in the submandibular triangle. It is placed posterior and superficial to the mylohyoid muscle with an extension folded around the posterior border of the mylohyoid to be above the muscle. The main excretory duct, Wharton's duct, runs forward above the mylohyoid muscle lying just below the mucosa of the floor of the mouth in its terminal portion. It opens at the sublingual papillae also called the caruncula sublingualis, lateral to the lingual frenum.
The gland receives blood supply from the lingual and facial arteries. The parasympathetic innervation is derived primarily from the VII cranial nerve reaching the gland through the lingual nerve after synapsing in the submandibular ganglion.
The lymphatic drainage is to the deep cervical and jugular chain of nodes.
The submandibular gland is enveloped by a well defined capsule. It is a branched tubuloacinar gland of mixed type.
The submandibular gland is a mixed gland, with both serous and mucous secretory units (Fig. 11.18B). The serous units predominate, but the proportions may vary from one lobule to the next. The mucous terminal portions are capped by demilunes of serous cells. The existence of demilunes has been recently questioned. Although they appear similar by light microscopy, notable differences between submandibular and parotid serous cells are observed in the electron microscope (Fig. 11.19). The basal and lateral plasma membranes are thrown into numerous folds, interdigitating with similar processes from adjacent cells. The serous granules exhibit a variable substructure, from a granular matrix with a dense core or crescent, to an irregular skein of dense material dispersed in the matrix. The intercalated ducts tend to be ducts that are somewhat shorter than those of the parotid, whereas the striated ducts are usually longer.
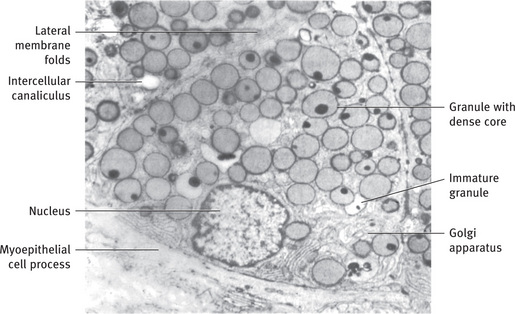
Fig. 11.19 Electron micrograph of serous cells of human submandibular gland, showing secretory granules with dense core. Immature granules with similar cores are seen in Golgi regions. Several intercellular canaliculi are cut in cross-section, and extensive folding of lateral cell membranes occurs between adjacent cells. Myoepithelial cell process is present at base of cell (.6600) From Tandler B and Erlandson RA: Am J Anat 135:419, 1972; reprinted by permission of the Wistar Institute Press.
Sublingual gland
Sublingual gland is the smallest of the major salivary glands which is almond shaped. The sublingual gland lies between the floor of the mouth, below the mucosa and above the mylohyoid muscle (Fig. 11.1). It is composed of one main gland with several small glands. The main duct; Bartholin's duct opens with or near the submandibular duct. Several smaller ducts; duct of Rivinus, open independently along the sublingual fold.
The sublingual is also a mixed gland, but the mucous secretory units greatly outnumber the serous units (Figs. 11.18C and 11.20). The mucous cells are usually arranged in a tubular pattern; serous demilunes may be present at the blind ends of the tubules. Pure serous acini are rare or absent. The intercalated and striated ducts are poorly developed; mucous tubules may open directly into ducts lined with cuboid or columnar cells without typical basal striations.
Fig. 11.20 Sublingual salivary gland lying beneath oral mucosa shows predominantly pale staining mucous acini with serous demilunes.
The sublingual gland receives its blood supply from the sublingual and the submental arteries. The parasympathetic nerve supply is also derived from the VII cranial nerve. It reaches the gland via the lingual nerve after synapsing in the submandibular ganglion. The lymphatic drainage is to the submandibular lymph nodes.
Minor salivary glands
The minor salivary glands are located beneath the epithelium in almost all parts of the oral cavity. These glands usually consist of several small groups of secretory units opening via short ducts directly into the mouth. They lack a distinct capsule, instead mixing with the connective tissue of the submucosa or muscle fibers of the tongue and cheek.
There are 600 to 1000 minor salivary glands lying in the oral cavity and the oropharynx.
The minor salivary glands are classified according to their anatomic location, e.g. labial glands, buccal glands, lingual glands, palatine and glossopalatine glands, etc. They are not present in the gingiva, anterior raphe region of the hard palate or the anterior two thirds of the dorsum of the tongue.
Labial and buccal glands
The glands of the lips and cheeks classically have been described as mixed, consisting of mucous tubules with serous demilunes. However, ultrastructural studies of the labial glands have revealed the presence of mucous cells only. Intercellular canaliculi have also been observed between the mucous cells. The intercalated ducts are variable in length, and the intralobular ducts possess only a few cells with basal striations. Although the buccal glands have not been examined by electron microscopy, they are usually described as a continuation of the labial glands with a similar structure.
Glossopalatine glands
The glossopalatine glands are pure mucous glands. They are principally localized to the region of the isthmus in the glossopalatine fold but may extend from the posterior extension of the sublingual gland to the glands of the soft palate.
Palatine glands
The palatine glands are also of the pure mucous variety. They consist of several hundred glandular aggregates in the lamina propria of the posterolateral region of the hard palate and in the submucosa of the soft palate and uvula. The excretory ducts may have an irregular contour with large distensions as they course through the lamina propria. The openings of the ducts on the palatal mucosa are often large and easily recognizable.
Lingual glands
The glands of the tongue can be divided into several groups. The anterior lingual glands (glands of Blandin and Nuhn) are located near the apex of the tongue. The anterior regions of the glands are chiefly mucous in character, whereas the posterior portions are mixed. The ducts open on the ventral surface of the tongue near the lingual frenum. The posterior lingual mucous glands (Fig. 11.21) are located lateral and posterior to the vallate papillae and in association with the lingual tonsil. They are purely mucous in character, and their ducts open onto the dorsal surface of the tongue. The posterior lingual serous glands (von Ebner's glands) are an extensive group of purely serous glands located between the muscle fibers of the tongue below the vallate papillae (Fig. 11.21). Their ducts open into the trough of the vallate papillae and at the rudimentary foliate papillae on the sides of the tongue.
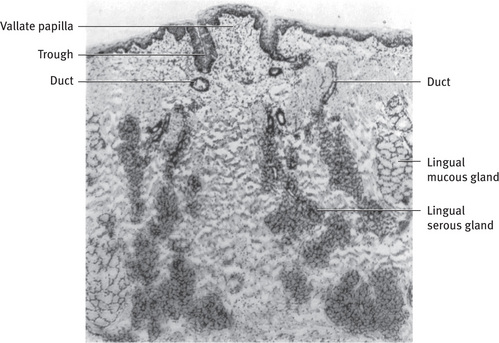
Fig. 11.21 Light micrograph of minor salivary glands of rat tongue. Lingual serous (von Ebner's) gland is located between muscle fibers of tongue below vallate papilla. Its ducts empty into trough around papilla. Posterior lingual mucous glands are located lateral to serous glands; their ducts open onto surface of tongue (.36) From Hand AR: J Cell Biol 44:340, 1970; reprinted by permission of the Rockefeller University Press.
Von Ebner's glands
Of all of the minor salivary glands, the posterior lingual serous glands (von Ebner's glands) are among the most interesting. Classically, their secretions have been described as serving to wash out the trough of the papillae and ready the taste receptors (located in the epithelium of the trough) for a new stimulus. Although this may be a part of their function, studies suggest that these glands have significant protective and digestive functions. Histochemical studies have localized the antibacterial enzymes peroxidase and lysozyme to these glands in humans. Biochemical studies of the lingual serous glands have demonstrated the presence of a secretory enzyme with lipolytic activity; similar lipolytic activity has been detected in aspirates from the esophagus and stomach. This lingual lipase has an acid pH optimum so that it is capable of hydrolyzing triglycerides in the stomach. The fatty acids, monoglycerides and diglycerides produced by lingual lipase, help to emulsify the remaining fat and increase the efficiency of pancreatic lipase in the intestine. In the newborn, when fat intake is high and levels of pancreatic lipase are low, lingual lipase probably plays a significant role in lipid digestion. Amylase activity has also been detected in the lingual serous glands of some species.
Development and growth
Salivary glands are specialized secretory apparatus present from the amphibian upward. They show varying differentiation, structure and arrangement in different species, to cite an example, the parotid is present only in mammals.
In human beings all the salivary glands arise from the ectoderm of the oral cavity and are comparable to other ectodermal derivatives such as sebaceous and mammary gland.
The minor salivary glands arise from the oral ectoderm and nasopharyngeal ectoderm. They develop after the major salivary glands.
During fetal life each salivary gland is formed at a specific location in the oral cavity through the growth of a bud of oral epithelium into the underlying mesenchyme. The primordial of the parotid and submandibular glands of humans appear during the 6th week, whereas the primordium of the sublingual gland appears after 7 to 8 weeks of fetal life. The minor salivary glands begin their development during the 3rd month. The epithelial bud grows into an extensively branched system of cords of cells that are first solid but gradually develop a lumen and become ducts. The secretory portions develop later than the duct system and form by repeated branching and budding of the finer cell cords and ducts.
The primordium of salivary glands is divided into pregland and preduct cells. The pregland cells take part in the formation of acini whereas the preduct cells are again divided into anterior and posterior domain. This gives rise to the common and the individual ducts respectively.
Three stages are seen in the development of the salivary gland. The 1st stage shows the formation of analogue and the development of the gland with dichromatic branched ducts. The second stage shows further differentiation of the gland with early formation of lobules and canalization of the ducts. This stage lasts till the 7th embryonal month and encompasses the period of formation of functional units. The 3rd stage begins in the 8th embryonal month and leads to further structural maturation of the gland with acinar cells and intercalated duct differentiation. The development of the glandular tissue is characterized by reduction of the abundant interstitial connective tissue.
Studies of embryonic salivary glands in vitro have provided considerable information on the mechanism of glandular morphogenesis. The mesenchyme into which the glandular rudiment grows produces a factor or factors that stimulate the growth of the gland. If the mesenchyme and epithelium are separated and cultured on opposite sides of a filter, the growth of the epithelium proceeds normally; in the absence of the mesenchyme, the epithelium fails to grow. The process of branching morphogenesis, that is, the formation of hollow, tubular glands from an initially flat epithelial surface, appears to be related to the presence of microfilaments in the epithelial cells. Microfilaments about 5 to 7 nm (50 to 70 Å) thick form a network beneath the cell membrane of almost all cells; they consist of the contractile protein actin. In developing salivary epithelium they are particularly prominent at the apical and basal ends of the cells; differential contraction could cause a group of cells to pucker outward or clefts to form in a solid cord or sheet of cells, similar to the effect of pulling a purse string.
The presence of a functional innervation is also essential to proper growth and maintenance of salivary gland structure. Parasympathetic denervation of adult animals results in a 30% loss in glandular weight within 2 to 3 weeks. Sympathetic denervation causes variable responses, from atrophy of some glands to hypertrophy of others. Parasympathectomy of the developing rat parotid prevents attainment of adult gland size, cell number and size, and DNA and RNA content; sympathectomy has a moderate effect on cell and gland size only. Normal physiologic activity is also important for the proper growth of developing glands, as well as maintenance of adult structure and enzyme content. Feeding of a liquid diet to rats greatly diminishes the reflexly mediated secretory activity; the parotid rapidly decreases in weight and amylase content, and the normal diurnal pattern of synthesis and secretion is eliminated.
Conversely, chronically increased stimulation can cause an increase in glandular size. For example, increasing the bulk content of the food, which necessitates increased masticatory activity, results in hypertrophy of the rat parotid.
Treatment of mice and rat with isoproterenol, a α-adrenergic drug causes rapid and complete discharge of the stored secretory products and stimulates protein synthesis; resulting in glandular enlargement up to 5 times to that of untreated animals.
These enlarged glands show enhanced production of certain proteins while others are reduced, additionally certain new proteins are also synthesized. The effect of these drugs on the growth of salivary gland has found wide application in experimental studies of cellular secretion protein and nucleic acid synthesis and regulation of gene expression.
The contractile myoepithelial cells which envelope each acinus as well as portions of the ductal system are derived from the neural crest cells and thus are ectodermal in origin. They become active between the 24th and 35th week of the prenatal development. It is important to note that most of the mesenchymal elements like the septa and the capsule are produced by the influence of the neural crest cells.
Control of secretion
The physiologic control of salivary gland secretion is mediated through the activity of the ANS; particularly parasympathetic nervous system. The control of secretion is also linked to changing taste and smell. Each of these is capable of modifying the amount and consistency of the salivary secretion though gustatory stimulus is more important than masticatory stimulus.
Postganglionic fibers of both the sympathetic (adrenergic) and parasympathetic (cholinergic) divisions innervate the secretory cells. Myoepithelial, arteriolar smooth muscle cells, intercalated and striated duct cells also receive direct innervation. Unmyelineated nerve invested by cytoplasmic processes of Schwann, forms a plexus in the connective tissue surrounding the terminal secretory units. Some instances show the pattern of innervation termed intraepithelial or hypolemmal. This type of innervation is seen in human submandibular gland and minor salivary glands of the lip whereas some areas show subepithelial or epilemmal innervation generally seen in human parotid gland.Both patterns pf innervations show varicosities which are swellings or varicosities along their length which contain neurotransmitter vesicles. These sites are the sites of neurotransmitter release called nerve terminals. A single axon may have several varicosities and may effect innervation at several different cells.
The release of neurotransmitter from the vesicle in the nerve terminals adjacent to parenchymal cells stimulates them to discharge their secretory granules, secretes water and electrolytes and contraction of myoepithelial cells. The molecular events that occur during this process is called stimulus secretion coupling.
Norepinephrine, the sympathetic transmitter activates both α- and β-adrenergic receptors, while the parasympathetic transmitters activate cholinergic receptors. Protein secretion is mediated mainly through the α-adrenergic receptor; stimulation of the β-adrenergic and the cholinergic receptors also causes low levels of protein secretion, but these two receptors mainly appear to be involved in the secretion of water and electrolytes. Receptors for the peptide transmitter substance P are also present on salivary gland cells; substance P stimulates secretion similar to that caused by α-adrenergic and cholinergic agonists. Vasoactive intestinal polypeptide is present in the nerve endings in the salivary glands and has shown to induce secretion by some glands.
Receptor stimulation results in increases in the intracellular concentration of “second messengers,” which trigger additional events leading to the cellular response. In the case of α-adrenergic, cholinergic, and substance P receptors, the membrane permeability to Ca++ is increased and a marked influx of Ca++ into the cell occurs. Recent experiments have linked the activation of these receptors to rapid changes in membrane phospholipid metabolism and release of Ca++ from intracellular stores such as the endoplasmic reticulum or the plasma membrane. The increased cytoplasmic Ca++ concentration causes K+ efflux, water and electrolyte secretion, and a low level of exocytosis. Stimulation of the β-adrenergic receptor activates the plasma membrane enzyme adenyl cyclase, which catalyzes the formation of 3′,5′- cyclic adenosine monophosphate (cyclic AMP) from adenosine triphosphate. The increased intracellular concentration of cyclic AMP activates cyclic AMP-dependent protein kinase, an enzyme that phosphorylates other proteins, which in turn may be involved in the process of exocytosis. Calcium activated K+ channels function through voltage independent intermediate single channels and Max-k channels. Cyclic AMP may also stimulate release of Ca++ from intracellular stores, thereby increasing its cytoplasmic concentration. Thus Ca++ may be the common intracellular mediator for all of the receptors; the different cellular responses may reflect the different sources of Ca++ or differing local concentration, or both. Ca++ may have additional effects, including stimulation of guanylate cyclase activity and an increase in the concentration of 3′,5′-cyclic guanosine monophosphate (cyclic GMP). However, the role of cyclic GMP in the secretory process has not yet been determined.
Adjacent secretory cells are joined to one another by specialized intercellular junctions called gap junctions. These junctions are permeable to ions and small molecules; thus changes in the intracellular concentration of these substances in one cell are reflected by parallel changes in the adjacent cells. Therefore physiologic stimulation probably results in a response by secretory units (acini) rather than individual cells.
Similarly these junctions presumably allow for a coordinated contraction of several myoepithelial cells associated with each secretory unit. In some instances the gap junctions become electrically coupled or have decreased permeability, thus the physiologic significance of gap junction in relation to control of secretion still remains to be established.
Finally the blood vessels in the salivary gland are innervated by sympathetic vasoconstrictor and parasympathetic vasodilator fibers. The vascular response elicited by the autonomic stimulation ultimately determines the availability of water, electrolytes and metabolic substances during sustained secretory activity.
The secretion of saliva depends on many other factors which also to an extent alter the composition of saliva, namely age, duration and nature of stimulus, etc. Secretion of saliva is minimum at birth and does not contain salivary amylase. The volume of saliva increases by 2–3 months and salivary amylase appears when the infant is given complex carbohydrates in diet. In old age the secretory reserve becomes decreased though the constituents appear to be stable.
The secretion of saliva can alter from 0.1 ml/min at rest to 4.0 ml/min at actively stimulated times.
It is believed that multiple methods of secretion coexist in the same acinar cells.
In the salivary glands, once the secretory cells are stimulated, the protein secretion in the salivary acini happens by two methods.
In the first and main pathway, the cells store and secrete protein by a process of stored granule exocytosis. The time taken from the synthesis to exocytosis is about 3–5 hours. In the second pathway, the cells do not store protein but secrete it continuously by vesicular mechanism i.e. vesicles traveling directly from the Golgi complex to the plasma membrane. This is called constitutive pathway. In this mechanism some proteins travel in the opposite direction; to the interstitial tissue. In addition, transcytosis is also seen which indicates passage of substances through the acinar cell like IgA, which passes from the interstitial tissue through the cell from the basolateral to the apical cell membrane (Fig. 11.4).
Composition of saliva
Saliva consists primarily of water accounting for 99% or more of saliva. Inorganic ions, secretory proteins glycoproteins ofserum constituents and other substances typically account for 1% or less. The main electrolytes of saliva are Na, K, Ca, Cl, HCO3 and HPO4. Other electrolytes present in smaller concentration are Mg, SO4, F, SCN, and I. The main organic substances found in saliva are secretory proteins. They include enzymes such as amylase, ribonuclease, kallikrein, esterase, nystatin, cystatin, peroxidase, lysozymes, lactoferrin and acid phosphatase; mucin containing large amounts of bound carbohydrates which have a similar composition to their specific blood group substance and other proline rich proteins and glycoproteins. Other normal organic constituents of saliva include secretory immunoglobulins like IgG and IgM, blood clotting factors, amino acids, urea, uric acid, glucose, various lipid and hormones. The minor salivary glands secrete proteins which play an important role in innate immunity. They are bacterial pattern recognition receptors.
The proportions of these components are variable depending upon the source of saliva, the nature and the intensity of the secretory stimulus and the time of the day.
The saliva produced by major salivary gland differ from one another in composition. The parotid gland secretes a watery saliva rich in enzymes such as amylase, protein rich protein and glycoproteins. The submandibular gland contains higher proportion of glycosylated substances such as mucin in addition to the components already listed. The sublingual gland produces a viscous saliva rich in mucin.
As can be seen the composition collected will reflect on the cellular make up of a particular gland.
The stimulated saliva collected from the mouth is a complex mixture. It is also known by the name mixed saliva. The secretion of all the minor and major salivary glands contribute to whole saliva. In addition to the components derived by the gland, the whole saliva also contains desquamated oral epithelial cells, microorganisms and their products, leukocytes, serum constituents and the fluid from the gingival crevice, and food remnants. The total volume of saliva secreted by humans is approximately 750 to 1000 ml daily, of which submandibular accounts for 60%, the parotid about 30% and the sublingual about 5% or less and about 1% of saliva is derived from the minor salivary glands.
Secretion elicited in response to sympathetic stimulation will differ in protein and electrolyte from that due to parasympathetic stimulation.
The resting flow rate of whole saliva is 0.2 to 0.4 ml/min, for the parotid is 0.4 ml/min, submandibular is 0.1 ml/min. On stimulation the rates increase to 0.2 to 0.4 ml/min for the whole saliva, 1.0 to 2.0 ml/min for parotid, and 0.8 ml/min for the submandibular salivary gland.
The concentration and proportion of different electrolytes depend in part on the flow rate of saliva.
The pH of whole saliva varies from 6.4 to 7.4, the parotid saliva varies over a greater range from pH of 6.0 to 7.8.
The time of the day also exerts an influence on the amount, source and composition of the saliva. For instance during sleep, very little saliva is secreted by the major salivary gland and minor salivary gland becomes much more significant.
Other important factors affecting the composition of saliva are flow rate, differential gland contribution, circadian rhythm, duration of stimulus, nature of stimulus and diet.
The concentration of constituents in saliva depends only on the rate of flow and not on the nature of stimulus.
Functions of saliva
The most important function of the salivary gland is the production and the secretion of the saliva.
Protection of the oral cavity environment is the major function of the saliva, the other functions being assisting in digestion, speech, mastication, taste and tissue repair.
Protection of the oral cavity and oral environment
Saliva extends protection to the oral cavity and its tissues in many ways. The constant secretion of saliva prevents desiccation of the oral tissues. The absence of which can cause the oral mucosa to degenerate and atrophy. Its fluid like nature provides a washing action to flush away debris and the nonadherent bacteria. The mucin and other glycoproteins provide lubrication for the movement of the oral tissues against each other allowing smooth and sliding movements.
Saliva protects the mucosa from chemical and thermal insults by reducing the concentration and lowering and buffering the temperature respectively.
The saliva causes dilution of detritus and oral acid neutralization. The saliva protects the oroesophageal mucosa. Saliva has high molecular weight glycoproteins responsible for oral, oropharyngeal and esophageal mucosal lubrication.
The primary buffering system of saliva is formed by bicarbonates (HCO3). To some extent phosphate ion (HPO4) and salivary proteins contribute to the buffering action. Additionally the metabolism of the salivary proteins and peptides provides urea and ammonia that help to increase the pH. This helps in maintaining a high pH, which is not conducive for cariogenic bacteria to survive, ferment carbohydrate and produce acid to cause tooth decay.
A large group of salivary proteins called proline rich proteins because of their high content of amino acid proline, and statherin, a small tyrosine rich protein, inhibit the precipitation of calcium phosphate from the saliva. Along with other salivary glycoproteins, statherin and certain of the proline rich proteins bind to the tooth surface, forming the acquiredenamel pellicle. The resulting supersaturation of the calcium and phosphate reduces dissolution and promotes remineralization of the tooth enamel. On the surface of the tooth, a high concentration of calcium and phosphate causes posteruptive maturation of the enamel, increases surface hardness and resistance to demineralization. Remineralization of the initial carious lesions can be enhanced by fluoride ions in saliva.
Saliva has many antibacterial features. They are chiefly produced by the serous secretor cells of both major and minor salivary glands. Some high molecular weight salivary glycoproteins aggregate specific strains of oral microorganisms and/or prevent their adhesion to oral tissues, thus facilitating their oral clearance.
The acinar cells secrete peroxidase and ductal system secretes thiocyanate, both of which establish a bactericidal system in saliva. Salivary peroxidase in presence of hydrogen peroxidase and thiocyanate, catalyses the formation of hypothiocyanate ion (OSCN) which inhibits bacterial growth.
Another antibacterial protein in saliva is lysozyme, an enzyme that hydrolyzes the polysaccharide of bacterial cell walls, resulting in cell lysis.
The antioxidant defense mechanism is exerted by uric acid and ascorbic acid. A profound amount of these antioxidants is secreted by the parotid during meal times. This helps in reducing oxidant stress and maintains oral integrity.
An important group of defensive substances in saliva are the immunoglobulins. The predominant salivary immunoglobulin is IgA. Salivary or secretory IgA differs from serum IgA that is produced locally by plasma cells in the connective stroma of the glands and consist of a dimer of two IgA molecules and a protein called J chain. There is an additional glycoprotein produced by the parenchymal cells, called secretory component, that is also a part of the secretory IgA molecule. Secretory component acting as a receptor in the parenchymal cell membrane for dimeric IgA, facilitates the transfer of the IgA to the lumen, either by translation in the cell or by the endocytosis and secretion along with the secretory products of the parenchymal cells. Secretory component may also increase the resistance of the IgA molecules to denaturation or proteolysis in the oral cavity. Small amounts of IgG and IgM have also been detected in saliva, and occasional plasma cells in the glandular stroma can be stained by fluorescent antibodies specific for these immunoglobulins. Salivary immunoglobulins may also enter the saliva through the gingival crevice. Salivary immunoglobulins may act primarily through their ability to inhibit the adherence of the microorganisms to the oral tissues. Another antibacterial substance found in the saliva is lactoferrin, an iron binding protein. In the presence of specific antibody, lactoferrin that is not saturated with iron enhances the inhibitory effect of the antibody on the microorganisms.
Saliva contains many antimicrobial substances. They include the antibacterial agents lysozymes, lactoferrins, calprotectin, lactoperoxidase, immunoglobulins, chromogranin A, cystatins, histatins, VEGP (von Ebner's gland protein), SLPI (secretory leukocyte proteinase inhibitor) and the antiviral agents; mainly cystatins, mucins, immunoglobulins, and SLPI. Antifungal action is exerted by histatins, chromogranin A and immunoglobulins.
Digestion
Saliva participates in digestion by providing a fluid environment for solubilization of food and taste substances, and through the action of the digestive enzymes, principally amylase have been identified in human beings; two of these, representing 25–30% of the total amylase protein, have small amounts of bound proteins. The action of amylase on ingested carbohydrates to produce glucose and maltose begins in the mouth and continues for up to 30 minutes in the stomach before the amylase is inactivated by the acid pH and proteolysis. Lingual lipase produced by lingual serous glands initiates the digestion of dietary lipids, hydrolyzing triglycerides to monoglycerides and diglycerides and fatty acids. Other hydrolytic enzymes have been detected in saliva, but their significance in food digestion has not been established.
Mastication and deglutition
Saliva moistens the food and helps its breakdown into smaller particles to initiate digestion. The moistening and lubricating properties of saliva allow the formation of bolus and facilitate deglutition.
Saliva not only moistens the dry food but also reduces the temperature of the hot foods.
Taste perception
The food taken into the oral cavity is emulsified in saliva and dissolved. This process is a prerequisite for the sense or perception of taste. This is due to the presence of water and lipocalins in saliva. It also helps in maintaining taste receptors.
Speech
Saliva keeps the oral tissue moist and well lubricated which facilitates speech. It helps in vocalization and communication ability.
Tissue repair
A variety of growth factors and trefoil proteins are present in small quantities in saliva. Under experimental conditions these promote tissue growth, differentiation and wound healing.
Excretion
The salivary glands have an excretory function as do pancreas and gastric glands. Saliva is generally not lost to metabolism since it is reabsorbed in the GIT. Many substances from blood reach the saliva, thus saliva can be considered as a route of excretion.
The low molecular weight serum constituents can be demonstrated in saliva as for example, electrolytes and drug concentrations which can be assessed in the saliva. Infective agents from blood can reach the saliva. This is particularly true for hepatitis B virus.
The nitrates in the food reach the saliva and are reduced to nitrites by microorganisms, which are considered to be important in carcinogenesis.
Clinical considerations
An understanding of the anatomy, histology, and physiology of the salivary glands is essential for good clinical practice. In all aspects of clinical practice salivary glands and saliva play an important role.
Saliva regulates the oral environment and has widespread distribution of the salivary glands in the oral cavity. Hence there is a great impact of salivary gland pathology on clinical practice in dentistry.
With the exception of a portion of the anterior part of the hard palate, salivary glands are seen everywhere in the oral cavity. Because of this feature salivary gland lesion can occur everywhere in the oral cavity. In a differential diagnosis of oral lesions therefore a salivary gland origin must always be kept in mind.
The salivary glands are subject to a number of pathologic conditions. These include inflammatory infective diseases such as viral, bacterial, or allergic sialadenitis, a variety of benign and malignant tumors, autoimmune diseases such as Sjögren's syndrome, and genetic diseases such as cystic fibrosis. One of the most common surface lesions of the oral mucosa is a vesicular elevation called mucocele. This is produced from the severance of the duct of a minor salivary gland and pooling of the saliva in the tissues. A blockage of a salivary gland duct may occur after formation of a mucous or calcified plug within the duct. If this occurs in a minor salivary gland, it usually causes no symptoms, but in major glands such obstruction can be very painful and may require surgical intervention.
Another lesion associated with salivary gland is nicotinic stomatitis. In this the hard palate is whitened by hyperkeratinization around the duct opening of minor salivary gland caused due to heat from tobacco use. The heat causes inflammation of the duct openings which become dilated. Hence clinically these are seen as red macules scattered on white background of the palatal mucosa.
The salivary glands may also be affected by a variety of systemic and metabolic diseases. The major glands, especially the parotid, may become enlarged during starvation, protein deficiency, alcoholism, pregnancy, diabetes mellitus, and liver disease. The association of the major salivary glands with the cervical lymph nodes, brought about by a common area of development, necessitates the differentiation of pathologic conditions of these lymph nodes from salivary gland diseases.
Alteration of salivary gland function during disease states may have profound influences on the oral tissue. Loss of salivary function or reduction in volume of saliva secreted is called xerostomia. This leads to dryness of the mouth. The causes of xerostomia include disease states like Sjögren's syndrome, effects of chemotherapy or radiation therapy or as a result of variety of medications.
The common drugs causing dry mouth are anticholinergics, antidepressants, antipsychotics, antihypertensives, anoretics and drugs used in the treatment of parkinsonism.
Decreased salivary volume leads to difficulty in speech, mastication and taste perception, and swallowing becomes painful. The teeth become susceptible to caries. Oral tissues become susceptible to frequent oral infection; inflammation and ulceration of oral mucosa is commonly seen. In some instances excessive salivary secretion is seen because of certain physiologic states and rare pathologies. This is called ptyalism or sialorrhea.
As seen saliva is the principal protector of the hard and soft oral tissues. Any alteration in the saliva thereby affects the quality of life. Thus signs and symptoms of salivary dysfunction should be accurately diagnosed and treated.
Age changes in the salivary glands, particularly prominent in the parotid, consist of a gradual replacement of parenchyma with fatty tissue. Since the parotid is the major source of serous saliva; with advancing age, patients often complain of dryness and an increase in the viscosity of saliva. Recent studies have shown that in the aged the flow of saliva is reduced during resting condition, but in composition and quantity stimulated saliva in healthy, aged individuals is similar to that of young adults.
Salivary gland and sialochemistry are often of value in the diagnosis of glandular and systemic diseases. It is valuable in diagnosing diseases like cystic fibrosis, Sjögren's syndrome and certain infectious diseases associated with H. pylori like peptic ulcer disease and chronic gastritis. Biopsy of the minor salivary gland of the lip is frequently used as an aid in the diagnosis of salivary gland disease. Sialochemistry is used to monitor plasma concentration of certain therapeutic drugs and toxic substances. Drug monitoring of a large variety of drugs is under trial. The therapeutic agents like antipyrine, carbamazepine, cyclosporin, digoxin, methadone, phenytoin, quinine and barbiturates are a few examples of drug monitoring. The blood type of individuals can be determined from salivary samples.The hormone levels can be monitored like in other body fluids, the efficacy of which needs to be established. Saliva can be used to monitor progesterone levels to find the ovulation time in females. The levels of testosterone, insulin and steroids can also be estimated in saliva. Hormonal levels of estrion and estradiol in saliva can give information about fetal growth. The determination of salivary electrolyte concentration and protein composition may aid in the diagnosis of salivary gland or systemic diseases, like alterations of salivary Na+:K+ ratio in patients with Addison's disease and Cushing's syndrome.
References
Amsterdam, A., Ohad, I., Schramm, M. Dynamic changes in the ultrastructure of the acinar cell of the rat parotid gland during the secretory cycle. J Cell Biol. 1969; 41:753.
Archer, F.L., Kao, V.C.Y. Immunohistochemical identification of actomyosin in myoepithelium of human tissues. Lab Invest. 1968; 18:669.
Nieuw Amerongen, Arie van, Veerman, Enno C.I. Saliva—the defender of the oral cavity. Oral Diseases. 2002; 8:12.
Aub, D.L., McKinney, J.S., Putney, J.W., Jr. Nature of the receptor-regulated calcium pool in the rat parotid gland. J Physiol. 1982; 331:557.
Ball, W.D. Development of the rat salivary glands III. Mesenchymal specificity in the morphogenesis of the embryonic submaxillary and sublingual glands of the rat. J Exp Zool. 1974; 188:277.
Barka, T. Biologically active polypeptides in submandibular glands. J Histochem Cytochem. 1980; 28:836.
Bath, M., Balogh, Fehrenbach, M.J., Head and neck structures. Rudolph Dental embryology histology anatomy. 2nd. Elsevier, St. Louis, 1997. [pp 162-168.].
Batzri, S., Selinger, Z., Schramm, M., et al. Potassium release mediated by the epinephrine α-receptor in rat parotid slices Properties and relation to enzyme secretion. J Biol Chem. 1973; 248:361.
Bdolah, A., Schramm, M. The function of 3’5’-cyclic AMP in enzyme secretion. Biochem Biophys Res Commun. 1965; 18:452.
Bennick, A. Salivary proline-rich proteins. Mol Cell Biochem. 1982; 45:83.
Bhaskar, S.N. Synopsis of oral pathology, 5. St. Louis: The CV Mosby Co; 1977.
Bhaskar, S.N. Radiographic interpretation for the dentist, 3. St. Louis: The CV Mosby Co; 1979.
Bienenstock, J., Tourville, D., Tomasi, T.B., Jr. The secretion of immunoglobulins by the human salivary glands. In: Botelho S.Y., Brooks F.P., Shelley W.B., eds. The exocrine glands. Philadelphia: University of Pennsylvania Press, 1969.
Blobel, G. Synthesis and segregation of secretory proteins: the signal hypothesis. In: Brinkley B.R., Porter K.R., eds. International cell biology,. New York: Rockefeller University Press, 1977. [1976-1977].
Brand, Isselhard, Salivary glands. Anatomy of Orofacial Structures. 7th. Mosby, St. Louis, 2003. [pp 305-310.].
Brandtzaeg, P. Mucosal and glandular distribution of immunoglobulin components: differential localization of free and bound SC in secretory epithelial cells. J Immunol. 1974; 112:1553.
Bullen, J.J., Rogers, H.J., Griffiths, E. Iron binding proteins and infection. Br J Haematol. 1972; 23:389.
Bundgaard, M., Mϕller, M., Poulsen, J.H. Localization of sodium pump sites in cat salivary glands. J Physiol. 1977; 273:339.
Case, R.M. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev. 1978; 53:211.
Castle, J.D., Jamieson, J.D., Palade, G.E. Radioautographic analysis of the secretory process in the parotid acinar cell of the rabbit. J Cell Biol. 1972; 53:290.
Clamp, J.R., Allen, A., Gibbons, R.A., Roberts, G.P. Chemical aspects of mucus. Br Med Bull. 1978; 34:25.
Code, C.F. Handbook of Physiology; 2. American Physiological Society, Washington, DC, 1967. [section 6].
Creutz, C.E., Pazoles, C.J., Pollard, H.B. Identification and purification of an adrenal medullary protein (synexin) that causes calciumdependent aggregation of isolated chromaffin granules. J Biol Chem. 1978; 253:2858.
Cutler, L.S., Gremski, W. Epithelial–mesenchymal interaction in the development of salivary gland. Crit Rev Oral Biol Med. 1991; 2(1):1.
Dardick, I., et al. Immunohistochemistry and ultrastructure of myoepithelium and modified myoepithelium of the ducts of human major salivary glands: histogenetic implications for salivary gland tumors. Oral Surg. 1987; 64:703.
Dawes, C., Jenkins, G.N. The effects of different stimuli on the composition of saliva in man. J Physiol. 1964; 170:86–100.
De Camilli, P., Peluchetti, D., Meldolesi, J. Dynamic changes of the luminal plasmalemma in stimulated parotid acinar cells A freeze-fracture study. J Cell Biol. 1976; 70:59.
Drenckhahn, D., Gröschel-Stewart, U., Unsicker, K. Immunofluorescencemicroscopic demonstration of myosin and actin in salivary glands and exocrine pancreas of the rat. Cell Tissue Res. 1977; 183:273.
Egdar, W.M. Saliva: its secretion, composition and function. Br Dent J. 1992; 172:305.
Ekfors, T.O., Hopsu-Havu, V.K. Immunofluorescent localization of trypsin-like esteropeptidases in the mouse submandibular gland. Histochem J. 1971; 3:415.
Emmelin, N., Garrett, J.R., Ohlin, P. Neural control of salivary myoepithelial cells. J Physiol. 1968; 196:381.
Erogchenko, V.P. Digestive system; oral cavity and salivary glands. Atlas of Histology, 10th. Philadelphia: Lippincott Williams and Wilkins; 2005. [pp 218-227].
Farquhar, M.G., Palade, G.E. The Golgi apparatus (complex)—(1954–1981)—from artifact to center stage. J Cell Biol. 1981; 91:77s.
Garrett, J.R. The innervation of normal human submandibular and parotid salivary glands Demonstrated by cholinesterase histochemistry, catecholamine fluorescence and electron microscopy. Arch Oral Biol. 1967; 12:1417.
Garrett, J.R. Neuro-effector sites in salivary glands. In: Emmelin N., Zotterman Y., eds. Oral physiology,. Oxford. England: Pergamon Press, 1972.
Gill, G. Metabolic and endocrine influences on the salivary glands. Otolaryngol Clin North Am. 1977; 10:363.
Gresik, E., Michelakis, A., Barka, T., et al. Immunocytochemical localization of renin in the submandibular gland of the mouse. J Histochem Cytochem. 1978; 26:855.
Grobstein, C. Epithelio-mesenchymal specificity in the morphogenesis of mouse submandibular rudiments in vitro. J Exp Zool. 1953; 124:383.
Hall, H.D., Schneyer, C.A. Salivary gland atrophy in rat induced by liquid diet. Proc Soc Exp Biol Med. 1964; 117:789.
Hall, H.D. Protective and maintenance function of human saliva. Quintessence International. 1993; 24:813.
Hammer, M.G., Sheridan, J.D. Electrical coupling and dye transfer between acinar cells in rat salivary glands. J Physiol. 1978; 275:495.
Hamosh, M., The role of lingual lipase in neonatal fat digestionHarries J.T., ed. Pre- and post-natal development of mammalian absorptive processes. Ciba Found Symp. 1979:69. [70].
Hamosh, M., Scow, R.O. Lingual lipase and its role in the digestion of dietary fat. J Clin Invest. 1973; 52:88.
Hand, A.R. The fine structure of von Ebner's gland of the rat. J Cell Biol. 1970; 44:340.
Hand, A.R. Morphology and cytochemistry of the Golgi apparatus of rat salivary gland acinar cells. Am J Anat. 1971; 130:141.
Hand, A.R. Synthesis of secretory and plasma membrane glycoproteins by striated duct cells of rat salivary glands as visualized by radioautography after 3H-fucose injection. Anat Ree. 1979; 195:317.
Hand, A.R., Ball, W.D. Ultrastructural immunocytochemical localization of secretory proteins in autophagic vacuoles of parotid acinar cells of starved rats. J Oral Pathol. 1988; 17:279.
Hand, A.R., Jungmann, R.A. Localization of cellular regulatory proteins using postembedding immunogold labeling. Am J Anat. 1989; 185:183.
Hand, A.R., Oliver, C. Cytochemical studies of GERL and its role in secretory granule formation in exocrine cells. Histochem J. 1977; 9:375.
Hand, A.R., Oliver, C. Basic mechanisms of cellular secretion: Methods in cell biology; 23. Academic Press, Inc, New York, 1981.
Hansson, H.A., Tunhall, S. Epidermal growth factor and insulin-like growth factor I are localized in different compartments of salivary gland duct cells Immunohistochemical evidence. Acta Physiol Scand. 1988; 134:383.
Ichikawa, M., Sasaki, K., Ichikawa, A. Immunocytochemical localization of amylase in gerbil salivary gland acinar cells processed by rapid freezing and freeze-substitution fixation. J Histochem Cytochem. 1989; 37:185.
Ito, Y. Parotin a salivary gland hormone. Ann NY Acad Sci. 1960; 85:228.
Jamieson, J.D., Palade, G.E. Intracellular transport of secretory proteins in the pancreatic exocrine cell I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 1967; 34:577.
Jamieson, J.D., Palade, G.E. Intracellular transport of secretory proteins in the pancreatic exocrine cell II. Transport to condensing vacuoles and zymogen granules. J Cell Biol. 1967; 34:597.
Jamieson, J.D., Palade, G.E. Production of secretory proteins in animal cells. In: Brinkley B.R., Porter K.R., eds. International cell biology,. New York: Rockefeller University Press, 1977. [1976-1977].
Johnson, D.A., Sreebny, L.M. Effect of food consistency and starvation on the diurnal cycle of the rat parotid gland. Arch Oral Biol. 1971; 16:177.
Johnson, D.A., Sreebny, L.M. Effect of increased mastication on the secretory process of the rat parotid gland. Arch Oral Biol. 1973; 18:1555.
Jonsson, R. Sjögren's syndrome more common than you think. Oral Diseases. 2002; 8:130.
Kauffman, D.L., Zager, N.I., Cohen, E., et al. The isoenzymes of human parotid amylase. Arch Biochem Biophys. 1970; 137:325.
Kaufman, D.L., Lamster, B. The diagnostic application of saliva. Crit Rev Oral Biol Med. 2002; 13:197.
Kim, S.K., Nasjleti, C.E., Han, S.S. The secretion processes in mucous and serous secretory cells of the rat's sublingual gland. J Ultrastruct Res. 1972; 38:371.
Klebanoff, S.J., Luebke, R.G. The antilactobacillus system of saliva Role of salivary peroxidase. Proc Soc Exp Biol Med. 1965; 118:483.
Kleinberg, I., Ellison, S.A., Mandel, I.D. Saliva and dental caries (special suppl) Microbiology Abstracts. New York: Information Retrieval Inc; 1979.
Korsrud, F.R., Brandtzaeg, P. Characterization of epithelial elements in human major salivary glands by functional markers: localization of amylase, lactoferrin, lysozyme, secretory component, and secretory immunoglobulins by paired immunofluorescence staining. J Histochem Cytochem. 1982; 30:657.
Kurth, B.E., et al. Cell culture and characterization of human minor salivary gland duct cells. J Oral Pathol. 1989; 18:214.
Lawrence, A.M., Tan, S., Hojvat, S., et al. Salivary gland hyperglycemic factor: an extrapancreatic source of glucagon-like material. Science. 1977; 195:70.
Lawson, D., Raff, M.C., Gomperts, B., et al. Molecular events during membrane fusion A study of exocytosis in rat peritoneal mast cells. J Cell Biol. 1977; 72:242.
Lawson, K.A. The role of mesenchyme in the morphogenesis and functional differentiation of rat salivary epithelium. J Embryol Exp Morphol. 1972; 27:497.
Leblond, C.P., Bennett, G. Role of the Golgi apparatus in terminal glycosylation. In: Brinkley B.R., Porter K.R., eds. International cell biology. New York: Rockefeller University Press, 1977. [1976-1977.].
Leon, C.P.M., Schenkels, Enno, C.I., et al. Biochemical composition of human saliva in relation to other mucosal fluids. Crit Rev Oral Biol Med. 1995; 6:161.
Leslie, B.A., Putney, J.W., Jr., Sherman, J.M. α-Adrenergic β-adrenergic and cholinergic mechanisms for amylase secretion by rat parotid gland in vitro. J Physiol. 1976; 260:351.
Levi-Montalcini, R., Angeletti, P.U. Nerve growth factor. Physiol Rev. 1968; 48:534.
Liang, T., Cascieri, M.A. Substance Preceptor on parotid cell membranes. J Neurosci. 1981; 1:1133.
Looms, D., Tritsaris, K., Pederson, M.A., et al. Nitric oxide signaling in salivary glands. J Oral Pathol Med. 2002; 31:569.
Lotti, L.V., Hand, A.R. Endocytosis of parotid salivary proteins by striated duct cells in streptozotocin-diabetic rats. Anat Rec. 1988; 221:802.
Mandel, I.D. Human submaxillary, sublingual and parotid glycoproteins and enamel pellicle. In: Horowitz, M.I., Pigman, W., eds. The glycoconjugates Mammalian glycoproteins and glycolipids, 1. New York: Academic Press Inc; 1977.
Mandel, I.D. Sialochemistry in diseases and clinical situations affecting salivary glands. CRC Grit Rev Clin Lab Sci. 1980; 12:321.
Mandel, I.D. The diagnostic uses of saliva. J Oral Pathol Med. 1990; 19:119.
Maria, O.M., Jung-Wan Martin Kim, Jonathan, A., et al. Distribution of Tight Junction Proteins in Adult Human Salivary Glands. J Histochem Cytochem. 2008; 56:1093.
Mason, D.K., Chisholm, D.M. Salivary glands in health and disease. London: WB Saunders Co; 1975.
Masson, P.L., Heremans, J.L., Dive, C. An iron-binding protein common to many external secretions. Clin Chim Acta. 1966; 14:735.
Mayo, J.W., Carlson, D.M. Protein composition of human submandibular secretions. Arch Biochem Biophys. 1974; 161:134.
Mayo, J.W., Carlson, D.M. Isolation and properties of four α-amylase isozymes from human submandibular saliva. Arch Biochem Biophys. 1974; 163:498.
Mazariegos, M.R., Hand, A.R. Regulation of tight junctional permeability in the rat parotid gland by autonomic agonists. J Dent Res. 1984; 63:1102.
Mc Vicar, A., Greenwood, C., Fewell, F., et al. Evaluation of anxiety, salivatory cortisol and melatonin secretion following reflexology treatment: a pilot study in healthy individuals. Complementary Therapies in Clinical Practice. 2007; 13:137.
Mednieks, M.I., Hand, A.R. Cyclic AMP-dependent protein kinase in stimulated rat parotid gland cells: compartmental shifts after in vitro treatment with isoproterenol. Eur J Cell Biol. 1982; 28:264.
Mednieks, M.I., Hand, A.R. Cyclic AMP binding proteins in saliva. Experientia. 1984; 40:945.
Mese, H., Matsuo, R. Salivary secretion, taste and hyposalivation. J Oral Rehab. 2007; 34:711.
Mestecky, J., Lawton, A.R. The immunoglobulin A system. New York: Plenum Press; 1974.
Moreira, J.E., et al. Light and electron microscopic immunolocalization of rat submandibular gland mucin glycoprotein and glutamine/glutamic acid-rich proteins. J Histochem Cytochem. 1989; 37:515.
Murakami, K., Tanaguchi, H., Baba, S. Presence of insulin-like immunoreactivity and its biosynthesis in rat and human parotid gland. Diabetologia. 1982; 22:358.
Murphy, R.A., Saide, J.D., Blanchard, M.H., et al. Molecular properties of the nerve growth factor secreted in mouse saliva. Proc Natl Acad Sci USA. 1977; 74:2672.
Myant, N.B. Iodine metabolism of salivary glands. Ann NY Acad Sci. 1960; 85:208.
Nager, R.M., Klien, I., Zarzhevsky, N., et al. Characterization of the differentiated antioxidant of human saliva free radical. Biology and Medicine. 2002; 32:286.
Nakamura, T., Nagura, H., Watanabe, K., et al. Immunocytochemical localization of secretory immunoglobulins in human parotid and submandibular glands. J Electron Microsc. 1982; 31:151.
Nauntoffe, B. Saliva as an essential component of GI tract function: speaking, swallowing, and digestion. Oral Diseases. 2002; 8:117.
Neutra, M., Leblond, C.P. Synthesis of the carbohydrate of mucus in the Golgi complex shown by electron microscope radioautography of goblet cells from rats injected with glucose-H3. J Cell Biol. 1966; 30:119.
Neuw, A.V. Amerongen salivary gland and saliva. Oral Diseases. 2002; 8:12.
Nustad, K., Ørstavik, T.B., Gautvik, K.M., et al. Glandular kallikreins. Gen Pharmacol. 1978; 9:1.
Oliver, C., Hand, A.R. Uptake and fate of luminally administered horseradish peroxidase in resting and isoproterenol stimulated rat parotid acinar cells. J Cell Biol. 1978; 76:207.
Ørstavik, T.B., Brandtzaeg, P., Nustad, K., et al. Cellular localization of kallikreins in rat submandibular and sublingual salivary glands. Acta Histochem. 1975; 54:183.
Osamu Katsumata, Yu-Ichi Sato, Yasuhiro Sakai, et al. Intercalated duct cells in the rat parotid gland may behave as tissue stem cells. Anat Sci Int. 2009; 84(3):148.
Palade, G. Intracellular aspects of the process of protein secretion. Science. 1975; 189:347.
Parks, H.F. On the fine structure of the parotid gland of mouse and rat. Am Anat. 1961; 108:303.
Pedessen, A.M., Bandow, A., Jensen, G.B., et al. Salivary gland and saliva No.5 saliva and GI functions of taste, mastication swallowing and digestion. Oral Diseases. 2002; 8:117.
Penny, R. Anxiety, salivary cortisol and melatonin secretion following reflexology treatment. J Australian Tradi-Med Soc,. 2007. [Dec].
Petersen, O.H. The electrophysiology of gland cells. London: Academic Press, Inc; 1980.
Pinkstaff, C.A. The cytology of salivary glands. Int Rev Cytol. 1980; 63:141.
Poggioli, J., Putney, J.W., Jr. Net calcium fluxes in rat parotid acinar cells Evidence for a hormone-sensitive calcium pool in or near the plasma membrane. Pflügers Arch. 1982; 392:239.
Provenza, V.D. Salivary gland histology inheritance and oral development, 2nd. Lea and Febiger; 1986. [pp 388-417].
Putney, J.W., Jr. Inositol lipids and cell stimulation in mammalian salivary gland. Cell Calcium. 1982; 3:369.
Putney, J.W., Jr., Weiss, S.J., Leslie, B.A., et al. Is calcium the final mediator of exocytosis in the rat parotid gland? J Pharmacol Exp Ther. 1977; 203:144.
Rasmussen, H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970; 170:404.
Richard BW and Isselhard DE: Salivary glands. Anatomy of Orofacial Structures, 7th ed. In: (Ed.)., Acciocca St. Louis, Mosby. pp 305–310.
Riva, A., Riva-Testa, F. Fine structure of acinar cells of human parotid gland. Anat Rec. 1973; 176:149.
Riva, A., Testa-Riva, F., Del Fiacco, M., et al. Fine structure and cytochemistry of the intralobular ducts of the human parotid gland. J Anat. 1976; 122:627.
Roland, J. Sjögren's syndrome more common than you think. Oral Diseases. 2002; 8:130.
Scott, B.L., Pease, D.C. Electron microscopy of the salivary and lacrimal glands of the rat. Am J Anat. 1959; 104:115.
Scott, J., Gradwell, E. A quantitative study of the effects of chronic hypoxia on the histological structure of the rat major salivary glands. Arch Oral Biol. 1989; 34:315.
Schneyer, C.A., Hall, H.D. Autonomic regulation of postnatal changes in cell number and size of rat parotid gland. Am J Physiol. 1970; 219:1268.
Schneyer, L.H., Young, J.A., Schneyer, C.A. Salivary secretion of electrolytes. Physiol Rev. 1972; 52:720.
Schramm, M., Selinger, Z. The functions of cyclic AMP and calcium as alternative second messengers in parotid gland and pancreas. J Cyclic Nucleotide Res. 1975; 1:181.
Scully, C. Drugs effect on salivary glands: dry mouth. Oral Diseases. 2003; 9:165.
Seifert, G., Mehmke, A., Haibrich, J. Diseases of the salivary glands pathology diagnosis and treatment. New York: Thieme; 1986. [pp 1-43, 305-10].
Selye, H., Veilleux, R., Cantin, M. Excessive stimulation of salivary gland growth by isoproterenol. Science. 1961; 133:44.
Shackleford, J.M., Klapper, C.E. Structure and carbohydrate histochemistry of mammalian salivary glands. Am J Anat. 1962; 111:25.
Ship, J.A. Diagnosing, management and preventing salivary gland disorder.In Salivary glands Saliva no 4. Oral Diseases. 2002; 8:77–89.
Simson, J.A.V., Hazen, D., Spicer, S.S., et al. Secretagogue-mediated discharge of nerve growth factor from granular tubules of male mouse submandibular glands: an immunocytochemical study. Anat Rec. 1978; 192:375.
Smith, P.H., Patel, D.G. Immunochemical studies of the insulin-like material in the parotid gland of rats. Diabetes. 1984; 33:661.
Smith, P.M. Mechanism of secretion. In Edgan W.M., Mullane D.M.O., eds.: Saliva and Oral Health, 2nd, London: British Dental Association, 1996. [pp 9-26].
Spooner, B.S., Wessells, N.K. An analysis of salivary gland morphogenesis: role of cytoplasmic microfilaments and microtubules. Dev Biol. 1972; 27:38.
Sreebny, L.M., Johnson, D.A., Robinovitch, M.R. Functional regulation of protein synthesis in the rat parotid gland. J Biol Chem. 1971; 246:3879.
Streckfus, C. Saliva as a diagnostic fluid. Oral Diseases. 2002; 8:69.
Suddick, R.P., Dowd, F.J. The microvascular architecture of the rat submaxillary gland: possible relationship to secretory mechanisms. Arch Oral Biol. 1969; 14:567.
Sumi, L.A., et al. Immunoelectron microscopical localization of immunoglobulins, secretory component and J chain in the human mipor salivary glands. J Oral Pathol. 1988; 17:390.
Tabak, L.A., Levine, M.J., Mandel, I.D., et al. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982; 11:1.
Tamarin, A., Sreebny, L.M. The rat submaxillary salivary gland A correlative study by light and electron microscopy. J Morphol. 1965; 117:295.
Tandler, B. Ultrastructure of the human submaxillary gland I. Architecture and histological relationships of the secretory cells. Am J Anat. 1962; 111:287.
Tandler, B. Ultrastructure of the human submaxillary gland III. Myoepithelium. Z Zellforsch. 1965; 68:852.
Tandler, B., Denning, C.R., Mandel, I.D., et al. Ultrastructure of human labial salivary glands I. Acinar secretory cells. J Morphol. 1969; 127:383.
Tandler, B., Erlandson, R.A. Ultrastructure of the human submaxillary gland IV. Serous granules. Am J Anat. 1972; 135:419.
Taubman, M.A., Smith, D.J. Secretory immunoglobulins and dental disease. In: Han S.S., Sreebny L., Suddick R., eds. Symposium on the mechanism of exocrine secretion. Ann Arbor: University of Michigan Press, 1973.
Taylor, T., Erlandsen, S.L. Peroxidase localization in von Ebner's gland of man. J Dent Res. 1973; 52:635.
Testa-Riva, F. Ultrastructure of human submandibular gland. J Submicrosc Cytol. 1977; 9:251.
Testa-Riva, F., Puxeddu, P., Riva, A., et al. The epithelium of the excretory duct of the human submandibular gland: a transmission and scanning electron microscope study. Am J Anat. 1981; 160:381.
Tomasi, T.B., Jr., Tan, E.M., Solomon, A., et al. Characteristics of an immune system common to certain external secretions. J Exp Med. 1965; 121:101.
Turner, R.J., Sugiya, H. Understanding salivary fluid and protein secretion Salivary Gland and Saliva No. 1. Oral Diseases. 2002; 8:3.
Victor, G.R., Nakamoto, T., Alaka, S., et al. Regulation of membrane potential and fluid secretion by Ca2+-activated K+ channels in mouse submandibular glands. J Physiol. 2007; 581(2):801.
Vigneswaran, N., Haneke, E., Hornstein, O.P. A comparative lectin histochemical study of major and minor salivary glands with special reference to the labial glands. Arch Oral Biol. 1989; 34:739.
Wells, H. Functional and pharmacological studies on the regulation of salivary gland growth. In: Schneyer L.H., Schneyer C.A., eds. Secretory mechanisms of salivary glands. New York: Academic Press, Inc, 1967.
Young, J.A., Schneyer, C.A. Composition of saliva in mammalia. Aust J Exp Biol Med Sci. 1981; 59:1.
Young, J.A., van Lennep, E.W. The morphology of salivary glands. London: Academic Press; 1978.
Young, J.A., van Lennep, E.W. Transport in salivary and salt glands. In: Giebisch, G., Tosteson, D.C., Ussing, H.H., eds. Membrane transport in biology,, 4B. Berlin: Springer-Verlag; 1979.