Anatomy and Kinesiology of the Shoulder
▪ Shoulder motion is a result of the complex interactions of the individual joints and muscles of the shoulder girdle.
▪ Scapulothoracic motion significantly affects measurements of glenohumeral motion.
▪ Shoulder motion is measured and described in multiple planes of motion.
▪ Stability of the shoulder is conferred by dynamic and static constraints.
▪ The clavicle serves as a strut and suspension between the thorax and scapula
▪ Scapular motion is a complex interaction of motion in three planes.
▪ The coordinated movement of the clavicle, scapula, and humerus involves a complex interaction of more than twenty muscles.
▪ Scapulohumeral rhythm is a dynamic state adapting to varying speed, load, and stability.
The shoulder is the most mobile joint in the body. Motion occurs through complex interactions of the individual joints of the shoulder girdle, including the glenohumeral joint, the sternoclavicular joint, the acromioclavicular joint, and the scapulothoracic articulation. Together the coordinated interaction of these structures allows for an extraordinary freedom of movement and function.
Traditionally, shoulder motion has been described by measuring the angle formed by the arm relative to trunk. Forward elevation, or flexion, of the shoulder is in the sagittal plane and may in some individuals reach 180 degrees. The normal range varies, but has been reported to be on average 165 to 170 degrees in men, and 170 to 172 degrees in women.1 Posterior elevation or extension in the sagittal plane has been found to be on average 62 degrees.2 Axial rotation of the arm is described by degrees of internal and external rotation. With the arm at the side an average external rotation is 67 degrees.3 Estimates of total axial rotation (the sum of internal and external rotation) with the arm at the side range from 150 degrees to 180 degrees. Total axial rotation with the arm abducted to 90 degrees is reduced to about 120 degrees. In the horizontal plane, when the arm is perpendicular to the trunk, motion is commonly described as horizontal abduction and adduction (or horizontal extension and flexion).
Range of motion is influenced by several factors, including the determination of the end-point, the plane in which the motion is tested, and whether the scapula is stabilized.3 By comparing the relative contribution of passive and active arcs of motion, McCully and colleagues concluded that scapulothoracic motion significantly influences glenohumeral range-of-motion measurements.3
Factors such as age, gender, and hand dominance also affect shoulder range of motion. Normal shoulder range of motion decreases with age. Boone and Azen reported on two groups of males with an age difference of 12.5 years. The younger group averaged 3.4 degrees more flexion and internal rotation, 8.4 degrees more external rotation, and 10.2 degrees more extension.4
Shoulder motion is rarely limited to one plane. Therefore, in describing the position of the arm in space it is necessary to use multiple planes of reference. The traditional methods of motion description are inadequate for complex motion because the final position of the arm is dependent on the motion sequence. This is illustrated by a concept known as the Codman’s paradox.5 If the arm is raised forward to the horizontal, then horizontally abducted, followed by a return adduction to the side, the final resting position of the arm is externally rotated axially 90 degrees, yet the arm was never specifically externally rotated. Serial angular rotations about orthogonal axes are not additive, but sequence-dependent. Rotation about the x-axis, followed by rotation about the y-axis results in a different end resting position from the reverse sequence.6
A central feature to the understanding of joint kinematics is the ability to measure and describe motion in a consistent and reproducible manner. One method of describing complex joint kinematics is to use a system of vertical planes of elevation, similar to the degrees of longitude used to describe global positioning6 (Fig. 4-1). Pure coronal abduction is defined as 0 degrees, and pure sagittal flexion as 90 degrees. At the horizontal, the maximum adduction is 124 degrees whereas the maximum abduction is −88 degrees, producing a total of 212 possible vertical planes of elevation.7 Humeral elevation is measured by the angle formed between the elevated arm and the unelevated arm. Isolated forward flexion to 90 degrees in this coordinate system is described as (90,90), whereas isolated abduction to 90 degrees is described as (0,90). Finally, axial rotation is described in reference to the plane of elevation by an angle formed by the forearm with the elbow flexed to 90 degrees. If the forearm is perpendicular to the plane of elevation, the rotation is 0 degrees. External rotation is positive, internal rotation is negative. A classic military salute in this system would be described as (+30, +80, −406).6
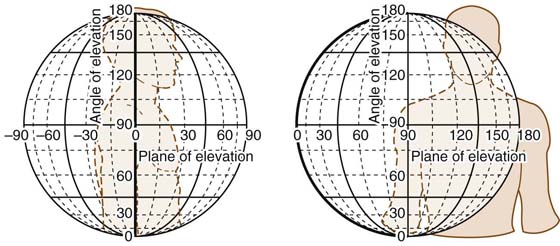
Figure 4-1 Range of motion of the shoulder is most similar to motion about a globe. (Reprinted from Rockwood CA Jr, Matsen FA III, The Shoulder. 3rd ed. Philadelphia: Saunders Elsevier, 2004.)
The glenoid arises laterally from the scapular neck at the junction of the coracoid, scapular spine, and lateral border of the scapular body. It is a pear-shaped structure forming a shallow socket that is retroverted on average 7 degrees with respect to the scapular plane, but maintains an overall anteversion of about 30 degrees with respect to the coronal plane of the body.8 The glenoid also maintains a superior tilt of about 5 degrees in the normal resting position of the scapula. It is thought that this superior inclination contributes to inferior stability via a cam effect that is a function of the tightening superior capsular structures.9
The glenoid surface area is about one third that of the humeral head. The depth of the glenoid measures about 9 mm in a superoinferior direction, but only 5 mm in an anteroposterior direction, half of which is constituted by the labrum.10 In addition, the glenoid cartilage is thicker peripherally than centrally, further deepening the socket. The glenoid socket is therefore significantly more concave and congruous with the humerus than the bony anatomy would suggest.
The humeral head is oriented with an upward tilt of about 45 degrees from the horizontal, and it is retroverted about 30 to 40 degrees with respect to the intercondylar axis of the distal humerus. The articular surface forms approximately one third of a sphere. Utilizing stereophotogrammetric studies, Soslowsky and associates demonstrated that the glenohumeral joint congruence is within 2 mm in 88% of cases, with a deviation from sphericity of less than 1% of the radius.11 Retroversion is greater in young children, with an average of 65 degrees between the ages of 4 months to 4 years.12 By 8 years of age most of the derotation has occurred with a more gradual derotation continuing until adulthood.
Glenohumeral laxity is a normal finding to varying degrees in all shoulders. In cadaveric shoulders, average passive humeral translation of 13.4 mm anteriorly and 10.4 mm posteriorly has been demonstrated with a 20-N force.13 In a study of healthy unanesthetized volunteers, passive humeral translation averaged 8 mm anteriorly, 9 mm posteriorly, and 11 mm inferiorly.14 Far less translation occurs with normal glenohumeral kinematics. Radiographic analysis of normal volunteers demonstrates that the humeral head is maintained precisely centered in the glenoid in all positions except simultaneous maximal horizontal abduction and external rotation.15 In this extreme position an average of 4 mm of posterior translation occurred. These studies demonstrate that despite the great potential for translational motion in the shoulder, the combined stabilizers of the glenohumeral joint act in concert to maintain centricity.
Shoulder laxity is a normal property that varies widely within the general population.16-19 It is often measured as increased passive translation of the humeral head on the glenoid and may be affected by several factors, including age, gender, and congenital factors.20 Instability is a pathologic condition involving active translation of the humeral head on the glenoid (Fig. 4-2). Unlike laxity, instability is usually symptomatic. It represents a failure of static and dynamic constraints to maintain the humeral head precisely centered within the glenoid. Instability may occur in one direction, such as anterior instability following a traumatic anterior dislocation, or it may be multidirectional, occurring in any combination of anterior, posterior, or inferior directions. Patients with multidirectional instability often have asymptomatic laxity of the contralateral shoulder.21 What distinguishes these shoulders from normally functioning shoulders is a complex interaction of muscular, neurologic, and structural factors.
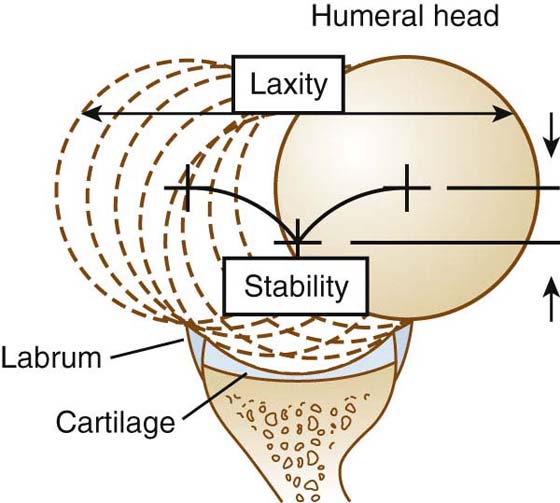
Figure 4-2 The difference between laxity and stability can be demonstrated graphically. Laxity is the translation permitted from one end of capsular tension to the other. Stability is the centered point of lowest potential energy and is related not only to capsular tension but also to joint congruency. [Modified from Lazarus MD, Sidles JA, Harryman DT 2nd, Matsen FA 3rd. Effect of chondral-labral defect on glenoid concavity and glenohumeral stability. A cadeveric model. J Bone Joint Surg Am. 1996;78A:94–102].
Shoulder dislocations are the most common form of joint dislocation, with an average incidence of 1.7%, demonstrating the great potential for instability that exists in the shoulder.22 The critical constraints for the control of shoulder stability may be divided into static and dynamic elements. The interaction of these constraining elements is complex. In the pathologic state, where one or more constraining factors is abnormal, instability may occur. Restoring these normal anatomic constraints is critical to the successful treatment and rehabilitation of the shoulder.
Early investigators focused on the articular components of glenohumeral stability. The humeral head retroversion roughly matches the glenoid orientation on the chest wall. Saha emphasized the contribution of this articular version to stability, noting that individuals with congenital anteversion of the glenoid had a greater tendency for recurrent dislocation.8 Subsequent studies have not confirmed this hypothesis, finding instead considerable variability in articular version and inclination.23-25 In any position of rotation only about 25% to 30% of the humeral head surface is in contact with the glenoid. The glenohumeral index, calculated by measuring the diameter of the humeral head relative to the glenoid, has been measured. It was hypothesized that individuals with larger heads relative to their glenoid would be unstable; however, investigators have made no such correlation.26
The relatively smaller surface area of the glenoid relative to the humeral head emphasizes the importance of soft tissues surrounding the joint, including the labrum, capsular, and ligamentous structures. The labrum is composed of dense collagen fibers that surround and attach to the glenoid rim, creating a deeper and broader glenoid surface. Functionally the labrum increases the articular contact of the glenoid with the humerus to about one third and improves the articular conformity and thereby stability27 (Fig. 4-3). Lippitt and Matsen demonstrated the contribution of the labrum to joint stability in cadavers.28 Excision of the labrum resulted in a 20% decrease in stability as measured using the stability ratio defined by Fukuda and coworkers.29
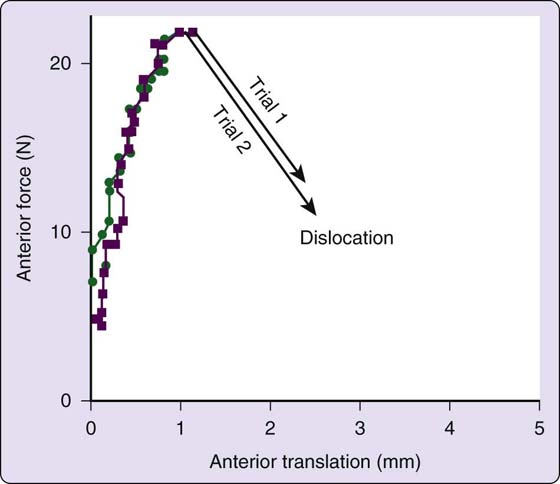
Figure 4-3 Even in older, cadaveric shoulders, in response to a translating force, humeral heads remain relatively well-centered until a threshold force is reached, resulting in sudden and explosive dislocation. [Modified from Lazarus MD, Sidles JA, Harryman DT 2nd, Matsen FA 3rd. Effect of chondral-labral defect on glenoid concavity and glenohumeral stability. A cadeveric model. J Bone Joint Surg Am. 1996;78A:94–102].
Slight mismatch of the articular surface diameter of curvature between the glenoid and humeral head may have a significant effect on glenohumeral stability. Saha initially described three types of glenohumeral articulations: type A had a shallow glenoid, type B had conforming surfaces, and type C had a humeral radius greater than that of the glenoid.8 Soslowsky and associates, using stereophotogrammetric studies of fresh frozen cadaveric shoulders, found that 88% of glenohumeral articulations are perfectly congruent.11 Kim and colleagues, however, recently analyzed MRI scans in patients with multidirectional instability (MDI) and compared them with normal MRIs. They determined that the diameter of curvature of the glenoid surface in the MDI patients was greater than the humeral diameter, suggesting that this loss of conformity may play a role in instability.30
A slightly negative intra-articular pressure of the glenohumeral joint also acts to maintain joint stability.31 Normally the shoulder contains about 1 mL of synovial fluid, which is maintained at a lower atmospheric pressure by high osmotic pressures in the surrounding tissues. Warner and coworkers demonstrated that in normal shoulders an inferiorly directed force of 16 N generated an inferior translation of 2 mm; however, if the capsule of the same shoulder is vented, the same force generates an inferior translation of 28 mm.32
The conformity of the glenohumeral joint combined with the presence of synovial fluid generates adhesion and cohesion between the humeral head and the glenoid in much the same fashion as a moist glass sticks to a coaster. Adhesion is due to the material properties of the synovial fluid, but cohesion is due to the conformity of the joint. The compliant labrum further potentiates these stabilizing effects.
The glenoid geometry and labrum in concert with muscle contractions of the rotator cuff are responsible for stability in the midranges of motion.3 The capsuloligamentous structures that remain lax during the midrange of motion are mainly responsible for stability at the end ranges of motion when all other stabilizing mechanisms have been overwhelmed.33,34
The superior ligaments comprise the coracohumeral ligament (CHL) and the superior glenohumeral ligament (SGHL) (Fig. 4-4). The CHL is broad, thin, extra-articular structure originating on the coracoid process and inserting broadly on the greater and lesser tuberosities, intermingling with the fibers of the supraspinatus and subscapularis. The SGHL, which lies deep to the CHL, is present in over 90% of cases, originating on the superior tubercle of the glenoid and inserting anteriorly just medial to the bicipital groove. Together these structures resist inferior translation with the arm in adduction.
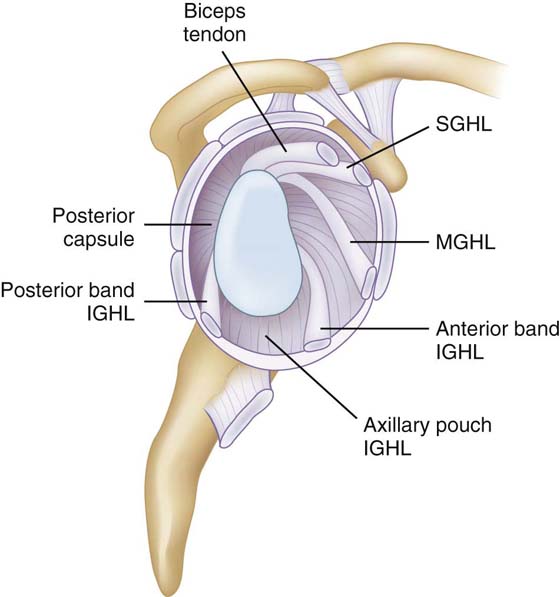
Figure 4-4 The glenohumeral joint is stabilized by discreet capsular ligaments, each of which has a separate role in maintaining stability. IGHL, inferior glenohumeral ligament; MGHL, middle glenohumeral ligament; SGHL, superior glenohumeral ligament.
The middle glenohumeral ligament (MGHL) has the greatest variation both in size and presence of all glenohumeral ligaments. It is absent in up to 30% of shoulders.35 The morphology of the MGHL may be sheetlike or cordlike, and it usually originates along with the SGHL on the superior glenoid tubercle, inserting just medial to the lesser tuberosity. Although the MGHL limits inferior translation in the adducted and externally rotated shoulder, the ligament primarily functions to limit anterior translation of the humerus on the glenoid with the shoulder abducted 45 degrees.36 In individuals with a more cordlike MGHL it may also function to limit anterior translation in the 60- to 90-degree abduction range with the arm externally rotated.36,37
The inferior glenohumeral ligament (IGHL) is likely the most important ligament complex of the glenohumeral joint. The IGHL is composed of thickened bands that form a sling, or “hammock,” that cradles the humerus inferiorly in what is referred to as the axillary pouch. Typically, the IGHL originates broadly at the equatorial to inferior half of the anterior glenoid adjacent to the labrum and inserts just inferior to the MGHL medial to the lesser tuberosity. In a histologic and anatomic study by O’Brien and coworkers, they demonstrated that the posterior and anterior portions of the IGHL contain thickened bands of dense collagen fibers.35 Gohlke and coworkers confirmed the existence of the anterior band, but found the posterior band to be present in only 62.8% percent of individuals.38 The stabilizing function of the IGHL complex increases as the arm is elevated in abduction. With external rotation of the arm in 90 degrees of abduction, the anterior band broadens and tightens, forming a taut sling that prevents anterior translation. Similarly, with internal rotation of the abducted arm, the posterior band fans out and tightens.39 The IGHL is also the primary restraint to inferior translation of the humerus with the arm in 90 degrees of abduction.
Ligaments only function under some degree of tension. However, during normal motion of the glenohumeral joint the ligaments remain under little to no tension. In addition a large amount of passive translation is commonly possible in multiple directions. Yet the humeral head remains perfectly centered in the glenoid during normal active motion. Therefore, it seems that factors other than the capsule and ligaments must be contributing to the lack of translation observed with normal motion. The factor responsible for maintaining the humeral head centered in the glenoid is therefore the interplay between the remaining static stabilizers (adhesion, cohesion, negative intra-articular pressure, and the congruency of the joint) and the dynamic stabilizers (the rotator cuff muscles, biceps brachii, the scapular rotators, and coordinated proprioceptive feedback) of the shoulder.
Despite the complex dynamic and static constraints that maintain the humeral head centered in the glenoid, pathologic translation of the humeral head does occur. In all but the most severe cases of laxity, shoulder dislocations result in tearing or fracturing of the glenohumeral architecture.40 Selective cutting experiments have demonstrated the potential instability that may result from sectioning individual capsular and ligamentous structures of the glenohumeral joint.
Cadaveric experiments have demonstrated the primary ligamentous constraints to translation of the humeral head on the glenoid in the anterior, inferior, and posterior directions. The anterosuperior band of the IGHL is the primary ligamentous constraint to anterior translation with the arm abducted and externally rotated.41 As abduction decreases, the MGHL is of increasing importance in resisting anterior translation.42 The primary constraints to inferior translation in the adducted arm are the superior structures, particularly the SGHL which is maximized by external rotation.36,43 With increasing abduction to 90 degrees, the IGHL becomes the primary constraint to inferior translation.44 The primary constraint to posterior translation is the posterior band of the IGHL. Although resection of the posterior capsule increases posterior translation, it is not sufficient for a posterior glenohumeral dislocation to occur.45 However, posterior dislocation is possible if the same shoulder is incised anterosuperiorly, cutting the SGHL and MGHL. Posterior translation increases with the arm in 30 degrees of extension if the anterior band of the IGHL is incised or detached from its glenoid insertion.44,46
The rotator cuff muscles improve joint stability by increasing the load necessary to translate the humeral head from its centered position in the glenoid. Lippitt and Matsen found that tangential forces as high as 60% of the compressive force were required to dislocate the glenohumeral joint in a cadaveric study,28 finding also that joint stability was reduced with removal of a portion of the anterior labrum. Similarly, Wuelker and associates noted a nearly 50% increase in anterior displacement of the humeral head in response to a 50% reduction in rotator cuff forces.47 The glenohumeral joint reaction force has been calculated in a cadaveric model to reach a maximum of 0.89 times body weight.48 Glenohumeral joint contact pressures measure a maximum of 5.1 MPa in cadavers using pressure-sensitive film with the arm in 90 degrees of abduction and 90 degrees of external rotation.49 Joint reaction forces increase with increasing abduction angle and peak at 90 degrees abduction.50 Increasing joint compression appears to increase the centering of the humeral head, thereby providing a stable fulcrum for arm elevation.51,52
Other factors may also contribute to dynamic glenohumeral stability. Ligament dynamization through attachment to the rotator cuff muscles has been postulated, whereby rotator cuff contraction may affect tensioning of the glenohumeral capsuloligamentous complex.53 Similarly, Pagnani and colleagues hypothesized that biceps contracture may tension the relatively mobile labrum and thereby tension the associated SGHL and MGHL, potentially enhancing stability.54 They also conclude that the long head of the biceps may itself stabilize the joint depending on shoulder position. Whether this is a true dynamic function is controversial. Yamaguchi and coworkers observed no biceps muscle activity with normal arm motion in both normal rotator cuffs and deficient cuffs.55
The capsuloligamentous structures may also provide propioceptive feedback on joint position. Vangsness and associates found low-threshold, rapid-adapting pacinian fibers in the glenohumeral ligaments.56 Others have found diminished proprioception in shoulders with instability, with subsequent improvement after repair.57 Proprioceptive feedback likely helps not only to tension the rotator cuff muscles, but also to position the scapula and clavicle appropriately in space.
The glenoid socket is relatively unconstrained compared with the acetabulum of the hip. The added mobility that this confers requires the coordinated movement and function of the muscles that position the scapula and clavicle in space. The glenoid may therefore be placed in a variety of positions that allow it to effectively resist the joint reaction forces generated by the muscles that power and position the arm.
The clavicle is a double-curved bone that functions as a strut and suspension between the thorax and the scapula while also protecting the underlying neurovascular structures (Fig. 4-5). While carrying a load at the side, for example, the clavicle functions as a strut, giving the muscles that elevate the clavicle and scapula a fulcrum to carry the load away from midline. Each end of the clavicle forms a diarthrodial articulation with an intervening fibrocartilagenous meniscus. The shape and mobile articulations of each joint allows for more motion than is typically observed. Muscles that power the shoulder cause compression across the glenohumeral joint. The force is transmitted to the trunk via the acromioclavicular (AC) and sternoclavicular (SC) joints. The conoid, trapezoid, and AC ligaments strongly suspend the scapula and the remaining upper extremity from the clavicle.
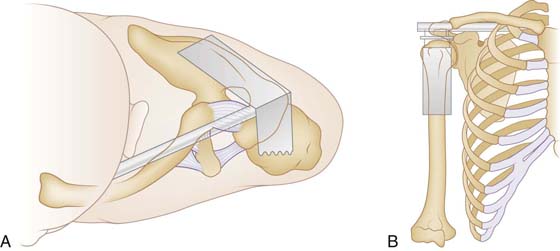
Figure 4-5 The clavicle serves as a strut (A) to position the scapula away from the thorax and as a suspension (B) for the shoulder girdle. (Reprinted from Lazarus MD. Fractures of the clavicle. In: Bucholz RW, Heckman JD, Court-Brown C, et al., eds. Rockwood and Green’s Fractures in Adults. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2001.)
The SC joint connects the axial skeleton to the upper extremity. The somewhat flattened bony articulation provides little inherent stability. Instead ligamentous structures both anteriorly and posteriorly confer stability (Fig. 4-6). Early studies demonstrated that the SC capsule was responsible for stability of the joint, but they did not isolate individual ligaments or regions of the capsule.58,59 Spencer and Kuhn demonstrated in a cadaveric selective cutting study that the posterior capsule is the primary restraint to both posterior and anterior translation;60 however, the anterior capsule is also important, particularly for restraint on anterior translation. Their research also showed that the costoclavicular and interclavicular ligaments are not crucial stabilizers of the SC joint.
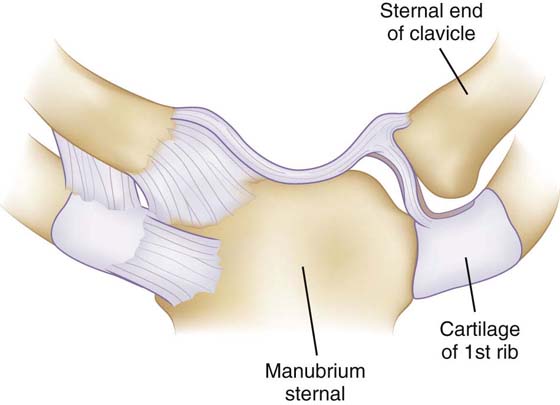
Figure 4-6 The sternoclavicular joint is stabilized by the strongest ligamentous complex in the body. (Reprinted from Gray’s Anatomy, 2007.)
The AC joint is a diarthrodial joint that allows articulation of the medial acromion with the lateral clavicle. Similarly to the SC joint, the AC joint has little inherent stability, instead relying on ligamentous support (Fig. 4-7). A capsule surrounds the joint, thickening superiorly to form the AC ligament. The scapula is suspended from the clavicle by way of the conoid and trapezoid ligaments connecting the distal clavicle to the coracoid process. Early investigators observed only minimal motion at the AC joint. However, a cadaveric selective cutting experiment by Fukuda and colleagues measured the relative constraint provided by the AC capsule, conoid, and trapezoid ligaments to small and large displacements.61 They concluded that in small displacements (10 N of force) the AC capsule is the primary restraint on both superior and posterior translation. With large displacements (90 N of force) there is a shift to the conoid ligament with respect to restraint to superior translation; however, 90% of the restraint on posterior translation is still maintained by the AC capsule. The coracoclavicular ligaments, especially the trapezoid, resist most of the load transmission in axial compression. The individual contributions of the AC ligament to resisting translation were further clarified by Klimkiewicz and coworkers in a cadaveric-sectioning study.62 They concluded that the posterior and superior ligaments are the most critical, resisting on average 25% and 56% of the posterior displacement, respectively. These results were confirmed by Debski and associates in a cadaveric study using in situ force measurements in three dimensions.42 They concluded that the superior AC ligament is the primary restraint on posterior translation and that the conoid is the primary restraint on superior translation. Moreover, they note that the constraints on the AC joint affect the resulting joint motion, but motion also affects the force on each ligament.
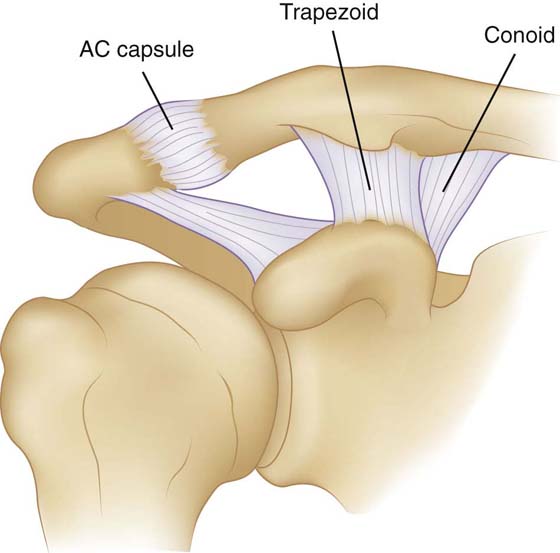
Figure 4-7 The ligamentous anatomy of the acromioclavicular joint. (Reprinted from Gray’s Anatomy, 2007.)
Clavicular motion occurs in anteroposterior and superoinferior directions as well as rotating on its longitudinal axis. Inman noted that about 30 degrees of clavicular elevation occurs with about 130 degrees of forward elevation of the upper extremity with relatively more motion occurring at the SC joint than the AC joint.63 Ten degrees of forward elevation also occurs with the first 40 degrees of elevation and an additional 15 to 20 degrees of forward elevation occurs with motion above 130 degrees of elevation.64 DePalma summarized this elevation and forward rotation by describing the motion of the distal clavicle as forming an angular cone of about 60 degrees.65
Dempster originally described six discrete motions at the SC joint (Table 4-1).58 The physiologic range of each type of motion has been defined by various studies.63,66 Additionally, anteroposterior rotation is greater than superoinferior motion by a ratio of 2 to 1.67 Motion at the AC joint is more limited than at the SC joint. The motion may be thought of as rotational, either axial (anterior and posterior) about the long axis of the clavicle, or hinging in an anteroposterior or superoinferior manner. Anteroposterior rotation is three times greater than superoinferior motion.58
Table 4-1 Range of Motion at the Sternoclavicular (SC) Joint (in Degrees)58
Action at the SC Joint |
Degrees of Motion |
Upward rotation |
35 |
Anterior rotation |
35 |
Posterior rotation |
35 |
Axial rotaion |
45–50 |
Downward rotation |
10 |
Upward rotation |
45 |
The scapula is predominately a thin sheet of bone loosely attached and congruent to the posterior chest wall, which serves to stabilize the upper extremity against the thorax. The scapula thickens along its borders at the site of muscle attachments and along its four projections: the spine of the scapula, the coracoid, the glenoid, and the acromion.
Although the scapulothoracic articulation is not a true joint, its motion is integral to positioning the arm in space. The scapula essentially glides over a muscle bed on the posterior chest wall, its shape conforming to the underlying ribs. At rest the scapula is rotated anteriorly about 30 degrees as viewed from above, upward about 3 degrees with respect to the sagittal plane, and tilted forward about 20 degrees as viewed from the side.
Although scapular motion has long been recognized as complex, descriptions of this motion have largely focused on elevation in the coronal or scapular plane. Using sensors attached to percutaneous pins McClure and associates demonstrated that scapular motion is three-dimensional and task-dependent.68 With arm elevation in the scapular plane, the scapula rotates upward on average 50 degrees, posteriorly about a mediolateral axis about 30 degrees, and externally about 24 degrees about a vertical axis (Fig. 4-8).
More than 20 muscles coordinate their function to move the shoulder joint complex. Several of these muscles have differing functional heads that further enhance shoulder function, including the three heads of the deltoid, the two heads of the biceps brachii, the three heads of the triceps brachii, the three portions of the trapezius, and the two portions of the pectoralis major. Based on these muscles’ origins and insertions, they may be categorized as glenohumeral, scapulothoracic, or thoracohumeral.
The relative function of each individual muscle depends on three factors: the cross-sectional area, the vector angle of pull, and the percentage recruitment of muscle fibers (or intensity of contraction). Electromyography (EMG) is useful in determining the relative level of activity within a particular muscle group, but it cannot measure the force of contraction. To understand the forces generated requires a calculation of the moment arm of the muscle as well as the physiologic cross-sectional area, both of which are dynamic, making accurate calculations challenging. Anatomic studies have calculated the cross-sectional area of several muscles of the shoulder girdle.69 Cross-sectional measurements and approximations of force vectors have been used to calculate glenohumeral joint reaction forces. Current research is focusing on in vivo calculations.
During active forward elevation of the arm, synchronous activity of the deltoid and rotator cuff muscles has been measured using a combination of stereophotogrammetry and EMG recordings. Inman and colleagues demonstrated that the deltoid and supraspinatus act synergistically during forward elevation of the arm.64 Synchronous function of the remaining rotator cuff muscles provides the humeral head depression necessary to prevent superior migration of the humeral head.52 The deltoid provides a substantial initial force nearly 90% of its total potential force.67 In massive rotator cuff tears the force required of the remaining rotator cuff to keep the glenohumeral joint centered increases experimentally by as much as 86%.70
The supraspinatus is thought to initiate abduction; however, the deltoid and all four rotator cuff muscles are active throughout the full range of forward arm elevation. The specific contributions of each muscle have been studied using selective nerve blocks in healthy volunteers. Blocks of either the suprascapular nerve or the axillary nerve demonstrate that both the deltoid and supraspinatus are responsible for generating torque during active forward elevation of the arm. Full abduction has been shown to be possible with an axillary nerve block with a reduction of strength of about 50% of normal.71 Similarly, suprascapular nerve block allowed full abduction with diminished strength. However, simultaneous axillary and suprascapular nerve blocks eliminated all active elevation, demonstrating that the deltoid, supraspinatus, and infraspinatus are essential for active shoulder elevation. With a suprascapular nerve block, strength is reduced about 50% at 30 degrees, 35% at 90 degrees, and 25% at 120 degrees of forward elevation.67
Classically the contributions to arm elevation are thought to be a 2:1 ratio of glenohumeral to scapulothoracic motion.64 More recent investigations suggest a more complex interaction with motion during the first 30 degrees as mostly glenohumeral,48,72,73 whereas the last 60 degrees comprises a near equal contribution by the glenohumeral and scapulothoracic joints. McClure and coworkers measured a ratio of 1.7 to 1 of glenohumeral motion to scapulothoracic motion with arm elevation in healthy volunteers.68 The speed of arm elevation also affects the relative contribution of each joint, with predominance of glenohumeral motion at high speeds.74 With age, the ratio of glenohumeral to scapulothoracic motion remains relatively constant; however, the range of motion is diminished.75
Maximal forward elevation of the arm requires external rotation of the humerus.76 Early observers concluded that external rotation was necessary for the tuberosity to clear the acromion, but more recent clinical and cadaveric studies suggest other factors play a significant role. Maximal external rotation may confer greater stability to the glenohumeral joint in the elevated position.77 Jobe and Iannotti conclude, based on a cadaveric range-of-motion study in three planes, that obligate external rotation makes more humeral head cartilage available for articulation with the glenoid.78 A cadaveric study using magnetic three-dimensional tracking devices determined maximal elevation was associated with approximately 35 degrees of external rotation.76 In vivo data suggest a greater amount of external rotation exists. Using a magnetic field around volunteers, Stokdijk and colleagues found an average external rotation of 55 degrees.79
Codman understood the complex and dependent relationships of the structures of the shoulder when he coined the term scapulohumeral rhythm to describe the coordinated motion.80 Inman noted the early phase of scapular motion as the setting phase, indicating the importance of positioning the scapula in an advantageous position for the rotator cuff muscles.63 More recent dynamic studies have confirmed this dependent relationship,68,81 defining more accurately the complex motion of the scapula in relation to the humerus in normal as well as pathologic states.82 Even with a 3-kg weight held in the hand, the scapulohumeral rhythm remains unchanged except in the midrange of elevation where the position of the scapula compensates for the increased load.81 These subtle coordinated adaptations in neuromuscular coordination contribute to the dynamic stability and unique function of the joint under a broad range of conditions.
Shoulder motion is a result of the complex interactions of the glenohumeral, acromioclavicular, sternoclavicular, and scapulothoracic joints. The shoulder is powered by the coordinated motion of more than twenty muscles interacting to confer stability under varying speeds and loads. Shoulder motion is measured and described in discrete planes of motion with motion at each joint affecting the measure of each other joint. The broad range of shoulder motion makes it vulnerable to instability and injury, including glenohumeral dislocation. Stability is maintained by the interaction of dynamic and static motion constraints. These include the bony anatomy, the soft-tissue constraints, and the dynamic coordinated muscle activity of the shoulder girdle.
1. Murray MP, Gore DR, Gardner GM, Mollinger LA. Shoulder motion and muscle strength of normal men and women in two age groups. Clin Orthop Relat Res. 1985 Jan-Feb; 192: 268–273.
2. Barnes CJ, Van Steyn SJ, Fischer RA. The effects of age, sex, and shoulder dominance on range of motion of the shoulder. J Shoulder Elbow Surg. 2001 May-Jun; 10 (3):242–246.
3. McCully SP, Kumar N, Lazarus MD, Karduna AR. Internal and external rotation of the shoulder: effects of plane, end-range determination, and scapular motion. J Shoulder Elbow Surg. 2005 Nov-Dec; 14 (6):602–610.
4. Boone DC, Azen SP. Normal range of motion of joints in male subjects. J Bone Joint Surg Am. 1979 Jul; 61 (5):756–759.
5. Pearl ML, Harris SL, Lippit SB, et al. Codman’s paradox: sixty years later. J Shoulder Elbow Surg. 1992; 1: 219–225.
6. Pearl ML, Harris SL, Lippit SB, et al. A system for describing positions of the humerus relative to the thorax and its use in the presentation of several functionally important arm positions. J Shoulder Elbow Surg. 1992; 1: 113–118.
7. Pearl ML, Jackins S, Lippit SB, et al. Humeroscapular positions in a shoulder range-of-motion examination. J Shoulder Elbow Surg. 1992; 1: 296–305.
8. Saha AK. Dynamic stability of the glenohumeral joint. Acta Orthop Scand. 1971; 42 (6):491–505.
9. Itoi E, Motzkin NE, Morrey BF, et al. Scapular inclination and inferior stability of the shoulder. J Shoulder Elbow Surg. 1992; 1: 131–139.
10. Howell SM, Galinat BJ. The glenoid-labral socket. A constrained articular surface. Clin Orthop Relat Res. 1989 Jun; 243: 122–125.
11. Soslowsky LJ, Flatow EL, Bigliani LU, Mow VC. Articular geometry of the glenohumeral joint. Clin Orthop Relat Res. 1992 Dec; 285: 181–190.
12. Edelson G. The development of humeral head retroversion. J Shoulder Elbow Surg. 2000 Jul-Aug; 9 (4):316–318.
13. Tibone JE, McMahon PJ, Shrader TA, et al. Glenohumeral joint translation after arthroscopic, nonablative, thermal capsuloplasty with a laser. Am J Sports Med. 1998 Jul-Aug; 26 (4):495–498.
14. Lippitt SB, Vanderhooft JE, Harris SL, et al. Glenohumeral stability from concavity-compression: A quantitative analysis. J Shoulder Elbow Surg. 1993; 2: 27–35.
15. Howell SM, Galinat BJ, Renzi AJ, Marone PJ. Normal and abnormal mechanics of the glenohumeral joint in the horizontal plane. J Bone Joint Surg Am. 1988 Feb; 70 (2):227–232.
16. Cofield RH, Irving JF. Evaluation and classification of shoulder instability. With special reference to examination under anesthesia. Clin Orthop Relat Res. 1987 Oct.(223):32–43.
17. Gerber C, Ganz R. Clinical assessment of instability of the shoulder. With special reference to anterior and posterior drawer tests. J Bone Joint Surg Br. 1984 Aug; 66 (4):551–556.
18. Harryman DT 2nd, Sidles JA, Clark JM, et al. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990 Oct; 72 (9):1334–1343.
19. Hawkins RJ, Schutte JP, Janda DH, Huckell GH. Translation of the glenohumeral joint with the patient under anesthesia. J Shoulder Elbow Surg. 1996 Jul-Aug; 5 (4):286–292.
20. Emery RJ, Mullaji AB. Glenohumeral joint instability in normal adolescents. Incidence and significance. J Bone Joint Surg Br. 1991 May; 73 (3):406–408.
21. O’Driscoll SW, Evans DC. Contralateral shoulder instability following anterior repair. An epidemiological investigation. J Bone Joint Surg Br. 1991 Nov; 73 (6):941–946.
22. Hovelius L. Incidence of shoulder dislocation in Sweden. Clin Orthop Relat Res. 1982 Jun; 166: 127–131.
23. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997 Sep; 79 (5):857–865.
24. Churchill RS, Brems JJ, Kotschi H. Glenoid size, inclination, and version: an anatomic study. J Shoulder Elbow Surg. 2001 Jul-Aug; 10 (4):327–332.
25. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996 Jul-Aug; 5 (4):320–326.
26. Randelli M, Gambrioli PL. Glenohumeral osteometry by computed tomography in normal and unstable shoulders. Clin Orthop Relat Res. 1986 Jul.(208):151–156.
27. Lazarus MD, Sidles JA, Harryman DT 2nd, Matsen FA 3rd. Effect of a chondral-labral defect on glenoid concavity and glenohumeral stability. A cadaveric model. J Bone Joint Surg Am. 1996 Jan; 78 (1):94–102.
28. Lippitt S, Matsen F. Mechanisms of glenohumeral joint stability. Clin Orthop Relat Res. 1993 Jun.(291):20–28.
29. Fukuda K, Chen CM, Cofield RH, Chao EY. Biomechanical analysis of stability and fixation strength of total shoulder prostheses. Orthopedics. 1988 Jan; 11 (1):141–149.
30. Kim SH, Noh KC, Park JS, et al. Loss of chondrolabral containment of the glenohumeral joint in atraumatic posteroinferior multidirectional instability. J Bone Joint Surg Am. 2005 Jan; 87 (1):92–98.
31. Kumar VP, Balasubramaniam P. The role of atmospheric pressure in stabilising the shoulder. An experimental study. J Bone Joint Surg Br. 1985 Nov; 67 (5):719–721.
32. Warner JJP, Deng X, Warren RF, et al. Superoinferior translation in the intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993; 2: 99–105.
33. Malicky DM, Soslowsky LJ, Blasier RB, Shyr Y. Anterior glenohumeral stabilization factors: progressive effects in a biomechanical model. J Orthop Res. 1996 Mar; 14 (2):282–288.
34. Schiffern SC, Rozencwaig R, Antoniou J, et al. Anteroposterior centering of the humeral head on the glenoid in vivo. Am J Sports Med. 2002 May-Jun; 30 (3):382–387.
35. O’Brien SJ, Neves MC, Arnoczky SP, et al. The anatomy and histology of the inferior glenohumeral ligament complex of the shoulder. Am J Sports Med. 1990 Sep-Oct; 18 (5):449–456.
36. Warner JJ, Deng XH, Warren RF, Torzilli PA. Static capsuloligamentous restraints to superior-inferior translation of the glenohumeral joint. Am J Sports Med. 1992 Nov-Dec; 20 (6):675–685.
37. O’Connell PW, Nuber GW, Mileski RA, Lautenschlager E. The contribution of the glenohumeral ligaments to anterior stability of the shoulder joint. Am J Sports Med. 1990 Nov-Dec; 18 (6):679–684.
38. Gohlke F, Essigkrug B, Schmitz F. The pattern of the collagen fiber bundles of the capsule of the glenohumeral joint. J Shoulder Elbow Surg. 1994; 3: 111–128.
39. O’Brien SJ, Schwartz RS, Warren RF, Torzilli PA. Capsular restraints to anterior-posterior motion of the abducted shoulder: a biomechanical study. J Shoulder Elbow Surg. 1995 Jul-Aug; 4 (4):298–308.
40. Speer KP, Deng X, Borrero S, et al. Biomechanical evaluation of a simulated Bankart lesion. J Bone Joint Surg Am. 1994 Dec; 76 (12):1819–1826.
41. Turkel SJ, Panio MW, Marshall JL, Girgis FG. Stabilizing mechanisms preventing anterior dislocation of the glenohumeral joint. J Bone Joint Surg Am. 1981 Oct; 63 (8):1208–1217.
42. Debski RE, Wong EK, Woo SL, et al. In situ force distribution in the glenohumeral joint capsule during anterior-posterior loading. J Orthop Res. 1999 Sep; 17 (5):769–776.
43. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000 Jul-Aug; 9 (4):336–341.
44. Schwartz E, Warren RF, O’Brien SJ, Fronek J. Posterior shoulder instability. Orthop Clin North Am. 1987 Jul; 18 (3):409–419.
45. Bowen MK, Warren RF. Ligamentous control of shoulder stability based on selective cutting and static translation experiments. Clin Sports Med. 1991 Oct; 10 (4):757–782.
46. Ovesen J, Nielsen S. Posterior instability of the shoulder. A cadaver study. Acta Orthop Scand. 1986 Oct; 57 (5):436–439.
47. Wuelker N, Korell M, Thren K. Dynamic glenohumeral joint stability. J Shoulder Elbow Surg. 1998 Jan-Feb; 7 (1):43–52.
48. Poppen NK, Walker PS. Normal and abnormal motion of the shoulder. J Bone Joint Surg Am. 1976 Mar; 58 (2):195–201.
49. Conzen A, Eckstein F. Quantitative determination of articular pressure in the human shoulder joint. J Shoulder Elbow Surg. 2000 May-Jun; 9 (3):196–204.
50. Apreleva M, Parsons IM 4th, Warner JJ, et al. Experimental investigation of reaction forces at the glenohumeral joint during active abduction. J Shoulder Elbow Surg. 2000 Sep-Oct; 9 (5):409–417.
51. Thompson WO, Debski RE, Boardman ND 3rd, et al. A biomechanical analysis of rotator cuff deficiency in a cadaveric model. Am J Sports Med. 1996 May-Jun; 24 (3):286–292.
52. McMahon PJ, Debski RE, Thompson WO, et al. Shoulder muscle forces and tendon excursions during glenohumeral abduction in the scapular plane. J Shoulder Elbow Surg. 1995 May-Jun; 4 (3):199–208.
53. Clark J, Sidles JA, Matsen FA. The relationship of the glenohumeral joint capsule to the rotator cuff. Clin Orthop Relat Res. 1990 May; 254: 29–34.
54. Pagnani MJ, Deng XH, Warren RF, et al. Role of the long head of the biceps brachii in glenohumeral stability: a biomechanical study in cadavers. J Shoulder Elbow Surg. 1996 Jul-Aug; 5 (4):255–262.
55. Yamaguchi K, Sher JS, Andersen WK, et al. Glenohumeral motion in patients with rotator cuff tears: a comparison of asymptomatic and symptomatic shoulders. J Shoulder Elbow Surg. 2000 Jan-Feb; 9 (1):6–11.
56. Vangsness CT Jr, Ennis M, Taylor JG, Atkinson R. Neural anatomy of the glenohumeral ligaments, labrum, and subacromial bursa. Arthroscopy. 1995 Apr; 11 (2):180–184.
57. Tibone JE, Fechter J, Kao JT. Evaluation of a proprioception pathway in patients with stable and unstable shoulders with somatosensory cortical evoked potentials. J Shoulder Elbow Surg. 1997 Sep-Oct; 6 (5):440–443.
58. Dempster WT. Mechanisms of shoulder movement. Arch Phys Med Rehabil. 1965 Jan; 46: 49–70.
59. Bearn JG. Direct observations on the function of the capsule of the sternoclavicular joint in clavicular support. J Anat. 1967 Jan; 101 (Pt 1):159–170.
60. Spencer EE Jr, Kuhn JE. Biomechanical analysis of reconstructions for sternoclavicular joint instability. J Bone Joint Surg Am. 2004 Jan; 86-A (1):98–105.
61. Fukuda K, Craig EV, An KN, et al. Biomechanical study of the ligamentous system of the acromioclavicular joint. J Bone Joint Surg Am. 1986 Mar; 68 (3):434–440.
62. Klimkiewicz JJ, Williams GR, Sher JS, et al. The acromioclavicular capsule as a restraint to posterior translation of the clavicle: a biomechanical analysis. J Shoulder Elbow Surg. 1999 Mar-Apr; 8 (2):119–124.
63. Inman V, Saunders JR, Abbott LC. Observations on the function of the shoulder joint. J Bone Joint Surg Am. 1944; 26: 1–31.
64. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. Clin Orthop Relat Res. 1944; 330: 3–12.
65. DePalma A. Degenerative Changes in the Sternoclavicular and Acromioclavicular Joints in Various Decades. Springfield, IL: Thomas; 1957.
66. Lucas D. Biomechanics of the shoulder joint. Arch Surg. 1973; 107: 425.
67. Colachis SC Jr, Strohm BR. Effect of suprascapular and axillary nerve blocks on muscle force in upper extremity. Arch Phys Med Rehabil. 1971 Jan; 52 (1):22–29.
68. McClure PW, Michener LA, Sennett BJ, Karduna AR. Direct 3-dimensional measurement of scapular kinematics during dynamic movements in vivo. J Shoulder Elbow Surg. 2001 May-Jun; 10 (3):269–277.
69. Bassett RW, Browne AO, Morrey BF, An KN. Glenohumeral muscle force and moment mechanics in a position of shoulder instability. J Biomech. 1990; 23 (5):405–415.
70. Hansen ML, Otis JC, Johnson JS, et al. Biomechanics of massive rotator cuff tears: implications for treatment. J Bone Joint Surg Am. 2008 Feb; 90 (2):316–325.
71. Howell SM, Kraft TA. The role of the supraspinatus and infraspinatus muscles in glenohumeral kinematics of anterior should instability. Clin Orthop Relat Res. 1991 Feb; 263: 128–134.
72. Doody SG, Freedman L, Waterland JC. Shoulder movements during abduction in the scapular plane. Arch Phys Med Rehabil. 1970 Oct; 51 (10):595–604.
73. Harryman DT, Walker ED, Harris SL, et al. Residual motion and function after glenohumeral or scapulothoracic arthrodesis. J Shoulder Elbow Surg. 1993; 2: 275–285.
74. Sugamoto K, Harada T, Machida A, et al. Scapulohumeral rhythm: relationship between motion velocity and rhythm. Clin Orthop Relat Res. 2002 Aug.(401):119–124.
75. Talkhani IS, Kelly CP. Movement analysis of asymptomatic normal shoulders: a preliminary study. J Shoulder Elbow Surg. 2001 Nov-Dec; 10 (6):580–584.
76. Browne AO, Hoffmeyer P, Tanaka S, et al. Glenohumeral elevation studied in three dimensions. J Bone Joint Surg Br. 1990 Sep; 72 (5):843–845.
77. Dvir Z, Berme N. The shoulder complex in elevation of the arm: a mechanism approach. J Biomech. 1978; 11 (5):219–225.
78. Jobe CM, Iannotti JP. Limits imposed on glenohumeral motion by joint geometry. J Shoulder Elbow Surg. 1995 Jul-Aug; 4 (4):281–285.
79. Stokdijk M, Eilers PH, Nagels J, Rozing PM. External rotation in the glenohumeral joint during elevation of the arm. Clin Biomech. 2003 May; 18 (4):296–302. (Bristol, Avon)
80. Codman EA. Rupture of the supraspinatus tendon. Clin Orthop Relat Res. 1911; 254: 23–26.
81. Kon Y, Nishinaka N, Gamada K, et al. The influence of handheld weight on the scapulohumeral rhythm. J Shoulder Elbow Surg. 2008 Nov-Dec; 17 (6):943–946.
82. Fayad F, Roby-Brami A, Yazbeck C, et al. Three-dimensional scapular kinematics and scapulohumeral rhythm in patients with glenohumeral osteoarthritis or frozen shoulder. J Biomech. 2008; 41 (2):326–332.