Pharmaceutical materials falling under the category of colloids include a wide range of substances such as suspensions of solids in liquids, emulsified liquids, clays, gels and solutions of soaps and proteins. The feature that is common to such a variety of systems is the fine state of subdivision of the dispersed phase. Early investigations were concerned with preparations such as colloidal suspen sions of sulphur and gold, and their characteristic properties were correctly thought to be due to the subdivision of the particles that were not in true solution. It is now recognized, however, that colloidality is a state of subdivision attainable with almost any material under suitable conditions, e.g. soaps form a colloidal solution in water due to aggregation of soap molecules, but form a non colloidal solution in alcohol. Some colloidal sys tems consist of true solutions of very large molecules, e.g. proteins, or colloidal aggregates of small molecules.
It is difficult to give a precise definition of the colloidal state but the following definition is frequently quoted: ‘A colloidal system is a two phase system, one phase dispersed as minute particles (disperse phase) throughout the other phase (continuous phase or dispersion medium). This, however, ignores such systems as soap films which are of colloidal dimensions as regards film thick ness. If the term ‘minute particles’ is taken to include particles which have at least one dimension lying in the range between 1
nm and 1
μm, the definition can be taken to include fibres and films as well as three-dimensional solid and liquid particles. Emulsions and suspensions used in pharmacy are somewhat coarser than 1
μm but are generally included because their behaviour is similar in many ways to that of true colloids (
Hiestand, 1964). When the dispersion medium is a liquid the colloidal systems are called
sols.
The term colloid (from Greek kolla, which means ‘glue’) has been applied first by Graham (1851–1861) to solutions of proteins such as albumin or gelatin and to naturally occurring gums. He found that crystalline substances could be separated from ‘colloids’ by placing the mixture inside a bag made of a thin membrane of animal gut and immersing it in water, since the colloids remained inside while simple salts diffused out through the fine pores in the membrane.
The extensive interface between two phases is a predominant feature of colloidal dispersions and it exerts a considerable influence on the physical and chemical properties of the material. In the interior of a solid, the ions, atoms or molecules are attracted equally to their neighbours by electrostatic, covalent bonds or van der Waals forces, respectively, but at the surface unsaturated forces are capable of interacting with forces emanating from molecules of other substances. Thus, finely divided solids can adsorb gas molecules on their surfaces and if the ratio of surface area to weight of solid is large, they will be effective adsorbents. It follows also that colloidal particles close to each other will be mutually attracted but there must be some mech anism responsible for stabilizing the particles, otherwise the colloidal suspension could only exist for a relatively short time before the particles agglomerated.
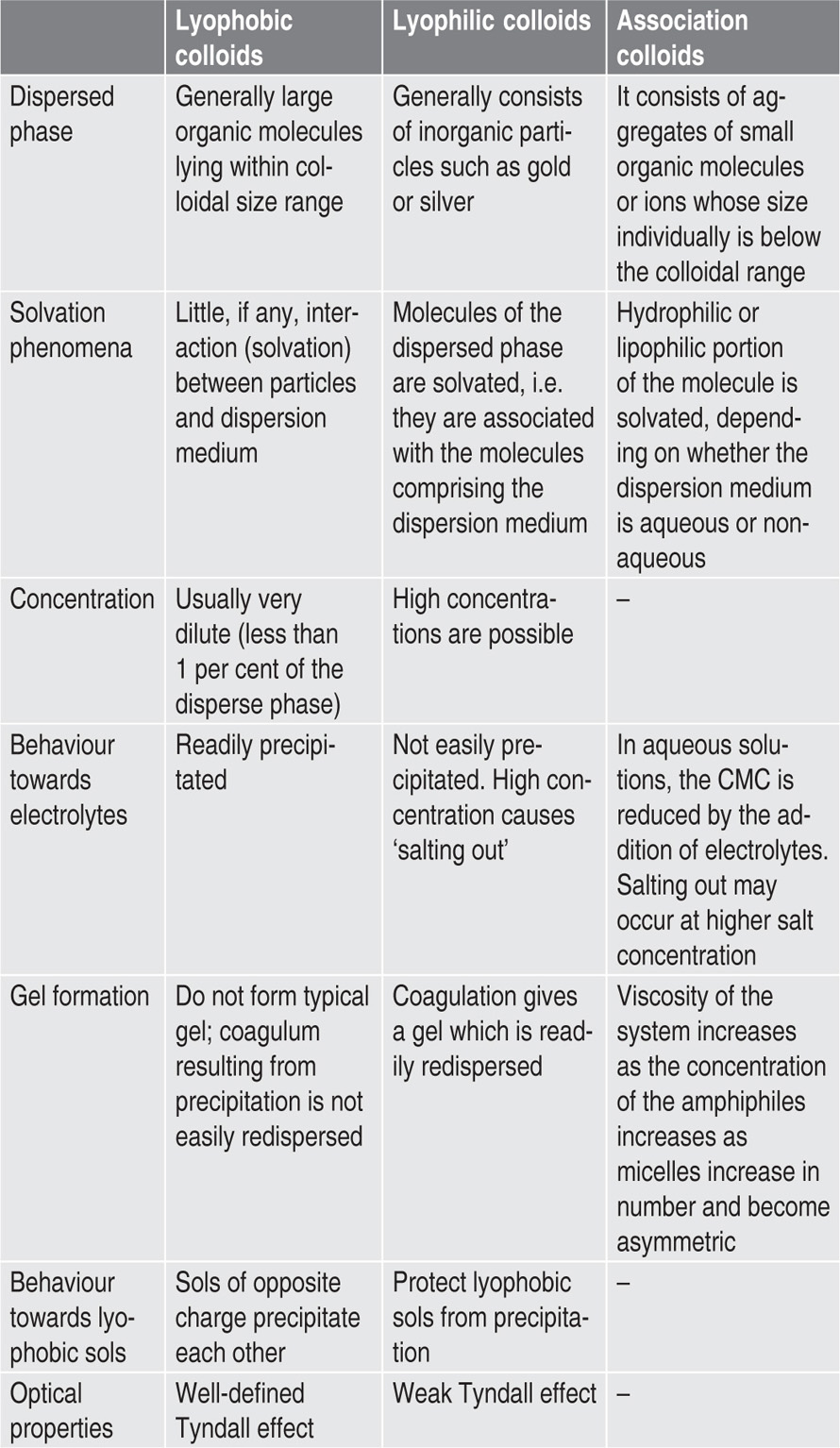
Before discussing the mechanism of stabilization of colloidal sols, it is convenient to classify colloidal systems into lyophobic, lyophilic colloids and associated colloids. By definition lyophobic colloids are mere suspensions and not solutions. There is little affinity between the solvent and particle, and the system is thermo dynamically unstable.
Lyophilic Colloids
Systems containing colloidal particles that interact to an appreciable extent with the dispersion medium (solvent loving) are referred to as lyophilic colloids. Lyophilic colloids or sol can be obtained by dissolving the material in the solvent being used. The properties of the lyophilic colloids are due to the attraction between the dispersion phase and the dispersion medium that leads to salvation. Salvation is the attachment of solvent molecules to the molecules of the dispersed phase. When water is used as a solvent or dispersion medium, in that case colloids are known as hydrophilic colloids. Gelatin, acacia, insulin, and albumin give hydrophilic colloid and rubber and polystyrene form lyophilic colloid, with nonaqueous solvent. Lyophilic colloids comprise true solutions either of very large molecules or of aggregates (micelles) of smaller molecules that achieve dimensions in the colloidal range. There is strong attraction between the solvent and particle and the solutions are thermodynamically stable. Lyophilic sols differ from solutions of smaller molecules only in the size of the molecules, and many molecules of biological interest fall in this category, e.g. proteins, nucleic acids, starches as well as synthetic polymers.
Lyophobic Colloids
Lyophobic colloids can be classified according to the nature of the disperse phase and dispersion medium (
Table 6.1).
Table 6.1 Nature of disperse phase and dispersion medium in lyophobic colloids |
| Dispersed phase |
Dispersion Medium |
Class |
| Solid |
Liquid |
Sol |
| Solid |
Gas |
Aerosol (smoke) |
| Liquid |
Liquid |
Emulsion |
| Liquid |
Gas |
Aerosol (fog) |
| Gas |
Liquid |
Foam |
| Gas |
Solid |
Solid foam (e.g. pumice) |
Particles in colloidal solution are subject to the same kind of random thermal motion as are individual molecules, except that their mean dis placement per unit time is smaller because of their larger size. The random movement, called Brownian movement, is observed for particles up to about 2μm, at which level the particles can be observed under an optical microscope. To observe particles small in comparison to the wavelength of light, e.g. <0.1μm, the ultramicroscope has been used. The suspension is illuminated by a narrow intense beam of light at right angles to the angle of viewing, when the particles appear as bright dots against a dark background in the same way that dust particles can be seen when a beam of sunlight enters a darkened room.
The beam of light appears as a whitish path as it passes through the suspension. This phenomenon was studied by Tyndall, and bears his name, the Tyndall effect.
Association Colloids (Amphiphilic Colloids)
This is a class three of the colloids which deals with interfacial phenomena, certain molecules or ions, called amphiphiles or surface active agents. Surface active agents are characterized by having two distinct regions of opposing solution affinities within the same molecule or ion. When surface active agents are present at lower concentration in a liquid medium, the amphiphiles exist separately and are of such a size as to be subcolloidal. As the concentration is increased, aggregation occurs over a narrow concentration range. These aggregations may contain 50 or more monomers and are called micelles. Concentration at which micelles are formed is termed critical micelle concentration (CMC). Formation of CMC can be explain as, at below the CMC, the concentration of amphiphiles undergoing adsorption at the air–water interface increases as the total concentration of amphiphiles is raised. A point where both interface and the bulk phase become saturated with monomers is termed as CMC.
Association colloids can be further classified as follows:
1. Anionic, e.g. sodium lauryl sulphate
2. Cationic, e.g. cetyl trimethylammonium bromide
3. Nonionic, e.g. polyoxyethylene lauryl ether
4. Ampholytic, e.g. dimethyl dodecylammonio propanesulphonate
The main differences between lyophobic, lyophilic and association colloids are summarized in
Table 6.2.
Table 6.2 Comparison between lyophobic, lyophilic and association colloids
Preparation of Lyophobic Sols
Dispersion methods: Grinding of coarse suspen sions in mills known as colloid mills can reduce particles in a liquid phase to 1μm and less. Some colloid mills consist of a high speed rotating smooth disc, separated from a stationary disc by about 25μm between the two surfaces. Great stresses are developed in the fluid film between the surfaces and these disrupt solid particles present in the gap. Rotor dimensions are such that speeds of between 1500 and 6500m/min are obtained at the working surfaces. Owing to the unstable nature of the sol, small amounts of lyophilic colloid or other stabiliz ing agents must be added to prevent agglomeration of the colloidal particles.
Condensation methods: Sols can be prepared from an initially molecular dispersion by controlled growth to colloidal size. The
experimental condi tions must be carefully controlled to prevent exces sive growth and hence precipitation of the particles. During the preparation of sols, von Weimarn found experimentally that small particles are formed by precipitation from either very concentrated or very dilute solutions, while coarse particles formed at intermediate concentra tions. In concentrated solution many nuclei are formed simultaneously and growth of these by deposition of solute present above the saturation point is rapid due to close proximity of solute molecules to the nuclei. The result is small colloidal particles of nearly uniform size. In less concentrated solution, the rate of formation of nuclei will be less but solute will still diffuse quite rapidly to the smaller number of nuclei and will produce a coarser sol. In very dilute solutions, the growth process will be slower, although new nuclei will still be forming from the supersaturated solution, and the resulting particles, again colloidal, will be less uniform in size.
Besides these precipitation methods for produc ing sols, other chemical processes can be used. For example, colloidal ferric hydroxide can be formed by boiling a solution of ferric chloride and allow ing the escape of the HCl produced by the hydrolysis
Colloidal sulphur sols can be produced by the decomposition of dilute solution of sodium thio sulphate in the presence of acids.
In the preparation of colloidal silicic acid, a solution of sodium silicate is added to excess hydrochloric acid and the sodium chloride removed by dialysis:
Colloidal sols of the noble metals can be obtained by striking an arc between electrodes of the noble metals immersed in the dispersion phase. The metal vapour, produced by passing a large current, condenses in the liquid phase, to which may be added traces of electrolyte in order to stabilize the colloidal particle.
Colloidal solution of gold, e.g. Gold (198Au) Injection BP is prepared by reducing a salt of gold-198 with a reducing agent such as dextrose in alkaline solution in the presence of gelatin.
Where a chemical or electrical method may not be applicable, sols may also be prepared by adding a solution of the substance to a second liquid which is a poor solvent for the original solute, e.g. sulphur in ethyl alcohol added to water.
In all these cases, the colloidal particles formed may tend to clump together or flocculate unless additional substances are added to stabilize the sols. The addition may be trace electrolytes or protective colloids. This is discussed later.
Purification by Dialysis
Low molecular weight impurities, e.g. electrolytes, can be separated from colloidal particles by dialysis. The process is most simply carried out by enclosing the solution in a cellophane sac which is immersed in a large vessel of water. Cellophane tubing, tied off at each end, is usually used. The pores in cellophane membranes are sufficiently large for low molecular weight solutes to pass through whilst retaining the larger colloidal particles. Other mem branes that are suitable for aqueous sols include parchment, collodion and cellulose acetate. If electrolyte is present in the colloidal sol it diffuses through the pores of the membrane and, by repeatedly changing the water, diffusion will continue until the dialysate remaining is substantially free from electrolyte.
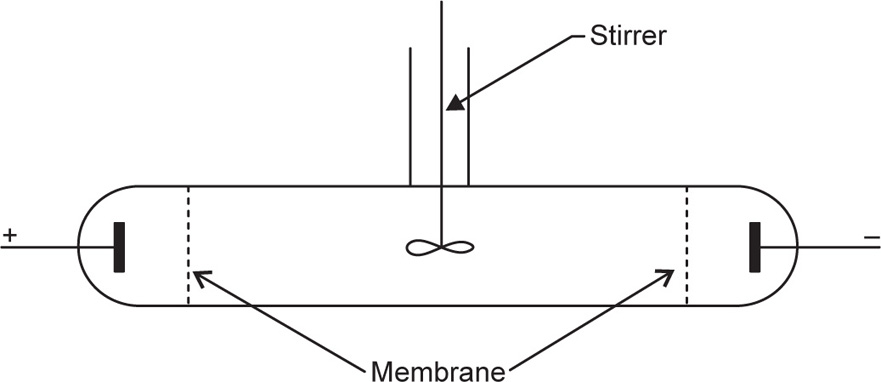
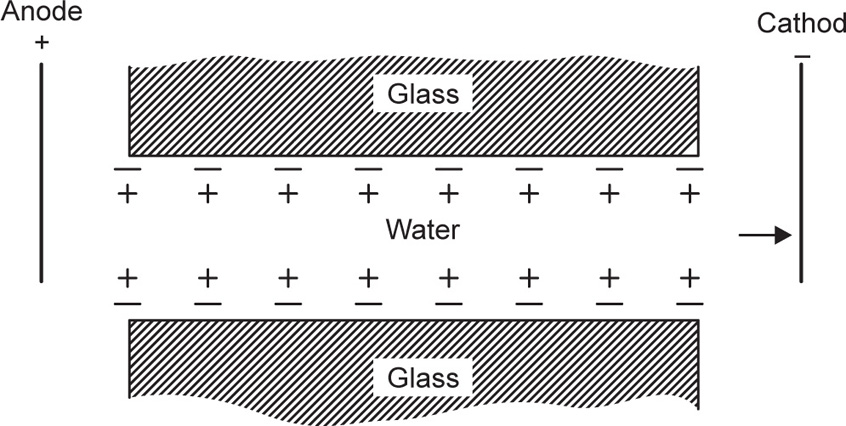
This process is relatively slow and the dialysis of electrolytes can be greatly facilitated by the applica tion of a direct current. For this purpose an apparatus illustrated in
Fig. 6.1 is used. This technique, known as
electrodialysis, is thus a combination of dialysis and electrolysis. The colloid to be purified is introduced into the middle section which is separated from the right and left sections by dialysis membranes. Provision is usually made for circulation of water on both sides of the colloid, and the removal of the electrolyte is achieved by direct current applied by means of electrodes.
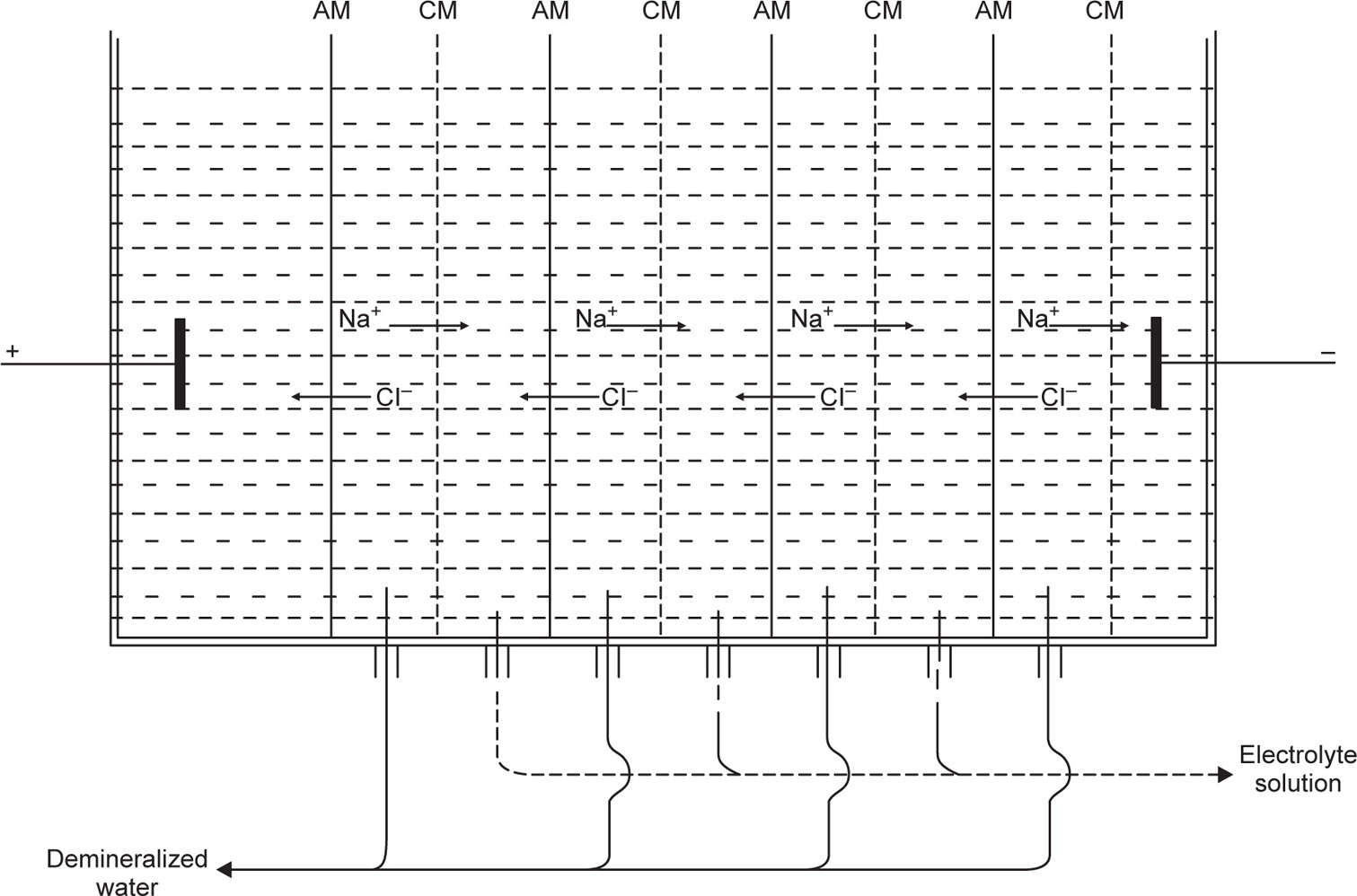
Recently electrodialysis has come into promi nence in connection with the desalination of water. The success of this operation is due to the develop ment of membranes which will pass only negatively charged or only positively charged ions. This enables multicompartment dialysis cells to be used where the cationic membranes are only permeable to cations and the anionic membrane only per meable to anions. The migration of anions and cations to the relative cell compartment enables deionized water to be collected in the alternate compartments as shown in
Fig. 6.2.
A modification of the electrodialysis technique is electrodecantation developed by Pauli. The colloid is not stirred and because of its charge will be pulled towards one of the membranes and hence the density near the membrane is increased and the dense solution sinks to the bottom. This provides a method of preparing concentrated colloids as the dense lower layer can be removed and more fresh solution supplied to the cell.
Electrophoresis convection is an extension of this method and is used to fractionate mixtures contain ing colloids of different mobilities. The component with the highest mobility will reach the membrane first, sink and become concentrated in the bottom of the cell while the less mobile will be enriched in the top of the cell. Suitably designed cells have been used to separate proteins into pure fractions, e.g.
γ-globulin.
Dialytic separations may also be effected by ultrafiltration (
Ferry, 1936). This process differs from dialysis only in that the passage of the electrolyte solution through the membrane is accelerated by applying pressure. The membrane needs to be supported on a sintered glass plate to prevent rupture as considerably high positive pressures are required to achieve reasonable rates of filtration. The pore size of the membranes can be increased to some extent by soaking them in solvents that cause swelling, e.g. cellophane swells in zinc chloride solutions, collodion membranes swell in alcohol. Although there is adequate information in the literature on methods of preparing collodion (nitrocellulose) membranes by controlled evapora tion of alcohol–ether solutions, it is difficult to obtain reproducible pore sizes. In practice it is more usual to use commercially available material such as cellophane which is available in tube or sheet form.
An example of the use of dialysis in pharmacy is the purification of enzymes such as pepsin. The form of pepsin known as precipitated pepsin is prepared by scraping off the mucous membrane of the lining of the stomach of the pig and steeping in diluted hydrochloric acid for some time. The extracted pepsin is then precipitated by adding a saturated solution of sodium chloride, separated by filtration, redissolved in water and dialysed. The sodium chloride and hydrochloric acid pass through the membrane; the pepsin remains in colloidal solution as the dialysate, from which it may be later pre cipitated by the addition of alcohol.
Fractionation of enzymes by fractional precipita tion from solution may be effected by ‘salting out’ with ammonium sulphate; dialysis or electrodialysis is then used to remove the contaminating electrolyte. Prolonged electrodialysis can sometimes cause inactivation owing to removal of a coenzyme. For instance, malt amylase becomes inactivated in this way and subsequent addition of salts to the deionized material is necessary to restore its activity.
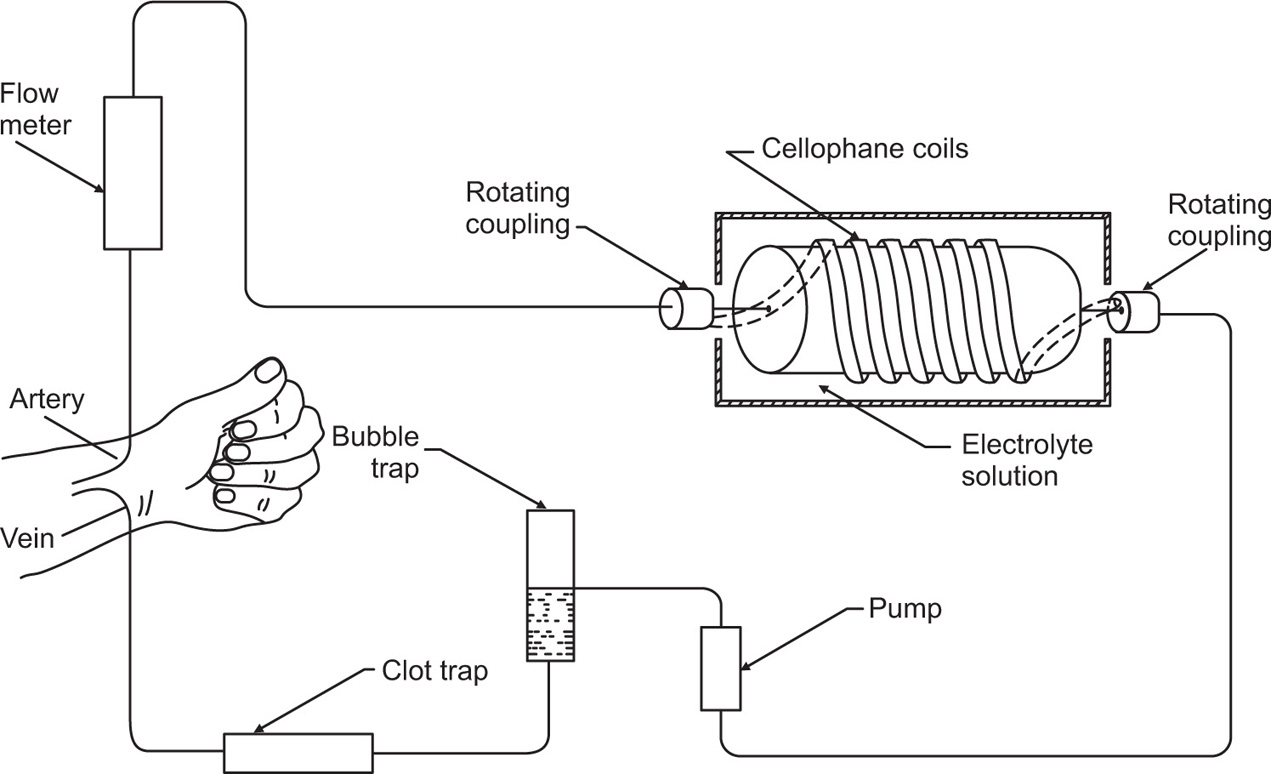
The success of the artificial kidney machine depends on the reduction of blood urea by passage of the patient’s blood through coils of cellophane tubing immersed in suitable rinsing fluid. The molecular composition is designed to create a diffusion gradient from blood to rinsing fluid of substances which are present in excess in the blood (e.g. urea) and from the rinsing fluid to blood of substances which are deficient in the body (e.g. bicarbonate), while the concentration of those diffusible substances which are present in normal amounts in the blood is kept unaltered by having them present in the same concentration as in the rinsing fluid. The length of the tubing is about 60m giving a dialysing surface area of just over 3m2. Other kidney dialysis units utilize sheets of dialysing membranes in place of coils.
Cellophane is an ideal semipermeable membrane for haemodialysis as its pore size is such that electrolytes, urea and glucose can all pass freely across it, while the larger molecules such as the plasma proteins, lipid fractions and blood cells, cannot pass. The cellophane tubing does not allow bacteria to pass across it, so that only the inside surface need be sterilized. The method of operation is discussed by
Anderson (1964) and shown schematically in
Fig. 6.3.
Properties of Colloidal Systems
Properties of the colloids can be classified into three categories such as optical properties, kinetic properties and electrochemical properties which are summarized in detail as follows.
Optical Properties
Ultramicroscope
The electron microscope is capable of giving actual pictures of the particles, and it is capable of yielding the size, shape and structure of colloidal particles. The success of the electron microscope is due to its high resolving power. Light passed in a convergent beam transversely through a sol is scattered and polarized by the dispersed particles and the path of the beam when examined laterally is seen as an illuminated cone. A true solution, examined similarly, appears opti cally empty, i.e. the Tyndall effect is absent. The ultramicroscope is simply a device for observing the Tyndall effect microscopically. The microscope is perpendicular to the beam of light passing through the sol in a shallow glass cell. The colloidal particles appear as bright spots against a dark background, similar to dust particles seen in a beam of sunlight in a darkened room. The actual shape cannot be seen but from the concentration of the sol and the dimensions of the cell, the number of particles per unit weight of dispersed phase can be calculated and hence the average particle size. The normal optical microscope method will not resolve particles less than wavelength of visible light (0.4–0.8μm).
Particle size of colloids can be roughly determined by ultrafiltration through membranes of known pore size.
Elford (1937) prepared nitrocellulose membranes with graded pore size by controlling the solvent composition in which the nitrocellulose was dissolved; good solvents favour dispersion and give dense membranes of small pore size while poor solvents produce more open membranes. Elford found that the size of large particles passing through the membranes corresponded fairly well with the pore size, but smaller particles (e.g. virus particles) were held back by membranes whose pore size was larger than the particle, probably due to adsorption effects. One big disadvantage of the electron microscope for viewing colloidal particles is that normally only dried samples can be examined. Consequently it usually gives no information on salvation or configuration in solution, and moreover, the particles may be affected by sample preparation. A recent development that overcomes these problems is environmental scanning electron microscopy (ESEM), which allows the observation of material in the wet state.
Light Scattering
When a light beam is passed through a colloidal solution, the intensity of the scattered beam depends upon the following factors: (1) intensity of the incident beam, (2) wavelength of the incident beam, (3) difference between the refractive index of the particle and the medium, (4) volume of the particles and (5) the number of particles which scatter the light. In some cases the intensity of light scattered at different angles may differ and can be used to give some indication of the shape of macro molecules. The work of Debye, Doty, Zimm and others (1944–1949) led to prediction of equations which correlate the molecular weight M with the intensity of scattered light. This relation, in the case of uncharged species, is expressed by the equation:
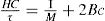
where τ is the turbidity measured at 90° to the incident beam, C the concentration of solute (grams per litre), M is the molecular weight and B is the interaction constant which, as in osmotic pressure measurements, depends on the degree of nonideality of the solution. The proportionality constant H is a complicated function of the wavelength of incident light, refractive index and rate of change of refractive index with concentration.
A plot of HC/τ versus C should give a straight line at low concentrations and extrapolation to infinite dilution will give the value of I/M and hence the molecular weight.
Kinetic Properties
Ultracentrifuge
Colloidal particles cannot be sedimented in the usual laboratory centrifuges because, being small and light, they are kept in random motion. If the particles (up to about 2μm diameter) are observed under a microscope or the light scattered by colloidal particles is viewed using an ultramicroscope, an erratic motion is seen. This movement is referred to as Brownian motion, after Robert Brown who first reported his observation of this phenomenon with pollen grains suspended in water in 1827.
Centrifugal force of upwards of 4,00,000g in the ultracentrifuge originated by Svedberg, is required to produce sedimentation at a reasonable rate. In this instrument the sol is placed in a glass cell in the centrifuge rotor and arranged so that light passing through the cell may be photographed. The centrifuge is rotated at 50,000 rev/min and higher and the rate of sedi mentation is derived from the change in light adsorption, or refractive index of the sol during centrifugation. It is possible not only to estimate molecular weight but also to determine the relative homogeneity of the system with respect to molecu lar weight. The molecular weight of proteins has been calculated from sedimentation velocity, e.g. pepsin 35,500, insulin 41,000, gelatin, 10,000–70,000.
Osmotic Pressure
The method is based on van’t Hoff’s law, according to which the osmotic pressure p depends on molar concentration of the solute and on temperature. This is shown in the following equation, where C represents concentration (in grams per litre), M molecular weight and T absolute temperature:
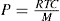
The equation is valid for very dilute solutions in which the molecules do not interact mutually. However, if association occurs, the plot of reduced osmotic pressure, p/C against C, will not be linear and it is necessary to extrapolate the curve to infinite dilution to obtain RT/M.
Osmotic pressure is inversely proportional to molecular weight, so that the pressures observed for, say, proteins of molecular weight 50,000 will be very low and special osmometers are employed. The dialysis membranes used for osmometry are permeable to small ions and the distribution of ions across membranes led to discrepancies in the measurement of the molecular weights of polyelectrolytes such as proteins. The protein molecule is a colloidal ion with several charges balanced by normally sized ions.
Donnan in 1911 found that the invariably low results for molecular weight obtained from osmotic pressure measurement were due to the unequal distribution of ions across the membrane. He found that by dissolving the protein in electrolyte solution (0.1
M or higher) and using the same salt solution outside the membrane, he was able to swamp out the Donnan effect.
Diffusion
Because of the Brownian motion, colloidal particles spontaneously diffuse from a region of higher concentration to one of lower concentration. The rate of diffusion is expressed by Fick’s first law:
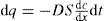
where D is the diffusion coefficient and has the dimensions of area per unit time. dq is the mass of substance diffusing in time dt across an area S under the influence of a concentration gradient dc/dx (the minus sign denotes that diffusion takes place in the direction of decreasing concentration).
It is assumed that if colloidal particles are to be approximately spherical, the following equation suggested by Sutherland and Einstein can be used to calculate the radius of the particle and the molecular weight.
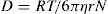
where D is diffusion coefficient, R is molar gas constant, T is absolute temperature, η is viscosity of the solvent, r is the radius of the spherical particle and N is Avogadro’s number. Molecular weight of an approximately spherical particle can be obtain by diffusion coefficient (such as egg albumin and haemoglobin) using the following equation:
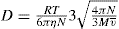
where
M is the molecular weight and
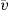
the partial specific volume of the colloidal material.
Sedimentation
Consider a spherical particle having radius a and density σ falling in a liquid of density ρ and viscosity η. Then velocity v of sedimentation is given by Stokes’ law:
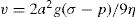 (6.1)
(6.1)
where
g is acceleration due to gravity. If the particles are subjected only to the force of gravity, then as a result of Brownian motion, the lower size limit of particles obeying
Eq. 6.1 is about 0.5
μm.
A stronger force than gravity is therefore needed for colloidal particles to sediment, and use is made of a high-speed centrifuge, usually termed an ultracentrifuge, which can produce a force of about 10
6g. In a centrifuge,
g is replaced by ω
2x, where ω is the angular velocity and
x the distance of the particle from the centre of rotation, and
Eq. 6.1 becomes:
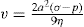 (6.2)
(6.2)
The ultracentrifuge is used in two distinct ways in investigating colloidal material, i.e. sedimentation velocity and sedimentation equilibrium. In the sedimentation velocity method a high centrifugal field is applied up to about 4 × 105g and the movement of the particles, monitored by changes in concentration, is measured at specified time intervals.
In the sedimentation equilibrium method the colloidal material is subjected to a much lower centrifugal field until sedimentation and diffusion tendencies balance one another, and an equilibrium distribution of particles throughout the sample is attained.
Sedimentation velocity: The velocity dx/dt of a particle in a unit centrifugal force can be expressed in terms of the Svedberg coefficient s:
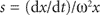 (6.3)
(6.3)
Under the influence of the centrifugal force, particles pass from position
xl at time
tl to position
x2 at time
t2: the differences in concentration with time can be measured using changes in refractive index and the application of the Schlieren optical arrangement, whereby photographs can be taken showing these concentrations as peaks. Integration of
Eq. 6.3 gives
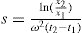 (6.4)
(6.4)
Using the above limits gives: By suitable manipulation of
Eqs. 6.2,
6.3 and
Eqs. 6.4 an expression giving molecular weight
M can be obtained:
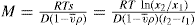
where
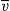
is the specific volume of the particle.
Sedimentation equilibrium: Equilibrium is established when sedimentation and diffusion forces balance. Sedimentation and diffusion equations are combined in the analysis, giving:
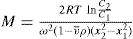
where C1 and C2 are the sedimentation equilibrium concentrations at distances x1 and x2 from the axis of rotation.
A disadvantage of the sedimentation equilibrium method is the length of time, often as long as several days required to attain equilibrium.
Viscosity
Viscosity is an expression of the resistance to flow of a fluid under an applied stress. An equation of flow applicable to colloidal dispersions of spherical particles was developed by Einstein:
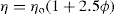 (6.5)
(6.5)
where ηo is the viscosity of the dispersion medium and η the viscosity of the dispersion when the volume fraction of colloidal particles present is ϕ.
A number of viscosity coefficients may be defined with respect to
Eq. 6.5These include relative viscosity
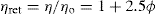 (6.6)
(6.6)
and specific viscosity as
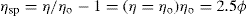 (6.7)
(6.7)
Or
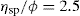 (6.8)
(6.8)
Because volume fraction is directly related to concentration,
Eq. 6.8 may be written as:
where C is the concentration expressed as grams of colloidal particles per 100ml of total dispersion. If η is determined for a number of concentrations of macromolecular material in solution and ηsp/C is plotted versus C, then the intercept obtained on extrapolation of the linear plot to infinite dilution is known as the intrinsic viscosity [η]. This constant may be used to calculate the molecular weight of the macromolecular material by making use of the Mark–Houwink equation:
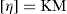
where K and α are constants characteristic of the particular polymer–solvent system. These constants are obtained initially by determining [η] for a polymer fraction whose molecular weight has been determined by another method, such as sedimentation, osmotic pressure or light scattering. The molecular weight of the unknown polymer fraction may then be calculated. This method is suitable for use with polymers such as the dextrans used as blood plasma substitutes.
Electrical Properties of Colloids
Most surfaces acquire a surface electric charge when brought into contact with an aqueous medium, the principal charging mechanisms being as follows. In ion dissolution ionic substances can acquire a surface charge by virtue of unequal dissolution of the oppositely charged ions of which they are composed. For example, the particles of silver iodide in a solution with excess [I
−] will carry a negative charge, but the charge will be positive if excess [Ag
+] is present. Because the concentrations of Ag
+ and I
− determine the electric potential at the particle surface, they are termed potential determining ions. In a similar way H
+ and OH
- are potential determining ions for metal oxides and hydroxides such as magnesium and aluminium hydroxides. The properties of the colloids that depend on, or are affected by, the
presence of the charge on the surface of a particle are discussed under the heading electric properties of the colloids
Ionization
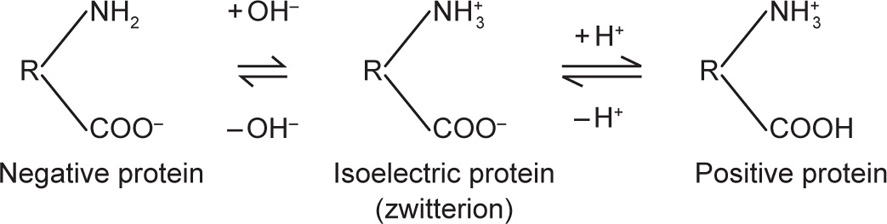
Soaps in aqueous solution form micelles of colloidal size, in which the molecules are orientated with their hydrocarbon chains forming the interior of the micelle and their polar groups forming the outer layer. Consider fatty acid soap, CnH2n + 1 COONa which ionizes to CnH2n + 1 COO−Na+ in water. The colloidal particle is thus a colloidal anion and the Na+ ions set free into the aqueous solution form the outer layer of the double layer. Proteins are another group of substances in which the electrical charge arises by ionization. Proteins contain both basic and acidic groups in the molecule and are termed ampholytes or amphoteric electrolytes. In alkaline solution the acidic group COOH will ionize to COO− so that the particle carries a negative charge. In acid solution the basic group NH2 will ionize to NH3+ so that the particle now carries a positive charge. At a certain definite pH, the total number of positive charges will equal the total number of negative charges and the net charge will, therefore, be zero. This pH is known as the isoelectric point. The protein is probably ionized at the isoelectric point, existing in the zwitterion form but the net charge is zero provided no other ions are adsorbed. The electrical state of the protein mol ecule can thus be depicted as:
(R denotes the residue of the protein molecule)
(A protein is least soluble at its isoelectric point and is readily precipitated. The protein, insulin, is pre cipitated from aqueous alcohol by adjusting the solution to the isoelectric point of insulin pH 5–6.)
Adsorption
The preferential adsorption of an ion by a particle is responsible for the charge on nonionizing particles, e.g. addition of Fe3+ ions to freshly precipi tated ferric hydroxide will produce a sol of hydroxide; the Fe3+ ions are adsorbed by the particles of ferric hydroxide and the Cl− ions form the outer shell of the electrical double layer. The spontaneous dispersion of a precipitate on the addition of a small amount of a third substance is known as peptization, a term introduced by Graham. The substance, generally an electrolyte, causing this dispersion is called a ‘peptizing agent’.
The presence of the electrical double layer gives rise to the electrokinetic effects electrophoresis, electro-osmosis and streaming potential.
Electrophoresis
Electrophoresis is the movement of colloidal particles with respect to the liquid when a potential difference is maintained across the sol. The zeta potential can be calculated from the mobility of the particle.
Spherical particles—small in comparison with the thick ness of the double layer. These include protein molecules, fine silver iodide sols, etc. The derivation made by Debye and Hückel was that if the exter nally applied potential gradient is E and the charge on the particle, Q (coulombs), the particle will experience a force QE tending to accelerate it in the direction of the field. The hydrodynamic resistance to its flow is given by Stokes’ Law as 6πηrv where η is viscosity, v the particle velocity and r the particle radius. The particle moves with a constant velocity as the two forces rapidly attain balance. The velocity of the particle in an electric field of 1 eV/cm is given by:
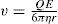
For spherical particles the relation between Q and the zeta potential ξ in a liquid of dielectric constant D is:
If ξ is expressed in millivolts, v in µm/sec per V/cm, the equation becomes ξ = 21.2v for water at 20ºC.
Particles—large compared with the thickness of the double layer. Particles visible under an optical microscope belong to this category. The particle and its double layer are more appropriately treated as a parallel plate condenser. The equation relating zeta potential to its measured mobility becomes:
This is generally referred to as Smoluchowski’s equation. For water at 25°C this expression is reduced to:
where ξ is expressed in millivolts and v in µm/sec per V/cm.
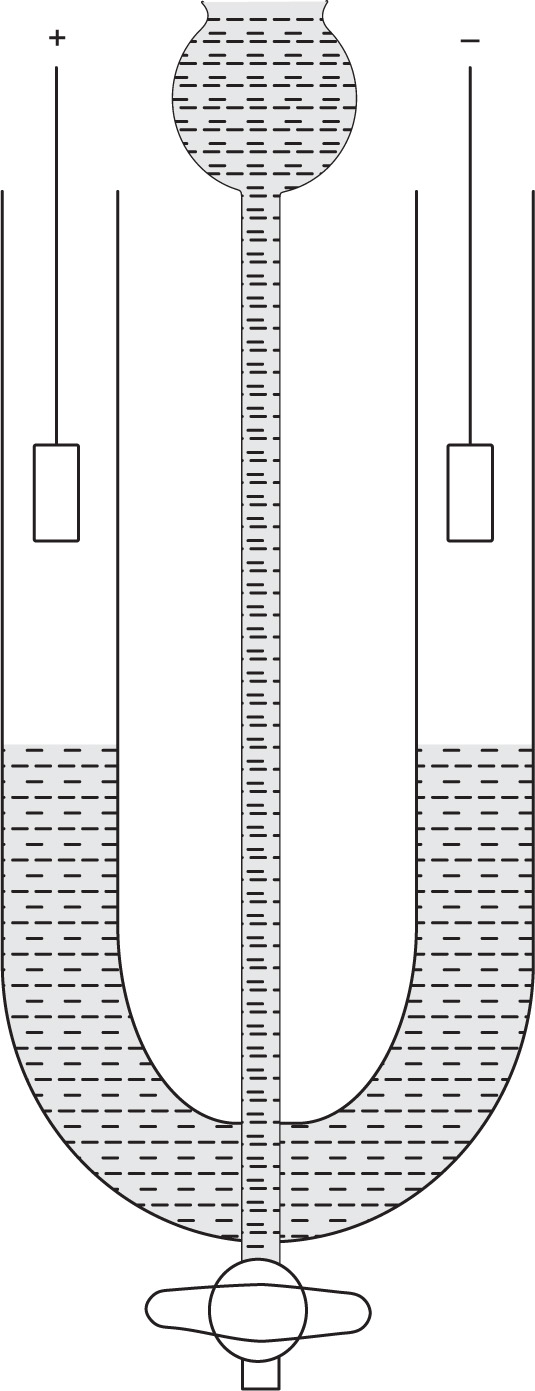
Experimental methods: The electrophoretic mo bility of colloidal particles can be measured from the rate of movement of the boundary of a sol using a simple U-tube apparatus shown in
Fig. 6.4.
The apparatus is designed so that a sharp boundary is formed between the sol and an electrolyte solution of the same concentration as that in the sol. If the sol is coloured or opaque, e.g. gold sols, emulsions, the position of the boundary can be followed visually. If the sol is colourless, then the boundary has to be observed by measuring the change in refractive index along the column using special optical equipment (Schlieren method). This technique is used for the studying of the electrophoretic mobility of components in mixtures, e.g. proteins.
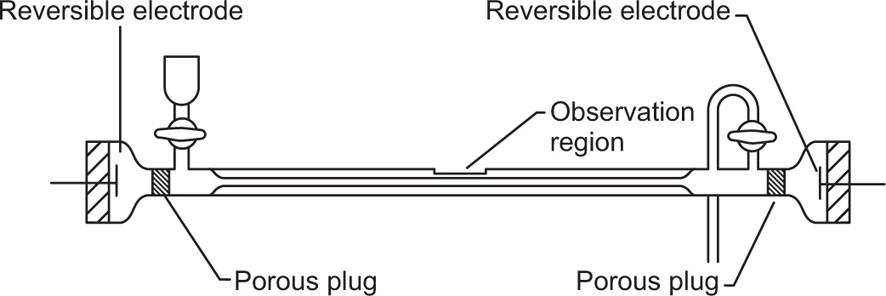
If the colloidal particles are visible under a microscope, an alternative method of electrophoresis is possible. This is microelectrophoresis, where direct observation is made of the movement of the particles. A cylindrical microelectrophoresis cell is shown in
Fig. 6.5. At each end of the tube is a porous plug leading to a reversible electrode (Ag–AgCl, Cu–CuSO
4, etc.) and a tap is provided for filling and cleaning. For low salt concentrations (below about
M/100) platinum electrodes may be used.
The cell is mounted horizontally on the microscope stage and on applying the electrical field (about 10
V/cm) the velocity of the particles is measured by timing their movement across the plane of a calibrated graticule placed in the microscope eye piece. In addition to the electrokinetic potential at the interface between the particles and the liquid, a similar potential usually exists between the walls of the tube and the liquid. Thus the particles are subject to both electrophoretic movement and electro-osmotic movement of the liquid near to the walls of the tube. In a closed cell, as shown in the figure, the liquid moving electro-osmotically at the wall must return along the centre of the tube. Thus at a certain level in the cell, the two opposing movements cancel each other out and the velocity of the particle in this stationary level will be the true electrophoretic mobility. Bacteria, emulsion droplets, suspensions, etc. have been studied by this method (
Alexander & Johnson, 1949).
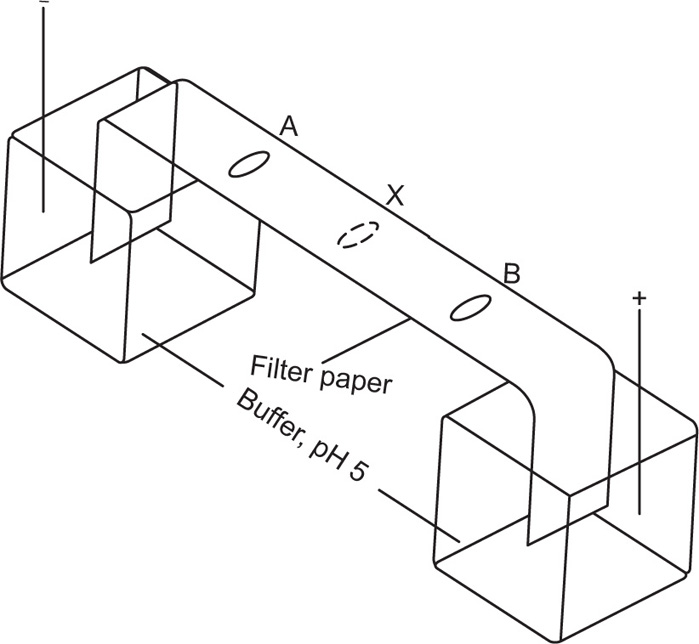
A simplified method for the electrophoresis of lyophilic colloids, e.g. proteins, nucleic acids, is paper electrophoresis. A strip of filter paper is moistened with a suitable buffer solution and supported horizontally with its ends dipping into separate reservoirs of buffer. A potential gradient is applied along the paper by electrodes inserted in the reservoirs. A drop of protein solution is spotted on to the paper and a direct current of about 10mA at 10V/cm of paper is passed for several hours. The filter paper acts merely as a support for the liquid phase through which the protein molecules migrate. At the conclusion of the experiment, the paper is removed and the position of the protein is detected by dipping the paper in a dye solution or spraying with a solution, staining the protein but not the paper. A mixture of proteins can be resolved into separate zones if the mobilities of the components are different.
The pH of the buffer solution is important; in alkaline and acid solutions the proteins will migrate in opposite directions, the ions being RCOO
− and RN
+ H
3, respectively. If the pH of the buffer solution corresponds to the isoelectric point of the protein, the latter molecule will be present as the zwitterion, N+H
3 ... COO
− and will not migrate. It is, therefore, possible to extend the scope of the method by choice of suitable buffers.
Fig. 6.6 shows the appearance of a filter paper strip after electrophoresis of two proteins A and B of isoelectric points pH 4 and 6, respectively when the paper is buffered at pH 5.
Electro-Osmosis
The atmosphere of counter ions around the particles confers a charge on the dispersion medium. The sign of this charge is opposite to that of the particles. Hence, as the particles move towards one pole, the liquid tends to move towards the opposite pole. This phenomenon can be observed if a tube is plugged in the middle with a porous material, e.g. glass powder, with electrodes immersed in dilute buffer solution on either side of the plug. A glass surface in contact with water bears a negative charge due to
the adsorption of OH
−. As the current passes, the liquid will move towards the positive electrode, and this can be best observed if the ends of the tubes are connected to capillary tubes, so that small displacements of the liquid are apparent.
Fig. 6.7 shows electro-osmotic flow of water in a glass capillary tube on application of a potential difference across the ends of the tube.
Streaming Potential
This is the converse of electro-osmosis. If the electrodes in the electro-osmosis apparatus (
Fig. 6.7) are replaced by a galvanometer in the circuit, no current would be detected when the liquid is stationary. However, if the liquid is forced through the tube the galvanometer will indicate a current. This streaming potential is due to the displacement of the charges equilibrated in the double layer around the solid. This technique can be used to measure the zeta potential of relatively coarse solids which would sediment rapidly in an electrophoresis cell. The liquid is forced through a plug of the powdered solid at a constant rate and the potential difference across the plug is measured.
Sedimentation Potential
The other electrokinetic phenomenon is sedimentation potential. Sedimentation potential is the reverse of electrophoresis, and is the electric field created when particles sediment.
Donnan Membrane Equilibrium
If sodium chloride is placed in solution on one side of a semipermeable membrane, and a negatively charged colloid, together with its counterions R
−Na
+, is placed on the other side, the sodium and chloride ions can pass freely across the barrier but not the colloidal anionic particles. At equilibrium state, R
− is the nondiffusible colloidal anion and the vertical line separating the various species represents the semipermeable membrane as shown in figure. The volumes of solution on the two sides of the membrane are considered to be equal.
| Outside (o) |
inside (i) |
| |
R− |
| Na+ |
Na+ |
| Cl− |
Cl− |
After equilibrium has been established the concentration in dilute solutions of NaCl must be the same on both sides of the membrane, according to the principle of escaping tendencies. Therefore,
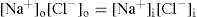 (6.9)
(6.9)
The condition of electroneutrality must also apply. That is concentration of positively charged ions in the solutions on either side of the membrane must balance the concentration of negatively charged ions. Therefore, on the outside:
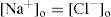 (6.10)
(6.10)
and inside,
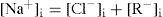 (6.11)
(6.11)
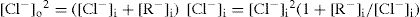 (6.12)
(6.12)
Eq. 6.11 gives the ratio of concentrations of the diffusible anion outside and inside the membrane at equilibrium (
Martin et al., 1997).
The unequal distribution of diffusible electrolyte ions on the two sides of the membrane will result in erroneous values for osmotic pressures of polyelectrolyte solutions.
The above equation (
Eq. 6.12) is used to demonstrate the use of the polyelectrolyte, sodium carboxymethyl cellulose, for enhancing the absorption of drugs such as sodium salicylate and benzyl penicillin (
Higuchi et al. 1954). If [Cl
−] in
equation 6.12 is replaced by the concentration of the diffusible drug, anion [D
−] at equilibrium and [R
−] is used to represent the concentration of sodium carboxymethylcellulose at equilibrium, we have
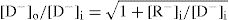
It will be observed that when +[R−]i/[D−]i = 8, the ratio
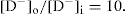
Therefore, the addition of an anionic polyelectrolyte to a diffusible drug anion should enhance the diffusion of the drug out of the chamber. By kinetic studies,
Higuchi et al. (1954) showed that the presence of sodium carboxymethylcellulose more than doubled the rate of transfer of the negatively charged dye, scarlet red sulphonate.
Precipitation of Lyophobic Colloids
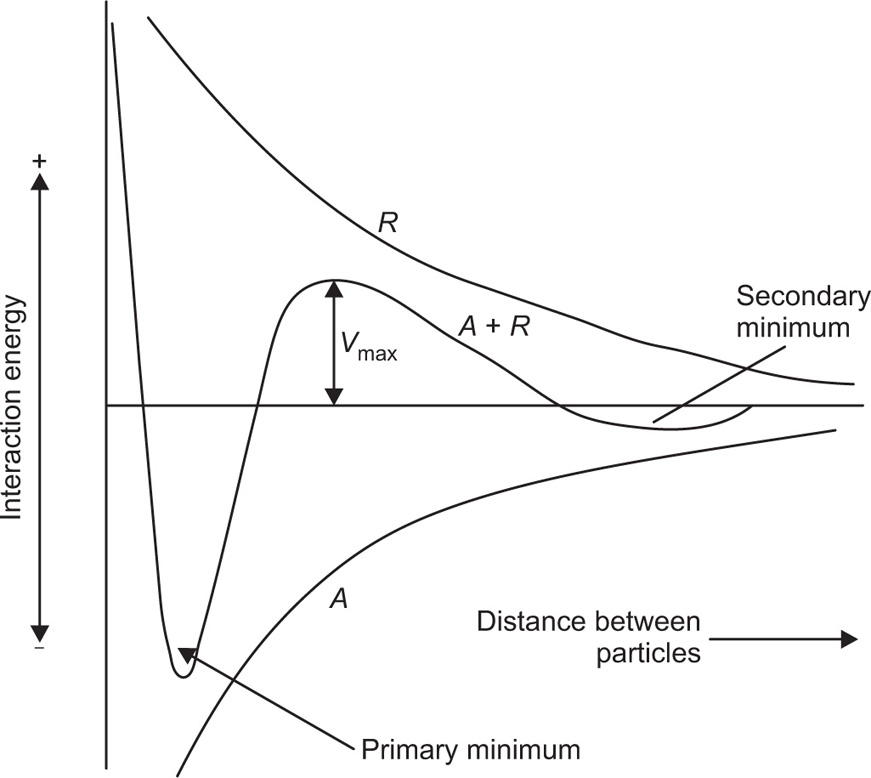
The stability of lyophobic colloids depends on the electrical repulsion exerted by the electrical charges on their surfaces. As discussed in a previous section, trace amounts of electrolytes adsorbed on to the colloidal surface may be responsible for the electrical charge and hence stability. However, if excess of electrolyte is added, this may cause reduction in the thickness of the double layer, lowering the zeta potential below the critical value. The reduction of
Vmax (see
Fig. 6.8) to a few
kT will lead to coagulation in the primary minimum. Ions of charge opposite to that of particles are most effective in coagulating colloids. Furthermore the valency of the ion is important. The coagulating effect of bivalent ions is not just twice that of monovalent ions but the activity increases between ten and a hundredfold on increasing the valency of the acting ion by one. This is known as the
Schulz–Hardy rule. Salts of monovalent cations have coagulation values for negative sols around 0.05
mole of cation per litre where the accompanying anion is Cl, SO
4 or NO
3. Acetates and citrate ions tend to be adsorbed even by negatively charged sols and higher concentrations of these salts are required to produce coagulation.
Precipitation of Lyophobic Colloids
The stability of lyophilic colloids depends mainly on interaction of the colloid with the solvent, the so-called solvation. With water as solvent, this interaction is called hydration; solvation is a more general term for any liquid. Rubber dissolves in benzene and a stable colloid is formed, as the macromolecules of rubber become solvated by the benzene. There is an enormous variety of electrically charged lyophilic colloids, e.g. proteins, pectins, agar, acacia, alginic acid and their stability in aqueous solution depends to a large extent on hydration arid to a lesser extent on electrical charge.
The electrolyte concentrations needed for the precipitation of hydrophilic colloids are many times greater than those needed for coagulation of hydrophobic sols. To precipitate the various proteins of blood plasma not less than 1.3–2.5mole/litre of ammonium sulphate is necessary. The effect of adding electrolytes to a hydrophilic colloid is two-fold: the electrical properties and degree of hydration are both affected. At low concentrations the electrical charge is affected by ions of opposite charge and at high concentrations both charge and hydration are affected, ions having the greatest affinity for water being the most effective in ‘dehydrating’ the colloid. The precipitation by the action of high concentrations of electrolyte is usually called ‘salting out’ and the order of effectiveness of various ions for this purpose can be arranged in a series known as the Hofmeister series. The most common ions arranged in order of decreasing salting out effect for, say, protein solutions, are as follows:
Sulphate/2 > Acetate> Chloride> Nitrate> Chlorate> Bromide> Iodide
For cations, the series is less definite and is as follows:
Lithium > Sodium > Potassium > Ammonium > Magnesium/2
Proteins are most readily precipitated if the pH of the solution is adjusted to the isoelectric point where the net charge is zero. The subsequent addition of a dehydrating agent, e.g. alcohol, acetone or electrolyte, will then precipitate the protein. For proteins of similar chemical constitution the salting out concentration of electrolyte decreases as the molecular weights of the proteins increase. Enzymes have been fractionated by repeatedly dissolving and salting out with ammonium sulphate or magnesium sulphate. The purified enzyme is then freed from electrolyte by dialysing against distilled water. Fractionation of human blood plasma into globulins, fibrinogen and thrombin, depends on selective precipitation by organic solvents under conditions of controlled pH, ionic strength and temperature.
Stabilizing Effect of Lyophilic Colloids
When a hydrophilic/lyophilic colloid is added to a hydrophobic sol in sufficient amount, the hydrophobic particles become coated with hydrophilic material and thus acquire the stable properties of a hydrophilic colloid. This protective action of hydrophilic colloids such as gelatin, acacia, cellulose ethers, polyvinylpyrrolidone, etc., has led to the term protective colloid to describe this type of colloid. Attempts have been made to express the protective action quantitatively in terms of the amount of colloid needed to protect a gold sol from precipitation, on the addition of sodium chloride. The gold number is the weight of protecting substance in milligrams which is just sufficient to prevent the coagulation of 10
ml of red gold sol when 1
ml of 10 per cent sodium chloride is added to it. The gold numbers for some common protective colloids are given in
Table 6.3. However, the effectiveness of protective colloids measured in this way must depend on the affinity of the hydrophilic colloid for the gold and will not necessarily correlate with the degree of protection in other systems. The binding of the protective colloid to the hydrophobic surface, may sometimes lead to flocculation, particularly if the two colloids are oppositely charged and are present in approximately equivalent amounts. In such a case, further addition of protective colloid may overcome this difficulty.
Table 6.3 The gold number of protective colloids |
| Protective colloid |
Gold number |
| Albumin |
0.100 |
| Acacia |
0.100–0.200 |
| Gelatin |
0.005–0.010 |
| Sodium oleate |
1.000–5.000 |
| Tragacanth |
2.000 |
A pharmaceutical example of sensitization and protective action is provided when bismuth subnitrate is suspended in tragacanth dispersion; the mixture forms a gel that sets to a hard mass in the bottom of the container. Bismuth subcarbonate, a compound that does not dissociate sufficiently to liberate the bismuth ions, is compatible with tragacanth. These phenomena probably involve a sensitization and coagulation of the gum by the Bi
3+ ions. The flocculated gum then aggregate with the bismuth subnitrate particles to form a gel or a hard cake. If phosphate, citrate or tartrate are added, they protect the gums from the coagulating influence of the Bi
3+ions, and by reducing the zeta potential on the bismuth particles, partially flocculated systems tend to cake considerably less than deflocculated system, and this effect is significant in the formulation of suspension (
Haines and Martin, 1961)
In pharmacy, there are numerous examples of protective colloids. Resinous tinctures can be dilu ted with a dilute solution of tragacanth or acacia to form a colloidal solution, even in the presence of electrolytes. In the absence of these gums, immediate coagulation of the resin occurs. Acacia and sodium carboxymethylcellulose are used to prepare stable emulsions of oil in water. Gelatin and serum albumin are used to stabilize silver oxide in the preparation of silver protein products (Silver Protein and Mild Silver Protein, BPC 1968). On addition to water, a colloidal silver oxide sol is formed which is suitable for application as eye drops. Gelatin is also used to maintain gold sol in a stable form for injection [Gold (198Au) Injection BP].
Stability of Colloids
All particles are attracted to one another by van der Waals forces. It is these forces that are primarily responsible for the liquefaction of gases, departure of gases from ideal behaviour, and adsorption of gases on to solids. The origin of the force is electrical and it can be explained easily in qualitative terms. The motion of electrons in their orbitals within the atom gives rise to a fluctuating electromagnetic field which exerts an influence upon the electrons in neighbouring matter, causing a net shift of the centre of positive (nuclear) and negative charges in such a direction that there is attraction between the two separate particles. This force falls off very rapidly with distance and is thus negligible except when the particles are quite close together (e.g. <100
nm). In the absence of repulsive forces between the particles in a colloidal sol they would rapidly aggregate together. The fact that stable lyophobic colloidal sols can be prepared is due to the electrical charges on the particle opposing the van der Waals attraction, and from a quantitative assessment of these opposing forces theories have been proposed notably by
Verwey and Overbeek (1948) in Holland and Derjaquin in Russia to explain and predict the stability of lyophobic colloids.
The electrical charge on particles dispersed in a liquid may arise from dissociation of substances which are an integral part of the crystal lattice of the particle, e.g. sodium ion of Na bentonite clay or Ba
2+ and
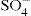
ions of barium sulphate. Fre quently the change arises from the adsorption of some specific ion with the surface. For example, Fe(OH)
3 sols become charged in the presence of excess Fe
3+ which is adsorbed on the surface.
This fixed layer of charges is called the
Stem layer. Ions of opposite charge to those in the Stem layer cluster around the particle, but random thermal motion prevents the existence of a rigid shell of opposite charges. The diagrammatic representation of this electrical double-layer is shown in
Fig.6.9.
The zeta potential, ξ, refers to the electrostatic charge on the particle which causes them to move in an electric field towards a pole of opposite charge. The zeta potential is measured at the surface of shear of the particle and this does not necessarily correspond with the Stern layer. In the absence of hydration the surface potential of the particles should be identical with the zeta potential. Zeta potential can be defined as the work necessary to bring unit charge from infinity to the surface of shear of the particle. The calculation of the decay of electrical potential with distance from the particle is very complex, but it can be solved fairly readily assuming that colloidal particles have an infinite uniform flat surface compared to the reference point where the potential is required. If the surface potential is small it falls to lie on its initial value (e is the base of natural logs 2.718) in a distance (δ given by):
M = molar concentration of counter ions,
z = valency of counter ions,
k = Boltzmann constant,
n = number of counter ions per cm of solution,
e = electronic charge.
δ is often called the thickness of the double-layer and is about 1nm in 0.1M monovalent electrolyte.
The basic concept underlying the stability of lyophobic colloids is the mutual repulsion due to interacting double layers and the van der Waals force of attraction. For infinitely large flat plates not too close together, the attractive energy VA between the particles per cm2, is given by:
The repulsive energy
VR, arising from the interaction of the double layers was calculated by
Verwey and Overbeek (1948) by considering the free energy of the system of the two double layers as a function of their distance of separation. From this knowledge of repulsive and attractive forces, it is possible to construct diagrams showing the interaction energy between a pair of colloidal particles as a function of the separation of their surfaces. In
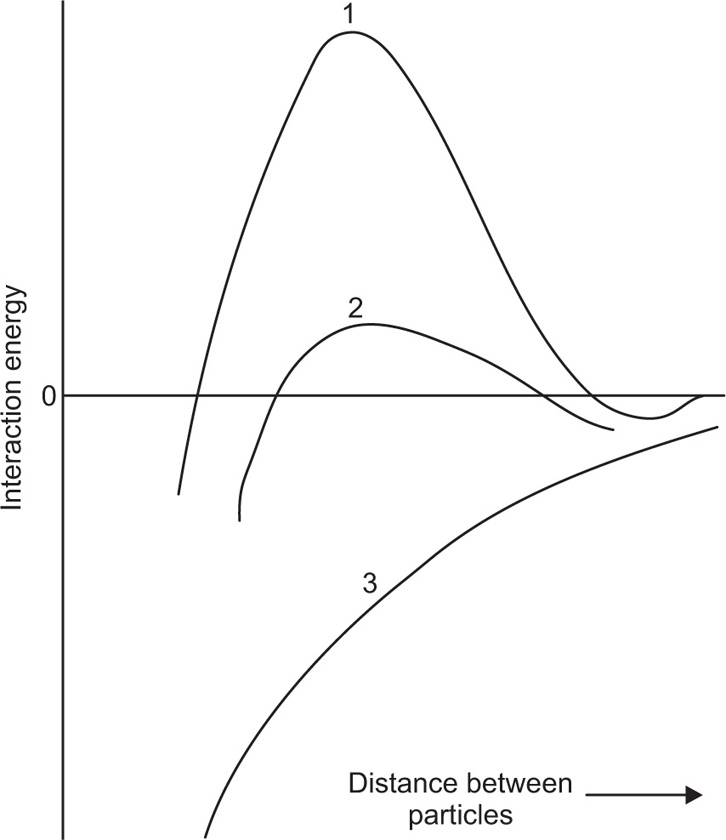
Fig. 6.8 the vertical axis represents the interaction energy, the horizontal axis the distance between two particles. From the summation of the attractive and repulsive forces, a net interaction curve is obtained.
When the particles are far apart, the interaction energy approaches zero, but becomes infinitely large when the particles touch, due to the mutual repulsion of the electrons in the outermost atoms of the particles. For particles to come together at the primary minimum they must surmount the energy barrier of magnitude Vmax. Particles in the primary minimum will be then irreversibly agglomerated.
As the repulsive forces fall off more rapidly with distance than do the attractive forces, a secondary minimum is produced when the particle surfaces are about 100
nm apart. This weak attraction causes slight flocculation of colloidal particles but the floccules can be redispersed on shaking. Experimental evidence indicates that flocculation in the secondary minimum is restricted to the larger colloidal sizes and is probably unimportant for particles below say 0.1
μm. The rate of coagulation of particles in a sol is obviously a function of the
Vmax Provided that
Vmax exceeds 20–25
kT, where
k is the Boltzmann constant and
T the absolute temperature, the particles are unable to gain sufficient energy to surmount the energy barrier and so they remain in a deflocculated condition. Three types of potential energy curve are shown in
Fig. 6.10.
Curve 1 has Vmax > 25 kT and represents a stable sol. Curve 2 has maximum of only low kT and the particle will agglomerate fairly rapidly due to Brownian movement producing collisions of sufficient energy. Curve 3 represents a sol that will agglomerate instantaneously as no net repulsive forces are operating.
Many attempts have been made to correlate the zeta potential with lyophobic colloid stability; a high zeta potential would give rise to a high
Vmax. The theoretical basis for the potential energy of interaction curves, deduced by
Verwey and Over beek (1948) depends on knowledge of the potential at the surface of the particle, i.e. at the Stern layer. The zeta potential which is derived from electrokinetic experiments such as electrophoresis is not identical with the surface potential. This is because the method involves the measurement of movement of particle through a liquid and the flow of liquid past the particle does not occur exactly at the particle–liquid interface but at a plane in the liquid some distance from the true interface. The zeta potential is the potential at this plane and is therefore generally much smaller than the surface potential. However, the zeta potential is readily determined experimentally and in the literature there is evidence that zeta potential can help in predicting whether or not sols will flocculate in the presence of electrolytes. The application to pharmaceutical suspensions is discussed by
Nash and Haeger (1966).
Lyophilic and associated colloids are thermodynamically stable and exist in true solution; because of that system constitutes a single phase. Addition of an electrolyte into lyophilic colloids in moderate amounts does not result in coagulation, but upon addition of electrolyte, coagulation is one of the distinct characteristic of lyophobic colloids. If more salt is added in lyophilic colloids, it may result in agglomeration and sedimentation of particles. This is known as salting out phenomena. This technique is used in the purification of antitoxins.
Lyophilic colloids can be considered to become lyophobic by the addition of solvents such as acetone and alcohol. The particles become desolvated and are then very sensitive to precipitation by added electrolyte.
Solutions of macromolecules, lyophilic colloidal sols, are stabilized by a combination of electrical double-layer interaction and salvation, and both of these factors must be sufficiently weakened before attraction predominates and the colloidal
particles coagulate. For example, gelatin has a sufficiently strong affinity for water to be soluble even at its isoelectric pH, where there is no double-layer interaction. When +ve and −ve charged hydrophilic colloids are mixed, the separation of particles from the dispersion medium takes place and forms a layer which is rich in the colloids aggregates. Formation of this layer is known as
coacervate and phenomenon is known as
coacervation; for example, +ve charge gelatin (at a pH below 4.7) with −ve charge acacia (unaffected by pH in acid range) are mixed in certain proportion, coacervation results. The viscosity of layer, now poor in colloid, is markedly decreased below that of the coacervate and in pharmacy it represents the physical incompatibility.
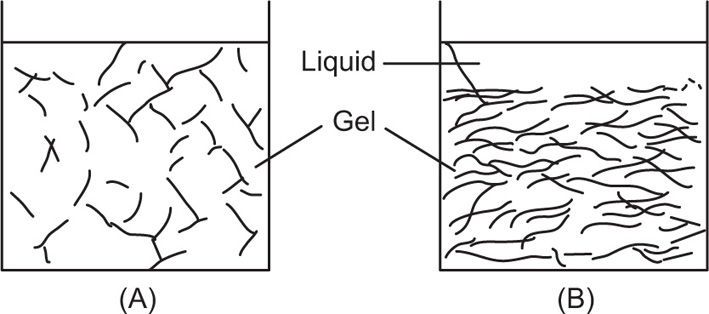
Gels
Lyophilic colloids such as gelatin, agar and pectin are soluble in hot water and on cooling give rise to a semisolid, jelly-like structure termed a gel. The mechanical strength of the gel is due to partial aggregation of the colloidal particles which form an interlaced network with the solvent held within the meshes. The gelation process can be regarded as a half-way stage between a colloidal sol and a completely precipitated sol. Thus electrolytes which promote precipitation of colloidal sols will also promote gelation, whereas peptizing agents will have the opposite effect. For instance, a low concentration of calcium or zinc ions tends to increase the gel strength of the negatively charged bentonite gel, whereas excess will result in precipitation and breakdown of the gel.
Preparation of Gels
Temperature effect: The solubility of most lyophilic colloids, e.g. gelatin, agar, sodium oleate, is reduced on lowering the temperature, so that cooling a concentrated hot sol will often produce a gel. In contrast to this, some materials such as the cellulose ethers owe their water solubility to hydrogen bonding with the water. Raising the temperature of these sols will disrupt the hydrogen bonding and the reduced solubility will cause gelation.
Flocculation with salts and nonsolvents: Gelation is produced by adding just sufficient precipitant to produce the gel state but insufficient to bring without complete precipitation. It is necessary to ensure rapid mixing to avoid local high concentrations of precipitant. Solutions of ethyl cellulose, polystyrene, etc. in benzene can be gelled by rapid mixing with suitable amounts of a nonsolvent such as petroleum ether.
The addition of salts to hydrophobic sols brings about coagulation, and gelation is rarely observed. However, the addition of suitable proportions of salts to moderately hydrophilic sols such as aluminium hydroxide, ferric hydroxide and bentonite, produces gels. As a general rule, the addition of about half of the amount of electrolyte needed for complete precipitation, is adequate. With positively charged hydroxide sols, divalent ions such as
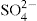
are more effective than univalent ions such as Cl
−. The gels formed are frequently thixotropic in behaviour.
Such hydrophilic colloids as gelatin, proteins and acacia are only affected by high concentrations of electrolytes, when the effect is to ‘salt out’ the colloid and gelation does not occur.
Chemical reaction: In the preparation of sols by precipitation from solution, e.g. Al(OH)3 sol prepared by interaction in aqueous solution of an aluminium salt and sodium carbonate, an increased concentration of reactants will produce a gel structure. Silica gel is another example and is produced by the interaction of sodium silicate and acids in aqueous solution.
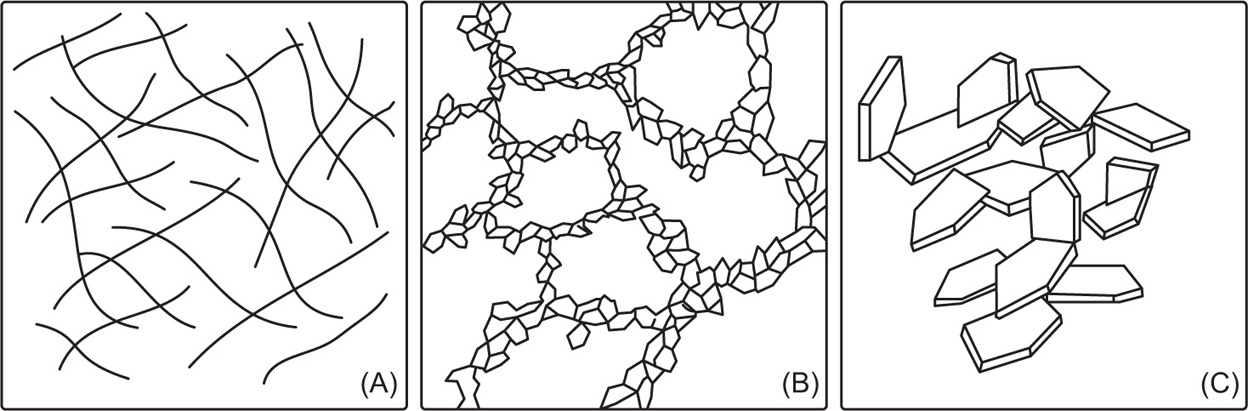
Structure of Gels
Elastic Gels
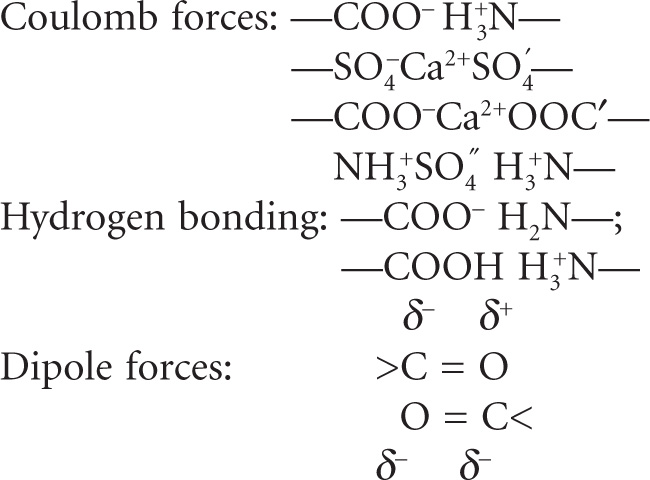
Gels of agar, pectin and gelatin are elastic, the fibrous molecules being linked at the points of junction by relatively weak bonds such as hydrogen bonds and dipole attraction. If the molecules pos sess free COOH groups, then additional bonding may take place in the presence of Ca
2+ when a salt bridge of the type –COO–Ca
2+O
−OC– is formed between two adjacent strands of the net work. The types of links that could be formed are shown below (
Alexander & Johnson, 1949).
Rigid Gels
In contrast to elastic gels rigid gels can be formed from macromolecules in which the framework is linked by primary valence bonds. Silica gel is such an example where silicic acid molecules are held together by –Si–O–Si–O– bonds to give a polymer structure possessing a network of pores, most of which are scarcely larger than a few molecular diameters in width. Because of the resultant large surface area, silica gel is a useful adsorbent particularly for water vapour and hence its use as a desiccant.
Thixotropic Gels
The bonds between particles in these gels are very weak and can be broken down by shaking or stirring. The resultant sol will revert back to a gel due to the particles colliding and linking together again; Brownian movement being responsible for the particle–particle collisions. This reversible isothermal sol–gel
transformation is termed
thixotropy. It is most likely to occur in colloidal systems with nonspherical particles such as rods or plates which can be easily built up into a scaffold-like structure. Many clay minerals dispersed in water show this effect, due not only to the plate-like structure of the particles, but also due to the faces and the edges of the platelets carrying charges of opposite sign.
Bentonite and acid-washed kaolin platelets have many positively charged edges while all the faces are negatively charged due to dissociation of positive counterions such as Na+, Ca2+. This results in a face to edge attraction giving a scaffold structure in which solvent is easily enmeshed. Mechanical shearing breaks the structure and liquefies the gels. The effect of adding citrate ions to clay dis persions may be to neutralize the positive charges on the edges of the platelets, thus minimizing the formation of three-dimensional gels. Other examples of thixotropic gels include aluminium hydroxide in water, many concentrated emulsions, particularly those prepared with emulsifying waxes, and oils gelled with aluminium monostearate.
The different possibilities of gel structure are presented schematically in
Fig. 6.11. Evidence for the coherent network structure has been obtained from a number of experiments. For instance a fluid dispersion of graphite in a nonconducting oil does not conduct electricity until the system is allowed to set to a gel, when it does conduct, indicating that the particles have linked together. Shaking this thixotropic gel will liquefy it when it will act as a nonconductor as before. The open structure of a gel is apparent if one considers the small proportion of solid phase present. For instance, water can be gelled by as little as 0.5 per cent agar, small molecules and ions are free to diffuse in the solvent through the relatively large meshes and it is therefore surprising that the diffusion of low molecular weight substances in gelatin and agar gels is the same as in water, provided no chemical interaction or adsorption occurs. If the concentration of gelling agent is increased the mesh size of the gel is reduced and there will be a limiting concentration that can be used before the diffusing molecule is restricted.
Syneresis
Swollen gels on storage may spontaneously contract with the liberation of dispersion medium e.g. pectin, starch gels. This exudation is probably due to increased particle–particle linkages of the gelling agent resulting in the squeezing out of the enmeshed liquid. This is shown schematically in
Fig. 6.12.
Pharmaceutical Applications of Colloids
A lot of food which we eat is colloidal in nature, such as milk. It is a colloid of ‘fat dispersed in water’ stabilized by Casein. We use a lot of jellies, jams, ice creams, etc., and all these are colloidal solutions. Similarly, solutions like calcium colloid are used for medicinal purposes. There are a lot of medicinal solutions which are prepared as a colloid for their easy absorption by the human body.
Therapeutic Effect
Colloidal systems are used as therapeutic agents in different areas.
Colloidal silver: It is the finest germ, virus and bacteria fighter available. Because of its super fine microscopic structure (0.10–0.01
micron) it is capable of incredible healing and also acts as defensive
power osteoporosis, rheumatism, arthritis, torn ligaments, swollen and sore joints, sprains, strains, stress, flu, colds, sore throat, etc. It is a phenomenal antibiotic alternative and germicide. It kills all known bacteria and viruses, stimulates immune system, speeds up healing and repair, accelerates bone and muscle repair, tones body and creates feeling of well-being, promotes healthy bones and joints, perfect skincare solution, safely kills infections in eyes, ears, nose and sinus, speeds healing of torn muscles and ligaments, and accelerates healing of stubborn skin ulcers.
Colloidal silver also promotes healthy plant growth (kills fungus and infection), promotes good health for dogs and most other pets (boosts immune system and accelerates healing), fights against the results of pollution and allergens, and together with zinc protects the prostrate.
Colloidal gold: It has amazing properties known to improve stress-related illness, and brain and arthritic conditions. It enhances, relaxes, relieves, interacts with nerves, stress, arthritis, certain brain disorders, neural, heart rhythm, neural transmitters, headache, migraine, brain, memory, glands, libido, upper back pain, numbness, relaxation, collagen, arthritis, rheumatism, inner ear, balance, enhances sexual function, assists in relaxation and blood pressure control, and controls attention deficit disorder in children.
Colloidal copper: It has been used for millennia by empresses, queens, princesses and the rich and famous of their day, together with gold for beauty and antiaging treatments. It is the roto-rooter of the blood arteries. It interacts, cleanses and defends the arteries. It stimulates collagen and promotes the skin’s elasticity. Thus it protects and defends the arteries and veins against aneurysm, arteriosclerosis, blocked arteries, deafness, inner-ear, lower-back pain, swollen disc, slipped disc, swollen soft tissue, inflammation, antioxidant, wrinkles, collagen, fungicide, damaged soft tissue, etc.
Colloidal copper also strengthens blood circulatory system, cleans arteries and veins, helps in resolving fine lines and wrinkles, increases synapse response within the brain leading to clearer brain clarity, improves skin elasticity, regulates varicose veins, is effective against parasites, can provide the missing element against osteoporosis, assists in preventing stroke, helps in preventing arteriosclerosis, assists in the reversal of greying hair and helps in retaining original natural youthful colour, relieves joint and muscle pain and oxygenizes the blood. It also brings more blood to afflicted areas where applied topically.
Colloidal mercury: It is used in many industrial applications and its salts have been employed therapeutically as purgatives, antisyphilitics, disinfectants and astringents. It can be absorbed through the skin and mucous membranes which leads to mercury poisoning. Because of its toxicity, the clinical use of mercury and mercurials is diminishing.
Proteins are natural colloids. Plasma protein may bind drugs and affect the therapeutic activity. Many synthetic polymers are used in coating to solid dosage form. Colloidal electrolyte surfactants are used as additives in pharmaceutical preparations.
Blood Plasma Substitutes
Hydrophilic colloids such as dextran, PVP and gelatin are used to restore or maintain blood volume, e.g. iron–dextran complex forms nonionic hydrophilic sols used for treatment of anaemia.
Stability
Charges play an important role in determining the stability of a colloidal system. Colloids, especially lyophobic colloids, having like charges on particle surface repel each other and prevent flocculation in suspensions, e.g. colloidal dispersion of gelatin is used in coating over tablets and granules which upon drying leaves a uniform dry film over them and protects them from adverse conditions of the atmosphere.
Absorption
As colloidal dimensions are small enough; they have a huge surface area. Hence, the drug constituted colloidal form is released into the vicinity in large amounts such as: (1) colloidal sulphur gives a large quantity of sulphur and this often leads to sulphur toxicity, (2) efficiency of kaolin in adsorbing toxins from GIT and (3) efficiency of aluminium hydroxide as antacid.
Dissolution
Due to huge surface area, the dissolution rate is very large as compared to coarse dispersion as stated by Noyes–Whitney equation.
Targeted Drug Delivery
Colloidal delivery systems include micelles, microemulsion, liposomes, parenteral emulsions, microspheres, nanoparticles and drug–polymer conjugates are preferentially taken up by the liver and spleen. Hence, principle of colloids is also used in targeted drug delivery system.
Colloidal microgels: It has recently received attention as environmentally responsive systems and now is increasingly used in applications as carriers for therapeutic drugs and diagnostic agents. Synthetic microgels consist of a crosslinked polymer network that provides a depot for loaded drugs, protection against environmental hazards and template for postsynthetic modification or vectorization of the drug carriers. Metal-containing microgels are used for diagnostic and therapeutic applications, and to advance approaches including the systemic administration of microgels.
Solubility Enhancement
Association colloids are used to increase solubility and stability of certain compounds in aqueous and oily pharmaceutical preparations.
Diagnostic Effect
Colloidal dispersions containing radioactive isotopes are being used as diagnostic and therapeutic agents in nuclear medicine. Colloidal gold is used for the diagnosis of paresis and colloidal mercury for syphilis.

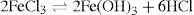
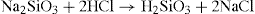
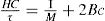
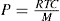
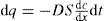
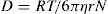
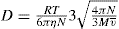
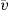 the partial specific volume of the colloidal material.
the partial specific volume of the colloidal material.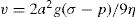 (6.1)
(6.1)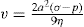 (6.2)
(6.2)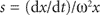 (6.3)
(6.3)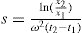 (6.4)
(6.4)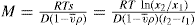
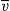 is the specific volume of the particle.
is the specific volume of the particle.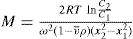
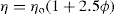 (6.5)
(6.5)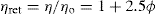 (6.6)
(6.6)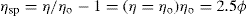 (6.7)
(6.7)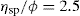 (6.8)
(6.8)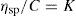
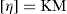
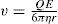
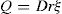
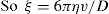
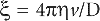
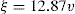
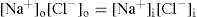 (6.9)
(6.9)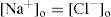 (6.10)
(6.10)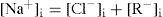 (6.11)
(6.11)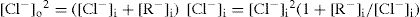 (6.12)
(6.12)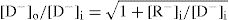
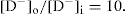
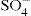 ions of barium sulphate. Fre quently the change arises from the adsorption of some specific ion with the surface. For example, Fe(OH)3 sols become charged in the presence of excess Fe3+ which is adsorbed on the surface.
ions of barium sulphate. Fre quently the change arises from the adsorption of some specific ion with the surface. For example, Fe(OH)3 sols become charged in the presence of excess Fe3+ which is adsorbed on the surface.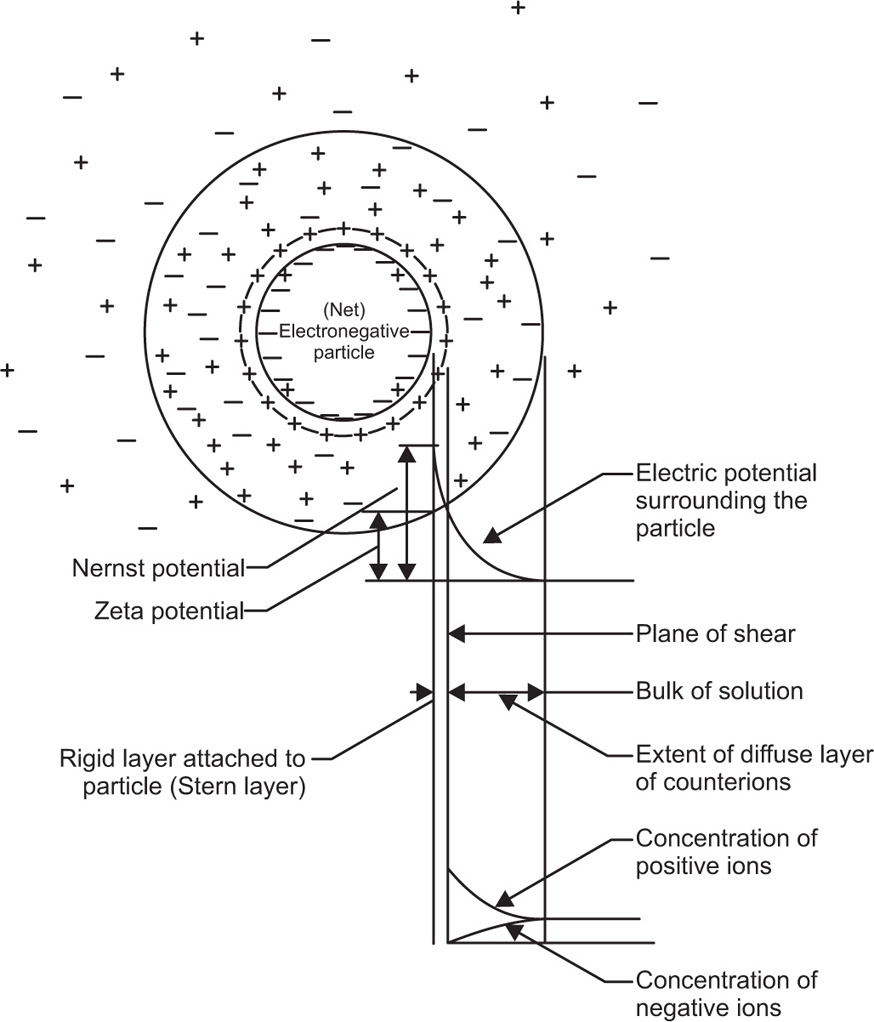
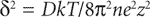
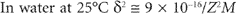
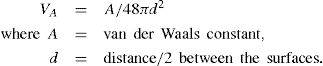
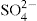 are more effective than univalent ions such as Cl−. The gels formed are frequently thixotropic in behaviour.
are more effective than univalent ions such as Cl−. The gels formed are frequently thixotropic in behaviour.