Chapter 40
Sutures and Ligatures
The use of strings made by twisting vegetable and animal materials is described in the most ancient of surviving records of the history of mankind. Animal skins, intestines and sinews were used for musical instruments, bows and many other items. Vegetable fibres, spun and woven, date back to prehistoric times. Linen as spun strand was well known by 5000 BC and probably was first prepared long prior to this date.
In surgery, susruta (1500 bc) records the use of ligatures for tying umbilical cord and celsus in the first century ad described the ligature as of ancient origin. In the highly developed civilization of ancient egypt, surgeons closed wounds with sutures. Galen (c. ad 200) used silk and hemp cords as ligatures and also recommended the use of animal gut. The term catgut is said to be derived from the gut used to string a musical instrument known as a kit, an Arabic word for a dancing master’s fiddle, hence kitgut. The Arabian surgeon rhazes (c. ad 900) used harp strings made from sheep intestine to repair abdominal wounds, but the use in surgery of twisted animal intestines was not generally practised as the patients nearly always became infected.
Ambroise Pare (1517–90) appears to have revived the use of ligatures and there are numerous references in his works to their use, particularly in amputations where he preferred them to the cautery. His valuable work, however, appears not to have been generally recognized and surgeons continued with the old methods of the hot knife and searing iron.
The credit for the reintroduction of catgut is given to P.S. Physick (1816) house surgeon to J. Hunter, and later Professor of Surgery in Philadelphia, but his observations, and publications of other workers of the same period, did not persuade surgeons to adopt the material.
It was not until 1869 that Joseph Lister, as a result of his Observations on the Ligature of Arteries on the Antiseptic System opened the way to the modern surgical suture techniques. The first step had been to show that sepsis was due to the growth of microorganisms from the site of infection. From that time onwards the search to find means of preventing infection has not ceased. The history of this work applied to surgical sutures and ligatures during the past seventy years is too large a subject for this chapter but the investigations by a host of dedicated workers into hundreds of different procedures has led to the highly efficient methods being employed today.
For further details on the historical aspect, see Fandre (1944).
Although the terms ligature and suture are often used in the same sense, and they are of the same material, there is nevertheless a technical difference. A ligature is a thread used to constrict and seal off a blood vessel, vein or artery—hence to ligate. The thread is a suture when it is used to stitch together the edges of various tissues, e.g. skin, fascia, muscle, tendon, peritoneum, etc. Hence a needle is always used for a suture (sewing) but not for a ligature.
Sutures and ligatures are classified as absorbable, or nonabsorbable, depending on the materials from which they are made.
Absorbable
Absorbable sutures and ligatures are absorbed by the tissues in which they are implanted, and the time taken for complete disappearance is dependent on a number of factors which will be treated more fully later in this chapter. Absorbable materials are catgut (nonboilable and boilable), reconstituted collagen, synthetic absorbable polymers, kangaroo tendon, ribbon gut and fascia lata.
Nonabsorbable
Nonabsorbable sutures and ligatures are not absorbed by tissue and, unless they are on the surface, remain in the body after the wound has healed. Some, notably silk, fragment after a long period of time; others are encapsulated by fibrous tissue, while others remain as inert implants. The most commonly used are silk, linen, nylon (polyamides), polyester, polyolefines and stainless steel wire, and to a very small extent: cotton, horsehair, human hair, silkworm gut and wires of other metals, e.g. tantalum, silver, phosphor bronze, etc.
The polymeric materials are also used in the form of woven meshes particularly for hernia repair and silk and nylon in floss form have specialist uses also.
Mention should also be made of the use of clips (Michel, Kifa, etc.) for surface application and the past few years have seen a good deal of development, particularly in Russia and the USA in the use of small wire staples (usually stainless steel) which are implanted by means of stapling guns.
Standards and Legal Requirements for Sutures and Ligatures
Although the national pharmacopoeias of most countries publish monographs on surgical sutures, the standards nowadays for EEC countries, Denmark, Sweden and Switzerland, i.e. the signatories to the convention, are established by the European Pharmacopoeia. Volume II of the first edition published in 1971 contained monographs for sterile catgut, sterile reconstituted collagen strings and sterile nonabsorbable strands including braided silk. Braided polyester, braided polyamides 6 and 6/6, monofilament polyamides 6 and 6/6 and linen thread. Some amendments were introduced in Volume III published in 1975 to approximate to the USP XIX (1975).
Both these compendia have adopted a metric numbering system whereby the gauge number applied to the suture represents the actual diameter in tenths of a millimetre. Previously a so-called conventional system was used and, although in the case of the UK and the USA the sizes represented were comparable, the values for diameter differed between absorbable and nonabsorbable materials. The conventional systems employed by other countries themselves differed and there was a lack of flexibility as finer sutures were developed for modern surgical techniques. (See table.)
| Former conventional size | ||
| Metri number | Catgut/collagen | Nonabsorbables synthetic absorbables |
| 0.1 | − | − |
| 0.2 | − | 10/0 |
| 0.3 | 9/0 | 9/0 |
| 0.4 | − | 8/0 |
| 0.5 | 8/0 | 7/0 |
| 0.7 | 7/0 | 6/0 |
| 1 | 6/0 | 5/0 |
| 1.5 | 5/0 | 4/0 |
| 2 | 4/0 | 3/0 |
| 3 | 3/0 | 2/0 |
| 3.5 | 2/0 | 0 |
| 4 | 0 | 1 |
| 5 | 1 | 2 |
| 6 | 2 | 3 and 4 |
| 7 | 3 | 5 |
| 8 | 4 | 6 |
Sutures have always been the odd man out as far as legislation is concerned and have been classed sometimes as ‘drugs’ and sometimes as ‘devices’. In the UK the manufacture and sterilization of catgut and other products of animal origin became subject to control by licensing under the Therapeutic substances Act in 1929 largely as a result of the reports of T.J. Mackie and of Bulloch et al. This control now extends to various ‘surgical materials’ of animal origin and to synthetic materials capable of being absorbed.
The Medicines Act is in process of taking over from the Therapeutic substances Act. Anomalies still remain however that in the meantime nonabsorbable materials of vegetable or synthetic origin are not subject to such control.
Absorbable Materials
Surgical Catgut
Sterilized surgical catgut consists of a strand prepared from collagen derived from healthy mammals purified and sterilized. The most widely used source is the submucosa of the small intestine from sheep or lambs and to a lesser extent, the serosa from beef cattle. The length of ovine intestine is about 20m and it is desirable in the preparation of surgical catgut that the diameter of the intestine should not be more than 18mm. A number of factors are important in the selection of suitable intestinal material. Obviously intestines will vary considerably depending on the age of the animal, the pasture, climate, etc., and it is not uncommon to find that intestines from some animals have been affected by scar tissue and are not suitable for preparing surgical catgut. Generally speaking the younger the animal the smaller its intestine, and less likely to be affected by feed.
A number of manufacturers of catgut use only the first 8m of intestine measured from the duodenum. In the meat trade such intestines are described as ligature casings or runners.
In the slaughterhouse the gut is removed from the animal by the gut pullers and is first of all cleaned to remove faecal matter after which it is inspected, measured and then preserved either in a frozen state or salted. The largest supplies of intestines come from Australia and New Zealand and the slaughterhouses in these countries are well equipped to deal with the vast number of animals involved. The intestine of any cadaver is the most vulnerable to bacterial attack and decomposition of this part of the body always begins earlier than any other. It is, therefore, vitally important that the slaughterhouse technique involves rapid cleaning and freezing or preservation by other methods in order to keep the bacterial growth as low as possible.
The ovine intestine consists of four layers (Fig. 40.1). The outermost is known as tunica serosa or serous layer, much of which is torn off in removal from the animal; next the tunica musculosa made up of two layers, one longitudinal and the other circular; the third layer is the tunica submucosa from which catgut is prepared, and the innermost layer is the tunica mucosa or mucous coat which is the wall to the lumen of the intestine. The intestine receives its blood supply from the mesenteric artery and being some 28 times the length of the sheep itself is twisted into a mass of convolutions until near its end where it ascends to join the colon.
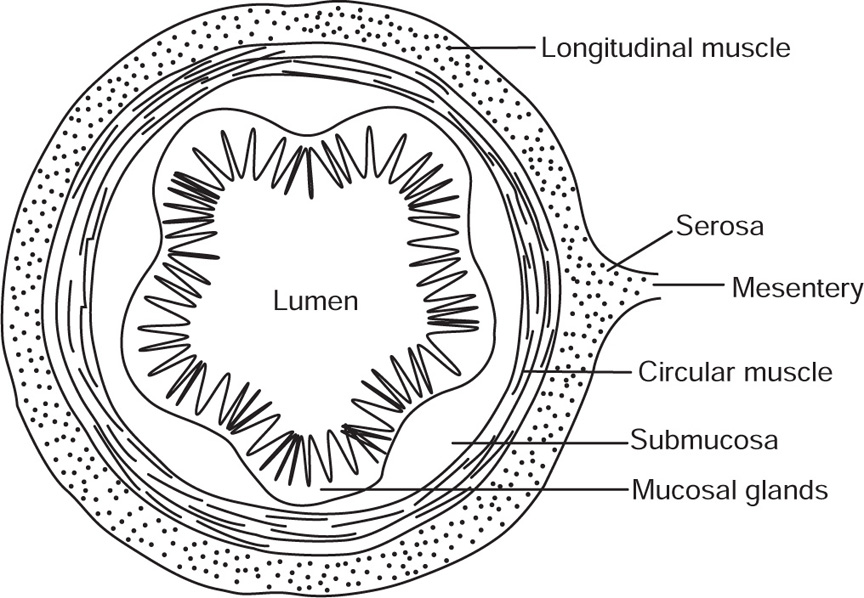
Fig. 40.1 Diagrammatic section of intestine.
The Medical research council Report on the preparation of catgut contains interesting microphotographs of the submucosal layer.
The manufacturer of surgical catgut receives intestines either intact or rough scraped at the abattoir, made up as knots or bundles which may be frozen, salted or in brine, and the first step is to soak these in water to thaw out or to remove salt and prepare them for splitting. It may be noted in passing that the intestines destined for sausage skins are not split but are cleaned and scraped in the tubular form and known as sausage casings. This cleaning usually takes place in the abattoir and the product is marketed in barrels or casts.
Splitting
The splitting or cutting operation is carried out by inserting the curved horn of a cutting tool into the end of the intestine and pulling the runner over cutting blades (Fig. 40.2). The number of ribbons produced can be varied but is usually two or three. The horn follows the curvature of the intestine and therefore can be said to locate down the track of the mesenteric vein, which is often called the rough side as distinct from the upper part known as the smooth side. The two parts of the intestine are kept separate throughout the process as they behave in different ways physically and chemically.
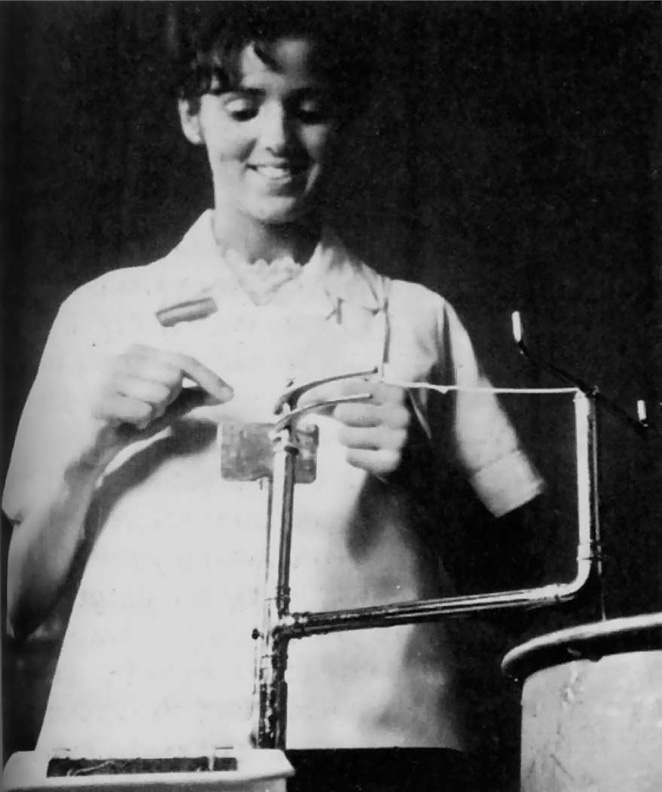
Fig. 40.2 Splitting of intestine (Ethicon Ltd., Edinburgh).
Cleaning of submucosa
The next step is to remove the mucosa, muscle and any remaining serosa, or, if the material has been cleaned in the abattoir; the remnants of these layers, and this is facilitated by treatment with alkaline solution. The general method of scraping is to fix the ribbons in a frame or on suitable flat surfaces so that the submucosa can be cleaned of unwanted material (Fig. 40.3). Often this is carried out by hand and is a very skilled operation. In other cases an apparatus known as a sliming machine is employed.
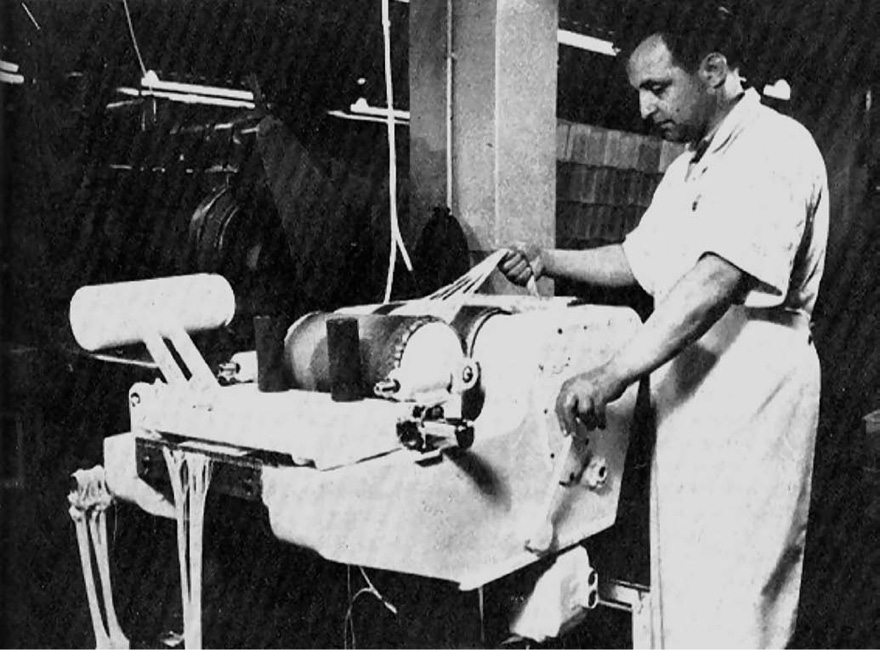
Fig. 40.3 Slimming machine for removal of mucosal and muscular coats from the submucosa of intestinal ribbon (Ethicon Ltd., Edinburgh).
When the ribbons are judged satisfactorily cleaned they are cut to a predetermined length and assembled in multiples of ribbons of the same type and mounted on to string loops at either end. The number of ribbons will determine the ultimate size of the spun strand.
Spinning
The apparatus used by manufacturers for spinning ribbons of catgut is largely dependent on the manufacturer’s choice. The number of hooks on the machine to which the string loops are attached varies from 2 to 20. In some cases spinning takes place from both ends of the strand and may take place immersed in water or alkaline solution. The spinning of catgut destined for surgical use is a highly skilled operation. Multiples of ribbons which are overspun will tend to lack elasticity and will curl when dry. On the other hand, if they are underspun the elasticity will be too great and the tensile strength reduced. The angle of ply to the horizontal is to some extent a guide as to whether a string has been properly prepared, but many other factors can affect the ultimate quality of the catgut. Because catgut is a biological material it must of necessity vary considerably and no one animal intestine is exactly the same as another. The spinning of catgut is, therefore, still largely on art rather than a mechanical operation. After spinning, the catgut is mounted on drying frames, the conditions of drying time and humidity being carefully controlled. The resulting strand of dried catgut is known at this stage as raw catgut and is usually between 3 and 5m long.
Polishing
strands of catgut can be prepared with such care and attention to manufacturing detail that they will vary only slightly in diameter and need only a light manual polishing, but more usually the manufacturer employs a technique which produces an approximate size and then polishes or grinds the string to a predetermined diameter. This process is achieved by means of machines, either by rotating the strings while a carriage bearing an abrasive paper moves to and fro along their length, or by using a pair of grinding wheels similar to those employed by engineers and setting the wheels the required distance apart. Both methods have to be very carefully controlled to avoid damage to the plies of the strand with consequent loss in tensile strength. The finish, moreover, must be such that the gut is neither ‘whiskery’ nor so smooth that the surgeon’s knots will slip and cause the wound to re-open.
Gauging
The methods of gauging carried out by various manufacturers vary with their own particular preferences, but the final control instrument for checking diameter is a gauge of the dial reading type, in which the details of pressor foot and weight loading are specified (Fig. 40.4). As the diameter of catgut will vary with the relative humidity of the atmosphere, the control test as laid down is the diameter in a relative humidity between 60 and 80 per cent and at a temperature between 16 and 21°C.

Fig. 40.4 Baty gauge (London Hospital Ligature Laboratories).
The normal gauges used in British surgery vary from the finest size 1 in ophthalmic work to the thickest, size 7, which is occasionally used for specialist surgery.
Standards
Length: This is determined immediately after removal of the strand from its container and is measured without stretching. The length must be not less than 90 per cent of the length stated on the label.
Tensile strength: The test is carried out on a machine of the deadweight type, having a movable jaw with a constant rate of traverse of 30cm/min, and a capacity so that when the strand breaks, the angle which the pendulum arm makes with the vertical is not less than 9 and not more than 45. The clamp heads are specially designed and any strands breaking within 12.5mm of the clamps are disregarded. The strands are tested within 15 min of removal from their container and in a temperature between 16 and 21°C and in an atmosphere in which the relative humidity is between 60 and 80. As catgut is inevitably knotted in the patient, the test is always carried out on strands in which a surgeon’s knot (Fig. 40.5) has been formed at a point midway between the two clamps. The knot strength is approximately half of the figure which would be obtained on an unknotted sample.
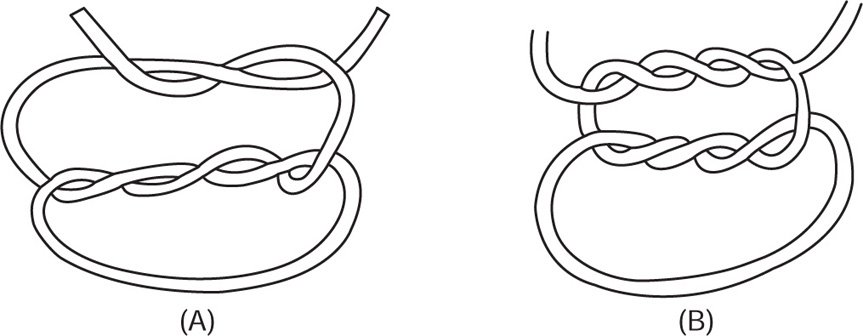
Fig. 40.5 (A) Surgeon’s knot (sample knot tied on double knot); (B) nylon knot.
Labelling: The label on or in the container must state by indelible marking or perforation the length of the strand, the gauge number, whether the strand is plain, hardened or chromicized, and that the container should not be subjected to heat treatment. The label on the box must state the name and percentage of any bactericide in the fluid in which the sutures are immersed.
Storage: sterilized surgical catgut should be protected from light and stored in a cool place.
Packaging: The British Pharmacopoeia states that sterilized surgical catgut is packed either in glass tubes sealed by fusion of the glass, or in other suitable containers which once opened cannot be resealed. Although glass tubes are probably the ideal method of packaging surgical sutures in that they are transparent, inert and impermeable, they are nevertheless regarded as a nuisance in the operating theatre because they have to be broken, and a large variety of flexible packages based on aluminium foil or plastic films which can be torn open, have in the past few years tended to replace glass. The bottle packs, which are still used to some extent in continental Europe, in which a continuous length in the form of a cocoon of catgut is packed in ingenious dispensing devices, is considered to introduce an element of risk of contamination, and this form of packing is no longer used in Great Britain.
Sterility testing: The Therapeutic Substances Act in Great Britain lays down the fundamental required for bacteriological testing of surgical catgut. Definitions are given of the term batch, the percentage of samples from each batch which are to be tested and broad definitions of the media and incubation times to be employed. The technique to be adopted where any bactericides present are liable to inhibit growth in the medium is specified. The medium is so designed that it will detect the presence both of aerobes and anaerobes. If after an incubation period of 14 days no growth or microorganism is found in any tube, the sample may be regarded as having passed the test. If growth of microorganisms is found in any tube a further sample may be taken from the batch and the test repeated. If no growth occurs the sample shall be regarded as having passed the test, but if any microorganism is found the batch is treated as not sterile.
Sterilization of Catgut
Lister’s work on catgut in 1869 using aqueous phenol and later phenol and olive oil was the forerunner of antiseptic technique applied to sutures. His experiments and resulting papers stimulated work in which a very large number of chemical compounds were investigated with varying success to bring about a reduction in the number of microorganisms present in surgical catgut.
The variety of substances employed was very numerous and is surveyed in detail in the Medical research council report on The Preparation of Catgut for Surgical Use (Bulloch et al., 1929). Bulloch checked the efficacy of a large number and found most to be of very doubtful value. As with most newly presented ideas each substance had its adherents and at a time when a large number of hospitals preferred to sterilize their own catgut sutures there were bound to be failures and many patients became infected as a result. The preparation of bulk sterile material by present day skilled technological methods was, before 1929, in its infancy and the problems of inactivating microorganisms in catgut were not then fully appreciated.
Mammalian intestine in the living animal is normally relatively free of microorganisms but immediately after death growth of microorganisms proceeds at a very rapid rate. Conditions in slaughterhouses, however clean, are, moreover, not conducive to aseptic techniques and the intestines inevitably carry a large bacterial population. The freezing or salting of the material inhibits further growth but does not kill these organisms. Staphylococci, streptococci, with a large proportion of Streptococcus faecalis, and Escherichia coli are commonly found in intestinal material but although a number may be pathologically significant they are relatively susceptible to sterilizing techniques. Even if they survive the manufacturing process, many would be killed by the alcohol in the final container. Sporing aerobes mostly nonpathogenic, such as Bacillus subtilis and its phage type, used to be known as B. mesentericus ruber, or the catgut bacillus, are very numerous, but the potentially dangerous organisms are Bacillus anthracis and the anaerobic spore bearing types, among them Clostridium sporogenes, C. tetani, and C. welchii. The spores of both aerobes and anaerobes are not easily killed, the aerobic types being generally more resistant. The fact that they revert to the spore form if conditions for growth are unsatisfactory further complicates the situation. The difficulty is exacerbated by the structure of the catgut strand where ribbons twisted together will carry any contamination throughout their whole cross section and at the centre of the string any organisms are well insulated. The successful means of attack on these microorganisms is by either chemicals (liquid or gaseous), heat or radiation. The choice of method must, however, be related to other desiderata. It is useless, for instance, to use a method to sterilize the material which will ruin its physical characteristics such as tensile strength and absorbability. Ideally, the aim will be to interfere as little as possible with the inherent structural advantages of collagen as a strong, flexible and elastic fibre that will be absorbed by the body in a surgically acceptable manner.
The three methods will now be discussed in further detail.
Chemical sterilization
The chemical method must employ a material which is sporicidal as distinct from bactericidal. Most inorganic or organic compounds in common use as antiseptics, disinfectants or bactericides are active against microorganisms in the vegetative phase. certain products such as the quaternary ammonium compounds are even more limited in that they are specific, i.e. they will kill Gram-positive organisms and leave Gram-negative types unaffected. Other preparations will be effective against most organisms but certain genera will not be killed and, most important of all, none of these compounds are sporicidal and are therefore useless in bringing about sterility in catgut.
The available sporicidal compounds are, in fact, relatively few. The main substances are formaldehyde, hydrogen peroxide, hypochlorite, glutaraldehyde, ethyl iodide, methyl bromide, iodine, ethylene oxide and β-propiolactone. Of these still fewer are suitable for commercial sterilization of catgut.
Histological techniques employing formalin introduced about 1893 led to its application to catgut sterilization by Cunningham (1895). Although a number of workers demonstrated that formaldehyde solutions of the order of 5 per cent for prolonged periods were necessary to destroy anthrax spores and that this treatment seriously affected catgut, the ‘formalin period’ lasted for about 15 years and there is little doubt that a great deal of unsterile material was used. The resulting catgut, moreover, was so hardened that its absorption in the body was considerably delayed and its tensile strength much reduced.
Hydrogen peroxide will effectively sterilize catgut but results in a material which is of poor quality. The compound has, however, sometimes been used to improve the colour of catgut by treating the collagen in the ribbon form, i.e. before spinning.
Solutions of sodium or potassium hypochlorites although sporicidal, particularly in acid solution, are not suitable for catgut as their penetrative power is very poor and the catgut is unduly swollen and of poor tensile strength.
Glutaraldehyde behaves very much in the same way and is only active in sodium bicarbonate buffered solutions. The solution only retains its sporicidal activity for about two weeks.
Iodine remained, as the only suitable chemical for large scale commercial sterilizing of catgut. Ethyl iodide and methyl bromide in alcohol and mixtures of iodine and iodine trichloride have also been used.
Basically the process consisted of immersion of standard lengths of catgut wound on frames or reels in a carefully standardized aqueous solution of iodine, potassium iodide and potassium iodate, the pH being rigidly controlled. The catgut must absorb about 12 per cent of its own weight of iodine and when this has taken place the catgut is transferred from the solution and excess iodine removed by sterile solvent often containing a bacteriostatic. The sterile strands are then transferred under aseptic conditions to their final container, sterile fluid is added (usually 95 per cent ethanol or 90 per cent isopropanol) and the container sealed. Processes involving ethyl iodide or iodine and iodine trichloride are carried out in alcoholic solution but in other respects the process is similar to the aqueous method.
Ethylene oxide may be employed as a sterilizing agent in the gaseous form or in solution. The important factors to be controlled are concentration, moisture time and temperature employed. It is an explosive gas and is usually supplied mixed with carbon dioxide or freon. The process is usually monitored with spore strips of B. subtilis var. niger (B. globigii). Care must be taken to limit the residual ethylene oxide in the suture material. It is usually necessary to carry out an aseptic procedure for filling and sealing the final container.
Heat Sterilization
Raw catgut contains 12–25 per cent by weight of combined moisture, depending on storage conditions. Heating above 80°C will result in hydrolysis of the collagen and the resulting gelatine or glue would render it useless for surgical purposes. All heat sterilizing processes, therefore, are based on the fundamental necessity of removing this combined moisture before the strings can be raised to sterilizing temperature of 150–165°C. The process varies with different manufacturers, but basically consists in inserting gauged standard lengths of coiled catgut into glass tubes which are then plugged, placed in ovens and the strings dehydrated by gradually raising the temperature. Baskets of tubes are then placed in a sterilizing chamber and the catgut covered with an organic fluid of high boiling point and the temperature raised to about 160°C.
Alternatively, the tubes are subjected to the vapour of the organic fluid usually toluene or xylol under pressure, the temperature again being held at about 160°C for a suitable time. Any condensed fluid is removed by a further heating period. Most variations in the methods have been aimed at reducing the fire and explosion hazards and at the same time producing a more acceptable catgut.
After sterilizing the tubes are allowed to cool and are then drained if necessary. The catgut at this stage is very brittle and could not be used in surgery. Under full aseptic conditions a suitably hydrated sterile alcoholic fluid is added in order to put back the necessary moisture and the tubes are sealed. This is the origin of the term tubing fluid, which is still used by many manufacturers even though the tube has been replaced with other forms of pack. After a few days the catgut is sufficiently rehydrated to be usable.
Boilable catgut: Although catgut in Great Britain is now all of the nonboilable type, up to a few years ago a boilable variety was prepared by some manufacturers. The term was used to indicate to operating theatre staff that the external surface of the tubes could be sterilized by boiling in water.
Boilable catgut was prepared by the heat sterilizing method outlined above but instead of adding a hydrated tubing fluid the strands were immersed in a nonaqueous compound such as toluene. The catgut, when removed from the tube, was hydrated by the operating theatre staff by soaking it in sterile saline or water some time before it was required for use.
Radiation Sterilization
Currently by far the most commonly employed method of sterilization for sutures is that of irradiation by electron particles or by gamma rays. More than 50 per cent of the suture companies in Europe use this process. Whilst the original commercial method in 1957 made use of electron machines, since 1961 most suture manufacturers now employ gamma radiation usually from cobalt 60 sources. The recognized sterilizing dose for sutures is 2.5 megarad. The method has the advantage that sterilization is effected in the final sealed container, indeed some manufacturers apply it to the complete sales package.
Reconstituted Collagen
The limitations on length imposed by the length of intestinal material combined with the natural biological variations in thickness and character have led manufacturers to search for many years for a means of overcoming these disadvantages. Collagen is available from a large number of sources. It is the major constituent of skin, tendon, ligament, etc. As a protein built up of some 11 amino acids, it is partially soluble in acids. The basic process has been to obtain an acidic solution of collagen prepared from hides or tendons which can be extruded into a coagulating solution and the resulting fibres oriented by stretching. The filaments can then either be spun or rolled to make up the necessary sizes of strand required. Reconstituted collagen is produced mainly in the finer sizes 0.5, 1 and 2 for ophthalmic and cuticular surgery.
Synthetic Absorbable Material
The search for evenness and continuous length has also been pursued among synthetic organic compounds. A number of patents have been obtained for filaments produced from the polymerization of certain lactides, hydroxy acids, etc. and two such products are gaining acceptance in surgery. The USP XIX describes absorbable surgical suture as ‘a sterile strand prepared from collagen derived from healthy mammals or from a synthetic polymer’.
Kangaroo Tendon
This absorbable material consists of the tail tendons of the wallaby. The tendons, which were usually preserved with naphthalene, were prepared and graded into various sizes, e.g. fine, medium, and stout. Lengths were 30–40cm. They were sterilized as for catgut and their main use was for hernia repair and bone surgery. This material is virtually unobtainable today.
Ribbon Gut
Ribbon gut as its name suggests is in the form of ribbon approximately 12mm wide and usually about 45cm long. Its use is limited but is preferred by some surgeons for the repair of large ventral herniae and in the closure of the kidney after nephrotomy. The material is prepared from bovine oesophagus and is sterilized in the same way as catgut.
Absorption of Catgut in the Body
Catgut is used in surgery because it is capable of being absorbed by animal and human tissues. This concept was propounded by Lister who recognized that the continued presence of a nonabsorbable material presented a focus for infection. The term absorption is often wrongly used to describe the loss of tensile strength of holding power of the suture in the wound and this is more rapid than the disappearance of the material itself. Formerly, terms such as 10 day, 20 day, 30 day and even 40 day were applied to catgut sutures. The basis for such claims was never stated and indeed in 1940 Holder showed that in the case of iodine sterilized catgut the terms were meaningless since the hardening effect of the iodine process was greater than that of any treatment applied to catgut. These descriptions are now obsolete although they occasionally occur in surgical papers. When the term 20 day is used it is taken to refer to the presentday ‘medium chromic material’. Medium chromic, now often abbreviated to ‘chromic’, or hardened material is catgut which has been subjected either in the ribbon form or as a string to a mild basic chrome tanning.
Lawrie (1955), working mainly with heat sterilized material implanted in the lumbar muscle of the rat, followed the rate of loss of tensile strength and evolved the concept of Half Strength Time (HST) which was the time required for the strength of the material to be reduced to 50 per cent of its original strength in vivo. Plain catgut was shown to possess a HST of 5–7 days and to reach zero strength in 3–4 weeks, while medium chromic catgut had a HST of 19 days and took about 5 weeks to reach zero strength.
The process which takes place in the body when catgut implants are made has been extensively studied by histological observation from the day after insertion of the material up to its complete disappearance in the tissue (Lawrie et al., 1959, 1960). It is undoubtedly the reaction of the tissues to the foreign body protein that brings about what is termed the absorption process. Although catgut prepared and sterilized by different methods tends to show different behaviour in the body, nevertheless the general histological picture is similar.
At first, around the site of the implant there is a fairly rapid gathering of polymorphonuclear leucocytes together with varying proportions of a fibrinous exudate. These cells are evident as early as one day after implantation of plain catgut which tends to excite a greater reaction in the tissue than chromicized material which does not begin to attract polymorph formations until up to 10 days after implantation. It should be understood that tissue reaction, i.e. inflammation, is the inevitable result of leucocytic concentrations (pus).
With plain catgut the polymorph phase begins to die down after about 5 days and there is an increasing accumulation of macrophages, by which time the catgut is considerably fragmented and any ultimate suture material is absorbed by phagocytosis.
With chromic catgut the mechanism is not necessarily exactly the same although with certain types of suture material very little difference can be observed. Where a difference is present the longer absorption usually involves fragmentation by phagocytes, histiocytes and foreign body giant cells. These observations do not, of course, explain why the catgut disappears and although a considerable amount of research has taken place, the actual proteolytic enzymes responsible have not been isolated. At present the leucocytes or histiocytes themselves are believed to carry their own proteolytic enzyme system, and at one time this was thought to be very closely related to α-chymotrypsin. The cellular reaction varies somewhat in different animals but the process in the rat is very similar to that which occurs in human tissue although absorption periods are somewhat longer. Very many attempts have been made by manufacturers of surgical catgut to try to relate the in vivo absorption of catgut to a standardized in vitro control. These latter methods have generally been based on exposing catgut to standard solutions of proteolytic enzymes such as trypsin, pepsin, papain, etc., and although the results obtained are of some practical use, it has not been possible to incorporate them into a reliable system of evaluation.
Nonabsorbable Sutures
Silk
Silk consists of strands prepared from filaments of the cocoon spun by the silkworm of the Bombyx family before it enters the chrysalis stage. Three forms are used in surgery—twisted (sometimes known as Chinese twist), floss and plaited or braided silk. Silk in its natural state contains up to 25 per cent of natural gum and strands prepared from this are described as undischarged. Twisted silk suture material is prepared from unbleached, undischarged filaments spun in multiples to the British Pharmaceutical Codex range of diameters and may be dyed with nontoxic dyestuffs. The surgical use of twisted silk has very much declined in favour of the braided type.
Floss silk is prepared from the coarser filaments on the outer surface of the silkworm cocoon and is used in its spun glossy white form mainly in the repair of herniae. Its use is diminishing fast as the plastic polymer meshes gain in popularity.
Plaited or braided silk is the material in large-scale use in modern surgery. It is prepared from discharged silk and the range of sizes is dependent on the number of strands braided together. As the gum has been removed it is not serum proof or noncapillary and for most surgical purposes it is therefore treated with proofing waxes or silicones.
Silk is identified chemically by warming with mercuric nitrate solution when a brick-red colour is produced and it is stained yellow by trinitrophenol solution. Silk, because of its strength, softness and general ease of handling is used in many sites of surgical operation, the fine strands being particularly suited to ophthalmic and neurosurgery. Silk sutures are classified as nonabsorbable in the body and are normally encapsulated by fibrous tissue. However, many cases have been reported where after a considerable length of time the silk has fragmented or even migrated from the original site of implantation.
Silk is sterilized either by autoclaving, which causes a certain loss in its tensile strength, or by radiation or ethylene oxide sterilization.
It is normally supplied by suture manufacturers to comply with the monographs published in the British Pharmaceutical Codex.
Linen
Linen sutures consist of selected fibres made into a twisted strand from flax (Linum usitatissimum). The strand is normally prepared by spinning three cords together, the size of the cords being chosen to produce the ultimate desired gauge of thread. For surgical use it must be firmly and evenly spun and free from fuzziness. Identification is by microscopic examination.
Linen may be dyed with any nontoxic dyestuff but although a certain amount of black thread is used the majority of surgeons prefer off-white or ivory colour.
It is extensively used in many surgical techniques and frequently needs to be rendered noncapillary and serum proof by treatment with suitable proofing agents similarly to braided silk.
It can be sterilized by autoclaving or ethylene oxide. Radiation sterilization does however cause a considerable loss of strength.
It is the subject of a monograph in the British Pharmaceutical Codex to which all British manufacturers conform.
Polyamides
In the UK these polymers are better known by the word nylon but as this is a registered trade mark in certain European countries, it is likely that the word polyamide will be used in the future. These compounds are formed from the polymerization of the reaction product of an acid and an amine. Hitherto, they have been known by suffixing the word nylon with a number, e.g. Nylon 6, Nylon 66, Nylon, 10, 11, 12, etc. Polyamide 66 is formed by the combination of hexamethylenediamine and adipic acid. Polyamide 6 is formed by the polymerization of caprolactam.
All the polyamides and suture materials are produced by an extrusion process, the size of the orifice on the extruder head determining the size of the filament. The bulk of the material used in surgery is produced in the form of monofilament. Its main use is in skin suturing although it is sometimes used internally. Polyamide mesh finds a use in hernia repair.
Finer filaments of polyamide are braided together to form braided nylon on nonabsorbable surgical sutures. Monofilament polyamide is normally coloured with distinctive nontoxic dye stuffs or pigments in order to improve its visibility. It may be sterilized by autoclaving, by ethylene oxide or by radiation treatment, but is incompatible with phenol and its homologues and other phenolic substances. The knotting of polyamide requires a special knot (Fig. 40.5).
Polyester
This suture material is usually prepared in the plaited or braided form and consists of filaments prepared by polymerizing the ester formed by a combination of ethylene glycol and terephthalic acid. In its commercial form it is known under the trade marks Terylene (ICI) and Dacron (Du Pont). The number of filaments in the braid determines the size of the completed strand.
The polymer has a softening temperature of not less than 255°C and may be sterilized by autoclaving, ethylene oxide or radiation treatments. In order to improve its visibility in tissue it is often dyed or pigmented with nontoxic materials.
Stainless Steel Wire
Stainless steel wire has largely replaced the various wires which have been used in surgery in past years, such as silver, tantalum, phosphor bronze, etc. It is supplied for surgical purposes in three forms, namely, monofilament, twisted and plaited or braided. The British Pharmaceutical Codex includes monographs on all three types and lays down standards for diameter and tensile strength.
In all three cases the wire is prepared from austenitic chromium–nickel stainless steel and is fully annealed. The austenitic steels are nonmagnetic and only capable of being hardened by cold working. The wire is made to British Standards Specification 4106:1967.
It may be sterilized by dry heat, autoclaving, ethylene oxide or radiation but it is important that the wire should be fully degreased when heat treatment is used.
Its main use is in orthopaedic work, but very fine wire is also used in plastic surgery and for repair of tendons.
Wire Staples
A considerable amount of experimental and clinical work is now being carried out, particularly in the USSR and the USA, on the use of very small wire staples, usually of stainless steel. The field of investigation is very wide and papers have been published on their use in orthopaedic, cardiovascular and arterial work and in the USSR. The results of many thousands of cases of gastric resection have been reported.
Surgical Needles
Whenever a suture is required to close a wound, needles to arm the suture material are necessary. The number of sizes and the variety of different shapes of needles run into many thousands. The range supplied by manufacturers is usually classified into types for specific surgery, as for example, arterial, general purpose, intestinal, obstetric, ophthalmic, plastic and retention. Shapes are designated as: straight, curved, 1/2-circle, 5/8-circle, etc., and the sections as round bodied, triangular cutting edge, triangular reverse cutting, cutting point and trocar point. In addition the length of the needle from point to hilt is specified in millimetres and the hilts are designed to take the various diameters of suture material employed. Needles are chosen by the surgeon to suit his operating technique and it is largely because of the surgeons interest in improving suture efficiency that the development of surgical needles has taken place. In very many cases the surgeon himself has caused needles to be made to his own design and a large number of commonly used needles are referred to by the name of the inventing surgeon.
Surgical needles are of two types; those which have an eye through which the suture is threaded, and eyeless needles where the suture is inserted into the hollow hilt and held in position by swaging the metal around it. The first patent on an eyeless needle was obtained by the late Sir Henry Souttar in 1921, but in the UK their use has only increased appreciably in the past few years. In the USA 70 per cent of the suture needles are of the eyeless type.
The needles themselves are made either of stainless steel or of carbon steel, the latter usually being plated to resist corrosion. At one time the carbon steel needle had a better resistance to bending than its counterpart in stainless steel, but recent technological advances have improved stainless steel needles and they now compete in strength with carbon steel and have the added advantage that they will not corrode and do not present the surgeon with the dreaded emergency of having to locate and extract the broken point of a needle embedded somewhere in the tissues of an unfortunate patient.
References
Bulloch W, Lampitt LH, Bushill JH. The Preparation of Catgut for Surgical Use. 1929. Medical Research Council Special Report, No. 138
Cunningham RH. New York Med. J. 1895;61:494.
Dawson JO, Roylance TW, Smith T. J. Pharm. Pharmacol. 1964.(16):121T.
Fandre A. Le Catgut. Paris: Masson et Cie; 1944.
Holder EJ. Desirable Factors in Surgical Sutures. William Blackwood & Sons Ltd: Edinburgh; 1946.
Lawrie P. Studies in the Absorption of Surgical Catgut. William Blackwood & Sons Ltd: Edinburgh; 1955.
Lawrif P, Angus EA, Reese AJM. Br. J. Surg. 1959;46:638.
Lawrif P, Angus EA, Reese AJM. Br. J. Surg. 1960;47:551.
Lister J. Lancet,. 1869;i:451.
Mackie TJ. An Inquiry into Post-operative Tetanus—A Rreport to the Sscottish Board of Health. 1928.