Chapter 20
Drugs Containing Tannins
20.1 Introduction
The name ‘tannin’ is derived from the French ‘tanin’ (tanning substance) and is used for a range of natural polyphenols. Tannins are complex organic, nonnitrogenous plant products, which generally have astringent properties. These compounds comprise a large group of compounds that are widely distributed in the plant kingdom. The term ‘tannin’ was first used by Seguin in 1796 to denote substances which have the ability to combine with animal hides to convert them into leather which is known as tanning of the hide. According to this, tannins are substances which are detected by a tanning test due to its absorption on standard hide powder. The test is known as Goldbeater’s skin test.
20.2 Classification
The tannin compounds can be divided into two major groups on the basis of Goldbeater’s skin test. A group of tannins showing the positive tanning test may be regarded as true tannins, whereas those, which are partly retained by the hide powder and fail to give the test, are called as pseudotannins.
Most of the true tannins are high molecular weight compounds. These compounds are complex polyphenolics, which are produced by polymerization of simple polyphenols. They may form complex glycosides or remains as such which may be observed by their typical hydrolytic reaction with the mineral acids and enzymes. Two major chemical classes of tannins are usually recognized based on this hydrolytic reaction and nature of phenolic nuclei involved in the tannins structure. The first class is referred to as hydrolysable tannins, whereas the other class is termed as condensed tannins.
Hydrolysable Tannins
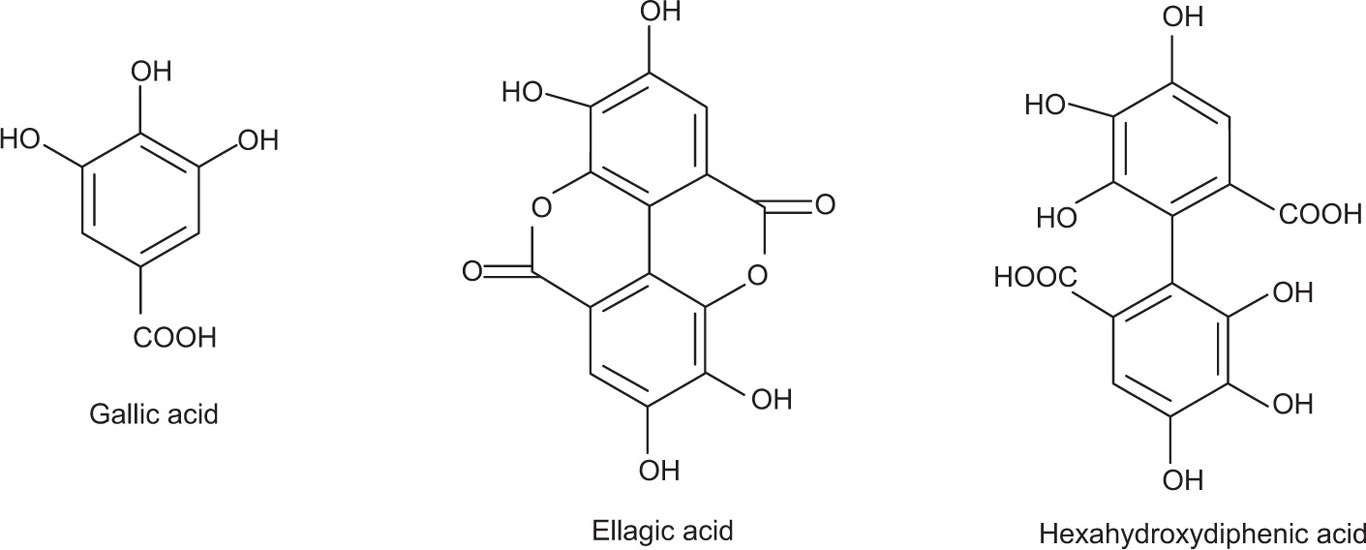
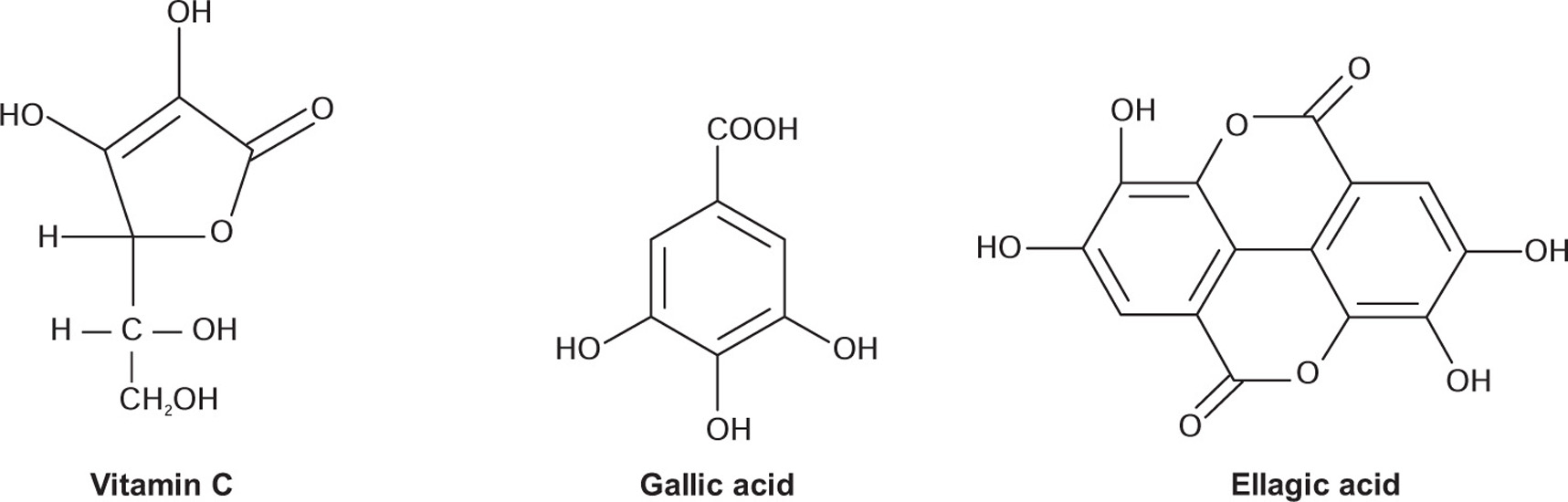
As the name implies, these tannins are hydrolysable by mineral acids or enzymes such as tannase. Their structures involve several molecules of polyphenolic acids such as gallic, hexahydrodiphenic, or ellagic acids, bounded through ester linkages to a central glucose molecule. On the basis of the phenolic acids produced after the hydrolysis, they are further categorized under gallotannins composed of gallic acid or ellagitannins which contains hexahydrodiphenic acid which after intraesterification produces ellagic acid.
Nonhydrolysable or Condensed Tannins
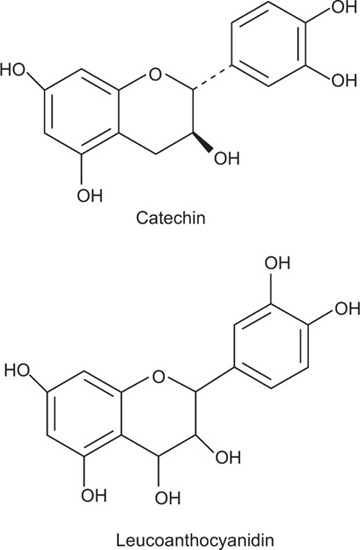
Condensed tannins, unlike the previously explained group are not readily hydrolysable to simpler molecules with mineral acids and enzymes, thus they are also referred to as nonhydrolysable tannins. The term proanthocyanidins is sometimes alternatively used for these tannins. The compounds containing condensed tannins contain only phenolic nuclei which are biosynthetically related to flavonoids. Catechin which is found in tannins is flavan-3-o1, whereas leucoanthocyanidins are flavan-3,4-diol structures. These phenolics are frequently linked to carbohydrates or protein molecules to produce more complex tannin compounds. When treated with acids or enzymes, they tend to polymerize yielding insoluble red coloured products known as phlobaphens. The phlobaphens give characteristic red colour to many drugs such as cinchona and wild cherry bark. On dry distillation, they yield catechol derivatives. Condensed tannins are also soluble in water and produces green colour with ferric chloride.
The families of the plants rich in both of the above groups of tannins include Rosaceae, Geraniaceae, Leguminosae, Combretaceae, Rubiaceae, Polygonaceae, Theaceae, etc. The members of families Cruciferae and Papaveraceae on the other hand are totally devoid of tannins. In the plants in which tannins are present, they exert an inhibitory effect on many enzymes due to their nature of protein precipitation and therefore contribute a protective function in barks and heartwood.
Pseudotannins
Pseudotannins are simple phenolic compounds of lower molecular weight. They do not respond to the tanning reaction of Goldbeater’s skin test. Gallic acid, Chlorogenic acid, or the simple phenolics such as catechin are pseudotannins which are abundantly found in plants, especially in dead tissues and dying cells.
20.3 Characteristics of Tannins
1. Tannins are colloidal solutions with water.
2. Non crystalline substance.
3. Soluble in water (exception of some high molecular weight structures), alcohol, dilute alkali, and glycerin.
4. Sparingly soluble in ethyl acetate.
5. Insoluble in organic solvents, except acetone.
6. Molecular weight ranging from 500 to >20,000.
7. Oligomeric compounds with multiple structure units with free phenolic groups.
8. Can bind with proteins and form insoluble or soluble tannin—protein complexes.
20.4 Biosynthesis of Tannins
Tannins belong to the phenolics class of secondary metabolites. All phenolic compounds; either primary or secondary are in one way or another formed through shikirnic acid pathway (phenylpropanoid pathway). Other phenolics such as isoflavones, coumarins, lignins, and aromatic amino acids (tryptophan, phenylalanine, and tyrosine) are also formed by the same pathway. Hydrolysable tannins (Hts) and condensed tannins (proanthocyanidins) are the two main categories of tannins that impact animal nutrition.
Common tannins are formed as follows:
• Gallic acid is derived from quinic acid.
• Ellagotannins are formed from hexahydroxydiphenic acid esters by the oxidative coupling of neighbouring gallic acid units attached to a D-glucose core.
• Further oxidative coupling forms the hydrolysable tannin polymers.
• Proanthocyanidin (PA) biosynthetic precursors are the leucocyanidins (flavan-3,4-diol and flavan-4-ol) which on autoxidation, in the absence of heat, form anthocyanidin and 3-deoxyanthocianidin, which, in turn, polymerize to form PAs.
20.5 Chemical Tests
1. Goldbeater’s skin test: Goldbeater’s skin is a membrane produced from the intestine of Ox. It behaves just like untanned animal hide. A piece of goldbeaters skin previously soaked in 2% hydrochloric acid and washed with distilled water is placed in a solution of tannin for 5 minutes. It is then washed with distilled water and transferred to 1% ferrous sulphate solution. A change of the colour of the goldbeater’s skin to brown or black indicates the presence of tannin. Hydrolysable and condensed tannins both give the positive goldbeater’s test, whereas pseudotannins show very little colour or negative test.
2. Phenazone Test: To 5ml of aqueous solution of tannin containing drug, add 0.5g of sodium acid phosphate. Warm the solution, cool, and filter. Add 2% phenazone solution to the filtrate. All tannins are precipitated as bulky, coloured precipitate.
3. Gelatin Test: To a 1% gelatine solution, add little 10% sodium chloride. If a 1% solution of tannin is added to the gelatine solution, tannins cause precipitation of gelatine from solution.
4. Test for Catechin (Matchstick Test): Catechin test is the modification of the well-known phloroglucinol test for lignin. Matchstick contains lignin. Dip a matchstick in the dilute extract of the drug, dry, moisten it with concentrated hydrochloric acid, and warm it near a flame. Catechin in the presence of acid produces phloroglucinol which stains the lignified wood pink or red.
5. Test for chlorogenic acid: A dilute solution of chlorogenic acid containing extract, if treated with aqueous ammonia and exposed to air, slowly turns green indicating the presence of chlorogenic acid.
6. Vanillin-hydrochloric acid test: Drug shows pink or red colour with a mixture of vanillin: alcohol : dilute HCl in the ratio 1:10:10. The reaction produces phloroglucinol which along with vanillin gives pink or red colour.
20.6 Isolation
Both hydrolysable and condensed tannins are highly soluble in water and alcohol but insoluble in organic solvents such as solvent ether, chloroform, and benzene. Tannin compounds can be easily extracted by water or alcohol. The general method for the extraction of tannic acid from various galls is either with water-saturated ether, or with mixture of water, alcohol, and ether. In such cases, free acids such as Gallic and ellagic acid go along with ether, whereas true tannin gets extracted in water. If the drug consists of chlorophyll or pigment, it may be removed by ether. After extraction, the aqueous and ethereal layers are separately concentrated, dried, and subjected to further isolation and purification using various separation techniques of chromatography.
20.7 Medicinal Properties and Uses
Tannins occur in crude drugs either as major active constituent as in oak bark, hammamelis leaves, and bearberry leaves, etc. or as a subsidiary component as in clove, cinnamon, peppermint, or garden sage. In many cases, they synergistically increase the effectiveness of active principles. Tannins are medicinally significant due to their astringent properties. They promote rapid healing and the formation of new tissues on wounds and inflamed mucosa. Tannins are used in the treatment of varicose ulcers, haemorrhoids, minor burns, frostbite, as well as inflammation of gums. Internally tannins are administered in cases of diarrhoea, intestinal catarrh, and in cases of heavy metal poisoning as an antidote. In recent years, these compounds have demonstrated their antiviral activities for treatment of viral diseases including AIDS. Tannins are used as mordant in dyeing, manufacture of ink, sizing paper and silk, and for printing fabrics. It is used along with gelatine and albumin for manufacture of imitation horn and tortoise shell. They are widely used in the leather industry for conversion of hide into leather, the process being known as tanning. Tannins are also used for clarifying beer or wine, in photography or as a coagulant in rubber manufacture. Tannins are used for the manufacture of gallic acid and pyrogallol, and sometimes as a reagent in analytical chemistry.
20.8 Hydrolysable Tannins
Myrobalan
Biological Sources
Myrobalan is the mature dried fruits of Terminalia chebula, belonging to family Combretaceae.
Geographical Source
Myrobalan trees are found at an elevation of 300 to 900m in North India, Satpura ranges of Madhya Pradesh, Maharashtra, and Panchamahal district in Gujarat. It is also found in Myanmar and Sri Lanka.
Collection and Preparation
T. chebula is a moderate-sized or large deciduous tree attaining a height of 25–30m. The plant lacks natural regeneration. The plant requires direct overhead light and cannot tolerate shady situations. It is a frost and draught resistant tree. The fruits ripen from November to March depending upon the locality, and fall soon after ripening. The mature fruits are collected from January to April by shaking the trees, and then drying by spreading in thin layers preferably in shades. The dried myrobalan fruits are graded under different trade names. Gradation is done on the basis of fruits colour, solidness, and freedom from insect attack.
Characteristics
| Colour | Yellowish brown to brown |
| Odour | Slight odour |
| Taste | Mucilaginous |
| Shape | Astringent and slightly bitter |
| Size | 2 to 3cm long and 1.5 to 3cm wide |
| Solubility | Ovate with longitudinal wrinkles |
| Extra features | Fruits are drupe. It is hard and stony with four to six longitudinal ribs. Seeds are pale yellow in colour and 1.6 to 2.3cm long |
Fig. 20.1 Terminalia chebula

Fig. 20.2 Myrobalan fruits
Chemical Constituents
Myrobalan contains about 30% of the hydrolysable tannins, which consists of chebulinic acid, chebulagic acid and D-galloyl glucose. It contains free tannic acid, gallic acid, ellagic acid, and resin myrobalanin. Anthraquinone glycosides, sennosides have been reported in myrobalan.
Uses
Myrobalan is reputed in Indian system of medicine as a drug for various types of diseases. Because of antiseptic and healing properties of tannins, it is used externally in chronic ulcers, wounds, piles, and as stomachic. It is one of the drugs of the well-known preparation ‘Triphala’. It has purgative properties. Fine powder of myrobalan is used in dental preparations. Commercially, it is used in dyeing and tanning industry and also in treatment of water used for locomotives.
Marketed Products
It is one of the ingredients of the preparation known as Constivac (Lupin Herbal Laboratory), a bowel regulator and relieves constipation. Also, it is one of the ingredients of the preparations known as Pilect (Aimil Pharmaceuticals), Abana, Bonnisan, Gariforte, Koflet, Menosan (Himalaya Drug Company), Haritakh churna, Triphala churna, Tentex forte (Baidyanath Company).
Bahera
Biological Source
It consists of dried ripe fruits of the plant Terminalia belerica Linn, belonging to family Combretaceae.
Cultivation and Collection
Cultivation of the drug, though not done on commercial scale, can be carried out by sowing the seeds. The seeds can retain the viability for a year and their rate of germination is about 80%. The plant can also be raised by transplantation. It takes about 15 to 30 days for germination of seed. Maximum height of the plant is about 40m and the girth is 2 to 3m. The stem of the plant is straight the leaves are broadly elliptic and clustered towards the end of the branches. Flowers are simple, solitary and in auxiliary spikes.
Morphology
| Colour | Fruits are dark brown to black |
| Odour | None |
| Taste | Astringent |
| Shape | Strips, flakes or coarse powder |
| Size | 1.3 to 2cm in length. |
| Shape | Fruits are globular and obscurely five-angled |
Fig. 20.3 Terminalia belerica
Microscopy
Transverse section shows an outer epicarp consisting of a layer of epidermis, most of the epidermal cells elongate to form hair like protuberance with swollen base; next to epidermis it contains a zone of parenchymatous cells, slightly tangentially elongated and irregularly arranged. Stone cells of varying shape and size are present in between these parenchymatous cells. Mesocarp traversed in various directions by numerous vascular bundles collateral, endarch; simple starch grains and rosettes of calcium oxalate crystals are present in parenchymatous cells.
Chemical Constituents
The fruits contain about 20 to 30% of tannins and 40 to 45% water-soluble extractives. It contains colouring matter. It contains gallic acid, ellagic acid, phyllemblin, ethyl gallate, and galloyl glucose. The seeds contain nonedible oil. The plant produces a gum. It also contains most of the sugars as reported in myrobalan.
Uses
Bahera is used as an astringent and in the treatment of dyspepsia and diarrhoea. It is a constituent of triphala. The purgative property of half ripe fruit is due to the presence of fixed oil. The oil on hydrolysis yields an irritant recipe. Gum is used as a demulcent and purgative. Oil is used for the manufacture of soap.
Arjuna
Biological Source
Arjuna consists of dried stem bark of the plant known as Terminalia arjuna Rob, belonging to family Combretaceae.
Geographical Source
The tree is common in Indian peninsula. It is grown by the side of streams and very common in Chotta Nagpur region.
Cultivation and Collection
Arjuna is found as naturally growing plant in the dense forests. It is very common in Baitul in Madhya Pradesh and also in Dehradun. Arjuna can be successfully raised by sowing seeds or by means of stumps. The seeds take about 21 days for germination. It needs moist fertile alluvial loam and rainfall in the range of 75 to 190cm. It grows satisfactorily up to 45°C. The bark is also collected from wild growing plants, and it is reported that yield per tree varies from 9 to 55kg.
Morphology
| Colour | Colour of the outer side, as well as, inner side of bark is greyish-brown. |
| Odour | None |
| Taste | Astringent |
| Shape | Flats |
| Size | The pieces of various-sizes, about 15×10×1cm |
| Extra features | The presence of the cork is not reported in the commercial drug. As arjuna is collected from the old trees, the cork gets removed due to exfoliation. The appearance of the transversely cut surface is dark brown with characteristic greyish shining patches. |
Fig. 20.4 Terminalia arjuna
Microscopy
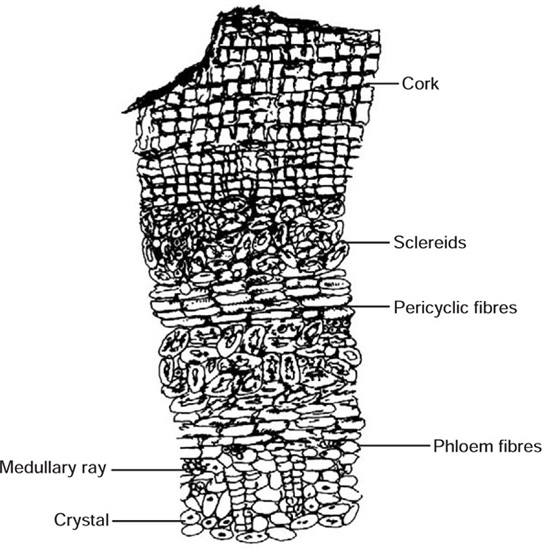
Transverse section of fresh bark shows cork composed of uniformly arranged several layers of small, tangentially elongated cells. Below cork is a region of cortex, composed of thin-walled, more or less brick-shaped parenchymatous cells containing cluster crystals of calcium oxalate. A few groups of sclerenchymatous pericyclic fibres are scattered in the cortex. Secondary phloem consists of phloem parenchyma composed of thin-walled, polygonal cells with wavy walls containing cluster crystals of calcium oxalate and pigmented cells. Phloem fibres, composed of sclerenchymatous cells, occur in groups and are scattered in the form of patches in parenchyma. Narrow and almost straight medullary rays are also present.
Fig. 20.5 Morphology of Arjuna bark
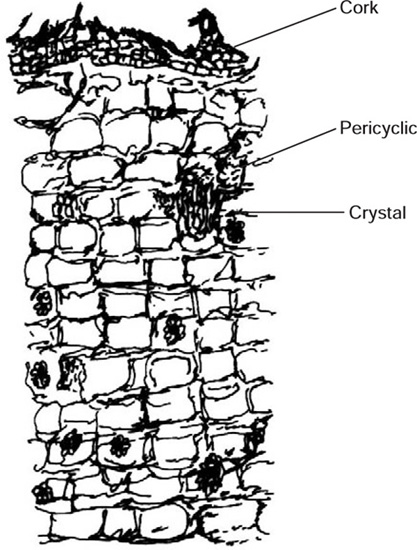
Fig. 20.6 T.S. of the outer part of the bark
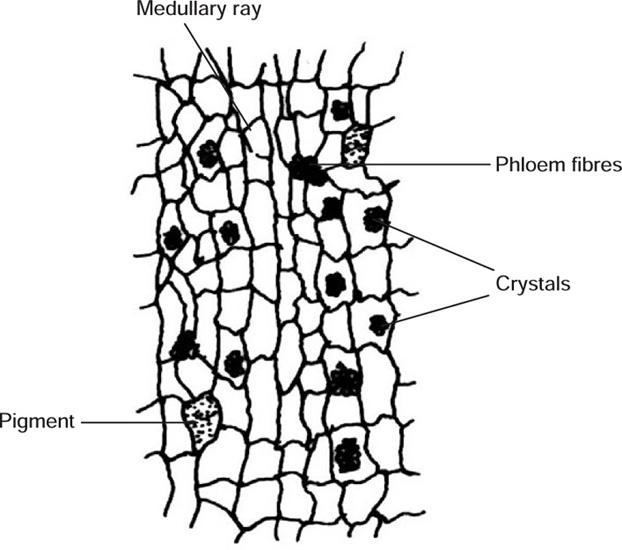
Fig. 20.7 T.S. of the inner part of bark
Chemical Constituents
The dry bark from the stem contains about 20 to 24% of tannin, whereas that of the bark obtained from the lower branches is up to 15 to 18%. The tannins present in arjuna bark are of mixed type consisting of both hydrolysable and condensed tannins. The tannins are reported to be present are (+) catechol, (+) gallocatechol, epicatechol, epigallocatechol, and ellgic acid. The flavonoids such as arjunolone, arjunone, and baicalein have been reported from the stem bark. The triterpenoid compounds arjunetin, arjungenin, arjunglucoside I and II, and terminoic acid have also been reported from the bark. The root contains number of triterpenoids such as arjunoside I and II, terminic acid, oleanolic acid, arjunic acid, arjunolic acid, etc. The fruits also contain 7 to 20% of tannins. A pentacyclic triterpenic glycoside arjunoglucoside III has been reported from the fruits along with hentriacontane, myristyl oleate and arachidic stearate.
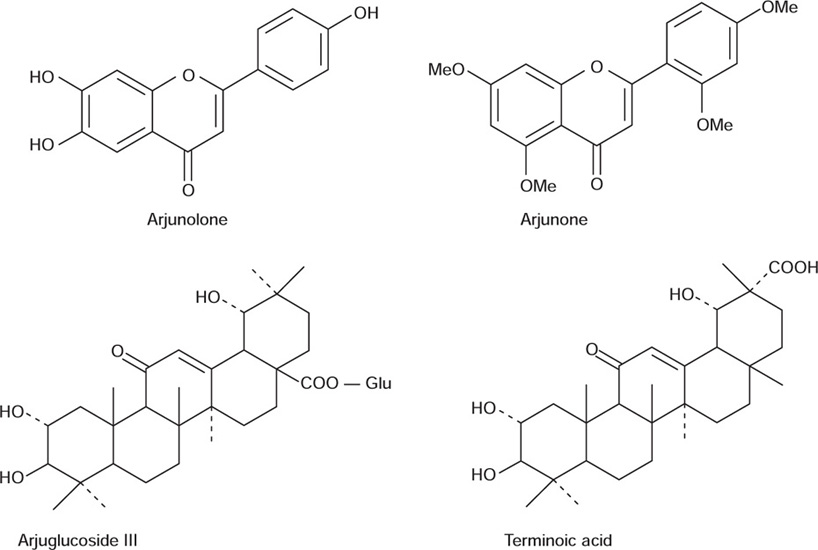
Uses
Arjuna bark is used as a diuretic and astringent. The diuretic properties can be attributed to the triterpenoids present in fruits. It causes decrease in blood pressure and heart rate. It is used in the treatment of various heart diseases in indigenous systems of medicines. The bark was extensively used in the past by the local tanneries for tanning animal hides. It yields a very firm leather of a colour which is similar babool tanned leather.
Amla
Biological Source
This consists of dried, as well as fresh fruits of the plant Emblica officinalis Gaerth (Phyllanthus emblica Linn.), belonging to family Euphorbiaceae.
Geographical Source
It is a small- or medium-sized tree found in all deciduous forests of India. It is also found in Sri Lanka and Myanmar. The leaves are feathery with small oblong pinnately arranged leaflets. The tree is characteristic greenish-grey and with smooth bark.
Cultivation and Collection
It is grown by seed germination. It can also be propagated by budding or cutting. It does not tolerate the frost or drought. It is normally found up to an altitude of 1500m. Commercially, it is collected from wild-grown plants.
Nowadays, the newly released varieties are selected for better yield. These are known as Banarasi, Kanchan, Anand-2, Balwant, NA6, NA7 and B5-1. Seeds or seedlings are placed at a distance of 4.5×4.5 meters in red loamy or coarse gravely soil. Proper arrangement for irrigation is required, Drip irrigation is most suitable. Fertilizers in the dose range of 750–900gm of urea, 1kg superphosphate, and 1 to 1.5kg of potash per annum depending upon the quality of soil are sufficient. The above dose is divided into two equal parts, one part is applied in September/October, whereas the other in April to May every year. Pruning is done regularly and only four to six branches about 0.75 to 1.0 meter above the ground are retained. Plant bears male and female flowers separately. Male flowers are reported in the axil of the leaf, in bunches, whereas female flowers in the axil of the branches are solitary. The extent of fertilization is 25–30% of flowers. Cultivated plants bear comparatively large fruits. The tree flowers in hot season and the fruits ripen during the winter.
Morphology
| Colour | Green changing to light yellow or brick red when matured. |
| Odour | None |
| Taste | Sore and astringent. |
| Shape | The fruits are depressed, globose. |
| Size | 1.5 to 2.5cm in diameter. |
| Extra features | Fruits are fleshy obscurely four-lobed with 6-trygonus seeds. They are very hard and smooth in appearance. |
Fig. 20.8 Twig of Emblica officinalis
Microscopy
Fruit shows an epicarp consisting of epidermis with a thick cuticle and two to four layers of hypodermis; the cells in hypodermis is tangentially elongated, thick-walled, smaller in dimension than epidermal cells; mesocarp consists of thin-walled isodiametric parenchymatous cells; several collateral fibrovascular bundles scattered throughout mesocarp; xylem composed of tracheal elements, fibre tracheids and xylem fibres; tracheal elements, show reticulate, scalariform, and spiral thickenings; mesocarp also contains large aggregates of numerous irregular silica crystals.
Chemical Constituents
It is highly nutritious and is an important dietary source of vitamin C, minerals, and amino acids. The edible fruit tissue contains protein concentration 3-fold and ascorbic acid concentration 160-fold compared to that of the apple. The fruit also contains considerably higher concentration of most minerals and amino acids than apples. The pulpy portion of fruit, dried and freed from the nuts contains: gallic acid 1.32%, tannin, sugar 36.10%; gum 13.75%; albumin 13.08%; crude cellulose 17.08%; mineral matter 4.12%; and moisture 3.83%. Tannins are the mixture of gallic acid, ellagic acid, and phyllembin. The alkaloidal constituents such as phyllantidine and phyllantine have also been reported in the fruits. An immature fruit contains indolacetic acid and four other auxins—a1, a3, a4, and a5 and two growth inhibitors R1 and R2.
Chemical Tests
1. Alcoholic or aqueous extract of the drug gives blue colour with ferric chloride solution.
2. To aqueous extract add gelatine and sodium chloride milky white colour is produced.
3. To the aqueous extract of amla add lead acetate remove precipitate by filtration. To the filtrate add solution of 2:6 dichlorophenol—indophenol, colour disappears.
Uses
The fruits are diuretic, acrid, cooling, refrigerant, and laxative. Dried fruit is useful in haemorrhage, diarrhoea, diabetes, and dysentery. They are useful in the disorders associated with the digestive system and are also prescribed in the treatment of jaundice and coughs. It has antioxidant, antibacterial, antifungal, and. antiviral activities. Amla is one of the three ingredients of the famous ayurvedic preparation, triphala, which is given to treat chronic dysentery, bilousness, and other disorders, and it is also an ingredient in chyavanprash.
Nutgalls
Biological Source
Nutgall consists of the pathological outgrowth obtained from the young twigs of the dyers oak, Quercus infectoria Olivier, belonging to family Fagaceae. Outgrowth is caused by the puncture of ovums of insect Cynips tinctoria or Adleria gallaetinctoriae Olivier Family Cynipidae.
Geographical Source
Oak galls are obtained principally from Asiatic Turkey. Dyers oak is found in Turkey, Syria, Iran, Cyprus, and Greece.
Collection and Preparation
Larvae of the insect C. tinctoria after emerging from the eggs, pierces the delicate epidermis near the growing point of the twigs where the eggs are deposited by the insect. The gall begins to enlarge, when the chrysalis stage is reached, starch disappears from the neighbourhood of insect and is replaced by gallic acid, whereas central cells consist of tannic acid. The insect passes through the larval and pupal stages. If the galls are not collected and dried at this stage the mature insect comes out of the gall and escapes, and during this stage galls changes the colour from a bluish grey, through olive-green to almost white. After the escape of the insect, a central cavity is formed, and the tannic acid is oxidized in the presence of moisture and air. The more porous gall is the white gall of commerce.
In Asiatic Turkey, galls are collected before the escape of the insect in the months of August and September. After drying, they are sorted out according to colour into three grades, that is, blue, green, and white and exported.
Characteristics
| Colour | Brown to greenish black or yellow |
| Odour | Odourless |
| Taste | Astringent |
| Shape | Round or globular |
| Size | 1 to 3cm in diameter |
Fig. 20.9 Twigs of Quercus infectoria
Microscopy
A transverse section through a nutgall show thin walled parenchymatous outer zone, which is quite larger as compared to inner zone. Parenchyma is followed by a ring of sclerenchyma composed of one or two layers of suberised cells. Inner zone is made up of thick walled parenchyma, which surrounds central cavity. Cells of parenchyma show the presence of numerous starch grains, calcium oxalate clusters and rosettes and tannins. Parenchyma also shows the bodies of lignified tissues, which stains with phloroglucinol and hydrochloric acid.
Chemical Constituents
Nutgalls contains about 50–70% tannin mainly gallotannic acid which is official tannic acid. It also consists of 2–4% gallic acid, ellagic acid, sitosterol, methyl belulate and methyl oleanolate which are methyl esters of betulic and oleanolic acid. Recently few more compounds such as Nyctanthic acid, roburic acid, and syringic acids have been reported from galls. It contains abundant starch.
Tannic acid of commerce is a hydrolysable tannin which yields gallic acid and glucose. The molecule of tannic acid may contain the gallic acid up to pentagalloyl glucose. It is isolated by fermentation and subsequent extraction of galls with water-saturated ether.
Allied Drugs
Various types of galls are produced on plants by insects of the genera Cynips and Aphis. Chinese and Japanese galls are of commercial interest. These galls are formed on Rhus chinensis Mill, family Anacardiaceae by an aphis, Schlectendalia chinensis. These galls are knoty, grey, irregular and breaks easily to show irregular cavities. They contain 57–77% of tannins. These drugs have been used in China and Japan since time immemorial as astringent and styptic.
20.9 Tannic Acid
Tannic acid is not a single constituent but a type of hydrolysable tannin that contains several units of gallic or ellagic acids esterified with the glucosyl OH to produce complex tannin compounds. Its exact composition varies according to its source. Turkish galls have a maximum complexity of hexa or heptagalloyl glucose, whereas Chinese galls are octa or nonagalloyl glucose, which affords methylgallate and pentagalloyl glucose on hydrolysis.
Tannic acid is extracted with a mixture of water, alcohol, and ether. The extracted liquid separates into two layers. The aqueous lower layer contains gallotannins, whereas the ethereal layer contains free gallic acid and other similar compounds. Aqueous and etheral extracts are treated separately for further purification.
Tannic acid occurs as amorphous powder containing brownish spongy masses. It has a faint odour and strong astringent taste. It is soluble in water, alcohol, and acetone but insoluble in organic solvents.
Tannic acid has strong astringent properties. It is used as an antidote in cases of alkaloidal poisoning as it precipitates alkaloids as tannate salts. It finds its uses in tanning, dyeing industries and for ink manufacture. Its preparation can be used topically for the treatment of bedsores and minor ulcerations. It is utilized in the laboratory as a reagent for detection of gelatine and proteins.
20.10 Condensed Tannins
Ashoka
Biological Source
Ashoka consists of dried stem bark of the plant Saraca indica Linn., belonging to family Leguminosae.
Geographical Source
It is distributed in South Asia, that is, in Malaysia, Indonesia, Sri Lanka, and India.
Cultivation and Collection
It is one of the most sacred trees of the Hindus. It is frequently grown as an ornamental and avenue tree in India. It is not found to be cultivated on commercial scale. It can be easily propagated from seeds. It is found growing suitably at an altitude of 750m in the Himalayas, Khasi, Garo, and Lushai hills. It is an evergreen tree, bearing dark red-coloured flowers reaching a maximum height of 9m. Bark is collected from the plant by making transverse and longitudinal incisions.
Morphology
| Colour | Outer side is dark brown or almost black with warty surface. Internally, it is reddish-brown with fine longitudinal striations. |
| Odour | None |
| Taste | Astringent and bitter |
| Shape | It occurs in the form of channels of various sizes |
| Size | up to 50cm length and 1cm in thickness. |
| Extra features | The bark is marked by bluish and ash white patches of lichens. |
Microscopy
Transverse section of bark shows cork cells, cork cambium, and phelloderm constituting periderm of bark. Pericycle is composed of sclereids (stone cells), parenchyma and scattered pericyclic fibres. Sclereids usually occur as densely packed zones, composed of thick-walled, tangentially elongated cells, which alternate with parenchyma. Parenchymatous cells are thick-walled, oval containing prismatic crystals of calcium oxalate. Sheath of prismatic crystals of calcium oxalate surrounds zone of sclereids. Secondary phloem is a wide region consisting of phloem parenchyma, traversed longitudinally by medullary rays and phloem fibres. Cells of phloem parenchyma contain prismatic crystals of calcium oxalate similar to that of parenchyma of Pericycle. Phloem fibres are arranged in small concentric groups of more than three on the radial rows of phloem elements. Medullary rays become much wider, dilated, and funnel shaped on reaching pericycle.

Fig. 20.10 Ashoka bark
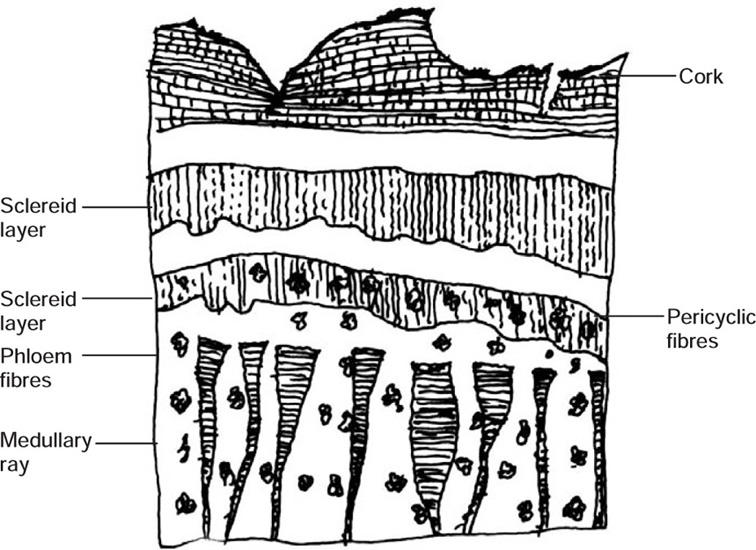
Fig. 20.11 T.S. (schematic) Ashoka bark
Chemical Constituents
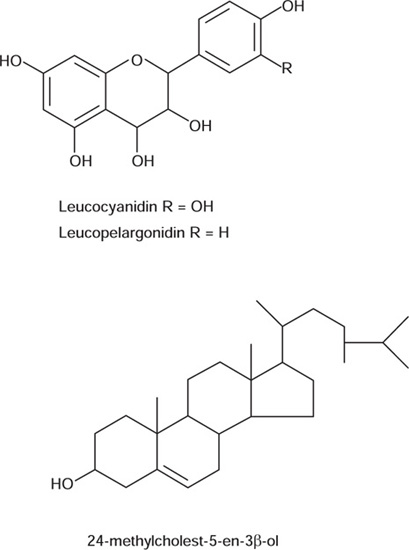
Ashoka stem bark contains about 6% of tannins and anthocyanin derivatives which includes leucopelargonidin-3-O-β-D-glucoside. leucopelargonidin and leucoanidin. It also contains waxy substance constituted of long chain alkanes, esters, alcohols and n-octacosanol. The steroidal components present in the bark includes 24-methylcholest- 5-en-3-β-ol, (ZZE)-24-ethylcholesta-5,22-dien-3-β-ol, 24-ethylcholest-5-en-3-β-ol and β-sitosterol.
The root bark contains (-) epicatechin, procyandin B2 and 11’-deoxyprocyanidin B. The pods consists of (+) catechol, (-) epicatechol, and leucocyanidin. The flowers are reported to have various anthocyanin pigments, kaempterol, quercetin and its glycoside, gallic acid, and β-sitosterol.
Uses
It is used as uterine tonic and also a sedative. It stimulates the uterus by the prolonged and frequent uterine contractions. It is also suggested in all cases of uterine bleeding, where ergot can also be used. It is reported to have a stimulant effect on the endometrium and ovarian tissue and useful in menorrhagia.
Marketed Products
It is the chief component of the preparation known as Pmensa (Lupin Herbal Laboratory) for symptomatic relief in painful and psychological symptoms associated with premenstrual syndrome. It is also an important ingredient of the preparation known as Femiplex (Charak Pharma Pvt. Ltd.), and Ashokarishta (Baidyanath).
Pale Catechu
Biological Source
Gambier or pale catechu is a dried aqueous extract produced from the leaves and young twigs of Uncaria gambier Roxburgh., belonging to family Rubiaceae.
Geographical Source
U. gambier is a native of erstwhile Malaya. It is cultivated in Indonesia, Malaysia, Sumatra, Bornea, and Singapore at elevation up to 150m. The plant is used mostly for the production of the drug, which is marketed through Singapore.
Cultivation, Collection, and Preparation
Propagation of U. gambier is done by seeds. Seeds are sown in the nursery to raise the seedlings, which after about 9 months are planted out in the clearing about 3 meters apart. Leaves and young shoots are collected as a first crop during second year’s growth. Later the crop is taken every year. The plant continues to give sufficient leaves and twigs up to 20 years, but the maximum yield is obtained during eighth year of growth.
The collected leaves and twigs are transported to the factory as loose material. The material is put into large drums with about three quarters of boiling water. It is boiled for about three hours with intermittent stirring. The marc is subsequently removed by large wooden forks and lodged on surface to drain the liquor back to the vessels. It is pressed and washed. The washing is added to the extract. The combined total aqueous extract is then concentrated for one and half-hour till it becomes thick, yellowish-green paste. It is transferred from the vessels to wooden tubs, stirred while it is hot, and cooling in a stream of water to crystallize tannins. Semicrystallized paste is again transferred to wooden trays in which it sets. They are cut into cubes by wooden knife and dried in sum. The drug is also made into large blocks in kerosene tins.
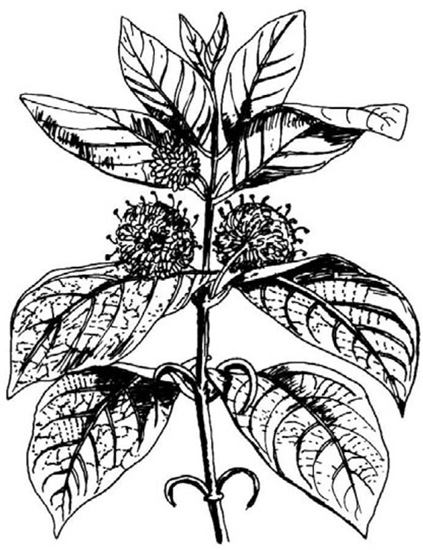
Fig. 20.13 Uncaria gambier flowering branch
Morphology
| Colour | Dull reddish brown colour externally and pale brown to buff colour internally. |
| Odour | Odourless |
| Taste | At first it is bitter and astringent but later it is sweet. |
| Shape | Strips, flakes or coarse powder |
| Size | Pale catechu comes in the form of cubes or rectangular blocks of 2 to 4cm length |
| Shape | Regular cubes or as rectangular blocks. |
Microscopy
The powdered drug, if mounted in the solution of lactophenol or water, shows the small circular crystals of catechu under microscope. The water insoluble part of the pale catechu under the microscope exhibits epidermal pieces, unicellular hairs, cork tissues, lignified fibres, etc. Alcohol insoluble part shows the absence of starch. The pale catechu from Indonesia is reported to have minute starch grains.
Chemical Constituents
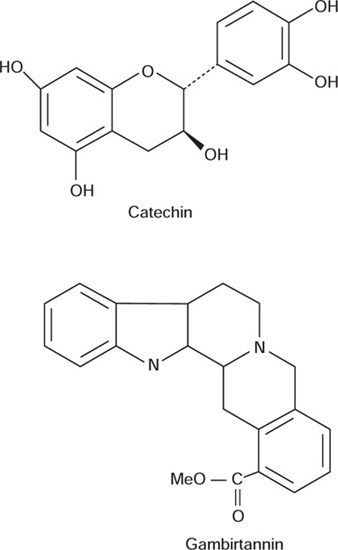
Pale catechu contain from about 7 to 30% of pseudotannin catechin and 22 to 55% of a phlobatannin catechutannic acid. Both of the about component constitute over 60% of the drug. It also contains catechu red, gambier fluorescin and quercetin. It contains indole alkaloid up to 0.05%, which includes gambirtannin and its derivatives. Gambirtannin gives a strong fluorescence under UV light. Catechin forms white, needle like crystals, which dissolves in alcohol and hot water. Catechutannic acid gives green colour with ferric chloride.
Chemical Tests
1. Gambier fluorescin test: Gambier fluorescin present in pale catechu gives the fluorescence. If to its alcohol extract, a little sodium hydroxide is added and shaken with petroleum ether. The petroleum ether layer shows green fluorescence. Black catechu gives negative test.
2. Vanillin-hydrochloric acid test: Drug shows pink or red colour with a mixture of vanillin:alcohol:dilute HCl in the ratio 1:10:10. The reaction produces phloroglucinol which along with vanillin gives pink or red colour.
3. A matchstick dipped in decoction of Pale catechu is air dried and again dipped into concentrated HCl and warmed near the burner. Pink or purple colour is produced.
4. Small quantity of powder is heated on water bath with 5ml chloroform and filtered. The filtrate is evaporated in white porcelain dish on a water bath. A greenishyellow residue is produced due to the presence of chlorophyll in the drug. Black catechu gives this test negative due to the absence of chlorophyll.
Uses
Pale catechu is medicinally used as local astringent. In diarrhoea, it is used as general astringent. It is largely used in various countries of east for chewing with betel leaf. Large proportion of gambier is used in dyeing and tanning industries. It is used for tanning of animal hides to convert it to leather.
Black Catechu
Biological Source
Black catechu is the dried aqueous extract prepared from the heartwood of Acacia catechu Willdenow, belonging to family, Leguminosae.
Geographical Source
A. catechu is common throughout the tract from Punjab to Assam ascending to an altitude of 300m. It is also quite common in drier regions of peninsula such as Madhya Pradesh, Maharashtra, Gujarat, Rajasthan, Bihar, and Tamil Nadu.
History
Possibly, the use of black catechu could be traced back in history from the time of chewing betel leaf, in which it has been used as adjuvant. In old days, it was used by women as a colouring agent for the feet. Since 15th century, this natural material has been exported to Europe. The old information about catechu is by a Portuguese writer Garcia de Orta in 1574. Dr. Wrath first used the scientific process to extract catechu, and showed that catechu consists of two parts, such as, kattha and cutch.
Collection and Preparation
A. catechu is a medium-sized tree with thorns. For preparation of the drug the tree is cut off from the ground. The main trunk and branches are cleared of foliage and thorns. The bark is stripped off, and the heartwood is made into chips. Heartwood is boiled in water in large earthen pots. The decoction is then strained and boiled in an iron pot with continuous stirring till it forms the syrupy mass. When the extract is cool enough, it is spread in the shallow wooden trays and kept for over night. When sufficiently dry, it is cut into pieces. Since the decoction is concentrated in iron vessels, the colour of the catechu becomes darker due to its reaction with iron salts. If the syrupy extract is stirred during cooling, it develops the shining crystals of catechin and produces translucent black catechu. Nowadays stainless steel vessels are used for the manufacture of catechu that produces a lighter coloured product.
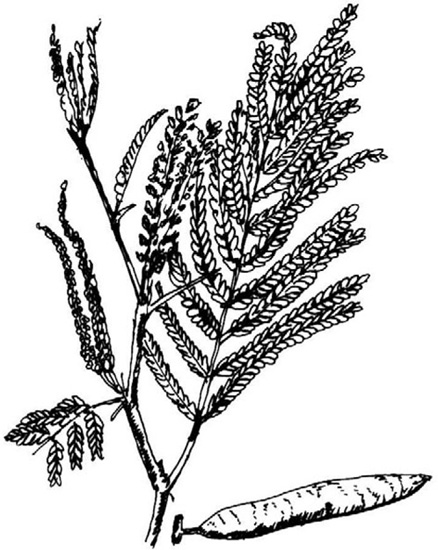
Fig. 20.14 Twig of black catechu
Microscopy
A transverse section of A. catechu heartwood shows numerous uniseriate and biseriate medullary rays, with vessels occurring isolated or in small groups of two or four. Xylem fibres with narrow lumen occupy major portion of wood and xylem parenchyma is usually predominantly paratracheal, forming a sheath around vessels. Wood consists of crystal fibres having prismatic crystals of calcium oxalate. A few tracheids with scalariform thickening and some cells including vessels are also present.
Chemical Constituents
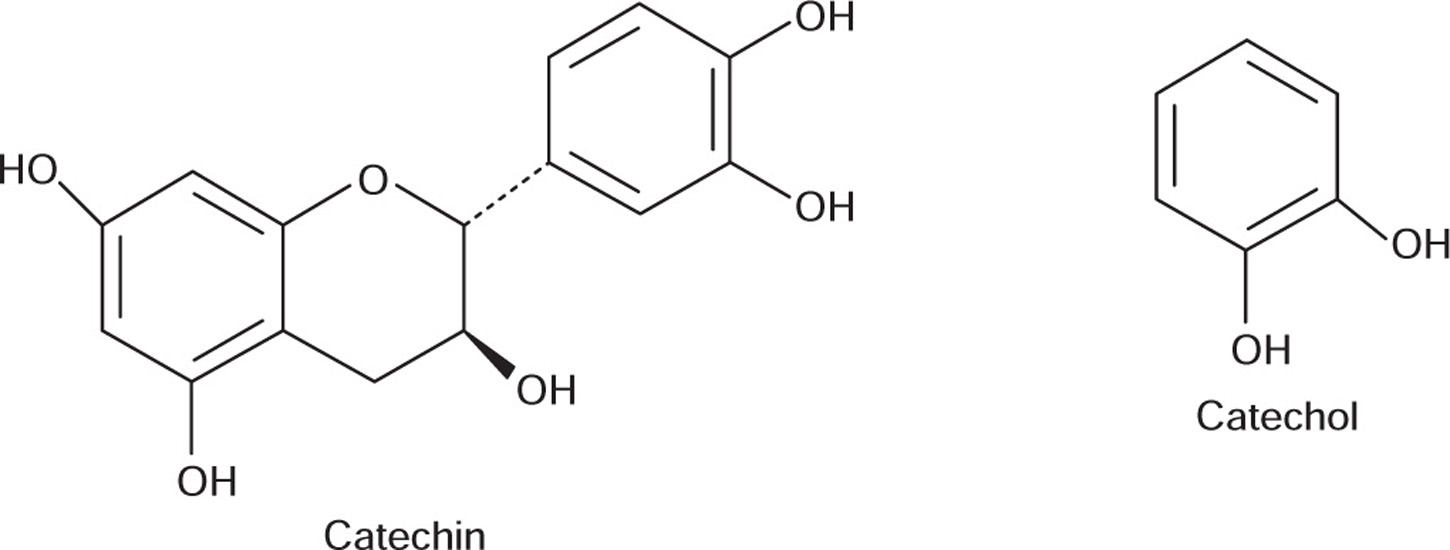
Cutch or black catechu resembles pale catechu or gambier in its composition. It contains about 2–12% of catechin and about 25 to 33% of phlobatannin catechutannic acid. The principle fraction of cutch has been identified as a mixture of catechin isomers which includes (-) epicatechin, acatechin, DL-acacatechin, L-acacatechin and D-isoacacatechin. It also contains 20–30% gummy matter, catechin red, quercetin and querecitin. It yields 2–3% of ash.
Chemical Tests
1. Because of the presence of catechin, black catechu gives pink or red colour with vanillin and HCl.
2. Catechin when treated with HCl produces phlorogucinol, which burns along with lignin to give purple or magenta colour. For this purpose, tannin extract is taken on match stick dipped in HCl and heated near the flame.
3. Lime water when added to aqueous extract of black catechu gives brown colour, which turns to red precipitate on standing for some time.
4. Green colour is produced when ferric ammonium sulphate is added to dilute solution of black catechu. By the addition of sodium hydroxide, the green colour turns to purple.
Uses
Cutch is used in medicine as astringent. It cures troubles of mouth, diseases of the throat and diarrhoea. It also increases appetite. In India and eastern countries, it is used in betel leaves for chewing. In dyeing industries, cutch is used for dyeing fabrics brown or black. It is also used in calico printing.
Pterocarpus
Biological Source
It consists of dried juice obtained by making vertical incisions to the stem bark of the plant Pterocarpus marsupium Linn., belonging to family Leguminosae.
Geographical Distribution
It is found in hilly regions of Gujarat, Madhya Pradesh, Uttar Pradesh, Bihar, and Orissa. It is also found in forests of Karnal, Kerala, West Bengal, and Assam.
Morphology
| Colour | Ruby-red |
| Odour | Odourless |
| Taste | Astringent |
| Shape | Angular grains |
| Size | 3 to 5 to 10mm granules |
| Solubility | It is partly soluble in water (about 80—90%), completely soluble in alcohol (90%). |
| Extra features | The pieces of kino are angular, glistening, transparent, breaking with vitreous fracture. |
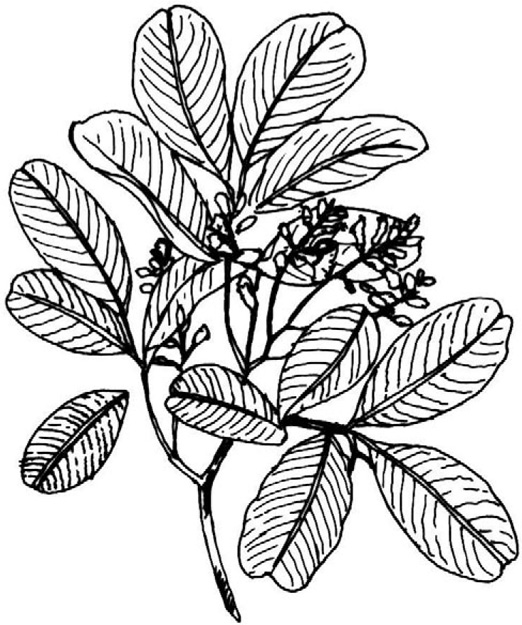
Fig. 20.15 Pterocarpus marsupium
Chemical Constituents
Kino contains about 70–80% of kinotannic acid, kino-red, k-pyrocatechin (catechol), resin and gallic acid. Kinotannic acid is glucosidal tannin, whereas kino-red is anhydride of kinoin. Kinoin is an insoluble phlobaphene and is produced by the action of oxydase enzyme. It is darker in colour than kinotannic acid.
Uses
Kino is used as powerful astringent and also in the treatment of diarrhoea and dysentery, passive haemorrhage, toothache, and in diabetes. It is used in dyeing, tanning, and printing. The aqueous infusion of the wood is considered to be of much use in diabetes. The alcoholic, as well as, aqueous extracts of heartwood are known to possess hypoglycaemic action. The cups made of wood are available with Khadi and Gramodyog commission for treatment of diabetes.