One of the most important properties of transition metals is that they form coordination or complex compounds. These compounds play a vital role in our lives. The importance of these compounds can be realized from the fact that life would not be possible without the existence of chlorophyll (Mg complex) in plants and haemoglobin (Fe complex) in the blood. One of the earliest known coordination compounds is Prussian blue, which was accidentally prepared in 1704 by a Berlin colour maker, Diesbach, by strongly heating animal wastes and sodium carbonate in an iron container. In 1753 Macquer prepared potassium ferrocyanide by treating Prussian blue with alkali. In 1799 Tassaert obtained an orange compound, CoCl3.6NH3, by allowing the mixture of colour chloride and aqueous ammonia to stand in air. The field of such compounds expanded very fast and today they are useful in many different fields, e.g. pharmacy, technology, analytical chemistry, polymerization reaction, metallurgy, refining of metals, organic synthesis, biochemistry, photography, etc. So, complexation may be defined as the reversible association of a substrate and ligand to form a new species. Intermolecular forces involved in the formation of complexes are the van der Waals forces of dispersion, dipolar and induced dipolar types. Hydrogen binding provides a significant force in some molecular complexes, whereas coordinate covalence is important in metal complexes.
Complexes or coordination compounds result from a donor–acceptor method or Lewis acid–base reaction among two or more chemical constituents. The donor may be any nonmetallic atom or ion, either free or contained in a neutral molecule or an ionic compound, which can donate an electron pair (Lewis base). The constituent that accepts an electron, like a metallic ion or a neutral atom, may serve as acceptor (Lewis acid).
Ligands serve as donors (Lewis bases), which are neutral molecules, anions or cations that are directly linked with the central metal atom or ion in a complex. With a few exceptions, free ligands have at least one electron pair that is not engaged in bonding and donate one or more electron pairs to the central metal atom or ion, which acts as an acceptor. So formation of complex ion involves the following two things:
1. Ligands should have lone pair or pairs of electrons, which can be donated to the central metal ion or atom.
2. The central atom or ion should have vacant orbital of nearly equivalent energy to accommodate the electrons donated by ligands. This condition is easily fulfilled by atoms or ions of transition metals.
Ligands can be classified on the basis of number of donor atoms present in them. Classification is as follows:
1. Mono- or unidentate ligands: These ligands have one donor atom or ion. F–, Cl–, Br–, H2O, CO, etc., are examples of monodentate ligands.
2. Bidentate ligands: Ligands that have two donor atoms and the ability to link with central metal ion at two positions are called bidentate ligands, e.g. ethylene diamine, oxalate, glycinate, etc.
3. Tridentate ligands: These ligands have three donor groups, e.g. ethylene triamine.
4. Tetradentate ligands: These ligands have four donor groups, e.g. nitriloacetate and triethylenetetramine.
5. Pentadentate ligands: They have five donor atoms such as ethylenediaminetriacetate ion.
6. Hexadentate ligands: They have six donor atoms, e.g. ethylenediaminetetraacetic acid (EDTA) ion.
Ligands with more than one potential donor atom are known as
ambidentate, such as the thiocyanate ion, NCS
–, which can bind to the metal centre with either nitrogen or sulphur atoms. Examples of ambidentate ligands include NO
2–/ONO
– (O and N) and
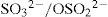
(O and S), where the first named atom refers to that which is bonded to the metal centre.
The ligands that attach with the centre ion or metals may be the same as or different from one another. When all the ligands are the same, the complex is called homoleptic. When they are not identical, the complex is called heteroleptic.
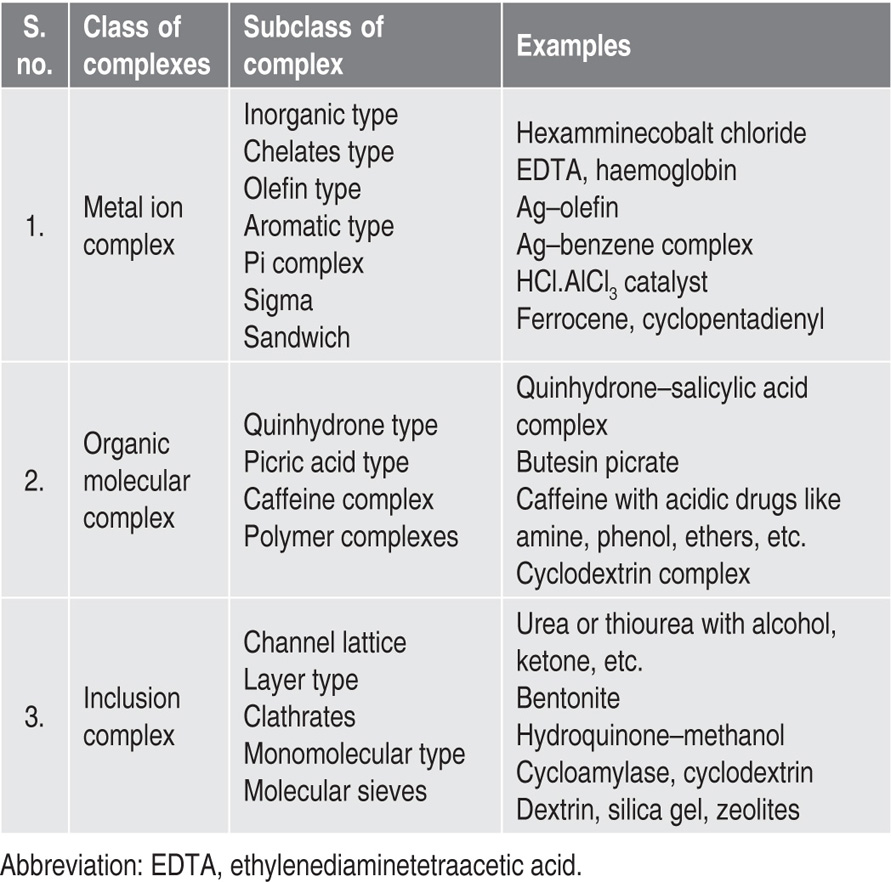
Complexes can be classified into two categories, depending on whether the component is a metal ion or an organic molecule. A third class, the inclusion/occlusion compounds, involves the entrapment of one compound in the molecular framework of another. The classification of complexes shown in
Table 4.1.
Table 4.1 Classification of complexes
Metal Ion Complexes
A metal complex (also known as
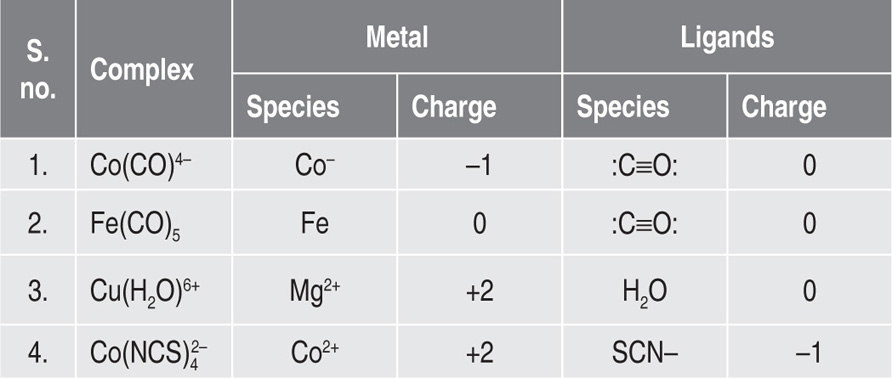
coordination complex) is a complex consisting of a central atom or ion usually metallic, bonded to a surrounding array of molecules or anions or ligands. Major intermolecular forces involved in the formation of complexes are the van der Waals forces of dispersion, dipolar and induced dipolar types. An adequate understanding of metal ion complexation is based on familiarity with atomic structure and molecular forces. Metal complexes include all metal compounds, aside from metal vapours, plasmas and alloys (
Table 4.2).
Table 4.2 Examples of metal–ligand complexes and the charges
Metal complexations can be classified into following categories:
1. Inorganic type
2. Olefin type
3. Chelates type
4. Aromatic type:
a. Sandwich compounds
b. Sigma complexes
c. Pi complexes
Inorganic-Type Complexes
Inorganic complexes are a group of simple complexes first described by Werner in 1891. Hexamminecobalt III chloride ([Co(NH3)6]3+Cl3–) is a type of inorganic complex. The ammonia molecule in hexamminecobalt III chloride acts as ligand and is said to be coordinated to the cobalt ion. The coordination number of the cobalt ion or number of ammonia groups coordinated to the metal ion is six. The coordination number is the number of ligands stuck to the metal. Common coordination numbers are 6 (octahedral), 5 (pentahedral) trigonal bipyramidal, but sometimes 4 (tetrahedral) square pyramidal, tetrahedral. Prefixes that are often used to refer to the coordination are: (1) monocoordinate, (2) dicoordinate, (3) tricoordinate, (4) tetracoordinate, (5) pentacoordinate, (6) hexacoordinate, (7) heptacoordinate, (8) octacoordinate and (9) nonacoordinate.
Ligand donates a pair of electrons to form a coordinate covalent link between itself and central ion having an incomplete electron shell. Normally, a lone pair of electrons on an atom within the ligand is shared with the metal cation, which does not have any valence electrons to share. Consider a chloride ion, for example. It has four lone pairs that can be shared. Now consider a gold ion, which has lost its 6s electrons and has an electronic configuration of [Xe]4f 5d. If a chloride ion sticks to a gold (III) ion, the 6s and 6p orbitals are available for bonding, but Au3+ has no electrons in them. Thus, chloride provides both the electrons for the bond. In fact, the Au3+ cation even rehybridizes to produce four sp3 orbitals and forms a tetrahedral complex with four chloride ions, i.e. AuCl4–.
Other complex ion belonging to the inorganic complex group include [Ag(NH3)2]+, [Fe(CN)6]4– and [Cr(H2O)6]3+.
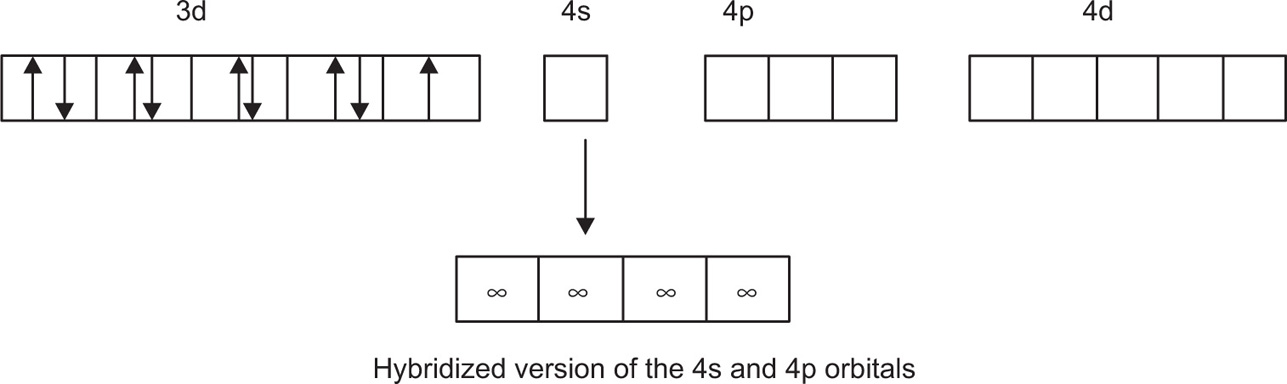
Hybridization plays very important part in coordination compounds in which adequate bonding orbitals are not usually available in the metal ion. In chemistry, hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridized orbitals are very useful in the explanation of the shape of molecular orbitals for molecules (
Fig. 4.1).
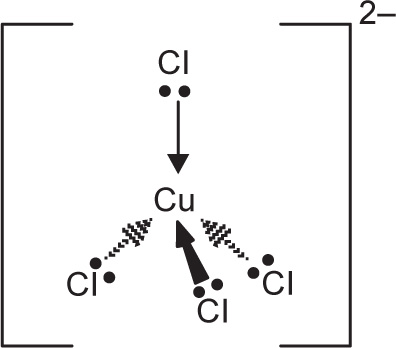
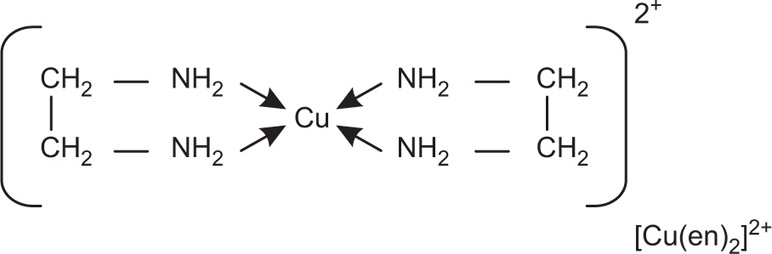
CuCl42– is a simple example of the formation of a complex ion with a negative charge.
Copper has the electronic structure 1s22s22p63s23p63d104s1.
When it forms a Cu
2+ ion, it loses the 4s electron and one of the 3d electrons to leave
1s22s02p63s23p63d9.
To bond the four chloride ions as ligands, the empty 4s and 4p orbitals are used (in a hybridized form) to accept a lone pair of electrons from each chloride ion.
Cu2+
Only one of the four lone pairs on each chloride ion is shown. The other three are pointing away from the copper ion and are not involved in the bonding.
That gives a complex ion shown in
Fig. 4.2.
The ion carries 2 negative charges overall. That comes from a combination of the 2 positive charges on the copper ion and the 4 negative charges from the 4 chloride ions. In this case, the coordination number of the copper is, of course, 4.
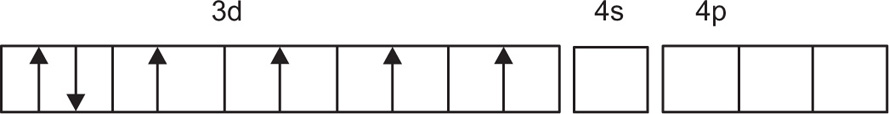
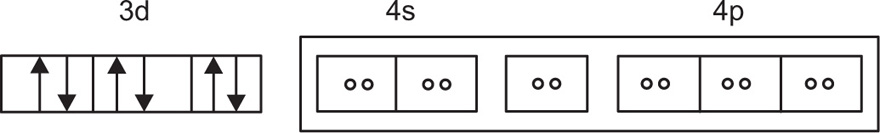
For estimation of hybridization in a metal ion complex is to select that complex in which the metal ion has its 3d and 4s orbitals primarily in the hybridization. For example, the trivalent cobalt ion Co(III) has the ground-state electronic configuration, as shown in
Fig. 4.3. Complex [Co(NH
3)
6]
3+ is a complex of cobalt with ammonia that shows the d
2sp
3 electronic configuration, as shown in
Fig. 4.4 (
Martin et al., 1991).
Olefin-Type Complexes
Olefin complexes are also referred to as
alkene complexes and usually pi bonding is involved in it. Zeise’s salt (K[PtCl
3(C
2H
4)]·H
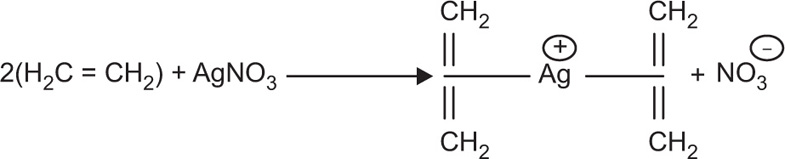
2O) is the first olefin and organometallic complex, which was discovered in 1827. A simple example of donation from a pi bond is the treatment of silver(I) salts with alkenes, shown in
Fig. 4.5.
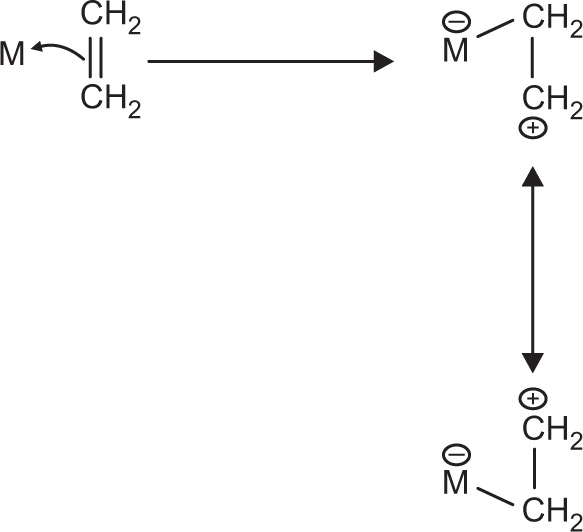
As shown in
Fig. 4.5, an alkene donates its pi bonding electrons to a 4s orbital of the silver metal. The electrons are shared between two carbon atoms in the alkene. Since a bond is generally thought of as a pair of electrons shared between two atoms, once the pi electrons are donated to the metal, they are shared between the metal and one of the carbons, but not both. During this process, one of the carbons is short of electrons. It must be a cation. Of course, either carbon could be the cationic one, so we can draw resonance structures showing both possible states (
Fig. 4.6).
1. Donation of pi electrons to a metal can ‘activate’ an alkene.
2. The alkene can become positive and electrophilic.
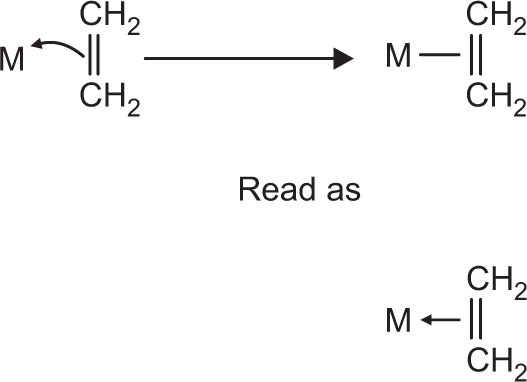
In alkene complexes, bonding is usually illustrated with a line between the pi bond and the metal, as shown in
Fig. 4.7. That line could be read as a pair of electrons, but it is not that way in fact. The pair of electrons is in the pi bond. They are being shared with the metal.
Olefin complexes are mostly used as catalysts and play a vital role in polymerization of unsaturated hydrocarbon, oxidation, hydrogenation, isomerization, cyclization and other industrial processes. They are also useful catalyst precursors, because the olefin ligand is relatively labile and is easily lost either through dissociation or via hydrogenation.
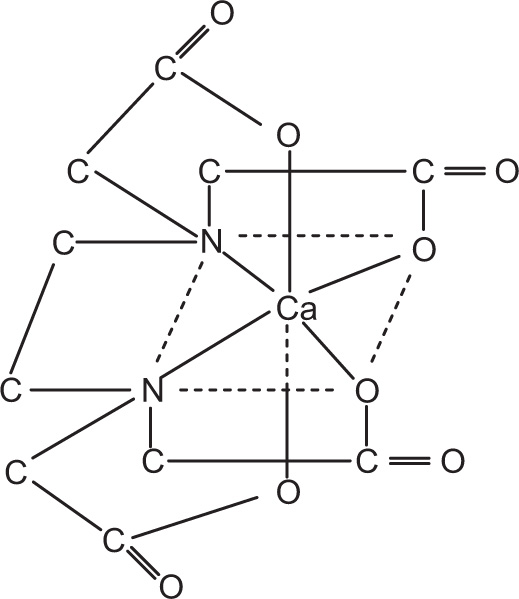
Chelates-Type Complexes
The word
chelates has been derived from the Greek word
chele meaning ‘claw’. There are many substances containing two or more donor groups that may combine with a metal to form a
special type of complex, which is known as
chelate. Simply put, if bidentate or polydentate ligands, which are known as
chelating ligands, on coordination result in the formation of a closed or cyclic ring structure complex, are called
chelates. The bonds in a chelate may be ionic or of the primary covalent type. Complex formed by Cu
2+ ion with ethylenediamine is an example of a chelate, as shown in
Fig. 4.8.
Some of the distinguished features of chelates-type complexes are as follows:
1. Chelating ligands form more stable complexes than monodentate ligands.
2. The chelates containing 5 or 6 members ring including metal atom are comparatively more stable. Chelating ligands that do not contain unsaturated groups in general form five-membered stable complexes while chelating ligands having unsaturated groups form six-membered stable complex.
3. Ligands with larger groups form more unstable rings than with smaller groups because of steric hindrance.
4. Polydentate ligands have the flexidentate characteristic; that is, it is not necessary that all the donor atoms present in the polydentate ligands should form coordinate bonds with central metal atom or ion. For example, EDTA, which has hexadentate ligand, can function as a pentadentate or tetradentate ligand with certain metal ions. Similarly, sulphate ion can also act as a monodentate ligand.
5. There are certain ligands that have two or more donor atoms but in forming complexes only one donor atom is attached to the metal ion. Such ligands are called ambidentate ligands.
Some examples of polydentate ligands are shown in
Table 4.3.
Table 4.3 Examples of polydentate ligands |
| Name |
Formula |
Dentate character |
| Carbonates |
CO32– |
Bidentate |
| Ethane 1,2-diamine |
NH2–CH2–CH2–NH2 |
Bidentate |
| Oxalates |
C2O42– |
Bidentate |
| Glycinate |
NH2–CH2–COO– |
Bidentate |
| Diethylenetriamine |
NH(NH2C2H4)2 |
Tridentate |
| Ethylenediaminetetraacetic acid |
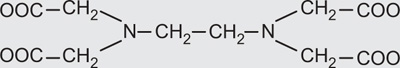 |
Hexadentate |
Two most important naturally occurring chelates are chlorophyll and haemoglobin. These are involved in the life processes of plants and animals, respectively. The synthetic chelating agents, e.g. EDTA, are being used to tie up or sequester iron and copper ions so that they cannot catalyze the oxidative degradation of ascorbic acid in fruit juices and drug formulations. The chelating agents and metal ions form a water-soluble compound during sequestration. EDTA is widely used sequester and also removes calcium ions from hard water (
Fig. 4.9). The process of chelation can also be applied during the assaying of drugs. For example, a colorimetric method to assay procainamide in injectable solutions is based on the formation of 1:1 complex of procainamide with cupric ion at pH 4–4.5 (
Martell & Calvin, 1952;
Martin et al., 1991).
Aromatic-Type Complexes
In recent years, there has been enhanced interest in the synthesis and characterization of aromatic metal complexes. Many platinum and nonplatinum metal complexes such as palladium, ruthenium, rhodium, copper and lanthanum, with these aromatic N-containing ligands, have shown very promising antitumour properties in vitro and in vivo in cisplatin-resistant model systems or against cisplatin-insensitive cell lines. For example, one Ru(III) compound, [ImH][
trans-Cl(4)(Me(2)SO) (Im)Ru(III)] (Im = imidazole, NAMI-A), successfully entered
phase I clinical trials. The aromatic metal complexes can further be subdivided into the following categories.
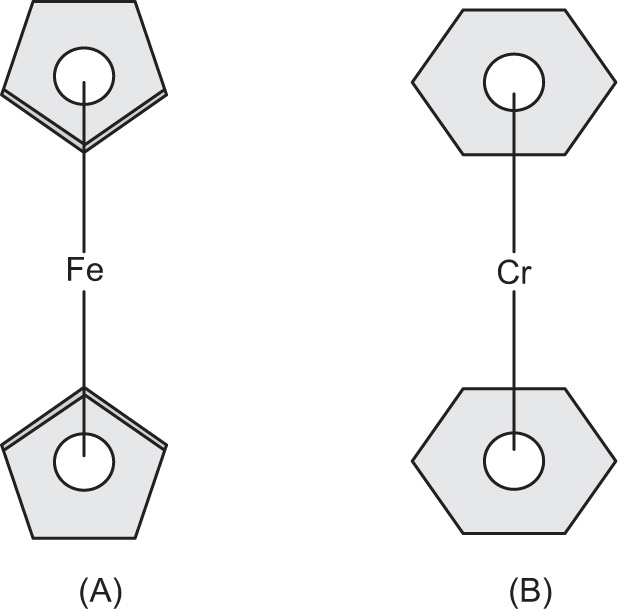
Sandwich Compounds
A sandwich compound in organometallic chemistry is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives (e.g. Cn(CH3)n) and heterocyclic derivatives (e.g. BCnHn+1). Because the metal is usually situated between the two rings, it is said to be sandwiched. A special class of sandwich complexes is the metallocenes. The formula of metallocenes is M(C5H5)2 where M = Cr, Fe, Co, Ni, Zr, Ru, Ti, V, Mo, W or Zn. These species are also called bis(cyclopentadienyl)metal complexes. These compounds are relatively stable and are considered to involve a delocalized covalent bond between the d orbital of a transition metal and a molecular orbital of the aromatic ring.
The term
sandwich compound was introduced in organometallic nomenclature during the mid-1950s in a report by J.D. Dunitz, L.E. Orgel and R.A. Rich, who confirmed the structure of ferrocene by X-ray crystallography. It is an orange-coloured crystalline solid with a camphor-like odour, melting point of 173°C and boils at 249°C without decomposition. It is slightly soluble in alcohol, ether and benzene.
Fig. 4.10a shows the structure of ferrocene and
Fig. 4.10b shows dibenzene chromium.
The sandwich compounds can be classified into three categories, as mentioned below:
1. Mixed cyclopentadienyl complexes: M(C5H5)(CnHn), e.g. Ti(C5H5)(C7H7)
2. Bis(benzene) complexes: M(C6H6)2, e.g. bis(benzene) chromium
3. Bis(cyclooctatetraenyl) complexes: U(C8H8)2
Types of sandwich complexes are discussed below:
Monometallic half-sandwich compounds: Metallocenes including just one facially bound planar organic ligand instead of two, e.g. methylcyclopentadienyl manganese tricarbonyl.
Dimetallic half-sandwich compounds: Compounds such as the cyclopentadienyliron dicarbonyl dimer and cyclopentadienylmolybdenumtricarbonyl dimer can be considered a special case of half sandwiches, except that they are dimetallic. A structurally related species is [Ru(C6H6)Cl2]2.
Multidecker sandwiches: The first multidecker sandwich complex was the dicationic tris(cyclopentadienyl) dinickel complex [Ni2Cp3](BF4)2. A versatile method involves the attachment of Cp.Ru+ to preform sandwich complexes.
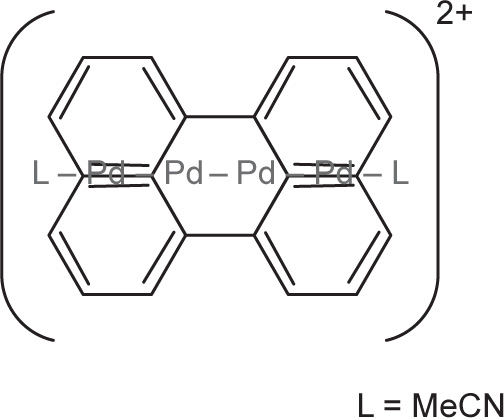
Di- and multimetallic sandwich compounds: Another family of sandwich compounds involves more than one metal sandwich between two polycyclic aromatics. Depicted below (
Fig. 4.11) is such a compound, which has four palladium atoms joined in a chain sandwiched between two perylene units. The counterions are tetraarylborates.
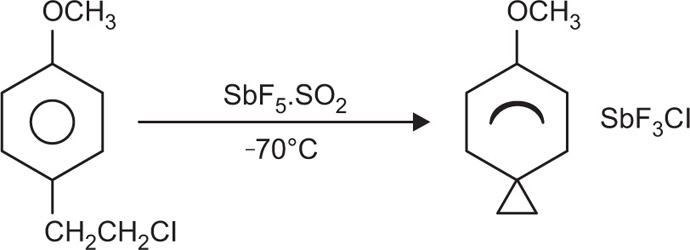
Sigma (σ) Complexes
Sigma complexes are molecular complexes resulting from the rupture of a sigma bond. The complex involves a sigma bond between an ion and a carbon of the aromatic ring. Examples of sigma complexes include H-AlCl
4 and ArCH
2CH
2-Cl; they also occur in Friedel—Crafts reactions. Because they are reactive towards water, no practical pharmaceutical used is made of these complexes. Aromatic compounds react with HCl.AlCl
3 or HF.BF
3 to produce salts that ionize in highly polar nonaqueous solvents. These coordination compounds are very reactive and difficult to isolate. Olah et al., detected the
p-anosonium and the 2,4,6-trimethyphenonium ions produced by ionizing beta-
p-anosylethyl chloride and beta-mesitylethyl chloride, respectively, in SbF
5–SO
2 at –70 to –60°C (
Fig. 4.12).
Pi (π) Complexes
Scientist Keefer et al. has shown that a number of aromatic compounds can be dissolved in aqueous solution of silver nitrate to form water-soluble complex. In case of silver—benzene complex, it is presumed that a cloud of pi bond (in doughnut shape) is over the benzene ring and silver ion is placed above the ring in the doughnut of pi electron. In a disilver ion, one
silver ion is placed over the ring and the other silver ion below the ring, stabilized by pi binding.
Weak molecular interactions such as those in pyridine—iodine, benzene—iodine and benzene—chloroform systems are some examples of pi-type complexes.
Fig. 4.13 shows the pi complex between benzene and protonated imidazole:
Organic Molecular Complexes
An organic molecular complex or organic coordination compound consists of components held together by weak forces of the donor—acceptor type or by hydrogen bonds. For example, dimethylaniline and 2,4,6-trinitroanisole are two compounds that react in the cold to give a molecular complex. Conversely, these two compounds react at a high temperature to form a salt in which the component molecules are held together by primary valence bond.
Numerous organic complexes are so weak that they cannot be separated from their solution as specific compounds, and they are frequently hard to identify by chemical or physical means. These molecular complexes are referred to as
charge transfer complexes. The difference between a charge transfer and a donor—acceptor complex is that in the former type, resonance makes the major contribution to complex action, while in the latter, dispersion forces and dipole—dipole interactions contribute more to the stability of the complex. These complexes are bound together by van der Waals force, dipole—dipole interaction and hydrogen bonding, but lacking charger transfer are known as
molecular complexes. The charge transfer complexes are of much importance in the field of pharmacy, e.g. 1:1 charge transfer complexes formed by iodine with drugs like clomethiazole, disulphiram and tolnaftate (
Andrews & Keefer, 1964;
Martin et al., 1991).
Each of these drugs holds a nitrogen—carbon—sulphur moiety and complex results from the transfer of charge from the pair of free electrons on the nitrogen and/or sulphur atoms of these drugs to antibonding orbital of iodine atom. Therefore, thyroid action in the body can be inhibited by molecules containing the N–C=S moiety via tying up iodine. The organic molecular complexes may further be divided into the following types:
1. Polymer-type complexes
2. Caffeine and other drug complexes
3. Picric acid-type complexes
4. Quinhydrone-type complexes
Polymer Complexes
Several types of polymers also form complexes with specific types of drugs. Carboxymethylcellulose, polyethylene glycols, polystyrene and many similar polymers containing nucleophilic oxygen might form complexes with various drugs. The incompatibilities of certain polyethers, such as the carbowaxes, pluronics and tweens with tannic acid, salicylic acid and phenol may also be due to these types of polymer complexes’ interactions. The incompatibility may be marked as flocculate, precipitate, delayed biological absorption, and loss of preservative action or other undesirable physical, chemical and pharmacological effects. Crosspovidone is a crosslinked insoluble polyvinyl pyrolidone (PVP). It is able to bind various drugs such as acetaminophene, benzocain, benzoic acid, tannic acid and papaverine hydrochloride to form polymer-type complexes. The interaction is mainly due to phenolic groups on the drugs and dipolar and porous organization of crosspovidone. Crosspovidone is used as a disintegrant in pharmaceutical granules and tablets. It does not obstruct the gastrointestinal absorption of drug because the binding of drug is reversible. Another example of polymer is hexylresorcinol, which shows extremely strong binding, but the interaction is less than 5% for most drugs. Polymer drug complexes are mainly employed to modify biopharmaceutical parameters of drugs, e.g. the dissolution rate of ajmaline is enhanced by complexation with PVP. This interaction is attributed to the aromatic ring of ajmaline and the amide group of PVP to yield a dipole–dipole induced polymer type of complex. Some polymer complexes with compound and/or drugs are mentioned in
Table 4.4.
Table 4.4 Polymer complexes with compound and/or drugs |
| Agent/drugs |
Polymers |
| Polyethylene glycol |
Resorcinol, catechol, salicylic acid, p-hydroxybenzoic acid, m-hydroxybenzoic acid |
| Povidone (polyvinyl pyrolidone, PVP) |
Salicylic acid, p-hydroxybenzoic acid, m-hydroxybenzoic acid, sodium salicylate, phenobarbital |
| Sodium carboxymethyl cellulose |
Procaine, quinine, pyribenzamine |
| Oxytetracycline and tetracycline |
N-methyl pyrrolidone, caffeine, sodium-p-aminobenzoate |
Caffeine and Other Drug Complexes
Complexation between drug and complexing agent can improve or impair drug absorption and bioavailability. Caffeine has been found to form complexes with many types of drugs. Higuchi and his associates were the first to investigate the complexation of caffeine with a number of acidic drugs. They pointed out the interaction between caffeine and a drug such as sulphonamide
or a barbiturate by a dipole–dipole force or a hydrogen bonding between the polarized carbonyl groups of caffeine and the hydrogen atom of the acid. Also a secondary interaction probably occurs between the nonpolar parts of the molecules, and the resultant complex is squeezed out of the aqueous phase due to the higher internal pressure of water. These two effects lead to a high degree of interaction.
The complexes formed between esters, amines, phenols, ethers and ketones have been attributed to the hydrogen bonding between a nucleophilic corbonyloxygen and active hydrogen. Complexation of esters such as benzocaine, procaine and tetracaine with caffeine has also been reported by Higuchi, and he suggested that, in the caffeine molecule, comparatively a positive centre exists that serves as a possible site of complexation. Caffeine makes complexes with organic acids’ anions, which are more soluble than the pure xanthine. But the complexes of caffeine made with organic acids, such as gentisic acid, are less soluble than caffeine alone. Such insoluble complexes provide caffeine in the form that masks its normally bitter taste and should serve as a suitable state for chewable tablets. These chewable tablets offer an extended release of the drug with improved taste.
Picric Acid Complexes
Picric acid is 2,4,6-trinitrophenol and its pKa is 0.38. Picric acid reacts with strong bases to form salts and with weak bases to form molecular complexes. Butesin picrate, a yellow powder most probably is a 2:1 complex, which is insoluble in water but soluble in organic solvents. The possible use of butesin picrate is as a 1% ointment for burns and painful skin abrasions. Butesin picrate combines the antiseptic property of picric acid and the anaesthetic property of butesin. It has been suggested that the stability of the complexes produced among carcinogenic agents and picric acid is related to carcinogenic activity, and any substitution on the carcinogen agent that hinders picrate complexation also reduces carcinogenicity. One more example of this category is anthranilic acid–picric acid complex (2C7H7NO2. C6H3N3O7). In this complex, picric acid is sandwiched between two crystallographically independent anthranilic acid molecules, with spacing of 3.29 and 3.33Å. The carboxyl and amino groups of anthranilic acid are neutral and the former group is hydrogen bonded to the polar nitro and phenol oxygen atoms of neighbouring picric acid molecules.
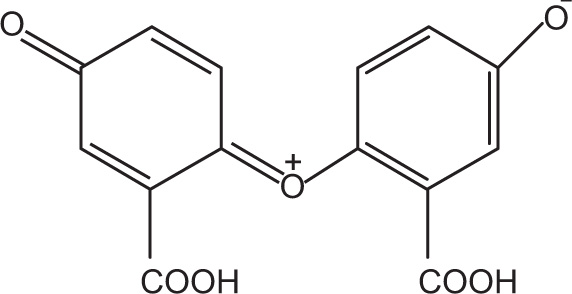
Quinhydron Complexes
Quinhydron complexes are formed by mixing alcoholic solutions of equimolar quantities of benzoquinone and hydroquinone and these complexes deposit as green crystals. The 1:1 complex formed between benzoquinone and hydroquinone might have resulted from the overlap of the pi-framework of the electron-deficient quinine molecule with the pi-framework of the electron-rich hydroquinone molecule. Highest overlap between the pi-frameworks is anticipated if the aromatic rings are parallel and are oriented in such a way as to have their centres directly above one another.
Once an aqueous solution is saturated with quinhydron, the complex dissociates into equal amount of quinine and hydroquinone. This is used as an electrode in the determination of pH. Hydrogen bonding may contribute in stabilizing this complex, but it is not the individual path of association, because hydroquinone dimethyl ether also forms a coloured adduct with quinone. A remarkable quinone is obtained from salicylic acid, which readily oxidizes and yields blue-black quinhydrone compound (
Fig. 4.14).
Quinhydrone compounds are valuable chemicals, which are useful as electron—donor—acceptor complexes in liquid crystal displays, as bactericides in the petroleum industry for inhibiting the growth of sulphate-reducing bacteria, as components of sulphuric acid—based pickling solutions for steel, components in oxidation—reduction electrodes, and as components in antifriction compositions based on polyethylene and powdered iron.
Inclusion/Occlusion Compound Complexes
The group of addition compounds is known as inclusion or occlusion compounds. These compounds are due to the architecture of molecules rather than from their chemical affinity. One of the constituents of the complex is trapped in the open lattice or cage-like crystal structure of the other to yield a stable arrangement. The inclusion/occlusion compound complexes may be divided into the following types:
Layer-Type Complexes
Some compounds such as the clay montmorillonite, the principal constituent of bentonite, may trap alcohols, hydrocarbons and glycols between the layers of lattices. Graphite can also intercalate compounds between its layers.
Channel Lattice-Type Complexes
The cholic acid (bile acids) may form a group of complexes principally involving deoxycholic acid in combination with esters, organic acids, paraffins, ketones and aromatic compounds and with solvents such as alcohol, ether and dioxane. The crystals of deoxycholic acids are arranged to form a channel into which the complexing molecule can fit. Actually, camphor has been partly resolved by complexation with deoxyhcholic acid and
dl-terpineol has been resolved by the use of digitonin, which occludes certain molecules in a manner similar to that of deoxycholic acid.
Urea (
Figs. 4.15 and
4.16) and thiourea also crystallize in a channel-like structure permitting enclosure of alcohols, ketones, unbranched paraffins, organic acids and other compounds.
The well-known starch—iodine solution is a channel-type complex consisting of iodine molecules entrapped within spirals of the glucose residues. Monoestearin, an interfering component in the assay of dienestrol, could be extracted easily from dermatologic creams by channel-type inclusion in urea.
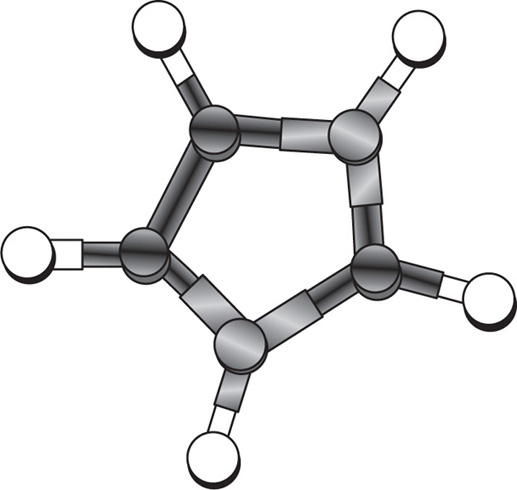
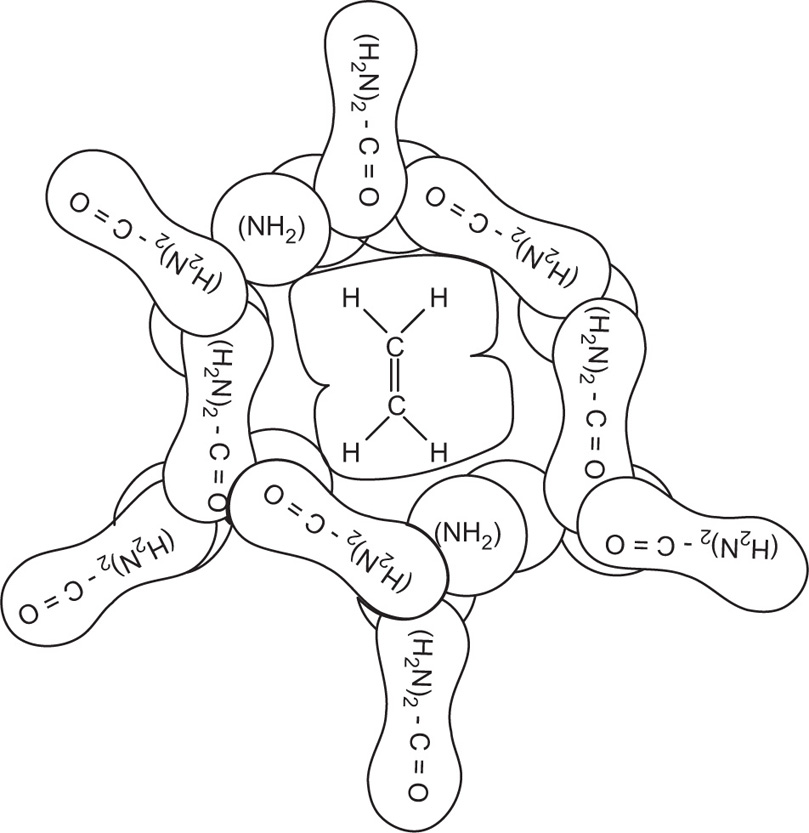
Monomolecular-Type Complexes
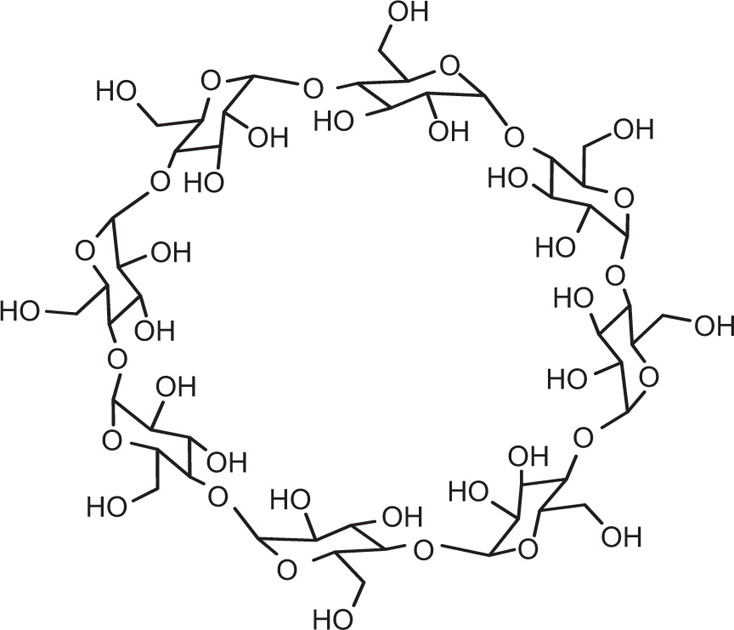
Apart from channel- and cage-type compounds, the inclusion compounds also include monomolecular and macromolecular inclusion compounds. Monomolecular inclusion compounds engross the entrapment of a single guest molecule in the cavity of one host molecule. Monomolecular host configurations are represented by the cyclodextrins (
Fig. 4.17). These cyclic compounds are oligosaccharides having a minimum of six
d-(+)-glucopyranose units attached by
α-1,4 linkages produced by the action on starch of
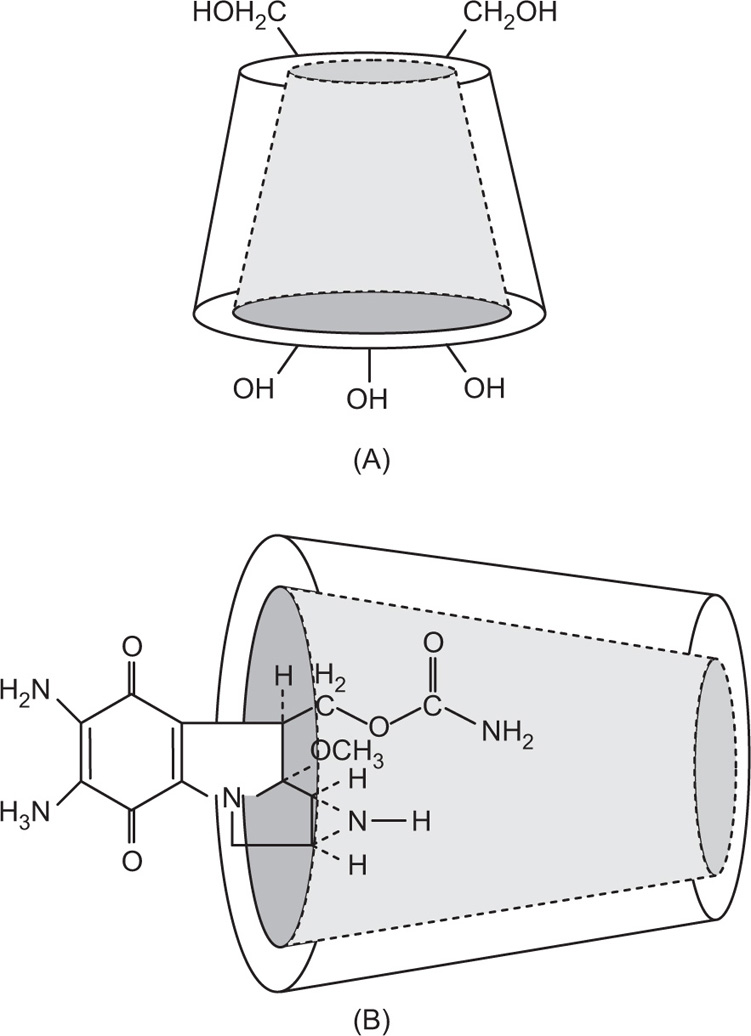
Bacillus macerans amylase. The ability of cyclodextrins to form the inclusion-type complex in the aqueous solution is attributed to the typical arrangement of the glucose units. The cyclodextrin structure forms a torus or doughnut ring. In fact, the molecule exists as a truncated cone. Cyclodextrins (CDs) have been studied as solubilizing and stabilizing agents in the pharmaceutical dosage formulations. They have been used to entrap, stabilize or solubilize morphine, tetracycline, retinoic acid, famotidine, tolbutamide, sulphonamides, reserpine, benzocaine, testosterone, ephedrine, etc.
One example of inclusion compound of cyclodextrin is with antibiotic mitomycin C. The interior cavity of the cyclodextrin is comparatively hydrophobic because of the CH2 groups, whereas the peripheries are hydrophilic because of the presence of primary and secondary hydroxyl groups. α-CDs have the smallest cavity, while β-cyclodextrins and γ-cyclodextrins are the most useful for pharmaceutical technology due to their larger cavity size. The molecules of the appropriate size and stereochemistry may be incorporated in the cyclodextrin cavity by hydrophobic interactions.
Mitomycin C interacts with γ-cyclodextrins at one side of the torus. Thus, the aziridine ring of the mitomycin C remains protected from degradation in the acidic medium (
Fig. 4.18 a and b). Complexation does not ordinarily involve the formation of covalent bonds. One more example of inclusion complex is indomethacin–cyclodextrin complex. The
p-chlorobenzoyl part of indomethacin enters the
β-cyclodextrin ring, whereas the substituted indol moiety of indomethacin is too large to enter the cyclodextrin cavity.
CDs may boost or diminish the reactivity of the guest molecule depending on the nature of the reaction and the orientation of the molecule inside the cyclodextrin cavity. Recently, derivatives of the natural crystalline cyclodextrin are being developed to enhance aqueous solubility and reduce toxicity. Controlled
partial methylation of some of the OH groups in cyclodextrin diminishes the intermolecular hydrogen bonding, which renders OH groups free to interact with water. The free OH groups increase the aqueous solubility of cyclodextrins.
Apart from hydrophilic derivatives, hydrophobic forms of β-cyclodextrins have also been instituted useful as sustained release carriers for many drugs. For example, release rate of diltiazem has been modified by complexation with ethylated β-cyclodextrins. Another example of sustaining the action of drug is of isosorbide dinitrate. CDs may also improve organoleptic characteristics of oral liquid formulations. For example, bitter taste of femoxetine can be suppressed by complexation with β-cyclodextrins.
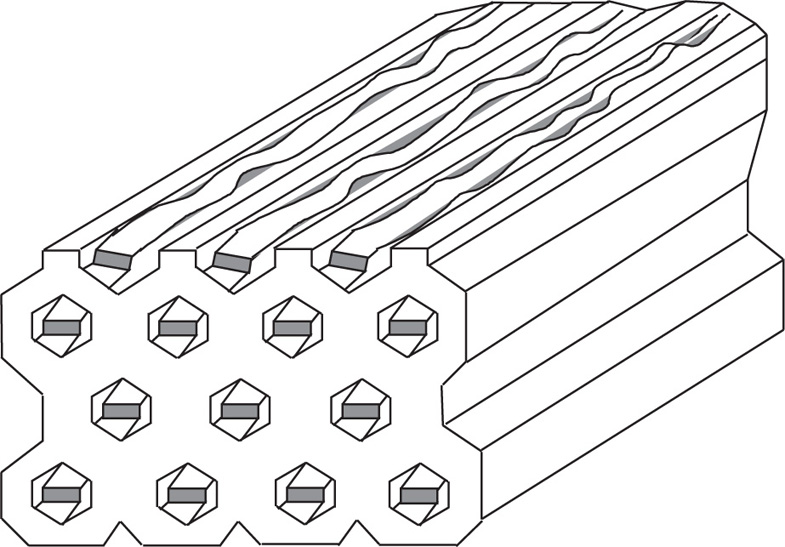
Macromolecular-Type Complexes
Macromolecular inclusion compounds are also called molecular sieves. They commonly include zeolites, dextrins, silica gels and related substances. The atoms are arranged in three dimensions to produce cages and channels. Synthetic zeolites may be made to a definite pore size so as to separate molecules of different dimensions. They are also capable of ion exchange.
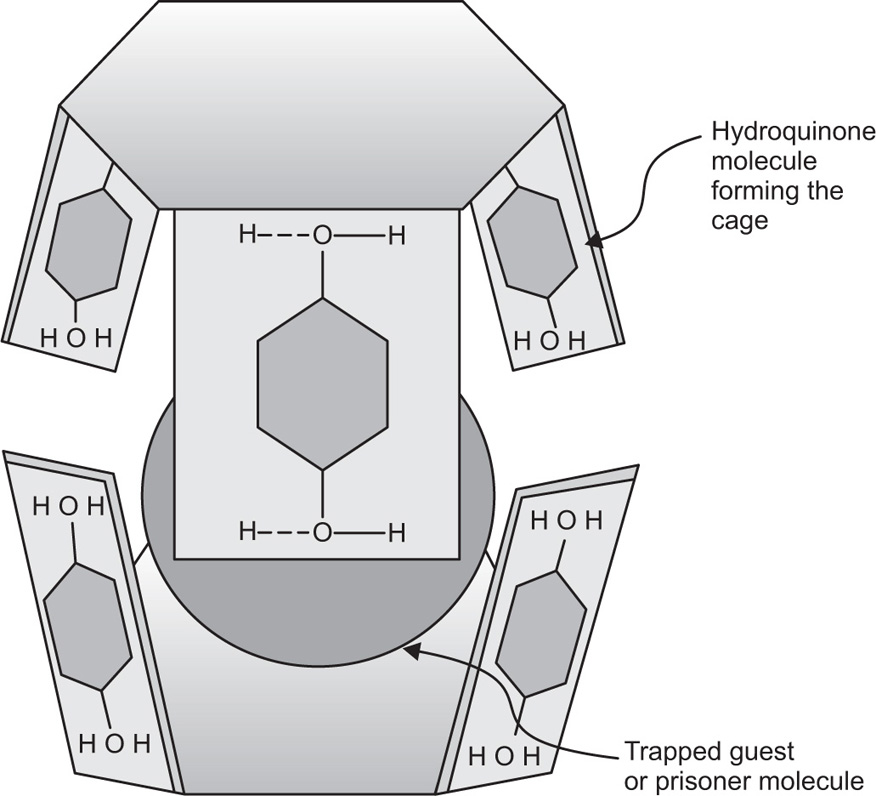
Clathrates-Type Complexes
The clathrates form complexes by crystallizing in the form of a cage-like lattice in which the coordinating compound is trapped. Chemical bonds are not involved in clathrates-type complexes and only the molecular size of the encaged component is of importance. The stability of the clathrates can be related to the confinement of the prisoner. The stability of clathrate might be due to the strength of the structure, that is, the high energy. This high energy must be expended to decompose the compound, just as prisoner is confined by the bars that prevent his escape. Warfarin sodium USP is a clathrate of isopropyl alcohol, water and sodium warfarin in the form of a white crystalline powder. Highly toxic hydroquinone (quinol) also forms clathrates-type complexes, which crystallize in a cage-like hydrogen-bonded structure (
Fig. 4.19).
Analysis Methods for Determination of Complexes
Quantitative expression of stability constants between the ligand and metal or donor and acceptor are extremely important in the study and application of coordination compounds. Stability constants are widely used in analytical chemistry, in devising new methods or estimating interfering effects. They must also be considered in such areas as the kinetics of reaction in solution involving metal complexes, biological effects of metal ions, etc.
Various methods for the determination of complexation are as follows:
1. Method of continuous variation
2. pH titration method
3. Distribution method
4. Solubility method
5. Spectrophotometric method
Method of Continuous Variation
There are several spectrophotometric methods used to evaluate the mole ratio and the formation constant (Kform) for complexes. The slope ratio method can only be used to find the mole ratio, but both the mole ratio method and the continuous variation methods can be used to find the mole ratio and the formation constant.
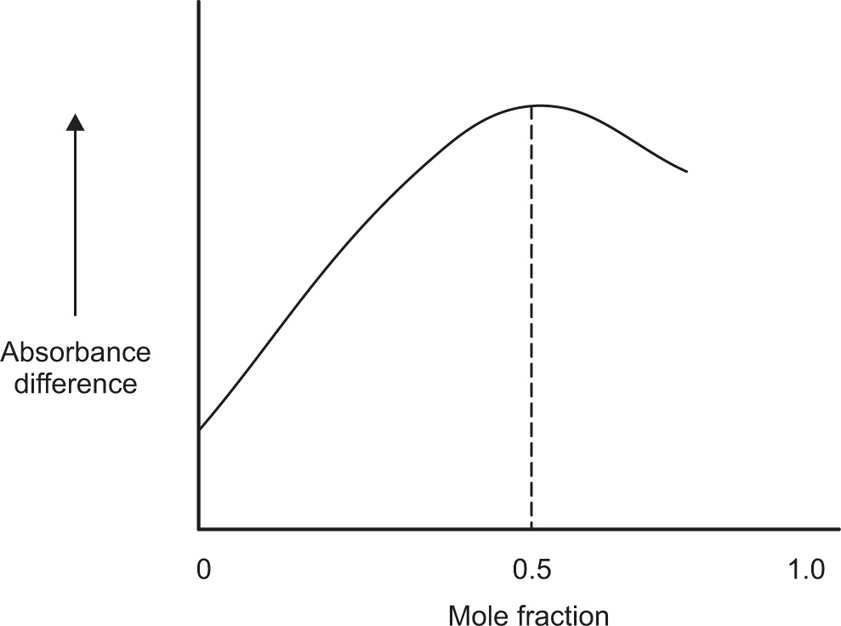
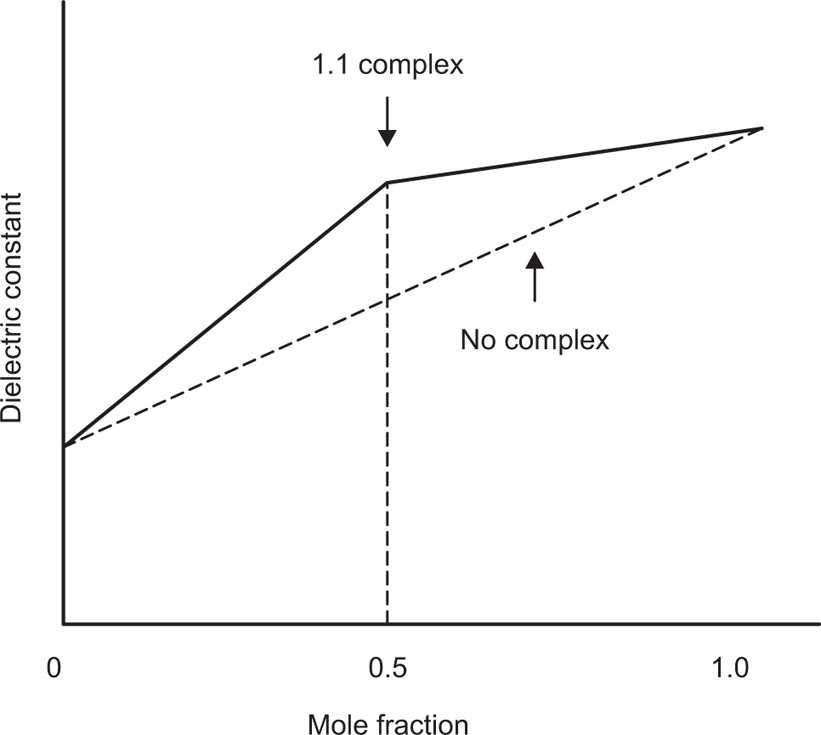
For the measurement of complexation, additive property such as spectrophotometric extinction coefficient is used. Dielectric constant or the square of the refractive index may also be used for the same. If the property for two species is sufficiently different and if no interaction occurs when the components are mixed, then the value of property is the weighted mean of the values of the separate species in the mixture. This means that, if the additive property, say dielectric constant, is plotted against the mole fraction from 0 to 1 for one of the components of a mixture where no complexation occurs, a linear relationship is observed. If solutions of two species A and B of equal molar concentration are mixed and a complex forms between the two species, the value of the additive property will pass through a maximum (or minimum). For a constant total concentration of A and B, the complex is at its greatest concentration at a point where the species A and B are combined in the ratio in which they occur in the complex (
Fig. 4.20).
When spectrophotometric absorbance is used as the physical property, the observed value obtained at various mole fractions when complexation occurs, is usually subtracted from the corresponding values that would have been expected had no complex resulted. This difference
D is then plotted against mole fraction. From such a curve, the molar ratio of the complex is obtained. By means of a calculation involving concentration, and the property being measured, the stability constant of the formation may be determined. Firstly, this method was restricted to the formation of a single complex. Nowadays this method is being used for the formation of higher complexes in the solution (
Fig. 4.21) (
Escander, 1999).
pH Titration Method
Direct pH method is based on the fact that the pH-metric titration of solutions of a protonated ligand, alone and in the presence of a metal ion, through the stepwise addition of a strong base allows evaluation of the constants for the ligand deprotonation equilibrium and for the proton displacement equilibrium due to the metal ion competition.
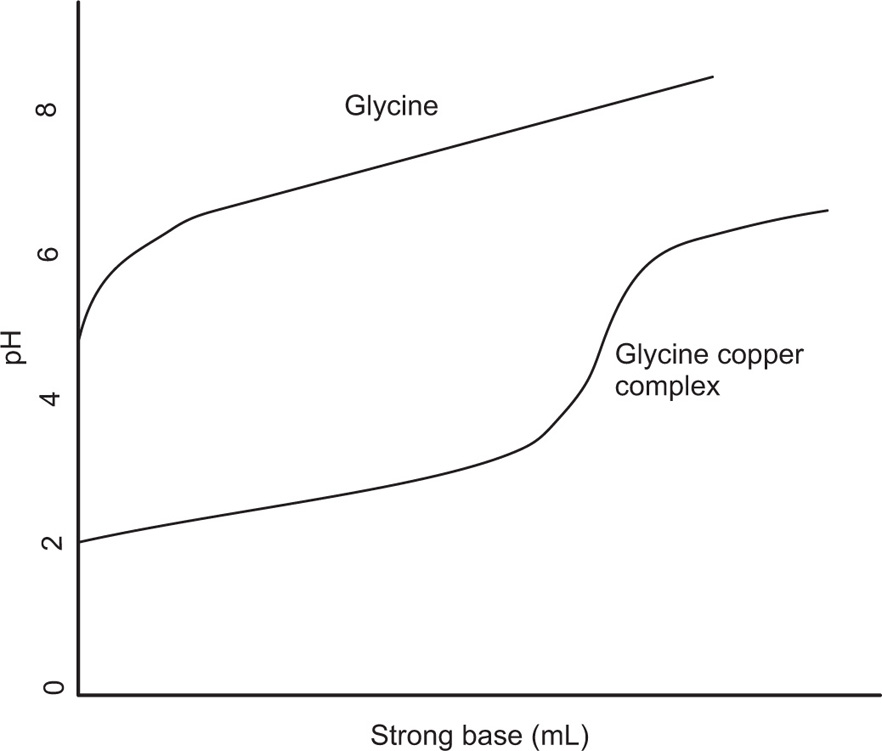
This is one of the most reliable methods and can be used whenever the complexation is followed by a change in pH. The chelation of the cupric ion by glycine, for example, may be represented as:
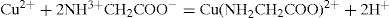
Since two protons are formed in this reaction. The addition of glycine to a solution containing cupric ions should result in a decrease in pH. Titration curves can be obtained by adding a strong base to a solution of glycine and to another solution containing glycine and a copper salt, and plotting the pH against the equivalents of base added. The curve (
Fig. 4.22) for the metal–glycine mixture is well below that for the glycine alone, and the decrease in pH shows that complexation is occurring throughout most of the neutralization range.
The quantitative estimation of stability constants for the complex, i.e. copper ion or metal M and glycine or the ligand A may be describe as:
And the overall reaction is M + 2A = MA
2; β =
K1K2 = [MA
2]/[M] [A]
2.
Where
K1 and
K2 are the formation constant, the equilibrium constant
β for the overall reaction is known as the
stability constant. The quantity
n can be defined as the number of ligand molecules bound to the number of metal ion. The average number of ligand groups bound per metal ion present is therefore designated as
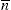
and is written as
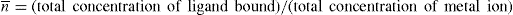
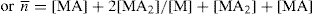
Similar results can be obtained with other zwitterions and weak acids (or bases), such as N,N′-diacetylethylenediamine diacetic acid, which has been studied for its complexing action with copper and calcium ions. This method can be used to study tendency of pyrrolidone 5-hydroxamic acid to bind the ferric ion to form mono, bis and tri chelates. Stability constant for lithium catecholamine complexes can be studied by potentiometer titration of free lithium ion. The lithium forms complexes with the zwitterionic species of catecholamines at pH 9–10 and with deprotonated forms at pH values above 10. The interaction with lithium depends on the dissociation of the phenolic oxygen of catecholamines. At physiological pH, the protonated species show no significant complexation. Some lithium salts such as lithium carbonate, lithium chloride and lithium citrate are used in psychiatry.
Distribution Method
The method of distributing a solute between two immiscible solutes can be used to determine the stability constant for certain complexes. The complexation of iodine by potassium iodine may be used as an example to illustrate the method. The equilibrium reaction in its simplest form is I2 + I– = I3–.
Addition steps also occur in polyiodine formation (
Dawsom, 1901); for example,
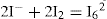
may occur at higher concentrations. Distribution coefficient of iodine when distributed between water (
w) and carbon sulphide as the organic phase (
o) is found to be 625. When it is distributed between a 0.1250 M solution of potassium iodide and carbon disulphide, the concentration of iodine is found to be 0.1896 mole/litre in organic phase and 0.02832 moles/litre in aqueous phase. In short, total concentration of KI (free and complex) = 0.1250 mole/litre.
Total concentration of I2 in aqueous phase = 0.02832 mole/ litre.
Total concentration of I2 in organic phase (free and complex) = 0.1896 mole/litre.
Distribution coefficient, K(o/w) = [I2]o/[I2]w = 625.
Higuchi et al. (1953) also investigated complexation of caffeine, PVP and PEG on number of acidic drugs using the same method, that is, distribution method. Zunk reported in 1953 the stability constant of benzoic acid with caffeine at temperature 0°C, and it was found to be 37.5.
Solubility Method
According to the solubility method, excess quantities of the drug are placed in well-stoppered containers, together with a solution of the complexing agent in various concentrations, and the bottles are agitated in a constant-temperature bath until equilibrium is attained. Aliquot portions of the supernatant liquid are removed and analyzed. The method can be employed to investigate the complexation of
p-aminobenzoic acid (PABA) by caffeine.
Higuchi and Lach (1954) used the solubility method of investigating the complexation of
p-aminobenzoic acid by caffeine. The results are shown in
Fig. 4.23 and explained by the curve, as follows.
The point A at which the line crosses the vertical axis is the solubility of the drug in water. With the addition of caffeine, the solubility of PABA rises linearly because of complexation. At point B, the solution is saturated with respect to the complex and to the drug itself. The complex continues to form and
to precipitate from the saturated system as more caffeine is added. At point C, all of the excess solid PABA has passed into solution and has been converted to the complex. Although the solid drug is exhausted and the solution is no longer saturated, some of the PABA remains uncomplexed in solution, and it combines further with caffeine to form higher complexes such as PABA-2 caffeine as shown in the curve at the right of the diagram. For obtaining the stoichiometric ratio of the complex, following calculations are made. The concentration of caffeine, corresponding to the plateau BC, equals the concentration of caffeine entering the complex over this range, and it is equal to 1.8 × 10
–2 mole/litre. The concentration of PABA entering the complex is obtained from the undissolved solid remaining at point B. It is obtained from subtracting the acid in solution at the saturation point B from the total acid initially added to the mixture since this is the amount yet undissolved that can form the complex. Amount of PABA is equal to 7.3 × 10
–2 – 5.5 × 10
–2 mole/litre or 1.8 × 10
–2 mole/litre. And the stoichiometric ratio = caffeine in complex/PABA in complex.
That is, 1.8 × 10–2/1.8 × 10–2 = 1
Complex formation can be written as
and stability constant for 1:1 complex can be written as
Value of stability constant can be determined as follows. The concentration of complex [PABA–caffeine] is equal to the total acid concentration at saturation less the solubility [PABA] of the acid in water. The concentration of [caffeine] in the solubility at equilibrium is equal to the caffeine added to the system less the concentration that has been converted to the complex. The total acid concentration at saturation is 4.58 × 10–2 mole/litre when no caffeine is added. And it is 5.312 × 10–2 mole/litre, when 1.00 × 10–2 mole/litre of caffeine is added.
Further, Higuchi et al. (1965) studied on various drug–caffeine complexes and stability constants of some caffeine complexes in water at 30°C, as shown below:
| Compound |
Stability constant (approximately) |
| Sulphadiazine |
07 |
| Picric acid |
08 |
| Sulphathiazole |
11 |
| Acetyl salicylic acid |
15 |
| Balicylic acid |
40 |
| Benzoic acid |
18 |
| Benzocaine |
59 |
Spectroscopy and Charge-Transfer Complexation
Absorption spectroscopy in the visible and ultraviolet regions of the spectrum is commonly used to investigate electron donor—acceptor or charger-transfer complexation. When iodine is analyzed in a noncomplexing solvent such as CCl4, a curve is obtained with a single peak at about 520nm. The solution is violet in colour. A solution of iodine in benzene exhibits a maximum shift to 475nm, and a new peak of considerably higher intensity for the charge-shifted band appears at 300nm. A solution of iodine in diethyl ether shows a still greater shift to lower wavelength and the appearance of a new maximum. These solutions are red to brown in colour.
In benzene and ether, iodine is the electron acceptor and the organic solvent is the donor; in CCl4, no complex is formed. The shift towards the ultraviolet becomes greater as the electron donor solvent becomes a stronger electron-releasing agent. These spectra arise from the transfer of an electron from the donor to the acceptor in close contact in the excited state of the complex. The more easily a donor such as benzene or diethyl ether releases its electron, as measured by its ionization potential, the stronger it is as a donor. Ionization potentials of a series of donors produce a straight line when plotted against the frequency maximum or charge-transfer energies for solutions of iodine in donor solvents.
Miscellaneous Methods
A number of other methods are available for studying the complexation of metal and organic molecular complexes. They include NMR, and infrared spectroscopy, polarography, circular dichroism, kinetics, X-ray diffraction and electron diffraction.
Complexation of caffeine with l-tryptophan in aqueous solution can be investigated using 1H NMR spectroscopy. Caffeine interacts with l-tryptophan at a molar ratio of 1:1 by parallel stacking. Complexation is a result of polarization and π–π interaction of aromatic rings. This study demonstrates that, tryptophan, which is presumed to be the binding site in serum albumin for certain drugs, can interact with caffeine even as free amino acid. However, caffeine does not interact with other aromatic amino acids such as l-valine or l-leucine.
Circular dichroism can be used to study the coil-helix transition of polyadenylic acid induced by the binding of the catecholamines, norepinephrine and isoproterenol. Most mRNA molecules contain regions of polyadenylic acid, which are thought to increase the stability of mRNA and to favour genetic code translation. The change of the circular dichroism spectrum of polyadenylic acid was interpreted as being due to intercalative binding of catecholamines between the stacked adenine bases.
Infrared spectroscopy can be used to investigate the hydrogen-bonded complexes involving polyfunctional bases such as proton donors. This is a very precise technique to determine the
thermodynamic parameters involved in the hydrogen bond formation and to characterize the interaction sites when the molecule has several groups available to form hydrogen bonds (
Kalinkova, 1999 a,
b &
Marino, 2000). Caffeine forms hydrogen-bonded complexes with various proton donors, e.g. phenol, phenol derivatives, aliphatic alcohols and water. From the infrared technique, the preferred hydrogen-bonding sites are the carbonyl functions of the caffeine. Seventy percent of the complexes are formed at the C=O(6) group and 30% of the complexes at the C=O(2) function of caffeine.
Protein Binding
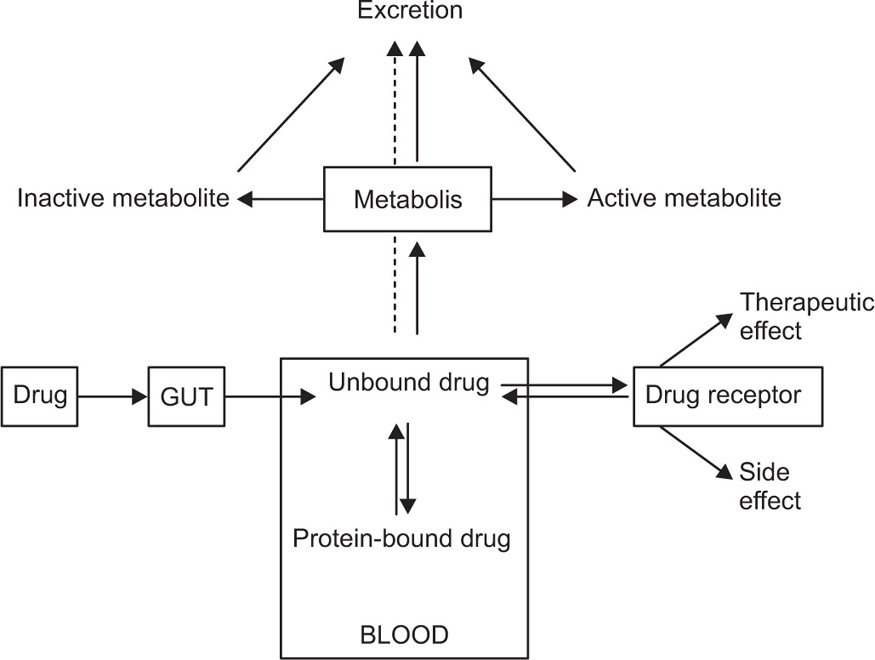
Binding to plasma proteins is both a help and a hindrance to the distribution of drugs through the body. Transport in the bloodstream by binding to albumin helps the drugs to reach regions remote from the site of administration. Because bound drug cannot readily leave the capillaries, however, the rate of distribution of drug into the tissues will be controlled by the concentration gradient produced by the concentration of unbound unionized drug. Usually, it is the unbound drug concentration that is considered to be pharmacologically and toxicologically active. The fraction of unbound drug can also influence the rate of drug elimination. In this manner, binding affects both the duration and the intensity of drug action. The binding and transport of endogenous and exogenous substances is one of several important functions of the plasma proteins (
Fig. 4.24). The endogenous substances include bilirubin, fatty acids, l-tryptophan, vitamins and many hormones. Most drugs also bind to one or other of the plasma proteins, at least to some extent. Drugs may bind to various macromolecular components in the blood, including albumin, acid glycoprotein, lipoproteins, immunoglobulins (IgGs) and erythrocytes (RBCs).
Albumin is quantitatively the major binding protein for acidic and neutral drugs. Bases are bound to a lesser extent by albumin, and there is growing evidence that globulins are the major binding proteins for basic drugs. Albumin has high-affinity binding sites, which are probably specific for bilirubin and the fatty acids, but the high-affinity sites for various hormones are located among the globulins. There is no precise definition of a ‘highly’ bound drug, but only when the percentage of a drug bound exceeds about 70% is binding likely to exert much influence on the distribution and pharmacokinetics of a drug. In the case of highly bound drugs, for instance, warfarin, carbenoxolone or phenylbutazone, the unbound fraction may be considerably less than 1% of the total plasma drug concentration. The interaction of most drugs with the plasma proteins is a dynamic, reversible process with dissociation of bound drug molecules from the drug–protein complex occurring very rapidly, probably within milliseconds or less. The rate of dissociation of drug molecules from plasma proteins is therefore not a limiting factor in the uptake of drug from the bloodstream by major organs, whose perfusion time may be several seconds or more.
Another plasma protein, α1-acid glycoprotein, has also shown to bind numerous drugs like propanolol, imipramine, lidocaine and globulin. This protein has shown greater affinity for basic drugs as compared to acidic drugs. Acidic drugs may also bind to lipoproteins if the albumin is saturated. Lipoprotein binding is not binding in the strict sense of the term; it is closer to dissolving and is common in lipid-soluble drugs. A drug that binds to tissue often binds to melanin-rich tissue or DNA. Globulins may be responsible for the plasma transport of certain endogenous substances such as corticosteroids. These globulins have a low capacity but high affinity for the binding of these endogenous substances.
Lipoproteins are macromolecular complexes of lipids and proteins and are classified according to their density and separation in the ultracentrifuge. The terms VLDL, LDL and HDL are abbreviations for very-low-density, low-density and high-density lipoproteins, respectively. Lipoproteins are responsible for the transport of plasma lipids to the liver and may be responsible for the binding of drugs if the albumin sites become saturated.
Erythrocytes, or red blood cells (RBCs), may bind both endogenous and exogenous compounds. RBCs consist of about 45% of the volume blood. Phenytoin, pentobarbital and amobarbital are known to have an RBC/plasma water ratio of 4:2, indicating preferential binding of drug to the erythrocytes over plasma water. Penetration into RBC is dependent on the free concentration of the drug. In the case of phenytoin, RBC drug level increases linearly with an increase in the plasma-free drug concentration. Increased, drug binding to plasma albumin reduces RBC drug concentration. With most drugs, however, binding of drug to RBC generally does not significantly affect the volume of distribution, because the drug is often bound to albumin in the plasma water. Even though phenytoin has a great affinity for RBC, only about 25% of the blood drug concentration is present in the blood cells and 75% in the plasma because the drug is also strongly bound to albumin. For drugs with strong erythrocyte binding, the haematocrit will influence the total amount of drug in the blood. For these drugs, the total whole-blood drug concentration should be measured.
The interaction of drugs with proteins present in the body can influence their action in a number of ways. Proteins may (1) enhance the distribution of drugs throughout the body, (2) inactivate the drug by not allowing a sufficient concentration of drug to accumulate or reach at the receptor site or (3) retard the excretion of drug. The interaction of the drug with proteins may cause (1) the displacement of body hormone or a coadministered agent (2) a configurational change in the protein, the structurally altered form of which is capable of binding a coadministered agent, or (3) the formation of drug–protein complex that itself is biologically active. A drug that extensively binds to plasma proteins is warfarin. Administration of another drug that has higher affinity for plasma proteins (e.g. several NSAIDs) will displace warfarin from its binding sites and cause serious haemorrhage to the patient.
Protein binding is usually reversible and thus creates a chemical equilibrium, in which the chemical reaction can go backwards and forwards, with no net change in reactants and products. This means that a cell that is effective at extracting the unbound drug may extract more of the drug as it disassociates in the course of achieving equilibrium. The equation for reversible protein binding is:
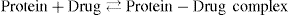
The amount of protein binding and the fraction unbound, written as the concentration of unbound drug over the total concentration of the drug, depends on several factors. It is determined by the drug’s affinity for the protein, the concentration of the binding protein and the concentration of the drug relative to the binding protein. This is important when considering other medications that a patient might be on because certain proteins may already be saturated, which would affect the amount of free drug and possibly change the desired pharmacological effects.
For example, if drug A saturated a certain binding protein and then drug B was not able to bind to that protein, there would be a higher concentration of unbound drug B. Drug B could also competitively displace drug A from the binding protein, thus raising the unbound fraction of drug A. This process happens fairly quickly, in minutes to hours, and both scenarios could have adverse effects. Many drugs, however, have different binding proteins or different binding sites on a protein, or they are not present in high enough relative concentration to saturate the proteins, and so, do not compete with the other drug or drugs in use. Likewise, the ability of the body to extract the drug can affect the drug’s clearance into the body. Renal failure and liver disease often negatively impact the body’s ability to extract the unbound drug. For these reasons, it is important to consider previous medical issues, the total concentration of the drug, the unbound fraction of the drug and any other medications a patient might be taking.
Mathematical analysis of protein binding – Binding equilibria
The interaction between a group or free receptor R in a protein and a drug molecule D is written as:
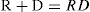 (1)
(1)
The equilibrium constant, disregarding the difference between activities and concentrations, is:
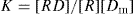 (2)
(2)
Or
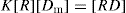 (3)
(3)
where K is the association constant, [R] is concentration of protein in terms of free binding sites or receptor, [Dm] is the concentration of free drug complex, usually given in moles. K may vary with temperature and might be better represented as KT. Bound drug may be written as [RD] and is sometimes written as [Db] and [D]; the free drug is written as [Dm].
If the total protein concentration is designated as [Rt], we can write
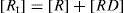 (4)
(4)
Or
Substituting the expression for [
P] from
Eq. 4 into
Eq. 3 gives:
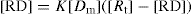 (5)
(5)
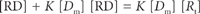 (6)
(6)
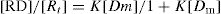 (7)
(7)
Let p is the number of moles of drug bound [RD] per moles of total protein [Rt]; then,
p = [RD]/[Rt] or
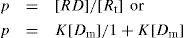 (8)
(8)
The ratio p can also be expressed in other dimensions, such as milligrams of drug bound per gram of protein. Equation is a form of Langmuir adsorption isotherm and quite useful for expressing protein-binding data. Expression of the equation can be converted to a linear form, convenient for plotting, by inverting it:
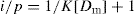 (9)
(9)
If
v independent binding sites are available, the expression for
p in
Eq. 8 is simply
v times that for a single site or
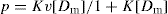 (10)
(10)
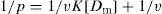 (11)
(11)
An alternative manner of writing
Eq. 10 is to rearrange it first to:
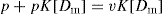 (12)
(12)
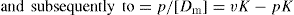 (13)
(13)
Data presented according to
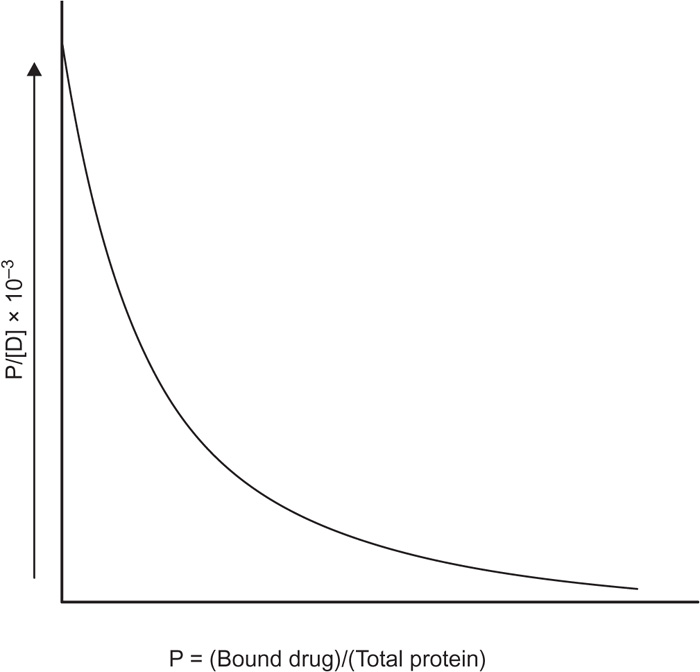
Eq. 13 are known as Scatchard plot. The binding of bishydroxycoumarin to human serum albumin is shown as a Scatchard plot in
Fig. 4.25.
A number of techniques are being used to determine the amount of drug bound to a protein. Equilibrium dialysis, ultrafiltration and electrophoresis are the classic techniques used. Recently, other methods like gel filtration and nuclear magnetic resonance have been used with satisfactory results. According to
equilibrium dialysis method, the serum albumin is placed in a cellulose membrane tube or similar dialyzing membrane. The tubes are tied securely and suspended in vessels containing the drug in various concentrations. Ionic strength and occasionally hydrogen ion concentration are adjusted to definite values. Control and blank samples are run to account for the adsorption of the drug and the protein on the membrane. If binding occurs, the drug concentration in the sac containing the protein is greater at equilibrium than the concentration of drug in the vessel outside the sac. Samples are removed and concentration of free and complexed drug obtained is analyzed. This is the oldest method for protein binding determination and continues to be the most accepted method. Some potential errors of this method are the possible binding of the drug to the membrane, transfer of the substantial amount of drug from plasma to the buffer side of the membrane and shifting of osmotic volume of the fluid to the plasma side.
Ultrafiltration methods are perhaps more convenient for the routine determination because they are relatively less time consuming. The ultrafiltration method is analogous to equilibrium dialysis method in that protein macromolecules such as serum albumin are separated from small drug molecules. Hydraulic pressure or centrifugation is employed in ultrafiltration to force the solvent and the small molecules of the unbound drug through the membrane while avoiding the passage of drug bound to the protein. This ultrafiltrate is then analyzed by spectrophotometry or other suitable techniques to determine the concentration of bound drug.
One more important method for quantification of protein binding is dynamic dialysis. This method has originated support in the recent years because it is relatively rapid, economical in terms of the amount of protein required and readily applied to the study of competitive inhibition of protein binding. Dynamic dialysis method is based on the rate of disappearance of drug from a dialysis cell, which is proportional to the concentration of unbound drug.
Applications of Complexation
Complexation means binding of the metal ion or organic molecules or any active molecules with ligands. By this way binding can affect the action of the agents or compounds either in terms of their solubility, stability or, maybe, their biological actions, etc. Some of the pharmaceutical and other applications of the complexation are mentioned below.
Complexation is used in the qualitative and quantitative estimation of the metals like silver, copper, mercury, aluminium, cobalt, etc. EDTA is used as a complexing agent in volumetric analysis of metals like calcium, magnesium, zinc, etc. Complex compounds of the transition metals exhibit variety of colours. This property is used in colorimetric estimation of drugs. Other analytical applications of coordination compounds include indication of oxidation–reduction, estimation of hardness of water, sequestration of agent and extraction of solvent.
Complexation has also played an important role in the field of metallurgical operations, e.g. silver and gold extracted by the use of complex formation with sodium cyanide solution. Nickel is extracted by converting it into a volatile complex nickel carbonyl by the use of carbon monoxide. The complex decomposed on heating again into nickel and carbon monoxide.
Complexation is also utilized in electroplating of metal. Cyano complexes of silver, gold, copper, etc., are used for electrodeposition of these metals.
Coordination compounds play many important roles in animal and plant life. They are essential in storage and transport of oxygen, as electron transfer agents, as catalysts and in photosynthesis. Haemoglobin is a protein present in the blood and is a complex of iron with porphyrin, which is known as haeme. It carries oxygen in the blood, from the lungs to the tissues where it delivers the oxygen molecules to myoglobin. Cytochrome is another example of the same. They act as electron carriers, which also play essential part in metabolic processes. In case of chlorophyll molecules, which also play a role in photosynthesis also contains the porphyrin ring but the metal ion there is magnesium ion rather than iron ion as in haemoglobin. Vitamin B12 is an example of complex of cobalt with a quandridentate ligand, and it is active when cobalt is present in +1 oxidation stage. Vitamin B12 is an essential growth factor for many microorganisms.
Organometallic compounds also have various applications, as follows: tetraethyl lead is used as antiknock compound in gasoline; silicons are used as polymers of unique properties; organoalkali and Grignard reagents are used in many organic synthetic reactions, etc. These compounds are also used as homogenous and heterogenous catalysts. Wilkinson’s catalyst [Rh(P.Ph
3)3Cl] is a homogenous catalyst and used in hydrogenation of alkenes, whereas Zeigler–Natta acts as a heterogenous complex and is used in polymerization of ethylene into polyethylene.
Cyclodextrin (CD) formulations are used to improve the oral bioavailability of the drugs when solubility and the rate of dissolution limit the availability of the drug for absorption. For example, the drug cefotiam hexetil hydrochloride forms a gel under the acidic conditions of gastric contents and shows poor dissolution, while α-CD complexation improves the dissolution and solubilization of the drug.
Complexation has been used to mask the unpleasant bitter taste of a number of drugs such as oxyphenonium bromide, propantheline bromide clofibrate and acetaminophen.
CD is also used as a stabilizing agent in pharmaceutical formulations. They protect drugs from hydrolysis, hydrolytic dehalogenation, oxidation, decarbozylation and isomerization, both in solution and the solid state. Inclusion complexes have also been prepared with a number of volatile substances including spices flavours, essential oils and several drugs. CD complexation has been shown to reduce volatility and improve the stability of many compounds. Examples include lemon oil and other flavouring agents, clofibrate isosorbide 5-mononitrate and nitroglycerine. In medical field, the complex of the calcium with EDTA is used for the treatment of lead poisoning. Lead readily replaces calcium in the complex, and lead–EDTA is finally eliminated from the body in urine. It is also used for the elimination of Cd, Co, Hg, U, It and Ce. Dimercaprol (BAL) is used for the treatment of battle poisoning along with the luisit (ClHC=CHAsCl2). The action of luisit is based on the binding with –SH groups. Salicylic acid is used in beryllium poisoning.
Ferric complex of oxine is used as antibacterial and antifungal agents. Oxine facilitates the penetration of iron into the membrane of bacterial cells. p-Aminosalicylic acid–copper complex showed much greater antitubercular activity than the cupric complex.
Penicillamine is used in the treatment of chronic copper accumulation (Wilson’s disease) and lead poisoning. It is also used in treatment of rheumatoid arthritis.
Platinum complexes, such as the square planer cis-dichlorodiammineplatinum (II), form a class of antitumor agents and have a large application in the treatment of cancer of the ovaries and testicles.
Diuretic drug preparations have promoted urine formation. They are derivatives of mercury propanol RCH2CH(OH) CH2HgX, where R is a polar hydrophilic group. The mercury diuretic preparations act as fermenter inhibiter. These properties lead to the use of bacterial infections. In these cases, they interact with –SH groups of the bacterial proteins. Many drug preparations can interact with metal ions. In some cases, the therapeutic activity of a drug compound can be inhibited through a chelated metal ion. For example, tetraethylethiouram disulphide is used in the treatment of chronic alcoholism, which inhibits aldehyde oxidase and interrupts the ethanol metabolism at the stage of formation of the acetaldehyse. The latter leads to an unpleasant feeling. It makes the alcohol nontransportable in its later use. Lithium itself is used in the treatment of bipolar disorder, i.e. manic depression. Thiomalate has also been used very successfully to treat severe cases of rheumatoid arthritis. Drugs obtained by using biotechnology have an important role in the pharmaceutical industry. These new products include peptides, proteins and antibodies and are used as therapeutics or diagnostics. Drugs such as interferons, interleukins, growth factors and plasminogen activators are also being used as diagnostics. They are very sensitive to many diseases, such as hepatitis, herpes and AIDS.
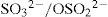 (O and S), where the first named atom refers to that which is bonded to the metal centre.
(O and S), where the first named atom refers to that which is bonded to the metal centre.


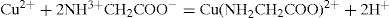
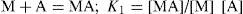
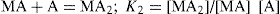
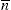 and is written as
and is written as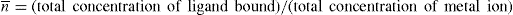
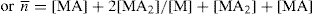
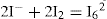 may occur at higher concentrations. Distribution coefficient of iodine when distributed between water (w) and carbon sulphide as the organic phase (o) is found to be 625. When it is distributed between a 0.1250 M solution of potassium iodide and carbon disulphide, the concentration of iodine is found to be 0.1896 mole/litre in organic phase and 0.02832 moles/litre in aqueous phase. In short, total concentration of KI (free and complex) = 0.1250 mole/litre.
may occur at higher concentrations. Distribution coefficient of iodine when distributed between water (w) and carbon sulphide as the organic phase (o) is found to be 625. When it is distributed between a 0.1250 M solution of potassium iodide and carbon disulphide, the concentration of iodine is found to be 0.1896 mole/litre in organic phase and 0.02832 moles/litre in aqueous phase. In short, total concentration of KI (free and complex) = 0.1250 mole/litre.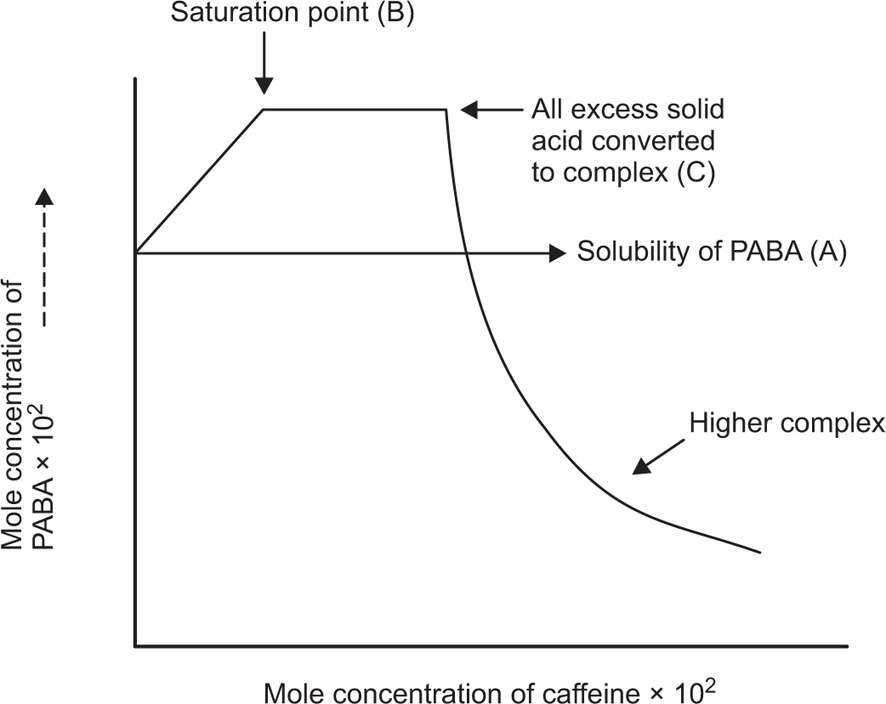
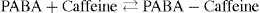
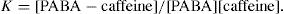
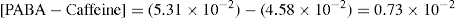
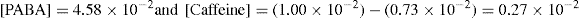
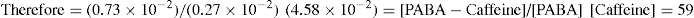
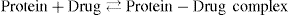
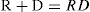 (1)
(1)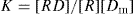 (2)
(2)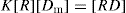 (3)
(3)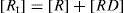 (4)
(4)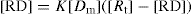 (5)
(5)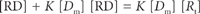 (6)
(6)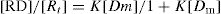 (7)
(7)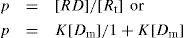 (8)
(8)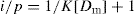 (9)
(9)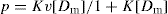 (10)
(10)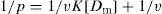 (11)
(11)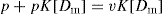 (12)
(12)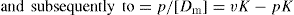 (13)
(13)