Controlling factors in growth of the skull
Growth of any part of the skull is coordinated with that of the other parts. Despite the shift from neurocranial dominance after the fifth year of life to orofacial dominance thereafter, with the emergence of the lower face from beneath the cranium, cephalometric analyses show that certain angular relations between various parts of the skull remain rather constant. The correlation of orofacial growth with the gradual growth and increasing use of the muscles of mastication is emphasized by Moss1 and will be discussed in detail later on in this chapter.
Controlling factors of craniofacial growth
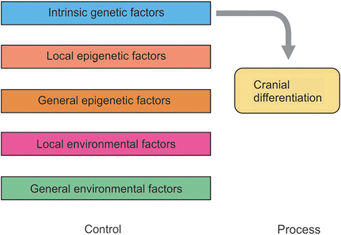
Analysis must be made of the more obvious controlling and modifying factors (Table 4.1). These are, first, the intrinsic genetic factors, or those inherent in the skull tissues themselves. Second are the epigenetic factors, which are genetically determined, but which manifest their influence in an indirect way by intermediary action on associated structures (i.e. eye, brain, and so forth). Structural or functional modifications of these associated structures would exert a modifying effect on the primary craniofacial complex. Third, local and general environmental factors are also controlling entities and require a value judgment in the overall picture. Just how these controlling factors work, separately and together, is not understood. The role of ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) at the cellular level, the establishment and maintenance of electric fields and the piezoelectric effect provide fertile fields for research activity at the genetic and epigenetic level. More readily interpretable are the local and general environmental factors.2, 3
TABLE 4.1
Van Limborgh’s factors
| Factor | Definition/Explanation |
| Intrinsic genetic factor | Genetic factors inherent to the craniofacial skeletal tissues |
| Local epigenetic factors (capsular matrix) | Genetically determined influences originating from adjacent structures and spaces (brain, eyes, etc.) |
| General epigenetic factors | Genetically determined influences originating from distant structures (sex hormones) |
| Local environmental factors (periosteal matrix) | Local non-genetic influences from external environment (muscle force, local external pressure) |
| General environmental factors | General non-genetic influences originating from the external environment (oxygen supply, food) |
Site vs center
A proper understanding of the terms growth site and growth center will help to clarify the differences between theories of growth. Baume had coined these two terminologies. According to him, ‘growth centers’ are places of endochondral ossification with tissue separating force, contributing to the increase in skeletal mass. Growth site has been defined as a region of periosteal or sutural bone formation and modeling resorption adaptive to environmental influences.
Proffit defines growth site as merely a location at which growth occurs, whereas center is a location at which independent or genetically controlled growth occurs. All growth centers are also sites, whereas all growth sites are not centers. Most of the theories of growth are based on where the growth center is expressed.
Enlow and Moyers use a common term ‘growth fields’, which includes both growth sites and centers. All surfaces of bone are covered by an irregular pattern of ‘growth fields’ comprising various soft-tissue osteogenic membranes or cartilages. Bone does not grow by itself; instead, it is grown by the environment. Growth ‘sites’ are growth fields having special roles in the growth of particular parts of bone. Examples of growth sites include mandibular condyle, maxillary tuberosity, synchondroses, sutures, alveolar process, etc. They do not cause the growth of the whole bone. ‘Growth center’ implies special areas that control the overall growth of bone. These growth centers have ‘force’ or ‘energy’ within them for bone growth. Refer Table 4.2 for differences between growth site and center. Based on where the growth center is expressed, different theories of growth have been put forward.
TABLE 4.2
Differences between growth site and center
| Growth Site | Growth Center/Growth Field |
| Is any location or place where growth takes place | Is any location or place where genetically controlled growth takes place |
| Is a region of periosteal or sutural bone formation and remodeling resorption adaptive to environment | Are places of ossification with tissue separating force |
| Sites of growth when transplanted to another area, does not continue to grow | Centers of growth when transplanted to another area, continues to grow |
| Marked response to external influences | Less response to external influence. More response to functional needs |
| They do not cause growth of the whole bone, instead they are simply places where exaggerated growth takes place | Cause growth of the major part of the bone |
| All growth sites are not growth centers | All growth centers are growth sites |
| Theories of growth are not based on growth site | Various theories of growth are based on the place where growth center is expressed |
| Growth sites do not control the overall growth of the bone | Growth center controls the overall growth of the bone |
Theories of growth
The various theories of growth are:
There are three major working hypotheses that have been advanced for skull growth, which we should analyze. These are associated primarily with investigators, such as Sicher,4 Scott5 and Moss6 or, based on tissue dominance concepts, sutural growth vs cartilaginous growth vs functional matrix growth.
Genetic theory by Allan Brodie
The genetic theory simply stated that genes determine and control the whole process of craniofacial growth. But the mechanism of action by the genetic unit and the mechanism by which the traits are transmitted were not understood until recently.
The classic idea that cranial differentiation is largely genetically determined (Fig. 4.1) seems be challenged by the high degree of individuality of certain parts of the cranium. Spontaneous and experimental malformations prove the very close relationships between the primordia of the other head structures and the development of the skull. Apparently, if there is no eye primordium, there will be no orbit. If there is only a single eye primordium, only one orbit develops.7 With the development of the field of molecular genetics, there is renewed interest in the genetic theory.
Van Limborgh hypothesis
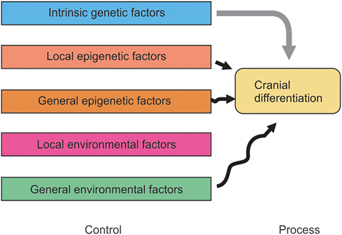
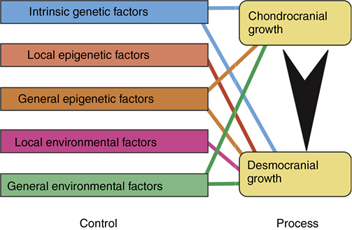
Limborgh says, “The role of local epigenetic factors is quite strong, despite the experiments which indicate the presence of the intrinsic factors in the condensed skull mesenchyme.”8 His view of embryonic skull differentiation is shown in Figure 4.2.
Van Limborgh after review of the sutural theory, cartilaginous theory and functional matrix theory has summarized the following features:
1. Intrinsic genetic factor controls chondrocranial growth
2. Epigenetic factors originating from skull cartilages and head tissues control desmocranial growth
3. Local environmental factors, like tension forces and pressure, influence the growth of desmocranial growth
4. General epigenetic and general environmental factors are less significant in craniofacial growth.
Sutural theory by Sicher and Weinmann
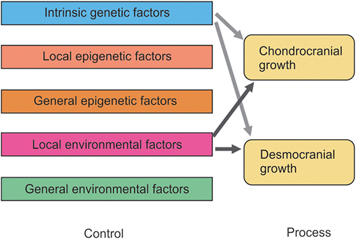
This traditional theory of skull growth is shown in Figure 4.3 which indicates that intrinsic genetic factors are the major concern, with only modeling resorptive and depository changes under the influence of muscles and other environmental factors.
Essence of the theory
According to Sicher, the sutures are the primary determinants of craniofacial growth. The craniofacial skeleton enlarges due the expansible forces exerted by the sutures as they separate.
Explanation of the theory
In this classic explanation, skull growth is largely independent of adjacent structure growth, or both are under the same genetic stimulus. Sicher gives equal value to all osteogenic tissues, cartilage, sutures and periosteum. However, his theory is generally referred to as the sutural dominance theory, with proliferation of connective tissue and its replacement by bone in the sutures being a primary consideration.4
Sicher postulated that bone growth within the various maxillary sutures produces pushing of the bone, which results in forward and downward movement of maxilla. It was believed that the stimulus for bone growth is tension, produced by the displacement of bones.
Evidences against sutural theory
1. Trabecular pattern in the bones at the suture changes with age, indicating the changes in the direction of growth. It cannot be accepted that sutures will have the necessary information for altering growth
2. Subcutaneous autotransplantation of the zygomatico-maxillary suture in the guinea pigs has not found to grow
3. Facial sutures extirpation has no perceptible effect on the dimensional skeleton growth
4. Suture’s shape depends upon functional stimulus on them
5. Suture closure appears to be determined extrinsically
6. Sutural growth can be halted by mechanical force, like clips placed across the sutures
7. The parallelism of circummaxillary suture so as to affect a forward and downward growth of maxilla is only superficial. Growth at zygomaticomaxillary suture occurs predominantly in lateral direction. The direction of growth of maxilla ranges from 0 to 82° in relation to the SN (Sella–Nasion) plane. It is practically impossible for the sutures running in the same direction to push the maxilla parallel to the reference plane. Present evidences indicate sutures as adaptive growth sites. Sutural tissues have no tissue separating force and they are not comparable to growth centers.
Conclusion: Present evidences indicate sutures as adaptive growth sites. Sutural tissues have no tissue separating force and they are not comparable to growth centers.
Scott hypothesis/nasal septum theory/cartilaginous theory/nasocapsular theory
Essence of the theory
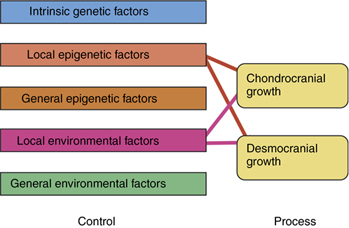
Figure 4.4 shows the Scott hypothesis, which emphasizes that the intrinsic growth-controlling factors are present in the cartilage and in the periosteum, with the sutures being only secondary and dependent on extrasutural influence.
Explanation of the theory
Scott feels that the cartilaginous parts of the skull must be recognized as primary centers of growth, with the nasal septum being a major contributor in maxillary growth perse.9 Sutural growth is responsive to synchondrosis proliferation and local environmental factors. Scott concluded that nasal septum is mostly active and vital for craniofacial growth both prenatally and postnatally.
The midface is pushed downward and forward by the anteroinferior growth of the nasal septal cartilage that is fortified against cranial base. The growth of cranial base is caused by its synchondroses and Scott compared the condylar cartilage with the cranial base cartilage.
Evidences supporting the theory
1. Histologic research validates much of the Scott hypothesis. Both pressure and tension have little effect on cartilaginous growth. On the contrary, intramembranous bone is immediately responsive.10 Thus, there is support for the contention that sutural growth is secondary to synchondral growth and occurs at the same time.11 Thus, early midface horizontal growth is tied to endochondrally induced anterior cranial base increase.
2. Supporting the Scott hypothesis is the research by Ohyama on rats.12 With experimental resection of the septum, using the most delicate and atraumatic procedures, significant interference with growth is still produced. The strong impression is gained in this research that the nasal septum is a primary growth center for nasal, frontal, premaxillary and maxillary bones.
3. In cleft palate cases, where maxillary growth has been retarded by scarified tissue, the nasal septum continues to grow and even bends on itself into the characteristic ‘S’ shape. The inhibition of sutural growth is considered a concomi tant lack of cartilage growth—no cartilage growth, no sutural growth, no proliferation of connective tissue. Scott has attributed an epiphysial plate-like effect to the nasal septum.13–19
4. Recent research indicates that the nasal septum seems to be more important in anteroposterior than in vertical growth.20, 21 Mandibular growth is considered more of an adaptive shift.12
Conclusion: Since endochondral growth process occurs in the posterior border of face at nasal septum, it can be considered essential for the anteroinferior growth of face. However, it is not regarded as an active contributor to the vertical growth and development of face.
Functional matrix hypothesis (FMH)—Melvin Moss
Essence of the theory
Figure 4.5 shows that the most popular current working hypothesis of Moss, which emphasizes that osseous growth of the skull, is entirely secondary. Based on the functional cranial component theory of van der Klaauw, Moss supports the concept of the role of the ‘functional matrix’.6, 22–25
Moss considers periosteal growth entirely secondary just as he does sutural growth.6, 26–28 He stresses the dominance of nonosseous structures of the craniofacial complex over the bony parts. Moss claims that the growth of the skeletal components, whether endochondral or intramembranous in origin, is largely dependent on the growth of the functional matrices. Moss recognizes no intrinsic regulatory mechanism in the growing skull tissues.
Definition: Functional matrix hypothesis (FMH) claims that the origin, growth, and maintenance of all skeletal tissues and organs are always secondary, compensatory, and obligatory responses to temporally and operationally prior events, or processes that occur in specifically related non-skeletal tissues, organs, or functioning spaces (functional Matrices).
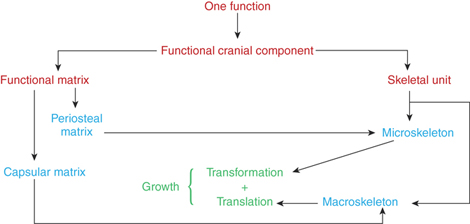
Functional cranial component
“The head is a composite structure, operationally consisting of a number of relatively independent functions: olfaction, respiration, vision, digestion, speech, audition, equilibration and neural integration. Each function is carried out by a group of soft tissues which are supported and/or protected by related skeletal elements. Taken together, the soft tissues and skeletal elements related to a single function are termed a functional cranial component.
The totality of the entire skeletal element associated with the single function is termed a skeletal unit. The totality of the soft tissues associated with a single function is termed the functional matrix. It may be further demonstrated that the origin, growth and maintenance of the skeletal unit depend almost exclusively upon its related functional matrix.”29
Each functional cranial component consists of a ‘skeletal unit’ and a ‘functional matrix’ (soft tissues and spaces). Any function is actually performed by the functional matrix, while the skeletal unit provides the necessary biomechanical role of providing protection and support to the soft-tissue matrix.
The skeletal unit may be composed of many bones, a single bone or a small portion of a bone. There are two types of skeletal units—microskeletal unit and macroskeletal unit.
The functional matrix consists of two distinct types: the ‘periosteal matrix’ and the ‘capsular matrix’. The activity of both the matrices is essential for craniofacial growth. Refer Box 4.1 for flowchart that depicts the organization of functional matrix theory.
Explanation of the theory
1. Functional matrix
The functional matrix refers to all the soft tissues and spaces that perform a given function (Fig. 4.6).
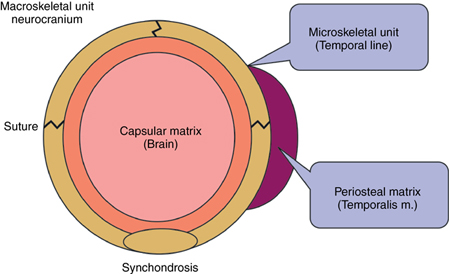
1. Periosteal matrix: The ‘periosteal matrix’ corresponds to the immediate local environment. Periosteal matrices are virtually self-defining. Examples of periosteal matrices include muscles, blood vessels, nerves, teeth, etc. The effects of periosteal matrices are best exemplified by the effect of muscles upon the skeletal units. Lack of contraction leads to atrophy of the bone. All periosteal matrices act homogeneously by means of osseous deposition and resorption. The muscles are attached either into the skeletal tissue or indirectly by fusion with the outer fibrous layer of the periosteum. Functioning muscles influence developmental changes in the form of skeletal tissues to which they are attached. This is achieved through muscle–bone interface.30 Sim and Kelly suggested that osseous blood flow modifies to osseous metabolism changes priorly. They further observed that resorption site had increased blood flow and depositary areas are poorly vascularized. The periosteal matrices stimulation causes growth of the microskeletal units. They act to alter the size, shape of both of the bones. The growth process that occurs due to periosteal matrix stimulation is called ‘transformation’.
2. Capsular matrix: The ‘capsular matrix’ is defined as the organs and spaces that occupy a broader anatomical complex. The functional cranial components arise, grow and are maintained within a series of capsules. Each capsule is an envelope that contains a series of functional cranial components, skeletal units and their related functional matrices and is sandwiched between two covering layers. This limiting layer consists of skin and dura mater in the neurocranial capsule and skin and mucosa in the orofacial capsule. The intervening spaces between themselves, between the functional components and that limiting the capsule are filled with indifferent loose connective tissue. Each capsule protects a capsular functional matrix by surrounding them.
The brain, leptomeninges and cerebrospinal fluid together constitute the neurocranial capsular matrix. It is easy to visualize the neurocranial capsule. On the other hand, orofacial capsular matrices or functioning spaces are difficult to visualize. The capsular matrices exist as volume.
Neurocranial capsule
The volume of total neural mass is morphogenetically significant in the neurocranium. In neurocranial capsule, the primary event in its expansion is the expansion of encompassed and guarded capsular matrix volume. When the capsule increases in size, the entire included and encompassed functional components (i. e. periosteal matrices and microskeletal units) are passively pushed outwards. The functional cranial components of whole calvarium are secondarily translated in space in a passive manner.
In pathologic or experimentally induced debilitating conditions, morphogenetic activity of the periosteal matrices are inhibited, e.g. hydrocephaly. Generally, the neurocranial capsule expansion is proportional to neural mass increase always. However, in hydrocephaly, rise in intracranial pressure obstructs capsule blood flow and thus prevents periosteal bone accumulation at sutural areas leading to characteristic large fontanelles and other sutural dehiscence. In simple words, the neural skull does not grow first to provide space for the secondary neural mass expansion but this expected secondary expansion becomes the primary event causing compensating neural skull growth as secondary event.
Orofacial capsular matrix
The orofacial capsular matrix or oropharyngeal functioning spaces are surrounded by the orofacial capsule. Limiting layers of this cavity are skin on the external aspect and mucous membrane internally. Establishment of the morphogenetic primacy of the orofacial functioning spaces will cause translation of all skeletal units embedded within the orofacial capsule.
The human oronasopharyngeal space increases in size from the third lunar month of pregnancy. This increase in volume causes compensatory increase in orofacial capsule size. The mitosis of both the epithelial and mesenchymal cellular elements and its resultant capsule expansion due to increase in intercellular elements leads to capsular growth. As the capsule enlarges, both the periosteal matrices along with the respective skeletal units are passively and secondarily translated to a new position in space.
Thus, the enclosed capsular matrices act indirectly on the macroskeletal units or on the entire functional cranial component. They only change their location in space and neither act by the osseous deposition or resorption process; nor by affecting cartilages directly; nor by altering the shape and size of the skeletal units. This type of growth process is called ‘translation’.
2. Skeletal unit
The skeletal unit refers to the bony structures which enable support to the functional matrix and these are essential or permissive for that function. The skeletal unit does not refer to the individual bone directly, but to the function it supports. There are two types of skeletal units:
1. Microskeletal units: They are part of the bone whose growth is modulated by the periosteal matrices. Functional variations in the periosteal matrices may be expressed within the microskeletal unit. The possible interaction between the periosteal matrix and microskeletal unit includes temporalis–coronoid process, masseter, medial pterygoid–gonial angle, and teeth–alveolar bone. The change in size and shape of microkeletal units occurs independently of the changes in spatial position. Moss uses two terms for this: ‘transformation’ or ‘intraosseous growth’.
2. Macroskeletal units: They are made of the core of the maxilla, mandible and neurocranium. Moss and Greenberg pointed out that the basic maxillary unit is the core that supports and protects the infraorbital neurovascular triad and in mandible, the basal tubular portion, which protects the mandibular canal. Through the neurotropic influence, the spatial constancy of the infraorbital canal with respect to anterior cranial base and mandibular canal from foramen ovale through mandibular foramen to mental foramen is maintained (unloaded nerve concept). The capsular matrix expansion causes the macro-skeletal unit to passively change the position. This process is called translational growth of skeletal structures. The overall skeletal growth is a combination of changes in microskeletal and macroskeletal units due to stimulation of periosteal and capsular matrices, respectively. This total growth changes are termed ‘interosseous growth’ by Moss.
3. Neurotropism
The sum of translation and changes in form comprises the totality of maxillary and mandibular growth. If the functional cranial analysis of Moss and his coworkers is correct, it is important to know how functional stimuli are translated at the skeletal unit interface and how functional matrices are regulated and controlled. This involves neurotropic processes.
Neurotropism “is a non-impulse transmittive neurofunc tion, involving axoplasmic transport, providing for the long-term interactions between neurons and innervated tissues which homeostatically regulate the morphological, compositional and functional integrity of those tissues”. The nature of neurotropic substances and the process of their introduction into the target tissue are unknown at present.30 Moss does indicate that there are three general categories: neuroepithelial, neurovisceral and neuromuscular.
Functional matrix revisited
Even though FMH gained popularity, it suffered from a major drawback. Moss was not able to explain clearly the process by which the functional stimuli could get converted into a signal and affect changes in bones. In his series of articles titled ‘Functional Matrix Hypothesis Revisited’,31, 32 Moss tried to explain the FMH in a more detailed and at microscopic level, and validate FMH. Moss tried to bridge the gap between hierarchical constraints and explained the operation from genome to organ level by two concepts:
1. Mechanotransduction occurring in single cells
2. Bone cells function multicellularly as a connected cellular network (osseous mechanotransduction).
Mechanotransduction is the process by which a mechanical stimulus is converted into a biologic signal to affect a cellular response. Whenever there is an alteration in the external environment, the vital cells are perturbed. Mechanosensing enables a cell to sense and respond to the external stimuli by using mechanoreception. After the signal is recovered, it is transferred to intracellular signal by mechanotransduction.
Subsequently, osseous mechanotransduction translates the periosteal functional stimulus into a skeletal unit cell signal by two skeletal cellular mechanotransductive processes namely ‘ionic’ and ‘mechanical’. The ionic (or electrical) process involves the ionic transport through the plasma membrane of the bone cell in some form. The possible ionic process includes stretch-activated ion channels, electromechanical, electrokinetic, and electric field strengths. This is made possible because the bone is viewed as an osseous connected cellular network (OCCN). The loaded tissue responds to the stimulus by the triad of bone cell adaptation. The triad includes bone deposition and maintenance and bone resorption. Both osteoblasts and osteocytes are competent for intracellular stimulus reception and transduction.
Conclusion
Moss concludes by saying that individually both genomic and epigenetic factors are necessary and satisfactory causes. Both factors together are necessary and satisfactory causes for controlling morphogenesis. Since epigenetic processes and its events are the immediate proximal cause of development, they are considered as the primary agencies of development.
Van limborgh’s composite hypothesis
The lumping together of both endochondral and intramembranous bone formation and response by Moss is questioned by others.33, 12–19 There is no doubt that the desmocranium (membranous calvarium) responds directly to pressure but the chondrocranium (endochondral base), which would seem to be under the same pressures or lack of pressures, remains practically normal. As Enlow has shown, the desmocranium shows a much greater incremental change than the chondrocranium during the normal growth process.34, 35 Yet, the brain rests on the chondrocranium and theoretically exerts the same amount of force downward as it would upward and outward. This apparent high degree of independence of endochondral bone growth is further substantiated by the fact that it is very difficult to distort the chondrocranium in contrast with the relative ease of deforming the desmocranium. Thus, there is apparent support for at least part of the Scott hypothesis while much research supports to a large measure the Moss functional matrix explanation. Limborgh lists the essential elements of the three hypotheses that seem to bear up under current research (Box 4.2).
Taking these six observations and constructing a diagram on the basis of their likely validity, Figure 4.7 shows the interrelationship of genetic, epigenetic and environmental controls with chondrocranium and desmocranium growth processes. Note also the influence of the chondrocranium on the membranous bone structures. Yet, the chondrocranium is primarily under the influence of intrinsic genetic factors with some lesser influence from general epigenetic factors and perhaps from general environmental factors.
This synthesis of parts from the three basic theories of craniofacial growth, while representing a logical interpretation, does not answer all the questions. The growth of the mandible, for example, is not explained completely. An added question of the possible difference between control of appositional cartilaginous growth and interstitial cartilaginous growth can be raised.33 Also, since the neurocranium is completed quite early and thus provides a stable base for continued membranous growth in other areas, a question is asked concerning the influence of these membranous bones on other membranous bones that are still growing. Thus, differential growth gradients are also a conditioning factor.
Servosystem theory
Petrovic36 using the language of cybernetics explained that the growth of various craniofacial regions is the result of interaction of a series of causal change and feedback mechanisms. Based on a series of experiments, Petrovic and coworkers have formulated a cybernetic model for the control of mandibular growth.
Servosystem theory starts with the explanation of cybernetics. Weiner defines cybernetics as the science of control and communication in the animal and machine. Cybernetics theory postulates that everything affects everything and, therefore, organized living systems never operate in an open-loop manner. Open loop is a type of mechanism that has no feedback loop or comparator. The other type of feedback is closed-loop mechanism. If a physiologic system is designed to maintain a specific correspondence between inputs and outputs, in spite of disturbances, it is called closed-loop system. It is characterized by the presence of a feedback loop and comparator. Closed loop has two variations, namely, regulator and servosystem.
• The regulator: The main input is a constant feature in this system. The comparator detects disturbances and their effects. It is a negative feedback system: disturbances cause changes that tend to restore the normal state of the disturbed system to the initial state.
• Feedback signal: It is the function of controlled variable that is compared to the reference input. It is negative in regulator and servosystem.
• The servosystem: It is also called follow-up system. The main input is not a constant in this system but varies across time.
Elements of servosystem theory
• Figure 4.8 depicts the various elements of servosystem theory. Command is a signal established independently of the feedback system under scrutiny. It affects the behavior of the controlled system without being affected by the consequences of this behavior, for example, secretion rates of growth hormone, testosterone, estrogen, and somatomedin. They are not modulated by variations of craniofacial growth.
• Reference input elements establish the relationship between the command and reference inputs. It includes septal cartilage, septo-premaxillary ligament, labionarinary muscles, and premaxillary and maxillary bones.
• Reference input is the signal established as a standard of comparison, e.g. sagittal position of maxilla. Ideally, it should be independent of the feedback.
• The controller is located between the deviation signal and the actuating signal.
• The confrontation between the position of the upper and lower dental arches is the comparator of the servosystem.
• Activity of the retrodiscal pad and lateral pterygoid constitutes the actuating signal. The elastic meniscotemporal and meniscomandibular frenum of the condylar disk form the retrodiscal pad.
• Controlled system is between the actuator and controlled variable, e.g. growth of condylar cartilage through the retrodiscal pad stimulation.
• Controlled variable is the output signal of the servosystem. The best example is the sagittal position of mandible.
• The gain of a system is the output divided by input. Gain value greater than one is called amplification and if it is less than one it is called attenuation. The pterygocondylar coupling is an example for gain.
• Any input other than the reference required is called a disturbance. Disturbance results in deviation of the output signal. For example, increase in hormone secretion results in supplemental lengthening of mandible.
• The attractor is the final structurally stable state in a dynamic system. It includes the full cusp class I molar relation.
• The repeller includes all unstable equilibrium states like cusp-to-cusp occlusal relationships.
Explanation of servosystem theory
According to servosystem theory,37 the midface (Fig. 4.9) grows downward and forward under the primary influence of the cartilaginous cranial base and nasal septum, influenced principally by the intrinsic cell–tissue-related properties common to all primary cartilages and mediated by the endocrine system. The influence of somatotropic hormone on the growth of cartilages of nasal septum, spheno-occipital synchondroses and other synchondroses follows that of a cybernetic form of command pattern. Related to this event, the maxillary dental arch is carried into a slightly more anterior position. This is the first and primary event. This causes a minuscule variation between the upper and lower arches which Petrovic36 referred as the ‘Comparator’, i.e. the constantly changing reference point between the positions of the upper and lower jaws. The upper dental arch is the constantly changing reference input. Second, within the periodontium and temporomandibular joint, the proprioceptors recognize even a tiny occlusal variation and activate the muscles for mandibular protrusion tonically. Petrovic says that the functional appliances will work in the same way when given to stimulate mandibular growth in class II malocclusions. Third, activation of jaw protruding muscles (retrodiscal pads and lateral pterygoid muscles) act on the condylar cartilage directly and through the vascular supply to the temporomandibular joint indirectly, thereby stimulating the growth of the condyle. The growth in secondary cartilages, like condyle, corresponds to local and environmental factors (epigenetic control). Lower arch constitutes the controlled variable.
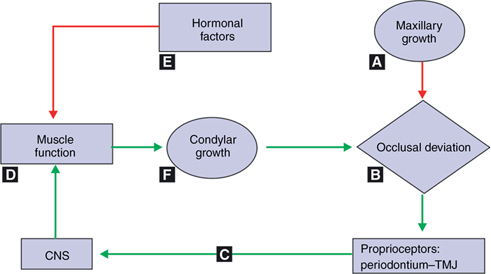
At last, hormonal factors acting principally on the condylar cartilage and the musculature get directly and indirectly influenced by the effect of the muscle function and receptivity of the condylar cartilage. As long as the midface-upper dental arch portion grows and matures with proper extrinsic, functional and hormonal factors being supportive, this complete cycle is activated incessantly as a servomotor. This affects the output signal. The output signal is the final sagittal position of the mandible. The sagittal position of the mandible depends on the modification of condylar growth by the activity of the retrodiscal pad and lateral pterygoid muscle stimulation. The major strength of the servosystem theory is that it provides a roadmap for future research and experimentation.